Introduction
Breast cancer (BC) currently ranks as the most
common malignancy among women worldwide, posing a significant
threat to their health, and in 2020, it accounted for 11.7% of all
new cancer cases (1). Previous
research has reported that BC progression is determined by tumor
cells and influenced by the tumor microenvironment (TME) (2). Furthermore, the TME significantly
influences the formation of the metabolic and chemical environment,
consequently impacting tumor development and progression (3). The complex and multi-level
interactions between BC cells and the stromal cells and immune
cells in the TME, through direct contact or secretion of factors,
can regulate tumor behavior, promoting metastasis and drug
resistance (4). For instance,
neutrophils have been shown to promote cancer cell EMT and drug
resistance, thereby encouraging metastasis (5).
Breast-conserving surgery (BCS) is a prevalent
method utilized for the treatment of early-stage BC (6). In 2018, over 266,000 women were
diagnosed with early-stage breast cancer in the US, ~60% of whom
chose BCS (7). However, surgery
can trigger an inflammatory response, modifying the local
microenvironment and increasing the invasiveness of residual cancer
cells post-surgery (8). In
addition, invasive surgical procedures and the related stress
responses may facilitate tumor metastasis through the stimulation
of angiogenic factor release and concurrent suppression of cellular
immunity (9). Thus, the
combination of surgery with chemotherapy, hormone therapy and
radiotherapy (RT) has become a common treatment approach for BC
(7). In 2018, 63% of stage I or II
BC patients underwent BCS and radiation therapy (RT) (7).
Currently, BCS combined with postoperative external
beam RT (EBRT) is the standard treatment modality and is typically
administered using conventional fractionation, with a total dose of
45–50 Gy. Most patients require a tumor bed boost of 10–16 Gy, with
the total treatment duration spanning 3–7 weeks (10). However, in comparison with
traditional whole-breast irradiation, intraoperative RT (IORT)
involves the delivery of high-dose internal brachytherapy radiation
therapy to the postoperative region, including the tumor bed and
any remaining lesions (11).
Compared to EBRT, IORT only requires a few minutes to deliver the
necessary radiation dose during surgery, with lower radioactive
side effects, reducing the risks of complications such as
infection, breast fibrosis, and dermatitis (12). Additionally, IORT can save about
$15,000 in total costs compared to EBRT, alleviating patients'
psychological stress and improving their psychological and quality
of life (12,13). Therefore, IORT is considered to be
an effective method for treating BC in clinical practice (12).
A study has reported that IORT not only eliminates
neoplastic cells using radiation but also remodels the TME
(14). This remodeling occurs via
changes in the constituents and biological roles of the exudate at
the surgical location, hindering the proliferation and invasion of
tumor cells, thereby diminishing local recurrence (14). The efficacy of IORT was reported to
be significantly lower in patients who received IORT after a
delayed secondary incision than in those who received IORT during
their primary surgery (12). This
may be due to factors such as the postoperative shift of the tumor
bed and the already established TME, highlighting the result that
IORT can influence the reshaping process of the postoperative TME
(12). Therefore, gaining a deeper
and more comprehensive understanding of the impact of IORT on the
TME, and using this knowledge as a foundation to seek more
personalized treatment plans or radiosensitizing drugs, may be key
to further leveraging the advantages of IORT. The present review
aims to summarize a range of biological effects and alterations
within the TME induced by IORT for BC. These aspects include direct
IORT effects, bystander effects, impacts on tumor angiogenesis,
miRNA expression and associated cytokines. The potential directions
for future research concerning IORT were also proposed, with the
goal of integrating these findings into relevant clinical
investigations for more precise personalized treatments. The main
content of this article is shown in Fig. 1.
Impact of IORT on angiogenesis
Angiogenesis is a vital process for cancer cell
proliferation and dissemination. Certain concentrations of
angiogenic factors within tumor tissue have been associated with
tumor aggressiveness and patient prognosis (15). Vascular endothelial growth factor
(VEGF) is a stimulator of vascular endothelial cell proliferation
and angiogenesis, and delta-like 4 (DLL4) serves as a key
angiogenesis inhibitor involved in the regulation of vascular
maturation and tumor angiogenesis (16). IORT can impact angiogenesis;
however, the effect of radiation on angiogenesis is complex, as it
can have both anti-angiogenic and pro-angiogenic effects (17–20).
A study by Nafissi et al (17) assessed the impact of IORT on
angiogenesis and reported that patients with BC who underwent BCS
and received 21 Gy of intraoperative electron RT (IOERT)
experienced a significant decrease in the blood levels of DLL4 and
an significant increase in VEGF levels compared with pre-surgery
levels. These findings suggest that IOERT may lead to an increase
in the formation of new blood vessels following BCS. However,
certain studies have reported contradictory results. For instance,
Belletti et al (18)
reported a decrease in the levels of angiopoietin and VEGF
receptor-3 in the wound fluid (WF) of patients following targeted
intraoperative RT (TARGIT) treatment, indicating a potential
decrease in neovascularization. Kulcenty et al (21) conducted a study on patients who
underwent IORT and reported a significant reduction in the
expression of the protein IL-8, which promotes the formation of
endothelial cells (20), in the
postoperative RT-WF of these patients. This finding suggests that
IORT exhibits anti-angiogenic effects. In a mouse model assessing
BC recurrence, a single high dose of radiation (20 Gy) was reported
to decrease the local vascular density within the breast compared
to normal breast tissue (22).
Furthermore, higher doses of radiation within the range of 2–15 Gy
have been reported to exhibit anti-angiogenic effects (23). However, tumors may also respond to
radiation by protecting their blood vessels from radiation-induced
damage, leading to a paradoxical pro-angiogenic effect (24).
The aforementioned differing results reported may be
a consequence of variations in the RT apparatus and the dosages of
RT. Thus, the relationship between IORT and angiogenesis in cancer
treatment remains to be fully elucidated. However, the research
indicates that IORT can potentially induce an anti-angiogenic
effect, which may lead to a decrease in proliferation and
metastasis of residual cancer cells. These findings provide new
directions for future research on IORT treatment for BC, such as
the determination of the impact of different radiation doses on
treatment efficacy. Currently, in clinical practice, the mainstream
radiation standards are based on TARGIT-A (20 Gy low-energy X-ray)
and intraoperative irradiation for early BC (ELIOT; 21 Gy electron
beam) (12,25). There are no consensus or guidelines
for adjusting the dose according to the specific circumstances of
the patient (26). In clinical
settings, the intricate relationship between IORT and angiogenesis
should be considered when deciding whether to use anti-angiogenic
drugs postoperatively, as it is crucial to clarify the potential
synergistic or attenuating effects it may have with anti-angiogenic
medications.
Effect of IORT on micro (mi)RNA
expression
miRNAs are a class of non-coding, single-stranded
RNA molecules. Certain miRNAs have been identified as oncogenes or
proto-oncogenes, making them important therapeutic cancer targets
(27). Research has reported that
ionizing radiation may lead to an increase or decrease in the miRNA
expression profiles in certain cells such as human lung cancer cell
lines, and pre-clinical models like mouse models, therefore making
miRNA potential therapeutic targets or biomarkers for the radiation
response of cancer (28,29).
