Introduction
Gastric cancer is one of the most common cancers
worldwide (1). Even though
Helicobacter pylori (H. pylori) infection is known to be the
major risk factor for the development of gastric cancer, H.
pylori eradication does not completely prevent gastric cancer,
and other genetic or environmental factors might influence gastric
cancer development (1–3).
Bile acids are cholesterol derivate and are required
for absorption and transport of fat (2). Bile acids exist in enterohepatic
organs such as the liver, gall bladder, and intestine, which
contain high levels of bile acids. We frequently observed
considerable bile colored fluid in the stomach of patients who had
undergone gastric surgery, cholecystectomy, or sphincterotomy, and
healthy controls with bile reflux who had no history of gastric
surgery. Recently, several clinical studies have suggested that
bile reflux is associated with premalignant gastric lesions such as
atrophic gastritis and intestinal metaplasia (3,4).
Furthermore, bile acid receptors, including G-protein-coupled bile
acid receptor 1 (TGR5) and farnesoid X receptor (FXR), have been
known to be expressed in the mucosa of patients with Barrett's
esophagus, esophageal adenocarcinoma, and advanced gastric cancer
(5–7). Acidified bile acids also activated
the transcription factor c-Myc, which is associated with tumor
progression and telomerase activity (2). However, the association between
intragastric bile acid and patients with early gastric cancer (EGC)
remains unelucidated. In addition, there is limited information
about the effects of bile acids on the molecular change in gastric
epithelial cells. Early growth response factor-1 (Egr-1) is a
transcription factor, which has been known to be implicated in
biological process including tissue injury, immune response, and
fibrosis. It is also related to the inflammation, cell
proliferation, cell differentiation, and initiation and progression
of cancer (8). Egr-1 can be
activated through stimuli by growth factors, tumor necrosis
factors, inflammatory factors, reactive oxygen species, and
bacteria such as H. pylori (9). Until now, there is limited
information whether bile acids activate Egr-1 in gastric epithelial
cells and which molecular mechanism is involved in the activation
of Egr-1 by bile acids.
In this study, we aimed to investigate the effect of
bile acids on the activation of Egr-1 and the related molecular
change in gastric epithelial cells and to evaluate the difference
in gastric bile acid concentration between controls and patients
with early gastric cancer.
Materials and methods
Cell culture
For our purposes, we required well-established,
acid-stable gastric cancer cell lines with comparable levels of
c-Myc expression (10).
Accordingly, we purchased AGS (ATCC®
CRL-1739™) and NCI-N87 (ATCC®
CRL-5822™) cell lines from the American Type Culture
Collection (Manassas, VA, USA). These gastric cancer cell lines
were grown in Dulbecco's modified Eagle's medium (DMEM) (GIBCO
Invitrogen) containing 4.5 mg/l glucose, 100 mg/l streptomycin, and
2 mM L-glutamine supplemented with 10% fetal bovine serum (FBS)
(GIBCO Invitrogen). They were maintained at 37°C under a humidified
5% CO2 atmosphere in a CO2 incubator (Sanyo).
Solutions of bile acids (Sigma-Aldrich) were prepared using
appropriate solvents according to the manufacturer's protocols
(Table SI). AGS and NCI-N87
cells were cultured in the growth medium for 24 h and then
transferred to fresh, serum-free medium containing 100 µM of a bile
acid for 48 h, with the bile acid being cholic acid (CA;
Sigma-Aldrich, C9377), chenodeoxycholic acid (CDCA; Sigma-Aldrichl,
C1129), taurocholic acid (TCA; Sigma-Aldrich, T4009), or
glycochenodeoxycholic acid (GCDCA; Sigma-Aldrich, G0759).
Afterward, we extracted the total RNA and total protein from the
cells.
Western blotting
We extracted proteins from the gastric cells treated
with bile acids using a radioimmunoprecipitation assay buffer
(#R0278, Sigma-Aldrich) containing protease and phosphatase
inhibitors (#p8340 and #p2850, respectively; Sigma-Aldrich).
