Introduction
Cataracts, which comprise opacification of the
lenses, are the leading cause of visual impairment worldwide. Due
to the global extended life expectancy and increased aging
population, the burden and impact of age-related cataracts are
expected to become more significant. The main causes of cataracts
are aging, ultraviolet radiation, inflammation, diabetes, smoking,
and application of steroid drugs, which result in the generation of
oxidative stress in the lenses (1–3). As
there is no protein turnover in the majority of lens tissues,
post-translational modifications occur in a number of lens
proteins, such as deamidation (4–6),
racemization (7), truncation
(8), oxidation (5,6), and
glycation (6). The lens has a high
concentration of protein, which helps it maintain a high refractive
index. The main and longest-living proteins in the human body are
crystallins (9). Among them,
α-crystallin is associated with chaperone activity that suppresses
the aggregation of denatured proteins. However, the
three-dimensional structure of α-crystallin changes with
post-translational modifications, which may cause reduced chaperone
activity and loss of lens transparency.
Advanced glycation end products (AGEs) in lens
proteins increase with aging through the formation of carbonyl
compounds attributable to reactions with decreasing sugars. AGEs in
the lens are initiated by ascorbate, methylglyoxal (MGO), and
glucose. Ascorbate undergoes oxidation to produce major glycation
agents, such as erythrulose and 3-deoxythreosone. Furthermore, it
has been reported that oxidative ascorbic acid increases because
glycation agents also increase with aging (10). Although a number of proteins in the
lens change to AGEs, there are no methodologies that explain all
AGEs. Therefore, most studies have used N(ε)-carboxymethyl-lysine
(CML) as an AGE indicator. The present study used the CML level as
an indicator of AGEs.
Presbyopia is directly correlated with
protein-linking AGE levels because of increasing lens stiffness
(11). Furthermore, presbyopia is
the earliest observable symptom of age-related nuclear cataracts
(12). Recently, dysfunctional
lens syndrome, which comprises natural lens changes, has become
more commonly observed (13).
Therefore, preventing AGE accumulation is one of the best
strategies for preventing cataracts and presbyopia.
Hesperetin (Hst), which is an abundant and
inexpensive plant flavanone largely derived from citrus species,
has a flavanone backbone structure and strong antioxidant activity.
It is a bioflavonoid because of its various biological activities,
including anti-inflammatory, anti-oxidative, anti-diabetic, and
anti-hypertensive activities (14,15).
The authors previously reported that Hst and its derivates could
prevent cataracts and presbyopia in mice and rats (16–20).
Hst can prevent AGE formation in vivo and in vitro
(21,22) and it can prevent the onset of
cataracts and presbyopia, thus preventing AGE formation. To
elucidate whether Hst could inhibit AGE formation in lens proteins,
the present study used in vitro human lens epithelial cell
lines and ex vivo mouse lens organ cultures.
Materials and methods
Materials
Experimental C57Black/6JJ (C57BL/6) mice were
purchased from Japan SLC Inc. (Shizuoka, Japan) and fed standard
rodent chow (cat. no. CE-2; Clea Japan Inc.). The AGE antibody was
obtained from Trans Genic Inc. (cat. no. KAL-KH001-01). The CML
antibody was obtained from Abcam (cat. no. ab27684). The
α-crystallin and β-actin antibodies were obtained from Santa Cruz
Biotechnology, Inc. (cat. nos. sc-28306 and sc-47778). Aldehyde
dehydrogenase (ALDH) was purchased from MilliporeSigma.
Paraformaldehyde was obtained from Nacalai Tesque, Inc.
Animals
Nine-week-old of twelve male mice (average weigh was
23 g), 10-week-old of eight male mice (average weigh was 23 g),
25-week-old of eight male mice (average weigh was 28 g), and
75-week-old of six male mice (average weigh was 32 g) were used in
this study. Animals were housed in a temperature-controlled
environment with a 12/12-h regular light/dark cycle. The mice were
sacrificed using 5% inhalational isoflurane for >3 min. The
lenses were removed after confirming that the animals had stopped
breathing and the heart had ceased to beat. All animal handling
procedures were performed in accordance with the ARVO statement for
the Use of Animals in Ophthalmic and Vision Research and the
National Institutes of Health guidelines for the care and use of
laboratory animals (arvo.org/About/policies/statement-for-the-use-of-animals-in-ophthalmic-and-vision-research/).
The Keio University Animal Research Committee approved all animal
experiments performed during this study [cat. no. 11014-(8)]. All
animal experiments performed during this study was completed by
October 2022.
