Introduction
Ovarian cancer is a prevalent malignancy of the
female reproductive system, carrying the highest mortality rate
among gynecological tumors. The overall 5-year relative survival
rate for ovarian cancer is <50% (1). The current standard of care for
ovarian cancer involves a multimodal approach consisting of
cytoreductive surgery and chemotherapy. The first-line chemotherapy
regimen for advanced ovarian cancer remains the combination of
paclitaxel (PTX) and cisplatin (2). Of patients who initially respond well
to anticancer treatment experience, ~75% relapse within 2 years as
they acquire resistance to existing chemotherapy drugs (3). Drug sensitivity in cells can be
influenced by various factors and pathways, including efflux
transporters, dysregulated apoptosis, autophagy, cancer stem cells,
epigenetics and the unfolded protein response signaling network
(4,5). Research has also revealed that the
utilization of poly-ADP-ribose polymerase inhibitors markedly
improves the prognosis of patients (6). Targeting a single region or pathway
alone is insufficient to reverse drug resistance. Therefore, it is
crucial to identify strategies that can effectively reduce drug
resistance in tumor cells (7,8).
An increasing number of studies have demonstrated
the inhibitory effects of Chinese medicine, including Chinese
patent medicine and single Chinese herbs, on tumor metastasis.
These studies have also investigated the molecular mechanisms
underlying the anti-metastatic effects of Chinese medicine
(9). Combining curcumin with
platinum chemotherapy has been demonstrated to improve the survival
rate of patients with non-small cell lung cancer (10). Ginsenoside has been found to
attenuate breast tumor growth by inhibiting angiogenesis (11). Combination therapy has the
potential to overcome drug resistance and reduce adverse reactions,
ultimately enhancing treatment efficacy (12). Tetramethylpyrazine (TMP), one of
the main bioactive components of ligustilide, exhibits inhibitory
effects on tumor cell growth through various mechanisms (13–15).
It has been demonstrated that TMP can reverse multidrug resistance
in BEl-7402/ADM and Pumc-91/ADM cells towards Adriamycin (16,17).
Danshensu is major bioactive ingredients from the Chinese herbs
Salvia miltiorrhiza Bge. Danshensu-Tetramethylpyrazine Conjugate
DT-010 has shown efficacy in overcoming doxorubicin resistance in
human breast cancer cells (18).
In lung cancer cells, TMP induces S-phase arrest and inhibits
pathological changes (19).
However, limitations such as suboptimal targeting and alterations
in drug elimination pathways, including liver and kidney
metabolism, have been observed, affecting the effectiveness of TMP
(20).
Exosomes (EXOs) are extracellular vesicles
characterized by a diameter range of 40–160 nm (average, ~100 nm)
and blast-like features (21).
These vesicles have the ability to enter cells, release cargo and
mediate various physiological and pathological processes. One of
the notable advantages of EXOs is their ‘natural’ properties, which
result in minimal, or even no, long-term accumulation in any organ
or tissue, thereby minimizing potential toxic effects on the whole
body (22). Compared with
synthetic drug delivery systems such as liposomes, micelles,
dendrimers and nanoparticles, EXOs, as membrane-derived vesicles
with diverse origins, exhibit higher biocompatibility and targeting
capabilities (23,24). Emerging studies have highlighted
the promising potential of tumor cell-derived EXOs as drug carriers
for cancer treatment. For instance, methotrexate-loaded
extracellular vesicles derived from tumor cells have been used to
alleviate biliary obstruction in patients with extrahepatic
cholangiocarcinoma, while doxorubicin-loaded EXOs derived from
liver cancer cells have demonstrated depletion of cancer stem cells
in subcutaneous, orthotopic and metastatic tumor models (25–29).
EXOs carrying LOC85009 regulate autophagy related 5-induced
autophagy via the ubiquitin specific peptidase 5/upstream
transcription factor 1 axis to suppress docetaxel resistance
(30). In our previous study, a
drug delivery system using folate-chitosan nanoparticles loaded
with TMP was developed and its effective reversal of adriamycin
resistance in human breast cancer cells was observed (31). Building upon these findings, the
present study aimed to harness the abundance and practicality of
tumor cell-derived EXOs to create a more efficient targeted
delivery platform.
In the present study, a novel TMP formulation based
on EXOs, termed EXOs-TMP, was successfully developed. The present
findings demonstrated that EXOs-TMP effectively reversed the
multidrug resistance of A2780T cells to PTX in vitro, as
illustrated in Fig. 1. The
incorporation of TMP into EXOs exhibited potent antitumor activity
by suppressing the growth of tumor cells and enhancing the efficacy
of PTX. Mechanistically, EXOs-TMP achieved this by downregulating
the expression of drug resistance proteins and isoenzymes, while
inducing cellular apoptosis.
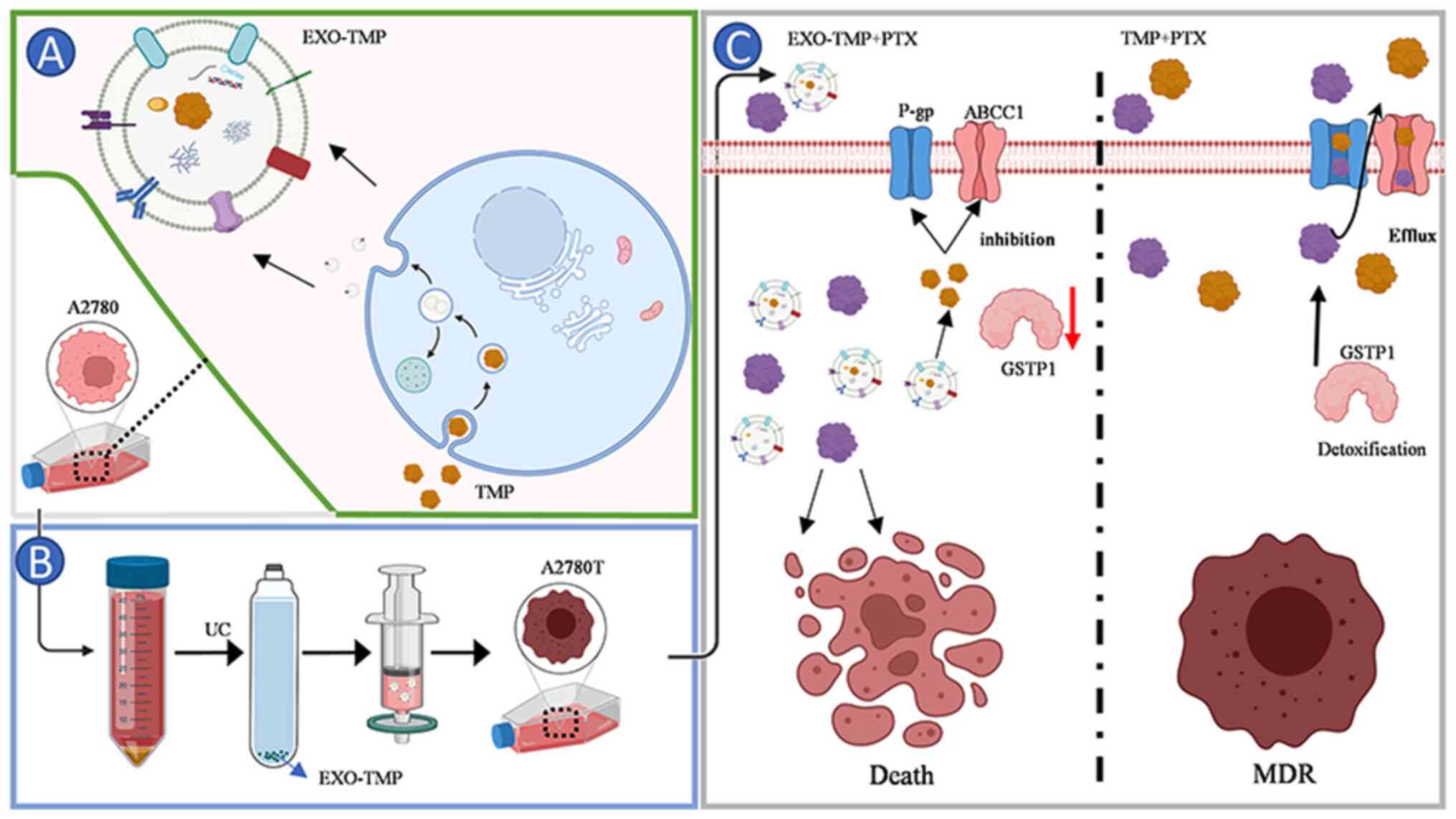 | Figure 1.Schematic illustration of EXO-TMP as
drug carriers for reversed tumor drug resistance. Schematic
illustration of the preparation of EXO-TMP. (A) TMP was
internalized by the cancer cells after incubation, then located in
MVBs. After MVBs fused with the cell membrane, EXOs-TMP were
exocytosed into the extracellular space. (B) Schematics showing
EXO-TMP acquisition. (C) Schematics showing how EXO-TMP efficiently
reversed tumor drug resistance. EXO-TMP bypassing the transporter
P-gp and ABCC1 to allow more TMP to enter the cell, and then
releasing TMP in the cell to close the transporter channel and
reduce GSTP1 to reverse the drug resistance of the cell, allowing
more PTX to remain in the cell and improving the effectiveness of
PTX. ABCC1, multidrug resistant-associated protein 1; EXO, exosome;
TMP, tetramethylpyrazine; MVB, multivesicular body; P-gp,
P-glycoprotein; ABC, ATP-binding cassette; GSTP1, glutathione
S-transferase π1; PTX, paclitaxel. |
Materials and methods
Cell culture
A2780 and A2780T human ovarian cancer cell lines and
the A549 lung cancer cell line were obtained from Type Culture
Collection of Shanghai Meixuan Biotechnology Co., Ltd (cat. nos.
