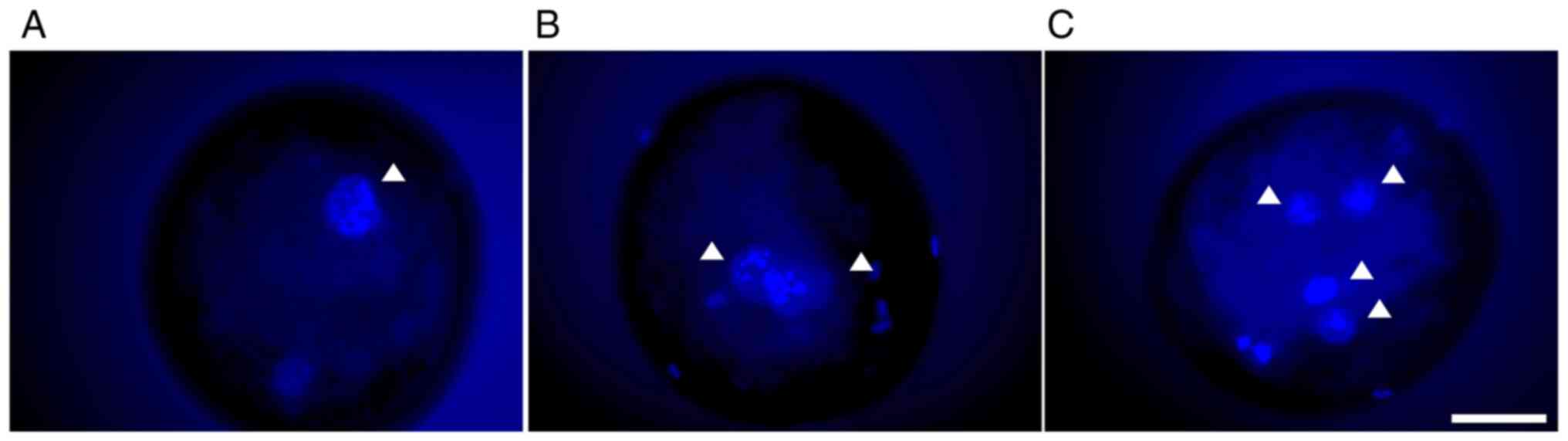
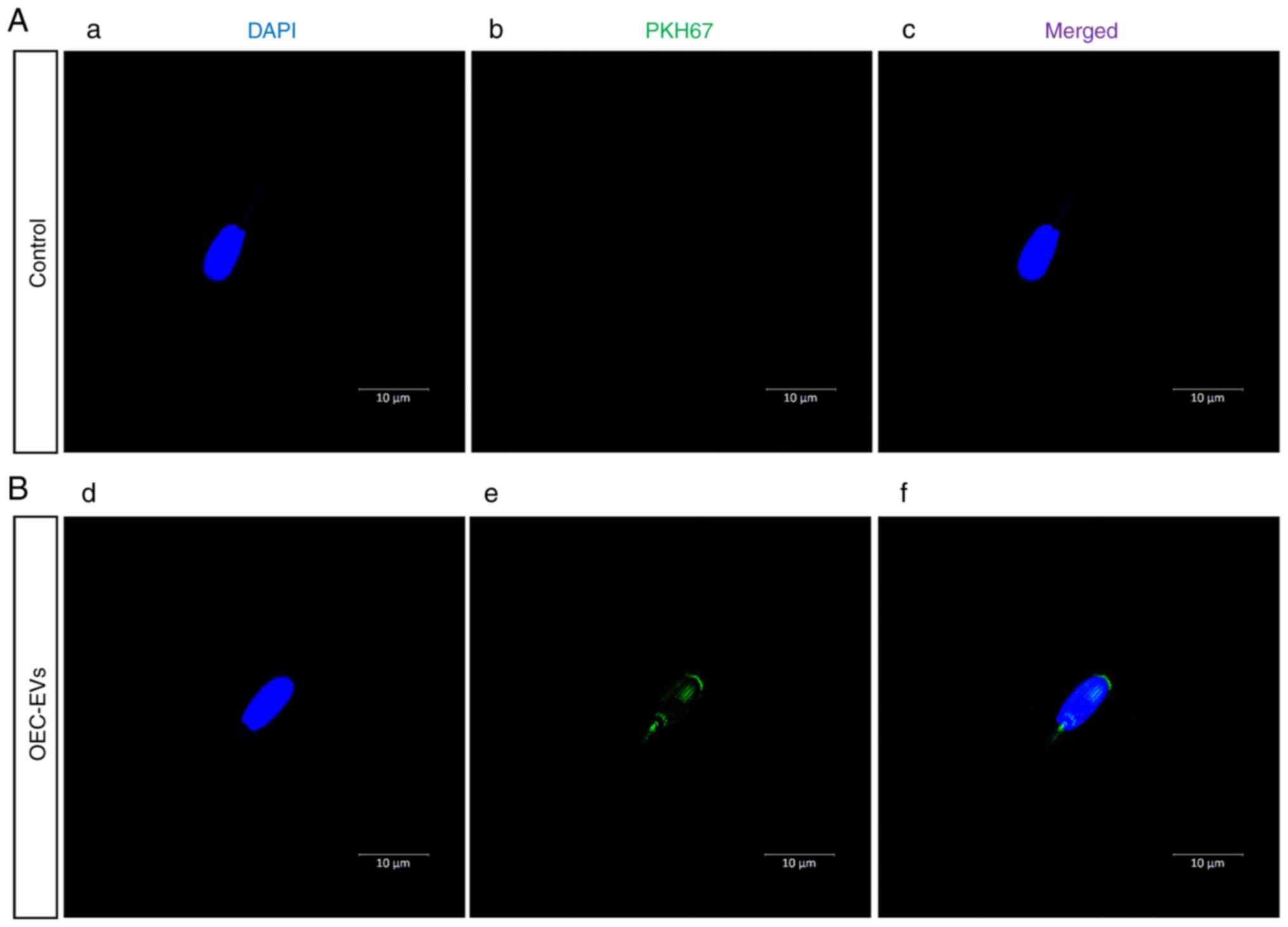
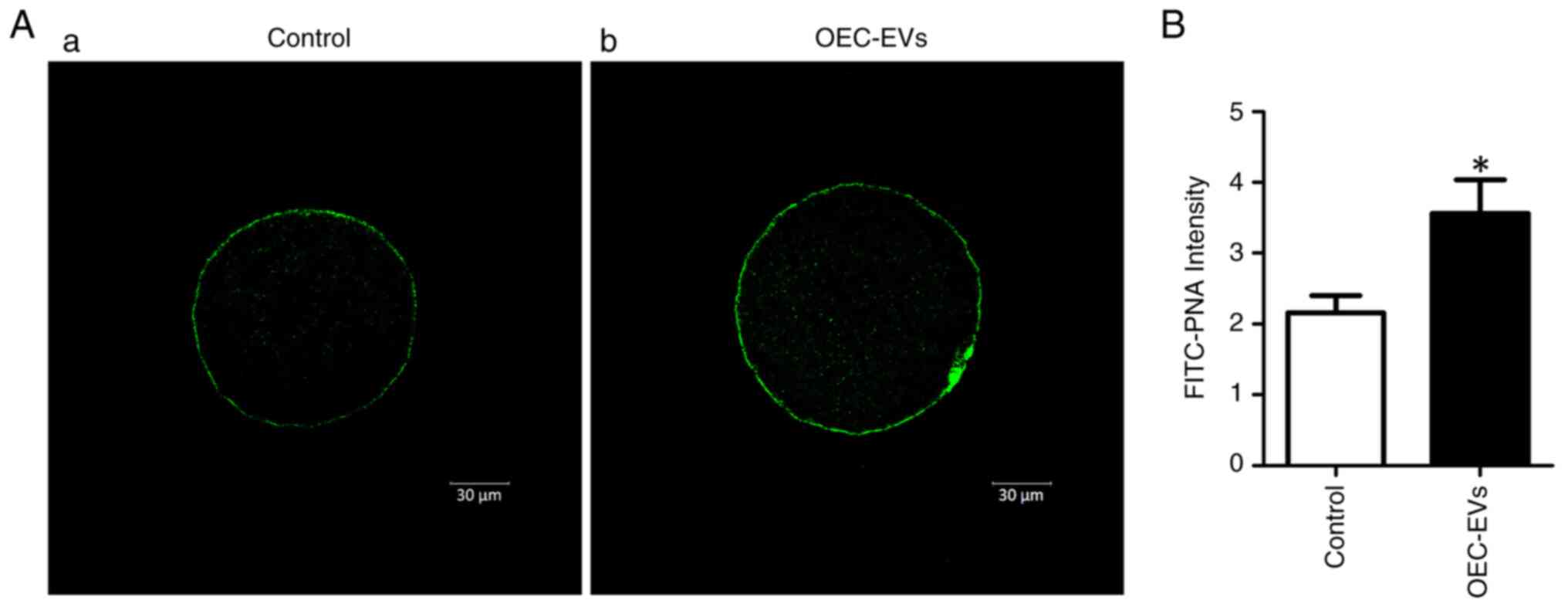
|
1
|
Brevini TAL, Antonini S, Cillo F, Crestan
M and Gandolfi F: Porcine embryonic stem cells: Facts, challenges
and hopes. Theriogenology. 68 (Suppl 1):S206–S213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martinez CA, Cambra JM, Maside C, Cuello
C, Roca J, Martinez EA, Parrilla I and Gil MA: High pre-freezing
sperm dilution improves monospermy without affecting the
penetration rate in porcine IVF. Theriogenology. 131:162–168. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tanihara F, Hirata M, Nguyen NT, Le QA,
Hirano T and Otoi T: Effects of concentration of CRISPR/Cas9
components on genetic mosaicism in cytoplasmic microinjected
porcine embryos. J Reprod Dev. 65:209–214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gil MA, Cuello C, Parrilla I, Vazquez JM,