High glucose stimulation to induce
AGEs
Immortalized human lens epithelial cells (ihLECs)
were established using transfection with SV40 large T antigen from
cataract patient (23). The ihLECs
were cultured under standard conditions in Dulbecco's modified
Eagle medium/nutrient mixture F-12 (Nacalai Tesque, Inc.)
containing glutamine (4 mM), penicillin-streptomycin antibiotic
mixture (100 U/ml and 100 mg/ml, respectively) and 10% fetal bovine
serum (Biosera) under the 5% CO2 culture condition. The
mice lenses were isolated after sacrifice and cultured in fresh
equilibrated medium 199 with Earle's salts (Thermo Fisher
Scientific, Inc.) with amphotericin B (2.5 mg/ml; FUJIFILM Wako
Pure Chemical Corporation) and the penicillin-streptomycin
antibiotic mixture containing 10% fetal bovine serum. To stimulate
AGE formation, ihLECs or lens organs were treated with 31 mM
glucose, 500 µM MGO, or 500 µM erythrose (ERT) for 2 days.
Immunoblot analysis
Lenses or cells were homogenized in ice-cold
radioimmunoprecipitation buffer inhibitor cocktail for general use
(Nacalai Tesque, Inc.). Protein concentrations were measured using
Bradford assay dye. Denatured 10 µg protein samples were separated
by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
and transferred to polyvinylidene difluoride membranes. Then,
membranes were incubated with 5% skim milk solution (Morinaga co.,
Tokyo) for 1 h at room temperature, followed by incubated with the
primary antibody. The primary antibodies in this study were
anti-AGE (1:1,000), anti-CML (1:10,000) and anti-β-actin (1:1,000).
At 1 h after incubation with the primary antibody at room
temperature, the membranes were washed with Tween20 (0.1%)
containing phosphate-buffered saline (T-PBS) more than three times.
The corresponding secondary antibodies, either horseradish
peroxidase-conjugated anti-mouse antibodies (cat. no. NXA931-1ML;
1:3,000; Cytiva) or horseradish peroxidase-conjugated anti-rabbit
antibodies (cat. no. 7074; 1:3,000; Cell Signaling Technology,
Inc.), were added and incubated at room temperature for 1 h. After
washing the membranes, the protein signals were visualized using an
enhanced chemiluminescence detection system (Cytiva). After
exposure, NIH ImageJ 1.44 was used for gray analysis (National
Institutes of Health).
Measurement of lens elasticity
Lens elasticity was measured using SoftMeasure
HG1003-SL (Horiuchi Electronics Co., Ltd.) as previously described
(19,20). Briefly, the lens was mounted soon
after euthanasia and then a load was applied to the top of the lens
to measure the force and indentation displacement. The mean strain
(%) under 0.05 N of force was assessed using Young's modulus.
Immunohistochemistry
To perform immunohistochemistry, proteins in
cultured ihLECs were fixed in 4% paraformaldehyde for 5 min at room
temperature, followed by incubation with 0.2% Triton X-100 for 5
min to permeabilize the cell membranes at room temperature. The
cells were incubated in a 3% bovine serum albumin/3% normal goat
serum blocking solution for 5 min at room temperature. After
blocking, the cells were first incubated with an anti-AGE antibody
(1:100) or anti-CML antibody (1:1,000). After 1 h of incubation at
room temperature, the cells were washed with phosphate-buffered
saline and incubated with either anti-mouse or anti-rabbit Alexa
Fluor 488 secondary antibody (cat. nos. A28175 and A11008,
respectively; Thermo Fisher Scientific, Inc.) with 0.125 mg/ml
4′,6′-diamidino-2-phenylindole (DAPI) for fluorescent labeling of
the nuclei for 1 h. Organ culturing was performed by fixing the
lenses in 0.75% paraformaldehyde and then they were prepared for
sectioning using established protocols (24,25).
The 12-µm-thick sections were obtained using a freezing microtome
(cat. no. CM1950; Leica Microsystems GmbH), transferred onto
microscope slides, incubated in blocking solution for 1 h, and
incubated further in α-AGE antibody (1:100) or α-CML antibody
(1:100) in blocking solution for 12 h at 4°C. The slides were
washed and incubated with either anti-mouse or anti-rabbit Alexa
Fluor 488 secondary antibodies with DAPI and wheat germ agglutinin.
Confocal images were obtained using a laser scanning microscope
(FV-3000; Olympus Corporation).
Chaperone activity measurement
Chaperone activity was measured as previously
reported, with minor modifications (26). Briefly, ALDH was aggregated by
heating (42°C) with 100 mM 1,10-phenanthroline (FUJIFILM Wako Pure
Chemical Corporation) in 50 mM sodium phosphate buffer containing
100 mM NaCl (pH 7.0). Water soluble proteins were added 10 min
before heat stimulation. The extent of aggregation was estimated by
monitoring light scattering at 360 nm using a microplate reader
(Infinite M200; Tecan Group, Ltd.). The rate of protein aggregation
was expressed as ΔA360/60 min.
