Pulmonary hypertension (PH) is a serious health
problem that affects ~1% of the global population (1). In the United States and Europe,
pulmonary arterial hypertension (PAH) is found in 15–50/million
individuals (2). Among them,
idiopathic, heritable and anorexigen-induced PH account for 52.6%
of total PH cases, of which 6–10% of patients have a family history
of PH (3,4). Furthermore, >70% of patients with
PAH are women aged 20–40 years, and its incidence is twice as high
as that in men (5–7). Although the advancement of medical
treatments has improved the survival rate, the prognosis of PH is
still poor, and its mortality rate remains high (8). The 5-year mortality rates of patients
diagnosed with idiopathic pulmonary arterial hypertension (IPAH) or
familiar PH were 31.8 and 46.3%, respectively in China as of 2014
(9). Increasing evidence has shown
that a variety of systemic metabolic derangements are associated
with PH with a number of studies on this topic focused on the role
of obesity, dyslipidemia, insulin resistance (IR), glucose
intolerance and metabolic disorder in the progression of pulmonary
circulation diseases (10–13). Hyperuricemia is an important
metabolic syndrome and is closely associated with gout, coronary
heart disease, hypertension, heart failure and atrial fibrillation
through oxidative stress, endothelial dysfunction, inflammatory
reactions and activation of the renin-angiotensin-aldosterone
system (14–17). These conditions may directly lead
to the occurrence or development of these diseases (18–25).
Whether hyperuricemia is an independent risk factor of PH and how
hyperuricemia promotes the occurrence of PH remains to be
determined. To the best of our knowledge, there has been no
systematic analysis of these issues to date. In the present review,
the complex relationship between hyperuricemia and PH is focused on
providing a novel viewpoint and strategy for the prevention and
treatment of PH.
UA can be derived exogenously and endogenously.
Exogenous UA, which accounts for 20% of the total UA, originates
from exogenous foods rich in purine compounds, nucleic acids and
nucleoproteins, such as animal viscera, seafood, mushrooms, beans,
wine and meat (42). Endogenous UA
accounts for 80% of the total sources and is derived from purine
products formed by the transformation, decomposition and metabolism
of amino acids, phosphoribosyl and nucleic acids in the body
(43,44).
There are numerous enzymes involved in the
conversion of adenine and guanine to UA. Xanthine oxidase is the
key rate-limiting enzyme in this process and it plays an important
role in purine metabolism. Xanthine oxidase is involved in two
important stages in the conversion of purines to UA: i) The
conversion of hypoxanthine to xanthine; and ii) the conversion of
xanthine to UA (45). Hypoxanthine
nucleotides (inosine monophosphate) and guanine nucleotides are
converted to xanthine by oxidation of xanthine oxidase-hypoxanthine
and deamination of guanine by guanine deaminase (46). Finally, xanthine is further
oxidized to UA by xanthine oxidase (46–48).
In most mammals, uricase further oxidizes UA to allantoin, but
humans cannot convert UA into allantoin, which is more soluble
owing to the lack of uricase (Fig.
1) (49–51). Therefore, human purine catabolism
ends in the UA stage.
Normal SUA concentrations are 89–357 µmol/l (1.5–6.0
mg/dl) in women and 149–417 µmol/l (2.5–7.0 mg/dl) in men (46). However, impaired purine metabolism
in the body, such as excessive purine food intake and disease
(e.g., obesity, diabetes and tumor), can lead to increased UA
production and/or decreased excretion, which further results in an
increase in SUA concentrations and even hyperuricemia (61). Hyperuricemia is usually defined as
an SUA concentration >417 µmol/l (7.0 mg/dl) in men and
postmenopausal women, or ≥357 µmol/l (6.0 mg/dl) in premenopausal
women with a normal purine diet (46). When the average SUA concentration
in humans is higher than its solubility limit of 405 µmol/l (6.8
mg/dl), urate crystals are formed and deposited in the kidneys,
tissues and joints (62), leading
to renal calculi, gout and other diseases (e.g., gouty
arthritis).
The variability of SUA concentrations is
multifactorial, and it is also affected by genetic and non-genetic
factors (63). Genome-wide
association studies have shown that the polymorphism and mutations
of genes encoding SLC22A12, SLC2A9 and adenosine triphosphate
binding cassette transporter 2 are related to hyperuricemia
(64). In addition, the
transporters URAT1, glucose transporter 9 (GLUT9) and breast cancer
resistance protein are associated with hyperuricemia and gout
(64–67). The concentration of UA is
influenced by non-genetic factors, mostly caused by excessive
intake and decreased excretion.
Biologically, UA can have not only pro-oxidative but
also anti-oxidative properties (68–72).UA has antioxidant effects under
physiological conditions. The antioxidant mechanism of UA is mainly
driven by the fact that UA is an oxygen radical scavenger,
scavenging superoxide anions, hydroxyl groups, singlet oxygen and
other reactive substances in vivo (73,74).
This protects the cardiovascular system from oxidative stress
damage. UA acts as a pro-oxidant in states with high levels of UA
or low levels of other antioxidants (68). The oxidative effects of UA mainly
manifest in mediating the immune response after cell injury
(75), increasing pro-inflammatory
immune activation (76) and
promoting low-density lipoprotein oxidation (77), the proliferation of smooth muscle
cells and activation and the adhesion of platelets (78). In the presence of Cu2+
in the in vitro environment, UA is susceptible to
antioxidant-oxidant interconversion (79,80).
In addition, UA can react with other oxidants (ONOO−,
OH−) and form pro-oxidants, which participate in lipid
metabolism and cause a chain reaction of lipophilic radical
oxidation (81,82). Therefore, UA exerts oxidative and
antioxidant effects at different concentrations (83,84).
In cardiovascular disease, UA is considered a ‘double-edged sword’
with beneficial and detrimental effects on cardiovascular disease
(17,85). So, is there a similar association
between UA and PH?.
Hyperuricemia is commonly found in patients with
secondary PH. Patients with PH and hemolytic diseases, such as
thalassemia (86), sickle cell
anemia (87), spherocytosis
(88) and paroxysmal sleep
hemoglobinuria (89,90), can develop erythrocyte lysis,
adenosine deaminase release (91),
tissue and organ hypoxia, reduced oxygen-carrying capacity and
increased UA metabolism (92). In
patients with PH and metabolic syndrome (93), hyperinsulinemia enhances the
reabsorption of urate in the proximal tubules and UA concentrations
increase (94). Inflammation,
hypoxia and endothelial damage caused by connective tissue
disease-related PH, such as systemic sclerosis, systemic lupus
erythematosus and Sjogren's syndrome, also play an important role
in the increase in UA concentrations (95). After inflammation is activated, the
release of cytokines promotes pulmonary artery vessel remodeling
and cell proliferation, resulting in insufficient lung perfusion,
tissue ischemia and hypoxia (96,97).
These findings suggest that patients with secondary PH are closely
associated with abnormal UA metabolism, and the SUA concentration
reflects the severity of the illness to a certain extent.
Therefore, UA may be useful as a potential biological marker of PH
and may be able to be applied to the clinical setting and
therefore, the importance of the application of UA in clinical
treatment is discussed in the present review.
Similar to IPAH, UA has high clinical value in
connective tissue disease-associated PH. In patients with PH
secondary to systemic sclerosis, elevated SUA concentrations are
negatively correlated with the 6-min walk test distance and
linearly correlated with pulmonary artery pressure (106–109). Serum uric acid concentrations
were significantly elevated in patients with systemic lupus
erythematosus (SLE) secondary to PH and were significantly
correlated with plasma NT-pro-B natriuretic peptide (NT-pro-BNP)
levels and resting pulmonary systolic pressure (sPAP), as well as
responding to the severity of SLE disease (110). When SUA is above the critical
concentration of 6.5 mg/dl, the incidence of PH in patients with
SLE can be reasonably and accurately predicted. Therefore, SUA
concentrations can be used as an alternative marker to screen for
PH in patients with SLE (111).
When the baseline SUA concentration is ≥416 µmol/l (7 mg/dl),
future development of PH secondary to SLE can be predicted
(112). A multifactorial analysis
showed that high UA concentrations were not only associated with
all-cause mortality from disease but also strongly associated with
death from PH and thus, UA concentrations may serve as an
independent predictor of survival in patients with connective
tissue-related PH (113).
Therefore, dynamic observation of SUA concentrations may be useful
for assessing the severity of the condition and serve as a
predictor of prognosis in connective tissue disease-associated
PH.
In conclusion, UA is not only a marker of metabolism
but also a representative independent risk factor and predictor of
PH. The aforementioned evidence suggests that UA is closely
associated with PH (114–117). However, the specific mechanisms
involved in hyperuricemia promoting the development and progression
of PH is unclear. In the present review, the effects of high UA
concentrations on PH and the molecular mechanisms of the effects of
high UA concentrations on endothelial cells, smooth muscle cells
and renin-angiotensin system (RAS) activation are described.
