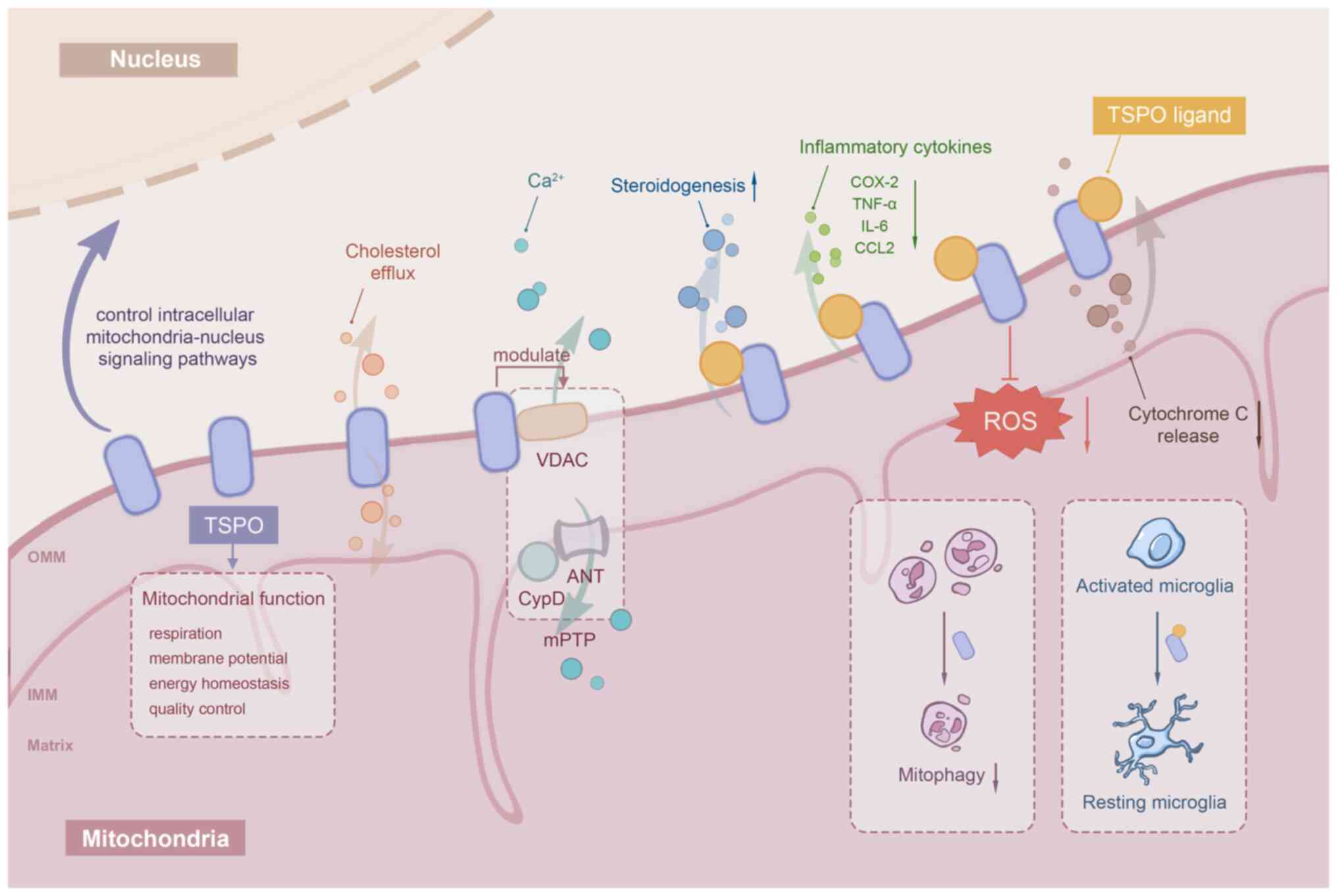
Translocator protein (TSPO; 18 kDa) is a highly
structurally conserved hydrophobic protein located on the outer
mitochondrial membrane (OMM) (1).
Discovered in the 1970s, TSPO was originally termed the ‘peripheral
benzodiazepine receptor’ due to its specific binding site for
certain benzodiazepines in peripheral tissues (2). In 2006, the name was changed to TSPO
to reflect the cholesterol binding and transportation functions of
the protein (3). TSPO is also
involved in a variety of cellular physiological activities such as
mitochondrial quality control, mitochondrial permeability
transition pore opening, voltage-dependent anion channel (VDAC)
opening, calcium transport, cellular proliferation, programmed
apoptosis and reactive oxygen species (ROS) production (4–8).
This extensive range of molecular functions has led to the
association of TSPO with the pathogenesis of multiple diseases,
including central nervous system diseases and cardiovascular
anomalies (9–12). Increasing research interest in TSPO
as a crucial pathogenic factor and therapeutic target has driven
the study of TSPO-specific, high-affinity binding molecules, known
as TSPO ligands. TSPO ligands have been shown to have a beneficial
effect by agonizing or inhibiting TSPO activity in diseases such as
Alzheimer's disease (AD), multiple sclerosis and cardiac
arrhythmias (13–15). It was not until the previous
decade, however, that the role of TSPO and its ligands in ocular
diseases became an area of active research, and a number of
promising developments have been identified (16–20).
The present article provided a review of the advances in the study
of TSPO and its ligands in ocular diseases. First, an overview of
TSPO function was presented, and a variety of classical and novel
TSPO ligands were described. Next, the expression of TSPO in ocular
tissues based on existing studies was summarized. Finally, the role
of TSPO and its ligands in different ocular conditions was
discussed, including ocular development and aging, age-related
macular degeneration (AMD), retinal ischemia, diabetic retinopathy
(DR) and glaucoma. The aim of the present review was to highlight
the research value of TSPO in ophthalmology and to suggest
perspectives on potential therapeutic targets.
TSPO is a highly hydrophobic protein that is
expressed in both prokaryotic and eukaryotic cells, from bacteria
to humans (21). TSPO is primarily
localized to the OMM, adjacent to the VDAC and adenine nucleotide
translocase (ANT) (1,22,23).
This 169-amino acid protein in humans is encoded by a nuclear gene
containing four exons (24) and
the mature protein contains five α-helical transmembrane domains
(TMs). A cholesterol-recognition amino acid consensus sequence is
located in the C-terminus of the TM structure, through which TSPO
binds to the lipid membrane (25,26).
The 3D structure of TSPO in complex with the TSPO-targeting ligand,
(R)-1-(2-chlorophenyl- N-methylpropyl)-3-isoquinolinecarboxamide
(PK11195), comprising five transmembrane helices (TM1-TM5) that
tightly pack together in the clockwise order TM1-TM2-TM5-TM4-TM3
viewed from the cytosol, has also been revealed (26). TSPO is highly expressed in a
variety of tissues, particularly in the gonads, adrenal glands,
placenta and brain, due to its high abundance in steroidogenic
cells (27). Therefore, the
pathogenic effects of TSPO and the potential therapeutic value of
TSPO ligands for anxiety disorders, AD and hypogonadism have been
thoroughly investigated in vitro and in vivo
(28–30).
TSPO ligands are classified as endogenous or
synthetic ligands. In previous years, TSPO-ligand interactions have
received increasing attention, with multiple studies reporting its
positive effects on diseases such as AD, multiple sclerosis,
anxiety and vasculopathy (13–15,41).
Steroidogenesis and anti-inflammation are the main pathways by
which TSPO ligands function (42).