Statistical analysis
All data are reported as the mean ± standard error
of the mean. The statistical analysis of data was performed using a
one-way analysis of variance with Tukey's multiple comparison test
using SPSS software (version 24; IBM Corp.). P<0.05 was
considered to indicate a statistically significant difference.
Results
AGEs and lens elasticity with
aging
The present study tested whether AGE modifications
and lens elasticity increased with age in 10, 25, and 75-week-old
mice. AGEs in mouse lenses were measured using an immunoblot
analysis, and lens elasticity was evaluated using SoftMeasure
(Horiuchi Electronics Co., Ltd.). Using immunohistochemistry,
AGE-modified proteins were detected in the lenses of 75-week-old
mice, but not in those of 10-week-old and 25-week mice. Similar to
AGE-modified proteins, CML-modified proteins were detected in the
lenses of 75-week-old mice, but not in those of 10 and 25-week-old
mice. The protein levels of α-crystallin or β-actin were not
significantly different in the lenses of 10, 25 and 75-week-old
mice (Fig. 1A). Subsequently, it
was elucidated that AGE formation could affect lens elasticity and
the lens stiffness was measured immediately following sacrifice.
The lenses of 75-week-old mice were harder than those of 10 and
25-week-old mice. There was no difference in the lens stiffness of
10 and 25-week-old mice (Fig. 1B).
Lens elasticity was assessed under 0.05 N of force and was
significantly increased in 75-week-old mice (Fig. 1C). These results suggested that
generating AGEs formation and changes in the elasticity of lens
with were observed in the mouse model.
Hst treatment and AGE formation in
vitro
The present study investigated whether Hst treatment
affected AGE formation in lens epithelial cell lines using ihLECs
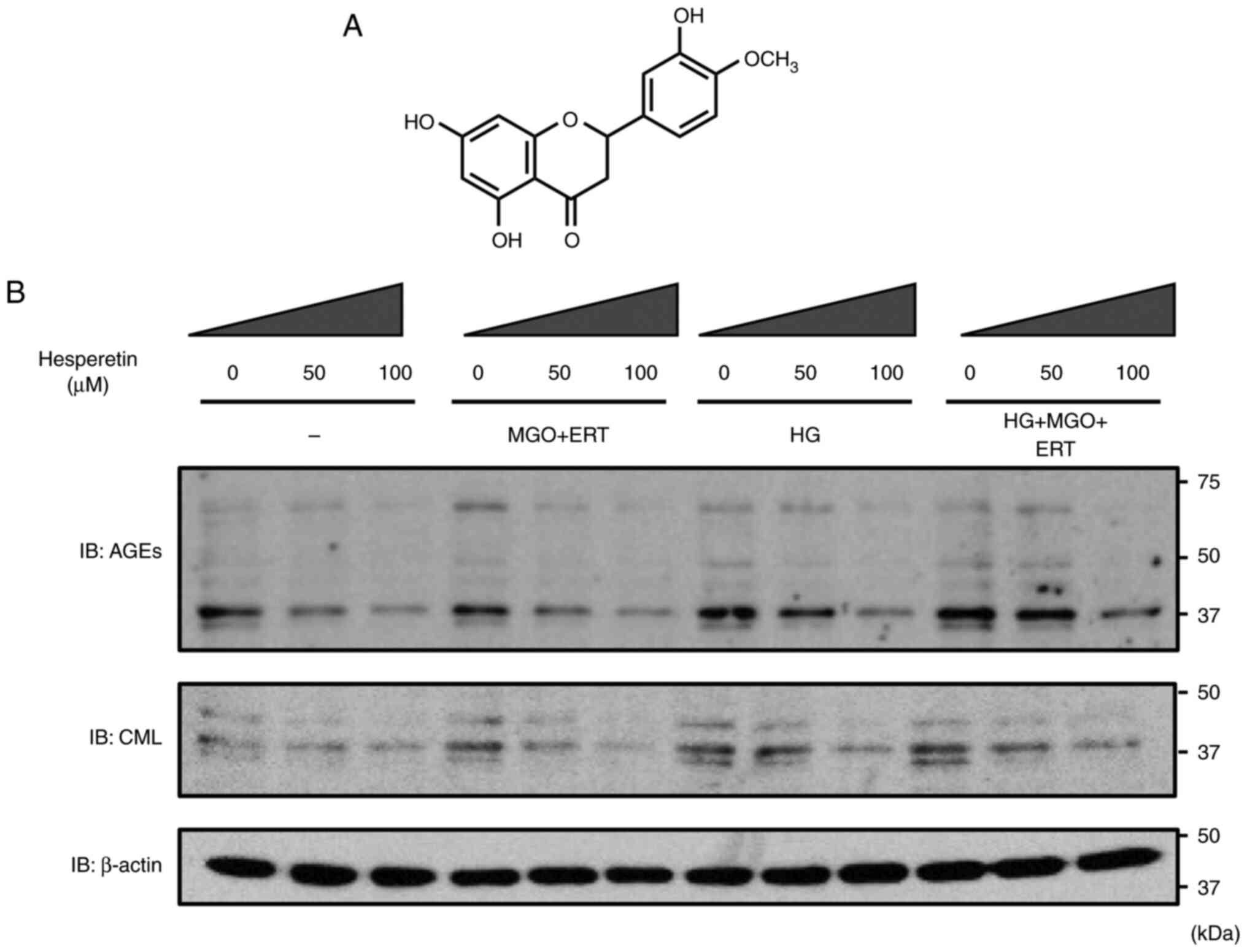
to assess AGE formation. The structural formula of Hst is shown in
Fig. 2A. CML, which is the major
AGE compounds that bind to proteins in ihLECs, was detected.