Proteins were separated by 10% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis, then blotted onto PVDF
membranes (Millipore) according to the manufacturer's protocol. We
then incubated the PVDF transfer membranes at 4°C overnight in
diluted solutions of primary antibodies: anti-phospho-AKT (#9271),
anti-AKT (#9272), anti-phospho-p42/44-MAPK (#9106),
anti-p42/44-MAPK (#4695), anti-Egr-1 (#4154), anti-β-actin (#4967),
anti-c-Jun (#9165), HRP-linked anti–rabbit IgG (#7074), and
HRP-linked anti-mouse IgG (#7076) (Cell Signaling Technology,
Inc.). We analyzed proteins using the Fujifilm LAS-3000 imaging
system (Fujifilm). The fold change in protein expression was
calculated by dividing the normalized signal intensity of the
target band in the experimental sample by that of the target band
in the control sample.
mRNA quantitation
Total RNA was extracted using TRIzol (Takara Bio
Inc.). Briefly, 1 ml of Trizol solution was added into each well
and the suspension was then moved to a 1.5 ml tube. After adding
200 µl of chloroform (Sigma-Aldrich Co. LLC) and vortex-mixing for
15 sec, the mixture was centrifuged at 20,000 × g for 20 min. The
supernatant was then collected and mixed with equal amounts of
99.9% isopropyl alcohol (Merck), followed by centrifugation at
20,000 × g for 20 min. The pellet was washed with 1 ml of 70% ethyl
alcohol (MERCK, Co. LLC), followed by centrifugation at 20,000 × g
for 5 min. After removing the remaining ethyl alcohol, the RNA
pellet was air dried at a 25°C. It was then resuspended in 50 µl of
diethyl pyrocarbonate water. Total RNAs were converted to cDNAs
using reverse transcription system (Promega Corporation). Real-time
PCR was performed with Applied Biosystems StepOnePlus™
Real time PCR System (Life Technologies Corporation) according to
the manufacturer's protocol. Glyceraldehyde 3-phosphate
dehydrogenase was used as a control, and ΔΔCT values were
calculated for cancer stem cell markers using the Taqman assay
probes (Twist1, HS01675818_s1; c-Myc, HS00153408_m1; c-Jun,
HS01103582_s1; Snail, HS00195591_m1; Thermo Fisher Scientific,
Inc.).
Subjects
A total of 39 subjects [20 controls and 19 EGC
patients] were enrolled in this study. We excluded patients with
secondary bile reflux, defined as bile reflux after gastric
surgery, patients with previous diagnosis of malignancy and
patients with taking any medicines which might affect bile acid
secretion or gastrointestinal motility such as steroid,
prokinetics, lipid lowering agents, bile acid sequestrants,
urodexoycholic acid and chenodexoycholic acid. This study was
approved by our institutional review board (CNUH-2020-085). We
explained the terms of participation in this study and obtained
written informed consent from patients before endoscopic
procedures.
Collection of gastric fluid
All endoscopic procedures were performed without
foaming mucus remover or antispasmotics by an experienced
endoscopist (SYP) in the early morning. Subjects were fasted for 12
h. First, we used distilled water to flush the endoscope clean; the
gastric fluid in the fundus and greater curvature of the gastric
body were aspirated into sterile collection traps immediately when
the endoscope was introduced into the stomach. The collected fluid
specimens were immediately cryopreserved at −80°C.
