Introduction
The genus Rhus (family Anacardiaceae) is
composed of >250 species of flowering plants, which are widely
distributed in temperate and tropical regions, including Saudi
Arabia (1). Globally, several
Rhus spp. are traditionally known for their medicinal value
(2,3). While Rhus tripartita has been
used to treat inflammatory, cardiovascular and gastrointestinal
diseases (4–6), R. glabra is known for its
anti-microbial activities (7) and
R. coriaria for its wound healing capacity (8). In addition, previous studies on R.
tripartita, R. verniciflua and R. retinorrhoea
identified several pharmacologically significant flavonoids and
anthocyanins (3,9–15).
Notably, three Rhus spp., R. abyssinica, R.
retinorrhoea and R. tripartita growing in Saudi Arabia
have been documented (16). It has
been reported that R. retinorrhoea exhibits anti-oxidative
(17), as well as weak
anti-malarial effects (11).
Further phytochemical analyses identified several other compounds,
such as persicogenin, velutin (VEL), trihydroxy-7-methoxyflavanone
and homoeriodictyol (18), as well
as di-O-methyltetrahydroamentoflavone,
7-O-methylnaringenin, 7,3′-O-dimethylquercetin,
7-O-methylapigenin and 7-O-methylluteolin (11). A previous study from our laboratory
reported the identification of SEK in aerial parts of R.
retinorrhoea by quantitative high-performance thin layer
chromatograph (19).
Liver diseases constitute a major public health
problem. Therefore, the use of herbal or plant products to treat
hepatic disorders has gained increasing attention in phytomedicine
(20). Globally, liver infection
by hepatitis B virus (HBV), which may progress to cirrhosis and
hepatocellular carcinoma, can cause fulminant and chronic
conditions in >350 million individuals (21,22).
Despite the efficacy of the currently available anti-HBV drugs,
drug-resistance mediated by prolonged therapy with HBV polymerase
(POL) inhibitors, such as lamivudine (LAM), acyclovir and adefovir,
remains a clinical challenge (23). In view of this, several bioactive
phytochemicals of different classes, such as alkaloids, flavonoids,
polyphenols, lignans, terpenes and anthraquinones have been
identified as promising and non-resistant anti-HBV drug candidates
(24–30). Notably, a previous study showed
that R. coriaria could inhibit the production of HBV
proteins in cultured hepatocytes (31). In addition, robustaflavone derived
from R. succedanea (32)
and catechins derived from R. tripartite (33) could serve as potential inhibitors
of HBV activities in HBV-reporter HepG2.2.15 cells. Notably, to the
best of the authors' knowledge, the anti-HBV efficacy of R.
retinorrhoea or its phytoconstituents remain unknown.
Therefore, the present study aimed to evaluate the inhibitory
potential of the flavonoids SEK and VEL isolated from R.
retinorrhoea against HBV in HepG2.2.15 cells, supported by
structure-based molecular docking studies.
Materials and methods
Plant material collection
The aerial parts of R. retinorrhoea Steud, ex
Olive, locally known as ‘Sumac/Heishar’ were collected from the
southern region of Saudi Arabia in March 2009. The plant material
was authenticated (voucher specimen no. 15371) by Dr. Mohammad
Yusuf, a plant taxonomist at College of Pharmacy, King Saud
University Riyadh.
Extraction, fractionation and
isolation of compounds from R. retinorrhoea
The ethanolic extract of the aerial parts of the
plant was further fractionated in dichloromethane following the
isolation of several known or new compounds belonging to different
classes of phytochemicals. The majority of these were either
obtained in very low quantity or were unsuitable candidate for
testing against HBV. Based on available literature on their
structural similarity and non-cytotoxic flavonoids reported against
other viruses, two compounds were finally selected, namely C251 and
C253, as previously described (11,18).
For structure elucidation, 1H and 13C, and 2D
nuclear magnetic resonance (NMR) spectroscopy of C251 and C253 were
recorded at 700 and 175 MHz, respectively, on the Bruker Avance
spectrometer (Bruker BioSpin GmbH)equipped with a 5-mm cryoprobe,
in deuterated DMSO, using standard pulse programs. All organic
solvents were purchased from Sigma-Aldrich (Merck KGaA).
Cell culture and drugs
HepG2.2.15 cells, which were established by stably
transfecting human hepatoma HepG2 cells with the full genome of
HBV, were generously provided by Dr S. Jameel (Virology Group,
ICGEB, New Delhi, India). HepG2.2.15 cells were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific Inc.)
supplemented with 10% bovine serum albumin (Gibco; Thermo Fisher
Scientific, Inc.) and 1X penicillin-streptomycin solution (HyClone;
Cytiva) at 37°Cin an incubator with 5% CO2. Prior to
treatment, cells at a density of 0.5×105/100 µl/well
were grown overnight in a 96-well plate (Corning, Inc.). LAM
triphosphate (or 3TC) and quercetin (QRC; both from Sigma-Aldrich;
Merck KGaA) served as positive controls, as previously described
(26–30). For consistency and reproducibility,
the assays were performed in duplicate.
Liver cell viability or toxicity
assay
Although the Rhus spp., including R.
tripartite, are known to be non-toxic (2,3), the
optimal non-cytotoxic doses of the isolated compounds were first
assessed in HepG2.2.15 cells. Briefly, compounds dissolved in DMSO
(Sigma-Aldrich; Merck KGaA) were prepared in RPMI-1640 to produce
four doses with 6.25, 12.5, 25 and 50 µg/ml of each compound.
Following HepG2.2.15 cell incubation overnight, the culture medium
was replaced with treatment media (in triplicate), including
negative control (0.1% DMSO) medium, followed by incubation at 37°C
for 72 h. The cells were periodically monitored directly under an
inverted microscope. Subsequently, cells were treated with MTT
solution (TACS MTT Cell Proliferation Assay Kit; Sigma-Aldrich;
Merck KGaA), according to the manufacturer's instructions. The
optical density at a wavelength of 570 nm was measured using the
Elx800 microplate reader (BioTek Instruments, Inc.). The results
were analyzed in Excel 2010 (Microsoft Corp.) and presented in
relation to the negative control.
HBV surface or envelop protein (HBsAg)
inhibition assay
Initially, HBsAgs were dose-dependently inhibited
(6.25, 12.5 and 25 µg/ml) by the isolated compounds to determine
the maximally active concentration. HepG2.2.15 cells were cultured
overnight and the culture medium was then replaced with treatment
medium, including negative and positive control media, and
incubated for an additional two days (a single time-point).
Following the determination of the maximal dose, the time-dependent
inhibition of HBsAg by the compounds was then assessed. HepG2.2.15
cells were treated with 25 µg/ml SEK or VEL and the corresponding
controls, and incubated for several days. The culture was directly
monitored every day under microscope and it was replenished with
treatment media every alternate day. The culture supernatants
collected and clarified (150 × g; 5 min; 22°C) on day 1, 3 and 5
were quantitatively analyzed for HBsAgs using the diagnostic HBsAg
ELISA kit (cat. no. 72348; Monolisa HBs Ag ULTRA assay; Bio-Rad
Laboratories Inc.) in a microplate, according to the manufacturer's
protocol. The optical density of the samples at a wavelength of 450
nm was measured and the results were then analyzed in relation to
the negative control (Excel software 2010; Microsoft Corp.) and
compared with the positive control.
