Introduction
Inflammatory bowel disease (IBD) is a chronic
inflammatory disease of the gastrointestinal tract, which includes
ulcerative colitis (UC) and Crohn's disease (CD). Due to the
increase of prevalence of IBD and the raised mortality during the
COVID-19 pandemic, IBD imposes a heavy economic and resource burden
on the society (1). Therefore, it
is imperative to explore the molecular mechanism of IBD and provide
a new direction for its treatment. Intestinal mucosal barrier
dysfunction is mainly manifested by increased intestinal mucosal
permeability, which is an important pathological characteristic
during the inflammatory process in IBD (2). Intestinal epithelial cells (IECs)
form this barrier to which they mainly contribute two parts:
Epithelial tight junction (TJ) proteins and the apical enterocyte
membrane. Elevation of proinflammatory cytokine production and
degradation of TJ proteins can lead to increased permeability in
the intestinal mucosa during IBD (3). In addition, contraction of the
intracellular actin cytoskeleton in the IECs can disrupt TJ
function between cells, which opens the intercellular space to
increase the permeability of the intestinal mucosa (4,5).
This process also requires myosin light chain (MLC) kinase (MLCK)
activity, which phosphorylates MLC (4,5).
Recent studies found that promoting mucosal healing is a novel
therapeutic strategy of UC (6,7).
The effects of 5-aminosalicyclic acid (5-ASA) on intestinal mucosal
healing remain controversial, since there have been marked
differences in outcomes among individuals (8). By contrast, salazosulfapyridine and
balsalazide have been previously demonstrated to improve intestinal
mucosal permeability (9).
Furthermore, although anti-TNF-α therapy has been reported to
confer promotional properties on mucosal healing, the specific
underlying mechanism remains unclear (10).
Aberrant TGF-β/Smad7 signaling may be important for
the pathogenesis of UC. In other cases, the TGF-β/Smad7 pathway has
been revealed to regulate MLCK expression (11–13). However, it remains unclear if the
TGF-β/Smad7 signaling pathway can regulate the expression of MLCK
and intestinal mucosal permeability in the small intestine
epithelium. Therefore, anti-TNF-α and 5-ASA were chosen in the
present study to investigate their effects on a mouse model of
dextran sulfate sodium (DSS)-induced colitis to observe their
influence on intestinal permeability and the possible underlying
mechanisms.
Materials and methods
Animals and reagents
A total of 32 specific pathogen-free grade C57BL/6J
mice (age, 8 weeks; weight, 18–22 g; male:female, 1:1) were
purchased from Shanghai SLAC Laboratory Animal Co., Ltd. They were
maintained under 20±2°C temperature, 50% humidity, 12-h light/dark
cycles and with tap water and a standard pellet diet. 5-ASA,
anti-MLCK antibodies (cat. nos. SAB1300116-100UG and
SAB1305415-40TST), FITC-dextran 4000 (FD-4; cat. no. 60842-46-8,
Evans blue (EB) and DSS were purchased from Sigma-Aldrich (Merck
KGaA). The Nanjing Jiancheng Biotechnology Institute supplied kits
for the detection of myeloperoxidase (MPO; cat. no. A044-1-1).
Anti-TNF-α (cat. no. AF-410-NA) was obtained from R&D Systems,
Inc. The MLCK ELISA kit (cat. no. ELA06754) was purchased from
RapidBio. Goat anti-rabbit IgG H&L (HRP; cat. no. ab205718) and
primary antibodies, including anti-zona occludens (ZO-1; cat. no.
ab216880), anti-E-cadherin (cat. no. ab76055), anti-occludin (cat.
no. ab168986), anti-TGF-β (cat. no. ab66043) and anti-Smad7 (cat.
no. ab90086) were purchased from Abcam. The RIPA buffer was
obtained from Beijing Solarbio Science & Technology Co., Ltd.
The SPI-PON 812 was purchased from SERVA Electrophoresis GmbH.
Chloral hydrate was obtained from Shanghai Jizhi Biochemical
Technology Co., Ltd. The protein concentration determination kit
(BCA reagent), 10% neutral formaldehyde solution, 1% osmium acid
and formamide were supplied by Beijing Solarbio Science &
Technology Co., Ltd., Wuxi Zhanwang Chemical Reagent Co., Ltd.,
Beijing Zhongjingkeyi Technology Co., Ltd., and Shanghai Sinopharm
Chemical Reagent Co., Ltd. The 25% glutaraldehyde solution (Johnson
Matthey) was made into a 2.5% glutaraldehyde solution with PBS
solution (pH 7.4) before use. Peroxidase blocking solution (cat.
no. ZLI-9311D) and the diaminobenzidine (DAB) Color kit (cat. no.
ZLI-9018) were purchased from Zsbio. Hematoxylin (cat. no. BA-4041)
was supplied by Baso. TBST buffer (0.05% Tween-20; cat. no. T1085)
was from Beijing Solarbio Science & Technology Co., Ltd.
Analytical grade methanol, formamide and isopropanol,
NaH2PO4 (Mw, 119.98), NaCl (Mw, 58.44),
phosphoric acid, glycerin, trichloroacetic acid, sulfosalicylic
acid, isopropanol and poly-lysine slides were provided by Sinopharm
Chemical Reagent Co., Ltd. Krebs solution consisted of 6.9 g NaCl,
0.35 g KCl, 0.28 g CaCl2, 2.1 g NaHCO3, 0.41
g MgSO4 and 0.16 g KH2PO4. In
total, 0.01 M PBS consisted of 8 g NaCl, 0.2 g KCl, 0.24 g
KH2PO4 and 1.44 g
NaH2PO4 dissolved in 800 ml distilled water
and adjusted to pH 7.4. The following instruments were applied in
the present study, including a light microscope (Olympus
Corporation), Ultra-violet spectrophotometer (752N; Shanghai
Precision Scientific Instruments Co., Ltd.), transmission electron
microscope (TEM; Hitachi, Ltd.), microplate reader (ELx800; BioTek
Instruments, Inc.) and a reverse transcription (RT)-PCR instrument
(LightCycler 480; Roche Diagnostics). The Ethics Committee of
Experimental Animals of Anhui Medical University (Hefei, China)
approved the present study (approval no. 20150044) and experiments
were conducted in accordance with laboratory animal management and
use guidelines.
Induction of DSS-colitis model
A mouse colitis model was induced by 5% (w/v) DSS
for 7 days of free drinking (14).
Experimental protocols
Mice were randomly classified into the normal,
DSS-treated, 5-ASA-treated and anti-TNF-α-treated groups
(n=8/group). Mice in the normal group did not receive DSS. The
5-ASA-treated and anti-TNF-α-treated groups were set as the
treatment groups. Anti-TNF-α was administered via intraperitoneal
injection at a dose of 5 mg/kg, once on day 1 and once on day 4
(15), whilst 5-ASA was
administered orally at a dose of 423 mg/kg/day (16). Mice in the normal and DSS-treated
groups were injected intraperitoneally with equivalent amounts of
normal saline daily. All groups were treated accordingly for 7
days.
Assessment of disease activity index
(DAI)
In total, two observers recorded the following
parameters daily: i) Body weight; ii) fecal blood; and iii)
consistency. The average daily DAI score per mouse was calculated
according to the standard method (14).
