Introduction
Endometriosis is a disease where the endometrial
cells move outside the uterus and grow at a secondary site, causing
dysmenorrhea and including abdominal pain. Endometriosis is
estimated to affect ~10% of women of reproductive age worldwide
(1,2). Current therapy against endometriosis
includes anti-inflammatory agents and hormonal therapy (3). Combined oral contraceptives are
frequently used by females with endometriosis to decrease the
severity of dysmenorrhea. However, the treatment of patients with
endometriosis who wish to become pregnant requires a therapy other
than hormonal treatment. Therefore, novel chemical drug treatments
that may be used for women who wish to become pregnant are
required.
There are various types of endometriosis and several
theories for its mechanism. Typically, endometrial cells, after
shedding from the uterine lining, travel through the fallopian
tubes to the ovaries and then to the peritoneal cavity, where they
migrate and invade various places such as the pouch of Douglas.
They may then attach, grow and cause various symptoms. Therefore,
the migration and invasion of endometrial cells are most commonly
involved in the mechanism of endometriosis.
Previously, our group found a selective NF-κB
inhibitor, dehydroxymethylepoxyquinimicin (DHMEQ), by molecular
design based on the structure of a natural compound (4,5).
DHMEQ covalently binds to the NF-κB components, such as p65, p50,
cRel and RelB, to inhibit mainly the DNA binding activity (6,7). It
has been shown to ameliorate various inflammatory (8) and neoplastic (9) disease models through intraperitoneal
(IP) administration. In addition, DHMEQ was shown to inhibit
inflammatory responses in patient-derived peritoneal cells
(10). No toxicity was observed in
any of the in vivo experiments. This finding is due to DHMEQ
not entering the systemic circulation; it instead acts only in the
peritoneal cavity (8).
DHMEQ (Fig. 1A) was
reported to inhibit cancer cell migration and invasion in breast
cancer cells in 2D and 3D analyses by lowering MMP-2 expression
(11,12). It also inhibited invasion of mouse
plasmacytoma cells by lowering the expression of the kisspeptin-1
receptor (13). Furthermore, it
inhibited the invasion of primary cultured mouse mast cells by
lowering MMP-2 expression (14).
In addition, intraperitoneal administration of DHMEQ inhibited
peritoneal metastasis of human pancreatic cancer cells in mice,
possibly by inducing anoikis (15). SEMBL is a stable analogue of DHMEQ
and it has also been shown to inhibit migration and invasion of
human ovarian carcinoma cells (16).
It may be possible for DHMEQ to inhibit the
migration and invasion of endometriosis cells. In the present
research, the effect of DHMEQ on the migration and invasion of
immortalized human endometriosis stromal cells (HESC) was studied
and the mechanisms of the inhibition were investigated.
Materials and methods
Materials
DHMEQ was synthesized in our laboratory as described
previously (8,17). Immortalized HESC (cat. no. T0533-C)
were purchased from Applied Biological Materials, Inc. These cells
possess insulin-like growth factor binding protein-1, prolactin,
tissue factor and plasminogen activator inhibitor-1 as markers for
decidualization endpoints (Supplier's manual). The cells were grown
in Prigrow IV medium (Applied Biological Materials, Inc.),
supplemented with 10% charcoal-stripped fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100
µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.) in a
humidified incubator with 5% CO2 at 37°C.
Cell viability assay
Cells (1×104) were seeded in 96-well
microplates and incubated for 24 h. Different concentrations of
DHMEQ were then added to each well and the cells incubated for
another 24 h. MTT solution (10 µl; Cayman Chemical Company) was
added to each well, followed by incubation for 2 h in the
incubator. Subsequently, 100 µl of DMSO was added to each well to
replace the culture supernatant. After the purple formazan crystals
were completely solubilized by DMSO, the absorbance of the samples
was measured at 570 nm using a microplate reader (Bio-Rad
Laboratories, Inc.).
Wound-healing assay
Cell migration was determined by the wound-healing
assay as previously described (18). Cells (1×105) were seeded
in a 24-well plate. After the cells became 90% confluent, a uniform
scratch wound across the center of each well was made with a 200-µl
pipette tip (Rikaken STAR Tips). The cells were then washed twice
with serum-free media to remove detached cells and growth media,
and the cells were replenished with fresh serum-free media and
cultured for 8 h. A phase contrast microscope at (magnification,
×4) was used to record the movement of the cells into the scratched
area.
Cell tracking analysis
Approximately 500 cells were seeded in 96-well
ImageLock plates (Essen BioScience) precoated with collagen type I
(Sigma-Aldrich; Merck KGaA). Images were taken every 15 min
continuously for 24 h using an Incucyte ZOOM (Essen BioScience).
The trajectories of motile cells were tracked manually using Image
J software (Image J 1.53e with Java 1.8.0_172; National Institutes
of Health) along with the plugin for manual tracking (Fabrice
Cordelieres, Institute Curie) (19).
Matrigel chamber invasion assay
The Matrigel chamber assay was performed as
described previously (18). BD
Matrigel Basement Membrane Matrix inserts (Corning Inc.) were
rehydrated in serum-free medium for 2 h at 37°C. After the
rehydration, HESC cells (4×104) suspension in 500 µl
serum-free medium containing DHMEQ or DMSO were seeded into the
inserts. Inserts were carefully transferred to the 24-well plate
filled with 750 µl medium containing 10% FBS and incubated for 24 h
at 37°C in an incubator. After the incubation, non-invading cells
were removed by scrubbing with a moistened cotton swab from the
upper surface of the membrane. Invading cells on the lower surface
of the membrane were stained with Diff-Quick (Sysmex Corporation)
and counted under a phase contrast microscope (magnification, ×10).
Both cell nuclei and the membrane hole were stained in purple. Only
the cell nuclei were counted, not the holes.
