Introduction
Stroke remains one of the leading causes of
morbidity and mortality, according to the 2022 Global Stroke Survey
released by the World Stroke Organization (1). In particular, ischemic strokes (IS)
accounts for 70% of all strokes. In general, cerebral
ischemia-reperfusion (I/R) injury is a transient or permanent
decrease in cerebral blood flow caused by thromboembolic artery
occlusion (2). When blood is
restored to ischemic tissue, oxidative stress injury and cell death
associated with autophagy, necrosis, and apoptosis may occur
(3). Although the treatment window
for mechanical thrombectomy has increased from a maximum of 6 to
≥24 h in the past decade, no post-stroke drug treatments have been
developed that can effectively enhance nerve repair and recovery
(4). Therefore, blood or brain
biomarkers and novel therapies are urgently needed to predict the
prognosis of stroke.
Para-hydroxybenzaldehyde (PHBA) is a neuroprotective
component of Gastrodia elata Blume and has neuroregulatory
activity (5). Given its fat
solubility and small molecular properties, PHBA can penetrate the
blood-brain barrier (BBB) and is used for the treatment of central
nervous system injury caused by cerebral ischemia reperfusion
(6,7). Additionally, studies have shown that
PHBA can protect against brain I/R injury and antioxidant stress
(7) and reduce inflammatory nerve
injury (5). Interestingly, PHBA
has a protective effect against ischemic neuronal death in the
hippocampal CA1 region and can increase the survival rate of
neurons (8). Pharmacokinetics
analysis showed that the half-life of PHBA (400 mg/kg) metabolites
was significantly prolonged in a model of middle cerebral artery
occlusion/reperfusion (MCAO/R) (9). Additionally, our previous study
showed that PHBA regulated the expression of apoptosis-related
proteins in IS rats, improved mitochondrial oxidative stress and
dysfunction, and thus played a neuroprotective role (10). The aim of the present study was to
further explore how PHBA regulated the metabolic mechanisms in the
blood after brain I/R to identify an effective therapeutic target
for the prevention and treatment of IS.
In recent years, metabonomics has received increased
attention. By analyzing metabolites in vivo by liquid
chromatography-mass spectrometry (LC-MS), researchers can associate
genotypes with phenotypes and identify biomarkers, thus enhancing
the understanding of the brain I/R damage process at the molecular
level (11,12). Metabonomics combined with
pharmacological verification is an advanced method for the
qualitative study of several target compounds of traditional
Chinese medicines and has been successfully used to evaluate the
overall therapeutic effects of these medicines (13,14).
Therefore, this study used liquid chromatography quadrupole
time-of-flight mass spectrometry (LC-QTOF/MS) technique to explore
the therapeutic mechanism of PHBA for brain I/R injury in rats,
with the aim of identifying potential disease biomarkers and
highlighting novel approaches for the prevention and treatment of
IS.
Materials and methods
Animals
A total of 48 male, pathogen-free, Sprague-Dawley
rats (15) [8–12 weeks old,
180–220 g in weight, provided by Changzhou Cavens Experimental
Animal Co., Ltd.; laboratory animal certificate no. SCXK (Su)
2021–0013] were used in the present study. Rats were raised in a
specific pathogen-free environment maintained at a temperature of
21–25°C and relative humidity of 40–60%, and rats were provided
ad libitum access to food and water. All animal experiments
were approved by the Animal Ethics Committee of Yunnan University
of Traditional Chinese Medicine (approval no. R-062019039). All
rats were handled according to the Guidelines for the Care and
Use of Laboratory Animals of the National Institute of Health
(16). Before the experiments, the
rats were randomly divided into a sham group (Sham), a model group
(MCAO/R), or a PHBA group (MCAO/R+PHBA) with 18 rats per group.
PHBA (≥98% purity) was purchased from Chengdu Alpha Biotechnology
Co., Ltd. According to our previous study (17) the effective dose for the treatment
group was 20 mg/kg PHBA and via gavage for 7 days, while the model
and Sham groups were administered an equivalent volume of distilled
water.
Establishment of the rat model of
MCAO/R
Although the risk factors for stroke in older rats
are similar to those in humans (15), aged rats were excluded due to
anatomical/pathological changes such as middle cerebral artery and
common carotid artery aberrations and luminal stenosis (15). Based on a previous preparation
method (10), emboli were prepared
using a 0.26 mm in diameter nylon thread (Ruibo Biotechnology Co.,
Ltd.), and then placed in heparin sodium (cat. no. 152104037A;
Qianhong Biopharma Co., Ltd.). The rats were anesthetized with a
small animal anesthesia machine (ZS-MV–IV; Beijing Zhongshidichuang
Science and Technology Development Co., Ltd.) using 5% isoflurane
(RWD Life Science Co., Ltd.) and maintained with 3% isoflurane, and
fixed in the supine position on the experimental table.
The left common carotid artery (CCA) was isolated
under a stereomicroscope through a median cervical incision. The
left external carotid artery (ECA) and internal carotid artery
(ICA) were separated upward, and the two external carotid artery
branches of the superior thyroid artery and occipital artery were
ligated to reduce the error of thread insertion. The ECA was doubly
ligated at 5–8 mm from the near CCA bifurcation and occluded with a
micro artery clip at the proximal ends of the ICA and CCA. A 0.2 mm
diameter V-shaped microincision was made at the proximal ligation
of the ECA. After the nylon thread bolt was gently inserted, the
nylon thread was tightened, the artery clamp was loosened, and the
nylon thread was moved into the brain along the ECA via the ICA.
The insertion depth was 18–20 mm and insertion was halted if slight
resistance was felt. The head end of the nylon thread was used to
block the blood flow of the MCA. Then, the incision was sutured,
and the ischemia time was recorded. After 2 h, the slow and light
pulling of the bolt caused the head end to return the CCA, at which
point the cerebral I/R was considered to be realized. Samples were
obtained from the rats 24 h after reperfusion. In the Sham group:
the carotid artery was exposed and isolated without I/R. The body
temperatures of all rats were maintained at 37°C throughout the
procedure. During this time, none of the animals exhibited symptoms
that would require termination, such as not eating, breathing
difficulties, convulsions, or hypothermia, nor did any die
prematurely. All rats in the study were euthanized before the end
of the study.
Neurological function score
After 24 h, the degree of neurological deficit was
evaluated using a score of 0–4 (18) as follows. 0, no neurological
deficit; 1, left forelimb flexion when lifting the tail in the air;
2, rats walked in circles; 3, hemiplegia causing rats to crawl; and
4, rats were unable to walk spontaneously and had a decreased level
of consciousness. A higher score indicated a more serious
behavioral disorder.
TTC staining
A total of 24 h after brain I/R, rats were injected
intraperitoneally with 2% pentobarbital sodium (150 mg/kg) to
induce deep anesthesia. After no response to a tail clip, the rats
were euthanized by decapitation. and death was confirmed by a lack
of response to tail clamping. The brain was frozen at −20°C for 20
min. The brain was sectioned from front to back with 2 mm coronal
sections on ice, stained with 2% TTC staining solution at 37°C for
20 min, and then fixed in 4% paraformaldehyde at room temperature
for 24 h. The experimental results were analyzed using ImageJ
version 1.52a (National Institutes of Health) software to obtain
the total infarcted area and the total area of brain slices. The
infarct rate (%) was calculated as the total infarct area/total
area of brain slices.