High-glucose medium with MGO and ERT can stimulate the binding of
AGEs and/or CML with proteins (10). AGE formation in proteins was
significantly increased in ihLECs treated with MGO and ERT in
high-glucose medium, and Hst treatment stopped AGE and CML
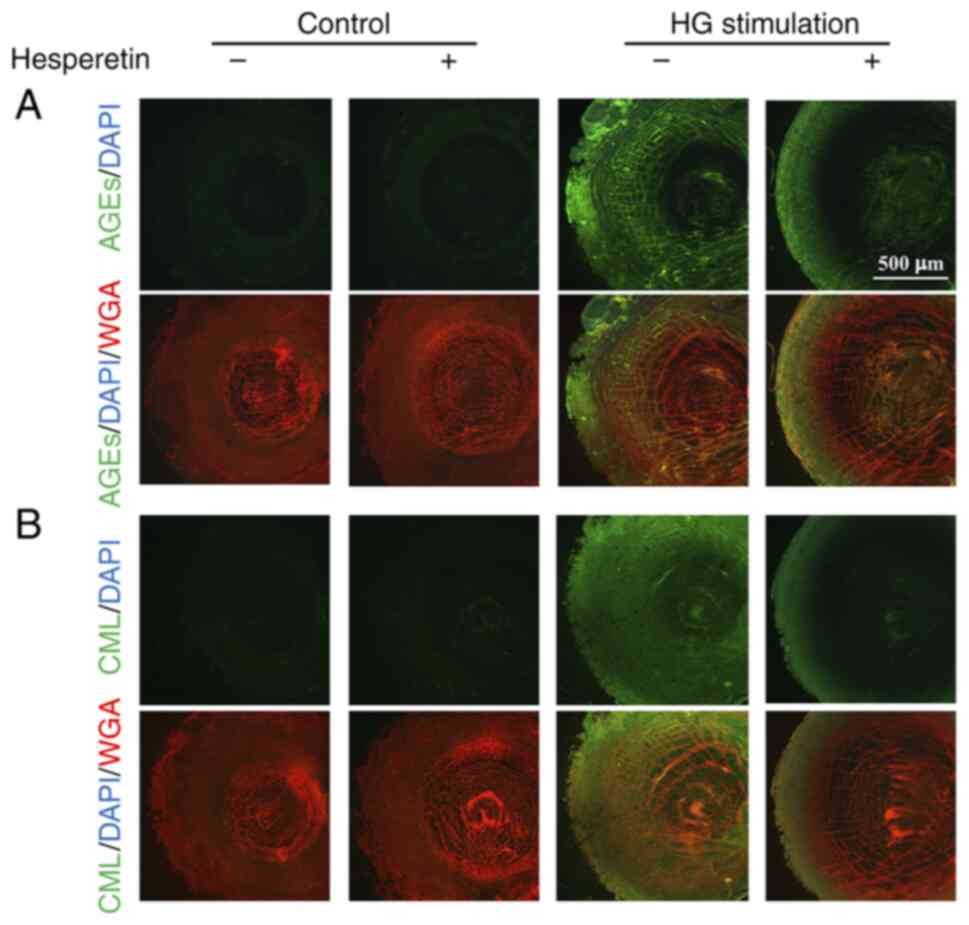
formation in a dose-dependent manner (Fig. 2B). Immunohistochemistry staining
with AGEs and CML also revealed that high-glucose stimulation
increased AGE and CML signals in ihLECs and that these were
cancelled by Hst treatment (Fig. 3A
and B). These results suggested that Hst could prevent AGE and
CML formation in the lens.
