Aryl hydrocarbon receptor (AhR) is a
ligand-activated nuclear transcription factor and a member of the
basic helix-loop-helix/Per AhR nuclear translocator (ARNT)-Sim
transcription factor family (9–12).
AhR has a complex ligand-binding domain that is activated by
numerous exogenous and endogenous ligands and natural compounds
with different structures and binding affinities (13–15).
Following ligand binding, AhR translocates into the nucleus
(16) to form a heterodimer with
ARNT and subsequently transactivate target genes (16,17).
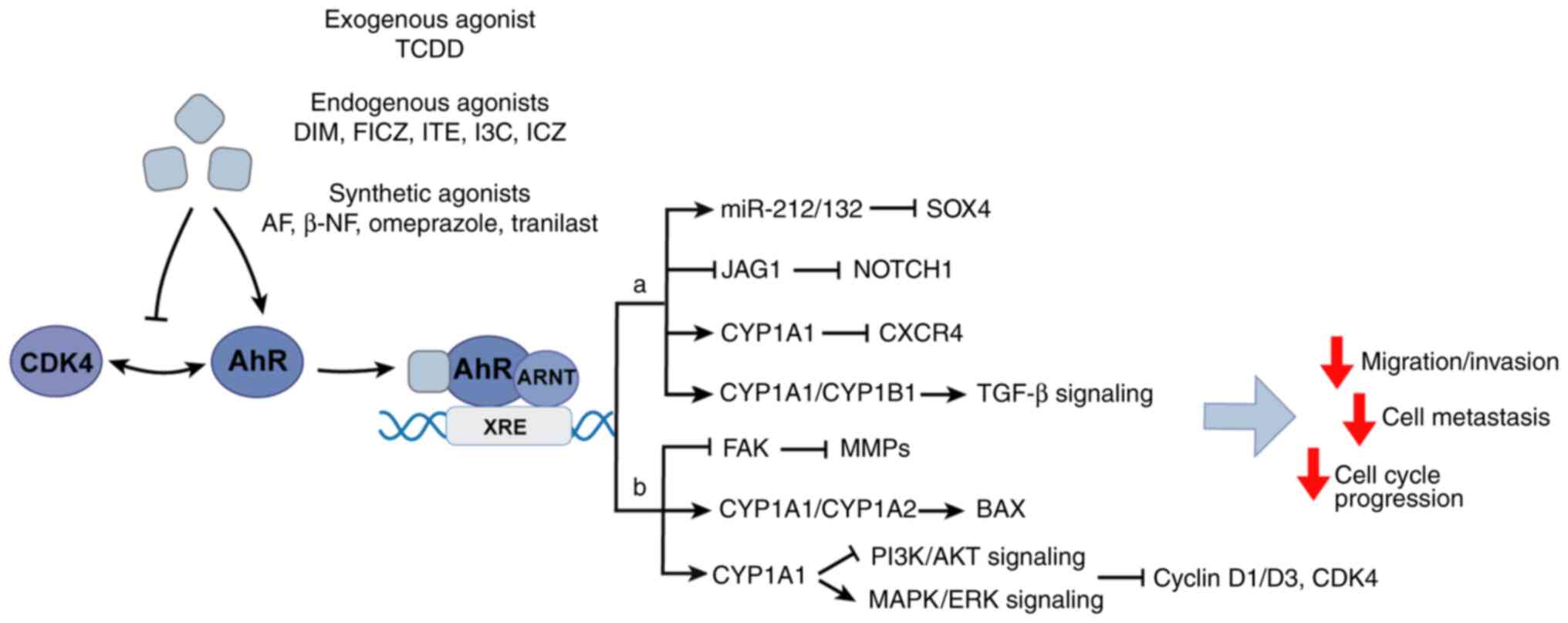
AhR is activated by numerous ligands, such as
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and β-naphthoflavone
(β-NF), and regulates different target genes depending on the type
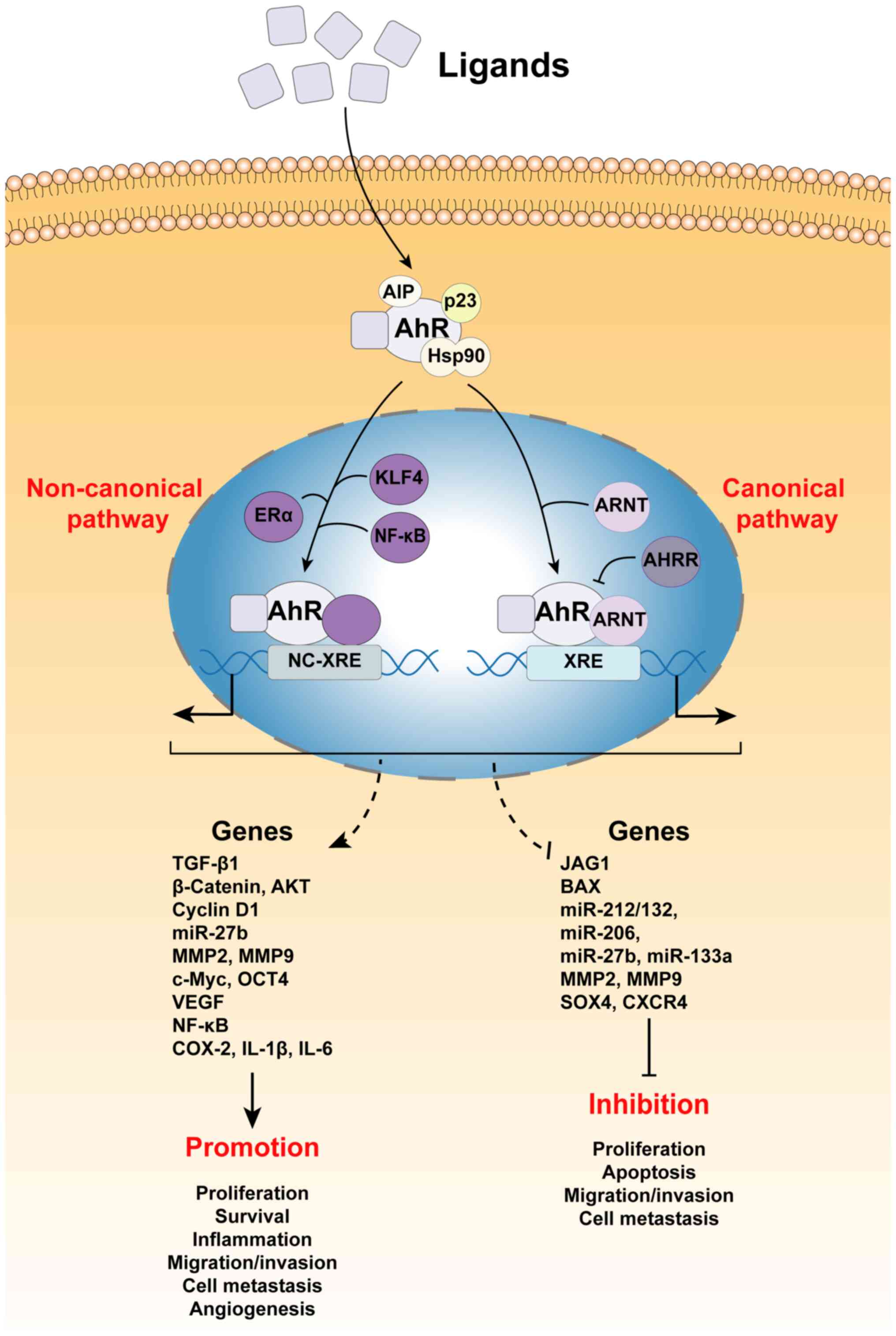
of ligand (Fig. 1). Exogenous AhR
ligands, endogenous ligands and natural products activate AhR
through genomic and non-genomic pathways (18). In the genomic pathways, activated
AhR acts as a transcription factor to bind dioxin reaction elements
(DREs) in promoters and regulate the expression of genes encoding
xenobiotic metabolic enzymes, such as cytochrome P450 family 1
subfamily A member 1 (CYP1A1), cytochrome P450 family 1 subfamily A
member 2 (CYP1A2), cytochrome P450 family 1 subfamily B member 1
(CYP1B1) and glutathione transferase and aldehyde dehydrogenase
(ALDH) (19–23). In non-genomic pathways, AhR exerts
non-transcriptional activities via the involvement of other
transcriptional regulators or signal transducers, such as c-Src,
NF-κB or estrogen receptor α (ERα) (24–28).
AhR regulates numerous important physiological and
pathological processes, such as immune response inhibition
(16,17,29–31),
homeostasis of the liver, vascular and cardiovascular systems
(32–34), tumor induction (11,31),
inflammation (17,31,35)
and intestinal barrier function (17,36).
AhR is also activated by environmental pollutants, such as
polycyclic aromatic hydrocarbons (PAHs) and halogenated aromatic
hydrocarbons (HAHs), which affect tumor formation (37–39).
Previous studies have reported the functional
interactions of AhR with certain signaling pathways, including the
ERα and the TGF-β pathways (25,40),
and its physiological role in regulating a number of cellular
processes related to cancer development, including cell
proliferation, the cell cycle, cell migration, pluripotency and
stemness (40). AhR expression is
upregulated in multiple types of cancer, including breast, lung,
liver, stomach, head, neck, cervical and ovarian cancer (41–45),
and its expression in these types of cancer is associated with the
stage of the disease (44,45). Research has demonstrated that AhR
mediates either pro- or anticancer activities in breast cancer
cells, with conflicting evidence linking AhR to breast cancer
progression or inhibition (15,46–48).
A number of structural AhR ligands, such as aminoflavone (AF) and
tranilast, can inhibit various aspects of breast carcinogenesis
(15). Conversely, AhR ligands
such as TCDD have also been reported to enhance the growth and
development of breast cancer (46–48).
In the present review, the roles of AhR and its
ligands as breast cancer inhibitors and promoters in vitro
and in vivo are summarized. The potential role of AhR as a
novel target for breast cancer therapy is also evaluated.
Breast cancer is a heterogeneous disease with
different molecular subtypes, and the molecular type of breast
cancer is closely related to prognosis (49,50).
Breast cancer is classified into three types on the basis of the
expression of specific hormone and growth factor receptors: Hormone
receptor (HR)-positive breast cancer, HER-2-positive breast cancer
and triple-negative breast cancer (TNBC; HR-negative and HER-2
negative) (49–52). HRs include ER and progesterone
receptor (PR).
Current treatments for breast cancer include
surgery, chemotherapy, radiotherapy, endocrine therapy and targeted
therapy. The treatment regimen depends on the tumor subtype, the
expression of HRs and HER-2, and whether the tumor is non-invasive
(carcinoma in situ) or invasive (4,49,50).
The choice of chemotherapy and endocrine therapy depends on the
presence of ER, PR and HER-2. HER-2-positive breast cancer can
often be successfully treated with trastuzumab, pertuzumab and
lapatinib (53). The most common
form of endocrine therapy for HR-positive cancer is selective ER
modulators (SERMs), such as tamoxifen and aromatase inhibitors,
both of which can inhibit estrogen biosynthesis (54). Aromatase inhibitors can improve
cancer-associated outcomes in the management of HR-positive breast
cancer, which may reduce the incidence of new primary breast cancer
but have less of an effect on more severe distant recurrences
(55). These drugs are usually
administered with CDK4/6 inhibitors to increase the sensitivity of
HR-positive and HER-2 negative metastatic breast tumors to
chemotherapy (56).