Assessment of inflammation
The mice were euthanized by cervical dislocation
following anesthesia with 5% chloral hydrate (0.1 ml/10 g). After
the use of chloral hydrate, the abdomen of the mice remained soft
with no significant resistance after touch. None of the mice showed
signs of peritonitis, pain or discomfort. After laparotomy, the
gross mucosal morphology of mouse colon was first examined before
two continuous pieces of the distal colon were collected. For
histological analysis, the present study used 10% neutral buffered
formalin to fix one piece of the colon at room temperature for 24 h
to maintain the original morphological structure of the cell,
followed by paraffin embedding for sections (thickness, 4 µm) and
finally H&E staining at room temperature for 17 min. The
severity of inflammation was assessed by light microscope in four
aspects using the histological index (HI) (14), with a total score range of 0–14.
The other piece of the colon tissue was homogenized for assessing
MPO activity (17).
TEM
A 0.5-cm distal ileal segment within 1 cm of the
ileocecal junction was fixed in 2.5% glutaraldehyde at 4°C for 6 h,
incubated in osmic acid at 4°C for 2 h and then embedded in SPI-PON
812 for 12 h at 45°C as the specimen for TEM.
Assessment of E-cadherin, ZO-1 and
occludin protein expression
Following isolation, 1 cm of the ileal tissue was
fixed in 10% neutral formalin and preserved in liquid nitrogen. The
expression of E-cadherin, occludin and ZO-1 in the ileal epithelial
cells was detected via immunohistochemistry (IHC).
Paraffin-embedded sections (4 µm) were incubated for 45 min at
60°C. Then the slices were deparaffinized in xylene, subsequently
dipped in alcohol. Sections were treated with hydrogen peroxide
(3%) at 37°C for 20 min, followed by incubation with goat serum
(10%; cat. no. G9023; Sigma-Aldrich; Merck KGaA) at 37°C for 30
min. The primary antibodies were added 37°C for 60 min and sections
were subsequently washed with PBS three times. The primary
antibodies included anti-E-cadherin (1:1,000; cat. no. ab76055;
Abcam), anti-zona occludens (ZO-1; 1:200; cat. no. ab216880; Abcam)
and anti-occludin (1:100; cat. no. ab168986; Abcam). The slices
were incubated with secondary antibodies (HRP labeled goat
anti-rabbit IgG H&L; 1:5,000; cat. no. ab205718; Abcam) at 37°C
for 20 min. Subsequently, the slices were exposed to
diaminobenzidine for 30 sec at 37°C, counterstained with
hematoxylin for 2 min at 37°C and viewed by light microscopy.
JD-801 system (Jiangsu Jetta Technology Development Co., Ltd.) was
used to evaluate the area and density of the staining areas, as
well as the integrated optical density (IOD) values of the IHC
sections. The intensity of protein staining in the image is
expressed as the mean densitometry (representing relative protein
expression level). Five fields were randomly selected, and the
signal density of tissue area was statistically analyzed by using
the blind method. To prevent false positives, negative control
groups with only secondary antibodies were included. Controls with
secondary antibodies were only performed while optimizing the
experiments, thus these pictures are not shown in the present
manuscript.
Intestinal permeability assay
According to a previously described method (18), EB and FD-4 were used to assay the
intestinal permeability. A 6-cm segment of the small intestine was
used as a sac by ligating both ends, before 0.2 ml 1.5% (w/v) EB
dissolved in PBS was injected into the sac. The sac was then
incubated in 20 ml Krebs buffer and removed after 30 min. The
intestinal lumen was rinsed with physiological saline until the
rinsing solution was clear and dried at 37°C for 24 h, before being
weighed to obtain the dry weight of the intestinal tissue and
finally incubated with formamide at 50°C for 24 h. Permeability of
the intestine was assessed using EB via Ultra-violet
spectrophotometer. The estimated wavelength of the dye eluting
amount was 655 nm and the amount of EB was calculated according to
the standard curve.
FD-4 was detected in vivo
The ileum of 6 cm was ligated at a distance of 2 and
8 cm from the ileocecal region after anesthesia and injected into
the cavity with 0.2 ml FD-4 solution, which consisted of PBS and 25
mg/kg FD-4. The portal vein blood (100 µl) was extracted after 30
min to determine the concentration of FITC in the plasma (18).
Assessment of MLCK enzymatic
activity
The intestinal mucosa homogenate was prepared at 4°C
from liquid nitrogen storage after adding extract buffer. MLCK
enzymatic activity in the small intestine homogenate was measured
according to the protocols of the ELISA kit.
Detection of MLCK via IHC
Intestinal mucosa specimens of the ileal were
collected, fixed with 10% formaldehyde overnight at room
temperature and embedded in paraffin for sectioning (thickness, 4
µm). The expression of MLCK protein in the ileal epithelial cells
was detected using the SP method of IHC. The experimental steps
were performed according to the kit protocols. The control group
was treated with PBS instead of the primary antibody. In total,
three visual fields were randomly selected from each slice under
the light microscope (magnification, ×40) and the distribution of
positive particles in the cells was observed under the light
microscope (magnification, ×200). To prevent false positives,
negative control groups with only secondary antibodies were
included. Controls with secondary antibodies were only performed
while optimizing the experiments, thus these images are not
included in the present manuscript.
Detection of MLCK, TGF-β and Smad7
expression via western blotting
The intestinal mucosa specimens of the ileal were
cut into pieces in an ice bath and treated with the RIPA buffer to
prepare the homogenate, frozen at −80°C and thawed three times to
fully release the MLCK, TGF-β and Smad7. The homogenate was then
centrifuged at 4°C for 15 min at 12,000 × g to extract the
supernatant. Protein concentration was determined. The extract was
then adjusted for protein concentration and mixed with the sample
buffer for 2X protein electrophoresis and boiled for 5 min.
Subsequently, the SDS-PAGE included 10% separated gel and 5%
concentrated gel. Equal amounts (50 µg) of samples were loaded into
each lane. After separation via SDS-PAGE, the samples were
transferred onto PVDF membranes, which were then blocked and
incubated with primary antibodies. After washing, the membranes
were incubated with secondary antibodies before exposure after
washing. BCA protein concentration determination kit was used to
determine the protein concentrations. The membranes were blocked at
room temperature in TBST (0.05% Tween-20) containing 5% BSA [cat.
no. A600903; Sangon Biotech (Shanghai) Co., Ltd.] for 1 h and
subsequently treated with primary antibodies overnight at 4°C:
anti-MLCK (1:1,000; cat. no. SAB1300116 and SAB1305415;
Sigma-Aldrich; Merck KGaA); anti-TGF-β (1:500; cat. no. ab66043;
Abcam); anti-Smad7 (1:1,000; cat. no. ab90086-Abcam). β-actin (cat.
no. 66009-1-Ig; Proteintech) was used as an endogenous control.
TBST buffer was used to rinse membranes, which were treated with
HRP labeled goat anti-rabbit IgG H&L (1:5,000; cat. no.
ab205718; Abcam) at 37°C for 1 h. The SuperSignal® West
Dura Extended Duration Substrate (cat. no. 34075; Thermo Pierce)
was used to visualize the protein bands, which were detected using
X-ray film (Huadong Medicine Co., Ltd.). ImageJ software version
1.8.0 (National Institutes of Health) was used to quantify the
protein expression. The experiments were repeated three times.