Hematoxylin and eosin (H&E)
staining
H&E staining was used to show the components of
cytoplasm, muscle fibers, and the general morphological structure
of lesions. Nissl is an important structure involved in protein
synthesis in neurons. When neurons are stimulated, the Nissl bodies
in the cell become disordered and crumpled. Therefore, detection
using H&E staining and Nissl staining provides mutual
verification of the pathological injury status of brain tissue
(19). Brain tissue sections
embedded in paraffin (thickness 5 µm, n=3) were heated at 60°C for
3 h, stained with a hematoxylin (using an H&E staining kit;
cat. no. KGA224; Nanjing KeyGen Biotech Co., Ltd.) for 2 min, and
then washed. Eosin staining was performed for 1 min at room
temperature. The samples were dehydrated using a gradient of
alcohol solutions, made transparent using xylene, and then sealed
with neutral gum. An inverted bright field phase contrast
microscope (IX83, Olympus Corporation) was used to obtain the
hippocampal images. Fields of view were randomly selected and
images were taken at a magnification of ×400, and the images were
analyzed using ImagePro Plus version 6.0 (Media Cybernetics,
Inc.).
Nissl staining
Brain tissue sections embedded in paraffin
(thickness 5 µm, n=3) were dewaxed, incubated in Nissl staining
solution (cat. no. G1430; Beijing Solarbio Science & Technology
Co., Ltd.) at 50–60°C for 40 min, washed with deionized water and
differentiated using the included differentiation solution. Then,
anhydrous ethanol was used for dehydration, xylene was used to
clear the tissue, and neutral gum was used for sealing. Images of
randomly selected fields of view of the hippocampus were obtained
using a phase contrast microscope at a magnification of ×400 and
were analyzed using ImagePro Plus 6.0 (Media Cybernetics,
Inc.).
Sample collection
Whole blood was collected with a heparin sodium
anticoagulant tube and centrifuged at room temperature at 3,000 × g
for 10 min. After the blood cells settled entirely to the bottom of
the tube, the upper plasma was taken. Plasma (50 µl) from the model
group and PHBA group was added to a methanol solution containing an
internal standard (4-chlorophenyl alanine, 1 µg/ml) and was then
shaken, mixed for 5 min, and centrifuged at 4°C (14,200 × g for 10
min). The supernatant (200 µl) was transferred to a centrifuge tube
and dried using a vacuum drier. The supernatant was then
redissolved in 100 µl ultra-pure water and methanol (1:1) and
centrifuged at 4°C (14,200 × g for 10 min). The supernatant volume
was 80 µl, and the sample volume injected was 5 µl.
Metabonomics analysis
The LC-QTOF/MS conditions were: Waters HSS T3 1.8
µm, 2.1×100 mm column; flow rate, 0.3 ml/min; column temperature
box, 40°C; mobile phase, water phase (ultra-pure water + 0.1%
formic acid); B organic phase (acetonitrile). The mass spectrometry
conditions were: Mass detection was performed in the positive ion
(4,000 V) and negative ion (−4,500 V) mode, respectively, and
scanned using a Turbo V electrospray ionization (ESI). The
parameters were set as follows: Ion spray voltage, 7 kV; turbine
spray temperature, 500°C; declustering potential, 70 V; collision
energy, 30 eV; atomizer gas, 55 psi; heater gas, 55 psi; curtain
gas, 35 psi. The atomizer and auxiliary gas are maintained by
nitrogen. The scanning range of the TOF MS was mCompz
100–1,200.
Total ion chromatographic (TIC) flow
analysis of Quality Control (QC) samples
Taking the mixed solution as the QC sample, in the
process of instrumental analysis, a quality control sample was
inserted every 10 tests and analysis samples. Through the
overlapping display and analysis of the TIC chromatogram of the
essential spectrum detection and analysis of the same quality
control sample, the stability of the instrument during the testing
was judged.
Metabonomics data analysis
The LC-MS data were processed using MS-DIAL (version
5.1.230719) (20). The ‘mixOmics’
(11) package in R (21) (www.r-project, version 4.2.1) was used for partial
least squares discriminant analysis (PLS-DA). The data were unit
variance scaled before analysis. The results of the systematic
cluster analysis of samples and metabolites are presented as a heat
map and tree map generated using the R package ‘pheatmap’ (11). The variable importance projection
(VIP) value was extracted from the PLS-DA results. An unpaired
Student's t-test was used to determine the significance.
Metabolites that were significantly differentially regulated
between groups were determined using the VIP and absolute
log2FC. The results are presented as a volcanic map
(‘ggplot2’ package) (22). The
KEGG database was used to annotate metabolites, which were then
mapped to the KEGG pathway database, and the Metaboanalyst website
(http://www.metaboanalyst.ca/) was used
for metabolic pathway analysis. Then, the pathways to which the
metabolites with significant regulatory effects were mapped and
metabolite concentration were analyzed. The P-value of the
hypergeometric test and the size of the pathway influence factors
were used to determine the importance of the metabolites.
Western blotting
First, the hippocampal tissues were lysed using RIPA
cleavage buffer (PSMF:RIPA lysate=1:100; cat. no. 051021210825;
Beyotime Institute of Biotechnology) on ice for 20 min, after which
the lysate was centrifuged at 4°C at 13,017 × g for 5 min. The
protein concentration was quantified using a bicinchoninic acid
assay. Samples of 80 µg protein were loaded on an 8% SDS gel,
resolved using SDS-PAGE at 80 V for ~30 min, followed by 120 V
until the target band reached a suitable position. The membrane was
then transferred to a PVDF membrane, which was subsequently blocked
for 1 h with 5% skimmed milk in TBST at room temperature. Membranes
were and then incubated with the primary antibody diluted in TBST
solution overnight at 4°C. The primary antibodies used were
anti-PSD-95 (1:1,000; cat. no. ab238135; Abcam), anti-Bcl-2
(1:1,000; cat. no. sc-7832; SantaCruz Biotechnology, Inc.),
anti-GluA1 (1:1,000; cat. no. 13185; Cell Signaling Technology,
Inc.), anti-caspase 3 (1:1,000; cat. no. 9662; Cell Signaling
Technology, Inc.), light chain-3 protein, LC3 II/I (1:1,000; cat.
no. 4108; Cell Signaling Technology, Inc.), Bax (1:2,000; cat. no.
50599-2-Ig; ProteinTech Group, Inc.), sequestosome-1 (SQSTM1/P62)
(1:5,000; cat. no. 18420-1-AP; ProteinTech Group, Inc.), and
autophagy effector protein 1 (Beclin1) (1:2,000; 66665-1-Ig;
ProteinTech Group, Inc.). The membranes were washed using TBST
(0.05% Tween) buffer then incubated with the secondary Goat
Anti-Rabbit IgG antibody (cat. no. ab6721) or Rabbit Anti-Mouse IgG
(cat. no. ab6728) at room temperature for 1 h (1:10,000; Abcam).
Signals were visualized using an ECL kit according to the
manufacturer's protocol (Thermo Fisher Scientific, Inc.) and a
Tanon 6600 luminous imaging workstation (Tanon Science &
Technology Co., Ltd.). Image ProPlus was used for densitometry
analysis.
TUNEL staining
TUNEL staining was performed using a TUNEL detection
kit (cat. no. C1090, Beyotime Institute of Biotechnology). After
heating at 60°C for 60 min, xylene was used to dewax the brain
slices twice, after which samples were hydrated in a series of
ethanol solutions (100, 95, 80, and 75%), with 5 min per solution.
A total of 20 µg/ml protease K without DNA enzymes was added to
each tissue section and allowed to react at 37°C for 30 min.
Subsequently, 50 µl TUNEL fluorescence detection solution was added
to each sample, which was then incubated at 37°C for 60 min, and
DAPI double staining was performed at room temperature for 5 min.
The hippocampus was observed under a laser confocal microscope
(Zeiss LSM). Randomly selected fields of view were captured at ×400
magnification.
Statistical analysis
Using GraphPad Prism Version 9.0.0 (GraphPad
Software, Inc.) for analysis and processing, if the data were
normally distributed and the variance (ANOVA F-test) was uniform, a
Bonferroni's multiple comparison test after a one-way ANOVA was
used. Data are presented as the mean ± the standard error.
