Introduction
Lung cancer is one of the most common types of
cancer worldwide, with the highest incidence and mortality rates
(1). According to statistics,
millions of new lung cancer cases and lung cancer-related deaths
are recorded annually (2). Lung
cancer is divided into two common types, namely small cell lung
cancer and non-small cell lung cancer (NSCLC) (3,4). The
latter accounts for ~85% of all lung cancer cases (5), and lung adenocarcinoma (LUAD) is the
most common subtype of NSCLC (6).
There are several treatment methods for lung cancer,
including routine surgery, chemotherapy and radiotherapy, as well
as the new methods of targeted therapy and immunotherapy (7); however, lung cancer is still the
leading cause of cancer-related mortality globally (8). Therefore, the identification of novel
biomarkers and the development of new treatment approaches for lung
cancer are of great importance.
Protein phosphatase, Mg2+/Mn2+
dependent 1G (PPM1G) is member of the metal-dependent protein
phosphatase (PPM) family. The PPM family consists of
serine/threonine phosphatases, which are involved in regulating the
cell cycle and differentiation (9,10).
Dysregulation of these phosphatases can result in the development
of several diseases, including cancer. It has been reported that
PPM1D, as a member of the PPM family, is associated with the onset
of several types of human tumors, including high-grade glioma
(11), colorectal cancer (12) and esophageal squamous cell
carcinoma (13). In addition,
previous studies have demonstrated that PPM1D upregulation is
associated with the poor prognosis of patients with NSCLC (14,15).
However, whether PPM1G, a significant member of the PPM family, is
associated with the prognosis of lung cancer and immune cell
infiltration, thus exerting a prognostic and immune potential,
remains to be elucidated. A previous study demonstrated that PPM1G
may serve a significant role in regulating the cell cycle (16). Another study showed that PPM1G is
involved in the dephosphorylation of pre-mRNA splicing factors
(17); pre-mRNA splicing serves a
critical role in the pathological process of cancer (18).
In the present study, multiple public online
platforms were used to detect the expression levels of PPM1G in
tumor sample data downloaded from The Cancer Genome Atlas (TCGA)
(https://cancergenome.nih.gov) and Gene
Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/), and to evaluate
the association between PPM1G expression, prognosis and immune cell
infiltration. In addition, the association between PPM1G and cell
cycle-related genes in LUAD was investigated. Overall, the current
study aimed to reveal the potential of PPM1G as a prognostic
biomarker and its effect on immune cell infiltration in patients
with LUAD, as well as to further investigate the role of PPM1G in
regulating the cell cycle.
Materials and methods
Patient datasets
The expression levels of PPM1G were detected in 33
types of human cancer using TCGA (https://portal.gdc.cancer.gov/) database (19). A Mann-Whitney U test was performed
to compare the differences in the expression levels of PPM1G
between tumor and normal tissues (tumor-adjacent tissue). The
cut-off P-value was set to <0.05 and the fold change was 1.5.
Subsequently, RNA-sequencing (RNA-seq) data and clinical data of
535 LUAD tumor and 59 normal tissue samples were downloaded from
the TCGA-LUAD dataset. Level 3 high-throughput sequencing
(HTSeq)-fragments per kilobase of exon model per million reads
mapped (FPKM) format of RNA-seq were downloaded from TCGA database,
and the RNA-seq data in FPKM format were converted into transcripts
per million (TPM) form and log2 conversion was then performed
(20). In addition, three LUAD
datasets, namely GSE30219 (21),
GSE116959 (22) and GSE10072
(23), were downloaded from the
Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/) (24). The aforementioned GEO datasets were
used to verify the differences in gene expression levels. Since the
clinical data in the GSE30219 dataset were more detailed, this
dataset was used to assess prognosis. Finally, the Human Protein
Atlas (HPA) database (http://www.proteinatlas.org/) was used to validate the
protein expression levels of PPM1G in LUAD.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
A total of 12 LUAD samples were collected from 6
male patients and 6 female patients (average age 60) who received
surgical treatment for lung cancer at the Yantai Affiliated
Hospital of Binzhou Medical University between March 2022 and
December 2022 and the adjacent lung tissues with a distance from
tumor tissue >2 cm were collected as negative controls. The
studies were conducted in accordance with the ethical standards
according to The Declaration of Helsinki, as well as national and
international guidelines (25).
The present study was approved by the Institutional Research Ethics
Committee of Yantai Affiliated Hospital of Binzhou Medical
University (protocol no. 20220215001; Yantai, China). The patients
provided written informed consent to participate in this study.
Total RNA was isolated from tissues using the GoldHi
Plasmid Mini kit (cat. no. CW0581; CoWin Biosciences). The Evo
M-MLV RT Master Mix (cat. no. AG11728) was used to reverse
transcribe RNA into cDNA, and the SYBR® Green Pro Taq HS
Premix kit (cat. no. AG11740; both from Accurate Biology) was used
for qPCR. The mRNA expression levels of PPM1G were analyzed by qPCR
on the iCycler system (Bio-Rad Laboratories, Inc.). The RNA
extraction, cDNA synthesis, and qPCR performed according to the
manufacturer's protocols. The qPCR was performed at 95°C for 5 min,
followed by 40 cycles at 95°C for 15 sec, 60°C for 30 sec and
extension at 72°C for 30 sec, with a final extension step at 72°C
for 10 min. GAPDH served as the internal control. The relative mRNA
expression levels were measured using the 2−∆∆Cq method
(26). The specific primer
sequences were as follows: PPM1G, forward
5′-GGACAGTGAGGATGAGTCAGATG-3′, and reverse
5′-TGGCACCATCATCTCTTCTTCTT-3′; GAPDH, forward
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′.
Survival analysis
The Kaplan-Meier plotter (http://kmplot.com/analysis/) was used to evaluate the
association between the expression levels of PPM1G and the overall
survival (OS) rate and clinical characteristics of patients with
LUAD from TCGA database (27).
Only RNAseq of LUAD patients with complete clinical data were
retained, while those without the corresponding clinical data and
from tumor-adjacent tissue were excluded.
Receiver operating characteristic
(ROC) analysis
ROC curves were plotted to evaluate the diagnostic
value of PPM1G in LUAD, and time-dependent ROC curves of PPM1G were
used to predict the 1-, 3- and 5-year survival rates.
Analysis of nomograms to assess
prognosis
The present study selected T stage, N stage, M
stage, the expression level of PPM1G and age from Cox regression
analysis to develop a prognostic nomogram chart to assess the 1-,
3- and 5-year OS rates of LUAD patients from TCGA database. The
nomogram prediction chart was calibrated and then its accuracy
verified by comparing the predicted probability of the line chart
with the observed actual probability through a calibration
curve.
LinkedOmics database analysis
The PPM1G-related co-expressed gene expression
profile of LUCA patients from TCGA data in the LinkedOmics database
were analyzed via gene set enrichment analysis (GSEA) in the Link
Interpreter module (http://www.linkedomics.org) (28). Gene Ontology (GO) (http://geneontology.org/) and Kyoto Encyclopedia of
Genes and Genomes (KEGG) (https://www.kegg.jp/) pathway enrichment analyses of
the PPM1G-related co-expressed genes were subsequently performed
for the functional enrichment analysis (29).
Functional enrichment analysis
The top 600 genes highly associated with PPM1G
identified by LinkedOmics database analysis, and the top 100
upregulated genes associated with the survival of patients with
LUAD identified by survival analysis were plotted into Venn
diagrams using the XIANTAO platform (www.xiantaozi.com) (30). A total of 29 intersecting genes
were obtained. GO and KEGG pathway enrichment analyses of these
genes were performed using DAVID software (http://david.ncifcrf.gov/) (31). P<0.05 was set as the cut-off
criterion.
Protein-protein interaction (PPI)
network analysis
The STRING (https://string-db.org/) (32) database was utilized to analyze the
PPIs. First, the ‘multiple proteins’ option was selected and the 29
intersecting genes were then entered into the STRING database.
‘Homo sapiens’ was selected in the species option. The
obtained PPI data were used to generate a high-level network
diagram using R package (33). A
correlation heat map of the 29 intersecting genes were visualized
by ‘ggplot2’ R package (34).
Correlation analysis between PPM1G,
immune cell infiltration and immune checkpoints
Single-sample gene set enrichment analysis(SSGSEA)
on the XIANTAO platform was performed to analyze the association
between PPM1G expression, immune cell infiltration and immune
checkpoints in LUAD. P<0.05 was set as the cut-off criterion.
ρ>-0.3 and <0.3 were regarded as very weak correlations.
TISIDB database analysis
TISIDB is an integrated knowledge-based portal
(http://cis.hku.hk/TISIDB/) (35). The association between PPM1G and
the expression of chemokines/chemokine receptors was evaluated via
evaluating the expression levels of chemokines/chemokine receptors
of tumor-infiltrating immune cells through the ‘chemokine’ module
in TISIDB.
Statistical analysis
The expression profile of PPM1G in patients with
LUAD was illustrated in boxplots and scatter plots. The data from
TCGA database were analyzed by Mann-Whitney U test for the unpaired
data and the paired Student's t-test for the paired data. The
unpaired data from the GEO database were analyzed by Mann-Whitney U
test. The RT-qPCR data was analyzed using Wilcoxon signed-ranks
test for paired data. The association between clinical parameters
and PPM1G expression was analyzed by Kruskal-Wallis test and Dunn's
test. The prognostic potential of PPM1G in patients with LUAD was
evaluated via univariate and multivariate Cox regression analyses.
The association between the expression levels of PPM1G and the OS
of patients with LUAD were analyzed with the Kaplan-Meier method
and log-rank test P-values were calculated. The contingency table
(age exclusion) was analyzed by χ2 test when satisfying
the assumption that the expected count in <20% of the cells of
the analyzed contingency table was ≤5, when the expected count
violated this assumption, Fisher's exact test was used to compare
the groups; thus Fisher's exact test was used to analyze N stage,
primary therapy outcome, ethnicity and residual tumor. The median
age variable in the contingency was analyzed by Wilcoxon
signed-ranks test. The patients were split into high and low
expression groups by the median. Finally, the correlation between
PPM1G and immune characteristics was analyzed by Spearman's rank
correlation coefficient. P<0.05 was considered to indicate a
statistically significant difference.