However, the independent mechanism, ‘mitohormesis’, defined as
subtoxic stress on mitochondria, may be responsible for the
protective effects of TSPO ligands. It is proposed that the ligands
suppress ATP synthesis through mitohormesis, leading to activation
of the cellular damage-response network. This, in turn, enhances
the tolerance of cells to the degeneration processes, including
oxidative damage, phototoxicity or DNA damage (43). A summary of classical and novel
ligands and their features and effects based on existing studies is
described below and in Table
I.
Porphyrins are high-affinity endogenous ligands for
TSPO, in the micromolar to nanomolar range (35). Mitochondria are critically involved
in porphyrin metabolism and TSPO is closely related to this process
(26,44). Porphyrins are involved in heme
synthesis, and protoporphyrin IX (PPIX), as a key intermediate in
the heme biosynthesis pathway, has been one of the most extensively
studied endogenous ligands since its discovery in 1987 (45,46).
Previous studies have reported that TSPO-PPIX interactions regulate
mitochondrial membrane permeability and thus exert cytoprotective
effects (46,47). Porphyrin-induced oxidative stress
is thought to be the major mechanism of porphyrin-mediated tissue
damage (39,48). Furthermore, Yamamoto et al
(49) demonstrated that TSPO
knockdown leads to the accumulation of PPIX and ROS in glioblastoma
cells. Specifically, TSPO possibly functions by binding or
sequestering PPIX rather than via TSPO-mediated transmembrane
transport (50). Unbound PPIX
inhibits heme oxygenase and prevents free heme degradation, thereby
maintaining the balance between free and bound heme and preventing
cytotoxic effects (51).
PK11195 was the first non-benzodiazepine TSPO
synthetic ligand discovered that acts in the nanomolar range
(42). PK11195 is considered one
of the best-characterized and prototypic ligands of TSPO (50), and is widely used to study the
roles and functions of TSPO in various diseases, particularly in
in vivo studies. [11C]PK11195 has been
additionally applied as a radioligand for positron emission
tomography (PET). Despite several limitations, such as poor brain
permeability, kinetic instability and signal-to-noise ratio issues
due to non-specific binding (52–54),
[11C]PK11195 remains the standard TSPO-based PET
radiotracer for assessing the activation of microglia or
macrophages in central nervous system disorders (55,56).
PK11195 has also been reported to have anti-neuroinflammation
effects in experimental models of AD (10) and pressure-induced retinal ganglion
cell (RGC) injury (57). However,
further research is needed to elucidate the specific mechanisms
underlying these protective effects, including whether they are
related to the stimulation effect on steroidogenesis in peripheral
tissues and the brain (10,58).
PK11195 downregulates the expression of the inflammatory cytokines,
tumor necrosis factor-α (TNF-α) and cyclooxygenase-2 (COX-2), in
lipopolysaccharide (LPS)-induced human microglia (59,60),
and modulation of Ca2+-mediated signaling pathways may
be involved in its anti-inflammatory actions (61). In prostate cancer cells, PK11195
can be used as a sensitizer to the chemotherapy agents mda-7/IL-24
(Ad.mda-7, a novel cytokine gene belonging to the IL-10 gene
superfamily) (62), both in
vitro and in vivo. PK11195 promotes cellular autophagy
by inactivating the oncoprotein, B-cell lymphoma-2, and by
targeting F1F0-ATP synthase (63), an essential component of mPTPs
(64). Notably, Gonzalez-Polo
et al (65) reported that
PK11195 promotes starvation-induced cell death through an
unexpected pathway independent of TSPO, which directly activates
the mitochondrial apoptotic pathway, as demonstrated by cytochrome
c release and caspase-3 activation. Although PK11195 is generally
considered to be a TSPO antagonist, there is evidence that PK11195
displays a partial agonistic effect on TSPO (66), which is possibly dependent on the
cell type and drug concentration.
Ro5-4864 is another classical synthetic TSPO ligand
that has been used to investigate the characteristics of TSPO in
health and disease due to its nanomolar selectivity (67,68).
However, the application of Ro5-4864 as a pharmacological agent
(67) is limited as its affinity
varies with species, from rat (high affinity) to human (low
affinity) (21). Another
limitation is that the affinity of [3H] Ro5-4864 can be
influenced by temperature, as found in brain membrane binding
assays (69). Gut et al
(70) reported that Ro5-4864 is
sufficient to reverse the glucose level fluctuations induced by
isoprenaline in zebrafish larvae, suggesting that Ro5-4864 may
influence glucose homoeostasis in response to altered mitochondrial
energy balance. Ro5-4864 also has certain features that are similar
to that of PK11195. For example, Ro5-4864 stimulates steroid
formation (10), contributes to
the neuroprotective effect (but differentiates from PK11195
activity under different experimental conditions) (71) and inhibits pro-inflammatory factors
and ROS production in human microglia incubated with LPS (16,59).
Furthermore, Ro5-4864 antagonizes the anxiolytic and
antidepressant-like effects of other TSPO ligands. For example, the
antidepressant properties of PK11195 induced in mice subjected to
the forced swimming test was blocked by Ro5-4864 administration
(72,73). Ro5-4864 has also been shown to
contribute to the maintenance of mitochondrial homeostasis,
reducing cytochrome c release and caspase-3 activation, thereby
inhibiting the mitochondrial apoptosis pathway (36). Furthermore, Baez et al
(74) demonstrated that Ro5-4864
maintains mitochondrial function in the T89G astrocyte cell line in
the presence of glucose deprivation damage by reducing the
production of free radicals.
XBD173 (AC-5216; Emapunil) is a novel high-affinity
and selective phenylpurine ligand of TSPO, which has been well
investigated and widely used in the research and therapy of anxiety
disorders (41,75,76).
GABA-mediated inhibitory postsynaptic currents were potentiated by
XBD173 in both animal experiments and clinical studies (75), and this neurotransmission appeared
to be mediated indirectly through the generation of neurosteroids
and could be prevented by the 5α-reductase inhibitor, finasteride
(76). According to previous
studies, the role of XBD173 in microglia has been well defined and
has been shown to alleviate the neurotoxic effect of microglia and
inhibit cell proliferation and migration (16,77).
The XBD173/TSPO axis appears to target the microglia/macrophage
marker, CD68, and inhibit the pro-inflammatory genes, C-C motif
chemokine receptor-2 and interleukin (IL-)6, to alleviate
apoptosis, thereby alleviating neurodegenerative dysfunction
(78). Mages et al
(18) further investigated the
exact response patterns of the major glial cell types of the retina
in a retinal ischemia mouse model. In the study it was reported
that XBD173 treatment resulted in lower microglia accumulation, and
the microglial activation profile showed a transformation towards
the anti-inflammatory M2 phenotype. However, the aforementioned
effects of XBD173 on microglia could be reversed to some extent,
since the reduced microglial cell number induced by XBD173 could
transiently rise again (18). In
addition, even with XBD173 intervention, cellular morphological
changes (from ramified to amoeboid) representing microglial
activation were observed, suggesting that XBD173 only partially
inhibits microglial activation (18).
To the best of our knowledge, Etifoxine
(etafenoxine; Stresam®) is currently the only commercially
available TSPO ligand (79).
Etifoxine was developed in the 1960s for anxiety disorders and is
administered for this purpose in >42 different countries
(80). However previous studies
have since reported its therapeutic potential in peripheral nerve
injury and demyelination, and chemotherapy-induced pain (14,80,81).
