Introduction
Endometriosis (EM) is an estrogen-dependent disorder
characterized by the presence of endometrioid epithelium and glands
outside the endometrium and myometrium (1). It affects 10–15% of women of
reproductive age worldwide and is one of the most frequently
occurring diseases among this group (1). The main clinical manifestation of EM
is pain, including typical but non-specific pain, such as
dysmenorrhea and chronic pelvic pain, and ~1/3 of patients have
infertility, with advanced EM potentially leading to gynecological
malignancy, such as ovarian cancer (2,3). The
lack of specific clinical manifestations of EM makes it difficult
to differentiate from conditions characterized by chronic pelvic
pain in clinical practice, which makes the diagnosis and treatment
of EM challenging (3). This
results in psychological burden to patients and affects physical
and mental health, as well as their quality of life (4,5).
The pathophysiology of EM disease is not thoroughly
understood. The potential pathogenesis of EM is hypothesized to
involve retrograde menstruation, uterine stem cells and somatic
epithelium (1). Among them, the
theory of retrograde menstruation is the leading theory of the
pathogenesis of EM (6). However,
development of EM is associated with immune alterations and a
proinflammatory peritoneal milieu, which determines the progression
to EM (7). It is noteworthy that
the formation and growth of ectopic lesions depends on nutrient
support, with neovascularization serving an important role in the
development of endothelial ectopic lesions (2,8).
Vascular permeability is notably increased in ectopic lesions of EM
and a large number of new blood vessels appear (2). This indicates that angiogenesis is a
key link in the pathogenesis of EM, which represents a potential
target for development of future diagnostic and therapeutic
strategies. Recent study of angiogenic mechanisms in EM has
revealed the importance and potential role of angiogenesis-related
pathways and markers of EM for diagnosis and treatment (9–12).
That has provided a new direction for the discovery of novel
targets of anti-angiogenic action and therapy and novel lines of
thought and strategies for clinical treatment of EM.
The present review aimed to summarize advances in
the investigation of EM angiogenesis, including availability of
non-invasive molecular markers for the diagnosis of EM, for the
potential clinical value of anti-angiogenic therapy as an
alternative to non-hormonal therapies for treatment of EM.
Search methods
Literature searches were performed in PubMed
(pubmed.ncbi.nlm.nih.gov/) and ISI Web of
Knowledge (webofscience.com) for English
articles with the key words ‘endometriosis’, ‘endometriotic
lesions’, ‘angiogenesis’, ‘vascularization’, ‘anti-angiogenic’,
‘anti-angiogenesis’, ‘anti-angiogenic therapy’ and ‘diagnosis’. The
search included animal and human studies focusing on EM
angiogenesis, diagnosis and treatment, and no restriction was set
regarding the publication date.
Angiogenesis in EM
Angiogenesis is defined as generating new blood
vessels from the germination of pre-existing vessels. This is
initiated by vascular endothelial growth factor (VEGF), which
activates resting endothelial cells in microvasculature to release
matrix metalloproteinase (MMP), thereby mediating degradation of
the vascular basal membrane so that endothelial cells migrate into
the surrounding tissue; additionally, tubular branches of
endothelial cells form vascular rings and novel basement membranes,
ultimately leading to new vessel formation (13). Angiogenesis is key for normal
physiological processes by spontaneous endometrial thickening and
development, serving an important role in the menstrual cycle
(14). In patients with EM, the
endometrium forces menstrual blood to the peritoneal cavity leading
to induction of oxidative stress and immune responses, releasing
related inflammatory factors and cytokines. This results in
disrupted homeostasis of the peritoneal microenvironment and a
relative increase in pro-angiogenic factors, which leads to
neovascularization of ectopic endometrial vessels and establishment
of the microvascular network (2,15).
EM lesions develop in a complex and dynamic environment that is
regulated by a number of molecules, cytokines and associated
signaling pathways (Table I)
(16).
 | Table I.Expression of pro-angiogenic factors
in intimal tissue, peritoneal fluid or serum/plasma of patients
with EM. |
Table I.
Expression of pro-angiogenic factors
in intimal tissue, peritoneal fluid or serum/plasma of patients
with EM.
| Factor | Source | Test sample | (Refs.) |
|---|
| VEGF | Endometrial
glandular epithelial and stromal cells, macrophages,
neutrophils | Intimal tissue,
serum, peritoneal fluid | (10,21,24,26,50) |
| FGF | Stromal cells,
macrophages | Intimal tissue | (10) |
| Ang | Endothelial
cells | Serum | (27) |
| HIF-1α | Stromal cells | Serum | (24) |
| IL-1β | Macrophages,
monocytes | Peritoneal
fluid | (20) |
|
|
| Serum | (26,27) |
| IL-6 | Macrophages, mast
cells, lymphocytes | Peritoneal
fluid | (21,23) |
|
|
| Serum | (104) |
| IL-8 | Endothelial cells,
macrophages, neutrophils | Peritoneal
fluid | (22) |
|
|
| Serum | (22,26,27) |
| IL-10 | Plasma cells,
dendritic cells, NK cells | Serum | (26) |
| IL-17 | Lymphocytes,
neutrophils | Peritoneal
fluid | (23) |
|
|
| Serum | (23) |
| TNF-α | Macrophages, NK
cells | Intimal tissue | (25) |
|
|
| Serum | (26,27) |
| MMIF | Lymphocytes,
macrophages | Serum | (24) |
| COX-2 | Macrophages | Serum | (26) |
Role of inflammatory cells in
angiogenesis progression in EM
At present, although the etiology of EM is unclear,
immune dysfunction has been cited as a key contributing factor in
the growth of ectopic lesions of endometrial debris following
retrograde menstruation (17,18).
Density of immunocompetent cells such as T lymphocytes, natural
killer cells, dendritic cells (DCs), macrophages and neutrophils is
markedly increased in EM lesions and the peritoneal cavity
(Fig. 1) (17,19).
Abnormal alterations in inflammatory and immune cells cause
alterations in cytokines in the peritoneal fluid of patients with
EM. Compared with healthy patients, the expression of interleukin
(IL) −1β, −6, −8, −10 and −17, tumor necrosis factor-α (TNF-α) and
macrophage migration inhibitory factor is notably elevated in the
peritoneal cavity or serum of patients with EM (20–27).
These cytokines are not only involved in the chronic inflammatory
response to EM, but also promote the implantation and growth of
ectopic lesions by upregulating expression of cyclooxygenase 2
(COX-2) and/or VEGF.
