Introduction
Sepsis is a major cause of admission to and death of
patients in the intensive care unit (ICU), and defined as a
systemic inflammatory response syndrome caused by abnormal host
response to infection (1).
Studies have indicated a high incidence and mortality of sepsis,
accounting for almost 20% of global deaths (2,3).
Notably, even patients who have survived sepsis, may still suffer
from severe sequelae, including long-term physical, psychological
and cognitive disabilities (4,5).
Sepsis is usually accompanied with abnormal immune response and
leads to sustained immunosuppression following recovery (6). In addition to immune suppression,
sepsis also causes other severe symptoms such as acute lung injury
(7), and myocardial injury
(8,9). When sepsis occurs, a dysregulated
immune response, including activated pro-inflammatory and
anti-inflammatory responses develop into an inflammatory cytokine
storm, extensive endothelial injury, subsequently sustained
immunosuppression, and even death in some cases (6). Confining the immunosuppression and
regulating the inflammatory response during sepsis are therefore
the main therapeutic methods for sepsis. Moreover, mesenchymal stem
cells (MSCs) are mobile in an inflammatory environment and modulate
inflammatory response, hence they are regarded as a promising
therapeutic approach for sepsis (10).
MSCs are derived from multipotent progenitor cells
of the mesoderm lineage during blast cytogenesis and may be
isolated from several tissues such as the bone marrow, adipose, and
umbilical cord (11,12). MSCs are characterized by
plasticity, adhesion, and the ability to differentiate into
osteocytes, adipocytes, and chondrocytes (13). MSCs are also regarded as a
potential new approach of immune modulatory therapy for diseases
(14,15). Adipose-derived MSCs (ADSCs), have
also shown reliable and promising clinical application potential
(16,17). In a lipopolysaccharide
(LPS)-induced acute respiratory distress syndrome (ARDS) model,
transplant of ADSCs decreased inflammatory neutrophil infiltration,
collagen deposition and lung injury, possibly by suppressing
classic inflammatory Toll-like receptor 4 (TLR4) signaling
(18,19). However, allogeneic immune
rejection is a great challenge for MSC transplantation. During
immune regulation, major histocompatibility complex class II (MHC
II), the immunogenicity-related gene, is essential for antigen
presentation to CD4+ Th cells, affects the selection,
immune tolerance of T cells, and the production of antibodies
(20). Therefore, handling the
survival of MSCs and immune rejection have become the primary
issues of stem cell transplantation.
A class of G protein-coupled receptors (GPCRs) exist
on the surface of antigen-presenting immune cells, namely the
hydroxy-carboxylic acid (HCA) receptor, among which
hydroxy-carboxylic acid receptor 1 (HCAR1) functions as the
receptor of lactate, and play important roles during the
development and differentiation of adipocytes, adipose metabolism,
and inflammatory response (21).
Hoque et al, found that lactate-HCAR1 signaling inhibited
TLR4-mediated inflammatory response (22). In addition, HCAR1 was revealed to
induce downregulation of MHC II activity and to promote
immunosuppression via lactate/HCAR1/ERK/STAT3/Arg-1 signaling,
protecting patients from intense inflammatory response-caused high
mortality in the early phase of sepsis (23).
The aim of the present study was to determine the
specific role of HCAR1 during immunoreaction following MSC
transplantation. An in vivo sepsis model was established and
the model rats were treated with an agonist or an antagonist and
then the levels of inflammatory factors and MHC II were detected.
Lung tissue damage was also observed. The present study provided a
novel approach for MSC transplantation-treated sepsis.
Materials and methods
Clinical samples
Patients diagnosed with sepsis (n=20) who were
hospitalized at the Third Affiliated Hospital of Inner Mongolia
Medical University (Baotou, China) from April 2014 to December
2019, and healthy donors (n=20) who underwent a physical exam, were
recruited for the present study. Each group included 10 females and
10 males, aged 55 to 70 years old. Blood samples (2 ml) were
collected and stored at −80°C. All participants provided signed
informed consent. All experiments were performed under the
authorization and guidelines of Ethics Committee of the Third
Affiliated Hospital of Inner Mongolia Medical University. The
standard for diagnosis of sepsis was according to the Sequential
Organ Failure Assessment (SOFA) score (24) guidelines of Sepsis 3.0 (Table I). The exclusion criteria were as
follows: i) aged <18 or >80 years old; ii) patients with
liver and kidney failure; iii) pregnant or breastfeeding women; iv)
malignant tumors or blood system diseases; v) granulocytes
<0.5×109/l; vi) immunodeficient patients; and vii)
participants who quit. The use of humans/human tissues followed the
guidelines of The Declaration of Helsinki.
 | Table I.SOFA score. |
Table I.
SOFA score.
|
|
| Score |
|---|
|
|
|
|
|---|
| System or
organ | Variables | 0 | 1 | 2 | 3 | 4 |
|---|
| Respiratory |
PaO2/FiO2
(mmHg) | >400 | 301-400 | <201-300 | 101-200 | ≤100 |
| Respiratory | Respiratory support
(yes/no) |
|
|
| Yes | Yes |
| Blood
coagulation | Platelets
(×109/l) | >150 | 101-150 | 51-100 | 21-50 | ≤20 |
| Liver | Bilirubin
(µmol/l) | <20 | 20-32 | 33-101 | 102-204 | >204 |
| Circulatory | Mean arterial
pressure (mmHg) | >70 | <70 |
|
|
|
| Circulatory | Dopamine
(µg/kg/min) |
|
| ≤5.0 or | >5 or | >15 or |
| Circulatory | Adrenaline
(µg/kg/min) |
|
|
| ≤0.1 or | >0.1 or |
| Circulatory | Noradrenaline
(µg/kg/min) |
|
|
| ≤0.1 | >0.1 |
| Circulatory | Dobutamine
(yes/no) |
|
| Yes |
|
|
| Central nervous
system | GCS score | 15 | 13-14 | 10-12 | 6-9 | <6 |
| Kidney | Creatinine
(µmol/l) | <110 | 110-170 | 171-299 | 300-440 | >440 |
| Kidney | Urine amount
(ml/d) |
|
|
| 201-500 | <200 |
Adipocyte-derived mesenchymal stem
cell (ADMSC) culture and transfection
Rat ADMSCs were purchased from Procell Life Science
& Technology Co., Ltd. and the cells were transplanted into a
25-cm2 plate with DMEM/F12 medium (Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin, and 100 µg/ml streptomycin
at 37°C in an incubator filled with 5% CO2. LVC-GFP
lentivirus vectors were co-transfected with packaging vector psPAX2
(0.1 µg; Addgene, Inc.) and envelope vector pMD2.G (0.9 µg;
Addgene, Inc.) (lentiviral plasmid:packaging vector:envelope
vector, 10:1:9) into 3rd generation 293T (CRL-3216; ATCC) cells
using Lipofectamine® 2000 (cat. no. 11668-019;
Invitrogen; Thermo Fisher Scientific, Inc.). Following transfection
at 25°C for 48 h, the lentiviral particles were collected via
ultracentrifugation at 55,000 × g, at 4°C for 2.5 h. After 24 h of
transfection, the cells were cultured for 24 h with serum-free
transfer solution as the complete medium. Cells were plated in
6-well plates (1×106 cells/ml) and incubated with
LVC-GFP lentivirus (MOI=10) under Polybrene (Thermo Fisher
Scientific, Inc.) (5 µg/ml) using Lipofectamine 2000 at 37°C for 24
h. The LVC-GFP lentivirus was synthesized and purchased from
Shanghai Genechem Co., Ltd. The medium was then replaced by fresh
medium and cultured for another 48 h.