Measurement of bile acids by liquid
chromatography with tandem mass spectrometry
We analyzed the gastric juice using a mass
spectrometer API 4000Q TRAP (AB Sciex), First, we diluted the
gastric juice 20- to 200-fold using distilled water. Then, diluted
100 µl of the gastric juice was mixed with an internal standard
solution (CA-d5 ng/ml in 50% methanol). The mixed solution was then
vortex-mixed for 3 sec, 200 µl of acetonitrile was added, and then
centrifuged at 20,000 × g for 2 min. We injected 20 µl of the
diluted supernatant that was diluted with 180 µl of 20 mM ammonium
acetate. We used the standard component of Sigma-Aldrich C9377,
G0759, C1129, T4009, L6250, D2510 for CA, CDCA, TCA, GCDCA,
lithochholic acid (LCA), and deoxycholic acid (DCA) (Sigma-Aldrich
Co. LLC). We analyzed LC-MS/MS data of each bile acid by the
Analyst software version 1.6.3 (AB Sciex Pte. Ltd.).
Diagnosis of H. pylori infection and
histology
Subjects were considered to be infected with H.
pylori if the results of at least one of four diagnostic tests
[rapid urease test, histologic results, H. pylori polymerase
chain reaction (PCR), and (13C.)-urea breath test] were positive.
All biopsy and resected specimens were evaluated for background
histology and tumor histology based on the Vienna classification
system by an expert pathologist (CYD) (11).
Statistical analysis
Statistical analysis was performed using SPSS
version 23.0 (SPSS, Inc.). Continuous data are shown as mean ±
standard deviation or median (interquartile range, IQR), while
categorical data are shown as absolute and relative frequencies.
Categorical data were examined using Fisher's exact test or the
chi-squared test. Variables with a skewed distribution were
performed using non-parametric tests (Mann-Whitney test, Kruskal
Wallis test) and Spearman non-parametric test. For adjustment of
variables, binary logistic regression models with enter were used
to evaluate the association between the levels of each bile acid
and EGC. The data in regression analysis were shown as adjusted
odds ratios (aOR) with 95% confidence interval (CIs). P<0.05 was
considered to indicate a statistically significant difference.
Results
Primary bile acids upregulate Egr-1
expression and oncogenes by modulating p42/44 MAPK signaling in
human gastric cancer cells
To investigate the effects of bile acids on the
expression of the bile acid receptor TGR5 and the transcription
factor Egr-1, human gastric cancer cells (AGS and NCI-N87) were
treated for 48 h with 100-µM solutions of several unconjugated and
conjugated primary bile acids, and the expression levels of TGR5
and Egr-1 were determined by western blotting. In AGS cells, Egr-1
expression was increased by both unconjugated and conjugated
primary bile acids, but in NCI-N87 cells, it was increased only by
unconjugated primary bile acids (CA and CDCA; Fig. 1A). In contrast, bile acids did not
induce TGR5 overexpression in AGS or NCI-N87 cells. Of all the bile
acids, CDCA was associated with the most significant increase in
Egr-1 expression in both AGS and NCI-N87 cells. Therefore, CDCA was
selected for further experiments, as presented in Figs. 1 and 2.
 | Figure 1.Upregulation of Egr-1 and oncogenes by
primary bile acids. (A) Egr-1 and TGR-5 protein expression levels
were measured in AGS and NCI-N87 cells using western blotting. CA
and CDCA increased the Egr-1 expression but did not affect the
TGR-5 expression in human gastric cancer cells. P-p42/44MAPK, AKT
and p38MAPK protein expression levels were measured in (B) AGS and
(C) NCI-N87 cells using western blotting. P-p42/44MAPK, AKT and
p38MAPK protein expression levels were measured in (D) AGS and (E)
NCI-N87 cells using quantitative reverse-transcription PCR. CDCA
significantly increased (>2-fold) the phosphorylation of
p42/44MAPK, AKT and p38MAPK in AGS and NCI-N87 cells. Fold change
in protein expression was calculated by dividing the normalized
signal intensity of the target band in the experimental sample by
that of the target band in the control sample. *P<0.05,
**P<0.01, and ***P<0.001. Egr-1, early growth response factor
1; TGR-5, G-protein coupled bile acid receptor 1; CA, cholic acid;
CDCA, chenodeoxycholic acid; p-, phosphorylated; t-, total; TCA,
taurocholic acid; GCDCA, glycochenodeoxycholic acid. |
To investigate the effect of bile acids on the
AKT-MAPK signaling activation, AGS and NCI-N87 were treated with
100 µM of CDCA for 48 h. Treatment with CDCA stimulated
phosphorylation of p42/44 MAPK, AKT, and p38 MAPK in both gastric
cancer cell lines. We also identified the upregulation of c-Jun and
c-Myc in AGS with 100 µM of CDCA for 48 h (Fig. 1B-E).