Hst affects lens chaperone activity
and elasticity
To investigate whether Hst treatment could
ameliorate lens sclerosis, which causes presbyopia, lens elasticity
was measured using SoftMeasure (Horiuchi Electronics Co., Ltd.),
and Young's modulus was calculated (Fig. 4A). Organ culturing of the lenses
was performed with high-glucose medium and/or Hst. Prior to glucose
stimulation, lenses were incubated at 42°C for 6 h to promote AGE
formation. There were no significant differences in the lenses
incubated under the control glucose condition, regardless of Hst
treatment. The elasticity of the lenses incubated with high-glucose
medium at 42°C was significantly decreased compared to those
incubated under the control glucose condition; however, Hst
treatment markedly inhibited the decrease in elasticity (Fig. 4B).
Lens proteins are associated with chaperone activity
that prevents protein aggregation and cataracts. Following
high-glucose stimulation, the lens chaperone activity was measured
by evaluating the light scattering with ALDH at 360 nm (Fig. 4C). An increase in light scattering
indicated ALDH aggregation and inhibition of protein aggregation,
depending on the chaperone activity. Light scattering with ALDH was
increased without lens proteins, and it was suppressed by the
addition of water-soluble lens proteins at 37° under the control
condition, normal glucose culture condition, and high-glucose
culture condition with Hst; however, light scattering in the lens
was not changed under the high-glucose culture condition without
Hst. Data are shown as the relative ΔA360/180 min values
of light scattering with ALDH without lens proteins (defined as
100%; Fig. 4D). Lens proteins
subjected to high-glucose stimulation completely lacked the
inhibition of light scattering; however, Hst treatment of lenses
subjected to high-glucose stimulation protected against increased
light scattering. These data suggested that Hst treatment prevents
protein aggregation by maintaining the chaperone activity and
preventing lens hardening and presbyopia.
Hst treatment reduces AGE formation ex
vivo
The present study investigated whether lens
hardening and decreased chaperone activity occurred based on AGE
formation and examined the results of immunohistochemical staining
with AGE-binding protein or CML-binding protein (Fig. 5). To stimulate AGE formation,
lenses were incubated at 42°C for 6 h before glucose, MGO and ERT
stimulation, with or without 100 µM Hst. As a result, AGE-binding
and CML-binding proteins were not detected in the lenses incubated
under the normal glucose condition either with or without Hst.
AGE-binding and CML-binding proteins were found in the lenses
stimulated under the high-glucose condition; however, these signals
were diminished in the lenses treated with Hst (Fig. 5A and B). These results suggested
that Hst treatment could attenuate lens sclerosis by preventing
chaperone activity and AGE formation.
Discussion
Presbyopia is an age-related physiological reduction
of accommodation that leads to unsatisfactory clarity of near
vision (27,28). This condition usually begins after
~ 45 years. In 2030, the number of individuals with presbyopia is
expected to increase to 2.1 billion globally (29). There are two age-related
biomechanical changes that have been hypothesized as causes of
presbyopia: aging of the ciliary muscle, which exerts the force
required to change the shape of the crystalline lens, and
stiffening of the lens itself, which increases with age (30,31).
Ostrin and Glasser (32) reported
that muscle function is normal beyond the onset of presbyopia in
the eyes of primates and humans. Furthermore, lens sclerosis is a
major inducer of presbyopia, thus demonstrating that the
replacement of a presbyopic lens with a soft polymer restores the
accommodative ability (33). The
present study revealed that AGE formation affects the lens
stiffness ex vivo. The cause of presbyopia is lens hardening
by several sources such as decreasing levels of α-crystallin water
solubility and chaperone activity and increasing lens disulfides
such as oxidative glutathione (34). The accumulation of denatured
proteins and disulfides makes the lens cloudy, which is termed a
cataract. It has also been reported that dysfunctional lens
syndrome includes three stages: Stage 1, presbyopia or cataracts
(age 42–50 years); stage 2, accommodation loss (from ≥50 years);
and stage 3, lens opacity, poor vision quality, and full cataracts
(from ≥65 years) (13). Therefore,
maintaining the accommodation ability of the lens and preventing
lens sclerosis are the best ways to improve the quality of vision
in elderly individuals and prevent cataracts.