MXC020, MXC021 and MXC026). All cells were cultured in RPMI 1640
medium (Gibco, C11875500BT) at 37°C in a 5% CO2
humidified incubator. All media contained 10% FBS(AusGeneX,
LV-FBSCN500S), 100 U/ml penicillin and 100 µg/ml streptomycin
(Beyotime, C0222). A2780T cells were cultured in a drug-free medium
for 7 days to avoid the interference of drug toxicity on the
experimental results. Logarithmic growth phase cells were taken for
the experiments.
EXO purification
EXOs were purified using the differential
ultracentrifugation method. First, FBS used for cell incubation was
centrifuged at 160,000 × g for 6 h at 4°C to wipe out the existing
EXOs. The precipitate was discarded. Subsequently, the supernatant
was filtered and sterilized with a 0.22-µm syringe filter in the
ultra-clean workbench, and frozen and stored at −20°C for later
use. A2780, A2780T or A549 cells were incubated in EXO-free RPMI
1640 for 48 h. The cell culture medium was collected and
sequentially centrifuged at 300 × g for 10 min, 2,000 × g for 10
min and 100,00 × g for 30 min at 4°C to remove cells and residual
cell debris. EXOs were pelleted and washed with PBS, and recovered
by centrifugation at 120,000 × g for 2 h at 4°C.
TMP-primed EXO collection and
characterization
The toxic effect of TMP on A2780 cells was
determined in a 48-h MTT assay (cytotoxicity test), and the cell
viability of TMP was not significantly decreased at all
concentrations, ranging between 0–200 µg/ml, and >85% of cells
survived. Based on these results, A2780 cells were treated with TMP
(50 µg/ml) for 48 h at 37°C. Culture media were collected and the
aforementioned method was used to concentrate EXO-TMP into a
pellet. Morphological characteristics of EXO and EXO-TMP were
observed after negative staining using a transmission electron
microscope (JEM-1200EX; JEOL, Ltd.). A single-drop suspension (500
µg/ml) of the sample was applied onto a carbon-coated, 300 mesh
copper grid and left to rest for 5 min or until it air-dried at
ambient temperature. Subsequently, the sample was stained using a 1
M solution of phosphotungstic acetate for 5 min, after which any
excess staining solution was carefully removed using filter paper.
Then, the samples were placed in a transmission electron microscope
for observation and photography. Nanoparticle tracking analysis
(NanoSight NS300; Malvern Instruments, Inc.) was used to measure
the concentration and size distribution of EXOs and EXOs-TMP. The
amount of TMP loaded into EXO was measured by dissolving with
methanol in ultrasound for 30 min to release TMP, and the content
of TMP in EXOs was determined by high-performance liquid
chromatography (HPLC). The chromatographic conditions were as
follows: The chromatographic column was a C18 column (Dalian Yilite
Analytical Instrument Co., Ltd.); mobile phase, methanol-water
(60:40); flow, 0.8 mg·min−1; ultraviolet detection
wavelength, 280 nm; room temperature; and sample volume, 10 µl. The
analysis time was 9 min and retention time of TMP was 5.69 min.
Exosomal proteins were analyzed using the BCA method and whole
cells, the purified EXOs and EXOs-TMP were lysed in RIPA lysis
buffer and then subjected to western blot analysis. The primary
antibodies used included anti-CD63 (ab216130; Abcam), anti-tumor
susceptibility 101 (TSG101; ab125011; Abcam) and anti-calnexin
(ab22595; Abcam). All primary antibodies were diluted to
1:2,000.
Cancer EXOs homing to the mother cell
line in vitro
A2780T or A549 cells were seeded into 24-well plates
at a density of 1×105 cells/ml. After 24 h of culture at
37°C, the medium was replaced with 1640 medium containing 100 µg/ml
Dil (C1991S-; Beyotime Institute of Biotechnology)-labeled A2780
EXOs or A549 EXOs. Cells were cultured for an additional 12 h,
washed three times with PBS, fixed with 4% paraformaldehyde for 10
min at 37°C and stained with Antifade Mounting Medium with DAPI
(P0131; Beyotime Institute of Biotechnology). A fluorescence
microscope (Leica DM6B THUNDER; Leica Microsystems GmbH) was used
to capture images. LAS AF Lite (Leica Microsystems GmbH, version
3.3.0-10134) was used for fluorometric measurements.
EXOs-TMP reduce the expression of
resistant proteins in PTX A2780T cells
The cytotoxic effect of EXOs-TMP on A2780T cells was
evaluated using an MTT assay at 37°C. The cells were inoculated at
1×105 cells per well on a 96-well dish and adhered to
the wall overnight. Subsequently, the cells were treated with
different concentrations of TMP, PTX and EXOs-TMP in the culture
medium for 48 h. Afterwards, 50 ml MTT was added and the resulting
formazan crystals were dissolved in 150 µl DMSO. The absorbance was
measured at 570 nm per well with a 96-well plate reader and cell
viability was expressed as the percentage of untreated
controls.
Western blot analysis
The cells were seeded in a 6-well plate with
2.5×105 cells/well and allowed to adhere overnight.
Subsequently, 8 µg/ml TMP, 0.6 µg/ml PTX, 8 µg/ml E-TMP (TMP 8
µg/ml), PTX + TMP, PTX + E-TMP and EXO + TMP + PTX was added to the
culture medium to treat the cells for 48 h. The medium in the
6-well plate was discarded after 48 h. Pre-cooled PBS was added to
rinse the cells twice. The prepared protein lysis solution
(RIPA:PMSF, 100:1) was added to lyse the cells for 30 min (shaking
the 6-well plate every 10 min). The cells were quickly scraped off
with a cell scraper to collect the cells and centrifuged using a
high-speed refrigerated centrifuge at 4°C at 12,000 g/min for 15
min. The supernatant was aspirated and the BCA protein assay kit
was used to determine the total protein concentration. Equal
quantities of protein samples (20 µg/lane) were added to each lane
and separated on 10% SDS-PAGE gels. Subsequently, the proteins were
transferred onto 0.45 µm PVDF membranes and blocked using 5%
non-fat milk for 60 min at room temperature. The PVDF membranes
were then incubated overnight at 4°C with primary antibodies. The
lysate was subjected to western blotting and incubated with
ATP-binding cassette sub-family C member 1 (ABCC1; diluted 1:1,000;
WL01027; Wanleibio Co., Ltd.), multidrug resistance protein 1
(P-gp; diluted 1:10,000; ab170904; Abcam), glutathione
S-transferase π (GSTP1; diluted 1:5,000; 66715-1-lg; Proteintech
Group, Inc.) and GAPDH (diluted 1:5,000; AB8245; Abcam) antibodies.
