Introduction
During the last decades, the effects of dietary fat
intake has provided more insight into the induction of metabolic
syndrome, such as obesity, type 2 diabetes mellitus and
cardiovascular disease (1,2). Evidence is emerging that elevated
levels of lipid substances evoke lipotoxicity and cell death
pathways, thereby contributing to the pathological process in the
body (3). In a variety of lipid
substances, saturated free fatty acid has the highest lipotoxicity,
whereas diary western diet often contains a great amount of free
fatty acid (4). Palmitic acid (PA)
is one of the most common free fatty acid, which stimulates
apoptosis in experimental systems (5,6).
However, the exact mechanism of PA has not been established and may
vary by cell type (7–9).
Macrophages are essential to the maintenance of the
organism homeostasis, immunological control, and pathogen defense
(10). Previous studies have
confirmed that macrophages apoptosis is a risk factor for lipid
metabolism diseases by controlling proliferation (11), lipoprotein efferocytosis and the
release of proinflammatory cytokines (12,13).
Oxidized low-density lipoprotein, cholesterol and fatty acid can
all induce apoptosis of macrophages, leading to the acceleration of
immunometabolism response. Strategies to protect macrophages from
lipotoxicity may provide a new therapeutic approach.
Insulin growth factor-1 (IGF-1), an endogenous
growth factor, has been extensively studied for its role in
physiological and pathological processes. IGF-1 and its receptor
are expressed in most cells in body, which are inversely related to
the risk of many metabolic disease (14,15).
For example, elevated IGF-1 levels reduce the progression of
cardiovascular disease (16,17).
IGF-1 also contributes to the immune homeostasis during metabolic
stresses (18). Notably,
overexpression of IGF-1 improves the prognosis in the mice fed with
high diet food by reducing apoptosis of macrophages. Evidence is
emerging that these effects depend on the protective role of IGF-1
on macrophages from external stimuli or internal signaling
imbalance (19–21). However, as an important contributor
of lipotoxicity, the influence of IGF-1 on PA-induced macrophages
remains to be elucidated.
Therefore, the present study investigated the effect
of IGF-1 on PA-induced macrophage apoptosis with the aim of
providing a scientific basis for further understanding of IGF-1 in
dyslipidemia diseases.
Materials and methods
Materials
THP-1 was obtained from the Cell Bank of the Typical
Culture Collection Committee of the Chinese Academy of Sciences;
Fetal bovine serum, 1640 medium from Gibco (Thermo Fisher
Scientific, Inc.); PBS buffer, trypsin–EDTA and penicillin from
Guangzhou Xinhe Technology Co., Ltd. IGF-1 and phorbol 12-myristate
13-acetate (PMA), Cell Counting Kit-8, mitochondrial membrane
potential assay kit, ECL chemiluminescence reagent were purchased
from MedChemExpress; GAPDH antibody, cytochrome c antibody,
phosphatase and tensin homolog-induced putative kinase protein 1
(PINK1) antibody and Parkin antibody was purchased from Affinity
Biosciences; Bax antibody was purchased from Cell Signaling
Technology, Inc.; caspase-3 antibody and Bcl-2 antibody were
purchased from Abcam; bovine serum albumin, DAPI, Hoechst 33342 and
Normal Goat Serum were purchased from Beijing Solarbio Science
& Technology Co., Ltd.; Annexin V-FITC/PI apoptosis assay kit
was purchased from Elabscience; BCA kit, caspase-3 activity assay
kit, mitochondrial isolation kit and horseradish
peroxidase-labelled goat anti-rabbit IgG were purchased from
Hangzhou Biyuntian Biotechnology Co., Ltd.
Cell culture
Human-derived THP-1 cells were grown and incubated
in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
containing 10% inactivated fetal bovine serum and 1%
penicillin/streptomycin at 37°C and 5% CO2. THP-1 cells
were treated for 48 h with 100 nmol/ml PMA to induce macrophages
differentiation, in which the cells transition from suspended to
adherent growth and from round to irregular. Then the cells were
treated with different doses of PA (0–800 µmol/l) dissolved in
bovine serum albumin.
Cell viability assay
The effect of IGF-1 on the activity of THP-1
macrophages was assessed by the Cell Counting Kit-8 assay (cat. no.
HY-K0301; MedChemExpress). THP-1 macrophages were co-treated with
IGF-1 with PA (cat. no. P0500; MilliporeSigma) at different
concentrations (1.0, 1.5, 2, 2.5 ng/ml) for 24 h. Then 10 µl of
cell counting kit-8 reagent was added to each well, incubated for 4
h and the OD value measured at 450 nm and plot the standard
curve.
Western blotting
Total protein was extracted using lysis buffer
including protease inhibitors (cat. no. HY-K1001; MedChemExpress).
Macrophages were washed with PBS after being lysed in an ice bath
for 30 min in cell lysis buffer. Collect the cells in a centrifuge
tube and centrifuge at 12,000 × g for 30 min at 4°C. The
supernatant is the total cell protein. Protein concentration was
determined using a BCA protein assay kit (cat. no. P0011; Hangzhou
Biyuntian Biotechnology Co., Ltd.). Using 10–15% SDS-PAGE, equal
amounts of protein (20 or 30 µg) were resolved and transferred to
polyvinylidene difluoride membranes. Membranes were blocked with 5%
skimmed milk for 1 h at room temperature followed by incubation
with the appropriate primary antibody: Rabbit anti-Bax (1:1,000;
cat. no. 41162S; Cell Signaling Technology, Inc.), rabbit
anti-Bcl-2 (1:1,000; cat. no. ab32124; Abcam), rabbit
anti-caspase-3 (1:1,000; cat. no. ab32042; Abcam), rabbit
anti-GAPDH (1:1,000; cat. no. AF7021; Affinity Biosciences), rabbit
anti-cytochrome c (1:1,000; cat. no. AF0146; Affinity Biosciences),
rabbit anti-PINK1 (1:1,000; cat. no. DF7742; Affinity Biosciences)
and rabbit anti-Parkin (1:1,000; cat. no. AF0235; Affinity
Biosciences) overnight at 4°C. Following that, secondary antibodies
(horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit
IgG) were incubated for 1 h at room temperature. Finally, the
Bio-Rad gel detection system (Bio-Rad Laboratories, Inc.) was used
to observe the fluorescence intensity detection bands and ImageJ
software (version 1.8.0; National Institutes of Health) used to
quantify their density, and the ratio of the target protein to the
internal reference protein was used as expression level of the
target protein.
Flow cytometry
Annexin V-FITC/PI cell apoptosis detection kit (cat.
no. E-CK-A211; Elabscience) was used for the analysis of cell
apoptosis (early + late apoptotic cells) in accordance with the
instructions. The treated cells were digested with trypsin solution
without EDTA, harvested, washed with PBS, resuspended in 500 µl of
1X Annexin V Binding Buffer; 5 µl of Annexin V-FITC and PI was
added and incubated in the dark for 15 min at room temperature. BD
Accuri C6 Plus cytometer (BD Biosciences) was used for data
collection. Flow.JoX (version 10.0.7; flowjo.com)
software was used for data analysis.
Caspase-3 activity assay
The activity of caspase-3 was assessed using the
caspase-3 kit assay (cat. no. C1115; Beyotime Institute of
Biotechnology). The cells were lysed after treatment and
Ac-DEVD-pNA was used as a substrate for caspase-3. Caspase-3
activity and absorbance were measured at OD 405.
Hoechst 33342 staining
Cells were stained with Hoechst 33342 (cat. no.
C0031; Beijing Solarbio Science & Technology Co., Ltd.) for 10
min at 37°C and then detected by fluorescence microscopy. When
apoptosis occurs in cells, the nuclei of the apoptotic cells can be
seen to be densely stained, or fragmented and densely stained.
