Introduction
Myocardial infarction (MI) is ischemic necrosis of
myocardial tissue (1). It is the
leading cause of death globally and ~20% of patients experiencing
an MI die within 1 year of the event (2). Thus, restoring blood supply as early
as possible is key to decrease infarction volume and protect
cardiac function. Restoring blood supply is termed reperfusion in
acute MI treatment (3). However,
reperfusion/reoxygenation worsens myocardial tissue injury, known
as ischemic-reperfusion (I/R) injury (4,5).
Long non-coding (lnc)RNAs, a class of ncRNA, are
more >200 bp in length. lncRNAs were once considered to exhibit
no biological function due to unclear functions and species
(6). With the development of high
throughput sequencing technology, the functions of lncRNAs have
been identified (7–9). lncRNAs are involved in a number of
regulatory processes, including chromosomal dose compensation,
genomic imprinting, epigenetic regulation, cell differentiation and
stem cell maintenance (10–12). Abnormalities in lncRNAs are
associated with cardiovascular disease in humans (13). Evidence suggests that lncRNAs are
involved in the regulation of myocardial apoptosis (14–16).
However, the effect of lncRNAs on regulation of myocardial
apoptosis remains unclear. Cardiac autophagy inhibitory factor
(CAIF) is a novel discovered lncRNA (17). CAIF suppresses MI by targeting the
p53/myocardin-dependent autophagy pathway (18). In addition, CAIF is downregulated in
end-stage cardiomyopathy (19).
However, the precise role and underlying mechanisms of CAIF remain
to be determined.
MicroRNAs (miRNAs/miRs) are single-stranded, nc
endogenous RNA 18–25 nucleotides in length that negatively regulate
gene expression post-transcription (20,21).
miRNAs serve an important role in a variety of physiological and
pathological processes, including embryonic development (22,23),
cell proliferation and differentiation (24,25),
apoptosis, metabolism and tumorigenesis (26). Studies have reported differential
expression of miRNAs in MI including, miR-19a/19b, miR-21, miR-206
and miR-483 (27,28).
Apoptosis and caspase activation inhibitor (AVEN) is
an anti-apoptotic protein that controls apoptosis partially by
abrogating caspase activation via binding to Bcl-xL and apoptotic
peptidase activating factor (Apaf)-1. AVEN was demonstrated to
mediate the protective effect of miR-30b-5p on hypoxia-induced
cardiomyocyte injury (29).
The present study aimed to explore the role of CAIF
in MI progression. Cardiomyocytes were treated with 200 µM
H2O2 for 4 h to establish cardiomyocyte
injury in vitro and female C57BL/6 mice were purchased for
MI model establishment in vivo. Flow cytometry and TUNEL
assays were performed to analyzed cell apoptosis. CAIF, miR-488-5p
and AVEN levels were measured by PCR or western blot. The present
study may provide a potential target for the diagnosis of infarct
heart diseases.
Materials and methods
Mouse model of MI
A total of 24 female C57BL/6 mice (weight,
20.12±1.28 g; age, 12 weeks) were purchased from Beijing Vital
River Laboratory Animal Technology Co., Ltd. (Beijing, China) and
housed in a 12/12-h light/dark cycle at 22–26°C and 50–70% humidity
with ad libitum access to food and water. The mice were
divided into Sham, I/R, I/R + Ad-nc and I/R + Ad-CAIF groups (all
n=6). For I/R treatment, mice were anesthetized with 1%
pentobarbital (60 mg/kg) and were intubated and attached to a small
animal ventilator. Following thoracotomy, the intercostal muscle
was separated in the 3rd and 4th intercostal space to expose the
heart. The middle of the left anterior descending coronary artery
was ligated with a 5–0 surgical suture. Following 30 min ischemia,
the surgical suture was removed for reperfusion. Mice were
euthanized with intraperitoneal injection of 3% pentobarbital (180
mg/kg). Cessation of breathing and loss of the righting reflex were
considered to indicate mortality. The mice in the I/R + Ad-nc group
were injected with 100 µl Ad-nc via the tail vein, and the mice in
the I/R + Ad-CAIF group were injected with 100 µl Ad-CAIF 7 days
before I/R. The mice in the sham group were only exposed by
thoracotomy without ligation. In the I/R group, the heart was
harvested at 0, 1, 4 or 8 h after reperfusion for the further
analysis. The study was approved by the Ethics Committee of Guilin
People's Hospital.
Isolation of primary
cardiomyocytes
A total of 10 female neonatal mice (weight,
19.58±1.34 g; age, 1–3 days) were purchased from Beijing Vital
River Laboratory Animal Technology Co., Ltd. and euthanized using
CO2 (displacement rate, 60% volume/min) followed by
cervical dislocation according to AVMA guidelines (30). Cessation of breathing and movement
were considered to indicate mortality. Then, the mice were
sterilized with alcohol and the heart was removed. Then, 1 0.10%
collagenase and 1 ml 0.08% trypsin were added into a 15 ml
centrifuge tube. The chopped tissue was digested at 37°C for 10 min
and centrifuged at 4°C (710.4 × g, 10 min). After that, the
supernatant was discarded to remove endothelial cells. Then, 1 ml
0.1% II collagen proteinase was added to the 15 ml tube and
digested for 10 min at 37°C. After centrifuging at 4°C (710.4 g, 10
min), the supernatant was transferred to a new 50 ml centrifuge
tube and 1 ml DMEM/F12, supplemented with 10% FBS (both Procell),
was added. This process was repeated 6–7 times. The cell suspension
was centrifuged at 4°C and 200 × g for 5 min, then cells were
collected and inoculated into a sterile 35-mm petri dish and
cultured for 120 min (37°C, 5% CO2). The non-adherent
cells were inoculated into a new gelatin-coated petri dish
(DMEM/F12 supplemented with 10% FBS) and cultured for 24 h (37°C,
5% CO2) to obtain cardiomyocytes.
