Introduction
Osteolysis is a pathological bone disorder primarily
caused by the abnormal activation of osteoclasts and is widely
reported in patients with dental implants and orthopedic
prostheses. The long-term use of titanium (Ti)-based implants may
produce wear debris, which subsequently causes osteolysis and
aseptic loosening associated with orthopedic implant failure and
dental peri-implantitis (1).
Wear particles are phagocytosed by monocytes/
macrophages around the prosthesis, which, in turn, release
pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α,
interleukin (IL)-1β and IL-6, resulting in the proliferation and
differentiation of bone marrow-derived macrophages (BMMs) (2,3). In
addition, wear particles can disrupt the balance of antioxidant
systems via the generation of large amounts of reactive oxygen
species (ROS) and the promotion of osteoclast formation. Endogenous
ROS generated following receptor activator of nuclear factor kB
(NF-κB) ligand (RANKL)-induced stimulation can activate the
NF-κB/mitogen-activated protein kinase (MAPK)/phosphoinositide
3-kinase pathway. Conversely, ROS also activate the nuclear factor
erythroid-2-related factor 2 (Nrf2)-Kelch-like ECH-associated
protein 1 pathway to relieve oxidative stress (4,5) and
suppress osteoclast formation and activity (6). In summary, these findings demonstrate
that wear particles promote osteoclast differentiation via the
secretion of inflammatory cytokines and the disruption of the
antioxidant system balance.
Osteoclasts are multinucleated cells derived from
the bone marrow macrophage/monocyte lineage (7). Two essential cytokines regulate
osteoclastogenesis: Macrophage colony-stimulating factor (M-CSF)
and RANKL. M-CSF plays a critical role in the survival and
proliferation of osteoclast progenitor cells. The binding of RANKL
to RANK in BMMs activates downstream targets, including MAPK, NF-κB
and c-Fos (8). Activation of NF-κB
and c-Fos induces the expression of nuclear factor of activated
T-cells cytoplasmic 1 (Nfatc1); subsequently, Nfatc1 functions in
conjunction with other transcription factors such as PU.1 activator
protein 1, microphthalmia-associated transcription factor and
cAMP-responsive element binding protein (9). Nfatc1 is a master transcriptional
regulator of osteoclast differentiation, which upregulates
osteoclast-specific genes such as cathepsin K (Ctsk),
tartrate-resistant acid phosphatase 5 (Acp5), matrix
metallopeptidase 9 (Mmp9) and dendritic cell-specific
transmembrane protein (Dc-stamp). Moreover, Nfatc1 regulates
the expression of B lymphocyte-induced maturation protein-1
(Blimp1), which is a transcriptional repressor of negative
regulators of osteoclastogenesis, including interferon regulatory
factor-8 (Irf8), v-maf musculoaponeurotic fibrosarcoma oncogene
family, protein B (MafB) and B-cell lymphoma 6 (Bcl6), during
osteoclast formation (10,11). Therefore, precise control of bone
turnover is pivotal for bone health and is an efficient strategy
for the inhibition of abnormal osteoclast formation and
activity.
Britanin is a natural product that has a
pseudoguaianolide sesquiterpene structure with an exomethylene
group, and has been shown to exert preventive effects against
inflammation, asthma and allergy (12–14).
Britanin also inhibits lipopolysaccharide-induced nitric oxide
production, prostaglandin E2 and pro-inflammatory cytokines via the
inactivation of NF-κB and MAPK in macrophages (15), and exerts antitumor effects by
inhibiting the proliferation and migration of breast, gastric and
pancreatic cancer cell lines (16,17).
In addition, britanin demonstrated the ability to inhibit tumor
growth in a pancreatic cancer xenograft mouse model (17). Furthermore, britanin has been
reported to protect organs against myocardial ischemia/reperfusion
injury and cerebral ischemic reperfusion injury via the activation
of Nrf2 signaling (18,19). These reports suggest that britanin
exhibits multiple biological activities, including
anti-inflammatory and anti-oxidative effects.
In the present study, the effects of britanin on
in vitro osteoclastogenesis and in vivo Ti
particle-mediated osteolysis were investigated in a mouse model.
The mechanism underlying the effect of britanin on osteoclast
formation was also investigated.
Materials and methods
Chemicals and reagents
α-modified Eagle's medium (α-MEM) was obtained from
Gibco; Thermo Fisher Scientific, Inc. Recombinant murine M-CSF and
RANKL were purchased from R&D Systems, Inc. The Leukocyte Acid
Phosphatase kit [tartrate-resistant acid phosphatase (TRAP)] kit,
3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT),
dimethyl sulfoxide (DMSO) and all other unspecified reagents were
purchased from Sigma-Aldrich; Merck KGaA. Specific antibodies
against phospho-extracellular signal-regulated kinase (ERK; cat.
no. 9101S), EKR1/2 (cat. no. 9102S) phospho-c-Jun N-terminal kinase
(JNK; cat. no. 9251S), JNK (cat. no. 9252S), phospho-p38 (cat. no.
9211S), p38 (cat. no. 9212S), phospho-AKT (cat. no. 4058S), AKT
(cat. no. 9272S) and phospho-inhibitor of nuclear factor kΒα (IκBα;
cat. no. 2859S) were purchased from Cell Signaling Technology, Inc.
Antibodies against IκBα (cat. no. sc-847), Irf8 (cat. no. sc-6058)
and Nfatc1 (cat. no. sc-7294) were purchased from Santa Cruz
Biotechnology, Inc. Antibodies against cathepsin K (cat. no.
MAB3324) and β-actin (cat. no. A5441) were purchased from
MilliporeSigma. Britanin, which is also known by the International
Union of Pure and Applied Chemistry name of
9-acetyloxy-8-hydroxy-5,8a-dimethyl-1-methylidene-2-oxo-4,5,5a,6,7,8,9,9a-octahydro-3aH-azuleno[6,5-b]furan-6-yl)
acetate, was isolated from Inula japonica (Inulae Flos), as
described previously (20) and its
purity was confirmed by one-dimensional nuclear magnetic resonance
data comparison. Britanin was dissolved in DMSO for further
experiments. Ti particles (purity, 99.9%; diameter, 30–50 nm; US
Research Nanomaterials, Inc.) for in vivo experiments were
prepared as previously described (21).
Cell culture and osteoclast
differentiation
BMMs were isolated from mouse bone marrow as
described previously (22).
Briefly, cells were extracted from the tibiae and femurs of 4- to
6-week-old ICR male mice. The mice were purchased from Dae Han Bio
Link Co., Ltd., and maintained under conventional housing
conditions at 22–24°C and 50–60% humidity, with a 12-h light/12-h
dark cycle and free access to water and food pellets. The mice were
euthanized by cervical dislocation following CO2
treatment. The flow rate of CO2 was maintained at 40–60%
chamber volume/min. Subsequently, the isolated cells were cultured
in α-MEM (cat. no. 11900) containing 10% fetal bovine serum (cat.
no. 16000; Gibco; Thermo Fisher Scientific, Inc.) for 24 h on a
Petri dish. Non-adherent cells were centrifuged on a Histopaque
density gradient medium (Sigma-Aldrich; Merck KGaA) and cultured
for 3 days in the presence of M-CSF (30 ng/ml) to obtain BMMs. The
BMMs were cultured with RANKL (20 ng/ml) and M-CSF (10 ng/ml) in
the presence of different concentrations of britanin (1 or 5 µM) in
96-well plates. After 4 days, the cells were fixed in 4%
paraformaldehyde for 15 min at room temperature and then stained
using a TRAP staining kit according to the manufacturer's
instructions. TRAP-positive multinucleated cells with ≥3 nuclei
were considered osteoclasts. To examine signaling pathway in
response to britanin, BMMs were stimulated with DMSO or 5 µM of
britanin for 0, 5, 15, and 30 min, then the reaction was terminated
using ice-cold PBS.
Cell viability assay
The cytotoxic effects of britanin were evaluated
using the MTT assay. BMMs were cultured with M-CSF (10 ng/ml) with
or without britanin (1 or 5 µM) for 3 days at 37°C. MTT reagent was
then added to each well, and the cells were incubated at 37°C for 2
h. The formazan was subsequently solubilized with DMSO. The
absorbance at 570 nm was measured using an Epoch 96-well microplate
reader (BioTek Instruments, Inc.).