To determine the signaling pathway by which CDCA
induced Egr-1 expression and upregulation of c-Jun and c-Myc,
signaling inhibitors of p42/44 MAPK (PD98509) were used. As shown
in Fig. 2, the expression of
Egr-1 was decreased by inhibitors of p42/44 MAPK, while the
inhibitor of p38 MAPK did not affect the expression of Egr-1 (data
not shown). These results suggest that the CDCA-induced
upregulation of Egr-1 was mediated through the p42/44MAPK signaling
pathway. Likewise, p42/44 MAPK inhibitors in AGS cells
downregulated CDCA-induced expression of c-Jun and c-Myc (all
P<0.05). In NCI-N87 cells, the expression of c-Myc was decreased
by p42/44 MAPK inhibitors (P<0.05, Fig. 2E and F).
Bile acids in gastric fluid
Baseline characteristics and
measurement of bile acids of subjects
A total of 39 subjects were enrolled in this study.
The purposes of endoscopic procedures were as follows: endoscopic
surveillance or evaluation of dyspepsia in 20 controls and
endoscopic resection, such as endoscopic mucosal resection or
endoscopic submucosal dissection for EGC in 19 patients. The median
age was 65 years (range, 24–85), with 26 males. H. pylori
infection was in 43.6% (17/39) patients. There was a significant
difference in age and histologic background of underlying gastric
mucosa (presence of atrophic gastritis with intestinal metaplasia)
among controls and patients with EGC (both P<0.05). However,
there were no difference in gender, hypertension, diabetes and
H. pylori status between 2 groups (Table SII).
We measured the concentration of bile acids from the
gastric fluid. The levels of conjugated primary bile acids were
higher than those of unconjugated primary bile acids (Fig. S1). The TCA level (median 1.71
µg/ml, IQR 0.00 µg/ml ~17.70 µg/ml) was higher than that of CA
(median 0.02 µg/ml, IQR 0.0 µg/ml ~0.17 µg/ml, P<0.001), and the
GCDCA level (median 2.36 µg/ml, IQR 0.00 µg/ml ~47.96 µg/ml) was
higher than that of CDCA (median 0.00 µg/ml, IQR 0.00 µg/ml ~0.22
µg/ml, P<0.001, Fig. S1). The
levels of secondary bile acids were lower than those of primary
bile acids (P=0.001 for CA and DCA, P<0.001 for CDCA and LCA).
The CA level correlated with CDCA level (rho=0.831, P<0.001) and
the TCA level correlated with GCDCA level (rho=0.967,
P<0.001).
Difference in bile acids from the
gastric fluid in controls and patients with early gastric
cancer
There were significant differences in CA, TCA, CDCA,
GCDCA, and DCA levels between controls and patients with EGC (all
P<0.05, Fig. 3). After
adjustment for age and background histology (presence of atrophic
gastritis with intestinal metaplasia), the levels of CA (aOR 4.3,
95% CI: 1.2~16.2, P=0.029) and GCDCA (aOR 9.9, 95% CI: 1.3~75.3,
P=0.027) were significantly higher in patients with EGC than in
controls.