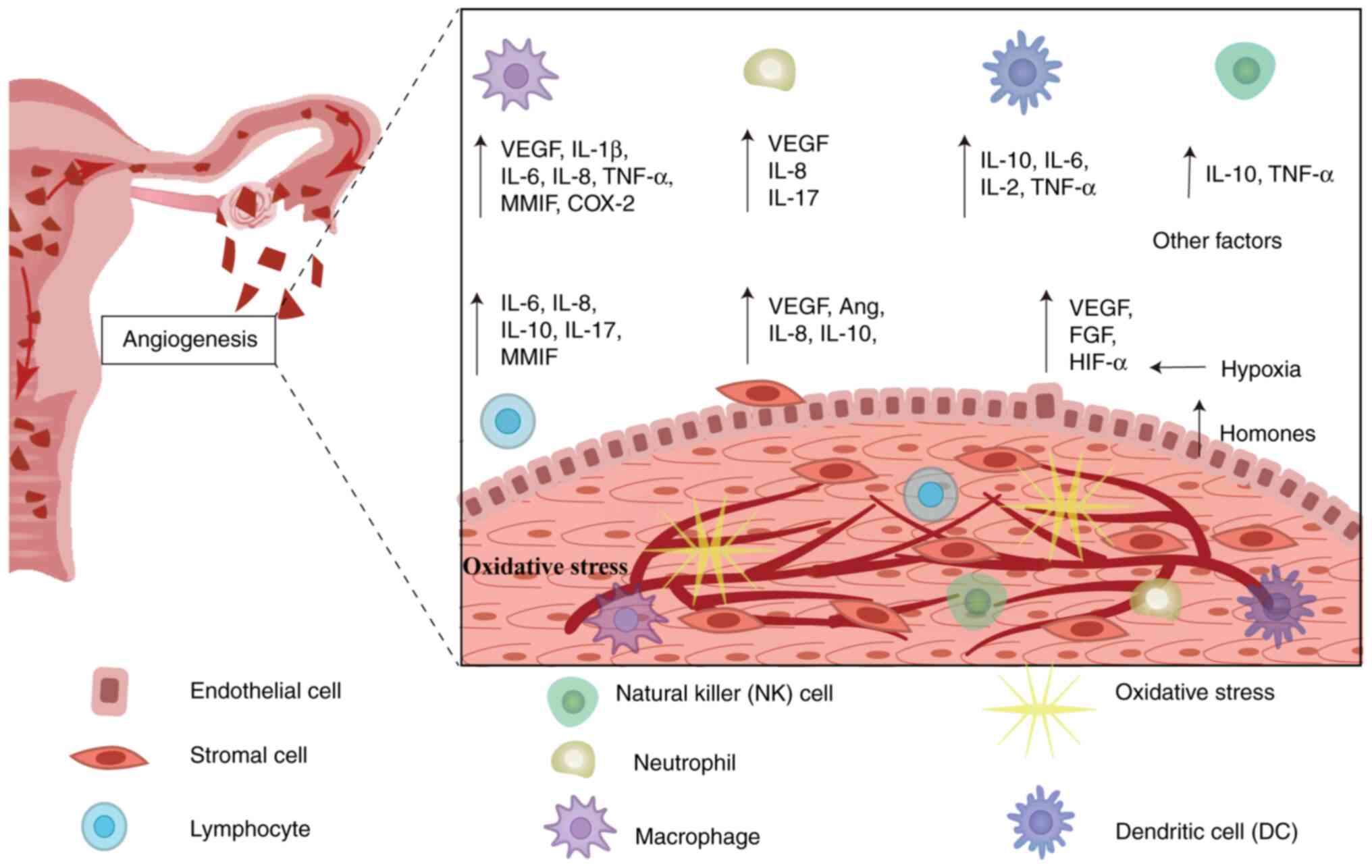 | Figure 1.Angiogenic microenvironment of EM
based on Sampson's retrograde menstruation theory, in which viable
endothelial cells reflux into the peritoneal cavity. Under
peritoneal hypoxia and altered immuno-inflammatory
microenvironment, defective immune surveillance occurs, leading to
imbalance of immune cells and secretion of relevant cytokines,
which promotes ectopic endothelial colonization, adhesion and
neovascularization. Ang, angiopoietin; IL, interleukin; FGF,
fibroblast growth factor; MMIF, macrophage migration inhibitory
factor; VEGF, vascular endothelial growth factor; COX-2,
cyclooxygenase 2; TNF-α, tumor necrosis factor-α. |
Previous studies have suggested that enhanced
concentration of neutrophils in the peritoneal fluid of patients
with EM may be the result of elevated concentrations of potent
neutrophil chemokines, such as IL-8, in plasma and peritoneal fluid
(28,29). Moreover, neutrophils secrete
cytokines such as VEGF and IL-8 and −17 to promote the
proliferation and invasion of endometriotic cells and angiogenesis
(30). In this process, estrogen
is involved in neutrophil activation, and it was found that in
vitro culture in the presence of IL-6, TNF-α,
lipopolysaccharide (LPS) and estrogen-enhanced VEGF release from
isolated peritoneal macrophages and neutrophils (31). Conversely, inhibition of estrogen
signaling activity decreases the number of neutrophils and
expression of proinflammatory cytokines, thereby inhibiting the
progression of EM (32).
Immune dysfunction characterized by hyperactive
peritoneal macrophages with altered phagocytic ability represents a
key point in EM angiogenesis (33,34).
Macrophages are hypothesized to be one of the sources of VEGF and
fibroblast growth factor (FGF), which promote the growth of
diseased blood vessels in EM and accelerate the process of EM
development (24). Previous
studies have found that accumulated macrophages are recruited to
the ectopic environment, primarily using the alternatively
activated macrophage (M2) phenotype, which promotes and enhances
proliferation and clonogenic capacity of endometrial stromal cells
(ESCs) (33–36). Additionally, M2 cells produce a
variety of stimulating factors to induce peritoneal inflammation,
thus forming a complex loop of regulatory mechanisms to maintain
the altered peritoneal microenvironment of EM to promote immune
escape, adhesion and angiogenesis of the ectopic endothelium
(31,37). By contrast, nanovesicles derived
from M1 macrophages directly or indirectly inhibit migration and
invasion of endometrial mesenchymal stromal cells (E-MSCs) to
decrease formation of blood vessels (35).
Contrary to macrophages, the role of DCs in
pathogenesis of EM is not yet clear. Suen et al (38) showed that IL-10 secreted by plasma
cell-like DCs enhances ESC migration and promotes angiogenesis via
the secretion of pro-angiogenic factors, leading to the growth of
endometriotic lesions. Furthermore, DCs may promote EM angiogenesis
by secreting cytokines such as IL-6 and −12 and transforming growth
factor-β (TGF-β) (39).
Regulatory T cells and helper T 17 (Th17) cells are
essential for immune defense and immune homeostasis (18). Imbalance in expression of Th2 and
Th1 cells is one of the essential promoters of ectopic endothelial
growth (40). Th17 cells produce
IL-17, a strong proinflammatory mediator that stimulates expression
of angiogenic and proinflammatory cytokines, and promotes
angiogenesis in the ectopic endothelium (30,41).
In vitro results suggest that IL-17 may be a stimulus that
induces pathogenic polarization of macrophages towards the M2
phenotype (42). This suggests a
synergistic role of inflammation and the Th17 immune response.
Molecules involved in angiogenesis in
EM
Previous studies have found numerous similarities
between EM angiogenesis and tumor pathologic angiogenesis (8,43).
Ectopic endometrial tissue initially has a degree of hypoxia
comparable with that of cells in growing tumor centers (16,44).
Hypoxia is one of the most potent stimuli for
upregulation of angiogenic growth factors; it prevents proteasomal
degradation of hypoxia-inducible factor-1α (HIF-1α) (44,45).
HIF-1α is required for oxygen-regulated transcriptional activation
of genes encoding VEGF to enhance hypoxia-induced angiogenesis.