Roca J and Martinez EA: Advances in swine in vitro embryo
production technologies. Reprod Domest Anim. 45 (Suppl 2):S40–S48.
2010. View Article : Google Scholar
|
|
5
|
Grupen CG: The evolution of porcine embryo
in vitro production. Theriogenology. 81:24–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nguyen HT, Dang-Nguyen TQ, Somfai T, Men
NT, Viet Linh N, Xuan Nguyen B, Noguchi J, Kaneko H and Kikuchi K:
Selection based on morphological features of porcine embryos
produced by in vitro fertilization: Timing of early cleavages and
the effect of polyspermy. Anim Sci J. 91:e134012020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nguyen HT, Dang-Nguyen TQ, Somfai T, Men
NT, Beck-Woerner B, Viet Linh N, Xuan Nguyen B, Noguchi J, Kaneko H
and Kikuchi K: Excess polyspermy reduces the ability of porcine
oocytes to promote male pronuclear formation after in vitro
fertilization. Anim Sci J. 92:e136502021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Romar R, Cánovas S, Matás C, Gadea J and
Coy P: Pig in vitro fertilization: Where are we and where do we go?
Theriogenology. 137:113–121. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Romero-Aguirregomezcorta J, Soriano-Úbeda
C and Matás C: Involvement of nitric oxide during in vitro oocyte
maturation, sperm capacitation and in vitro fertilization in pig.
Res Vet Sci. 134:150–158. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li S and Winuthayanon W: Oviduct: Roles in
fertilization and early embryo development. J Endocrinol.
232:R1–R26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Harris EA, Stephens KK and Winuthayanon W:
Extracellular vesicles and the oviduct function. Int J Mol Sci.
