Introduction
Parkinson's disease (PD) and Alzheimer's disease are
the most prevalent degenerative neurological disorders (1). As the aging population in most
countries increases, the incidence of PD is expected to surge,
posing a considerable personal and economic burden on families and
the larger society (2). The main
pathological features of PD are progressive loss of dopaminergic
(DA) neurons and decreased dopamine levels (3). To date, the compound l-dopa is
considered as the optimal treatment for PD in clinical practice;
however, long-term use of this compound causes complications such
as efficacy attenuation, motor fluctuation, and dysmotility, and it
cannot prevent or delay disease progression (4). Therefore, there is an urgent need to
understand the pathogenesis of PD in greater detail in order to
facilitate the search for new safe and effective drugs that can
delay disease progression.
The pathogenesis of PD is complex, involving
misfolding and abnormal aggregation of α-synuclein (α-syn),
oxidative stress, and mitochondrial dysfunction (5). DA neurons have high oxygen
consumption and metabolic rates and generate large quantities of
reactive oxygen species (ROS) that can damage mitochondria.
Analysis of the brains of PD patients at autopsy have shown that
the activity of mitochondrial complex I (the main site of ROS
generation) in the substantia nigra is selectively decreased and
mitochondrial function is impaired, suggesting that oxidative
stress and mitochondrial dysfunction play a key role in the
selective destruction of DA neurons (6). Additional studies have shown that
oxidative stress and mitochondrial dysfunction can induce α-syn to
form soluble oligomers, which then undergo misfolding to form
insoluble fibers (7–9). In turn, abnormal aggregation of
α-syn can amplify oxidative stress and mitochondrial dysfunction,
creating a vicious cycle that eventually triggers degeneration of
DA neurons in the substantia nigra. Therapeutic agents that reduce
ROS levels may maintain mitochondrial function and break the
destructive cycle of events, thereby delaying the loss of DA
neurons in PD.
Activation of apoptosis can occur through various
mechanisms, including via the phosphatidylinositol 3-kinase
(PI3K)/protein kinase B (AKT) and extracellular regulated protein
kinase (ERK) signaling pathways, which also play important roles in
cell proliferation and differentiation (10). Previous studies have found that
the PI3K/AKT and ERK pathways are associated with and may activate
the downstream transcription factor nuclear factor erythroid
2-related factor 2 (Nrf2), leading to expression of its antioxidant
target gene heme oxygenase-1 (HO-1) (11–13). Therefore, drugs that regulate
these pathways and protect from oxidative stress-induced apoptosis
may have neuroprotective activity.
Panax ginseng C.A. Meyer is a traditional
herbal medicine in China and has been demonstrated to have numerous
pharmacological effects on the nervous system (14). The antioxidant and neuroprotective
properties are mediated by ginsenosides, the main active
ingredients in ginseng. In particular, these properties have been
demonstrated for ginsenoside Re (Re) in a variety of
neurodegenerative disease models in vivo and in vitro
(15). However, the mechanism of
action of Re in PD remains unclear.
Rotenone (Rot), a naturally occurring isoflavone
that inhibits the mitochondrial electron transport chain complex I,
is commonly used to model PD by virtue of its ability to reproduce
numerous features of PD in animal models. Moreover, epidemiological
studies have shown that chronic exposure of individuals to Rot
confers an increased risk for PD (16). In the present study, the effects
of Re on Rot-induced PD models in vitro and in vivo
were analyzed and it was established that Re had potent
neuroprotective effects. The molecular mechanism of Re-mediated
neuroprotection was further examined and it was determined that it
acts by countering oxidative stress and maintaining mitochondrial
function. The results of the present study, thus lay the foundation
for further development of traditional Chinese medicines as
treatments to prevent or delay PD.
Materials and methods
Drugs
The ginsenosides Re, Rg1, Rg2, Rg3 and Rh2
(monomeric compounds identified by HPLC method, purity ≥98%; batch
nos. DSTDR001401, DSTDR000901, DSTDR001001, DSTDR001101 and
DSTDS003601) were all purchased from Chengdu Desite Biological
Technology Co., Ltd. All drugs were dissolved in dimethyl sulfoxide
(DMSO), and each experimental and control group contained ≤0.1%v/v
DMSO.
Cell culture
SH-SY5Y cells were obtained from the American Type
Culture Collection (cat. no. CRL-2266) and cultured in DMEM/F12
complete medium (supplemented with 10% fetal bovine serum and 1%
penicillin-streptomycin; all from Hyclone; Cytiva) at 37°C in a 5%
CO2 atmosphere.
Cell Counting Kit-8 (CCK-8) cell
viability assay
Cell viability was analyzed using a CCK-8 assay kit
(Boster Biological Technology) according to the manufacturer's
instructions. SH-SY5Y cells were seeded in 96-well plates
(3×103 cells/well) and incubated at 37°C for 24 h. The
cells were then co-treated with 0.3 µM Rot (Dalian Meilun
Biotechnology Co., Ltd.; cat. no. MB5842) and Re (1, 2.5, 5, or 10
µM), Rg1 (2.5 µM), Rg2 (5 µM), Rg3 (10 µM), Rh2 (10 µM), or
L-dopamine (Beijing Solarbio Science & Technology Co., Ltd.;
cat. no. ID0360; 5 or 10 µM) for 24 h. CCK-8 solution was added at
20 µl/well and the plates were incubated in the dark at 37°C for 30
min. The absorbance at 450 nm was measured with a microplate reader
(Tecan Group, Ltd.).
Lactate dehydrogenase (LDH) release
assay
LDH was measured using an LDH cytotoxicity assay kit
(Nanjing Jiancheng Bioengineering Institute; cat. no. A020-2-2)
according to the manufacturer's guidelines. Cells were seeded at
5×104 cells/well and co-treated with Re (1, 2.5, 5, or
10 µM) and Rot (0.3 µM) at 37°C for 24 h. The absorbance at 490 nm
was then measured with a microplate reader (Tecan Group, Ltd.) at
room temperature.
Apoptosis assay
Cells undergoing apoptosis were detected by flow
cytometry after staining with Annexin V-FITC and PI (Becton
Dickinson and Company). Cells were seeded at 5×104
cells/well and co-treated with Rot (0.3 µM) and Re (5 µM) at 37°C
for 24 h. The cells were collected, washed twice with
phosphate-buffered saline (PBS), resuspended in 1X Binding Buffer,
and mixed with Annexin V-FITC and PI staining solution. The cells
were incubated at room temperature in the dark for 15 min and then
analyzed using an flow cytometer (Amnis Corporation), and
quantified using IDEAS software v6.1 (Amnis Corporation).
Fluorescence microscopy
Cells were seeded at 5×104 cells/well and
co-treated with Rot (0.3 µM) and Re (5 µM) at 37°C for 24 h. The
cells were then fixed in 4% paraformaldehyde (PFA) solution for 0.5
h at room temperature, washed twice with PBS, and incubated with
Hoechst 33342 (Beyotime Institute of Biotechnology) staining
solution at 1 ml/well. The plates were incubated at 37°C in the
dark for 30 min, washed twice with PBS, and visualized using with
an EVOS fluorescence microscope (Thermo Fisher Scientific,
Inc.).
Caspase assay
Caspase activities were measured using Caspase-3,
Caspase-8 and Caspase-9 (cat nos. C1115, C1151; C1157; Beyotime
Institute of Biotechnology) Activity assay kits according to the
manufacturers' instructions. Cells were washed with PBS,
centrifuged at 4°C (530 × g, 5 min) and 100 µl lysate was used per
2×105 cells and incubated on ice for 15 min.
Centrifugation at 4,246 × g at 4°C for 10 min. Protein
concentration in supernatant was measured using a Bradford assay
kit (Tiangen Biotech Co., Ltd.). Then 50 µl cell lysate supernatant
and 10 µl AC-DevD-PNA (2 mM) for caspase-3, AC-IETD-PNA (2 mM) for
caspase-8 and AC-LEHD -pNA (2 mM) for caspase-9 was mixed in a 40
µl detection buffer at 37°C for 4 h and then analyzed using a
microplate reader (Tecan Group, Ltd.).
Detection of adenosine triphosphate
(ATP) content
ATP was measured using a chemiluminescence ATP assay
kit (Beyotime Institute of Biotechnology; cat. no. S0026).
Following incubation of SH-SY5Y cells (5×104 cells/well)
with Rot (0.3 µM) and Re (5 µM) at 37°C for 24 h, the medium was
discarded, 200 µl of ATP detection solution was added, and the
plates were centrifuged at 4,246 × g for 5 min at 4°C. The
supernatant was transferred to a new tube. A total of 100 µl of ATP
detection buffer was added to each assay well and incubate at 37°C
for 3 min to deplete background, followed by addition of 20 µl of
supernatant to the assay wells. Optical density values were
recorded using a microplate reader (Tecan Group, Ltd.) and ATP
content was converted according to a standard curve.
Mitochondrial membrane potential (MMP)
analysis by fluorescence microscopy
Cells were seeded at 5×104 cells/well and
were incubated with Rot (0.3 µM) with or without Re (5 µM) at 37°C
for 24 h, washed twice with PBS, mixed with 1 ml of JC-1 solution
(Beijing Solarbio Science & Technology Co., Ltd.; cat. no.
J8030) and incubated in the dark for 20 min (maintained at 37°C).
The cells were washed twice with JC-1 staining buffer. The cells
were then processed and examined with an EVOS fluorescence
microscope (Thermo Fisher Scientific, Inc.).