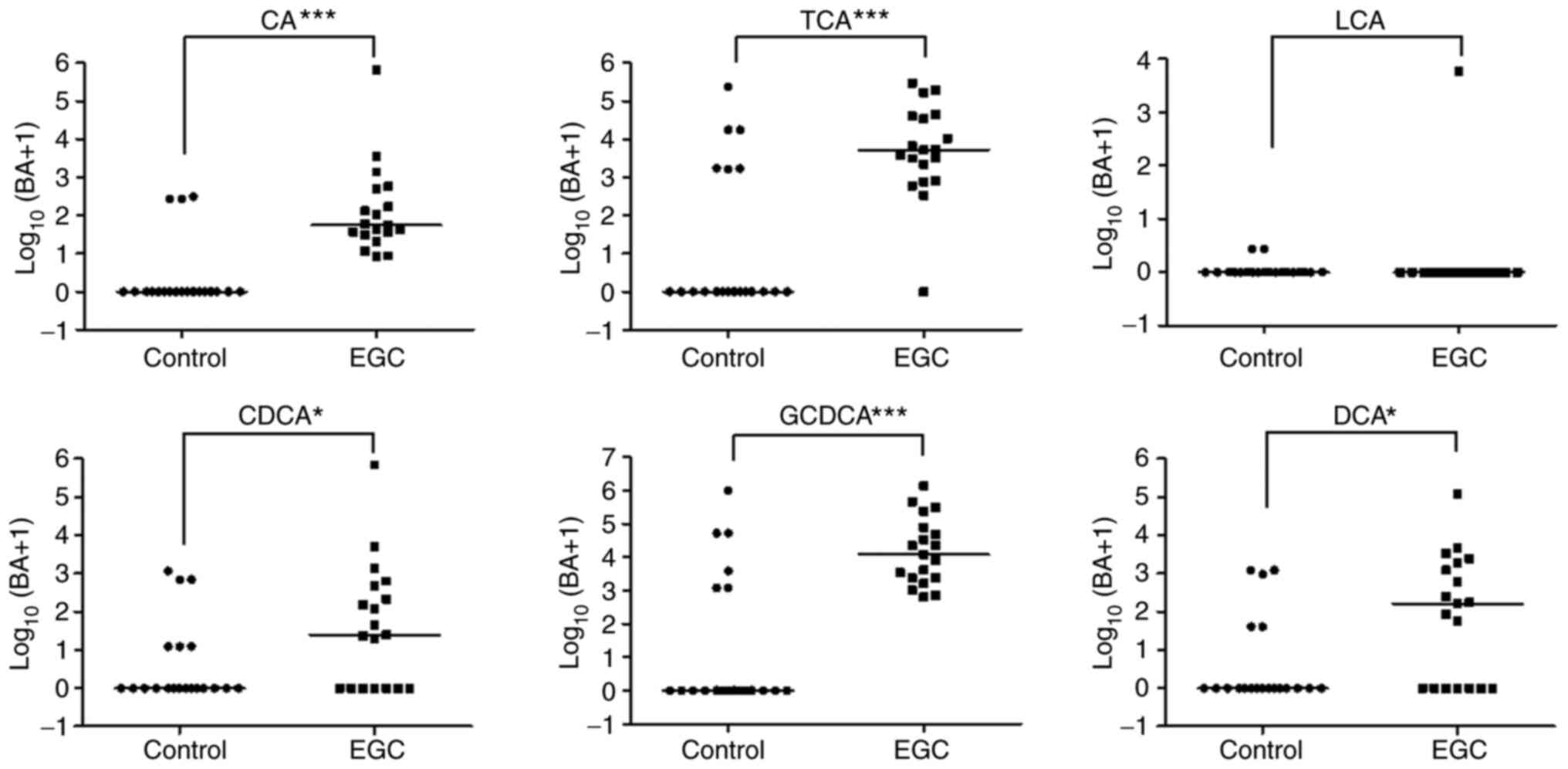 | Figure 3.Gastric bile acids concentration in
controls and patients with EGC. Concentrations of CA, TCA, CDCA,
GCDCA and DCA in controls and patients with EGC measured using
liquid chromatography with tandem mass spectrometry. *P<0.05 and
***P<0.001. BA, bile acids; CA, cholic acid; TCA, taurocholic
acid; LCA, lithocholic acid; CDCA, chenodeoxycholic acid; GCDCA,
glycochenodeoxycholic acid; DCA, deoxycholic acid; LGD, low grade
dysplasia; EGC, early gastric cancer. |
Discussion
In this study, we found that bile acid induced the
upregulation of Egr-1 and oncogenes through MAPK signaling in
gastric cancer cells. We also identified the presence of several
primary and secondary bile acids in human gastric fluid, with
gastric levels of primary bile acids (both conjugated and
unconjugated) being higher in patients with EGC than in
controls.