P<0.05 was considered to indicate a statistically significant
difference.
Results
PHBA ameliorates brain injury and
pathological damage of hippocampal neurons in MCAO/R rats
The results of TTC staining showed that there were
notable white infarcted areas following brain I/R. Compared with
the model group, PHBA significantly reduced the size of infarct
sizes [F (2,6) 1.822, P=0.413] (Fig. 1A and B) and improved the
neurological function of rats [F (2,12)
1.333, P=0.300] (Fig. 1C). It has
been shown that PHBA can effectively reduce brain injury in MCAO/R
rats (10). To further explore the
effects of PHBA on the pathological changes of the brain following
I/R injury, HE and Nissl staining were used in this study. The
results showed that the number of cells [F (2,15)
1.273, P=0.529] in the sham group was higher, the nuclei and cell
bodies of neurons in the sham group were clear, and the Nissl
bodies [F (2,15) 0.121, P=0.887] were abundant.
Conversely, in the model group, hippocampal ischemic neuronal
injury, nuclear pyknosis, a disorderly arrangement of neurons, and
sparsity of Nissl bodies were observed. PHBA reversed these changes
(Fig. 1D-G). The above data
suggest that PHBA had a protective effect on hippocampal neuronal
death after I/R.
Screening and identification of
differential metabolites between the model and PHBA groups
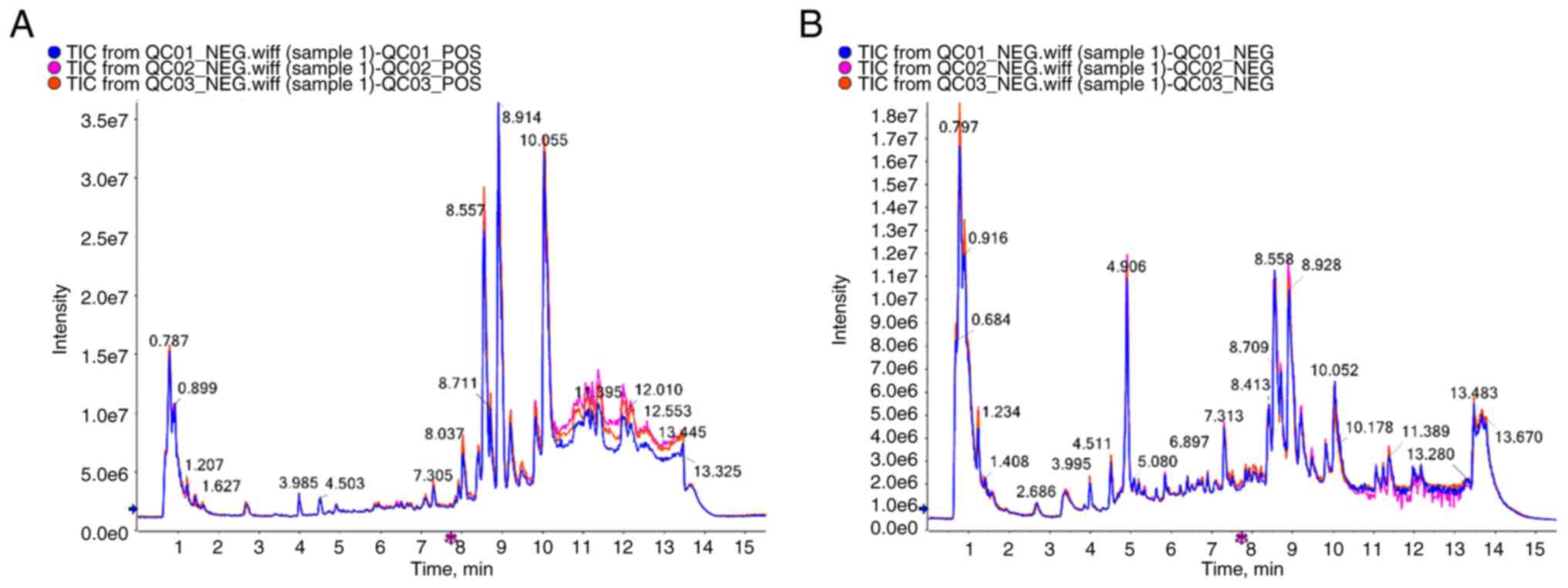
The high stability of the LC-QTOF/MS instrument
provides an essential guarantee for the repeatability and
reliability of data. The curve overlaps of the total ion currents
of the QC samples were high, suggesting that the experimental data
were stable (Fig. 2). To ascertain
the changes of metabolites following PHBA treatment, the plasma of
rats in the model and PHBA groups (n=6) were collected. In
addition, based on the LC-QTOF/MS detection platform and database,
856 metabolites were detected under positive and negative ions,
including purines, pyrimidines, amino acids, and other metabolites.
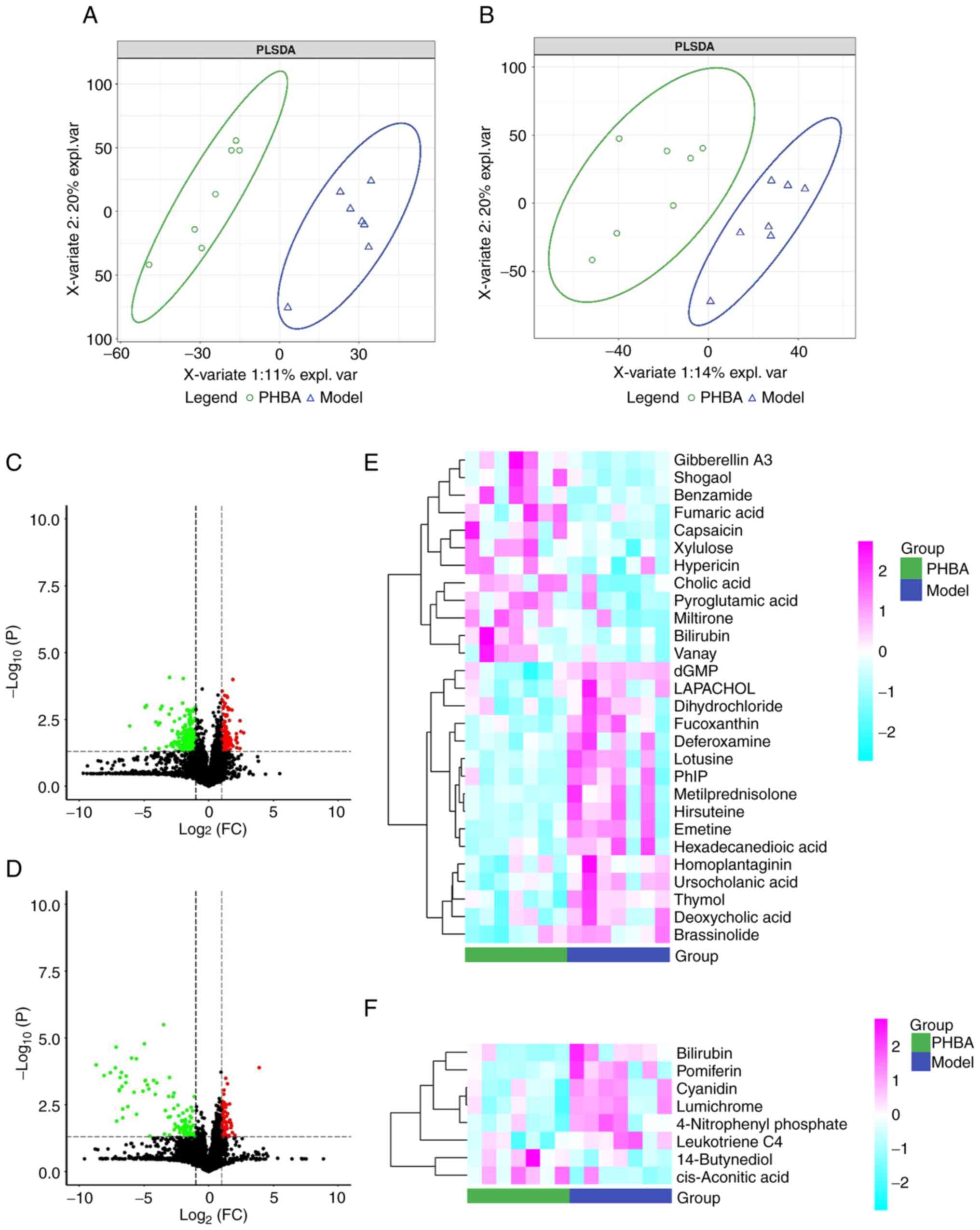
To further determine the differences between groups, the
multivariate statistical analysis method PLS-DA was used to analyze
the two groups in pairs. The results showed there was a significant
separation between the model and the PHBA group, and the internal
correlation between each group was very high (Fig. 3A and B). The screening results are
shown as a volcano map (Fig. 3C and
D). In addition, the stratified cluster heat map was used to
show the changes in metabolites more directly. The heat map showed
that the model and PHBA group could be divided into two parts
according to the identified metabolites (Fig. 3E and F). Finally, 13 differential
metabolites were identified, of which 6 metabolites were
upregulated: benzamide, pyroglutamic acid, fumaric acid,
d-Xylulose, cholic acid, and cis-Aconitic acid, and 7 metabolites
were downregulated: 2′-Deoxyguanosine 5′-monophosphate (dGMP),
hexadecanedioic acid, deoxycholic acid, deferoxamine, 4-Nitrophenyl
phosphate, bilirubin, leukotriene C4 (Table I). Among these metabolites, the
P-value of dGMP was the lowest (0.018).