Western blotting
BMMs were lysed using RIPA lysis buffer
(Pro-Prep™; Intron Biotechnology, Inc.) containing
protease and phosphatase inhibitors. Protein concentrations were
determined using the Pierce BCA Protein Assay Kit (Thermo Fisher
Scientific, Inc.). Proteins (30 µg/lane) were separated on 10% gels
using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred to 0.2-µm nitrocellulose membranes (Whatman plc;
Cytiva). Before incubation with the primary antibody, the membranes
were blocked with a blocking buffer [3% non-fat milk in
Tris-buffered saline containing 0.1% Tween 20 (TBS-T)] for 1 h at
room temperature. The membranes were incubated with specific
primary antibodies (1:1,000 by volume) overnight at 4°C in blocking
buffer. The membranes were then washed with TBS-T and incubated
with a secondary antibody (HRP-conjugated; cat. no. PA1-74362;
1:5,000 by volume; Thermo Fisher Scientific, Inc.) in blocking
buffer for 1 h at room temperature. Immunoreactive bands were
analyzed using a WesternBright ECL kit (Advansta, Inc.) and
recorded using a chemiluminescent imager (cSeries Capture Software,
version 1.9.5.0606; Azure Biosystems, Inc.).
Immunofluorescence staining
BMMs were plated on glass coverslips and cultured
with RANKL (20 ng/ml) and M-CSF (10 ng/ml) in the presence or
absence of britanin (5 µM). After 4 days of culture, the cells were
fixed with 4% paraformaldehyde for 15 min at room temperature and
then treated with 0.25% Triton X-100 in phosphate-buffered saline
(PBS). The cells were blocked with 5% normal goat serum (S-1000;
Vector Laboratories, Inc.) in PBS for 1 h at room temperature,
after which the cells were incubated with Nfatc1 primary antibody
(1:500) overnight at 4°C, followed by Alexa Fluor 488-conjugated
secondary antibody (1:100; cat. no. A-11059; Thermo Fisher
Scientific, Inc.) for 1 h at room temperature. Rhodamine-conjugated
phalloidin (1:1,000; Cytoskeleton, Inc.) and
4′,6-diamidino-2-phenylindole dihydrochloride (1:10,000; Santa Cruz
Biotechnology, Inc.) were used to stain actin and nuclei,
respectively. Fluorescence images were obtained using a Leica
DM2500 microscope (Leica Microsystems GmbH). The images were
aligned using ImageJ software (version 1.52a; National Institutes
of Health).
Bone resorption pit assay
Briefly, 2.5×104 BMMs were placed on each
bone slice (IDS Nordic) and incubated with RANKL (20 ng/ml) and
M-CSF (10 ng/ml) for 3 days. After osteoclast formation, the cells
were treated with or without 5 µM britanin for 2 days at 37°C. The
bone slices were washed and soaked in hematoxylin solution for 30
sec at room temperature to visualize the resorption pits. The pit
area was quantified using ImageJ software (version 1.52a).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from britanin or
DMSO-treated cells for 1 day using TRI-Solution™ (Bio
Science Technology), and 1 µg RNA was converted into cDNA using
SuperScript II reverse transcriptase according to the
manufacturer's protocol (Invitrogen; Thermo Fisher Scientific,
Inc.). The cDNA and each primer set were mixed with SYBR Premix Ex
Taq (Takara Bio, Inc.). qPCR was performed using a LightCycler 1.5
Real-Time PCR System (Roche Diagnostics). The amplification
conditions consisted of an initial denaturation step at 95°C,
followed by 40 cycles of denaturation at 95°C, annealing at 60°°C
and extension at 72°C. The primers used for the qPCR are listed in
Table I and included Gapdh
as the reference gene. The relative gene expression data were
calculated using the 2−ΔΔCq method (23).
 | Table I.PCR primer sequences. |
Table I.
PCR primer sequences.
| Gene | Primer sequence (5′
to 3′) | Amplicon size
(bp) | RefSeq NCBI
no. | Amplification
factor (E) |
|---|
| Nfatc1 | F:
ACCACCTTTCCGCAACCA | 72 | NM_001164112.1 | 2.03 |
|
| R:
TTCCGTTTCCCGTTGCA |
|
|
|
| Ctsk | F:
GGCTGTGGAGGCGGCTAT | 66 | NM_007802.4 | 1.94 |
|
| R:
AGAGTCAATGCCTCCGTTCTG |
|
|
|
| Mmp9 | F:
AAAGACCTGAAAACCTCCAACCT | 77 | NM_013599.5 | 1.97 |
|
| R:
GCCCGGGTGTAACCATAGC |
|
|
|
| Acp5 | F:
TCCCCAATGCCCCATTC | 63 | NM_001102405.1 | 2.10 |
|
| R:
CGGTTCTGGCGATCTCTTTG |
|
|
|
|
Dc-stamp | F:
CTTCCGTGGGCCAGAAGTT | 64 | NM_029422.4 | 2.13 |
|
| R:
AGGCCAGTGCTGACTAGGATGA |
|
|
|
| TNF-α | F:
GGTGCCTATGTCTCAGCCTCTT | 139 | NM_013693.3 | 2.03 |
|
| R:
GCCATAGAACTGATGAGAGGGAG |
|
|
|
| Nrf2 | F:
CAGCATAGAGCAGGACATGGAG | 107 | NM_010902.5 | 1.89 |
|
| R:
GAACAGCGGTAGTATCAGCCAG |
|
|
|
| Nqo1 | F:
GCCGAACACAAGAAGCTGGAAG | 120 | NM_008706.5 | 2.08 |
|
| R:
GGCAAATCCTGCTACGAGCACT |
|
|
|
| HO-1 | F:
CACTCTGGAGATGACACCTGAG | 115 | NM_010442.2 | 2.14 |
|
| R:
GTGTTCCTCTGTCAGCATCACC |
|
|
|
| TRAF6 | F:
AACTGTGCTGTGTCCATGGC | 246 | NM_001303273.1 | 1.89 |
|
| R:
CAGTCTCATGTGCAACTGGG |
|
|
|
| Blimp1 | F:
TTCTTGTGTGGTATTGTCGGGACT | 148 | NM_001405929.1 | 1.90 |
|
| R:
TTGGGGACACTCTTTGGGTAGAGTT |
|
|
|
| Irf8 | F:
GATCGAACAGATCGACAGCA | 214 | NM_001301811.1 | 1.91 |
|
| R:
AGCACAGCGTAACCTCGTCT |
|
|
|
| Bcl6 | F:
ATGAGATTGCCCTGCATTTC | 202 | NM_009744.5 | 1.89 |
|
| R:
TTCTTCCAGTTGCAGGCTTT |
|
|
|
| Gapdh | F:
ATGACATCAAGAAGGTGGTG | 177 | NM_001411843.1 | 1.90 |
|
| R:
CATACCAGGAAATGAGCTTG |
|
|
|
Ti particle-induced calvarial
osteolysis model
Animal experiments were approved by the Committee on
the Care and Use of Animals in Research at Kyungpook National
University (Daegu, Korea; approval no. 2021-0071). As previously
described, a mouse calvarial osteolysis model was established to
determine the protective effect of britanin on osteolysis in
vivo (24). Briefly,
6-week-old C57BL/J6 male mice (n=20) were divided into four groups:
Sham (DMSO control; n=4), Ti + vehicle (DMSO) (15 mg Ti particles;
n=6), Ti + 2 mg britanin (15 mg Ti particles plus 2 mg/kg/day
britanin; n=4) and Ti + 25 mg britanin (15 mg Ti particles plus 25
mg/kg/day britanin; n=6). The mice were purchased from Dae Han Bio
Link Co., Ltd., and maintained under conventional housing
conditions at 22–24°C, with 50–60% humidity, a 12-h light/12-h dark
cycle, and free access to water and food pellets. For mouse
anesthesia, 240 mg/kg avertin was injected intraperitoneally. The
mouse heads were shaved and disinfected using a 10% povidone-iodine
solution under anesthesia. A periosteal pocket was created using a
28-gauge needle, followed by an injection of pure Ti particles (15
mg) dissolved in 30 µl PBS and embedded in the center of the
calvarium under the periosteum. Britanin (2 or 25 mg/kg) was
administered intraperitoneally daily for 9 days, while DMSO was
administered daily to the mice in the sham and vehicle groups. No
adverse events were detected during the animal experiments, and the
mice were sacrificed at the end of the experimental period (10
days). To euthanize the mice, 480 mg/kg avertin was injected
intraperitoneally, and cervical dislocation was performed under
anesthesia. The calvariae were isolated and fixed for 1 day in 4%
paraformaldehyde at 4°C for micro-computed tomography (CT) and
histological analyses.
Micro-CT scanning
Harvested mouse calvariae were fixed and then
analyzed using a high-resolution micro-CT scanner (Skyscan 1272;
Bruker Corporation). The scan was set to a resolution of 10 µm with
a voltage source of 70 kV and current of 142 µA. The
three-dimensional image was reconstructed using CTvox software
(version 3.0.0 r1114; Bruker), and a rectangular region of interest
(3.2×2.1×0.1 mm) around the midline suture was assessed for further
quantitative analysis as previously described (25). Bone volume as a percentage of
tissue volume and the number of pores in each sample were measured
as previously reported (26).
Histological and histomorphometric
analysis
Calvarial samples were decalcified in 10% EDTA for
10 days and then embedded in paraffin. The paraffinized samples
were cut in the coronal direction to a section thickness of 6 µm.