HIF-1α enters the nucleus to form hypoxia response elements and
binds the promoter region of the VEGF gene, which in turn
upregulates VEGF expression and promotes angiogenesis (9). In the hypoxic microenvironment of the
peritoneal cavity, HIF-1α downregulates expression of chicken
ovalbumin upstream of the promoter transcription factor II, which
leads to elevated angiopoietin expression and promotes
neovascularization of ectopic lesions in EM (46). Additionally, HIF-1α is involved in
immune regulation and inflammatory responses and it can upregulate
expression of IL-8 and COX-2, thus initiating IL-8- and
COX-2-mediated angiogenic mechanisms and indirectly promoting
ectopic endothelial neovascularization (47). The involvement of HIF-1α in
regulating disease is complex and HIF-1α is also involved in
cellular autophagy. HIF-1α promotes mesenchymal cell migration and
invasion in EM via upregulation of autophagy (45). Reactive oxygen species (ROS) are a
key element in stabilizing HIF-1α. The release of ROS and the
expression of VEGF are enhanced under hypoxic conditions, which
forms a positive feedback mechanism between ROS and VEGF in
enhancing angiogenesis (48). As a
consequence, HIF-1α is an important regulator that promotes
angiogenesis and is involved in angiogenesis in EM via the complex
regulation of multiple pathways.
VEGF is an important pro-angiogenic factor that
induces vascular endothelial cell neogenesis and cell proliferation
by binding to vascular endothelial cells, increases vascular
permeability and promotes blood vessel formation (43,49).
VEGF protein family includes VEGF-A, -B and -C, virus-encoded
VEGF-D and placental growth factor, with VEGF-A playing pivotal
roles in regulation of angiogenesis (43). The VEGF signaling pathway regulates
activity of multiple kinases by binding to cell surface tyrosine
kinase receptors, VEGF receptor (R)-1, VEGFR-2, and VEGFR-3,
resulting in different biological effects that ultimately result in
angiogenesis (43). In recent
years, expression of VEGF has been studied in different
experimental EM models and tissue samples from patients with EM
(10,50–52).
Numerous researchers have found that increased expression of VEGF
in the serum and peritoneal fluid of patients with EM can serve as
an auxiliary diagnostic indicator of EM (9,10,50).
Li et al (10) found that
angiogenic factors released from ESCs may be influenced by
expression of fibrinogen α chain (FGA) and that the expression of
pro-angiogenic factors, such as VEGF, platelet-derived growth
factor (PDGF), FGF and MMP-2 and −9, is reduced in FGA-knockdown
hEM15A cells. FGA may activate the VEGFA/VEGFR-2/FAK signaling
pathway in EM and promote angiogenesis by regulating expression of
VEGF-A and MMPs in EM ESCs. In addition, VEGF-C expression is
upregulated in EM cells, and it has been demonstrated that VEGF-C
enhances lymphangiogenic capacity of lymphatic endothelial cells
via extracellular vesicle transport in an autograft mouse model of
EM, whereas blocking the VEGF-C signaling pathway attenuates
development of local chronic inflammation and EM (50). The expression of VEGF in EM is
regulated by expression of multiple factors and related pathways
and plays a vital regulatory role in the process of
neovascularization in EM.
The stimulation of endometrial and endometriotic
cells leads to activation of different intracellular pathways and
associated signaling molecules. Nuclear factor-κB (NF-κB) is a
transcription factor that mediates inflammatory signaling pathways
and is a driver of inflammation and plays a crucial role in the
development and regulation of inflammation (2). NF-κB is expressed in trace amounts in
normal endometrium, but it is expressed at high levels in EM,
suggesting that activated NF-κB serves an important role in
regulating the development of ectopic endometrium (26,53).
Gou et al (33) showed that
estrogen receptor β (ERβ) regulates production of C-C motif
chemokine ligand 2 through activation of the NF-κB signaling
pathway in B cells, thereby recruiting macrophages to ectopic
lesions in ESCs to promote pathogenesis. Additionally, ERβ
stimulates expression of genes associated with the unfolded protein
response in normal endometrium, inhibits the IL-6/JAK/STAT3
signaling pathway and suppresses the TNFα/NF-κB signaling pathway,
leading to endometrial dysfunction associated with EM (54). In addition, oxidative stress is
associated with development of EM and is a key inducer of the NF-κB
signaling pathway in EM cells, extracellular high mobility group
box-1 (HMGB-1), a prototypical molecule of damage-associated
molecular patterns, inducing an inflammatory response via
HMGB-1/TLR4/NF-κB axis (2,53). Excessive production of ROS in the
pelvis of patients with EM is an important inducer of
NF-κB-mediated chronic inflammatory responses, and NF-κB activation
is primarily regulated by ROS, regulating the expression of
cytokines, such as IL-10, in EM by activating the NF-κB pathway
(26). Oxidative damage to DNA in
EM increases DNA fragmentation and activates NF-κB signaling to
promote transcription and expression of inflammatory factors such
as TNF-α, and IL-1, −8 and −6 (26,55),
while NF-κB is activated by TNF-α, IL-1β and LPS (56), thus creating positive feedback to
promote alteration of the abdominal inflammatory microenvironment
and the development of ectopic endothelial lesions. Additionally,
the NF-κB signaling pathway decreases expression of antioxidant
enzymes by activating the nitric oxide synthase (NOS)/NO signaling
pathway, which is involved in oxidative stress (53).
In addition to VEGF, other factors have been shown
to be involved in angiogenesis in EM. COX-2 serves as a
rate-limiting enzyme for prostaglandins (PGs), induces the
production of PGE2 and E2, which increase VEGF expression (57). COX-2 is rapidly upregulated in
response to proinflammatory and pathogenic stimuli, and it is able
to induce VEGF synthesis upon stimulation by cytokines (26,57)
Furthermore, COX-2 has been shown to serve a vital role in the
pathological process of diseases such as endometrial carcinoma and
endometrial carcinoma (53,58).
COX-2, which is either not expressed or expressed at low levels in
normal endometrium, is aberrantly expressed in patients with EM and
may regulate VEGF to serve a role in the process of disease
development (54,57). MMPs play a key role in various
pathophysiological processes, such as adhesion and invasion of
ectopic endothelium (13,59). Furthermore, COX-2 can regulate
angiogenesis via regulation of MMP-2 activity in EM by the
COX-2/PGE2/phosphorylated AKT axis (60). Blocking the expression of COX-2
and/or AKT inhibits MMP-2 activity and endothelial tube formation
and inhibition of MMP-2 and COX-2 notably decreases the number of
lesions in a mouse model of EM (60).
In conclusion, the pathogenesis of EM is complex and
its angiogenesis is driven by a variety of cytokines mediating pro-
and anti-angiogenic effects, such as hormonal, immune and
inflammatory oxidative stress. These cytokines interact with each
other to form a complex network of regulatory mechanisms and are
involved in development of neovascularization in EM via multiple
cellular pathways (Fig. 2).
Further studies on pathogenesis of EM will contribute to
identification of biomarkers or biomarker groups to improve and the
diagnostic process in a non-invasive manner.