H2O2
treatment
As oxidative stress injury is an important part of
I/R injury (31).
H2O2 was used to simulate oxidative injury in
cardiomyocytes. Cardiomyocytes were treated with 200 µM
H2O2 for 4 h at 37°C to establish
cardiomyocyte injury, as previously described (32,33).
Oligonucleotide transfection
Adenovirus overexpressing CAIF (Ad-CAIF), small
interfering CAIF (si-CAIF) and their negative control were obtained
from Hanbio Biotechnology Co., Ltd. miR-488-5p mimic and mimic
control (50 nM), inhibitor and inhibitor control (50 nM) were
purchased from Shanghai GenePharma Co., Ltd. Oligonucleotides were
transfected using a Lipofectamine® 3000 kit (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions at room temperature. At 48 h post-transfection, cells
were harvested for subsequent experiments. Sequences were as
follows: miR-488-5p mimic: 5′CCCAGATAATGGCACTCTCAA3′; mimic
control: 5′CCTGAGTCCGACAATTACGTAC3′; miR-488-5p inhibitor:
5′TTGAGAGTGCCATTATCTGGG3′ and inhibitor control,
5′CTAGGGATACCGTTTATCATAAC3′.
Reverse transcription-quantitative
(RT-q)PCR
RNA was extracted from tissue and cells using an
Beyozol RNA extraction kit (Beyotime Institute of Biotechnology)
according to the manufacturer's instructions. A total of 500 ng RNA
was reverse-transcribed into cDNA using a Reverse Transcription kit
(Takara Bio, Inc.) according to the manufacturer's instructions.
RT-qPCR was performed using a SYBR-Green kit (Takara) and a PCR
Detection system (Applied Biosystems) according to the
manufacturer's instructions. The RT-qPCR conditions consisted of
initial denaturation at 95°C for 30 sec, followed by 40 cycles of
denaturation at 95°C for 5 sec and annealing at 60°C for 30 sec.
The expression of miR-488-5p was normalized to U6, while the
expression of CAIF and AVEN mRNA were normalized to GAPDH. The
2−ΔΔCq method (34) was
applied to calculate the relative expression levels. The primer
sequences were as follows: CAIF forward,
5′-CTTCACTCCTGCAAATGTGTT-3′, reverse, 5′-TTATAGTGGGATGGGCAGTTT-3′;
miR-488-5p forward: 5′-CCCAGATAATGGCACTC-3′, reverse,
5′-GAACATGTCTGCGTATCTC-3′; AVEN forward: 5′-GCGCCGGTTGAAGATGACA-3′,
reverse: 5′-TGCAGAGCTAAGGAGGACACT-3′; GAPDH forward:
5′-TCGGAGTGAACGGATTTGGC-3′, reverse, 5′-TGACAAGCTTCCCGTTCTCC-3′; U6
forward, 5′-AGTAAGCCCTTGCTGTCAGTG-3′ and reverse:
5′-CCTGGGTCTGATAATGCTGGG-3′.
Western blotting
Total protein was extracted from cells with RIPA
buffer (Beyotime). BCA kit was used to detect protein concentration
and 12% SDS-PAGE was performed to separate proteins (40 µg) in
samples. Then, the separated proteins were transferred to PVDF
membranes and blocked with 5% non-fat milk at room temperature for
2 h. The proteins on PVDF membranes were labeled with primary
antibodies (AVEN, ab133285, 1:800; GAPDH, ab8245, 1:2500; both
Abcam) at 4°C overnight and horseradish peroxidase-conjugated
secondary goat-anti-rabbit IgG (1:1,000, cat. no. ab150077, Abcam)
at room temperature for 2 h. ECL kit (Beyotime Institute of
Biotechnology) was used to visualize the blots. Images were
recorded with the Luminescent Image Analyzer LAS-4000 system
(Fujifilm) and quantified by the Gel-Pro Analyzer 4.0 software
(Media Cybernetics, Inc.).
Flow cytometry
A total of ~1×106 cardiomycoytes/ml were
collected and centrifuged at centrifuged at 4°C and 710.4 g for 5
min and the culture medium was discarded. The cells were washed
with 3 ml PBS and centrifuged at 4°C and 710.4 g for 5 min, cells
were fixed with precooled 70% ethanol at 4°C for 2 h. Then, cells
were incubated with FITC-Annexin V (300 ng/ml; 4°C) for 10 min to
label apoptotic cells. The samples were further incubated with PI
(Procell Life Science & Technology Co., Ltd.) at 4°C for 5 min.
The apoptotic cells were detected using a Fortessa flow cytometer
(BD Biosciences). FlowJo10 (BD Biosciences) was used to analyze the
flow cytometry data.
Dual luciferase reporter assay
Bioinformatics analysis using mirDB (mirdb.org/) and
TargetScan 7.1 (targetscan.org/vert_71/) was used to predict
binding sites. The predicted binding sites of miR-488-5p on CAIF
and AVEN mRNA were cloned into pGL3 luciferase reporter vector
(Promega Corporation) and named CAIF-wild-type (WT) and AVEN-WT,
respectively. Vectors with mutated sequences at the predicted
binding sites were also synthesized and named CAIF-mutant (MUT) and
AVEN-MUT, respectively. The cells were co-transfected with
miR-488-5p mimics or miR-control and MUT or WT vectors using a
Lipofectamine® 3000 kit (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions at
room temperature. TRL-SV40 vector served as an internal reference.