Assessment of mRNA expression of TGF-β
and Smad7
The mRNA expression levels of TGF-β and Smad7 mRNA
in the IECs of the ileal were detected via RT-quantitative (q)PCR.
RNA was extracted from the samples using TRIzol® (Thermo
Fisher Scientific, Inc.) to establish the RT-qPCR reaction system
and conditions. The samples were first mixed with TRIzol, placed in
static conditions and then centrifuged at 4°C for 15 min at 12,000
× g to obtain the supernatant. The supernatant was then mixed with
equal volumes of isopropanol and centrifuged at 4°C for 10 min at
12,000 × g to obtain the precipitate. These samples were washed
with 75% alcohol, dried, dissolved and frozen at −80°C. The
concentration, purity, quantity and quality of the RNA were all
determined. cDNA samples were prepared by reverse transcription.
The subsequent PCR reaction system consisted of 4 µl cDNA, 1.96 µl
10X PCR buffer, 2.4 µl MgCl2 (25 mM), 1 µl upstream
primer (20 pM), 1 µl downstream primer (20 pM), 0.36 µl dNTP (10
mM), 0.1 µl Taq DNA polymerase (5 IU/µl) and 10.08 µl deionized
water. cDNA samples were prepared by PrimeScript™ RT Master Mix
(Perfect Real Time; Takara; cat. no. RR036A) according to the
manufacturer's instructions. qPCR was conducted using the TB Green
Premix Ex Taq II (Tli RNaseH Plus; TAKARA; cat. no. RR820A) on a
StepOnePlus Real-Time PCR System (ABI). Thermocycling conditions
was as following: denaturation for 30 sec at 95°C; then 40 cycles
of 5 sec at 95°C and 30 sec at 60°C. The internal references was
GAPDH. The primer sequences included TGF-β, F
5′-GCGTGCTAATGGTGGAAAC-3′ and R 5′-CGGTGACATCAAAAGATAACCAC-3′;
Smad7, F 5′-AGAGGCTGTGTTGCTGTGAATC-3′ and R
5′-GCAGAGTCGGCTAAGGTGATG−3′; and GAPDH, F
5′-TGACTTCAACAGCGACACCCA−3′ and R 5′-CACCCTGTTGCTGTAGCCAAA−3′. All
experiments were performed in triplicate. The 2−ΔΔCq
method was used to calculate expressions of target genes (19).
Statistical analysis
Stata 16.0 software (StataCorp LP) was used to
analyze ordinal variables (DAI and HI) by using the Kruskal-Wallis
test followed by Dunn's post hoc test. SPSS 20.0 software (IBM
Corp.) was used to analyze the other data, and one-ANOVA followed
by Dunnett's post hoc test was performed to determine statistical
differences between the three groups compared with the DSS group.
All experiments were repeated three times. All results are
presented as the mean ± standard error of the mean. P<0.05 was
considered to indicate a statistically significant difference.
Results
General condition of the mice
In the DSS group, mice exhibited weight loss. At the
end of the experiment, there were different degrees of blood in
feces, the appearance of which was soft or thin-shaped. The DAI
scores gradually increased with time (Fig. 1A). The mice in the treatment
groups also showed weight loss, but the decrease was less than that
of DSS group (data not shown). Meanwhile, a few mice of the
treatment groups produced slightly bloody feces or positive fecal
occult blood test. DAI scores of mice in the treatment groups were
lower than DSS groups (P<0.05 at day 7; Fig. 1A).
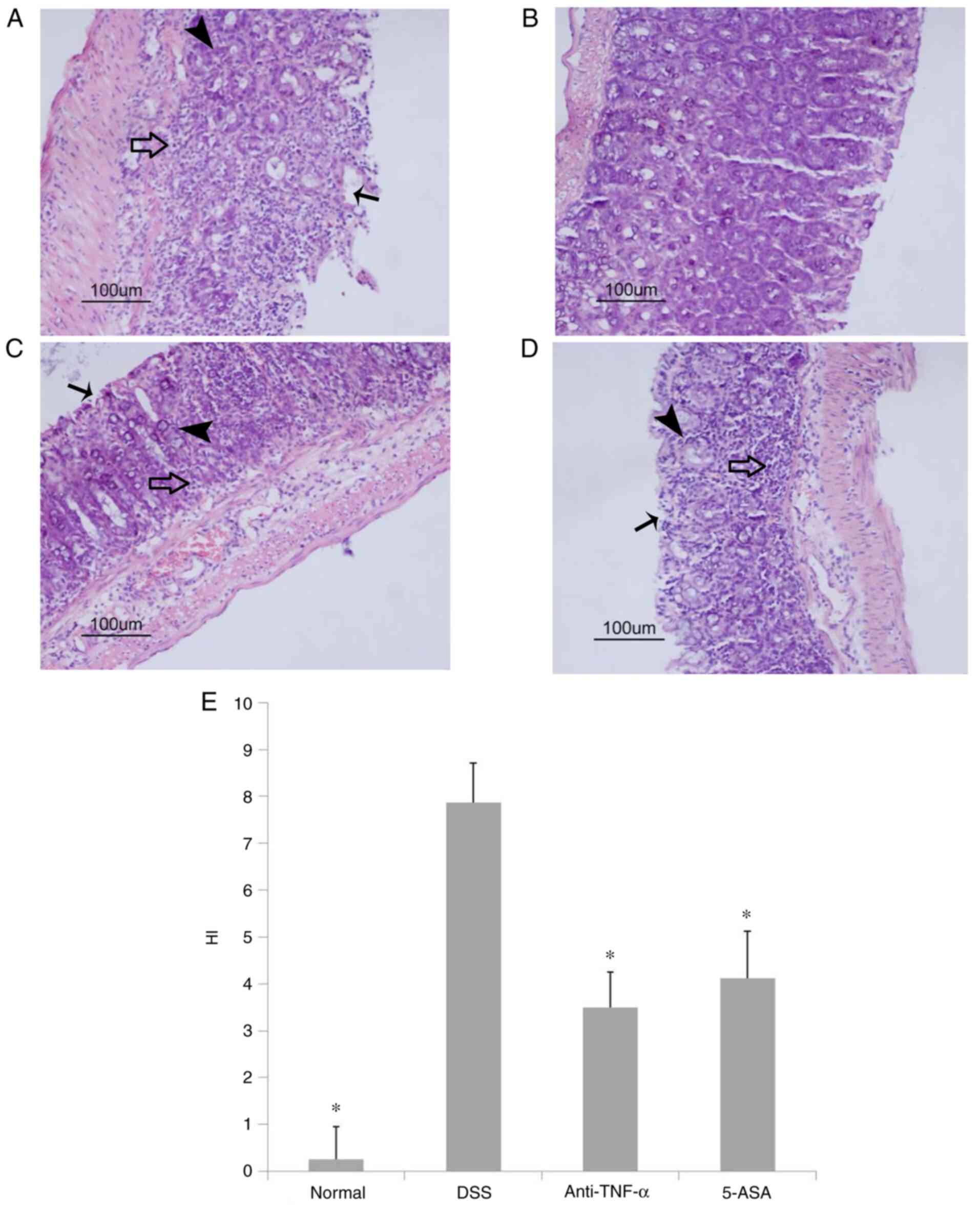
Gross observation and pathological
examination of the colonic tissue
The colon mucosa in the DSS group was characterized
by extensive hyperemia and edema. In addition, multiple erosion,
bleeding spots and superficial ulcers were observed (data not
shown). However, no obvious abnormalities could be found in tissues
from mice in the normal group. By contrast, only scattered
hyperemia and erosion were observed without obvious bleeding or
ulcers in treatment groups.