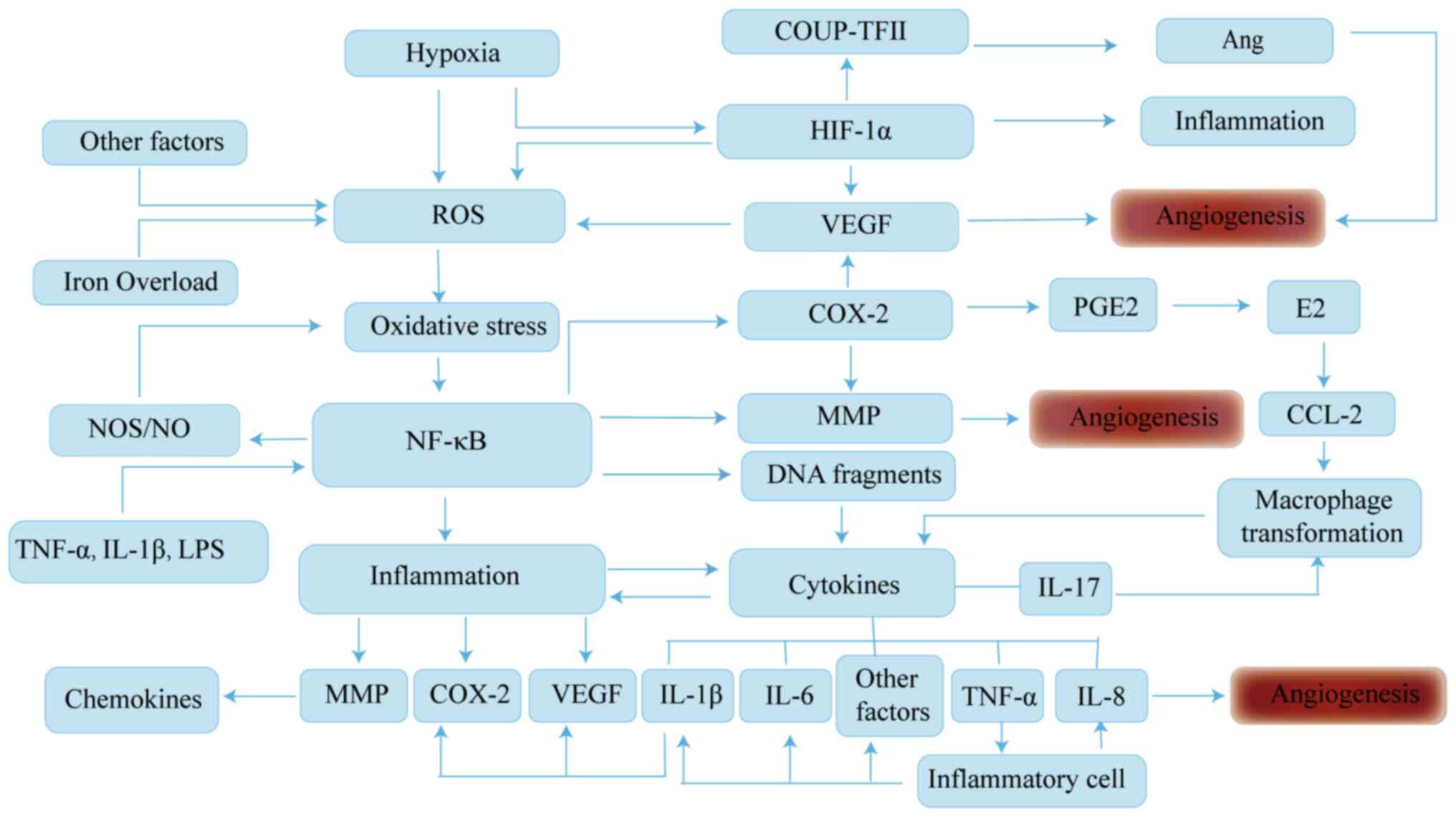 | Figure 2.Molecular pathways of angiogenic
signaling in EM. Angiogenesis is associated with a variety of
factors, including abdominal microenvironment and immunomodulatory
imbalance. In hypoxic conditions, ROS release and VEGF expression
are enhanced. ROS can induce oxidative stress, which drives the
activation of genes downstream of NF-κB signaling, induces
inflammatory responses and regulates the expression of cytokines
such as IL-8 and COX-2, which regulates angiogenesis. In addition,
there is a positive feedback mechanism between ROS and VEGF, which
contributes to angiogenesis. The interaction of the inflammatory
response with cytokines can mediate differentiation of immune
cells, which in turn exacerbates imbalance of the abdominal
microenvironment and angiogenesis of the ectopic endothelium. EM,
endometriosis; ROS, reactive oxygen species; VEGF, vascular
endothelial growth factor; NF-κB, nuclear factor-κB; IL,
interleukin; COX-2, cyclooxygenase 2; COUP-TFII, COUP transcription
factor 2; Ang, angiopoietin; HIF-1α, hypoxia-inducible factor-1α;
PGE2, prostaglandin E2; NOS, nitric oxide synthase; MMP, matrix
metalloproteinase; CCL-2, C-C motif chemokine ligand 2; TNF-α,
tumor necrosis factor-α; LPS, lipopolysaccharide. |
New non-invasive biomarkers in EM
At present, reliable laboratory biomarkers for EM
pathology are difficult to obtain and there are no uniform
international standards (1). The
gold standard for diagnosis of EM remains the pathological findings
following surgical treatment (61,62).
There is often a 6–12-year hiatus between onset and diagnosis of
EM, with the delay being caused by multiple factors, including
economic deprivation and uneven distribution of health services
(63,64). Reliable biomarkers found in the
biological fluids of affected patients may be an expedient
diagnostic tool for EM, which would also facilitate an objective
assessment of treatment efficacy.
Although lacking both specificity and sensitivity
for this pathology, the most representative glycoproteins used as
biomarkers for EM include cancer antigen CA-125 and CA-199, which
have similar specificity and reflect the severity of disease
(65,66). However, in total laparoscopic
ectopic lesion removal, high CA-199 expression is markedly
associated with a high postoperative recurrence rate; monitoring
the expression of CA-199 may help predict the progression of EM
(67).
Vascular cell adhesion molecule-1 (VCAM-1) and
intercellular adhesion molecule-1 (ICAM-1) are members of the
integrin adhesion protein family, expressed by endothelial and
other cells, and are involved in inflammatory responses, immunity,
tumor immune escape and other processes (68,69),
potentially due to involvement of the NF-κB pathway in
TNF-α-induced upregulation of ICAM-1 and VCAM-1 in vascular
endothelial cells (70,71). Kuessel et al (72) showed that the mRNA levels of both
VCAM-1 and ICAM-1 are high in normal peritoneal samples of patients
with EM and that serum soluble VCAM-1 (sVCAM-1) levels are not
influenced by the entity of the lesion, the stage of menstrual
cycle or the severity of the disease. The aforementioned study
further indicated that the specificity and sensitivity of serum
VCAM-1 for diagnosis of EM is 80 and 84%, respectively. It is
noteworthy that the diagnostic specificity and sensitivity of
sVCAM-1/sICAM-1 ratio, which further improved this predictive
performance, were 86.7 and 90.3%, respectively. In addition,
elevated levels of sICAM-1 may impair natural killer cell activity,
which in turn accelerates the progression of EM (70).
VEGF, one of the most potent pro-angiogenic factors,
is expressed at elevated levels in patients with EM and may be a
potential biological molecule for the diagnosis of EM (10,50).
A previous study demonstrated that degree of VEGF elevation is
associated with the stage of EM, with additional marked elevation
in advanced stages (24).
Serum microRNA (miRNA/miR) is a non-coding RNA
consisting of 21–23 nucleotides widely found in eukaryotes, which
bind exosomes or to specific protein complexes to protect against
endogenous RNase degradation, playing an active role in the
regulation of gene expression and cell cycle (73,74).
