Introduction
The dermal-epidermal junction (DEJ), located between
the epidermis and dermis, provides a specific niche that mediates
numerous signals such as MAPK in addition to providing structural
support to keratinocytes (1,2).
The DEJ serves an important role in skin cohesion, resistance to
mechanical stress and exchange of the signals between the dermis
and epidermis (3). As skin ages,
the wavelike structure of the DEJ becomes thinner and appears
flattened with a loss of rete ridges (4–6).
Furthermore, previous studies have reported that production of
protein components of the DEJ, including collagen IV, VII and XVII,
nidogen, integrin β4 and laminin-332, decreases with age (6–9).
Laminins are the most abundant glycoproteins of the basement
membrane (BM) extracellular matrix (ECM) and are key for supporting
tissue architecture and stability (10). Laminins are involved in skin
reepithelization and wound healing via regulation of adhesion,
proliferation, migration, apoptosis and differentiation (11). Collagen IV is a primary component
of anchoring fibrils, which provide mechanical support for
keratinocytes (12). Collagen
XVII, another structural component of anchoring fibrils, serves
important roles in assembly and function of the cell-matrix
adhesion structure, signal transduction and keratinocyte
differentiation (13). Based on
the key roles of the DEJ in skin homeostasis, modulation of DEJ
components has been suggested as a potential strategy to mitigate
skin aging (14–16).
Our previous study demonstrated that
palmitoyl-Arg-Gly-Asp (PAL-RGD) decreases the appearance of human
facial wrinkles, skin elasticity and dermal density via stimulation
of procollagen synthesis and inhibition of matrix metalloproteinase
(MMP)-1 expression in human dermal fibroblasts (17). Although collagen degradation
within the dermis is considered the primary cause of wrinkles,
biological changes in the epidermis and DEJ, such as decreased
collagen IV, collagen VII, collagen XVII, integrin β4, and
laminin-332, are also reported to affect wrinkle formation
(6). In the present study, the
effects of PAL-RGD on expression of DEJ components, including
laminin-332, collagen IV and collagen XVII in HaCaT keratinocytes
was investigated.
Materials and methods
Reagents
PAL-RGD was purchased from Celltrion Chemical
Research Institute. MTT was purchased from Sigma-Aldrich (Merck
KGaA). Dulbecco's Modified Eagle's Medium (DMEM), penicillin,
streptomycin, PBS, fetal bovine serum (FBS) and trypsin/EDTA were
purchased from Gibco (Thermo Fisher Scientific, Inc.).
Cell culture
The human keratinocyte HaCaT cell line, kindly
gifted by Dr Norbert Fusenig of the German Cancer Research Center
(Heidelberg, Germany), was cultured in DMEM supplemented with 10%
FBS and 1% penicillin/streptomycin at 37°C in a humidified
atmosphere with 5% CO2.
Cell viability assay
Cell viability was determined using MTT assay.
Briefly, HaCaT keratinocytes were seeded into a 24-well plate at
5×104 cells/well and incubated at 37°C for 24 h.
Following serum starvation at 37°C for 24 h, cells were treated
with PAL-RGD (0, 1, 5, 10, 15 and 20 µg/ml) for 48 h in fresh
serum-free DMEM at 37°C. MTT was added and cells were incubated at
37°C for 4 h to allow reduction of the MTT reagent to formazan
dissolved with DMSO. The absorbance was measured at 570 nm using a
VersaMax microplate reader (Molecular Devices, LLC).
Western blotting
HaCaT cells were seeded into a 6-well plate at
1×106 cells/well and incubated at 37°C for 24 h.
Following serum starvation at 37°C for 24 h, the cells were treated
with PAL-RGD (0.0, 5.0, 7.5, 10.0, 12.5 and 15.0 µg/ml) at 37°C for
48 h in fresh serum-free DMEM. Cell lysates were prepared using
PRO-PREP™ Protein Extraction Solution (Intron
Biotechnology, Inc.) and conditioned medium was collected. Protein
concentration was determined with the BCA method (Pierce
Biotechnology). The 20 µg protein from cell lysate and conditioned
media were separated on 4–15% Mini-PROTEAN®
TGX™ Precast Gels (Bio-Rad Laboratories, Inc.) and
transferred to a nitrocellulose membrane. The membranes were
blocked with 5% skimmed milk in Tris-buffered saline for 1 h at
room temperature and incubated with primary antibodies overnight at
4°C against laminin α3 (1:500; cat. no. ab151715; Abcam), laminin
β3 (1:500; cat. no. sc-133178; Santa Cruz Biotechnology, Inc.),
laminin γ2 (1:500; cat. no. MAB19562; MilliporeSigma), collagen
type IV (COL4; 1:500; cat. no. AB769; MilliporeSigma), COL17
(1:500; cat. no. ab184996; Abcam) and β-tubulin (1:1,000; cat. no.