MMP analysis by flow cytometry
Cells were seeded at 5×104 cells/well and
were incubated with Rot (0.3 µM) with or without Re (5 µM) at 37°C
for 24 h, washed twice with PBS, mixed with Rhodamine 123 (2 µM)
(Beijing Solarbio Science & Technology Co., Ltd.; cat. no.
R8030), and incubated at 37°C for 30 min in the dark. The cells
were then centrifuged at 530 × g for 5 min at room temperature,
resuspended in PBS, and analyzed using a flow cytometer (Amnis
Corporation), and quantified using IDEAS software v6.1 (Amnis
Corporation).
Intracellular ROS assay
Intracellular ROS levels were detected by flow
cytometry (Amnis Corporation) and quantified using IDEAS software
v6.1 (Amnis Corporation) or fluorescence microplate reader. Cells
were seeded at 5×104 cells/well and were incubated with
Rot (0.3 µM) and Re (5 µM) at 37°C for 24 h, washed twice with PBS,
mixed with 100 µl of 2,7-dichlorofluorescein diacetate (DCFH-DA;
Beijing Solarbio Science & Technology Co., Ltd.; cat. no.
CA1410) diluted in serum-free DMEM/F12 medium (final concentration,
10 µM), and incubated in the dark for 20 min at 37°C. The cells
were then centrifuged at 530 × g for 5 min at room temperature,
resuspended in PBS, and analyzed using an Amnis® flow
cytometer. Alternatively, cells were seeded at 3×103
cells/well and incubated with Rot (0.3 µM) and Re (5 µM) at 37°C
for 24 h, after being washed by PBS were inoculated into black
96-well plates, and a 10-µl probe was added before detection. The
cells were incubated at 37°C for 20 min under dark conditions, and
then detected using a fluorescence microplate reader. The
excitation wavelength was 488 nm, and the emission wavelength was
530 nm.
Oxidative stress marker assays
Cells were seeded at 5×104 cells/well and
incubated with Rot (0.3 µM) in the presence or absence of Re (5 µM)
at 37°C for 24 h. Glutathione (GSH), malondialdehyde (MDA),
superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px)
were assayed using kits (Nanjing Jiancheng Bioengineering
Institute; cat. no. A006-2-1, A003-4-1, A001-3-2, A005-1-2)
according to the manufacturer's instructions and measured with a
microplate reader (Tecan Group, Ltd.).
Inhibitor treatment and small
interfering RNA (siRNA) transfection
Experiments involving the following inhibitors
included cell viability, ROS detection and western blotting: 15 µM
PD98059 (ERK1/2 signal inhibitor, cat. no. HY-12028), 15 µM
LY294002 (PI3K/AKT inhibitor, cat. no. HY-10108), 5 µM Akt
inhibitor IV (cat. no. HY14971). 5 µM SB203580 [p38
mitogen-activated protein kinase (MAPK) inhibitor, cat. no.
HY10256], 10 µM SP600125 [Jun N-terminal kinase (JNK) inhibitor,
cat. no. HY12041], and 10 µM Compound C [CC; Adenosine
5′-monophosphate (AMP)-activated protein kinase (AMPK) inhibitor,
cat. no. HY13418A], were only used in cell viability assays to
determine which signalling pathways mediate Nrf2 activation. The
inhibitors were added to cells before Re and/or Rot treatment for 1
h at 37°C and all inhibitors were purchased from Med Chem Express
corporation. A customized siRNA reagent system (Guangzhou RiboBio
Co., Ltd.) was used for cell viability or ROS assays as previously
described (17). The target
sequence of the negative control for RNA interference was
5′-CGUACGCGGAAUACUUCGA-3′ and the interference target sequences for
Nrf2 were as follows: NRF2 siRNA-1, 5′-GAATGGTCCTAAAACACCA-3′; NRF2
siRNA-2; 5′-GAGAAAGAATTGCCTGTAA-3′; and NRF2 siRNA-3,
5′-GCTACGTGATGAAGATGGA-3′.
Western blot analysis
Cells were washed twice with PBS and centrifuged at
530 × g at 4°C to obtain cells pellet, cells were lysed with RIPA
lysis buffer (Beyotime Institute of Biotechnology) and total
protein was measured using a BCA kit (Beijing Solarbio Science
& Technology Co., Ltd.; cat. no. P0012S). Subcellular fractions
were produced using a Cell Mitochondria Isolation Kit (Beyotime
Institute of Biotechnology; cat. no. C3601) for cytosolic and
mitochondrial fractions and a Nuclear and Cytoplasmic Protein
Extraction Kit (Beyotime Institute of Biotechnology; cat. no.
P0027) for nuclear and cytosolic fractions. Proteins (20 µg/lane)
were separated by SDS-PAGE and electron transfer onto a
nitrocellulose membrane. Non-specific protein binding was blocked
by incubation of the membranes with 5% (v/v) non-fat dried milk in
PBS for 1 h at 25°C and the membranes were incubated overnight at
4°C with anti-B-cell lymphoma-2 (Bcl-2; 1:1,000; cat. no. BS1511),
anti-Bcl-2 associated X protein (Bax; 1:1,000; cat. no. BS6420),
anti-cleaved caspase-3 (1:1,000; cat. no. BS7004), anti-Nrf2
(1:1,000; cat. no. BS1258), anti-glutamate cysteine ligase modifier
(GCLM; 1:1,000; cat. no. BS2927), anti-Akt (1:1,000; cat. no.
BS90043), anti-PI3K (1:1,000; cat. no. BS9852M), anti-p-PI3K
(1:1,000; cat. no. AP0153), anti-ERK (1:1,000; cat. no. BS1968),
anti-p-ERK (1:1,000; cat. no. BS4759), anti-glyceraldehyde
3-phosphate dehydrogenase (GAPDH; 1:1,000; cat. no. AP0063),
anti-cytochrome c oxidase IV (COX IV; 1:600; cat. no.
AP0705), anti-histone 3 (H3; 1:1,000; cat. no. BS1161), and
anti-β-actin (1:1,000; cat. no. AP0060), all from Bioworld
Technology, Inc.. Anti-HO-1 (1:1,000; cat. no. 66743-1),
anti-NAD(P)H:quinone oxidoreductase 1 (NQO1; 1:1,000; cat. no.
11451-1-AP), from Proteintech Group, Inc.. Anti-phosphorylated
(p)-Akt (1:1,000; cat. no. #4060s; Cell Signaling Technology,
Inc.), anti-cytochrome c (cyt-c, 1:600; cat. no. AF2047;
Beyotime Institute of Biotechnology). Following washing with PBS
containing 0.05% (v/v) Tween-20 (PBST), the membranes were
incubated with horseradish peroxidase-conjugated anti-mouse IgG
(1:0000; cat. no. #7076; Cell Signaling Technology Inc.) or
horseradish peroxidase-conjugated anti-rabbit IgG (1:0000; cat. no.
SA00001-2; ProteinTech Group, Inc.), and bands were visualized
using Enhanced Chemiluminescence Reagent kit (Beyotime Institute of
Biotechnology; cat. no. P0018S). Membranes were imaged using the
iBright FL1000 Imaging System (Invitrogen; Thermo Fisher
Scientific, Inc.). Image J software 1.53a (National Institutes of
Health) was used to quantify the gray value of western blot bands
and protein expression level was normalized as the gray value of
the target protein/the loading control protein.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using a Total RNA extraction
kit (Tiangen Biotech Co., Ltd.) according to the manufacturer's
instructions, and perform reverse transcribed according to Prime
Script RT reagent kit instructions (Takara Biotechnology Co.,
Ltd.). Aliquots of cDNA were subjected to qPCR analysis with SYBR
Green PCR Master Mix (Takara Biotechnology Co., Ltd.) and a
Real-Time PCR system equipped with a CFX 96 Connect™ Optics Module
(Bio-Rad Laboratories, Inc.). Reaction program [94°C
pre-denaturation for 30 sec; 40 cycles (94°C denaturation for 5
sec, 60°C annealing for 1 min)]. Primer sets specific to GAPDH
(reference gene) and NRF2 were as follows: GAPDH forward,
5′-ACCACAGTCCATGCCATCAC-3′ and reverse, 5′-TCCACCACCCTGTTGCTGTA-3′;
NRF2 forward, 5′-CAGTCAGCGACGGAAAGAGT-3′ and reverse,
5′-ACGTAGCCGAAGAAACCTCA-3′. Data were analyzed according to the
2−∆∆cq method as previously described (18).
Drosophila stocks, husbandry, and
lifespan analysis
Drosophila (including W−,
UAS-CncC, UAS-CncC RNAi and da-Gal4) were obtained from Dr
Yufeng Yang (Institute of Life Sciences, Fuzhou University, Fuzhou,
China). UAS-CncC RNAi × da-Gal4 was obtained by mating
UAS-CncC RNAi and da-Gal4. Flies were grown on
sugar-yeast-agar medium with or without Re (final concentration 0.4
mM in DMSO) and Rot (final concentration 515 µM in DMSO), which was
replenished daily and the number of deaths of flies was recorded.
Flies were incubated in a humidified (50% relative humidity),
temperature-controlled (29°C) incubator with a 12-h on/off light
cycle. The significance of the survival curve was calculated using
the Log-rank (Mantel-Cox) test.