The pharmacological treatment of presbyopia has been
studied recently. In November 2021, the United States Food and Drug
Administration approved the use of 1.25% pilocarpine hydrochloride
ophthalmic solution as the first eye drop treatment for presbyopia
(35). This eye drop effectively
increases the depth of focus and causes the pupils of the eye to
constrict, thus producing a small pupil and creating a pinhole
effect for ~6 h. However, retinal detachments and vitreofoveal
traction were reported following pilocarpine eye drop treatment
(36–38). Other candidate eye drops for
presbyopia comprise the choline ester of lipoic acid (EV-06), which
can replenish glutathione and decrease disulfide linkages in lens
proteins of aged lenses (39). The
study did not report any serious adverse effect from use of EV-06,
drug development had been abandoned following phase 2b clinical
trial in which the drug did not achieve a statistically significant
dose response. In addition, mercaptoethylguanidine could increase
lens stiffness by preventing AGE formation in lens proteins and
increasing lens glutathione levels (40). It is suggested that oxidative
stress and AGE formation with age may promote lens hardening and
cause presbyopia (11). The
authors previously reported that Hst and its derivatives have
antioxidant and anti-cataract effects (18) and in the present study, it was
found that Hst treatment could prevent AGE generation and AGE
formation in the lens protein and lens hardening caused by
presbyopia.
The lens is a large avascular tissue that has
evolved an internal microcirculation system that compensates for
the absence of a blood supply by generating circulating fluxes of
ions and water that deliver nutrients and remove metabolic waste.
This microcirculation system is generated by a circulating flux of
sodium, which drives the flow of isotonic fluid that enters the
lens at both the anterior and posterior poles via an extracellular
pathway. Sodium and water cross the membranes of deeper fiber cells
before returning to the surface via an intracellular pathway
mediated by gap junction channels that direct sodium and water to
the lens equator, which is regulated by a dual feedback system that
utilizes transient receptor potential vanilloid (TRPV)1 and TRPV4
(41). Moreover, TRPV1 and/or
TRPV4 in peripheral fiber cells of the lens alter their membrane
trafficking by accommodating changes, meaning that TRPV channels
located in the cytoplasm comprise a protein pool that meets
physiological needs and accommodates changes. The authors
previously reported that treatment with oral a-glucosyl hesperidin,
which is Hst derivatives with a water solubility ~10,000 times
higher than that of Hst, altered TRPV4 localization in the
peripheral fibre cells of the lens to control water
microcirculation (20).
Additionally, oral treatment ameliorated lens fluid influx and
osmotic imbalance in the lenses of individuals with diabetes and
cataracts (42). These results
suggest that TRPV channels could contribute to lens sclerosis and
might be a target for presbyopia treatment.
The limitation to this study was in vitro and
ex vivo experiments. In this study, we experienced Het
treated culture cells in vitro study and mouse lens organ
culture ex vivo study. In vivo experiments such as
the suppression of AGEs formation by experimental animals fed Het
or Hst derivatives will be needed.
Hst treatment attenuates lens hardening ex
vivo and prevents AGE generation in vitro. The results
of the present study revealed that Hst treatment may prevent lens
sclerosis and cataract formation attributable to water influx, TRPV
channel distribution and AGE generation. Further research is needed
to evaluate the onset of presbyopia attributable to oxidative
stress, AGE generation and TRPV channel localization.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the Japan
Society for the Promotion of Science KAKENHI (grant no. 20K07184)
to YN and from the Keio Gijuku Fukuzawa Memorial Fund for the
Advancement of Education and Research.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contribution
YN, HT, and MFT defined the study themes. YN, NN,
MFT and HT designed the study. YD and YN performed laboratory
experiments. YN, NM, SE, NN, NY and HT analyzed and interpreted the
data. YD, YN and HT confirm the authenticity of all the raw data.
YN was a major contributor in writing the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Keio
University Animal Research Committee [approval no. 11014-(8)].
Patient consent for publication
Not applicable.
Competing interests
NM and SE are employees of Hayashibara Co., Ltd.
(Okayama, Japan). The funders had no role in the design of the
study; collection, analysis, or interpretation of data; writing of
the manuscript; or decision to publish the results. The other
authors also declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
AGE
|
advanced glycation end product
|
|
ALDH
|
aldehyde dehydrogenase
|
|
CML
|
N(ε)-carboxymethyl-lysine
|
|
ERT
|
erythrulose
|
|
Hst
|
hesperetin
|
|
MGO
|
methylglyoxal
|
|
TRPV
|
transient receptor potential
vanilloid
|
References
|
1
|
Vasvada AR and Raj SM: Cataract treatment
where resources are scarce. Lancet. 365:550–551. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Little MP, Kitahara CM, Cahoon EK, Bernier
MO, Velazquez-Kronen R, Doody MM, Borrego D, Miller JS, Alexander
BH, Simon SL, et al: Occupational radiation exposure and risk of
cataract incidence in a cohort of US radiologic technologists. Eur
J Epidemiol. 33:1179–1191. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Little MP, Cahoon EK, Kitahara CM, Simon
SL, Hamada N and Linet MS: Occupational radiation exposure and
excess additive risk of cataract incidence in a cohort of US
radiologic technologists. Occup Environ Med. 77:1–8. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Voorter CE, de Haard-Hoekman WA, van den
Oetelaar PJ, Bloemendal H and de Jong WW: Spontaneous peptide bond
cleavage in aging alpha-crystallin through a succinimide
intermediate. J Biol Chem. 263:19020–19023. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takemoto L, Horwitz J and Emmons T:
Oxidation of the N-terminal methionine of lens alpha-A crystallin.