 | Table I.Screening results of differential
metabolites in the Para-hydroxybenzaldehyde group compared with the
model group. |
Table I.
Screening results of differential
metabolites in the Para-hydroxybenzaldehyde group compared with the
model group.
| Compound | Formula | KEGG | Variable importance
projection | Fold change | P-value | Change in
expression |
|---|
| dGMP |
C10H14N5O7P | C00362 | 2.498 | 1.278 |
1.76×10−3 | Down |
| Cholic acid |
C24H40O5 | C00695 | 2.132 | 0.424 |
1.41×10−2 |
Up |
| Benzamide |
C7H7NO | C09815 | 2.070 | 0.322 |
1.65×10−2 |
Up |
| 4-Nitrophenyl
phosphate |
C6H6NO6P | C03360 | 1.895 | 1.553 |
2.36×10−2 | Down |
| cis-Aconitic
acid |
C6H6O6 | C00417 | 1.887 | 0.600 |
2.43×10−2 |
Up |
| Leukotriene C4 |
C30H47N3O9S | C02166 | 1.991 | 1.303 |
2.65×10−2 | Down |
| Bilirubin |
C33H36N4O6 | C00486 | 1.991 | 2.155 |
2.69×10−2 | Down |
| Pyroglutamic
acid |
C5H7NO3 | C01879 | 1.942 | 0.687 |
2.70×10−2 |
Up |
| D-Xylulose |
C5H10O5 | C00310 | 1.904 | 0.767 |
3.20×10−2 |
Up |
| Deferoxamine |
C25H48N6O8 | C06940 | 1.913 | 1.835 |
3.20×10−2 | Down |
| Fumaric acid |
C4H4O4 | C00122 | 1.890 | 0.646 |
3.43×10−2 |
Up |
| Hexadecanedioic
acid |
C16H30O4 | C19615 | 1.874 | 1.847 |
3.43×10−2 | Down |
| Deoxycholic
acid |
C24H40O4 | C04483 | 1.925 | 1.319 |
3.60×10−2 | Down |
Enrichment analysis of differential
metabolites using KEGG
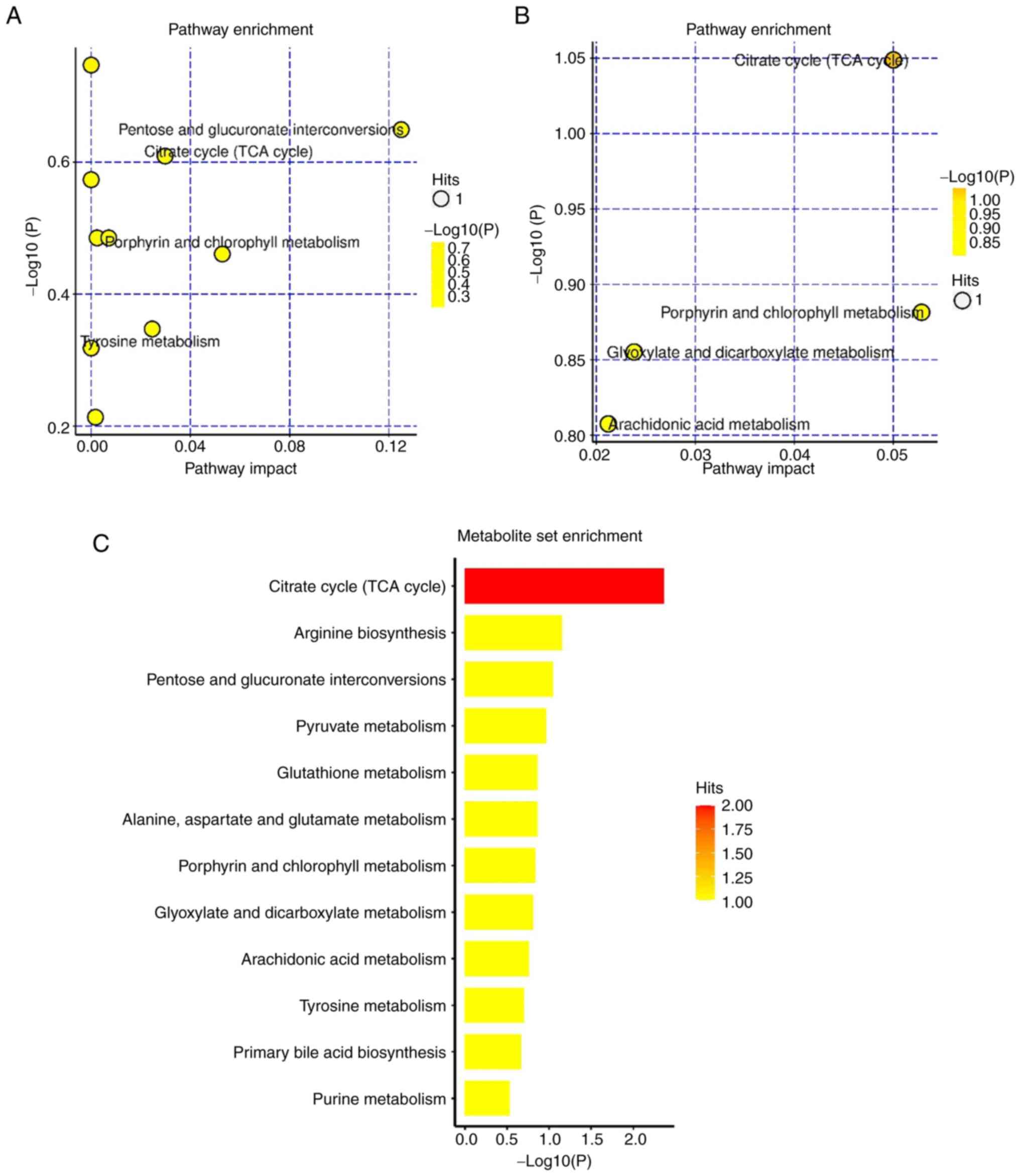
To comprehensively observe the changes in metabolic
pathways, pathway enrichment analysis of all differential
metabolites was performed. In positive ion mode, the primary ways
that the model group and the PHBA group were affected by the
differential metabolites were: The Tricarboxylic acid (TCA) cycle',
‘glutathione metabolism’, and ‘mutual transformation of pentose and
glucuronates’ (Fig. 4A). This may
be related to the improvement of energy metabolism and the
antioxidant effect of glutathione metabolic pathway by PHBA, which
has been reported in the pathogenesis of glucose metabolic diseases
(23). In negative ion mode, the
primary pathways affected by the differential metabolites were: The
‘TCA cycle’ and ‘arachidonic acid metabolism’, amongst others
(Fig. 4B). This suggests that
MCAO/R inhibits energy production and induces inflammation. These
pathways interact with each other and are closely related to I/R.