Animal experiments
Sprague-Dawley (SD) male rats weighing 100–150 g
(n=30) were purchased from Beijing Vital River Laboratory Animal
Technology Co., Ltd. Rats were randomly divided into five groups:
i) The normal control that received regular feeding without any
treatment; ii) the LPS group (25) which was the sepsis group that
received intraperitoneal injection of LPS (L; 5 mg/kg) (26,27); iii) the L + M group in which rats
received ADMSCs (1×106) transplanted 30 min following
LPS treatment; iv) the L + Gi (HCAR1 receptor agonist) + M group,
in which rats received intraperitoneal injection of the HCAR1
receptor agonist (30 mg/kg, 3-chloro-5-hydroxy BA; APeXBIO
Technology LLC) 30 min after LPS treatment, followed by intravenous
injection of ADMSCs 1 h later; and v) the L + Gk (HCAR1 receptor
antagonist) + M group, in which rats received intraperitoneal
injection of HCAR1 receptor antagonist (30 mg/kg,
[(R)-3-hydroxybutaboic acid]; MedChemExpress) 30 min after LPS
treatment, followed by intravenous injection of ADMSCs 1 h later.
HCAR1 is a GPCR, and the agonist was named according to the
‘inducer’ and the antagonist according to the ‘blocker’ in the
initial application. For ADMSC transplant, ADMSCs transfected with
LVC-GFP lentivirus were collected, suspended in saline
(1×106 cell/ml) and were intravenously injected into the
tail vein of rats. To identify the successful inoculation of ADMSCs
in rats, 72 h after injection, rats were anesthetized with
phenobarbital sodium (50 ml/kg), and observed on an in vivo
optical two-dimensional imaging system (Lumina II; PerkinElmer,
Inc.). After 5 days of ADMSC injection, the rats were euthanized
with an overdose of pentobarbital sodium (150 mg/kg, intravenous
injection). To investigate the mechanism, the NF-κB inhibitor
antioxidant pyrrolidine dithiocarbamate (PDTC; MilliporeSigma) was
used (intragastrical injection, 100 mg/kg) 30 min after LPS
treatment There were six rats per group (n=6/group). Animal care
and method procedures were authorized by the Animal Ethics
Committee (approval no. 2020-0618-37) of the Third Affiliated
Hospital of Inner Mongolia Medical University.
Tissue collection and histological
analysis
After establishment of a rat model, the rats were
anesthetized by an overdose of pentobarbital sodium. In brief, the
abdomen of rats was opened to expose the abdominal aorta, then the
blood was collected (2 ml/rat) and centrifuged at 2,000 × g at 4°C
for 15 min to obtain serum. The serum was stored at −20°C.
Subsequently, the chest of rats was opened to isolate the lungs.
The lung tissues were partially stored at −80°C for RNA and protein
analysis. For hematoxylin and eosin (H&E) staining and
fluorescence imaging, lung tissues were embedded in optimal cutting
temperature (OCT) gel (Agar Scientific, Ltd.), and cut into 5-µm
slices at −20°C For H&E staining, the slices were fixed with 4%
paraformaldehyde (PFA) overnight at 4°C, stained with H&E
(hematoxylin staining at 25°C for 3 min and eosin staining at 25°C
for 3 min) and observed under an optical microscope. For
fluorescence imaging, the nucleus was stained with DAPI for 10 min
at 4°C, and the GFP and DAPI were observed under LSM 710 NLO/7 MP
confocal microscope.
Reverse transcription quantitative-PCR
(RT-qPCR)
Total RNA of peripheral blood (1 ml) and lung tissue
(0.2 g) was extracted using TRIzol reagent (Thermo Fisher
Scientific, Inc.), reversed to cDNA using ReverTra Ace PCR RT
(Toyobo Life Science) according to the manufacturer's instructions.
Gene expression was quantified using THUNDERBIRD SYBR qPCR Mix
(Toyobo Life Science) according to the manufacturer's instructions,
following the 2−ΔΔCq method (8). The following thermocycling
conditions were used for qPCR: Initial denaturation at 95°C for 30
sec; followed by 39 cycles at 95°C for 5 sec and 60°C for 30 sec;
and a final extension at 72°C for 5 min. GAPDH was used as an
internal control. Primers were synthesized by Takara Bio, Inc., and
the sequences are listed in Table
II.
 | Table II.Primers for reverse transcription
quantitative-PCR. |
Table II.
Primers for reverse transcription
quantitative-PCR.