21:82802020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alcântara-Neto AS, Fernandez-Rufete M,
Corbin E, Tsikis G, Uzbekov R, Garanina AS, Coy P, Almiñana C and
Mermillod P: Oviduct fluid extracellular vesicles regulate
polyspermy during porcine in vitro fertilisation. Reprod Fertil
Dev. 32:409–418. 2020. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim KM, Abdelmohsen K, Mustapic M,
Kapogiannis D and Gorospe M: RNA in extracellular vesicles. Wiley
Interdiscip Rev RNA. 8:10.1002/wrna.1413. 2017. View Article : Google Scholar
|
|
14
|
Jeppesen DK, Fenix AM, Franklin JL,
Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q,
Evans R, et al: Reassessment of exosome composition. Cell.
177:428–445.e18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mathieu M, Martin-Jaular L, Lavieu G and
Théry C: Specificities of secretion and uptake of exosomes and
other extracellular vesicles for cell-to-cell communication. Nat
Cell Biol. 21:9–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
D'Souza A, Burch A, Dave KM, Sreeram A,
Reynolds MJ, Dobbins DX, Kamte YS, Zhao W, Sabatelle C, Joy GM, et
al: Microvesicles transfer mitochondria and increase mitochondrial
function in brain endothelial cells. J Control Release.
338:505–526. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Willis GR, Reis M, Gheinani AH,
Fernandez-Gonzalez A, Taglauer ES, Yeung V, Liu X, Ericsson M, Haas
E, Mitsialis SA and Kourembanas S: Extracellular vesicles protect
the neonatal lung from hyperoxic injury through the epigenetic and
transcriptomic reprogramming of myeloid cells. Am J Respir Crit
Care Med. 204:1418–1432. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mannavola F, D'Oronzo S, Cives M, Stucci
LS, Ranieri G, Silvestris F and Tucci M: Extracellular vesicles and
epigenetic modifications are hallmarks of melanoma progression. Int
J Mol Sci. 21:522019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bridi A, Perecin F and Silveira JCD:
Extracellular vesicles mediated early embryo-maternal interactions.
Int J Mol Sci. 21:11632020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Almiñana C and Bauersachs S: Extracellular
vesicles in the oviduct: Progress, challenges and implications for
the reproductive success. Bioengineering (Basel). 6:322019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Almiñana C and Bauersachs S: Extracellular
vesicles: Multi-signal messengers in the gametes/embryo-oviduct
cross-talk. Theriogenology. 150:59–69. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Almiñana C, Corbin E, Tsikis G,
Alcântara-Neto AS, Labas V, Reynaud K, Galio L, Uzbekov R, Garanina
AS, Druart X and Mermillod P: Oviduct extracellular vesicles
protein content and their role during oviduct-embryo cross-talk.
Reproduction. 154:153–168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saadeldin IM, Oh HJ and Lee BC:
Embryonic-maternal cross-talk via exosomes: Potential implications.
Stem Cells Cloning. 8:103–107. 2015.PubMed/NCBI
|
|
24
|
Fang X, Tanga BM, Bang S, Seong G,
Saadeldin IM, Lee S and Cho J: Oviduct epithelial cells-derived
extracellular vesicles improve preimplantation developmental
competence of in vitro produced porcine parthenogenetic and cloned
embryos. Mol Reprod Dev. 89:54–65. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mazzarella R, Bastos NM, Bridi A, Del
Collado M, Andrade GM, Pinzon J, Prado CM, Silva LA, Meirelles FV,
Pugliesi G, et al: Changes in oviductal cells and small
extracellular vesicles mirnas in pregnant cows. Front Vet Sci.
8:6397522021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fang X, Tanga BM, Bang S, Seo C, Kim H,
Saadeldin IM, Lee S and Cho J: Oviduct epithelial cell-derived
extracellular vesicles improve porcine trophoblast outgrowth. Vet
Sci. 9:6092022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roy PK, Qamar AY, Tanga BM, Bang S, Seong
G, Fang X, Kim G, Edirisinghe SL, De Zoysa M, Kang DH, et al:
Modified spirulina maxima pectin nanoparticles improve the
developmental competence of in vitro matured porcine oocytes.