Results
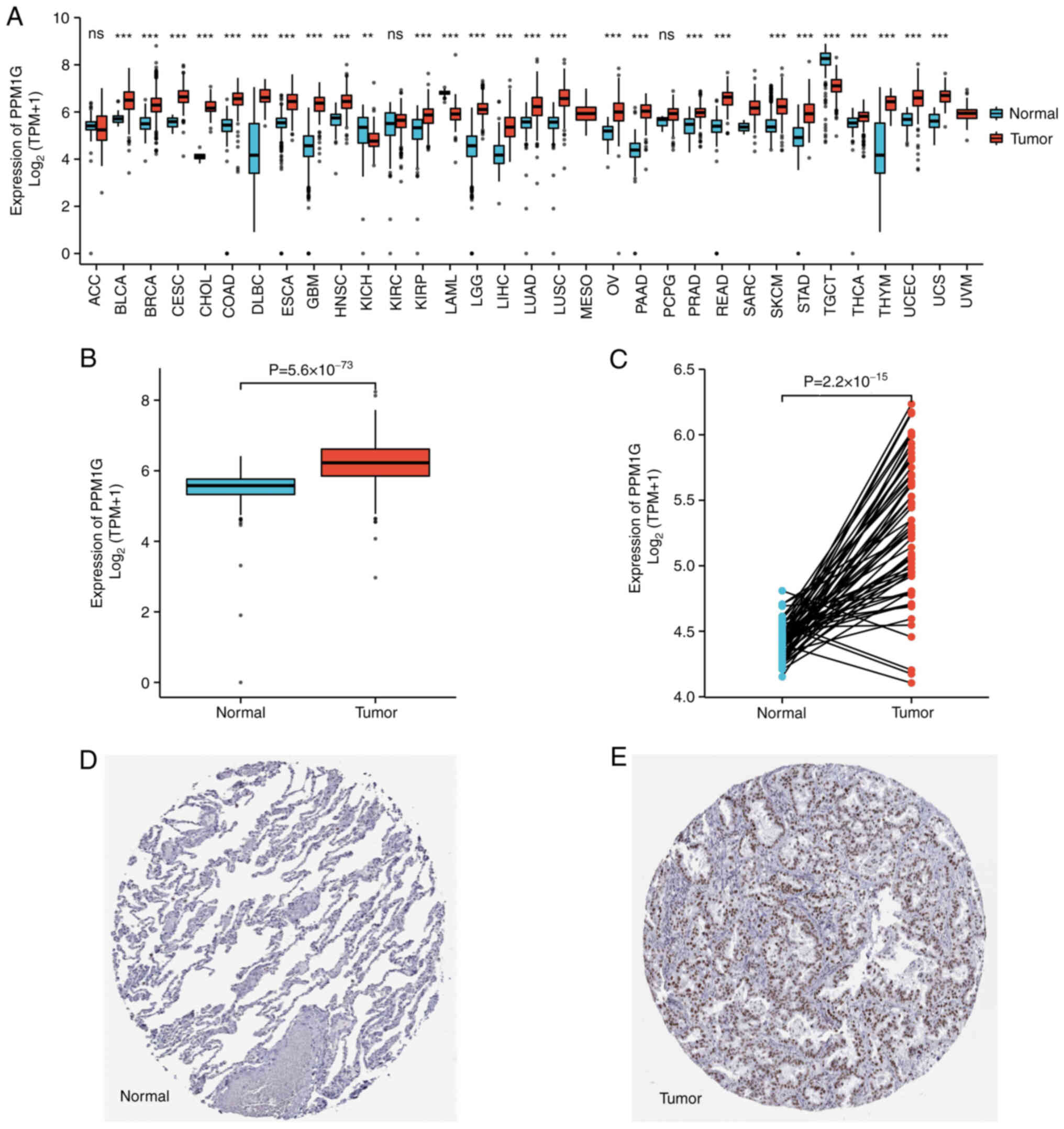
PPM1G is upregulated in cancer tissues
compared with in non-cancer tissues
To explore the possible role of PPM1G, its
expression in 33 types of cancer was detected. Fig. 1A summarizes the expression levels
of PPM1G in pan-cancer data (33 cancer types) obtained from TCGA
database. The results showed that, compared with in normal tissues,
PPM1G was upregulated significantly in 24 tumor tissues, including
LUAD, clear cell carcinoma, bladder urothelial carcinoma, breast
invasive carcinoma, cholangiocarcinoma, colon cancer, esophageal
cancer, hepatocellular carcinoma, lung squamous cell carcinoma,
prostate cancer, rectal adenocarcinoma, gastric cancer, thyroid
cancer, endometrial carcinoma, adrenal cortical carcinoma and
pancreatic cancer, etc. PPM1G was downregulated significantly in
kidney chromophobe, testicular germ cell tumors and acute myeloid
leukemia. The unpaired (normal=347 and tumor=515; P=5.6 ×
10−73; Fig. 1B) and
paired (normal=57 and tumor=57; P=2.2 × 10−15; Fig. 1C) TCGA-LUAD data presented in
boxplots further demonstrated that the expression of PPM1G was
increased in LUAD tissues compared with that in normal lung
tissues. In addition, in the HPA database, immunohistochemical
staining revealed that the expression levels of PPM1G in normal
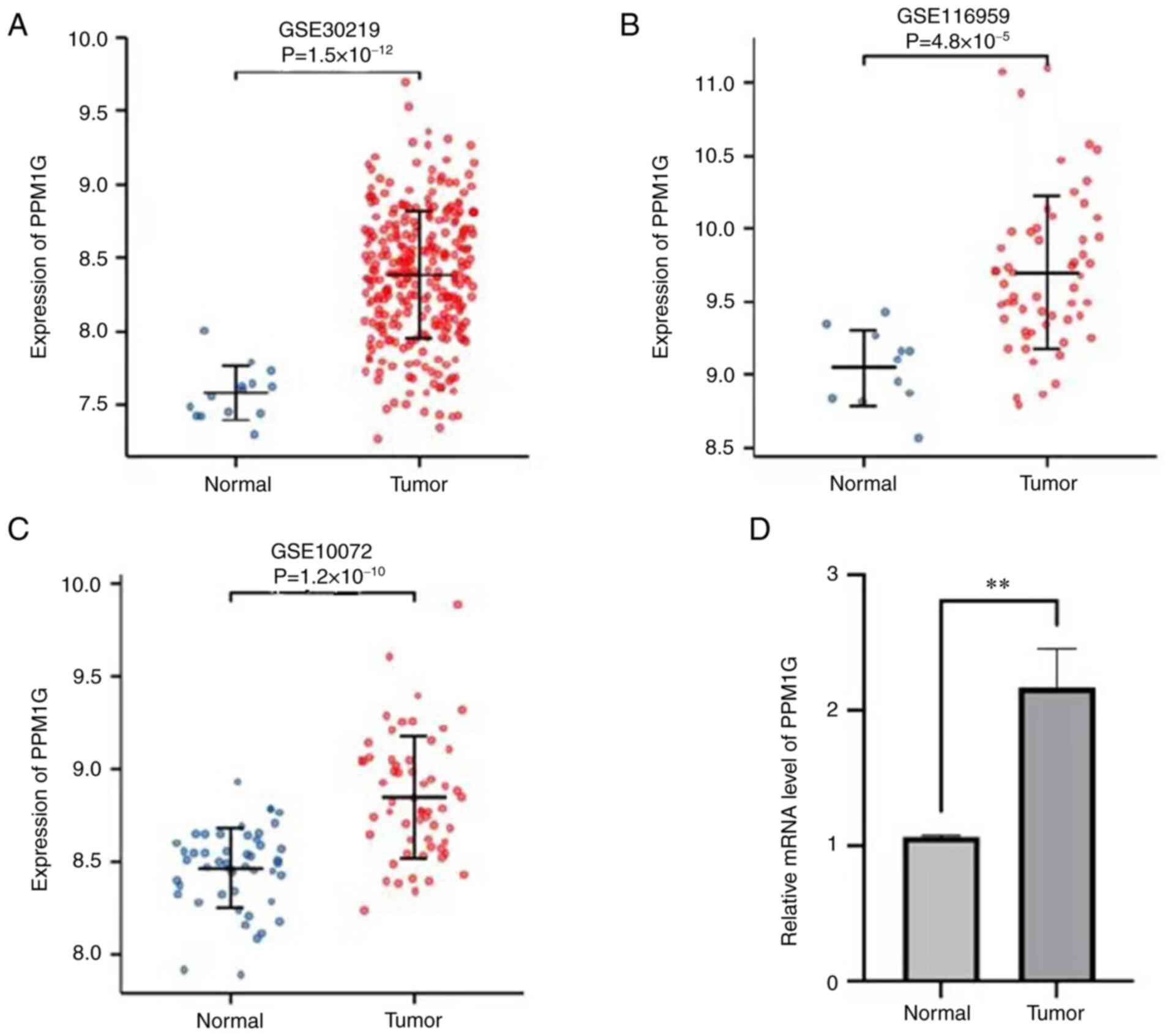
lung tissues were lower than those in tumor tissues (Fig. 1D and E). Additionally, three GEO
datasets, namely GSE30219, GSE116959 and GSE10072, were selected to
further verify the expression levels of PPM1G in LUAD. The results
demonstrated that PPM1G was significantly upregulated in LUAD
tissues compared with that in non-tumoral lung samples in all three
GEO datasets (Fig. 2A-C). Finally,
RT-qPCR verified that the relative mRNA expression levels of PPM1G
were significantly higher in LUAD compared with in normal tissues
(Fig. 2D).
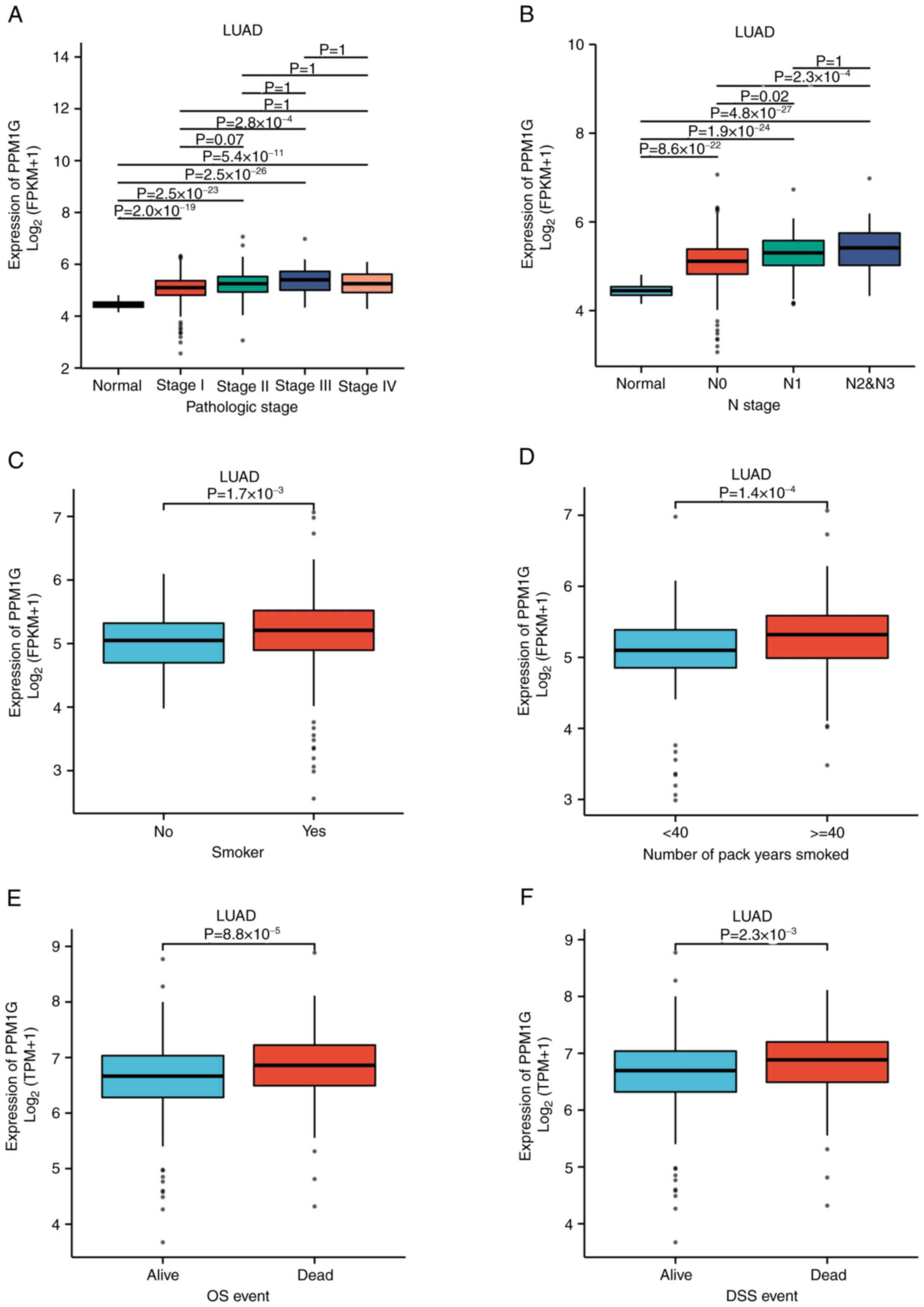
PPM1G expression is associated with
the clinical features of patients with LUAD
To evaluate the association between PPM1G expression
and the clinical characteristics of patients with LUAD, the
expression levels of PPM1G were detected in different clinical
categories in TCGA database (Table
I). The results showed that high expression of PPM1G was
significantly associated with pathologic stage (P<0.001), N
stage (P<0.001), smoking status (smoker; P=0.019), number of
pack years smoked (P=0.001), OS (P<0.001) and disease-specific
survival (DSS; P=0.007 Table I;
Fig. 3A-F). In DSS assessment,
mortality caused by LUAD was counted as outcomes, and fatalities
not caused by LUAD were not counted as outcomes.
 | Table I.Relationship between PPM1G expression
and clinical characteristics in lung adenocarcinoma. |
Table I.
Relationship between PPM1G expression
and clinical characteristics in lung adenocarcinoma.
| Characteristic | Low expression of
PPM1G (n=267) | High expression of
PPM1G (n=268) | P-value |
|---|
| T stage, n (%) |
|
| 0.010 |
| T1 | 105 (19.7) | 70 (13.2) |
|
| T2 | 128 (24.1) | 161 (30.3) |
|
| T3 | 24 (4.5) | 25 (4.7) |
|
| T4 | 8 (1.5) | 11 (2.1) |
|
| N stage, n (%) |
|
| <0.001 |
| N0 | 198 (38.2) | 150 (28.9) |
|
| N1 | 34 (6.6) | 61 (11.8) |
|
| N2 | 21 (4) | 53 (10.2) |
|
| N3 | 1 (0.2) | 1 (0.2) |
|
| M stage, n (%) |
|
| 0.304 |
| M0 | 176 (45.6) | 185 (47.9) |
|
| M1 | 9 (2.3) | 16 (4.1) |
|
| Primary therapy
outcome, n (%) |
|
| 0.014 |
| PD | 25 (5.6) | 46 (10.3) |
|
| SD | 22 (4.9) | 15 (3.4) |
|
| PR | 2 (0.4) | 4 (0.9) |
|
| CR | 180 (40.4) | 152 (34.1) |
|
| Pathologic stage, n
(%) |
|
| <0.001 |
| Stage
I | 175 (33.2) | 119 (22.6) |
|
| Stage
II | 48 (9.1) | 75 (14.2) |
|
| Stage
III | 27 (5.1) | 57 (10.8) |
|
| Stage
IV | 10 (1.9) | 16 (3.0) |
|
| Sex, n (%) |
|
| 0.514 |
|
Female | 147 (27.5) | 139 (26.0) |
|
|
Male | 120 (22.4) | 129 (24.1) |
|
| Ethnicity, n
(%) |
|
| 0.817 |
|
Asian | 4 (0.9) | 3 (0.6) |
|
| Black
or African American | 30 (6.4) | 25 (5.3) |
|
|
Caucasian | 206 (44.0) | 200 (42.7) |
|
| Age, n (%) |
|
| 0.791 |
| ≤65
years | 125 (24.2) | 130 (25.2) |
|
| >65
years | 132 (25.6) | 129 (25.0) |
|
| Residual tumor, n
(%) |
|
| 0.510 |
| R0 | 175 (47.0) | 180 (48.4) |
|
| R1 | 5 (1.3) | 8 (2.2) |
|
| R2 | 1 (0.3) | 3 (0.8) |
|
| Anatomic neoplasm
subdivision, n (%) |
|
| 0.223 |
|
Left | 109 (21.0) | 96 (18.5) |
|
|
Right | 149 (28.7) | 166 (31.9) |
|
| Anatomic neoplasm
subdivision 2, n (%) |
|
| 0.932 |
| Central
lung | 28 (14.8) | 34 (18) |
|
|
Peripheral lung | 55 (29.1) | 72 (38.1) |
|
| Number of pack
years smoked, n (%) |
|
| 0.001 |
|
<40 | 107 (29.0) | 81 (22.0) |
|
|
≥40 | 72 (19.5) | 109 (29.5) |
|
| Smoker, n (%) |
|
| 0.019 |
| No | 47 (9.0) | 28 (5.4) |
|
|
Yes | 211 (40.5) | 235 (45.1) |
|
| OS event, n
(%) |
|
| <0.001 |
|
Survived | 192 (35.9) | 151 (28.2) |
|
|
Succumbed | 75 (14.0) | 117 (21.9) |
|
| DSS event, n
(%) |
|
| 0.007 |
|
Survived | 204 (40.9) | 175 (35.1) |
|
|
Succumbed | 47 (9.4) | 73 (14.6) |
|
| PFI event, n
(%) |
|
| 0.072 |
|
Survived | 165 (30.8) | 144 (26.9) |
|
|
Succumbed | 102 (19.1) | 124 (23.2) |
|
| Age, median
(IQR) | 66 (60, 73) | 65 (58, 72) | 0.225 |
High PPM1G expression is an
independent prognostic factor for OS in LUAD
To determine the effect of PPM1G expression on OS,
patients with LUAD, based on TCGA database, were divided into high
and low PPM1G expression groups by the median. Kaplan-Meier
survival analysis revealed that high PPM1G expression was
associated with poor prognosis in patients with LUAD (HR=1.82;
P<0.001; Fig. 4A). In addition,
subgroup analysis showed that high PPM1G expression was
significantly associated with poor prognosis in patients with T1
stage (HR=2.24; P<0.012; Fig.