| Primer | Sequence |
|---|
| Human-HCAR1 | Forward,
5′-AGAACCATCTCTGCGTGCAA-3′ |
|
| Reverse,
5′-GCGACCGAGGTTCGAAATTG-3′ |
| Human-MHC II
(HLA-DRB1) | Forward,
5′-CTCCTGCATGGCAGTTCTGA-3′ |
|
| Reverse,
5′-ATTGTGGATCAGGCCTGTGG-3′ |
| Human-TLR4 | Forward,
5′-GGTGCCTCCATTTCAGCTCT-3′ |
|
| Reverse,
5′-ACTGCCAGGTCTGAGCAATC-3′ |
| Human-NLRP3 | Forward,
5′-CACTTCCAGTTTTTGCCGGG-3′ |
|
| Reverse,
5′-CAAGGCATTCTCCCCCACAT-3′ |
| Human-GAPDH | Forward,
5′-GAGAAGGCTGGGGCTCATTT-3′ |
|
| Reverse,
5′-AGTGATGGCATGGACTGTGG-3′ |
| Rat-HCAR1 | Forward,
5′-GTGTTGGCGAGGCTCTACTT-3′ |
|
| Reverse,
5′-AACACACTTGGAGACCCCAC-3′ |
| Rat-MHC II
(RT1-Db2) | Forward,
5′-TCCGGAATGGTGAGGAGGAA-3′ |
|
| Reverse,
5′-GTAGATGAACAGCCCCACCG-3′ |
| Rat-TLR4 | Forward,
5′-TCAGCTTTGGTCAGTTGGCT-3′ |
|
| Reverse,
5′-GTCCTTGACCCACTGCAAGA-3′ |
| Rat-NLRP3 | Forward, 5′-
ACGGCAAGTTCGAAAAAGGC-3′ |
|
| Reverse, 5′-
AGACCTCGGCAGAAGCTAGA-3′ |
| Rat-GAPDH | Forward,
5′-GCGAGATCCCGCTAACATCA-3′ |
|
| Reverse,
5′-CTCGTGGTTCACACCCATCA-3′ |
Enzyme-linked immunosorbent assay
(ELISA)
The levels of interleukin (IL)-1β (IL-1β ELISA kit;
cat. no. E-EL-R0012), IL-10 (IL-10 ELISA kit; cat. no. E-EL-R0016;
Elabscience Biotechnology, Inc.), tumor necrosis factor-α (TNF-α;
TNF-α ELISA kit; cat. no. E-EL-R2856), and IL-18 (IL-18 ELISA Kit;
cat. no. E-EL-R0567), in blood were determined by ELISA using
commercial kits (all from Elabscience Biotechnology, Inc.)
following the manufacturer's instructions. In brief, the blood
samples were centrifuged at 2,000 × g at 4°C for 15 min, and the
supernatants were added to test plates and incubated for 1 h at
37°C. Biotin-labeled antibodies (1:100; cat. nos. A18821, PA1-29608
and A27035; Thermo Fisher Scientific, Inc.) were then added,
followed by incubation for 1 h at 4°C. The absorbance values at 450
nm were detected on a microplate reader (Beijing Liuyi
Biotechnology Co., Ltd.).
Immunofluorescence assay
The identification of ADMSCs was performed using
immunofluorescence assays. In brief, cells (1×106) were
plated on coverslips, fixed with 4% PFA overnight at 4°C,
penetrated with 0.5% Triton X-100, blocked with 10% goat serum
(Thermo Fisher Scientific, Inc.) for 2 h at 4°C, followed by
incubation with specific primary antibodies against CD44 (cat. no.
bs-2507R), CD90 (cat. no. bs-0778R), CD34 (cat. no. bs-0646R) and
CD45 (cat. no. bs-0522R; all from BIOSS) at 4°C overnight. The
following day, the cells were incubated with Cy2- (Cy™2 AffiniPure;
1:50; code no. 111-225-144) or Cy3-conjugated secondary anti-rabbit
antibodies (Cy™3 AffiniPure; 1:50; code no. 111-165-003; both from
Jackson ImmunoResearch Laboratories, Inc.) at 25°C for 1 h in dark.
The nucleus was stained with DAPI (Beyotime Institute of
Biotechnology) at 25°C for 5 min. The images were obtained under a
fluorescence microscope (Nikon Corporation).
Western blotting
Proteins were extracted from lung tissues using
ice-cold RIPA lysis buffer, and quantified by BCA kit (both from
Beyotime Institute of Biotechnology). An equal amount of protein (2
µg) was separated on 10% gels using SDS-PAGE and then transferred
to PVDF membranes. The membranes were blocked with 5% skimmed milk
for 1 h at 25°C, followed by incubation with primary antibodies,
including HCAR1 (1:1,000; product code ab106942), MHC II (1:1,000;
product code ab55152), TLR4 (1:1,000; product code ab13556), NLRP3
(1:1,000; product code ab263899), GFP (1:1,000; product code
ab290), and GAPDH (1:1,000; product code ab8245; all from Abcam) at
4°C overnight. The following day, the membranes were incubated with
HRP-conjugated secondary antibodies (1:1,000; product codes
ab150077, ab150113 and ab6741; all Abcam) at 25°C for 1 h, and
visualized using ECL reagent (Millipore; Merck KGaA). Images were
obtained on a gel imaging system (Bio-Rad Laboratories, Inc.). ALL
antibodies were purchased from Abcam and were used according to the
manufacturer's protocols. The densitometric analysis was performed
using ImageJ (1.8.0; National Institutes of Health).
Statistical analysis
Statistical analysis was conducted by using SPSS
21.0 software (IBM Corp.). Differences between two groups were
analyzed using unpaired Student's t-test and differences among
multiple groups were analyzed using one-way ANOVA followed by
Tukey's post hoc test. Correlation analysis was performed using
Pearson's correlation analysis. The diagnostic value of HCAR1,
TLR4, MHC II, NLRP3, IL-1β, TNF-α, IL-10, and IL-18 in patients
with sepsis was assessed using receiver operating characteristic
(ROC) curves. In this method, a perfect biomarker has 100%
sensitivity, shows no false positives (100% specificity), and
produces an area under the curve (AUC) of 1.0, while a biomarker
with no diagnostic value has an AUC of 0.5. Youden's index with the
highest sum of sensitivity and specificity was used to determine
the optimal cut-off value for differentiation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression levels of HCAR1, TLR4, MHC
II, NLRP3, and inflammatory factors in the peripheral blood of
patients with sepsis
The expression levels of HCAR1, TLR4, MHC II, NLRP3,
and inflammatory factors in the peripheral blood of patients with
sepsis were first evaluated. The results revealed that the mRNA
expression of HCAR1 was reduced while the mRNA expression levels of
TLR4, MHC II, and NLRP3 were enhanced in the peripheral blood of
patients with sepsis compared with those in the healthy controls
(P<0.01, P<0.001 and P<0.0001; Fig. 1A-D and Table I). In addition, the levels of
IL-1β, TNF-α, IL-10, and IL-18 were elevated in the peripheral
blood of patients with sepsis compared with those in the healthy
controls (P<0.01 and P<0.0001; Fig. 1E-H).
 | Figure 1.Expression levels of HCAR1, TLR4, MHC
II, NLRP3, and inflammatory factors (IL-1β, TNF-α, IL-10, and
IL-18) in the peripheral blood of patients with sepsis. The levels
of HCAR1 (A), TLR4 (B) MHC II (C), and (D) NLRP3 were assessed by
quantitative PCR in the peripheral blood of patients with sepsis
and healthy controls. The levels of (E) IL-1β, (F) TNF-α, (G)
IL-10, and (H) IL-18 were detected by ELISA assays. **P<0.01,
***P<0.001 and ****P<0.0001. HCAR1, hydroxy-carboxylic acid
receptor 1; TLR4, Toll-like receptor 4; MHC II, major
histocompatibility complex class II; NLRP3, NOD-like receptor
family pyrin domain containing 3; IL, interleukin; TNF-α, tumor
necrosis factor-α; NC, normal control. |
Correlation of the expression levels
of HCAR1, TLR4, MHC II, NLRP3, and inflammatory factors in the
peripheral blood of patients with sepsis
Next, the correlation of HCAR1, TLR4, MHC II, NLRP3,
and inflammatory factors in the peripheral blood of patients with
sepsis was evaluated. It was observed that the expression of TLR4
was positively associated with NLRP3 (r=0.641), IL-1β (r=0.666),
TNF-α (r=0.606), and IL-18 (r=0.624) levels in the peripheral blood
of patients with sepsis (Fig.