SC-9104; Santa Cruz Biotechnology, Inc.). After rinsing with TBS +
0.05% Tween-20, the membranes were incubated at room temperature
for 2 h with horseradish peroxidase-conjugated anti-goat (cat. no.
#605-4302), anti-mouse (cat. no. #610-4302) and anti-rabbit
antibodies (all 1:5,000, #611-1302, all Rockland Immunochemicals,
Inc.). Membranes were developed with ECL solution
(SuperSignal™ West Femto Maximum Sensitivity Substrate,
Thermo Fisher Scientific, Inc.). Signals were visualized using the
ChemiDoc XRS system (Bio-Rad Laboratories, Inc.) and analyzed with
Image Lab 5.0 (Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative
(RT-q)PCR
Cells were incubated with PAL-RGD (0.0, 5.0, 7.5,
10.0, 12.5 and 15.0 µg/ml) for 2, 4, 8 and 24 h. Total RNA was
isolated from cells using RNeasy Mini Kit (Qiagen GmbH) according
to the manufacturer's protocol. First-strand complementary DNA
(cDNA) synthesis using 1 µg total RNA was performed using
SuperScript™ IV First-Strand Synthesis System (Thermo
Fisher Scientific, Inc.). qPCR was performed using SYBR Green and
StepOnePlus™ Real-Time PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The final reaction mixture
contained 10 ng cDNA, 100 nmol each primer, 10 µl Power SYBR Green
PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.)
and RNase-free water to a final volume of 20 µl. PCR was performed
an initial denaturation step at 95°C for 10 min, followed by 40
cycles at 95°C for 15 sec and 60°C for 1 min. Dissociation curve
was generated to verify the specificity of each reaction. Data were
analyzed using the 2−ΔΔCq method and expressed as
percent change in gene expression relative to 36B4 (18). The primer sequences are presented
in Table I.
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Sequence, 5′-3′ |
|---|
| LAMA3 | F:
CAATTCTGAAGACCCCAGGA |
|
| R:
TCCAAGACCCAAAGATCAGG |
| LAMB3 | F:
CAGATTGGGTTGGAATGCTT |
|
| R:
CCCAGCTTCCTTGACTTGAG |
| LAMC2 | F:
GGCTGGTCTTACTGGAGCAG |
|
| R:
TATGGCAGCTTCACTGTTGC |
| Col4A1 | F:
CTGGTCCAAGAGGATTTCCA |
|
| R:
TCATTGCCTTGCACGTAGAG |
| COL17A1 | F:
TCTGCCTACAGCAACGTGAC |
|
| R:
CTCACGGCTTGACAGCAATA |
| 36B4 | F:
TCGACAATGGCAGCATCTAC |
|
| R:
TGATGCAACAGTTGGGTAGC |
Statistical analysis
Data are presented as the mean ± SD of ≥3
independent experiments. Statistical analysis was performed using
SAS 9.4 (SAS Institute, Inc.) software. Statistical significance
was calculated using one-way ANOVA followed by two-tailed post hoc
Dunnett's multiple comparisons test. P<0.05 was considered to
indicate a statistically significant difference.