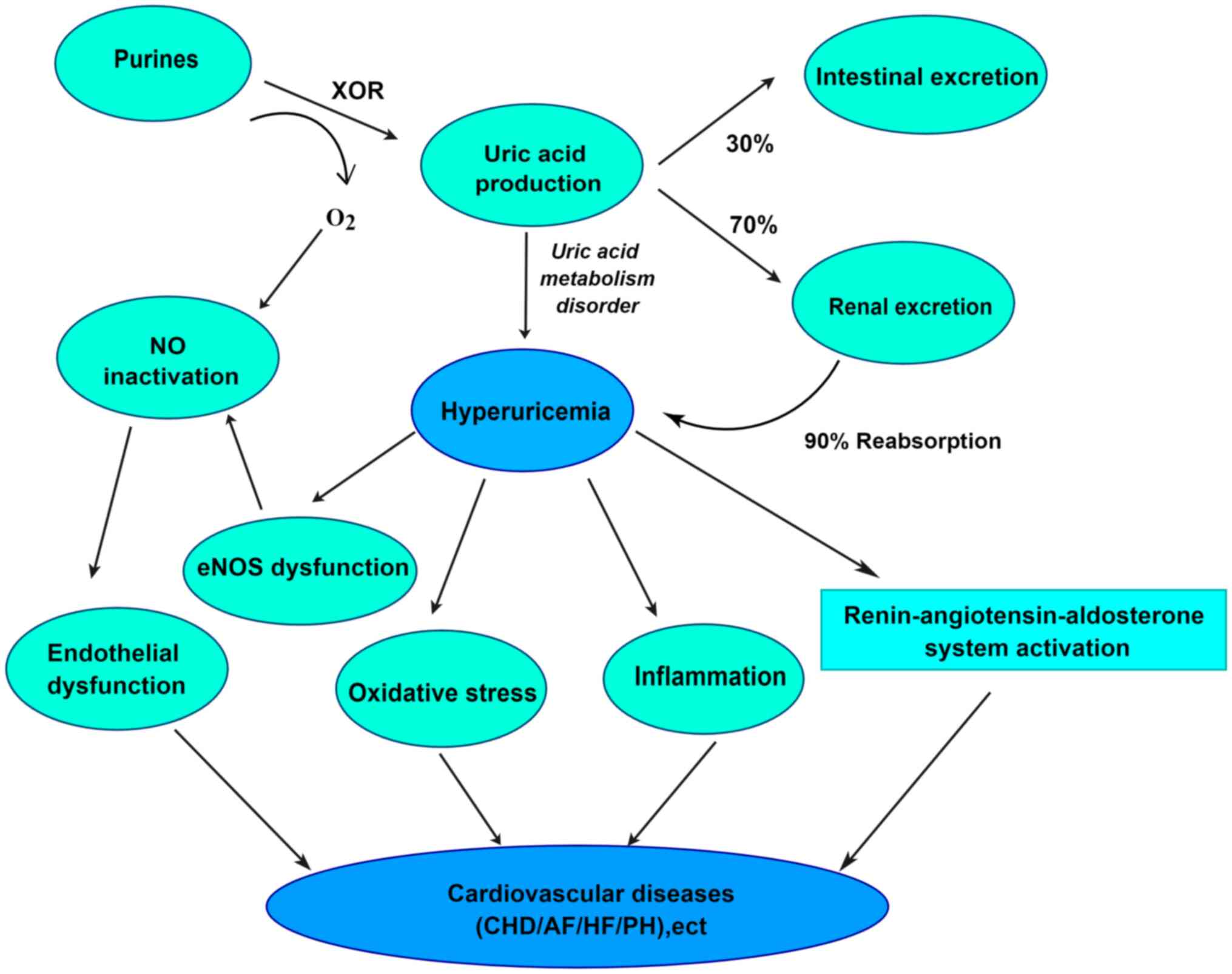
Hyperuricemia can mediate the development of
cardiovascular disease by inducing endothelial dysfunction,
oxidative stress, inflammatory responses and activation of the RAS
(Fig. 3) (118–122). On a pathophysiological basis, UA
also induces pulmonary vascular endothelial dysfunction and
promotes the transformation of smooth muscle cell proliferation
(123,124), thereby possibly promoting the
development of PH. The series of molecular mechanisms whereby UA
affects the course of PH through a series of molecular mechanisms
are described in the present review.
Endothelial cells are in direct contact with blood
flow and act as a permeability barrier to maintain the exchange
between the tissues of the vessel wall and blood (125,126). Furthermore, endothelial cells
secrete vasoactive substances and cytokines, which also play an
important role in regulating vasoconstriction, vascular
inflammation, platelet aggregation and adhesion (127). Therefore, the integrity of
endothelial function plays a major role in maintaining
cardiovascular homeostasis.
Nitric oxide (NO) is an endothelium-derived relaxing
factor and it regulates vascular tension, inhibits platelet
activation and causes intimal hyperplasia (132). High UA concentrations are
hypothesized to result in endothelial dysfunction by affecting the
production of NO, which may contribute to PH (133). UA may affect the formation of NO
in two ways. Firstly, UA can be directly oxidized with NO to form
superoxide anion, which consumes high levels of NO. Secondly, there
are various pathways by which UA inhibits NO production which are
described in the present review.
Endothelial NO synthase (eNOS) is a key enzyme for
NO synthesis in endothelial cells. This enzyme catalyzes the
hydrolysis of L-arginine to produce NO (134). UA can enter endothelial cells
through URAT1 on the cell membrane (135), inducing intracellular reactive
oxygen species production, endoplasmic reticulum stress and protein
kinase C activation (136).
Activated protein kinase C inactivates the inhibitory site of eNOS,
Thr495, by phosphorylating it and rendering it unable to bind
calmodulin and catalyzes NO synthesis (136). In addition to regulating glucose
homeostasis, insulin activates the signal of phosphatidylinositol
3-kinase (PI3K)-protein kinase B (Akt), which promotes the
activation of eNOS phosphorylation and NO production, thus inducing
vasodilation (137).
Hyperuricemia antagonizes insulin receptor substrates and blocks
insulin-dependent eNOS phosphorylation in the PI3K/Akt/eNOS
pathway, thereby inhibiting NO production (137,138). Elevated UA concentrations in
patients with metabolic syndrome (MS) can trigger endothelial
dysfunction by decreasing endothelial NO bioavailability, while
reduced NO production in this pathway may be associated with
hyperinsulinemia and insulin resistance (IR) (139), which lead to increased monocyte
adhesion and impaired cellular energy metabolism (140,141). However, allopurinol may restore
the effect of insulin on NO production and vasodilation by reducing
SUA concentrations, thereby improving the associated clinical
symptoms (142,143).
UA also increases the expression of the inflammatory
cytokines interleukin-6 and interleukin-8, tumor necrosis factor-α
and miR-155 by activating nuclear factor-κB (NF-κB) (144,145). Overexpression of miR-155 leads to
decreased eNOS stability, reduced NO production and endothelial
dysfunction (146). By contrast,
the use of NF-κB inhibitor II can prevent the UA-induced decrease
in NO and the inflammatory reaction (145). Furthermore, arginase competes
with eNOS to bind L-arginine and catalyze its hydrolysis to
ornithine and urea (147).
However, UA reduces NO production in endothelial cells by
increasing arginase activity and promoting competition between
arginine and eNOS for L-arginine (41,147). Mitochondrial damage is also a
major feature of endothelial dysfunction. UA can trigger
mitochondrial calcium overload and reactive oxygen species
production by activating the mitochondrial
Na+/Ca2+ exchanger (148). This process can inhibit the
tricarboxylic acid cycle and damage mitochondrial DNA, thus leading
to endothelial dysfunction (149). These findings suggest that UA
induces reduced NO production and vascular endothelial dysfunction,
which in turn causes abnormal pulmonary vasoconstriction and
provides a pathophysiological basis for the development of PH.
UA can enter vascular smooth muscle cells (VSMCs)
via URAT1 (SLC22A12, a member of the organic anion transporter
superfamily) (150,151), stimulating specific
mitogen-activated protein kinases (MAPKs), ERK 1/2 and p38 MAPK
(152,153). This stimulation induces
cyclooxygenase-2 production and local coagulation, promotes
platelet-derived growth factor (PDGF)-A and PDGF-C chain secretion
and upregulates PDGF-A receptor mRNA expression, promotes VSMC
proliferation, increases cell survival and reduces apoptosis
(123,153–159). However, angiotensin II (Ang II)
type I receptor inhibits UA-induced activation of p38 MAPK and ERK
1/2, thereby blocking the proliferative pathway of VSMCs (153,160). In addition, UA may also regulate
the proliferation of smooth muscle cells by inducing inflammatory
responses and activation of the chemokine monocyte chemoattractant
protein 1, transcription factor activator protein-1, NF-κB and
inflammasome NOD-like receptor protein 3 (153,161,162). Xanthine oxidase and URAT1 were
up-regulated in remodeled pulmonary artery walls in patients of
IPAH, monocrotaline (MCT) and Sugen-hypoxia rats, increasing
intracellular UA production, which promotes the proliferation of
pulmonary artery smooth muscle cells, leading to further
deterioration of PH (163). Thus,
UA promotes smooth muscle cell proliferation and may play an
important role in vascular remodeling in PH.
The RAS is an important and complex endocrine system
in the body. It not only plays an important role in regulating
blood pressure and maintaining extracellular fluid homeostasis, but
also affects the normal development of the cardiovascular system
and maintains homeostasis of cardiovascular function (164). Several studies have shown that
elevated SUA concentrations may be associated with activation of
the RAS (121,161,165–168). In animal studies, high UA
concentrations inhibited NOS-1 activity in glomerular dense
plaques, downregulated NO production and activated the RAS
(157,160,169–171), leading to elevated blood
pressure. These findings are consistent with human studies
suggesting that UA activates the RAS to mediate an elevation in
blood pressure (172,173). Usually, the activation of RAS
begins with the decrease of blood flow through renal artery
(174). The production of
angiotensin peptides is first initiated by the synthesis and
processing of preprorenin in juxtaglomerular cells neighboring the
renal glomerulus with subsequent proteolytic cleavage of the signal
peptide, intracellular sorting of prorenin to dense-core secretory
vesicles, and cleavage of the prosegment, producing catalytically
active renin that is secreted in the systemic circulation (164,175,176). Renin hydrolyzes angiotensinogen
secreted by the liver to produce angiotensin I (Ang I) (177). In PAECs, Ang I is cleaved to Ang
II by angiotensin-converting enzyme (178). In the mechanism of high
UA-induced endothelial dysfunction, excess UA can be rapidly taken
up by vascular smooth muscle cells, and intracellular UA
upregulates angiotensinogen mRNA expression, thereby promoting Ang
II production and Ang II type 1 receptor (main effector peptide of
RAS) expression (179). These
findings suggest that UA upregulates Ang II expression, activates
the RAS system, produces oxidative stress, and leads to endothelial
cell senescence and apoptosis (179,180). Ang II, which is a pleiotropic
endocrine and paracrine hormone, upregulates vasopressin released
by the central nervous system and induces VSMC contraction in the
pulmonary circulation and systemic arterial and venous circulation
(176,181). In addition, Ang II stimulates the
release of aldosterone, which stimulates mineralocorticoid
receptors in PAECs, inducing hypertrophy of PASMCs and pulmonary
artery vascular remodeling (182–185). However, the vascular remodeling
effects caused by UA and Ang II stimulation of VSMC proliferation
and hypertrophy is inhibited by losartan [an angiotensin receptor
blocker (ARB)] and captopril [an angiotensin-converting enzyme
inhibitor (ACEI)] (157,186). Ang II also promotes
vasoconstriction, proliferation, inflammation and fibrosis in the
pulmonary vascular system and lung parenchyma by stimulating ANG II
type 1 receptor (187,188). All of these studies suggest that
UA mediates the relationship between the RAS and PH, promoting
pulmonary vascular remodeling, enhancing pulmonary vasoconstriction
and ultimately exacerbating the progression of PH.