Drosophila negative geotaxis
(climbing) assay
On the day of the assay, flies were anesthetized
with CO2 and transferred into a glass pipette (height 22
cm, diameter 1.0 cm, capacity 25 ml) capped with a cotton plug.
Following recovery, the flies were left for 15 min and the glass
pipette was then placed vertically and flies were shaken down to
the bottom of the tube. Data were recorded by taking photographs at
5 sec intervals for a total of 1 min. The climbing index was
evaluated as follows. The fly movement area (18 cm from the bottom
of the pipette to the lower end of the plug) was divided into 2-cm
zones (1, 2, 3, 4, 5, 6, 7, 8, 9) from the bottom to the top. The
climbing index was calculated as the total number of flies in each
area multiplied by the number of regions.
Determination of hydrogen peroxide
(HP) level in Drosophila
To evaluate the HP level in Drosophila a
hydrogen peroxide detection kit (cat. no. BC3590; Beijing Solarbio
Science & Technology Co., Ltd.) Weigh about 0.1 g flies tissue
and add 1 ml working solution for ice bath homogenization;
centrifuged 8,000 × g at 4°C for 10 min, all supernatant was taken
and placed on ice for testing. Add corresponding reagents according
to the kit instructions, and use a microplate reader (Tecan Group,
Ltd.) to detect at 415 nm.
ATP analysis, respirometry analysis
and ROS production
ATP analysis and respiration measurement analysis
methods were performed as previously described (19). Flies were homogenized in 500 µl
PBS by a cell disruptor, centrifuged at 530 × g for 5 min at room
temperature, and incubated in DCFH-DA (final concentration 20 µM;
cat. no. CA1410; Beijing Solarbio Science & Technology Co.,
Ltd.) for 30 min at 37°C. ROS was measured with an
Infinite® 200 Pro microplate reader (Tecan Group, Ltd.)
using excitation at 488 nm and emission at 530 nm (20).
Immunofluorescence microscopy of
Drosophila
Flies were anesthetized by CO2 and next
fixed in 4% paraformaldehyde at 25°C for 2.5 h, washed with PBST,
and the brains were dissected and placed in blocking agent (PBS
buffer; 0.1% Triton X-100; 10% heat-inactivated fetal bovine serum)
at 25°C for 90 min. The blocking agent was removed and replaced
with anti-tyrosine hydroxylase (TH) antibody (1:500; cat. no.
AB152; Millipore corporation) overnight at 4°C in the dark. The
brains were washed with pre-cooled PBST buffer, incubated with
Rabbit anti-goat IgG antibody, FITC conjugate (1:200; cat. no.
AP106F; Millipore corporation) for 2 h at 25°C in the dark, and
washed again. Finally, the brains were mounted onto slides and
imaged immediately with a laser confocal microscope.
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD). Data were evaluated using GraphPad Prism 6.0 software
(GraphPad Software, Inc.). All experiments in the present study
were repeated three times. Group means were compared using one-way
analysis of variance (ANOVA) test followed by Tukey's post hoc
tests. P≤0.05 was considered to indicate a statistically
significant difference.
Results
Re protects SH-SY5Y cells against the
cytotoxic effects of Rot
Rot has been shown to reproduce numerous features of
PD in animal models (21);
therefore, its effects in vitro were examined using the
human neuroblastoma cell line SH-SY5Y. The results revealed that
Rot alone significantly decreased cell viability (Fig. S1). The antioxidant and
neuroprotective effects of ginseng are mediated by ginsenosides,
the main active components of ginseng (22). To assess these compounds, the
optimal nontoxic concentrations of five representative
ginsenosides: Rg1, Rg2, Rg3 and Rh2 (Fig. S2), as well as Re (Figs. 1A and B, and S3A) were first determined using the
CCK-8 cell viability assay. The protective effect of each monomer
saponin on Rot-treated SH-SY5Y cells was then examined (Figs. S4, 1C and S3B). Among the ginsenosides assessed,
Re had the most significant protective effect on Rot-induced
SH-SY5Y cytotoxicity (Figs. 1D
and S3C; Table SI). Re was protective at 2.5, 5,
and 10 µM, with maximal protection observed at 5 µM Re (Figs. 1C and S3B). Moreover, there was no significant
difference between the protective effects of Re at 5 µM and l-dopa
at 5 or 10 µM (Figs. 1E and
S3D). Re also displayed
significant protection when Rot-induced cytotoxicity was measured
using a LDH release assay (Fig.
1F).
 | Figure 1.Re attenuates Rot-induced
cytotoxicity in SH-SY5Y cells. (A) Chemical structure of Re. (B)
Detection of Re cytotoxicity in SH-SY5Y cells assessed by CCK-8
assay. (C) Effect of Re on the viability of SH-SY5Y cells treated
with Rot assessed by CCK-8 assay. (D) Comparison of the effects of
ginsenoside monomers (Re, Rg1, Rg2, Rg3, and Rh2; purity ≥98%) on
the viability of SH-SY5Y cells treated with Rot. (E) Analysis of
the effects of Re and l-dopamine on the viability of SH-SY5Y cells
treated with Rot. The above experiments calculated the relative
cell viability using an endpoint time of 24 h. (F) LDH release
cytotoxicity assay. Data are expressed as the mean ± SD; n=3.
*P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001 vs. the
control; #P<0.05, ##P<0.01 and
###P<0.001 vs. Rot treatment alone. Re, ginsenoside
Re; Rot, rotenone; CCK-8, Cell Counting Kit-8; l-dopa, l-dopamine;
LDH, lactate dehydrogenase. |
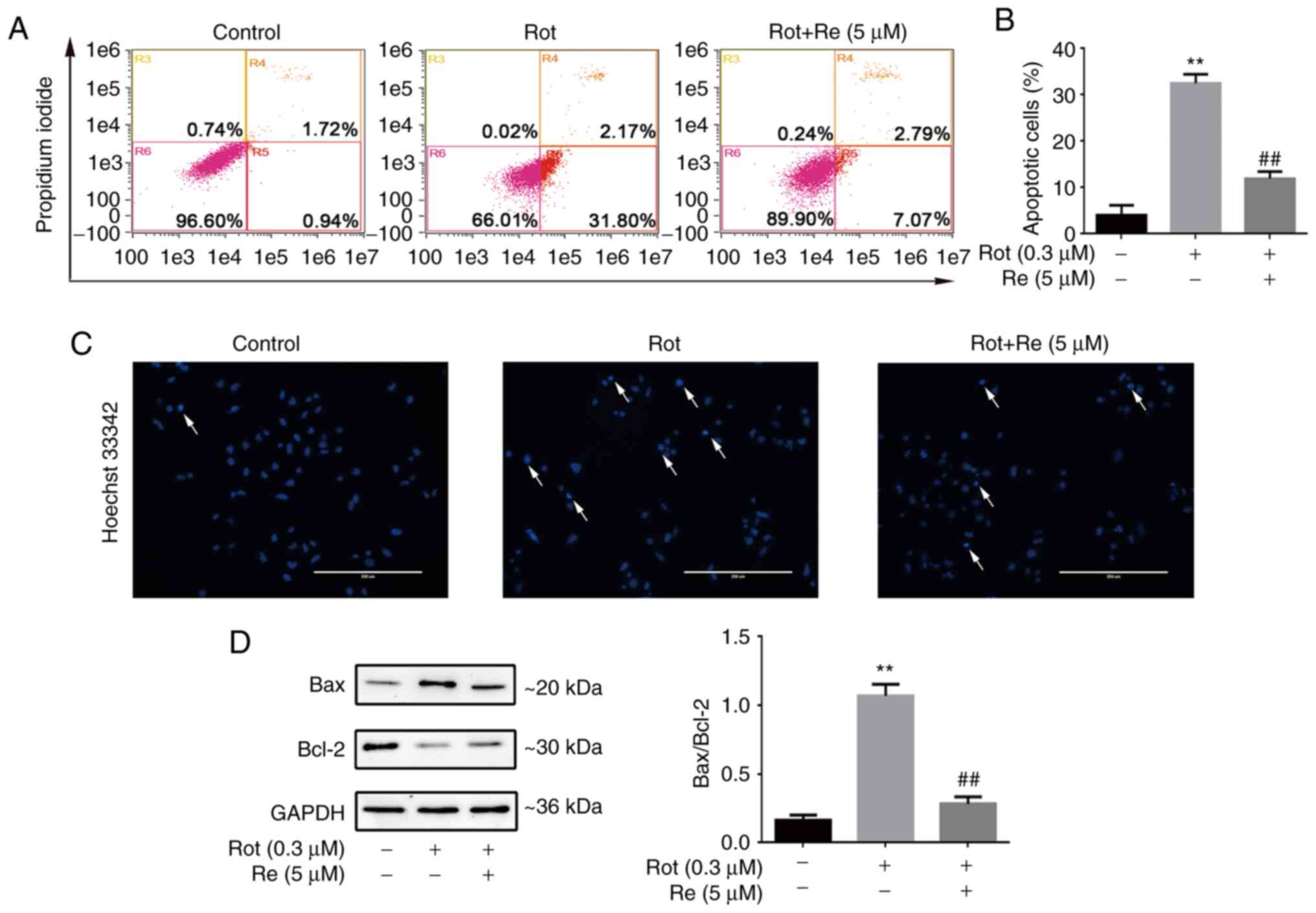
Re attenuates Rot-induced apoptosis of
SH-SY5Y cells
To determine whether Rot-induced cell death was
mediated by triggering of apoptosis, the cells were stained with
Annexin V-FITC and propidium iodide (PI) and analyzed by flow
cytometry. The results showed that exposure to Rot (0.3 µM) alone
increased the abundance of cells in early [Annexin
V-FITC+, PI– (R4)] and late [Annexin
V-FITC+, PI+ (R5)] apoptosis (total up to
33.97%), and that Re co-treatment significantly reduced the
apoptotic rate (Fig. 2A and B).