H&E pathological examination revealed that in
the DSS group, the colonic mucosa had multiple superficial ulcers,
where a large number of the crypt glands were destroyed with
extensive inflammatory cell infiltration (Fig. 2A). By contrast, in the normal
group the colonic IECs remained intact such that the crypt glands
were neatly arranged with no inflammatory cell infiltration
(Fig. 2B). In the two treatment
groups, colon mucosal tissues from only a few mice exhibited
superficial ulcers, where there were also signs of crypt gland
destruction compared with that of the normal group. However, the
extent of ulceration and crypt destruction was markedly lower
compared with that in the DSS group. Additionally, the degree of
infiltration by inflammatory cells in the mucosa and submucosa was
mild in treatment groups (Fig. 2C and
D). Compared with those in the DSS group, the HI scores in the
treated groups were significantly lower (P<0.05; Fig. 2E).
MPO activity in the colon
The activity of MPO in the colonic homogenates from
the DSS group was higher compared with that in the normal group,
whilst the MPO activity in the treatment groups was decreased
compared with that in the DSS group. This suggested that the degree
of colon inflammation in the DSS group was significant (P<0.05;
Fig. 1B), which could be
alleviated by anti-TNF-α and 5-ASA treatment (P<0.05; Fig. 1B).
Ultrastructure of the intestinal
mucosal barrier
TEM was used to observe the ultrastructure of the
IECs in the ileum of mice. It was observed that in the normal
group, the IECs within the tissue were intact, where the surface
microvilli were long and dense and the arrangement was regular. The
cells were also closely connected. However, in the DSS group, edema
or even shedding of IECs could be observed, which was accompanied
by atrophy and sparseness of the surface microvilli, enlargement of
the intercellular space and the opening of a number of TJs. In the
treatment groups, although edema of some IECs, reduction of the
number of microvilli and the opening of TJs could also be observed,
the severity was lower compared with that in the DSS group
(Fig. 3A-D).
Intestinal mucosal barrier
function
Compared with those in the normal group, the
intestinal EB content and plasma FITC levels in the DSS group were
significantly increased (P<0.05; Fig. 3E and F). This suggested that the
intestinal mucosal barrier in DSS group was damaged, which allowed
the high molecular weight EB to enter the intestinal mucosa through
the expanded TJs, which could also have resulted in the absorption
of FITC into the portal vein system. Anti-TNF-α and 5-ASA treatment
could significantly decrease the level of intestinal EB and plasma
FITC by varying degrees (P<0.05; Fig. 3E and F).
Protein expression of TJs and adhesion
junctions (AJs)
According to the results of IHC, it was found that
the expression of occludin, ZO-1 and E-cadherin in the small
intestinal mucosal epithelial cells of mice in the treated groups
were lower compared with those in the control group, but were
higher compared with those in the DSS group (Fig. 4,Fig.
5,6 and Table I).
 | Table I.Mean density of occludin, ZO-1,
E-cadherin and MLCK in different groups according to the results of
immunohistochemistry. |
Table I.
Mean density of occludin, ZO-1,
E-cadherin and MLCK in different groups according to the results of
immunohistochemistry.
| Groups | Occludin | ZO-1 | E-cadherin | MLCK |
|---|
| Normal | 0.331 | 0.429 | 0.321 | 0.333 |
| DSS | 0.295 | 0.300 | 0.192 | 0.370 |
| Anti-TNF-α | 0.316 | 0.336 | 0.283 | 0.348 |
| 5-ASA | 0.308 | 0.309 | 0.248 | 0.353 |
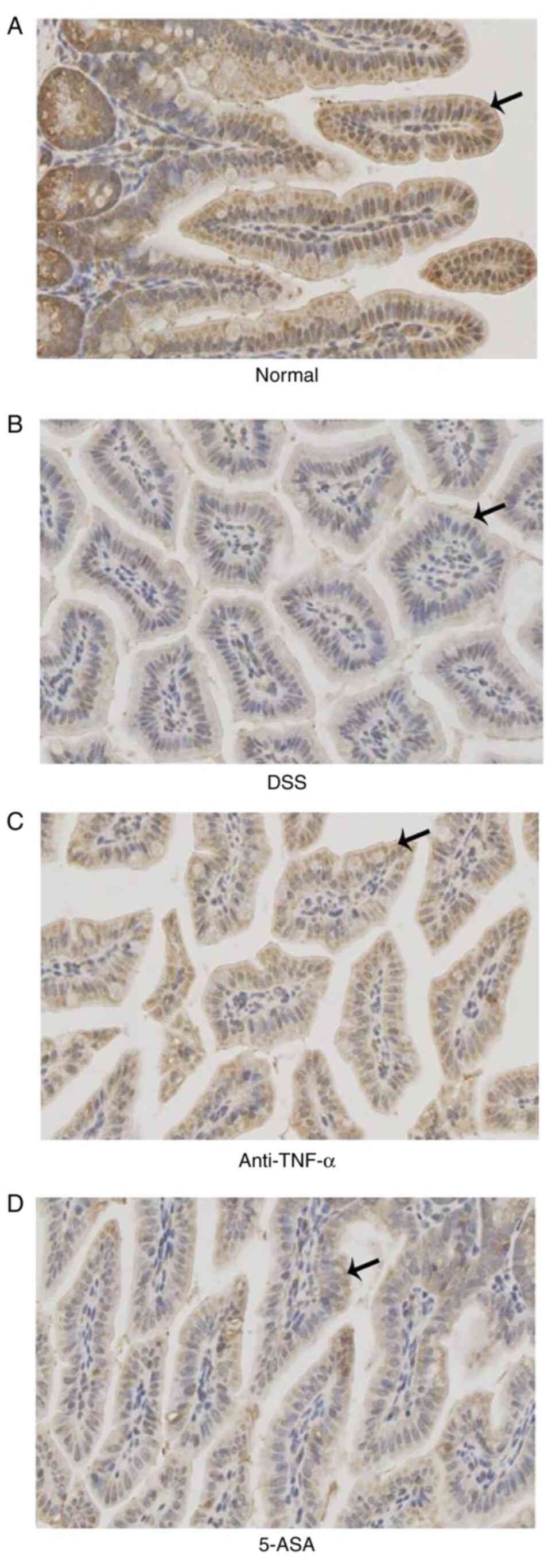
Expression, distribution and activity
of the MLCK protein
The expression and activity of the MLCK protein in
small intestinal mucosal epithelial cells were higher in the DSS
group compared with those in the normal group, but were lower in
the treated group compared with those in the DSS group (P<0.05;
Fig. 7). The results of IHC
(Fig. 7A and Table I) were consistent with those
observed following western blotting (Fig. 7B). Although there were significant
differences in MLCK expression and activity between the treatment
and DSS groups (P<0.05; Fig.
7C).