PH affects UA concentrations in two main ways.
First, an elevation in SUA concentrations in patients with PH is
mainly due to tissue ischemia/hypoxia and oxidative stress
(30,189,190). When oxygen metabolism is abnormal
in the body, tissue ischemia or hypoxia and oxidative stress can
lead to elevated UA concentrations (191,192). For example, PH is associated with
chronic heart failure and chronic obstructive pulmonary disease,
tissue hypoxia, increased anaerobic metabolism, decreased adenosine
triphosphate synthesis and accelerated purine degradation, leading
to increased uric acid production (193–195). In addition, patients with heart
failure are often associated with renal insufficiency or even renal
failure, which can reduce UA excretion and lead to increased UA
concentrations (196). As SUA
concentrations rise, free radicals released by xanthine oxidase may
activate inflammatory cells (197). When UA concentrations exceed the
threshold, hyperuricemia enhances intracellular urate accumulation
via down-regulation of cell-surface BCRP/ABCG2 expression in
vascular endothelial cells (198), leading to endothelial
dysfunction, leukocyte recruitment, cytokine release, and
stimulation of activation and proliferation of VSMCs, as well as
vasoconstriction and diastolic dysfunction (199) and ultimately, exacerbates tissue
hypoxia (200). Moreover,
hyperuricemia is involved in oxidative metabolism, platelet
adhesion, blood rheology and platelet aggregation (201,202). These processes can increase
platelet adhesion and make patients with PH more susceptible to
pulmonary vascular thrombosis (203). Hypoxia also leads to impaired
pulmonary vascular perfusion, and the release of additional
cytokines further accelerates vascular remodeling and fibrosis
(191,204,205). The effect of the use of drugs,
such as diuretics in the setting of heart failure, on UA
concentrations should not be overlooked. Borghi et al
(206) reported that tab
diuretics, thiazides and aspirin may increase SUA concentrations.
When PH is combined with underlying diseases, such as renal
insufficiency, hypermetabolic syndrome, obesity, hyperlipidemia,
hypertension, coronary artery disease and diabetes mellitus, it can
also result in hyperuricemia (11,93,131). These diseases mainly cause
dysfunction of UA excretion/increased UA synthesis (199). Additionally, the use of clinical
medications in these conditions can interfere with UA
concentrations. Examples of these medications include calcium
channel blockers (e.g., amlodipine and cilnidipine) (207), angiotensin-converting enzyme
inhibitors (e.g., captopril, enalapril and ramipril) (208,209), angiotensin-converting enzyme II
receptor antagonists (e.g., losartan) (210), lipid-lowering agents (e.g.,
atorvastatin, simvastatin, ezetimibe and fenofibrate) (211), weight loss medications (e.g.,
orlistat) (212) and hypoglycemic
agents (e.g., metformin) (213).
Additionally, sodium glucose transporter protein 2 reduces UA
concentrations (214,215). Therefore, PH with hypoxia leads
to elevated UA concentrations. However, UA, as a risk marker,
exacerbates the severity of PH and increases the risk of death due
to PH.
Currently, UA-lowering drugs mainly include the
following categories: i) Drugs that inhibit UA production (xanthine
oxidase inhibitors, such as allopurinol and febuxostat) (216,217); ii) drugs that promote UA
excretion (drugs that inhibit the production of the UA reabsorption
proteins URAT1 and GLUT9, such as benzbromarone and probenecid)
(218,219); iii) drugs that promote UA
catabolism (UA enzymes, such as rasburicase and pegloticase)
(220,221); and iv) antihypertensive drugs
(ACEIs such as enalapril, and ARBs such as irbesartan and losartan)
(208,210). Based on the role of UA in PH,
some of these drugs (e.g., allopurinol and benzbromarone) have been
shown to reduce SUA concentrations and has a certain protective
effects against arterial hypertension (163,222–224). Therefore, lowering SUA
concentration has the potential to serve as a target for the
treatment of PH.
Increasing evidence has shown that UA is
inextricably associated with PH and may serve as a circulating
marker of PH (189) (Fig. 4). UA may be involved in PH by
mediating inflammatory responses, oxidative stress, RAS activation
and endothelial dysfunction (131). PH leads to tissue
ischemia/hypoxia and oxidative stress, and impaired UA metabolism,
which lead to an increase in SUA concentrations (225,226). However, the causal relationship
between UA and PH is not completely clear. Hyperuricemia may be
considered a risk factor/independent risk factor for PH and a
predictor of disease onset, progression and prognosis (115,116,227), but whether SUA can be used as a
circulating marker for PH needs to be validated by additional
clinical and basic research. In addition, to determine whether
lowering SUA concentrations improves the clinical symptoms of PH,
further investigation and clinical studies are required.
Not applicable.
The present study was supported by The National Natural Science
Foundation of China (grant no. 81970056), Discipline Construction
Project of Guangdong Medical University (grant no. 4SG21233G), Key
platform of Department of Education of Guangdong (grant no.
2021LSYS007) and Zhanjiang Science and Technology Development
Special Funding Competitive Allocation Project (grant nos.
2022E05011, 2022A01196, 2021A05158, 2021A05058, 2021A05056,
2020A01020 and 2020A06004).
Not applicable.
YZ and WL conceived and designed the entire review
and wrote the paper. YZ assisted with the figures. MC, JZ, YH, HL,
XS and WL reviewed and edited the manuscript. All authors read and
approved the final version of the manuscript. Data authentication
is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Hoeper MM, Humbert M, Souza R, Idrees M,
Kawut SM, Sliwa-Hahnle K, Jing ZC and Gibbs JS: A global view of
pulmonary hypertension. Lancet Respir Med. 4:306–322. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beshay S, Sahay S and Humbert M:
Evaluation and management of pulmonary arterial hypertension.
Respir Med. 171:1060992020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morrell NW, Aldred MA, Chung WK, Elliott
CG, Nichols WC, Soubrier F, Trembath RC and Loyd JE: Genetics and
genomics of pulmonary arterial hypertension. Eur Respir J.
53:18018992019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levine DJ: Pulmonary arterial
hypertension: Updates in epidemiology and evaluation of patients.
Am J Manag Care. 27 (3 Suppl):S35–S41. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rich S, Dantzker DR, Ayres SM, Bergofsky
EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM,
Koerner SK, et al: Primary pulmonary hypertension. A national
prospective study. Ann Intern Med. 107:216–223. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Girerd B, Montani D, Eyries M, Yaici A,
Sztrymf B, Coulet F, Sitbon O, Simonneau G, Soubrier F and Humbert
M: Absence of influence of gender and BMPR2 mutation type on
clinical phenotypes of pulmonary arterial hypertension. Respir Res.
11:732010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ventetuolo CE, Praestgaard A, Palevsky HI,
Klinger JR, Halpern SD and Kawut SM: Sex and haemodynamics in
pulmonary arterial hypertension. Eur Respir J. 43:523–530. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jing ZC, Xu XQ, Han ZY, Wu Y, Deng KW,
Wang H, Wang ZW, Cheng XS, Xu B, Hu SS, et al: Registry and
survival study in chinese patients with idiopathic and familial
pulmonary arterial hypertension. Chest. 132:373–379. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu X, Sun M, Jiang X, Zhang R, Zhao Q,
Wang Y, Sun K, Wang X, Peng F, Zheng L, et al: Comparison of
clinical characteristics and survival on patients with idiopathic
pulmonary arterial hypertension and familial pulmonary arterial
hypertension during conventional therapy era and targeted therapy
era. Zhonghua Xin Xue Guan Bing Za Zhi. 42:465–468. 2014.(In
Chinese). PubMed/NCBI
|
|
10
|
Hansmann G, Wagner RA, Schellong S, Perez
VA, Urashima T, Wang L, Sheikh AY, Suen RS, Stewart DJ and
Rabinovitch M: Pulmonary arterial hypertension is linked to insulin
resistance and reversed by peroxisome proliferator-activated
receptor-gamma activation. Circulation. 115:1275–1284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zamanian RT, Hansmann G, Snook S,
Lilienfeld D, Rappaport KM, Reaven GM, Rabinovitch M and Doyle RL:
Insulin resistance in pulmonary arterial hypertension. Eur Respir
J. 33:318–324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fessel JP, Hamid R, Wittmann BM, Robinson
LJ, Blackwell T, Tada Y, Tanabe N, Tatsumi K, Hemnes AR and West
JD: Metabolomic analysis of bone morphogenetic protein receptor
type 2 mutations in human pulmonary endothelium reveals widespread
metabolic reprogramming. Pulm Circ. 2:201–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zare E, Kafshbani P, Chenaghlou M, Noori
M, Ghaemmaghami Z, Amin A, Taghavi S and Naderi N: Prognostic
significance of insulin resistance in pulmonary hypertension. ESC
Heart Fail. 9:318–326. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feig DI, Kang DH and Johnson RJ: Uric acid
and cardiovascular risk. N Engl J Med. 359:1811–1821. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gagliardi AC, Miname MH and Santos RD:
Uric acid: A marker of increased cardiovascular risk.