Mitochondrial and cytoplasmic protein
isolation
Mitochondria were isolated from THP-1 macrophages
according to the instructions of the Cellular Mitochondrial
Isolation Kit (cat. no. C3601; Beyotime Institute of
Biotechnology). THP-1 macrophages were digested with trypsin,
harvested, resuspended in wash buffer, and centrifuged at 200 × g
for 5 min at 4°C to collect the cellular sediment. Cells were
resuspended in mitochondrial isolation reagent and incubated in an
ice bath for 15 min, after which a glass homogenizer was used to
obtain cell homogenates. The mitochondrial precipitates were
separated by gradient centrifugation for 10 min at 1,000 × g and
4°C, then gently transfer the supernatant to another centrifuge
tube. The supernatant was 3,500 × g again, centrifuged at 4°C for
10 min, and then transferred to a centrifuge tube. The collected
supernatant was again centrifuged at 12,000 × g for 10 min at a
4°C. The cytoplasmic protein concentration was determined by the
BCA method (Hangzhou Biyuntian Biotechnology Co., Ltd.).
Detection of mitochondrial membrane
potential by JC-1
The mitochondrial membrane potential was measured
using the fluorescent dye JC-1 (cat. no. HY-K0601; MedChemExpress).
THP-1 cells were seeded in a small confocal dish, the number of
seeded cells being approximately 2×105. They were
cultured in an incubator at 37°C for 48 h to induce differentiation
into macrophages, then treated with drugs for 24 h, stained with
JC-1 for 20 min at 37°C, stained with DAPI (cat. no. C0065; Beijing
Solarbio Science & Technology Co., Ltd.)-labelled nuclei for 10
min at 37°C and 5% CO2, washed with PBS and 500 µl of
10% FBS RPMI-1640 medium added. The changes in cell membrane
potential were observed under a laser confocal microscope (Carl
Zeiss AG).
Detection of mitochondrial reactive
oxygen species (ROS) generation
Mitochondrial ROS production was detected according
to the mitochondrial ROS kit (cat. no. BB-46091, BestBio). The
treated cells were digested with trypsin solution without EDTA,
washed with PBS and incubated with mitochondrial reactive oxygen
species staining solution at 37°C in the dark for 20 min.
Pre-cooled 500 µl 1X PBS was added to the centrifuge tubes to
resuspend the cells and immediately detected with a BD Accuri C6
Plus cytometer (version 1.0.23.1; BD Biosciences). Flow.JoX
(version 10.0.7; flowjo.com) software was used for
data analysis.
Immunofluorescence staining
Treated cells were stained with green fluorescent
MitoTracker Green FM (200 nM; cat. no. HY-135056; MedChemExpress)
for 30 min, then fixed with pre-cooled methanol for 30 min,
permeabilized with 0.1% Triton X-100 for 1 min and incubated
blocked with 10% goat serum for 2 h at room temperature. Cells were
incubated with LC3 primary antibody (1:1,000; cat. no. 3868; Cell
Signaling Technology, Inc.) overnight at 4°C. After three washes
with PBS, cells were incubated with secondary antibody Alexa Fluor
594 goat anti-rabbit IgG (1:500; cat. no. S0006; Affinity
Biosciences) for 1 h. After staining with DAPI for 10 min at 37°C,
cells were observed using a confocal microscope (Carl Zeiss AG).
Colocalization was assessed by line scanning using ImageJ software
(version 1.8.0; National Institutes of Health) and line plots were
generated using GraphPad (version 8.4.3; Dotmatics).
Mitochondrial-lysosome colocalization
analysis
Mitophagy was detected by co-localization of
mitochondria with lysosome. Cells were incubated with LysoTracker
(100 nM; cat. no. L7528; Beijing Solarbio Science & Technology
Co., Ltd.) and MitoTracker (300 nM; cat. no. 8778; Cell Signaling
Technology, Inc.) for 30 min at 37°C and 5% CO2. The
cells were then washed with PBS. Cells were fixed with pre-cooled
methanol in the dark and incubated in an ice bath for 30 min.
Bright green fluorescence represented mitochondria and bright red
fluorescence represented lysosomes. Cell images were acquired using
a confocal microscope (Carl Zeiss AG).
Statistical analysis
SPSS 16.0 software (SPSS, Inc.) was used for
statistical analysis of the data. Data normality was tested using
the Kolmogorov-Smirnov test. Normally distributed quantitative
variables are described as mean ± standard deviation. Normally
distributed continuous variables were compared between groups using
one-way analysis of variance. The LSD method was used for
homogeneity of variance, and Games-Howell method was used for
non-homogeneity of variance. While non-normally distributed
variables were compared using the rank sum test. Qualitative
variables were described by frequency (percentage) and
χ2 test was used for comparison between groups.
Pearson's product-moment correlation coefficient was used to
analyze the correlation between the indicators following normal
distribution, and Spearman's rank correlation coefficient was used
to analyze the correlation between the indicators not following
normal distribution. P<0.05 was considered to indicate a
statistically significant difference.
Results
IGF-1 increases survival in
PA-stimulated macrophages
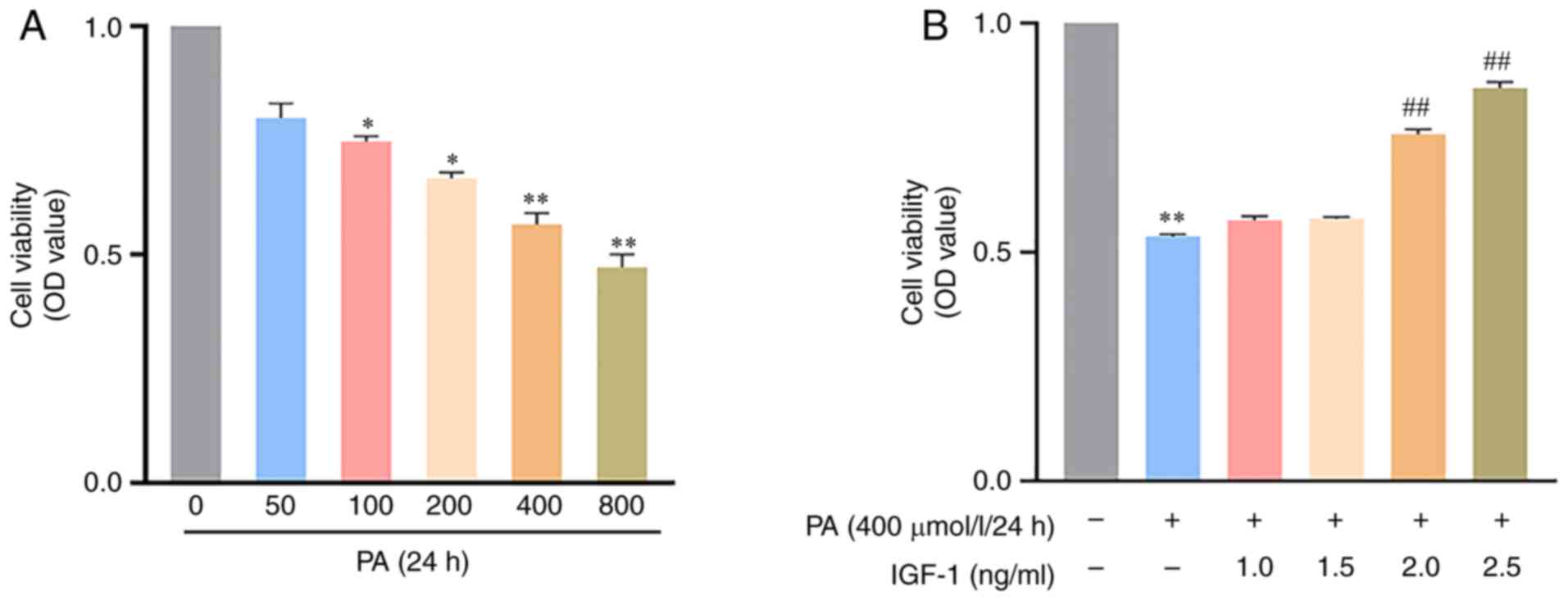
To assess the effect of IGF-1 on cell viability,
Cell Counting Kit-8 assay was performed. Cell viability was
examined at different PA concentrations (0–800 µmol/l) and 400
µmol/l was selected for subsequent experiments (Fig. 1A). Co-treatment of IGF-1 with PA at
different concentrations (1.0, 1.5, 2, 2.5 ng/ml) for 24 h
increased cell viability in a dose-dependent manner (Fig. 1B). Based on the results, 2.5 ng/ml
IGF-1 was selected as the working concentration for subsequent
experiments.