Curr Eye Res. 11:651–655. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miesbauer LR, Zhou X, Yang Z, Yang Z, Sun
Y, Smith DL and Smith JB: Post-translational modifications of
water-soluble human lens crystallins from young adults. J Biol
Chem. 269:12494–12502. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujii N, Momose Y, Yamasaki M, Yamagaki T,
Nakanishi H, Uemura T, Takita M and Ishii N: The conformation
formed by the domain after alanine-155 induces inversion of
aspartic acid-151 in alpha A-crystallin from aged human lenses.
Biochem Biophys Res Commun. 239:918–923. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Argirova MD and Breipohl W: Glycated
proteins can enhance photooxidative stress in aged and diabetic
lenses. Free Radic Res. 36:1251–1259. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McAvoy JW, Chamberlain CG, de Iongh RU,
Hales AM and Lovicu FJ: Lens development. Eye (Lond). 13:425–437.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nemet I and Monnier VM: Vitamin C
degradation products and pathways in the human lens. J Biol Chem.
286:37128–37136. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nandi SK, Nahomi RB, Rankenberg J, Glomb
MA and Nagaraj RH: Glycation-mediated inter-protein cross-linking
is promoted by chaperone-client complexes of α-crystallin:
Implications for lens aging and presbyopia. J Biol Chem.
295:5701–5716. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McGinty SJ and Truscott RJW: Presbyopia:
The first stage of nuclear cataract? Ophthalmic Res. 38:137–148.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fernández J, Rodríguez-Vallejo M, Martínez
J, Tauste A and Piñero DP: From presbyopia to cataracts: A critical
review on dysfunctional lens syndrome. J Ophthalmol.
2018:43184052018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Akiyama S, Katsumata S, Suzuki K, Nakaya
Y, Ishimi Y and Uehara M: Hypoglycemic and hypolipidemic effects of
hesperidin and cyclodextrin-clathrated hesperetin in Goto-Kakizaki
rats with type 2 diabetes. Biosci Biotechnol Biochem. 73:2779–2782.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alu'datt MH, Rababah T, Alhamad MN,
Al-Mahasneh MA, Ereifej K, Al-Karaki G, Al-Duais M, Andrade JE,
Tranchant CC, Kubow S and Ghozlan KA: Profiles of free and bound
phenolics extracted from citrus fruits and their roles in
biological systems: Content, and antioxidant, anti-diabetic and
anti-hypertensive properties. Food Funct. 8:3187–3197. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakazawa Y, Oka M, Bando M and Takehana M:
Hesperetin prevents selenite-induced cataract in rats. Mol Vis.
21:804–810. 2015.PubMed/NCBI
|
|
17
|
Nakazawa Y, Oka M, Tamura H and Takehana
M: Effect of hesperetin on chaperone activity in selenite-induced
cataract. Open Med (Wars). 11:183–189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakazawa Y, Pauze M, Fukuyama K, Nagai N,
Funakoshi-Tago M, Sugai T and Tamura H: Effect of hesperetin
derivatives on the development of selenite-induced cataracts in
rats. Mol Med Rep. 18:1043–1050. 2018.PubMed/NCBI
|
|
19
|
Nakazawa Y, Aoki M, Ishiwa S, Morishita N,
Endo S, Nagai N, Yamamoto N, Funakoshi-Tago M and Tamura H: Oral
intake of α-glucosyl-hesperidin ameliorates selenite-induced
cataract formation. Mol Med Rep. 21:1258–1266. 2020.PubMed/NCBI
|
|
20
|
Nakazawa Y, Doki Y, Sugiyama Y, Kobayashi
R, Nagai N, Morishita N, Endo S, Funakoshi-Tago M and Tamura H:
Effect of alpha-glucosyl-hesperidin consumption on lens sclerosis
and presbyopia. Cells. 10:3822021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ouyang A, Garner TB and Fleenor BS:
Hesperidin reverses perivascular adipose-mediated aortic stiffness
with aging. Exp Gerontol. 97:68–72. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khan MS, Rehman MT, Ismael MA, AlAjmi MF,
Alruwaished GI, Alokail MS and Khan MR: Bioflavonoid (hesperidin)
restrains protein oxidation and advanced glycation end product
formation by targeting AGEs and glycolytic enzymes. Cell Biochem
Biophys. 79:833–844. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamamoto N, Takeda S, Hatsusaka N,
Hiramatsu N, Nagai N, Deguchi S, Nakazawa Y, Takata T, Kodera S,
Hirata A, et al: Effect of a lens protein in low-temperature
culture of novel immortalized human lens epithelial cells
(iHLEC-NY2). Cells. 9:26702020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jacobs MD, Donaldson PJ, Cannell MB and
Soeller C: Resolving morphology and antibody labeling over large
distances in tissue sections. Microsc Res Tech. 61:83–91. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakazawa Y, Donaldson PJ and Petrova RS:
Verification and spatial mapping of TRPV1 and TRPV4 expression in
the embryonic and adult mouse lens. Exp Eye Res. 186:1077072019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakazawa Y, Nagai N, Ishimori N, Oguchi J
and Tamura H: Administration of antioxidant compounds affects the
lens chaperone activity and prevents the onset of cataracts. Biomed
Pharmacother. 95:137–143. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Atchison DA: Accommodation and presbyopia.