Atherosclerosis. 202:11–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Borghi C and Cicero AFG: Serum uric acid
and acute coronary syndrome: Is there a role for functional markers
of residual cardiovascular risk. Int J Cardiol. 250:62–63. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ndrepepa G: Uric acid and cardiovascular
disease. Clin Chim Acta. 484:150–163. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krishnan E, Kwoh CK, Schumacher HR and
Kuller L: Hyperuricemia and incidence of hypertension among men
without metabolic syndrome. Hypertension. 49:298–303. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brodov Y, Behar S, Boyko V and Chouraqui
P: Effect of the metabolic syndrome and hyperuricemia on outcome in
patients with coronary artery disease (from the Bezafibrate
Infarction Prevention Study). Am J Cardiol. 106:1717–1720. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galassi FM and Borghi C: A brief history
of uric acid: From gout to cardiovascular risk factor. Eur J Intern
Med. 26:3732015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li M, Hu X, Fan Y, Li K, Zhang X, Hou W
and Tang Z: Hyperuricemia and the risk for coronary heart disease
morbidity and mortality a systematic review and dose-response
meta-analysis. Sci Rep. 6:195202016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuwabara M, Niwa K, Nishihara S, Nishi Y,
Takahashi O, Kario K, Yamamoto K, Yamashita T and Hisatome I:
Hyperuricemia is an independent competing risk factor for atrial
fibrillation. Int J Cardiol. 231:137–142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sanchez-Lozada LG, Rodriguez-Iturbe B,
Kelley EE, Nakagawa T, Madero M, Feig DI, Borghi C, Piani F,
Cara-Fuentes G, Bjornstad P, et al: Uric acid and hypertension: An
update with recommendations. Am J Hypertens. 33:583–594. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Si K, Wei C, Xu L, Zhou Y, Lv W, Dong B,
Wang Z, Huang Y, Wang Y and Chen Y: Hyperuricemia and the Risk of
Heart Failure: Pathophysiology and Therapeutic Implications. Front
Endocrinol (Lausanne). 12:7708152021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang X, Hou Y, Wang X, Li Z, Wang X, Li H,
Shang L, Zhou J and Zhang Y, Ren M and Zhang Y: Relationship
between serum uric acid levels and different types of atrial
fibrillation: An updated meta-analysis. Nutr Metab Cardiovasc Dis.
31:2756–2765. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gomes MT, Bai Y, Potje SR, Zhang L,
Lockett AD and Machado RF: Signal transduction during metabolic and
inflammatory reprogramming in pulmonary vascular remodeling. Int J
Mol Sci. 23:24102022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Perera FP, Rauh V, Whyatt RM, Tang D, Tsai
WY, Bernert JT, Tu YH, Andrews H, Barr DB, Camann DE, et al: A
summary of recent findings on birth outcomes and developmental
effects of prenatal ETS, PAH, and pesticide exposures.
Neurotoxicology. 26:573–587. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Humbert M, Kovacs G, Hoeper MM,
Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS,
Escribano-Subias P, Ferrari P, et al: 2022 ESC/ERS Guidelines for
the diagnosis and treatment of pulmonary hypertension. Eur Heart J.
43:3618–3731. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Humbert M, Kovacs G, Hoeper MM,
Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS,
Escribano-Subias P, Ferrari P, et al: 2022 ESC/ERS Guidelines for
the diagnosis and treatment of pulmonary hypertension. Eur Respir
J. 61:22008792023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Galiè N, Humbert M, Vachiery JL, Gibbs S,
Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A,
Beghetti M, et al: 2015 ESC/ERS Guidelines for the diagnosis and
treatment of pulmonary hypertension: The Joint Task Force for the
Diagnosis and Treatment of Pulmonary Hypertension of the European
Society of Cardiology (ESC) and the European Respiratory Society
(ERS): Endorsed by: Association for European Paediatric and
Congenital Cardiology (AEPC), International Society for Heart and
Lung Transplantation (ISHLT). Eur Respir J. 46:903–975. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Galiè N, Humbert M, Vachiery JL, Gibbs S,
Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A,
Beghetti M, et al: 2015 ESC/ERS Guidelines for the diagnosis and
treatment of pulmonary hypertension: The Joint Task Force for the
Diagnosis and Treatment of Pulmonary Hypertension of the European
Society of Cardiology (ESC) and the European Respiratory Society
(ERS): Endorsed by: Association for European Paediatric and
Congenital Cardiology (AEPC), International Society for Heart and
Lung Transplantation (ISHLT). Eur Heart J. 37:67–119. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simonneau G, Robbins IM, Beghetti M,
Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin
MT, Jing ZC, et al: Updated clinical classification of pulmonary
hypertension. J Am Coll Cardiol. 54 (1 Suppl):S43–S54. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gelzinis TA: Pulmonary Hypertension in
2021: Part I-Definition, Classification, Pathophysiology, and
Presentation. J Cardiothorac Vasc Anesth. 36:1552–1564. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Badesch DB, Champion HC, Gomez Sanchez MA,
Hoeper MM, Loyd JE, Manes A, McGoon M, Naeije R, Olschewski H,
Oudiz RJ and Torbicki A: Diagnosis and assessment of pulmonary
arterial hypertension. J Am Coll Cardiol. 54 (1 Suppl):S55–S66.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Assad TR and Hemnes AR: Metabolic
dysfunction in pulmonary arterial hypertension. Curr Hypertens Rep.
17:202015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Satoh T, Wang L, Espinosa-Diez C, Wang B,
Hahn SA, Noda K, Rochon ER, Dent MR, Levine AR, Baust JJ, et al:
Metabolic Syndrome Mediates ROS-miR-193b-NFYA-Dependent
downregulation of soluble guanylate cyclase and contributes to
exercise-induced pulmonary hypertension in heart failure with
preserved ejection fraction. Circulation. 144:615–637. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nicolls MR and Voelkel NF: Hypoxia and the
lung: Beyond hypoxic vasoconstriction. Antioxid Redox Signal.
9:741–743. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Langleben D, Orfanos SE, Giovinazzo M,
Hirsch A, Baron M, Senécal JL, Armaganidis A and Catravas JD:
Pulmonary capillary endothelial metabolic dysfunction: Severity in
pulmonary arterial hypertension related to connective tissue
disease versus idiopathic pulmonary arterial hypertension.
Arthritis Rheum. 58:1156–1164. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jones PL, Cowan KN and Rabinovitch M:
Tenascin-C, proliferation and subendothelial fibronectin in
progressive pulmonary vascular disease. Am J Pathol. 150:1349–1360.
1997.PubMed/NCBI
|
|
40
|
Stacher E, Graham BB, Hunt JM, Gandjeva A,
Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool
CD and Tuder RM: Modern age pathology of pulmonary arterial
hypertension. Am J Respir Crit Care Med. 186:261–272. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Watanabe T, Ishikawa M, Abe K, Ishikawa T,
Imakiire S, Masaki K, Hosokawa K, Fukuuchi T, Kaneko K, Ohtsubo T,
et al: Increased Lung Uric Acid Deteriorates Pulmonary Arterial
Hypertension. J Am Heart Assoc. 10:e0227122021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lippi G, Montagnana M, Franchini M,
Favaloro EJ and Targher G: The paradoxical relationship between
serum uric acid and cardiovascular disease. Clin Chim Acta.
392:1–7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yamaoka T and Itakura M: Metabolism of
purine nucleotides and the production of uric acid. Nihon Rinsho.
54:3188–3194. 1996.(In Japanese). PubMed/NCBI
|
|
44
|
El Ridi R and Tallima H: Physiological
functions and pathogenic potential of uric acid: A review. J Adv
Res. 8:487–493. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Benn CL, Dua P, Gurrell R, Loudon P, Pike
A, Storer RI and Vangjeli C: Physiology of hyperuricemia and
urate-lowering treatments. Front Med (Lausanne). 5:1602018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Maiuolo J, Oppedisano F, Gratteri S,
Muscoli C and Mollace V: Regulation of uric acid metabolism and
excretion. Int J Cardiol. 213:8–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chaudhary K, Malhotra K, Sowers J and
Aroor A: Uric Acid-key ingredient in the recipe for cardiorenal
metabolic syndrome. Cardiorenal Med. 3:208–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gherghina ME, Peride I, Tiglis M, Neagu
TP, Niculae A and Checherita IA: Uric acid and oxidative
stress-relationship with cardiovascular, metabolic, and renal
impairment. Int J Mol Sci. 23:31882022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sánchez-Lozada LG, Nakagawa T, Kang DH,
Feig DI, Franco M, Johnson RJ and Herrera-Acosta J: Hormonal and
cytokine effects of uric acid. Curr Opin Nephrol Hypertens.
15:30–33. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen C, Lü JM and Yao Q:
Hyperuricemia-Related Diseases and Xanthine Oxidoreductase (XOR)
Inhibitors: An Overview. Med Sci Monit. 22:2501–2512. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Furuhashi M: New insights into purine
metabolism in metabolic diseases: Role of xanthine oxidoreductase
activity. Am J Physiol Endocrinol Metab. 319:E827–E834. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shima Y, Teruya K and Ohta H: Association
between intronic SNP in urate-anion exchanger gene, SLC22A12, and
serum uric acid levels in Japanese. Life Sci. 79:2234–2237. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Caulfield MJ, Munroe PB, O'Neill D,
Witkowska K, Charchar FJ, Doblado M, Evans S, Eyheramendy S,
Onipinla A, Howard P, et al: SLC2A9 is a high-capacity urate
transporter in humans. PLoS Med. 5:e1972008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wright AF, Rudan I, Hastie ND and Campbell
H: A ‘complexity’ of urate transporters. Kidney Int. 78:446–452.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ma Q, Fang L, Su R, Ma L, Xie G and Cheng
Y: Uric acid stones, clinical manifestations and therapeutic
considerations. Postgrad Med J. 94:458–462. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lipkowitz MS: Regulation of uric acid
excretion by the kidney. Curr Rheumatol Rep. 14:179–188. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ichida K, Matsuo H, Takada T, Nakayama A,
Murakami K, Shimizu T, Yamanashi Y, Kasuga H, Nakashima H, Nakamura
T, et al: Decreased extra-renal urate excretion is a common cause
of hyperuricemia. Nat Commun. 3:7642012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Eckenstaler R and Benndorf RA: The Role of
ABCG2 in the pathogenesis of primary hyperuricemia and Gout-An
Update. Int J Mol Sci. 22:66782021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Homolya L: Medically Important Alterations
in Transport Function and Trafficking of ABCG2. Int J Mol Sci.