As a non-benzodiazepine anxiolytic, etifoxine appears to exercise
its functions by binding to the β2 or β3 subunits of the
GABAA receptor complex (82). Etifoxine also regulates
GABAA receptors indirectly by stimulating neurosteroid
production in both the central and peripheral nervous systems
(24,83). Furthermore, Biswas et al
(84) suggested that etifoxine
modulates cholesterol homeostasis, promoting cholesterol efflux and
transporting cholesterol to the liver, which is the most important
function of this ligand. The authors also found that etifoxine
enhanced the expression of cholesterol metabolism and transport
enzymes in high-fat diet mice and significantly decreased the total
cholesterol, triglycerides and phospholipid mass in the retinal
pigment epithelium (RPE). This finding was consistent with that of
a study by Ibrahim et al (85), which suggested that serum lipid
levels were downregulated in etifoxine-treated mice. Furthermore,
etifoxine leads to a higher conversion of cholesterol into
high-density lipoprotein while preventing intracellular lipid
accumulation, and therefore protecting cells against apoptosis by
inhibiting oxidized low-density lipoprotein-induced ROS production
and inflammatory cytokine secretion (17,25).
In addition, etifoxine stimulates neurosteroid synthesis; however,
Costa et al (86) suggested
that this effect depends more on residence time at the TSPO binding
site rather than the binding affinities.
Next, the expression of TSPO in ocular tissues, the
mechanisms by which TSPO is involved in the progression of various
ocular diseases (Fig. 2) and the
role of TSPO ligands in therapeutic interventions are discussed,
based on existing studies.
In recent years, the role of TSPO in ocular diseases
has gained attention. However, it must be acknowledged that the
number of studies on TSPO expression in ocular cells and tissues
has been limited. Biswas et al (17,25)
reported high TSPO expression in the RPE and choroidal endothelium
of mice. This observation was consistent with that of a study by
Mages et al (18), in which
specific cell types were enriched via immunomagnetic separation and
then protein expression levels were measured using mass
spectrometry analysis. It was noted that TSPO was most highly
expressed in RPE cells, vascular cells and Müller cells, while
expression was lower in microglia and almost undetectable in
retinal neurons (16,18,77).
By contrast, Biswas et al (17) reported strong TSPO signals in the
ganglial cell layer by immunofluorescence analysis in mouse eye
sections. Differences in TSPO expression and localization may be
due to differences in sample collection and immunoassay approaches.
In addition, TSPO expression has been detected in human retina and
RPE (77,92,93),
with both containing high expression at the mRNA level. When the
central and peripheral sections of the retina and RPE were isolated
separately, the peripheral RPE exhibited higher TSPO expression
than the central RPE (92). It
remains unclear whether this infers any functional implications. To
the best of our knowledge, there have been no further reports of
TSPO expression in other ocular structures such as the cornea,
conjunctiva, lens, meibomian gland and lacrimal gland.
Retinal ischemia is a major cause of visual
impairment, often leading to irreversible morphological and
functional injuries with depleted ATP stores (117). Extensive research has been
devoted to clarifying the mechanisms of ischemia-induced neuronal
damage, although debates persist. Retinal ischemic injury involves
a cascade of self-reinforcing and destructive events, including
neuronal depolarization, calcium imbalance and oxidative stress due
to increased energy depletion and enhanced glutamate stimulation
(118–121). TSPO was discovered to be
upregulated in microglia during the early stages of retinal
ischemia injury, which then decreased 4 days later (16). TSPO expression increases over time
in Müller cells, which is the primary immune cell type in the
retina, indicating an association between TSPO and the active
retinal immune response during ischemic injury (16,18).
A number of studies have shown that TSPO is closely associated with
microglial activation and microglial energy metabolism (98,122). Thus, the transient increase in
TSPO expression may be due to a microglia-mediated immune response
or an acute response to an imbalanced energy supply in the
postischemic phase. Furthermore, mice treated with the TSPO ligand,
XBD173, have a significant reduction in neuron death in the
postischemic retina. In addition, they have a decreased microglial
number accompanied by phenotypic transformation from the M1 type to
the M2 type, indicating anti-inflammatory activation (18). This finding concurred with those of
Wang et al (16) and
Karlstetter et al (77),
who found that XBD173 inhibited microglial neurotoxicity and
proliferation. The effect of XBD173 on Müller cells was also
notable since it increased the level of DBI in postischemic Müller
cells (18). As aforementioned,
DBI an endogenous TSPO ligand, can alleviate neuroinflammation and
induce microglia to favor the resting state (16). XBD173 regulates glutamine
synthetase expression, thereby maintaining the metabolism of a
‘neuron-supportive microenvironment’ and protecting against
glutamate-overload induced neurotoxic injury (123–126). It should be noted that the
aforementioned effect of XBD173 was incomplete, as the transient
upregulation of inflammatory markers as well as the downregulation
of glutamine synthetase were still detected following XBD173
therapy. Given the high abundance of TSPO in the RPE, its role in
retinal ischemia injury should not be overlooked. However, to the
best of our knowledge, no relevant studies on the association
between TSPO and RPE injury in retinal ischemia have been published
to date. In summary, TSPO is involved in the pathogenic reactions
of specific cell types to retinal ischemia and XBD173 treatment
increased retinal neuron survival, to a certain extent.
Glaucoma is a leading cause of irreversible
blindness worldwide and is characterized by progressive optic nerve
injury and visual field loss (142,143). Glaucoma is a multifactorial
disorder and elevated intraocular pressure is thought to be a major
risk factor, leading to morphological deformation of the lamina
cribrosa and disturbance of axoplasmic transport in RGC axons
(144). Acute or chronic
deficiency of these RGC neurotrophic signals ultimately leads to
apoptosis (144). Other related
pathogeneses of glaucoma include ocular hemodynamic abnormalities,
low intracranial pressure, autoimmune dysfunction and mitochondrial
dysfunction, while further studies are needed to investigate the
detailed mechanisms of RGC injury (145). The neurosteroid, allopregnanolone
(AlloP), has been shown to synthesize and exert neuroprotective
effects on the retina through the GABAA receptor pathway
in a rat model of acute glaucoma in vitro (146,147). As a major mitochondrial
cholesterol carrier, TSPO mediates the transport of cholesterol
into the mitochondria, which is a key process in the synthesis of
pregnenolone (57). Pregnenolone
then functions as the starting material for AlloP synthesis
(83,148). Under elevated pressure (75 mmHg),
TSPO expression is found to be upregulated and TSPO colocalizes
with AlloP and 5α-reductase (5αRD; a rate-limiting enzyme in the
AlloP synthesis pathway) in RGCs, suggesting that RGCs are the
principal site of AlloP synthesis (57). Furthermore, the TSPO antagonist,
atriol, significantly inhibits the production of AlloP (149), suggesting that TSPO is important
in AlloP synthesis. In addition, atriol induces changes in retinal
excitotoxicity, including edema-like changes in the inner plexiform
layer and bull's eye formation in the inner nuclear layer (150,151). Moreover, the TSPO ligand,
PK11195, acts as a selective agonist that promotes TSPO expression
and inhibits pressure-induced RGC apoptosis (57). Atriol and 5αRD inhibitors (24) reverse this effect, suggesting that
PK11195 may exert neuroprotective effects by promoting the
TSPO-5αRD-mediated AlloP synthesis pathway, as aforementioned.
Hence, TSPO agonists may have therapeutic potential for ocular
hypertension injury.
Overall, the present review concluded that TSPO is
broadly involved in multiple cellular processes as well as
mitochondrial functions. TSPO participates in the occurrence and
development of various ocular diseases, including AMD, DR, retinal
ischemia and glaucoma, with evidence of both protective and
aggravating effects in the observed pathology of the diseases.