From the above results, it can be inferred that PHBA components are
absorbed into the blood and achieve their neuroprotective effects
by regulating various metabolic processes in the body, especially
those related to the TCA cycle (Fig.
4C).
Effect of PHBA on the expression of
PSD-95 and NMDAR in the hippocampus
The most significantly different metabolite induced
by PHBA was dGMP. Guanosine kinase (GK) can phosphorylate dGMP to
dGDP as a central enzyme in the guanine rescue pathway (24). The changes in PSD-95 levels in the
membrane-associated guanosine kinase (MAGUK) family are related to
the content of synaptic AMPAR (GluA1), which regulates the
intensity of hippocampal synaptic activity and is associated with
neuroplasticity (25). Here, the
protein expression levels of PSD-95 and GluA1 were determined.
Western blotting results showed that the expression of PSD-95 [F
(2,6) 0.035, P=0.966] and GluA1 [F (2,6)
1.684, P=0.263] increased significantly compared with the model
group following PHBA treatment (Fig.
5A-C). This suggested that PHBA increased the levels of dGMP in
the brain following IS by promoting the interaction between PSD-95
and AMPAR.
Effect of PHBA on autophagy in
hippocampal neurons
To verify whether PHBA ameliorates I/R by activating
autophagy, western blotting was used to detect the effects of PHBA
on the expression of the autophagy marker LC3 and essential
autophagy proteins p62 and Beclin1 (Fig. 6A). The results showed that compared
with the model group, PHBA significantly increased the expression
of LC3-II/LC3I [F (2,6) 0.463, P=0.650] and Beclin1 [F
(2,6) 1.730, P=0.255] (Fig. 6B and C), and decreased the protein
expression levels of p62 [F (2,6)
0.103, P=0.903] (Fig. 6D). This
suggests that autophagy in the hippocampus is significantly
activated following PHBA treatment, which may be related to the
interaction between PSD-95 and AMPAR.
Effect of PHBA on the apoptosis of
hippocampal neurons
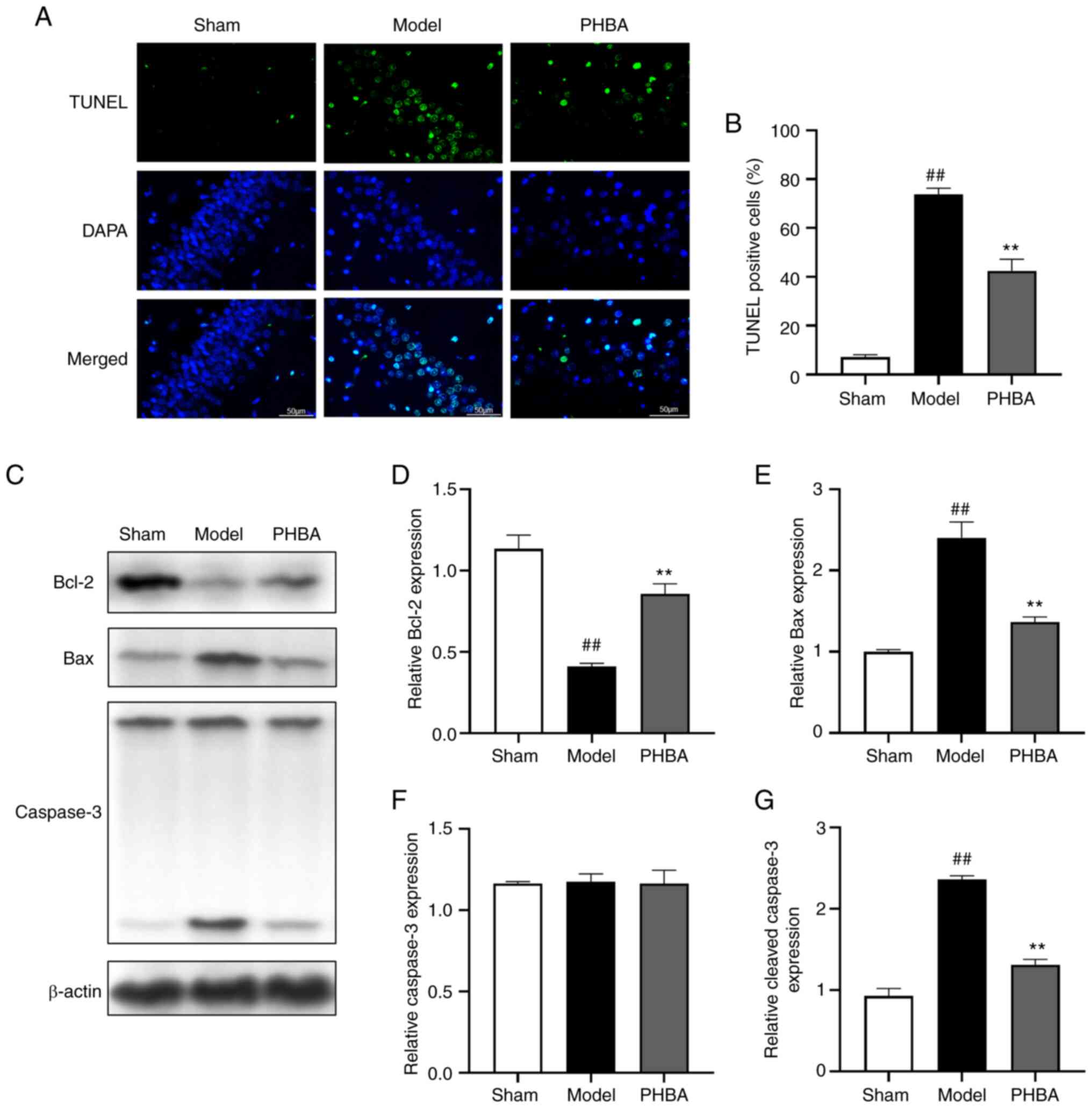
It is well-established that brain I/R is often
associated with neuronal injury and apoptosis (18,19).
TUNEL staining showed that the number of positive cells in the
hippocampus of the model group was significantly higher than that
of the sham group. After treatment with PHBA, the number of
positive cells in the hippocampus decreased significantly [F
(2,6) 1.169, P=0.373] (Fig. 7A and B). In addition, the protein
expression levels of apoptosis-related genes were analyzed by
western blotting (Fig. 7C-G).
MCAO/R resulted in the upregulation of Bax [F (2,6)
1.207, P=0.363] and cleaved-caspase-3 [F (2,6)
0.349, P=0.719] expression and downregulation of Bcl-2 [F (2,6)
0.734, P=0.519] expression. However, PHBA partially reversed the
MCAO/R-induced apoptosis. These results suggested that PHBA
inhibited I/R-induced apoptosis of hippocampal cells and reduced
the loss of neurons.
Discussion
Several studies have shown that PHBA has significant
potential for treating IS (10,17).