22:27862021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ohashi Y, Toyoda M, Saito N, Koizumi M,
Kanai G, Komaba H, Kimura M, Wada T, Takahashi H, Takahashi Y, et
al: Evaluation of ABCG2-mediated extra-renal urate excretion in
hemodialysis patients. Sci Rep. 13:932023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Su J, Wei Y, Liu M, Liu T, Li J, Ji Y and
Liang J: Anti-hyperuricemic and nephroprotective effects of Rhizoma
Dioscoreae septemlobae extracts and its main component dioscin via
regulation of mOAT1, mURAT1 and mOCT2 in hypertensive mice. Arch
Pharm Res. 37:1336–1344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wu XH, Zhang J, Wang SQ, Yang VC, Anderson
S and Zhang YW: Riparoside B and timosaponin J, two steroidal
glycosides from Smilax riparia, resist to hyperuricemia based on
URAT1 in hyperuricemic mice. Phytomedicine. 21:1196–1201. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Nath SD, Voruganti VS, Arar NH, Thameem F,
Lopez-Alvarenga JC, Bauer R, Blangero J, MacCluer JW, Comuzzie AG
and Abboud HE: Genome scan for determinants of serum uric acid
variability. J Am Soc Nephrol. 18:3156–3163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Anzai N, Jutabha P, Amonpatumrat-Takahashi
S and Sakurai H: Recent advances in renal urate transport:
Characterization of candidate transporters indicated by genome-wide
association studies. Clin Exp Nephrol. 16:89–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Dehghan A, van Hoek M, Sijbrands EJ,
Hofman A and Witteman JC: High serum uric acid as a novel risk
factor for type 2 diabetes. Diabetes Care. 31:361–362. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Okada Y, Sim X, Go MJ, Wu JY, Gu D,
Takeuchi F, Takahashi A, Maeda S, Tsunoda T, Chen P, et al:
Meta-analysis identifies multiple loci associated with kidney
function-related traits in east Asian populations. Nat Genet.
44:904–909. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Major TJ, Dalbeth N, Stahl EA and Merriman
TR: An update on the genetics of hyperuricaemia and gout. Nat Rev
Rheumatol. 14:341–353. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sautin YY and Johnson RJ: Uric acid: The
oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids.
27:608–619. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Jakše B, Jakše B, Pajek M and Pajek J:
Uric acid and plant-based nutrition. Nutrients. 11:17362019.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shi Y, Chen W, Jalal D, Li Z, Chen W, Mao
H, Yang Q, Johnson RJ and Yu X: Clinical outcome of hyperuricemia
in IgA nephropathy: A retrospective cohort study and randomized
controlled trial. Kidney Blood Press Res. 35:153–160. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Joosten LAB, Crişan TO, Bjornstad P and
Johnson RJ: Asymptomatic hyperuricaemia: A silent activator of the
innate immune system. Nat Rev Rheumatol. 16:75–86. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Miao Y, Ottenbros SA, Laverman GD, Brenner
BM, Cooper ME, Parving HH, Grobbee DE, Shahinfar S, de Zeeuw D and
Lambers Heerspink HJ: Effect of a reduction in uric acid on renal
outcomes during losartan treatment: A post hoc analysis of the
reduction of endpoints in non-insulin-dependent diabetes mellitus
with the Angiotensin II Antagonist Losartan Trial. Hypertension.
58:2–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ames BN, Cathcart R, Schwiers E and
Hochstein P: Uric acid provides an antioxidant defense in humans
against oxidant- and radical-caused aging and cancer: A hypothesis.
Proc Natl Acad Sci USA. 78:6858–6862. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zou H, Wang H, Liu T, Li X, Zhu X and Wang
Z: Protective role of α-lipoic acid in hyperuricemia-induced
endothelial dysfunction. Exp Ther Med. 13:3047–3054. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Shi Y, Evans JE and Rock KL: Molecular
identification of a danger signal that alerts the immune system to
dying cells. Nature. 425:516–521. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Netea MG, Kullberg BJ, Blok WL, Netea RT
and van der Meer JW: The role of hyperuricemia in the increased
cytokine production after lipopolysaccharide challenge in
neutropenic mice. Blood. 89:577–582. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bagnati M, Perugini C, Cau C, Bordone R,
Albano E and Bellomo G: When and why a water-soluble antioxidant
becomes pro-oxidant during copper-induced low-density lipoprotein
oxidation: A study using uric acid. Biochem J. 340((Pt 1)):
143–152. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kang DH, Park SK, Lee IK and Johnson RJ:
Uric acid-induced C-reactive protein expression: Implication on
cell proliferation and nitric oxide production of human vascular
cells. J Am Soc Nephrol. 16:3553–3562. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Patterson RA, Horsley ET and Leake DS:
Prooxidant and antioxidant properties of human serum ultrafiltrates
toward LDL: Important role of uric acid. J Lipid Res. 44:512–521.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Samocha-Bonet D, Lichtenberg D and Pinchuk
I: Kinetic studies of copper-induced oxidation of urate, ascorbate
and their mixtures. J Inorg Biochem. 99:1963–1972. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Sautin YY, Nakagawa T, Zharikov S and
Johnson RJ: Adverse effects of the classic antioxidant uric acid in
adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am
J Physiol Cell Physiol. 293:C584–C596. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhang JX, Zhang YP, Wu QN and Chen B: Uric
acid induces oxidative stress via an activation of the
renin-angiotensin system in 3T3-L1 adipocytes. Endocrine.
48:135–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang JG, Staessen JA, Fagard RH,
Birkenhäger WH, Gong L and Liu L: Prognostic significance of serum
creatinine and uric acid in older Chinese patients with isolated
systolic hypertension. Hypertension. 37:1069–1074. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kuzkaya N, Weissmann N, Harrison DG and
Dikalov S: Interactions of peroxynitrite with uric acid in the
presence of ascorbate and thiols: Implications for uncoupling
endothelial nitric oxide synthase. Biochem Pharmacol. 70:343–354.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Nagahama K, Inoue T, Iseki K, Touma T,
Kinjo K, Ohya Y and Takishita S: Hyperuricemia as a predictor of
hypertension in a screened cohort in Okinawa, Japan. Hypertens Res.
27:835–841. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Morris CR, Kuypers FA, Kato GJ, Lavrisha
L, Larkin S, Singer T and Vichinsky EP: Hemolysis-associated
pulmonary hypertension in thalassemia. Ann N Y Acad Sci.
1054:481–485. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Castro O, Hoque M and Brown BD: Pulmonary
hypertension in sickle cell disease: Cardiac catheterization
results and survival. Blood. 101:1257–1261. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Verresen D, De Backer W, Van Meerbeeck J,
Neetens I, Van Marck E and Vermeire P: Spherocytosis and pulmonary
hypertension coincidental occurrence or causal relationship. Eur
Respir J. 4:629–631. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Heller PG, Grinberg AR, Lencioni M, Molina
MM and Roncoroni AJ: Pulmonary hypertension in paroxysmal nocturnal
hemoglobinuria. Chest. 102:642–643. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Devalet B, Mullier F, Chatelain B, Dogné
JM and Chatelain C: Pathophysiology, diagnosis, and treatment of
paroxysmal nocturnal hemoglobinuria: A review. Eur J Haematol.
95:190–198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Tofovic SP, Jackson EK and Rafikova O:
Adenosine deaminase-adenosine pathway in hemolysis-associated
pulmonary hypertension. Med Hypotheses. 72:713–719. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Cerqueira BA, Boas WV, Zanette AD, Reis MG
and Goncalves MS: Increased concentrations of IL-18 and uric acid
in sickle cell anemia: Contribution of hemolysis, endothelial
activation and the inflammasome. Cytokine. 56:471–476. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Robbins IM, Newman JH, Johnson RF, Hemnes
AR, Fremont RD, Piana RN, Zhao DX and Byrne DW: Association of the
metabolic syndrome with pulmonary venous hypertension. Chest.
136:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Quiñones Galvan A, Natali A, Baldi S,
Frascerra S, Sanna G, Ciociaro D and Ferrannini E: Effect of
insulin on uric acid excretion in humans. Am J Physiol. 268((1 Pt
1)): E1–E5. 1995.PubMed/NCBI
|
|
95
|
Gashouta MA, Humbert M and Hassoun PM:
Update in systemic sclerosis-associated pulmonary arterial
hypertension. Presse Med. 43((10 Pt 2)): e293–e304. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kherbeck N, Tamby MC, Bussone G, Dib H,
Perros F, Humbert M and Mouthon L: The role of inflammation and
autoimmunity in the pathophysiology of pulmonary arterial
hypertension. Clin Rev Allergy Immunol. 44:31–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ferreira NS, Tostes RC, Paradis P and
Schiffrin EL: Aldosterone, inflammation, immune system, and
hypertension. Am J Hypertens. 34:15–27. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Nagaya N, Uematsu M, Satoh T, Kyotani S,
Sakamaki F, Nakanishi N, Yamagishi M, Kunieda T and Miyatake K:
Serum uric acid levels correlate with the severity and the
mortality of primary pulmonary hypertension. Am J Respir Crit Care
Med. 160:487–492. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Editorial. Major changes made by Criteria
Committee of the New York Heart Association. Circulation.
49:3901974. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Voelkel MA, Wynne KM, Badesch DB, Groves
BM and Voelkel NF: Hyperuricemia in severe pulmonary hypertension.