In a study by Zaleska et al (30), postoperative WF obtained from BCS
alone was compared to the RT-WF from patients who received IORT,
and a significant downregulation in the expression of miR-21,
miR-221 and miR-155 in BC cells treated with RT-WF was reported.
Another study indicated that miR-223 was involved in mediating
inflammatory responses and also functioned as an oncogene in tumor
cells (31). In addition, Fabris
et al (32) reported that
41 miRNAs exhibited differential expression in peritumor tissues
between patients with BC who only underwent surgery and those who
received IORT. Furthermore, the study reported that miR-223
targeted EGF, and overexpression of miR-223 inhibited residual
tumor cell proliferation by reducing post-surgical EGF levels and
subsequently suppressing the EGFR signal transduction activation,
as demonstrated in Fig. 2.
Overexpression of miR-21, an miRNA commonly associated with
inflammation, has been reported to promote BC cell proliferation
and metastasis in vivo (33). Furthermore, low levels of miR-21
are associated with a more favorable prognosis in patients who are
HER-2 positive (34). In addition
to miR-21, overexpression of miR-221 and miR-155 has also been
associated with tumor growth, invasion and metastasis (35–37).
In a previous review on miRNA and radiation
(28), it was reported that miR-21
expression was upregulated in BC, esophageal cancer and lung cancer
tissue samples after RT. While changes in the expression of miR-221
reported were inconsistent among studies, it tended to decrease
according to most studies. Qu et al (38) reported that after undergoing
surgery, chemotherapy and RT, the plasma levels of miR-155
significantly decreased in patients with BC compared to before
treatment. However, the specific effects of RT could not be
determined. Based on these data, it may be inferred that IORT
serves a pivotal role in regulating miRNA to exert its antitumor
effects. In particular, miR-223 is a noteworthy miRNA. It has been
reported to serve a key role in BC proliferation, drug resistance
and metastasis (38). Therefore,
miR-223 is a potential target in BC therapy. However, current
research on the relationship between miR-223 and radiation is
limited. In summary, the study by Zaleska et al (30) offers a new clinical therapeutic
perspective, suggesting that IORT may have synergistic or
antagonistic effects when combined with certain miRNA-targeted
treatments. Furthermore, the combined use of IORT with drugs
targeting miR-223 may enhance the therapeutic effect of IORT,
thereby advancing its application.
Epithelial-mesenchymal transition (EMT) of
BC cells using IORT elimination of WF
EMT is a biological process where epithelial or
endothelial cells transform into mesenchymal cells. During this
transition, cells lose their polarity and exhibit downregulation of
epithelial markers, including cadherin (CDH)1, epithelial cell
adhesion molecule and keratin, whilst upregulating mesenchymal
markers such as CDH2, snail family transcriptional repressor 1
(SNAI1) and vimentin (VIM). EMT confers stem cell-like
characteristics to mesenchymal cells, enhances tumor cell
aggressiveness and promotes tumor dissemination and metastasis
(39). Furthermore, EMT is closely
associated with the aggressive phenotype of cancer stem cells
(CSCs). Kulcenty et al (40) reported that treatment of BC cell
lines with WF resulted in an upregulation of mesenchymal markers
(CDH2, SNAI1 and VIM) and downregulation of the epithelial marker
CDH1 in vitro. Hence, it can be inferred that WF may have
the potential to induce EMT in BC cells. However, in the
IORT-treated group, both the BC cells and the induced WF group
demonstrated higher levels of epithelial markers and lower levels
of mesenchymal markers compared to the RT-WF group. These results
indicate that IORT attenuated the EMT process induced by
postoperative WF in BC cells. In addition, the study reported that
radiation-induced bystander effects counteracted the stimulatory
influence of WF on the CSC phenotype and EMT in BC cells.
Impact of IORT on adipose stromal cells
Adipocytes are an essential component of the TME
both before and after BCS (41). A
study reported that adipocytes can interact with tumor cells and
contribute to the development, progression and metastasis of BC
(42). The mesenchymal stem cells
(MSCs) derived from adipose tissue in the breast are referred to as
breast adipose stromal cells (bASC) (43). bASCs have the ability to
differentiate into cancer-associated fibroblasts and actively
participate in the regulation of the TME for tumor cells (44).
In a study by Uhlig et al (45), bASCs exposed to IORT demonstrated a
senescent-like morphology, indicating a loss of their ability to
proliferate when cultured in vitro. This suggests that IORT
may have eliminated the capacity of bASCs to adhere and
proliferate, demonstrating the radiosensitivity of bASCs. These
findings indicate that IORT may influence the active components of
stem cells in the tumor bed, thereby reducing tumor recurrence. A
further study by the same group reported that IORT-stimulated
mammary bASCs demonstrated significantly reduced proliferation,
migration and wound-healing compared with that of the group treated
with WF alone (46). In addition,
the stimulated MSCs demonstrated significantly lower levels of
secretory ‘regulated on activation, normal T cell expressed and
secreted’ (RANTES), growth-regulated oncogene α (GROα) and VEGF,
suggesting a potential mechanism by which IORT influenced the TME.
GROα, one of the secretory factors assessed, has been reported to
increase the aggressiveness of triple-negative BC when
overexpressed, whilst knockdown of GROα attenuated these effects
(47). RANTES, also known as
chemokine ligand (CCL)5, serves a significant role in the
interaction between MSCs and the tumor stroma, thereby facilitating
the metastasis of BC cells (41).
The mechanism by which IORT affects MSC function may be associated
with the bystander effect it produces.
IORT alters WF composition, expression of
associated factors and inflammation
Several studies have demonstrated that surgical
removal of the primary tumor from the breast induced a
wound-healing response and inflammatory process (8,9).
This, in turn, altered the local TME and stimulated the
proliferation of remaining cancer cells, promoting tumor recurrence
and metastasis. Conversely, IORT mitigated these effects.
IORT reduces the stimulatory effect of
WF on tumor cell proliferation
Agresti et al (8) reported that the composition of WF
varied based on the pathological type of tumors. The study analyzed
34 cytokines, growth factors and chemokines in the WF of 27
patients undergoing BCS. The increased expression of macrophage
inflammatory protein (MIP)-1α, MIP-1β, interferon (IFN)γ-induced
protein 10 (IP-10), IL-6, granulocyte colony-stimulating factor
(G-CSF), monocyte chemoattractant protein-1/monocyte chemotactic
and activating factor and bone bridge proteins were reported in
more aggressive tumors, specifically those with HER-2
overexpression. In addition, patients who underwent mastectomy had
significantly higher levels of IL-1 receptor antagonist, IL-1β,
IFN-γ, IL-6, G-CSF, bone bridging protein, IP-10 and MIP-1β in
their WF compared with that of those who underwent partial
mastectomy. These factors may promote cancer development and
progression, and a study by Belletti et al (18) on the effects of postoperative WF on
BC cells reported that the fluid promoted the proliferation,
movement and invasiveness of the cells. However, when the patients
underwent IORT, the fluid collected from their wounds did not
demonstrate these effects. It was reported that the WF obtained
from patients treated with IORT had diminished levels of numerous
proteins linked to tumor development and movement, such as
hepatocyte growth factor, leptin and RANTES. This reduction would
have resulted in impaired activation of the signaling pathways
leading to STAT3 and p70S6 kinases, ultimately inhibiting tumor
growth and metastasis. These results demonstrate that IORT may
modulate the abundance of growth factors and cytokines within WF,
resulting in antitumor effects. Therefore, IORT may be recommended
for highly invasive subtypes of BC.