Histological sections were stained with hematoxylin and eosin
(H&E; hematoxylin for 5 min and eosin for 1 min at room
temperature) or a TRAP kit (cat. no. 387A; MilliporeSigma)
according to the manufacturer's instructions. The stained area was
examined using a bright-field light microscope (Leica Microsystems
GmbH), and the number of TRAP-positive osteoclasts in each sample
was quantified using ImageJ software.
Statistical analysis
Experiments were performed in triplicate, and all
data are presented as the mean ± standard deviation. Statistical
analyses were performed using a two-tailed unpaired Student's
t-test or one-way analysis of variance with Tukey's multiple
comparison post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Britanin inhibits RANKL-induced
osteoclastogenesis
Britanin was originally identified through the
screening of natural product-derived compounds in an osteoclast
differentiation model, with testing conducted at a concentration of
5 µM (22,27,28).
Once its inhibitory effect on osteoclast differentiation was
confirmed, its cytotoxicity was assessed across a range of
concentrations from 1 to 10 µM. No cytotoxic effects were observed
on BMMs at these concentrations, as determined by MTT assay. In
addition, a 5-µM concentration of britanin significantly inhibited
osteoclast differentiation in the presence of M-CSF (30 ng/ml) when
applied for 3 days in vitro. As a result, concentrations of
1 and 5 µM were selected for further investigation to demonstrate
the inhibitory effect on osteoclasts in vitro in the present
study. Moreover, 5 µM britanin treatment significantly increased
cell growth by 19% at 24 h (data not shown) and 11.5% at 72 h in
BMMs when compared with that in the control group (Fig. S1).
The effect of britanin on osteoclast differentiation
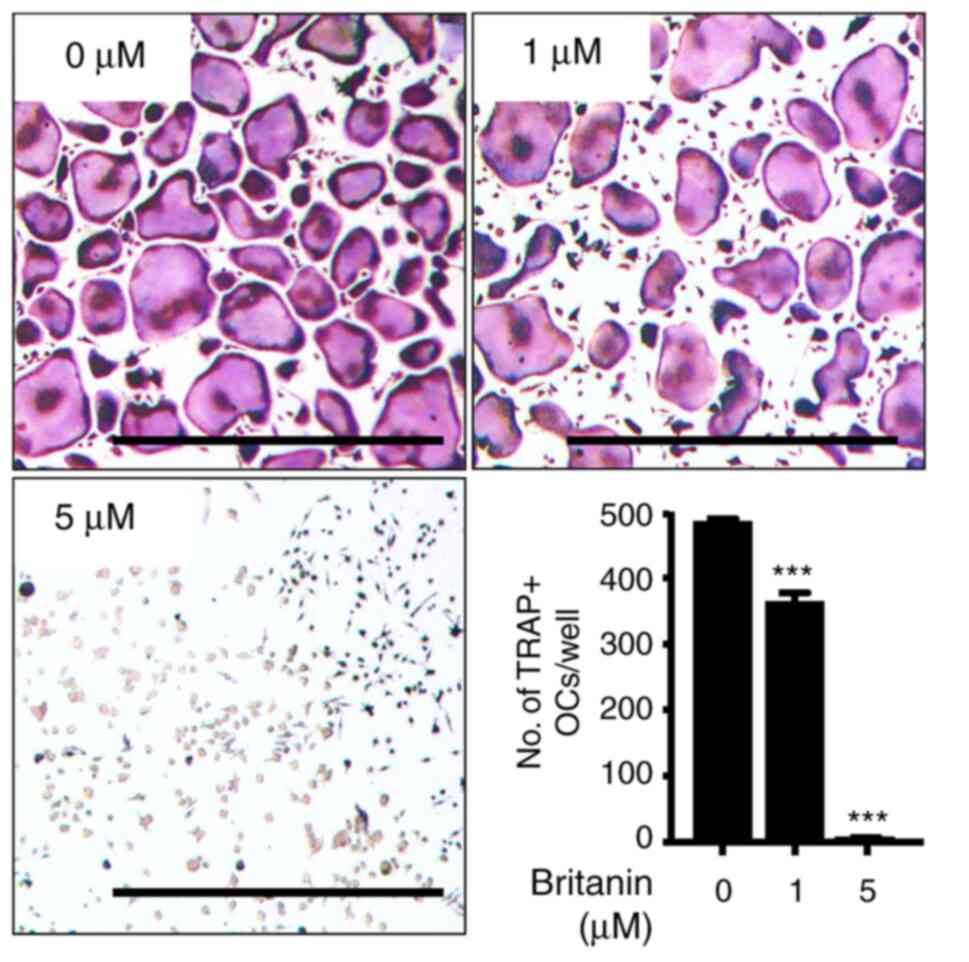
in BMMs was evaluated using TRAP staining. The BMMs were incubated
with RANKL (20 ng/ml) and M-CSF (10 ng/ml) in the presence or
absence of britanin. Multinucleated osteoclasts were formed in the
control group, whereas britanin significantly inhibited osteoclast
formation in a concentration-dependent manner, even when cell
growth was enhanced. Compared with the control group, treatment
with 1 and 5 µM britanin significantly reduced the number of
TRAP-positive multinucleated cells by 25 and 98.7%, respectively
(Fig. 1). These results suggest
that britanin directly suppressed osteoclast formation without
affecting cell viability.
Britanin suppresses the expression of
osteoclast-specific genes but induces the expression of antioxidant
marker genes
To further confirm that britanin inhibits osteoclast
differentiation, the mRNA expression levels of
osteoclast-associated genes were examined by RT-qPCR. The results
revealed that the expression of Nfatc1 was increased in the
RANKL-treated group, and britanin significantly suppressed this
expression (Fig. 2A). Likewise,
britanin significantly suppressed the RANKL-induced expression of
osteoclast marker genes, namely Acp5, Dc-stamp, Mmp9 and
Ctsk (Fig. 2B-E). Following
3 days of treatment, britanin markedly decreased the protein levels
of Nfatc1 and Ctsk when compared with those in the
osteoclastogenesis control (RANKL-treated) group (Fig. 2K). Inflammation and oxidative
stress are major events that mediate osteolysis. Therefore, the
effect of britanin on the expression of genes associated with
inflammation and oxidative stress, namely TNF receptor-associated
factor 6 (TRAF6) and TNF-α, was examined. The results
demonstrated a significant reduction in the mRNA expression levels
of TRAF6 and TNF-α in the RANKL-induced cells upon
treatment with britanin (Fig. 2F and
G).
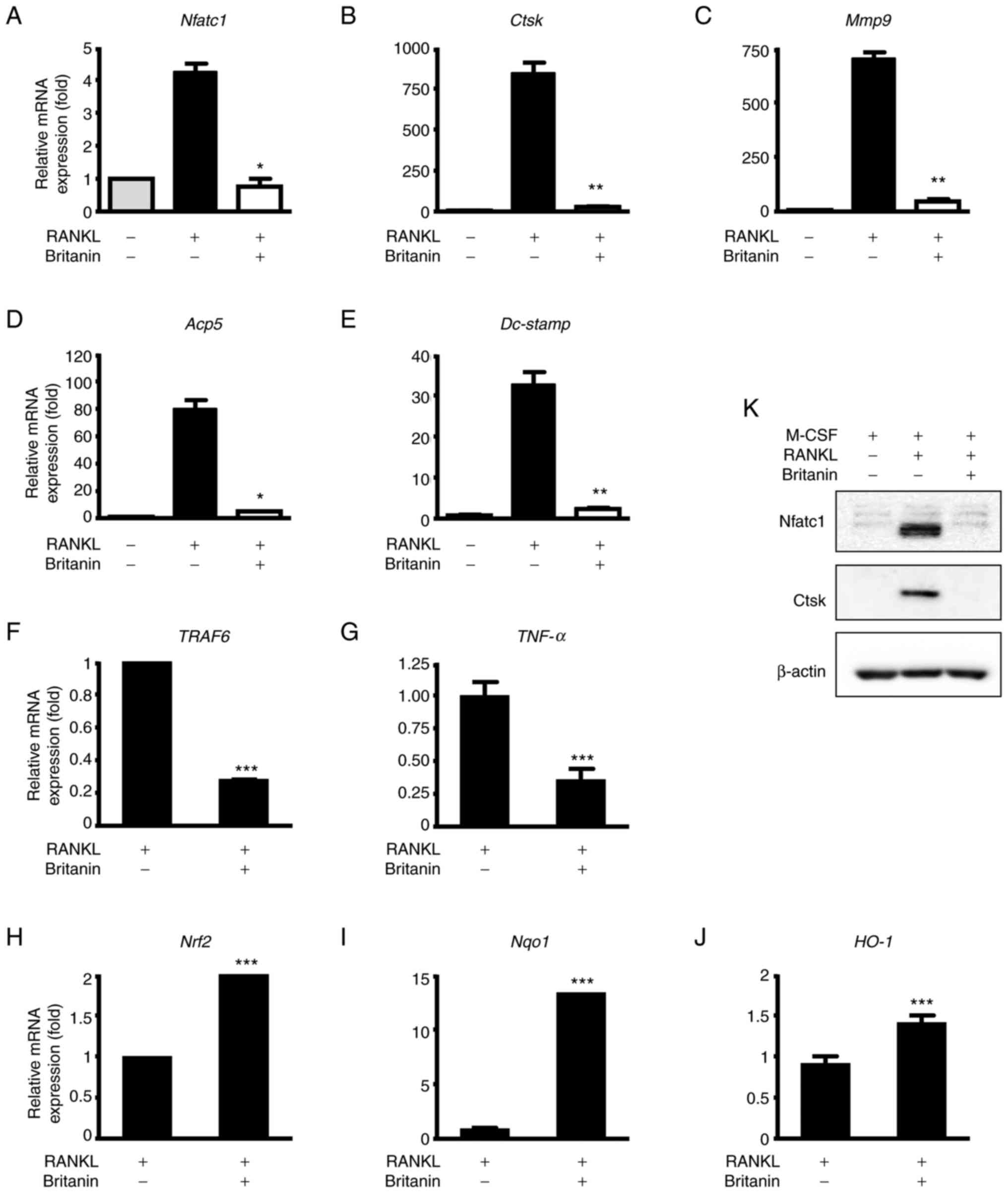 | Figure 2.Britanin suppresses the expression of
osteoclast and Nrf2-associated markers during osteoclastogenesis.