2A). In addition, it was further observed that the expression
of HCAR1 was negatively correlated with TLR4 (r=−0.666), MHC II
(r=−0.587), and NLRP3 (r=−0.621) expression in the peripheral blood
of patients with sepsis (Fig.
2B).
 | Figure 2.Correlation of the expression levels
of HCAR1, TLR4, MHC II, NLRP3, and inflammatory factors (IL-1β,
TNF-α, IL-10, and IL-18) in the peripheral blood of patients with
sepsis. (A and B) The correlation of the expression levels of
HCAR1, TLR4, MHC II, NLRP3, IL-1β, TNF-α, IL-10, and IL-18 were
analyzed in peripheral blood of patients with sepsis. HCAR1,
hydroxy-carboxylic acid receptor 1; TLR4, Toll-like receptor 4; MHC
II, major histocompatibility complex class II; NLRP3, NOD-like
receptor family pyrin domain containing 3; IL, interleukin; TNF-α,
tumor necrosis factor-α. |
Diagnostic value of HCAR1, TLR4, MHC
II and NLRP3 mRNA expression and IL-1β, IL-18, TNF-α and IL-10
levels for predicting sepsis
HCAR1, TLR4, MHC II and NLRP3 mRNA expression and
IL-1β, IL-18, TNF-α and IL-10 levels were then assessed to
determine their diagnostic value for predicting sepsis. The AUC
values of IL-1β, IL-18, TNF-α, and IL-10 were 0.751, 0.841, 0.924
and 0.729, respectively, in which TNF-α exhibited the highest
diagnostic value (Fig. 3A). ROC
curve analysis revealed that the AUC values of HCAR1, TLR4, MHC II
and NLRP3 mRNA expression were 0.830, 0.853, 0.735 and 0.945,
respectively, in which NLRP3 exhibited the highest diagnostic value
(Fig. 3B).
 | Figure 3.Diagnostic value of HCAR1, TLR4, MHC
II and NLRP3 mRNA expression and IL-1β, IL-18, TNF-α and IL-10
levels for predicting sepsis. (A and B) The diagnostic value of
HCAR1, TLR4, MHC II and NLRP3 mRNA expression and IL-1β, IL-18,
TNF-α and IL-10 levels for predicting sepsis was revealed using ROC
curve analysis. HCAR1, hydroxy-carboxylic acid receptor 1; TLR4,
Toll-like receptor 4; MHC II, major histocompatibility complex
class II; NLRP3, NOD-like receptor family pyrin domain containing
3; IL, interleukin; TNF-α, tumor necrosis factor-α; ROC, receiver
operating characteristic. |
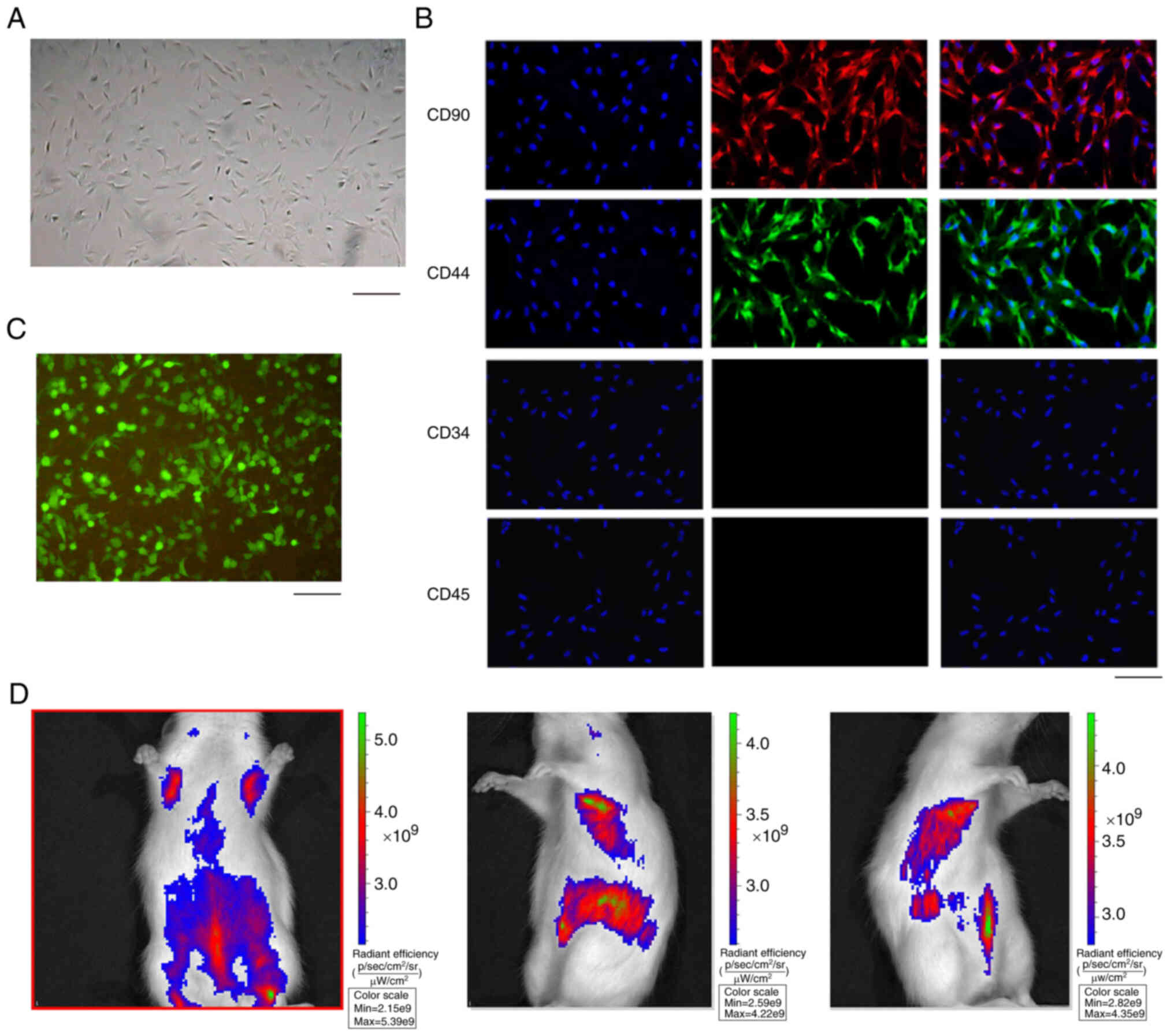
Isolation and identification of
ADSCs
In order to analyze the effect of ADSC-derived HCAR1
on sepsis in vivo, the ADSCs were isolated and the
morphology of ADSCs was observed (Fig. 4A). The majority of the adherent
cells were fibroblast-like cells, but showed heterogenic
morphology, including spindle-shaped or small round cells (Fig. 4A). In addition, the ADSCs were
identified by the positive markers, including CD90 and CD44, while
the negative markers, such as CD34 and CD45, were undetectable
(Fig. 4B). Notably, the
GFP-labeled ADSCs were validated (Fig. 4C) and GFP-labeled ADSCs were
identified in the rats by in vivo fluorescence tracing
(Fig. 4D), suggesting that ADSCs
can be colonized in the lungs through blood circulation after
transplantation.