IORT induces tumor cell apoptosis and
inhibits their expansion and spread
Tumor necrosis factor (TNF) is a small protein
molecule mainly produced by activated macrophages, NK cells, and T
lymphocytes (48). This can lead
to natural killer cell dysfunction in BC, resulting in the failure
of immunotherapy. Death receptor 5 (DR5; also known as TRAIL
receptor 2) is a receptor in TNF that induces cancer cell death via
exogenous pathway activation by cystatin proteases (49). Kulcenty et al (19) reported that DR5 protein expression
was significantly induced in BC cells treated with RT-WF compared
to BC cells treated with WF. By contrast, a previous study
demonstrated notably diminished levels of IL-6 in the WF of
patients following IORT (18), and
this is closely associated with tumor stem cell proliferation and
drug resistance (50). In
addition, IL-8 is an inflammatory chemokine, associated with the
EMT and CSC phenotypes of BC cells (51,52).
A notable decrease was observed in RT-WF in the protein expression
of both IL-6 and IL-8 after treatment (19,21).
This reduction in IL-6 expression led to the inhibition of STAT 3
activity, thereby affecting tumor growth, and it also resulted in
decreased EMT and CSC phenotypes (21).
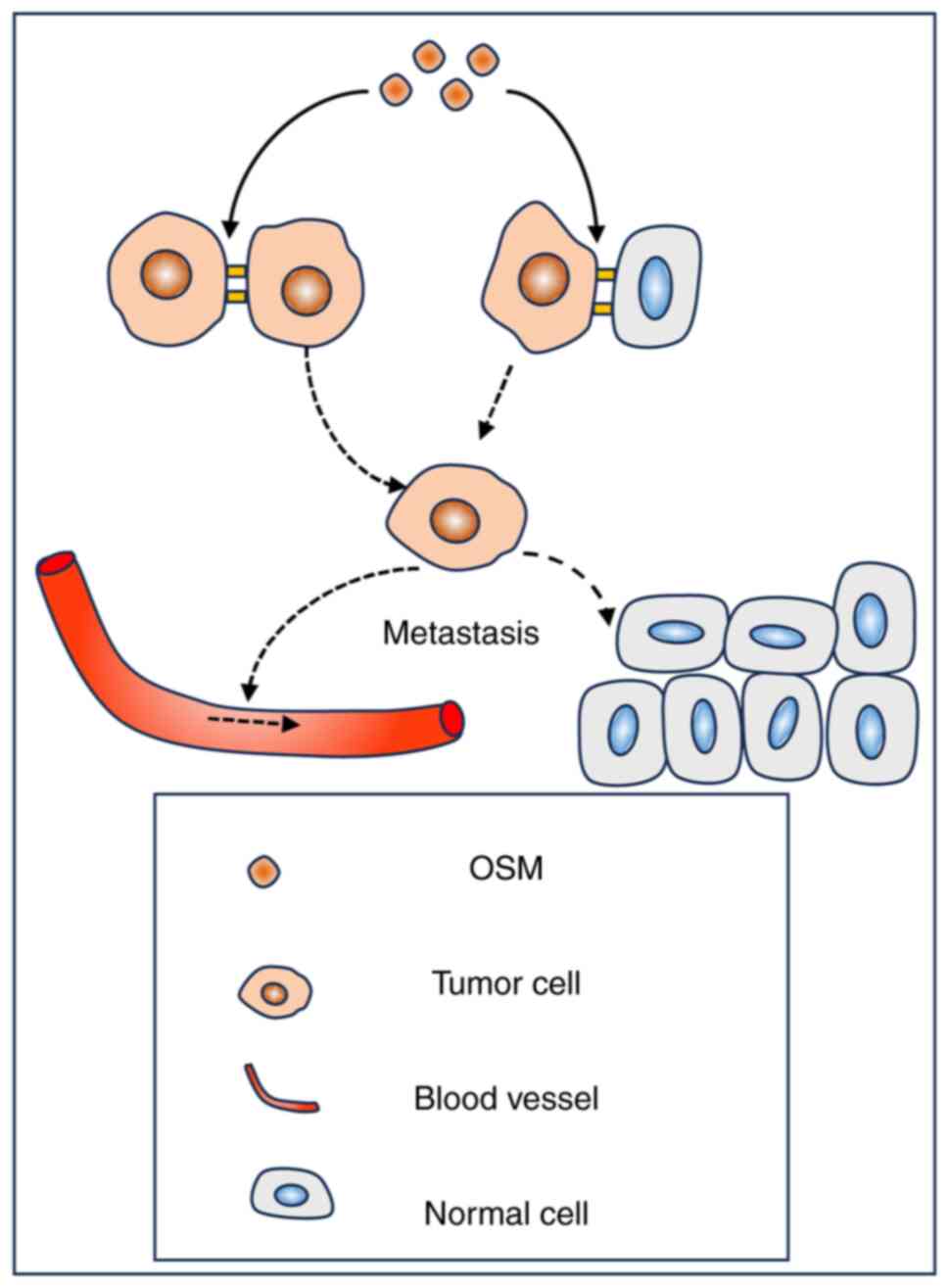
Oncostatin M (OSM) is a key factor in the
reprogramming of the TME in BC, promoting tumor progression
(53). A study reported that OSM
can loosen cell-cell and cell-matrix junctions in BC cells,
ultimately leading to increased aggressiveness of tumor cells
(54), as demonstrated in Fig. 3. IL-1β exhibits a context-dependent
function in cancer progression. A study reported that it promotes
the development of bone metastases (55), whilst another has reported that it
may hinder the colonization of tumor cells that induce metastasis
(56). Therefore, the precise role
of IL-1β may depend on its specific context. Wuhrer et al
(46) reported a significant
decrease in OSM levels and a significant increase in leptin and
IL-1β levels in BC cells that underwent RT-WF, compared to BC cells
treated with WF. The differences in leptin were hypothesized to be
due to the adipose tissue being exposed to radiation (18). In addition, it was reported that
RT-WF not only reduced the proliferation of MSCs, but also
attenuated wound healing.
The aforementioned findings suggest that IORT has
the potential to induce apoptosis in tumor cells and prevent their
proliferation and metastasis by influencing the composition of WF
constituents, modulating the expression of cytokines and affecting
signaling pathways. However, the possibility that these effects may
coincide with a prolonged wound recovery process following the
surgical procedure should be considered and the specific mechanisms
underlying this remain to be elucidated through more in-depth
research. Furthermore, more research should be conducted on the
relationship between OSM and RT, as the OSM/OSM receptor signaling
pathway has been reported to be a critical avenue for remodeling
the TME of BC and therefore, inhibition of this pathway may offer a
new strategy for treating BC (53). The suppressive effect of IORT on
OSM could perhaps be an effective measure to realize this
therapeutic strategy.
