Introduction
The main cause of advanced renal disease is diabetic
nephropathy globally (1). Renal
failure caused by nephropathy involving end-stage diabetes is
increasingly becoming one of the principal causes of chronic renal
failure (2,3). As an important component of the
glomerular filtration barrier, renal podocytes is involved in the
progression of diabetic nephropathy (DN). Their damage is
associated with proteinuria and severe renal insufficiency
(4,5). DN is a metabolic disease mediated by
multiple risk factors, such as lipid metabolism disorder,
hyperglycemia, advanced glycosylation products and inflammatory
response (3). At present,
inflammatory diseases are considered to be immune-involved, due to
the comprehensive action of various factors, such as hemodynamics,
cytokines and growth factors caused by glucose metabolism disorder
(6,7).
Dapagliflozin is a relatively novel drug for the
treatment of diabetes that acts as a sodium-glucose cotransporter 2
inhibitor (SGLT2i), which can reduce the glucose absorption in
renal tubules, targeting the kidneys to delay the occurrence and
development of diabetic complications. It can also prevent renal
function damage and failure in diabetic patients (8,9). A
study reported that SGLT2 inhibitors (empagliflozin, canagliflozin
and dapagliflozin) generally lowered the risk of death from
transplant or dialysis complications (8). An observation report showed that the
hazard of a continuous decrease in glomerular filtration rate was
reduced by at least 50% among patients with chronic nephrosis
(9). As compared with the placebo
group, the incidence of terminal kidney disease and mortality rates
was significantly reduced in the dapagliflozin group (10). Other studies suggested that SGLT2
inhibitors may improve DN by inhibiting inflammatory response,
oxidative stress and autophagy (11). Therefore, SGLT2 inhibitors play an
important role in delaying DN (12,13).
Heme oxygenase 1 (HO-1) is a type of antioxidant
enzyme that can combat oxidative stress; it is widely distributed
in tissues and is more expressed in organs such as the liver,
spleen, kidney and heart (14,15).
Its metabolic process consumes oxygen (O2), in addition,
NADPH is used to provide hydrogen proton, which catalyzes heme
catabolism to produce Fe2+, biliverdin and carbon
monoxide (CO). The metabolism of the heme group is beneficial to
preventing oxidation. Biliverdin and the product of its metabolism,
bilirubin, not only have powerful antioxidant and anti-inflammatory
effects but can also effectively scavenge reactive oxygen species
activity to defend against peroxide, peroxynitrite, hydroxyl and
superoxide free radicals (14,16).
As a necessary endogenous gas messenger molecule, CO has also
anti-inflammatory, vasodilator and microcirculation metabolic roles
(16,17). A previous study reported that
dapagliflozin as an SGLT2i enhanced the expression of the nuclear
factor erythroid 2-related factor 2 (Nrf2)/HO-1 pathway and reduced
the levels of oxidative stress biomarkers, leading to amelioration
of colitis (18). Dapagliflozin
also increased HO-1 expression by increasing Nrf2 protein levels in
the rat brain of an Alzheimer's disease model (19).
Pyroptosis is a novel type of programmed cell death
during which some immunocompetent cells are activated by caspase-1
under the stimulation of pathogens and inflammatory agents, forming
pores in the cell membrane and releasing a large number of
inflammatory molecules to the external environment (20,21).
In addition, during pyroptosis, the nucleotide oligomerization
domain-like receptor thermal protein domain associated protein 3
(NLRP3) inflammasome can cleave caspase-1, causing a cascade
reaction and inducing renal inflammatory injury (22,23).
Previous studies showed that pyroptosis is widely involved in DN,
atherosclerosis, Alzheimer's disease and cardiovascular diseases
(24,25).
However, there are only a few studies on the effect
of dapagliflozin on cell pyroptosis in diabetic kidney injury and
its protection mechanism remains unclear. On this basis, the
present study established an in vitro high-fat model of
mouse podocyte clone 5 (MPC5) cell line (26) to investigate the improvement effect
on renal podocyte of dapagliflozin in palmitic acid (PA)-induced
renal podocyte pyroptosis (27,28)
and to explore the protective mechanism of dapagliflozin in cell
pyroptosis, to provide new ideas for diabetes prevention and
treatment.
Materials and methods
Cell culture and transfection
The MPC5 cell line was purchased from the China
Center for Type Culture Collection. The basic culture medium for
this cell line was composed of 89% RPMI-1640 Medium (Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin (Wuhan Servicebio
Technology Co., Ltd.). The MPC5 cell line was maintained at a
temperature of 37°C with 5% CO2.
Cells were added to the culture medium following
mixing in Opti-MEM for 15 min at room temperature (Thermo Fisher
Scientific, Inc.), followed by the addition of antibiotic-free
culture medium. pcDNA3.1-HO-1 was then transfected into MPC5 cells
using EZ Cell Transfection Reagent (Life-iLab Biotech Co., Ltd.) at
a 3:1 (µl/µg) ratio of reagent to DNA; the concentration of nucleic
acid was 1,000 ng/µl and pcDNA3.1 was used as the negative control.
pcDNA3.1-HO-1 was synthesized by Tsingke Biotech Co., Ltd., with 5′
HindIII and 3′ EcoRV restriction sites.
Small interfering (si)RNA targeting HO-1 (siHO-1)
was transfected into MPC5 cells using Lipofectamine™ RNAiMAX
(Thermo Fisher Scientific, Inc.). The final amount of siHO-1 used
per well was 25 pmol, while the amount of transfection reagent was
7.5 µl. After 4–6 h of transfection at 37°C, the medium containing
the EZ Trans complex was replaced with fresh medium, the cells were
further cultured for 12 h and then treated with or without 0.2 mmol
PA (Merck Corp) for 24 h. When the drug treatment was complete, the
cell samples were used for protein and total RNA extraction. The
following sequences were used: siRNA-HO-1 forward,
5′-GUUCAAACAGCUCUAUCGUTT-3′ and reverse,
5′-ACGAUAGAGCUGUUUGAACTT-3′; and siRNA negative control forward,
5′-UUCUCCGACAGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACUGUCGGAGAATT-3′.
Cells were cultured without penicillin/streptomycin
in 6-well plates for 24 h, then the culture medium was changed by
RPMI-1640 Medium. Added 2 µl MCC950 (MedChemExpress) for 2 h, then
2 µl dapagliflozin were added for 4 h. After 48 h, the cells were
used for fluorescent staining.
ELISA
Following treatment with PA (0, 0.1, 0.2, 0.3 and
0.4 mmol) for 24 h at 37°C, the cell culture was centrifuged at
1,000 × g at 4°C for 20 min. Cell-free supernatant was collected
for NLRP3 detection. NLRP3 content in the supernatant was measured
using an ELISA kit (ELK Biotechnology Co., Ltd.), according to the
manufacturer's instructions. Each sample was assayed in more than
three technical replicates.