Several studies (75,76) have shown that multiple miRNAs
regulate angiogenesis and inflammatory responses in the ectopic
endometrium by regulating expression of molecules such as VEGF,
MMPs, IL and TGF-β1. Consequently, dysregulation of their
expression serves an essential role in the pathogenesis of EM
(75,77). Compared with conventional
ultrasound, miRNAs have the advantages of improved stability and
specificity, simpler detection methods and lower detection costs,
they are becoming potential molecular indicators for non-invasive
identification of EM (Table
II).
 | Table II.Studies reporting the sensitivity and
specificity of dysregulated miRNAs in EM. |
Table II.
Studies reporting the sensitivity and
specificity of dysregulated miRNAs in EM.
| miRNA | Sensitivity, % | Specificity, % | Alteration | (Refs.) |
|---|
| miRNA-125b-5p | 100.0 | 96.0 | ↑ | (74) |
| miRNA-34a-5p | 79.0 | 49.0 | ↓ | (95) |
| miRNA-122 | 96.5 | 94.1 | ↑ | (86) |
| miRNA-141 | 71.9 | 70.8 | ↓ | (94) |
| miRNA-185-5p | 81.2 | 90.0 | ↓ | (78) |
| miRNA-199a | 100.0 | 100.0 | ↑ | (86) |
| miRNAs-199b-3p | 96.0 | 80.0 | ↑ | (99) |
| miRNA-200a | 90.6 | 62.5 | ↓ | (94) |
| miRNA-200c | 100.0 | 96.0 | ↑ | (95) |
| miRNA-224-5p | 84.0 | 80.0 | ↓ | (99) |
| miRNA-342 | 90.0 | 91.2 | ↑ | (79) |
| miRNA-451a | 85.4 | 84.6 | ↑ | (84) |
| miRNA-3613 | 92.7 | 61.0 | ↓ | (79) |
| let-7d | 83.3 | 100.0 | ↓ | (80) |
| miRNA-199a,
miRNA-122, miRNA-145 and miRNA-542-3p | 93.2 | 96.0 | - | (98) |
| miRNAs-199b-3p,
miRNA-224-5p and let-7d-3p | 96.0 | 100.0 | - | (99) |
| miRNA-155,
miRNA574-3p and miRNA139-3p | 83.0 | 51.0 | - | (97) |
An increasing number of studies have been conducted
to detect expression of one or more specific miRNAs in plasma by
reverse transcription-quantitative PCR to investigate their
diagnostic value in EM and it has been hypothesized that the
miR-125b, −451 and let-7 families may serve as potential
circulating biomarkers for EM (78–80).
A previous study showed that serum miR-125b-5p has a sensitivity
and specificity of 100 and 96%, respectively, for the diagnosis of
EM (74). In patients with EM,
upregulation of miR-125b-5p and downregulation of let-7b-5p
expression may be associated with an increase in cytokines such as
TNF-α and IL-1β and −6 (81,82).
These cytokines are hypothesized to be important components
involved in angiogenesis (31,39).
Migration inhibitory factor (MIF) is a pleiotropic inflammatory
cytokine with upstream regulatory roles in immunity and has been
shown to be involved in the development of EM. miRNA-451, which is
hypothesized to target MIF, is a mitogenic cytokine that creates a
proliferative and angiogenic phenotype for growth of ectopic
endothelium (76). Compared with
the healthy population, miRNA-451 expression is decreased in
patients with endometrial co-infertility, suggesting that miRNA-451
expression levels are negatively associated with co-infertility in
patients with EM and may be used as a marker for disease prognosis
(83,84). In addition, elevated miR-145-5p
expression levels in EM are associated with upregulation of VEGF-A
and decreased levels of epidermal growth factor 2, phosphatase and
tensin homolog and C-X-C chemokine receptor type 4 (75).
MiR-199a is another possible suitable biomarker for
EM. miR-199a downregulates the expression of VEGF-A in ESCs under
hypoxic conditions and partially attenuates angiogenesis in
hematopoietic stem cells under hypoxic conditions by inhibiting the
HIF-1α/VEGF-A pathway (85). Maged
et al (86) showed that
serum miR-199a and −122 have high sensitivity and specificity and
miR-199a has 100% sensitivity and specificity for the diagnosis of
EM. Another study showed that VEGF-A is a direct and functional
target of miR-199a-5p and elevated miR-199a-5p expression inhibits
the proliferation and angiogenesis of MSCs, as confirmed in
vitro experiments and EM female C57BL/6j mouse models (87). Notably, HIF-1 upregulates miR-20a;
miR-20a not only increases the expression of the VEGF-A angiogenic
gene, but also downregulates anti-angiogenic thrombospondin 1 to
promote angiogenesis (76,88–90).
However, miR-20 as a biomarker for the diagnosis of EM remains to
be investigated. In addition, dysregulation of miRNA-202-3p
(91), miRNA-126 (92), miRNA-200 (75,93–95)
and other genes mediate VEGF expression to promote angiogenesis and
invasion of ectopic endothelium by promoting cell proliferation and
angiogenesis. The aforementioned studies suggest that epigenetic
abnormalities and dysregulation of miRNAs may play a key role in
the formation of EM by affecting different physiological processes,
which may also be beneficial for further studies on mechanisms of
EM.
With the progress of miRNA research, diagnostic
value of miRNAs has been further confirmed in the clinical setting.
Moustafa et al (79) first
demonstrated in a prospective study that plasma miRNAs accurately
distinguish EM from other gynecological diseases, and miR-125b-5p,
−150-5p, −342-3p and 451a are notably higher in patients with EM,
while miR-3613-5p and let-7b are expressed at lower levels, miRNA
expression levels are not markedly associated with menstrual cycle
or hormonal drug use. To improve the diagnostic value of miRNA,
co-diagnostic methods have been applied (96–99).
Results of several studies remain controversial and
even contradictory due to the irreproducible nature of the studies
(12,100). At present, no single or groups of
miRNA biomarkers has sufficient specificity and sensitivity in the
diagnosis of EM (101). Future
studies need to adopt uniform and standardized methods to clarify
the value of miRNA as a biological marker for early diagnosis and
prognosis prediction of EM and for clinical translation and
application.
The Internet of Medical Thing (IoMT) is the most
emerging era of the Internet of Things (102,103). IoMT can generate large amounts of
data without human intervention through connected smart medical
devices, allowing healthcare professionals to facilitate disease
detection by learning complex models and extracting meaningful
information from large amounts of data (103). Based on this, researchers have
trained on pulmonary embolism detection datasets and obtained
satisfactory results in terms of sensitivity and specificity
(102). To the best of our
knowledge, however, its application in EM has not been reported
yet. EM is a chronic inflammatory disease and using IoMT to
establish a safe and effective system may contribute to the
examination and treatment of the disease.
Numerous studies reveal (69,104,105) the importance and effectiveness of
extracting various putative biomarkers from the biological fluids
of affected patients. Nevertheless, due to the large heterogeneity
between different studies, there is no uniform conclusion and
further studies with larger sample sizes are needed to identify
sensitive and specific indicators for transition from the
laboratory to the clinic. As vascular mechanisms of ectopic
endometrium are refined, newer and more accurate diagnostic markers
for EM are expected to be discovered.
Anti-angiogenic treatment in EM
The current medical treatment for EM focuses on
either hormonal modulation to induce a low estrogen state or
surgical treatment to remove the ectopic lesions. However, even if
the ectopic lesions are removed by surgery, certain patients will
still have recurrence and even face the possibility of reoperation
(106). Nevertheless, targeted
therapeutic strategies based on anti-angiogenic therapy have been
demonstrated in oncology and ophthalmology and have been widely
used in clinical practice (8,43).