HBV pre-core protein (HBeAg)
inhibition assay
The treated culture supernatants collected and
clarified (150 × g; 5 min; 22°C) on day 1, 3 and day 5 were also
quantitatively analyzed for HBeAg production using a HBeAg ELISA
kit (cat. no. KAPG4BNE3; HBeAg/Anti-HBe Elisa Kit; DIAsource
ImmunoAssays SA) according to the manufacturer's instructions. The
recorded optical density (λ=450 nm) of the samples were analyzed in
relation to the negative control (Excel software 2010; Microsoft
Corp.), and compared with the positive control. All samples were
tested in triplicate and the experiment was repeated for two
times.
Molecular docking analysis
Based on their promising anti-HBV activities in
cultured cells, VEL and SAK were further subjected to virtual
structure-activity analysis to uncover the potential mechanisms
underlying their inhibitory effects. The viral POL and CORE
proteins served as target drugs, while their respective inhibitor
molecules LAM and heteroaryldihydropyrimidine (HAP) acted as
standard ligands (28,34). Notably, in the absence of
crystallographic data or 3D model for HBV POL, an in-house
constructed POL structure was used, as previously described
(28). The available 3D structures
of HBV CORE (PDB code, 5E0I; http://www.rcsb.org/) and the ligands LAM, VEL and SAK
(https://pubchem.ncbi.nlm.nih.gov/)
were retrieved. The target proteins were prepared by removing any
solvent molecules or co-crystallized ligands and via adding
hydrogen atoms and Kollman charges (28). For docking, the published catalytic
or active residues of LAM (28)
and CORE (34) were confirmed
using the SEINA program (35). The
two target proteins were prepared and energy-minimized in Maestro
software (36). The ligand-target
interactions were visualized using the 2D (Maestro) and 3D (UCSF
ChimeraX) modes (37). The ligands
were docked onto their corresponding target binding pocket or
active site using AutoDock Vina 1.2.3 software (38,39).
Statistical analysis
All data were analyzed using SPSS 17.0 (SPSS Inc.).
Data are expressed as the mean ± SEM of three independent
experiments. The results were compared with the negative control
group using one-way ANOVA followed by Dunnett's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Structure determination of the
compounds isolated from R. retinorrhoea
The two isolated compounds, 251 and 253, which were
subjected to 1H and 13C, and 2D NMR analyses
(Table I; Fig. S1, Fig. S2, Fig. S3, Fig. S4, Fig. S5, Fig. S6, Fig. S7, Fig. S8, Fig. S9, Fig. S10, Fig. S11, Fig. S12), were identified as the
structurally-related flavonoids SEK
(4′,5-dihydroxy-7-methoxyflavanone) and VEL
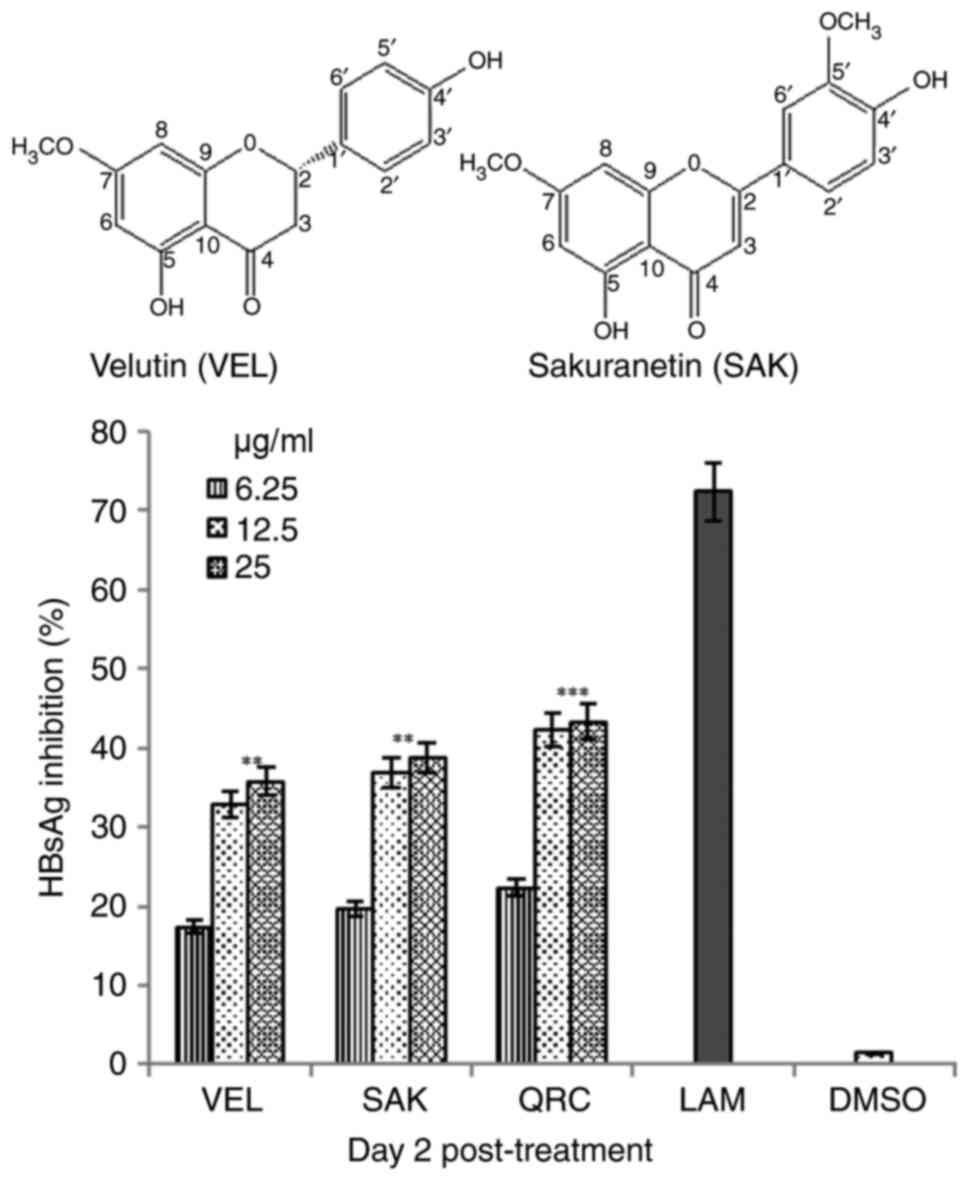
(5,4′-dihydroxy-7,3′-dimethoxyflavone), respectively (Fig. 1; upper panel).
 | Table I.The 1H and 13C
nuclear magnetic resonance spectroscopy data for the isolated
compounds C251 and C253 in deuterated DMSO. |
Table I.