IGF-1 attenuates apoptosis in
PA-stimulated macrophages
An increasing body of research suggests that PA
triggers a number of relatively distinct mechanisms underlying
apoptosis, including endoplasmic reticulum stress, ceramide and
mitochondrial malfunction (22,23).
However, the mechanism by which PA induces apoptosis in macrophages
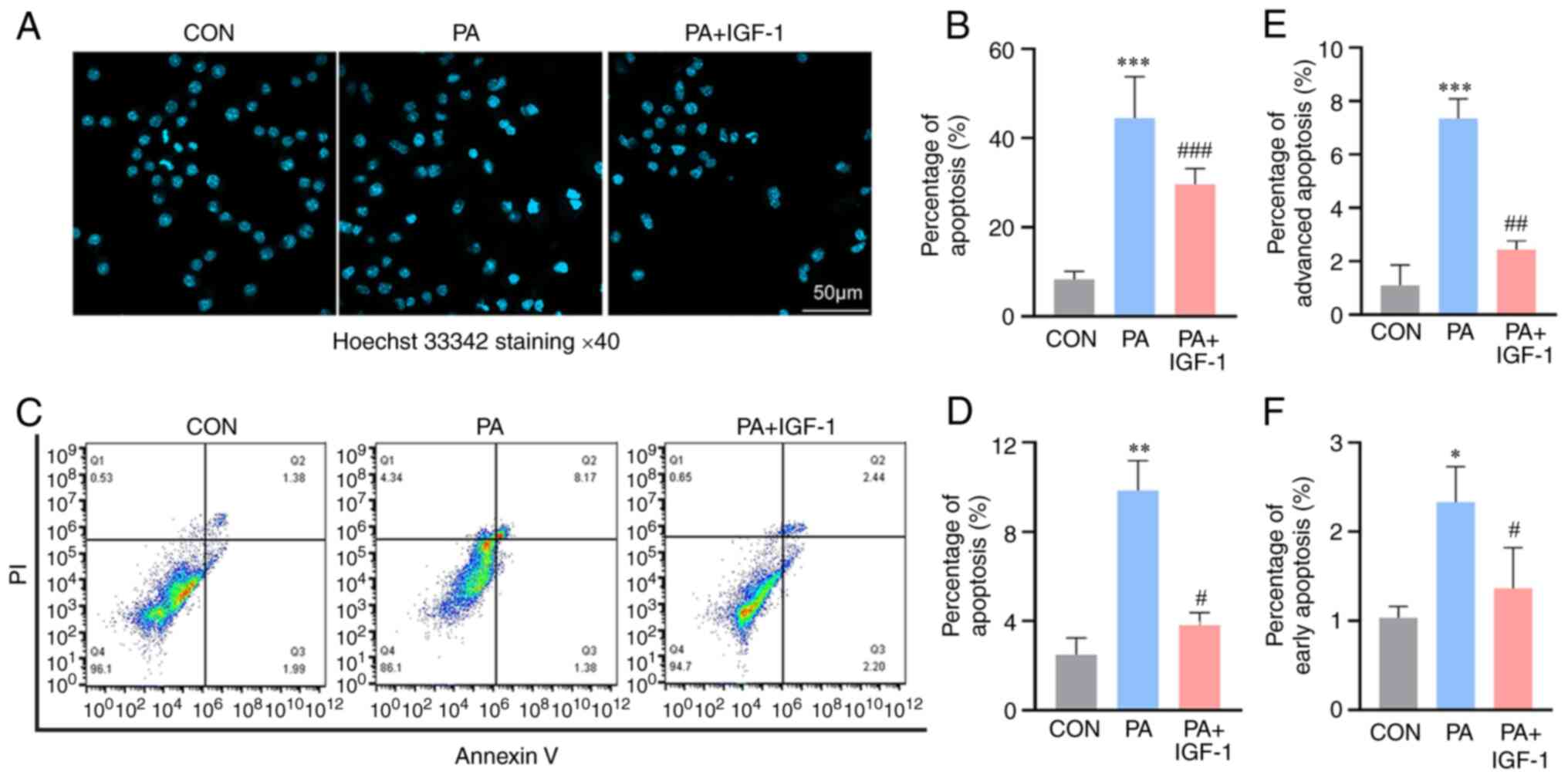
has not been fully elucidated. The present study investigated
PA-induced apoptosis in macrophages. Apoptosis level was observed
when cells were treated with PA at a concentration of 400 µmol/l.
However, following co-treatment with IGF-1, PA-induced apoptosis
was inhibited (Fig. 2). The
results of Hoechst 33342 staining showed that compared with the
control group, macrophages nuclei were deformed in the PA group and
the apoptosis rate was obviously increased. Compared with the PA
group, the IGF-1 and PA co-treated group reduced the apoptosis of
macrophages (Fig. 2A and B). Flow
cytometry analysis using Annexin V/PI double staining also
confirmed that apoptosis was markedly increased in the PA group,
while apoptosis was significantly reduced by co-treatment with
IGF-1 (Fig. 2C-F). The above
results suggested that IGF-1 attenuates PA damage to
macrophages.
IGF-1 reduces caspase-3 expression in
PA-stimulated macrophages
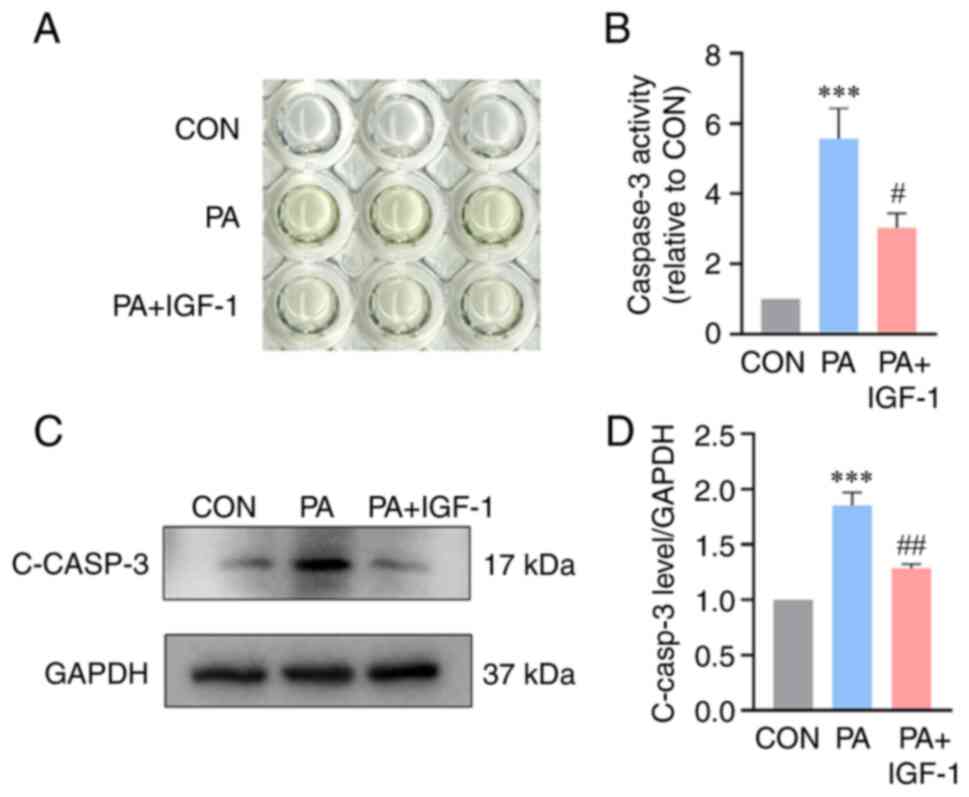
Caspase-3 is an indication of apoptosis in the late
process. For the purpose of determining how IGF-1 affected the
expression of caspase-3 in macrophages, a caspase-3 activity kit
and western blotting was employed. The findings demonstrated that
macrophages in the PA group had considerably higher caspase-3
expression (Fig. 3A and B).