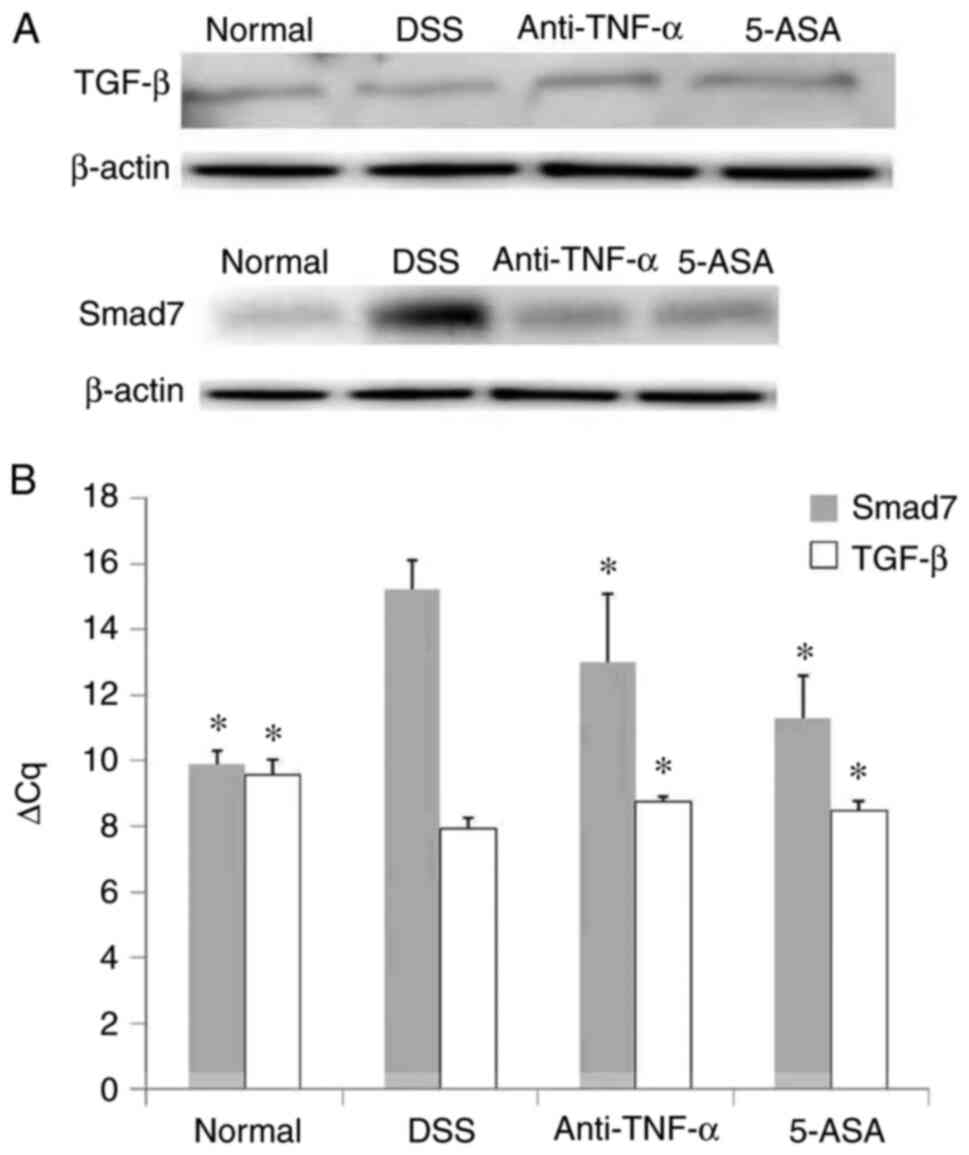
mRNA and protein expression of TGF-β
and Smad7
Compared with those in the normal group, the mRNA
and protein expression levels of TGF-β and Smad7 were decreased and
increased in the DSS group, respectively. Intervention with
anti-TNF-α and 5-ASA reversed the aforementioned effects mediated
by DSS (P<0.05, Fig. 8A and
B). The purity of TGF-β1 RNA was between 1.86–1.98, whereas the
concentration was 843.6–1421.6 µg/µl (data not shown). The purity
of Smad7 RNA was between 1.86–1.97, whilst the concentration was
821.3–1895.8 µg/µl.
Discussion
UC is a recurrent, non-specific inflammatory
disease. Intestinal mucosal barrier damage may be an important
cause for the onset and recurrence of UC (2). Previous studies on human samples and
animal models found that intestinal mucosal permeability increased
significantly before the manifestation of intestinal inflammation
(20,21).
In the present study, anti-TNF-α and 5-ASA were
found to improve the clinical symptoms of UC, reduce the DAI and
colonic mucosal HI scores and decrease MPO activity in mice with
DSS-induced UC, suggesting that anti-TNF-α and 5-ASA could
alleviate inflammatory injury in the colon. Subsequently, TEM was
used to observe the ultrastructure in the small intestine, and EB
and FITC were used to detect the permeability of the small
intestinal mucosa. Although TEM results suggested that anti-TNF-α
and 5-ASA could improve the structure and function of the small
intestinal mucosa, their specific mechanism remains unclear.
TJs are expressed at the top of the IECs, the
components of which include occludin, claudin, ZO and junction
adhesion molecule (JAM)-1 (22).
The AJ is a structure that lies adjacent to TJs, which includes
E-cadherin and β-catenin. In transgenic animal models, absence of
E-cadherin expression can result in the dysfunction of AJs during
the pathophysiological process of IBD (21). By blocking the adjacent
intercellular spaces within the intestinal epithelium, TJs can
prevent bacteria, antigens and other harmful substances from
entering the intestinal mucosal lamina propria to activate immune
cells (23). TJ assembly is
largely dependent on AJ formation (9,24).
Previously, it was found that a number of IBD-associated loci could
regulate the expression of E-cadherin and stability of AJs
(25,26), where it was subsequently confirmed
genetically that TJs and AJs both serve a role in intestinal
barrier function (25,26). In the present study, the
expression of occludin, ZO-1 and E-cadherin in the mucosal
epithelium of the small intestine of mice with DSS was decreased.
Consistent with these results from the present study, Clayburgh
et al (5) found that in
patients with IBD, TJs were significantly damaged, which increased
the permeability of the intestinal mucosa.
TNF-α treatment has been reported to lead to the
internalization and disruption of junctional proteins, such as
occludin and E-cadherin. Following 5-ASA pretreatment, membranous
localization of proteins of TJs and AJs was maintained (9). In addition, 5-ASA could increase
intercellular adhesion through the restoration of AJ proteins onto
the cell membrane, such as β-catenin and E-cadherin, in turn
promoting mucosal healing. 5-ASA can also alter the transcriptional
regulation of proteins, including JAMs, claudins and epithelial
cytoskeletal proteins (9). The
present study also found that 5-ASA increased the expression of
occludin, ZO-1 and E-cadherin in the intestinal mucosal epithelium
of DSS mice.
Anti-TNF-α has been documented to decrease
neutrophil infiltration in inflammatory mucosa of patients with IBD
and reduce the activity of T cells and inflammatory mediators
(27,28). In addition, anti-TNF-α can inhibit
neutrophils from producing proinflammatory mediators, including
reactive oxygen species, TNF-α and IL-8 (27,28). In particular, by binding to the
antibody, TNF-α receptor activation is blocked, leading to the
reduction of intestinal permeability due to the decrease in
paracellular permeability across the TJs and decreased apoptosis of
IECs (28). The present study
showed that anti-TNF-α increased the expression of occludin, ZO-1
and E-cadherin in the epithelium of small intestinal mucosa from
mice with DSS.
