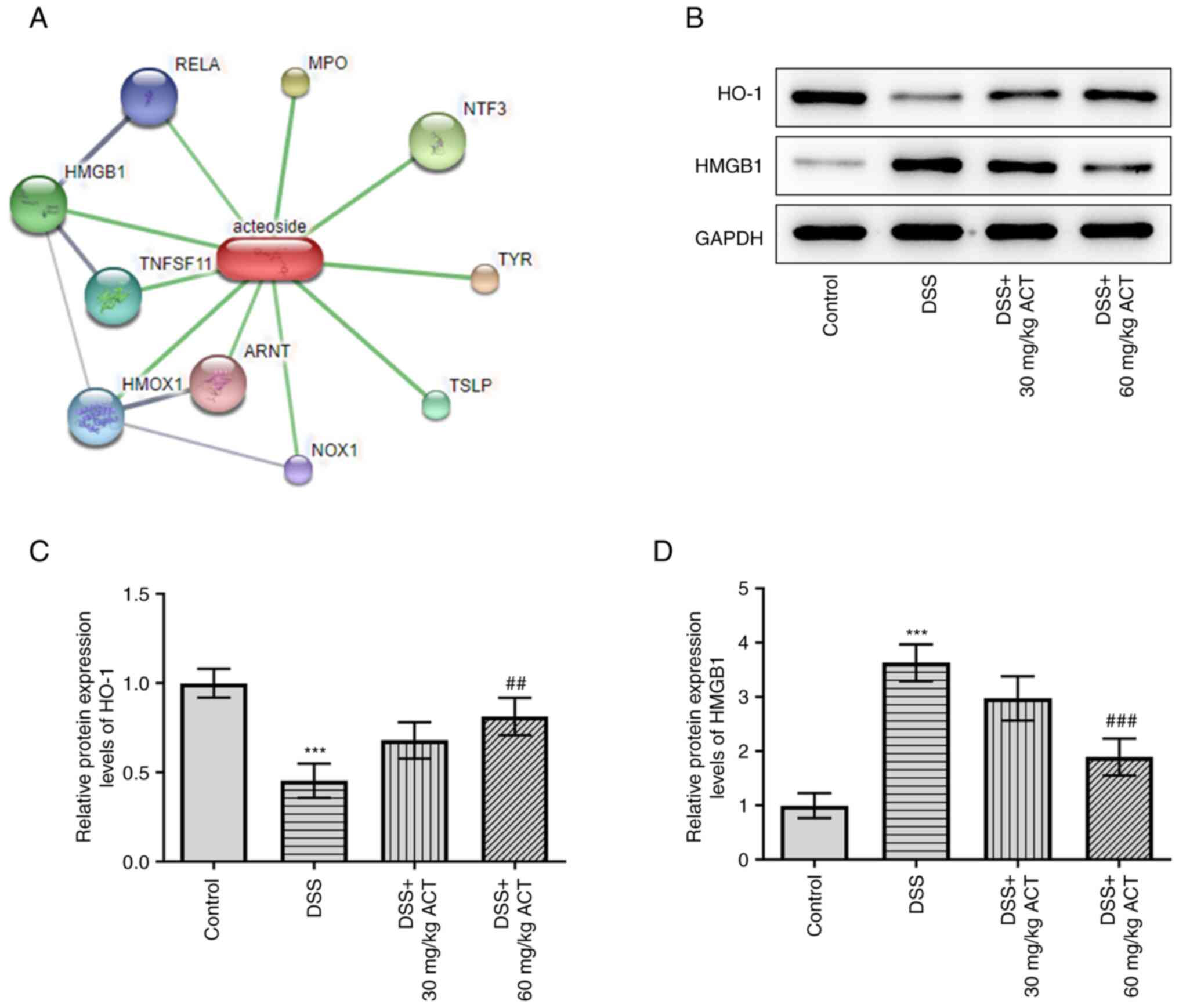
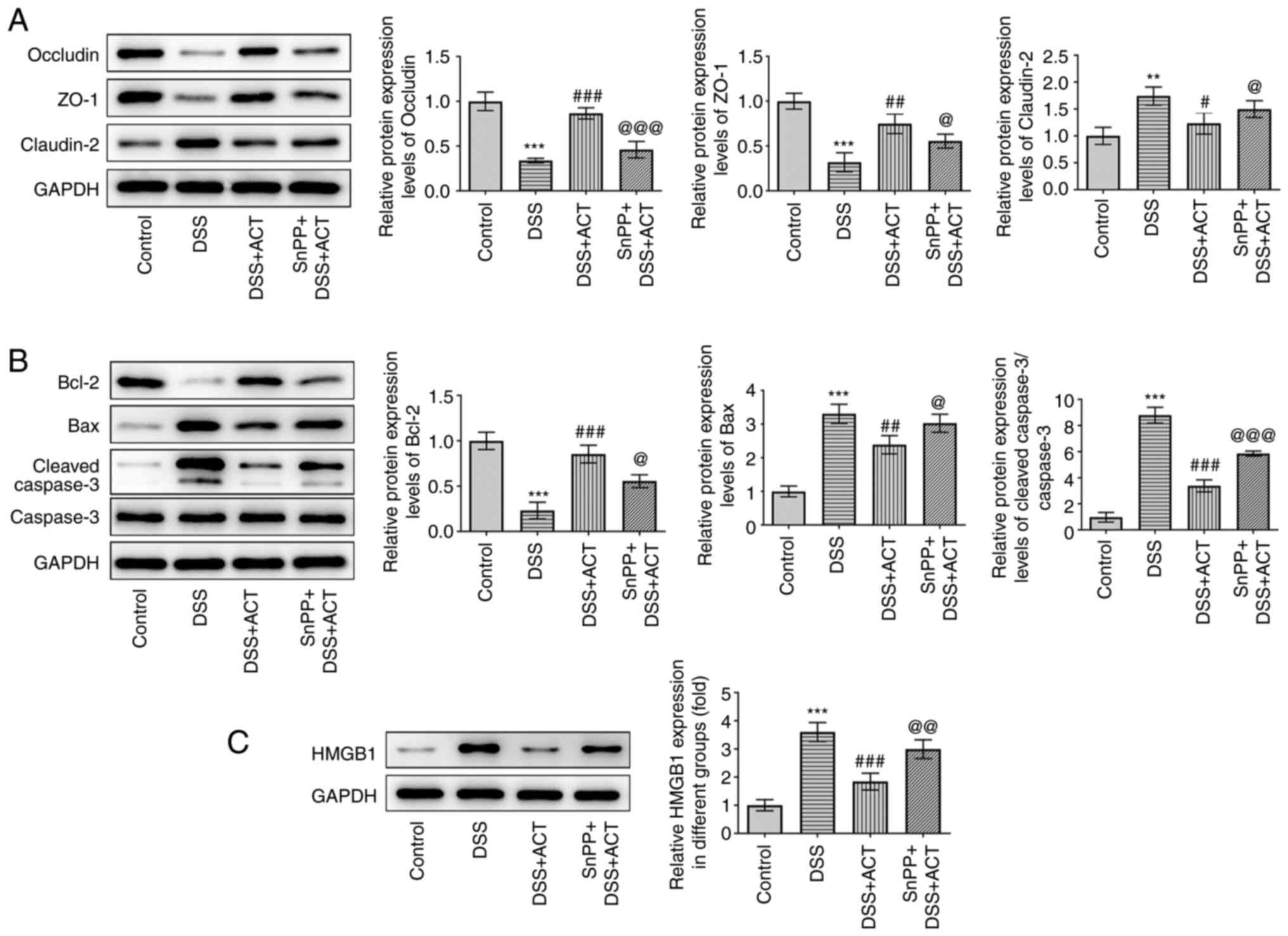
|
1
|
Hodson R: Inflammatory bowel disease.
Nature. 540:S972016. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chu H, Khosravi A, Kusumawardhani IP, Kwon
AH, Vasconcelos AC, Cunha LD, Mayer AE, Shen Y, Wu WL, Kambal A, et
al: Gene-microbiota interactions contribute to the pathogenesis of
inflammatory bowel disease. Science. 352:1116–1120. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neurath MF: Cytokines in inflammatory
bowel disease. Nat Rev Immunol. 14:329–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang YZ and Li YY: Inflammatory bowel
disease: Pathogenesis. World J Gastroenterol. 20:91–99. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ungar B and Kopylov U: Advances in the
development of new biologics in inflammatory bowel disease. Ann
Gastroenterol. 29:243–248. 2016.PubMed/NCBI
|
|
6
|
Ng SC, Tang W, Ching JY, Wong M, Chow CM,
Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, et al: Incidence and
phenotype of inflammatory bowel disease based on results from the
Asia-pacific Crohn's and colitis epidemiology study.