Chest. 117:19–24. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Li ZN, He JG, Liu ZH, Gu Q, Ni XH, Cheng
XS and Xiong CM: Relationship between serum uric acid levels and
patient conditions and prognosis in idiopathic pulmonary arterial
hypertension. Zhonghua Yi Xue Za Zhi. 92:3261–3264. 2012.(In
Chinese). PubMed/NCBI
|
|
102
|
Zhang CY, Ma LL and &Wang LX:
Relationship between serum uric acid levels and ventricular
function in patients with idiopathic pulmonary hypertension. Exp
Clin Cardiol. 18:e37–3e9. 2013.PubMed/NCBI
|
|
103
|
Seyyedi SR, Malekmohammad M, Chitsazan M,
Behzadnia N, Sadr M, Hashemian SM and Sharif-Kashani B:
Relationship between Serum Uric Acid Levels and the Severity of
Pulmonary Hypertension. Tanaffos. 16:283–288. 2017.PubMed/NCBI
|
|
104
|
Yan L, Huang Z, Zhao Z, Zhao Q, Tang Y,
Zhang Y, Li X, Duan A, Luo Q and Liu Z: The Prognostic Impact of
Serum Uric Acid on Disease Severity and 5-Year mortality in
patients with idiopathic pulmonary artery hypertension. Front Med
(Lausanne). 9:8054152022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Dhaun N, Vachiery JL, Benza RL, Naeije R,
Hwang LJ, Liu X, Teal S and Webb DJ: Endothelin antagonism and uric
acid levels in pulmonary arterial hypertension: Clinical
associations. J Heart Lung Transplant. 33:521–527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Dimitroulas T, Giannakoulas G, Dimitroula
H, Sfetsios T, Parcharidou D, Karvounis H and Settas L:
Significance of serum uric acid in pulmonary hypertension due to
systemic sclerosis: A pilot study. Rheumatol Int. 31:263–267. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Denton CP: Systemic sclerosis: From
pathogenesis to targeted therapy. Clin Exp Rheumatol. 33 (4 Suppl
92):S3–S7. 2015.PubMed/NCBI
|
|
108
|
Gigante A, Barbano B, Barilaro G, Quarta
S, Gasperini ML, Di Mario F, Romaniello A, Amoroso A, Cianci R and
Rosato E: Serum uric acid as a marker of microvascular damage in
systemic sclerosis patients. Microvasc Res. 106:39–43. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Pagkopoulou E, Soulaidopoulos S,
Triantafyllidou E, Malliari A, Kitas GD, Garyfallos A and
Dimitroulas T: Association Between Uric Acid and Worsening
Peripheral Microangiopathy in Systemic Sclerosis. Front Med
(Lausanne). 8:8069252021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Aghdashi M, Behnemoon M, Mahmoodi Rad J
and Rabiepour M: Evaluation of serum uric acid level in systemic
lupus erythematosus patients with normal and high pulmonary
arterial hypertension. Biomedicine (Taipei). 8:162018. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Kim KJ, Baek IW, Park YJ, Yoon CH, Kim WU
and Cho CS: High levels of uric acid in systemic lupus
erythematosus is associated with pulmonary hypertension. Int J
Rheum Dis. 18:524–532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Castillo-Martínez D, Marroquín-Fabián E,
Lozada-Navarro AC, Mora-Ramírez M, Juárez M, Sánchez-Muñoz F,
Vargas-Barrón J, Sandoval J and Amezcua-Guerra LM: Levels of uric
acid may predict the future development of pulmonary hypertension
in systemic lupus erythematosus: A seven-year follow-up study.
Lupus. 25:61–66. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Njaman W, Iesaki T, Iwama Y, Takasaki Y
and Daida H: Serum uric Acid as a prognostic predictor in pulmonary
arterial hypertension with connective tissue disease. Int Heart J.
48:523–532. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Luo DL, Zhang CJ, Huang YG, Huang T and Li
HZ: Serum uric acid is associated with disease severity and an
important predictor for clinical outcome in patients with pulmonary
hypertension. Zhonghua Xin Xue Guan Bing Za Zhi. 45:496–500.
2017.(In Chinese). PubMed/NCBI
|
|
115
|
Simpson CE, Damico RL, Hummers L, Khair
RM, Kolb TM, Hassoun PM and Mathai SC: Serum uric acid as a marker
of disease risk, severity, and survival in systemic
sclerosis-related pulmonary arterial hypertension. Pulm Circ.
9:20458940198594772019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Uk Kang T, Park KY, Kim HJ, Ahn HS, Yim SY
and Jun JB: Association of hyperuricemia and pulmonary
hypertension: A systematic review and meta-analysis. Mod Rheumatol.
29:1031–1041. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Hong JW, Noh JH and Kim DJ: Association
between serum uric acid and spirometric pulmonary function in
Korean adults: The 2016 Korea National Health and Nutrition
Examination Survey. PLoS One. 15:e02409872020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Johnson RJ, Kang DH, Feig D, Kivlighn S,
Kanellis J, Watanabe S, Tuttle KR, Rodriguez-Iturbe B,
Herrera-Acosta J and Mazzali M: Is there a pathogenetic role for
uric acid in hypertension and cardiovascular and renal disease.
Hypertension. 41:1183–1190. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Khosla UM, Zharikov S, Finch JL, Nakagawa
T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S and Johnson RJ:
Hyperuricemia induces endothelial dysfunction. Kidney Int.
67:1739–1742. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
O'Riordan E, Chen J, Brodsky SV, Smirnova
I, Li H and Goligorsky MS: Endothelial cell dysfunction: The
syndrome in making. Kidney Int. 67:1654–1658. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
van Thiel BS, van der Pluijm I, te Riet L,
Essers J and Danser AH: The renin-angiotensin system and its
involvement in vascular disease. Eur J Pharmacol. 763((Pt A)):
3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Podkowińska A and Formanowicz D: Chronic
kidney disease as oxidative stress- and inflammatory-mediated
cardiovascular disease. Antioxidants (Basel). 9:7522020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Rao GN, Corson MA and Berk BC: Uric acid
stimulates vascular smooth muscle cell proliferation by increasing
platelet-derived growth factor A-chain expression. J Biol Chem.
266:8604–8608. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kanbay M, Segal M, Afsar B, Kang DH,
Rodriguez-Iturbe B and Johnson RJ: The role of uric acid in the
pathogenesis of human cardiovascular disease. Heart. 99:759–766.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Potente M, Gerhardt H and Carmeliet P:
Basic and therapeutic aspects of angiogenesis. Cell. 146:873–887.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Eelen G, Treps L, Li X and Carmeliet P:
Basic and therapeutic aspects of angiogenesis updated. Circ Res.
127:310–329. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Krüger-Genge A, Blocki A, Franke RP and
Jung F: Vascular endothelial cell biology: An update. Int J Mol
Sci. 20:44112019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Dai Z, Zhu MM, Peng Y, Jin H, Machireddy
N, Qian Z, Zhang X and Zhao YY: Endothelial and smooth muscle cell
interaction via FoxM1 signaling mediates vascular remodeling and
pulmonary hypertension. Am J Respir Crit Care Med. 198:788–802.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Evans CE, Cober ND, Dai Z, Stewart DJ and
Zhao YY: Endothelial cells in the pathogenesis of pulmonary
arterial hypertension. Eur Respir J. 58:20039572021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Liu B, Peng Y, Yi D, Machireddy N, Dong D,
Ramirez K, Dai J, Vanderpool R, Zhu MM, Dai Z and Zhao YY:
Endothelial PHD2 deficiency induces nitrative stress via
suppression of caveolin-1 in pulmonary hypertension. Eur Respir J.
60:21026432022. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zharikov SI, Swenson ER, Lanaspa M, Block
ER, Patel JM and Johnson RJ: Could uric acid be a modifiable risk
factor in subjects with pulmonary hypertension? Med Hypotheses.
74:1069–1074. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Komaszyło K, Zalewska R, Mariak Z and
Wiśniewska RJ: Biosynthesis of nitric oxide and its function in
organism. Klin Oczna. 108:99–102. 2006.(In Polish). PubMed/NCBI
|
|
133
|
Gersch C, Palii SP, Kim KM, Angerhofer A,
Johnson RJ and Henderson GN: Inactivation of nitric oxide by uric
acid. Nucleosides Nucleotides Nucleic Acids. 27:967–978. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Förstermann U: Janus-faced role of
endothelial NO synthase in vascular disease: Uncoupling of oxygen
reduction from NO synthesis and its pharmacological reversal. Biol
Chem. 387:1521–1533. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Mishima M, Hamada T, Maharani N, Ikeda N,
Onohara T, Notsu T, Ninomiya H, Miyazaki S, Mizuta E, Sugihara S,
et al: Effects of Uric Acid on the NO Production of HUVECs and its
Restoration by Urate Lowering Agents. Drug Res (Stuttg).
66:270–274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Li P, Zhang L, Zhang M, Zhou C and Lin N:
Uric acid enhances PKC-dependent eNOS phosphorylation and mediates
cellular ER stress: A mechanism for uric acid-induced endothelial
dysfunction. Int J Mol Med. 37:989–997. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Choi YJ, Yoon Y, Lee KY, Hien TT, Kang KW,
Kim KC, Lee J, Lee MY, Lee SM, Kang DH and Lee BH: Uric acid
induces endothelial dysfunction by vascular insulin resistance
associated with the impairment of nitric oxide synthesis. FASEB J.
28:3197–3204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Bahadoran Z, Mirmiran P, Kashfi K and
Ghasemi A: Hyperuricemia-induced endothelial insulin resistance:
The nitric oxide connection. Pflugers Arch. 474:83–98. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Roy D, Perreault M and Marette A: Insulin
stimulation of glucose uptake in skeletal muscles and adipose
tissues in vivo is NO dependent. Am J Physiol. 274:E692–E699.
1998.PubMed/NCBI
|
|
140
|
Nakagawa T, Hu H, Zharikov S, Tuttle KR,
Short RA, Glushakova O, Ouyang X, Feig DI, Block ER, Herrera-Acosta
J, et al: A causal role for uric acid in fructose-induced metabolic
syndrome. Am J Physiol Renal Physiol. 290:F625–F631. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Lee TS, Lu TM, Chen CH, Guo BC and Hsu CP:
Hyperuricemia induces endothelial dysfunction and accelerates
atherosclerosis by disturbing the asymmetric
dimethylarginine/dimethylarginine dimethylaminotransferase 2
pathway. Redox Biol. 46:1021082021. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Deedwania PC: Mechanisms of endothelial
dysfunction in the metabolic syndrome. Curr Diab Rep. 3:289–292.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Yu W and Cheng JD: Uric acid and
cardiovascular disease: An update from molecular mechanism to
clinical perspective. Front Pharmacol. 11:5826802020. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Lee KS, Kim J, Kwak SN, Lee KS, Lee DK, Ha
KS, Won MH, Jeoung D, Lee H, Kwon YG and Kim YM: Functional role of
NF-κB in expression of human endothelial nitric oxide synthase.