At 48 h post-transfection, the luciferase activity was detected
using a dual-luciferase assay kit and normalized to Renilla
luciferase activity (Promega Corporation). The sequences were as
follows: miR-488-5p sequences: mimic, CCCAGATAATGGCACTCTCAA;
inhibitor: TTGAGAGTGCCATTATCTGGG.
2,3,5-Triphenyltetrazolium chloride
(TTC) staining and evaluation of infarcted and viable
myocardium
Hearts were harvested following reperfusion
treatment. Perpendicular to the long axis of the heart, the heart
was cut into short-axis slices (4 mm) from the apex to the bottom
of the heart, then the slices were placed in a 37°C water bath
within a mass fraction of 1% TTC phosphate buffer for 20 min and
fixed in a volume fraction of 10% formaldehyde at room temperature
for 6 h. The fixed sections were removed, dried and photographed
(Canon EOS 800D).
TUNEL staining
To detect apoptotic cells in cultured myocardial
cells and tissue, TUNEL staining was performed. The cells and
tissue slices were fixed with paraformaldehyde (4%) at room
temperature for 1 h. TUNEL kit (Sigma-Aldrich; Merck KGaA) was
applied to label apoptotic cells according to the manufacturer's
instruction. TUNEL staining was performed at 37°C for 2 h. PBS was
used for rinsing. Then, 200 ml DAPI (1 µg/ml, Sigma-Aldrich; Merck
KGaA) was applied to label nuclei at room temperature for 20 min.
Neutral gum was used to mounting. Images were captured in six
fields of view/sample using an inverted fluorescence microscope
(Nikon Corporation; scale bar, 100 µm). The apoptotic cells were
counted manually.
Reactive oxygen species (ROS)
detection
2′,7′-dichlorofluorescin diacetate (DCFH)
(Sigma-Aldrich; Merck KGaA) was used to detect the levels of ROS.
Briefly, at the indicated time points, cardiomyocytes were rinsed
with PBS (4°C). Then, the cells (3×104) were incubated
with DCFH (10 µM) at 37°C for 30 min in the dark. The fluorescence
intensity of harvested cells was detected via flow cytometry. The
cells were stimulated with 488 nm excitation light by flow
cytometry. The cells were fully washed with fresh culture medium
without serum three times to fully remove DCFH that did not enter
the cells. The fluorescence intensity was detected on the flow
cytometer within 1 h. The acquired data were analyzed with
Flowjo_V10 (35).
MDA and LDH levels determination
The MDA and LDH levels of the cells were determined
using Malondialdehyde (MDA) assay kit (cat. A003-1-2; Jiancheng)