Gastroenterology. 145:158–165.e152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaser A, Zeissig S and Blumberg RS:
Inflammatory bowel disease. Annu Rev Immunol. 28:573–621. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maloy KJ and Powrie F: Intestinal
homeostasis and its breakdown in inflammatory bowel disease.
Nature. 474:298–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cho JH: The genetics and
immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol.
8:458–466. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khor B, Gardet A and Xavier RJ: Genetics
and pathogenesis of inflammatory bowel disease. Nature.
474:307–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang R, Luo Y, Lu Y, Wang D, Wang T, Pu W
and Wang Y: Maggot extracts alleviate inflammation and oxidative
stress in acute experimental colitis via the activation of Nrf2.
Oxid Med Cell Longev. 2019:47032532019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saito M, Chen-Yoshikawa TF, Suetsugu K,
Okabe R, Takahagi A, Masuda S and Date H: Pirfenidone alleviates
lung ischemia-reperfusion injury in a rat model. J Thorac
Cardiovasc Surg. 158:289–296. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Shou Z, Fan H, Xu M, Chen Q, Tang
Q, Liu X, Wu H, Zhang M, Yu T, et al: Protective effects of
oxymatrine against DSS-induced acute intestinal inflammation in
mice via blocking the RhoA/ROCK signaling pathway. Biosci Rep. Jul
18–2019.(Epub ahead of print). View Article : Google Scholar
|
|
14
|
Wu X, He W, Zhang H, Li Y, Liu Z and He Z:
Acteoside: A lipase inhibitor from the Chinese tea Ligustrum
purpurascens kudingcha. Food Chem. 142:306–310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Esposito E, Mazzon E, Paterniti I, Dal
Toso R, Pressi G, Caminiti R and Cuzzocrea S: PPAR-alpha
contributes to the anti-inflammatory activity of verbascoside in a
model of inflammatory bowel disease in mice. PPAR Res.
2010:9173122010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao H, Cui Y, Kang N, Liu X, Liu Y, Zou Y,
Zhang Z, Li X, Yang S, Li J, et al: Isoacteoside, a
dihydroxyphenylethyl glycoside, exhibits anti-inflammatory effects
through blocking toll-like receptor 4 dimerization. Br J Pharmacol.
174:2880–2896. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qiao Z, Tang J, Wu W, Tang J and Liu M:
Acteoside inhibits inflammatory response via JAK/STAT signaling
pathway in osteoarthritic rats. BMC Complement Altern Med.
19:2642019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jing W, Chunhua M and Shumin W: Effects of
acteoside on lipopolysaccharide-induced inflammation in acute lung
injury via regulation of NF-kappaB pathway in vivo and in vitro.
Toxicol Appl Pharmacol. 285:128–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hausmann M, Obermeier F, Paper DH, Balan
K, Dunger N, Menzel K, Falk W, Schoelmerich J, Herfarth H and
Rogler G: In vivo treatment with the herbal phenylethanoid
acteoside ameliorates intestinal inflammation in dextran sulphate
sodium-induced colitis. Clin Exp Immunol. 148:373–381. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Committee for the Update of the Guide for
the Care and Use of Laboratory Animals, . Guide for the Care and
Use of Laboratory Animals. 8th edition. The National Academies
Press; Washington, DC: 2011
|
|
21
|
Liu YJ, Tang B, Wang FC, Tang L, Lei YY,
Luo Y, Huang SJ, Yang M, Wu LY, Wang W, et al: Parthenolide
ameliorates colon inflammation through regulating Treg/Th17 balance
in a gut microbiota-dependent manner. Theranostics. 10:5225–5241.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eichele DD and Kharbanda KK: Dextran
sodium sulfate colitis murine model: An indispensable tool for
advancing our understanding of inflammatory bowel diseases
pathogenesis. World J Gastroenterol. 23:6016–6029. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park YH, Kim N, Shim YK, Choi YJ, Nam RH,
Choi YJ, Ham MH, Suh JH, Lee SM, Lee CM, et al: Adequate dextran
sodium sulfate-induced colitis model in mice and effective outcome
measurement method. J Cancer Prev. 20:260–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Z, Yu K, Zhu F and Gorczynski R:
Over-expression of CD200 protects mice from dextran sodium sulfate
induced colitis. PLoS One. 11:e01466812016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dutton JW III, Artwohl JE, Huang X and
Fortman JD: Assessment of pain associated with the injection of
sodium pentobarbital in laboratory mice (mus musculus). J Am Assoc
Lab Anim Sci. 58:373–379. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ren J, Su D, Li L, Cai H, Zhang M, Zhai J,
Li M, Wu X and Hu K: Anti-inflammatory effects of aureusidin in
LPS-stimulated RAW264.7 macrophages via suppressing NF-κB and
activating ROS- and MAPKs-dependent Nrf2/HO-1 signaling pathways.