(A-E) BMMs were cultured with M-CSF (10 ng/ml) and RANKL (20 ng/ml)
in the absence or presence of britanin for 4 days and RT-qPCR was
performed to analyze the expression of (A) Nfatc1, (B)
Ctsk, (C) Mmp9, (D) Acp5 and (E)
Dc-stamp. (K) BMMs were incubated in an osteoclastogenic
medium with vehicle or britanin for 3 days and the protein
expression levels of Nfatc1 and Ctsk were determined using western
blotting. BMMs were cultured with M-CSF (10 ng/ml) and RANKL (20
ng/ml) in the absence or presence of britanin for (F and G) 1 day
and (H and I) 4 days, and RT-qPCR was performed to analyze the
expression of (F) TRAF6, (G) TNF-α, (H) Nrf2,
(I) Nqo1 and (J) HO-1. *P<0.05, **P<0.01 and
***P<0.001 vs. RANKL-treated control as analyzed by (A-E) ANOVA
with Tukey's multiple comparison post hoc test and (F-J) two-tailed
unpaired Student's t-test. Nrf2, nuclear factor erythroid-2-related
factor 2; BMMs, bone marrow-derived macrophages; M-CSF, macrophage
colony-stimulating factor; RANKL, receptor activator of nuclear
factor kB ligand; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; Nfatc1, nuclear factor of activated
T-cells, cytoplasmic 1; Ctsk, cathepsin K; Mmp9, matrix
metallopeptidase 9; Acp5, tartrate-resistant acid phosphatase 5;
Dc-stamp, dendritic cell-specific transmembrane protein; TRAF6,
tumor necrosis factor receptor-associated factor 6; TNF-α, tumor
necrosis factor-α; Nqo1, NAD(P)H quinone oxidoreductase 1; HO-1,
heme oxygenase 1. |
Nrf2 is a transcription factor that regulates
cellular defense against oxidative stress, and its deficiency has
been shown to promote RANKL-induced osteoclast differentiation
(29,30). In BMMs undergoing
osteoclastogenesis, britanin increased the mRNA expression of
Nrf2, NAD(P)H quinone oxidoreductase 1 (Nqo1) and
heme oxygenase 1 (HO-1) on day 4 (Fig. 2H-J). Taken together, these findings
suggest that britanin may inhibit osteoclast differentiation via
downregulation of the expression levels of genes associated with
inflammatory cytokines and oxidative stress in vitro.
Britanin suppresses F-actin ring
formation
RANKL-induced mature osteoclasts attach to the bone
matrix, reorganize the cytoskeleton and form circular actin ring
structures that provide a sealing zone for osteoclast bone
resorption (31,32). Frequently, large osteoclasts
indicate more bone resorption, but in certain cases, small
osteoclasts can actively resorb bone (33). Therefore, the effect of britanin on
F-actin ring formation was investigated using immunofluorescence
staining. While the control group exhibited large F-actin rings,
the formation of F-actin rings was impaired in the britanin-treated
group (Figs. 3A and S2). Compared with the RANKL control
group, britanin significantly reduced the number of F-actin rings
and nuclear Nfatc1-positive cells by 85.1 and 87.1%, respectively
(Fig. 3B and C). In addition, bone
resorption was monitored to determine whether it was influenced by
the effect of britanin on F-actin ring formation. Britanin
treatment was performed after initial osteoclast formation, and
under these conditions, britanin markedly reduced the resorption
ability of bone slices compared with that in the RANKL control
group (Fig. 3D). Collectively,
these findings suggest that britanin may attenuate osteoclast
function.
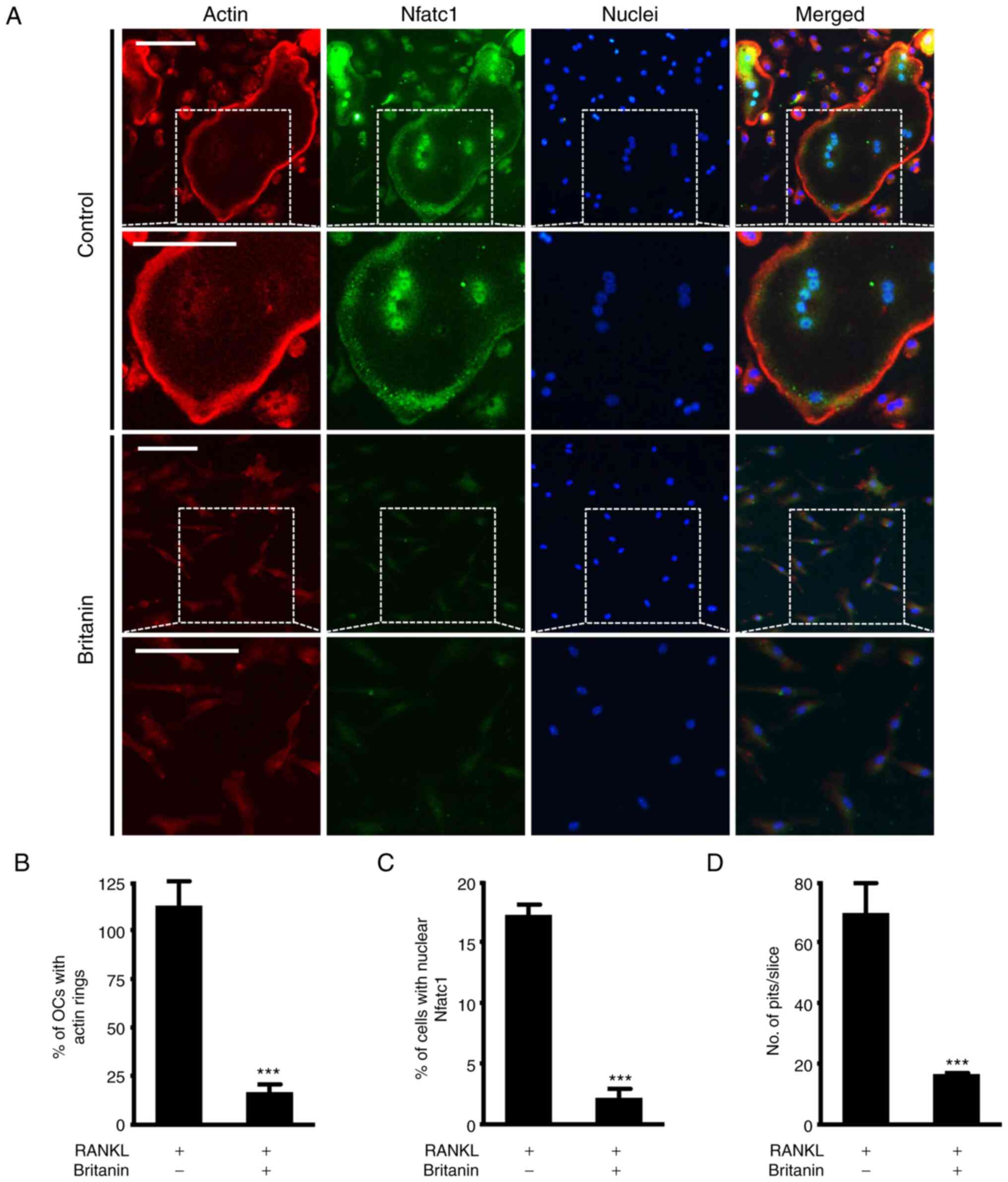 | Figure 3.Britanin attenuates the formation of
actin rings and resorption pits. (A) BMMs were incubated on glass
coverslips with M-CSF (10 ng/ml) and RANKL (20 ng/ml) for 4 days in
the absence or presence of britanin. Actin, nuclei and Nfatc1 were
stained with rhodamine-phalloidin (red),
4′,6-diamidino-2-phenylindole dihydrochloride (blue) and
anti-Nfatc1 antibody (green), respectively. The white dashed box
indicates the magnified region. Scale bar, 50 µm. The percentage of
(B) OCs displaying actin rings and (C) nuclear Nfatc1-positive
cells was assessed. (D) BMMs were seeded on bone slices and
cultured with an OC-inducing medium for 3 days. The cells were then
treated with or without britanin for 2 days. Bone slices were
stained with hematoxylin to observe the resorption pits. The number
of resorption pits per bone slice was counted using ImageJ
software. ***P<0.001 vs. RANKL-treated control as analyzed using
two-tailed unpaired Student's t-test. BMMs, bone marrow-derived
macrophages; M-CSF, macrophage colony-stimulating factor; RANKL,
receptor activator of nuclear factor kB ligand; Nfatc1, nuclear
factor of activated T-cells, cytoplasmic 1; OC, osteoclast. |
Britanin inhibits osteoclast
differentiation via the repression of transcriptional negative
regulators
To elucidate the molecular mechanism underlying the
inhibitory effect of britanin on RANKL-induced osteoclastogenesis,
RANKL downstream signaling pathways, including ERK, JNK, p38, AKT
and NF-κB, were analyzed using western blotting. The
phosphorylation of ERK was notably decreased by britanin at 5 and
15 min; however, the phosphorylation levels of p38, JNK, AKT and
IκBα were not markedly altered (Fig.