4B), T2 stage (HR=1.74; P=0.005; Fig. 4C), N0 stage (HR=1.99; P=0.002;
Fig. 4D), M0 stage (HR=1.64;
P=0.005; Fig. 4E) and pathologic
stage I (HR=1.72; P=0.027; Fig.
4F). Additionally, high PPM1G expression was associated with
poor prognosis in patients of both sexes (female, HR=1.75; P=0.007;
Fig. 4G and male, HR=1.82;
P=0.006; Fig. 4H), in smokers
(HR=1.74; P=0.001; Fig. 4I),
number of pack years smoked <40 (HR=2.61; P=0.001; Fig. 4J), and in patients aged ≤65 years
(HR=1.88; P=0.004; Fig. 4K) and
>65 years (HR=1.77; P=0.006; Fig.
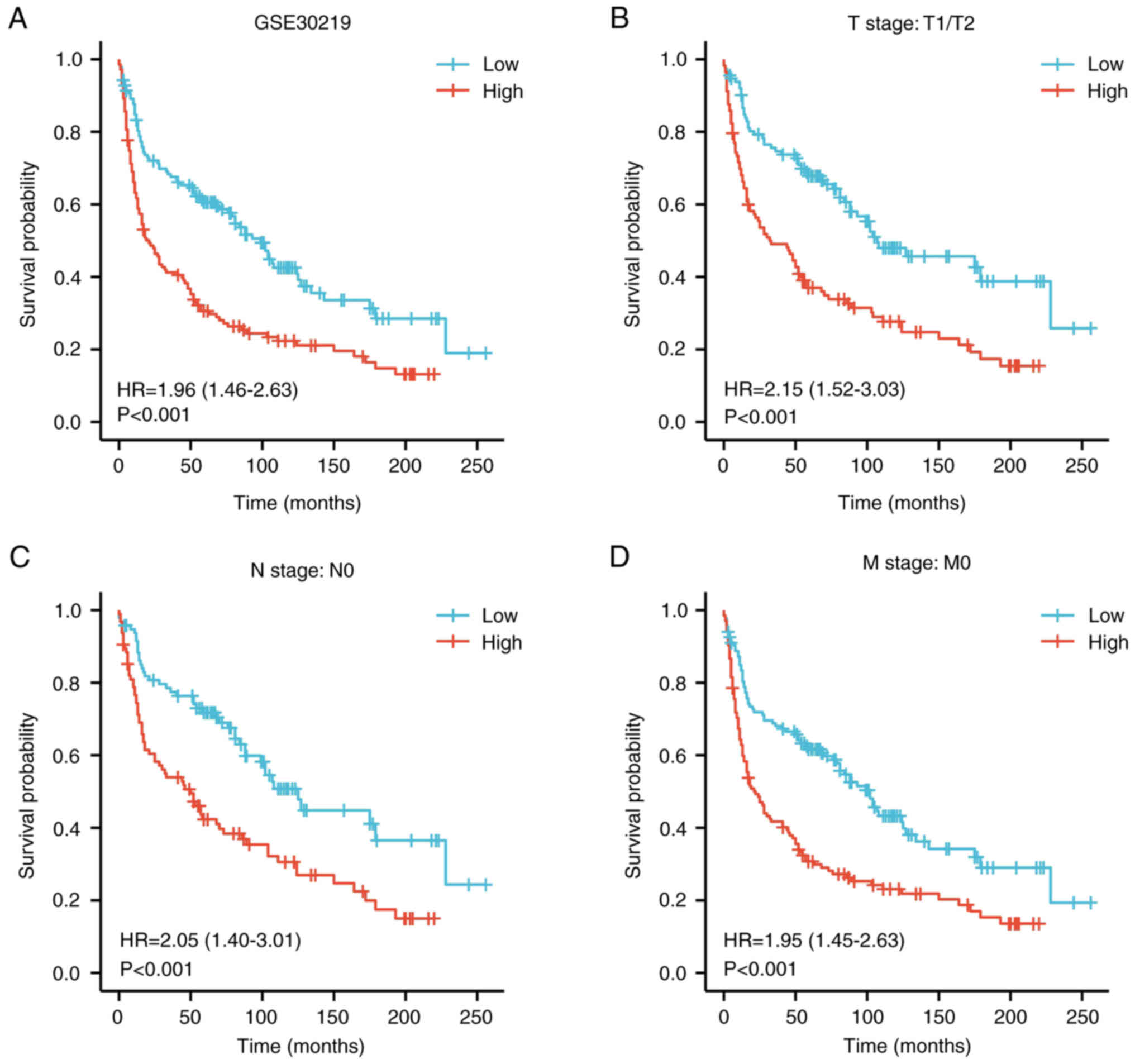
4L). In addition, the effect of PPM1G expression on the
prognosis of patients with LUAD was verified using the GSE30219 GEO
dataset. Kaplan-Meier survival analysis of this dataset
demonstrated that patients with increased PPM1G expression had a
shorter OS (Fig. 5A-D). Univariate
Cox regression analysis of the OS-related clinical features of
patients with LUAD in TCGA showed that T stage, N stage, M stage,
pathologic stage, residual tumor and high PPM1G expression were
notably associated with poor OS; while multivariate Cox regression
analysis showed that only T stage was notably associated with poor
OS (Table II). These data
suggested that the high expression levels of PPM1G could be an
independent prognostic factor for OS in patients with LUAD.
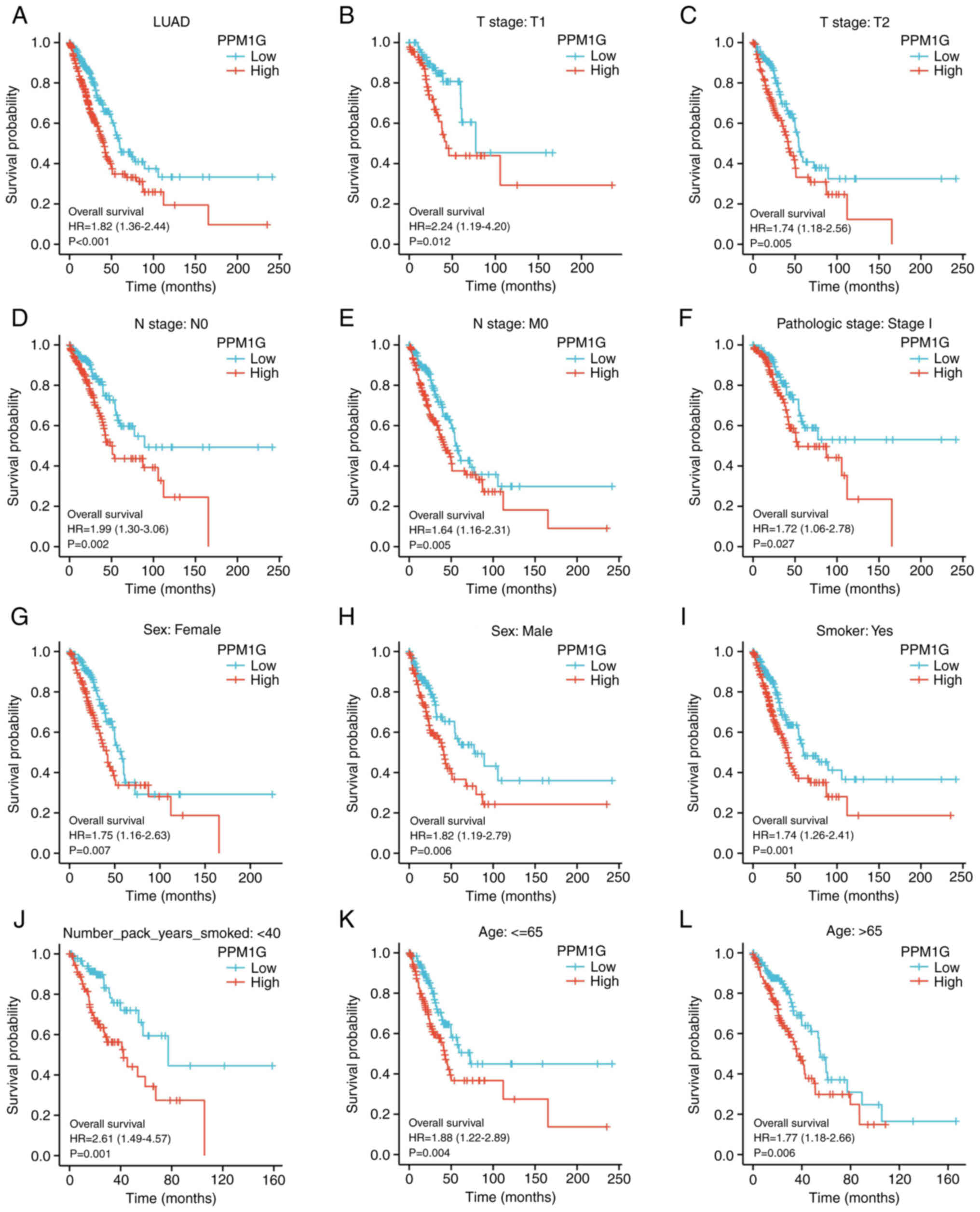 | Figure 4.Kaplan-Meier curve for overall
survival in LUAD. (A) Kaplan-Meier curve for PPM1G in all tumor
patients. Subgroup analysis for patients with (B) T1, (C) T2, (D)
N0, (E) M0 and (F) pathologic stage I LUAD, and for (G) female
patients, (H) male patients, (I) smokers, (J) smokers with <40
pack years, and patients aged (K) ≤65 and (L) >65 years. PPM1G,
protein phosphatase, Mg2+/Mn2+ dependent 1G;
LUAD, lung adenocarcinoma. |
 | Table II.Univariate and multivariate Cox
regression analyses of the clinical characteristics associated with
OS in lung adenocarcinoma in The Cancer Genome Atlas. |
Table II.
Univariate and multivariate Cox
regression analyses of the clinical characteristics associated with
OS in lung adenocarcinoma in The Cancer Genome Atlas.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Characteristic | Total, n | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| T stage | 523 |
|
|
|
|
|
T1&T2 | 457 | Reference |
|
|
|
|
T3&T4 | 66 | 2.317
(1.591–3.375) |
<0.001a | 2.111
(1.156–3.853) | 0.015a |
| N stage | 510 |
|
|
|
|
| N0 | 343 | Reference |
|
|
|
| N1 | 94 | 2.382
(1.695–3.346) |
<0.001a | 1.863
(0.879–3.949) | 0.104 |
|
N2&N3 | 73 | 2.968
(2.040–4.318) |
<0.001a | 2.174
(0.809–5.845) | 0.124 |
| M stage | 377 |
|
|
|
|
| M0 | 352 | Reference |
|
|
|
| M1 | 25 | 2.136
(1.248–3.653) | 0.006a | 1.219
(0.462–3.217) | 0.689 |
| Pathologic
stage | 518 |
|
|
|
|
| Stage
I | 290 | Reference |
|
|
|
| Stage
II | 121 | 2.418
(1.691–3.457) |
<0.001a | 0.929
(0.427–2.020) | 0.852 |
| Stage
III | 81 | 3.544
(2.437–5.154) |
<0.001a | 1.251
(0.432–3.621) | 0.679 |
| Stage
IV | 26 | 3.790
(2.193–6.548) |
<0.001a |
|
|
| Sex | 526 |
|
|
|
|
|
Female | 280 | Reference |
|
|
|
|
Male | 246 | 1.070
(0.803–1.426) | 0.642 |
|
|
| Age | 516 |
|
|
|
|
| ≤65
years | 255 | Reference |
|
|
|
| >65
years | 261 | 1.223
(0.916–1.635) | 0.172 |
|
|
| Residual tumor | 363 |
|
|
|
|
| R0 | 347 | Reference |
|
|
|
| R1 | 13 | 3.255
(1.694–6.251) |
<0.001a | 2.208
(0.943–5.168) | 0.068 |
| R2 | 3 | 11.085
(3.443–35.689) |
<0.001a | 3.324
(0.658–16.796) | 0.146 |
| Anatomic neoplasm
subdivision | 512 |
|
|
|
|
|
Left | 200 | Reference |
|
|
|
|
Right | 312 | 1.037
(0.770–1.397) | 0.810 |
|
|
| Anatomic neoplasm
subdivision 2 | 182 |
|
|
|
|
| Central
lung | 62 | Reference |
|
|
|
|
Peripheral lung | 120 | 0.913
(0.570–1.463) | 0.706 |
|
|
| Number of pack
years smoked | 363 |
|
|
|
|
|
<40 | 183 | Reference |
|
|
|
|
≥40 | 180 | 1.073
(0.753–1.528) | 0.697 |
|
|
| Smoker | 512 |
|
|
|
|
| No | 72 | Reference |
|
|
|
|
Yes | 440 | 0.894
(0.592–1.348) | 0.591 |
|
|
| PPM1G | 526 |
|
|
|
|
|
Low | 262 | Reference |
|
|
|
|
High | 264 | 1.821
(1.357–2.442) |
<0.001a | 1.183
(0.794–1.762) | 0.409 |
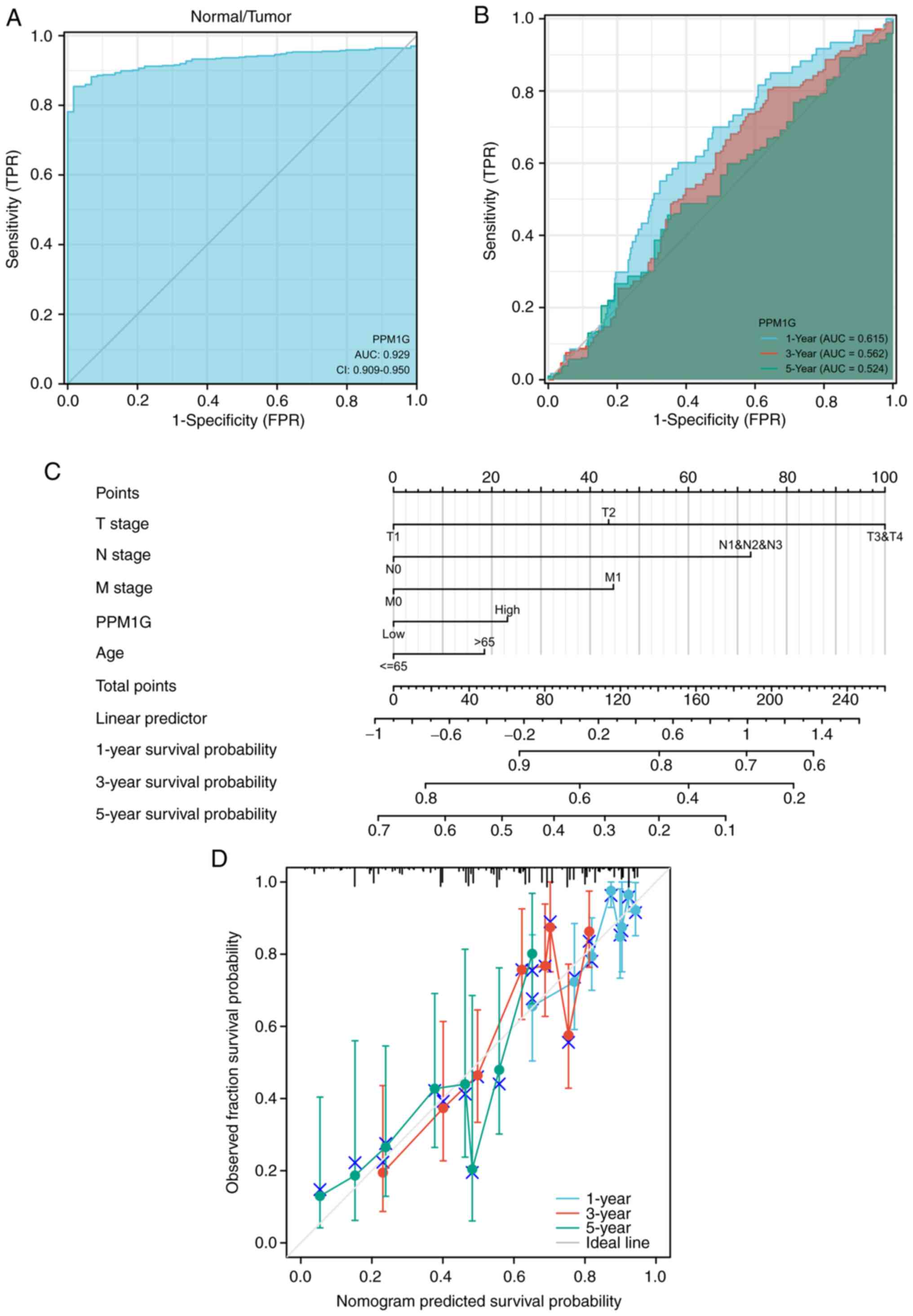
Diagnostic value of PPM1G in LUAD
ROCs and nomograms were plotted to evaluate the
diagnostic value of PPM1G in LUAD. The diagnostic ROC curve showed
that the area under the curve (AUC) value was 0.929 (Fig. 6A), thus indicating that PPM1G could
exert an accurate diagnostic effect. Time-dependent ROC curves of
PPM1G were used to predict the 1-, 3- and 5-year survival rates.