Caspase-3 activity and protein levels were considerably reduced
with the co-treatment with IGF-1 compared with the PA group
(Fig. 3C and D).
Effect of IGF-1 on PA-induced
expression of Bcl-2, Bax in macrophages
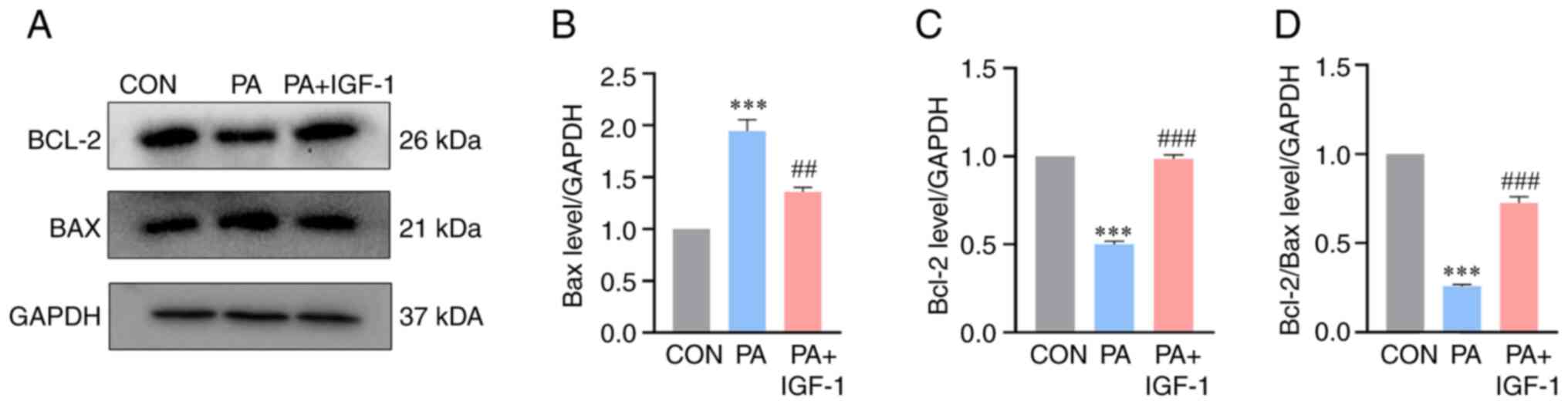
Bcl-2 and Bax genes are among the main genes
involved in apoptosis. Studies have shown that Bcl-2 has a direct
effect on mitochondrial membrane proteins and can directly prevent
the opening of channels in the outer mitochondrial membrane, thus
preventing the release of cytochrome c and achieving inhibition of
apoptosis (24–26). Western blotting results showed that
IGF-1 upregulated the expression of anti-apoptotic protein Bcl-2
and downregulated the expression of pro-apoptotic protein Bax
compared with the PA group, while the Bcl-2/Bax ratio was
significantly higher (Fig. 4).
IGF-1 attenuates PA-induced
mitochondrial apoptosis pathway in macrophages
The ability of IGF-1 ability to inhibit macrophages
apoptosis was further studied. Previous research findings revealed
that IGF-1 might regulate mitochondrial activity, which is crucial
for cell survival (27,28). The current study examined the
levels of mitochondrial apoptosis. According to the flow cytometry
findings, macrophages in the IGF-1 group had considerably lower
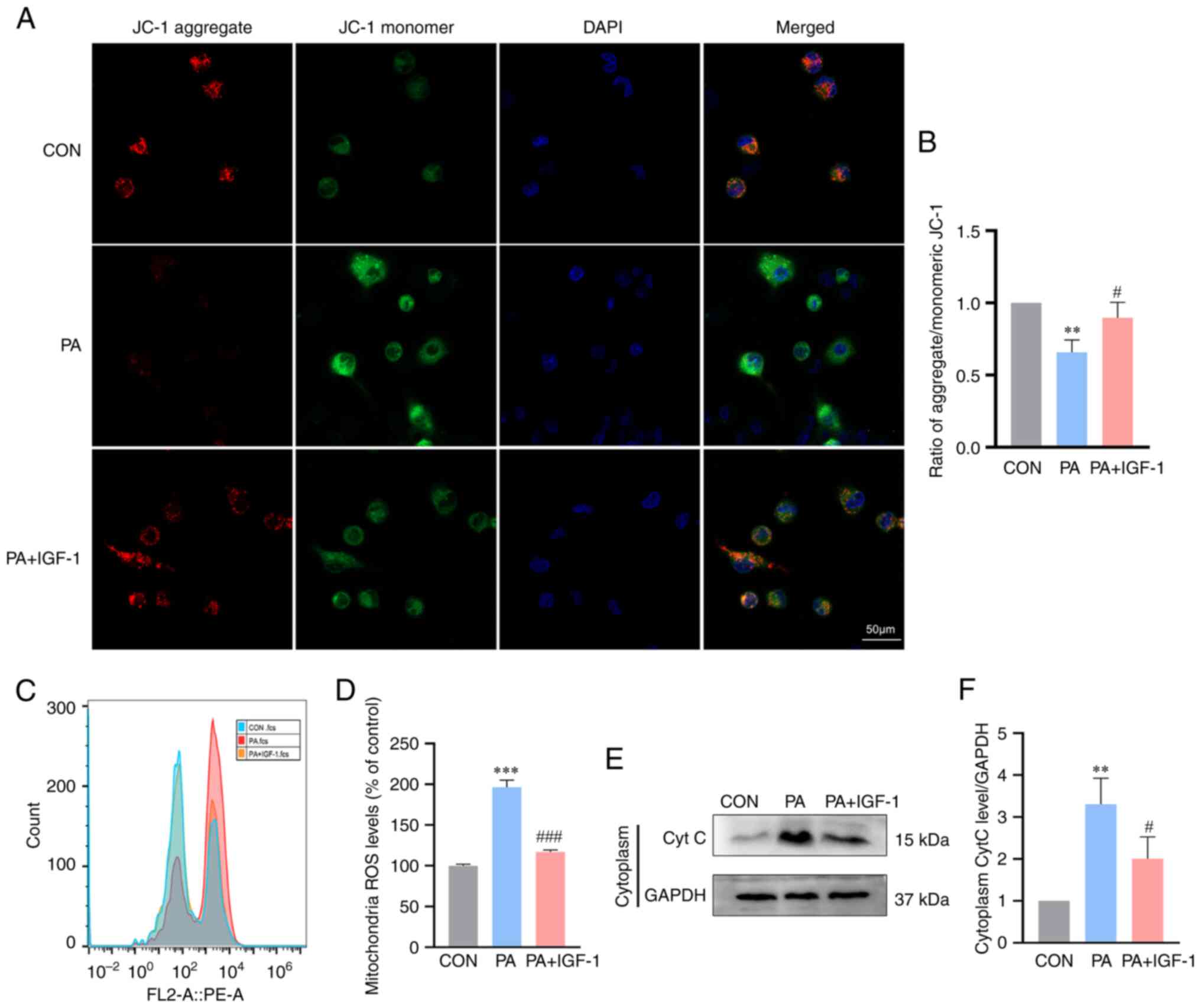
levels of mitochondrial ROS than those in the PA group (Fig. 5C and D). Changes in the potential
of the mitochondrial membrane are related to ROS levels. Longer
mitochondrial permeability transition pore openings might result in
ROS bursts that disturb mitochondria at higher ROS levels (29). JC-1 staining demonstrated that PA
treatment reduced the mitochondrial membrane potential of
macrophages, but IGF-1 administration increased the membrane
potential (Fig. 5A and B).