There is increasing evidence that endogenous or synthetic TSPO
ligands, which modulate TSPO functions, play beneficial roles in
the aforementioned diseases primarily by promoting steroidogenesis,
maintaining cholesterol homeostasis, exerting anti-inflammatory
effects, decreasing ROS production and regulating microglial
activation. These findings suggest that TSPO may be a key potential
therapeutic target. Despite the progress made in understanding the
role of TSPO in ocular diseases, the expression of TSPO in ocular
surface tissues, the lens, the lacrimal gland and the optic nerve
and whether TSPO plays a key role in these diseases remain to be
investigated. Moreover, whether TSPO is an essential protein for
ocular tissue in healthy individuals is another question that
requires more extensive study. At present, the use of TSPO ligands
in the ocular environment is limited and there is a lack of
comprehensive evaluation of their potential adverse effects. Only
by addressing these issues can TSPO and its ligands be applied on a
larger scale.
Not applicable.
The present research was funded by Tianjin Key Medical
Discipline (Specialty) Construction Project (grant no.
TJYXZDXK-037A).
Not applicable.
MY and SZ designed the study; MY performed the
figure preparation and manuscript draft; and SZ reviewed and edited
the manuscript. All authors read and approved the final version of
the manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Shoshan-Barmatz V, Pittala S and Mizrachi
D: VDAC1 and the TSPO: Expression, Interactions, and associated
functions in health and disease states. Int J Mol Sci. 20:33482019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Braestrup C and Squires RF: Specific
benzodiazepine receptors in rat brain characterized by
high-affinity (3H)diazepam binding. Proc Natl Acad Sci USA.
74:3805–3809. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Papadopoulos V, Baraldi M, Guilarte TR,
Knudsen TB, Lacapère JJ, Lindemann P, Norenberg MD, Nutt D, Weizman
A, Zhang MR and Gavish M: Translocator protein (18kDa): New
nomenclature for the peripheral-type benzodiazepine receptor based
on its structure and molecular function. Trends Pharmacol Sci.
27:402–409. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tu LN, Zhao AH, Hussein M, Stocco DM and
Selvaraj V: Translocator Protein (TSPO) affects mitochondrial fatty
acid oxidation in steroidogenic cells. Endocrinology.
157:1110–1121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bonsack F and Sukumari-Ramesh S: TSPO: An
evolutionarily conserved protein with elusive functions. Int J Mol
Sci. 19:16942018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fan J and Papadopoulos V: Mitochondrial
TSPO deficiency triggers retrograde signaling in MA-10 mouse tumor
leydig cells. Int J Mol Sci. 22:2522020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vainshtein A, Veenman L, Shterenberg A,
Singh S, Masarwa A, Dutta B, Island B, Tsoglin E, Levin E,
Leschiner S, et al: Quinazoline-based tricyclic compounds that
regulate programmed cell death, induce neuronal differentiation,
and are curative in animal models for excitotoxicity and hereditary
brain disease. Cell Death Discov. 1:150272015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sileikyte J, Blachly-Dyson E, Sewell R,
Carpi A, Menabò R, Di Lisa F, Ricchelli F, Bernardi P and Forte M:
Regulation of the mitochondrial permeability transition pore by the
outer membrane does not involve the peripheral benzodiazepine
receptor (Translocator Protein of 18 kDa (TSPO)). J Biol Chem.
289:13769–13781. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ilkan Z and Akar FG: The mitochondrial
translocator protein and the emerging link between oxidative stress
and arrhythmias in the diabetic heart. Front Physiol. 9:15182018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arbo BD, Ribeiro MF and Garcia-Segura LM:
Development of new treatments for Alzheimer's disease based on the
modulation of translocator protein (TSPO). Ageing Res Rev.
54:1009432019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mitro N, Cermenati G, Giatti S, Abbiati F,
Pesaresi M, Calabrese D, Garcia-Segura LM, Caruso D and Melcangi
RC: LXR and TSPO as new therapeutic targets to increase the levels
of neuroactive steroids in the central nervous system of diabetic
animals. Neurochem Int. 60:616–621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Veenman L and Gavish M: The
peripheral-type benzodiazepine receptor and the cardiovascular
system. Implications for drug development. Pharmacol Ther.
110:503–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barron AM, Garcia-Segura LM, Caruso D,
Jayaraman A, Lee JW, Melcangi RC and Pike CJ: Ligand for
translocator protein reverses pathology in a mouse model of
Alzheimer's disease. J Neurosci. 33:8891–8897. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Daugherty DJ, Selvaraj V, Chechneva OV,
Liu XB, Pleasure DE and Deng W: A TSPO ligand is protective in a
mouse model of multiple sclerosis. EMBO Mol Med. 5:891–903. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu LP, Gong ZF, Wang H, Zhou ZS, Zhang MM,
Liu C, Ren HM, Yang J, Han Y and Zeng CY: TSPO ligands prevent the
proliferation of vascular smooth muscle cells and attenuate
neointima formation through AMPK activation. Acta Pharmacol Sin.
41:34–46. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang M, Wang X, Zhao L, Ma W, Rodriguez
IR, Fariss RN and Wong WT: Macroglia-microglia interactions via
TSPO signaling regulates microglial activation in the mouse retina.
J Neurosci. 34:3793–3806. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Biswas L, Zhou X, Dhillon B, Graham A and
Shu X: Retinal pigment epithelium cholesterol efflux mediated by
the 18 kDa translocator protein, TSPO, a potential target for
treating age-related macular degeneration. Hum Mol Genet.
26:4327–4339. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mages K, Grassmann F, Jagle H, Rupprecht
R, Weber BHF, Hauck SM and Grosche A: The agonistic TSPO ligand
XBD173 attenuates the glial response thereby protecting inner
retinal neurons in a murine model of retinal ischemia. J
Neuroinflammation. 16:432019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo Y, Sun Z, Wang L, Jiang R, Shu Q and
Xu G: Increased expression of TSPO-VDAC complex is correlated with
NLRP3 inflammasome activation in diabetic retinopathy. Mol Med Rep.
26:3532022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Y, Ou Y, Ju Z, Zhang X, Zheng L, Li
J, Sun Y and Liu X: Visualization of translocator protein (18 kDa)
(TSPO) in the retina of diabetic retinopathy rats using
fluorine-18-DPA-714. Ann Nucl Med. 34:675–681. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lacapere JJ, Duma L, Finet S, Kassiou M
and Papadopoulos V: Insight into the Structural Features of TSPO:
Implications for drug development. Trends Pharmacol Sci.
41:110–122. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Papadopoulos V, Aghazadeh Y, Fan J,
Campioli E, Zirkin B and Midzak A: Translocator protein-mediated
pharmacology of cholesterol transport and steroidogenesis. Mol Cell
Endocrinol. 408:90–98. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jaremko M, Jaremko L, Jaipuria G, Becker S
and Zweckstetter M: Structure of the mammalian TSPO/PBR protein.
Biochem Soc Trans. 43:566–571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rupprecht R, Papadopoulos V, Rammes G,
Baghai TC, Fan J, Akula N, Groyer G, Adams D and Schumacher M:
Translocator protein (18 kDa) (TSPO) as a therapeutic target for
neurological and psychiatric disorders. Nat Rev Drug Discov.
9:971–988. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Biswas L, Farhan F, Reilly J, Bartholomew
C and Shu X: TSPO ligands promote cholesterol efflux and suppress
oxidative stress and inflammation in choroidal endothelial cells.
Int J Mol Sci. 19:37402018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jaremko L, Jaremko M, Giller K, Becker S
and Zweckstetter M: Structure of the mitochondrial translocator
protein in complex with a diagnostic ligand. Science.