Results
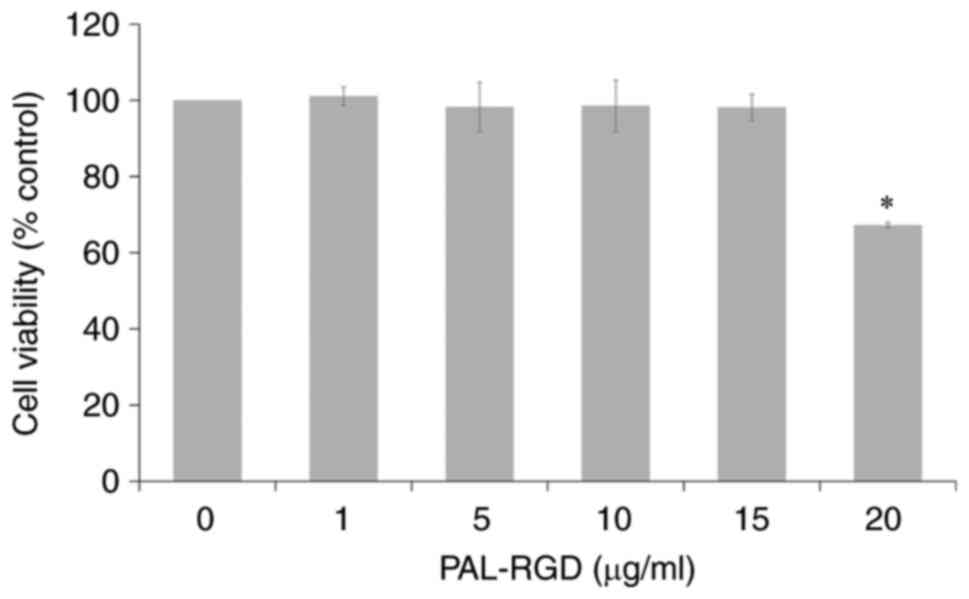
Effect of PAL-RGD on viability in
HaCaT cells
MTT assay was performed to determine optimal PAL-RGD
concentration for treatment of cells. PAL-RGD did not induce a
significant effect on cell viability at concentrations <15
µg/ml. The cell viability significantly decreased to 67% only at 20
µg/ml Pal-RGD (Fig. 1). For
subsequent experiments, the maximum concentration used was 15
µg/ml.
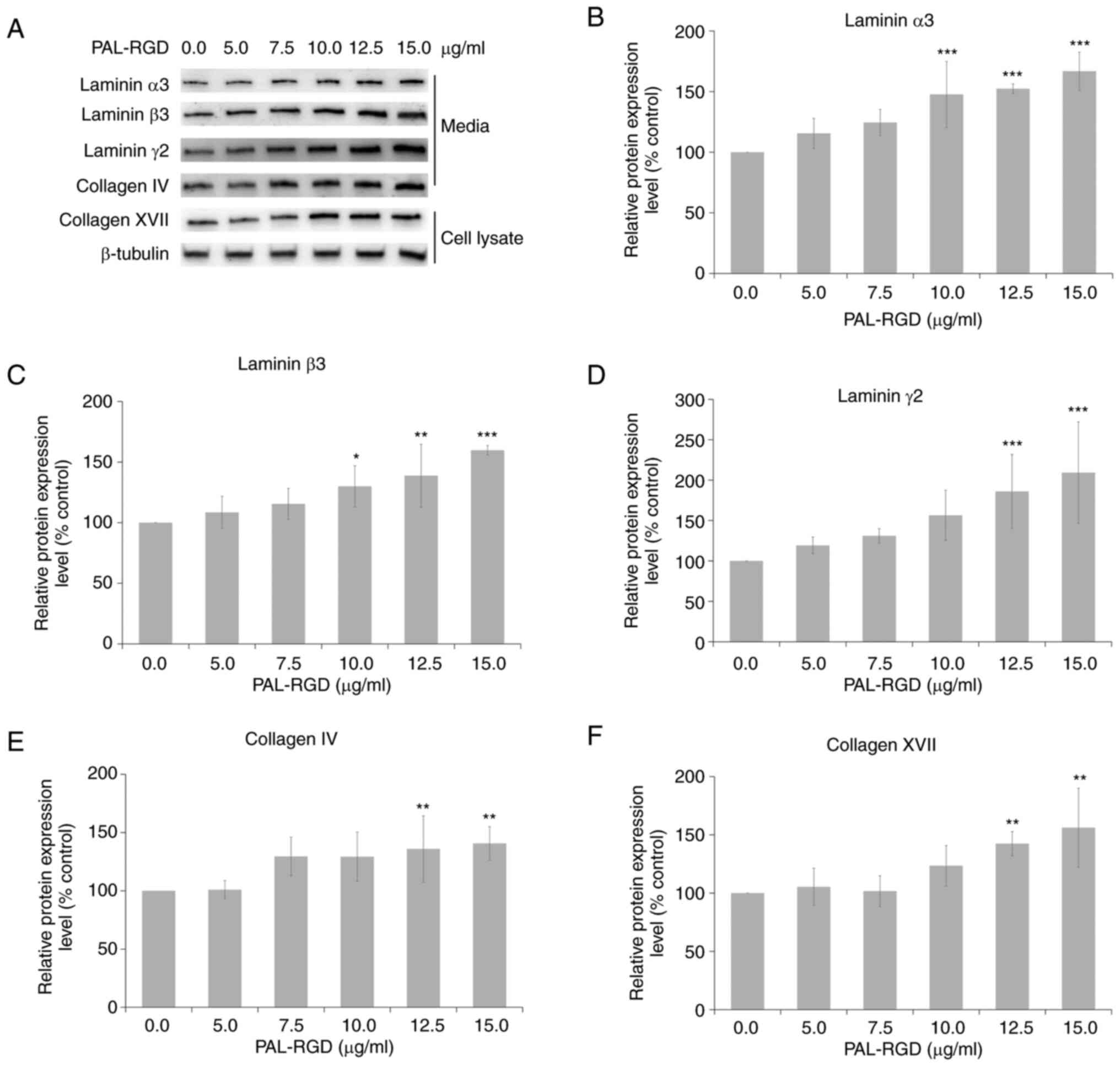
Effect of PAL-RGD on DEJ protein
expression in HaCaT cells
HaCaT cells were treated with PAL-RGD for 48 h.
Total protein extracted from cell lysate and conditioned media was
analyzed using western blotting. PAL-RGD dose-dependently increased
the expression of DEJ proteins (Fig.
2). Treatment with 15 µg/ml PAL-RGD significantly increased
protein expression of laminin α3 (167%), laminin β3 (160%), laminin
γ2 (209%), collagen IV (141%) and collagen XVII (156%) compared
with the control.
Effect of PAL-RGD on laminin subunit
(LAM)A3, LAMB3 and LAMC2 mRNA expression in HaCaT cells
Treatment with PAL-RGD enhanced mRNA expression
levels of LAMA3, LAMB3 and LAMC2 in HaCaT cells
(Fig. 3). LAMA3 mRNA
expression increased in a dose-dependent manner at 2, 4 and 8 h,
then decreased to the basal level at 24 h. LAMA3 mRNA
expression levels significantly increased at 4 h for all
concentrations of PAL-RGD compared with the control. LAMB3