Furthermore, the concentration of cytochrome c protein in the
cytoplasm increased in the PA group at the same time and IGF-1
prevented the release of cytochrome c into the cytoplasm (Fig. 5E and F). According to the findings,
IGF-1 prevented macrophages apoptosis via the mitochondrial
pathway.
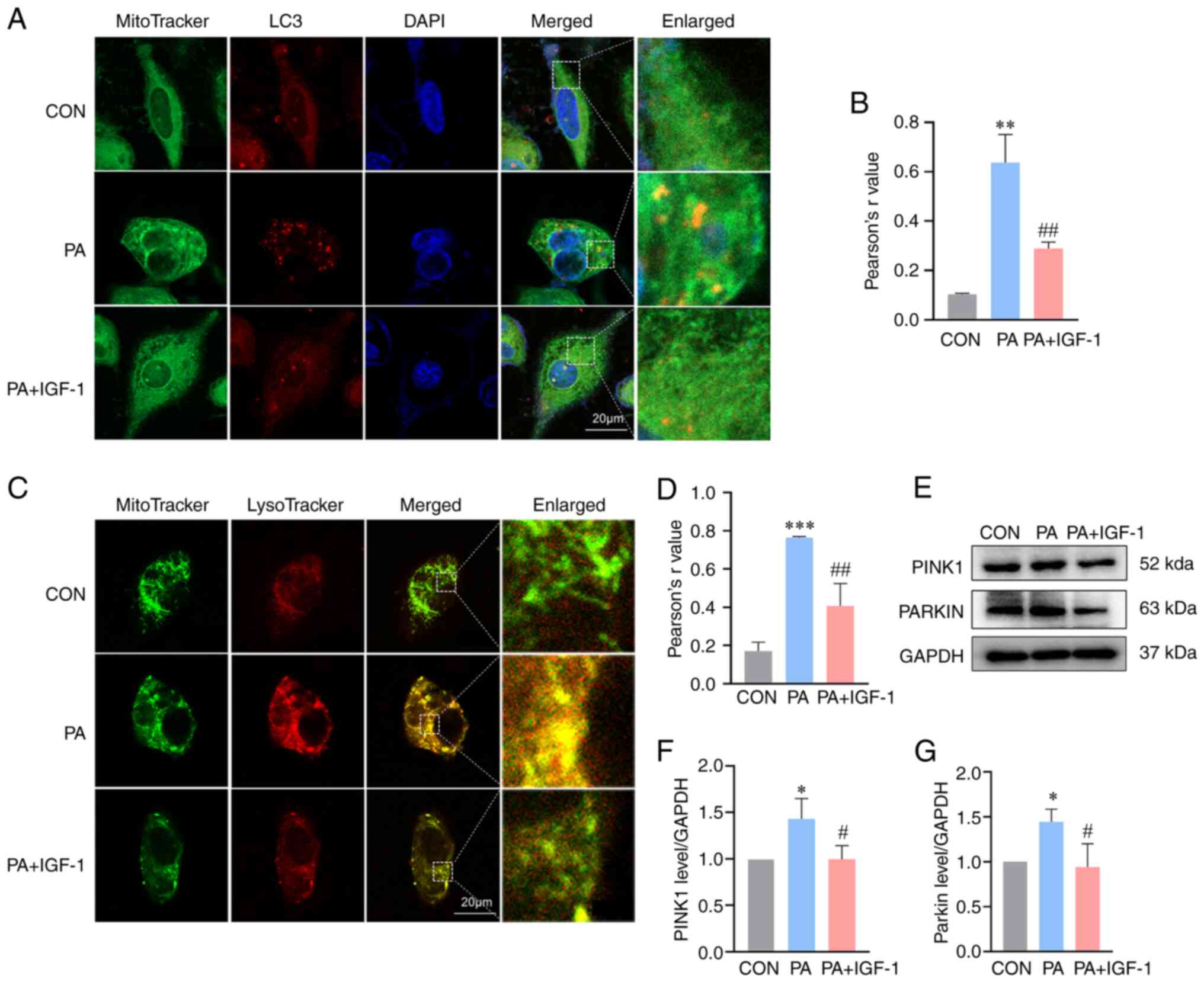
IGF-1 diminishes PA-induced mitophagy
in macrophages
Previous studies have suggested that excessive
mitophagy might result in mitochondrial damage (30–32).
To evaluate whether IGF-1 protect mitochondrial damage based on
mitophagy, the present study initially measured the engulfing
mitochondria using co-localization of autophagosomes and
mitochondria. The combined fluorescence signal of LC3 and
MitoTracker showed that PA significantly enhanced the
colocalization of autophagosomes with mitochondria (Fig. 6A and B). Under confocal microscopy,
mitophagy and lysosome fusion was observed in the PA group. IGF-1
co-treatment significantly reduced mitochondrial lysosomal fusion,
implying that IGF-1 inhibited mitophagy (Fig. 6C and D). The PINK1/Parkin pathway
has recently been recognized as a crucial signaling pathway driving
mitophagy in mammalian cells (33–35).
Thus, PINK1 and Parkin expression levels were evaluated. Treatment
with PA increased PINK1 and Parkin in the macrophages and the
co-treatment with IGF-1 decreased the expression of both proteins
(Fig. 6E-G). Taken together, these
findings suggested that IGF-1 diminished PA-induced mitophagy.
Discussion
Lipotoxicity is the term for excessive lipid
accumulation in non-adipose tissue, which can lead to cell death
and altered immune responses, particularly in macrophages (36,37).
There is increasing evidence that lipid disorders can have a direct
or indirect effect on the immune cells. Data from the present study
showed that treatment of macrophages with PA significantly reduced
cell viability and induction of apoptosis.
IGF-1 is an endocrine and autocrine/paracrine growth
factor widely expressed in human tissues and organs (38,39).
Early research has shown that IGF-1 is associated with the
protective role on immune cells. For example, IGF-1 may improve
cell metabolism and survival by controlling the expression of genes
via apoptotic pathways (40,41).
A recent study found that overexpression of IGF-1 in macrophages
reduced high-fat diet-induced macrophages apoptosis in a murine
model (42). The effect of IGF-1
on PA-induced macrophages apoptosis in dyslipidemia pathophysiology
is still an unexplored field. The present study observed that IGF-1
restored PA-induced nuclear deformation by Hoechst 33342 staining
and IGF-1 inhibited PA-induced macrophages apoptosis by Annexin
V/PI staining. As a downstream signal of apoptosis, the expression
of caspase-3 was positively correlated with cell apoptosis.
According to the analysis of caspase-3 activity, PA
treatment-induced caspase-3 activity and protein expression were
markedly reduced by IGF-1 treatment. These results suggested that
IGF-1 attenuated macrophages apoptosis.
Apoptosis is a key intracellular homeostasis
regulatory process. The endogenous mitochondrial pathway, the
endoplasmic reticulum pathway and exogenous death receptor pathway
are considered the basic pathways of apoptosis (43). The endogenous mitochondrial pathway
is activated by permeabilization of the outer mitochondrial
membrane (44). The Bcl-2 protein
family is found in mitochondria and regulates the permeabilization
of the outer mitochondrial membrane (25,45,46).