NF-κB-DNA binding assay
After the treatment with DHMEQ, the nuclear extract
was prepared with the Nuclear Extract Kit (Active Motif, Inc.)
following the supplier's protocol. DNA binding activity of the
extract was measured with the TransAM NF-κB p65 Transcription
Factor Assay Kit (Active Motif, Inc.). Nuclear extract (5 µg) and
96-well plates pre-coated with an immobilized oligonucleotide
containing the NF-κB consensus site (5′-GGGACTTTCC-3′) were used
for the assay. Detection of the DNA binding activity of NF-κB was
performed as described previously (20).
Whole-genome array analysis
Whole-genome array analysis was performed as
previously described (21). Total
RNA was extracted with the RNeasy Mini Kit (Qiagen GmbH) and 250 ng
of total RNA was used for cDNA synthesis. The Agilent Low Input
Quick Amp Labeling Kit (Agilent Technologies, Inc.) was applied for
cDNA synthesis and cRNA labeling with cyanine 3 (Cy3) dye.
Cy3-labeled cRNA was purified and quantified with the Nanodrop One
(Thermo Fisher Scientific, Inc.). The purified Cy3-labeled cRNA was
then fragmented and hybridized on a Human Gene Expression 4× 44K v2
Microarray Chip containing 27,958 Entrez Gene RNAs using a Gene
Expression Hybridization kit (Agilent Technologies, Inc.). After
the microarray chip was washed twice in gene expression wash
buffer, microarray slides were scanned with the Agilent G2565BA
Microarray Scanner (International Equipment Trading Ltd.). Raw and
normalized microarray data were submitted to the Gene Expression
Omnibus database at the National Center for Biotechnology
Information (accession no. GSE216255; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE216255).
Gene set enrichment analysis was performed according to the
tutoring [Gene Set Enrichment Analysis (GSEA) User Guide:
https://www.gsea-msigdb.org/gsea/doc/GSEAUserGuideFrame.html].
Metastasis PCR array
Total RNA from HESC was purified by spin column
using the RNeasy Mini Kit (Qiagen GmbH) and 0.5 µg of total RNA was
used for reverse transcription (RT) with the RT2 First
Strand Kit (Qiagen GmbH) according to the manufacturer's
instructions. The cDNA was added to the qPCR Master Mix (Qiagen
GmbH). The PCR components mix was dispensed into the Human Tumor
Metastasis PCR Array format (Qiagen GmbH) according to the