ERα is a transcription factor that is activated in
>70% of patients with breast cancer (68,69).
Tamoxifen, an antagonist of ERα, is the first-line treatment for
HR-positive breast cancer. It inhibits the activity of ERα, thereby
interfering with aberrant ERα transcriptional activity and
prolonging patient survival (70,71).
AhR is expressed in ER-positive and -negative breast cancer cells
(58,59). Certain studies have reported that
the AhR target gene CYP1A1 is activated by TCDD only in ER-positive
breast cancer cells (24,25,72).
TCDD is a well-studied environmental pollutant, an effective
immunosuppressant and one of the most potent exogenous agonists of
AhR (9,11,73,74).
Transfection into MDA-MB-231 cells with an ERα overexpression
vector confers AhR ligand sensitivity, whereas ERα knockdown in
MCF-7 cells confers resistance to the same ligand (72,75).
Therefore, ER expression may affect the activity of AhR in breast
cancer. Additionally, several studies on the anticancer effects of
AhR ligands have reported that the crosstalk between the AhR
pathway and ERα can influence the selectivity and resistance of
different molecular subtypes of breast cancer cells, such as MCF-7
and MDA-MB-231 cells, to AhR ligands (25,75–77).
Botanical estrogens (BEs), although not estrogens,
are natural phytocompounds that bind to ER and are commonly used in
hormone replacement therapy for menopausal women (81). A study on the effects of BEs and
estradiol (E2) on liver cells and ER-positive breast cancer cells
reported that both treatments cause the upregulation of ERα
activity and enhance the proliferation of breast cancer cells,
whilst E2 has no significant effect on the stimulation of AhR
(82). Additionally, it has been
reported that BEs act via the AhR pathway to bind to xenobiotic
response elements and upregulate CYP1A1 and CYP1B1, whereas E2 only
acts through ER (82). This
indicates that the crosstalk between AhR and ER is ligand- and
cell-specific.
Among the genetic factors that drive breast cancer,
the BRCA1 and BRCA2 tumor suppressor genes serve an important role
in breast cancer susceptibility (27,83).
BRCA proteins are involved in cell cycle progression, apoptosis,
DNA repair and transcription (84). BRCA1 interacts with the estrogen
pathway at the transcriptional and post-transcriptional levels to
limit the effects of estrogen on the promotion of mammary gland
growth. BRCA-regulated transcription occurs via protein-protein
interactions, the most important of which is via a complex
formation with ERα, leading to the transactivation of ERs (27,85).
The absence of this control, through a BRCA1 gene mutation, is a
well-known risk factor for TNBC development (84).
In ER-positive breast cancer cells, BRCA1 has been
associated with the AhR pathway. A study reported that upon ligand
activation, BRCA1 was recruited to the promoter regions of CYP1A1
and CYP1B1, together with ARNT and AhR. However, this was not
observed in ER-negative cells, suggesting an association between ER
and BRCA1 presence (27,86,87).
In the mammary glands, BRCA1 limits aromatase expression, and thus
estrogen production, and AhR ligand-induced BRCA1 inhibition
results in the increase of aromatase and E2 in tumor cells, thereby
maintaining cell proliferation (84,85).
In breast cancer cells treated with AhR agonists, activation of the
aromatase gene and an increase in E2 production have been reported
(88). Previous studies have
reported that AhR inhibits ER-dependent signaling by recruiting the
proteasome complex (79,89). Furthermore, BRCA1 activates the
ESR1 gene, which encodes ERα (90). Therefore, the paradoxical effects
of activated AhR on the inhibition of ERα and the increased
expression of E2 induced by activated AhR may be associated with
the inhibition of BRCA1 by AhR.
The anticancer effects of the AhR agonist TCDD and
structurally related HAHs in breast cancer in vivo and in
vitro models are summarized in Table II. In one study, seven ER-negative
breast cancer cell lines were treated with six ligands, including
TCDD and 6-methyl-1,3,8-trichlorodibenzofuran (MCDF), and it was
reported that these ligands inhibited the proliferation of
ER-negative breast cancer cells (91). Other studies reported that TCDD
inhibited the invasion of different types of breast cancer cell
lines, including ER-positive (MCF-7 and ZR75), ER-negative
(MDA-MB-231) and HER-2-positive (BT474 and SKBR3) breast cancer
cells (92–94). In a study of AhR regulation of cell
cycle progression in human breast cancer cells, disruption of the
interaction of AhR with CDK4 by TCDD inhibited cell cycle
progression in MCF-7 and MDA-MB-231 cells (95). In another study of 4T1 murine
breast cancer cells in a syngeneic mouse model, TCDD was reported
to inhibit lung metastasis of the primary tumor but did not impact
primary tumor growth (96). MCDF,
a partial AhR antagonist, has been reported to inhibit CYP1A1
induction by TCDD in cell culture (97). Zhang et al (92) reported that MCDF inhibited the
proliferation and invasion of HER-2-positive (BT474) and
ER-negative (MDA-MB-231) cells and inhibited lung metastasis in an
athymic nude mouse xenograft model bearing tumors from MDA-MB-231
cells. Additionally, MCDF has been reported to inhibit tumor growth
in an athymic nude mouse xenograft model bearing tumors from
MDA-MB-468 cells (91). These
results obtained using TCDD and structurally related HAHs as AhR
ligands suggest that these compounds exhibit anticancer effects in
breast cancer.
Several potential AhR ligands have been identified
and designed. Some of these compounds are categorized as selective
AhR modulators (105), exhibiting
low to medium affinity to AhR. These ligands activate AhR in both
genomic and non-genomic pathways to influence breast cancer
tumorigenesis and metastasis (15)
(Table IV).
Aminoflavone (AF) has been reported to potently
inhibit the proliferation of ER-positive MCF-7 cells (106,107), and it has been clinically tested
in patients with breast cancer (108). Mechanistic studies reported that
AF activated the AhR pathway and induced the expression of CYP1A1
and CYP1A2 (109,110), forming metabolites that
covalently bonded to DNA. These metabolites inhibited DNA synthesis
by inducing S-phase arrest and phosphorylation of H2AX (a
replication dependent histone), leading to DNA double-strand breaks
and ultimately cytotoxicity (77,111,112). Previous studies have reported
that AF inhibits α6-integrin expression (113–115). Upregulation of this cell adhesion
molecule is associated with tumor-initiating cell proliferation,
malignant breast cancer progression and poor prognosis (115). Furthermore, α6-integrin
upregulation is associated with radiotherapy resistance and
tamoxifen resistance in breast cancer (114,116). In tamoxifen-resistant MCF-7 and
BT474 cells, AF has been reported to decrease α6-integrin
expression, inhibit α6-integrin-Src-AKT signaling and inhibit
tamoxifen resistance of the ER-positive cells (114). A study reported that β-NF, a
strong inducer of CYP1A1, had antitumor activity in vitro
against ER-positive (MCF-7) (117). A study reported that β-NF
mediates cell cycle arrest in ER-positive breast cancer cells via
AhR-dependent regulation of PI3K/AKT and MAPK/ERK signaling,
leading to cellular senescence (118). This inhibition of proliferation
was not observed in MDA-MB-231 cells and was reported to be
AhR-dependent (118).
Furthermore, a report identified 5,6,7,8-tetrahydrocarcinolin-5-ol
(NK150460) as a noncompetitive inhibitor of E2-dependent
transcriptional regulation for the potential treatment of
ER-positive breast cancer (119).
Simultaneous treatment of MCF-7 cells with NK150460 and AhR
antagonists demonstrated that inhibition of ER transcriptional
regulation by NK150460 was mediated by AhR pathway modulation and
CYP1A1 induction. In addition, the study also reported that
NK150460 not only inhibited the proliferation of several
ER-positive breast cancer cell lines such as MCF-7 and T47D cells,
but also some ER-negative cell lines such as MDA-MB-453, MDA-MB-468
and SKBR3 cells (119). Another
study reported that (Z)-2-(3,4-dichlorophenyl)-3-(1H-pyrrol-2-yl)
acrylonitrile (ANI-7), a member of the acrylonitrile family,
exhibited good cytotoxic activity (120). This compound inhibited the
proliferation of different breast cancer cell lines, including
ER-positive (MCF-7) breast cancer cells, TNBC (MDA-MB-231) cells
and HER-2-positive (SKBR3) breast cancer cells (121). MDA-MB-468 cells treated with
ANI-7 exhibited S phase and G2 + M phase cell cycle
arrest, and this effect was mediated by the AhR pathway and
specifically increased CYP1A1 expression levels (121).
Several studies have assessed the repurposing of
drugs to identify novel compounds to target the AhR pathway.
Consequently, drugs with agonistic activities for AhR have been
identified (22,122). These drugs included the
antiosteoporosis drug raloxifene (58), the proton pump inhibitor omeprazole
(123) and the antiallergen drug
tranilast (124,125).
Raloxifene is a selective ER-targeted drug that is a
second generation SERM and has been approved for the prevention of
osteoporosis in postmenopausal women (54), to whom it is frequently
administered. It has been reported to reduce the risk of breast
cancer, and it has been reported to have high efficacy, comparable
to that of tamoxifen (126–129). Raloxifene exhibits estrogenic
properties at low concentrations, and in vivo studies have
reported that the administration of 1–20 mg/kg/day raloxifene
inhibits mammary tumor growth in rats (130,131). In a study that screened novel
activators of the AhR pathway, raloxifene was reported to induce
AhR nuclear localization in MDA-MB-231 (ER-negative) and Hepa1
cells at levels similar to that of TCDD and induce apoptosis in
ER-negative breast cancer cells in an AhR-dependent manner
(58). In a previous study, the
raloxifene analog Y134, which serves as an AhR ligand, induced
apoptosis in TNBC (MDA-MB-231 and MDA-MB-436) cells in an
AhR-dependent manner, and also inhibited the proliferation of
ER-positive (MCF-7, T47D) breast cancer cells and ER-negative
(MDA-MB-231) breast cancer cells (132,133). The study also reported a low
toxicity profile in a zebrafish embryo model (133). As aforementioned, SERMs, such as
raloxifene and aromatase inhibitors, are currently also used to
treat osteoporosis; however, this does not interfere with the role
of SERMs as cancer drugs (54,134). Thus, there is potential for the
use of raloxifene and Y134 via the AhR pathway for the treatment of
breast cancer.
The proton pump inhibitor omeprazole is used
clinically to primarily treat peptic ulcers. A number of studies
have reported that omeprazole acts on the AhR pathway (22,123,135), while in liver and pancreatic
cancer cells, it does not bind directly to AhR but activates it
through a nongenomic pathway (14,136,137). Omeprazole, identified as an AhR
activator and inducer of CYP1A1, promotes the expression of
AhR-induced DREs (22). The
propensity of omeprazole to displace TCDD has additionally been
demonstrated by AhR competitive ligand binding experiments
(22). Another study reported the
upregulation of CYP1A1 via the AhR pathway in omeprazole-treated
BT474 and MDA-MB-468 cells (125). Another study by the same group
reported that omeprazole inhibited the lung metastasis of
MDA-MB-231 cells in athymic nude mice (138). Treatment of MDA-MB-231 cells with
omeprazole in vitro inhibited cell migration and invasion by
upregulating CYP1A1 and downregulating C-X-C motif chemokine
receptor 4 via the AhR pathway (138). Omeprazole is the most commonly
used drug in digestive diseases and its good overall safety
profile, combined with its inhibition of breast cancer invasion and
metastasis via the AhR pathway, suggests that it may be a promising
targeted breast cancer drug (15,125,138).