and Lactate dehydrogenase assay kit (cat. A020-1-2; Jiancheng)
according to the instructions.
RNA pull-down assay
AmpliScribe™ T7-Flash™
Biotin-RNA Transcription kit (cat. no. ASB71110; Epicentre;
Illumina, Inc.) was used to transcribe and purify biotin-labeled
RNA probe in vitro according to manufacturer's protocol.
1×107 cardiomycoytes was subjected to RNA extraction
using RNA extraction kit (Beyotime Institute of Biotechnology) at
room temperature for 2 h. The biotin-labeled probe was incubated
with 500 µl cell RNA. The mixture was incubated with streptavidin
agarose beads (Invitrogen; Thermo Fisher Scientific, Inc.) at room
temperature for 1 h. Following strict washing with
detergent/combined buffer, the complex were collected and
centrifuged at 4°C and 10,000 g for 15 min and the recovered
samples were analyzed. The results were determined using
RT-qPCR.
Statistical analysis
Data are expressed as the mean ± SD of three
independent repeats. GraphPad 6.0 software (GraphPad Software,
Inc.) was used for statistical analysis. Comparisons between two
groups were performed by unpaired t-test. Comparisons between
multiple groups were performed by one way ANOVA followed by post
hoc Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
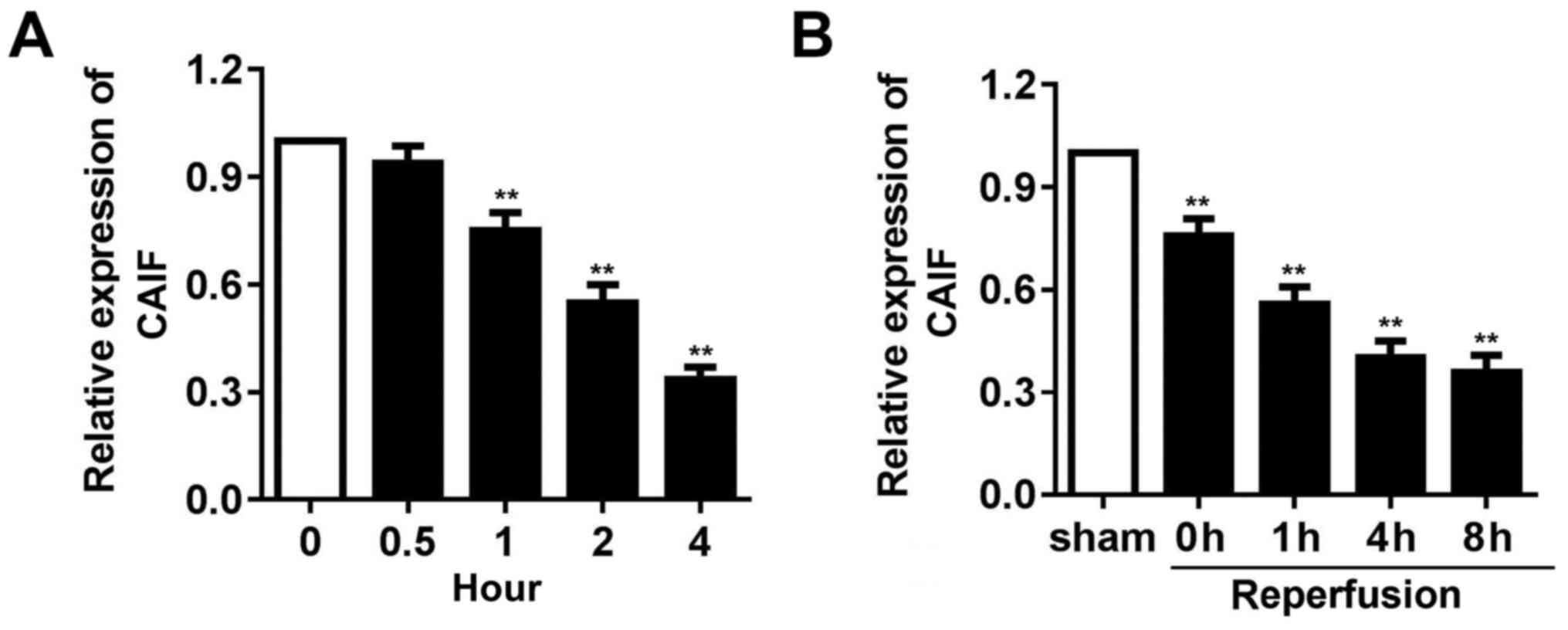
CAIF expression is decreased in
myocardium following I/R and in cardiomyocytes following
H2O2 treatment
To detect whether CAIF serves a role in cardiac I/R
injury, mouse I/R and oxidative stress models using
H2O2 treated cardiomyocytes were established.
RT-qPCR indicated that CAIF expression in cardiomyocytes
significantly decreased from 1 h post-H2O2
treatment (Fig. 1A). CAIF levels in
the myocardium of mice decreased during ischemia and reperfusion
further inhibited its expression (Fig.
1B). These results indicated that CAIF was downregulated in
MI.
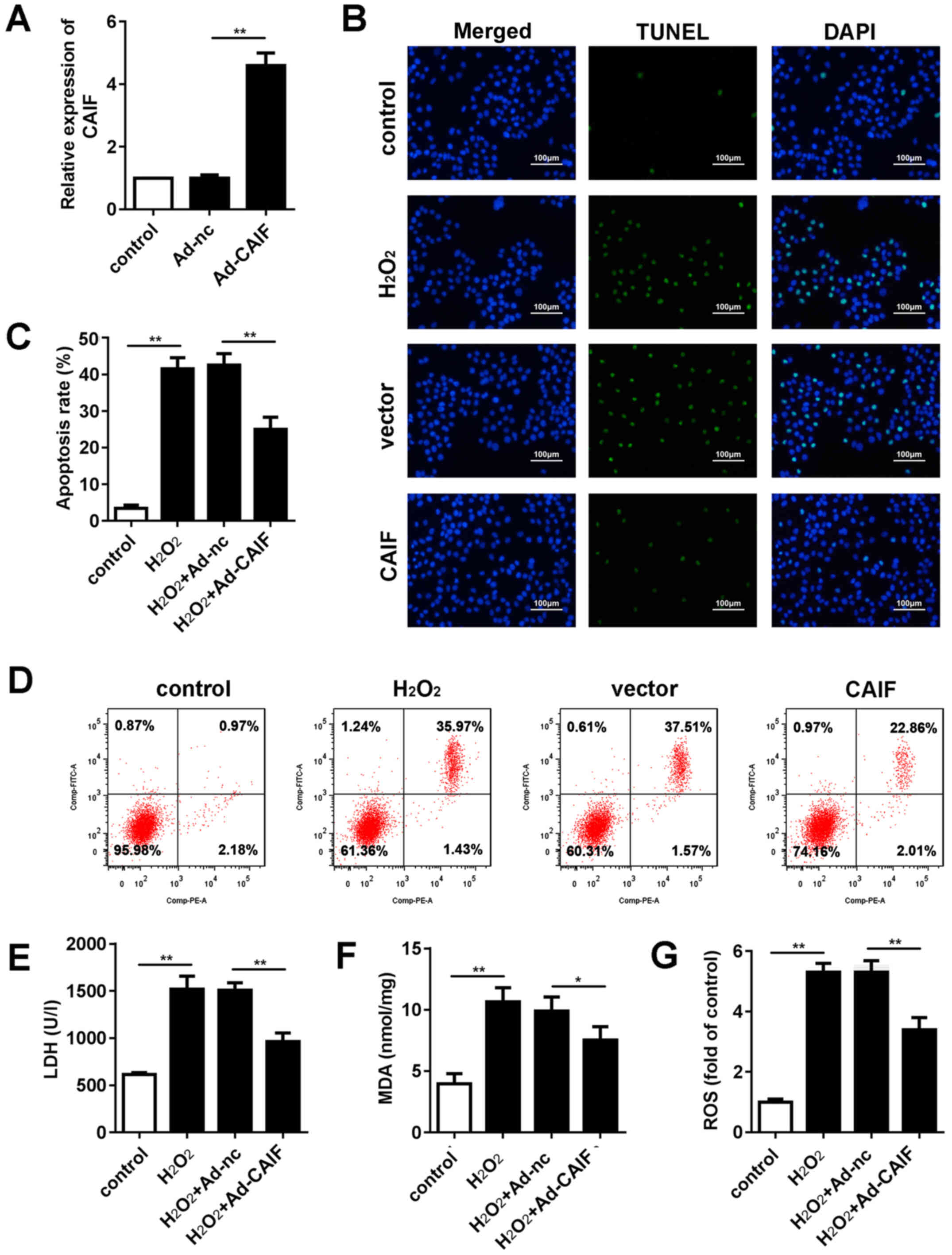
CAIF overexpression inhibits
H2O2-induced oxidative stress injury in
cardiomyocytes
MI is accompanied by oxidative stress and
cardiomyocyte apoptosis (36). An
oxidative stress injury model was established in vitro via
H2O2 treatment. The efficiency of Ad
overexpressing CAIF was assessed; RT-qPCR indicated that Ad-CAIF
significantly promoted CAIF expression (Fig. 2A). Flow cytometry and TUNEL assay
were performed to evaluate apoptosis of cardiomyocytes. CAIF
significantly inhibited apoptosis of cardiomyocytes compared with
the negative control group (Fig.