mRNA expression was increased in a dose-dependent manner at 2 h
prior to dropping to the basal level at 8 h. The 2 h increase of
LAMB3 mRNA expression was significant from 7.5 to 15 µg/ml
of PAL-RGD used compared with the control. Furthermore, mRNA
expression of LAMC2 significantly increased 2 h after
PAL-RGD treatment. At 2 h increase of LAMC3 mRNA expression
was significantly for all concentrations of PAL-RGD used compared
with the control. The maximum increases in LAMA3, LAMB3 and
LAMC2 in response to 15 µg/ml PAL-RGD treatment were
significant by 201% (4 h, P<0.0001), 146% (2 h, P=0.0003) and
460% (4 h, P<0.0001) respectively, compared with the
control.
Effect of PAL-RGD on COL4 α 1 chain
(COL4A1) and COL17A1 mRNA expression in HaCaT cells
The mRNA expression of COL4A1 and
COL17A1 was increased by PAL-RGD treatment in HaCaT cells
(Fig. 4). Although unchanged
following exposure to PAL-RGD for 2 and 8 h, mRNA expression levels
increased in a dose-dependent manner at 24 h. Treatment with 12.5
µg/ml PAL-RGD significantly induced COL4A1 and
COL17A1 mRNA levels by 146 and 136%, respectively. The mRNA
expression of COL4A1 and COL17A1 in HaCaT cells
treated with 15 µg/ml PAL-RGD significantly increased by 153 and
136%, respectively, compared with the control.
Discussion
In the present study, PAL-RGD treatment increased
both mRNA and protein expression levels of DEJ components, such as
laminin-332, collagen IV and collagen XVII, in HaCaT keratinocytes.