The anti-allergic drug tranilast is commonly used to
treat bronchial asthma and allergic rhinitis (139,140). Its AhR-inducing activity was
first revealed in a study that reported its inhibition of the
activity of breast CSCs. The study also reported that tranilast was
effective in vivo, as it inhibited lung metastasis in mice
injected with triple-negative (MDA-MB-231) mitoxantrone-selected
cells (124). AhR knockdown or
treatment with the AhR antagonist α-naphthoflavone (α-NF)
completely abolished the anti-CSC activity of tranilast (124). CSCs are a type of pluripotent
cell that express stem cell marker genes, such as the OCT4 and ALDH
genes, and exhibit self-renewal ability, making them immortal
(141). Chakrabarti et al
(142) reported that tranilast
has no cytotoxicity on 4T1 cells (an estrogen-independent mouse
breast cancer cell) and inhibited the proliferation of certain
breast cancer cells, such as MDA-MB-231 and MCF-7 cells, in
vitro. The study also reported that tranilast inhibited tumor
growth and lung metastasis in vivo, while tranilast
inhibited EMT and cell cycle progression of 4T1 cells through the
TGF-β signaling pathway in vitro. In another study,
tranilast inhibited the proliferation, migration and colony
formation, and stimulated apoptosis of HER-2-positive (BT474) and
triple-negative (MDA-MB-231) cells. BT474 cells were more
responsive to treatment with tranilast than MDA-MB-231 cells
(143). Following treatment with
tranilast, the expression of CYP1A1 in BT474 cells was induced,
whereas CYP1B1 expression was induced in MDA-MB-468 cells (125). CSCs serve key roles in tumor
metastasis, and AhR may be a potential target for the inhibition of
CSCs (41,124). Thus, the use of tranilast for the
treatment of breast cancer requires further evaluation.
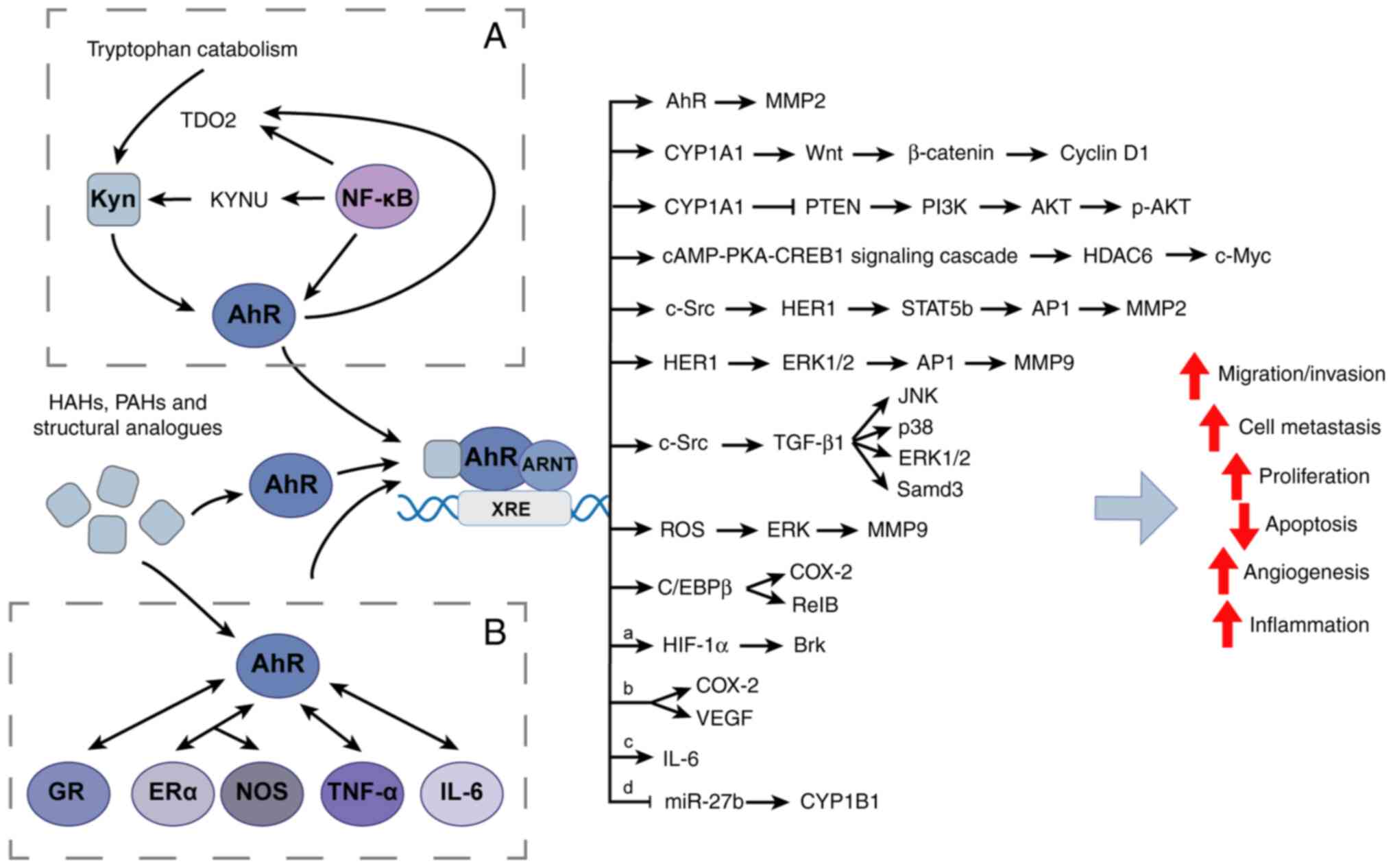
In summary, structurally diverse AhR ligands have
been reported to inhibit breast carcinogenesis in multiple breast
cancer cell lines and xenograft models (Table II, Table III, Table IV). AhR ligands have distinct
actions that may be governed by different mechanisms (Figs. 1 and 2), depending on the ligand structure and
cell context. For example, the AhR ligand mediated antitumor effect
is associated with the TGF-β signaling pathway or PI3K/AKT
signaling pathway or MAPK/ERK signaling pathway. However, AhR
ligand mediated pro-cancer activity is associated with the
Wnt/β-catenin signaling pathway or PTEN-PI3K/AKT signaling pathway
or several molecules such as the glucocorticoid receptor (GR). The
binding affinity of AhR for the same AhR ligand varies among
species, likely due to species-specific biochemical and
physiological properties of AhR resulting from differences in the
amino acid sequence of the ligand-binding domain (145). Furthermore, both AhR inhibitors
and agonists may lead to similar outcomes if the inhibitors block
signaling pathways driven by the endogenous ligands, whereas the
exogenous ligands drive different pathways, effectively ‘diverting’
the signaling (146).
Although AhR ligands of different structures
exhibit anticancer effects, the expression and function of AhR in
breast cancer cells are variable (Fig.
2). Several studies have reported that exogenous AhR ligands,
such as PAHs and HAHs, act as AhR agonists to exert
cancer-promoting effects in breast cancer (Table V). These ligands, which exhibit AhR
agonistic activity, maintain or even promote malignant
transformation phenotypes in breast cancer cells and xenograft
models by activating the AhR pathway (48,62,102,117,147–164). The ligands exert numerous
effects, such as enhancing the migratory and invasive capacity of
breast cancer cells, inhibiting apoptosis, stimulating CSC
generation, promoting angiogenesis and inducing inflammatory
responses.
Numerous environmental toxicants, such as TCDD,
butyl benzyl phthalate (BBP), di-n-butyl phthalate (DBP),
hexachlorobenzene (HCB) and benzo[a]pyrene (B[a]P) enhance cell
motility by activating AhR, which promotes cell migration and organ
invasion (48,94,149,151,153–156,161–163). Most of these environmental
toxicants serve as AhR agonists in different breast cancer cell
lines, including ER-positive (T47D, MCF-7 and ZR-75), ER-negative
(MDA-MB-231, MDA-MB-436, MCF-10A, SUM149 and Hs578T) and
HER-2-positive (SKBR3) cells. However, the use of AhR antagonists,
AhR silencing or AhR knockout reversed this effect (48,153,165–167). Among them, the effects of AhR
antagonists used in a number of studies are listed in Table VI. These synthetic antagonists
suppress the effect of AhR activation on breast cancer cells
(48,124,149,150,152,153,157,165–167). In a breast cancer cell xenograft
zebrafish model, AhR knockdown or AhR antagonist (CH223191)
impaired cell invasion and migration, and suppressed metastasis of
TNBC and IBC cells by decreasing the expression of
invasion-associated genes and increasing the expression of
E-cadherin (48). However, AhR
agonists may also exhibit anti-tumorigenic effects, for example,
TCDD inhibited the formation of irregular colonies of TNBC cells,
including SUM149, Hs578T and BP1 cells, as presented in Table V (48). In another study, MCF-7 cells were
co-treated with TCDD and mono 2-ethylhexyl phosphate (MEHP). MEHP
was reported to be a potential AhR agonist, and MEHP and TCDD alone
both induced cell migration and invasion. The promotion was
partially dependent on AhR, and this effect mediated by MEHP may be
related to the AhR-MMP2 pathway (153). Another study also reported that
following the co-treatment with MEPH and TCDD, MEHP antagonized
TCDD to reduce AhR-mediated CYP1A1 expression and inhibit the
migration and invasion of in MCF-7 cells (Table V) (149).
Several studies have reported that exogenous
ligands that activate AhR, such as TCDD, suppress apoptosis induced
by stimuli, including chemotherapeutic drugs in ER-positive (MCF-7
and T47D), ER-negative (MDA-MB-231, Hs578T and MCF-10A) and HER-2
positive (SKBR3) cells (65,150,157,170). When the AhR pathway was blocked
using AhR silencing (RNA interference), AhR knockout cell lines or
AhR antagonists (CH223191 or α-NF or 3′ methoxy-4′nitroflavone), an
increase in cell death was observed (150,157). Treatment with an endogenous AhR
ligand (Kyn) inhibited anoikis (a type of epithelial cell
programmed death) in ER-negative (BT549 and SUM159) cells in forced
suspension culture (Table III),
whereas AhR knockdown or AhR inhibitor (CH223191) treatment
promoted anoikis (Table VI)
(166). Similarly, Kyn inhibited
apoptosis in ER-negative breast cancer cells (150). Goode et al (65) reported that AhR knockout in athymic
nude mouse xenograft models reduced tumor growth by increasing
apoptosis. However, the exact biological mechanism linking the
activation of AhR and the reduction of apoptosis remains unclear.
Bekki et al (150)
proposed that TCDD induces the expression of inflammatory genes,
such as the genes encoding cyclooxygenase 2 (COX-2) and NF-κB
subunit RelB, to prevent apoptosis. Another possible mechanism,
proposed by Anderson et al (170), is that exposure of TNBC cells to
chemotherapeutic agents, such as paclitaxel, induces the expression
of breast tumor kinase via the AhR/GR/hypoxia-inducible factor
signaling axis, which is involved in the inhibition of
apoptosis.