Following washing with TBS buffer containing 0.1% Tween-20
(Beyotime Institute of Biotechnology; cat. no. T1082), the PVDF
membranes were incubated with horseradish peroxidase-conjugated
secondary antibodies (Thermo, XD345904, WE324878) at 37°C for 60
min. The Odyssey CLX (LI-COR) was used to capture images of the
protein bands. Image Studio software (LI-COR,4.0.21) was used for
the optical density measurement of band intensity.
Apoptosis induction by EXO-TMP in
ovarian cancer cells
A2780T cells were cultured in 6-well dishes
(4×105 cells/well) overnight and then treated with 8
µg/ml TMP, 0.6 µg/ml PTX, PTX + TMP mixture, 8 µg/ml EXOs-TMP, PTX
+ E-TMP mixture or PTX + EXO + TMP mixture for 48 h. Next, the
cells were collected by trypsinization, rinsed, resuspended in
binding buffer and double stained using an Annexin V-FITC/PI kit
(KeyGEN BioTECH, KGA108). Cell apoptosis was measured by flow
cytometry using a BD FACSCalibur (BD Biosciences) flow cytometer
according to the manufacturer's instructions. A total of
1×104 gated events were recorded per sample. Data were
analyzed using BD FACSDiva 8.0.1 software (BD Biosciences).
Statistical analysis
The data are expressed as mean ± standard error of
the mean. Statistical analyses were performed using GraphPad Prism
8.0 (GraphPad Software; Dotmatics). To compare differences between
two groups, a two-way independent-sample t-test was employed. For
comparisons involving three or more groups, we conducted one-way
ANOVA) followed by Tukey's post-hoc test to assess significance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
EXOs of tumor cells specifically home
to their parental cells
To test whether tumor cell EXOs return to their
parent cells in vitro, EXOs produced by A2780 and
drug-resistant A2780T cells were selected as experimental subjects.
A2780T cells were cocultured with Dil-labeled A2780 EXOs or
Dil-labeled A2780T EXOs for 12 h and fluorescence microscopy was
used to quantify EXO uptake in A2780T cells. No significant
difference was observed in the uptake of the two EXOs in A2780T
cells (Fig. 2A and B).
Subsequently, Dil-labeled A2780 EXOs or Dil-labeled A549 EXOs were
selected for co-culture with A2780T cells for 12 h, and
fluorescence microscopy was used to quantify EXO uptake in A2780T
cells. A2780T cells took ≥4-fold more A2780-derived EXOs compared
with A459-derived EXOs (Fig. 2C and
D). To ensure that this finding was not an A2780T-specific
phenomenon, it was examined whether EXOs derived from A549 cells
would also exhibit parental cell tropism. A549 cells were
co-cultured with fluorescently labeled A549 EXOs and A2780 EXOs for
12 h. A549 cells were ~3 times more efficient in taking up A549
EXOs than A2780 EXOs (Fig. 2E and
F). Thus, the present data suggested that EXOs from tumor cells
preferentially returned to parental cells.
EXOs-TMP isolation and
characterization
After confirming the homing ability of tumor
cell-derived EXOs, A2780 cell-derived EXOs were selected as the
drug delivery vehicle of TMP in order to inhibit ovarian cancer
drug resistance. The EXOs were encapsulated with TMP
endocytotically.
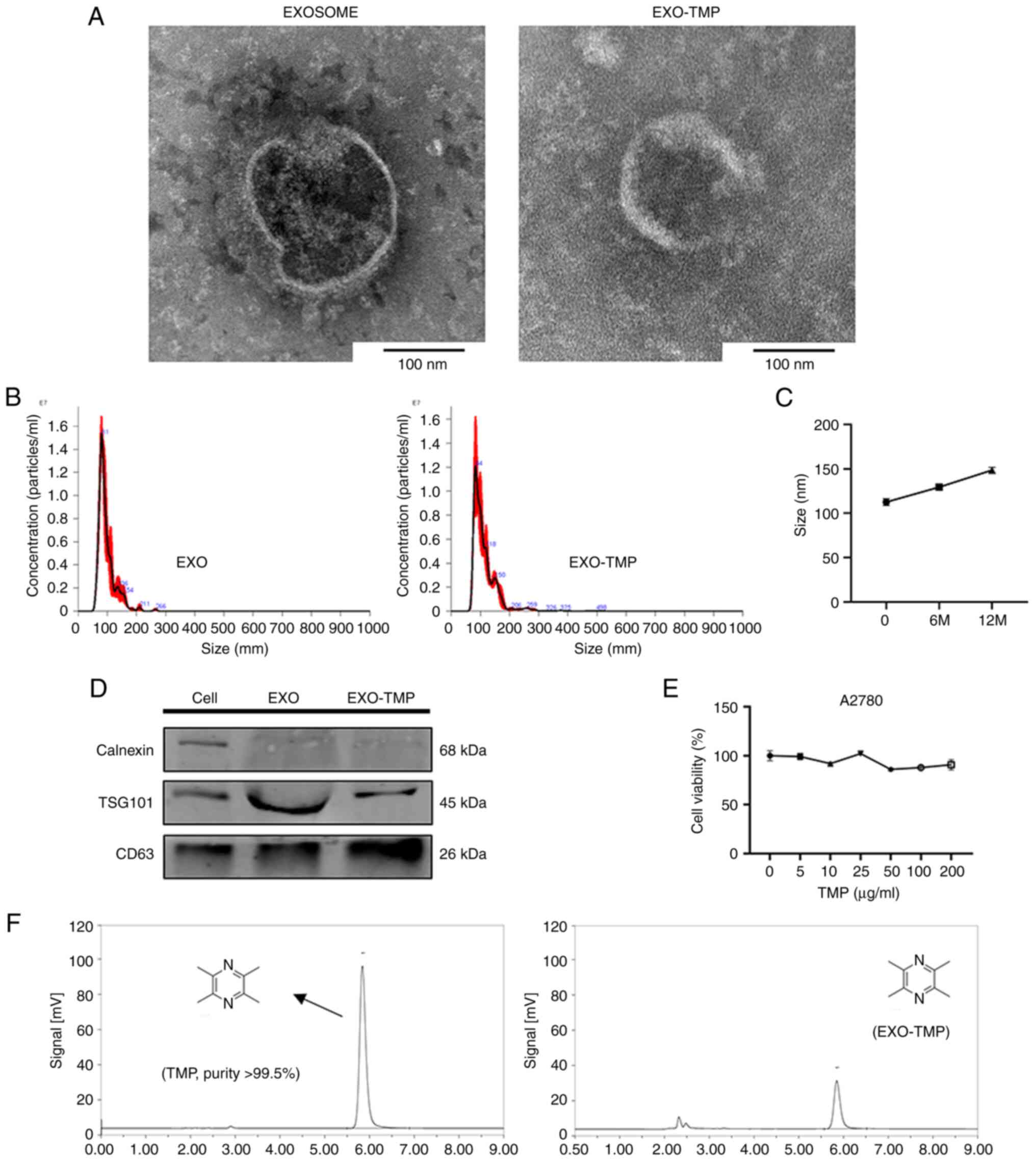
The typical structures of EXOs and EXOs-TMP were
observed by transmission electron microscopy. EXOs and EXOs-TMP had
normal morphological features, being cup-round in shape and
measuring ~100 nm in diameter (Fig.
3A). Nanoparticle tracking analysis showed that the average
size of EXOs and EXOs-TMP was 97.0±2.8 and 112.6±3.8 nm,
respectively (Fig. 3B). These
features indicated that the properties of the EXOs were not
affected by the loaded drugs. The loading rate of the preparation
was measured using HPLC and a loading efficiency of 20% was
obtained (Fig. 3F). When the
preparation was stored at −80°C for 6 and 12 months, stability was
demonstrated by nanoparticle tracking analysis showing that the
average size remained <160 nm (Fig.