IORT reduces the incidence of
radiation inflammation
In a study with a 4-year follow-up period, a single
application of IORT did not have a significant impact on the
leukocyte count in peripheral blood samples in comparison with
conventional external irradiation (57). Meng et al (58) reported that a single high-dose
irradiation was more effective in treating cancer compared with
multiple irradiations. It was elucidated that a single high-dose
radiation treatment diminished the activation cycle of the
autotaxin-lysophosphatidic acid axis. Conversely, multiple
irradiations facilitated inflammatory responses, which may have
protected cancer cells from cell death induced by radiation. In an
in vivo mouse model study, Krall et al (59) reported that the systemic
inflammatory response initiated after BCS could augment tumor cell
proliferation and this process could potentially lead to recurrent
metastasis. However, a reduction in tumor resurgence and relapse
among patients with BC who received anti-inflammatory treatment
during the perioperative period was also reported.
Direct, bystander and cancer cell metabolism
effects of IORT
IORT inhibits the division of cancer
cells
A study by Pan et al (60) reported that after in vitro
irradiation of BC cells with a single dose of 2/4/6 Gy, the
proportion of normal BC cells decreased and the proportion of cells
undergoing apoptosis or necrosis increased with increasing doses of
irradiation. Furthermore, among the examined cells, there was a
rise in the proportion of cells arrested in the G1 phase and a
decline in the proportion of cells in the S and G2 phases,
indicating an inhibition of the mitotic process. In addition, the
total number of newly dead cells gradually increased on days 2 and
3 after RT. Based on the observation of cancer cell survival after
four weeks, it was hypothesized that a single dose of brachytherapy
may have a long-term inhibitory effect on proliferation and
invasion, and a pro-apoptotic effect. Additionally, it was reported
that this effect increased with higher doses of brachytherapy.
IORT affects unirradiated cells
through bystander effects
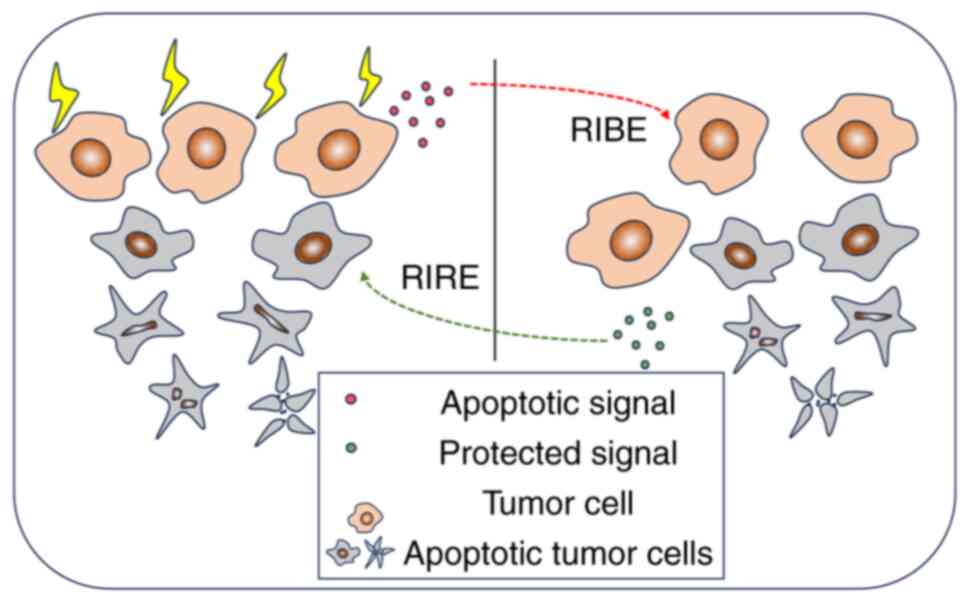
In addition to its direct effects, ionizing
radiation has the capacity to influence non-irradiated cells
situated adjacent to those exposed to radiation. This occurrence is
recognized as the radiation-induced bystander effect (RIBE) and is
facilitated by intercellular gap junctions, along with the
secretion of cytokines and chemokines (61), as illustrated in Fig. 4. Kulcenty et al (40) reported that co-culturing WF-treated
BC cells with RT-WF-treated BC cells eliminated the original
EMT-inducing effect. Moreover, in vitro scratch assays
demonstrated a reduction in the migration of BC cells treated with
WF after undergoing RT-WF.
During RT, bystander cells that are not directly
exposed to radiation can display effects akin to those of directly
irradiated cells, including heightened genomic damage, modified
apoptosis rates, increased mutation frequency, DNA damage,
diminished cloning efficiency and oncogenic transformation
(61). However, there is a
distinct difference between the radiation-induced bystander effect
and the direct effect of radiation. Al-Abedi et al (62) reported that radiation-generated
RIBE enhanced the EMT phenotype of bystander cells and increased
the invasiveness of bystander MCF-7 cells. By contrast, Feghhi
et al (63) reported that
using culture media from electron beam-irradiated cells reduced the
survival rate of non-irradiated MCF-7 cells but promoted their
migration. It has been hypothesized that, to produce this bystander
effect, the radiation dose needs to reach a certain threshold,
resulting in an ‘all-or-nothing’ state (61).
The radiation-induced rescue effect (RIRE) is a
phenomenon bearing a strong association with RIBE. The occurrence
of RIRE permits cells exposed to radiation to derive advantages
from signals emitted by nearby, non-irradiated cells. Chen et
al (64) reported an improved
survival rate for radiation-exposed fibroblasts when grown
alongside non-irradiated cells in a shared environment. Similarly,
when radiation-exposed HeLa cells were cultivated together with
non-irradiated fibroblasts, a reduction in the formation of
micronuclei within the HeLa cells was reported. This indicated that
bystander cells may rescue irradiated tumor cells through the RIRE,
as illustrated in Fig. 4.
Consequently, this effect could potentially contribute to the
recurrence of residual tumor cells.
In addition, when exposed to radiation stress
conditions such as RT, cells can display autophagy-inducing
behavior. The induction of autophagy may result in tumor cells
transitioning into a reversible dormant condition, allowing them to
survive instead of undergoing apoptosis (65,66).
However, this behavior may result in later tumor recurrence
(66). A study reported that
markers of autophagy are notably increased in bystander
hepatocellular carcinoma cells exposed to 3 Gy of γ-rays (67). This demonstrates that bystander
hepatocellular carcinoma cells can produce an autophagic
response.
The mechanism of the RIBE produced during RT is
complex. The aforementioned studies demonstrated that diverse cells
could generate different biological effects when exposed to varying
doses of radiation. However, despite the advantages of IORT, the
specific RIBE produced has remained to be fully determined
(14,40). Therefore, further research is
required to provide an improved understanding of the clinical
implications associated with IORT.
Effect of IORT on DNA damage and
glucose metabolism in cancer cells
Ionizing radiation primarily damages DNA in cells.
Studies conducted by Piotrowski et al (14) and Kulcenty et al (68) reported that both RT with and
without RIBE stimulation in cancer cells induced DNA double-strand
breaks and heightened the expression of genes responsible for DNA
damage repair [such as the genes ERCC excision repair 2, TFIIH core
complex helicase subunit (ERCC2), ERCC8 and RAD51 recombinase].