Bile acid has been known to be associated with the
pathomechanism of gastric carcinogenesis. Several studies suggested
the involvement of bile acid receptors in gastric carcinogenesis.
Cao et al showed that TGR5 was overexpressed in gastric
intestinal-type adenocarcinomas and was associated with poor
prognosis in gastric cancer patients (5,12).
Yu et al suggested that FXR was associated with Caudal type
homeobox 2 (CDX2) and Mucin 2 (MUC2) expression, leading to gastric
intestinal metaplasia (7). We
identified the expression of TGR5 in gastric cancer cells in
vitro. However, bile acids did not promote the expression of
TGR5 in gastric cancer cell lines. Instead, we identified the
overexpression of Egr-1 by the bile acid in gastric cancer cells.
Until now, there is little information about the involvement of
Egr-1 induced by bile acids in gastric carcinogenesis. A previous
study demonstrated that Egr-1 was overexpressed in precancerous
lesions of the stomach (13).
Egr-1 has been implicated in biological processes, including
inflammation, cell proliferation, cell differentiation, and cancer
progression (8). In our study,
the upregulation of Egr-1 in gastric cancer cells in vitro
by bile acids require MAPK signaling, not the activation of TGR5.
Allen et al also demonstrated that the activation of MAPK
signaling is required for the upregulation of Egr-1 by bile acids
in cholestasis liver injury models (14). This study also showed that primary
bile acids increased the expression levels of the c-Myc and c-Jun
genes through MAPK signaling in gastric cancer cell lines, which
were involved in the initiation and development of gastric cancer
(15,16). Our results suggest that continuous
exposure of gastric epithelial cells to primary bile acids may be a
factor in gastric carcinogenesis. Future research are needed to
know the roles of bile acids in other gastric cancer cell lines
with variable characteristics.
Recent studies used the concentration of total bile
acids or each bile acid component to evaluate bile reflux status
(3,17,18). We measured the levels of variable
bile acids in the stomach. The levels of primary bile acids were
higher than those of secondary bile acids. Among primary bile
acids, conjugated bile acids were more abundant than unconjugated
bile acids, consistent with a recent study (17). In addition, the levels of bile
acids were correlated to each other.
Several studies reported that bile reflux was
associated with reflux esophagitis, the proliferation of esophageal
squamous cells, Barrett's adenocarcinoma, and intestinal metaplasia
in the cardia (19,20). Moreover, recent studies suggested
the association between bile acids and the risk of precancerous
gastric lesions such as atrophic gastritis and intestinal
metaplasia (3,7,21).
Matsuhisa et al demonstrated that the total bile acid
concentration was correlated with the grade of glandular atrophy
and intestinal metaplasia of the stomach (3,22).
Li et al showed that endoscopic bile reflux grading in
patients with chronic gastritis and precancerous lesions was lower
than that in patients with gastric cancer (4). Xu et al showed that
DCA-induced macrophage-derived exosomes increased the expression of
spasmolytic polypeptide expressing metaplasia markers in gastric
organoids leading to intestinal metaplasia of gastric mucosa
(23,24). In our study, the levels of primary
bile acid (conjugated and unconjugated) in the gastric fluid were
still higher in patients with EGC than in controls after adjustment
of age and background gastric mucosa status. Previous studies also
demonstrated that the levels of total bile acids in the gastric
fluid was higher in patients with precancerous lesion such as
intestinal metaplasia (3,22). Suggesting that a high
concentration of bile acid may be involved in early steps of
gastric carcinogenesis.