Cell proliferation and cytotoxicity
assays
Cell Counting Kit-8 (CCK-8; Biosharp Life Sciences)
assay was used to measure cell viability. When the cells had grown
to 80–90% confluence, digestion was performed with 0.25%
Trysin-EDTA. A total of 1×105 MPC5 cell suspension (100
µl) was transferred into 96-well plates followed by treatment with
different concentrations of PA or dapagliflozin for 24 h at 37°C.
Subsequently, 10 µl CCK-8 solution was added to each well for 2 h.
For the controls, the same concentration of CCK-8, culture medium
and PA were added to a 96-well plate without the cells (29). Optical density was measured at 450
nm using a microplate reader (Synergy 2; BioTek China).
Western blotting
Total protein was extracted from 2.5×106
MPC5 cells using RIPA lysis buffer (MedChemExpress) supplemented
with 1% protease and phosphatase inhibitors for 20 min on ice,
followed by centrifugation at 15,520 × g for 10 min at 4°C
(30). The protein concentration
was quantified using a BCA protein assay kit (Biosharp Life
Sciences) and 25 µg protein/lane was separated by SDS-PAGE on a 12%
gel. The separated proteins were then transferred onto a PVDF
membrane (MilliporeSigma) and blocked with 5% nonfat dry milk for 1
h at room temperature. The membrane was incubated with primary
antibodies against NLRP3 (cat. no. A12694, ABclonal Biotech Co.,
Ltd.), caspase-1 (cat. no. A0964, ABclonal Biotech Co., Ltd.),
IL-18 (cat. no. A16737, ABclonal Biotech Co., Ltd.), IL-1β (cat.
no. A11396&A16288, ABclonal Biotech Co., Ltd.) and HO-1 (cat.
no. A19062, ABclonal Biotech Co., Ltd.), as well as a β-actin
antibody (GB11001-100, Wuhan Servicebio Technology Co., Ltd.) used
as the internal reference, for 10 h at 4°C. All antibodies were
diluted at 1:1,000 in western blot primary antibody dilution buffer
(ABclonal Biotech Co., Ltd.). Following primary incubation, the
PVDF membrane was incubated with anti-rabbit IgG secondary antibody
(cat. no. BL003A; 1:10,000; Biosharp Life Sciences) at room
temperature for 1 h and then developed using a Pierce™ ECL
chemiluminescence kit (Thermo Fisher Scientific, Inc.). The protein
bands were exposed and observed in a multifunctional digital gel
imaging analysis system and images were obtained (Bio-Rad GelDoc™
XR and ChemiDoc™ XRS System; Bio-Rad Laboratories, Inc.). Finally,
ImageJ v1.51 (National Institutes of Health) was used for target
protein expression analysis.
Reverse transcription-quantitative PCR
(RT-qPCR)
The cell culture medium was removed and the
2.5×106 cells from 6-well plates were washed with 2 ml
of 4°C pre-cooled PBS. The PBS was then thoroughly removed and 1 ml
RNA extraction solution (Wuhan Servicebio Technology Co., Ltd.) was
added to each sample in the 6-well plate, the bottom cells were
gently shaken off the plate to fully contact the RNA extraction
solution and the RNA extraction solution was then transferred to a
1.5-ml Eppendorf tube at room temperature for 5 min.
cDNA was synthesized using a reverse transcription
kit (Novoprotein Scientific, Inc.), according to the manufacturer's
instructions. The 2X SYBR Green qPCR Master Mix Kit (Wuhan
Servicebio Technology Co., Ltd.) was used to quantify the relative
mRNA levels of ASC, NLRP3, IL-18 and IL-1β (31). qPCR was performed using the qPCR
System (CFX Connect; Bio-Rad Laboratories, Inc.) for 40 cycles,
with GAPDH serving as internal controls. The sequences of primer
pairs used for qPCR were as follows: NLRP3 forward,
5′-GCAGCCTCACCTCACACAGCT-3′ and reverse,
5′-TTTCACCCAACTGTAGGCTCT-3′; ASC forward,
5′-CTGACTGAAGGACAGTACCAG-3′ and reverse,
5′-TCCTGACTTTGTATACACAAT-3′; IL-1β forward,
5′-CTCACAAGCAGAGCACAAGC-3′ and reverse, 5′-AGCTGTCTGCTCATTCACGA-3′;
IL-18 forward, 5′-GACTCTTGCGTCAACTTCAAGG-3′ and reverse,
5′-CAGGCTGTCTTTTGTCAACGA-3′; HO-1 forward,
5′-AAGGGAGAATCTTGCCTGGCT-3′ and reverse,
5′-ACCCCTCAAAAGATAGCCCCA-3′; GAPDH forward,
5′-AAGAAGGTGGTGAAGCAGGC-3′ and reverse,
5′-TCCACCACCCTGTTGCTGTA-3′.
Hoechst 33342/propidium iodide (PI)
fluorescent staining
The cultured cells were washed twice with PBS,
followed by the addition of 1 ml cell staining buffer in the
Hoechst 33342/PI double stain kit (Solarbio Science &
Technology Co., Ltd.). Subsequently, 5 µl Hoechst 33342 staining
solution and 5 µl PI staining solution were mixed and kept at 4°C
for 20 min (32). Following
staining, the cells were washed twice with PBS and observed with a
10Χ objective lens using a fluorescent microscope (IX71; Olympus
Corporation).
Statistical analysis
GraphPad Prism 9 software (GraphPad Software;
Dotmatics) was used for drawing and statistical analysis.
Differences between the control group and each other group in
Fig. 1A and B were analyzed using
one-way ANOVA followed by Dunnett's post hoc test. An unpaired
t-test was used for comparisons between two groups in Fig. 1C and D. Differences between
multiple groups were analyzed using one-way ANOVA followed by
Tukey's post hoc test (Fig. 2,
Fig. 3, Fig. 4). P<0.05 was considered to
indicate a statistically significant difference (33).