Effect of ADSC-derived HCAR1 on lung
injury of rats with sepsis
Next, the rat model of sepsis was established by
injecting LPS and the rats were randomly divided into 5 groups,
including the normal control group (NC group; n=6), the sepsis
model group (LPS group; n=6), the ADSC transplantation group (L + M
group; n=6), the combined HCAR1 receptor agonist group (L + Gi + M
group; n=6), and the combined HCAR1 receptor inhibitor group (L +
Gk + M group; n=6). Using H&E staining, the comparison between
the L + M group and the LPS group revealed that there were still
neutrophils and inflammatory cell infiltrates in the L + M group,
but congestion and edema were reduced and the alveolar cavity and
interstitial structures were partially intact. In the L + Gi + M
group inflammatory cell infiltrates were markedly reduced in
comparison with the L + M and LPS groups, with no obvious
congestion and edema and clear alveolar and interstitial structures
(Fig. 5A). The L + Gk + M group
compared with the L + Gi + M and L + M groups revealed a marked
increase of inflammatory cell infiltration, with visible congestion
and edema, and partially clear alveoli and interstitial structures,
but the degree of pathological damage was weaker than that of the
LPS group (Fig. 5A). In addition,
it was determined that the L + M group exhibited an increase in the
number of ADSCs in the lung tissue compared with the LPS group
(Fig. 5B). The L + Gi + M group
showed an enhanced number of ADSCs compared with the L + M group
(Fig. 5B). The L + Gk + M group
showed a marked decrease in the number and distribution of ADSCs
compared with the L + Gi + M and L + M groups (Fig. 5B). Notably, it was identified that
the L + Gk + M group demonstrated a marked increase of inflammatory
cell infiltration, with visible congestion and edema, and partially
clear alveoli and interstitial structures, but the degree of
pathological damage was weaker than that of the LPS group, while
the treatment with the NF-κB inhibitor PDTC could reverse the
effect, implying that ADSC-derived HCAR1 may regulate sepsis
through NF-κB signaling (Fig.
5C).
 | Figure 5.Effect of ADSC-derived HCAR1 on lung
injury of sepsis rats. (A) The lung injury was analyzed using
H&E staining in the rats with sepsis. Scale bar, 50 µm. (B) The
number and distribution of ADSCs in the lung tissues of rats with
sepsis was detected by immunofluorescence analysis. Scale bar, 50
µm. (C) The lung injury was analyzed using H&E staining in the
rats with sepsis. The following groups were observed: The normal
control group (NC), the sepsis model group (LPS), the adipocyte
mesenchymal stem cell transplantation group (L + M), the combined
HCAR1 receptor agonist group (L + Gi + M), the combined HCAR1
receptor inhibitor group (L + Gk + M), and the combined PDTC (NF-κB
inhibitor group; L + Gk + M + PDTC); n=6 per group. The
inflammatory cell infiltration was identified by pathologists and
indicated by black arrows. Scale bar, 50 µm. ADSC, adipose-derived
mesenchymal stem cell; HCAR1, hydroxy-carboxylic acid receptor 1;
H&E, hematoxylin and eosin; NC, normal control; LPS,
lipopolysaccharide. |
Effect of ADSC-derived HCAR1 on the
expression levels of HCAR1, TLR4, MHC II, NLRP3, and inflammatory
factors in rats with sepsis
Furthermore, it was observed that the expression
levels of HCAR1 were reduced while those of TLR4, MHC II, and NLRP3
were enhanced in the rats with sepsis, while the L + M treatment
produced the opposite results. In addition, the treatment of L + Gi
+ M further enforced the effect but L + Gk + M treatment rescued
the effect in the system (P<0.05; Fig. 6A-E). In addition, the expression
of IL-1β, IL-18 and TNF-α in the peripheral blood of the rats was
significantly higher in the LPS group compared with the NC group.
The expression of IL-1β, IL-18 and TNF-α in the L + M group was
significantly lower compared with the LPS group. The L + Gi + M
group exhibited decreased expression compared with the L + M and
LPS groups, but still exhibited increased expression compared with
the NC group. In addition, the L + Gk + M group exhibited
significantly higher expression compared with the L + Gi + M, L + M
groups. The expression of IL-10 was significantly higher in the LPS
group compared with the NC group, in the L + M group compared with
the LPS group, and in the L + Gi + M group compared with both the L
+ M and NC groups. The L + Gk + M group exhibited a significant
decrease in expression of IL-10 compared with the L + Gi + M, L + M
groups, but still higher than the LPS group (P<0.05; Fig. 6F-I).