Similarly, microscopic analysis showed that SH-SY5Y cells treated
with Rot exhibited the typical morphological characteristics of
apoptosis, whereas Re co-treated cells exhibited a lower level of
Hoechst 33342 staining and had uniformly stained chromatin,
comparable to the untreated control cells (Fig. 2C). Cell apoptosis is regulated in
part by a balance between the expression of the anti-apoptotic and
pro-apoptotic regulatory proteins Bcl-2 and Bax (23). Therefore, the expression of these
proteins in Rot and Re co-treated cells was examined by western
blot analysis. Notably, the ratio of Bax to Bcl-2 protein was
increased by Rot treatment and decreased by co-incubation of
Rot-treated cells with Re (Fig.
2D).
Re reduces Rot-induced mitochondrial
dysfunction and suppresses activation of the mitochondrial
apoptotic pathway in SH-SY5Y cells
Mitochondrial function was assessed in SH-SY5Y cells
following Rot and/or Re treatment using several methods. First, the
cells were labeled with JC-1, an MMP probe. In healthy cells,
green-fluorescent JC-1 monomers accumulate in mitochondria and form
red-fluorescent aggregates. Loss of MMP results in formation of
JC-1 monomers; thus, the ratio of green/red fluorescence is a
marker of MMP integrity and reflects mitochondrial health (24). Rot-treated cells exhibited an
increase in green fluorescence compared with the red JC1 aggregates
in control cells, which was reduced by co-treatment with Re
(Fig. 3A). As a second method of
MMP analysis, the cells were labeled with the fluorescent dye
rhodamine 123. In healthy cells, rhodamine 123 selectively enters
the mitochondrial matrix and emits bright yellow-green
fluorescence; however, cells undergoing apoptosis or necrosis
experience disruption of the MMP, which leads to opening of the
mitochondrial permeability transition pore, release of rhodamine
123 from the mitochondria, and a reduction in mitochondrial
fluorescence (25). Notably, the
results obtained when MMP was assessed by rhodamine 123 labeling
(Fig. 3B) were similar to those
obtained using the JC-1 labeling method. Finally, ATP generation,
as a third indicator of mitochondrial health in SH-SY5Y cells, was
also detected. As expected, Rot-treated cells contained less ATP
than the control cells, and Re significantly reversed these
deleterious effects of Rot (Fig.
3C).
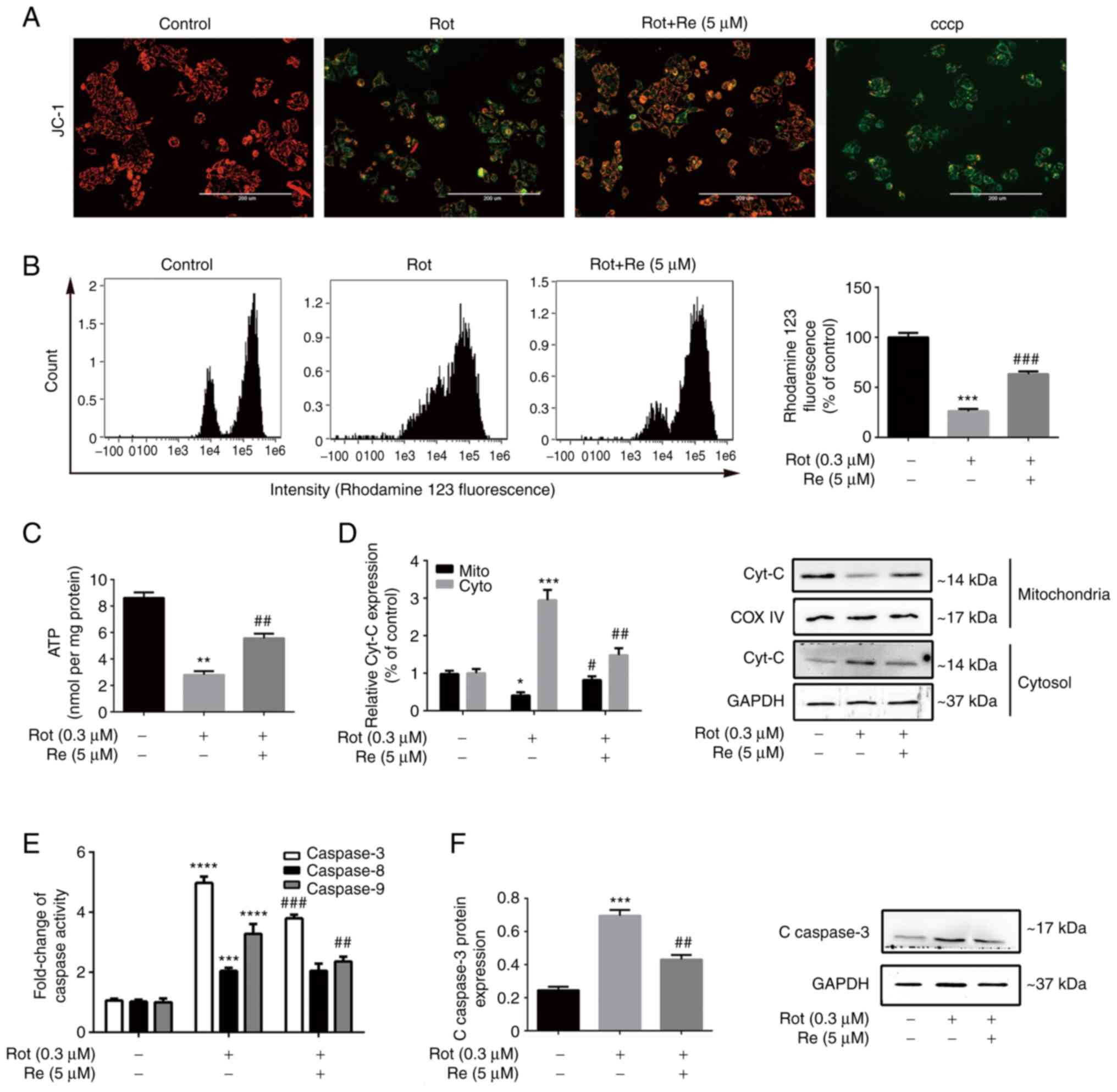 | Figure 3.Re alleviates Rot-induced
mitochondrial dysfunction in SH-SY5Y cells. (A) Fluorescence
microscopic images of JC-1-stained cells to assess the MMP. Scale
bar, 200 µm. (B) Flow cytometric analysis of loss of MMP integrity.
(C) Quantification of ATP production. (D) Western blot analysis of
cytochrome c subcellular localization. (E) Quantification of
caspase-3, caspase-8, and caspase-9 activities. (F) Western blot
analysis of cleaved caspase-3 expression. Data are expressed as the
mean ± SD; n=3. *P<0.05, **P<0.01, ***P<0.001 and
****P<0.0001 vs. the control; #P<0.05,
##P<0.01 and ###P<0.001 vs. Rot
treatment alone. Re, ginsenoside Re; Rot, rotenone; MMP,
mitochondrial membrane potential; ATP, adenosine triphosphate;
Cyt-c, cytochrome c; COX IV, cytochrome c oxidase
IV. |
To determine whether the observed increase in
SH-SY5Y cell apoptosis induced by Rot (Fig. 2) was mediated by the exogenous
receptor-activated pathway or the endogenous mitochondrial pathway,
the release of the mitochondrial enzyme cyt-c into the cytosol, a
key feature of endogenous apoptosis (26), was examined, as well as the
activity of caspases, which are the main effector enzymes of
apoptosis (27). As expected, Rot
treatment increased cyt-c release and stimulated the activities of
caspase-3, −8, and −9; however, co-treatment with Re attenuated the
effects of Rot on each of these parameters except activation of
caspase-8 (Fig. 3D-F).
Collectively, these results suggest that Re-mediated protection
against Rot-induced cell injury involves attenuation of
mitochondrial dysfunction and inhibition of the mitochondrial
apoptotic pathway.
Re reduces Rot-induced oxidative
stress in SH-SY5Y cells
To determine whether Re affects Rot-induced
oxidative stress in SH-SY5Y cells, several metabolites that undergo
characteristic changes in expression upon cell exposure to
oxidative stress were assessed. These included ROS, the fatty acid
oxidation product, MDA, and the antioxidants GSH-Px, an enzyme that
catalyzes reduction of lipid peroxides by GSH, and SOD, which
catalyzes the dismutation of superoxide anions and is an important
antioxidant enzyme (28). Rot
treatment alone resulted in increased levels of ROS and MDA,
decreased levels of GSH, and reduced SOD and GSH-Px activities
(Fig. 4). Notably, each of these
Rot-induced effects was reversed in cells co-treated with Re
(Fig. 4), demonstrating that Re
is able to counteract Rot-induced oxidative stress.