manufacturer's instructions. Data analysis was accomplished by the
comparative Cq method (22).
RNA isolation and semi-quantitative
RT-PCR analysis
RNA isolation and semi-quantitative RT-PCR analysis
were performed as previously described (18). The primer, number of PCR cycles and
the annealing temperature were as follows: Myosin light chain
kinase (MLCK), 5′-CAACAGAGAAGACGGTGACCA-3′ (forward) and
5′-TCACAAGGCTGAAAGTCCCC-3′ (reverse), 32 cycles, 58°C; β-actin,
5′-CTTCTACAATGAGCTGCGTG-3′ (forward) and 5′-TCATGAGGTAGTCAGTCAGG-3′
(reverse), 21 cycles, 58°C. PCR products were electrophoresed on 2%
agarose gels stained with ethidium bromide and visualized with a UV
illuminator (FAS-V; NIPPON Genetics co., Ltd.) and analyzed by
Image J 1.53e with Java 1.8.0 _172 (National Institutes of
Health).
Knockdown by small interfering
(si)RNA
siMLCK (cat. no. sc-365352) and control siRNA-A
(cat. no. sc-37007) were purchased from Santa Cruz Biotechnology,
Inc. Transfection of siRNAs into cells was carried out using the
Lipofectamine® RNAiMax transfection reagent (Thermo
Fisher Scientific, Inc.) following the manufacturer's protocols.
The mRNA expression of MLCK was analyzed to measure the knockdown
efficiency.
Statistical analysis
All experiments were repeated at least three times.
Values are expressed as mean ± standard error of the mean. The
significance of differences between groups was analyzed by ANOVA
followed by Dunnett's post-hoc test and/or the Student's t-test
where appropriate. P<0.05 was considered to indicate a
statistically significant difference.
Results
Inhibition of cellular migration and
invasion by DHMEQ
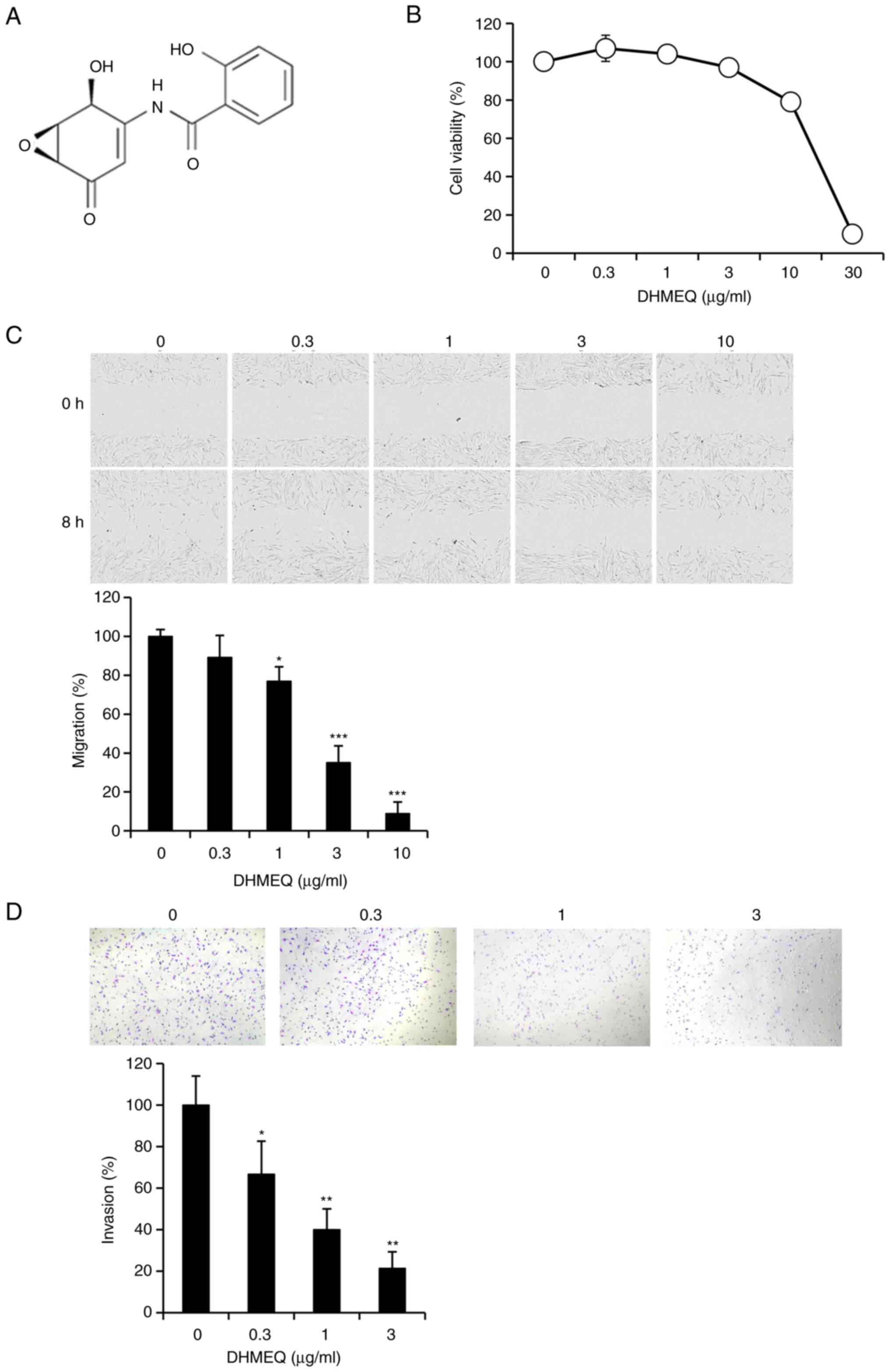
DHMEQ (Fig. 1A)
exhibited no prominent toxicity below 10 µg/ml in HESC (Fig. 1B). Cellular migration was assessed
by a wound-healing assay. DHMEQ inhibited the migration of HESC at
nontoxic concentrations (Fig. 1C).
Cellular invasion was assessed by a Matrigel® chamber
assay. DHMEQ inhibited cellular invasion at the nontoxic
concentrations (Fig. 1D).
Inhibition of cellular migration and
invasion by SEMBL
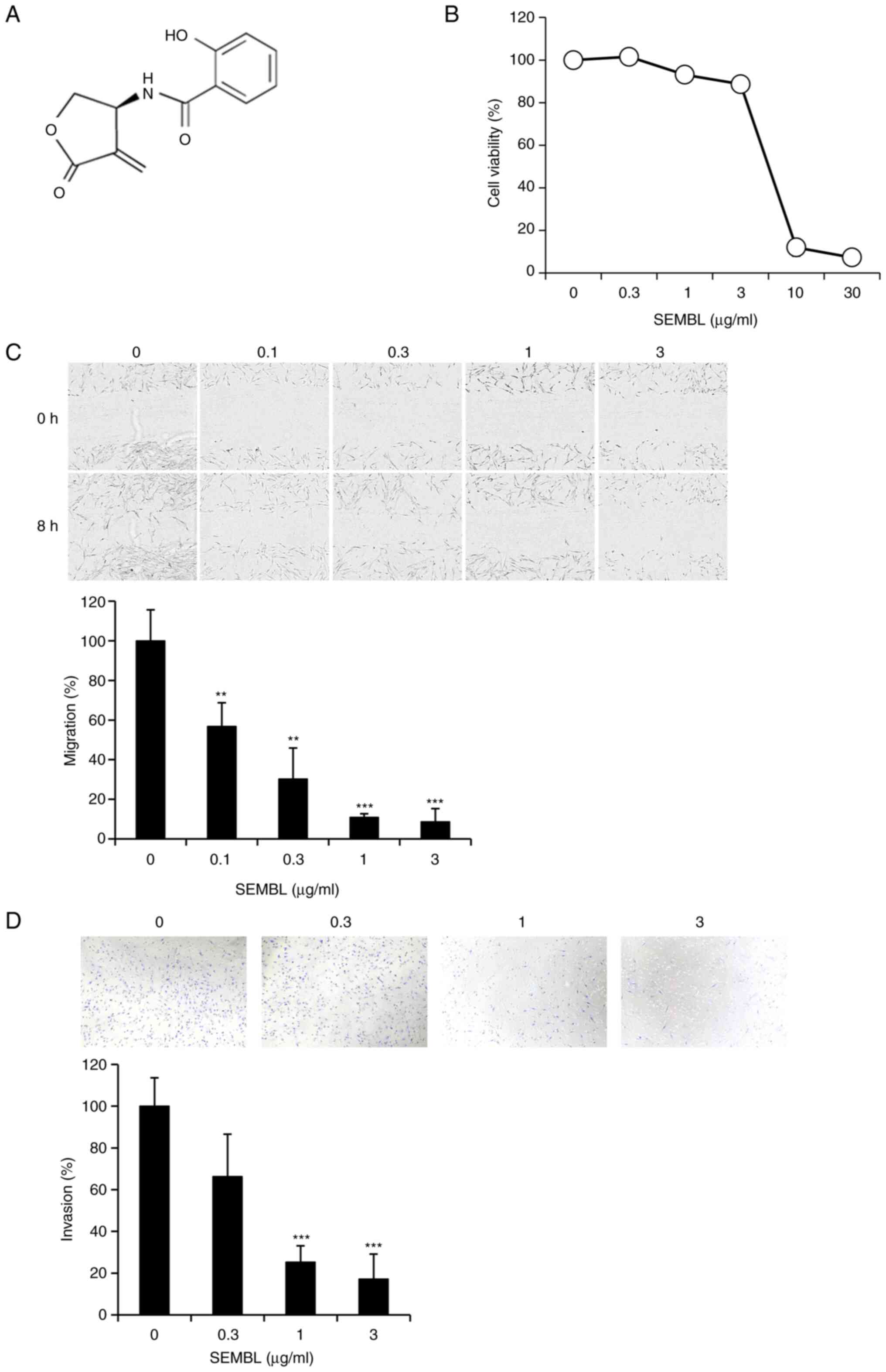
(S)-β-salicyloylamino-α-exo-methylene-γ-butyrolactone (SEMBL;
Fig. 2A) is a stable analog of
DHMEQ and it is also known to inhibit DNA binding of the NF-κB
component p65 directly (16).