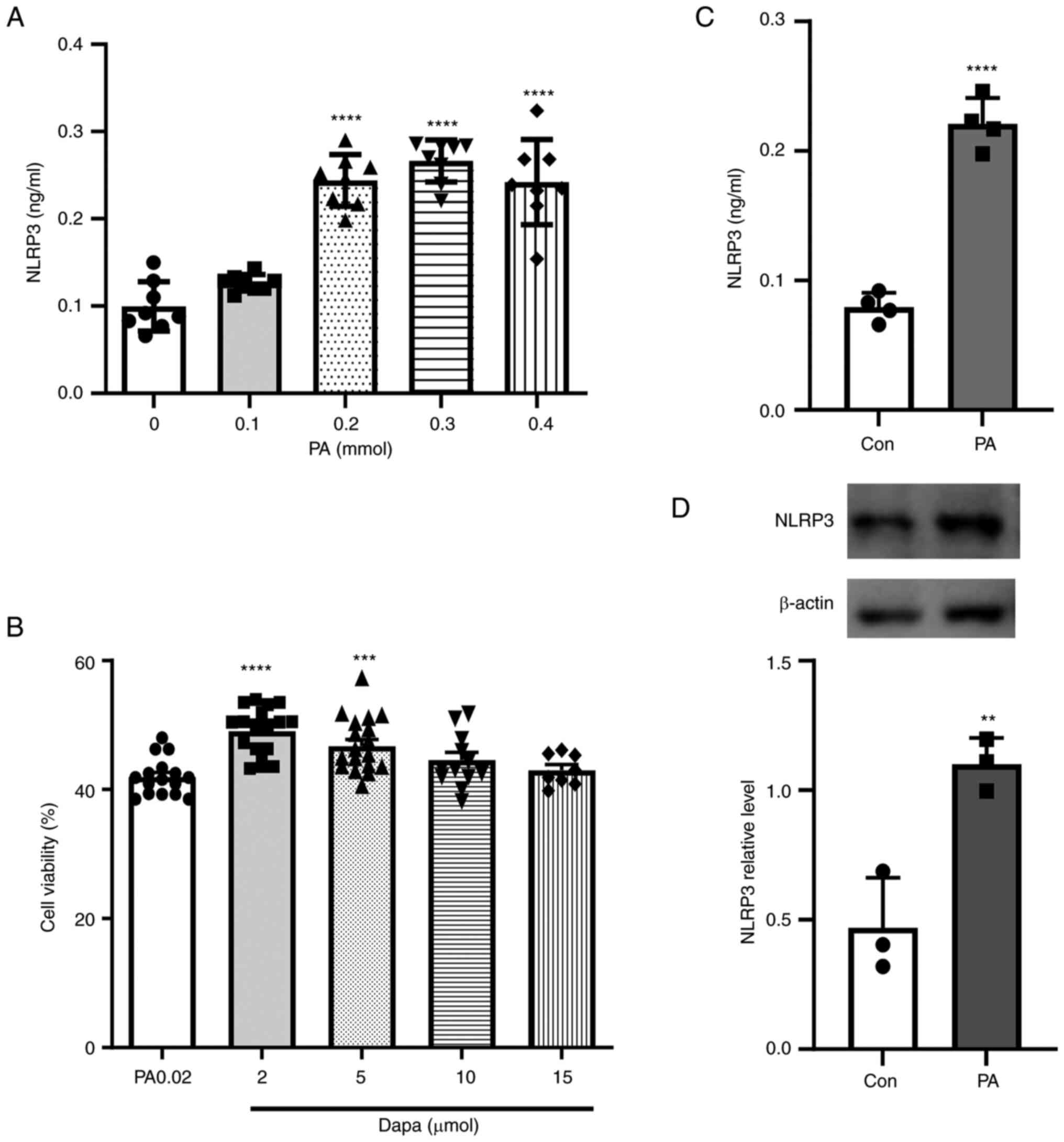 | Figure 1.Effect of PA on the NLRP3
inflammasome in MPC5 cells and cell viability assay following
dapagliflozin treatment. (A) NLRP3 inflammatory production in MPC5
cells following treatment with different PA concentrations (0, 0.1,
0.2, 0.3 and 0.4 mmol). (B) NLRP3 inflammasome production
significantly increased in MPC5 cells treated with 0.2 mmol PA. (C)
Cell Counting Kit-8 cytotoxicity assay following treatment with
different concentrations of dapagliflozin (2, 5, 10 and 15 µmol).
(D) NLRP3 inflammatory protein expression increased in MPC5 cells
following treatment with 0.2 mmol PA. **P<0.001, ***P<0.001,
****P<0.0001 vs. control group. PA, palmitic acid; NLRP3,
NOD-like receptor thermal protein domain associated protein 3;
MPC5, mouse podocyte clone 5; Con, control; dapa,
dapagliflozin. |
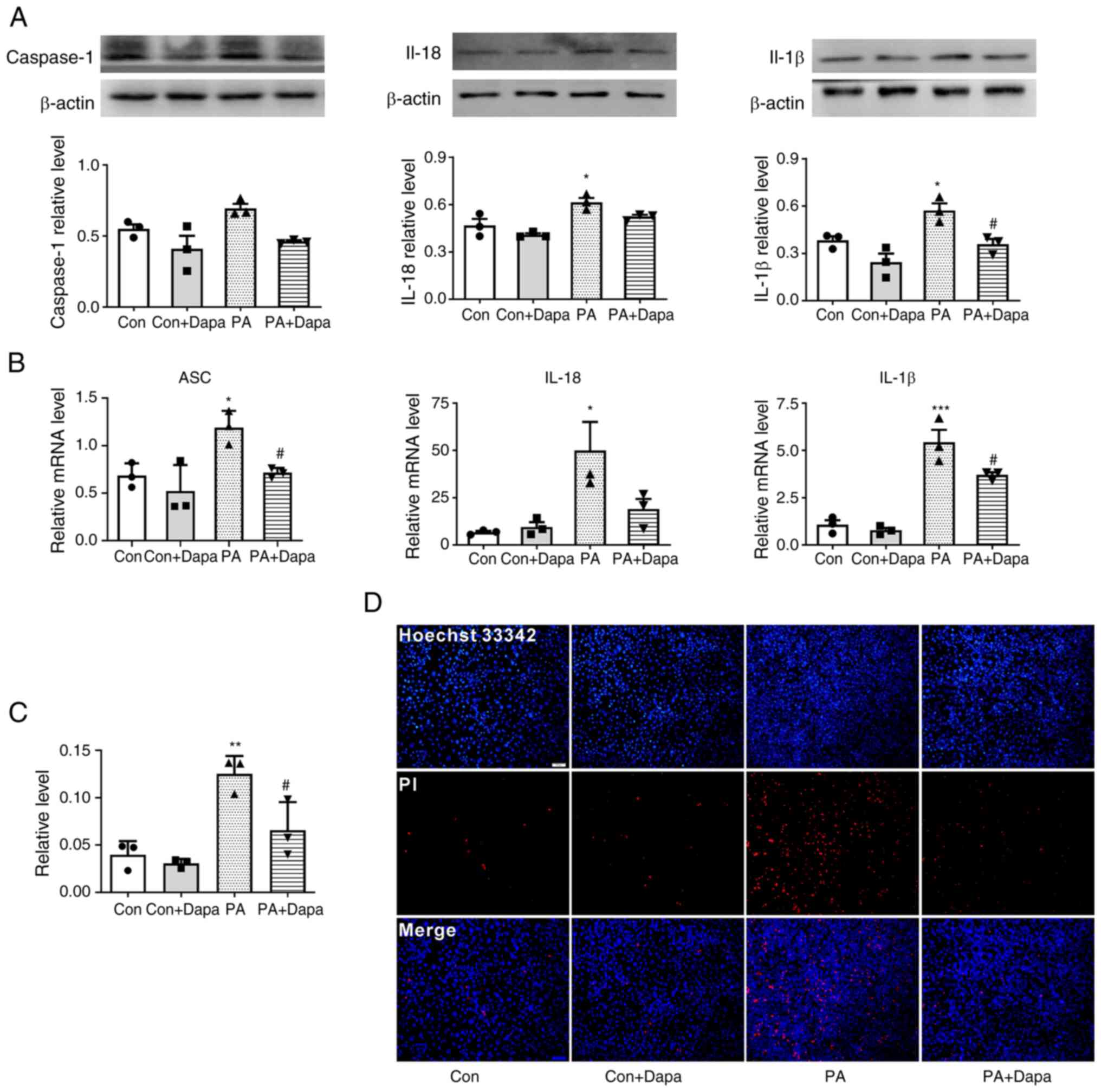 | Figure 2.Dapagliflozin inhibits PA-induced
pyroptosis in MPC5 cells. (A) The protein expression of caspase-1,
IL-18 and IL-1β decreased following dapagliflozin treatment. (B)
The relative mRNA level of ASC, IL-18 and IL-1β decreased following
dapagliflozin treatment in MPC5 cells. (C) Relative cell count
ratio of PI uptake. (D) Photomicrographs of double-fluorescent
staining with Hoechst 33342/PI. PA, 0.2 mmol; Dapa, 2 µmol; scale
bar, 100 µm. *P<0.05, **P<0.01 vs. control group;