The environmental pollutants TCDD, HCB and
chlorpyrifos (CPF) promote angiogenesis in breast cancer models by
activating AhR (150,151,160). TCDD induces expression of the
inflammatory marker COX-2 in mammalian cells through an alternative
AhR pathway involving CCAAT/enhancer binding protein β (150). This promotes angiogenesis by
upregulating vascular endothelial growth factor (VEGF) (171,172). Pontillo et al (151) reported that HCB stimulated
angiogenesis and increased VEGF expression in a breast cancer
xenograft mouse model. HCB also induced neovasculogenesis of the
HMEC-1 human microvascular endothelial cell line in vitro,
enhanced the expression of VEGF-receptor 2 and activated the
downstream pathways p38 and ERK1/2 (Table V), whereas an AhR inhibitor (α-NF)
suppressed these effects (Table
VI) (151). Another study
reported that VEGF-A expression, induced by HCB and CPF, was
mediated by ER and nitric oxide (NO), whilst the increase of COX-2
was mediated via AhR and the NO pathway in MCF-7 cells. In
vivo, HCB and CPF stimulated the angiogenic switch (160).
A number of studies have reported that activation
of AhR leads to increased expression of numerous inflammatory
markers, including COX-2, IL-6 and IL-8, in numerous tumors and
cancer types (46,62,150,152,160), including breast cancer (46,173). Furthermore, a more aggressive,
chemoresistant breast cancer phenotype in ER-positive cells can
reside in the inflammatory microenvironment (174,175). Epidemiological evidence indicates
that IBC has a poor prognosis and that patients with IBC have a
shortened survival compared with patients with non-IBC (176,177). The NF-κB pathway is a key pathway
linking AhR activation to cellular inflammation (28,148,152,178). NF-κB is a hub molecular in a
number of inflammatory pathways. Kim et al (179) reported that the activity of NF-κB
(p65/p50) increased during neoplastic transformation in murine
normal mammary cells treated with DMBA and in human non-transformed
mammary cells treated with DMBA or B[a]P. When non-transformed
breast cells MCF-10F (ER-negative, PR-negative and HER-2 negative)
were treated with DMBA or B[a]P, the AhR interacted with NF-κB
subunit RelA to activate transcription of the proto-oncogene and
expression of the protein c-Myc, a master regulator of cell
proliferation and neoplastic transformation (180). Furthermore, in DMBA-induced
murine mammary tumors, high expression of AhR, c-Myc and cyclin D1
was associated with NF-κB and Wnt signaling pathways (181). Another inflammatory marker, IL-6,
which is regulated by NF-κB, is involved in the immune function,
hematopoiesis, acute phase response and inflammatory response
(182). In mammary tissue, IL-6,
along with its downstream transcription factor, STAT3, has been
reported to stimulate cell proliferation and migration during
ontogeny, and be involved in gland remodeling during aging
(173,183). A previous study reported that
IL-6 enhances B[a]P and 2-amino-1-methyl-6-phenylimidazo [4,5-b]
pyridine-induced micronuclei (MN) formation in breast cancer cells
via the miR-27b-CYP1B1 signaling pathway, which leads to DNA damage
(159).
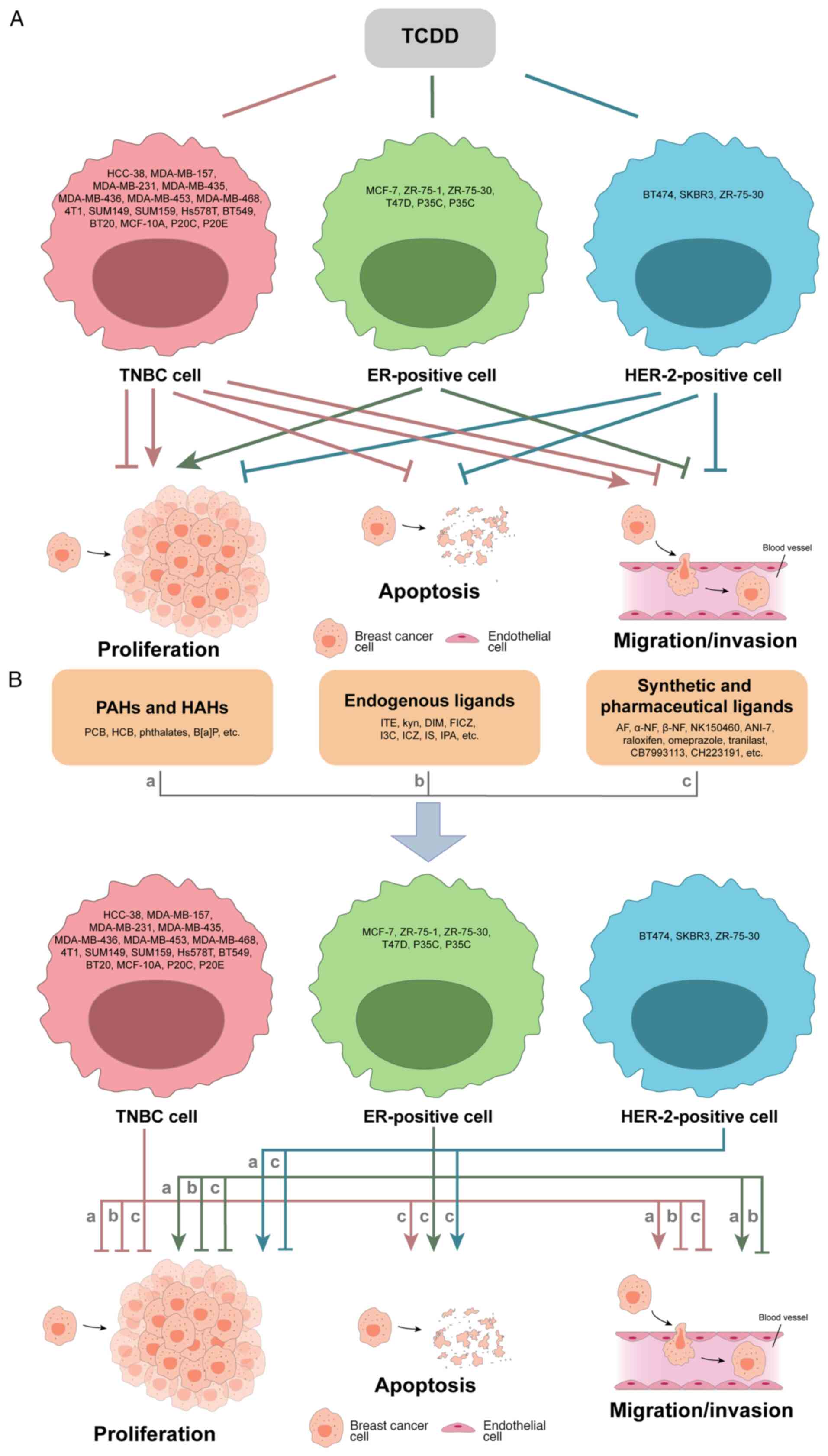
AhR is a receptor with complex functions, as its
activation is ligand- and cell-dependent. AhR is not only bound by
multiple ligands, but it can also interact and function with
numerous molecules. A number of AhR agonists also target other
molecules; therefore, ligand-induced activation of AhR leads to the
altered expression of hundreds of genes. Additionally, breast
cancer cells of the same molecular subtype have different
regulatory effects and mechanisms associated with the same AhR
ligand (Fig. 3). Moreover, breast
cancer has different molecular subtypes, which makes the studies
more complicated. Most AhR ligands demonstrate tumor-suppressive
characteristics; however, under specific circumstances, different
types of ligands also show different regulation patterns. For
example, the exogenous AhR ligand TCDD is involved in the promotion
of mammosphere formation, and inhibition of migration/invasion and
cell cycle progression in ER-positive (MCF-7) breast cancer cells,
whilst it inhibits proliferation and invasion in HER-2-positive
(BT474) breast cancer cells (Tables
II and V). Additionally, TCDD
exhibits dual regulatory roles in proliferation and
migration/invasion in TNBC cells (Fig.
3A). Numerous endogenous, synthetic and medicinal AhR ligands
also exhibit tumor-suppressive characteristics (Fig. 3B). All three categories of AhR
ligands exhibit mainly inhibitory roles in TNBC cell proliferation
and promote apoptosis in the three types of breast cancer, while
their regulatory roles in cell migration/invasion are inconsistent.
AhR mediates either pro-cancer or anticancer activities in breast
cancer cells (Fig. 4); however,
the underlying mechanisms are still unclear. AhR regulation in
cancer appears to be dependent on the types and levels of AhR
ligands. However, quantifying each of these ligands in patients
with different types of cancer, including breast cancer, may be
challenging as patients may be exposed to a number of AhR ligands
over a prolonged period (66). For
example, the half-life of the environmental carcinogen TCDD in
humans is 7–10 years (184).
Thus, directly targeting AhR rather than AhR ligands may be a more
feasible option for cancer treatment. However, a number of AhR
agonists are too toxic at high levels to be used clinically or have
not been tested in humans, necessitating the development and
exploration of non-toxic, clinically applicable alternatives, such
as omeprazole and tranilast.
Current research has demonstrated that AhR and its
ligands serve important roles in breast cancer progression and
metastasis, possibly via the regulation of apoptosis, migration,
invasion, inflammation and angiogenesis. Several synthetic AhR
ligands and widely used drugs with affinity to AhR exhibit
selectivity for breast cancer cells with different molecular types,
and regulate breast cancer cell functions. This suggests their
potential as novel strategies for breast cancer therapy.
Not applicable.
The present study was supported by the Characteristic Innovation
Projects of Universities in Guangdong Province (grant no.
2021KTSCX037) and Zhanjiang Science and Technology Project (grant
nos. 2020A06004, 2021A05056 and 2022A01190).
Not applicable.
CC, WL and XS designed the study. CC completed the
first draft of this manuscript. CC, YZ, ZL and ZW collected and
analyzed the data and revised the manuscript. Data authentication
is not applicable. All authors have read and approved the final
version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller KD, Nogueira L, Devasia T, Mariotto
AB, Yabroff KR, Jemal A, Kramer J and Siegel RL: Cancer treatment
and survivorship statistics, 2022. CA Cancer J Clin. 72:409–436.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Verrill M: Metastatic disease of the
breast and local recurrence. Surgery (Oxford). 37:181–185. 2019.
View Article : Google Scholar
|
|
5
|
Flatley MJ and Dodwell DJ: Adjuvant
treatment for breast cancer. Surgery (Oxford). 34:43–46. 2016.
View Article : Google Scholar
|
|
6
|
Burstein HJ, Curigliano G, Thürlimann B,
Weber WP, Poortmans P, Regan MM, Senn HJ, Winer EP and Gnant M;
Panelists of the St Gallen Consensus Conference, : Customizing
local and systemic therapies for women with early breast cancer:
The St. Gallen international consensus guidelines for treatment of
early breast cancer 2021. Ann Oncol. 32:1216–1235. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harbeck N and Gnant M: Breast cancer.