Toxicol Appl Pharmacol. 387:1148462020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lien GS, Wu MS, Bien MY, Chen CH, Lin CH
and Chen BC: Epidermal growth factor stimulates nuclear factor-κB
activation and heme oxygenase-1 expression via c-Src, NADPH
oxidase, PI3K, and Akt in human colon cancer cells. PLoS One.
9:e1048912014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsoyi K, Lee TY, Lee YS, Kim HJ, Seo HG,
Lee JH and Chang KC: Heme-oxygenase-1 induction and carbon
monoxide-releasing molecule inhibit lipopolysaccharide
(LPS)-induced high-mobility group box 1 release in vitro and
improve survival of mice in LPS- and cecal ligation and
puncture-induced sepsis model in vivo. Mol Pharmacol. 76:173–182.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reinke D, Kritas S, Polychronopoulos P,
Skaltsounis AL, Aligiannis N and Tran CD: Herbal substance,
acteoside, alleviates intestinal mucositis in mice. Gastroenterol
Res Pract. 2015:3278722015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tian T, Wang Z and Zhang J:
Pathomechanisms of oxidative stress in inflammatory bowel disease
and potential antioxidant therapies. Oxid Med Cell Longev.
2017:45351942017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xia D, Zhang Z and Zhao Y: Acteoside
attenuates oxidative stress and neuronal apoptosis in rats with
focal cerebral ischemia-reperfusion injury. Biol Pharm Bull.
41:1645–1651. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peerzada KJ, Faridi AH, Sharma L, Bhardwaj
SC, Satti NK, Shashi B and Tasduq SA: Acteoside-mediates
chemoprevention of experimental liver carcinogenesis through STAT-3
regulated oxidative stress and apoptosis. Environ Toxicol.
31:782–798. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakov R: New markers in ulcerative
colitis. Clin Chim Acta. 497:141–146. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Palone F, Vitali R, Cucchiara S,
Pierdomenico M, Negroni A, Aloi M, Nuti F, Felice C, Armuzzi A and
Stronati L: Role of HMGB1 as a suitable biomarker of subclinical
intestinal inflammation and mucosal healing in patients with
inflammatory bowel disease. Inflamm Bowel Dis. 20:1448–1457. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen Y, Wu D and Sun L: Clinical
significance of high-mobility group box 1 protein (HMGB1) and
Nod-like receptor protein 3 (NLRP3) in patients with ulcerative
colitis. Med Sci Monit. 26:e9195302020.PubMed/NCBI
|
|
36
|
Hu Z, Wang X, Gong L, Wu G, Peng X and
Tang X: Role of high-mobility group box 1 protein in inflammatory
bowel disease. Inflamm Res. 64:557–563. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Long H, Ruan J, Zhang M, Wang C and Huang
Y: Gastrodin alleviates Tourette syndrome via
Nrf-2/HO-1/HMGB1/NF-small ka, CyrillicB pathway. J Biochem Mol
Toxicol. 33:e223892019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang J, Hu X, Xie J, Xu W and Jiang H:
Beta-1-adrenergic receptors mediate Nrf2-HO-1-HMGB1 axis regulation
to attenuate hypoxia/reoxygenation-induced cardiomyocytes injury in
vitro. Cell Physiol Biochem. 35:767–777. 2015. View Article : Google Scholar : PubMed/NCBI
|