2B-D). Furthermore, release of lactate dehydrogenase (LDH) and
malonaldehyde (MDA) in cell culture medium was assessed.
H2O2 treatment significantly elevated the
levels of LDH and MDA, while CAIF overexpression suppressed this
(Fig. 2E and F). ROS activity was
evaluated. H2O2 significantly promoted ROS
activity of cardiomyocytes; this effect was decreased by CAIF
overexpression (Fig. 2G). These
results indicated that CAIF overexpression relieved the oxidative
stress injury in MI.
CAIF decreases MI and apoptosis
Animals were injected with Ad-CAIF via the tail vein
and I/R was performed 7 days later. RT-qPCR showed that the levels
of CAIF significantly increased in the myocardium following Ad-CAIF
administration (Fig. 3A). Compared
with sham group, the infarct area was significantly increased in
the I/R group; this effect was decreased by CAIF (Fig. 3B). TUNEL staining was used to
evaluate apoptosis. CAIF significantly inhibited the I/R-induced
increase in myocardium apoptosis (Fig.
3C). These results indicated that CAIF overexpression relieved
MI progression in vivo.
CAIF sponges miR-488-5p
mirDB was used to predict the potential target genes
of CAIF; the results showed that there was a complementary binding
site with miR-488-5p in the sequence of CAIF (Fig. 4A). Luciferase reporter assay showed
that miR-448-5p decreased the luciferase activity of cardiomyocytes
transfected with reporter vector carrying CAIF-WT but not CAIF-MUT
(Fig. 4B). RNA pull-down assay
further demonstrated that CAIF directly bound to miR-488-5p
(Fig. 4C). Expression levels of
miR-488-5p were evaluated following CAIF overexpression or
knockdown. The results indicated that CAIF overexpression
significantly decreased miR-488-5p levels, whereas CAIF knockdown
increased them (Fig. 4D). These
results indicated a direct interaction between miR-488-5p and CAIF.
These results indicated that miR-488-5p was negatively regulated by
CAIF in MI.
miR-488-5p overexpression reverses the
protective effect of CAIF
To confirm the interaction between miR-488-5p and
CAIF, rescue experiments were performed. RT-qPCR indicated that
miR-488-5p mimic restored expression levels of miR-488-5p decreased
by CAIF (Fig. 5A). Apoptosis assay
showed that CAIF treatment decreased
H2O2-induced cardiomyocyte apoptosis, but
this effect was partially reversed by miR-488-5p overexpression
(Fig. 5B-D). The levels of LDH and
MDA as well as ROS activity were detected. CAIF inhibited release
of LDH and MDA as well as ROS activity, while miR-488-5p partially
reversed this effect (Fig. 5E-G).
These results confirmed the interaction between miR-488-5p and
CAIF. These results indicated that CAIF relieved the oxidative
stress injury in MI via regulating miR-488-5p expression.
miR-488-5p directly targets AVEN
To elucidate the precise mechanism of CAIF and
miR-488-5p in I/R injury, the downstream genes regulated by
miR-488-5p were investigated. AVEN was predicted to be a potential
target of miR-488-5p via Targetscan. Fig. 6A shows the binding site between
miR-488-5p and CAIF. Luciferase assay showed that miR-448-5p
decreased luciferase activity of cardiomyocytes transfected with
reporter vector carrying AVEN-WT but not AVEN-MUT (Fig. 6B). RT-qPCR and western blotting
indicated that miR-488-5p inhibited mRNA and protein expression
levels of AVEN, whereas miR-488-5p promoted AVEN expression
(Fig. 6C and D). RNA pull-down
assay further demonstrated that AVEN can directly bound to
miR-488-5p (Fig. 6E). These results
indicated that miR-488-5p serves role in MI via targeting AVEN.
Discussion
MI, which is caused by blockage of coronary arteries
and leads to apoptosis or necrosis of myocardial cells due to
persistent ischemia and hypoxia, has one of the highest mortality
rates in the world (2). Apoptosis
is a process of spontaneous cell death controlled by genes
(7). Following MI, myocardial cell
apoptosis is an important factor leading to ventricular function
weakening and myocardial remodeling (37). Therefore, inhibiting apoptosis of
cardiomyocytes in the early stage of MI may effectively control its
progression.