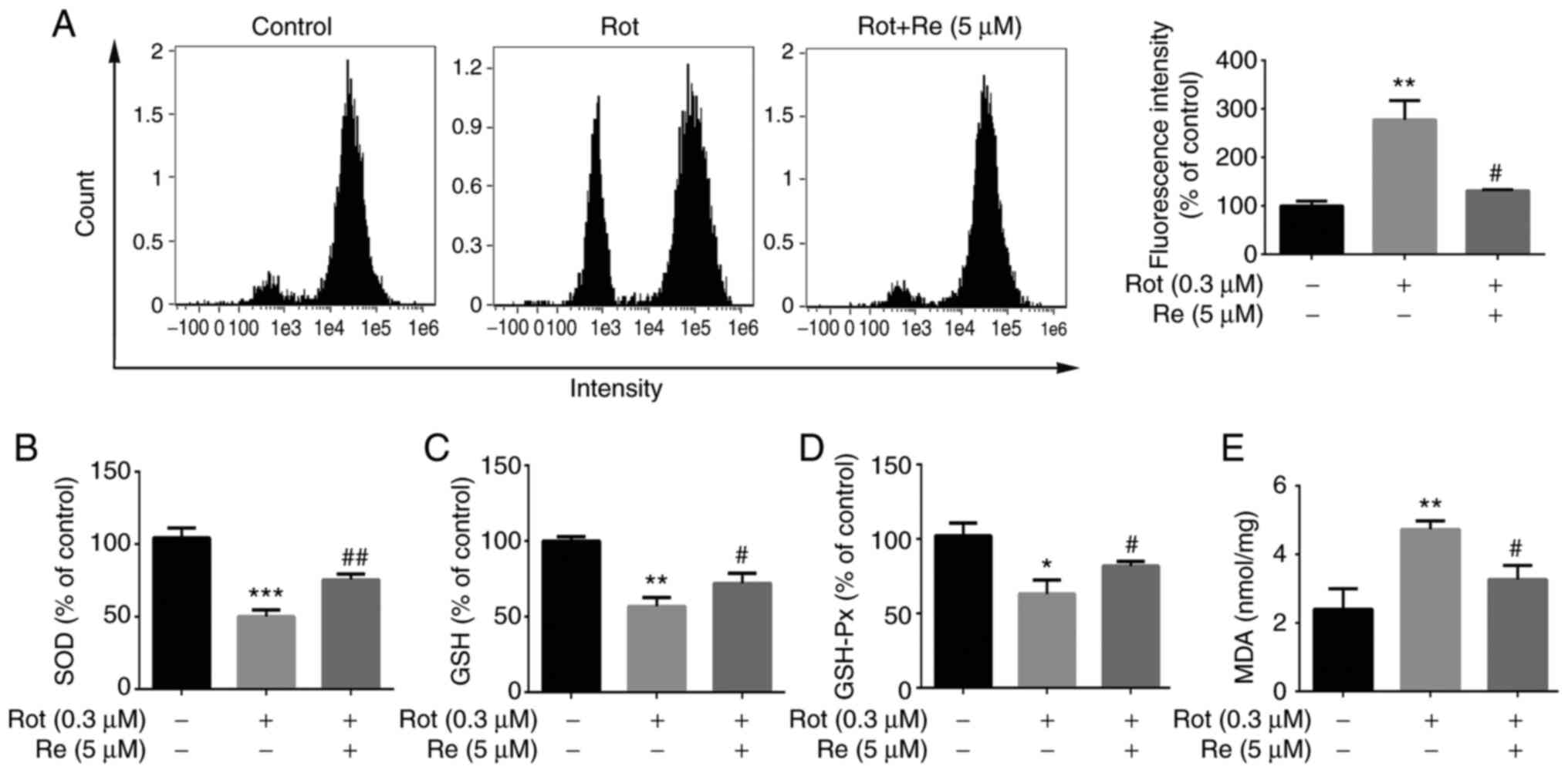 | Figure 4.Re reduces Rot-induced oxidative
stress in SH-SY5Y cells. Measurement of intracellular (A) ROS
levels, (B) SOD activity, (C) GSH concentration, (D) GSH-Px
activity and (E) MDA concentration. Data are expressed as the mean
± SD; n=3. *P<0.05, **P<0.01 and ***P<0.001 vs. the
control; #P<0.05 and ##P<0.01 vs. Rot
treatment alone. Re, ginsenoside Re; Rot, rotenone; ROS, reactive
oxygen species; SOD, superoxide dismutase; GSH, glutathione;
GSH-Px, glutathione peroxidase; MDA, malondialdehyde. |
Re reduces Rot-induced oxidative
stress via effects on the antioxidant transcription factor Nrf2 in
SH-SY5Y cells
Nrf2 is a key component of the oxidative stress
response and regulates the expression of antioxidant and
cytoprotective genes (29). To
determine whether Nrf2 is modulated by Rot and/or Re treatment,
western blot analysis of SH-SY5Y subcellular fractions was
performed. Compared with the untreated control cells, Rot treatment
induced no significant change in the distribution of Nrf2 in the
cytoplasm and nucleus, whereas Re-co-treated cells showed an
increase in nuclear localization of Nrf2 (Fig. 5A). Nrf2 is generally cytosolic but
translocates to the nucleus in response to oxidative stress, where
it binds to antioxidant response elements and induces transcription
of genes such as HO-1, GCLM, NQO1 (30). Consistent with this, Re treatment
also significantly increased the expression of HO-1, GCLM, and NQO1
proteins (Fig. 5B).
 | Figure 5.Re reduces Rot-induced cytotoxicity
and ROS accumulation via Nrf2. (A and B) Western blot analysis of
the expression of (A) Nrf2 and (B) HO-1, GCLM and NQO1. (C)
Silencing of Nrf2 expression by transfection with gene-specific
siRNA. Knockdown efficiencies were 41% for siRNA-1, 31% for
siRNA-2, and 22% for siRNA-3. Further cell viability and ROS level
determinations were performed using the most efficient siRNA,
siRNA-1. (D) CCK-8 assay of cell viability after transfection of
SH-SY5Y cells with Nrf2 siRNA-1. (E) The intracellular ROS
quantification and (F) flow cytometric plots of Nrf2 siRNA-1
transfected cells. Data are expressed as the mean ± SD; n=3.
*P<0.05, **P<0.01 and ***P<0.001 vs. the control;
#P<0.05 and ###P<0.001 vs. Rot
treatment alone. Re, ginsenoside Re; Rot, rotenone; ROS, reactive
oxygen species; Nrf2, nuclear factor erythroid 2-related factor 2;
HO-1, heme oxygenase-1; GCLM, glutamate cysteine ligase modifier;
NQO1, NAD(P)H:quinone oxidoreductase 1; siRNA, small interfering
RNA; CCK-8, Cell Counting Kit-8. |
To determine the importance of Nrf2 in Re-mediated
protection against Rot, the cells were transfected with a control
siRNA or Nrf2-targeting siRNA (Fig.
5C). The beneficial effects of Re on Rot-induced cytotoxicity
(Fig. 5D) and ROS production
(Fig. 5E and F) were reduced in
siNrf2-transfected cells compared with control cells. Collectively,
these results indicated that Nrf2 plays an essential role in
Re-mediated neuroprotection against oxidative stress.
Re induces Nrf2 nuclear transport via
the PI3K/AKT and ERK signaling pathways in SH-SY5Y cells
To determine which signaling pathways mediate
Re-induced Nrf2 activation, SH-SY5Y cells were pretreated with
inhibitors of PI3K/AKT (LY294002, 15 µM), JNK (SP600125, 10 µM),
ERK (PD98059, 15 µM), AMPK (CC, 10 µM), and p38 MAPK (SB203580, 5
µM) and then the effects on Re activity were analyzed in cell
viability assays. Compared with cells incubated under control
conditions, LY294002 and PD98059 treatment significantly suppressed
the protective effect of Re on Rot-induced cell viability (Fig. 6A) and ROS production (Fig. 6B; P<0.05), whereas the
remaining inhibitors had no effect. The ERK and PI3K/AKT inhibitors
prevented Re-stimulated nuclear localization of Nrf2 in SH-SY5Y
cells (Fig. 6C). In addition, ROS
levels, Nrf2 expression in the nucleus, and cell viability were all
significantly altered by cell treatment with AKT inhibitor IV
(Fig. S5). Confirming these
associations, it was determined that Rot downregulated the
expression of p-AKT (Fig. 6D and
E), p-ERK (Fig. 6D and F) and
phosphorylated (activated) PI3K (Fig.
6D and G), and these effects were significantly reversed by
treatment with Re.
 | Figure 6.Re-induced Nrf2 accumulation is
mediated by PI3K/AKT and ERK. (A) Cell viability assessed by CCK-8
assay. (B) Quantification of ROS levels assessed by the DCFH-DA
assay and detected by fluorescence microplate reader. The
excitation wavelength was 488 nm, and the emission wavelength was
530 nm. (C) Western blot analysis of nuclear Nrf2 protein levels.
Cells were pretreated with 15 µM PD98059 (ERK1/2 inhibitor), 15 µM
LY294002 (PI3K/AKT inhibitor), 5 µM SB203580 (p38 MAPK inhibitor),
10 µM SP600125 (JNK inhibitor), or 10 µM CC (AMPK inhibitor) for 1
h and then treated with 0.3 µM Rot and 5 µM Re for 24 h. (D)
Representative western blot images of PI3K/AKT and ERK pathways.