Lancet. 389:1134–1150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pondé NF, Zardavas D and Piccart M:
Progress in adjuvant systemic therapy for breast cancer. Nat Rev
Clin Oncol. 16:27–44. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Poland A, Glover E and Kende AS:
Stereospecific, high affinity binding of
2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence
that the binding species is receptor for induction of aryl
hydrocarbon hydroxylase. J Biol Chem. 251:4936–4946. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kung T, Murphy KA and White LA: The aryl
hydrocarbon receptor (AhR) pathway as a regulatory pathway for cell
adhesion and matrix metabolism. Biochem Pharmacol. 77:536–546.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murray IA, Patterson AD and Perdew GH:
Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat
Rev Cancer. 14:801–814. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bersten DC, Sullivan AE, Peet DJ and
Whitelaw ML: bHLH-PAS proteins in cancer. Nat Rev Cancer.
13:827–841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Opitz CA, Litzenburger UM, Sahm F, Ott M,
Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller
M, et al: An endogenous tumour-promoting ligand of the human aryl
hydrocarbon receptor. Nature. 478:197–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Denison MS and Nagy SR: Activation of the
aryl hydrocarbon receptor by structurally diverse exogenous and
endogenous chemicals. Annu Rev Pharmacol Toxicol. 43:309–334. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baker JR, Sakoff JA and McCluskey A: The
aryl hydrocarbon receptor (AhR) as a breast cancer drug target. Med
Res Rev. 40:972–1001. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rothhammer V and Quintana FJ: The aryl
hydrocarbon receptor: An environmental sensor integrating immune
responses in health and disease. Nat Rev Immunol. 19:184–197. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stockinger B, Di Meglio P, Gialitakis M
and Duarte JH: The aryl hydrocarbon receptor: Multitasking in the
immune system. Annu Rev Immunol. 32:403–432. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Denison MS, Soshilov AA, He G, DeGroot DE
and Zhao B: Exactly the same but different: Promiscuity and
diversity in the molecular mechanisms of action of the aryl
hydrocarbon (dioxin) receptor. Toxicol Sci. 124:1–22. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mimura J, Ema M, Sogawa K and
Fujii-Kuriyama Y: Identification of a novel mechanism of regulation
of Ah (dioxin) receptor function. Genes Dev. 13:20–25. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Whitlock JP Jr: Induction of cytochrome
P4501A1. Annu Rev Pharmacol Toxicol. 39:103–125. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brauze D, Widerak M, Cwykiel J, Szyfter K
and Baer-Dubowska W: The effect of aryl hydrocarbon receptor
ligands on the expression of AhR, AhRR, ARNT, Hif1alpha, CYP1A1 and
NQO1 genes in rat liver. Toxicol Lett. 167:212–220. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu W, Sorrentino C, Denison MS, Kolaja K
and Fielden MR: Induction of cyp1a1 is a nonspecific biomarker of
aryl hydrocarbon receptor activation: Results of large scale
screening of pharmaceuticals and toxicants in vivo and in vitro.
Mol Pharmacol. 71:1475–1486. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao X, Xie C, Wang Y, Luo Y, Yagai T, Sun
D, Qin X, Krausz KW and Gonzalez FJ: The antiandrogen flutamide is
a novel aryl hydrocarbon receptor ligand that disrupts bile acid
homeostasis in mice through induction of Abcc4. Biochem Pharmacol.
119:93–104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Swedenborg E and Pongratz I: AhR and ARNT
modulate ER signaling. Toxicology. 268:132–138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tarnow P, Tralau T and Luch A: Chemical
activation of estrogen and aryl hydrocarbon receptor signaling
pathways and their interaction in toxicology and metabolism. Expert
Opin Drug Metab Toxicol. 15:219–229. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Göttel M, Le Corre L, Dumont C, Schrenk D
and Chagnon MC: Estrogen receptor α and aryl hydrocarbon receptor
cross-talk in a transfected hepatoma cell line (HepG2) exposed to
2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Rep. 1:1029–1036.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang HJ, Kim HJ, Kim SK, Barouki R, Cho
CH, Khanna KK, Rosen EM and Bae I: BRCA1 modulates xenobiotic
stress-inducible gene expression by interacting with ARNT in human
breast cancer cells. J Biol Chem. 281:14654–14662. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tian Y, Rabson AB and Gallo MA: Ah
receptor and NF-kappaB interactions: Mechanisms and physiological
implications. Chem Biol Interact. 141:97–115. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marshall NB and Kerkvliet NI: Dioxin and
immune regulation: Emerging role of aryl hydrocarbon receptor in
the generation of regulatory T cells. Ann N Y Acad Sci. 1183:25–37.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gutiérrez-Vázquez C and Quintana FJ:
Regulation of the immune response by the Aryl hydrocarbon receptor.
Immunity. 48:19–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Esser C and Rannug A: The aryl hydrocarbon
receptor in barrier organ physiology, immunology, and toxicology.
Pharmacol Rev. 67:259–279. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee JH, Wada T, Febbraio M, He J,
Matsubara T, Lee MJ, Gonzalez FJ and Xie W: A novel role for the
dioxin receptor in fatty acid metabolism and hepatic steatosis.
Gastroenterology. 139:653–663. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lahvis GP, Lindell SL, Thomas RS, McCuskey
RS, Murphy C, Glover E, Bentz M, Southard J and Bradfield CA:
Portosystemic shunting and persistent fetal vascular structures in
aryl hydrocarbon receptor-deficient mice. Proc Natl Acad Sci USA.
97:10442–10447. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang S, Shui X, He Y, Xue Y, Li J, Li G,
Lei W and Chen C: AhR expression and polymorphisms are associated
with risk of coronary arterial disease in Chinese population. Sci
Rep. 5:80222015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Neavin DR, Liu D, Ray B and Weinshilboum
RM: The role of the Aryl hydrocarbon receptor (AHR) in immune and
inflammatory diseases. Int J Mol Sci. 19:38512018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stockinger B, Shah K and Wincent E: AHR in
the intestinal microenvironment: Safeguarding barrier function. Nat
Rev Gastroenterol Hepatol. 18:559–570. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bock KW: Aryl hydrocarbon or dioxin
receptor: Biologic and toxic responses. Rev Physiol Biochem
Pharmacol. 125:1–42. 1994.PubMed/NCBI
|
|
38
|
Bradshaw TD and Bell DR: Relevance of the
aryl hydrocarbon receptor (AhR) for clinical toxicology. Clin
Toxicol (Phila). 47:632–642. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Knerr S and Schrenk D: Carcinogenicity of
2,3,7,8-tetrachlorodibenzo-p-dioxin in experimental models. Mol
Nutr Food Res. 50:897–907. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Roman ÁC, Carvajal-Gonzalez JM, Merino JM,
Mulero-Navarro S and Fernández-Salguero PM: The aryl hydrocarbon
receptor in the crossroad of signalling networks with therapeutic
value. Pharmacol Ther. 185:50–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Stanford EA, Wang Z, Novikov O, Mulas F,
Landesman-Bollag E, Monti S, Smith BW, Seldin DC, Murphy GJ and
Sherr DH: The role of the aryl hydrocarbon receptor in the
development of cells with the molecular and functional
characteristics of cancer stem-like cells. BMC Biol. 14:202016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stanford EA, Ramirez-Cardenas A, Wang Z,
Novikov O, Alamoud K, Koutrakis P, Mizgerd JP, Genco CA,
Kukuruzinska M, Monti S, et al: Role for the Aryl hydrocarbon
receptor and diverse ligands in oral squamous cell carcinoma
migration and tumorigenesis. Mol Cancer Res. 14:696–706. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu Z, Wu X, Zhang F, Han L, Bao G, He X
and Xu Z: AhR expression is increased in hepatocellular carcinoma.
J Mol Histol. 44:455–461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chang JT, Chang H, Chen PH, Lin SL and Lin
P: Requirement of aryl hydrocarbon receptor overexpression for
CYP1B1 up-regulation and cell growth in human lung adenocarcinomas.
Clin Cancer Res. 13:38–45. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Koliopanos A, Kleeff J, Xiao Y, Safe S,
Zimmermann A, Büchler MW and Friess H: Increased arylhydrocarbon
receptor expression offers a potential therapeutic target for
pancreatic cancer. Oncogene. 21:6059–6070. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Guarnieri T: Aryl hydrocarbon receptor
connects inflammation to breast cancer. Int J Mol Sci. 21:52642020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Benoit L, Jornod F, Zgheib E, Tomkiewicz
C, Koual M, Coustillet T, Barouki R, Audouze K, Vinken M and
Coumoul X: Adverse outcome pathway from activation of the AhR to
breast cancer-related death. Environ Int. 165:1073232022.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Narasimhan S, Stanford Zulick E, Novikov
O, Parks AJ, Schlezinger JJ, Wang Z, Laroche F, Feng H, Mulas F,
Monti S and Sherr DH: Towards resolving the pro- and anti-tumor
effects of the Aryl hydrocarbon receptor. Int J Mol Sci.
19:13882018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Waks AG and Winer EP: Breast cancer
treatment: A review. JAMA. 321:288–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Loibl S, Poortmans P, Morrow M, Denkert C
and Curigliano G: Breast cancer. Lancet. 397:1750–1769. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yu F, Quan F, Xu J, Zhang Y, Xie Y, Zhang
J, Lan Y, Yuan H, Zhang H, Cheng S, et al: Breast cancer prognosis
signature: Linking risk stratification to disease subtypes. Brief
Bioinform. 20:2130–2140. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Eroles P, Bosch A, Pérez-Fidalgo JA and
Lluch A: Molecular biology in breast cancer: Intrinsic subtypes and
signaling pathways. Cancer Treat Rev. 38:698–707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Loibl S and Gianni L: HER2-positive breast
cancer. Lancet. 389:2415–2429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Patel HK and Bihani T: Selective estrogen
receptor modulators (SERMs) and selective estrogen receptor
degraders (SERDs) in cancer treatment. Pharmacol Ther. 186:1–24.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Goodwin PJ: Extended aromatase inhibitors
in hormone-receptor-positive breast cancer. N Engl J Med.
385:462–463. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Goel S, Bergholz JS and Zhao JJ: Targeting
CDK4 and CDK6 in cancer. Nat Rev Cancer. 22:356–372. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hushka LJ, Williams JS and Greenlee WF:
Characterization of 2,3,7,8-tetrachlorodibenzofuran-dependent
suppression and AH receptor pathway gene expression in the
developing mouse mammary gland. Toxicol Appl Pharmacol.