SEMBL showed no prominent toxicity below 3 µg/ml in HESC (Fig. 2B). It inhibited the migration of
HESC at nontoxic concentrations (Fig.
2C). SEMBL again inhibited cellular invasion at the nontoxic
concentrations (Fig. 2D).
Inhibition of cellular NF-κB by
DHMEQ
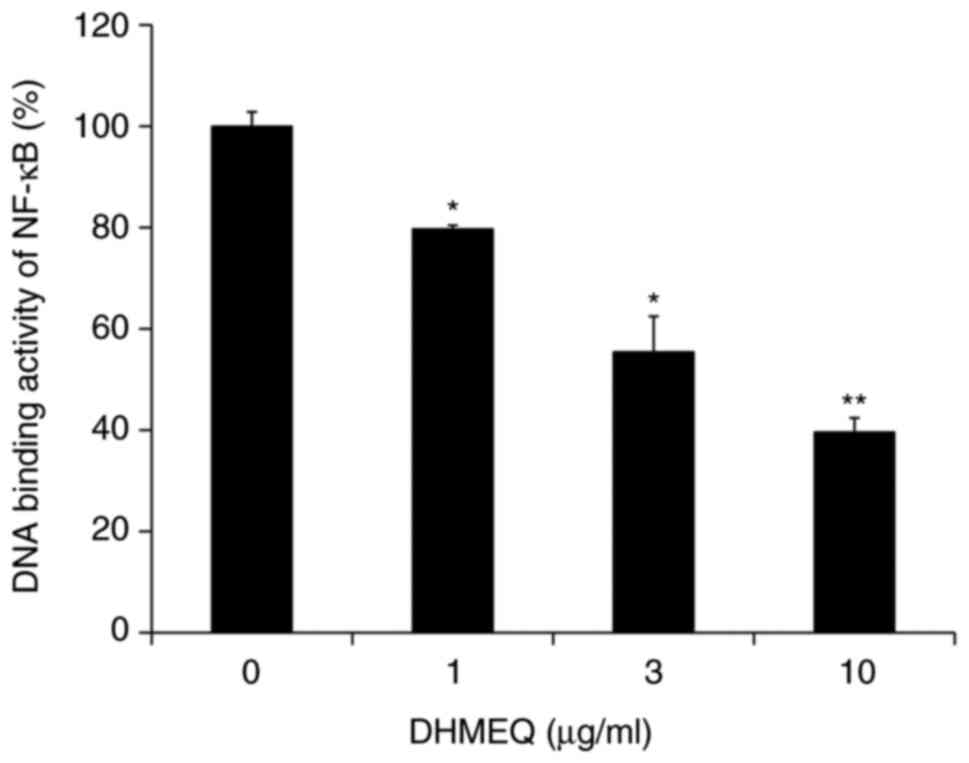
DHMEQ was employed for further mechanistic studies,
as it has been more extensively studied in terms of biological
activities and is being developed into a drug (8). Endometriosis cells often possess
elevated NF-κB (23). Experiments
performed by our group have indicated that HESC also possess
constitutively activated NF-κB activity (data not shown). DHMEQ
inhibited cellular NF-κB activity in HESC (Fig. 3).