343:1363–1366. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morohaku K, Pelton SH, Daugherty DJ,
Butler WR, Deng W and Selvaraj V: Translocator protein/peripheral
benzodiazepine receptor is not required for steroid hormone
biosynthesis. Endocrinology. 155:89–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tu LN, Morohaku K, Manna PR, Pelton SH,
Butler WR, Stocco DM and Selvaraj V: Peripheral benzodiazepine
receptor/translocator protein global knock-out mice are viable with
no effects on steroid hormone biosynthesis. J Biol Chem.
289:27444–27454. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tu LN, Zhao AH, Stocco DM and Selvaraj V:
PK11195 effect on steroidogenesis is not mediated through the
translocator protein (TSPO). Endocrinology. 156:1033–1039. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao AH, Tu LN, Mukai C, Sirivelu MP,
Pillai VV, Morohaku K, Cohen R and Selvaraj V: Mitochondrial
translocator protein (TSPO) function is not essential for heme
biosynthesis. J Biol Chem. 291:1591–1603. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Casellas P, Galiegue S and Basile AS:
Peripheral benzodiazepine receptors and mitochondrial function.
Neurochem Int. 40:475–486. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Corsi L, Geminiani E and Baraldi M:
Peripheral benzodiazepine receptor (PBR) new insight in cell
proliferation and cell differentiation review. Curr Clin Pharmacol.
3:38–45. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gatliff J, East D, Crosby J, Abeti R,
Harvey R, Craigen W, Parker P and Campanella M: TSPO interacts with
VDAC1 and triggers a ROS-mediated inhibition of mitochondrial
quality control. Autophagy. 10:2279–2296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Varga B, Marko K, Hadinger N, Jelitai M,
Demeter K, Tihanyi K, Vas A and Madarász E: Translocator protein
(TSPO 18kDa) is expressed by neural stem and neuronal precursor
cells. Neurosci Lett. 462:257–262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Veenman L and Gavish M: The role of 18 kDa
mitochondrial translocator protein (TSPO) in programmed cell death,
and effects of steroids on TSPO expression. Curr Mol Med.
12:398–412. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bernardi P, Carraro M and Lippe G: The
mitochondrial permeability transition: Recent progress and open
questions. FEBS J. 289:7051–7074. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Loth MK, Guariglia SR, Re DB, Perez J, de
Paiva VN, Dziedzic JL, Chambers JW, Azzam DJ and Guilarte TR: A
novel interaction of translocator protein 18 kDa (TSPO) with NADPH
oxidase in microglia. Mol Neurobiol. 57:4467–4487. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gatliff J, East DA, Singh A, Alvarez MS,
Frison M, Matic I, Ferraina C, Sampson N, Turkheimer F and
Campanella M: A role for TSPO in mitochondrial Ca2+
homeostasis and redox stress signaling. Cell Death Dis.
8:e28962017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zeno S, Veenman L, Katz Y, Bode J, Gavish
M and Zaaroor M: The 18 kDa mitochondrial translocator protein
(TSPO) prevents accumulation of protoporphyrin IX. Involvement of
reactive oxygen species (ROS). Curr Mol Med. 12:494–501. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wolf A, Herb M, Schramm M and Langmann T:
The TSPO-NOX1 axis controls phagocyte-triggered pathological
angiogenesis in the eye. Nat Commun. 11:27092020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rupprecht R, Rammes G, Eser D, Baghai TC,
Schüle C, Nothdurfter C, Troxler T, Gentsch C, Kalkman HO, Chaperon
F, et al: Translocator protein (18 kD) as target for anxiolytics
without benzodiazepine-like side effects. Science. 325:490–493.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Barresi E, Robello M, Costa B, Da Pozzo E,
Baglini E, Salerno S, Da Settimo F, Martini C and Taliani S: An
update into the medicinal chemistry of translocator protein (TSPO)
ligands. Eur J Med Chem. 209:1129242021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gut P: Targeting mitochondrial energy
metabolism with TSPO ligands. Biochem Soc Trans. 43:537–542. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Taketani S, Kohno H, Furukawa T and
Tokunaga R: Involvement of peripheral-type benzodiazepine receptors
in the intracellular transport of heme and porphyrins. J Biochem.
117:875–880. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Snyder SH, Verma A and Trifiletti RR: The
peripheral-type benzodiazepine receptor: A protein of mitochondrial
outer membranes utilizing porphyrins as endogenous ligands. FASEB
J. 1:282–288. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Veenman L, Vainshtein A, Yasin N, Azrad M
and Gavish M: Tetrapyrroles as endogenous TSPO ligands in
eukaryotes and prokaryotes: Comparisons with synthetic ligands. Int
J Mol Sci. 17:8802016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tonon MC, Vaudry H, Chuquet J, Guillebaud
F, Fan J, Masmoudi-Kouki O, Vaudry D, Lanfray D, Morin F, Prevot V,
et al: Endozepines and their receptors: Structure, functions and
pathophysiological significance. Pharmacol Ther. 208:1073862020.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ma Z, An R, Chen M, Wang X and Zhu M:
Random versus Block Glycopolymers bearing betulin and porphyrin for
enhanced photodynamic therapy. Biomacromolecules. 23:5074–5083.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yamamoto M, Arimura H, Fukushige T, Minami
K, Nishizawa Y, Tanimoto A, Kanekura T, Nakagawa M, Akiyama S and
Furukawa T: Abcb10 role in heme biosynthesis in vivo: Abcb10
knockout in mice causes anemia with protoporphyrin IX and iron
accumulation. Mol Cell Biol. 34:1077–1084. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Batoko H, Veljanovski V and Jurkiewicz P:
Enigmatic Translocator protein (TSPO) and cellular stress
regulation. Trends Biochem Sci. 40:497–503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Korolnek T and Hamza I: Like iron in the
blood of the people: The requirement for heme trafficking in iron
metabolism. Front Pharmacol. 5:1262014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chauveau F, Boutin H, Van Camp N, Dolle F
and Tavitian B: Nuclear imaging of neuroinflammation: A
comprehensive review of [11C]PK11195 challengers. Eur J Nucl Med
Mol Imaging. 35:2304–2319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dolle F, Luus C, Reynolds A and Kassiou M:
Radiolabelled molecules for imaging the translocator protein (18
kDa) using positron emission tomography. Curr Med Chem.
16:2899–2923. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fan Z, Calsolaro V, Atkinson RA,
Femminella GD, Waldman A, Buckley C, Trigg W, Brooks DJ, Hinz R and
Edison P: Flutriciclamide (18F-GE180) PET: First-in-Human PET study
of novel third-generation in vivo marker of human translocator
protein. J Nucl Med. 57:1753–1759. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chiabrando D, Marro S, Mercurio S, Giorgi
C, Petrillo S, Vinchi F, Fiorito V, Fagoonee S, Camporeale A, Turco
E, et al: The mitochondrial heme exporter FLVCR1b mediates
erythroid differentiation. J Clin Invest. 122:4569–4579. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ni R, Rojdner J, Voytenko L, Dyrks T,
Thiele A, Marutle A and Nordberg A: In vitro characterization of
the regional binding distribution of amyloid PET tracer florbetaben
and the glia tracers deprenyl and PK11195 in Autopsy Alzheimer's
brain tissue. J Alzheimers Dis. 80:1723–1737. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ishikawa M, Yoshitomi T, Covey DF,
Zorumski CF and Izumi Y: TSPO activation modulates the effects of
high pressure in a rat ex vivo glaucoma model. Neuropharmacology.