All AUC values were >0.5 (Fig.
6B). To construct a nomogram, the expression levels of
PPM1G were combined with several clinical variables,
including T stage, N stage, M stage and age, to predict the
survival probability of patients at 1, 3 and 5 years (Fig. 6C). In addition, the calibration and
accuracy verification of the nomogram were evaluated via
calibration curve (Fig. 6D).
PPM1G is closely associated with
regulation of the cell cycle in LUAD
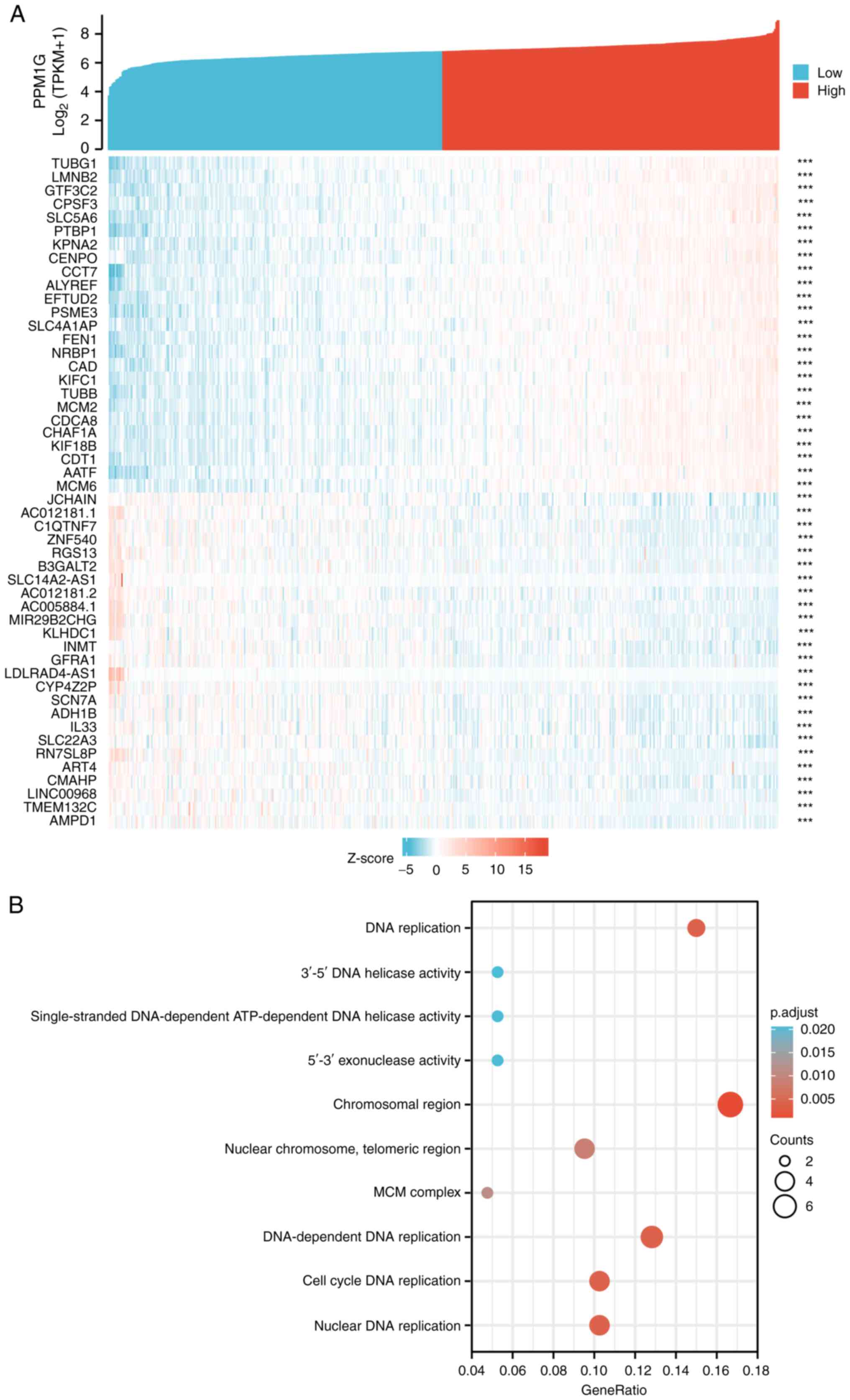
The Link Interpreter module of the LinkedOmics
website was applied to detect the co-expression pattern of PPM1G in
TCGA-LUAD to verify the biological function of PPM1G in LUAD. A
total of 25 genes with the highest Spearman correlation coefficient
and negative correlation were selected. Subsequently, a single gene
co-expression heatmap was constructed (Fig. 7A). GO function and KEGG pathway
enrichment analyses of the top 600 PPM1G-related genes were
performed using DAVID Functional Annotation Bioinformatics
Microarray analysis. The results showed that the 600 genes were
mainly enriched in the GO terms ‘cell cycle DNA replication’ and
‘DNA replication’ (Fig. 7B).
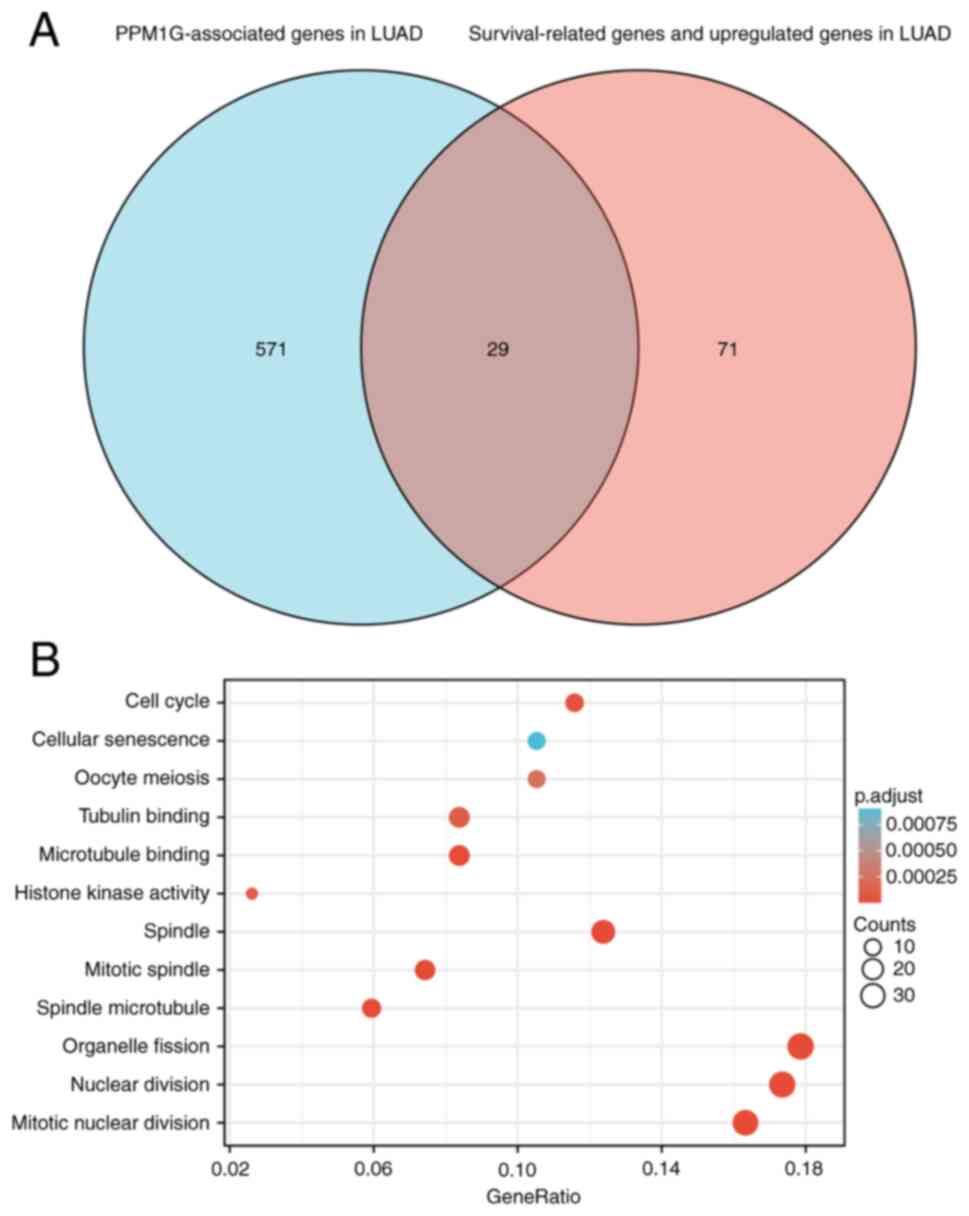
Furthermore, all genes that were significantly associated with
survival in LUAD were screened. The top 100 upregulated genes were
selected. The top 600 genes significantly associated with PPM1G
expression with the 100 survival-associated genes were entered into
a Venn diagram and the 29 common genes associated with PPM1G
expression and survival in LUAD were obtained (Fig. 8A). These 29 genes were subjected to
GO enrichment and KEGG pathway analyses, and the results showed
that the differentially expressed genes were significantly enriched
in the GO term ‘cell cycle’ (Fig.
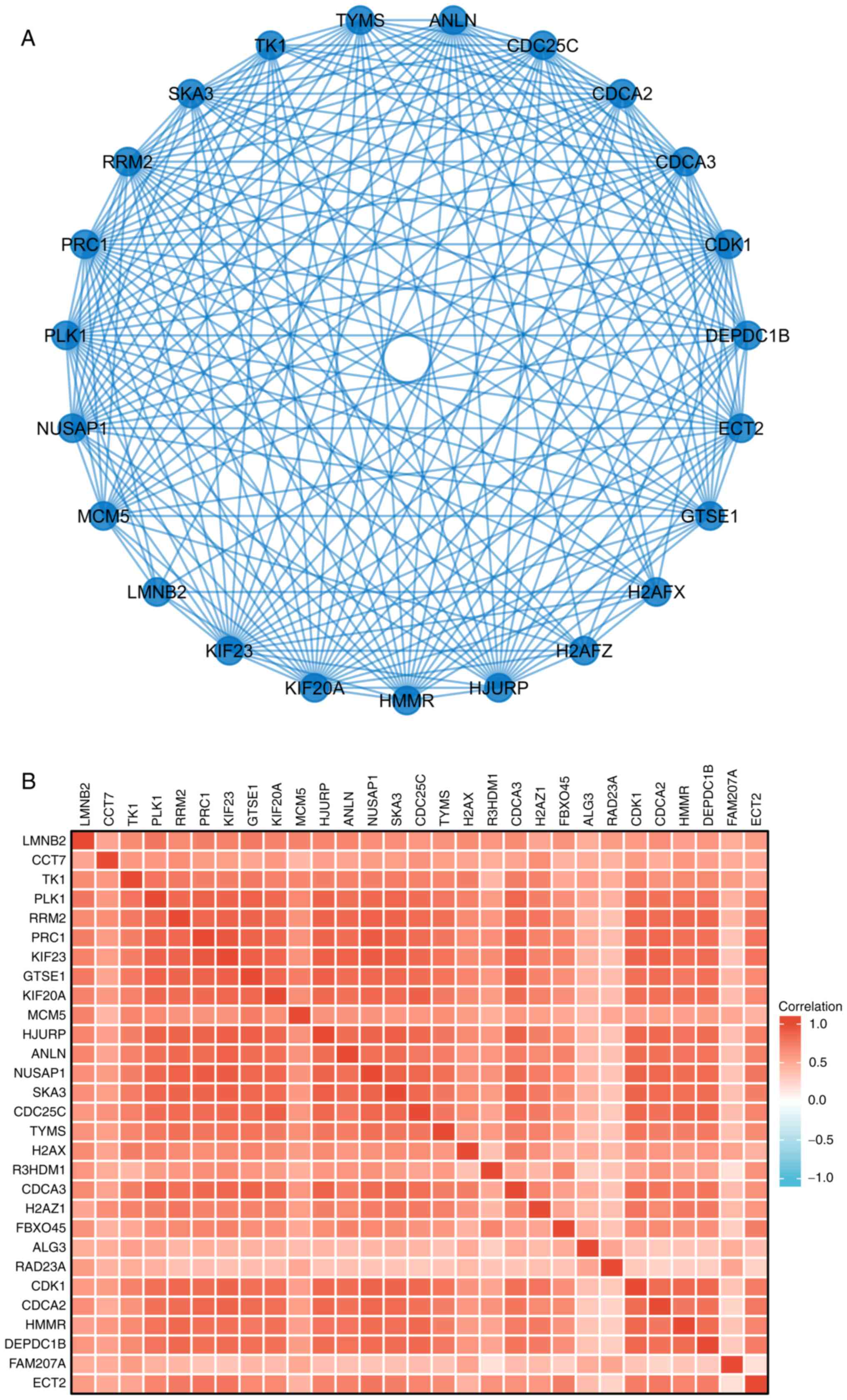
8B). A PPI network was then constructed to analyze the
interactions between the 29 proteins. The network diagram revealed
that these proteins were strongly associated with each other and
all genes were closely associated with the cell cycle (Fig. 9A). The gene co-expression
correlation heatmap of the 29 proteins also demonstrated that the
majority of proteins were positively associated with each other
(Fig. 9B). These results indicated
that these PPM1G-associated cell cycle-related genes were closely
associated and could be used as polygenic biomarkers to predict the
survival of patients with LUAD.