3C). EXOs-TMP were isolated from the collected cell culture
supernatants using differential ultracentrifugation. EXO marker
proteins were detected by western blotting and the results
demonstrated that TSG101 and CD63 were positively expressed in
cells and EXOs, while calnexin was expressed in cells but not
detected in EXOs (Fig. 3D),
confirming that EXOs-TMP were indeed isolated from cells. TMP and
A2780 cells were co-cultured in 96-well plates for 48 h, and the
toxicity of TMP in A2780 cells was detected by an MTT assay. The
experimental results revealed that when the drug concentration
gradient of TMP was 0–200 µg/ml, the survival rate of A2780 cells
was >90%, which demonstrated that TMP had very low or no
toxicity (Fig. 3E).
EXOs-TMP reduce cell resistance to
PTX
After the EXOs-TMP drug delivery system was
successfully constructed, in order to test whether TMP would affect
the absorption of EXOs, EXOs and EXOs-TMP were incubated with
A2780T cells for 12 h. Fluorescence microscopy demonstrated that
there was no significant difference in the fluorescence intensity
of EXOs and EXOs-TMP absorption by A2780T cells. It was
demonstrated that loading TMP into EXOs did not affect cellular
uptake (Fig. 4A). The
IC50 of PTX in A2780T cells was 11 µg/ml, and that in
A2780 cells was 0.65 µg/ml, and the drug resistance fold was ~17
times (Fig. 4B). The expression of
P-gb was positive in A2780T cells but not in A2780 cells (Fig. 4C). The drug resistance stability of
A2780T cells was confirmed. The cells were incubated with EXOs,
free TMP and EXOs-TMP at drug concentrations of 3 and 8 µg/ml for
48 h and the cytotoxicity of EXOs-TMP was examined using an MTT
assay. TMP had no effect on cell viability at the concentrations of
3 and 8 µg/ml. EXOs-TMP reduced the viability of A2780T cells by
~75% when the concentration of TMP was 8 µg/ml. Neither EXOs nor
TMP alone was cytotoxic, while TMP was cytotoxic when loaded into
EXOs, suggesting that the EXOs confer a novel therapeutic effect of
TMP rather than just reducing cell resistance (Fig. 4D). The cytotoxicity of EXOs-TMP
when combined with PTX in ovarian cancer resistant cells was next
verified. Cells were incubated with PTX and TMP, EXO-TMP treatment
for 48 h, and viability was examined using an MTT assay. The effect
of TMP on drug resistance was very weak when a low concentration of
TMP was combined with PTX. EXOs-TMP (E-TMP8) containing 8 µg/ml TMP
in combination with PTX markedly inhibited cell proliferation
compared with free PTX and PTX + TMP mixtures (Fig. 4E). It was hypothesized that the
reversal effect of free TMP was not obvious due to the high drug
resistance of A2780T cells. Next, the mechanism of its cytotoxicity
will be investigated.
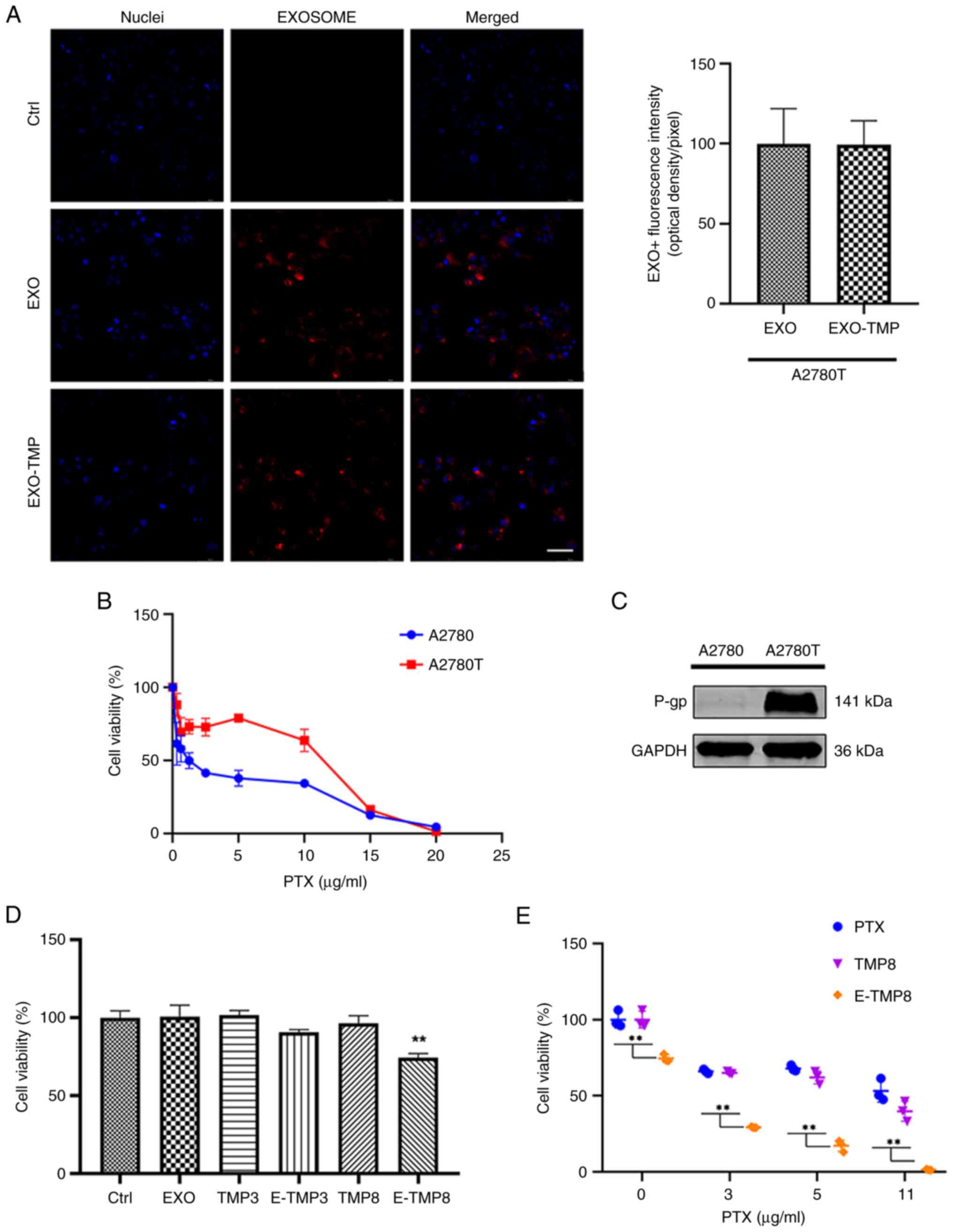 | Figure 4.EXOs-TMP reduce resistance to PTX in
drug-resistant cells. (A) Representative micrographs showing
absorption of Dil-labeled EXO and EXO-TMP by A2780T cells. The
endocytic EXOs are seen around the nucleus (red). Scale bar, 50 µm.
(B) PTX (0–20 µg/ml) was added to A2780 and A2780T cells, which
were 17 times more resistant. (C) P-gp expression of A2780T cells.