The mRNA expression levels of laminin-332 components increased
before 8 h and returned to normal at 24 h; however, protein
expression levels in media were significantly increased at 48 h.
Laminin-332 is a secreted protein that matures via specific
proteolytic processing, which may contribute to the time lag
between mRNA expression in cells and protein accumulation in media
(19) Previous studies have
reported that transforming growth factor-β (TGF-β) upregulates
expression of LAMA3, LAMB3, LAMC2, COL4A1 and COL17A1
(20–22). PAL-RGD may induce TGF-β signaling;
further studies are required to investigate the signaling pathway
underlying the PAL-RGD-mediated upregulation of laminin-332,
collagen IV and collagen XVII.
Laminins are glycoproteins composed of three chains
(α, β and γ). At least 15 different isoforms exist with a
combination of five distinct α, three β and three γ subunits
(23,24). The functional domains of LAMs
serve distinct roles, such as assembly into trimeric molecules,
binding to other ECM molecules and interaction with cell surface
receptors. Laminins are involved in various cellular functions,
such as cell adhesion, migration, proliferation and differentiation
(25). In particular, laminin-332
activates the signaling through integrin α3β1 and α6β4 (26). The binding of laminin-332 and
integrin α3β1 activates the mitogen-activated protein kinase
signaling pathway, which regulates keratinocyte proliferation
(27). Moreover, the interaction
between laminin-332 and integrin α3β1 regulates Src kinase
signaling through focal adhesion kinase, promoting polarized
lamellipodium extension, which regulates keratinocyte migration
(28). The interaction between
laminin-332 and integrin α6β4 mediates assembly of the
hemidesmosome cytoskeleton and recruitment of adaptor proteins
Shc/growth factor receptor-bound protein 2 (29). Therefore, PAL-RGD may promote
proliferation and migration of keratinocytes by increasing
laminin-332 expression.
Collagen IV is a non-fibrillar collagen that
constitutes ~50% of the BM, forming a structural scaffold for
interactions between BM components, such as laminin networks,
perlecan and proteoglycans, during BM assembly (30,31). Other extracellular molecules,
including growth factors, laminins, proteoglycans and nidogens, are
attached to the scaffolds, where they serve various biological
roles, such as cell adhesion, migration, tissue regeneration, wound
healing, immobilization of growth factors and enzymes and molecular
sieving (32,33). Given these features of collagen
and related molecules, PAL-RGD may strengthen BM scaffolds by
promoting synthesis of collagen IV.
Collagen XVII is a hemidesmosomal transmembrane
protein involved in epidermal-dermal attachment (34). Congenital collagen XVII deficiency
results in junctional epidermolysis bullosa and acquired
autoimmunity to collagen XVII leads to bullous pemphigoid (35). Furthermore, collagen XVII binds to
laminin-332 in hemidesmosomes and collagen IV in the ECM, linking
cytoplasmic structural components with ECM (36). Based on these functions of
collagen XVII, PAL-RGD may enhance BM assembly by inducing collagen
XVII synthesis.
In summary, the present study suggested that PAL-RGD
may serve a role in strengthening the DEJ by promoting expression
of laminin-332, collagen IV and collagen XVII in keratinocytes.