Animals (Basel). 11:24832021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mehdiani A, Maier A, Pinto A, Barth M,
Akhyari P and Lichtenberg A: An innovative method for exosome
quantification and size measurement. J Vis Exp.
509742015.PubMed/NCBI
|
|
29
|
Simonsen JB: Pitfalls associated with
lipophilic fluorophore staining of extracellular vesicles for
uptake studies. J Extracell Vesicles. 8:15822372019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takov K, Yellon DM and Davidson SM:
Confounding factors in vesicle uptake studies using fluorescent
lipophilic membrane dyes. J Extracell Vesicles. 6:13887312017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wheeler MB, Clark SG and Beebe DJ:
Developments in in vitro technologies for swine embryo production.
Reprod Fertil Dev. 16:15–25. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim JW, Park HJ, Yang SG and Koo DB:
Anti-oxidative effects of exogenous ganglioside GD1a and GT1b on
embryonic developmental competence in pigs. J Anim Reprod
Biotechnol. 35:347–356. 2020. View Article : Google Scholar
|
|
33
|
Park SH, Jeong PS, Joo YE, Kang HG, Kim
MJ, Lee S, Song BS, Kim SU, Cho SK and Sim BW: Luteolin
orchestrates porcine oocyte meiotic progression by maintaining
organelle dynamics under oxidative stress. Front Cell Dev Biol.
9:6898262021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiao X, Ding Z, Meng F, Zhang X, Wang Y,
Chen F, Duan Z, Wu D, Zhang S, Miao Y and Huo L: The toxic effects
of Fluorene-9-bisphenol on porcine oocyte in vitro maturation.
Environ Toxicol. 35:152–158. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Saadeldin IM, Gad A and Mermillod P:
Editorial: Biofluid extracellular vesicles and their involvement in
animal reproductive physiology. Front Vet Sci. 8:7471382021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alcântara-Neto AS, Schmaltz L, Caldas E,
Blache MC, Mermillod P and Almiñana C: Porcine oviductal
extracellular vesicles interact with gametes and regulate sperm
motility and survival. Theriogenology. 155:240–255. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Batista RITP, Moro LN, Corbin E, Alminana
C, Souza-Fabjan JMG, de Figueirêdo Freitas VJ and Mermillod P:
Combination of oviduct fluid and heparin to improve monospermic
zygotes production during porcine in vitro fertilization.
Theriogenology. 86:495–502. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Coy P, Lloyd R, Romar R, Satake N, Matas
C, Gadea J and Holt WV: Effects of porcine pre-ovulatory oviductal
fluid on boar sperm function. Theriogenology. 74:632–642. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Coy P and Avilés M: What controls
polyspermy in mammals, the oviduct or the oocyte? Biol Rev Camb
Philos Soc. 85:593–605. 2010.PubMed/NCBI
|
|
40
|
Coy P, Cánovas S, Mondéjar I, Saavedra MD,
Romar R, Grullón L, Matás C and Avilés M: Oviduct-specific
glycoprotein and heparin modulate sperm-zona pellucida interaction
during fertilization and contribute to the control of polyspermy.
Proc Natl Acad Sci USA. 105:15809–15814. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Avilés M, Coy P and Rizos D: The oviduct:
A key organ for the success of early reproductive events. Anim
Front. 5:25–31. 2015. View Article : Google Scholar
|
|
42
|
Kulus M, Kranc W, Jeseta M,
Sujka-Kordowska P, Konwerska A, Ciesiółka S, Celichowski P,
Moncrieff L, Kocherova I, Józkowiak M, et al: Cortical granule
distribution and expression pattern of genes regulating cellular
component size, morphogenesis, and potential to differentiation are
related to oocyte developmental competence and maturational
capacity in vivo and in vitro. Genes (Basel). 11:8152020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Körschgen H, Kuske M, Karmilin K,
Yiallouros I, Balbach M, Floehr J, Wachten D, Jahnen-Dechent W and
Stöcker W: Intracellular activation of ovastacin mediates
pre-fertilization hardening of the zona pellucida. Mol Hum Reprod.