Zhuo et al (38) found an association between CAIF and
MI and demonstrated that lncRNAs inhibit autophagy of myocardial
cells and attenuate MI by blocking p53-mediated myocardin
transcription. The expression of myocardin is upregulated by
H2O2 and I/R and myocardin knockdown inhibits
autophagy and weakens MI. p53 regulates autophagy and myocardial
I/R injury by regulating expression of myocardin (39). CAIF binds directly to p53 protein
and blocks p53-mediated myocardin transcription, which leads to
decreased myocardin expression (40,41).
Myocardial cell apoptosis and oxidative stress are involved in a
series of pathological changes, including ventricular remodeling
and heart failure, following MI (42–44).
Among these, myocardial cell apoptosis is an important factor in
MI, which leads to the infarction but also promotes myocardial
remodeling (45). In the present
study, CAIF inhibited the infarct area and apoptosis of
cardiomyocytes in vivo, and protected cultured
cardiomyocytes from H2O2-induced
apoptosis.
lncRNAs serve as sponges of miRNA molecules, binding
with miRNA via endogenous competition to inhibit degradation of
mRNA (46,47). Abnormally expressed miRNAs are
widely used in clinical diagnosis and treatment of tumors. miRNAs
affect cardiovascular development, diseases and other
pathophysiological processes (48,49) by
regulating cell differentiation, migration and proliferation. In
the present study, miR-488-5p was sponged by CAIF in MI, indicating
that miRNAs serve an important role in MI and may be a promising
research direction. To the best of our knowledge, the number of
studies of miR-488-5p is low. miR-488-5p is expressed at low levels
and sponged by small nucleolar host gene 1 in acute myeloid
leukemia (50). In addition,
miR-488-5p acts as a tumor suppressor and is lost during melanoma
development (51).
miRNAs target downstream mRNAs to inhibit their
expression, thus participating in the regulation of cell processes.
For example, downregulated miR-26a-5p induced the apoptosis of
endothelial cells in coronary heart disease via targeting PTEN
(52). MiR-103b promoted apoptosis
in stored platelets via targeting ITGB3 (53). Here, bioinformatics analysis
predicted AVEN as a potential target of miR-488-5p. AVEN binds with
the apoptosis regulator Bcl-xL and Apaf-1 (29). AVEN impairs Apaf-1-mediated
activation of caspases, thus inhibiting proteolytic activation of
caspases and suppressing apoptosis. Moreover, a previous study
indicated that AVEN is involved in hypoxia-induced cardiomyocyte
injury as a target gene of miR-30b-5p (54). However, the present study did not
perform rescue experiments to confirm the interaction between AVEN
and miR-488-5p and CAIF; this should be assessed in future.
In conclusion, the present study observed
downregulated CAIF in the myocardium and cardiomyocytes following
I/R and H202 treatment, respectively. CAIF
inhibited I/R injury via the miR-488-5p/AVEN signaling axis. It
remains to be verified whether the diagnostic and therapeutic
potential of CAIF can be applied to clinical treatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL and RC performed in vitro experiments. XL
and LW performed in vivo experiments. ZL performed
bioinformatics analysis. YL performed statistical analysis. DT
designed the study and wrote the manuscript. XL and DT confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Guilin People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barnett R: Acute myocardial infarction.
Lancet. 393:25802019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ketchum ES, Dickstein K, Kjekshus J, Pitt
B, Wong MF, Linker DT and Levy WC: The seattle post myocardial
infarction model (SPIM): Prediction of mortality after acute
myocardial infarction with left ventricular dysfunction. Eur Heart
J Acute Cardiovasc Care. 3:46–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Villiers C and Riley PR: Mouse models
of myocardial infarction: Comparing permanent ligation and
ischaemia-reperfusion. Dis Model Mech. 13:dmm0465652020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blankenberg S, Neumann JT and Westermann
D: Diagnosing myocardial infarction: A highly sensitive issue.
Lancet. 392:893–894. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Isaaz K and Gerbay A: Deferred stenting in
acute ST elevation myocardial infarction. Lancet. 388:13712016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hombach S and Kretz M: Non-coding RNAs:
Classification, biology and functioning. Adv Exp Med Biol.
937:3–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu BY and Dong B: LncRNA H19 regulates
cardiomyocyte apoptosis and acute myocardial infarction by
targeting miR-29b. Int J Cardiol. 271:252018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yin Y, Lv L and Wang W: Expression of
miRNA-214 in the sera of elderly patients with acute myocardial
infarction and its effect on cardiomyocyte apoptosis. Exp Ther Med.
17:4657–4662. 2019.PubMed/NCBI
|
|
9
|
Xin B, Liu Y, Li G, Xu Y and Cui W: The
role of lncRNA SNHG16 in myocardial cell injury induced by acute
myocardial infarction and the underlying functional regulation
mechanism. Panminerva Med. 63:388–389. 2019.PubMed/NCBI
|
|
10
|
Zhang Y, Jiao L, Sun L, Li Y, Gao Y, Xu C,
Shao Y, Li M, Li C, Lu Y, et al: LncRNA ZFAS1 as a SERCA2a
inhibitor to cause intracellular Ca(2+) overload and contractile
dysfunction in a mouse model of myocardial infarction. Circ Res.
122:1354–1368. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liao J, He Q, Li M, Chen Y, Liu Y and Wang
J: LncRNA MIAT: Myocardial infarction associated and more. Gene.