However, whether PHBA has a protective effect against brain I/R via
regulation of metabonomic characteristics has not been reported
previously to the best of our knowledge. To elucidate the
therapeutic impact of PHBA for the management of brain I/R injury,
metabonomic analysis and pharmacodynamic experiments were
performed, and biochemical and histopathological indices were
measured. The results showed that the differential metabolites in
plasma exerted a neuroprotective effect against IS via the
regulation of numerous metabolic pathways such as energy, glucose,
and oxidative metabolism. The results also suggested that
interactions between PSD-95 and AMPAR may promote synaptic
plasticity in hippocampal neurons and reduce brain damage mediated
by autophagy deficiency in MCAO/R rats, thus inhibiting apoptosis
of hippocampal neurons. Therefore, PHBA promoted the activity of
PSD-95-AMPAR and thus played a neuroprotective role.
The results of metabonomics showed the metabolites
regulated by PHBA that were involved in brain I/R, which primarily
included plasma purines, pyrimidines, and amino acids. The primary
pathways these differential metabolites were involved in were the
‘TCA cycle’, ‘arginine biosynthesis’, and ‘mutual transformation of
pentose and glucuronates’. It is worth noting that compared with
the model group, PHBA increased the levels of benzamide,
pyroglutamic acid, fumaric acid, d-xylulose, cholic acid, and
cis-aconitic acid, whilst decreasing hexadecanedioic acid,
deoxycholic acid, deferoxamine, 4-nitrophenyl phosphate, bilirubin,
leukotriene C4, and dGMP levels.
Benzamides exhibit brain region specificity, can
cross the BBB, and increase the levels of histone acetylation in
the hippocampal regions involved in behavior and cognition
(26). In a model of mouse brain
I/R, benzamide treatment significantly reduced the extent of
delayed neuronal apoptosis in the hippocampus of mice, thus
improving neuronal survival and memory (27). Pyroglutamic acid is an important
molecule involved in glutathione metabolism. The decrease in
pyroglutamic acid in patients with cerebral infarction indicates a
decrease in reducing substances caused by oxidative stress
(28,29). D-xylulose is the product of a
dehydrogenase reaction when xylitol is used as a substrate. In rat
brains, d-xylulose, similar to glucose, stimulates hormone
secretion through a nicotinamide nucleotide-dependent mechanism
(16). Fumaric acid is the
precursor of L-malic acid in the TCA cycle and is formed by the
oxidation of succinic acid (30).
Cis-aconitic acid is an intermediate of the TCA cycle in
mitochondria that is synthesized by aconitase and can affect cell
energy metabolism (31). A
catalase modified via maleic anhydride has been developed that can
inhibit reactive oxygen species-mediated apoptosis, thereby
reducing the cerebral infarction volume of MCAO mice (32). The primary active components of
bile acid biosynthesis include cholic acid, deoxycholic acid, and
taurine deoxycholic acid (33).
Among these, the mechanism of cholic acid in protecting against IS
may involve the BDNF-TrkB pathway by protecting the integrity of
the BBB of the neurovascular unit BBB in vitro,
downregulating apoptosis, and finally, reducing the oxidative
stress and inflammatory injury of the neurovascular unit following
oxygen-glucose deprivation/reoxygenation (34). In contrast, an abnormal increase in
the bile acid-related metabolite, deoxycholic acid, significantly
affected BBB permeability following brain injury (35). In the present study, deoxycholic
acid levels decreased following PHBA treatment, as well as
downstream metabolites of molecules affected by PHBA. A previous
study reported that hexadecanedioic acid was associated with blood
pressure levels and all-cause mortality (36). Sun et al (37) performed a metabolomics study of 114
patients with IS and 112 healthy controls. The results showed that
the pathways related to intracellular hexadecanedioic acid
synthesis were involved in the occurrence of IS. A previous study
showed that deferoxamine, an iron-chelating agent, can quickly
enter the brain tissue through the BBB to counteract an overload of
iron ions in the brain, but it had no effect on the clinical
symptoms of IS (38). Bilirubin is
the primary product of heme catabolism (39). Its toxicity involves a variety of
pathological mechanisms, and neurons and glial cells are
susceptible to bilirubin toxicity. Bilirubin also affects brain
circuits, particularly those that affect cognition, learning,
behavior, and sensation (40).
Clinically high serum bilirubin levels were positively correlated
with the severity of IS and the degree of disability 3 months after
stroke onset (41). 4-nitrophenyl
phosphate is the substrate of alkaline phosphatase and can inhibit
the phagocytosis of macrophages in the nervous system (42). Leukotriene C4 is a metabolite of
arachidonic acid found in the forebrain (43), and increases in leukotriene C4 in
the hippocampus are greatest after I/R. Accumulation of leukotriene
C4 may alter the membrane permeability, cause BBB dysfunction and
edema, and eventually, neuronal death (44).
It Is worth noting that the most significantly
differentially expressed metabolite induced by PHBA was dGMP.
Deoxyguanosine, in the pathway from exogenous guanine to DNA, can
readily produce toxic cellular dGMP (45). Interestingly, the results of the
present study showed that PHBA treatment significantly reduced
plasma dGMP levels. When further examining the mechanism of dGMP
following treatment, it was found that GK could phosphorylate dGMP
to produce dGDP, which is a central enzyme in the guanine rescue
pathway (24). GK is a domain of
the PSD-95 family of membrane-associated guanylate kinases and is
largely found in the MAGUK family, especially in neuronal tissues
(46). PSD-95 is an important
postsynaptic membrane protein involved in synaptic plasticity. It
exhibits neuroplasticity and contributes to brain repair, thus
improving the prognosis of IS (25). In addition, studies have shown that
PSD-95 mediates the postsynaptic localization of ~40% of AMPARs
(the GluA1 protein is an essential subunit of AMPAR) in hippocampal
CA1 pyramidal cells (47). The
hippocampal damage caused by I/R leads to cognitive and memory
impairment in animals, and the increased expression of PSD-95 is a
marker of recovery of neurological function (48). Therefore, it was hypothesized that
following cerebral I/R injury, PHBA may accelerate dGMP metabolism
by promoting the interaction between PSD-95 and AMPAR resulting in
synaptic structural changes that inhibit the dGMP levels in the
plasma following MCAO/R, thus reducing neurotoxicity.
Interestingly, the results showed that PHBA significantly increased
the protein expression levels of PSD-95 and GluA1 in the
hippocampus and decreased the levels of dGMP in the plasma of I/R
rats. These results suggested that the neuroprotective effects of
PHBA in cerebral I/R injury were associated with promoting synaptic
recovery and neural plasticity.
It is well established that structural autophagy is
responsible for neuronal survival and protects neurons from
nutritional starvation (49–51).
There is growing evidence that autophagy provides neuroprotection
and improves clinical symptoms by significantly reducing ischemic
damage to neurons, glial cells, and endothelial cells (52,53).
Several key molecular components are involved in autophagy, such as
Beclin1 and LC3 (54), that
promote autophagy. In addition, p62, as a selective autophagy
substrate, is degraded during autophagy activation (55). It is worth noting that the
decreased expression of PSD-95 in hippocampal neurons following
experimental brain injury was accompanied by reduced inhibition of
LC3-positive autophagosomes and mitochondrial mass, indicating
autophagy dysfunction (56).
Consistent with previous reports, it was found that the expression
of the autophagy markers LC3 and Beclin1 decreased and expression
of the autophagy-associated protein p62 increased in the
hippocampus of rats in the model group, indicating that autophagy
was inhibited. However, this result was reversed following PHBA
treatment, suggesting that the potential neuroprotective effect of
PHBA in the pathological mechanism of cerebral I/R injury was
related to its promotion of autophagy and reduction of ischemic
synaptic damage.