Semi-quantitative analysis of (E) p-Akt, (F) p-ERK and (G) p-PI3K
protein expression in SH-SY5Y cells. Data are presented as the mean
± SD; n=3. *P<0.05, **P<0.01 and ***P<0.001 vs. the
control; #P<0.05 and ##P<0.01 vs. Rot
treatment alone; &P<0.05 vs. Re+Rot treatment.
Re, ginsenoside Re; Nrf2, nuclear factor erythroid 2-related factor
2; PI3K, phosphatidylinositol 3-kinase; AKT, protein kinase B; ERK,
extracellular regulated protein kinase; CCK-8, Cell Counting Kit-8;
DCFH-DA, 2,7-dichlorofluorescein diacetate; MAPK, p38
mitogen-activated protein kinase; JNK, Jun N-terminal kinase; CC,
Compound C; AMPK, adenosine 5′-monophosphate (AMP)-activated
protein kinase; Rot, rotenone; p-, phosphorylated. |
Re attenuates Rot-induced locomotor
defects and neuron loss in Drosophila
Exposure of Drosophila to Rot mimics numerous
of the features of human PD and is widely used as a model for
investigating the pathogenesis of PD (31). Consistent with the aforementioned
study, it was determined that exposure of flies to 515 µM Rot for 7
days before analysis caused a loss of dopaminergic neurons, as
measured by staining of Drosophila brains for the marker
protein TH (Fig. 7A and B).
However, flies that had been administered Rot plus Re (0.4 mM)
showed partial reversal of neuron loss (Fig. 7C). Since Rot-induced dopaminergic
neurotoxicity has been reported to cause locomotor deficits
(32), the effects of Re
treatment on fly movement were also examined. Exposure of flies to
515 µM Rot reduced their climbing ability to 39.3% compared with
control flies, as measured by a quantitative climbing index
(Fig. 7D). However, co-treatment
of flies with Re (0.4 mM) significantly reversed the effect of Rot
and increased the climbing index to 61.3% of that observed for the
control flies (Fig. 7D).
Re rescues Rot-induced oxidative
damage and mitochondrial dysfunction in Drosophila
Finally, whether reversal of Rot-induced oxidative
stress and mitochondrial dysfunction contribute to the protective
effects of Re were determined in the Drosophila PD model.
Mitochondria are the predominant site of ATP production in cells,
and changes in cellular ATP content can indicate impaired
mitochondrial function (33).
Accordingly, a reduction in ATP levels in Rot-treated flies was
observed that was reversed by Re co-treatment (Fig. 8A). Consistent with a previous
study, mitochondrial respiratory chain complex I (NADH oxidase) has
been demonstrated to be related to the occurrence of PD (34). However, mitochondrial respiratory
chain complex II (succinate dehydrogenase), which is the main
element of electrons entering the mitochondrial electron transport
chain (35), also exhibited a
positive effect of Re in flies (Fig.
8B and C). Re also reduced the Rot-induced increase in ROS
(Fig. 8D) and HP (Fig. 8E). However, Re had no significant
effect on the survival curve of Rot-treated flies (UAS-CncC RNAi ×
da-Gal4) (Fig. 8F).
Overall, these results indicated that the neuroprotective effect of
Re in the Drosophila PD model was consistent with that
detected using the SH-SY5Y cell model in vitro.
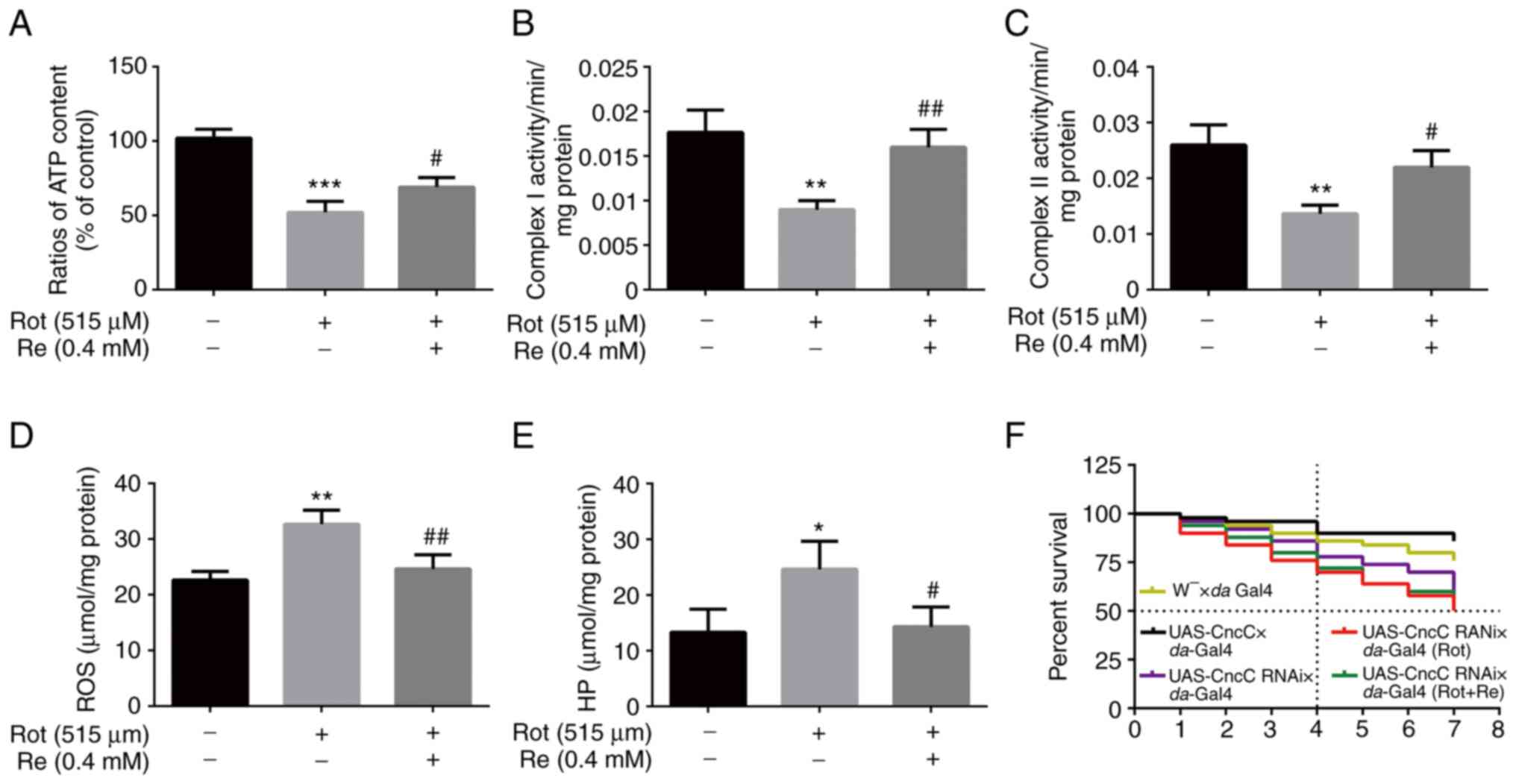 | Figure 8.Re rescues Rot-induced oxidative
stress and mitochondrial dysfunction in Drosophila. Flies
were treated with 515 µM Rot with or without 0.4 mM Re and analyzed
for (A) ATP levels, (B) mitochondrial respiratory chain complex I
levels, (C) mitochondrial respiratory chain complex II levels, (D)
ROS levels, and (E) HP levels. (F) Life survival curve of
Drosophila; n=50 flies per condition. *P<0.05,
**P<0.01 and ***P<0.001 vs. the control;
#P<0.05 and ##P<0.01 vs. Rot treatment
alone. Re, ginsenoside Re; Rot, rotenone; ATP, adenosine
triphosphate; ROS, reactive oxygen species; HP, hydrogen
peroxide. |
Discussion
Re was previously shown to have neuroprotective
properties in several models of neurological diseases, including
PD. In the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model
of PD, Re protected nigral neurons from apoptosis, reduced carbon
tetrachloride-induced cell loss and degeneration, and maintained
TH+ cell and neurite numbers (32). Re had previously been shown to
cross the blood-brain barrier in adult rats (36), further demonstrating that Re has a
better application prospect in neuroprotection and PD treatment or
prevention. In the present study, the protective effects of Re and
other ginsenosides against Rot-induced toxicity were analyzed in a
human neuroblastoma cell line and a Drosophila functional PD
model. The ginsenosides assessed were selected because they have
been reported to be protective in PD models in vivo or in
vitro (37). At optimal
concentrations, Re had the most potent effects among the compounds
examined. Notably, no significant differences between the
cytoprotective effects of Re and l-dopa were observed. The
neuroprotective effects of Re in vitro were also observed in
the in vivo Drosophila model, in which Re co-treatment
reduced Rot-induced loss of TH+ neurons and partially
restored the defects in motor behavior. Thus, the results of the
present study further confirm previous suggestions that Re may be
the main active ingredient in ginseng and its effects on PD.
Multiple studies have described the neuroprotective
effect of ginsenosides such as Re, including their ability to
inhibit apoptosis (38–40). The results of the present study
confirmed this in SH-SY5Y cells, and additionally demonstrated that
Re reduces the characteristic changes in morphology associated with
apoptosis. Two major apoptotic pathways have been described. In the
exogenous pathway, apoptosis is triggered by extracellular signals
that initiate a cascade of events leading to caspase-8 activation;
in the endogenous pathway, mitochondria release factors that lead
to activation of caspases-3 and −9 (41). In the present study it was
demonstrated that Rot induced loss of MMP integrity, cyt-c release
into the cytoplasm, activation of caspases-3 and −9, and
additionally decreased the ratio of the pro-/anti-apoptotic
proteins Bax/Bcl-2. Re inhibited these apoptosis-related events,
including the activation of caspase-3 and −9, but had no
significant effect on caspase-8 activity. These results suggest
that Re inhibits activation of the endogenous mitochondrial
apoptotic pathway in this PD model and raises the possibility that
this pathway could be a therapeutic target for PD. Notably, the
protective effect of Re on mitochondrial function was also observed
in the Drosophila PD model.