When several apoptotic factors (for example, cytochrome c) are
released from the mitochondrial intermembrane space, they induce
the formation of the apoptosome together with caspase-9, which then
activates caspase-3, thereby activating the common pathway of
apoptosis (47,48). When the endoplasmic reticulum
stress is excessive or lasts too long, the unfolded protein
response fails to hydrolyze unfolded or misfolded proteins in time,
then the apoptotic-signaling molecules are activated, causing
apoptosis (49). In the death
receptor pathway, tumor necrosis factor-related apoptosis-inducing
ligand combines with cell surface death receptors such as death
receptor-4 and death receptor-5 to form a death-inducing signaling
complex, leading to the recruitment of caspase-8 ultimately leading
to apoptosis (50). Studies have
reported that IGF-I has strong anti-apoptotic activity in a variety
of cell types and can protect cells through different apoptotic
mechanisms (51–53). IGF-1 regulates PI3K/AKT/Forkhead
box O signaling to reduce cytochrome c levels in the cytoplasm,
thereby suppressing cleaved caspase-3 formation and apoptosis
(41,54,55).
Another study demonstrated that IGF-1 can reduce
lipopolysaccharide-induced neuronal apoptosis through the
mitochondrial pathway (41).
Kurshan et al (56) found
that IGF-1 protects cells from endoplasmic reticulum stress-induced
apoptosis via enhancement of the adaptive capacity of endoplasmic
reticulum. In addition, inhibition of the IGF-1 receptor increases
death receptor-mediated apoptosis in colon cancer cells (57). The present study demonstrated that
IGF-1 can regulate mitochondrial apoptosis induced by PA in
macrophages, although the other apoptotic mechanisms cannot be
excluded. The precise mechanisms need to be investigated
further.
The Bcl-2 family is involved in the transmission and
reception of apoptotic signals in the mitochondrial apoptotic
pathway, as well as modulating apoptosis upstream to regulate
caspase-3 expression levels (48).
Bcl-2 and Bax are essential proteins in this family that play
opposing and complementary roles in the mitochondrial apoptotic
pathway of cells. Bax proteins play a major role in facilitating
the evolution of apoptosis by heterodimerizing with the
anti-apoptotic protein Bcl-2 upon activation and altering the
permeability of the cellular mitochondrial membrane, resulting in a
homeostatic imbalance that promotes apoptosis (45,58).
The Bcl-2/Bax ratio is an important indicator of cell apoptotic
susceptibility (59) and directly
determines the degree of opening of various channels in the outer
mitochondrial membrane (60,61).
The present study demonstrated that IGF-1 increased the expression
of Bcl-2 protein, inhibited the expression of Bax protein and
elevated the Bcl-2/Bax ratio. These results suggested that IGF-1
might improve the mitochondrial apoptosis by regulating the
proportion of Bcl-2 family members and mitochondrial outer membrane
channels.
Mitochondria are key organelles of eukaryotic cells
that undertake important processes, such as cellular metabolite
conversion and oxidative phosphorylation, as well as ATP synthesis
(62). Mitochondria are organelles
that produce ROS, a major factor in the induction of apoptosis.
When the level of ROS reaches a certain point, the accumulated ROS
will activate the opening of the mitochondrial permeability
transition pore, leading to a decrease in mitochondrial membrane
potential (63). This allows
cytochrome c, located in the lumen between the inner and outer
mitochondrial membranes, to be released from the mitochondria into
the cytoplasm, which in turn mediates the onset of apoptosis via
the caspase-3 (64). PA can induce
mitochondrial apoptosis in macrophages, which was confirmed by the
present study. Furthermore, it was found that PA treatment
increased mitochondrial ROS production, decreased mitochondrial
membrane potential, and increased cytoplasmic cytochrome c protein
aggregation, whereas IGF-1 reversed these effect. This indicated
that IGF-1 can reverse PA-induced mitochondrial apoptosis.
Mitophagy is an important process that regulates
mitochondrial dynamics. A major cause of mitochondrial dysfunction
is dysregulation of mitophagy (32). When mitochondria are degraded in
significant amounts due to over-activated mitophagy, it results in
mitochondrial dysfunction and continuing production of ROS
(65). Hence, it was hypothesized
that a critical molecular mechanism by which IGF-1 prevents
macrophages apoptosis involves the reduction of mitophagy. Confocal
microscopy showed that colocalization of lysosomes and mitochondria
was significantly downregulated by IGF-1 compared with PA treated
group. This suggested that IGF-1 might partly prevent autophagosome
from fusion with lysosomes. The PINK1/Parkin pathway is a classic
signaling pathway during mitophagy (33,34).
When mitochondria are depolarized, PINK1 is prevented from entering
the inner membrane, resulting in the accumulation of PINK1 on the
surface of damaged mitochondria. At this stage, PINK1 on the
mitochondrial surface recruits Parkin from the cytoplasm to the
damaged mitochondria, inducing and promoting mitophagy (35). In the present study, expression of
PINK1 and Parkin was inhibited when IGF-1 treatment in
vitro. The above results suggested that IGF-1 inhibited
excessive mitophagy induced by PA via the PINK1/Parkin pathway.
There are some limitations to the current study. It
demonstrated that IGF-1 had a significant inhibitory effect on
PA-induced macrophages apoptosis (Hoechst 33342 staining and flow
cytometry) and mitochondrial apoptosis (Bcl-2/Bax ratio). Although
the other apoptotic pathways could not be excluded, these results
indicated that mitochondrial apoptosis is involved in the action of
IGF-1. It is worth noting that the effect of PA 400 µmol/l appeared
to be partly blocked by IGF-1 2.5 ng/ml, suggesting that other
apoptotic pathways may also be involved in the pro-apoptotic effect
of PA. Moreover, the inhibition of mitochondrial apoptosis by IGF-1
was not complete, which might partially explain the inability of
IGF-1 to completely inhibit the effect of PA. Another limitation
was the use of only one cell line, THP-1. Therefore, the mechanism
of IGF-1 anti-apoptotic effect and its effect on macrophages
apoptosis in other cell lines remain to be further studied.
In summary, the present study demonstrated that
IGF-1 can partly inhibit macrophages apoptosis by protecting
mitochondria. The present study provided a further scientific basis
for understanding the therapeutic effects of IGF-1 on PA-induced
dyslipidemia macrophages apoptosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by Natural Science Foundation of
China (grant no. 82170485), Natural Science Foundation of Hunan
Province, China (grant nos. 2019JJ40249 and 2023JJ30426) and
University-Industry Cooperation Education Project of Education
Department (grant no. 202002138007).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WT, MZ, YW, LJ, YO and WJ were involved in
conception and design and manuscript writing. DM, MH, HL, YZ, GZ,
PH and YO performed experiments, data analysis and interpretation.
LJ, YO and WJ wrote the manuscript or revising it critically for
important intellectual content. GH, PH and YO given final approval
of the version to be published. WT and MZ confirm the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The Sixth Affiliated Hospital of Guangzhou Medical
University waives the requirement for authors to obtain ethical
approval for the use of commercially available cells.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
References
|
1
|
Ma XM, Geng K, Law BY, Wang P, Pu YL, Chen
Q, Xu HW, Tan XZ, Jiang ZZ and Xu Y: Lipotoxicity-induced mtDNA
release promotes diabetic cardiomyopathy by activating the
cGAS-STING pathway in obesity-related diabetes. Cell Biol Toxicol.