(D) A2780T cells were co-incubated with free TMP (3 or 8 µg/ml),
EXO and EXO-TMP (TMP 3 or 8 µg/ml) for 48 h, and cell viability was
determined using the MTT method (n=3). (E) A2780T cells were
co-incubated with free PTX, TMP (8 µg/ml) + PTX and EXO-TMP (TMP 8
µg/ml) + PTX for 48 h, and cell viability was determined using the
MTT method (n=3). All data are expressed as mean ± standard error
of the mean. **P<0.01. EXO, exosome; TMP, tetramethylpyrazine;
PTX, paclitaxel; P-gp, multidrug resistance protein 1. |
EXOs-TMP reduce the expression of
resistant proteins in PTX A2780T cells
There is substantial evidence that the expression of
ATP-binding cassette (ABC) transporters, especially P-gp and ABCC1,
can confer resistance to cytotoxic and targeted chemotherapy
(32). A2780T cells were incubated
with PTX, free TMP (8 µg/ml) and EXOs-TMP for 48 h, and the
expression of drug resistance proteins was detected by western
blotting. In the present study, a low dose of TMP could not reduce
the expression of P-gp and ABCC1, while EXOs loaded with TMP could
reduce the expression of P-gp and ABCC1 to 68.2 and 72.9%,
respectively. When TMP and PTX were combined, the protein
expression levels of P-gp and ABCC1 were decreased, and the protein
expression levels of the EXO-TMP + PTX group were decreased from
72.9 and 81.8% to 51.5 and 55.2% in the free TMP + PTX group. The
efficacy of EXOs-TMP in reversing the multidrug resistance of
A2780T cells was demonstrated. GSTP1 is involved in the
anti-apoptosis and metabolism of numerous chemotherapeutic drugs.
Platinum drugs have been found to be metabolized by GSTP1,
resulting in the expression of GSTP1 in ovarian tumors (33). Therefore, GSTP1 can be used as a
target gene and candidate response biomarker for platinum-based
chemotherapy. In addition, it has been found that GSTP1 serves a
role in the metabolism of PTX in ovarian cancer cells. However, to
the to the best of the authors' knowledge, the effect of TMP on the
expression of GSTP1 has not yet been reported. In the present
study, EXOs-TMP were found to reduce the expression of GSTP1 and
the combination of EXOs-TMP with PTX was more effective than free
TMP. Together, these results suggested that EXOs-TMP reversed PTX
resistance by reducing GSTP1 expression (Fig. 5A and B).
 | Figure 5.EXOs-TMP reduce the expression of
drug resistance proteins in PTX-resistant A2780T cells. (A and B)
A2780T cells were co-incubated with free TMP (3 or 8 µg/ml), EXO
and EXO-TMP (TMP concentration is 3 or 8 µg/ml) for 48 h,
respectively. The protein levels of ABCC1, P-gp and GSTP1 were
determined by western blot analysis, and the optical density of the
proteins was calculated. All data are expressed as mean ± standard
error of the mean. *P<0.05 vs. untreated controls;
+P<0.05 vs. TMP; #P<0.05 vs. EXO-TMP.
EXO, exosome; TMP, tetramethylpyrazine; PTX, paclitaxel; ABC,
ATP-binding cassette; P-gp, P-glycoprotein; GSTP1, glutathione
S-transferase π1. |
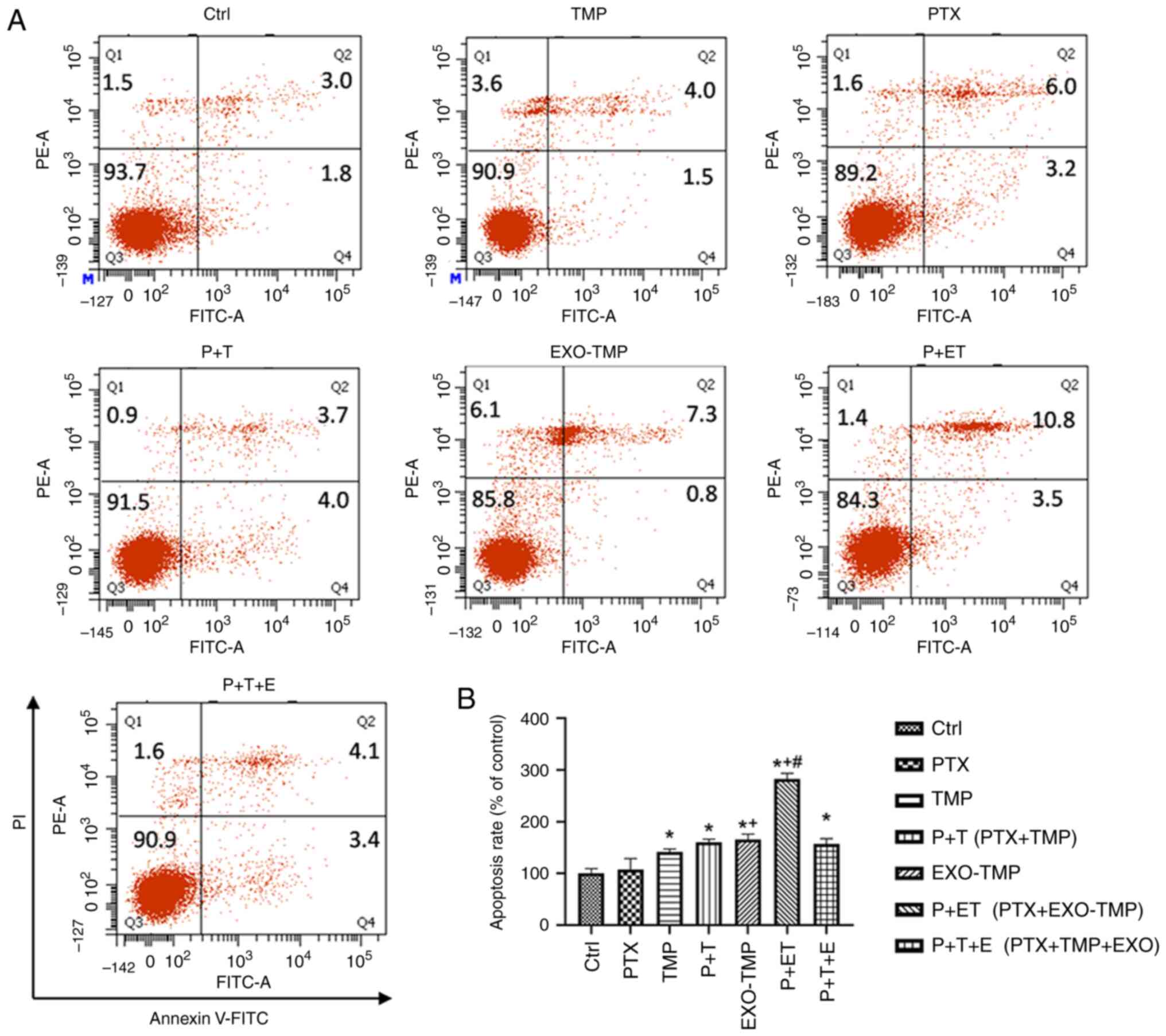
Apoptosis induction by EXOs-TMP in
ovarian cancer cells
Induction of apoptosis can increase the sensitivity
of drug-resistant cells to PTX (34). TMP combined with PTX treatment
markedly promoted the apoptosis induced by PTX in A2780T ovarian
cancer cells. To demonstrate the apoptotic effect of EXOs-TMP on
drug-resistant cells, apoptosis was assessed by flow cytometry in
A2780T cells treated with 0.6 µg/ml PTX in coculture with TMP or
EXOs-TMP for 48 h. The treated cells were double stained with
Annexin V-FITC/PI. The results demonstrated that the apoptosis
rates induced by each treatment (calculated by adding the values in
the upper and lower right quadrants) were consistent with the
cytotoxicity results. There was no apoptotic effect of TMP on
A2780T cells, but the apoptosis rate of A2780T cells treated with
PTX was increased by 1.9 times. EXOs-TMP increased the apoptosis
rate of A2780T cells by 1.6 times. These results indicated that the
EXO loading of TMP bestowed a novel antitumor effect to TMP in
inducing apoptosis of drug-resistant ovarian cancer cells.
Furthermore, EXOs-TMP + PTX exhibited a much higher apoptosis rate
than both free PTX and PTX + TMP mixtures. The apoptosis rate
induced by P + ET was ~3 times higher than that of the control
group, and ~2 times higher than that induced by P + T, indicating
the superiority of the EXOs-TMP drug delivery system. The results
demonstrated the enhanced apoptotic effect of EXOs-TMP, and in
particular, EXOs conferred an apoptotic effect of TMP on ovarian
cancer cells (Fig. 6A and B).