The 1H and 13C
nuclear magnetic resonance spectroscopy data for the isolated
compounds C251 and C253 in deuterated DMSO.
|
| C251
(Sakuranetin) | C253 (Velutin) |
|---|
|
|
|
|
|---|
| Carbon no. | 1H(J in
Hz) | 13C | 1H(J in
Hz) | 13C |
|---|
| 2 | 5.51 dd
(2.8,2.8) | 79.1 | - | 164.3 |
| 3 | 2.7dd (2.8,
2.8) | 42.4 | 6.98 s | 103.8 |
|
| 2.53 (merge in
solvent peak) |
|
|
|
| 4 | - | 197.5 | - | 182.4 |
| 5 | - | 163.4 | - | 161.6 |
| 6 | 6.09 d (2.8) | 95.1 | 6.38 d (2.1) | 98.4 |
| 7 | - | 167.9 | - | 165.6 |
| 8 | 6.11 d (2.8) | 94.3 | 6.38 d (2.8) | 93.1 |
| 9 | - | 163.7 | - | 157.7 |
| 10 | - | 103.1 | - | 105.1 |
| 1′ | - | 129.2 | - | 121.8 |
| 2′ | 7.34 d (8.4) | 128.9 | 7.60 brs | 110.7 |
| 3′ | 6.81 d (8.4) | 115.6 | - | 148.5 |
| 4′ | - | 158.3 | - | 151.3 |
| 5, | 6.81 d (8.4) | 115.6 | 6.94d (8.4) | 116.2 |
| 6′ | 7.34 d (8.4) | 128.9 | 7.61d (2.1) | 120.9 |
| OCH3 | 3.79 s | 56.5 | 3.88 s | 56.5 |
| OCH3 | - | - | 3.91 s | 56.4 |
| 5-OH | 12.12 | - | 12.98 | - |
| 4′-OH | 9.61 | - | 10.01 | - |
Non-cytotoxic effects of SEK and
VEL
MTT assay demonstrated that the flavonoids SAK and
VEL did not show any hepatotoxicity in cells cultured for 72 h even
at the maximal dose tested (Fig.
S13). This was consistent with the microscopic observation of
cells, as treated cells exhibited intact morphology as with
negative cells. Therefore, the 50% cytotoxicity concentration
(CC50) values could not be determined.
SEK and VEL inhibit HBsAg
synthesis
Optimal dose assessment revealed that SAK and VEL at
a dose of 25 µg/ml showed the maximal inhibition of HBsAg on day 2
(Fig. 1; lower panel). However, at
a dose of 50 µg/ml, no significant increase in the inhibitory
activities of VEL and SAK was observed (data not shown). Therefore,
a dose of 25 µg/ml was selected as the optimally active dose for
the time-course study. Among the three selected time-points (day 1,
3 and 5), the maximal inhibition rate of SAK and VEL on HBsAg
synthesis was ~58.8 and ~56.4%, respectively, on day 5 (Fig. 2). In comparison, LAM and QRC
inhibited HBsAg by ~86.4 and ~84.5%, respectively. Notably, since
cell treatment with flavonoids at the maximal dose also enhanced
cell proliferation and overgrowth-mediated apoptosis (data not
shown), the assay was carried out at day 5.
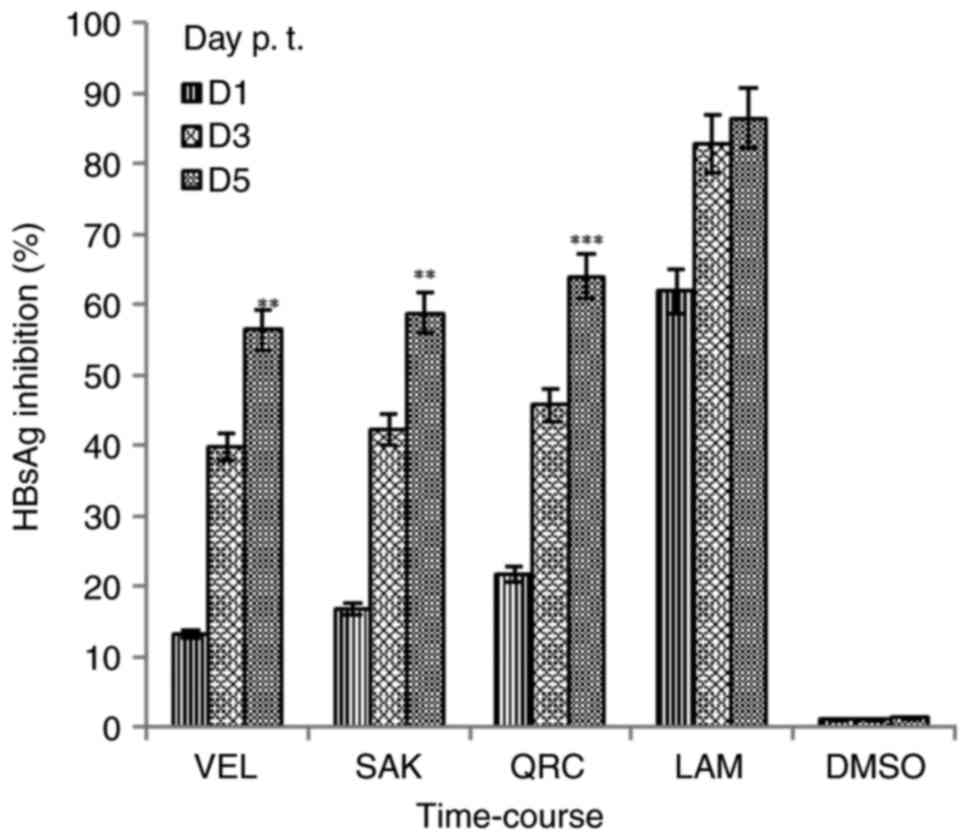 | Figure 2.Time-course inhibitions of HBV
surface or HBsAg by Rhus retinorrhoea-derived VEL and SAK at
the optimal selected dose (12.5 µg/ml, each) at day 1, 3 and 5 p.
t. in HepG2.2.15 cells. QRC; 12.5 µg/ml and LAM; 2 µM served as
positive controls while DMSO (0.1%) acted as negative or vehicle
control. Data are presented as the mean ± standard error of the
mean (n=3). **P<0.01, ***P<0.001 vs. LAM. HBV, hepatitis B
virus; sAg, ‘s’ antigen; VEL, velutin; SAK, sakuranetin; p. t.,
post-treatment; QRC, quercetin; LAM, lamivudine; D, day. |
SEK and VEL suppress HBV
replication
Synthesis of HBeAg is a serological gold marker of
HBV DNA replication in patients with HBV (21). Therefore, the inhibitory effect of
SEK and VEL (25 µg/ml, each) on HBeAg expression in treated
HepG2.2.15 cells was further analyzed. Of the analyzed time-points
(day 1, 3 and 5), the maximal inhibition rate in HBeAg production
was ~55.5% by SAK and ~52.4% by VEL on day 5 (Fig. 3). Comparatively, LAM and QRC
suppressed HBeAg generation by ~64 and ~62%, respectively. As
aforementioned, since flavonoids could promote cell overgrowth and
apoptotic death, the assay was performed on day 5.