111:142–159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Papadopoulos V and Lecanu L: Translocator
protein (18 kDa) TSPO: An emerging therapeutic target in
neurotrauma. Exp Neurol. 219:53–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Girard C, Liu S, Adams D, Lacroix C,
Sinéus M, Boucher C, Papadopoulos V, Rupprecht R, Schumacher M and
Groyer G: Axonal regeneration and neuroinflammation: Roles for the
translocator protein 18 kDa. J Neuroendocrinol. 24:71–81. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ravikumar B, Crawford D, Dellovade T,
Savinainen A, Graham D, Liere P, Oudinet JP, Webb M and Hering H:
Differential efficacy of the TSPO ligands etifoxine and XBD-173 in
two rodent models of Multiple Sclerosis. Neuropharmacology.
108:229–237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Choi HB, Khoo C, Ryu JK, van Breemen E,
Kim SU and McLarnon JG: Inhibition of lipopolysaccharide-induced
cyclooxygenase-2, tumor necrosis factor-alpha and [Ca2+]i responses
in human microglia by the peripheral benzodiazepine receptor ligand
PK11195. J Neurochem. 83:546–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lebedeva IV, Su ZZ, Sarkar D, Kitada S,
Dent P, Waxman S, Reed JC and Fisher PB: Melanoma differentiation
associated gene-7, mda-7/interleukin-24, induces apoptosis in
prostate cancer cells by promoting mitochondrial dysfunction and
inducing reactive oxygen species. Cancer Res. 63:8138–8144.
2003.PubMed/NCBI
|
|
63
|
Seneviratne MS, Faccenda D, De Biase V and
Campanella M: PK11195 inhibits mitophagy targeting the
F1Fo-ATPsynthase in Bcl-2 knock-down cells. Curr Mol Med.
12:476–482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bonora M, Giorgi C and Pinton P: Molecular
mechanisms and consequences of mitochondrial permeability
transition. Nat Rev Mol Cell Biol. 23:266–285. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gonzalez-Polo RA, Carvalho G, Braun T,
Decaudin D, Fabre C, Larochette N, Perfettini JL, Djavaheri-Mergny
M, Youlyouz-Marfak I, Codogno P, et al: PK11195 potently sensitizes
to apoptosis induction independently from the peripheral
benzodiazepin receptor. Oncogene. 24:7503–7513. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Choi J, Ifuku M, Noda M and Guilarte TR:
Translocator protein (18 kDa)/peripheral benzodiazepine receptor
specific ligands induce microglia functions consistent with an
activated state. Glia. 59:219–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Awad M and Gavish M: Binding of [3H]Ro
5-4864 and [3H]PK 11195 to cerebral cortex and peripheral tissues
of various species: Species differences and heterogeneity in
peripheral benzodiazepine binding sites. J Neurochem. 49:1407–1414.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Marangos PJ, Patel J, Boulenger JP and
Clark-Rosenberg R: Characterization of peripheral-type
benzodiazepine binding sites in brain using [3H]Ro 5-4864. Mol
Pharmacol. 22:26–32. 1982.PubMed/NCBI
|
|
69
|
Kreisl WC, Kim MJ, Coughlin JM, Henter ID,
Owen DR and Innis RB: PET imaging of neuroinflammation in
neurological disorders. Lancet Neurol. 19:940–950. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gut P, Baeza-Raja B, Andersson O,
Hasenkamp L, Hsiao J, Hesselson D, Akassoglou K, Verdin E, Hirschey
MD and Stainier DY: Whole-organism screening for gluconeogenesis
identifies activators of fasting metabolism. Nat Chem Biol.
9:97–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Da Pozzo E, Giacomelli C, Barresi E, Costa
B, Taliani S, Passetti Fda S and Martini C: Targeting the 18-kDa
translocator protein: recent perspectives for neuroprotection.
Biochem Soc Trans. 43:559–565. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gavioli EC, Duarte FS, Bressan E, Ferrara
P, Farges RC and De Lima TC: Antidepressant-like effect of
Ro5-4864, a peripheral-type benzodiazepine receptor ligand, in
forced swimming test. Eur J Pharmacol. 471:21–26. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kita A, Kohayakawa H, Kinoshita T, Ochi Y,
Nakamichi K, Kurumiya S, Furukawa K and Oka M: Antianxiety and
antidepressant-like effects of AC-5216, a novel mitochondrial
benzodiazepine receptor ligand. Br J Pharmacol. 142:1059–1072.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Baez E, Guio-Vega GP, Echeverria V,
Sandoval-Rueda DA and Barreto GE: 4′-chlorodiazepam protects
mitochondria in T98G astrocyte cell line from glucose deprivation.
Neurotox Res. 32:163–171. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Nothdurfter C, Rammes G, Baghai TC, Schüle
C, Schumacher M, Papadopoulos V and Rupprecht R: Translocator
protein (18 kDa) as a target for novel anxiolytics with a
favourable side-effect profile. J Neuroendocrinol. 24:82–92. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kita A, Kinoshita T, Kohayakawa H,
Furukawa K and Akaike A: Lack of tolerance to anxiolysis and
withdrawal symptoms in mice repeatedly treated with AC-5216, a
selective TSPO ligand. Prog Neuropsychopharmacol Biol Psychiatry.
33:1040–1045. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Karlstetter M, Nothdurfter C, Aslanidis A,
Moeller K, Horn F, Scholz R, Neumann H, Weber BH, Rupprecht R and
Langmann T: Translocator protein (18 kDa) (TSPO) is expressed in
reactive retinal microglia and modulates microglial inflammation
and phagocytosis. J Neuroinflammation. 11:32014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Scholz R, Caramoy A, Bhuckory MB, Rashid
K, Chen M, Xu H, Grimm C and Langmann T: Targeting translocator
protein (18 kDa) (TSPO) dampens pro-inflammatory microglia
reactivity in the retina and protects from degeneration. J
Neuroinflammation. 12:2012015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Schlichter R, Rybalchenko V, Poisbeau P,
Verleye M and Gillardin J: Modulation of GABAergic synaptic
transmission by the non-benzodiazepine anxiolytic etifoxine.
Neuropharmacology. 39:1523–1535. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Choi YM and Kim KH: Etifoxine for pain
patients with anxiety. Korean J Pain. 28:4–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Girard C, Liu S, Cadepond F, Adams D,
Lacroix C, Verleye M, Gillardin JM, Baulieu EE, Schumacher M and
Schweizer-Groyer G: Etifoxine improves peripheral nerve
regeneration and functional recovery. Proc Natl Acad Sci USA.
105:20505–20510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Olsen RW and Li GD: GABA(A) receptors as
molecular targets of general anesthetics: Identification of binding
sites provides clues to allosteric modulation. Can J Anaesth.
58:206–215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Gunn BG, Brown AR, Lambert JJ and Belelli
D: Neurosteroids and GABA(A) Receptor Interactions: A focus on
stress. Front Neurosci. 5:1312011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Biswas L, Ibrahim KS, Li X, Zhou X, Zeng
Z, Craft J and Shu X: Effect of a TSPO ligand on retinal pigment
epithelial cholesterol homeostasis in high-fat fed mice,
implication for age-related macular degeneration. Exp Eye Res.
208:1086252021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ibrahim KS, Craft JA, Biswas L, Spencer J
and Shu X: Etifoxine reverses weight gain and alters the colonic
bacterial community in a mouse model of obesity. Biochem Pharmacol.