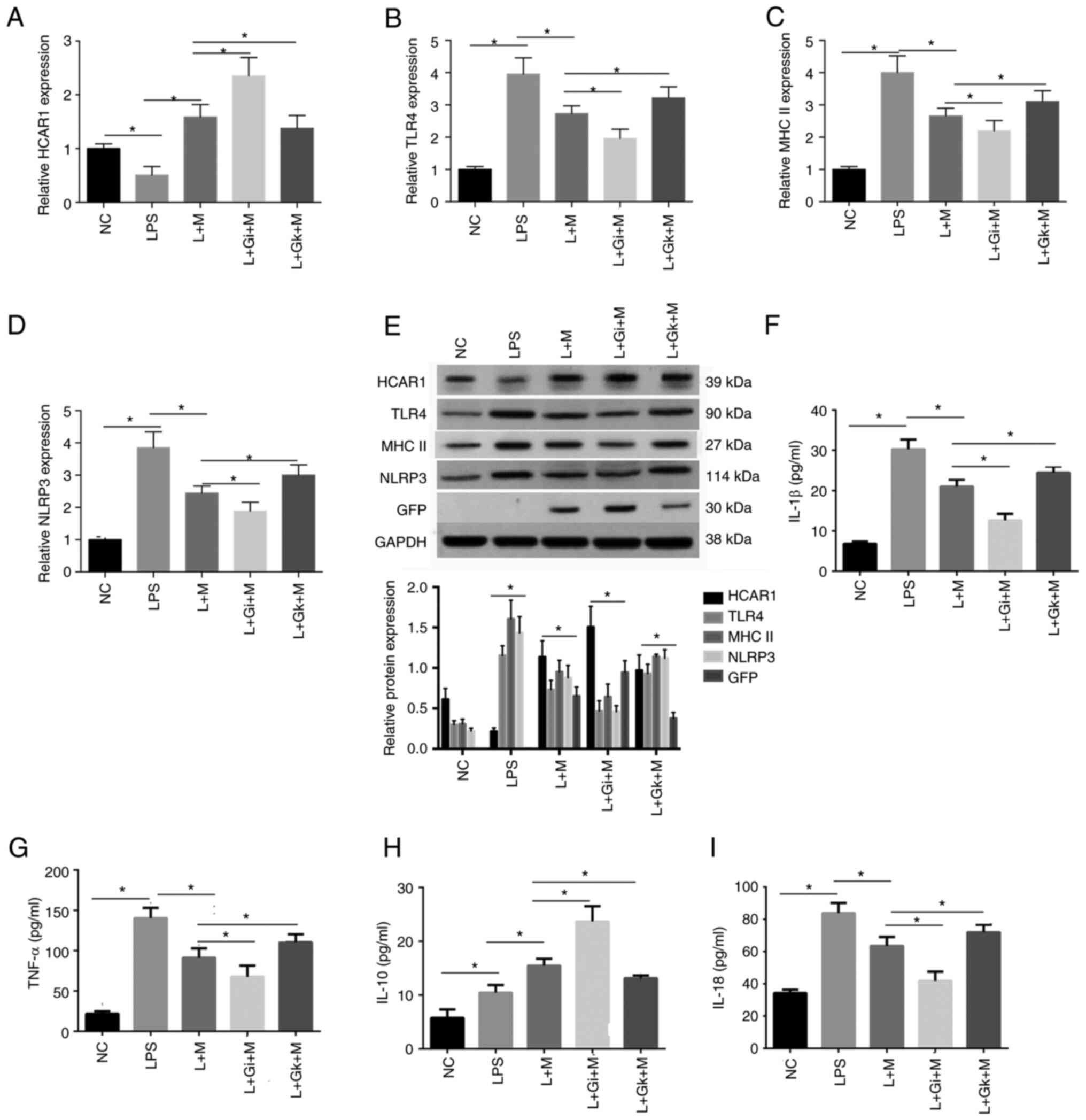 | Figure 6.Effect of ADSC-derived HCAR1 on the
expression levels of HCAR1, TLR4, MHC II, NLRP3, and inflammatory
factors (IL-1β, TNF-α, IL-10, and IL-18) in rats with sepsis. (A-D)
The levels of HCAR1 (A), TLR4 (B), MHC II (C), and NLRP3 (D) were
assessed by quantitative PCR in the peripheral blood and lung
tissues of rats with sepsis. (E) The protein levels of HCAR1, TLR4,
MHC II and NLRP3 were analyzed by western blotting in the lung
tissues of rats with sepsis. (F-I) The levels of IL-1β, TNF-α,
IL-10, and IL-18 were detected by ELISA assays. The following
groups were assessed: The normal control group (NC), the sepsis
model group (LPS), the adipocyte mesenchymal stem cell
transplantation group (L + M group), the combined HCAR1 receptor
agonist group (L + Gi + M group), the combined HCAR1 receptor
inhibitor group (L + Gk + M group); n=6 per group. Results were
analyzed using ANOVA; *P<0.05. ADSC, adipose-derived mesenchymal
stem cell; HCAR1, hydroxy-carboxylic acid receptor 1; TLR4,
Toll-like receptor 4; MHC II, major histocompatibility complex
class II; NLRP3, NOD-like receptor family pyrin domain containing
3; IL, interleukin; TNF-α, tumor necrosis factor-α; NC, normal
control. |
Discussion
Sepsis is a life-threatening disease in which the
body normally produces an organismic immune response to bacterial
infection, which can cause tissue and organ damage when the immune
response is too intense (28). As
a key receptor molecule in the classical inflammatory signaling
pathway, TLR4 can be activated and activate downstream NLRP3
inflammatory vesicles to participate in the inflammatory response,
and TLR-4 receptors are activated by exogenous signaling molecules
to cause the secretion of cytokines such as IL-1β, IL-18 and TNF-α
(29). In the present study, the
expression levels of HCAR1, TLR4, MHC II, NLRP3, and inflammatory
factors were assessed in the peripheral blood of patients with
sepsis. It was determined that the mRNA expression of HCAR1 was
reduced while the mRNA expression levels of TLR4, MHC II, and
NLRP3, and the levels of IL-1β, TNF-α, IL-10, and IL-18 were
enhanced in the peripheral blood of patients with sepsis compared
with those in the healthy controls. These data indicated that the
tissue cell damage caused by sepsis is the result of an intense
inflammatory response involving multiple inflammatory cytokines
activated by TLR4/NLRP3. This is similar to what was aforementioned
in previous research.
HCAR1 is a receptor for lactate and has an important
role in the regulation of inflammatory responses (30). It has been demonstrated that in
RAW264.7 cells, lactate inhibits TLR4-mediated NF-κB activation and
suppresses pro-IL-1β, NLRP3 and CASP1 gene expression (31). It has also been reported that
lactate can promote immunosuppression by macrophage M2-type
polarization through signaling pathways such as
HCAR1/PI3K/AKT/CEBPβ/IL-10 and HCAR1/ERK/STAT3/Arg1, thus the
discovery of HCAR1, makes lactate no longer an inert molecule but a
bioactive molecule (32,33). In the present study, it was found
that the expression levels of HCAR1 in clinical peripheral blood
and animal peripheral blood and lung tissues were significantly
negatively associated with the expression of TLR4, MHC II and NLRP3
in both the clinical sepsis group and the LPS group of the animal
model, indicating that HCAR1 expression was suppressed in the
septic state, attenuating the inhibitory effect on TLR4/NLRP3
signaling activation with upregulation of MHC II expression and
promoting the inflammatory response.