Hippocampal neuronal damage caused by apoptosis is
the focus of treatment after cerebral I/R injury, therefore,
inhibition of apoptosis is a neuroprotective strategy in IS therapy
(57). Further analysis of the
biological function of PSD-95 and AMPARs showed that the
interaction between overexpression of PSD-95 and AMPARs reduced the
expression of the pro-apoptotic protein Bax and increased the
expression of the anti-apoptotic protein Bcl2, thus blocking the
activation of the apoptosis signal Caspase-3 and playing an
anti-apoptotic role. Similarly, the results of the TUNEL assays
showed that PHBA could reduce hippocampal neuronal apoptosis
induced by brain injury in MCAO/R rats following stroke, which may
be an important metabolic mechanism of PHBA in treating IS and
improving the neurological dysfunction caused by cerebral I/R.
In summary, according to the results of
histopathology and metabonomics analysis, the present study showed
that PHBA protected against cerebral I/R injury. This may have been
achieved via the activation of autophagy and by increasing the
interaction between PSD-95 and AMPAR, which are related to synaptic
plasticity in the hippocampus. Therefore, PHBA may serve as a
promising therapeutic strategy for improving the prognosis of
IS.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 81960733), the Xingdian Talent
Support Program-Special for Young Talent (grant no.
XDYC-QNRC-2022-0284) and the National Administration of Traditional
Chinese Medicine High-level Key Discipline Construction Project
‘Dai Medicine’ and ‘Dai Pharmacy’.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request. The relevant data sets have been submitted to the public
database, https://www.ebi.ac.uk/metabolights/editor/study/MTBLS7870.
Authors' contributions
LY and XD designed the experiments. YL and XY
conducted a preliminary analysis of animal experiments and
metabolomics results. XY and XD revised the manuscript. XD and LY
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Ethics Committee of Yunnan University of Traditional Chinese
Medicine (Kunming, China; approval no. R-062019039).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
No authors listed. Corrigendum to: World
stroke organization (WSO): Global stroke fact sheet 2022. Int J
Stroke. 17:4782022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng
YL, Cheng PW, Li CY and Li CJ: Current mechanistic concepts in
ischemia and reperfusion injury. Cell Physiol Biochem.
46:1650–1667. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun MS, Jin H, Sun X, Huang S, Zhang FL,
Guo ZN and Yang Y: Free radical damage in ischemia-reperfusion
injury: An obstacle in acute ischemic stroke after
revascularization therapy. Oxid Med Cell Longev. 2018:38049792018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Messmer SJ, Salmeron KE, Frank JA, McLouth
CJ, Lukins DE, Hammond TC, Lin AL, Fraser JF and Pennypacker KR:
Extended middle cerebral artery occlusion (MCAO) model to mirror
stroke patients undergoing thrombectomy. Transl Stroke Res.
13:604–615. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ha JH, Lee DU, Lee JT, Kim JS, Yong CS,
Kim JA, Ha JS and Huh K: 4-Hydroxybenzaldehyde from Gastrodia
elata B1. is active in the antioxidation and GABAergic
neuromodulation of the rat brain. J Ethnopharmacol. 73:329–333.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He F, Duan X, Dai R, Wang W, Yang C and
Lin Q: Protective effects of ethyl acetate extraction from
Gastrodia elata blume on blood-brain barrier in focal
cerebral ischemia reperfusion. Afr J Tradit Complement Altern Med.
13:199–209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu YP, Li X, Du Y, Zhang L, Ran L and
Zhou NN: Protective effect and mechanism of p-hydroxybenzaldehyde
on blood-brain barrier. Zhongguo Zhong Yao Za Zhi. 43:1021–1027.
2018.(In Chinese). PubMed/NCBI
|
|
8
|
Kim HJ, Hwang IK and Won MH: Vanillin,
4-hydroxybenzyl aldehyde and 4-hydroxybenzyl alcohol prevent
hippocampal CA1 cell death following global ischemia. Brain Res.
1181:130–141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng J, Yang JG, Yang QY, Xu Y and He F:
Pharmacokinetics of metabolites of p-hydroxybenzaldehyde in rats.
China pharmaceuticals. 29:9–12. 2020.(In Chinese).
|
|
10
|
Xiao T, Yang L, Chen P and Duan X:
Para-hydroxybenzaldehyde against transient focal cerebral ischemia
in rats via mitochondrial preservation. Exp Ther Med. 24:7162022.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo Y, Chen P, Yang L and Duan X:
Metabolomic analysis and pharmacological validation of the cerebral
protective effect of 3,4-dihydroxybenzaldehyde on cerebral
ischemia-reperfusion injury. Mol Med Rep. 27:92023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramana P, Adams E, Augustijns P and Van
Schepdael A: Metabonomics and drug development. Methods Mol Biol.
1277:195–207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu Z, Qian S, Zhao L, Zhang Z, Song C,
Chen L, Gao H and Zhu W: Metabolomics-based study of the potential
interventional effects of Xiao-Xu-Ming Decoction on cerebral
ischemia/reperfusion rats. J Ethnopharmacol. 295:1153792022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang D, Wang Q, Chen R, Yang S, Li Z and
Feng Y: Exploring the effects of Gastrodia elata Blume on
the treatment of cerebral ischemia-reperfusion injury using
UPLC-Q/TOF-MS-based plasma metabolomics. Food Funct. 10:7204–7215.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang P, Huang Z, Yan HQ, Su LL, Gui YK,
Lv HX, Zhu B and Li T: Improvement of the suture-occluded method in
rat models of focal cerebral ischemia-reperfusion. Exp Ther Med.
7:657–662. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the care and use of laboratory animals. 8th
edition. Washington (DC): National Academies Press (US); 2011
|
|
17
|
Liu J, Yang L, Niu Y, Su C, Wang Y, Ren R,
Chen J and Ma X: Potential therapeutic effects of Mi-Jian-Chang-Pu
Decoction on neurochemical and metabolic changes of cerebral
ischemia-reperfusion injury in rats. Oxid Med Cell Longev.
2022:73195632022.PubMed/NCBI
|
|
18
|
Li H, Peng D, Zhang SJ, Zhang Y, Wang Q
and Guan L: Buyang Huanwu Decoction promotes neurogenesis via
sirtuin 1/autophagy pathway in a cerebral ischemia model. Mol Med
Rep. 24:7912021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsugawa H, Cajka T, Kind T, Ma Y, Higgins
B, Ikeda K, Kanazawa M, VanderGheynst J, Fiehn O and Arita M:
MS-DIAL: Data-independent MS/MS deconvolution for comprehensive
metabolome analysis. Nat Methods. 12:523–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heinemann J: Cluster analysis of
untargeted metabolomic experiments. Methods Mol Biol. 1859:275–285.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu S, Xie X, Lei H, Zou B and Xie L:
Identification of key circRNAs/lncRNAs/miRNAs/mRNAs and pathways in
preeclampsia using bioinformatics analysis. Med Sci Monit.
25:1679–1693. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sandholm N, Van Zuydam N, Ahlqvist E,
Juliusdottir T, Deshmukh HA, Rayner NW, Di Camillo B, Forsblom C,
Fadista J, Ziemek D, et al: The genetic landscape of renal
complications in type 1 diabetes. J Am Soc Nephrol. 28:557–574.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kumar V, Spangenberg O and Konrad M:
Cloning of the guanylate kinase homologues AGK-1 and AGK-2 from
Arabidopsis thaliana and characterization of AGK-1. Eur J Biochem.
267:606–615. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan BC, Park JH, Ahn JH, Lee JC, Won MH
and Kang IJ: Postsynaptic density protein (PSD)-95 expression is
markedly decreased in the hippocampal CA1 region after experimental
ischemia-reperfusion injury. J Neurol Sci. 330:111–116. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Simonini MV, Camargo LM, Dong E, Maloku E,
Veldic M, Costa E and Guidotti A: The benzamide MS-275 is a potent,
long-lasting brain region-selective inhibitor of histone
deacetylases. Proc Natl Acad Sci USA. 103:1587–1592. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kumaran D, Udayabanu M, Nair RU, R A and
Katyal A: Benzamide protects delayed neuronal death and behavioural
impairment in a mouse model of global cerebral ischemia. Behav
Brain Res. 192:178–184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Geenen S, Guallar-Hoyas C, Michopoulos F,
Kenna JG, Kolaja KL, Westerhoff HV, Thomas P and Wilson ID:
HPLC-MS/MS methods for the quantitative analysis of 5-oxoproline
(pyroglutamate) in rat plasma and hepatic cell line culture medium.