4A and B). These data suggest that britanin suppresses the ERK
signaling pathway, which may underlie its ability to attenuate
osteoclast differentiation and function.
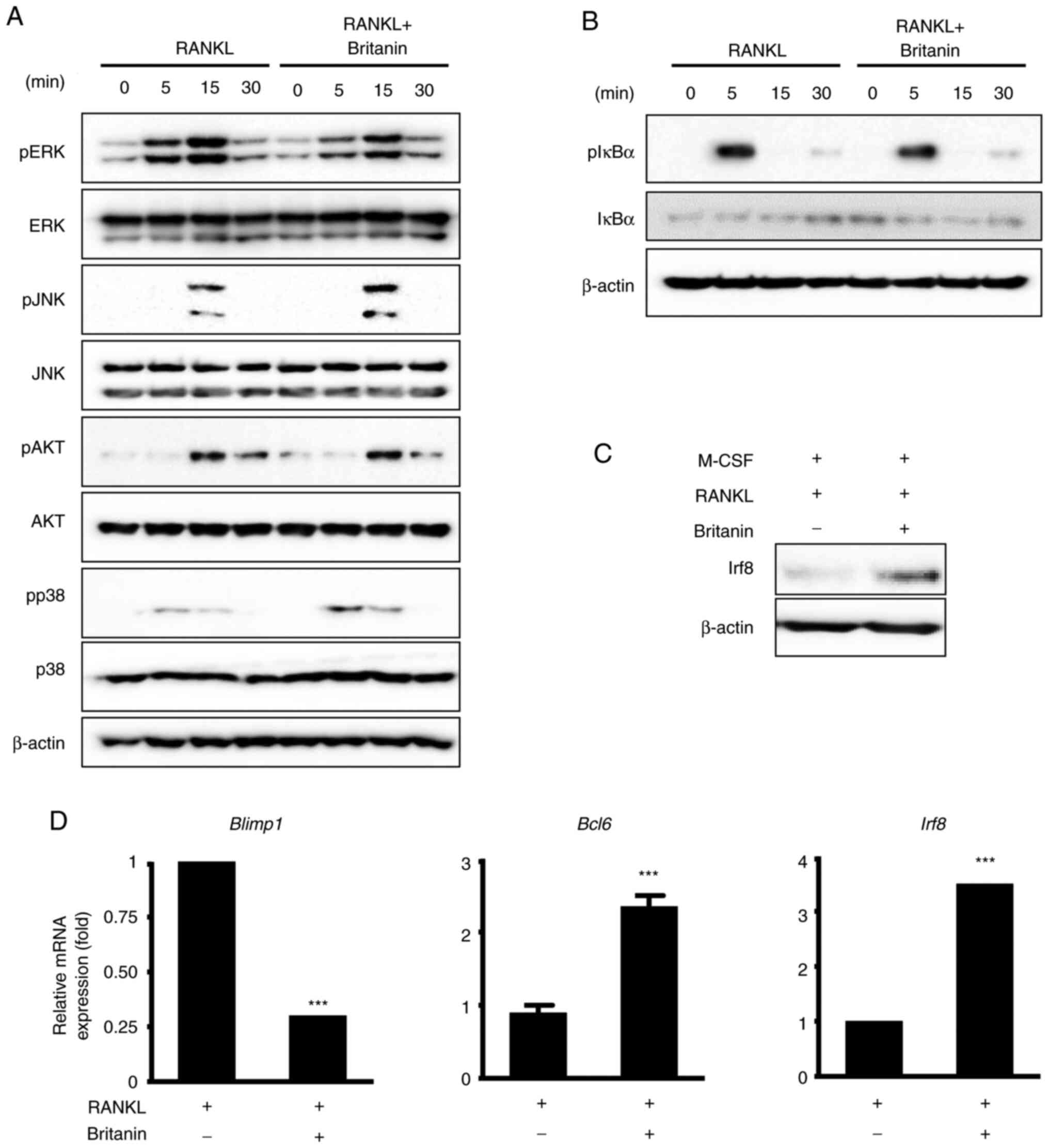 | Figure 4.Britanin inhibits the suppression of
negative mediators of RANKL-induced osteoclast differentiation. (A
and B) BMMs incubated in serum-free medium were pretreated with
britanin or vehicle for 1 h. Next, several time points were
examined following treatment with RANKL (50 ng/ml). Phosphorylation
levels of ERK, JNK, Akt, p38 and IκBα were determined using western
blotting. (C) BMMs were incubated in an osteoclastogenic medium
with vehicle or britanin for 3 days. The protein expression of Irf8
was examined using western blotting. (D) BMMs were cultured with
M-CSF (10 ng/ml) and RANKL (20 ng/ml) in the absence or presence of
britanin for 4 days. Reverse transcription-quantitative PCR was
performed to analyze the expression of Blimp1, Bcl6 and
Irf8. ***P<0.001 vs. RANKL-treated control as analyzed
using two-tailed unpaired Student's t-test. RANKL, receptor
activator of nuclear factor kB ligand; BMMs, bone marrow-derived
macrophages; ERK, extracellular signal-regulated kinase; JNK, c-Jun
N-terminal kinase; IκBα, inhibitor of nuclear factor kBa; Irf8,
interferon regulatory factor-8; M-CSF, macrophage
colony-stimulating factor; Blimp1, B lymphocyte-induced maturation
protein-1; Bcl6, B-cell lymphoma 6; p-, phosphorylated. |
The expression of Nfatc1 can also be suppressed by
anti-osteoclastogenic genes such as Irf8, Bcl6 and
MafB, and the expression levels of these anti-osteoclastic
genes can be downregulated by Blimp1 (11–13).
Britanin treatment for 4 days was confirmed to induce Irf8 protein
expression associated with M-CSF and RANKL (Fig. 4C). In the case of mRNA expression,
compared with the control group, britanin significantly decreased
the expression of Blimp1 and significantly increased the
expression of Bcl6 and Irf8 (Fig. 4D). These findings suggest that
britanin may suppress osteoclast differentiation via the
upregulation of negative regulators, including Irf8 and Bcl6.
Britanin exhibits inhibitory effects
on a Ti particle-induced mouse calvarial osteolysis model
After confirming that britanin reduced osteoclast
formation and function in vitro, the effect on osteolysis
was investigated in a Ti particle-induced osteolysis mouse model
(Fig. 5). In previous experiments
(21), compounds that affected
cell responses at a concentration of ~5 µM in vitro also
demonstrated effective results in vivo within the range of
20–30 mg/kg. Consequently, an initial dose of 25 mg/kg was selected
for in vivo testing, whereas an additional dose of 5 mg/kg
was selected to assess the dose-dependent efficacy of britanin. To
induce osteolysis, Ti particles were implanted into mouse calvaria.