#P<0.05 vs. PA group. Con, control; Dapa,
dapagliflozin; MPC5, mouse podocyte clone 5; PA, palmitic acid;
ASC, apoptosis-associated speck-like protein containing a caspase
activation and recruitment domain; PI, propidium iodide. |
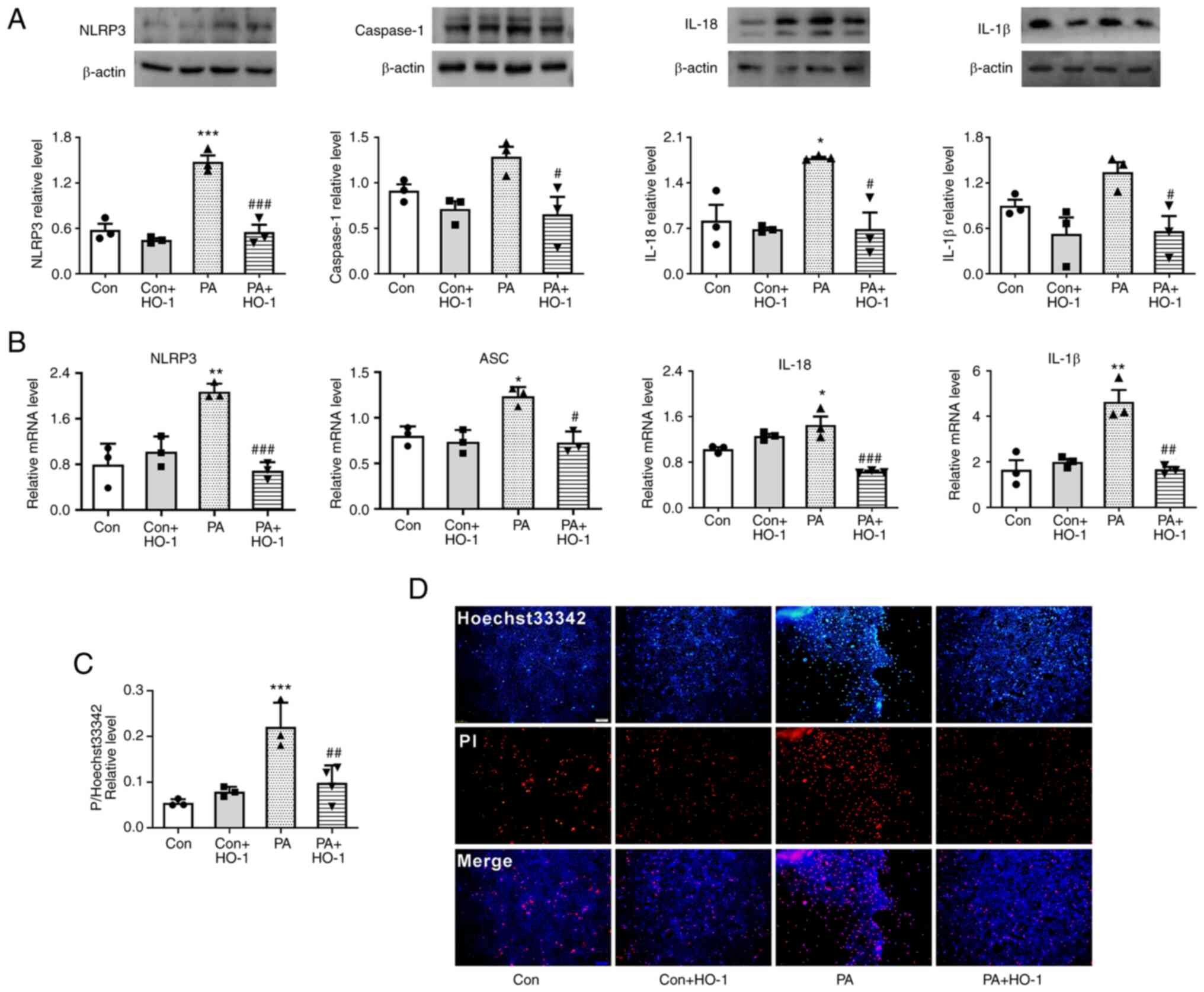 | Figure 4.HO-1 overexpression inhibits
PA-induced pyroptosis in MPC5 cells. (A) HO-1 overexpression
decreased the expression of NLRP3, caspase-1, IL-18 and IL-1β in
MPC5 cells. (B) HO-1 overexpression decreased the relative mRNA
level of NLRP3, caspase-1, IL-18 and IL-1β in MPC5 cells. (C)
Relative cell count ratio of PI uptake. (D) Photomicrographs of
double-fluorescent staining with Hoechst 33342/PI. PA, 0.2 mmol;
scale bar, 100 µm. *P<0.05, **P<0.001, ***P<0.001 vs.
control group; #P<0.05, ##P<0.01,
###P<0.001 vs. PA group. HO-1, heme oxygenase 1;
MPC5, mouse podocyte clone 5; NLRP3, NOD-like receptor thermal
protein domain associated protein 3; PA, palmitic acid; Con,
control; Dapa, dapagliflozin. |
Results
NLRP3 inflammasome increases in MPC5
cells under PA
The pathophysiology of diabetes is complicated and
involves glucose and lipid metabolism disorders. Fat deposits in
the kidneys have been suggested to be instrumental to DN, so
different concentrations of PA were used to pretreat cells in lipid
metabolism-related experiments (34–36).