Ophthalmic Physiol Opt. 15:255–272. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wolffsohn JS and Davies LN: Presbyopia:
Effectiveness of correction strategies. Prog Retin Eye Res.
68:124–143. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Holden BA, Fricke TR, Wilson DA, Jong M,
Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ and Resnikoff S:
Global prevalence of myopia and high myopia and temporal trends
from 2000 through 2050. Ophthalmology. 123:1036–1042. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Strenk SA, Semmlow JL, Strenk LM, Munoz P,
Gronlund-Jacob J and DeMarco JK: Age-related changes in human
ciliary muscle and lens: A magnetic resonance imaging study. Invest
Ophthalmol Vis Sci. 40:1162–1169. 1999.PubMed/NCBI
|
|
31
|
Hermans EA, Pouwels PJW, Dubbelman M,
Kuijer JPA, van der Heijde RGL and Heethaar RM: Constant volume of
the human lens and decrease in surface area of the capsular bag
during accommodation: An MRI and Scheimpflug study. Invest
Ophthalmol Vis Sci. 50:281–289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ostrin LA and Glasser A: Edinger-Westphal
and pharmacologically stimulated accommodative refractive changes
and lens and ciliary process movements in rhesus monkeys. Exp Eye
Res. 84:302–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koopmans SA, Terwee T, Barkhof J, Haitjema
HJ and Kooijman AC: Polymer refilling of presbyopic human lenses in
vitro restores the ability to undergo accommodative changes. Invest
Ophthalmol Vis Sci. 44:250–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pathai S, Shiels PG, Lawn SD, Cook C and
Gibert C: The eye as a model of ageing in translational
research-molecular, epigenetic and clinical aspects. Ageing Res
Rev. 12:490–508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jackson MA, Giyanani J, Shabaik Y, Penzner
J, Gore AV, Robinson MR and Waring GOW IV: In vitro and in-eye
comparison of commercial pilocarpine ophthalmic solution and an
optimized, reformulated pilocarpine for presbyopia treatment.
Ophthalmol Ther. 11:869–879. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Al-Khersan H, Flynn HW Jr and Townsend JH:
Retinal detachments associated with topical pilocarpine use for
presbyopia. Am J Ophthalmol. 242:52–55. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eton EA, Zhao PY, Johnson MW, Rao RC and
Huvard MJ: Rhegmatogenous retinal detachment following initiation
of pilocarpine hydrochloride ophthalmic solution 1.25% for
treatment of presbyopia. Retin Cases Brief Rep. Aug 12–2022.(Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Amarikwa L, Michalek SM, Caul S,
Mruthyunjaya P and Rahimy E: Vitreofoveal traction associated with
pilocarpine for presbyopia. Ophthalmic Surg Lasers Imaging Retina.
53:410–411. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Garner WH and Garner MH: Protein disulfide
levels and lens elasticity modulation: Applications for presbyopia.
Invest Ophthalmol Vis Sci. 57:2851–2863. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nandi SK, Rankenberg J, Rakete S, Nahomi
RB, Glomb MA, Linetsky MD and Nagaraj RH: Glycation-mediated
protein crosslinking and stiffening in mouse lenses are inhibited
by carboxitin in vitro. Glycoconj J. 38:347–359. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gao J, Sun X, White TW, Delamere NA and
Mathias RT: Feedback regulation of intracellular hydrostatic
pressure in surface cells of the lens. Biophys J. 109:1830–1839.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Umran NSS, Mohamed S, Lau SF and Mohd
Ishak NI: Citrus hystrix leaf extract attenuated diabetic-cataract
in STZ-rats. J Food Biochem. 44:e132582020. View Article : Google Scholar : PubMed/NCBI
|