Upon exposure to RT-WF and WF + RIBE stimuli, two DNA repair
mechanisms were activated, nucleotide excision repair and
homologous recombination.
Moreover, it was reported that BC cells exposed to
RT-WF and WF + RIBE treatment exhibited increased oxidative
phosphorylation levels in comparison with those treated solely with
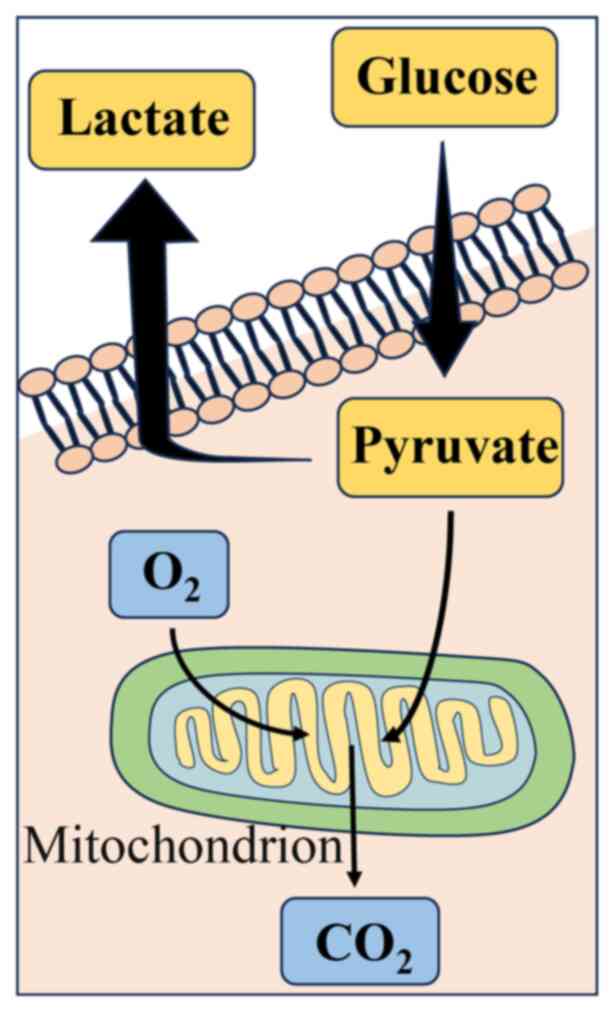
WF. It has been reported that cancer cells may be more adapted to
aerobic glycolysis as their primary means of glucose metabolism
instead of oxidative phosphorylation and this phenomenon is known
as the Warburg effect (69).
During this metabolic process in cancer cells, glucose molecules
are converted into pyruvate via glycolysis and then reduced to
lactate with the help of lactate dehydrogenase, ultimately leading
to a decrease in the pH of the TME (69), as illustrated in Fig. 5. This reduction in pH can enhance
the metastatic capacity of cancer cells (70,71).
Kulcenty et al (68)
hypothesized that it is therefore possible that IORT facilitates a
metabolic shift in BC cells from glycolysis to the oxidative
phosphorylation pathway, due to direct and bystander effects. The
metabolic shift may contribute to a decrease in glucose consumption
and lactate secretion in BC cells, which consequently modifies both
the cellular metabolism and the pH of the TME. These findings
suggest that the alteration of metabolism in cancer cells is
another role served by IORT.
Discussion
Recent research from the TARGIT-A and ELIOT trials
have reported that the effectiveness of IORT for patients with BC
is not inferior to traditional EBRT (12,25).
The data from the TARGIT-A trial demonstrated that over an average
follow-up period of 8.6 years, the overall mortality rate in the
IORT group was significantly lower than that in the EBRT group (3.9
vs. 5.3%; hazard ratio, 0.76), with no significant differences in
BC mortality rate and distant metastasis (12). The local recurrence rate in the
IORT group was markedly higher than that in the EBRT group (3.3 vs.
1.3%; hazard ratio, 2.55), but this was within an acceptable range.
In the TARGIT-A trial, the non-inferiority margin for the
difference in local recurrence rates between the two groups was set
at 2.5%. In the ELIOT trial (25),
after a median follow-up of 12.4 years, the ipsilateral breast
tumor recurrence rate was significantly higher in patients
receiving single-dose IORET compared to those receiving
whole-breast external radiotherapy (12.6 vs. 2.4% at 15 years;
hazard ratio, 4.62). However, there was no significant difference
in overall survival between the two groups (83.4 vs. 82.4% at 15
years).
The application of IORT has yet to be standardized
internationally, primarily due to insufficient large-scale clinical
trials and foundational research on this technique. IORT is an RT
technique that has become more commonly used over the last 30 years
and has filled certain gaps between conventional surgical
treatments and traditional EBRT (72). For instance, in the traditional
surgical + EBRT treatment, patients are required to endure long
periods of psychological distress and complications, whereas
implementing IORT is able to markedly shorten patients' treatment
cycles, enhance their quality of life and reduce economic losses
(73).
However, for optimal therapeutic and economic
benefits, whilst ensuring patient quality of life and mental
health, integrating IORT with EBRT may be a novel paradigm worth
exploring. The ongoing TARGIT-B trial is a large-scale clinical
trial evaluating the combination of IORT with post-operative tumor
bed boost (74). However, there is
still insufficient research supporting the wider clinical
application of IORT and therefore, understanding the potential
mechanisms of IORT is pivotal for the advancement of this
technology.
Furthermore, the present review demonstrated
variations in the composition of RT-WF across different studies,
possibly due to differences in IORT equipment or radiation doses.
Belletti et al (18)
reported a notable decrease in hepatocyte growth factor (HGF) and
MIP-1α levels and an increase in IL-13 concentration in RT-WF,
whereas Kulcenty et al (21) reported a significant increase in
HGF and MIP-1α levels and a decrease in the IL-13 concentration in
RT-WF, as illustrated in Fig. 6.
Thus, it is hypothesized that these changes in the TME may explain
the differences in the results of the TARGIT-A and ELIOT trials.