578:158–161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hao K, Lei W, Wu H, Wu J, Yang Z, Yan S,
Lu XA, Li J, Xia X, Han X, et al: LncRNA-Safe contributes to
cardiac fibrosis through Safe-Sfrp2-HuR complex in mouse myocardial
infarction. Theranostics. 9:7282–7297. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Viereck J, Bührke A, Foinquinos A,
Chatterjee S, Kleeberger JA, Xiao K, Janssen-Peters H, Batkai S,
Ramanujam D, Kraft T, et al: Targeting muscle-enriched long
non-coding RNA H19 reverses pathological cardiac hypertrophy. Eur
Heart J. 41:3462–3474. 2020.PubMed/NCBI
|
|
14
|
Yang J, Huang X, Hu F, Fu X, Jiang Z and
Chen K: LncRNA ANRIL knockdown relieves myocardial cell apoptosis
in acute myocardial infarction by regulating IL-33/ST2. Cell Cycle.
18:3393–3403. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu SY, Dong B, Fang ZF, Hu XQ, Tang L and
Zhou SH: Knockdown of lncRNA AK139328 alleviates myocardial
ischaemia/reperfusion injury in diabetic mice via modulating
miR-204-3p and inhibiting autophagy. J Cell Mol Med. 22:4886–4898.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang SM, Liu GQ, Xian HB, Si JL, Qi SX and
Yu YP: LncRNA NEAT1 alleviates sepsis-induced myocardial injury by
regulating the TLR2/NF-κB signaling pathway. Eur Rev Med Pharmacol
Sci. 23:4898–4907. 2019.PubMed/NCBI
|
|
17
|
Wang Y and Zhang Y: LncRNA CAIF suppresses
LPS-induced inflammation and apoptosis of cardiomyocytes through
regulating miR-16 demethylation. Immun Inflamm Dis. 9:1468–1478.
2021. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu CY, Zhang YH, Li RB, Zhou LY, An T,
Zhang RC, Zhai M, Huang Y, Yan KW, Dong YH, et al: LncRNA CAIF
inhibits autophagy and attenuates myocardial infarction by blocking
p53-mediated myocardin transcription. Nat Commun. 9:292018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leads from the MMWR. Recommendations for
protection against viral hepatitis. JAMA. 254:197–198. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cramer DW and Elias KM: A prognostically
relevant miRNA signature for epithelial ovarian cancer. Lancet
Oncol. 17:1032–1033. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krell J, Stebbing J, Frampton AE,
Carissimi C, Harding V, De Giorgio A, Fulci V, Macino G, Colombo T
and Castellano L: The role of TP53 in miRNA loading onto AGO2 and
in remodelling the miRNA-mRNA interaction network. Lancet. 385
(Suppl 1):S152015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng R, Sang Q, Zhu Y, Fu W, Liu M, Xu Y,
Shi H, Xu Y, Qu R, Chai R, et al: MiRNA-320 in the human follicular
fluid is associated with embryo quality in vivo and affects mouse
embryonic development in vitro. Sci Rep. 5:86892015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Collignon J: miRNA in embryonic
development: The taming of Nodal signaling. Dev Cell. 13:458–460.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhong YX, Li WS, Liao LS and Liang L:
LncRNA CCAT1 promotes cell proliferation and differentiation via
negative modulation of miRNA-218 in human DPSCs. Eur Rev Med
Pharmacol Sci. 23:3575–3583. 2019.PubMed/NCBI
|
|
25
|
Hara ES, Ono M, Eguchi T, Kubota S, Pham
HT, Sonoyama W, Tajima S, Takigawa M, Calderwood SK and Kuboki T:
miRNA-720 controls stem cell phenotype, proliferation and
differentiation of human dental pulp cells. PLoS One. 8:e835452013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen N, Liu S, Cui J, Li Q, You Y, Zhong
Z, Cheng F, Guo AY, Zou P, Yuan G and Zhu X: Tumor necrosis factor
α knockout impaired tumorigenesis in chronic myeloid leukemia cells
partly by metabolism modification and miRNA regulation. Onco
Targets Ther. 12:2355–2364. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu A, Lou L, Zhai J, Zhang D, Chai L, Nie
B, Zhu H, Gao Y, Shang H and Zhao M: miRNA expression profile and
effect of Wenxin granule in rats with ligation-induced myocardial
infarction. Int J Genomics. 2017:21758712017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang ZH, Sun XY, Li CL, Sun YM, Li J, Wang
LF and Li ZQ: miRNA-21 expression in the serum of elderly patients
with acute myocardial infarction. Med Sci Monit. 23:5728–5734.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang L and Jia X: Down-regulation of
miR-30b-5p protects cardiomyocytes against hypoxia-induced injury
by targeting Aven. Cell Mol Biol Lett. 24:612019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
American Veterinary Medical Association, .
AVMA guidelines for complementary and alternative veterinary
medicine. J Am Vet Med Assoc. 218:17312001.PubMed/NCBI
|
|
31
|
Zeng J, Zhu L, Liu J, Zhu T, Xie Z, Sun X
and Zhang H: Metformin protects against oxidative stress injury
induced by ischemia/reperfusion via regulation of the
lncRNA-H19/miR-148a-3p/Rock2 axis. Oxid Med Cell Longev.