Correlation analysis between PPM1G and
cell cycle-related regulatory genes in LUAD
The aforementioned analysis showed that PPM1G was
closely associated with regulation of the cell cycle in LUAD.
Therefore, the association between PPM1G and cell cycle-related
genes in LUAD was subsequently investigated. The results showed
that the expression levels of the cell cycle-related genes LMNB2
(ρ=0.750; P<0.001; Fig. 10A),
CCT7 (ρ=0.734; P<0.001; Fig.
10B), PLK1 (ρ=0.699; P<0.001; Fig. 10C), PRC1 (ρ=0.678; P<0.001;
Fig. 10D), RRM2 (ρ=0.692;
P<0.001; Fig. 10E), TK1
(ρ=0.699; P<0.001; Fig. 10F),
KIF20A (ρ=0.657; P<0.001; Fig.
10G), GTSE1 (ρ=0.670; P<0.001; Fig. 10H), KIF23 (ρ=0.674; P<0.001;
Fig. 10I), MCM5 (ρ=0.638;
P<0.001; Fig. 10J), HJURP
(ρ=0.636, P<0.001; Fig. 10K),
ANLN (ρ=0.636; P<0.001; Fig.
10L), NUSAP1 (ρ=0.630; P<0.001; Fig. 10M), CDC25C (ρ=0.624; P<0.001;
Fig. 10N) and SKA3 (ρ=0.625;
P<0.001; Fig. 10O) were
positively associated with those of PPM1G. The aforementioned
findings indicated that the expression of PPM1G was closely
associated with that of cell cycle-related genes in LUAD.
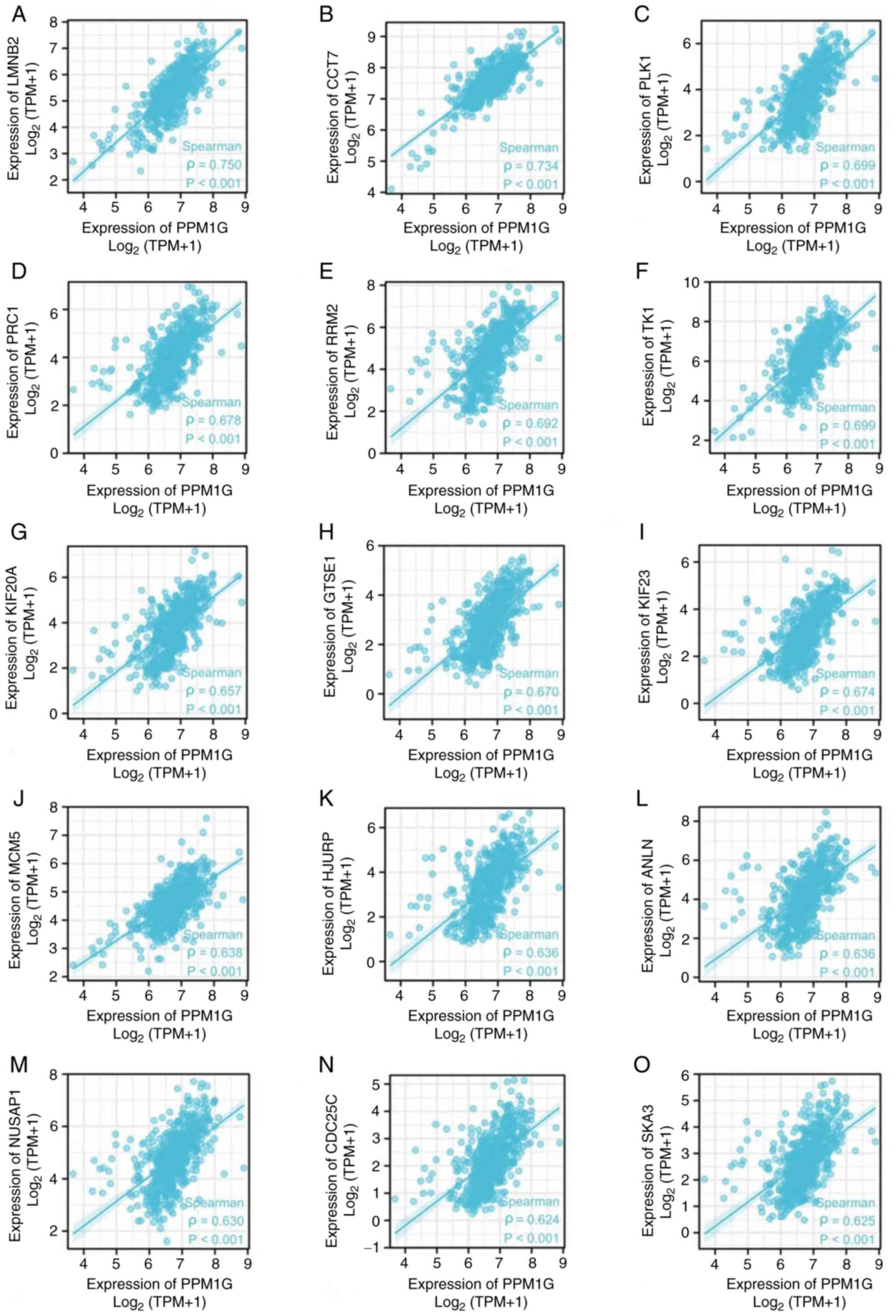 | Figure 10.Correlation of cell cycle regulatory
genes with PPM1G in lung adenocarcinoma. (A) LMNB2, (B) CCT7, (C)
PLK1, (D) PRC1, (E) RRM2, (F) TK1, (G) KIF20A, (H) GTSE1, (I)
KIF23, (J) MCM5, (K) HJURP, (L) ANLN, (M) NUSAP1, (N) CDC25C and
(O) SKA3. PPM1G, protein phosphatase,
Mg2+/Mn2+ dependent 1G. |
Correlation analysis between PPM1G
expression and immune cells
Subsequently, the association between PPM1G
expression and tumor immune response was explored. The lollipop
chart in Fig. 11 illustrates the
association between PPM1G expression and immune cell infiltration
in LUAD. The results of the assessment of the differential
distribution of immune cells in patients with high and low PPM1G
expression levels showed that the numbers of T helper (Th)2 and
γδ-T cells in patients with LUAD and low PPM1G expression were
significantly lower compared with those in patients with high PPM1G
expression. Additionally, the numbers of T follicular helper (TFH)
cells, central memory T (Tcm) cells, Th cells, T cells,
plasmacytoid dendritic cells (pDCs), mast cells, macrophages,
immature DCs, eosinophils, DCs, cytotoxic cells, CD8 T cells and B
cells were notably lower in LUAD patients with high PPM1G
expression compared with those in patients with low PPM1G
expression (Fig. 12A-O).
Furthermore, the correlation between the expression levels of PPM1G
and immune cell infiltration in LUAD was evaluated. The results
demonstrated that the expression levels of PPM1G were positively
correlated with the infiltration degree of Th2 cells (ρ=0.462;
P<0.001; Fig. 13A), natural
killer CD56dim cells (ρ=0.152; P<0.001; Fig. 13B) and γδ-T cells (ρ=0.151;
P<0.001; Fig. 13C). By
contrast, the expression levels of PPM1G were negatively correlated
with the infiltration degree of B cells (ρ=−0.273; P<0.001;
Fig. 13D), mast cells (ρ=−0.316;
P<0.001; Fig. 13E), pDCs
(ρ=−0.171; P<0.001; Fig. 13F),
Th cells (ρ=−0.127; P=0.003; Fig.
13G), macrophages (ρ=−0.159; P<0.001; Fig. 13H), T cells (ρ=−0.224; P<0.001;
Fig. 13I), CD8 T cells (ρ=−0.158,
P<0.001; Fig. 13J), Tcm cells
(ρ=−0.171; P<0.001; Fig. 13K),
effector memory T cells (ρ=−0.110, P<0.001; Fig. 13L), TFH cells (ρ=−0.183;
P<0.001; Fig. 13M) and Th1
cells (ρ=−0.103; P=0.018; Fig.
13N). Chemokines and chemokine receptors play a key role in the
infiltration of immune cells into the tumor. Therefore, the
association between PPM1G expression and chemokines/chemokine
receptors in LUAD was analyzed using TISIDB. The heatmap revealed
that there was a clear association between the expression of
several chemokines and PPM1G expression in LUAD (Fig. 14A). Therefore, the correlation
between the expression of PPM1G and that of chemokines in LUAD was
further analyzed. The results showed that the expression of PPM1G
was negatively correlated with CCL13 (ρ=−0.259;
P=2.62×10−9; Fig.
14B), CCL14 (ρ=−0.442; P<2.2×10−16; Fig. 14C), CCL15 (ρ=−0.191;
P=1.22×10−5; Fig.
14D), CCL19 (ρ=−0.34; P=2.47×10−15; Fig. 14E), CXCL2 (ρ=−0.119; P=0.00666;
Fig. 14F), CCL22 (ρ=−0.191;
P=1.33×10−5; Fig.
14G), CCL23 (ρ=−0.294; P=1.11×10−11; Fig. 14H), CXCL14 (ρ=−0.242;
P=2.95×10−8; Fig.
14I), CXCL16 (ρ=−0.285; P=4.64×10−11; Fig. 14J), CCL17 (ρ=−0.302;
P=2.8×10−12; Fig.
14K), CXCL17 (ρ=−0.3; P=4.22×10−12; Fig. 14L), CXCL13 (ρ=−0.148; P=0.000742;
Fig. 14M), CXCL12 (ρ=−0.338;
P=3.44×10−15; Fig.
14N) and CX3CL1 (ρ=−0.205; P=2.8×10−6; Fig. 14O). The heatmap of the association
between PPM1G expression and the expression of multiple chemokine
receptors in LUAD revealed that there was also a significant
association between the two factors (Fig. 15A). More specifically, the results
demonstrated that the expression of PPM1G was negatively correlated
with that of CCR1 (ρ=−0.185; P=2.38×10−5; Fig. 15B), CCR2 (ρ=−0.38;
P<2.2×10−16; Fig.
15C), CCR3 (ρ=−0.093; P=0.0349; Fig. 15D), CCR5 (ρ=−0.203;
P=3.36×10−6; Fig.
15E), CCR4 (ρ=−0.425; P<2.2×10−16; Fig. 15F), CCR6 (ρ=−0.521;
P<2.2×10−16; Fig.
15G), CCR7 (ρ=−0.341; P=1.91×10−15; Fig. 15H), CCR8 (ρ=−0.215;
P=8.51×10−7; Fig.
15I), CX3CR1 (ρ=−0.457; P<2.2×10−16; Fig. 15J), CXCR1 (ρ=−0.199;
P=5.08×10−6; Fig.
15K), CXCR2 (ρ=−0.282; P=8.66×10−11; Fig. 15L), CXCR4 (ρ=−0.256;
P=3.98×10−9; Fig.
15M), CXCR5 (ρ=−0.255; P=4.46×10−9; Fig. 15N) and CXCR6 (ρ=−0.187;
P=1.99×10−5; Fig.
15O). These results indicated that the expression of PPM1G was
negatively associated with that of chemokines/chemokine receptors
in LUAD.