It has been reported that in the respiratory system,
allogeneic and allogeneic MSCs effectively protected the lung from
ischemia-reperfusion injury by downregulating inflammatory response
signaling pathways in rats, which involved the inhibition of
TLR4/MyD88/TAK1/IKK/I-κB/NF-κB signaling pathways by
adipose-derived MSCs, resulting in a reduction in Cox-2, TNF-α and
IL-1. TNF-α, IL-1β as well as other cytokines as a result of the
aforementioned studies suggest that MSC transplantation therapy is
used in life science research (33,34). As for the treatment of acute lung
injury due to sepsis, it has been shown that bone marrow MSC
transplantation can reduce the functional impairment of organs
caused by inflammation in rats (35). In the present study, the crucial
effect of ADSC-derived HCAR1 on lung injury of rats with sepsis was
identified. ADSCs can reduce the inflammatory response and improve
lung tissue injury, similar to the results of the aforementioned
study. Moreover, it has been reported that miR-17 is inhibited in
sepsis and MSC-derived extracellular vesicles transfer miR-17 to
attenuate LPS-induced sepsis (36). In the present study, it was also
revealed that the mRNA expression of HCAR1 was reduced in sepsis
and that ADSC-derived HCAR1 regulated the immune response in the
attenuation of sepsis. The data of the present is consistent with
the previous aforementioned study. Some negative feedback mechanism
may be involved in the decrease of the expression level of HCAR1 in
sepsis and should be explored in future investigations. However, in
the present study, it was revealed that ADSCs failed to completely
repair the damaged lung tissues after transplantation and did not
achieve the expected results. The immune rejection involved is
mainly related to the antigen presentation-related molecule MHC II
on the surface of APCs, which, on the one hand, upregulates the
expression of inflammatory factors such as IL-6, IL-12 and TNF-α,
and on the other hand promotes the TLR4/MyD88/NF-κB signaling
pathway and increases the production of inflammatory factors, while
it also presents alloantigens. It can also present allogeneic
antigens to T lymphocytes, which promote cellular immunity to
produce a large number of cytokines and chemokines, thus promoting
humoral immunity to remove allogeneic substances (37,38). In the present study, it was
determined that activation of the lactate receptor HCAR1 inhibited
the maturation of antigen-presenting peripheral blood dendritic
cells (DC) and also promoted the polarization of antigen-presenting
macrophages to the M2 type in tissues, both of which were
characterized by a downregulation of the expression of MHC II
molecules on the cell surface. Thus, in the experimental results,
it was found that in the septic state, the results of genetic
testing of peripheral blood and lung tissue of clinical patients
and model rats showed that HCAR1 expression was significantly
downregulated in the sepsis and LPS groups compared with the normal
and NC groups, whereas MHC II expression was significantly
increased, which is involved in the septic inflammatory
response.
There are still some limitations in the present
study. Further in vitro experiments are needed to verify the
modulatory effect of HCAR1 on this immune rejection of MHC II and
its effect on stem cell transplantation survival. In addition, the
function of HCAR1 derived from ADSCs, in the attenuation of
LPS-induced lung injury in sepsis was identified. However, which
types of HCAR are present in ADSCs and further investigation with
regard to HCAR1 protein expression in ADSCs should be performed to
characterize the receptor of interest. In addition, the sample size
for the ROC analysis was small and in future a larger sample size
is warranted. In addition, the experimental results did not reveal
a dose-effect or time-effect association, and there were some
contradictions in the experimental results. Furthermore, the
investigation into the mechanism showed that the treatment of NF-κB
inhibitor PDTC could reverse the effect of L + Gk + M on sepsis,
indicating that ADSC-derived HCAR1 may regulate sepsis through
NF-κB signaling. The specific mechanism should be confirmed with
more studies in the future.
In summary, it was concluded that ADSC-derived HCAR1
regulates the immune response in the attenuation of sepsis.
ADSC-derived HCAR1 may be a promising therapeutic strategy for
sepsis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
HW, PX and LQ designed the study. HW, PX, HT, XH, JY
and XX performed the experiments in Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, 6. HW, PX, JPY and LQ wrote the
manuscript. XX and LQ confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were performed under the
authorization and guidelines of the Ethics Committee of the Third
Affiliated Hospital of Inner Mongolia Medical University (Baotou,
China). All participants provided signed informed consent. Animal
care and method procedures were authorized by the Animal Ethics
Committee (approval no. 2020-0618-37) of the Third Affiliated
Hospital of Inner Mongolia Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (sepsis-3). JAMA.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rudd KE, Johnson SC, Agesa KM, Shackelford
KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer
S, et al: Global, regional, and national sepsis incidence and
mortality, 1990–2017: Analysis for the global burden of disease
study. Lancet. 395:200–211. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perman SM, Goyal M and Gaieski DF: Initial
emergency department diagnosis and management of adult patients
with severe sepsis and septic shock. Scand J Trauma Resusc Emerg
Med. 20:412012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iwashyna TJ, Ely EW, Smith DM and Langa
KM: Long-term cognitive impairment and functional disability among
survivors of severe sepsis. JAMA. 304:1787–1794. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang CY, Daniels R, Lembo A, Hartog C,
O'Brien J, Heymann T, Reinhart K and Nguyen HB; Sepsis Survivors
Engagement Project (SSEP), : Life after sepsis: an international
survey of survivors to understand the post-sepsis syndrome. Int J
Qual Health Care. 31:191–198. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim EY, Ner-Gaon H, Varon J, Cullen AM,
Guo J, Choi J, Barragan-Bradford D, Higuera A, Pinilla-Vera M,
Short SA, et al: Post-sepsis immunosuppression depends on NKT cell
regulation of mTOR/IFN-γ in NK cells. J Clin Invest. 130:3238–3252.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koh H, Sun HN, Xing Z, Liu R, Chandimali
N, Kwon T and Lee DS: Wogonin influences osteosarcoma stem cell
stemness through ROS-dependent signaling. In Vivo. 34:1077–1084.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun W, Li H and Gu J: Up-regulation of
microRNA-574 attenuates lipopolysaccharide- or cecal ligation and
puncture-induced sepsis associated with acute lung injury. Cell
Biochem Funct. 38:847–858. 2020. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qiu N, Xu X and He Y: LncRNA TUG1
alleviates sepsis-induced acute lung injury by targeting
miR-34b-5p/GAB1. BMC Pulm Med. 20:492020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Laroye C, Gibot S, Reppel L and Bensoussan
D: Concise review: Mesenchymal stromal/stem cells: A new treatment
for sepsis and septic shock? Stem Cells. 35:2331–2339. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kariminekoo S, Movassaghpour A, Rahimzadeh
A, Talebi M, Shamsasenjan K and Akbarzadeh A: Implications of
mesenchymal stem cells in regenerative medicine. Artif Cells
Nanomed Biotechnol. 44:749–757. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thomas ED: Bone marrow transplantation
from the personal viewpoint. Int J Hematol. 81:89–93. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The international society for cellular
therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Perlee D, de Vos AF, Scicluna BP, Mancheño
P, de la Rosa O, Dalemans W, Nürnberg P, Lombardo E and van der
Poll T: Human adipose-derived mesenchymal stem cells modify lung
immunity and improve antibacterial defense in pneumosepsis caused
by Klebsiella pneumoniae. Stem Cells Transl Med. 8:785–796. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hackstein H, Lippitsch A, Krug P,
Schevtschenko I, Kranz S, Hecker M, Dietert K, Gruber AD, Bein G,
Brendel C and Baal N: Prospectively defined murine mesenchymal stem
cells inhibit Klebsiella pneumoniae-induced acute lung injury and
improve pneumonia survival. Respir Res. 16:1232015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Argentati C, Morena F, Bazzucchi M,
Armentano I, Emiliani C and Martino S: Adipose stem cell
translational applications: From bench-to-bedside. Int J Mol Sci.