According to the results of the microplate reader
detection of cell-released NLRP3 inflammasome, the present study
showed that, under the induction of PA, MPC5 cells released a large
amount of NLRP3 inflammasome (Fig.
1B). The protein expression of NLRP3 was also increased in MPC5
cells (Fig. 1D). However,
inflammasome release was not significantly increased with
increasing PA content. When the PA concentration reached 0.3 mmol,
the release amount of NLRP3 inflammasome decreased (Fig. 1A) and a large number of dead cells
floated in the culture dish at this time, which indicated that a
high PA concentration had a detrimental effect on cell viability.
In brief, 0.2 mmol was selected as the PA concentration in this
experiment. Based on various relevant studies, concentrations of 2,
5, 10 and 15 µmol dapagliflozin were selected to verify the most
suitable treatment methods, following treatment with 0.2 mmol PA.
The drug effect was examined through a CCK-8 assay, which showed
that when the concentration of dapagliflozin was >5 µmol, the
number of viable cells began to decrease. Thus, the CCK-8 assay
results showed that 2 µmol dapagliflozin was appropriate as the
protective concentration for subsequent experiments (Fig. 1C).
Dapagliflozin inhibits PA-induced
pyroptosis in MPC5 cells
Western blot results suggested that the expression
of pyroptosis-related proteins NLRP3 and caspase-1 was increased
compared with that in the control group. The expression of
inflammatory cytokines IL-18 and IL-1β was increased by PA
treatment. Following the addition of dapagliflozin, the expression
of pyroptosis-related proteins decreased. At the same time, the
content of intracellular inflammatory factors was also decreased
(Fig. 2A).
qPCR results indicated that the mRNA levels of NLRP3
inflammasome, ASC, and inflammatory factors IL-18 and IL-1β were
increased following the treatment of MPC5 cells with PA. Following
the addition of dapagliflozin, the mRNA levels of inflammatory and
pyroptosis-related factors were significantly decreased (Fig. 2B).
Dapagliflozin reduces the level of
membrane rupture in MPC5
Hoechst 33342/PI fluorescence staining results
indicated that the uptake of PI dye by MPC5 cells was increased
following PA treatment and significantly decreased following the
addition of dapagliflozin (Fig. 2C and
D).
MCC950 is an effective and selective NLRP3
inhibitor, which can specifically inhibit activation of NLRP3 and
reduced IL-1β production in vivo. After MCC950 was added,
the uptake of PI dye by MPC5 cells also decreased. When both MCC950
and dapagliflozin were added to the cells, the uptake of PI dye
decreased (Fig. S1). This result
indicated that cell membrane damage was attenuated when NLRP3 was
inhibited and dapagliflozin might also reduce the membrane damage
by inhibiting the activity of NLRP3.
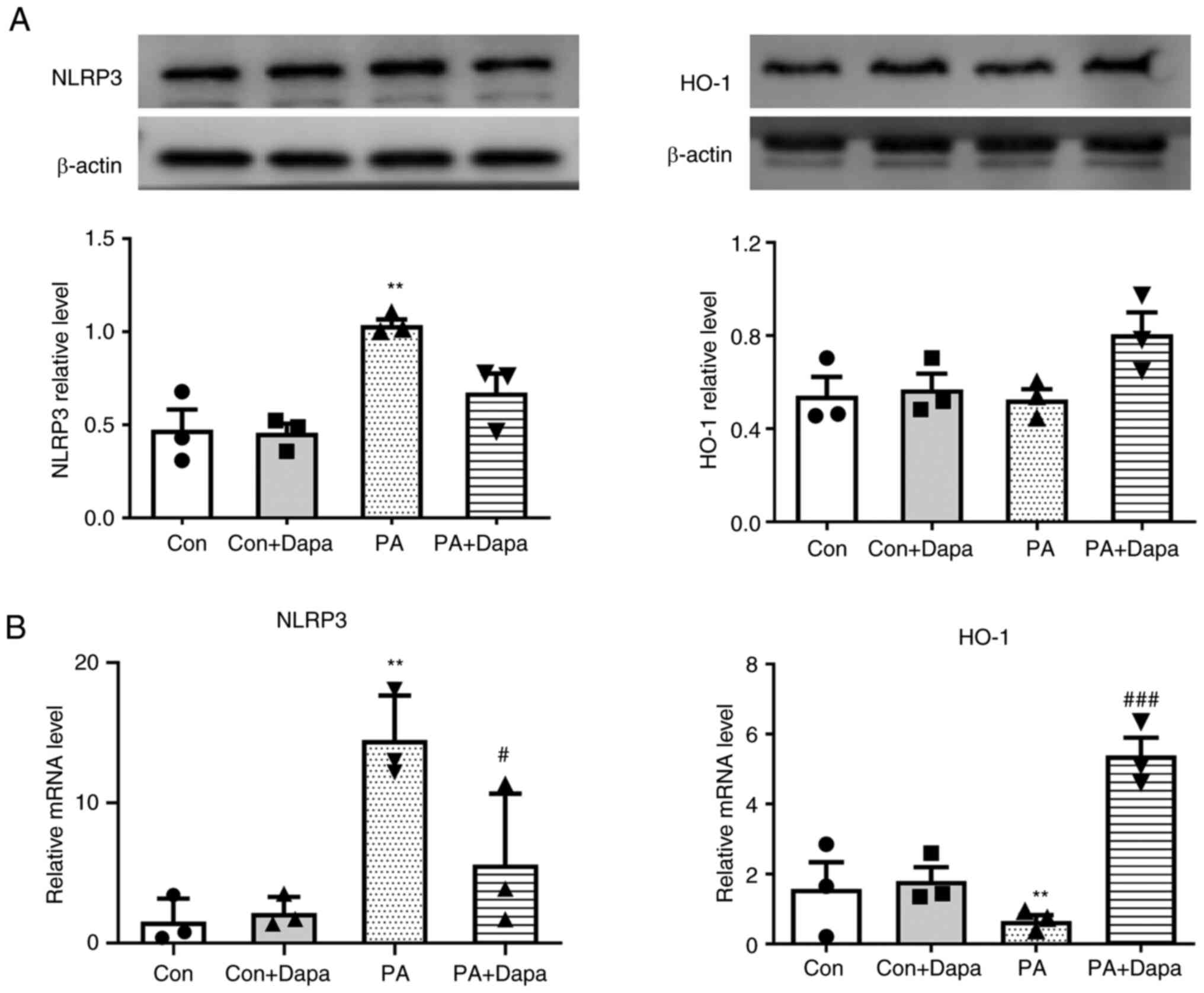
HO-1 is associated with the
anti-pyroptosis effect of dapagliflozin in MPC5
The protein expression of HO-1 decreased following
the treatment of MPC5 cells with PA, which was consistent with the
qPCR results. However, following dapagliflozin treatment, HO-1
protein and mRNA expression levels were both increased (Fig. 3).