In conclusion, bile acids activated Egr-1 expression
in gastric cancer cells through the MAPK signaling pathway, and
higher gastric concentrations of primary bile acids were associated
with EGC. These findings suggest that exposure of gastric cells to
primary bile acids may play a role in gastric carcinogenesis.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was supported by The National Research Foundation
of Korea (NRF) grant funded by the Korea government (grant nos.
2018R1C1B5043483 and 2020R1I1A1A01068428), The Chonnam National
University Hospital Research Institute of Clinical Medicine (grant
nos. CRI 18018-1 and BCRI19258) and Korean College of Helicobacter
and Upper Gastrointestinal Research Foundation Grant.
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors have read and approved this manuscript.
SYP conceptualized, designed and supervised the study, analyzed and
interpreted data and drafted and finalized the manuscript. SYP and
SML confirm the authenticity of all the raw data. SML and MSP
conducted the study, collected and interpreted data and drafted the
manuscript. YDC analyzed and reviewed the pathological findings.
JOC analyzed and interpreted the collected clinical data and
drafted and reviewed the manuscript. YDJ analyzed and interpreted
the experimental data. DHK and HSK analyzed electronic medical
records about clinical data including demographic factors,
endoscopic findings from enrolled patients.
Ethics approval and consent to
participate
This study was approved by our institutional review
board (approval no. CNUH-2020-085). The terms of participation in
this study were explained and written informed consent was obtained
from patients before endoscopic procedures.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Di Ciaula A, Wang DQ, Molina-Molina E,
Lunardi Baccetto R, Calamita G, Palmieri VO and Portincasa P: Bile
acids and cancer: Direct and environmental-dependent effects. Ann
Hepatol. 16 (Suppl 1: s3-e105):S87–S105. 2017. View Article : Google Scholar
|
|
3
|
Matsuhisa T, Arakawa T, Watanabe T,
Tokutomi T, Sakurai K, Okamura S, Chono S, Kamada T, Sugiyama A,
Fujimura Y, et al: Relation between bile acid reflux into the
stomach and the risk of atrophic gastritis and intestinal
metaplasia: A multicenter study of 2283 cases. Dig Endosc.
25:519–525. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li D, Zhang J, Yao WZ, Zhang DL, Feng CC,
He Q, Lv HH, Cao YP, Wang J, Qi Y, et al: The relationship between
gastric cancer, its precancerous lesions and bile reflux: A
retrospective study. J Dig Dis. 21:222–229. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao W, Tian W, Hong J, Li D, Tavares R,
Noble L, Moss SF and Resnick MB: Expression of bile acid receptor
TGR5 in gastric adenocarcinoma. Am J Physiol Gastrointest Liver
Physiol. 304:G322–G327. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hong J, Behar J, Wands J, Resnick M, Wang
LJ, DeLellis RA, Lambeth D, Souza RF, Spechler SJ and Cao W: Role
of a novel bile acid receptor TGR5 in the development of
oesophageal adenocarcinoma. Gut. 59:170–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu JH, Zheng JB, Qi J, Yang K, Wu YH, Wang
K, Wang CB and Sun XJ: Bile acids promote gastric intestinal
metaplasia by upregulating CDX2 and MUC2 expression via the
FXR/NF-κB signalling pathway. Int J Oncol. 54:879–892.
2019.PubMed/NCBI
|
|
8
|
Thiel G and Cibelli G: Regulation of life
and death by the zinc finger transcription factor Egr-1. J Cell
Physiol. 193:287–292. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang B, Guo H, Yu H, Chen Y, Xu H and Zhao
G: The role of the transcription factor EGR1 in cancer. Front
Oncol. 11:6425472021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Steele NG, Chakrabarti J, Wang J, Biesiada
J, Holokai L, Chang J, Nowacki LM, Hawkins J, Mahe M, Sundaram N,
et al: An organoid-based preclinical model of human gastric cancer.