Together with previous studies demonstrating that PAL-RGD decreases
facial wrinkles by increasing collagen I and decreasing MMP-1, this
compound could improve skin aging by increasing production of
components of the DEJ and BM. A limitation of the present study was
lack of data on the biological effect of PAL-RGD on the function of
the DEJ. Further studies are needed to determine the biological
effect of PAL-RGD on the function of the DEJ.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JHL conceived and designed the study, performed
experiments, collected the data and wrote the manuscript. JSB
designed the study, performed experiments and revised the
manuscript. JHL and JSB confirm the authenticity of all the raw
data. SKL conceived the study and revised the manuscript. DHL
interpreted the data and revised the manuscript. All authors
discussed the results. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Burgeson RE and Christiano AM: The
dermal-epidermal junction. Curr Opin Cell Biol. 9:651–658. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roig-Rosello E and Rousselle P: The human
epidermal basement membrane: A shaped and cell instructive platform
that aging slowly alters. Biomolecules. 10:16072020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marionnet C, Vioux-Chagnoleau C, Pierrard
C, Sok J, Asselineau D and Bernerd F: Morphogenesis of
dermal-epidermal junction in a model of reconstructed skin:
Beneficial effects of vitamin C. Exp Dermatol. 15:625–633. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lavker RM, Zheng P and Dong G: Aged skin:
A study by light, transmission electron, and scanning electron
microscopy. J Invest Dermatol. 88 (3 Suppl):44s–51s. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vázquez F, Palacios S, Alemañ N and
Guerrero F: Changes of the basement membrane and type IV collagen
in human skin during aging. Maturitas. 25:209–215. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Langton AK, Halai P, Griffiths CE,
Sherratt MJ and Watson RE: The impact of intrinsic ageing on the
protein composition of the dermal-epidermal junction. Mech Ageing
Dev. 156:14–16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mondon P, Hillion M, Peschard O, Andre N,
Marchand T, Doridot E, Feuilloley MG, Pionneau C and Chardonnet S:
Evaluation of dermal extracellular matrix and epidermal-dermal
junction modifications using matrix-assisted laser
desorption/ionization mass spectrometric imaging, in vivo
reflectance confocal microscopy, echography, and histology: Effect
of age and peptide applications. J Cosmet Dermatol. 14:152–160.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Contet-Audonneau JL, Jeanmaire C and Pauly
G: A histological study of human wrinkle structures: Comparison
between sun-exposed areas of the face, with or without wrinkles,
and sun-protected areas. Br J Dermatol. 140:1038–1047. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Varlet BL, Chaudagne C, Barré P, Sauvage
C, Berthouloux B, Meybeck A, Dumas M, Saunois A and Bonté F:
Age-related functional and structural changes in human
dermo-epidermal junction components. Journal of Investigative
Dermatology Symposium Proceedings. Vol. 3. Elsevier; pp. 172–179.
1998, View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishihara J, Ishihara A, Fukunaga K, Sasaki
K, White MJV, Briquez PS and Hubbell JA: Laminin heparin-binding
peptides bind to several growth factors and enhance diabetic wound
healing. Nat Commun. 9:21632018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iorio V, Troughton LD and Hamill KJ:
Laminins: Roles and utility in wound repair. Adv Wound Care (New
Rochelle). 4:250–263. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chung HJ and Uitto J: Type VII collagen:
The anchoring fibril protein at fault in dystrophic epidermolysis
bullosa. Dermatol Clin. 28:93–105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watanabe M, Natsuga K, Nishie W, Kobayashi
Y, Donati G, Suzuki S, Fujimura Y, Tsukiyama T, Ujiie H, Shinkuma
S, et al: Type XVII collagen coordinates proliferation in the
interfollicular epidermis. Elife. 6:e266352017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeong S, Yoon S, Kim S, Jung J, Kor M,
Shin K, Lim C, Han HS, Lee H, Park KY, et al: Anti-wrinkle benefits
of peptides complex stimulating skin basement membrane proteins
expression. Int J Mol Sci. 21:732019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amano S: Possible involvement of basement
membrane damage in skin photoaging. Journal of Investigative
Dermatology Symposium Proceedings. Vol. 14. Elsevier; pp. 2–7.
2009, View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kusumaningrum N, Oh JH, Lee DH, Shin CY,
Jang JH, Kim YK and Chung JH: Topical treatment with a cathepsin G
inhibitor, β-keto-phosphonic acid, blocks ultraviolet
irradiation-induced basement membrane damage in hairless mouse
skin. Photodermatol Photoimmunol Photomed. 35:148–156. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bae JS, Kim JM, Kim JY, Choi CH, Kim JY,
Moon WK, Lee MS, Moon SH, Lim JH, Park SJ, et al: Topical
application of palmitoyl-RGD reduces human facial wrinkle formation
in Korean women. Arch Dermatol Res. 309:665–671. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peters BP, Hartle RJ, Krzesicki RF, Kroll
TG, Perini F, Balun JE, Goldstein IJ and Ruddon RW: The
biosynthesis, processing, and secretion of laminin by human
choriocarcinoma cells. J Biol Chem. 260:14732–1442. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Korang K, Christiano AM, Uitto J and
Mauviel A: Differential cytokine modulation of the genes LAMA3,
LAMB3, and LAMC2, encoding the constitutive polypeptides, alpha 3,
beta 3, and gamma 2, of human laminin 5 in epidermal keratinocytes.