Biochem Biophys Res Commun. 448:101–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Zhen H and Gui F: The role of
hyperuricemia on vascular endothelium dysfunction. Biomed Rep.
7:325–330. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Zhang X, Hong Q, Hou K, Wang Y, Wu D and
Chen X: High concentration uric acid regulates endothelial function
via miR-155. Nan Fang Yi Ke Da Xue Xue Bao. 33:1141–1145. 2013.(In
Chinese). PubMed/NCBI
|
|
147
|
Zharikov S, Krotova K, Hu H, Baylis C,
Johnson RJ, Block ER and Patel J: Uric acid decreases NO production
and increases arginase activity in cultured pulmonary artery
endothelial cells. Am J Physiol Cell Physiol. 295:C1183–C1190.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Hong Q, Qi K, Feng Z, Huang Z, Cui S, Wang
L, Fu B, Ding R, Yang J, Chen X and Wu D: Hyperuricemia induces
endothelial dysfunction via mitochondrial Na+/Ca2+
exchanger-mediated mitochondrial calcium overload. Cell Calcium.
51:402–410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Sánchez-Lozada LG, Lanaspa MA,
Cristóbal-García M, García-Arroyo F, Soto V, Cruz-Robles D,
Nakagawa T, Yu MA, Kang DH and Johnson RJ: Uric acid-induced
endothelial dysfunction is associated with mitochondrial
alterations and decreased intracellular ATP concentrations. Nephron
Exp Nephrol. 121:e71–e78. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Kang DH, Han L, Ouyang X, Kahn AM,
Kanellis J, Li P, Feng L, Nakagawa T, Watanabe S, Hosoyamada M, et
al: Uric acid causes vascular smooth muscle cell proliferation by
entering cells via a functional urate transporter. Am J Nephrol.
25:425–433. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Price KL, Sautin YY, Long DA, Zhang L,
Miyazaki H, Mu W, Endou H and Johnson RJ: Human vascular smooth
muscle cells express a urate transporter. J Am Soc Nephrol.
17:1791–1795. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Oğuz N, Kırça M, Çetin A and Yeşilkaya A:
Effect of uric acid on inflammatory COX-2 and ROS pathways in
vascular smooth muscle cells. J Recept Signal Transduct Res.
37:500–505. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Kırça M, Oğuz N, Çetin A, Uzuner F and
Yeşilkaya A: Uric acid stimulates proliferative pathways in
vascular smooth muscle cells through the activation of p38 MAPK,
p44/42 MAPK and PDGFRβ. J Recept Signal Transduct Res. 37:167–173.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Bowen-Pope DF, Ross R and Seifert RA:
Locally acting growth factors for vascular smooth muscle cells:
Endogenous synthesis and release from platelets. Circulation.
72:735–740. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Berk BC: Vascular smooth muscle growth:
Autocrine growth mechanisms. Physiol Rev. 81:999–1030. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Kang DH, Nakagawa T, Feng L, Watanabe S,
Han L, Mazzali M, Truong L, Harris R and Johnson RJ: A role for
uric acid in the progression of renal disease. J Am Soc Nephrol.
13:2888–2897. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Mazzali M, Kanellis J, Han L, Feng L, Xia
YY, Chen Q, Kang DH, Gordon KL, Watanabe S, Nakagawa T, et al:
Hyperuricemia induces a primary renal arteriolopathy in rats by a
blood pressure-independent mechanism. Am J Physiol Renal Physiol.
282:F991–F997. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Watanabe S, Kang DH, Feng L, Nakagawa T,
Kanellis J, Lan H, Mazzali M and Johnson RJ: Uric acid, hominoid
evolution, and the pathogenesis of salt-sensitivity. Hypertension.
40:355–360. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Doğru S, Yaşar E and Yeşilkaya A: Uric
acid can enhance MAPK pathway-mediated proliferation in rat primary
vascular smooth muscle cells via controlling of mitochondria and
caspase-dependent cell death. J Recept Signal Transduct Res.
42:293–301. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Messerli FH, Frohlich ED, Dreslinski GR,
Suarez DH and Aristimuno GG: Serum uric acid in essential
hypertension: An indicator of renal vascular involvement. Ann
Intern Med. 93:817–821. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Kanellis J, Watanabe S, Li JH, Kang DH, Li
P, Nakagawa T, Wamsley A, Sheikh-Hamad D, Lan HY, Feng L and
Johnson RJ: Uric acid stimulates monocyte chemoattractant protein-1
production in vascular smooth muscle cells via mitogen-activated
protein kinase and cyclooxygenase-2. Hypertension. 41:1287–1293.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Li H, Qian F, Liu H and Zhang Z: Elevated
Uric Acid Levels Promote Vascular Smooth Muscle Cells (VSMC)
Proliferation via an Nod-Like Receptor Protein 3
(NLRP3)-Inflammasome-Dependent Mechanism. Med Sci Monit.
25:8457–8464. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Savale L, Akagi S, Tu L, Cumont A,
Thuillet R, Phan C, Le Vely B, Berrebeh N, Huertas A, Jaïs X, et
al: Serum and pulmonary uric acid in pulmonary arterial
hypertension. Eur Respir J. 58:20003322021. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Forrester SJ, Booz GW, Sigmund CD, Coffman
TM, Kawai T, Rizzo V, Scalia R and Eguchi S: Angiotensin II Signal
Transduction: An update on mechanisms of physiology and
pathophysiology. Physiol Rev. 98:1627–1738. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Satou R, Penrose H and Navar LG:
Inflammation as a Regulator of the Renin-Angiotensin System and
Blood Pressure. Curr Hypertens Rep. 20:1002018. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Saxena T, Ali AO and Saxena M:
Pathophysiology of essential hypertension: An update. Expert Rev
Cardiovasc Ther. 16:879–887. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Wang XD, Liu J, Zhang YC, Wang Y, Wang Y
and Ma D: Correlation between the elevated uric acid levels and
circulating renin-angiotensin-aldosterone system activation in
patients with atrial fibrillation. Cardiovasc Diagn Ther. 11:50–55.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Sankrityayan H, Rao PD, Shelke V, Kulkarni
YA, Mulay SR and Gaikwad AB: Endoplasmic reticulum stress and
renin-angiotensin system crosstalk in endothelial dysfunction. Curr
Mol Pharmacol. 16:139–146. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Saito I, Saruta T, Kondo K, Nakamura R,
Oguro T, Yamagami K, Ozawa Y and Kato E: Serum uric acid and the
renin-angiotensin system in hypertension. J Am Geriatr Soc.
26:241–247. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Cappuccio FP, Iacone R and Strazzullo P:
Serum uric acid and proximal sodium excretion: An independent
association in man (the Olivetti Study). J Hypertens. (Suppl
9):S280–S281. 1991.
|
|
171
|
Welch WJ, Wilcox CS and Thomson SC: Nitric
oxide and tubuloglomerular feedback. Semin Nephrol. 19:251–262.
1999.PubMed/NCBI
|
|
172
|
Perlstein TS, Gumieniak O, Hopkins PN,
Murphey LJ, Brown NJ, Williams GH, Hollenberg NK and Fisher ND:
Uric acid and the state of the intrarenal renin-angiotensin system
in humans. Kidney Int. 66:1465–1470. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Feig DI, Kang DH, Nakagawa T, Mazzali M
and Johnson RJ: Uric acid and hypertension. Curr Hypertens Rep.
8:111–115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Brewster UC and Perazella MA: The
renin-angiotensin-aldosterone system and the kidney: Effects on
kidney disease. Am J Med. 116:263–272. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Sparks MA, Crowley SD, Gurley SB, Mirotsou
M and Coffman TM: Classical Renin-Angiotensin system in kidney
physiology. Compr Physiol. 4:1201–1228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Nehme A, Zouein FA, Zayeri ZD and Zibara
K: An Update on the Tissue Renin Angiotensin System and Its Role in
Physiology and Pathology. J Cardiovasc Dev Dis. 6:142019.PubMed/NCBI
|
|
177
|
Laghlam D, Jozwiak M and Nguyen LS:
Renin-Angiotensin-Aldosterone System and Immunomodulation: A
State-of-the-Art Review. Cells. 10:17672021. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Bernstein KE, Khan Z, Giani JF, Cao DY,
Bernstein EA and Shen XZ: Angiotensin-converting enzyme in innate
and adaptive immunity. Nat Rev Nephrol. 14:325–336. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Corry DB, Eslami P, Yamamoto K, Nyby MD,
Makino H and Tuck ML: Uric acid stimulates vascular smooth muscle
cell proliferation and oxidative stress via the vascular
renin-angiotensin system. J Hypertens. 26:269–275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Yu MA, Sánchez-Lozada LG, Johnson RJ and
Kang DH: Oxidative stress with an activation of the
renin-angiotensin system in human vascular endothelial cells as a
novel mechanism of uric acid-induced endothelial dysfunction. J
Hypertens. 28:1234–1242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Basso N and Terragno NA: History about the
discovery of the renin-angiotensin system. Hypertension.
38:1246–1249. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Lipworth BJ and Dagg KD: Vasoconstrictor
effects of angiotensin II on the pulmonary vascular bed. Chest.
105:1360–1364. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Orte C, Polak JM, Haworth SG, Yacoub MH
and Morrell NW: Expression of pulmonary vascular
angiotensin-converting enzyme in primary and secondary plexiform
pulmonary hypertension. J Pathol. 192:379–384. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Abraham WT, Raynolds MV, Badesch DB, Wynne
KM, Groves BM, Roden RL, Robertson AD, Lowes BD, Zisman LS, Voelkel
NF, et al: Angiotensin-converting enzyme DD genotype in patients
with primary pulmonary hypertension: Increased frequency and
association with preserved haemodynamics. J Renin Angiotensin
Aldosterone Syst. 4:27–30. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Chung WK, Deng L, Carroll JS, Mallory N,
Diamond B, Rosenzweig EB, Barst RJ and Morse JH: Polymorphism in
the angiotensin II type 1 receptor (AGTR1) is associated with age
at diagnosis in pulmonary arterial hypertension. J Heart Lung
Transplant. 28:373–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Berk BC and Rao GN: Angiotensin II-induced
vascular smooth muscle cell hypertrophy: PDGF A-chain mediates the
increase in cell size. J Cell Physiol. 154:368–380. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Morrell NW, Atochina EN, Morris KG,
Danilov SM and Stenmark KR: Angiotensin converting enzyme
expression is increased in small pulmonary arteries of rats with
hypoxia-induced pulmonary hypertension. J Clin Invest.