23:607–616. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Saadeldin IM, Kim SJ, Choi YB and Lee BC:
Improvement of cloned embryos development by co-culturing with
parthenotes: A possible role of exosomes/microvesicles for embryos
paracrine communication. Cell Reprogram. 16:223–234. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Babayev E and Seli E: Oocyte mitochondrial
function and reproduction. Curr Opin Obstet Gynecol. 27:175–181.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Niu YJ, Zhou W, Nie ZW, Shin KT and Cui
XS: Melatonin enhances mitochondrial biogenesis and protects
against rotenone-induced mitochondrial deficiency in early porcine
embryos. J Pineal Res. 68:e126272020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hao ZD, Liu S, Wu Y, Wan PC, Cui MS, Chen
H and Zeng SM: Abnormal changes in mitochondria, lipid droplets,
ATP and glutathione content, and Ca(2+) release after
electro-activation contribute to poor developmental competence of
porcine oocyte during in vitro ageing. Reprod Fertil Dev.
21:323–332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Clapier CR, Iwasa J, Cairns BR and
Peterson CL: Mechanisms of action and regulation of ATP-dependent
chromatin-remodelling complexes. Nat Rev Mol Cell Biol. 18:407–422.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Singhal N, Graumann J, Wu G, Araúzo-Bravo
MJ, Han DW, Greber B, Gentile L, Mann M and Schöler HR:
Chromatin-remodeling components of the BAF complex facilitate
reprogramming. Cell. 141:943–955. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Russell AE, Jun S, Sarkar S, Geldenhuys
WJ, Lewis SE, Rellick SL and Simpkins JW: Extracellular vesicles
secreted in response to cytokine exposure increase mitochondrial
oxygen consumption in recipient cells. Front Cell Neurosci.
13:512019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Machtinger R, Laurent LC and Baccarelli
AA: Extracellular vesicles: Roles in gamete maturation,
fertilization and embryo implantation. Hum Reprod Update.
22:182–193. 2016.PubMed/NCBI
|
|
52
|
Qamar AY, Fang X, Bang S, Mahiddine FY,
Kim MJ and Cho J: The interplay between exosomes and spermatozoa.
Alzahrani FA and Saadeldin IM: Role of Exosomes in Biological
Communication Systems. Springer; Singapore: pp. 115–139. 2021
|
|
53
|
Saadeldin IM: Extracellular vesicles
mediate the embryonic-maternal paracrine communication. Alzahrani
FA and Saadeldin IM: Role of Exosomes in Biological Communication
Systems. Springer; Singapore: pp. 77–97. 2021, View Article : Google Scholar
|
|
54
|
Du J, Shen J, Wang Y, Pan C, Pang W, Diao
H and Dong W: Boar seminal plasma exosomes maintain sperm function
by infiltrating into the sperm membrane. Oncotarget. 7:58832–58847.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kumaresan A, Johannisson A, Humblot P and
Bergqvist AS: Effect of bovine oviductal fluid on motility,
tyrosine phosphorylation, and acrosome reaction in cryopreserved
bull spermatozoa. Theriogenology. 124:48–56. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bathala P, Fereshteh Z, Li K, Al-Dossary
AA, Galileo DS and Martin-DeLeon PA: Oviductal extracellular
vesicles (oviductosomes, OVS) are conserved in humans: Murine OVS
play a pivotal role in sperm capacitation and fertility. Mol Hum
Reprsod. 24:143–157. 2018.PubMed/NCBI
|
|
57
|
Ferraz MAMM, Carothers A, Dahal R, Noonan
MJ and Songsasen N: Oviductal extracellular vesicles interact with
the spermatozoon's head and mid-piece and improves its motility and
fertilizing ability in the domestic cat. Sci Rep. 9:94842019.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Al-Dossary AA, Strehler EE and
Martin-DeLeon PA: Expression and secretion of plasma membrane
Ca2+-ATPase 4a (PMCA4a) during murine estrus: association with
oviductal exosomes and uptake in sperm. PLoS One. 8:e801812013.
View Article : Google Scholar : PubMed/NCBI
|