HO-1 overexpression restrains
PA-induced pyroptosis in MPC5 cells
Western blot analysis indicated that the expression
levels of pyroptosis-related proteins NLRP3 and caspase-1, as well
as inflammatory factors IL-18 and IL-1β, decreased following HO-1
overexpression in the PA group (Fig.
4A). Meanwhile, NLRP3 inflammasome, IL-18 and IL-1β increased
following transfection with siHO1. Moreover, when dapagliflozin was
added, NLRP3 inflammasome, IL-18 and IL-1β decreased (Fig. S2A).
qPCR results indicated that the mRNA levels of NLRP3
inflammasome and ASC, as well as inflammatory factors IL-18 and
IL-1β, significantly decreased following HO-1 overexpression in
MPC5 cells (Fig. 4B).
HO-1 overexpression reduces the level
of membrane rupture in MPC5
According to Hoechst 33342/PI fluorescence staining,
when HO-1 was overexpressed in the PA group, the PI uptake of MPC5
cells was significantly reduced (Fig.
4C and D). This result suggested that a large number of cell
membranes were damaged following treatment with PA, while HO-1
overexpression reduced this damage.
Discussion
SGLT2 inhibitors are a type of anti-diabetic agent
that can lower glucose reabsorption and lead to urinary glucose
excretion (37–39). Their therapeutic effects include
lowering lipid markers, assisting weight loss and delaying diabetic
vascular complications. Specifically, dapagliflozin has been proven
to be effective in preventing and slowing renal function loss and
failure in diabetic patients (10). Previous studies suggested that the
protective effect of SGLT2 inhibitors is closely associated with
the reduction of inflammatory markers including TNF-α, IL-1β and
IL-6, and the inhibition of oxidative stress. In addition, SGLT2
inhibitors can help lower blood sugar and lipids, rendering
dapagliflozin an ideal drug for the treatment of metabolic
abnormalities (40,41). As a recently discovered type of
programmed cell death, pyroptosis was shown to be extensively
involved in diseases such as diabetes and atherosclerosis, and its
key features are loss of cytoplasmic membrane integrity and release
of several inflammatory cytokines (20,22).
Therefore, it was found that PA can induce NLRP3 inflammasomes in
renal podocytes and inflammasome activation can then increase
insulin resistance in diabetic patients and significantly increase
blood glucose and insulin levels, which are linked to hyperglycemia
and hyperinsulinemia. The NLRP3 inflammasome is an intracellular
platform that converts the proinflammatory cytokines IL-1β and
IL-18 to their active forms in response to ‘danger’ signals, which
can be either host or pathogen-derived (20,42,43).
Thus, the present authors chose inflammatory cytokines of IL-18 and
IL-1β for detection and verification of NLRP3 activation
levels.
Pyroptosis-related signals were also detected in
MPC5 cells following PA treatment; the expression of NLRP3 and
caspase-1 was found to be significantly increased, as well as the
inflammatory factors IL-18 and IL-1β. A significant increase in
pyrototic pores was observed in the Hoechst 33342/PI staining
assay. Based on this, we hypothesized that PA acts as a stimulating
factor to induce NLRP3 upregulation in MPC5 cells, further
resulting in the formation and activation of caspase-1. The mature
inflammatory factors pro-IL-18 and pro-IL-1β were activated by
active caspase-1, which led to the generation of the inflammatory
reaction in cells and the phenomenon of pyroptosis. Following
dapagliflozin treatment, the expression of NLRP3, caspase-1 and
inflammatory cytokines IL-18 and IL-1β decreased. Meanwhile, the
degree of cell plasma membrane rupture decreased. Compared with
previous studies, the aforementioned experimental results indicated
that the activation of NLRP3 inflammatory signaling may serve a
role in diabetic cell injury and dapagliflozin may slow down the
phenomenon of pyroptosis by inhibiting the NLRP3
inflammasome-mediated inflammatory response. These results provide
scientific validation for further exploration of the therapeutic
effect of dapagliflozin on PA-induced renal cell pyroptosis.
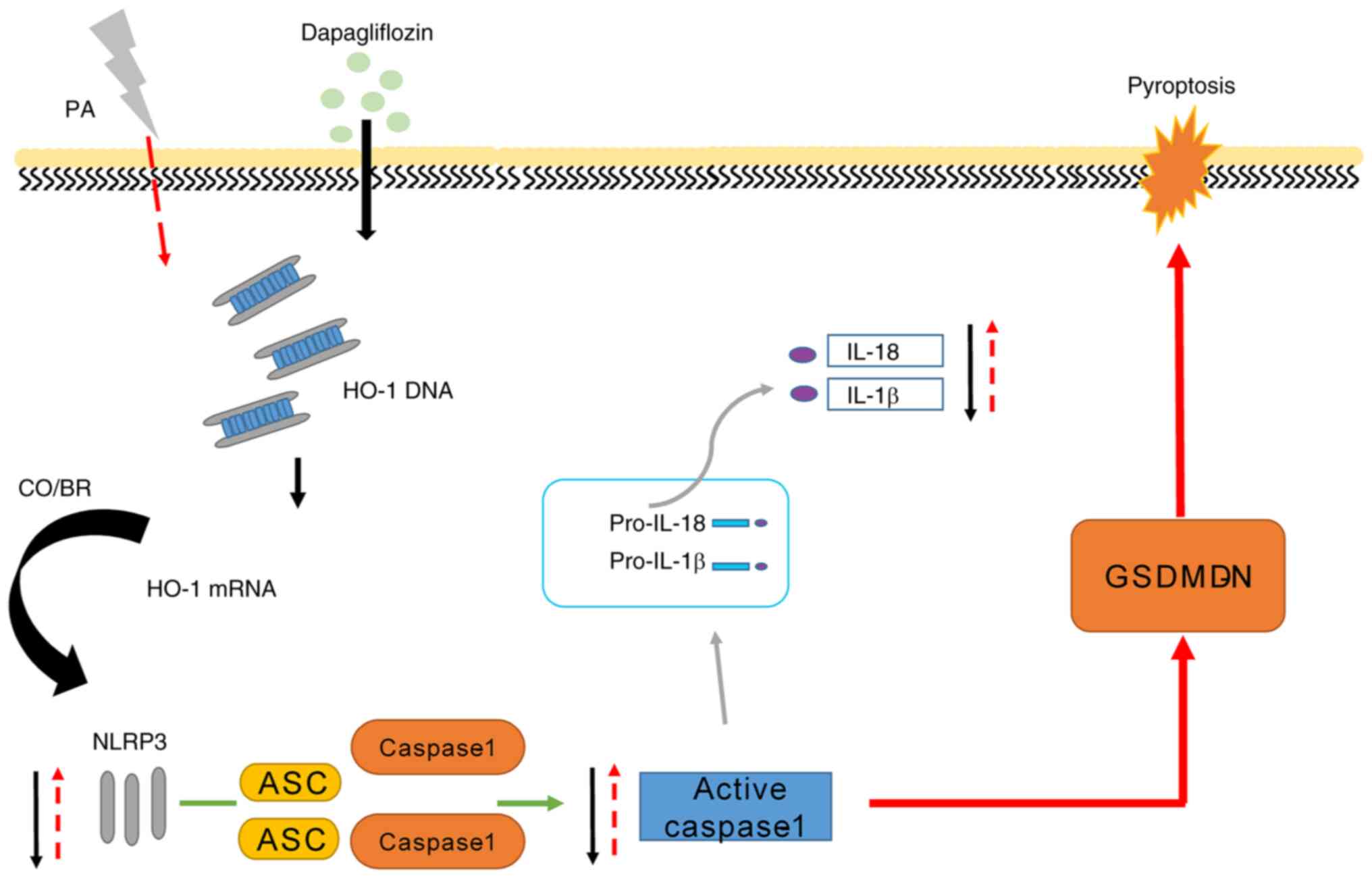
HO-1 is an important rate-limiting enzyme in the
process of heme catabolism, which plays a key role in oxidative
stress and inflammatory damage (15). The present study found that HO-1
expression decreased following MPC5 induction by PA and increased
following dapagliflozin treatment. Moreover, HO-1 overexpression in
MPC5 significantly reduced the uptake of PI and the level of
cytoplasmic membrane rupture. The above experimental results
confirmed that HO-1 could reduce cell damage in high-fat in
vitro models and that dapagliflozin may mediate the expression
of HO-1 to inhibit cell pyroptosis, hence exerting a renal
protective effect (Fig. 5). This
result suggests that the protective role of dapagliflozin might be
mediated by the regulation of HO-1 expression.