Whole genome and metastasis PCR array
analyses
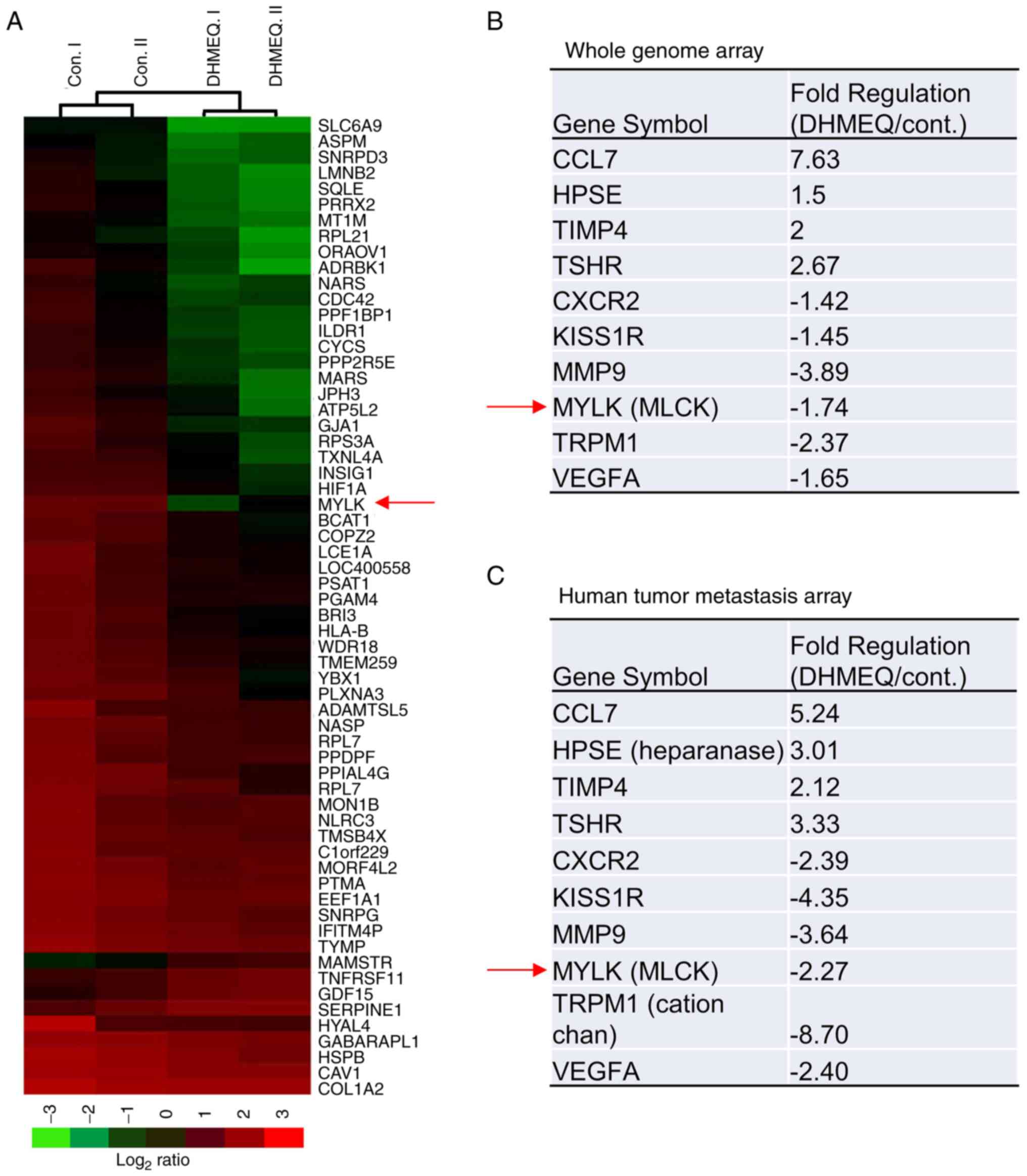
Next, the mediator of the inhibition of migration
and invasion by DHMEQ was investigated using whole-genome (Fig. 4A and B) and metastasis PCR
(Fig. 4C) array analyses. A large
number (27,958) of genes were analyzed in the whole-genome array,
while a limited number of genes involved in metastasis were
analyzed in the metastasis PCR array. Both array analyses were
performed with HESC treated with or without 3 µg/ml of DHMEQ for 24
h. The expression of several genes was changed. Of note, both the
whole-genome (Fig. 4B) and
metastasis PCR (Fig. 4C) analyses
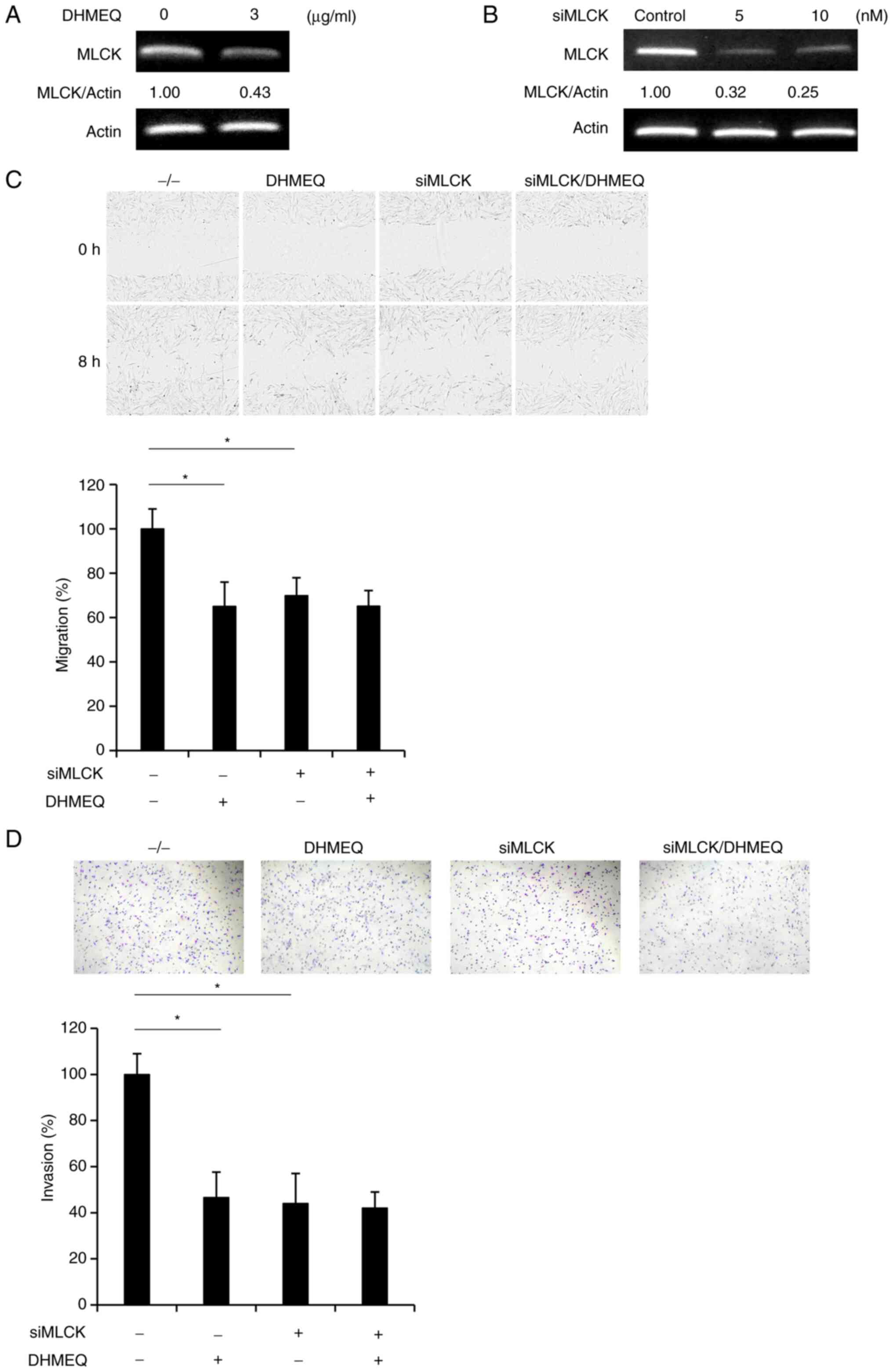
indicated a decrease in MLCK expression.