152:200–210. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
O'Donnell EF, Koch DC, Bisson WH, Jang HS
and Kolluri SK: The aryl hydrocarbon receptor mediates
raloxifene-induced apoptosis in estrogen receptor-negative hepatoma
and breast cancer cells. Cell Death Dis. 5:e10382014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Romagnolo DF, Papoutsis AJ, Laukaitis C
and Selmin OI: Constitutive expression of AhR and BRCA-1 promoter
CpG hypermethylation as biomarkers of ERα-negative breast
tumorigenesis. BMC Cancer. 15:10262015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mohamed HT, Gadalla R, El-Husseiny N,
Hassan H, Wang Z, Ibrahim SA, El-Shinawi M, Sherr DH and Mohamed
MM: Inflammatory breast cancer: Activation of the aryl hydrocarbon
receptor and its target CYP1B1 correlates closely with
Wnt5a/b-β-catenin signalling, the stem cell phenotype and disease
progression. J Adv Res. 16:75–86. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Jeschke U, Zhang X, Kuhn C, Jalaguier S,
Colinge J, Pfender K, Mayr D, Ditsch N, Harbeck N, Mahner S, et al:
The prognostic impact of the Aryl hydrocarbon receptor (AhR) in
primary breast cancer depends on the lymph node status. Int J Mol
Sci. 20:10162019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Vacher S, Castagnet P, Chemlali W,
Lallemand F, Meseure D, Pocard M, Bieche I and Perrot-Applanat M:
High AHR expression in breast tumors correlates with expression of
genes from several signaling pathways namely inflammation and
endogenous tryptophan metabolism. PLoS One. 13:e01906192018.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Tryggvadottir H, Sandén E, Björner S,
Bressan A, Ygland Rödström M, Khazaei S, Edwards DP, Nodin B,
Jirström K, Isaksson K, et al: The prognostic impact of
intratumoral Aryl hydrocarbon receptor in primary breast cancer
depends on the type of endocrine therapy: A population-based cohort
study. Front Oncol. 11:6427682021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Goode G, Pratap S and Eltom SE: Depletion
of the aryl hydrocarbon receptor in MDA-MB-231 human breast cancer
cells altered the expression of genes in key regulatory pathways of
cancer. PLoS One. 9:e1001032014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Goode GD, Ballard BR, Manning HC, Freeman
ML, Kang Y and Eltom SE: Knockdown of aberrantly upregulated aryl
hydrocarbon receptor reduces tumor growth and metastasis of
MDA-MB-231 human breast cancer cell line. Int J Cancer.
133:2769–2780. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Koual M, Tomkiewicz C, Cano-Sancho G,
Antignac JP, Bats AS and Coumoul X: Environmental chemicals, breast
cancer progression and drug resistance. Environ Health. 19:1172020.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li Y, Qin HZ, Song Q, Wu XD and Zhu JH:
Lack of association between the aryl hydrocarbon receptor rs2066853
polymorphism and breast cancer: A meta-analysis on Ahr polymorphism
and breast cancer. Genet Mol Res. 14:16162–16168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ali S and Coombes RC: Endocrine-responsive
breast cancer and strategies for combating resistance. Nat Rev
Cancer. 2:101–112. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
69
|
Xu Y, Huangyang P, Wang Y, Xue L,
Devericks E, Nguyen HG, Yu X, Oses-Prieto JA, Burlingame AL,
Miglani S, et al: ERα is an RNA-binding protein sustaining tumor
cell survival and drug resistance. Cell. 184:5215–5229.e17. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shiau AK, Barstad D, Loria PM, Cheng L,
Kushner PJ, Agard DA and Greene GL: The structural basis of
estrogen receptor/coactivator recognition and the antagonism of
this interaction by tamoxifen. Cell. 95:927–937. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Metcalfe C, Friedman LS and Hager JH:
Hormone-targeted therapy and resistance. Annu Rev Cancer Biol.
2:291–312. 2018. View Article : Google Scholar
|
|
72
|
Safe S and Wormke M: Inhibitory aryl
hydrocarbon receptor-estrogen receptor alpha cross-talk and
mechanisms of action. Chem Res Toxicol. 16:807–816. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Niwa A, Kumaki K, Nebert DW and Poland AP:
Genetic expression of aryl hydrocarbon hydroxylase activity in the
mouse. Distinction between the ‘responsive’ homozygote and
heterozygote at the Ah locus. Arch Biochem Biophys. 166:559–564.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hankinson O: Single-step selection of
clones of a mouse hepatoma line deficient in aryl hydrocarbon
hydroxylase. Proc Natl Acad Sci USA. 76:373–376. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Stark K, Burger A, Wu J, Shelton P, Polin
L and Li J: Reactivation of estrogen receptor α by vorinostat
sensitizes mesenchymal-like triple-negative breast cancer to
aminoflavone, a ligand of the aryl hydrocarbon receptor. PLoS One.
8:e745252013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Go RE, Hwang KA and Choi KC: Cytochrome
P450 1 family and cancers. J Steroid Biochem Mol Biol. 147:24–30.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Luzzani GA, Callero MA, Kuruppu AI,
Trapani V, Flumian C, Todaro L, Bradshaw TD and Loaiza Perez AI: In
vitro antitumor effects of AHR ligands aminoflavone (AFP 464) and
benzothiazole (5F 203) in human renal carcinoma cells. J Cell
Biochem. 118:4526–4535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Brunnberg S, Pettersson K, Rydin E,
Matthews J, Hanberg A and Pongratz I: The basic
helix-loop-helix-PAS protein ARNT functions as a potent coactivator
of estrogen receptor-dependent transcription. Proc Natl Acad Sci
USA. 100:6517–6522. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ohtake F, Takeyama K, Matsumoto T,
Kitagawa H, Yamamoto Y, Nohara K, Tohyama C, Krust A, Mimura J,
Chambon P, et al: Modulation of oestrogen receptor signalling by
association with the activated dioxin receptor. Nature.
423:545–550. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kociba RJ, Keyes DG, Beyer JE, Carreon RM,
Wade CE, Dittenber DA, Kalnins RP, Frauson LE, Park CN, Barnard SD,
et al: Results of a two-year chronic toxicity and oncogenicity
study of 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats. Toxicol Appl
Pharmacol. 46:279–303. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Low Dog T: Menopause: A review of
botanical dietary supplements. Am J Med. 118 (Suppl 12B):S98–S108.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Gong P, Madak-Erdogan Z, Flaws JA, Shapiro
DJ, Katzenellenbogen JA and Katzenellenbogen BS: Estrogen
receptor-alpha and aryl hydrocarbon receptor involvement in the
actions of botanical estrogens in target cells. Mol Cell
Endocrinol. 437:190–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Moynahan ME: The cancer connection: BRCA1
and BRCA2 tumor suppression in mice and humans. Oncogene.
21:8994–9007. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Baek HJ, Kim SE, Choi EK, Kim JK, Shin DH,
Park EJ, Kim TH, Kim JY, Kim KG, Deng CX and Kim SS: Inhibition of
estrogen signaling reduces the incidence of BRCA1-associated
mammary tumor formation. Int J Biol Sci. 14:1755–1768. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wang L and Di LJ: BRCA1 and
estrogen/estrogen receptor in breast cancer: Where they interact?
Int J Biol Sci. 10:566–575. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kang HJ, Kim HJ, Cho CH, Hu Y, Li R and
Bae I: BRCA1 transcriptional activity is enhanced by interactions
between its AD1 domain and AhR. Cancer Chemother Pharmacol.
62:965–975. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Tapia T, Smalley SV, Kohen P, Muñoz A,
Solis LM, Corvalan A, Faundez P, Devoto L, Camus M, Alvarez M and
Carvallo P: Promoter hypermethylation of BRCA1 correlates with
absence of expression in hereditary breast cancer tumors.
Epigenetics. 3:157–163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Papoutsis AJ, Selmin OI, Borg JL and
Romagnolo DF: Gestational exposure to the AhR agonist
2,3,7,8-tetrachlorodibenzo-p-dioxin induces BRCA-1 promoter
hypermethylation and reduces BRCA-1 expression in mammary tissue of
rat offspring: Preventive effects of resveratrol. Mol Carcinog.
54:261–269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wormke M, Stoner M, Saville B, Walker K,
Abdelrahim M, Burghardt R and Safe S: The aryl hydrocarbon receptor
mediates degradation of estrogen receptor alpha through activation
of proteasomes. Mol Cell Biol. 23:1843–1855. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Crimini E, Repetto M, Aftimos P,
Botticelli A, Marchetti P and Curigliano G: Precision medicine in
breast cancer: From clinical trials to clinical practice. Cancer
Treat Rev. 98:1022232021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhang S, Lei P, Liu X, Li X, Walker K,
Kotha L, Rowlands C and Safe S: The aryl hydrocarbon receptor as a
target for estrogen receptor-negative breast cancer chemotherapy.
Endocr Relat Cancer. 16:835–844. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhang S, Kim K, Jin UH, Pfent C, Cao H,
Amendt B, Liu X, Wilson-Robles H and Safe S: Aryl hydrocarbon
receptor agonists induce microRNA-335 expression and inhibit lung
metastasis of estrogen receptor negative breast cancer cells. Mol
Cancer Ther. 11:108–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Hanieh H: Aryl hydrocarbon
receptor-microRNA-212/132 axis in human breast cancer suppresses
metastasis by targeting SOX4. Mol Cancer. 14:1722015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Hall JM, Barhoover MA, Kazmin D, McDonnell
DP, Greenlee WF and Thomas RS: Activation of the aryl-hydrocarbon
receptor inhibits invasive and metastatic features of human breast
cancer cells and promotes breast cancer cell differentiation. Mol
Endocrinol. 24:359–369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Barhoover MA, Hall JM, Greenlee WF and
Thomas RS: Aryl hydrocarbon receptor regulates cell cycle
progression in human breast cancer cells via a functional
interaction with cyclin-dependent kinase 4. Mol Pharmacol.
77:195–201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wang T, Wyrick KL, Meadows GG, Wills TB
and Vorderstrasse BA: Activation of the aryl hydrocarbon receptor
by TCDD inhibits mammary tumor metastasis in a syngeneic mouse
model of breast cancer. Toxicol Sci. 124:291–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Safe S and Zhang L: The role of the Aryl
hydrocarbon receptor (AhR) and its ligands in breast cancer.
Cancers (Basel). 14:55742022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ho JN, Jun W, Choue R and Lee J: I3C and
ICZ inhibit migration by suppressing the EMT process and FAK
expression in breast cancer cells. Mol Med Rep. 7:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Nguyen CH, Brenner S, Huttary N, Atanasov
AG, Dirsch VM, Chatuphonprasert W, Holzner S, Stadler S, Riha J,
Krieger S, et al: AHR/CYP1A1 interplay triggers lymphatic barrier
breaching in breast cancer spheroids by inducing 12(S)-HETE
synthesis. Hum Mol Genet. 25:5006–5016. 2016.PubMed/NCBI
|
|
100
|
Yerushalmi R, Bargil S, Ber Y, Ozlavo R,
Sivan T, Rapson Y, Pomerantz A, Tsoref D, Sharon E, Caspi O, et al:
3,3-Diindolylmethane (DIM): A nutritional intervention and its
impact on breast density in healthy BRCA carriers. A prospective
clinical trial. Carcinogenesis. 41:1395–1401. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Mobini K, Tamaddon G, Fardid R, Keshavarzi
M and Mohammadi-Bardbori A: Aryl hydrocarbon-estrogen alpha
receptor-dependent expression of miR-206, miR-27b, and miR-133a
suppress cell proliferation and migration in MCF-7 cells. J Biochem
Mol Toxicol. 33:e223042019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Piwarski SA, Thompson C, Chaudhry AR,
Denvir J, Primerano DA, Fan J and Salisbury TB: The putative
endogenous AHR ligand ITE reduces JAG1 and associated NOTCH1
signaling in triple negative breast cancer cells. Biochem
Pharmacol. 174:1138452020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Sári Z, Mikó E, Kovács T, Boratkó A,
Ujlaki G, Jankó L, Kiss B, Uray K and Bai P: Indoxylsulfate, a
metabolite of the microbiome, has cytostatic effects in breast
cancer via activation of AHR and PXR receptors and induction of
oxidative stress. Cancers (Basel). 12:29152020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Sári Z, Mikó E, Kovács T, Jankó L, Csonka
T, Lente G, Sebő É, Tóth J, Tóth D, Árkosy P, et al:
Indolepropionic acid, a metabolite of the microbiome, has
cytostatic properties in breast cancer by activating AHR and PXR
receptors and inducing oxidative stress. Cancers (Basel).