In conclusion, the present findings suggested that
ovarian cancer EXOs had the ability to return to their parent
cells. Compared with free TMP, the loading of TMP into EXOs not
only showed novel characteristics of toxicity to ovarian cancer
cells, but also markedly promoted the antitumor effect of PTX.
After investigating the mechanism of action of EXOs-TMP, it was
revealed that EXOs-TMP could markedly reduce the expression of drug
resistance proteins, including P-gp, ABCC1 and GSTP1, to enhance
the sensitivity of drug-resistant cells to PTX. Furthermore,
EXO-TMP itself exhibited cytotoxicity because EXOs-TMP caused
apoptosis and also enhanced the pro-apoptotic effect of PTX. Based
on the present results, EXOs-TMP enhanced the antitumor activity of
PTX, and thus, should be further developed as a potential
anticancer candidate.
Discussion
Ovarian cancer is a disease with high incidence and
mortality rate, posing a significant threat to human health
(35). Chemotherapy and
radiotherapy still represent the common and established methods for
treating patients with advanced cancer (36). As a complementary or alternative
therapy, the combination of Chinese herbal medicine with other
drugs shows promise (37). To
address the side effects, drug resistance and unsatisfactory
treatment outcomes in clinical cancer treatment, numerous studies
on combination therapy have been conducted, with strategies aimed
at improving efficacy or reducing toxicity gaining increased
attention (38). Combined
treatment is a feasible strategy for the development of Chinese
herbal medicine. For example, Ginkgo biloba extract inhibits
the proliferation, invasion and migration of gastric cancer cells
(39). Dihydroartemisinin, a
sesquiterpene lactone extracted from Artemisia annua, can
induce apoptosis and inhibit proliferation, invasion and migration
of ovarian cancer cells by inhibiting the hedgehog signaling
pathway (40). Baicalin, a
flavonoid extracted from Scutellaria baicalensis, inhibits
epithelial-mesenchymal transition and angiogenesis through the
PI3K/Akt/mTOR signaling pathway (41).
TMP is an alkaloid monomer extracted from
Ligusticum chuanxiong. A series of studies have demonstrated
that TMP had a variety of antitumor effects, including the
inhibition of tumor cell proliferation, invasion and drug
resistance. For example, TMP inhibits angiogenesis and tumor growth
in lung cancer in a dose- and time-dependent manner by blocking the
bone morphogenetic protein/Smad/inhibitor of DNA binding 1
signaling pathway (42).
Tmp-betulinic acid derivative inhibits the growth and metastasis of
bladder cancer cells (T24) by interfering with glutathione
metabolism and activating glycerophosphatidylcholine metabolism to
block angiogenesis (43). TMP can
inhibit the proliferation and migration of ovarian cancer cells by
regulating miR-211 (44). In
addition, we have confirmed that TMP can reduce the expression of
multidrug-resistance-1 and GST-π at the mRNA level (45). However, due to the different
pharmacokinetics of the drugs and their nonspecific biodistribution
and membrane transport properties, combination therapy is far from
ideal (46). Therefore, the
preparation of a novel drug delivery regimen by loading TMP into
EXOs was considered.
A variety of synthetic drug delivery systems have
been developed over the past decades (47). However, the application of such
systems is limited due to inefficiency, cytotoxicity and/or
immunogenicity (48). EXOs are
small vesicles produced by fusion and exocytosis between
multivesicles (MVBs) and the plasma membrane, with a diameter range
of 40–160 nm (49). The negative
charge on the surface of EXOs ensures stability in the circulation
system and the ability to deliver biomolecules to recipient cells
makes them suitable drug delivery carriers (50). For example, PTX encapsulated in
EXOs derived from human bone marrow mesenchymal stem cells
exhibited marked cytotoxic and tumor growth inhibition effects
against triple-negative breast cancer cells in in vitro and
in vivo experiments (51).
Gemcitabine-loaded EXOs were evaluated in mice with pancreatic
tumors and were associated with inhibition of tumor growth, minimal
damage to normal tissues and prolonged mouse survival (52). A study found that EXOs produced by
HeLa and HT1080 cells had the property of homing to maternal
tumors, and drug-loaded cancer EXOs could be used for targeted
cancer therapy (53). In the
present study, the selection of cell lines was based on validating
the homing effect of exosomes derived from ovarian cancer cells on
parental drug-resistant ovarian cancer cells. The choice of A549
cells was merely based on the consideration that they are not of
the same lineage as ovarian cancer cells. Therefore, EXOs from
A2780 ovarian cancer cells and A549 lung cancer cells were selected
to observe whether they also have homing properties. When the
mother cells were A2780T cells, the uptake rate of A2780 cell EXOs
was three times higher than that of A549 EXOs and vice
versa, confirming the ability of cancer-derived EXOs to home to
their maternal tumors. Nevertheless, EXOs as drug carriers offer
numerous advantages, but they also come with certain disadvantages
and limitations. For example, they have limited drug-loading
capacity, pose challenges in production and purification and are
relatively fragile, making them susceptible to damage during
storage and transportation. The research objective of this project
is to develop an EXO-TMP drug delivery system and investigate its
in vitro targeted drug delivery capabilities. The absence of
validation through in vivo experiments is a limitation of
this study. In future research, it is planned to enhance the purity
of exosomes and conduct comprehensive in vivo
experiments.
In the present study, cytotoxicity of TMP in A2780
and A2780T ovarian cancer cell lines was investigated. Serial
concentrations up to 200 µg/ml of TMP did not show any inhibitory
effect, even at the concentration of 200 µg/ml. This is consistent
with a previous study (54).
However, treatment of EXOs-TMP with TMP reduced the cell survival
rate to <80% at the concentration of 8 µg/ml. To the to the best
of the authors' knowledge, this is the first report of TMP
inhibiting ovarian cancer cells. It is possible that EXOs confer a
novel anticancer effect of TMP. It was found that 11 µg/ml PTX
reduced the cell viability to ~50%, and the survival rate of A2780T
cells was almost zero when combined with EXO-TMP. Its role and
mechanism are worthy of further study.
Resistance to chemotherapy remains one of the most
important obstacles to successful treatment of cancer. One of the
most intensively studied mechanisms of multiresistance is the
upregulation of expression of proteins from the ABC transporter
superfamily (55). P-gp and ABCC1,
in particular, efflux widely used chemotherapy drugs such as PTX,
vincristine and doxorubicin (32).
P-gp and ABCC1 in tumor cells cause multidrug resistance by pumping
drugs out of the cell and altering drug metabolism. These efflux
transporters diminish drug efficacy by reducing intracellular drug
concentrations. Previous work has demonstrated that TMP could
reverse the resistance of cancer cells to chemotherapeutic drugs by
reducing drug resistance proteins (16). The present study revealed that the
protein expression of P-gp and ABCC1 decreased to 72.9 and 81.8%
when PTX was combined with TMP. A low dose of TMP did not reduce
the expression of drug resistance proteins. However, EXOs-TMP
reduced the resistance protein to ~70%. It was hypothesized that
EXOs are composed of cell membranes, which can fuse with the plasma
membrane or endocytic membrane and deliver TMP, bypassing
P-gp-mediated efflux. Thus, the TMP worked. Furthermore, when
EXOs-TMP were combined with PTX, the expression of drug resistance
proteins was reduced to 50–60%. The effect was more significant
than that of free TMP + PTX. GSTP1 serves an important regulatory
role in detoxification, anti-oxidative damage and the occurrence of
various diseases (56). GSTP1 is
involved in the anti-apoptosis and metabolism of numerous
chemotherapeutic drugs. In ovarian cancer, highly expressed GSTP1
serves a major role in the metabolism of cisplatin and carboplatin
(57). To the to the best of the
authors' knowledge, the present study was the first to demonstrate
that EXO-TMP also exerted an inhibitory effect on GSTP1. The trend
was consistent with the resistance proteins. Therefore, loading TMP
into EXOs can exert an improved effect on drug-resistant cells.
As one of the most crucial mechanisms for triggering
cell death, the effective elimination of cancer cells through
apoptosis has been a primary objective in clinical cancer therapy.