96:1823–1833. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
de Man FS, Tu L, Handoko ML, Rain S,
Ruiter G, François C, Schalij I, Dorfmüller P, Simonneau G, Fadel
E, et al: Dysregulated renin-angiotensin-aldosterone system
contributes to pulmonary arterial hypertension. Am J Respir Crit
Care Med. 186:780–789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Pezzuto B, Badagliacca R, Poscia R, Ghio
S, D'Alto M, Vitulo P, Mulè M, Albera C, Volterrani M, Fedele F and
Vizza CD: Circulating biomarkers in pulmonary arterial
hypertension: Update and future direction. J Heart Lung Transplant.
34:282–305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Foris V, Kovacs G, Tscherner M, Olschewski
A and Olschewski H: Biomarkers in pulmonary hypertension: What do
we know? Chest. 144:274–283. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Ozanturk E, Ucar ZZ, Varol Y, Koca H,
Demir AU, Kalenci D, Halilcolar H and Ozacar R: Urinary uric acid
excretion as an indicator of severe hypoxia and mortality in
patients with obstructive sleep apnea and chronic obstructive
pulmonary disease. Rev Port Pneumol (2006). 22:18–26.
2016.PubMed/NCBI
|
|
192
|
Deng S, Zhu T, Wang C, Gu X, Zhang J, Lu
Q, Yang Y and Ma X: Analysis of Correlation Between Serum
Hypoxia-Inducible Factor-1α, Uric Acid, and Inflammatory Factor
Levels and Lung Function in Patients with AECOPD. Altern Ther
Health Med. AT8122:Aug 11–2023.(Epub ahead of print). PubMed/NCBI
|
|
193
|
Leyva F, Anker S, Swan JW, Godsland IF,
Wingrove CS, Chua TP, Stevenson JC and Coats AJ: Serum uric acid as
an index of impaired oxidative metabolism in chronic heart failure.
Eur Heart J. 18:858–865. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Horodinschi RN, Bratu OG, Dediu GN, Pantea
Stoian A, Motofei I and Diaconu CC: Heart failure and chronic
obstructive pulmonary disease: A review. Acta Cardiol. 75:97–104.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Nathan SD, Barbera JA, Gaine SP, Harari S,
Martinez FJ, Olschewski H, Olsson KM, Peacock AJ, Pepke-Zaba J,
Provencher S, et al: Pulmonary hypertension in chronic lung disease
and hypoxia. Eur Respir J. 53:18019142019. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Kaufman M and Guglin M: Uric acid in heart
failure: A biomarker or therapeutic target? Heart Fail Rev.
18:177–186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Richette P, Frazier A and Bardin T: Impact
of anti-inflammatory therapies, xanthine oxidase inhibitors and
other urate-lowering therapies on cardiovascular diseases in gout.
Curr Opin Rheumatol. 27:170–174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Komori H, Yamada K and Tamai I:
Hyperuricemia enhances intracellular urate accumulation via
down-regulation of cell-surface BCRP/ABCG2 expression in vascular
endothelial cells. Biochim Biophys Acta Biomembr. 1860:973–980.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Yanai H, Adachi H, Hakoshima M and
Katsuyama H: Molecular biological and clinical understanding of the
pathophysiology and treatments of hyperuricemia and its association
with metabolic syndrome, cardiovascular diseases and chronic kidney
disease. Int J Mol Sci. 22:92212021. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Lokmic Z, Musyoka J, Hewitson TD and Darby
IA: Hypoxia and hypoxia signaling in tissue repair and fibrosis.
Int Rev Cell Mol Biol. 296:139–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Alderman M and Aiyer KJ: Uric acid: Role
in cardiovascular disease and effects of losartan. Curr Med Res
Opin. 20:369–379. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Cai H and Harrison DG: Endothelial
dysfunction in cardiovascular diseases: The role of oxidant stress.
Circ Res. 87:840–844. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Țăpoi L, Șalaru DL, Sascău R and Stătescu
C: Uric Acid-An emergent risk marker for thrombosis? J Clin Med.
10:20622021. View Article : Google Scholar
|
|
204
|
Schober A: Chemokines in vascular
dysfunction and remodeling. Arterioscler Thromb Vasc Biol.
28:1950–1959. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Voelkel NF, Mizuno S and Bogaard HJ: The
role of hypoxia in pulmonary vascular diseases: A perspective. Am J
Physiol Lung Cell Mol Physiol. 304:L457–L465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Borghi C, Verardi FM, Pareo I, Bentivenga
C and Cicero AF: Hyperuricemia and cardiovascular disease risk.
Expert Rev Cardiovasc Ther. 12:1219–1225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Das A, Kumar P, Kumari A, Chandra S, Gari
M, Singh N and Dey D: Effects of cilnidipine on heart rate and uric
acid metabolism in patients with essential hypertension. Cardiol
Res. 7:167–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Qin X, Li Y, He M, Tang G, Yin D, Liang M,
Wang B, Nie J, Huo Y, Xu X and Hou FF: Folic acid therapy reduces
serum uric acid in hypertensive patients: A substudy of the China
Stroke Primary Prevention Trial (CSPPT). Am J Clin Nutr.
105:882–889. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Chida R, Hisauchi I, Toyoda S, Kikuchi M,
Komatsu T, Hori Y, Nakahara S, Sakai Y, Inoue T and Taguchi I:
Impact of irbesartan, an angiotensin receptor blocker, on uric acid
level and oxidative stress in high-risk hypertension patients.
Hypertens Res. 38:765–769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Kim EJ, Song WH, Lee JU, Shin MS, Lee S,
Kim BO, Hong KS, Han SW, Park CG and Seo HS: Efficacy of losartan
and carvedilol on central hemodynamics in hypertensives: a
prospective, randomized, open, blinded end point, multicenter
study. Hypertens Res. 37:50–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Newman CB: Safety of statins and
nonstatins for treatment of dyslipidemia. Endocrinol Metab Clin
North Am. 51:655–679. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Noori S, Mirzababaei A, Amini MR, Clark
CCT and Mirzaei K: Effect of orlistat on serum uric acid level in
adults: A systematic review and meta-analysis of randomised
controlled trials. Int J Clin Pract. 75:e146742021. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Zhang G, Ma Y, Xi D, Rao Z, Sun X and Wu
X: Effect of high uric acid on the disposition of metformin: in
vivo and in vitro studies. Biopharm Drug Dispos. 40:3–11. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Katsiki N, Karagiannis A, Athyros VG and
Mikhailidis DP: Hyperuricaemia: More than just a cause of gout. J
Cardiovasc Med (Hagerstown). 14:397–402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Thomas MC: Renal effects of dapagliflozin
in patients with type 2 diabetes. Ther Adv Endocrinol Metab.
5:53–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
George J, Carr E, Davies J, Belch JJ and
Struthers A: High-dose allopurinol improves endothelial function by
profoundly reducing vascular oxidative stress and not by lowering
uric acid. Circulation. 114:2508–2516. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Tani S, Nagao K and Hirayama A: Effect of
febuxostat, a xanthine oxidase inhibitor, on cardiovascular risk in
hyperuricemic patients with hypertension: A prospective,
open-label, pilot study. Clin Drug Investig. 35:823–831. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Lin HC, Daimon M, Wang CH, Ho Y, Uang YS,
Chiang SJ and Wang LH: Allopurinol, benzbromarone and risk of
coronary heart disease in gout patients: A population-based study.
Int J Cardiol. 233:85–90. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Kim SC, Neogi T, Kang EH, Liu J, Desai RJ,
Zhang M and Solomon DH: Cardiovascular risks of probenecid versus
allopurinol in older patients with gout. J Am Coll Cardiol.
71:994–1004. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Moss JD, Wu M, Axelrod DM and Kwiatkowski
DM: Rasburicase versus intravenous allopurinol for
non-malignancy-associated acute hyperuricemia in paediatric
cardiology patients. Cardiol Young. 29:1160–1164. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Sundy JS, Baraf HS, Yood RA, Edwards NL,
Gutierrez-Urena SR, Treadwell EL, Vázquez-Mellado J, White WB,
Lipsky PE, Horowitz Z, et al: Efficacy and tolerability of
pegloticase for the treatment of chronic gout in patients
refractory to conventional treatment: Two randomized controlled
trials. JAMA. 306:711–720. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Theilmann AL and Ormiston ML: Repurposing
benzbromarone for pulmonary arterial hypertension: Can channelling
the past deliver the therapy of the future? Eur Respir J.
53:19005832019. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Liu-Shiu-Cheong PSK, Lipworth BJ,
Weir-McCall JR, Houston JG and Struthers AD: Allopurinol in
patients with pulmonary hypertension associated with chronic lung
disease. Int J Chron Obstruct Pulmon Dis. 15:2015–2024. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Gokcen T, Inci K, Inci EE, Sevgen O and
Serdar U: Allopurinol treatment reduced vascular remodeling and
improved vascular functions in monocrotaline-induced pulmonary
hypertensive rats. Pulm Pharmacol Ther. 77:1021662022. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
De Scheerder IK, van de Kraay AM, Lamers
JM, Koster JF, de Jong JW and Serruys PW: Myocardial
malondialdehyde and uric acid release after short-lasting coronary
occlusions during coronary angioplasty: Potential mechanisms for
free radical generation. Am J Cardiol. 68:392–395. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Friedl HP, Till GO, Trentz O and Ward PA:
Role of oxygen radicals in tourniquet-related ischemia-reperfusion
injury of human patients. Klin Wochenschr. 69:1109–1112. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Many A, Hubel CA and Roberts JM:
Hyperuricemia and xanthine oxidase in preeclampsia, revisited. Am J
Obstet Gynecol. 174((1 Pt 1)): 288–291. 1996. View Article : Google Scholar : PubMed/NCBI
|