This model allowed the measurement of bone resorption in
vivo owing to Ti particle-induced activated osteoclasts, which
are typically generated in the Ti-implanted sites of patients
(34). Despite excluding the
load-bearing effect that can induce additional osteoclast-mediated
bone resorption, Ti-particle-induced inflammation and osteoclast
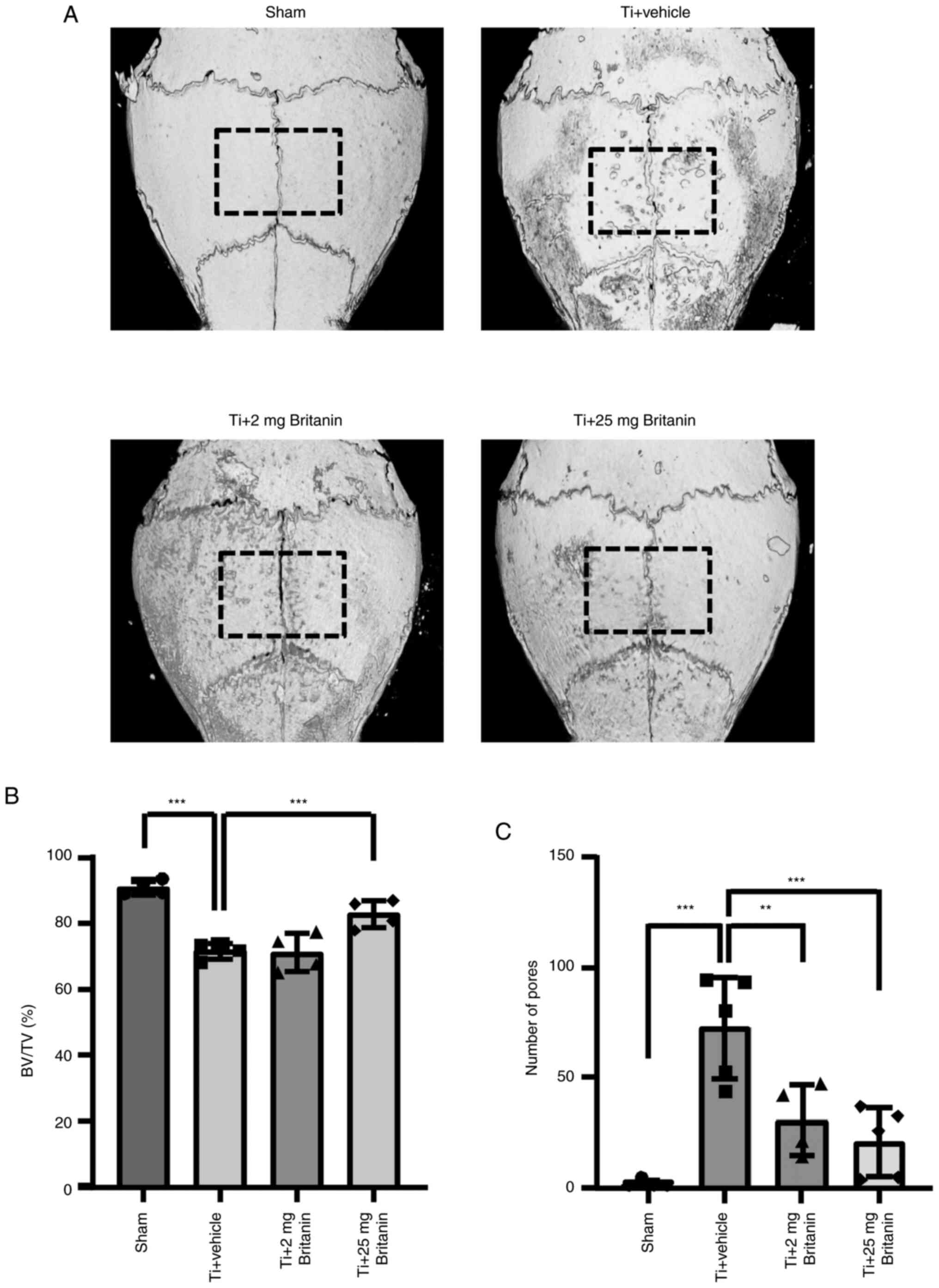
differentiation were detected. Micro-CT analysis was performed in
Ti particle-loaded sites, revealing the presence of extensive bone
resorption in the Ti particle-implanted group (vehicle). However,
britanin appeared to reduce bone resorption in a dose-dependent
manner (Fig. 5A). Based on
quantitative analysis, the administration of high-dose britanin (Ti
+ britanin; 25 mg/kg/day) significantly preserved bone volume
compared with that in the Ti-loading group (Fig. 5B). The number of bone-resorbing
pits was significantly elevated in the Ti group, and the effect of
Ti was reduced in the britanin-treated groups in a dose-dependent
manner (Fig. 5C).
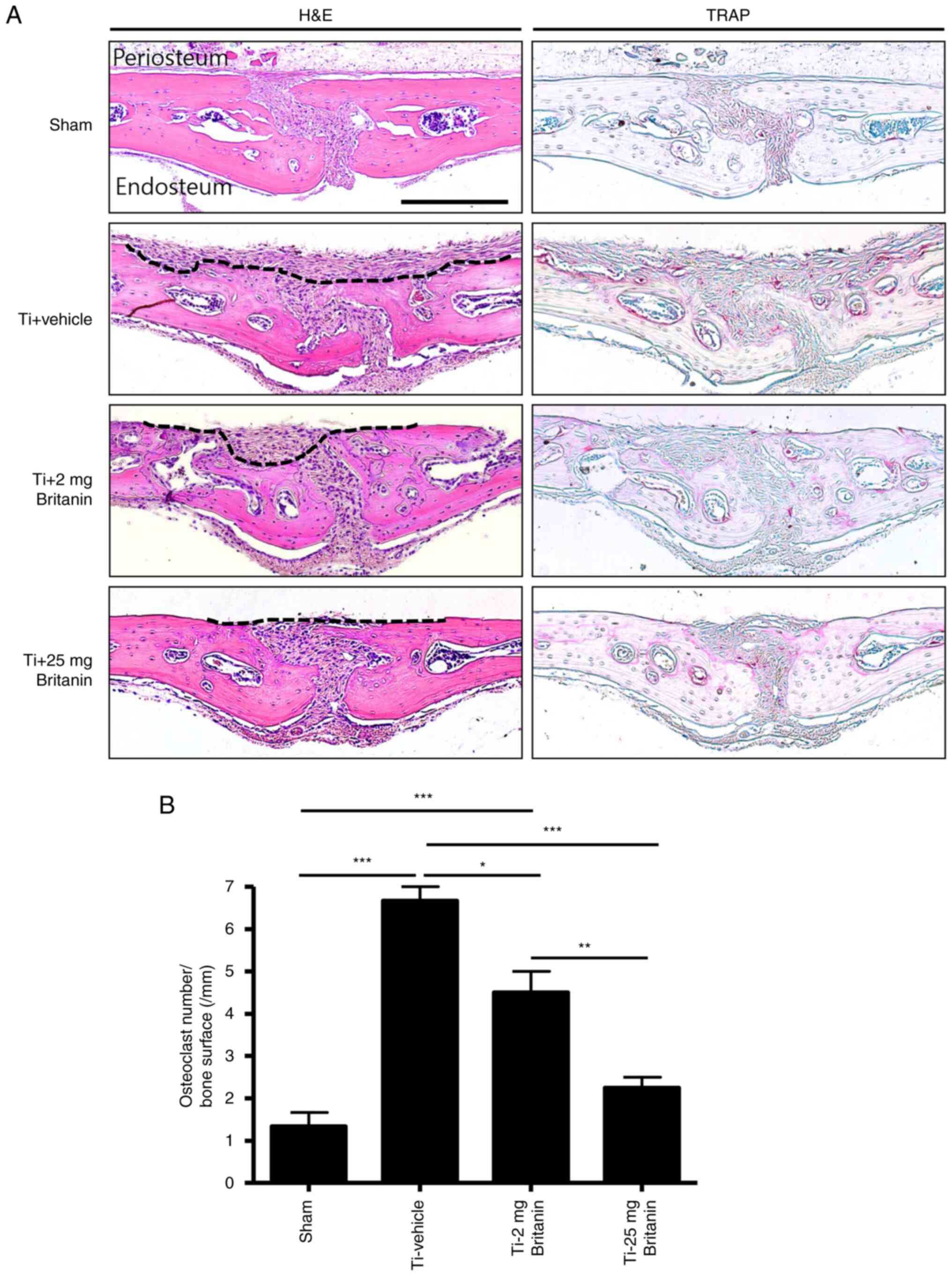
Histochemical analysis demonstrated that britanin
protected bone from Ti particle-induced resorption in a
dose-dependent manner (Fig. 6A).
In the H&E stained tissue, the periosteum layers of the
calvarial bone were clean and flat in the sham group. By contrast,
a deep and wide area of periosteum side bone was resorbed in the Ti
+ vehicle group. Treatment with britanin substantially and
dose-dependently reduced the Ti particle-induced resorbed area.
Moreover, TRAP staining showed that the number of TRAP-positive
osteoclasts was reduced by 29 and 56% in the Ti + britanin 2 mg (2
mg/kg/day) and Ti + britanin 25 mg (25 mg/kg/day) groups,
respectively, compared with that in the Ti-vehicle group (Fig. 6B). These results suggest that
britanin inhibits in vivo osteoclast formation and indicate
that britanin may protect the bone against Ti particle-induced
osteolysis via the suppression of osteoclast formation in mice.