Inhibition of migration and invasion
by knockdown of MLCK
Downregulation of MLCK was confirmed by independent
semi-quantitative PCR analysis (Fig.
5A). Knockdown of MLCK by siRNA (Fig. 5B) inhibited cellular migration
(Fig. 5C) and invasion (Fig. 5D) of HESC. Since the addition of
DHMEQ to the MLCK-knockdown cells did not further inhibit migration
and invasion, the NF-κB/MLCK pathway was considered to be involved
mainly in the mechanism of migration and invasion.
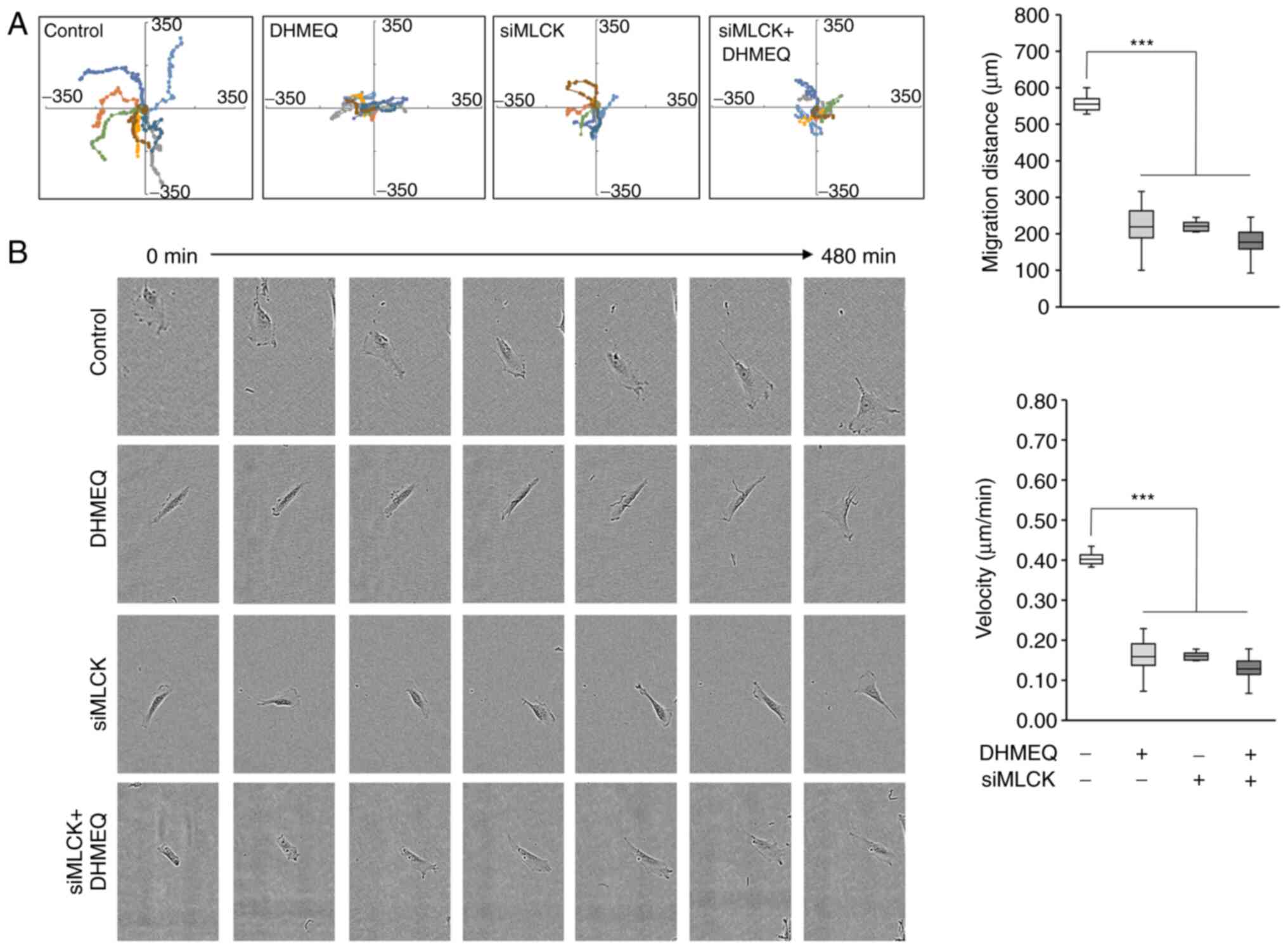
The inhibition of migration was further studied by
cell tracking analysis. DHMEQ and knockdown of MLCK both clearly
lowered the mean distance of movement (Fig. 6A). Not only the distance but also
the speed of movement was lowered by DHMEQ or MLCK knockdown
(Fig. 6B). When DHMEQ was added to
the siMLCK cells, there was again no further inhibition
observed.
Discussion
The present study indicated that NF-κB is involved
in the migration and invasion of endometriosis cells. NF-κB
activates the transcription of numerous inflammatory cytokines,
anti-apoptotic molecules and matrix metalloproteases. Constitutive
activation of NF-κB has been demonstrated in endometriotic lesions.
Activation of NF-κB in endometriotic cells and environmental
macrophages may maintain inflammatory reactions in endometriosis.
NF-κB is considered to contribute to the increased ability of
endometriotic cells to invade and adhere to the peritoneal surface
by regulating the expression of matrix metalloproteases (24). Several factors, including estrogen,
progesterone, oxidative stress and noncoding RNAs, may regulate
NF-κB signaling in endometriosis (25).
Whole-genome PCR array, metastasis PCR array and
siRNA knockdown analyses suggested that MLCK is involved in the
mechanism of inhibition. Matrix metalloproteases are often
essential for cancer cell migration (11,12),
and MMP9 was downregulated in both analyses. Regulation of cellular
migration by MLCK has been reported. A critical role of MLCK in
cell migration involves regulating the cell membrane tension and
protrusion necessary for migration, thereby stabilizing the
membrane skeleton through F-actin-binding activity. Phosphorylation
by MLCK on Thr18 and Ser19 of MLC has been shown to be critical for
the activation of myosin ATPase activity and the contractile
functions in smooth muscle and non-muscle cells (25,26).