2019:87683272019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dhalla NS, Elmoselhi AB, Hata T and Makino
N: Status of myocardial antioxidants in ischemia-reperfusion
injury. Cardiovasc Res. 47:446–456. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang D, Yu J, Liu HB, Yan XQ, Hu J, Yu Y,
Guo J, Yuan Y and Du ZM: The long non-coding RNA TUG1-miR-9a-5p
axis contributes to ischemic injuries by promoting cardiomyocyte
apoptosis via targeting KLF5. Cell Death Dis. 10:9082019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kroeze A, van Hoeven V, Verheij MW,
Turksma AW, Weterings N, van Gassen S, Zeerleder SS, Blom B,
Voermans C and Hazenberg MD: Presence of innate lymphoid cells in
allogeneic hematopoietic grafts correlates with reduced
graft-versus-host disease. Cytotherapy. 24:302–310. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yoshimura C, Nagasaka A, Kurose H and
Nakaya M: Efferocytosis during myocardial infarction. J Biochem.
168:1–6. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
González A, Fortuño MA, Querejeta R,
Ravassa S, López B, López N and Díez J: Cardiomyocyte apoptosis in
hypertensive cardiomyopathy. Cardiovasc Res. 59:549–562. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhuo LA, Wen YT, Wang Y, Liang ZF, Wu G,
Nong MD and Miao L: LncRNA SNHG8 is identified as a key regulator
of acute myocardial infarction by RNA-seq analysis. Lipids Health
Dis. 18:2012019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Su Q, Lv XW, Xu YL, Cai RP, Dai RX, Yang
XH, Zhao WK and Kong BH: Exosomal LINC00174 derived from vascular
endothelial cells attenuates myocardial I/R injury via p53-mediated
autophagy and apoptosis. Mol Ther Nucleic Acids. 23:1304–1322.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou T, Qin G, Yang L, Xiang D and Li S:
LncRNA XIST regulates myocardial infarction by targeting
miR-130a-3p. J Cell Physiol. 234:8659–8667. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang G, Sun H, Zhang Y, Zhao H, Fan W, Li
J, Lv Y, Song Q, Li J, Zhang M and Shi H: Characterization of
dysregulated lncRNA-mRNA network based on ceRNA hypothesis to
reveal the occurrence and recurrence of myocardial infarction. Cell
Death Discov. 4:352018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sun F, Zhuang Y, Zhu H, Wu H, Li D, Zhan
L, Yang W, Yuan Y, Xie Y, Yang S, et al: LncRNA PCFL promotes
cardiac fibrosis via miR-378/GRB2 pathway following myocardial
infarction. J Mol Cell Cardiol. 133:188–198. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Y, Hou YM, Gao F, Xiao JW, Li CC and
Tang Y: lncRNA GAS5 regulates myocardial infarction by targeting
the miR-525-5p/CALM2 axis. J Cell Biochem. 120:18678–186788. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Su T and Shao X, Zhang X, Yang C and Shao
X: Value of circulating miRNA-1 detected within 3 h after the onset
of acute chest pain in the diagnosis and prognosis of acute
myocardial infarction. Int J Cardiol. 307:146–151. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yaoita H, Ogawa K, Maehara K and Maruyama
Y: Apoptosis in relevant clinical situations: Contribution of
apoptosis in myocardial infarction. Cardiovasc Res. 45:630–641.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Song Y, Zhang C, Zhang J, Jiao Z, Dong N,
Wang G, Wang Z and Wang L: Localized injection of miRNA-21-enriched
extracellular vesicles effectively restores cardiac function after
myocardial infarction. Theranostics. 9:2346–2360. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fan PC, Chen CC, Peng CC, Chang CH, Yang
CH, Yang C, Chu LJ, Chen YC, Yang CW, Chang YS and Chu PH: A
circulating miRNA signature for early diagnosis of acute kidney
injury following acute myocardial infarction. J Transl Med.
17:1392019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bejerano T, Etzion S, Elyagon S, Etzion Y
and Cohen S: Nanoparticle delivery of miRNA-21 mimic to cardiac
macrophages improves myocardial remodeling after myocardial
infarction. Nano Lett. 18:5885–5891. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gidlöf O, van der Brug M, Ohman J, Gilje
P, Olde B, Wahlestedt C and Erlinge D: Platelets activated during
myocardial infarction release functional miRNA, which can be taken
up by endothelial cells and regulate ICAM1 expression. Blood.
121:39083917S1–S26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bao XL, Zhang L and Song WP: LncRNA SNHG1
overexpression regulates the proliferation of acute myeloid
leukemia cells through miR-488-5p/NUP205 axis. Eur Rev Med
Pharmacol Sci. 23:5896–5903. 2019.PubMed/NCBI
|
|
51
|
Arnold J, Engelmann JC, Schneider N,
Bosserhoff AK and Kuphal S: miR-488-5p and its role in melanoma.
Exp Mol Pathol. 112:1043482020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jing R, Zhong QQ, Long TY, Pan W and Qian
ZX: Downregulated miRNA-26a-5p induces the apoptosis of endothelial
cells in coronary heart disease by inhibiting PI3K/AKT pathway. Eur
Rev Med Pharmacol Sci. 23:4940–4947. 2019.PubMed/NCBI
|
|
53
|
Dahiya N and Atreya C: MiRNA-103b
downregulates ITGB3 and mediates apoptosis in ex vivo stored human
platelets. Microrna. 10:123–129. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Melzer IM, Fernández SB, Bösser S, Lohrig
K, Lewandrowski U, Wolters D, Kehrloesser S, Brezniceanu ML, Theos
AC, Irusta PM, et al: The Apaf-1-binding protein aven is cleaved by
cathepsin D to unleash its anti-apoptotic potential. Cell Death
Differ. 19:1435–1445. 2012. View Article : Google Scholar : PubMed/NCBI
|