Discussion
Inflammation and oxidative stress are known to
mediate periprosthetic osteolysis (24). Ti is widely used in medical
devices, including dental and joint implants, which are used to
recover the function of damaged tissues. However, weight or load
bearing can gradually generate wear particles of various sizes from
the implants over time. These nano- to micro-sized wear particles
induce inflammation, oxidative stress and periprosthetic
osteolysis, and may subsequently result in implant failure
(34–36). To the best of our knowledge, the
present study is the first to demonstrate that britanin exerts
antiosteoclastic activity, indicating its therapeutic potential
against wear particle-induced osteolysis.
Oxidative stress is typically involved in osteoclast
differentiation (37). Wear
particles induce ROS-mediated immune responses (24) and are crucial for RANKL-induced
osteoclast differentiation (38).
Elevated ROS levels decrease Nrf2 expression, thereby contributing
to increased Nfatc1 expression during osteoclast formation
(39,40). Accordingly, ROS signaling is
indicated to be a suitable target for the pharmacological treatment
of bone loss (30). The
pathogenesis of periprosthetic osteolysis involves several
inflammatory events. Specifically, Ti particles induce inflammatory
cytokines such as TNF-α and ILs (1). TNF-α promotes osteoclast
differentiation in the presence of ROS, and the expression of TNF-α
is increased by ROS in the presence of large quantities of
iron-like wear particles. In the present study, britanin not only
upregulated the expression of ROS defense molecules, including
Nrf2, Nqo1 and HO-1, in the absence of excess iron
in vitro, but also significantly inhibited the RANKL-induced
expression of TNF-α.
Considering the anti-inflammatory and antioxidative
properties of britanin, it was speculated that britanin would be
able to suppress abnormal osteoclast differentiation effectively,
in vitro and in vivo. The inhibition of inflammation
and ROS can suppress osteoclastogenesis; however, the regulation of
osteoclast function can be challenging. Therefore,
osteoclastogenesis is an important therapeutic target in
periprosthetic osteolysis. The present study examined the effects
of britanin on the differentiation and function of osteoclasts to
explore its potential mode of action. The binding of RANK to RANKL
leads to osteoclast differentiation and activation. The RANK-RANKL
interaction activates the NF-κB and MAPK downstream signaling
pathways by recruiting the signaling molecule TRAF6 and
subsequently inducing the expression of the master osteoclast
regulator Nfatc1 (41–44).
In the present study, britanin significantly reduced the expression
of Nfatc1 and its target genes, as well as ERK phosphorylation,
without impacting NF-κB activity. In previous studies, britanin was
found to exhibit antitumor effects via the inhibition of NF-κB
activity in pancreatic, gastric and prostate cancer cell lines
(16,17,45).
NF-κB inhibition has also been shown to mediate mast cell-mediated
inflammatory responses (12,13).
However, in the present study, the results indicated that britanin
inhibited osteoclast differentiation via suppression of the ERK
pathway. Accordingly, these results suggest that the effects of
britanin on NF-κB and MAPK may differ according to the cell type or
environment.
Blimp1 is a transcriptional repressor that
suppresses the expression of anti-osteoclastogenic genes, including
Irf8, MafB and Bcl6. Blimp1 deletion has been shown
to increase Bcl6 expression, resulting in osteopetrosis due
to failure of osteoclast function (10,46).
Conversely, Irf8 binds to TRAF6 through physical interactions and
induces TRAF6 ubiquitination (47). Irf8 deficiency activates the
expression of Nfatc1, resulting in osteoporosis in vivo
owing to excessive osteoclast formation (48). In the present study, britanin
inhibited Blimp1 gene expression and increased the
expression of Bcl6 and Irf8, suggesting that its
inhibitory effect on osteoclast differentiation may be partially
associated with an attenuating effect on the Blimp1-mediated
repression of anti-osteoclastogenic genes.
In addition, the present study evaluated the
therapeutic potential of britanin in periprosthetic osteolysis in a
mouse calvarial model. Ti particles significantly induced
osteolysis in the examined mouse model, whereas britanin reduced
bone resorption. Histological analysis revealed that britanin
prevented Ti particle-induced osteolysis. In addition, TRAP
staining indicated that britanin suppressed osteoclast formation
in vivo. These results strongly suggest that britanin not
only inhibits osteoclast differentiation but also suppresses
inflammatory signals, such as those mediated by TNF-α and ROS.
These britanin-mediated activities effectively inhibited osteolysis
in vivo. Although the inhibitory effect of britanin on
osteolysis in vivo was examined using a mouse calvaria
osteolysis model, this may not be the most suitable model for fully
mimicking human osteolysis. In this model, the absence of
load-bearing prevents the gradual generation of titanium particles.
Moreover, it does not replicate physiological dynamic environments
such as the movement of joints in activities such as running and
jumping.
In summary, the present study indicated that
britanin regulates inflammation and oxidative stress signaling and
inhibits osteoclast differentiation by the downregulation of
Blimp1-Nfatc1 in vitro, thereby suppressing osteoclast
differentiation and the expression of osteoclast-specific marker
genes. In addition, britanin was demonstrated to protect bone from
Ti particle-induced calvarial osteolysis in vivo. These
results strongly suggest that britanin is a potential candidate for
the treatment of osteoclast-associated diseases and wear
particle-induced osteolysis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by a National Research Foundation of
Korea grant funded by the Korean government (MSIT) (grant nos.
2017R1A5A2015391 and 2020M3A9I4039539).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JAK, SL and EKP were responsible for
conceptualization. JAK, SL and HJI designed the study methodology.
Validation was performed by JAK, SL and HJI. Formal analysis was
performed by JAK, SL and EKP. JEK, KY and HC carried out the
investigation. JM and HC contributed resources, and extracted and
analyzed the britanin. The original draft of the manuscript was
prepared and written by JAK and SL, and was reviewed and edited by
HC and EKP. HC and EKP supervised the study. HC and EKP confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Animal experiments were approved by the Committee on
the Care and Use of Animals in Research at Kyungpook National
University (Daegu, Korea; approval no. 2021-0071).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eger M, Hiram-Bab S, Liron T, Sterer N,
Carmi Y, Kohavi D and Gabet Y: Mechanism and prevention of titanium
particle-induced inflammation and osteolysis. Front Immunol.
9:29632018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holding CA, Findlay DM, Stamenkov R, Neale
SD, Lucas H, Dharmapatni AS, Callary SA, Shrestha KR, Atkins GJ,
Howie DW and Haynes DR: The correlation of RANK, RANKL and TNFalpha
expression with bone loss volume and polyethylene wear debris
around hip implants. Biomaterials. 27:5212–5219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Landgraeber S, Jäger M, Jacobs JJ and
Hallab NJ: The pathology of orthopedic implant failure is mediated
by innate immune system cytokines. Mediators Inflamm.
2014:1851502014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tao H, Ge G, Liang X, Zhang W, Sun H, Li M
and Geng D: ROS signaling cascades: Dual regulations for osteoclast
and osteoblast. Acta Biochim Biophys Sin (Shanghai). 52:1055–1062.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Samelko L, Caicedo MS, Lim SJ, Della-Valle
C, Jacobs J and Hallab NJ: Cobalt-Cobalt-alloy implant debris
induce HIF-1α hypoxia associated responses: A mechanism for
metal-specific orthopedic implant failure. PLoS One. 8:e671272013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun YX, Xu AH, Yang Y and Li J: Role of
Nrf2 in bone metabolism. J Biomed Sci. 22:1012015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Udagawa N: The mechanism of osteoclast
differentiation from macrophages: Possible roles of T lymphocytes
in osteoclastogenesis. J Bone Miner Metab. 21:337–343. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Negishi-Koga T and Takayanagi H:
Ca2+-NFATc1 signaling is an essential axis of osteoclast
differentiation. Immunol Rev. 231:241–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishikawa K, Nakashima T, Hayashi M,
Fukunaga T, Kato S, Kodama T, Takahashi S, Calame K and Takayanagi
H: Blimp1-mediated repression of negative regulators is required
for osteoclast differentiation. Proc Natl Acad Sci USA.
107:3117–3122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park SJ, Huh JE, Shin J, Park DR, Ko R,
Jin GR, Seo DH, Kim HS, Shin HI, Oh GT, et al: Sirt6 cooperates
with Blimp1 to positively regulate osteoclast differentiation. Sci
Rep. 6:261862016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu Y, Li X, Park YN, Kwon O, Piao D, Chang
YC, Kim CH, Lee E, Son JK and Chang HW: Britanin suppresses
IgE/Ag-induced mast cell activation by inhibiting the Syk pathway.