180:1141512020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Costa B, Cavallini C, Da Pozzo E, Taliani
S, Da Settimo F and Martini C: The anxiolytic etifoxine binds to
TSPO Ro5-4864 binding site with long residence time showing a high
neurosteroidogenic activity. ACS Chem Neurosci. 8:1448–1454. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kondo D, Saegusa H, Yabe R, Takasaki I,
Kurihara T, Zong S and Tanabe T: Peripheral-type benzodiazepine
receptor antagonist is effective in relieving neuropathic pain in
mice. J Pharmacol Sci. 110:55–63. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Tsukagoshi E, Kawaguchi M, Shinomiya T,
Yoshikawa M, Kawano T, Okubo M and Sawaki K: Diazepam enhances
production of diazepam-binding inhibitor (DBI), a negative saliva
secretion regulator, localized in rat salivary gland. J Pharmacol
Sci. 115:221–229. 2011. View Article : Google Scholar
|
|
89
|
Morin D, Musman J, Pons S, Berdeaux A and
Ghaleh B: Mitochondrial translocator protein (TSPO): From
physiology to cardioprotection. Biochem Pharmacol. 105:1–13. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Leducq N, Bono F, Sulpice T, Vin V, Janiak
P, Fur GL, O'Connor SE and Herbert JM: Role of peripheral
benzodiazepine receptors in mitochondrial, cellular, and cardiac
damage induced by oxidative stress and ischemia-reperfusion. J
Pharmacol Exp Ther. 306:828–837. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Santoro A, Mattace Raso G, Taliani S, Da
Pozzo E, Simorini F, Costa B, Martini C, Laneri S, Sacchi A,
Cosimelli B, et al: TSPO-ligands prevent oxidative damage and
inflammatory response in C6 glioma cells by neurosteroid synthesis.
Eur J Pharm Sci. 88:124–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Klee K, Storti F, Barben M, Samardzija M,
Langmann T, Dunaief J and Grimm C: Systemic knockout of Tspo in
mice does not affect retinal morphology, function and
susceptibility to degeneration. Exp Eye Res. 188:1078162019.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Storti F, Klee K, Todorova V, Steiner R,
Othman A, van der Velde-Visser S, Samardzija M, Meneau I, Barben M,
Karademir D, et al: Impaired ABCA1/ABCG1-mediated lipid efflux in
the mouse retinal pigment epithelium (RPE) leads to retinal
degeneration. Elife. 8:e451002019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Santos AM, Calvente R, Tassi M, Carrasco
MC, Martín-Oliva D, Marín-Teva JL, Navascués J and Cuadros MA:
Embryonic and postnatal development of microglial cells in the
mouse retina. J Comp Neurol. 506:224–239. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lee MA, Sitko AA, Khalid S, Shirasu-Hiza M
and Mason CA: Spatiotemporal distribution of glia in and around the
developing mouse optic tract. J Comp Neurol. 527:508–521. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Stevens B, Allen NJ, Vazquez LE, Howell
GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman
AD, Stafford B, et al: The classical complement cascade mediates
CNS synapse elimination. Cell. 131:1164–1178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Prinz M and Mildner A: Microglia in the
CNS: Immigrants from another world. Glia. 59:177–187. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Banati RB, Middleton RJ, Chan R, Hatty CR,
Kam WW, Quin C, Graeber MB, Parmar A, Zahra D, Callaghan P, et al:
Positron emission tomography and functional characterization of a
complete PBR/TSPO knockout. Nat Commun. 5:54522014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Barron AM, Ji B, Kito S, Suhara T and
Higuchi M: Steroidogenic abnormalities in translocator protein
knockout mice and significance in the aging male. Biochem J.
475:75–85. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Grassmann F, Fauser S and Weber BH: The
genetics of age-related macular degeneration (AMD)-Novel targets
for designing treatment options? Eur J Pharm Biopharm. 95:194–202.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Csader S, Korhonen S, Kaarniranta K and
Schwab U: The effect of dietary supplementations on delaying the
progression of age-related macular degeneration: A systematic
review and meta-analysis. Nutrients. 14:42732022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Fleckenstein M, Keenan TDL, Guymer RH,
Chakravarthy U, Schmitz-Valckenberg S, Klaver CC, Wong WT and Chew
EY: Age-related macular degeneration. Nat Rev Dis Primers.
7:312021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Pikuleva IA and Curcio CA: Cholesterol in
the retina: The best is yet to come. Prog Retin Eye Res. 41:64–89.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Curcio CA, Presley JB, Malek G, Medeiros
NE, Avery DV and Kruth HS: Esterified and unesterified cholesterol
in drusen and basal deposits of eyes with age-related maculopathy.
Exp Eye Res. 81:731–741. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Bhutto I and Lutty G: Understanding
age-related macular degeneration (AMD): Relationships between the
photoreceptor/retinal pigment epithelium/Bruch's
membrane/choriocapillaris complex. Mol Aspects Med. 33:295–317.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Biesemeier A, Taubitz T, Julien S, Yoeruek
E and Schraermeyer U: Choriocapillaris breakdown precedes retinal
degeneration in age-related macular degeneration. Neurobiol Aging.
35:2562–2573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Fritsche LG, Igl W, Bailey JN, Grassmann
F, Sengupta S, Bragg-Gresham JL, Burdon KP, Hebbring SJ, Wen C,
Gorski M, et al: A large genome-wide association study of
age-related macular degeneration highlights contributions of rare
and common variants. Nat Genet. 48:134–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Toomey CB, Johnson LV and Bowes Rickman C:
Complement factor H in AMD: Bridging genetic associations and
pathobiology. Prog Retin Eye Res. 62:38–57. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Malek G, Johnson LV, Mace BE, Saloupis P,
Schmechel DE, Rickman DW, Toth CA, Sullivan PM and Bowes Rickman C:
Apolipoprotein E allele-dependent pathogenesis: A model for
age-related retinal degeneration. Proc Natl Acad Sci USA.
102:11900–11905. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Toomey CB, Kelly U, Saban DR and Bowes
Rickman C: Regulation of age-related macular degeneration-like
pathology by complement factor H. Proc Natl Acad Sci USA.
112:E3040–E3049. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Langmann T: Microglia activation in
retinal degeneration. J Leukoc Biol. 81:1345–1351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Zeng H, Ding M, Chen XX and Lu Q:
Microglial NADPH oxidase activation mediates rod cell death in the
retinal degeneration in rd mice. Neuroscience. 275:54–61. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zhao L, Zabel MK, Wang X, Ma W, Shah P,
Fariss RN, Qian H, Parkhurst CN, Gan WB and Wong WT: Microglial
phagocytosis of living photoreceptors contributes to inherited
retinal degeneration. EMBO Mol Med. 7:1179–1197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Farhan F, Almarhoun M, Wong A, Findlay AS,
Bartholomew C, Williams MTS, Hurd TW and Shu X: Deletion of TSPO
causes dysregulation of cholesterol metabolism in mouse retina.
Cells. 10:30662021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Luckoff A, Scholz R, Sennlaub F, Xu H and
Langmann T: Comprehensive analysis of mouse retinal mononuclear
phagocytes. Nat Protoc. 12:1136–1150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Nagineni CN, Kommineni VK, William A,
Detrick B and Hooks JJ: Regulation of VEGF expression in human
retinal cells by cytokines: implications for the role of
inflammation in age-related macular degeneration. J Cell Physiol.