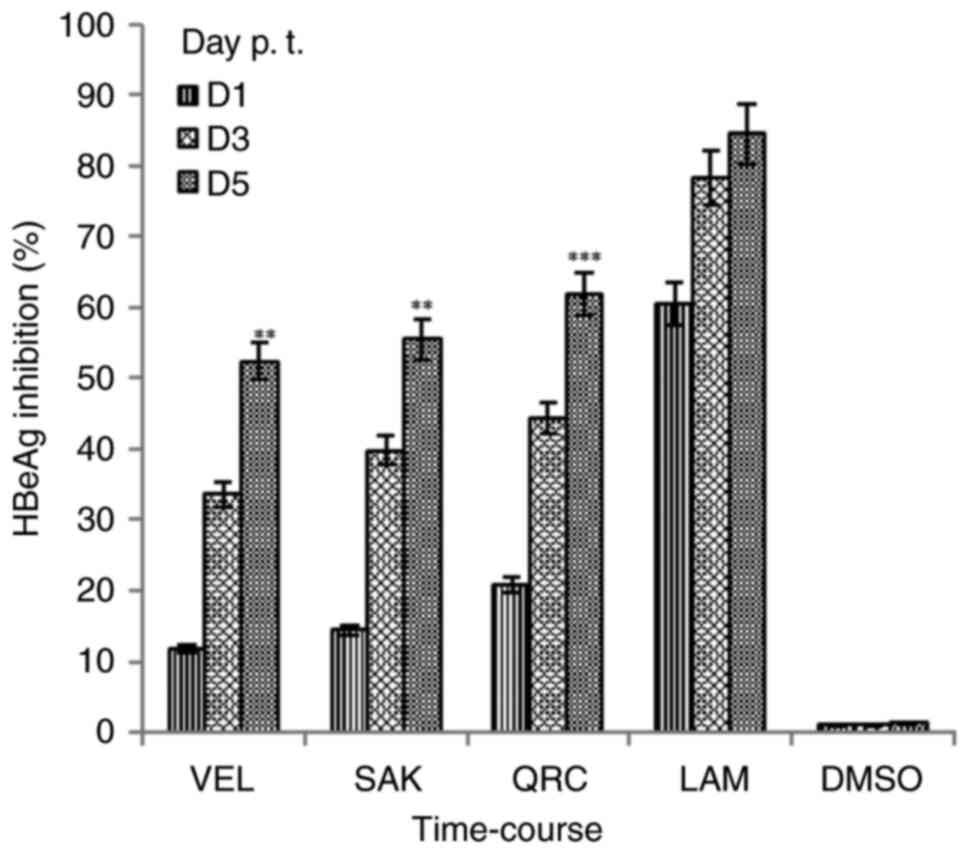 | Figure 3.Time-course inhibitions of HBV
pre-core or HBeAg by Rhus retinorrhoea-derived VEL and SAK
at the optimal selected dose (12.5 µg/ml, each) at day 1, 3 and 5
p. t. in HepG2.2.15 cells. QRC; 12.5 µg/ml and LAM; 2 µM served as
positive controls while DMSO (0.1%) acted as negative or vehicle
control. Data are presented as the mean ± standard error of the
mean (n=3). **P<0.01, ***P<0.001 vs. LAM. HBV, hepatitis B
virus; eAg, ‘e’ antigen; VEL, velutin; SAK, sakuranetin; p. t.,
post-treatment; QRC, quercetin; LAM, lamivudine; D, day. |
Structure-based interactions of the
isolated flavonoids with HBV proteins
The two isolated anti-HBV active flavonoids, VEL and
SAK, were virtually docked into the binding pocket of POL and CORE
proteins. The results revealed good re-alignments of the ligands.