In conclusion, dapagliflozin was found to
significantly improve dyslipidemia damage by restraining pyroptosis
in MPC5 cells. These results showed that HO-1 mediates the
anti-pyroptosis effects of dapagliflozin. However, the detailed
molecular mechanism of dapagliflozin affects HO-1 in pyroptosis
protective pathway was not verified in the present study and will
be further investigated in future studies. Further studies are also
required to verify the present results in vivo.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This present study was supported by the Foundation of Hubei
Educational Committee (grant no. Q20202803) and the Special Project
on Diabetes and Angiopathy (grant nos. 2020TNB10 and 2022TNB11) and
the Doctoral Scientific Research Foundation of Hubei University of
Science and Technology (grant no. BK202010).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BZ and CL conceived and designed the experiments; ZZ
and BZ drafted the manuscript; ZZ, PN, MT and YS acquired and
analyzed the data, and interpretated the results; PN and CL revised
the draft critically. ZZ and BZ confirm the authenticity of all the
raw data. All the authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ASC
|
apoptosis-associated speck-like
protein containing a caspase activation and recruitment domain
|
|
NLRP3
|
nucleotide oligomerization domain-like
receptor thermal protein domain associated protein 3
|
|
caspase-1
|
cysteinyl aspartate specific
proteinase
|
|
Dapa
|
dapagliflozin
|
|
DN
|
diabetic nephropathy
|
|
HO-1
|
heme oxygenase 1
|
|
IL-1β
|
interleukin-1β
|
|
IL-18
|
interleukin-18
|
|
PA
|
palmitic acid
|
|
SGLT2
|
sodium-glucose cotransporter 2
|
|
MPC5
|
mouse podocyte clone 5
|
References
|
1
|
Shi Y and Hu FB: The global implications
of diabetes and cancer. Lancet. 383:1947–1948. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee HB, Ha H, Kim SI and Ziyadeh FN:
Diabetic kidney disease research: Where do we stand at the turn of
the century? Kidney Int Suppl. 77:S1–S2. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Samsu N: Diabetic nephropathy: Challenges
in pathogenesis, diagnosis, and treatment. Biomed Res Int.
2021:14974492021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin J, Hu K, Ye M, Wu D and He Q:
Rapamycin reduces podocyte apoptosis and is involved in autophagy
and mTOR/P70S6K/4EBP1 signaling. Cell Physiol Biochem. 48:765–772.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin X, Zhen X, Huang H, Wu H, You Y, Guo
P, Gu X and Yang F: Role of MiR-155 signal pathway in regulating
podocyte injury induced by TGF-β1. Cell Physiol Biochem.
42:1469–1480. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tuttle KR, Bakris GL, Bilous RW, Chiang
JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K,
Narva AS, Navaneethan SD, et al: Diabetic kidney disease: A report
from an ADA consensus conference. Diabetes Care. 37:2864–2883.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wada J and Makino H: Inflammation and the
pathogenesis of diabetic nephropathy. Clin Sci (Lond). 124:139–152.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cassis P, Locatelli M, Cerullo D, Corna D,
Buelli S, Zanchi C, Villa S, Morigi M, Remuzzi G, Benigni A and
Zoja C: SGLT2 inhibitor dapagliflozin limits podocyte damage in
proteinuric nondiabetic nephropathy. JCI Insight. 3:e987202018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heerspink HJL, Stefánsson BV,
Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray
JJV, Lindberg M, Rossing P, et al: Dapagliflozin in patients with
chronic kidney disease. N Engl J Med. 383:1436–1446. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Neuen BL, Young T, Heerspink HJL, Neal B,
Perkovic V, Billot L, Mahaffey KW, Charytan DM, Wheeler DC, Arnott
C, et al: SGLT2 inhibitors for the prevention of kidney failure in
patients with type 2 diabetes: A systematic review and
meta-analysis. Lancet Diabetes Endocrinol. 7:845–854. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ala M: SGLT2 inhibition for cardiovascular
diseases, chronic kidney disease, and NAFLD. Endocrinology.
162:bqab1572021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Neumiller JJ, White JR Jr and Campbell RK:
Sodium-glucose co-transport inhibitors: Progress and therapeutic
potential in type 2 diabetes mellitus. Drugs. 70:377–385. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
El-Rous MA, Saber S, Raafat EM and Ahmed
AAE: Dapagliflozin, an SGLT2 inhibitor, ameliorates acetic
acid-induced colitis in rats by targeting NFκB/AMPK/NLRP3 axis.
Inflammopharmacology. 29:1169–1185. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takahashi T, Morita K, Akagi R and Sassa
S: Heme oxygenase-1: A novel therapeutic target in oxidative tissue
injuries. Curr Med Chem. 11:1545–1561. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kozakowska M, Dulak J and Józkowicz A:
Heme oxygenase-1-more than the cytoprotection. Postepy Biochem.