Dioscin inhibits the growth of human osteosarcoma by inducing
apoptosis in in vitro and in vivo settings (58). TMP can induce apoptosis in a
variety of tumor cells (59,60).
However, no apoptotic effects in A2780T ovarian cancer cells were
observed in the present study. While TMP has been found to enhance
the apoptosis-inducing effect of PTX in A2780 cells, the present
experiments demonstrated that free TMP did not enhance the
apoptotic effect of PTX on drug-resistant cells (54). It was hypothesized that TMP was
lost to induce apoptosis through ABC transporter and its isoenzyme
GSTP1, which was consistent with the failure of free TMP to reduce
the expression of drug resistance proteins in resistant cells.
Nonetheless, EXOs-TMP itself could promote the apoptosis of A2780T
cells. TMP alone has not been shown to induce apoptosis in ovarian
cancer cells. The combination of EXO-TMP and PTX increased the
apoptosis rate by ~threefold, highlighting the potential of EXO-TMP
as a reversal agent for overcoming drug resistance in tumor cells.
Due to the stability in bodily fluids, good biocompatibility, and
strong targeting capability of EXOs, the present study confirmed
their potential as carriers for traditional Chinese medicine
formulations. This provided a novel drug delivery pathway for
overcoming multidrug resistance in tumors.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Dalian Traditional
Chinese Medicine Scientific Research Project (grant no. 21Z12008),
the Dalian Key Field Innovation Team Project (grant no. 2021RT14)
and In-Hospital Cultivation Project of the Second Hospital of
Dalian Medical University (grant no. dy2yynpy202210).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CZ, LQ, DW, HL, LC contributed to the study
conception and design. CZ, LQ, LC wrote the manuscript and
collected and analyzed data. CZ and LC critically revised the final
manuscript. MZ, WX interpreted data, CG was responsible for
photographing and organizing data images. CZ, LQ, DW, MZ, WX, CG,
HL and LC confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EXO
|
exosome
|
|
EXO-TMP
|
exosome-tetramethylpyrazine
|
|
P-gp
|
P-glycoprotein
|
|
PTX
|
paclitaxel
|
References
|
1
|
Miller KD, Nogueira L, Mariotto AB,
Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL and Siegel
RL: Cancer treatment and survivorship statistics, 2019. CA Cancer J
Clin. 69:363–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bodurka-Bevers D, Sun CC and Gershenson
DM: Pharmacoeconomic considerations in treating ovarian cancer.
Pharmacoeconomics. 17:133–150. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang ZJ, Zhao BB and Li L: The
significance of the change pattern of serum CA125 level for judging
prognosis and diagnosing recurrences of epithelial ovarian cancer.
J Ovarian Res. 9:572016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiao R, You L, Zhang L, Guo X, Guo E, Zhao
F, Yang B, Li X, Fu Y, Lu F, et al: Inhibiting the IRE1α axis of
the unfolded protein response enhances the antitumor effect of
AZD1775 in TP53 mutant ovarian cancer. Adv Sci (Weinh).
9:e21054692022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miller EM, Samec TM and Alexander-Bryant
AA: Nanoparticle delivery systems to combat drug resistance in
ovarian cancer. Nanomedicine. 31:1023092021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chiappa M, Guffanti F, Bertoni F, Colombo
I and Damia G: Overcoming PARPi resistance: Preclinical and
clinical evidence in ovarian cancer. Drug Resist Updat.
55:1007442021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Norouzi-Barough L, Sarookhani MR, Sharifi
M, Moghbelinejad S, Jangjoo S and Salehi R: Molecular mechanisms of
drug resistance in ovarian cancer. J Cell Physiol. 233:4546–4562.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eisenhauer EA: Real-world evidence in the
treatment of ovarian cancer. Ann Oncol. 28 (Suppl 8):viii61–viii65.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang MY, Zhang LL, Ding J and Lu JJ:
Anticancer drug discovery from Chinese medicinal herbs. Chin Med.
13:352018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Liu Z, Sui X, Wu Q, Wang J and Xu
C: Elemene injection as adjunctive treatment to platinum-based
chemotherapy in patients with stage III/IV non-small cell lung
cancer: A meta-analysis following the PRISMA guidelines.
Phytomedicine. 59:1527872019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang E, Shi H, Yang L, Wu X and Wang Z:
Ginsenoside Rd regulates the Akt/mTOR/p70S6K signaling cascade and
suppresses angiogenesis and breast tumor growth. Oncol Rep.
38:359–367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Malyutina A, Majumder MM, Wang W, Pessia
A, Heckman CA and Tang J: Drug combination sensitivity scoring
facilitates the discovery of synergistic and efficacious drug
combinations in cancer. PLoS Comput Biol. 15:e10067522019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo SK, Chen KJ, Qian ZH, Weng WL and Qian
MY: Tetramethylpyrazine in the treatment of cardiovascular and
cerebrovascular diseases. Planta Med. 47:891983. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Z, Zhang C, Gao F, Fu Q, Fu C, He Y
and Zhang J: A systematic review on the rhizome of Ligusticum
chuanxiong Hort. (Chuanxiong). Food Chem Toxicol. 119:309–325.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao Y, Liu Y and Chen K: Mechanisms and
clinical application of tetramethylpyrazine (an interesting natural
compound isolated from Ligusticum Wallichii): Current status
and perspective. Oxid Med Cell Longev. 2016:21246382016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang S, Lei T and Zhang M: The reversal
effect and its mechanisms of tetramethylpyrazine on multidrug
resistance in human bladder cancer. PLoS One. 11:e01577592016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang XB, Wang SS, Zhang QF, Liu M, Li HL,
Liu Y, Wang JN, Zheng F, Guo LY and Xiang JZ: Inhibition of
tetramethylpyrazine on P-gp, MRP2, MRP3 and MRP5 in multidrug
resistant human hepatocellular carcinoma cells. Oncol Rep.
23:211–215. 2010.PubMed/NCBI
|
|
18
|
Zhou X, Wang A, Wang L, Yin J, Wang L, Di
L, Hoi MP, Shan L, Wu X and Wang Y: A danshensu-tetramethylpyrazine
conjugate DT-010 overcomes multidrug resistance in human breast
cancer. Front Pharmacol. 10:7222019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang HH, Liu FB, Ruan Z, Zheng J, Su YJ
and Wang J: Tetramethylpyrazine (TMPZ) triggers S-phase arrest and
mitochondria-dependent apoptosis in lung cancer cells. Neoplasma.
65:367–375. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alotaibi BS, Buabeid M, Ibrahim NA,
Kharaba ZJ, Ijaz M, Noreen S and Murtaza G: Potential of
nanocarrier-based drug delivery systems for brain targeting: A
current review of literature. Int J Nanomedicine. 16:7517–7533.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367:eaau69772020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Antimisiaris SG, Mourtas S and Marazioti
A: Exosomes and exosome-inspired vesicles for targeted drug
delivery. Pharmaceutics. 10:2182018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim MS, Haney MJ, Zhao Y, Mahajan V,
Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O,
et al: Development of exosome-encapsulated paclitaxel to overcome
MDR in cancer cells. Nanomedicine. 12:655–664. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elsharkasy OM, Nordin JZ, Hagey DW, de
Jong OG, Schiffelers RM, Andaloussi SE and Vader P: Extracellular
vesicles as drug delivery systems: Why and how? Adv Drug Deliv Rev.
159:332–343. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chinnappan M, Srivastava A, Amreddy N,
Razaq M, Pareek V, Ahmed R, Mehta M, Peterson JE, Munshi A and
Ramesh R: Exosomes as drug delivery vehicle and contributor of
resistance to anticancer drugs. Cancer Lett. 486:18–28. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma J, Zhang Y, Tang K, Zhang H, Yin X, Li
Y, Xu P, Sun Y, Ma R, Ji T, et al: Reversing drug resistance of
soft tumor-repopulating cells by tumor cell-derived
chemotherapeutic microparticles. Cell Res. 26:713–727. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao Y, Zhang H, Zhou N, Xu P, Wang J, Gao
Y, Jin X, Liang X, Lv J, Zhang Y, et al: Methotrexate-loaded
tumour-cell-derived microvesicles can relieve biliary obstruction
in patients with extrahepatic cholangiocarcinoma. Nat Biomed Eng.