Docking of LAM and HAP generated complexes with good docking
energies and orientations, thus indicating a good docking protocol
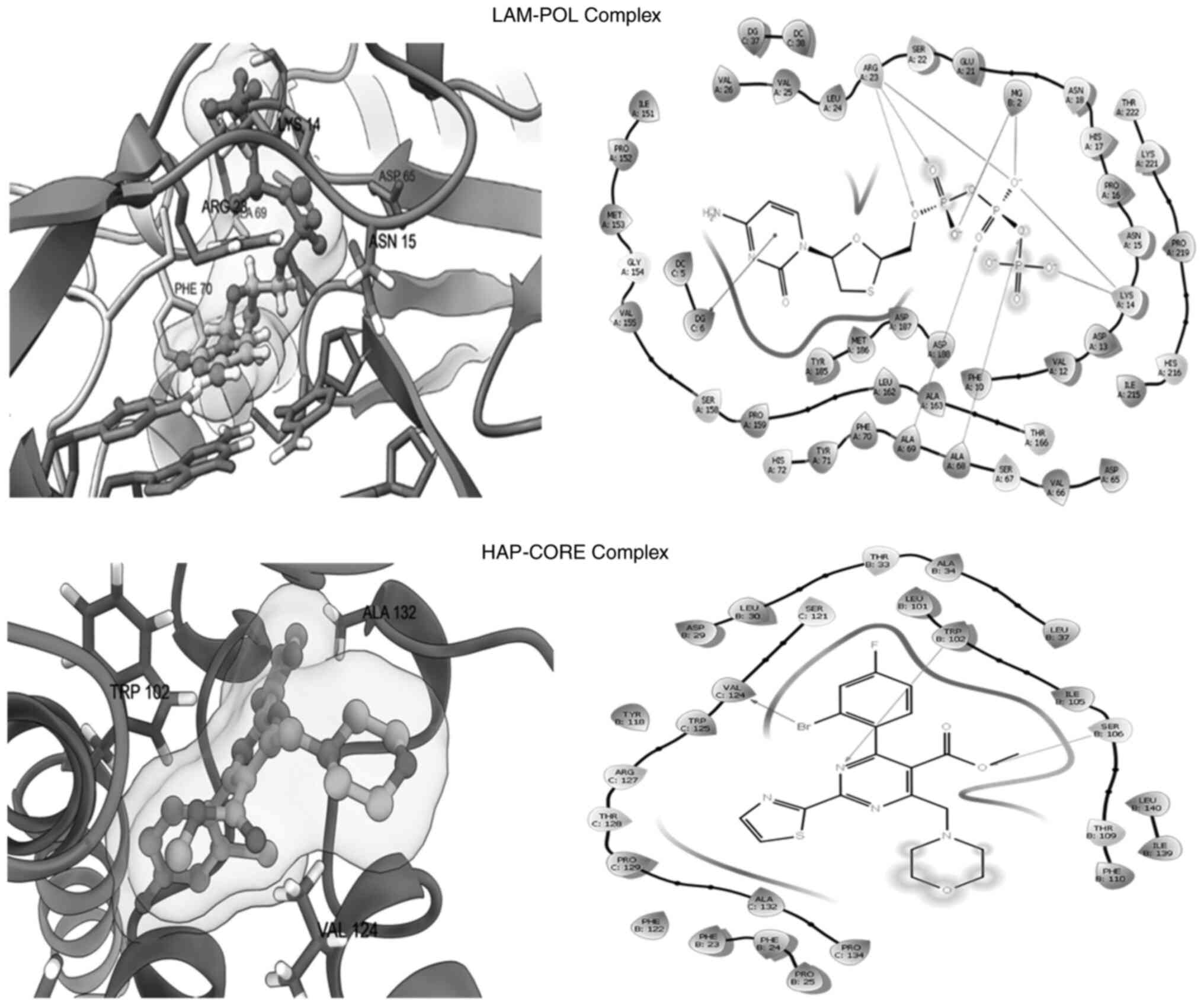
(Fig. 4; Table II). Owing to their common
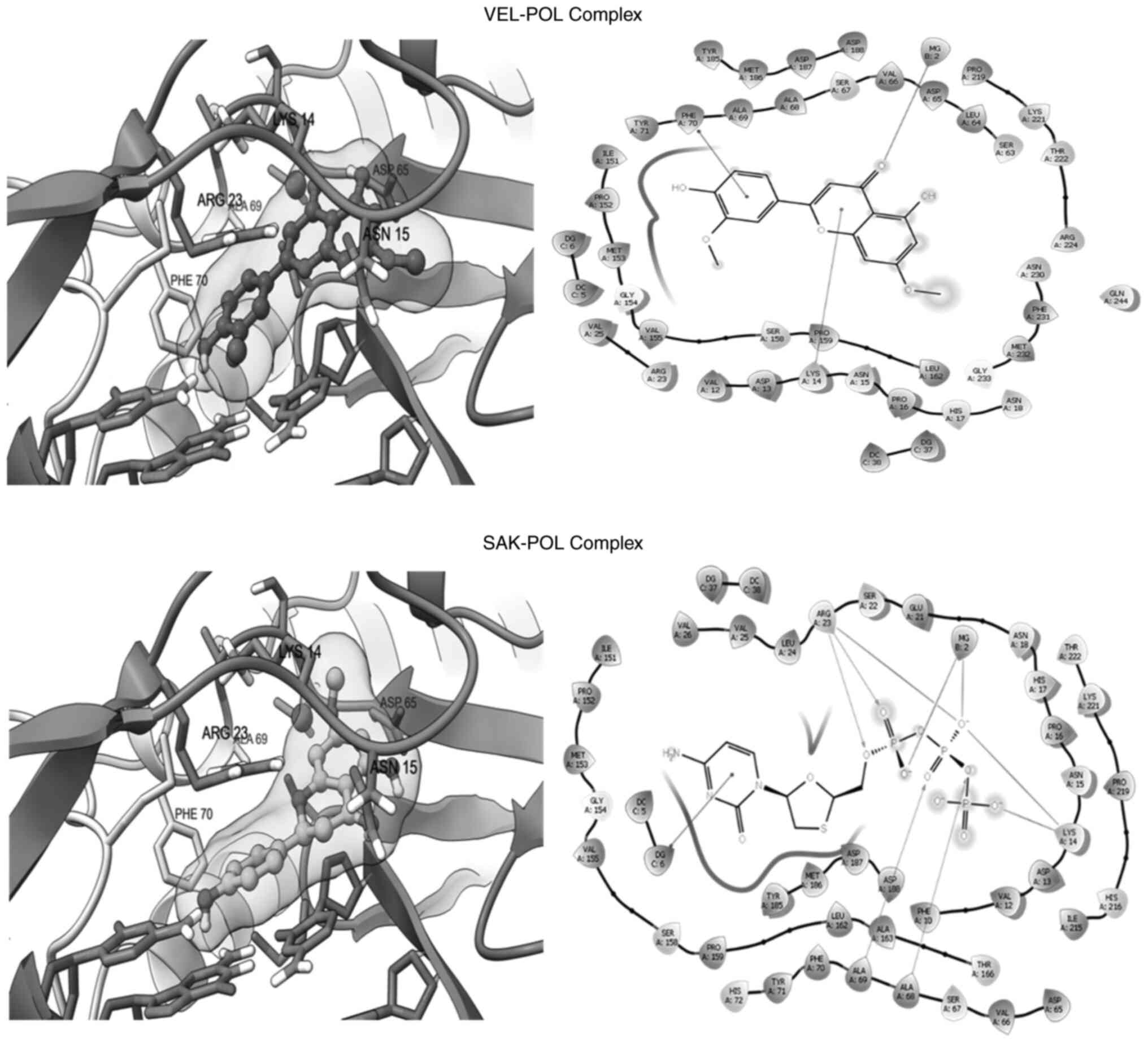
flavonoid structure, VEL and SEK acquired relatively similar
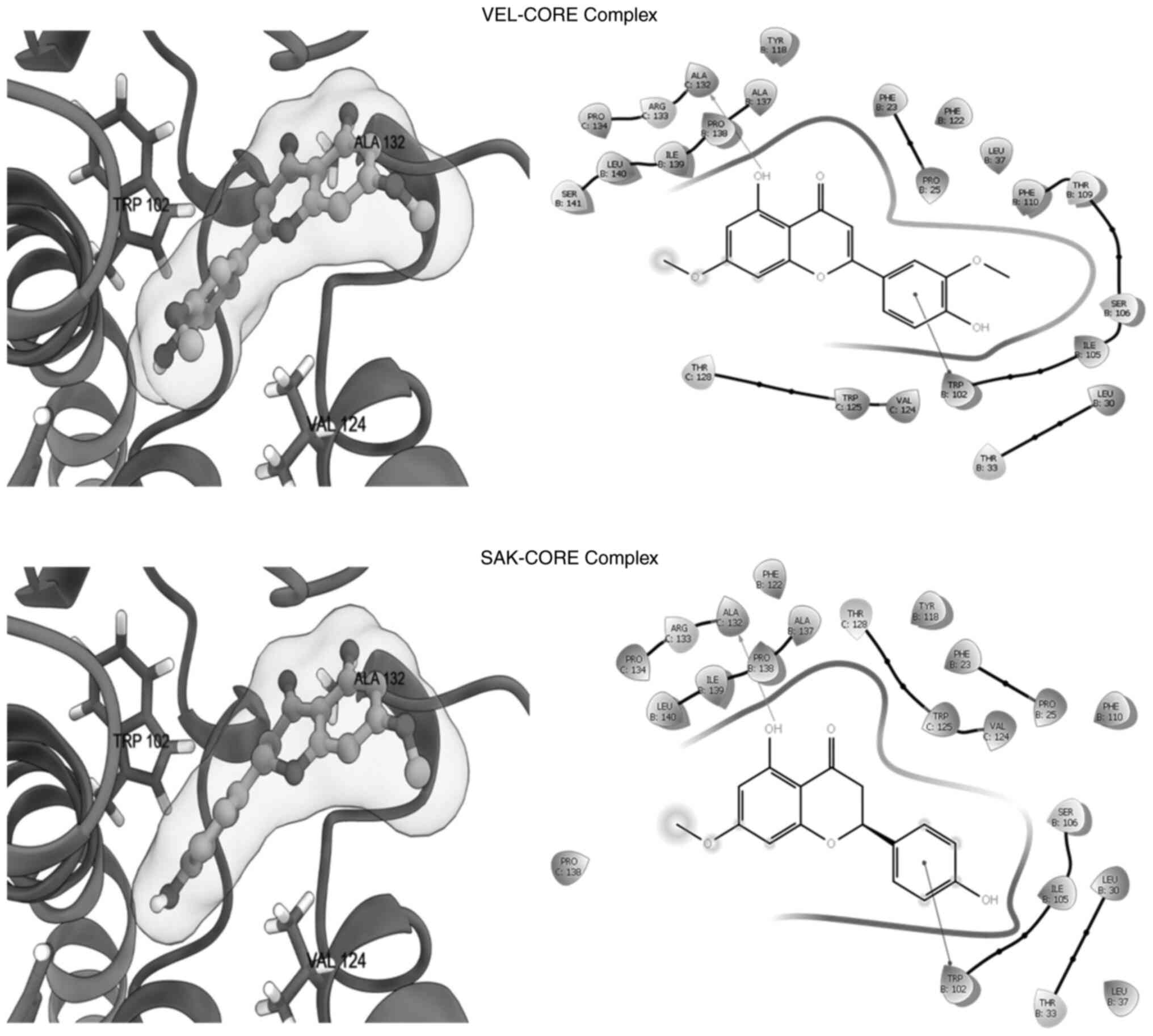
alignment and orientations inside the binding site of POL (Fig. 5; Table II) and CORE (Fig. 6; Table II). In addition, both flavonoids
shared interactions with key active residues of the target
proteins. Notably, similar to the negative charges of the
triphosphate group, which significantly interacted with positive
charged residues at Arg23 and Lys14 in the LAM-POL complex, and in
coordination with Mg+2 (Fig. 4; upper panel), the oxygen atoms of
VEL and SEK showed the same interactions in the VEL-POL and SAK-POL
complexes, respectively (Fig. 5).