Oxidative stress is one of the main causes of human
PD and Rot-mediated damage in animal PD models (42). A previous study by the authors
demonstrated that Re prevents ROS generation in Aβ-induced SH-SY5Y
cells (17), and findings in the
present study revealed that Re suppressed Rot-triggered ROS
production, which was consistent with that observation. GSH-Px,
SOD, and GSH are important cellular antioxidants and have been
widely studied due to their ability to remove oxygen free radicals
in vivo. MDA content in cells indirectly reflects the lipid
peroxidation rate and thus the degree of oxidative stress damage
(43). The results of the present
study suggest that Re strengthens the cellular antioxidant response
by increasing SOD, GSH-Px, and GSH expression and reducing MDA
content. Re also significantly improved Rot-induced compromise of
the antioxidant capacity in the PD model in flies. These data
clearly established that Re suppresses Rot-induced oxidative
stress.
The Nrf2 activation pathway plays an important role
in regulating the cellular response to oxidative stress, mainly by
regulating the expression of antioxidant, anti-inflammatory, and
detoxifying proteins (44).
Modulation of this pathway therefore represents a potential target
to prevent and treat PD. Re promotes the transport of Nrf2 into the
nucleus and consequently increases the expression of oxidative
stress response genes such as HO-1. Increased expression of HO-1
has been shown to protect against Rot-induced neurotoxicity
(45). In the present study, it
was determined that Rot modestly increased the nuclear transport of
Nrf2 but the addition of Re to Rot-treated cells significantly
increased Nrf2 nuclear localization and upregulated expression of
HO-1. Moreover, siRNA-mediated Nrf2 silencing confirmed that Nrf2
activation is an essential component of the antioxidant and
anti-apoptotic effects of Re. It was also observed that Re promoted
phosphorylation of PI3K/AKT and ERK and that PI3K/AKT and ERK
inhibitors significantly diminished the ability of Re to suppress
apoptosis and ROS production in Rot-treated cells. Based on these
results, it is theorized that Re may regulate Nrf2 activation and
HO-1 expression via activation of the PI3K/AKT and ERK signaling
pathways.
In summary, the key findings in the present study
include: i) Re alleviates Rot-induced dopaminergic neuron loss and
locomotor deficits in Drosophila and protects SH-SY5Y cells
against Rot-induced apoptotic death in vitro; ii) inhibition
of Rot-induced oxidative stress and increased expression of
antioxidant enzymes are likely to be the dominant mechanisms by
which Re exerts its neuroprotective effects; iii) the Nrf2
activation pathway contributes to Re-mediated neuroprotection
against Rot; and iv) Re-induced Nrf2 activation may be mediated, at
least in part, via activation of PI3K/AKT and ERK dual pathways
(Fig. 9). In future, RNA-seq
technology may be further used to comprehensively explore other
potential signaling pathways of Re to delay the progression of
PD.
 | Figure 9.Scheme summarizing the
neuroprotective effects of Re. In vitro and in vivo
experimental models of PD showed that treatment with Re prevents
apoptosis induced by Rot-stimulated oxidative stress and
mitochondrial dysfunction via a mechanism involving the PI3K/AKT,
ERK, and Nrf2/HO-1 pathways. Re, ginsenoside Re; PD, Parkinson's
disease; Rot, rotenone; PI3K, phosphatidylinositol 3-kinase; AKT,
protein kinase B; ERK, extracellular regulated protein kinase;
Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme
oxygenase-1; ROS, reactive oxygen species; p-, phosphorylated; MDA,
malondialdehyde; GSH, glutathione; SOD, superoxide dismutase;
GSH-Px, glutathione peroxidase; GCLM, glutamate cysteine ligase
modifier; NQO1, NAD(P)H:quinone oxidoreductase 1; siRNA, small
interfering RNA; Cyt-c, cytochrome c; Bax, Bcl-2 associated
X protein; B-cell lymphoma-2; siRNA, small interfering RNA. |
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like thank Dr Yufeng Yang from the
Institute of Life Sciences, Fuzhou University, Fuzhou, China for
providing us with the Drosophila for the experiment.
Funding
The present study was supported by the Key Project at Central
Government Level: The Ability of Establishment of Sustainable Use
for Valuable Chinese Medicine Resources (grant no. 2060302). In
addition, it was also supported by the Science and Technology
Project of Jilin Provincial Education Department (grant nos.
JJKH20210966KJ and JJKH20210967KJ).
Availability of date and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JQ, SL and ML conceived and designed the
experiments. YL, SZ and WZ analyzed the data, prepared the figures,
and helped with the writing of the manuscript. JQ and YZ performed
the experiments and wrote the manuscript. SL and ML provided
technical expertise and edited the article. JQ and ML confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript, and agree to be accountable for all aspects of
the research in ensuring that the accuracy or integrity of any part
of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang Y, Ren R, Sanford LD, Yang L, Zhou
J, Tan L, Li T, Zhang J, Wing YK, Shi J, et al: Sleep in
Parkinson's disease: A systematic review and meta-analysis of
polysomnographic findings. Sleep Med Rev. 51:1012812020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ou Z, Pan J, Tang S, Duan D, Yu D, Nong H
and Wang Z: Global trends in the incidence, prevalence, and years
lived with disability of parkinson's disease in 204
countries/territories from 1990 to 2019. Front Public Health.
9:7768472021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choong CJ and Mochizuki H: Neuropathology
of α-synuclein in Parkinson's disease. Neuropathology. 42:93–103.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kurihara K, Mishima T, Fujioka S and
Tsuboi Y: Efficacy and safety evaluation of safinamide as an add-on
treatment to levodopa for parkinson's disease. Expert Opin Drug
Saf. 21:137–147. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uzuegbunam BC, Librizzi D and Hooshyar
Yousefi B: PET radiopharmaceuticals for alzheimer's disease and
parkinson's disease diagnosis, the current and future landscape.
Molecules. 25:9772020. View Article : Google Scholar
|
|
6
|
Tabassum R and Jeong NY: Potential for
therapeutic use of hydrogen sulfide in oxidative stress-induced
neurodegenerative diseases. Int J Med Sci. 16:1386–1396. 2019.
View Article : Google Scholar
|
|
7
|
Wilkaniec A, Cieślik M, Murawska E, Babiec
L, Gąssowska-Dobrowolska M, Pałasz E, Jęśko H and Adamczyk A: P2X7
receptor is involved in mitochondrial dysfunction induced by
extracellular alpha synuclein in neuroblastoma SH-SY5Y cells. Int J
Mol Sci. 21:39592020. View Article : Google Scholar
|
|
8
|
Feng ST, Wang ZZ, Yuan YH, Sun HM, Chen NH
and Zhang Y: Update on the association between alpha-synuclein and
tau with mitochondrial dysfunction: Implications for parkinson's
disease. Eur J Neurosci. 53:2946–2959. 2021. View Article : Google Scholar
|
|
9
|
Lin KJ, Lin KL, Chen SD, Liou CW, Chuang
YC, Lin HY and Lin TK: The overcrowded crossroads: Mitochondria,
Alpha-Synuclein, and the Endo-Lysosomal system interaction in
parkinson's disease. Int J Mol Sci. 20:53122019. View Article : Google Scholar
|
|
10
|
Minato T, Nakamura N, Saiki T, Miyabe M,
Ito M, Matsubara T and Naruse K: β-Aminoisobutyric acid, L-BAIBA,
protects PC12 cells from hydrogen peroxide-induced oxidative stress
and apoptosis via activation of the AMPK and PI3K/Akt pathway. IBRO
Neurosci Rep. 12:65–72. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li C, Tang B, Feng Y, Tang F, Pui-Man Hoi
M, Su Z and Ming-Yuen Lee S: Pinostrobin exerts neuroprotective
actions in neurotoxin-induced parkinson's disease models through
Nrf2 induction. J Agric Food Chem. 66:8307–8318. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brasil F, de Almeida F, Luckachaki M,
Dall'Oglio E and de Oliveira M: The C-glucosyl flavone isoorientin
pretreatment attenuates the methylglyoxal-induced mitochondrial
dysfunction in the human neuroblastoma SH-SY5Y cells: Role for the
AMPK-PI3K/Akt/Nrf2/γ-GCL/GSH axis. Metab Brain Dis. Mar
22–2022.(Epub ahead of print). View Article : Google Scholar
|
|
13
|
Chiang NN, Lin TH, Teng YS, Sun YC, Chang
KH, Lin CY, Hsieh-Li HM, Su MT, Chen CM and Lee-Chen GJ: Flavones
7,8-DHF, quercetin, and apigenin against tau toxicity via
activation of TRKB signaling in ΔK280 TauRD-DsRed SH-SY5Y Cells.