12:24112020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Safe S, Jin UH, Park H, Chapkin RS and
Jayaraman A: Aryl hydrocarbon receptor (AHR) ligands as selective
AHR modulators (SAhRMs). Int J Mol Sci. 21:66542020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Akama T, Ishida H, Shida Y, Kimura U, Gomi
K, Saito H, Fuse E, Kobayashi S, Yoda N and Kasai M: Design and
synthesis of potent antitumor 5,4′-diaminoflavone derivatives based
on metabolic considerations. J Med Chem. 40:1894–1900. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Akama T, Shida Y, Sugaya T, Ishida H, Gomi
K and Kasai M: Novel 5-aminoflavone derivatives as specific
antitumor agents in breast cancer. J Med Chem. 39:3461–3469. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Kenz S, Haas CS, Werth SC, Bohnet S and
Brabant G: High sensitivity to tolvaptan in paraneoplastic syndrome
of inappropriate ADH secretion (SIADH). Ann Oncol. 22:26962011.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Kuffel MJ, Schroeder JC, Pobst LJ, Naylor
S, Reid JM, Kaufmann SH and Ames MM: Activation of the antitumor
agent aminoflavone (NSC 686288) is mediated by induction of tumor
cell cytochrome P450 1A1/1A2. Mol Pharmacol. 62:143–153. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Loaiza-Perez AI, Kenney S, Boswell J,
Hollingshead M, Alley MC, Hose C, Ciolino HP, Yeh GC, Trepel JB,
Vistica DT and Sausville EA: Aryl hydrocarbon receptor activation
of an antitumor aminoflavone: Basis of selective toxicity for MCF-7
breast tumor cells. Mol Cancer Ther. 3:715–725. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Loaiza-Perez AI, Kenney S, Boswell J,
Hollingshead M, Hose C, Linehan WM, Worrell R, Rubinstein L,
Sausville EA and Vistica DT: Sensitivity of renal cell carcinoma to
aminoflavone: role of CYP1A1. J Urol. 171:1688–1697. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Meng LH, Shankavaram U, Chen C, Agama K,
Fu HQ, Gonzalez FJ, Weinstein J and Pommier Y: Activation of
aminoflavone (NSC 686288) by a sulfotransferase is required for the
antiproliferative effect of the drug and for induction of histone
gamma-H2AX. Cancer Res. 66:9656–9664. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Mavingire N, Campbell P, Liu T, Wooten J,
Khan S, Chen X, Matthews J, Wang C and Brantley E: Aminoflavone
upregulates putative tumor suppressor miR-125b-2-3p to inhibit
luminal A breast cancer stem cell-like properties. Precis Clin Med.
5:pbac0082022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Campbell PS, Mavingire N, Khan S, Rowland
LK, Wooten JV, Opoku-Agyeman A, Guevara A, Soto U, Cavalli F,
Loaiza-Pérez AI, et al: AhR ligand aminoflavone suppresses
α6-integrin-Src-Akt signaling to attenuate tamoxifen resistance in
breast cancer cells. J Cell Physiol. 234:108–121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Brantley E, Callero MA, Berardi DE,
Campbell P, Rowland L, Zylstra D, Amis L, Yee M, Simian M, Todaro
L, et al: AhR ligand Aminoflavone inhibits α6-integrin expression
and breast cancer sphere-initiating capacity. Cancer Lett.
376:53–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Hu T, Zhou R, Zhao Y and Wu G: Integrin
α6/Akt/Erk signaling is essential for human breast cancer
resistance to radiotherapy. Sci Rep. 6:333762016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zhao S, Kanno Y, Nakayama M, Makimura M,
Ohara S and Inouye Y: Activation of the aryl hydrocarbon receptor
represses mammosphere formation in MCF-7 cells. Cancer Lett.
317:192–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Wang C, Xu CX, Bu Y, Bottum KM and
Tischkau SA: Beta-naphthoflavone (DB06732) mediates estrogen
receptor-positive breast cancer cell cycle arrest through
AhR-dependent regulation of PI3K/AKT and MAPK/ERK signaling.
Carcinogenesis. 35:703–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Fukasawa K, Kagaya S, Maruyama S, Kuroiwa
S, Masuda K, Kameyama Y, Satoh Y, Akatsu Y, Tomura A, Nishikawa K,
et al: A novel compound, NK150460, exhibits selective antitumor
activity against breast cancer cell lines through activation of
aryl hydrocarbon receptor. Mol Cancer Ther. 14:343–354. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Tarleton M, Gilbert J, Robertson MJ,
McCluskey A and Sakoff JA: Library synthesis and cytotoxicity of a
family of 2-phenylacrylonitriles and discovery of an estrogen
dependent breast cancer lead compound†. Med Chem Commun. 2:31–37.
2011. View Article : Google Scholar
|
|
121
|
Gilbert J, De Iuliis GN, Tarleton M,
McCluskey A and Sakoff JA: (Z)-2-(3,4-Dichlorophenyl)-3-(1
H-Pyrrol-2-yl)acrylonitrile exhibits selective antitumor activity
in breast cancer cell lines via the aryl hydrocarbon receptor
pathway. Mol Pharmacol. 93:168–177. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Corsello SM, Bittker JA, Liu Z, Gould J,
McCarren P, Hirschman JE, Johnston SE, Vrcic A, Wong B, Khan M, et
al: The drug repurposing Hub: A next-generation drug library and
information resource. Nat Med. 23:405–408. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Quattrochi LC and Tukey RH: Nuclear uptake
of the Ah (dioxin) receptor in response to omeprazole:
Transcriptional activation of the human CYP1A1 gene. Mol Pharmacol.
43:504–508. 1993.PubMed/NCBI
|
|
124
|
Prud'homme GJ, Glinka Y, Toulina A, Ace O,
Subramaniam V and Jothy S: Breast cancer stem-like cells are
inhibited by a non-toxic aryl hydrocarbon receptor agonist. PLoS
One. 5:e138312010. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Jin UH, Lee SO and Safe S: Aryl
hydrocarbon receptor (AHR)-active pharmaceuticals are selective AHR
modulators in MDA-MB-468 and BT474 breast cancer cells. J Pharmacol
Exp Ther. 343:333–341. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Cummings SR, Tice JA, Bauer S, Browner WS,
Cuzick J, Ziv E, Vogel V, Shepherd J, Vachon C, Smith-Bindman R and
Kerlikowske K: Prevention of breast cancer in postmenopausal women:
Approaches to estimating and reducing risk. J Natl Cancer Inst.
101:384–398. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Moen MD and Keating GM: Raloxifene: A
review of its use in the prevention of invasive breast cancer.
Drugs. 68:2059–2083. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Bevers TB: Raloxifene and the prevention
of breast cancer. Expert Opin Pharmacother. 7:2301–2307. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Martino S, Cauley JA, Barrett-Connor E,
Powles TJ, Mershon J, Disch D, Secrest RJ and Cummings SR; CORE
Investigators, : Continuing outcomes relevant to Evista: Breast
cancer incidence in postmenopausal osteoporotic women in a
randomized trial of raloxifene. J Natl Cancer Inst. 96:1751–1761.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Clemens JA, Bennett DR, Black LJ and Jones
CD: Effects of a new antiestrogen, keoxifene (LY156758), on growth
of carcinogen-induced mammary tumors and on LH and prolactin
levels. Life Sci. 32:2869–2875. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Kleinberg DL, Todd J and Babitsky G:
Inhibition by estradiol of the lactogenic effect of prolactin in
primate mammary tissue: Reversal by antiestrogens LY 156758 and
tamoxifen. Proc Natl Acad Sci USA. 80:4144–4148. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Ning M, Zhou C, Weng J, Zhang S, Chen D,
Yang C, Wang H, Ren J, Zhou L, Jin C and Wang MW: Biological
activities of a novel selective oestrogen receptor modulator
derived from raloxifene (Y134). Br J Pharmacol. 150:19–28. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Jang HS, Pearce M, O'Donnell EF, Nguyen
BD, Truong L, Mueller MJ, Bisson WH, Kerkvliet NI, Tanguay RL and
Kolluri SK: Identification of a raloxifene analog that promotes
AhR-mediated apoptosis in cancer cells. Biology (Basel).
6:412017.PubMed/NCBI
|
|
134
|
Hyder T, Marino CC, Ahmad S, Nasrazadani A
and Brufsky AM: Aromatase inhibitor-associated musculoskeletal
syndrome: Understanding mechanisms and management. Front Endocrinol
(Lausanne). 12:7137002021. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Dzeletovic N, McGuire J, Daujat M,
Tholander J, Ema M, Fujii-Kuriyama Y, Bergman J, Maurel P and
Poellinger L: Regulation of dioxin receptor function by omeprazole.
J Biol Chem. 272:12705–12713. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Jin UH, Kim SB and Safe S: Omeprazole
inhibits pancreatic cancer cell invasion through a nongenomic Aryl
hydrocarbon receptor pathway. Chem Res Toxicol. 28:907–918. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Lesca P, Peryt B, Larrieu G, Alvinerie M,
Galtier P, Daujat M, Maurel P and Hoogenboom L: Evidence for the
ligand-independent activation of the AH receptor. Biochem Biophys
Res Commun. 209:474–482. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Jin UH, Lee SO, Pfent C and Safe S: The
aryl hydrocarbon receptor ligand omeprazole inhibits breast cancer
cell invasion and metastasis. BMC Cancer. 14:4982014. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Koda A, Nagai H, Watanabe S, Yanagihara Y
and Sakamoto K: Inhibition of hypersensitivity reactions by a new
drug, N(3′,4′-dimethoxycinnamoyl) anthranilic acid (N-5′). J
Allergy Clin. Immunol. 57:396–407. 1976.
|
|
140
|
Azuma H, Banno K and Yoshimura T:
Pharmacological properties of N-(3′,4′-dimethoxycinnamoyl)
anthranilic acid (N-5′), a new anti-atopic agent. Br J Pharmacol.
58:483–488. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Batlle E and Clevers H: Cancer stem cells
revisited. Nat Med. 23:1124–1134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Chakrabarti R, Subramaniam V, Abdalla S,
Jothy S and Prud'homme GJ: Tranilast inhibits the growth and
metastasis of mammary carcinoma. Anticancer Drugs. 20:334–345.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Subramaniam V, Ace O, Prud'homme GJ and
Jothy S: Tranilast treatment decreases cell growth, migration and
inhibits colony formation of human breast cancer cells. Exp Mol
Pathol. 90:116–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Ciolino HP, MacDonald CJ, Memon OS, Bass
SE and Yeh GC: Sulindac regulates the aryl hydrocarbon
receptor-mediated expression of phase 1 metabolic enzymes in vivo
and in vitro. Carcinogenesis. 27:1586–1592. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Hahn ME: Aryl hydrocarbon receptors:
Diversity and evolution. Chem Biol Interact. 141:131–160. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Schlezinger JJ, Liu D, Farago M, Seldin
DC, Belguise K, Sonenshein GE and Sherr DH: A role for the aryl
hydrocarbon receptor in mammary gland tumorigenesis. Biol Chem.