As aforementioned, angiogenesis serves a key role in the
development of EM and is essential for survival of ectopic
endothelial implants. This shows that inhibition of ectopic
endothelial angiogenesis is an important method to treat EM and if
the mechanism can be regulated or inhibited or its pathway
expression blocked, ectopic endothelial angiogenesis can be
inhibited, improving the symptoms of patients and having value for
clinical treatment. Currently, the primary anti-angiogenic drugs
used in targeted therapy include anti-VEGF antibodies, VEGFR
tyrosine kinase and COX-2 inhibitors and dopamine agonists (D2-ags;
Table III).
 | Table III.Research progress of anti-angiogenic
drugs in EM. |
Table III.
Research progress of anti-angiogenic
drugs in EM.
| Class of drug | Drug | Experimental
design | Mechanism of
action | Outcome | (Refs.) |
|---|
| Anti-VEGF
antibody | Bevacizumab | Rat model | Anti-VEGF | Significant
decrease in lesion area | (52) |
|
| Ranibizumab | Rat model | Anti-VEGF | Significant
decrease in lesion area | (51) |
| Tyrosine
kinase | Sorafenib | Mouse model | Inhibition of
VEGF | Decreased lesion
implantation | (109) |
| inhibitor | Sunitinib | Mouse model | Promotes maturation
of peritoneal fluid MDSCs and suppresses immunosuppressive
function | Significant
decrease in the size and weight of EM lesions | (108) |
|
| Pazopanib | Rat model | Inhibits VEGF and
CD117 expression | Decreased lesion
implantation | (109) |
| Dopamine receptor
agonist | Cabergoline | Clinical trial | Inactivates VEGFR-2
to exert anti-angiogenic effects | Significant
decrease in size of endometriomas | (112) |
|
| Quinagolide | In
vitro | Inhibition of AKT
signaling pathway | Significant
inhibition of ectopic E-MSC | (110) |
| COX-2
Inhibitor | Celecoxib | BALB/c mouse
model | Inhibition of COX-2
signaling pathway | Decreased lesion
implant volume and vascular density | (114) |
|
| Parecoxib | C57BL/6 mouse
model | Inhibition of COX-2
signaling pathway | Decreased volume of
diseased implant volume | (117) |
| PPARγ
activator | Rosiglitazone | BALB/c mouse and
rat model | Activation of PPARγ
signaling pathway | Inhibited lesion
growth, cell proliferation and vascularization; increased
apoptosis | (114,115) |
| Angiotensin II
receptor blocker | Telmisartan | C57BL/6 mouse
model | Combined blockade
of AT1R and activation of PPARγ | Significant
inhibition of the growth of lesions and decreased density of blood
vessels | (116,117) |
| NF-κB
inhibitor | BAY11-7085 | In
vitro | Inhibition of NF-κB
signaling pathway and expression of anti-apoptotic proteins | Inhibition of the
viability of ectopic endothelial cells | (118) |
|
| Pyrrolidine
dithiocarbamate | In
vitro | Inhibition of NF-κB
signaling pathway | Inhibition of COX-2
expression, decreased PGE2 production, inhibition of EM cell
proliferation, angiogenesis and inflammatory response | (119) |
| Other | Ginsenoside
Rg3 | In
vitro | Regulation of
apoptosis and angiogenesis via nuclear factor/NF-κB signaling
pathway | Significantly
decreased levels of VEGF | (120) |
|
| Curcumin | In
vitro | Inhibition of NF-κB
pathway activation | Decreased secretion
of chemokines and cytokines | (121,122) |
|
| Nobiletin | C57BL/6 mouse
model | Inhibition of NF-κB
pathway activation | Significantly
decreased lesion size and pain in EM mice | (123) |
|
| Dienogest | Clinical trial | Inhibition of NF-κB
pathway | Effective decrease
in endometrial tumor size and EM-associated pain | (124) |
|
Immunomodulator | Etanercept | Wistar Albino rat
model | Decreased
expression and activity of serum VEGF, IL-6 and TNF-α | Effective decrease
in the volume of ectopic lesions | (125) |
|
| Rapamycin | Mouse model | Inhibition of VEGF
expression | Significant
decrease in the volume of ectopic lesions | (126) |
|
| Interleukin
(IFN) | In
vitro | Interference with
the cell cycle | Inhibition of ESC
cell proliferation and migration | (128) |
| Antioxidant | Melatonin | Clinical trial | Antioxidation | Improved patient
sleep quality and decreased pain associated with EM | (129,131) |
|
| Vitamins E and
C | Clinical trial | Antioxidation | Decreased
inflammatory markers in peritoneal fluid and chronic pelvic
pain | (130) |
|
| Resveratrol | Clinical trial | Antioxidation | Decreased
expression levels of VEGF and TNF-α | (25) |
| Iron death
inducer | Erastin | C57BL/6 mouse
model | Induces ectopic
endometrial stromal cell death | Significant
decrease in ectopic lesions | (133) |
Anti-VEGF and its receptor signaling
pathway
Since VEGF is the most potent angiogenic factor and
has an important role in EM (24),
VEGF and its signaling pathway are considered to be the most
effective targets for anti-angiogenic therapy, and in treatment of
kidney, lung, rectal and cervical cancer (13,107). Bevacizumab and ranibizumab are
monoclonal antibodies that bind and selectively neutralize VEGF
activity by inhibiting binding of VEGF to VEGFR, primarily VEGFR2
or kinase insertion domain receptor, thereby reducing angiogenesis;
additionally, both antibodies are effective against EM (51,52).
Blocking VEGF signaling and inhibiting tyrosine kinase activity are
potential targets for anti-angiogenic therapy (107–109). Research on VEGFR tyrosine kinase
inhibitors has been ongoing and tyrosine kinase inhibitors such as
sorafenib and sunitinib have been shown to be effective against EM
(108,109). In comparing the effects of
pazopanib, sorafenib and sunitinib on the VEGF/VEGFR protein kinase
pathway and their role in EM, Yildiz et al (109) noted that pazopanib is more
effective than control and other treatments, reducing EM lesions by
≥45%, but sorafenib exhibited improved modulation of VEGF.
Anlotinib is a novel oral multitarget tyrosine kinase inhibitor
developed independently in China that can effectively inhibit
VEGFR, platelet-derived growth factor receptor, FGFR and c-Kit and
exert antitumor angiogenesis effects and has been applied in
gynecological tumors such as ovarian cancer; to the best of our
knowledge however, no studies have reported on its application in
EM (107).
D2-ags
E-MSCs express D2, with notably higher levels of
ectopic endometrial expression relative to normal endometrial
tissue (110). D2-ags decrease
tumor size by targeting abnormal angiogenesis in pathological
tissue, showing that D2-ags could be used to treat EM (111,112). Cabergoline, a dopamine agonist,
exerts anti-angiogenic effects by inducing endocytosis of VEGFR-2
in endothelial cells, leading to decreased and inactivated
VEGF-VEGFR-2 binding (112).