FEBS Lett. 368:556–558. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feru J, Delobbe E, Ramont L, Brassart B,
Terryn C, Dupont-Deshorgue A, Garbar C, Monboisse JC, Maquart FX
and Brassart-Pasco S: Aging decreases collagen IV expression in
vivo in the dermo-epidermal junction and in vitro in dermal
fibroblasts: Possible involvement of TGF-β1. Eur J Dermatol.
26:350–360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huilaja L, Hurskainen T, Autio-Harmainen
H, Hofmann SC, Sormunen R, Räsänen J, Ilves M, Franzke CW,
Bruckner-Tuderman L and Tasanen K: Pemphigoid gestationis
autoantigen, transmembrane collagen XVII, promotes the migration of
cytotrophoblastic cells of placenta and is a structural component
of fetal membranes. Matrix Biol. 27:190–200. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sugawara K, Tsuruta D, Ishii M, Jones JC
and Kobayashi H: Laminin-332 and −511 in skin. Exp Dermatol.
17:473–480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miner JH and Yurchenco PD: Laminin
functions in tissue morphogenesis. Annu Rev Cell Dev Biol.
20:255–284. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsuruta D, Kobayashi H, Imanishi H,
Sugawara K, Ishii M and Jones JC: Laminin-332-integrin interaction:
A target for cancer therapy? Curr Med Chem. 15:1968–1975. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kariya Y and Gu J: Roles of laminin-332
and alpha6beta4 integrin in tumor progression. Mini Rev Med Chem.
9:1284–1291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gonzales M, Haan K, Baker SE, Fitchmun M,
Todorov I, Weitzman S and Jones JC: A cell signal pathway involving
laminin-5, alpha3beta1 integrin, and mitogen-activated protein
kinase can regulate epithelial cell proliferation. Mol Biol Cell.
10:259–270. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choma DP, Milano V, Pumiglia KM and
DiPersio CM: Integrin alpha3beta1-dependent activation of FAK/Src
regulates Rac1-mediated keratinocyte polarization on laminin-5. J
Invest Dermatol. 127:31–40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mainiero F, Pepe A, Wary KK, Spinardi L,
Mohammadi M, Schlessinger J and Giancotti F: Signal transduction by
the alpha 6 beta 4 integrin: Distinct beta 4 subunit sites mediate
recruitment of Shc/Grb2 and association with the cytoskeleton of
hemidesmosomes. EMBO J. 14:4470–4481. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pöschl E, Schlötzer-Schrehardt U,
Brachvogel B, Saito K, Ninomiya Y and Mayer U: Collagen IV is
essential for basement membrane stability but dispensable for
initiation of its assembly during early development. Development.
131:1619–1628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brown KL, Cummings CF, Vanacore RM and
Hudson BG: Building collagen IV smart scaffolds on the outside of
cells. Protein Sci. 26:2151–2161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Good MC, Zalatan JG and Lim WA: Scaffold
proteins: Hubs for controlling the flow of cellular information.
Science. 332:680–686. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Trappmann B, Gautrot JE, Connelly JT,
Strange DG, Li Y, Oyen ML, Cohen Stuart MA, Boehm H, Li B, Vogel V,
et al: Extracellular-matrix tethering regulates stem-cell fate. Nat
Mater. 11:642–649. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nishimura M, Nishie W, Shirafuji Y,
Shinkuma S, Natsuga K, Nakamura H, Sawamura D, Iwatsuki K and
Shimizu H: Extracellular cleavage of collagen XVII is essential for
correct cutaneous basement membrane formation. Hum Mol Genet.
25:328–339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Natsuga K, Watanabe M, Nishie W and
Shimizu H: Life before and beyond blistering: The role of collagen
XVII in epidermal physiology. Exp Dermatol. 28:1135–1141. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kamaguchi M, Iwata H, Nishie W, Toyonaga
E, Ujiie H, Natsuga K, Kitagawa Y and Shimizu H: The direct binding
of collagen XVII and collagen IV is disrupted by pemphigoid
autoantibodies. Lab Invest. 99:48–57. 2019. View Article : Google Scholar : PubMed/NCBI
|