Biomol Ther (Seoul). 22:193–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park HH, Kim SG, Park YN, Lee J, Lee YJ,
Park NY, Jeong KT and Lee E: Suppressive effects of britanin, a
sesquiterpene compound isolated from Inulae flos, on mast
cell-mediated inflammatory responses. Am J Chin Med. 42:935–947.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim SG and Lee E, Park NY, Park HH, Jeong
KT, Kim KJ, Lee YJ, Jin M and Lee E: Britanin attenuates
ovalbumin-induced airway inflammation in a murine asthma model.
Arch Pharm Res. 39:1006–1012. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park HH, Kim MJ, Li Y, Park YN, Lee J, Lee
YJ, Kim SG, Park HJ, Son JK, Chang HW and Lee E: Britanin
suppresses LPS-induced nitric oxide, PGE2 and cytokine production
via NF-κB and MAPK inactivation in RAW 264.7 cells. Int
Immunopharmacol. 15:296–302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi K, Liu X, Du G, Cai X and Zhan Y: In
vivo antitumour activity of britanin against gastric cancer through
nuclear factor-κB-mediated immune response. J Pharm Pharmacol.
72:607–618. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li K, Zhou Y, Chen Y, Zhou L and Liang J:
A novel natural product, britanin, inhibits tumor growth of
pancreatic cancer by suppressing nuclear factor-κB activation.
Cancer Chemother Pharmacol. 85:699–709. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu G, Zhu L, Yuan X, Chen H, Xiong R,
Zhang S, Cheng H, Shen Y, An H, Li T, et al: Britanin ameliorates
cerebral ischemia-reperfusion injury by inducing the Nrf2
protective pathway. Antioxid Redox Signal. 27:754–768. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu H, Xiao H, Dai M, Xue Y and Zhao R:
Britanin relieves ferroptosis-mediated myocardial
ischaemia/reperfusion damage by upregulating GPX4 through
activation of AMPK/GSK3β/Nrf2 signalling. Pharm Biol. 60:38–45.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Piao D, Kim T, Zhang HY, Choi HG, Lee CS,
Choi HJ, Chang HW, Woo MH and Son JK: DNA topoisomerase inhibitory
activity of constituents from the flowers of Inula japonica.
Chem Pharm Bull (Tokyo). 64:276–281. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee YE, Park KS, Park EK, Im SU, Choi YH
and Song KB: Polycan suppresses osteoclast differentiation and
titanium particle-induced osteolysis in mice. J Biomed Mater Res B
Appl Biomater. 104:1170–1175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ihn HJ, Lee T, Kim JA, Lee D, Kim ND, Shin
HI, Bae YC and Park EK: OCLI-023, a novel pyrimidine compound,
suppresses osteoclastogenesis in vitro and alveolar bone resorption
in vivo. PLoS One. 12:e01701592017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen W, Li Z, Guo Y, Zhou Y, Zhang Z,
Zhang Y, Luo G, Yang X, Liao W, Li C, et al: Wear particles promote
reactive oxygen species-mediated inflammation via the nicotinamide
adenine dinucleotide phosphate oxidase pathway in macrophages
surrounding loosened implants. Cell Physiol Biochem. 35:1857–1867.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ihn HJ, Kim K, Cho HS and Park EK:
Pentamidine inhibits titanium particle-induced osteolysis in vivo
and receptor activator of nuclear factor-κB ligand-mediated
osteoclast differentiation in vitro. Tissue Eng Regen Med.
16:265–273. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu X, Qu X, Wu C, Zhai Z, Tian B, Li H,
Ouyang Z, Xu X, Wang W, Fan Q, et al: The effect of enoxacin on
osteoclastogenesis and reduction of titanium particle-induced
osteolysis via suppression of JNK signaling pathway. Biomaterials.
35:5721–5730. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deng W, Huang Y, Li H, Chen C, Lin Y, Wang
M, Huang H, Liu T, Qin Q, Shao Y, et al: Dehydromiltirone inhibits
osteoclast differentiation in RAW264.7 and bone marrow macrophages
by modulating MAPK and NF-κB activity. Front Pharmacol.
13:10156932022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu Q, Zhan P, Li X, Mo F, Xu H, Liu Y, Lai
Q, Zhang B, Dai M and Liu X: Bisphosphonate-enoxacin inhibit
osteoclast formation and function by abrogating RANKL-induced JNK
signalling pathways during osteoporosis treatment. J Cell Mol Med.
25:10126–10139. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hyeon S, Lee H, Yang Y and Jeong W: Nrf2
deficiency induces oxidative stress and promotes RANKL-induced
osteoclast differentiation. Free Radic Biol Med. 65:789–799. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Agidigbi TS and Kim C: Reactive oxygen
species in osteoclast differentiation and possible pharmaceutical
targets of ROS-mediated osteoclast diseases. Int J Mol Sci.
20:35762019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miyamoto T: Regulators of osteoclast
differentiation and cell-cell fusion. Keio J Med. 60:101–105. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matsubara T, Kinbara M, Maeda T, Yoshizawa
M, Kokabu S and Takano Yamamoto T: Regulation of osteoclast
differentiation and actin ring formation by the cytolinker protein
plectin. Biochem Biophys Res Commun. 489:472–476. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang D, Jing J, Lou F, Li R, Ping Y, Yu
F, Wu F, Yang X, Xu R, Li F, et al: Evidence for excessive
osteoclast activation in SIRT6 null mice. Sci Rep. 8:109922018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tarpada SP, Loloi J and Schwechter EM: A
case of titanium pseudotumor and systemic toxicity after total hip
arthroplasty polyethylene failure. Arthroplast Today. 6:710–715.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tribst JPM, Werner A and Blom EJ: Failed
dental implant: When titanium fractures. Diagnostics (Basel).
13:21232023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim KT, Eo MY, Nguyen TTH and Kim SM:
General review of titanium toxicity. Int J Implant Dent. 5:102019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Suda N, Morita I, Kuroda T and Murota S:
Participation of oxidative stress in the process of osteoclast
differentiation. Biochim Biophys Acta. 1157:318–323. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee NK, Choi YG, Baik JY, Han SY, Jeong
DW, Bae YS, Kim N and Lee SY: A crucial role for reactive oxygen
species in RANKL-induced osteoclast differentiation. Blood.
106:852–859. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee HI, Lee GR, Lee J, Kim N, Kwon M, Kim
HJ, Kim NY, Park JH and Jeong W: Dehydrocostus lactone inhibits
NFATc1 via regulation of IKK, JNK, and Nrf2, thereby attenuating
osteoclastogenesis. BMB Rep. 53:218–222. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang W, Liang X, Liu X, Bai J, Zhang W, Li
W, Wang T, Li M, Wu Z, Chen L, et al: NOX4 blockade suppresses
titanium nanoparticle-induced bone destruction via activation of
the Nrf2 signaling pathway. J Nanobiotechnology. 20:2412022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tomomura M, Suzuki R, Shirataki Y,
Sakagami H, Tamura N and Tomomura A: Rhinacanthin C inhibits
osteoclast differentiation and bone resorption: Roles of
TRAF6/TAK1/MAPKs/NF-κB/NFATc1 signaling. PLoS One. 10:e01301742015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Teitelbaum SL: Bone resorption by
osteoclasts. Science. 289:1504–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Takayanagi H, Kim S, Koga T, Nishina H,
Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, et al:
Induction and activation of the transcription factor NFATc1 (NFAT2)
integrate RANKL signaling in terminal differentiation of
osteoclasts. Dev Cell. 3:889–901. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shi JH and Sun SC: Tumor tumor necrosis
factor receptor-associated factor regulation of nuclear factor κB
and mitogen-activated protein kinase pathways. Front Immunol.
9:18492018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zeng Q, Zeng Y, Nie X, Guo Y and Zhan Y:
Britanin exhibits potential inhibitory activity on human prostate
cancer cell lines through PI3K/Akt/NF-κB signaling pathways. Planta
Med. 86:1401–1410. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Miyauchi Y, Ninomiya K, Miyamoto H,
Sakamoto A, Iwasaki R, Hoshi H, Miyamoto K, Hao W, Yoshida S,
Morioka H, et al: The Blimp1-Bcl6 axis is critical to regulate
osteoclast differentiation and bone homeostasis. J Exp Med.
207:751–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao J, Kong HJ, Li H, Huang B, Yang M,
Zhu C, Bogunovic M, Zheng F, Mayer L, Ozato K, et al:
IRF-8/interferon (IFN) consensus sequence-binding protein is
involved in Toll-like receptor (TLR) signaling and contributes to
the cross-talk between TLR and IFN-gamma signaling pathways. J Biol
Chem. 281:10073–10080. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhao B, Takami M, Yamada A, Wang X, Koga
T, Hu X, Tamura T, Ozato K, Choi Y, Ivashkiv LB, et al: Interferon
regulatory factor-8 regulates bone metabolism by suppressing
osteoclastogenesis. Nat Med. 15:1066–1071. 2009. View Article : Google Scholar : PubMed/NCBI
|