Cell Mol Gastroenterol Hepatol. 7:161–184. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schlemper RJ, Riddell RH, Kato Y, Borchard
F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF,
Geboes K, et al: The Vienna classification of gastrointestinal
epithelial neoplasia. Gut. 47:251–255. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carino A, Graziosi L, D'Amore C, Cipriani
S, Marchianò S, Marino E, Zampella A, Rende M, Mosci P, Distrutti
E, et al: The bile acid receptor GPBAR1 (TGR5) is expressed in
human gastric cancers and promotes epithelial-mesenchymal
transition in gastric cancer cell lines. Oncotarget. 7:61021–61035.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park SY, Kim JY, Lee SM, Chung JO, Lee KH,
Jun CH, Park CH, Kim HS, Choi SK, Rew JS, et al: Expression of
early growth response gene-1 in precancerous lesions of gastric
cancer. Oncol Lett. 12:2710–2715. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Allen K, Kim ND, Moon JO and Copple BL:
Upregulation of early growth response factor-1 by bile acids
requires mitogen-activated protein kinase signaling. Toxicol Appl
Pharmacol. 243:63–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shibata W, Maeda S, Hikiba Y, Yanai A,
Sakamoto K, Nakagawa H, Ogura K, Karin M and Omata M: c-Jun
NH2-terminal kinase 1 is a critical regulator for the development
of gastric cancer in mice. Cancer Res. 68:5031–5039. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen R, Masuo K, Yogo A, Yokoyama S,
Sugiyama A, Seno H, Yoshizawa A and Takaishi S: SNAIL regulates
gastric carcinogenesis through CCN3 and NEFL. Carcinogenesis.
42:190–201. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao A, Wang S, Chen W, Zheng X, Huang F,
Han X, Ge K, Rajani C, Huang Y, Yu H, et al: Increased levels of
conjugated bile acids are associated with human bile reflux
gastritis. Sci Rep. 10:116012020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee W, Um J, Hwang B, Lee YC, Chung BC and
Hong J: Assessing the progression of gastric cancer via profiling
of histamine, histidine, and bile acids in gastric juice using
LC-MS/MS. J Steroid Biochem Mol Biol. 197:1055392020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goldman A, Shahidullah M, Goldman D,
Khailova L, Watts G, Delamere N and Dvorak K: A novel mechanism of
acid and bile acid-induced DNA damage involving Na+/H+ exchanger:
Implication for Barrett's oesophagus. Gut. 59:1606–1616. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Souza RF: The role of acid and bile reflux
in oesophagitis and Barrett's metaplasia. Biochem Soc Trans.
38:348–352. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hyun JJ, Yeom SK, Shim E, Cha J, Choi I,
Lee SH, Chung HH, Cha SH and Lee CH: Correlation between bile
reflux gastritis and biliary excreted contrast media in the
stomach. J Comput Assist Tomogr. 41:696–701. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsuhisa T and Tsukui T: Relation between
reflux of bile acids into the stomach and gastric mucosal atrophy,
intestinal metaplasia in biopsy specimens. J Clin Biochem Nutr.
50:217–221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu X, Cheng J, Luo S, Gong X, Huang D, Xu
J, Qian Y, Wan X and Zhou H: Deoxycholic acid-stimulated
macrophage-derived exosomes promote spasmolytic
polypeptide-expressing metaplasia in the stomach. Biochem Biophys
Res Commun. 524:649–655. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weis VG, Sousa JF, LaFleur BJ, Nam KT,
Weis JA, Finke PE, Ameen NA, Fox JG and Goldenring JR:
Heterogeneity in mouse spasmolytic polypeptide-expressing
metaplasia lineages identifies markers of metaplastic progression.
Gut. 62:1270–1279. 2013. View Article : Google Scholar : PubMed/NCBI
|

