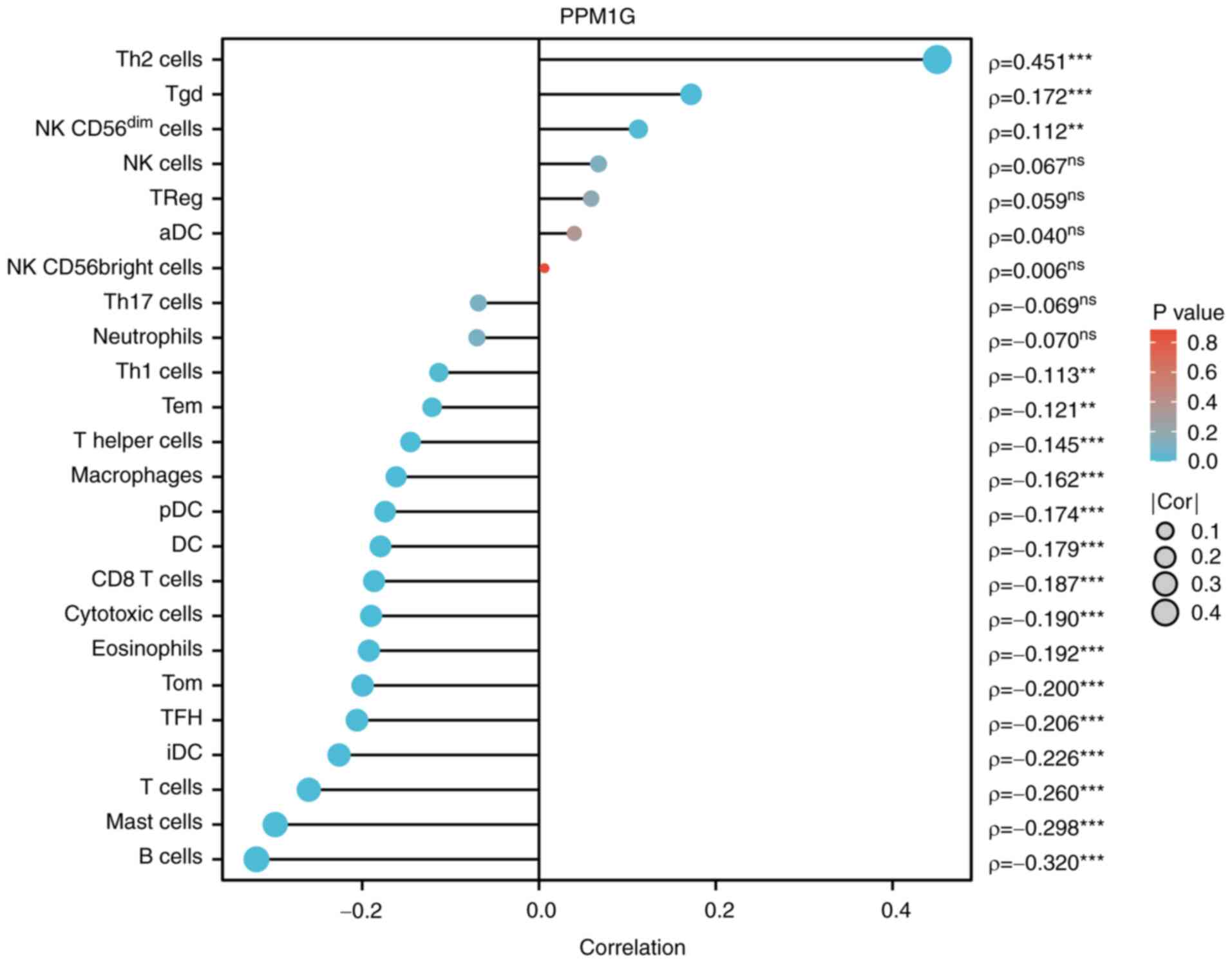 | Figure 11.Correlation between PPM1G expression
and immune infiltration in LUAD. **P<0.01 and ***P<0.001.
PPM1G, protein phosphatase, Mg2+/Mn2+
dependent 1G; LUAD, lung adenocarcinoma; Th, T helper; Tgd, γδ-T;
NK, natural killer; DC, dendritic cell; Tem, effector memory T;
pDC, plasmacytoid DC; Tcm, central memory T; TFH, T follicular
helper; iDC, immature DC; aDC, activated DC. |
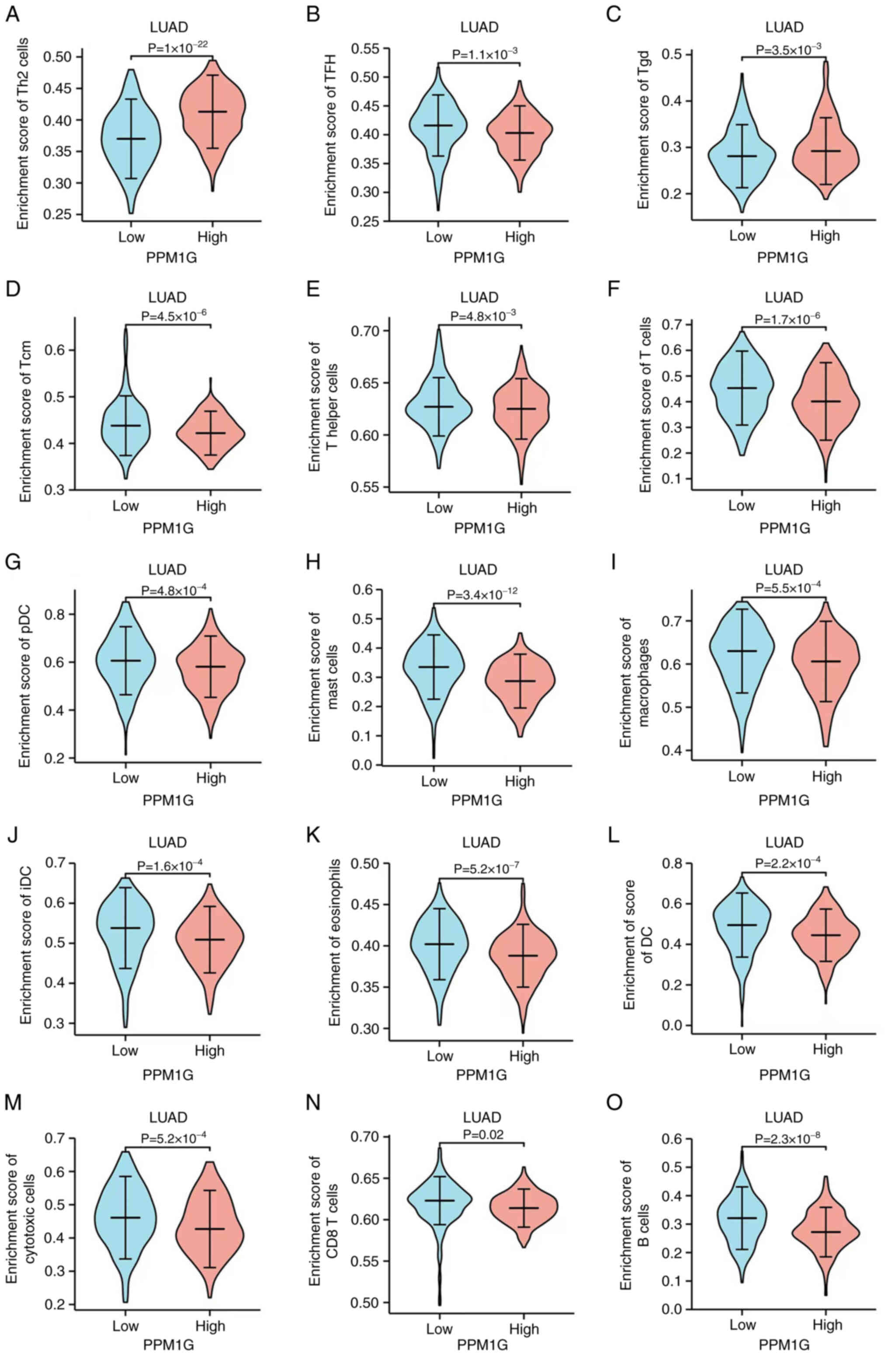 | Figure 12.Differential distribution of immune
cells in patients with high and low PPM1G expression. (A) Th2, (B)
TFH, (C) Tgd, (D) Tcm, (E) T helper and (F) T cells, (G) pDCs, (H)
mast cells, (I) macrophages, (J) iDCs, (K) eosinophils, (L) DCs,
(M) cytotoxic cells, (N) CD8 T cells and (O) B cells. PPM1G,
protein phosphatase, Mg2+/Mn2+ dependent 1G;
Th, T helper; Tgd, γδ-T; DC, dendritic cell; Tem, effector memory
T; pDC, plasmacytoid DC; Tcm, central memory T; TFH, T follicular
helper; iDC, immature DC. |
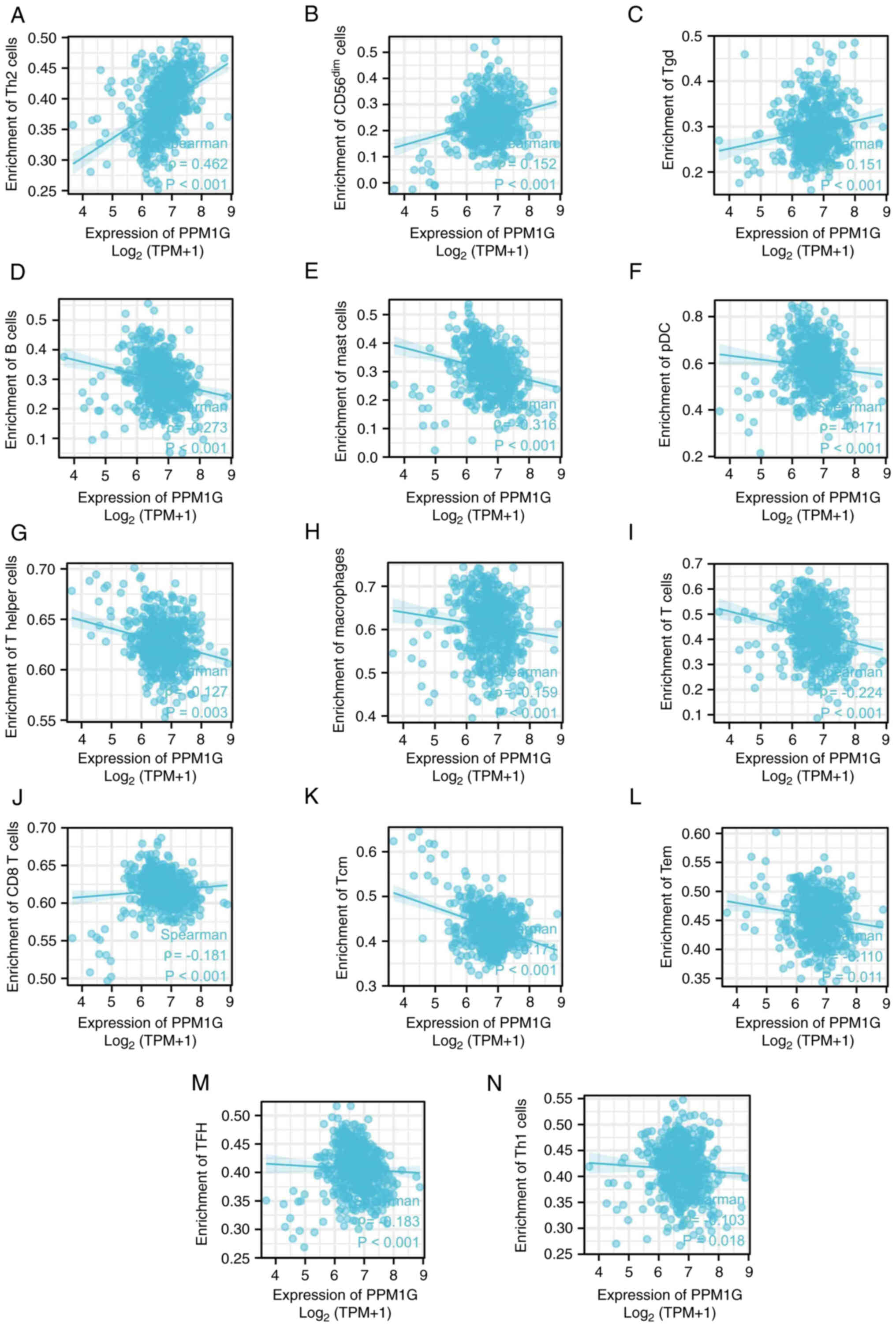 | Figure 13.Correlation between PPM1G expression
and immune infiltration in LUAD. (A) Th2, (B) natural killer
CD56dim, (C) Tgd, (D) B and (E) mast cells, (F) pDCs,
(G) Th cells, (H) macrophages, (I) T, (J) CD8 T, (K) Tcm and (L)
Tem cells, (M) TFH and (N) Th1 cells. PPM1G, protein phosphatase,
Mg2+/Mn2+ dependent 1G; LUAD, lung
adenocarcinoma; Th, T helper; Tgd, γδ-T; Tem, effector memory T;
pDC, plasmacytoid dendritic cell; Tcm, central memory T; TFH, T
follicular helper. |
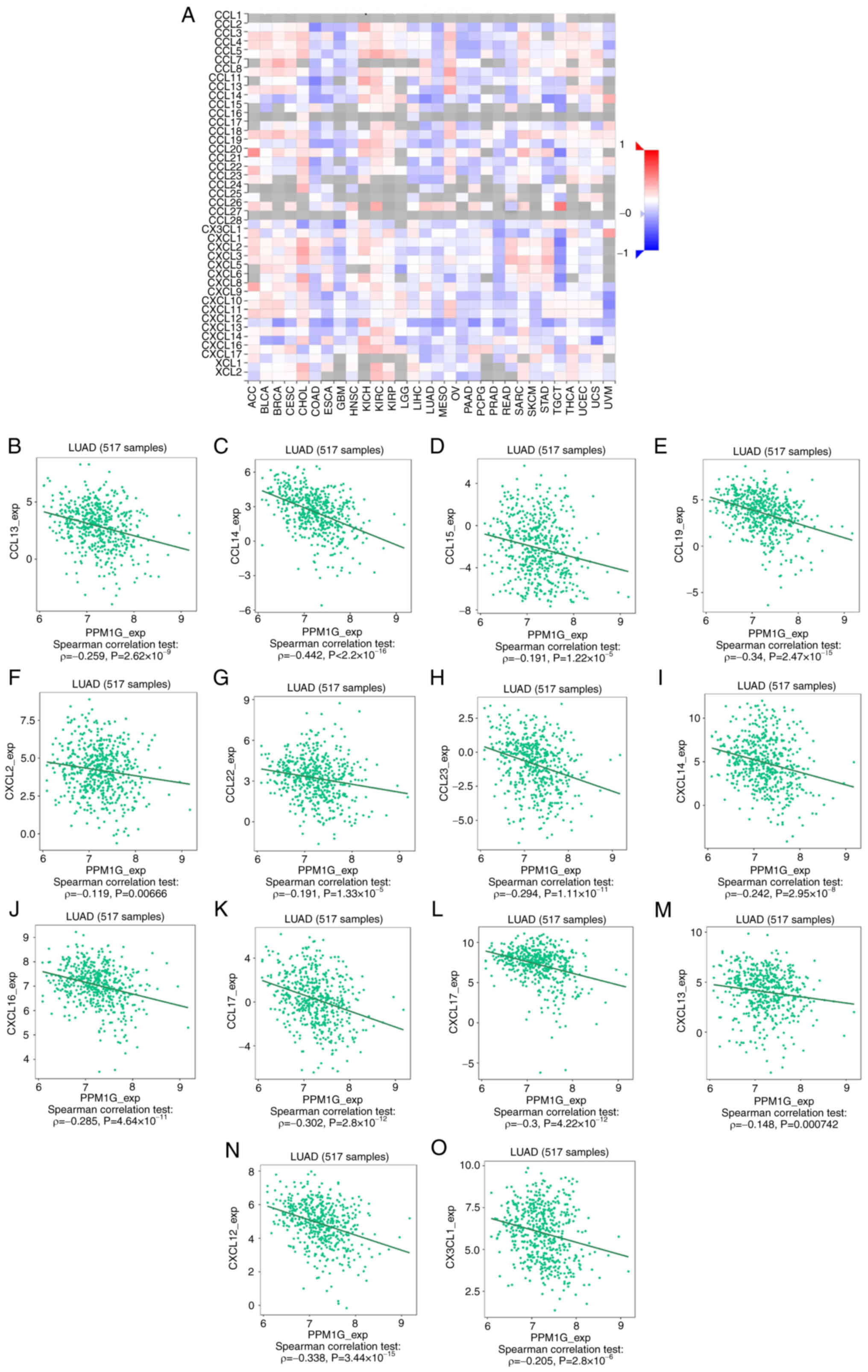 | Figure 14.Correlation analysis between PPM1G
expression and chemokines. (A) Heatmap analysis of the correlation
between PPM1G and chemokines in tumors. PPM1G expression in lung
adenocarcinoma was negatively correlated with (B) CCL13, (C) CCL14,
(D) CCL15, (E) CCL19, (F) CXCL2, (G) CCL22, (H) CCL23, (I) CXCL14,
(J) CXCL16, (K) CCL17, (L) CXCL17, (M) CXCL13, (N) CXCL12 and (O)
CX3CL1. PPM1G, protein phosphatase, Mg2+/Mn2+
dependent 1G. |
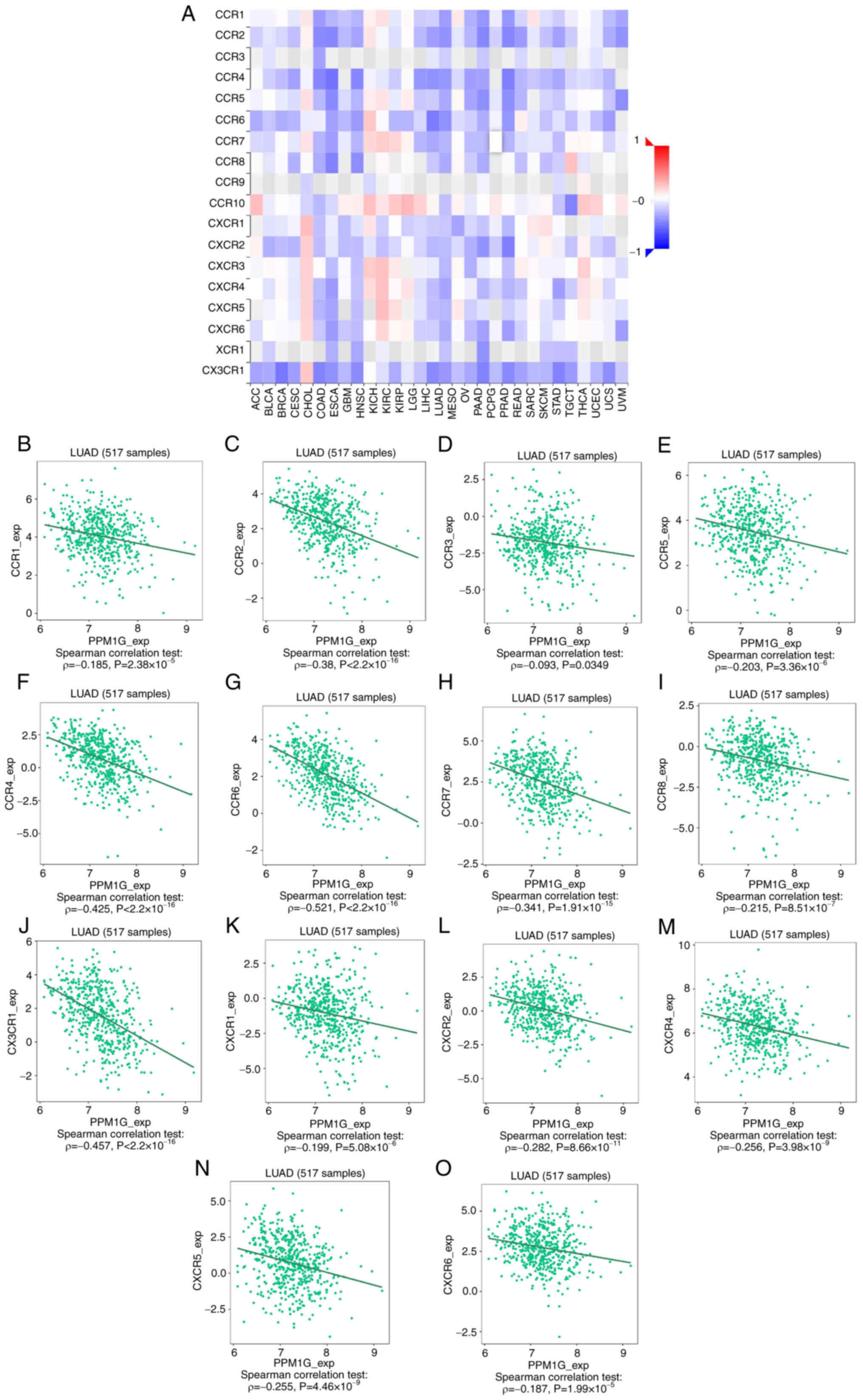 | Figure 15.Correlation analysis between PPM1G
expression and chemokine receptors. (A) Heatmap analysis of the
correlation between PPM1G and chemokine receptors in tumors. PPM1G
expression in lung adenocarcinoma was negatively correlated with
(B) CCR1, (C) CCR2, (D) CCR3, (E) CCR5, (F) CCR4, (G) CCR6, (H)
CCR7, (I) CCR8, (J) CX3CR1, (K) CXCR1, (L) CXCR2, (M) CXCR4, (N)
CXCR5 and (O) CXCR6. PPM1G, protein phosphatase,
Mg2+/Mn2+ dependent 1G. |
PPM1G expression is associated with
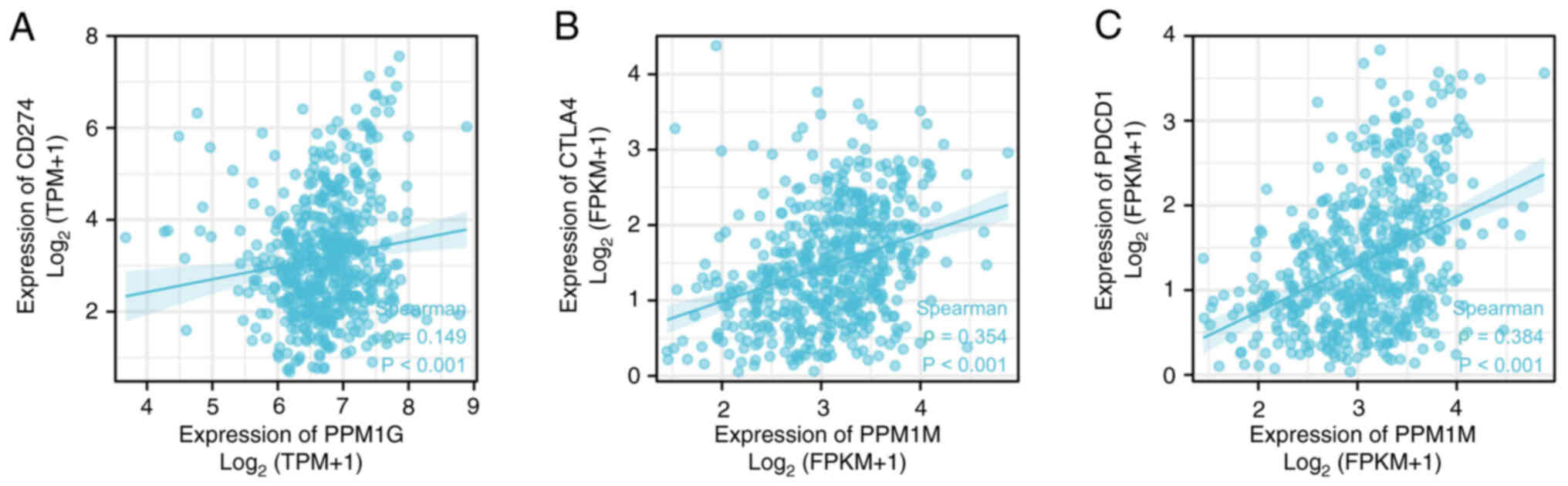
immune checkpoints in LUAD
Programmed death ligand 1 (PD-L1; gene, CD274),
cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed cell death
1 (PD-1; gene, PDCD1) are significant immune checkpoints involved
in tumor immune escape (36).
Considering the potential carcinogenic effect of PPM1G on LUAD, the
association between PPM1G and PD-L1, CTLA-4 and PD-1 was further
investigated by SSGSEA. The results showed that PPM1G was
positively correlated with PD-L1 (ρ=0.149; P<0.001; Fig. 16A), CTLA-4 (ρ=0.354; P<0.001;
Fig. 16B) and PD-1 (ρ=0.384;
P<0.001; Fig. 16C) in
LUAD.
Discussion
With the recent development of targeted therapy and
immunotherapy, it is necessary to identify new biomarkers of lung
cancer and explore novel treatments.
PPM1G, a significant member of the PPM family, is an
Mg2+/Mn2+-dependent serine/threonine
phosphatase (37). It has been
reported that PPM1G is involved in the dephosphorylation of several
histones and in different cellular biological processes, including
cell cycle regulation and immune response (10). Previous studies have also suggested
that PPM1G may be involved in pre-mRNA splicing (38), DNA damage response and
tumorigenesis (39). Previous
studies have focused on the association between PPM1G and liver
cancer, and have concluded that the PPM1G gene may be a potential
immunotherapy target and prognostic marker of liver cancer
(17,40). However, to the best of our
knowledge, there are few reports on the role of PPM1G in LUAD
(41). No studies, to the best of
our knowledge, have reported mutations or single nucleotide
polymorphisms of PPM1G in LUAD. Therefore, to investigate the
association between PPM1G expression and immune cell infiltration,
cell cycle and prognosis in LUAD, bioinformatics analysis was
performed using several online databases.