Furthermore, WF had significantly higher concentrations of HGF and
MIP-1α, and lower levels of IL-13 in both studies. Kulcenty et
al (21) additionally noted a
marked disparity in the levels of HGF in the RT-WF between patients
with luminal A and B BC. The concentrations of small molecules,
including IL-9, platelet-derived growth factor-BB, RANTES, TNF-β,
CCL2 and CCL7, were reported to differ between the WFs of the two
groups. This suggested that there may be differences in how
different types of BC respond to IORT.
 | Figure 6.Changes in cytokines, genes and other
compounds reported in numerous studies. The highlighted parts
represent different components in different studies. IORT,
intraoperative radiotherapy; IOERT, intraoperative electron
radiotherapy; EGFR, epidermal growth factor receptor; FAS/TNFRSF6,
FAS cell surface death receptor/tumor necrosis factor receptor
superfamily member 6; FGF, fibroblast growth factor; G-CSF,
granulocyte-colony stimulating factor; IGFBP-6, insulin like growth
factor binding protein 6; IL, interleukin; Mip, macrophage
inflammatory protein; MCP, monocyte chemoattractant protein;
PDGF-BB, platelet-derived growth factor B; GRO, growth regulated
oncogene; HGF, hepatocyte growth factor; sTNFR. soluble tumor
necrosis factor receptor; uPAR, urokinase-type plasminogen
activator receptor; Tie, tyrosine kinase with immunoglobulin like
and EGF like domains; OSM, oncostatin M; SCGF, stem cell growth
factor; CCL, C-C motif chemokine ligand; CTACK, cutaneous T
cell-attracting chemokine; MIF, macrophage migration inhibitory
factor. |
The aforementioned studies illustrate the intricate
biological effects that result from direct irradiation of the tumor
bed and surrounding tissues through IORT during surgery, which may
be the primary mechanism underlying its antitumor effects. However,
the entire biological foundation underlying this phenomenon has
remained to be fully elucidated, necessitating additional studies.
Detailed molecular-level information obtained from these studies
would form a foundation for further investigations into tumor
recurrence and metastasis, and for the identification of new
therapeutic targets and treatment modalities. Through comprehending
the interplay between BC cells and their TME following IORT, novel
approaches to BC therapy may be identified.
Lastly, it is noteworthy to mention some limitations
in this review. Firstly, due to time constraints, we may not have
incorporated the most recent research findings. Secondly, the
heterogeneous quality of reviews might lead to a lack of depth or
comprehensiveness in the interpretation of certain literature.
Additionally, given the constraints in length and focus, this
review may not have encompassed all pertinent literature, thereby
possibly omitting some crucial information or research.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural Science
Foundation of China (grant nos. 82060543 and 82060538), the Yunnan
Provincial Science and Technology Plan Kunming Medical Joint
Special Project (grant no. 202001AY070001 079) and the Middle-aged
and Young Academic and Technical Leader Reserve Talent Program of
Yunnan Province Science and Technology Department (grant no.
202205AC160008).
Availability of data and materials
Not applicable.
Authors' contributions
YY, XH, SK, ZZ, MH, CL and NL searched the
literature. YY, XH and SK wrote the manuscript. ZZ, MH, CL and NL
critically revised the manuscript. FG and WC conceived the idea for
the review and provided the final approval. All authors have read
and approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo S and Deng CX: Effect of stromal cells
in tumor microenvironment on metastasis initiation. Int J Biol Sci.
14:2083–2093. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simmons A, Burrage PM, Nicolau DV Jr,
Lakhani SR and Burrage K: Environmental factors in breast cancer
invasion: A mathematical modelling review. Pathology. 49:172–180.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Terceiro LEL, Edechi CA, Ikeogu NM, Nickel
BE, Hombach-Klonisch S, Sharif T, Leygue E and Myal Y: The breast
tumor microenvironment: A key player in metastatic spread. Cancers
(Basel). 13:47982021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Güç E and Pollard JW: Redefining
macrophage and neutrophil biology in the metastatic cascade.
Immunity. 54:885–902. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Si J, Guo R, Lu X, Han C, Xue L, Xing D
and Chen C: Decision aids on breast conserving surgery for early
stage breast cancer patients: A systematic review. BMC Med Inform
Decis Mak. 20:2752020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giaquinto AN, Sung H, Miller KD, Kramer
JL, Newman LA, Minihan A, Jemal A and Siegel RL: Breast cancer
statistics, 2022. CA Cancer J Clin. 72:524–541. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Agresti R, Triulzi T, Sasso M, Ghirelli C,
Aiello P, Rybinska I, Campiglio M, Sfondrini L, Tagliabue E and
Bianchi F: Wound healing fluid reflects the inflammatory nature and
aggressiveness of breast tumors. Cells. 8:1812019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim R: Effects of surgery and anesthetic
choice on immunosuppression and cancer recurrence. J Transl Med.
16:82018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng K, Meng X, Liu J, Xing Z, Zhang M and
Wang X, Feng Q and Wang X: Update on intraoperative radiotherapy
for early-stage breast cancer. Am J Cancer Res. 10:2032–2042.
2020.PubMed/NCBI
|
|
11
|
Stoll A, van Oepen A and Friebe M:
Intraoperative delivery of cell-killing boost radiation-a review of
current and future methods. Minim Invasive Ther Allied Technol.
25:176–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vaidya JS, Bulsara M, Baum M, Wenz F,
Massarut S, Pigorsch S, Alvarado M, Douek M, Saunders C, Flyger HL,
et al: Long term survival and local control outcomes from single
dose targeted intraoperative radiotherapy during lumpectomy
(TARGIT-IORT) for early breast cancer: TARGIT-A randomised clinical
trial. BMJ. 370:m28362020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eisavi M, Rezapour A, Alipour V, Mirzaei
HR and Arabloo J: Cost-effectiveness analysis of intraoperative
radiation therapy versus external beam radiation therapy for the
adjuvant treatment of early breast cancer: A systematic review. Med
J Islam Repub Iran. 34:1672020.PubMed/NCBI
|
|
14
|
Piotrowski I, Kulcenty K, Murawa D and
Suchorska W: Surgical wound fluids from patients treated with
intraoperative radiotherapy induce radiobiological response in
breast cancer cells. Med Oncol. 36:142018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee E, Lee EA, Kong E, Chon H,
Llaiqui-Condori M, Park CH, Park BY, Kang NR, Yoo JS, Lee HS, et
al: An agonistic anti-Tie2 antibody suppresses the normal-to-tumor
vascular transition in the glioblastoma invasion zone. Exp Mol Med.