Treatment with MLCK inhibitors resulted in a marked reduction of
invasiveness, which was mainly due to reduced cellular motility
(27). On the other hand, Chen
et al (28) showed that
deletion of MLCK increased cellular migration independent of MLCK
phosphorylation.
The relationship between NF-κB and MLCK has been
studied. Activation of NF-κB induced by TNF-α leads to increased
MLCK expression in human pancreatic ductal adenocarcinoma cell
lines, resulting in tight junction degradation and high
permeability of the cells (29).
Inhibitors of NF-κB, such as BAY 11 and sulfasalazine, were found
to inhibit TNF-α-induced MLCK upregulation in Caco-2 monolayer
cells (30). A subsequent study
also suggested that NF-κB would be critical to TNF-α-induced MLCK
upregulation (31,32). Furthermore, NF-κB was shown to
activate the MLCK promoter (33).
It has been reported that there are multiple κB sites in the
promoter region of MLCK (34).
Since IP administration of DHMEQ is effective in
various in vivo disease models (8,9),
DHMEQ IP therapy is being developed for the treatment of
inflammation and cancer. This therapy may also be useful for the
treatment of endometriosis, particularly for those women who wish
to conceive.
Acknowledgements
Not applicable.
Funding
This work was financially supported in part by the Japan Society
for the Promotion of Science Kakenhi (grant no. 22H03062) and the
Japan Agency for Medical Research and Development (grant no.
JP18fk0310118JSPS).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. Raw and normalized microarray data were submitted to the
Gene Expression Omnibus database at the National Center for
Biotechnology Information (accession no. GSE216255; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE216255).
Authors' contributions
YL, SK, KT, KU and AW contributed to the
experimental plan and manuscript preparation. HM and JM contributed
to the experimental plan. YL, SK, AI, YM and YT carried out the
experiments. All authors read and approved the final manuscript. YL
and KU confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
JM is affiliated with Shenzhen Wanhe Pharmaceutical
Company (Shenzhen, China). The Department of Molecular Target
Medicine, to which KU belongs, is a fund-donated laboratory. It is
supported financially by Shenzhen Wanhe Pharmaceutical Company
(Shenzhen, China), Meiji Seika Pharma (Tokyo, Japan), Fukuyu
Medical Corporation (Nisshin, Japan) and Brunaise Co., Ltd.
(Nagoya, Japan). This study was partly supported by Shenzhen Wanhe
Pharmaceutical Company.
References
|
1
|
World Health Organization (WHO), .
International Classification of Diseases. 11th Revision (ICD-11).
Geneva: 2018
|
|
2
|
Zondervan KT, Becker CM and Missmer SA:
Endometriosis. N Engl J Med. 382:1244–1256. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johnson NP and Hummelshoj L: World
endometriosis society montpellier consortium. Consensus on current
management of endometriosis. Hum Reprod. 28:1552–1568. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsumoto N, Ariga A, To-e S, Nakamura H,
Agata N, Hirano S, Inoue J and Umezawa K: Synthesis of NF-kappaB
activation inhibitors derived from epoxyquinomicin C. Bioorg Med
Chem Lett. 10:865–869. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ariga A, Namekawa J, Matsumoto N, Inoue J
and Umezawa K: Inhibition of TNF-α-induced nuclear translocation
and activation of NF-κB by dehydroxymethyl-epoxyquinomicin. J Biol
Chem. 277:27625–27630. 2002. View Article : Google Scholar
|
|
6
|
Yamamoto M, Horie R, Takeiri M, Kozawa I
and Umezawa K: Inactivation of nuclear factor kappa B components by
covalent binding of (−)-dehydroxymethylepoxyquinomicin to specific
cysteine residues. J Med Chem. 51:5780–5788. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takeiri M, Horie K, Takahashi D, Watanabe
M, Horie R, Simizu S and Umezawa K: Involvement of DNA binding
domain in the cellular stability and importin affinity of NF-κB
component RelB. Org Biomol Chem. 10:3053–3059. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma J, Zhang Y, Sugai T, Kubota T, Keino H,
El-Salhy M, Ozaki M and Umezawa K: Inhibition of cellular and
animal inflammatory disease models by NF-κB inhibitor DHMEQ. Cells.
10:22712021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Umezawa K, Breborowicz A and Gantsev S:
Anticancer activity of novel NF-κB inhibitor DHMEQ by
intraperitoneal administration. Oncol Res. 28:541–550. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sosińska P, Maćkowiak B, Staniszewski R,
Umezawa K and Bręborowicz A: Inhibition of NF-κB with
dehydroxyepoxiquinomicin modifies function of human peritoneal
mesothelial cells. Am J Transl Res. 8:5756–5765. 2016.PubMed/NCBI
|
|
11
|
Ukaji T, Lin Y, Okada S and Umezawa K:
Inhibition of MMP-2-mediated cellular invasion by NF-κB inhibitor
DHMEQ in 3D culture of breast carcinoma MDA-MB-231 cells: A model
for early phase of metastasis. Biochem Biophys Res Commun.
485:76–81. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Umezawa K and Lin Y: Inhibition of matrix
metalloproteinase expression and cellular invasion by NF-κB
inhibitors of microbial origin. Biochim Biophys Acta Proteins
Proteom. 1868:1404122020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin Y, Sidthipong K, Ma J, Koide N,
Umezawa K and Kubota T: Designed NF-κB inhibitor, DHMEQ, inhibits
KISS1R-mediated invasion and increases drug-sensitivity in mouse
plasmacytoma SP2/0 cells. Exp Ther Med. 22:10922021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Noma N, Asagiri M, Takeiri M, Ohmae S,
Takemoto K, Iwaisako K, Simizu S and Umezawa K: Inhibition of
MMP-2-mediated mast cell invasion by NF-κB inhibitor DHMEQ in mast
cells. Int Arch Allergy Immunol. 166:84–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sato M, Nakanishi K, Haga S, Fujiyoshi M,
Baba M, Mino K, Yimin, Niwa H, Yokoo H, Umezawa K, et al: Anoikis
induction and inhibition of peritoneal metastasis of pancreatic
cancer cells by a nuclear factor-kappa B inhibitor, (−)-DHMEQ.