In the present study, the expression levels and
prognostic potential of PPM1G in LUAD were explored. By performing
a pan-cancer analysis of the expression of PPM1G using TCGA
database, increased PPM1G expression levels were detected in cancer
tissues compared with those in noncancer tissues, and PPM1G was
revealed to be significantly upregulated in LUAD. Its increased
expression was associated with poor OS and different
clinicopathological features of patients with LUAD, including
pathologic stage, N stage, smoking status, number of pack years
smoked and DSS event. The Kaplan-Meier survival analysis, and
univariate and multivariate Cox regression analyses showed that
high expression of PPM1G was an independent prognostic factor for
the OS of patients with LUAD, and TCGA database and the GEO
datasets also suggested that patients with increased PPM1G
expression had poor OS. In addition, the diagnostic significance of
PPM1G in LUAD was evaluated. To analyze the diagnostic value of
PPM1G expression in LUAD, ROC curve and nomogram analyses were
performed on the PPM1G gene expression data of TCGA database. The
AUC was 0.929, suggesting a high diagnostic value, indicating that
it could be an independent diagnostic marker for LUAD
progression.
At present, there are few studies on the substrate
of PPM1G. PPM1G has been shown to bind to the Tat protein of the
human immunodeficiency virus as well as to NF-κB (9). In addition, PPM1G can bind to the RNA
of small nuclear ribonucleoproteins (42). PPM1G forms a PPP-type phosphatase
holoenzyme with B56δ that maintains adherens junction integrity by
acting directly on substrate α-catenin (43). This indicates that PPM1G may affect
cell migration. In LUAD, it has been shown that PPM1G is closely
related to the regulation of the cell cycle by inhibiting p38
activation via dephosphorylation of MEK6 (41). Lin et al (10) suggested that PPM1G could affect the
prognosis of patients with hepatocellular carcinoma via regulating
the cell cycle, based on the results of data analysis. Cell cycle
disorders are commonly characterized by the uncontrolled growth,
proliferation, differentiation and apoptosis of tumor cells
(44). In the present study, KEGG
pathway enrichment analysis and GO functional annotation of the
PPM1G-related genes showed a positive relationship with cell
cycle-related genes, including LMNB2, CCT7, PLK1, PRC1 and RRM2.
However, no definite PPM1G substrate has been assessed in the study
of other tumors; therefore, verification of the direct substrate of
PPM1G, especially cell cycle-related proteins, should be an aim in
future work.
The tumor microenvironment (TME) includes immune
cells, extracellular matrix components, mesenchymal cells and
inflammatory mediators, which are involved in tumor development,
metastasis and recurrence (45).
The type and aggregation of immune cells serve a key role in tumor
development (46). Previous
studies on lung cancer have demonstrated that the immunological
analysis results regarding immune cell type and density are more
valuable in predicting clinical outcomes than TNM staging (47–50).
It has been reported that CD4+ T-cell and
CD8+ T-cell infiltration is enhanced in NSCLC, and that
these two immune cell types are associated with prolonged patient
survival (46,51). CTLA-4 and PD-1/PD-L1 inhibitors are
common immune checkpoint inhibitors that can markedly prolong the
survival of patients with cancer. A previous study showed that the
number of CD4+ T cells in the blood of patients with
NSCLC was associated with the clinical effect of immune checkpoint
inhibitors (52). Th1 and Th2
cells are common subsets of CD4 T cells. Frafjord et al
(53) demonstrated that in NSCLC,
the number of Th2 cells is increased in the tumor matrix and tumor
epithelium. By contrast, Th1 cells are mainly enriched in distal
normal lung tissue. In the present study, the association between
PPM1G expression, the degree of immune cell infiltration and immune
checkpoints in LUAD was evaluated. The results revealed that the
increased expression levels of PPM1G were positively associated
with the infiltration level of Th2 cells. By contrast, PPM1G
expression was negatively associated with the infiltration level of
T cells, CD8+ T cells and Th1 cells. These findings
indicated that there are different degrees of correlation between
the expression of PPM1G and immune cell infiltration.