4:743–753. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rao Q, Zuo B, Lu Z, Gao X, You A, Wu C, Du
Z and Yin H: Tumor-derived exosomes elicit tumor suppression in
murine hepatocellular carcinoma models and humans in vitro.
Hepatology. 64:456–472. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yong T, Zhang X, Bie N, Zhang H, Zhang X,
Li F, Hakeem A, Hu J, Gan L, Santos HA and Yang X: Tumor
exosome-based nanoparticles are efficient drug carriers for
chemotherapy. Nat Commun. 10:38382019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu Z, Tang H, Chen S, Xie Y, Shi L, Xia S,
Jiang M, Li J and Chen D: Exosomal LOC85009 inhibits docetaxel
resistance in lung adenocarcinoma through regulating ATG5-induced
autophagy. Drug Resist Updat. 67:1009152023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shieh MJ, Hsu CY, Huang LY, Chen HY, Huang
FH and Lai PS: Reversal of doxorubicin-resistance by
multifunctional nanoparticles in MCF-7/ADR cells. J Control
Release. 152:418–425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bruckmueller H and Cascorbi I: ABCB1,
ABCG2, ABCC1, ABCC2, and ABCC3 drug transporter polymorphisms and
their impact on drug bioavailability: What is our current
understanding? Expert Opin Drug Metab Toxicol. 17:369–396. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cui J, Li G, Yin J, Li L, Tan Y, Wei H,
Liu B, Deng L, Tang J, Chen Y and Yi L: GSTP1 and cancer:
Expression, methylation, polymorphisms and signaling (review). Int
J Oncol. 56:867–878. 2020.PubMed/NCBI
|
|
34
|
Jiang L and Hou R: Tetrandrine reverses
paclitaxel resistance in human ovarian cancer via inducing
apoptosis, cell cycle arrest through β-catenin pathway. Onco
Targets Ther. 13:3631–3639. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang Z, Zhang Q, Yu L, Zhu J, Cao Y and
Gao X: The signaling pathways and targets of traditional Chinese
medicine and natural medicine in triple-negative breast cancer. J
Ethnopharmacol. 264:1132492021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Meyer CT, Wooten DJ, Paudel BB, Bauer J,
Hardeman KN, Westover D, Lovly CM, Harris LA, Tyson DR and Quaranta
V: Quantifying drug combination synergy along potency and efficacy
axes. Cell Syst. 8:97–108.e116. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fu Z, Lin L, Liu S, Qin M, He S, Zhu L and
Huang J: Ginkgo biloba extract inhibits metastasis and
ERK/nuclear factor kappa B (NF-κB) signaling pathway in gastric
cancer. Med Sci Monit. 25:6836–6845. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Y, Gao S, Zhu J, Zheng Y, Zhang H and
Sun H: Dihydroartemisinin induces apoptosis and inhibits
proliferation, migration, and invasion in epithelial ovarian cancer
via inhibition of the hedgehog signaling pathway. Cancer Med.
7:5704–5715. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li CY, Wang Q, Wang X, Li G, Shen S and
Wei X: Scutellarin inhibits the invasive potential of malignant
melanoma cells through the suppression epithelial-mesenchymal
transition and angiogenesis via the PI3K/Akt/mTOR signaling
pathway. Eur J Pharmacol. 858:1724632019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yi M, Jiao D, Qin S, Chu Q, Wu K and Li A:
Synergistic effect of immune checkpoint blockade and
anti-angiogenesis in cancer treatment. Mol Cancer. 18:602019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cui H, Guo W, Zhang B, Li G, Li T, Yuan Y,
Zhang N, Yang Y, Feng W, Chu F, et al: BA-12 inhibits angiogenesis
via glutathione metabolism activation. Int J Mol Sci. 20:40622019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang H, Ding S and Xia L: Ligustrazine
inhibits the proliferation and migration of ovarian cancer cells
via regulating miR-211. Biosci Rep. 41:BSR202001992021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ma H, Deng C, Zong X, He Y, Cheng L, Fan
Q, Shao M, Lin Y, Zhao C, Li G and Zhang C: Reversal of
doxorubicin-resistance by delivering tetramethylprazine via
folate-chitosan nanoparticles in MCF-7/ADM cells. Int J Clin Exp
Med. 9:5439–5448. 2016.
|
|
46
|
Li FS and Weng JK: Demystifying
traditional herbal medicine with modern approach. Nat Plants.
3:171092017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shi J, Kantoff PW, Wooster R and Farokhzad
OC: Cancer nanomedicine: Progress, challenges and opportunities.
Nat Rev Cancer. 17:20–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
He H, Liu L, Morin EE, Liu M and
Schwendeman A: Survey of clinical translation of cancer
nanomedicines-lessons learned from successes and failures. Acc Chem
Res. 52:2445–2461. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Namee NM and O'Driscoll L: Extracellular
vesicles and anti-cancer drug resistance. Biochim Biophys Acta Rev
Cancer. 1870:123–136. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Batrakova EV and Kim MS: Using exosomes,
naturally-equipped nanocarriers, for drug delivery. J Control
Release. 219:396–405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kalimuthu S, Gangadaran P, Rajendran RL,
Zhu L, Oh JM, Lee HW, Gopal A, Baek SH, Jeong SY, Lee SW, et al: A
new approach for loading anticancer drugs into mesenchymal stem
cell-derived exosome mimetics for cancer therapy. Front Pharmacol.
9:11162018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li YJ, Wu JY, Wang JM, Hu XB, Cai JX and
Xiang DX: Gemcitabine loaded autologous exosomes for effective and
safe chemotherapy of pancreatic cancer. Acta Biomater. 101:519–530.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Qiao L, Hu S, Huang K, Su T, Li Z,
Vandergriff A, Cores J, Dinh PU, Allen T, Shen D, et al: Tumor
cell-derived exosomes home to their cells of origin and can be used
as Trojan horses to deliver cancer drugs. Theranostics.
10:3474–3487. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zou L, Liu X, Li J, Li W, Zhang L, Li J
and Zhang J: Tetramethylpyrazine enhances the antitumor effect of
paclitaxel by inhibiting angiogenesis and inducing apoptosis. Front
Pharmacol. 10:7072019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang JQ, Yang Y, Cai CY, Teng QX, Cui Q,
Lin J, Assaraf YG and Chen ZS: Multidrug resistance proteins
(MRPs): Structure, function and the overcoming of cancer multidrug
resistance. Drug Resist Updat. 54:1007432021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lei X, Du L, Yu W, Wang Y, Ma N and Qu B:
GSTP1 as a novel target in radiation induced lung injury. J Transl
Med. 19:2972021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sawers L, Ferguson MJ, Ihrig BR, Young HC,
Chakravarty P, Wolf CR and Smith G: Glutathione S-transferase P1
(GSTP1) directly influences platinum drug chemosensitivity in
ovarian tumour cell lines. Br J Cancer. 111:1150–1158. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ding Q, Zhang W, Cheng C, Mo F, Chen L,
Peng G, Cai X, Wang J, Yang S and Liu X: Dioscin inhibits the
growth of human osteosarcoma by inducing G2/M-phase arrest,
apoptosis, and GSDME-dependent cell death in vitro and in vivo. J
Cell Physiol. 235:2911–2924. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mohammad RM, Muqbil I, Lowe L, Yedjou C,
Hsu HY, Lin LT, Siegelin MD, Fimognari C, Kumar NB, Dou QP, et al:
Broad targeting of resistance to apoptosis in cancer. Semin Cancer
Biol. 35 (Suppl):S78–S103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wu X, Wang Z, Wu G, Xu X, Zhang J, Li Y,
Zhang H and Guo S: Tetramethylpyrazine induces apoptosis and
inhibits proliferation of hypertrophic scar-derived fibroblasts
via inhibiting the phosphorylation of AKT. Front Pharmacol.
11:6022020. View Article : Google Scholar : PubMed/NCBI
|