39:277–299. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Unger RH and Scherer PE: Gluttony, sloth
and the metabolic syndrome: A roadmap to lipotoxicity. Trends
Endocrinol Metab. 21:345–352. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van der Rijt S, Leemans JC, Florquin S,
Houtkooper RH and Tammaro A: Immunometabolic rewiring of tubular
epithelial cells in kidney disease. Nat Rev Nephrol. 18:588–603.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Plotz T, von Hanstein AS, Krummel B,
Laporte A, Mehmeti I and Lenzen S: Structure-toxicity relationships
of saturated and unsaturated free fatty acids for elucidating the
lipotoxic effects in human EndoC-βH1 beta-cells. Biochim Biophys
Acta Mol Basis Dis. 1865:1655252019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tao L, Yi Y, Chen Y, Zhang H, Orning P,
Lien E, Jie J, Zhang W, Xu Q, Li Y, et al: RIP1 kinase activity
promotes steatohepatitis through mediating cell death and
inflammation in macrophages. Cell Death Differ. 28:1418–1433. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yen CC, Lii CK, Chen CC, Li CC, Tseng MH,
Lo CW, Liu KL, Yang YC and Chen HW: Andrographolide inhibits
lipotoxicity-induced activation of the NLRP3 inflammasome in bone
marrow-derived macrophages. Am J Chin Med. 51:129–147. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu D, Liu L, Zhao Y, Yang L, Cheng J, Hua
R, Zhang Z and Li Q: Melatonin protects mouse testes from palmitic
acid-induced lipotoxicity by attenuating oxidative stress and DNA
damage in a SIRT1-dependent manner. J Pineal Res. 69:e126902020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He Y, Zhou L, Fan Z, Liu S and Fang W:
Palmitic acid, but not high-glucose, induced myocardial apoptosis
is alleviated by N-acetylcysteine due to attenuated
mitochondrial-derived ROS accumulation-induced endoplasmic
reticulum stress. Cell Death Dis. 9:5682018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang JS, Guo BB, Wang GH, Zeng HM, Hu YH,
Wang T and Wang HY: DGAT1 inhibitors protect pancreatic β-cells
from palmitic acid-induced apoptosis. Acta Pharmacol Sin.
42:264–271. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Biswas SK and Mantovani A: Orchestration
of metabolism by macrophages. Cell Metab. 15:432–437. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bao J, He Y, Yang C, Lu N, Li A, Gao S,
Hosyanto FF, Tang J, Si J, Tang X, et al: Inhibition of
mycobacteria proliferation in macrophages by low cisplatin
concentration through phosphorylated p53-related apoptosis pathway.
PLoS One. 18:e2811702023. View Article : Google Scholar
|
|
12
|
Guo X, Chai Y, Zhao Y, Wang D, Ding P and
Bian Y: Correlation between mechanism of oxidized-low density
lipoprotein-induced macrophage apoptosis and inhibition of target
gene platelet derived growth factor receptor-β expression by
microRNA-9. Bioengineered. 12:11716–11725. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cash JG, Kuhel DG, Basford JE, Jaeschke A,
Chatterjee TK, Weintraub NL and Hui DY: Apolipoprotein E4 impairs
macrophage efferocytosis and potentiates apoptosis by accelerating
endoplasmic reticulum stress. J Biol Chem. 287:27876–27884. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hanwright PJ, Qiu C, Rath J, Zhou Y, von
Guionneau N, Sarhane KA, Harris TGW, Howard GP, Malapati H, Lan MJ,
et al: Sustained IGF-1 delivery ameliorates effects of chronic
denervation and improves functional recovery after peripheral nerve
injury and repair. Biomaterials. 280:1212442022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsushita M, Fujita K, Hatano K, De
Velasco MA, Uemura H and Nonomura N: Connecting the dots between
the Gut-IGF-1-Prostate Axis: A Role of IGF-1 in prostate
carcinogenesis. Front Endocrinol (Lausanne). 13:8523822022.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Laughlin GA, Barrett-Connor E, Criqui MH
and Kritz-Silverstein D: The prospective association of serum
insulin-like growth factor I (IGF-I) and IGF-binding protein-1
levels with all cause and cardiovascular disease mortality in older
adults: The Rancho Bernardo Study. J Clin Endocrinol Metab.
89:114–120. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang WB, Aleksic S, Gao T, Weiss EF,
Demetriou E, Verghese J, Holtzer R, Barzilai N and Milman S:
Insulin-like growth factor-1 and IGF binding proteins predict
all-cause mortality and morbidity in older adults. Cells.
9:13682020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang HR, Kim HJ, Xu X and Ferrante AW Jr:
Macrophage and adipocyte IGF1 maintain adipose tissue homeostasis
during metabolic stresses. Obesity (Silver Spring). 24:172–183.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Hou L, Yuan X, Xu N, Zhao S, Yang
L and Zhang N: miR-483-3p promotes cell proliferation and
suppresses apoptosis in rheumatoid arthritis fibroblast-like
synoviocytes by targeting IGF-1. Biomed Pharmacother.
130:1105192020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo L, Santos A, Konganti K, Hillhouse A,
Lambertz IU, Zheng Y, Gunaratna RT, Threadgill DW and Fuchs-Young
RS: Overexpression of IGF-1 during early development expands the
number of mammary stem cells and primes them for transformation.
Stem Cells. 40:273–289. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bhalla S, Mehan S, Khan A and Rehman MU:
Protective role of IGF-1 and GLP-1 signaling activation in
neurological dysfunctions. Neurosci Biobehav Rev. 142:1048962022.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alnahdi A, John A and Raza H: Augmentation
of glucotoxicity, oxidative stress, apoptosis and mitochondrial
dysfunction in HepG2 cells by palmitic acid. Nutrients.
11:19792019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu G, Luo H, Zhang N, Wang Y, Li Y, Huang
H, Liu Y, Hu Y, Liu H, Zhang J, et al: Loss of p53 sensitizes cells
to palmitic acid-induced apoptosis by reactive oxygen species
accumulation. Int J Mol Sci. 20:62682019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Antonsson B: Bax and other pro-apoptotic
Bcl-2 family ‘killer-proteins’ and their victim the mitochondrion.
Cell Tissue Res. 306:347–361. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Czabotar PE and Garcia-Saez AJ: Mechanisms
of BCL-2 family proteins in mitochondrial apoptosis. Nat Rev Mol
Cell Biol. 24:732–748. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chipuk JE, Bouchier-Hayes L and Green DR:
Mitochondrial outer membrane permeabilization during apoptosis: The
innocent bystander scenario. Cell Death Differ. 13:1396–1402. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamamura T, Otani H, Nakao Y, Hattori R,
Osako M and Imamura H: IGF-I differentially regulates Bcl-xL and
Bax and confers myocardial protection in the rat heart. Am J
Physiol Heart Circul Physiol. 280:H1191–H1200. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hao CN, Geng YJ, Li F, Yang T, Su DF, Duan
JL and Li Y: Insulin-like growth factor-1 receptor activation
prevents hydrogen peroxide-induced oxidative stress, mitochondrial
dysfunction and apoptosis. Apoptosis. 16:1118–1127. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zorov DB, Juhaszova M and Sollott SJ:
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS
release. Physiol Rev. 94:909–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li JJ, Wang YJ, Wang CM, Li YJ, Yang Q,
Cai WY, Chen Y and Zhu XX: Shenlian extract decreases mitochondrial
autophagy to regulate mitochondrial function in microvascular to
alleviate coronary artery no-reflow. Phytother Res. 37:1864–1882.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen Y, Chen HN, Wang K, Zhang L, Huang Z,
Liu J, Zhang Z, Luo M, Lei Y, Peng Y, et al: Ketoconazole
exacerbates mitophagy to induce apoptosis by downregulating
cyclooxygenase-2 in hepatocellular carcinoma. J Hepatol. 70:66–77.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang M, Linn BS, Zhang Y and Ren J:
Mitophagy and mitochondrial integrity in cardiac
ischemia-reperfusion injury. Biochim Biophys Acta Mol Basis Dis.