55:470–484. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baharlou R, Tajik N, Habibi-Anbouhi M,
Shokrgozar MA, Zarnani AH, Shahhosseini F and Behdani M: Generation
and characterization of an anti-delta like ligand-4 nanobody to
induce non-productive angiogenesis. Anal Biochem. 544:34–41. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nafissi N, Mohammadlou M, Akbari ME,
Mahdavi SR, Sheikh M, Borji M, Babaee E and Baharlou R: The impact
of intraoperative radiotherapy on breast cancer: Focus on the
levels of angiogenic factors. World J Surg Oncol. 20:1912022.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Belletti B, Vaidya JS, D'Andrea S,
Entschladen F, Roncadin M, Lovat F, Berton S, Perin T, Candiani E,
Reccanello S, et al: Targeted intraoperative radiotherapy impairs
the stimulation of breast cancer cell proliferation and invasion
caused by surgical wounding. Clin Cancer Res. 14:1325–1332. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kulcenty KI, Piotrowski I, Zaleska K,
Murawa D and Suchorska WM: Wound fluids collected from patients
after IORT treatment activates extrinsic apoptotic pathway in MCF7
breast cancer cell line. Ginekol Pol. 89:175–182. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Végran F, Boidot R, Michiels C, Sonveaux P
and Feron O: Lactate influx through the endothelial cell
monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway
that drives tumor angiogenesis. Cancer Res. 71:2550–2560. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kulcenty K, Piotrowski I, Wróblewska JP,
Wasiewicz J and Suchorska AWM: The composition of surgical wound
fluids from breast cancer patients is affected by intraoperative
radiotherapy treatment and depends on the molecular subtype of
breast cancer. Cancers (Basel). 12:112019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuonen F, Laurent J, Secondini C, Lorusso
G, Stehle JC, Rausch T, Faes-Van't Hull E, Bieler G, Alghisi GC,
Schwendener R, et al: Inhibition of the Kit ligand/c-Kit axis
attenuates metastasis in a mouse model mimicking local breast
cancer relapse after radiotherapy. Clin Cancer Res. 18:4365–4374.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abdollahi A, Griggs DW, Zieher H, Roth A,
Lipson KE, Saffrich R, Gröne HJ, Hallahan DE, Reisfeld RA, Debus J,
et al: Inhibition of alpha(v)beta3 integrin survival signaling
enhances antiangiogenic and antitumor effects of radiotherapy. Clin
Cancer Res. 11:6270–6279. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goedegebuure RSA, de Klerk LK, Bass AJ,
Derks S and Thijssen VLJL: Combining radiotherapy with
anti-angiogenic therapy and immunotherapy; a therapeutic triad for
cancer? Front Immunol. 9:31072019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Orecchia R, Veronesi U, Maisonneuve P,
Galimberti VE, Lazzari R, Veronesi P, Jereczek-Fossa BA, Cattani F,
Sangalli C, Luini A, et al: Intraoperative irradiation for early
breast cancer (ELIOT): Long-term recurrence and survival outcomes
from a single-centre, randomised, phase 3 equivalence trial. Lancet
Oncol. 22:597–608. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harris EER and Small W Jr: Intraoperative
radiotherapy for breast cancer. Front Oncol. 7:3172017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dhawan A, Scott JG, Harris AL and Buffa
FM: Pan-cancer characterisation of microRNA across cancer hallmarks
reveals microRNA-mediated downregulation of tumour suppressors. Nat
Commun. 9:52282018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mueller AK, Lindner K, Hummel R, Haier J,
Watson DI and Hussey DJ: MicroRNAs and their impact on radiotherapy
for cancer. Radiat Res. 185:668–677. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Metheetrairut C and Slack FJ: MicroRNAs in
the ionizing radiation response and in radiotherapy. Curr Opin
Genet Dev. 23:12–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zaleska K, Przybyla A, Kulcenty K,
Wichtowski M, Mackiewicz A, Suchorska W and Murawa D: Wound fluids
affect miR-21, miR-155 and miR-221 expression in breast cancer cell
lines, and this effect is partially abrogated by intraoperative
radiation therapy treatment. Oncol Lett. 14:4029–4036. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jeffries J, Zhou W, Hsu AY and Deng Q:
miRNA-223 at the crossroads of inflammation and cancer. Cancer
Lett. 451:136–141. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fabris L, Berton S, Citron F, D'Andrea S,
Segatto I, Nicoloso MS, Massarut S, Armenia J, Zafarana G, Rossi S,
et al: Radiotherapy-induced miR-223 prevents relapse of breast
cancer by targeting the EGF pathway. Oncogene. 35:4914–4926. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang H, Tan Z, Hu H, Liu H, Wu T, Zheng C,
Wang X, Luo Z, Wang J, Liu S, et al: microRNA-21 promotes breast
cancer proliferation and metastasis by targeting LZTFL1. BMC
Cancer. 19:7382019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Badr M, Said H, Louka ML, Elghazaly HA,
Gaballah A and Atef Abd El Mageed M: MicroRNA-21 as a predictor and
prognostic factor for trastuzumab therapy in human epidermal growth
factor receptor 2-positive metastatic breast cancer. J Cell
Biochem. 120:3459–3466. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Di Martino MT, Arbitrio M, Caracciolo D,
Cordua A, Cuomo O, Grillone K, Riillo C, Caridà G, Scionti F,
Labanca C, et al: miR-221/222 as biomarkers and targets for
therapeutic intervention on cancer and other diseases: A systematic
review. Mol Ther Nucleic Acids. 27:1191–1224. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang J, Wang Q, Guan Y, Sun Y, Wang X,
Lively K, Wang Y, Luo M, Kim JA, Murphy EA, et al: Breast cancer
cell-derived microRNA-155 suppresses tumor progression via
enhancing immune cell recruitment and antitumor function. J Clin
Invest. 132:e1572482022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Khalighfard S, Alizadeh AM, Irani S and
Omranipour R: Plasma miR-21, miR-155, miR-10b, and Let-7a as the
potential biomarkers for the monitoring of breast cancer patients.
Sci Rep. 8:179812018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qu H, Zhu F, Dong H, Hu X and Han M:
Corrigendum: Upregulation of CCT-3 induces breast cancer cell
proliferation through miR-223 competition and Wnt/b-catenin
signaling pathway activation. Front Oncol. 12:9173782022.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Park M, Kim D, Ko S, Kim A, Mo K and Yoon
H: Breast cancer metastasis: Mechanisms and therapeutic
implications. Int J Mol Sci. 23:68062022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kulcenty K, Piotrowski I, Zaleska K,
Wichtowski M, Wróblewska J, Murawa D and Suchorska WM: Wound fluids
collected postoperatively from patients with breast cancer induce
epithelial to mesenchymal transition but intraoperative
radiotherapy impairs this effect by activating the
radiation-induced bystander effect. Sci Rep. 9:78912019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao C, Wu M, Zeng N, Xiong M, Hu W, Lv W,
Yi Y, Zhang Q and Wu Y: Cancer-associated adipocytes: Emerging
supporters in breast cancer. J Exp Clin Cancer Res. 39:1562020.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Iwase T, Wang X, Shrimanker TV, Kolonin MG
and Ueno NT: Body composition and breast cancer risk and treatment:
Mechanisms and impact. Breast Cancer Res Treat. 186:273–283. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bunnell BA, Martin EC, Matossian MD, Brock
CK, Nguyen K, Collins-Burow B and Burow ME: The effect of obesity
on adipose-derived stromal cells and adipose tissue and their
impact on cancer. Cancer Metastasis Rev. 41:549–573. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Eckel-Mahan K, Ribas Latre A and Kolonin
MG: Adipose stromal cell expansion and exhaustion: Mechanisms and
consequences. Cells. 9:8632020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Uhlig S, Wuhrer A, Berlit S, Tuschy B,
Sutterlin M and Bieback K: Intraoperative radiotherapy for breast
cancer treatment efficiently targets the tumor bed preventing
breast adipose stromal cell outgrowth. Strahlenther Onkol.
196:398–404. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wuhrer A, Uhlig S, Tuschy B, Berlit S,
Sperk E, Bieback K and Sütterlin M: Wound fluid from breast cancer
patients undergoing intraoperative radiotherapy exhibits an altered
cytokine profile and impairs mesenchymal stromal cell function.