The degree of MLC phosphorylation depends on the
activity of MLCK (4). TNF-α has
been previously found to upregulate the distribution and expression
of NF-κB p65 (29–31). NF-κB can bind to the promoter
region of the MLCK gene to increase its transcription (31). By contrast, 5-ASA has been
reported to regulate intestinal epithelial homeostasis by
inhibiting the ERK1/2, Wnt/β-catenin and NF-κB signaling pathways,
whilst inducing cell cycle arrest (31). In addition, 5-ASA pretreatment was
revealed to alleviate the increase in NF-κB p65 mediated by TNF-α
(9). Blair et al (32) previously documented that the
expression of MLCK in IECs of 26 patients with IBD was increased
according to the results of an immunofluorescence assay. However,
since intestinal mucosal permeability was not simultaneously
measured, the potential association between MLCK and intestinal
mucosal barrier function could not be proven. In the present study,
the expression, distribution and activity of the MLCK protein in
IECs were also measured. Compared with that in the normal group,
the expression of MLCK in the DSS group was increased and the
activity was enhanced. Intervention with anti-TNF-α and 5-ASA could
decrease the expression and activity of MLCK, which could reduce
the permeability of the intestinal mucosa in mice with UC. However,
no significant difference between anti-TNF-α and 5-ASA could be
observed.
Aberrant TGF-β/Smad7 signaling may be an important
mechanism of IBD. In particular, increased expression of Smad7 and
the imbalance in the homeostasis between Smad7, Smad2 and Smad3 can
lead to the loss of the anti-inflammatory effects of TGF-β,
resulting in chronic inflammation in the intestinal tract during UC
(33). Abnormalities in TGF-β and
TGF-β receptor 2 are key to the pathogenesis of IBD. TGF-β can
maintain the integrity of the tissue structure by regulating the
proliferation and differentiation of T lymphocytes (33).
There is evidence that during the active stage of
IBD, the number of regulatory T (Treg) cells, which regulate
lymphocyte activity by secreting anti-inflammatory cytokines, such
as IL-10 and TGF-β, is lower compared with that in healthy
individuals (34). Treg cell
disorders, which are of importance to the development of various
diseases, can maintain the vicious cycle of inflammation and
disease aggravation, resulting in impaired barrier function
(35). Anti-TNF-α has been shown
to upregulate the number of Treg cells (28). In addition, Smad7 induced by TGF-β
is considered to be one of the key negative regulatory factors in
the TGF-β/Smad signal transduction pathway. Activated Smad7 can
inhibit the phosphorylation and activation of Smad2/3 by binding to
TGF-β receptor 1, in addition to accelerating its inactivation by
activating protein phosphatase PP1 and degradation by the ubiquitin
ligase SMAD-specific E3 ubiquitin protein ligase 2 (36). Furthermore, Smad7 can enter the
nucleus and block Smad2-3/Smad4 complex binding to target genes
(37). The level of Smad7
expression is the main regulatory factor for determining TGF-β
transcription (36). Knockdown of
Smad7 expression was found to enhance endogenous TGF-β signaling
(38). Increased expression of
TGF-β in the colon was previously shown in various murine colitis
models and patients with UC (39). Sedda et al (40) revealed that, although the
production of TGF-β1 is increased, deficiency in the TGF-β1/Smad
signaling pathway sustains the chronic inflammation of IBD.
However, in the present study, TGF-β was mainly studied in the
small intestine, which showed that TGF-β mRNA and protein
expression in the DSS group was reduced. Consistent with these
results, Vieira et al (41) also found a reduction in TGF-β
expression in the duodenum of mice with DSS.
A previous study reported that Smad7 inhibition in
patients with CD conferred endoscopic and clinical improvements
during phase 1 and 2 clinical trials (42). However, the corresponding phase 3
trial was suspended due to the lack of efficacy (43). A recent study has indicated that
rigorous experimental design assist in furthering the understanding
in the significance of Smad7 as a therapeutic target for IBD
(36). In addition, Wu-Mei-Wan is
a classic Chinese medicine for treating digestive diseases and
pervious study has shown that the antifibrotic effects of it may
result from the inhibition of the TGF-β/Smad pathway (44), whilst knocking down Smad7
expression was found to be beneficial in preventing the
post-operative recurrence of CD (45). Therefore, understanding the
molecular characteristics of IBD will assist in the identification
of novel candidates for the inhibition of Smad7. Nevertheless, the
mechanism of Smad7 in IBD remains to be fully elucidated (43,46).
In the present study, compared with that in the
normal group, Smad7 protein and mRNA expression was increased in
the IECs of mice with DSS. This was consistent with findings from
previous studies. Monteleone et al (47) showed that the levels of
phosphorylated Smad3 in lamina propria mononuclear cells in the
intestinal mucosa were significantly decreased, whereas Smad7
expression was markedly increased in patients with CD and UC
compared with those in the normal group. It has been suggested that
the overexpression of Smad7 can inhibit the TGF-β signaling
pathway, where inflammatory cytokines in the intestinal mucosa,
such as TNF-α and IFN-γ, can be continuously increased during IBD
(48). In addition, IFN-γ can
inhibit the TGF-β/Smad signaling pathway by upregulating the
expression of Smad7, whilst TNF-α can directly interfere with the
formation of the Smad2/3-Smad4 complex with DNA through the
inducible protein activator (49,50). However, Monteleone et al
(51) analyzed the expression of
Smad7 in the mucosal samples from patients with IBD and found that
Smad7 expression was increased at the protein levels, but not the
mRNA levels, suggesting the post-transcriptional regulation of
Smad7. The mucous membrane specimens of patients with UC in this
previous study were mainly taken from the colon, whilst the present
study was performed on the small intestine. Intervention with
anti-TNF-α and 5-ASA was demonstrated to increase the expression of
TGF-β whilst weakening Smad7, suggesting that both may regulate
MLCK activity through the TGF-β/Smad7 signaling pathway. This in
turn can alter the expression levels of TJ and AJ proteins in IECs
before finally regulating intestinal permeability.