Furthermore, the correlation between PPM1G
expression and immune checkpoints in LUAD was analyzed. It has been
reported that chemokines/chemokine receptors serve a significant
role in directional immune cell migration (54–56).
In the present study, TISIDB was used to analyze the association
between PPM1G expression and chemokines/chemokine receptors in
LUAD. The results revealed that PPM1G expression was negatively
associated with that of multiple chemokines/chemokine receptors,
and was positively associated with those of PD-1/PD-L1 and CTLA-4
in LUAD. This finding suggested that PPM1G upregulation could be
associated with the occurrence and progression of LUAD and may have
a critical role in inhibiting immune cell migration to TME.
In conclusion, the bioinformatics analysis was used
to extract data from a variety of online databases and strict
statistical methods were used to analyze and verify the results,
which indicated that PPM1G may have prognostic potential and
diagnostic value in LUAD. It was further inferred that PPM1G has
the potential as a novel prognostic biomarker and therapeutic
target in patients with LUAD.
The present study filled the gap of the effect of
PPM1G in LUAD, provided a certain research basis for further
verifying the prognostic and immunological potential of PPM1G in
lung adenocarcinoma through in vivo or in vitro
studies, and provided ideas for exploring novel targeted therapy
and immunotherapy to treat lung cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Shandong Natural Science
Fund of Shandong Province (grant no. ZR2020MH080), The Projects of
Medical and Health Technology Development Program in Shandong
Province (grant no. 2019WS310), the Clinical Research Fund of
Shandong Medical Association (grant no. YXH2022ZX033), and the
Shandong Province Traditional Chinese Medicine Science and
Technology Project (grant no. M-2022234).
Availability of data and materials
The datasets used and/or analyzed during the
current study available from the corresponding author on reasonable
request.
Authors' contributions
RY performed the bioinformatics analysis, wrote and
revised the manuscript. LQ performed analysis and wrote the
manuscript. ZW, JT, HG, XW and DY analyzed the data. PD and MD
performed the experiments and drafted and edited the manuscript. RY
and MD confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The current study was approved by the Scientific
Research Ethics Committee of Yantai Affiliated Hospital of Binzhou
Medical University (Yantai, China; protocol no. 20220215001). The
patients provided written informed consent to participate in this
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang X, Chen X and Liu H: Expression and
bioinformatics-based functional analysis of UAP1 in lung
adenocarcinoma. Cancer Manag Res. 12:12111–12121. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cho JH: Immunotherapy for non-small-cell
lung cancer: Current status and future obstacles. Immune Netw.
17:378–391. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O'Brien TD, Jia P, Caporaso NE, Landi MT
and Zhao Z: Weak sharing of genetic association signals in three
lung cancer subtypes: Evidence at the SNP, gene, regulation, and
pathway levels. Genome Med. 10:162018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Friedlaender A, Banna G, Malapelle U,
Pisapia P and Addeo A: Next generation sequencing and genetic
alterations in squamous cell lung carcinoma: Where are we today?
Front Oncol. 9:1662019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
AbdulJabbar K, Raza SEA, Rosenthal R,
Jamal-Hanjani M, Veeriah S, Akarca A, Lund T, Moore DA, Salgado R,
Al Bakir M, et al: Geospatial immune variability illuminates
differential evolution of lung adenocarcinoma. Nat Med.
26:1054–1062. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Avancini A, Sartori G, Gkountakos A,
Casali M, Trestini I, Tregnago D, Bria E, Jones LW, Milella M,
Lanza M and Pilotto S: Physical activity and exercise in lung
cancer care: Will promises be fulfilled? Oncologist. 25:e555–e569.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kamada R, Kudoh F, Ito S, Tani I, Janairo
JIB, Omichinski JG and Sakaguchi K: Metal-dependent Ser/Thr protein
phosphatase PPM family: Evolution, structures, diseases and
inhibitors. Pharmacol Ther. 215:1076222020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin YR, Yang WJ and Yang GW: Prognostic
and immunological potential of PPM1G in hepatocellular carcinoma.
Aging (Albany NY). 13:12929–12954. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang M, Xu E, Zhang J and Chen X: PPM1D
phosphatase, a target of p53 and RBM38 RNA-binding protein,
inhibits p53 mRNA translation via dephosphorylation of RBM38.
Oncogene. 34:5900–5911. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peng TS, He YH, Nie T, Hu XD, Lu HY, Yi J,
Shuai YF and Luo M: PPM1D is a prognostic marker and therapeutic
target in colorectal cancer. Exp Ther Med. 8:430–434. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li K, Liu Y, Xu S and Wang J: PPM1D
functions as oncogene and is associated with poor prognosis in
esophageal squamous cell carcinoma. Pathol Oncol Res. 26:387–395.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang C, Chen Y, Wang M, Chen X, Li Y,
Song E, Liu X, Kim S and Peng H: PPM1D silencing by RNA
interference inhibits the proliferation of lung cancer cells. World
J Surg Oncol. 12:2582014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang H, Gao XY, Li P and Jiang TS: PPM1D
overexpression predicts poor prognosis in non-small cell lung
cancer. Tumour Biol. 36:2179–2184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun C, Wang G, Wrighton KH, Lin H,
Songyang Z, Feng XH and Lin X: Regulation of p27Kip1
phosphorylation and G1 cell cycle progression by protein
phosphatase PPM1G. Am J Cancer Res. 6:2207–2220. 2016.PubMed/NCBI
|
|
17
|
Chen D, Zhao Z, Chen L, Li Q, Zou J and
Liu S: PPM1G promotes the progression of hepatocellular carcinoma
via phosphorylation regulation of alternative splicing protein
SRSF3. Cell Death Dis. 12:7222021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Di C, Syafrizayanti, Zhang Q, Chen Y, Wang
Y, Zhang X, Liu Y, Sun C, Zhang H and Hoheisel JD: Function,
clinical application, and strategies of Pre-mRNA splicing in
cancer. Cell Death Differ. 26:1181–1194. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vasaikar SV, Straub P, Wang J and Zhang B:
LinkedOmics: Analyzing multi-omics data within and across 32 cancer
types. Nucleic Acids Res. 46(D1): D956–D963. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pachter L: Models for transcript
quantification from RNA-Seq. arXiv preprint arXiv:11043889.
2011.
|
|
21
|
Rousseaux S, Debernardi A, Jacquiau B,
Vitte AL, Vesin A, Nagy-Mignotte H, Moro-Sibilot D, Brichon PY,
Lantuejoul S, Hainaut P, et al: Ectopic activation of germline and
placental genes identifies aggressive metastasis-prone lung
cancers. Sci Transl Med. 5:186ra1662013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moreno Leon L, Gautier M, Allan R, Ilié M,
Nottet N, Pons N, Paquet A, Lebrigand K, Truchi M, Fassy J, et al:
The nuclear hypoxia-regulated NLUCAT1 long non-coding RNA
contributes to an aggressive phenotype in lung adenocarcinoma
through regulation of oxidative stress. Oncogene. 38:7146–7165.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Landi MT, Dracheva T, Rotunno M, Figueroa
JD, Liu H, Dasgupta A, Mann FE, Fukuoka J, Hames M, Bergen AW, et
al: Gene expression signature of cigarette smoking and its role in
lung adenocarcinoma development and survival. PLoS One.
3:e16512008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu
JS, Li B and Liu XS: TIMER: A web server for comprehensive analysis
of tumor-infiltrating immune cells. Cancer Res. 77:e108–e110. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stockhausen K: The declaration of
Helsinki: Revising ethical research guidelines for the 21st
century. Med J Aust. 172:252–253. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lánczky A, Nagy Á, Bottai G, Munkácsy G,
Szabó A, Santarpia L and Győrffy B: miRpower: A web-tool to
validate survival-associated miRNAs utilizing expression data from
2178 breast cancer patients. Breast Cancer Res. 160:439–446. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kanehisa M, Furumichi M, Tanabe M, Sato Y
and Morishima K: KEGG: New perspectives on genomes, pathways,
diseases and drugs. Nucleic Acids Res. 45(D1): D353–D361. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang J, Liu W, Li JC, Li M, Li B and Zhu
R: Hepcidin downregulation correlates with disease aggressiveness
and immune infiltration in liver cancers. Front Oncol.
11:7147562021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43((Database Issue)): D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Prates L, Lemes RB, Hünemeier T and
Leonardi F: Population-based change-point detection for the
identification of homozygosity islands. Bioinformatics.
39:btad1702023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wickham H: ggplot2: Elegant graphics for
data analysis. New York, NY: Springer; 2009, View Article : Google Scholar
|
|
35
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Darvin P, Toor SM, Sasidharan Nair V and
Elkord E: Immune checkpoint inhibitors: Recent progress and
potential biomarkers. Exp Mol Med. 50:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ge MX, Liu HT, Zhang N, Niu WX, Lu ZN, Bao
YY, Huang R, Yu DK, Shao RG and He HW: Costunolide represses
hepatic fibrosis through WW domain-containing protein 2-mediated
Notch3 degradation. Br J Pharmacol. 177:372–387. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pan C, Liu HD, Gong Z, Yu X, Hou XB, Xie
DD, Zhu XB, Li HW, Tang JY, Xu YF, et al: Cadmium is a potent
inhibitor of PPM phosphatases and targets the M1 binding site. Sci
Rep. 3:23332013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chaudhary N and Maddika S: WWP2-WWP1
ubiquitin ligase complex coordinated by PPM1G maintains the balance
between cellular p73 and ΔNp73 levels. Mol Cell Biol. 34:3754–3764.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xiao Q, Cheng Z, Kuang W, Wu H, Luo X and
Wang R: Clinical value of PPM1G gene in survival prognosis and
immune infiltration of hepatocellular carcinoma. Appl Bionics
Biomech. 2022:89262212022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen J, Li J, Sun H, Hu T, Wang Y, Kang G,
Cao M and Li X: PPM1G promotes the progression of lung
adenocarcinoma by inhibiting p38 activation via dephosphorylation
of MEK6. Carcinogenesis. 44:93–104. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gudipaty SA, McNamara RP, Morton EL and
D'Orso I: PPM1G binds 7SK RNA and hexim1 To block P-TEFb assembly
into the 7SK snRNP and sustain transcription elongation. Mol Cell
Biol. 35:3810–3828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kumar P, Tathe P, Chaudhary N and Maddika
S: PPM1G forms a PPP-type phosphatase holoenzyme with B56δ that
maintains adherens junction integrity. EMBO Rep. 20:e469652019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dyer DP, Medina-Ruiz L, Bartolini R,
Schuette F, Hughes CE, Pallas K, Vidler F, Macleod MKL, Kelly CJ,
Lee KM, et al: Chemokine receptor redundancy and specificity are
context dependent. Immunity. 50:378–389.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang M, Chang M, Li C, Chen Q, Hou Z, Xing
B and Lin J: Tumor-microenvironment-activated reactive oxygen
species amplifier for enzymatic cascade cancer
starvation/chemodynamic /immunotherapy. Adv Mater. 34:e21060102022.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Stankovic B, Bjørhovde HAK, Skarshaug R,
Aamodt H, Frafjord A, Müller E, Hammarström C, Beraki K, Bækkevold
ES, Woldbæk PR, et al: Immune cell composition in human non-small
cell lung cancer. Front Immunol. 9:31012019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Welsh TJ, Green RH, Richardson D, Waller
DA, O'Byrne KJ and Bradding P: Macrophage and mast-cell invasion of
tumor cell islets confers a marked survival advantage in
non-small-cell lung cancer. J Clin Oncol. 23:8959–8967. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kilic A, Landreneau RJ, Luketich JD,
Pennathur A and Schuchert MJ: Density of tumor-infiltrating
lymphocytes correlates with disease recurrence and survival in
patients with large non-small-cell lung cancer tumors. J Surg Res.
167:207–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen X, Wan J, Liu J, Xie W, Diao X, Xu J,
Zhu B and Chen Z: Increased IL-17-producing cells correlate with
poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer.
69:348–354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Horne ZD, Jack R, Gray ZT, Siegfried JM,
Wilson DO, Yousem SA, Nason KS, Landreneau RJ, Luketich JD and
Schuchert MJ: Increased levels of tumor-infiltrating lymphocytes
are associated with improved recurrence-free survival in stage 1A
non-small-cell lung cancer. J Surg Res. 171:1–5. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Al-Shibli KI, Donnem T, Al-Saad S, Persson
M, Bremnes RM and Busund LT: Prognostic effect of epithelial and
stromal lymphocyte infiltration in non-small cell lung cancer. Clin
Cancer Res. 14:5220–5227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zuazo M, Arasanz H, Fernández-Hinojal G,
García-Granda MJ, Gato M, Bocanegra A, Martínez M, Hernández B,
Teijeira L, Morilla I, et al: Functional systemic CD4 immunity is
required for clinical responses to PD-L1/PD-1 blockade therapy.
EMBO Mol Med. 11:e102932019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Frafjord A, Buer L, Hammarström C, Aamodt
H, Woldbæk PR, Brustugun OT, Helland Å, Øynebråten I and Corthay A:
The immune landscape of human primary lung tumors Is Th2 skewed.
Front Immunol. 12:7645962021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Phua SC, Chiba S, Suzuki M, Su E, Roberson
EC, Pusapati GV, Schurmans S, Setou M, Rohatgi R, Reiter JF, et al:
Dynamic remodeling of membrane composition drives cell cycle
through primary cilia excision. Cell. 178:2612019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kanemitsu N, Ebisuno Y, Tanaka T, Otani K,
Hayasaka H, Kaisho T, Akira S, Katagiri K, Kinashi T, Fujita N, et
al: CXCL13 is an arrest chemokine for B cells in high endothelial
venules. Blood. 106:2613–2618. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Pallandre JR, Krzewski K, Bedel R, Ryffel
B, Caignard A, Rohrlich PS, Pivot X, Tiberghien P, Zitvogel L,
Strominger JL and Borg C: Dendritic cell and natural killer cell
cross-talk: A pivotal role of CX3CL1 in NK cytoskeleton
organization and activation. Blood. 112:4420–4424. 2008. View Article : Google Scholar : PubMed/NCBI
|