D2-ags are as powerful as standard anti-angiogenic compounds in
interfering with angiogenesis and lesion size (111,113). In addition, in a prospective
clinical trial, D2-ags were shown to be superior to luteinizing
hormone-releasing hormone agonists in decreasing size of
endometrial tumors, with fewer drug side effects, easier
administration and lower price (112). Quinagolide is a non-ergot-derived
D2-ag that has been reported to reduce or eliminate peritoneal EM
lesions in patients with EM (110). Another study found notable
inhibition of E-MSCs by quinagolide, which decreases invasion and
endothelial differentiation via the AKT signaling pathway, further
supporting the theoretical basis for the use of this drug in
treatment of EM (110). The role
of quinagolide in EM is currently undergoing phase 2 clinical
trials (trial nos. NCT03749109 and NCT03692403), which are expected
to provide a novel treatment strategy for the future treatment of
EM (110).
COX-2 inhibitor
Celecoxib, a potent COX-2 inhibitor, has been shown
to inhibit EM lesions by markedly decreasing the size of ectopic
lesions and vascular density in a study exploring the effects of
celecoxib and rosiglitazone on implantation and growth of EM-like
lesions in mice with EM (114).
Additionally, treatment with peroxisome proliferator-activated
receptor γ (PPARγ) activator rosiglitazone has also been shown to
inhibit the growth of EM implants, and the combination of celecoxib
and rosiglitazone is more potent than single application of
celecoxib in the inhibition of ectopic EM lesions (114,115). Telmisartan, a partial agonist of
PPARγ, also blocks angiotensin II type 1 receptor (AT1R); combined
blockade of AT1R and activation of PPARγ markedly inhibits
angiogenesis and growth in EM-like lesions in mice (116). In addition, Nenicu et al
(117) found that combination of
telmisartan and parecoxib in the treatment of endometrioid lesions
increases the rate of lesion regression and notably improves
therapeutic effects compared with treatment with telmisartan alone.
This suggests that the combination of ≥2 drugs may achieve more
desirable therapeutic effects than a single drug against
angiogenesis.
NF-κB inhibitor
NF-κB, a key factor in signaling, can regulate
growth of ectopic endothelium through multiple pathways (53). Therefore, NF-κB is considered an
important target for anti-angiogenic therapy. BAY11-7085, an NF-κB
inhibitor, inhibits the viability of EM cells and induces apoptosis
in endometriotic cyst stromal cells by inhibiting anti-apoptotic
proteins in an in vitro experimental study; its effect on
normal endometrial cells is not notable, but, to the best of our
knowledge, clinical studies for its treatment have not been
reported (118). Pyrrolidine
dithiocarbamate is another potent NF-κB inhibitor that inhibits
NF-κB signaling in EM cells, suppresses COX-2 expression, decreases
PGE2 production and suppresses EM cell proliferation, angiogenesis
and inflammatory responses (119). In addition, natural extracts such
as ginsenoside Rg3 and curcumin decrease EM by regulating apoptosis
and inhibiting VEGF expression and angiogenesis via the NF-κB
signaling pathway (120–122). The low risk of adverse effects of
these natural products indicates their potential clinical value in
the treatment of EM. It has been demonstrated that the inhibitory
effect of nobiletin on EM is achieved by inhibiting activation of
the NF-κB pathway, which markedly decreases the regulation of the
expression of angiogenic factors, including VEGF and E-cadherin, in
ectopic endometrium (123).
Dienogest is an artificially developed, fourth-generation progestin
with strong anti-proliferative effects on EM implants, as well as
anti-angiogenic and anti-inflammatory properties (124). Its mechanism of action may be
associated with inhibition of the NF-κB pathway, which is effective
in decreasing the size of endometriomas and EM-related pain and has
a good safety and tolerability profile, with improved clinical
efficacy in young patients with endometriomas who have not yet had
children (124). Moreover,
2-cyano-3,12-dioxooleana1,9-dien-28-oic acid effectively inhibit EM
by increasing ectopic cell apoptosis and decreasing angiogenesis by
modulating Nrf2 and NF-κB pathways and has anti-fibrotic,
anti-inflammatory and antioxidant effects in EM (55).
Immunomodulators
Numerous researchers have noted involvement of
immune inflammatory factors in ectopic endothelial vascularization
(18,27,104). Therefore, modulation of the
immune system may also serve a role in EM treatment. Etanercept, a
fusion protein consisting of the human recombinant soluble TNF
receptor 2 bound to human Fc antibody subunits, is effective in
decreasing the volume of ectopic lesions in rats by decreasing
serum VEGF and IL-6 levels, and TNF-α activity (125). An experiment investigating the
effect of rapamycin (RAPA) on EM lesions in severe combined
immunodeficient mice found that RAPA inhibits the growth of EM
lesions, potentially by suppressing expression of VEGF in the
lesions and thus angiogenesis (126). In addition, similar
pharmacological efficacy of interferons (IFNs) and pentoxifylline
for treatment of EM has been observed, IFN-β 1a has a notably
stronger in vitro inhibitory effect on ESC proliferation and
migration than IFN-α 2b, while the efficacy and safety of
pentoxifylline in treatment of EM has been reported, but there is
insufficient evidence to support its effectiveness in the treatment
of infertility and pain in EM (127,128). The data from the aforementioned
studies provide a theoretical basis for future clinical trials.
Antioxidant therapy
With in-depth research on development and targets of
EM, the important role of oxidative stress in EM has become
increasingly recognized (2,53).
In parallel, studies have demonstrated that antioxidants inhibit
the development of ectopic endothelium and achieve efficacy in the
treatment of EM (25,129–131). The effects of antioxidant
melatonin and vitamins E and C on EM are all demonstrated in
clinical randomized placebo-controlled trials; they notably improve
the reduction of chronic pelvic pain and decrease expression of
inflammatory markers in peritoneal fluid in patients with EM
(129–132). Resveratrol is a naturally
occurring synthetic polyphenolic compound with antitumor,
anti-inflammatory, antioxidant and anti-angiogenic properties
(25). In a randomized controlled
clinical trial study, resveratrol was found to decrease
angiogenesis and inflammation in endometrial tissue of patients
with EM by downregulating expression of VEGF and TNF-α (25). Although melatonin, resveratrol and
combined vitamin C and E supplements have shown good results in
treatment of EM, their use requires further investigation.
Recently, Li et al (133)
demonstrated that the iron death inducer erastin could induce
ectopic endometrial stromal cell death in C57BL/6 female mouse
model of EM, and the ectopic lesions are reduced after treatment
with erastin, suggesting that erastin may be a potential drug for
treatment of EM, which provides a new idea for the targeted
treatment of EM.
In conclusion, anti-angiogenic therapy has become a
focal point of medical research and has an improved effect in the
treatment of EM, which may provide a potential novel treatment for
EM (Fig. 3). However, most of the
drug application research results are at the stage of in
vitro and animal experiments; although some drugs are at the
stage of clinical experiments, there is still lack of drug safety
and efficacy assessment. Therefore, more clinical trials are needed
to assess the value of these drugs clinically.