To conclude, the present study evaluated the effects
of anti-TNF-α and 5-ASA on the TGF-β/Smad7 signaling pathway in a
mouse experimental model of colitis. Similar studies were performed
in the colon (40,52–57), peripheral blood (58) or in chronic pathologies (53,56), whereas the present experiment
studied the small intestine and acute response to injury. It was
found that anti-TNF-α and 5-ASA both improved the permeability of
the intestinal epithelium on this model of UC. The mechanism was
partly due to the increase in TGF-β expression or the decrease in
Smad7 expression, which may inhibit epithelial MLCK expression and
activity, leading to the reduction of intestinal mucosal
permeability in UC. The present study may provide novel evidence
for the treatment of IBD by either upregulating TGF-β expression or
downregulating Smad7 expression.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China (grant no. 81500403) and The Education
Department of Anhui (grant no. Y2016).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BB designed and performed the experiments and data
interpretation. HL performed experiments and data analysis. BB and
HL wrote and revised the manuscript. LH, YM and CH assisted in the
completion of animal experiments and some molecular biology
experiments. QM and XL designed and guided all experiments. JX
assisted in guiding experimental design and data analysis. BB, HL
and XL confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Experimental Animals of
Anhui Medical University (Hefei, China) approved the present study
(approval no. 20150044) and experiments were conducted in
accordance with laboratory animal management and use
guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kaplan GG and Windsor JW: The four
epidemiological stages in the global evolution of inflammatory
bowel disease. Nat Rev Gastroenterol Hepatol. 18:56–66. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ramos GP and Papadakis KA: Mechanisms of
disease: Inflammatory bowel diseases. Mayo Clin Proc. 94:155–165.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vergnolle N: Protease inhibition as new
therapeutic strategy for GI diseases. Gut. 65:1215–1224. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang S, Fu Y, Xu B, Liu C, Wang Q, Luo S,
Nong F, Wang X, Huang S, Chen J, et al: Wogonoside alleviates
colitis by improving intestinal epithelial barrier function via the
MLCK/pMLC2 pathway. Phytomedicine. 68:1531792020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clayburgh DR, Shen L and Turner JR: A
porous defense: The leaky epithelial barrier in intestinal disease.
Lab Invest. 84:282–291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li K, Marano C, Zhang H, Yang F, Sandborn
WJ, Sands BE, Feagan BG, Rubin DT, Peyrin-Biroulet L, Friedman JR
and De Hertogh G: Relationship between combined histologic and
endoscopic endpoints and efficacy of ustekinumab treatment in
patients with ulcerative colitis. Gastroenterology. 159:2052–2064.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ungaro R, Colombel JF, Lissoos T and
Peyrin-Biroulet L: A treat-to-target update in ulcerative colitis:
A systematic review. Am J Gastroenterol. 114:874–883. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rubin DT, Ananthakrishnan AN, Siegel CA,
Sauer BG and Long MD: ACG clinical guideline: Ulcerative colitis in
adults. Am J Gastroenterol. 114:384–413. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khare V, Krnjic A, Frick A, Gmainer C,
Asboth M, Jimenez K, Lang M, Baumgartner M, Evstatiev R and Gasche
C: Mesalamine and azathioprine modulate junctional complexes and
restore epithelial barrier function in intestinal inflammation. Sci
Rep. 9:28422019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scarallo L, Bolasco G, Barp J, Bianconi M,
di Paola M, Di Toma M, Naldini S, Paci M, Renzo S, Labriola F, et
al: Anti-tumor necrosis factor-alpha withdrawal in children with
inflammatory bowel disease in endoscopic and histologic remission.
Inflamm Bowel Dis. Apr 9–2021.(Epub ahead of print). PubMed/NCBI
|
|
11
|
Yang H, Zhang L, Weakley SM, Lin PH, Yao Q
and Chen C: Transforming growth factor-beta increases the
expression of vascular smooth muscle cell markers in human
multi-lineage progenitor cells. Med Sci Monit. 17:BR55–BR61. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu B, Zhai J, Zhu H and Kyprianou N:
Prohibitin regulates TGF-beta induced apoptosis as a downstream
effector of Smad-dependent and -independent signaling. Prostate.
70:17–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sinpitaksakul SN, Pimkhaokham A,
Sanchavanakit N and Pavasant P: TGF-beta1 induced MMP-9 expression
in HNSCC cell lines via Smad/MLCK pathway. Biochem Biophys Res
Commun. 371:713–718. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kihara N, de la Fuente SG, Fujino K,
Takahashi T, Pappas TN and Mantyh CR: Vanilloid receptor-1
containing primary sensory neurones mediate dextran sulphate sodium
induced colitis in rats. Gut. 52:713–719. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scheinin T, Butler DM, Salway F, Scallon B
and Feldmann M: Validation of the interleukin-10 knockout mouse
model of colitis: Antitumour necrosis factor-antibodies suppress
the progression of colitis. Clin Exp Immunol. 133:38–43. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu XC, Mei Q, Xu JM and Hu J: Balsalazine
decreases intestinal mucosal permeability of dextran sulfate
sodium-induced colitis in mice. Acta Pharmacol Sin. 30:987–993.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kannengiesser K, Maaser C, Heidemann J,
Luegering A, Ross M, Brzoska T, Bohm M, Luger TA, Domschke W and
Kucharzik T: Melanocortin-derived tripeptide KPV has
anti-inflammatory potential in murine models of inflammatory bowel
disease. Inflamm Bowel Dis. 14:324–331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X, Xu J, Mei Q, Han L and Huang J:
Myosin light chain kinase inhibitor inhibits dextran sulfate
sodium-induced colitis in mice. Dig Dis Sci. 58:107–114. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arrieta MC, Madsen K, Doyle J and Meddings
J: Reducing small intestinal permeability attenuates colitis in the
IL10 gene-deficient mouse. Gut. 58:41–48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martini E, Krug SM, Siegmund B, Neurath MF
and Becker C: Mend your fences: The epithelial barrier and its
relationship with mucosal immunity in inflammatory bowel disease.
Cell Mol Gastroenterol Hepatol. 4:33–46. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Buckley A and Turner JR: Cell biology of
tight junction barrier regulation and mucosal disease. Cold Spring
Harb Perspect Biol. 10:a0293142018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Otani T and Furuse M: Tight junction
structure and function revisited: (Trends in cell biology 30,
805–817, 2020). Trends Cell Biol. 30:10142020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rajasekaran AK, Hojo M, Huima T and
Rodriguez-Boulan E: Catenins and zonula occludens-1 form a complex
during early stages in the assembly of tight junctions. J Cell
Biol. 132:451–463. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mohanan V, Nakata T, Desch AN, Lévesque C,
Boroughs A, Guzman G, Cao Z, Creasey E, Yao J, Boucher G, et al:
C1orf106 is a colitis risk gene that regulates stability of
epithelial adherens junctions. Science. 359:1161–1166. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
UK IBD Genetics Consortium, ; Barrett JC,
Lee JC, Lees CW, Prescott NJ, Anderson CA, Phillips A, Wesley E,
Parnell K, Zhang H, et al: Genome-wide association study of
ulcerative colitis identifies three new susceptibility loci,
including the HNF4A region. Nat Genet. 41:1330–1334. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang C, Shu W, Zhou G, Lin J, Chu F, Wu H
and Liu Z: Anti-TNF-α therapy suppresses proinflammatory activities
of mucosal neutrophils in inflammatory bowel disease. Mediators
Inflamm. 2018:30218632018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Olesen CM, Coskun M, Peyrin-Biroulet L and
Nielsen OH: Mechanisms behind efficacy of tumor necrosis factor
inhibitors in inflammatory bowel diseases. Pharmacol Ther.
159:110–119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Al-Sadi R, Guo S, Ye D, Rawat M and Ma TY:
TNF-α modulation of intestinal tight junction permeability is
mediated by NIK/IKK-α axis activation of the canonical NF-κB
pathway. Am J Pathol. 186:1151–1165. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen S, Zhu J, Chen G, Zuo S, Zhang J,
Chen Z, Wang X, Li J, Liu Y and Wang P: 1,25-Dihydroxyvitamin D3
preserves intestinal epithelial barrier function from TNF-α induced
injury via suppression of NF-kB p65 mediated MLCK-P-MLC signaling
pathway. Biochem Biophys Res Commun. 460:873–878. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ye D and Ma TY: Cellular and molecular
mechanisms that mediate basal and tumour necrosis
factor-alpha-induced regulation of myosin light chain kinase gene
activity. J Cell Mol Med. 12:1331–1346. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Blair SA, Kane SV, Clayburgh DR and Turner
JR: Epithelial myosin light chain kinase expression and activity
are upregulated in inflammatory bowel disease. Lab Invest.