19:34752018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kapur SK and Katz AJ: Review of the
adipose derived stem cell secretome. Biochimie. 95:2222–2228. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Horie S, Gaynard S, Murphy M, Barry F,
Scully M, O'Toole D and Laffey JG: Cytokine pre-activation of
cryopreserved xenogeneic-free human mesenchymal stromal cells
enhances resolution and repair following ventilator-induced lung
injury potentially via a KGF-dependent mechanism. Intensive Care
Med Exp. 8:82020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jung YJ, Park YY, Huh JW and Hong SB: The
effect of human adipose-derived stem cells on
lipopolysaccharide-induced acute respiratory distress syndrome in
mice. Ann Transl Med. 7:6742019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Turesson C: Endothelial expression of MHC
class II molecules in autoimmune disease. Curr Pharm Des.
10:129–143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brooks GA: Lactate as a fulcrum of
metabolism. Redox Biol. 35:1014542020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hoque R, Farooq A, Ghani A, Gorelick F and
Mehal WZ: Lactate reduces liver and pancreatic injury in Toll-like
receptor- and inflammasome-mediated inflammation via GPR81-mediated
suppression of innate immunity. Gastroenterology. 146:1763–1774.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raychaudhuri D, Bhattacharya R, Sinha BP,
Liu CSC, Ghosh AR, Rahaman O, Bandopadhyay P, Sarif J, D'Rozario R,
Paul S, et al: Lactate induces pro-tumor reprogramming in
intratumoral plasmacytoid dendritic cells. Front Immunol.
10:18782019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Z, Meng Z, Li Y, Zhao J, Wu S, Gou S
and Wu H: Prognostic accuracy of the serum lactate level, the SOFA
score and the qSOFA score for mortality among adults with Sepsis.
Scand J Trauma Resusc Emerg Med. 27:512019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kalyanaraman B, Darley-Usmar V, Davies KJ,
Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ II
and Ischiropoulos H: Measuring reactive oxygen and nitrogen species
with fluorescent probes: Challenges and limitations. Free Radic
Biol Med. 52:1–6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vicente-Dueñas C, González-Herrero I,
García Cenador MB, García Criado FJ and Sánchez-García I: Loss of
p53 exacerbates multiple myeloma phenotype by facilitating the
reprogramming of hematopoietic stem/progenitor cells to malignant
plasma cells by MafB. Cell Cycle. 11:3896–3900. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rosengarten B, Wolff S, Klatt S and
Schermuly RT: Effects of inducible nitric oxide synthase inhibition
or norepinephrine on the neurovascular coupling in an endotoxic rat
shock model. Crit Care. 13:R1392009. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang Y, Su Y, Xu C, Zhang N, Liu D, Li G,
Tong T and Chen J: Protein kinase D1 phosphorylation of KAT7
enhances its protein stability and promotes replication licensing
and cell proliferation. Cell Death Discov. 6:892020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Salomão R, Ferreira BL, Salomão MC, Santos
SS, Azevedo LCP and Brunialti MKC: Sepsis: Evolving concepts and
challenges. Braz J Med Biol Res. 52:e85952019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Korbecki J and Bajdak-Rusinek K: The
effect of palmitic acid on inflammatory response in macrophages: An
overview of molecular mechanisms. Inflamm Res. 68:915–932. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khatib-Massalha E, Bhattacharya S,
Massalha H, Biram A, Golan K, Kollet O, Kumari A, Avemaria F,
Petrovich-Kopitman E, Gur-Cohen S, et al: Lactate released by
inflammatory bone marrow neutrophils induces their mobilization via
endothelial GPR81 signaling. Nat Commun. 11:35472020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xiang P, Chen T, Mou Y, Wu H, Xie P, Lu G,
Gong X, Hu Q, Zhang Y and Ji H: NZ suppresses TLR4/NF-κB signalings
and NLRP3 inflammasome activation in LPS-induced RAW264.7
macrophages. Inflamm Res. 64:799–808. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee YJ, Shin KJ, Park SA, Park KS, Park S,
Heo K, Seo YK, Noh DY, Ryu SH and Suh PG: G-protein-coupled
receptor 81 promotes a malignant phenotype in breast cancer through
angiogenic factor secretion. Oncotarget. 7:70898–70911. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xie Q, Zhu Z, He Y, Zhang Z, Zhang Y, Wang
Y, Luo J, Peng T, Cheng F, Gao J, et al: A lactate-induced
Snail/STAT3 pathway drives GPR81 expression in lung cancer cells.
Biochim Biophys Acta Mol Basis Dis. 1866:1655762020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Popp FC, Piso P, Schlitt HJ and Dahlke MH:
Therapeutic potential of bone marrow stem cells for liver diseases.
Curr Stem Cell Res Ther. 1:411–418. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang W, Zheng Y, Sun S, Li W, Song M, Ji
Q, Wu Z, Liu Z, Fan Y, Liu F, et al: A genome-wide CRISPR-based
screen identifies KAT7 as a driver of cellular senescence. Sci
Transl Med. 13:eabd26552021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fu YJ, Xu B, Huang SW, Luo X, Deng XL, Luo
S, Liu C, Wang Q, Chen JY and Zhou L: Baicalin prevents LPS-induced
activation of TLR4/NF-κB p65 pathway and inflammation in mice via
inhibiting the expression of CD14. Acta Pharmacol Sin. 42:88–96.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bauer AK and Kleeberger SR: Genetic
mechanisms of susceptibility to ozone-induced lung disease. Ann NY
Acad Sci. 1203:113–119. 2010. View Article : Google Scholar : PubMed/NCBI
|