The VEL-POL complex was further stabilized by π-cation with Lys14
and π-stacking with Phe70 (Fig. 5;
upper panel). In addition to the POL catalytic ‘Tyr-Met-Asp-Asp’
motif residues, other surrounding residues, such as those at Ser67
and Ala68, could also be involved in the stability of the VEL-POL
and SAK-POL complexes. Nonetheless, LAM (standard) showed a more
potent binding affinity compared with both VEL and SAK, which could
be due to its more efficient electrostatic interactions (Table II).
 | Table II.Estimated docking energies (kcal
M−1) of anti-hepatitis B virus active flavonoids and
astandards. |
Table II.
Estimated docking energies (kcal
M−1) of anti-hepatitis B virus active flavonoids and
astandards.
|
| Hepatitis B virus
target proteins |
|---|
|
|
|
|---|
| Ligands | Polymerase | Capsid |
|---|
| Velutin | −8.092 | −9.079 |
| Sakuranetin | −7.502 | −8.526 |
| Lamuvidine
triphosphatea | −9.245 |
|
|
Heteroaryldihydropyrimidinea |
| −8.876 |
Regarding docking with the HBV-CORE protein, both
VEL and SAK formed complexes with very close poses (Fig. 6), while VEL displayed a higher
binding affinity compared with SEK (Table II). The two ligands shared
H-bonding with Ala132 and π-stacking with Trp102. Notably, HAP
(standard) could also interact with Trp102 through H-bonds. Other
surrounding residues, such as those at Val124 and Ser106 could also
contribute to the VEL-HAP and SAK-HAP complex stabilities. Taken
together, the molecular docking data suggested that the activities
of VEL and SEK against HBV could be mediated by the inhibition of
the viral POL and CORE proteins.
Discussion
Flavonoids are a class of phytochemical polyphenols,
which are further subclassified into flavonols, flavanones,
flavones, chalcones, anthocyanidins and catechins (40). In addition to their known health
benefits, it has been reported that several flavonoids exhibit
therapeutic potentials against several viruses (41,42),
including HBV (24,25,27–30).
In the current study, the anti-HBV activities of the two
structurally-similar R. retinorrhoea-derived flavonoids,
namely SEK and VEL, were evaluated using a HBV-reporter cell
culture model. Notably, since several therapeutic plant products or
isolated compounds can cause liver toxicity (43), prior to anti-HBV assays, both SEK
and VEL were first assessed for hepatotoxicity.
SEK is one of the best characterized and most
studied flavanones, which is also the derivative of naringenin. In
SEK, the hydroxy group at C7 is swapped by a methoxy group
(44). It has been reported that
SEK has several pharmacological properties, including antioxidant,
anti-inflammatory and chemopreventive activities (44–49).
Notably, a study revealed that SEK derived from Sorbus
commixta exerted a marked activity against influenza B virus
(IBV) in MDCK cells, at the non-cytotoxic concentration of 100
µg/ml (50). Additionally, a dose
of 100 µg/ml SEK isolated from S. commixta could inhibit the
replication of human rhinovirus (RV3) in cultured HeLa cells, with
no cytotoxicity (51). Consistent
with the above findings, the results showed that the optimal
concentration of R. retinorrhoea-derived SEK against HBV
activity was at 12.5 µg/ml, which was comparatively 1/8 of that
used against IBV and RV3.
VEL is a dimethoxyflavone, where the hydroxy groups
at C7 and C3′ are swapped by methoxy groups (52). VEL has several pharmacological
activities, such as antioxidant, anti-allergic, anti-inflammatory
and anti-microbial properties (52,53).
A previous study demonstrated that VEL derived from marine seaweeds
displayed enhanced anti-microbial and anti-protozoal activities
in vitro (54). To the best
of the authors' knowledge, there is currently no published data on
the antiviral activity of VEL. However, a previous in silico
study suggested that mushroom-derived VEL could significantly
inhibit the main protease of SARS-CoV-2 (55). The present study demonstrated that
the optimal inhibitory activity of R. retinorrhoea-derived
VEL against HBV was at 12.5 µg/ml, which was comparatively 1/8 of
the structurally-similar SEK, which was used against IBV and
RV3.
Structure-based docking is a widely used
computational tool in drug research. It is most commonly applied to
more accurately predict how a small molecule could interact with a
macromolecule to form a stable complex via evaluating their
potential energies through a scoring tool. To further uncover the
supportive mechanism of the in vitro observed anti-HBV
activities of VEL and SEK, the aforementioned flavonoids were
docked against viral POL and CORE. The HBV non-structural protein
POL remains the most favored antiviral target. It has been reported
that its inhibition can block its DNA replication (23). By contrast, the HBV CORE protein, a
structural protein that has been recently emerged as a potential
anti-HBV target, can destabilize or disrupt the formation of viral
nucleocapsid (34). In the present
study, both VEL and SEK formed stable complexes with HBV POL, as
well as with CORE, with very good docking scores. Notably, owing to
their structural similarity, both flavonoids exhibited very similar
alignments and orientations inside the active sites of the HBV
target proteins. Blocking or inhibition of HBV POL activity leads
to downregulation or cessation of viral sub-genomic (sg)RNA and
mRNA transcriptions resulting in suppressions of HBV proteins
syntheses. Therefore, in HBV infected individuals or polymerase
inhibitor-treated patients, serological test (quantitative) for
HBsAg and HBeAg levels is a routine and ‘indirect’ diagnostic
method to monitor HBV replication. Further, both the CORE (HBcAg)
and pre-core (HBeAg) proteins are synthesized from a common
bicistronic mRNA, and its downregulated transcription due to
‘direct-acting’ polymerase-inhibitors leads to ‘indirect’
inhibitions of HBcAg and HBeAg production. In addition, there are
limited anti-HBV molecules (e.g., HAP) that ‘directly’ interfere
with CORE assembly and capsid formation with HBV DNA in
experimental settings. This ‘direct’ interference leads to failing
to capsid maturation, virus morphogenesis and production of
infectious virions or HBV DNA replication. In view of this, the
‘indirect’ anti-HBV activity measured by suppressions of both HBsAg
and HBeAg in cell culture models is a well and universally accepted
assay for evaluating the ‘direct-acting’ POL or CORE inhibitors.