Oncology Res. 21:333–343. 2014. View Article : Google Scholar
|
|
16
|
Sidthipong K, Ma J, Yu W, Wang Y,
Kobayashi S, Kishino S, Koide N, Yokochi T, Kato K, Okada S and
Umezawa K: Rational design, synthesis and in vitro evaluation of
novel exo-methylene butyrolactone salicyloylamide as NF-κB
inhibitor. Bioorg Med Chem Lett. 27:562–566. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suzuki Y, Sugiyama C, Ohno O and Umezawa
K: Preparation and biological activities of optically active
dehydroxymethylepoxyquinomicin, a novel NF-κB inhibitor.
Tetrahedron. 60:7061–7066. 2004. View Article : Google Scholar
|
|
18
|
Lin Y, Chen Y, Ukaji T, Okada S and
Umezawa K: Isolation of ketomycin from Actinomycetes as an
inhibitor of 2D and 3D cancer cell invasion. J Antibiot (Tokyo).
72:148–157. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ito H, Tsunoda T, Riku M, Inaguma S, Inoko
A, Murakami H, Ikeda H, Matsuda M and Kasai K: Indispensable role
of STIL in the regulation of cancer cell motility through the
lamellipodial accumulation of ARHGEF7-PAK1 complex. Oncogene.
39:1931–1943. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin Y, Sidthipong K, Ma J, Koide N,
Umezawa K and Kubota T: The designed NF-κB inhibitor, DHMEQ,
inhibits KISS1R-mediated invasion and increases drug-sensitivity in
mouse plasmacytoma SP2/0 cells. Exp Ther Med. 22:10922021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karnan S, Ota A, Murakami H, Rahman ML,
Hasan MN, Wahiduzzaman M, Hanamura I, Quang Vu L, Inoko A, Hyodo T,
et al: Identification of CD24 as a potential diagnostic and
therapeutic target for malignant pleural mesothelioma. Cell Death
Discovry. 6:1272020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vandesompele J, De Preter K, Pattyn F,
Poppe B, Van Roy N, De Paepe A and Speleman F: Accurate
normalization of real-time quantitative RT-PCR data by geometric
averaging of multiple internal control genes. Genome Biol.
3:RESEARCH00342002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Banu SK, Lee J, Speights VO Jr,
Starzinski-Powitz A and Arosh JA: Selective inhibition of
prostaglandin E2 receptors EP2 and EP4 induces apoptosis of human
endometriotic cells through suppression of ERK1/2, AKT, NFkappaB,
and beta-catenin pathways and activation of intrinsic apoptotic
mechanisms. Mol Endocrinol. 23:1291–1305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaponis A, Uwabe T, Taniguchi F, Ito M,
Deura I, Decavalas G, Terakawa N and Harada T: The role of NF-κB in
endometriosis. Front Biosci (Schol Ed). 4:1213–1234.
2012.PubMed/NCBI
|
|
25
|
Somlyo A and Somlyo A: Signal transduction
by G-proteins, rho-kinase and protein phosphatase to smooth muscle
and non-muscle myosin II. J Physiol. 522:177–185. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tan J, Ravid S and Spudich J: Control of
nonmuscle myosins by phosphorylation. Annu Rev Biochem. 61:721–759.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tohtong R, Phattarasakul K and
Jiraviriyakul A: Dependence of metastatic cancer cell invasion on
MLCK-catalyzed phosphorylation of myosin regulatory light chain.
Prostate Cancer Prostatic Dis. 6:212–216. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen C, Tao T, Wen C, He W, Qiao Y, Gao Y,
Chen X, Wang P, Chen C, Zhao W, et al: Myosin light chain kinase
(MLCK) regulates cell migration in a myosin regulatory light chain
phosphorylation-independent mechanism. J Biol Chem.
289:28478–28488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Su Z, Gong Y, Yang H, Deng D and Liang Z:
Activation of the nuclear factor-kappa B signaling pathway damages
the epithelial barrier in the human pancreatic ductal
adenocarcinoma cell line HPAF-II. Pancreas. 48:1380–1385. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang F, Graham W, Wang Y, Witkowski E,
Schwarz B and Turner J: Interferon-gamma and tumor necrosis
factor-alpha synergize to induce intestinal epithelial barrier
dysfunction by up-regulating myosin light chain kinase expression.
Am J Pathol. 166:409–419. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma T, Boivin M, Ye D, Pedram A and Said H:
Mechanism of TNF-(alpha) modulation of Caco-2 intestinal epithelial
tight junction barrier: Role of mysion light-chanin kinase protein
expression. Am J Physiol Gastrointest Liver Physiol. 288:G422–G430.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma T, Iwamoto G, Hoa N, Akotia V, Pedram
A, Boivin M and Said H: TNF-alpha-induced increase in intestinal
epithelial tight junction permeability requires NF-kappa b
activation. Am J Physiol Gastrointest Liver Physiol. 286:G367–G376.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Graham W, Wang F, Clayburgh D, Cheng J,
Yoon B, Wang Y, Lin A and Turner J: Tumor necrosis factor-induced
long myosin light chain kinase transcription is regulated by
differentiation-dependent signaling events. Characterization of the
human long myosin light chain kinase promoter. J Biol Chem.
281:26205–26215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ye D and Ma TY: Cellular and molecular
mechanisms that mediate basal and tumour necrosis
factor-alpha-induced regulation of myosin light chain kinase gene
activity. J Cell Mol Med. 12:1331–1346. 2008. View Article : Google Scholar : PubMed/NCBI
|