387:1175–1187. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Vogel CFA, Lazennec G, Kado SY, Dahlem C,
He Y, Castaneda A, Ishihara Y, Vogeley C, Rossi A,
Haarmann-Stemmann T, et al: Targeting the Aryl hydrocarbon receptor
signaling pathway in breast cancer development. Front Immunol.
12:6253462021. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Kolasa E, Houlbert N, Balaguer P and
Fardel O: AhR- and NF-κB-dependent induction of interleukin-6 by
co-exposure to the environmental contaminant benzanthracene and the
cytokine tumor necrosis factor-α in human mammary MCF-7 cells. Chem
Biol Interact. 203:391–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Shan A, Leng L, Li J, Luo XM, Fan YJ, Yang
Q, Xie QH, Chen YS, Ni CS, Guo LM, et al: TCDD-induced antagonism
of MEHP-mediated migration and invasion partly involves aryl
hydrocarbon receptor in MCF7 breast cancer cells. J Hazard Mater.
398:1228692020. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Bekki K, Vogel H, Li W, Ito T, Sweeney C,
Haarmann-Stemmann T, Matsumura F and Vogel CF: The aryl hydrocarbon
receptor (AhR) mediates resistance to apoptosis induced in breast
cancer cells. Pestic Biochem Physiol. 120:5–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Pontillo C, Español A, Chiappini F, Miret
N, Cocca C, Alvarez L, Kleiman de Pisarev D, Sales ME and Randi AS:
Hexachlorobenzene promotes angiogenesis in vivo, in a breast cancer
model and neovasculogenesis in vitro, in the human microvascular
endothelial cell line HMEC-1. Toxicol Lett. 239:53–64. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Vogel CFA, Li W, Wu D, Miller JK, Sweeney
C, Lazennec G, Fujisawa Y and Matsumura F: Interaction of aryl
hydrocarbon receptor and NF-κB subunit RelB in breast cancer is
associated with interleukin-8 overexpression. Arch Biochem Biophys.
512:78–86. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Novikov O, Wang Z, Stanford EA, Parks AJ,
Ramirez-Cardenas A, Landesman E, Laklouk I, Sarita-Reyes C,
Gusenleitner D, Li A, et al: An Aryl hydrocarbon receptor-mediated
amplification loop that enforces cell migration in
ER-/PR-/Her2-human breast cancer cells. Mol Pharmacol. 90:674–688.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Hsieh TH, Tsai CF, Hsu CY, Kuo PL, Lee JN,
Chai CY, Wang SC and Tsai EM: Phthalates induce proliferation and
invasiveness of estrogen receptor-negative breast cancer through
the AhR/HDAC6/c-Myc signaling pathway. FASEB J. 26:778–787. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Miret N, Pontillo C, Ventura C, Carozzo A,
Chiappini F, Kleiman de Pisarev D, Fernández N, Cocca C and Randi
A: Hexachlorobenzene modulates the crosstalk between the aryl
hydrocarbon receptor and transforming growth factor-β1 signaling,
enhancing human breast cancer cell migration and invasion.
Toxicology. 366–367. 20–31. 2016.
|
|
156
|
Pontillo CA, Rojas P, Chiappini F,
Sequeira G, Cocca C, Crocci M, Colombo L, Lanari C, Kleiman de
Pisarev D and Randi A: Action of hexachlorobenzene on tumor growth
and metastasis in different experimental models. Toxicol Appl
Pharmacol. 268:331–342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Al-Dhfyan A, Alhoshani A and Korashy HM:
Aryl hydrocarbon receptor/cytochrome P450 1A1 pathway mediates
breast cancer stem cells expansion through PTEN inhibition and
β-catenin and Akt activation. Mol Cancer. 16:142017. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Jung JW, Park SB, Lee SJ, Seo MS, Trosko
JE and Kang KS: Metformin represses self-renewal of the human
breast carcinoma stem cells via inhibition of estrogen
receptor-mediated OCT4 expression. PLoS One. 6:e280682011.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Malik DE, David RM and Gooderham NJ:
Interleukin-6 selectively induces drug metabolism to potentiate the
genotoxicity of dietary carcinogens in mammary cells. Arch Toxicol.
93:3005–3020. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Zárate LV, Pontillo CA, Español A, Miret
NV, Chiappini F, Cocca C, Álvarez L, de Pisarev DK, Sales ME and
Randi AS: Angiogenesis signaling in breast cancer models is induced
by hexachlorobenzene and chlorpyrifos, pesticide ligands of the
aryl hydrocarbon receptor. Toxicol Appl Pharmacol. 401:1150932020.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Pontillo CA, Garcia MA, Peña D, Cocca C,
Chiappini F, Alvarez L, Kleiman de Pisarev D and Randi AS:
Activation of c-Src/HER1/STAT5b and HER1/ERK1/2 signaling pathways
and cell migration by hexachlorobenzene in MDA-MB-231 human breast
cancer cell line. Toxicol Sci. 120:284–296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Castillo-Sanchez R, Villegas-Comonfort S,
Galindo-Hernandez O, Gomez R and Salazar EP: Benzo-[a]-pyrene
induces FAK activation and cell migration in MDA-MB-231 breast
cancer cells. Cell Biol Toxicol. 29:303–319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Guo J, Xu Y, Ji W, Song L, Dai C and Zhan
L: Effects of exposure to benzo[a]pyrene on metastasis of breast
cancer are mediated through ROS-ERK-MMP9 axis signaling. Toxicol
Lett. 234:201–210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Cirillo F, Lappano R, Bruno L, Rizzuti B,
Grande F, Guzzi R, Briguori S, Miglietta AM, Nakajima M, Di Martino
MT and Maggiolini M: AHR and GPER mediate the stimulatory effects
induced by 3-methylcholanthrene in breast cancer cells and
cancer-associated fibroblasts (CAFs). J Exp Clin Cancer Res.
38:3352019. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Yamashita N, Saito N, Zhao S, Terai K,
Hiruta N, Park Y, Bujo H, Nemoto K and Kanno Y: Heregulin-induced
cell migration is promoted by aryl hydrocarbon receptor in
HER2-overexpressing breast cancer cells. Exp Cell Res. 366:34–40.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
D'Amato NC, Rogers TJ, Gordon MA, Greene
LI, Cochrane DR, Spoelstra NS, Nemkov TG, D'Alessandro A, Hansen KC
and Richer JK: A TDO2-AhR signaling axis facilitates anoikis
resistance and metastasis in triple-negative breast cancer. Cancer
Res. 75:4651–4664. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Parks AJ, Pollastri MP, Hahn ME, Stanford
EA, Novikov O, Franks DG, Haigh SE, Narasimhan S, Ashton TD, Hopper
TG, et al: In silico identification of an aryl hydrocarbon receptor
antagonist with biological activity in vitro and in vivo. Mol
Pharmacol. 86:593–608. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Nassar D and Blanpain C: Cancer stem
cells: Basic concepts and therapeutic implications. Annu Rev
Pathol. 11:47–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Peitzsch C, Tyutyunnykova A, Pantel K and
Dubrovska A: Cancer stem cells: The root of tumor recurrence and
metastases. Semin Cancer Biol. 44:10–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Regan Anderson TM, Ma S, Perez Kerkvliet
C, Peng Y, Helle TM, Krutilina RI, Raj GV, Cidlowski JA, Ostrander
JH, Schwertfeger KL, et al: Taxol induces Brk-dependent prosurvival
phenotypes in TNBC cells through an AhR/GR/HIF-driven signaling
axis. Mol Cancer Res. 16:1761–1772. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Toomey DP, Murphy JF and Conlon KC: COX-2,
VEGF and tumour angiogenesis. Surgeon. 7:174–180. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Kirkpatrick K, Ogunkolade W, Elkak A,
Bustin S, Jenkins P, Ghilchik M and Mokbel K: The mRNA expression
of cyclo-oxygenase-2 (COX-2) and vascular endothelial growth factor
(VEGF) in human breast cancer. Curr Med Res Opin. 18:237–241. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Sansone P, Storci G, Tavolari S, Guarnieri
T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P,
Marcu KB, et al: IL-6 triggers malignant features in mammospheres
from human ductal breast carcinoma and normal mammary gland. J Clin
Invest. 117:3988–4002. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Baumgarten SC and Frasor J: Minireview:
Inflammation: An instigator of more aggressive estrogen receptor
(ER) positive breast cancers. Mol Endocrinol. 26:360–371. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Sasser AK, Sullivan NJ, Studebaker AW,
Hendey LF, Axel AE and Hall BM: Interleukin-6 is a potent growth
factor for ER-alpha-positive human breast cancer. FASEB J.
21:3763–3770. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Fouad TM, Barrera AMG, Reuben JM, Lucci A,
Woodward WA, Stauder MC, Lim B, DeSnyder SM, Arun B, Gildy B, et
al: Inflammatory breast cancer: A proposed conceptual shift in the
UICC-AJCC TNM staging system. Lancet Oncol. 18:e228–e232. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Wang Z, Wang H, Ding X, Chen X and Shen K:
A large-cohort retrospective study of metastatic patterns and
prognostic outcomes between inflammatory and non-inflammatory
breast cancer. Ther Adv Med Oncol. 12:17588359209326742020.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Sovak MA, Bellas RE, Kim DW, Zanieski GJ,
Rogers AE, Traish AM and Sonenshein GE: Aberrant nuclear
factor-kappaB/Rel expression and the pathogenesis of breast cancer.
J Clin Invest. 100:2952–2960. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Kim DW, Sovak MA, Zanieski G, Nonet G,
Romieu-Mourez R, Lau AW, Hafer LJ, Yaswen P, Stampfer M, Rogers AE,
et al: Activation of NF-kappaB/Rel occurs early during neoplastic
transformation of mammary cells. Carcinogenesis. 21:871–879. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Kim DW, Gazourian L, Quadri SA,
Romieu-Mourez R, Sherr DH and Sonenshein GE: The RelA NF-kappaB
subunit and the aryl hydrocarbon receptor (AhR) cooperate to
transactivate the c-myc promoter in mammary cells. Oncogene.
19:5498–5506. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Currier N, Solomon SE, Demicco EG, Chang
DL, Farago M, Ying H, Dominguez I, Sonenshein GE, Cardiff RD, Xiao
ZX, et al: Oncogenic signaling pathways activated in DMBA-induced
mouse mammary tumors. Toxicol Pathol. 33:726–737. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Vyas D, Lopez-Hisijos N, Shah P, Deshpande
KS, Basson MD, Vyas A and Chaturvedi LS: A second-generation
proteasome inhibitor and doxorubicin modulates IL-6, pSTAT-3 and
NF-kB activity in MDA-MB-231 breast cancer cells. J Nanosci
Nanotechnol. 17:175–185. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Sakamoto K, Wehde BL, Yoo KH, Kim T,
Rajbhandari N, Shin HY, Triplett AA, Rädler PD, Schuler F,
Villunger A, et al: Janus kinase 1 is essential for inflammatory
cytokine signaling and mammary gland remodeling. Mol Cell Biol.
36:1673–1690. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Poland A and Knutson JC:
2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated
aromatic hydrocarbons: Examination of the mechanism of toxicity.
Annu Rev Pharmacol Toxicol. 22:517–554. 1982. View Article : Google Scholar : PubMed/NCBI
|