Since the two isolated flavonoids showed inhibitions of both HBsAg
and HBeAg in cell culture, the in silico molecular docking
was performed against viral polymerase or core proteins. The strong
binding of the flavonoids with both proteins further supports our
in vitro data, suggesting their possible mechanism of
antiviral activities. In conclusion, the in silico data of
the current study strongly endorsed the in vitro anti-HBV
activities of VEL and SEK.
Supplementary Material
Supporting Data
Acknowledgement
Not applicable.
Funding
The authors acknowledge the Researchers Supporting Project (no.
RSP2023R379), King Saud University, Riyadh, Saudi Arabia for
supporting this work.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SA collected and extracted the plant material,
isolated compounds and participated in structural analysis and
manuscript writing. MKP conceived, designed and supervised the
research, performed in vitro assays, collected and analyzed
data, and wrote the manuscript. MSA statistically analyzed the data
and participated in manuscript writing. MASA performed molecular
docking and data analysis. TAA and AJR participated in plant
collection, structural analysis of compounds, and manuscript
review. SA and MKP confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rayne S and Mazza G: Biological activities
of extracts from sumac (Rhus spp.): A review. Plant Foods Hum Nutr.
62:165–175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Wyk BE and Wink M: Medicinal plants of
the world. Portland, Oregon, USA: Timber Press; pp. pp4252004
|
|
3
|
Opiyo SA, Njoroge PW, Ndirangu EG and
Kuria KM: A review of biological activities and phytochemistry of
Rhus species. Am J Chem. 11:28–36. 2021.
|
|
4
|
Itidel C, Chokri M, Mohamed B and Yosr Z:
Antioxidant activity, total phenolic and flavonoid content
variation among Tunisian natural populations of Rhus
tripartite (Ucria) Grande and Rhus pentaphylla desf. Ind
Crops Prod. 51:171–177. 2013. View Article : Google Scholar
|
|
5
|
El-Mokasabi FM: The state of the art of
traditional herbal medicine in the eastern mediterranean coastal
region of Libya. Middle East J Sci Res. 21:575–582. 2014.
|
|
6
|
Shahat AA, Alsaid MS, Rafatullah S,
Al-Sohaibani MO, Parvez MK, Al-Dosari MS, Exarchou V and Pieters L:
Treatment with Rhus tripartita extract curtails
isoproterenol-elicited cardiotoxicity and oxidative stress in rats.
BMC Complement Altern Med. 16:3512016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Erichsen-Brown C: Medicinal and other uses
of North American plants: A historical survey with special
reference to the Eastern Indian Tribes. Mineola, New York, USA:
Dover Publications; 1989
|
|
8
|
Sezik E, Tabata M, Yeşilada E, Honda G,
Goto K and Ikeshiro Y: Traditional medicine in Turkey. I. Folk
medicine in northeast Anatolia. J Ethnopharmacol. 35:191–196. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jang JY, Shin H, Lim JW, Ahn JH, Jo YH,
Lee KY, Hwang BY, Jung SJ, Kang SY and Lee MK: Comparison of
antibacterial activity and phenolic constituents of bark, lignum,
leaves and fruit of Rhus verniciflua. PLoS One.
13:e02002572018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang SY, Kang JY and Oh MJ: Antiviral
activities of flavonoids isolated from the bark of Rhus
verniciflua stokes against fish pathogenic viruses in vitro. J
Microbiol. 50:293–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ahmed MS, Galal AM, Ross SA, Ferreira D,
ElSohly MA, Ibrahim AS, Mossa JS and El-Feraly FS: A weakly
antimalarial biflavanone from Rhus retinorrhoea.
Phytochemistry. 58:599–602. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mahjoub MA, Ammar S and Mighri Z: A new
biflavonoid and an isobiflavonoid from Rhus tripartitum. Nat
Prod Res. 19:723–729. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alimi H, Mbarki S, Barka ZB, Feriani A,
Bouoni Z, Hfaeidh N, Sakly M, Tebourbi O and Rhouma KB:
Phytochemical, antioxidant and protective effect of Rhus
tripartitum root bark extract against ethanol-induced ulcer in
rats. Gen Physiol Biophys. 32:115–127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mohammed AESI: Phytoconstituents and the
study of antioxidant, antimalarial and antimicrobial activities of
Rhus tripartite growing in Egypt. J Pharmacogn Phytochem.
4:276–281. 2015.
|
|
15
|
Alqahtani AS, Abdel-Mageed WM, Shahat AA,
Parvez MK, Al-Dosari MS, Malik A, Abdel-Kader MS and Alsaid MS:
Proanthocyanidins from the stem bark of Rhus tripartita
ameliorate methylgloxal-induced endothelial cell apoptosis. J Food
Drug Anal. 27:758–765. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Collenetle S: An illustrated guide to the
flowersof Saudi Arabia. Scorpion Publishing LTD; London: pp. 45–49.
1985
|
|
17
|
Mothana RA, Gruenert R, Bednarski PJ and
Lindequist U: Evaluation of the in vitro anticancer, antimicrobial
and antioxidant activities of some Yemeni plants used in folk
medicine. Pharmazie. 64:260–268. 2009.PubMed/NCBI
|
|
18
|
Mossa JS, Abdel Sattar E, Abou-Shoer M and
Galal AM: Free flavonoids from Rhus retinorrhoea steud, ex
olive. Int J Pharmacog. 34:198–201. 1996. View Article : Google Scholar
|
|
19
|
Alam P, Parvez MK, Arbab AH, Siddiqui NA,
Al-Dosary MS, Al-Rehaily AJ, Ahmed S, Kalam MA and Ahmad MS:
Inter-species comparative antioxidant assay and HPTLC analysis of
sakuranetin in the chloroform and ethanol extracts of aerial parts
of Rhus retinorrhoea and Rhus tripartita. Pharm Biol.
55:1450–1457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adewusi E and Afolayan AJ: A review of
natural products with hepatoprotective activity. J Med Plants Res.
4:1318–1334. 2010.
|
|
21
|
Tang LSY, Cover E, Wilson E and Kottilil
S: Chronic hepatitis B infection: A review. JAMA. 319:1802–1813.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
World Health Organisation, . Hepatitis B.
https://www.who.int/news-room/fact-sheets/detail/hepatitis-bFebruary
18–2023
|
|
23
|
Devi U and f Locarnini S, . Hepatitis B
antivirals and resistance. Curr Opin Virol. 3:495–500. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang G, Zhang L and Bonkovsky HL: Chinese
medicine for treatment of chronic hepatitis B. Chin J Integr Med.