J Pharm Biomed Anal. 56:655–663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang Z, Sun J, Liang Q, Cai Y, Li S,
Huang Y, Wang Y and Luo G: A metabonomic approach applied to
predict patients with cerebral infarction. Talanta. 84:298–304.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deery DJ and Taylor KW: Effect of
phenylpyruvate on enzymes involved in fatty acid synthesis in rat
brain. Biochem J. 134:557–563. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang T, Jiao J, Shang J, Bi L, Wang H,
Zhang C, Wu H, Cui Y, Wang P and Liu X: The differences of
metabolites in different parts of the brain induced by shuxuetong
injection against cerebral ischemia-reperfusion and its
corresponding mechanism. Evid Based Complement Alternat Med.
2022:94650952022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Du C, Cao S, Shi X, Nie X, Zheng J, Deng
Y, Ruan L, Peng D and Sun M: Genetic and biochemical
characterization of a gene operon for trans-aconitic acid, a novel
nematicide from bacillus thuringiensis. J Biol Chem. 292:3517–3530.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang C, Ling CL, Pang L, Wang Q, Liu JX,
Wang BS, Liang JM, Guo YZ, Qin J and Wang JX: Direct macromolecular
drug delivery to cerebral ischemia area using neutrophil-mediated
nanoparticles. Theranostics. 7:3260–3275. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen XL, Su SL, Liu R, Qian DW, Chen LL,
Qiu LP and Duan JA: Chemical constituents and pharmacological
action of bile acids from animal: A review. Zhongguo Zhong Yao Za
Zhi. 46:4898–4906. 2021.(In Chinese). PubMed/NCBI
|
|
34
|
Li C, Wang X, Yan J, Cheng F, Ma X, Chen
C, Wang W and Wang Q: Cholic acid protects in vitro neurovascular
units against oxygen and glucose deprivation-induced injury through
the BDNF-TrkB signaling pathway. Oxid Med Cell Longev.
2020:12016242020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Quinn M, McMillin M, Galindo C, Frampton
G, Pae HY and DeMorrow S: Bile acids permeabilize the blood brain
barrier after bile duct ligation in rats via Rac1-dependent
mechanisms. Dig Liver Dis. 46:527–534. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Menni C, Graham D, Kastenmüller G, Alharbi
NH, Alsanosi SM, McBride M, Mangino M, Titcombe P, Shin SY, Psatha
M, et al: Metabolomic identification of a novel pathway of blood
pressure regulation involving hexadecanedioate. Hypertension.
66:422–429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun D, Tiedt S, Yu B, Jian X, Gottesman
RF, Mosley TH, Boerwinkle E, Dichgans M and Fornage M: A
prospective study of serum metabolites and risk of ischemic stroke.
Neurology. 92:e1890–e1898. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dong Y and Li HF: Research status of
inherited disorders of metal metabolism in nervous system and the
challenges faced. Chin J Pract Intern Med. 42:278–282. 2022.
|
|
39
|
Mendez NV, Wharton JA, Leclerc JL,
Blackburn SL, Douglas-Escobar MV, Weiss MD, Seubert CN and Doré S:
Clinical implications of bilirubin-associated neuroprotection and
neurotoxicity. Int J Clin Anesthesiol. 1:10132013.PubMed/NCBI
|
|
40
|
Amin SB, Smith T and Timler G:
Developmental influence of unconjugated hyperbilirubinemia and
neurobehavioral disorders. Pediatr Res. 85:191–197. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kurzepa J, Bielewicz J, Stelmasiak Z and
Bartosik-Psujek H: Serum bilirubin and uric acid levels as the bad
prognostic factors in the ischemic stroke. Int J Neurosci.
119:2243–2249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Siebert H, Engelke S, Maruschak B and
Brück W: Concentration-dependent effects of the esterase inhibitor
BNPP on macrophage migration and myelin phagocytosis. Brain Res.
916:159–164. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Batirel HF, Aktan S, Aykut C, Yeğen BC and
Coşkun T: The effect of aqueous garlic extract on the levels of
arachidonic acid metabolites (leukotriene C4 and prostaglandin E2)
in rat forebrain after ischemia-reperfusion injury. Prostaglandins
Leukot Essent Fatty Acids. 54:289–292. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rao AM, Hatcher JF, Kindy MS and Dempsey
RJ: Arachidonic acid and leukotriene C4: Role in transient cerebral
ischemia of gerbils. Neurochem Res. 24:1225–1232. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nguyen BT and Sadée W: Compartmentation of
guanine nucleotide precursors for DNA synthesis. Biochem J.
234:263–269. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim E and Sheng M: PDZ domain proteins of
synapses. Nat Rev Neurosci. 5:771–781. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Buonarati OR, Hammes EA, Watson JF, Greger
IH and Hell JW: Mechanisms of postsynaptic localization of
AMPA-type glutamate receptors and their regulation during long-term
potentiation. Sci Signal. 12:eaar68892019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mardones MD, Jorquera PV, Herrera-Soto A,
Ampuero E, Bustos FJ, van Zundert B and Varela-Nallar L: PSD95
regulates morphological development of adult-born granule neurons
in the mouse hippocampus. J Chem Neuroanat. 98:117–123. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Poels J, Spasić MR, Callaerts P and Norga
KK: An appetite for destruction: From self-eating to cell
cannibalism as a neuronal survival strategy. Autophagy.
8:1401–1403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Overhoff M, De Bruyckere E and Kononenko
NL: Mechanisms of neuronal survival safeguarded by endocytosis and
autophagy. J Neurochem. 157:263–296. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lou G, Palikaras K, Lautrup S,
Scheibye-Knudsen M, Tavernarakis N and Fang EF: Mitophagy and
neuroprotection. Trends Mol Med. 26:8–20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jiang T, Yu JT, Zhu XC, Zhang QQ, Tan MS,
Cao L, Wang HF, Shi JQ, Gao L, Qin H, et al: Ischemic
preconditioning provides neuroprotection by induction of
AMP-activated protein kinase-dependent autophagy in a rat model of
ischemic stroke. Mol Neurobiol. 51:220–229. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dai SH, Chen T, Li X, Yue KY, Luo P, Yang
LK, Zhu J, Wang YH, Fei Z and Jiang XF: Sirt3 confers protection
against neuronal ischemia by inducing autophagy: Involvement of the
AMPK-mTOR pathway. Free Radic Biol Med. 108:345–353. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tran S, Fairlie WD and Lee EF: BECLIN1:
Protein structure, function and regulation. Cells. 10:15222021.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lamark T, Svenning S and Johansen T:
Regulation of selective autophagy: The p62/SQSTM1 paradigm. Essays
Biochem. 61:609–624. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ritzel RM, Li Y, He J, Khan N, Doran SJ,
Faden AI and Wu J: Sustained neuronal and microglial alterations
are associated with diverse neurobehavioral dysfunction long after
experimental brain injury. Neurobiol Dis. 136:1047132020.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li Z, Xiao G, Wang H, He S and Zhu Y: A
preparation of Ginkgo biloba L. leaves extract inhibits the
apoptosis of hippocampal neurons in post-stroke mice via regulating
the expression of Bax/Bcl-2 and caspase-3. J Ethnopharmacol.
280:1144812021. View Article : Google Scholar : PubMed/NCBI
|