Cancers (Basel). 13:21402021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bhat K, Sarkissyan M, Wu Y and Vadgama JV:
GROα overexpression drives cell migration and invasion in triple
negative breast cancer cells. Oncol Rep. 38:21–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Slattery K, Woods E, Zaiatz-Bittencourt V,
Marks S, Chew S, Conroy M, Goggin C, MacEochagain C, Kennedy J,
Lucas S, et al: TGFβ drives NK cell metabolic dysfunction in human
metastatic breast cancer. J Immunother Cancer. 9:e0020442021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pan L, Fu TM, Zhao W, Zhao L, Chen W, Qiu
C, Liu W, Liu Z, Piai A, Fu Q, et al: Higher-order clustering of
the transmembrane anchor of DR5 drives signaling. Cell.
176:1477–1489.e14. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang T, Fahrmann JF, Lee H, Li YJ,
Tripathi SC, Yue C, Zhang C, Lifshitz V, Song J, Yuan Y, et al:
JAK/STAT3-regulated fatty acid β-oxidation is critical for breast
cancer stem cell self-renewal and chemoresistance. Cell Metab.
27:136–150.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Valeta-Magara A, Gadi A, Volta V, Walters
B, Arju R, Giashuddin S, Zhong H and Schneider RJ: Inflammatory
breast cancer promotes development of M2 tumor-associated
macrophages and cancer mesenchymal cells through a complex
chemokine network. Cancer Res. 79:3360–3371. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Deng F, Weng Y, Li X, Wang T, Fan M and
Shi Q: Overexpression of IL-8 promotes cell migration via PI3K-Akt
signaling pathway and EMT in triple-negative breast cancer. Pathol
Res Pract. 223:1528242021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Araujo AM, Abaurrea A, Azcoaga P,
López-Velazco JI, Manzano S, Rodriguez J, Rezola R, Egia-Mendikute
L, Valdés-Mora F, Flores JM, et al: Stromal oncostatin M cytokine
promotes breast cancer progression by reprogramming the tumor
microenvironment. J Clin Invest. 132:e1486672022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Junk DJ, Bryson BL, Smigiel JM,
Parameswaran N, Bartel CA and Jackson MW: Oncostatin M promotes
cancer cell plasticity through cooperative STAT3-SMAD3 signaling.
Oncogene. 36:4001–4013. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tulotta C and Ottewell P: The role of
IL-1B in breast cancer bone metastasis. Endocr Relat Cancer.
25:R421–R434. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Castaño Z, San Juan BP, Spiegel A, Pant A,
DeCristo MJ, Laszewski T, Ubellacker JM, Janssen SR, Dongre A,
Reinhardt F, et al: IL-1β inflammatory response driven by primary
breast cancer prevents metastasis-initiating cell colonization. Nat
Cell Biol. 20:1084–1097. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wersal C, Keller A, Weiss C, Giordano FA,
Abo-Madyan Y, Tuschy B, Sütterlin M, Wenz F and Sperk E: Long-term
changes in blood counts after intraoperative radiotherapy for
breast cancer-single center experience and review of the
literature. Transl Cancer Res. 8:1882–1903. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Meng G, Wuest M, Tang X, Dufour J, Zhao Y,
Curtis JM, McMullen TPW, Murray D, Wuest F and Brindley DN:
Repeated fractions of X-radiation to the breast fat pads of mice
augment activation of the autotaxin-lysophosphatidate-inflammatory
cycle. Cancers (Basel). 11:18162019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Krall JA, Reinhardt F, Mercury OA,
Pattabiraman DR, Brooks MW, Dougan M, Lambert AW, Bierie B, Ploegh
HL, Dougan SK and Weinberg RA: The systemic response to surgery
triggers the outgrowth of distant immune-controlled tumors in mouse
models of dormancy. Sci Transl Med. 10:eaan34642018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Pan L, Wan M, Zheng W, Wu R, Tang W, Zhang
X, Yang T and Ye C: Intrabeam radiation inhibits proliferation,
migration, and invasiveness and promotes apoptosis of MCF-7 breast
cancer cells. Technol Cancer Res Treat. 18:15330338198407062019.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tang H, Cai L, He X, Niu Z and Huang H, Hu
W, Bian H and Huang H: Radiation-induced bystander effect and its
clinical implications. Front Oncol. 13:11244122023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Al-Abedi R, Tuncay Cagatay S, Mayah A,
Brooks SA and Kadhim M: Ionising radiation promotes invasive
potential of breast cancer cells: The role of exosomes in the
process. Int J Mol Sci. 22:115702021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Feghhi M, Rezaie J, Mostafanezhad K and
Jabbari N: Bystander effects induced by electron beam-irradiated
MCF-7 cells: A potential mechanism of therapy resistance. Breast
Cancer Res Treat. 187:657–671. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chen S, Zhao Y, Han W, Chiu SK, Zhu L, Wu
L and Yu KN: Rescue effects in radiobiology: Unirradiated bystander
cells assist irradiated cells through intercellular signal
feedback. Mutat Res. 706:59–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Amaravadi RK and Thompson CB: The roles of
therapy-induced autophagy and necrosis in cancer treatment. Clin
Cancer Res. 13:7271–7279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lu Z, Luo RZ, Lu Y, Zhang X, Yu Q, Khare
S, Kondo S, Kondo Y, Yu Y, Mills GB, et al: The tumor suppressor
gene ARHI regulates autophagy and tumor dormancy in human ovarian
cancer cells. J Clin Invest. 118:3917–3929. 2008.PubMed/NCBI
|
|
67
|
Wang X, Zhang J, Fu J, Wang J, Ye S, Liu W
and Shao C: Role of ROS-mediated autophagy in radiation-induced
bystander effect of hepatoma cells. Int J Radiat Biol. 91:452–458.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kulcenty K, Piotrowski I, Rucinski M,
Wroblewska JP, Jopek K, Murawa D and Suchorska WM: Surgical wound
fluids from patients with breast cancer reveal similarities in the
biological response induced by intraoperative radiation therapy and
the radiation-induced bystander effect-transcriptomic approach. Int
J Mol Sci. 21:11592020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Vaupel P and Multhoff G: Revisiting the
Warburg effect: Historical dogma versus current understanding. J
Physiol. 599:1745–1757. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Vaupel P, Schmidberger H and Mayer A: The
Warburg effect: Essential part of metabolic reprogramming and
central contributor to cancer progression. Int J Radiat Biol.
95:912–919. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Estrella V, Chen T, Lloyd M, Wojtkowiak J,
Cornnell HH, Ibrahim-Hashim A, Bailey K, Balagurunathan Y, Rothberg
JM, Sloane BF, et al: Acidity generated by the tumor
microenvironment drives local invasion. Cancer Res. 73:1524–1535.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wenz F: Keynote address at the american
society of breast surgeons 18th annual meeting: Current and future
application of intraoperative radiotherapy (IORT) in the curative
and palliative treatment of breast cancer. Ann Surg Oncol.
24:2811–2817. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Omosule M, De Silva-Minor S and Coombs N:
Case report: Intraoperative radiotherapy as the new standard of
care for breast cancer patients with disabling health conditions or
impairments. Front Oncol. 13:11566192023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hochhertz F, Hass P, Röllich B, Ochel HJ
and Gawish A: A single-institution retrospective analysis of
intraoperative radiation boost during breast-conservation treatment
for breast cancer. J Cancer Res Clin Oncol. 149:5743–5749. 2023.
View Article : Google Scholar : PubMed/NCBI
|