18:253–255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Parvez MK, Arbab AH and Al-Dosari MS: An
update on natural or herbal drugs against hepatitis B virus. In
Hepatitis B: Diagnosis, Prevention and Treatment. NOVA Science
Publishers; USA: pp. 159–184. 2021
|
|
26
|
Parvez MK, Rehman MT, Alam P, Al-Dosari
MS, Alqasoumi SI and Alajmi MF: Plant-derived antiviral drugs as
novel hepatitis B virus inhibitors: Cell culture and molecular
docking study. Saudi Pharm J. 27:389–400. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Parvez MK, Al-Dosari MS, Alam P, Rehman
MT, Alajmi MF and Alqahtani AS: The anti-hepatitis B virus
therapeutic potential of anthraquinones derived from Aloe
vera. Phytother Res. 33:1960–1970. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Parvez MK, Al-Dosari MS, Arbab AH,
Al-Rehaily AJ and Abdelwahid MAS: Bioassay-guided isolation of
anti-hepatitis B virus flavonoid myricetin-3-O-rhamnoside along
with quercetin from Guiera senegalensis leaves. Saudi Pharm
J. 28:550–559. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Parvez MK, Ahmed S, Al-Dosari MS,
Abdelwahid MAS, Arbab AH, Al-Rehaily AJ and Al-Oqail MM: Novel
anti-hepatitis B virus activity of Euphorbia schimperi and
its quercetin and kaempferol derivatives. ACS Omega. 6:29100–29110.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ahmed S, Parvez MK, Zia K, Nur-e-Alam M,
Ul-Haq Z, Al-Dosari MS and Al-Rehaily AJ: Natural anti-hepatitis B
virus flavones isolated from Stachys schimperi Vatke growing
in Saudi Arabia. Pharmacog Mag. 18:386–392. 2022.
|
|
31
|
Gharabolagh AF, Sabahi F, Karimi M,
Kamalinejad M, Mirshahabi H, Dawood S, Nasab M and Ahmadi NA:
Effects of Rhus Coriaria L. (Sumac) extract on hepatitis B
virus replication and HBs Ag secretion. J Rep Pharm Sci. 7:100–107.
2018.
|
|
32
|
Zembower DE, Lin YM, Flavin MT, Chen FC
and Korba BE: Robustaflavone, a potential non-nucleoside
anti-hepatitis B agent. Antiviral Res. 39:81–88. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Parvez MK, Al-Dosari MS, Abdelwahid MAS,
Alqahtani AS and Alanzi AR: Novel anti-hepatitis B virus-active
catechin and epicatechin from Rhus tripartita. Exp Ther Med.
23:3982022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou Z, Hu T, Zhou X, Wildum S,
Garcia-Alcalde F, Xu Z, Wu D, Mao Y, Tian X, Zhou Y, et al:
Heteroaryldihydropyrimidine (HAP) and sulfamoylbenzamide (SBA)
inhibit hepatitis B virus replication by different molecular
mechanisms. Sci Rep. 7:423742017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bertoletti N, Chan AH, Schinazi RF, Yin YW
and Anderson KS: Structural insights into the recognition of
nucleoside reverse transcriptase inhibitors by HIV-1 reverse
transcriptase: First crystal structures with reverse transcriptase
and the active triphosphate forms of lamivudine and emtricitabine.
Protein Sci. 28:1664–1675. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bietz S and Rarey M: SIENA: Efficient
compilation of selective protein binding site ensembles. J Chem Inf
Model. 56:248–259. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maestro, . Schrödinger Release 2021-3.
Maestro, Schrödinger, LLC; New York, NY: 2021
|
|
38
|
Pettersen EF, Goddard TD, Huang CC, Meng
EC, Couch GS, Croll TI, Morris JH and Ferrin TE: UCSF ChimeraX:
Structure visualization for researchers, educators, and developers.
Protein Sci. 30:70–82. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Eberhardt J, Santos-Martins D, Tillack AF
and Forli S: AutoDock Vina 1.2.0: New docking methods, expanded
force field, and python bindings. J Chem Inf Model. 61:3891–3898.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hollman PCH: Absorption, bioavailability
and metabolism of flavonoids. Pharm Biol. 42:74–83. 2004.
View Article : Google Scholar
|
|
41
|
Badshah SL, Faisal S, Muhammad A, Poulson
BG, Emwas AH and Jaremko M: Antiviral activities of flavonoids.
Biomed Pharmacother. 140:1115962021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zakaryan H, Arabyan E, Oo A and Zandi K:
Flavonoids: Promising natural compounds against viral infections.
Arch Virol. 162:2539–2551. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Parvez MK and Rishi V: Herb-drug
interactions and hepatotoxicity. Curr Drug Metab. 20:275–282. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang L, Song J, Liu A, Xiao B, Li S, Wen
Z, Lu Y and Du G: Research progress of the antiviral bioactivities
of natural flavonoids. Nat Prod Bioprospect. 10:271–283. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stompor M: A review on sources and
pharmacological aspects of sakuranetin. Nutrients. 12:5132020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Soarse DG, Andreazza AC and Salvador M:
Evaluation of compounds with antioxidant activity in
Sachhromyces cerevisiae yeast cells. Rev Bras Cienc Farm.
41:95–100. 2005.
|
|
47
|
Zhang X, Hung TM, Phuong PT, Ngoc TM, Min
BS, Song KS, Seong YH and Bae K: Anti-inflammatory activity of
flavonoids from Populus davidiana. Arch Pharm Res.
29:1102–1108. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cruz MP, Andrade CM, Silva KO, de Souza
EP, Yatsuda R, Marques LM, David JP, David JM, Napimoga MH and
Clemente-Napimoga JT: Antinoceptive and anti-inflammatory
activities of the ethanol extract, fractions and flavones isolated
from Mimosa tenuiflora (Willd.) Poir (Leguminosae). PLoS One.
11:e01508392016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Charles C, Nachtergael A, Ouedraogo M,
Belayew A and Duez P: Effects of chemopreventive natural products
on non-homologous end-joining DNA double-strand break repair. Mutat
Res Genet Toxicol Environ Mutagen. 768:33–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kwon DH, Ji JH, Yim SH, Kim BS and Choi
HJ: Suppression of influenza B virus replication by sakuranetin and
mode of its action. Phytother Res. 32:2475–2479. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Choi HJ: In vitro antiviral activity of
sakuranetin against human rhinovirus 3. Osong Public Health Res
Perspect. 8:415–420. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kang J, Xie C, Li Z, Nagarajan S, Schauss
AG, Wu T and Wu X: Flavonoids from acai (Euterpe oleracea
Mart.) pulp and their antioxidant and anti-inflammatory activities.
Food Chem. 128:152–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xie CH, Kang J, Li ZM, Schauss AG, Badger
TM, Nagarajan S, Wu T and Wu XL: The açaí flavonoid velutin is a
potent anti-inflammatory agent: Blockade of LPS-mediated TNF-α and
IL-6 production through inhibiting NF-κB activation and MAPK
pathway. J Nutr Biochem. 23:1184–1191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hassan S, Hamed S, Almuhayawi M, Hozzein
W, Selim S and AbdElgawad H: Bioactivity of ellagic acid and
velutin: Two phenolic compounds isolated from marine algae. Egypt J
Botany. 16:219–231. 2012.
|
|
55
|
Rangsinth P, Sillapachaiyaporn C, Nilkhet
S, Tencomnao T, Ung AT and Chuchawankul S: Mushroom-derived
bioactive compounds potentially serve as the inhibitors of
SARS-CoV-2 main protease: An in silico approach. J Tradit
Complement Med. 11:158–172. 2021. View Article : Google Scholar : PubMed/NCBI
|