227:116–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Osborne NN, Casson RJ, Wood JP, Chidlow G,
Graham M and Melena J: Retinal ischemia: Mechanisms of damage and
potential therapeutic strategies. Prog Retin Eye Res. 23:91–147.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Osborne NN, Ugarte M, Chao M, Chidlow G,
Bae JH, Wood JP and Nash MS: Neuroprotection in relation to retinal
ischemia and relevance to glaucoma. Surv Ophthalmol. 43 (Suppl
1):S102–S128. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Youngblood H, Robinson R, Sharma A and
Sharma S: Proteomic biomarkers of retinal inflammation in diabetic
retinopathy. Int J Mol Sci. 20:47552019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Khayat M, Williams M and Lois N: Ischemic
retinal vein occlusion: Characterizing the more severe spectrum of
retinal vein occlusion. Surv Ophthalmol. 63:816–850. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Heyck M, Bonsack B, Zhang H, Sadanandan N,
Cozene B, Kingsbury C, Lee JY and Borlongan CV: The brain and eye:
Treating cerebral and retinal ischemia through mitochondrial
transfer. Exp Biol Med (Maywood). 244:1485–1492. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Liu GJ, Middleton RJ, Kam WW, Chin DY,
Hatty CR, Chan RH and Banati RB: Functional gains in energy and
cell metabolism after TSPO gene insertion. Cell Cycle. 16:436–447.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ,
Tanase D and Strother JM: Glial reactivity and impaired glutamate
metabolism in short-term experimental diabetic retinopathy. Penn
State Retina Research Group. Diabetes. 47:815–820. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Wagner L, Pannicke T, Rupprecht V,
Frommherz I, Volz C, Illes P, Hirrlinger J, Jägle H, Egger V,
Haydon PG, et al: Suppression of SNARE-dependent exocytosis in
retinal glial cells and its effect on ischemia-induced
neurodegeneration. Glia. 65:1059–1071. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Dkhissi O, Chanut E, Wasowicz M,
Savoldelli M, Nguyen-Legros J, Minvielle F and Versaux-Botteri C:
Retinal TUNEL-positive cells and high glutamate levels in vitreous
humor of mutant quail with a glaucoma-like disorder. Invest
Ophthalmol Vis Sci. 40:990–995. 1999.PubMed/NCBI
|
|
126
|
Delyfer MN, Forster V, Neveux N, Picaud S,
Leveillard T and Sahel JA: Evidence for glutamate-mediated
excitotoxic mechanisms during photoreceptor degeneration in the rd1
mouse retina. Mol Vis. 11:688–696. 2005.PubMed/NCBI
|
|
127
|
Vujosevic S, Aldington SJ, Silva P,
Hernández C, Scanlon P, Peto T and Simó R: Screening for diabetic
retinopathy: New perspectives and challenges. Lancet Diabetes
Endocrinol. 8:337–347. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Wang W and Lo ACY: Diabetic retinopathy:
Pathophysiology and treatments. Int J Mol Sci. 19:18162018.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Fung TH, Patel B, Wilmot EG and Amoaku WM:
Diabetic retinopathy for the non-ophthalmologist. Clin Med (Lond).
22:112–116. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Brownlee M: The pathobiology of diabetic
complications: A unifying mechanism. Diabetes. 54:1615–1625. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Naruse K, Nakamura J, Hamada Y, Nakayama
M, Chaya S, Komori T, Kato K, Kasuya Y, Miwa K and Hotta N: Aldose
reductase inhibition prevents glucose-induced apoptosis in cultured
bovine retinal microvascular pericytes. Exp Eye Res. 71:309–315.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Romeo G, Liu WH, Asnaghi V, Kern TS and
Lorenzi M: Activation of nuclear factor-kappaB induced by diabetes
and high glucose regulates a proapoptotic program in retinal
pericytes. Diabetes. 51:2241–2248. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Abcouwer SF: Muller cell-microglia cross
talk drives neuroinflammation in diabetic retinopathy. Diabetes.
66:261–263. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Sorrentino FS, Allkabes M, Salsini G,
Bonifazzi C and Perri P: The importance of glial cells in the
homeostasis of the retinal microenvironment and their pivotal role
in the course of diabetic retinopathy. Life Sci. 162:54–59. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Podesta F, Romeo G, Liu WH, Krajewski S,
Reed JC, Gerhardinger C and Lorenzi M: Bax is increased in the
retina of diabetic subjects and is associated with pericyte
apoptosis in vivo and in vitro. Am J Pathol. 156:1025–1032. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Tien T, Zhang J, Muto T, Kim D, Sarthy VP
and Roy S: High glucose induces mitochondrial dysfunction in
retinal muller cells: Implications for diabetic retinopathy. Invest
Ophthalmol Vis Sci. 58:2915–2921. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Sasaki M, Ozawa Y, Kurihara T, Kubota S,
Yuki K, Noda K, Kobayashi S, Ishida S and Tsubota K:
Neurodegenerative influence of oxidative stress in the retina of a
murine model of diabetes. Diabetologia. 53:971–979. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Li J and Papadopoulos V: Translocator
protein (18 kDa) as a pharmacological target in adipocytes to
regulate glucose homeostasis. Biochem Pharmacol. 97:99–110. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Musman J, Paradis S, Panel M, Pons S,
Barau C, Caccia C, Leoni V, Ghaleh B and Morin D: A TSPO ligand
prevents mitochondrial sterol accumulation and dysfunction during
myocardial ischemia-reperfusion in hypercholesterolemic rats.
Biochem Pharmacol. 142:87–95. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Ciudin A, Simo-Servat O, Hernandez C,
Arcos G, Diego S, Sanabria Á, Sotolongo Ó, Hernández I, Boada M and
Simó R: Retinal Microperimetry: A new tool for identifying patients
with type 2 diabetes at risk for developing Alzheimer disease.
Diabetes. 66:3098–3104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Kinuthia UM, Wolf A and Langmann T:
Microglia and inflammatory responses in diabetic retinopathy. Front
Immunol. 11:5640772020. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Flaxman SR, Bourne RRA, Resnikoff S,
Ackland P, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J,
Kempen JH, et al: Global causes of blindness and distance vision
impairment 1990–2020: A systematic review and meta-analysis. Lancet
Glob Health. 5:e1221–e1234. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Tham YC, Li X, Wong TY, Quigley HA, Aung T
and Cheng CY: Global prevalence of glaucoma and projections of
glaucoma burden through 2040: A systematic review and
meta-analysis. Ophthalmology. 121:2081–2090. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Zukerman R, Harris A, Oddone F, Siesky B,
Verticchio Vercellin A and Ciulla TA: Glaucoma heritability:
Molecular mechanisms of disease. Genes (Basel). 12:11352021.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Quigley HA: Glaucoma. Lancet.
377:1367–1377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Ishikawa M, Yoshitomi T, Zorumski CF and
Izumi Y: Neurosteroids are endogenous neuroprotectants in an ex
vivo glaucoma model. Invest Ophthalmol Vis Sci. 55:8531–8541. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Belelli D and Lambert JJ: Neurosteroids:
endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci.
6:565–575. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Weir CJ, Ling AT, Belelli D, Wildsmith JA,
Peters JA and Lambert JJ: The interaction of anaesthetic steroids
with recombinant glycine and GABAA receptors. Br J Anaesth.
92:704–711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Midzak A, Akula N, Lecanu L and
Papadopoulos V: Novel androstenetriol interacts with the
mitochondrial translocator protein and controls steroidogenesis. J
Biol Chem. 286:9875–9887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Izumi Y, Benz AM, Kurby CO, Labruyere J,
Zorumski CF, Price MT and Olney JW: An ex vivo rat retinal
preparation for excitotoxicity studies. J Neurosci Methods.
60:219–225. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Izumi Y, Kirby CO, Benz AM, Olney JW and
Zorumski CF: Muller cell swelling, glutamate uptake, and
excitotoxic neurodegeneration in the isolated rat retina. Glia.
25:379–389. 1999. View Article : Google Scholar : PubMed/NCBI
|