61:147–158. 2015.(In Polish). PubMed/NCBI
|
|
16
|
Ryter SW, Alam J and Choi AMK: Heme
oxygenase-1/carbon monoxide: From basic science to therapeutic
applications. Physiol Rev. 86:583–650. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Otterbein L, Chin BY, Otterbein SL, Lowe
VC, Fessler HE and Choi AM: Mechanism of hemoglobin-induced
protection against endotoxemia in rats: A ferritin-independent
pathway. Am J Physiol. 272:L268–L275. 1997.PubMed/NCBI
|
|
18
|
Arab HH, Al-Shorbagy MY and Saad MA:
Activation of autophagy and suppression of apoptosis by
dapagliflozin attenuates experimental inflammatory bowel disease in
rats: Targeting AMPK/mTOR, HMGB1/RAGE and Nrf2/HO-1 pathways. Chem
Biol Interact. 335:1093682021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Samman WA, Selim SM, El Fayoumi HM,
El-Sayed NM, Mehanna ET and Hazem RM: Dapagliflozin ameliorates
cognitive impairment in aluminum-chloride-induced Alzheimer's
disease via modulation of AMPK/mTOR, oxidative stress and glucose
metabolism. Pharmaceuticals (Basel). 16:7532023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fuchs Y and Steller H: Programmed cell
death in animal development and disease. Cell. 147:742–758. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bertheloot D, Latz E and Franklin BS:
Necroptosis, pyroptosis and apoptosis: An intricate game of cell
death. Cell Mol Immunol. 18:1106–1121. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hutton HL, Ooi JD, Holdsworth SR and
Kitching AR: The NLRP3 inflammasome in kidney disease and
autoimmunity. Nephrology (Carlton). 21:736–744. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cookson BT and Brennan MA:
Pro-inflammatory programmed cell death. Trends Microbiol.
9:113–114. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Al Mamun A, Ara Mimi A, Wu Y, Zaeem M,
Abdul Aziz M, Aktar Suchi S, Alyafeai E, Munir F and Xiao J:
Pyroptosis in diabetic nephropathy. Clin Chim Acta. 523:131–143.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He X, Fan X, Bai B, Lu N, Zhang S and
Zhang L: Pyroptosis is a critical immune-inflammatory response
involved in atherosclerosis. Pharmacol Res. 165:1054472021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tu Q, Li Y, Jin J, Jiang X, Ren Y and He
Q: Curcumin alleviates diabetic nephropathy via inhibiting podocyte
mesenchymal transdifferentiation and inducing autophagy in rats and
MPC5 cells. Pharm Biol. 57:778–786. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chu SG, Villalba JA, Liang X, Xiong K,
Tsoyi K, Ith B, Ayaub EA, Tatituri RV, Byers DE, Hsu FF, et al:
Palmitic acid-rich high-fat diet exacerbates experimental pulmonary
fibrosis by modulating endoplasmic reticulum stress. Am J Respir
Cell Mol Biol. 61:737–746. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Urso CJ and Zhou H: Palmitic acid
lipotoxicity in microglia cells is ameliorated by unsaturated fatty
acids. Int J Mol Sci. 22:90932021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu B, Liu Q, Han Q, Zeng B, Chen J and
Xiao Q: Downregulation of Krüppel-like factor 1 inhibits the
metastasis and invasion of cervical cancer cells. Mol Med Rep.
18:3932–3940. 2018.PubMed/NCBI
|
|
30
|
Zhang L, Jiang B, Zhu N, Tao M, Jun Y,
Chen X, Wang Q and Luo C: Mitotic checkpoint kinase Mps1/TTK
predicts prognosis of colon cancer patients and regulates tumor
proliferation and differentiation via PKCα/ERK1/2 and PI3K/Akt
pathway. Med Oncol. 37:52019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dong D, Wu J, Sheng L, Gong X, Zhang Z and
Yu C: FUNDC1 induces apoptosis and autophagy under oxidative stress
via PI3K/Akt/mTOR pathway in cataract lens cells. Curr Eye Res.
47:547–554. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu J, Li QQ, Zhou H, Lu Y, Li JM, Ma Y,
Wang L, Fu T, Gong X, Weintraub M, et al: Selective tumor cell
killing by triptolide in p53 wild-type and p53 mutant ovarian
carcinomas. Med Oncol. 31:142014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gao ZD, Yan HD, Wu NH, Yao Q, Wan BB, Liu
XF, Zhang ZW, Chen QJ and Huang CP: Mechanistic insights into the
amelioration effects of lipopolysaccharide-induced acute lung
injury by baicalein: An integrated systems pharmacology study and
experimental validation. Pulm Pharmacol Ther. 73–74.
1021212022.
|
|
34
|
Huang B, Wen W and Ye S: Dapagliflozin
ameliorates renal tubular ferroptosis in diabetes via SLC40A1
stabilization. Oxid Med Cell Longev. 2022:97355552022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen X, Han Y, Gao P, Yang M, Xiao L,
Xiong X, Zhao H, Tang C, Chen G, Zhu X, et al: Disulfide-bond A
oxidoreductase-like protein protects against ectopic fat deposition
and lipid-related kidney damage in diabetic nephropathy. Kidney
Int. 95:880–895. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Du Q, Wu X, Ma K, Liu W, Liu P, Hayashi T,
Mizuno K, Hattori S, Fujisaki H and Ikejima T: Silibinin alleviates
ferroptosis of rat islet β cell INS-1 induced by the treatment with
palmitic acid and high glucose through enhancing
PINK1/parkin-mediated mitophagy. Arch Biochem Biophys.
743:1096442023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pafili K, Maltezos E and Papanas N: The
potential of SGLT2 inhibitors in phase II clinical development for
treating type 2 diabetes. Expert Opin Investig Drugs. 25:1133–1152.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pafili K and Papanas N: Luseogliflozin and
other sodium-glucose cotransporter 2 inhibitors: No enemy but time?
Expert Opin Pharmacother. 16:453–456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Plosker GL: Dapagliflozin: A review of its
use in patients with type 2 diabetes. Drugs. 74:2191–2209. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kimura T, Obata A, Shimoda M, Okauchi S,
Kanda-Kimura Y, Nogami Y, Moriuchi S, Hirukawa H, Kohara K,
Nakanishi S, et al: Protective effects of the SGLT2 inhibitor
luseogliflozin on pancreatic β-cells in db/db mice: The earlier and
longer, the better. Diabetes Obes Metab. 20:2442–2457. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Daems C, Welsch S, Boughaleb H,
Vanderroost J, Robert A, Sokal E and Lysy PA: Early treatment with
empagliflozin and GABA improves β-cell mass and glucose tolerance
in streptozotocin-treated mice. J Diabetes Res. 2019:28134892019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jorgensen I, Rayamajhi M and Miao EA:
Programmed cell death as a defence against infection. Nat Rev
Immunol. 17:151–164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jourdan T, Godlewski G, Cinar R, Bertola
A, Szanda G, Liu J, Tam J, Han T, Mukhopadhyay B, Skarulis MC, et
al: Activation of the Nlrp3 inflammasome in infiltrating
macrophages by endocannabinoids mediates beta cell loss in type 2
diabetes. Nat Med. 19:1132–1140. 2013. View Article : Google Scholar : PubMed/NCBI
|