1865:2293–2302. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eldeeb MA, Thomas RA, Ragheb MA, Fallahi A
and Fon EA: Mitochondrial quality control in health and in
Parkinson's disease. Physiol Rev. 102:1721–1755. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nguyen TN, Padman BS and Lazarou M:
Deciphering the molecular signals of PINK1/Parkin mitophagy. Trends
Cell Biol. 26:733–744. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matsuda N, Sato S, Shiba K, Okatsu K,
Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, et al:
PINK1 stabilized by mitochondrial depolarization recruits Parkin to
damaged mitochondria and activates latent Parkin for mitophagy. J
Cell Biol. 189:211–221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Joles JA, Kunter U, Janssen U, Kriz W,
Rabelink TJ, Koomans HA and Floege J: Early mechanisms of renal
injury in hypercholesterolemic or hypertriglyceridemic rats. J Am
Soc Nephrol. 11:669–683. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Unger RH: Lipotoxicity in the pathogenesis
of obesity-dependent NIDDM. Genetic and clinical implications.
Diabetes. 44:863–870. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Frater J, Lie D, Bartlett P and McGrath
JJ: Insulin-like Growth Factor 1 (IGF-1) as a marker of cognitive
decline in normal ageing: A review. Ageing Res Rev. 42:14–27. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Clemmons DR: Modifying IGF1 activity: An
approach to treat endocrine disorders, atherosclerosis and cancer.
Nat Rev Drug Discov. 6:821–833. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Higashi Y, Sukhanov S, Anwar A, Shai SY
and Delafontaine P: IGF-1, oxidative stress and atheroprotection.
Trends Endocrinol Metab. 21:245–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang F, Wang L, Sui G, Yang C, Guo M,
Xiong X, Chen Z and Lei P: IGF-1 alleviates mitochondrial apoptosis
through the GSK3β/NF-κB/NLRP3 signaling pathway in LPS-Treated
PC-12 Cells. J Mol Neurosci. 71:1320–1328. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Snarski P, Sukhanov S, Yoshida T, Higashi
Y, Danchuk S, Chandrasekar B, Tian D, Rivera-Lopez V and
Delafontaine P: Macrophage-Specific IGF-1 overexpression reduces
CXCL12 chemokine levels and suppresses atherosclerotic burden in
Apoe-deficient mice. Arterioscler Thromb Vasc Biol. 42:113–126.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Singh P and Lim B: Targeting apoptosis in
cancer. Curr Oncol Rep. 24:273–284. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bock FJ and Tait SWG: Mitochondria as
multifaceted regulators of cell death. Nat Rev Mol Cell Biol.
21:85–100. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pena-Blanco A and Garcia-Saez AJ: Bax, Bak
and beyond-mitochondrial performance in apoptosis. FEBS J.
285:416–431. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Spitz AZ and Gavathiotis E: Physiological
and pharmacological modulation of BAX. Trends Pharmacol Sci.
43:206–220. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang D, He L, Ma S, Li S, Zhang Y, Hu C,
Huang J, Xu Z, Tang D and Chen Z: Pharmacological Targeting of
Bcl-2 Induces Caspase 3-Mediated Cleavage of HDAC6 and regulates
the autophagy process in colorectal cancer. Int J Mol Sci.
24:66622023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu Q, Si T, Xu X, Liang F, Wang L and Pan
S: Electromagnetic radiation at 900 MHz induces sperm apoptosis
through bcl-2, bax and caspase-3 signaling pathways in rats. Reprod
Health. 12:652015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Samanta S, Yang S, Debnath B, Xue D, Kuang
Y, Ramkumar K, Lee AS, Ljungman M and Neamati N: The
hydroxyquinoline analogue YUM70 Inhibits GRP78 to Induce ER
stress-mediated apoptosis in pancreatic cancer. Cancer Res.
81:1883–1895. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Qiao L and Wong BC: Targeting apoptosis as
an approach for gastrointestinal cancer therapy. Drug Resist
Update. 12:55–64. 2009. View Article : Google Scholar
|
|
51
|
Hou JM, Chen EY, Wei SC, Lin F, Lin QM,
Lan XM, Xue Y and Wu M: Lactoferrin inhibits apoptosis through
insulin-like growth factor I in primary rat osteoblasts. Acta
Pharmacol Sin. 35:523–530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Oh SH, Jin Q, Kim ES, Khuri FR and Lee HY:
Insulin-like growth factor-I receptor signaling pathway induces
resistance to the apoptotic activities of SCH66336 (lonafarnib)
through Akt/mammalian target of rapamycin-mediated increases in
survivin expression. Clin Cancer Res. 14:1581–1589. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Han Y, Wang S, Wang Y and Zeng S: IGF-1
inhibits apoptosis of porcine primary granulosa cell by targeting
degradation of BimEL. Int J Mol Sci. 20:53562019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liao R, Yan F, Zeng Z, Farhan M, Little P,
Quirion R, Srivastava LK and Zheng W: Amiodarone-Induced retinal
neuronal cell apoptosis attenuated by IGF-1 via counter regulation
of the PI3k/Akt/FoxO3a pathway. Mol Neurobiol. 54:6931–6943. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cui F and He X: IGF-1 ameliorates
streptozotocin-induced pancreatic β cell dysfunction and apoptosis
via activating IRS1/PI3K/Akt/FOXO1 pathway. Inflamm Res.
71:669–680. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Novosyadlyy R, Kurshan N, Lann D,
Vijayakumar A, Yakar S and LeRoith D: Insulin-like growth factor-I
protects cells from ER stress-induced apoptosis viaenhancement of
the adaptive capacity of endoplasmic reticulum. Cell Death Differ.
15:1304–1317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pennarun B, Kleibeuker JH, Oenema T,
Stegehuis JH, de Vries EG and de Jong S: Inhibition of
IGF-1R-dependent PI3K activation sensitizes colon cancer cells
specifically to DR5-mediated apoptosis but not to rhTRAIL. Anal
Cell Pathol (Amst). 33:229–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tang Q, Chen H, Mai Z, Sun H, Xu L, Wu G,
Tu Z, Cheng X, Wang X and Chen T: Bim- and Bax-mediated
mitochondrial pathway dominates abivertinib-induced apoptosis and
ferroptosis. Free Radic Biol Med. 180:198–209. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cory S, Huang DC and Adams JM: The Bcl-2
family: Roles in cell survival and oncogenesis. Oncogene.
22:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Harris MH and Thompson CB: The role of the
Bcl-2 family in the regulation of outer mitochondrial membrane
permeability. Cell Death Differ. 7:1182–1191. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Autret A and Martin SJ: Emerging role for
members of the Bcl-2 family in mitochondrial morphogenesis. Mol
Cell. 36:355–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Akbari M, Kirkwood TBL and Bohr VA:
Mitochondria in the signaling pathways that control longevity and
health span. Ageing Res Rev. 54:1009402019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jou MJ, Peng TI and Reiter RJ: Protective
stabilization of mitochondrial permeability transition and
mitochondrial oxidation during mitochondrial Ca2+ stress
by melatonin's cascade metabolites C3-OHM and AFMK in RBA1
astrocytes. J Pineal Res. 66:e125382019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhou T, Mo J, Xu W, Hu Q, Liu H, Fu Y and
Jiang J: Mild hypothermia alleviates oxygen-glucose
deprivation/reperfusion-induced apoptosis by inhibiting ROS
generation, improving mitochondrial dysfunction and regulating DNA
damage repair pathway in PC12 cells. Apoptosis. 28:447–457. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gero D, Torregrossa R, Perry A, Waters A,
Le-Trionnaire S, Whatmore JL, Wood M and Whiteman M: The novel
mitochondria-targeted hydrogen sulfide (H2S) donors
AP123 and AP39 protect against hyperglycemic injury in
microvascular endothelial cells in vitro. Pharmacol Res.
113:186–198. 2016. View Article : Google Scholar : PubMed/NCBI
|