86:191–201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stolfi C, Troncone E, Marafini I and
Monteleone G: Role of TGF-beta and Smad7 in gut inflammation,
fibrosis and cancer. Biomolecules. 11:172020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maul J, Loddenkemper C, Mundt P, Berg E,
Giese T, Stallmach A, Zeitz M and Duchmann R: Peripheral and
intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel
disease. Gastroenterology. 128:1868–1878. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fan L, Qi Y, Qu S, Chen X, Li A, Hendi M,
Xu C, Wang L, Hou T, Si J and Chen S: B. adolescentis ameliorates
chronic colitis by regulating Treg/Th2 response and gut microbiota
remodeling. Gut Microbes. 13:1–17. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
de Ceuninck van Capelle C, Spit M and Ten
Dijke P: Current perspectives on inhibitory SMAD7 in health and
disease. Crit Rev Biochem Mol Biol. 55:691–715. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang S, Fei T, Zhang L, Zhang R, Chen F,
Ning Y, Han Y, Feng XH, Meng A and Chen YG: Smad7 antagonizes
transforming growth factor beta signaling in the nucleus by
interfering with functional Smad-DNA complex formation. Mol Cell
Biol. 27:4488–4499. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fantini MC, Rizzo A, Fina D, Caruso R,
Sarra M, Stolfi C, Becker C, Macdonald TT, Pallone F, Neurath MF
and Monteleone G: Smad7 controls resistance of colitogenic T cells
to regulatory T cell-mediated suppression. Gastroenterology.
136:1308–1316, e1-e3. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Olsen T, Rismo R, Cui G, Goll R,
Christiansen I and Florholmen J: TH1 and TH17 interactions in
untreated inflamed mucosa of inflammatory bowel disease, and their
potential to mediate the inflammation. Cytokine. 56:633–640. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sedda S, Marafini I, Dinallo V, Di Fusco D
and Monteleone G: The TGF-β/Smad system in IBD pathogenesis.
Inflamm Bowel Dis. 21:2921–2925. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vieira EL, Leonel AJ, Sad AP, Beltrão NR,
Costa TF, Ferreira TM, Gomes-Santos AC, Faria AM, Peluzio MC, Cara
DC and Alvarez-Leite JI: Oral administration of sodium butyrate
attenuates inflammation and mucosal lesion in experimental acute
ulcerative colitis. J Nutr Biochem. 23:430–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ardizzone S, Bevivino G and Monteleone G:
Mongersen, an oral Smad7 antisense oligonucleotide, in patients
with active Crohn's disease. Therap Adv Gastroenterol. 9:527–532.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Feagan BG, Sands BE, Rossiter G, Li X,
Usiskin K, Zhan X and Colombel JF: Effects of mongersen (GED-0301)
on endoscopic and clinical outcomes in patients with active Crohn's
disease. Gastroenterology. 154:61–64.e6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu F, Shao Q, Hu M, Zhao Y, Dong R, Fang
K, Xu L, Zou X, Lu F, Li J and Chen G: Wu-Mei-Wan ameliorates
chronic colitis-associated intestinal fibrosis through inhibiting
fibroblast activation. J Ethnopharmacol. 252:1125802020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zorzi F, Calabrese E, Di Fusco D, De
Cristofaro E, Biancone L, Casella S, Palmieri G and Monteleone G:
High Smad7 in the early post-operative recurrence of Crohn's
disease. J Transl Med. 18:3952020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Izzo R, Bevivino G, De Simone V, Sedda S,
Monteleone I, Marafini I, Di Giovangiulio M, Rizzo A, Franzè E,
Colantoni A, et al: Knockdown of Smad7 with a specific antisense
oligonucleotide attenuates colitis and colitis-driven colonic
fibrosis in mice. Inflamm Bowel Dis. 24:1213–1224. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Monteleone G, Kumberova A, Croft NM,
McKenzie C, Steer HW and MacDonald TT: Blocking Smad7 restores
TGF-beta1 signaling in chronic inflammatory bowel disease. J Clin
Invest. 108:601–609. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Barreiro-de Acosta M, Lorenzo A, Mera J
and Dominguez-Muñoz JE: Mucosal healing and steroid-sparing
associated with infliximab for steroid-dependent ulcerative
colitis. J Crohns Colitis. 3:271–276. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wen FQ, Liu X, Kobayashi T, Abe S, Fang Q,
Kohyama T, Ertl R, Terasaki Y, Manouilova L and Rennard SI:
Interferon-gamma inhibits transforming growth factor-beta
production in human airway epithelial cells by targeting Smads. Am
J Respir Cell Mol Biol. 30:816–822. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Schiffer M, von Gersdorff G, Bitzer M,
Susztak K and Böttinger EP: Smad proteins and transforming growth
factor-beta signaling. Kidney Int Suppl. 77:S45–S52. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Monteleone G, Del Vecchio Blanco G,
Monteleone I, Fina D, Caruso R, Gioia V, Ballerini S, Federici G,
Bernardini S, Pallone F and MacDonald TT: Post-transcriptional
regulation of Smad7 in the gut of patients with inflammatory bowel
disease. Gastroenterology. 129:1420–1429. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Troncone E, Marafini I, Stolfi C and
Monteleone G: Involvement of Smad7 in inflammatory diseases of the
gut and colon cancer. Int J Mol Sci. 22:39222021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Latella G: Redox imbalance in intestinal
fibrosis: Beware of the TGFβ-1, ROS, and Nrf2 connection. Dig Dis
Sci. 63:312–320. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cui Y, Zhu C, Ming Z, Cao J, Yan Y, Zhao
P, Pang G, Deng Z, Yao Y and Chen Q: Molecular mechanisms by which
casein glycomacropeptide maintains internal homeostasis in mice
with experimental ulcerative colitis. PLoS One. 12:e01810752017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Laudisi F, Dinallo V, Di Fusco D and
Monteleone G: Smad7 and its potential as therapeutic target in
inflammatory bowel diseases. Curr Drug Metab. 17:303–306. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Speca S, Rousseaux C, Dubuquoy C, Rieder
F, Vetuschi A, Sferra R, Giusti I, Bertin B, Dubuquoy L, Gaudio E,
et al: Novel PPARγ modulator GED-0507-34 levo ameliorates
inflammation-driven intestinal fibrosis. Inflamm Bowel Dis.
22:279–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Marafini I, Zorzi F, Codazza S, Pallone F
and Monteleone G: TGF-beta signaling manipulation as potential
therapy for IBD. Curr Drug Targets. 14:1400–1404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yamashita A, Inamine T, Suzuki S, Fukuda
S, Unoike M, Kawafuchi Y, Machida H, Isomoto H, Nakao K and
Tsukamoto K: Genetic variants of SMAD2/3/4/7 are associated with
susceptibility to ulcerative colitis in a Japanese genetic
background. Immunol Lett. 207:64–72. 2019. View Article : Google Scholar : PubMed/NCBI
|