Front Aging Neurosci. 13:7588952021. View Article : Google Scholar
|
|
14
|
Wang Y, Yang G, Gong J, Lu F, Diao Q, Sun
J, Zhang K, Tian J and Liu J: Ginseng for Alzheimer's Disease: A
systematic review and meta-analysis of randomized controlled
trials. Curr Top Med Chem. 16:529–536. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Wang Y, Ma C, Yan Y, Yang Y, Wang
X and Rausch WD: Ginsenoside Rd and ginsenoside Re offer
neuroprotection in a novel model of parkinson's disease. Am J
Neurodegener Dis. 5:52–61. 2016.PubMed/NCBI
|
|
16
|
Rai SN and Singh P: Advancement in the
modelling and therapeutics of parkinson's disease. J Chem
Neuroanat. 104:1017522020. View Article : Google Scholar
|
|
17
|
Liu M, Bai X, Yu S, Zhao W, Qiao J, Liu Y,
Zhao D, Wang J and Wang S: Ginsenoside Re inhibits ROS/ASK-1
dependent mitochondrial apoptosis pathway and activation of
Nrf2-antioxidant response in beta-Amyloid-challenged SH-SY5Y cells.
Molecules. 24:26872019. View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu M, Yu S, Wang J, Qiao J, Liu Y, Wang S
and Zhao Y: Ginseng protein protects against mitochondrial
dysfunction and neurodegeneration by inducing mitochondrial
unfolded protein response in Drosophila melanogaster PINK1 model of
parkinson's disease. J Ethnopharmacol. 247:1122132020. View Article : Google Scholar
|
|
20
|
Ahmad S, Hussain A, Ullah F, Jamil M, Ali
A, Ali S and Luo Y: 60Co-γ radiation alters developmental stages of
zeugodacus cucurbitae (Diptera: Tephritidae) through apoptosis
pathways gene expression. J Insect Sci. 21:162021. View Article : Google Scholar
|
|
21
|
Ramalingam M, Huh Y and Lee Y: The
impairments of α-Synuclein and mechanistic target of rapamycin in
rotenone-induced SH-SY5Y cells and mice model of parkinson's
disease. Front Neurosci. 13:10282019. View Article : Google Scholar
|
|
22
|
Ren TT, Yang JY, Wang J, Fan SR, Lan R and
Qin XY: Gisenoside Rg1 attenuates cadmium-induced neurotoxicity in
vitro and in vivo by attenuating oxidative stress and inflammation.
Inflamm Res. 70:1151–1164. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu C, Wu A, Zhu H, Fang H, Xu L, Ye J and
Shen J: Melatonin is involved in the apoptosis and necrosis of
pancreatic cancer cell line SW-1990 via modulating of Bcl-2/Bax
balance. Biomed Pharmacother. 67:133–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yatchenko Y and Ben-Shachar D: Update of
mitochondrial network analysis by imaging: Proof of technique in
schizophrenia. Methods Mol Biol. 2277:187–201. 2021. View Article : Google Scholar
|
|
25
|
Zorova LD, Demchenko EA, Korshunova GA,
Tashlitsky VN, Zorov SD, Andrianova NV, Popkov VA, Babenko VA,
Pevzner IB, Silachev DN, et al: Is the mitochondrial membrane
potential (∆Ψ) correctly assessed? Intracellular and
intramitochondrial modifications of the ∆Ψ probe, Rhodamine 123.
Int J Mol Sci. 23:4822022. View Article : Google Scholar
|
|
26
|
Pessoa J: Live-cell visualization of
cytochrome c: A tool to explore apoptosis. Biochem Soc Trans.
49:2903–2915. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chehade H, Fox A, Mor GG and Alvero AB:
Determination of caspase activation by western blot. Methods Mol
Biol. 2255:1–12. 2021. View Article : Google Scholar
|
|
28
|
Yüksel M, Nazıroğlu M and Özkaya MO:
Long-term exposure to electromagnetic radiation from mobile phones
and Wi-Fi devices decreases plasma prolactin, progesterone, and
estrogen levels but increases uterine oxidative stress in pregnant
rats and their offspring. Endocrine. 52:352–362. 2016. View Article : Google Scholar
|
|
29
|
Shahcheraghi SH, Salemi F, Peirovi N,
Ayatollahi J, Alam W, Khan H and Saso L: Nrf2 regulation by
curcumin: Molecular aspects for therapeutic prospects. Molecules.
28:1672021. View Article : Google Scholar
|
|
30
|
Kanno T, Tanaka K, Yanagisawa Y, Yasutake
K, Hadano S, Yoshii F, Hirayama N and Ikeda JE: A novel small
molecule, N-(4-(2-pyridyl)
(1,3-thiazol-2-yl))-2-(2,4,6-trimethylphenoxy) acetamide,
selectively protects against oxidative stress-induced cell death by
activating the Nrf2-ARE pathway: Therapeutic implications for ALS.
Free Radic Biol Med. 53:2028–2042. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu X, Wang C, Liu W, Song S, Fu J,
Hayashi T, Mizuno K, Hattori S, Fujisaki H and Ikejima T: Oral
administration of silibinin ameliorates cognitive deficits of
parkinson's disease mouse model by restoring mitochondrial
disorders in hippocampus. Neurochem Res. 46:2317–2332. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Y, Tang J, Khatibi NH, Zhu M, Chen D,
Tu L, Chen L and Wang S: Treatment with ginsenoside rb1, a
component of panax ginseng, provides neuroprotection in rats
subjected to subarachnoid hemorrhage-induced brain injury. Acta
Neurochir Suppl. 110:75–79. 2011.
|
|
33
|
Chen X, Zhang Z, Li H, Zhao J, Wei X, Lin
W, Zhao X, Jiang A and Yuan J: Endogenous ethanol produced by
intestinal bacteria induces mitochondrial dysfunction in
non-alcoholic fatty liver disease. J Gastroenterol Hepatol.
35:2009–2019. 2020. View Article : Google Scholar
|
|
34
|
Gibson GE, Kingsbury AE, Xu H, Lindsay JG,
Daniel S, Foster OJ, Lees AJ and Blass JP: Deficits in a
tricarboxylic acid cycle enzyme in brains from patients with
parkinson's disease. Neurochem Int. 43:129–135. 2003. View Article : Google Scholar
|
|
35
|
Ahn EH, Lei K, Kang SS, Wang ZH, Liu X,
Hong W, Wang YT, Edgington-Mitchell LE, Jin L and Ye K:
Mitochondrial dysfunction triggers the pathogenesis of parkinson's
disease in neuronal C/EBP β transgenic mice. Mol Psychiatry.
26:7838–7850. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shi J, Xue W, Zhao WJ and Li KX:
Pharmacokinetics and dopamine/acetylcholine releasing effects of
ginsenoside Re in hippocampus and mPFC of freely moving rats. Acta
Pharmacol Sin. 34:214–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Espinosa-Oliva AM, García-Revilla J,
Alonso-Bellido IM and Burguillos MA: Brainiac caspases: Beyond the
wall of apoptosis. Front Cell Neurosci. 13:5002019. View Article : Google Scholar
|
|
38
|
Madhi I, Kim JH, Shin JE and Kim Y:
Ginsenoside Re exhibits neuroprotective effects by inhibiting
neuroinflammation via CAMK/MAPK/NF-κB signaling in microglia. Mol
Med Rep. 24:6982021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang X, Chu SF, Wang ZZ, Li FF, Yuan YH
and Chen NH: Ginsenoside Rg1 exerts neuroprotective effects in
3-nitropronpionic acid-induced mouse model of Huntington's disease
via suppressing MAPKs and NF-κB pathways in the striatum. Acta
Pharmacol Sin. 42:1409–1421. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Y, Yu S, Xing X, Qiao J, Yin Y, Wang
J, Liu M and Zhang W: Ginsenoside Rh2 stimulates the production of
mitochondrial reactive oxygen species and induces apoptosis of
cervical cancer cells by inhibiting mitochondrial electron transfer
chain complex. Mol Med Rep. 24:8732021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ilie OD, Ciobica A, McKenna J, Doroftei B
and Mavroudis I: Minireview on the relations between Gut microflora
and parkinson's disease: Further biochemical (oxidative stress),
inflammatory, and neurological particularities. Oxid Med Cell
Longev. 2020:45180232020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li Q, Qiu Z, Wang Y, Guo C, Cai X, Zhang
Y, Liu L, Xue H and Tang J: Tea polyphenols alleviate hydrogen
peroxide-induced oxidative stress damage through the Mst/Nrf2 axis
and the Keap1/Nrf2/HO-1 pathway in murine RAW264.7 cells. Exp Ther
Med. 22:14732021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Terada K, Murata A, Toki E, Goto S,
Yamakawa H, Setoguchi S, Watase D, Koga M, Takata J, Matsunaga K
and Karube Y: Atypical antipsychotic drug ziprasidone protects
against rotenone-induced neurotoxicity: An in vitro study.
Molecules. 25:42062020. View Article : Google Scholar
|
|
44
|
He YB, Liu YL, Yang ZD, Lu JH, Song Y,
Guan YM and Chen YM: Effect of ginsenoside-Rg1 on experimental
parkinson's disease: A systematic review and meta-analysis of
animal studies. Exp Ther Med. 21:5522021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Han Y, Wang T, Li C, Wang Z, Zhao Y, He J,
Fu L and Han B: Ginsenoside Rg3 exerts a neuroprotective effect in
rotenone-induced parkinson's disease mice via its anti-oxidative
properties. Eur J Pharmacol. 909:1744132021. View Article : Google Scholar
|