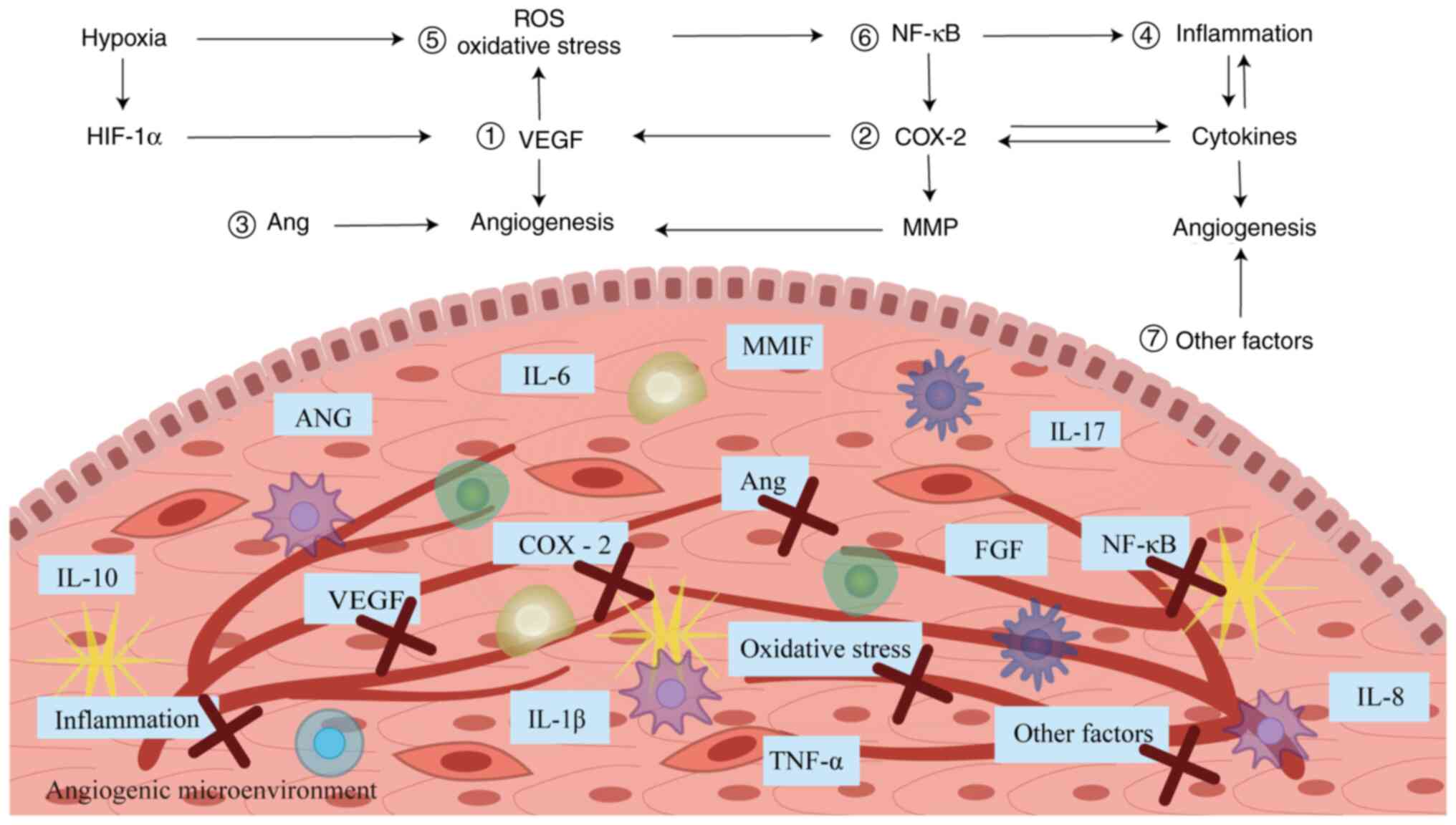 | Figure 3.Basis of action of anti-angiogenic
drugs in EM. (1) Anti-VEGF drugs
such as bevacizumab and sorafenib. (2) COX-2 inhibitors such as celecoxib and
parecoxib. (3) Angiotensin II
receptor blockers such as telmisartan. (4) Immunomodulators such as etanercept and
rapamycin. (5) Antioxidants such
as melatonin and resveratrol. (6)
NF-κB inhibitors such as BAY11-7085 and pyrrolidine
dithiocarbamate. (7) Other drugs
such as cabergoline and quinagolide. EM, endometriosis; VEGF,
vascular endothelial growth factor; COX-2, cyclooxygenase 2; NF-κB,
nuclear factor-κB; IL, interleukin; ANG, angiotensin; TNF-α, tumor
necrosis factor-α; MMIF, macrophage migration inhibitory factor;
Ang, angiopoietin; FGF, fibroblast growth factor; HIF-1α,
hypoxia-inducible factor-1α. |
Conclusions
Although EM is a benign lesion, the mechanism of its
angiogenesis has similarities with pathologic angiogenesis
mediating tumor and metastasis, which is complicated by multiple
factors and mechanisms. The improvement of understanding of the EM
angiogenic network regulation system has brought new hope for its
diagnosis and treatment. However, the majority of studies are still
at the laboratory stage, and to the best of our knowledge, there
are no satisfactory clinical trials due to differences in research
data and non-reproducibility, making it challenging to apply
non-invasive biomarkers and anti-angiogenic drugs in the
clinic.
In addition, clinical evidence for the efficacy of
anti-angiogenic treatment strategies in EM is lacking.
Anti-angiogenic therapy may adversely affect normal physiological
angiogenesis such as ovulation and wound healing (134,135). Research is required to elucidate
the interactions between factors in the angiogenic microenvironment
to discover more effective targets for drug therapy. In addition,
clinical trials are needed to evaluate the therapeutic value of
these serum markers and anti-vascular drugs and to strengthen
multi-targeted combined diagnosis and treatment to inhibit
angiogenesis.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National Natural Science
Foundation of China (grant no. 81502255), Shandong Provincial
Traditional Chinese Medicine Science and Technology Development
Project (grant no. M-2022242) and the Key R&D Program of Jining
(grant nos. 2020YXNS026 and 2022YXNS007).
Availability of data and materials
Not applicable.
Authors' contributions
CB conceived the study, performed the literature
review, wrote the manuscript and constructed figures. YW conceived
and supervised the study and wrote and edited the manuscript. Both
authors have read and approved the final manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AT1R
|
angiotensin II type 1 receptor
|
|
COX-2
|
cyclooxygenase 2
|
|
DC
|
dendritic cell
|
|
D2-ag
|
dopamine receptor agonist
|
|
E-MSC
|
endometrial mesenchymal stromal
cell
|
|
EM
|
endometriosis
|
|
ESC
|
endometrial stromal cell
|
|
ERβ
|
estrogen receptor β
|
|
FGA
|
fibrinogen α chain
|
|
FGF
|
fibroblast growth factor
|
|
Th17
|
helper T 17
|
|
HIF-1α
|
hypoxia-inducible factor-1α
|
|
ICAM-1
|
intercellular adhesion molecule-1
|
|
LPS
|
lipopolysaccharide
|
|
MMP
|
matrix metalloproteinase
|
|
NF-κB
|
nuclear factor-κB
|
|
PPARγ
|
peroxisome proliferator-activated
receptor γ
|
|
PDGF
|
platelet-derived growth factor
|
|
PGE2
|
prostaglandin E2
|
|
RAPA
|
rapamycin
|
|
ROS
|
reactive oxygen species
|
|
TNF-α
|
tumor necrosis factor-α
|
|
TFG-β
|
transforming growth factor-β
|
|
sVCAM-1
|
soluble vascular cell adhesion
molecule-1
|
|
VEGF
|
vascular endothelial growth
factor
|
References
|
1
|
Smolarz B, Szyłło K and Romanowicz H:
Endometriosis: Epidemiology, classification, pathogenesis,
treatment and genetics (Review of Literature). Int J Mol Sci.
22:105542021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Samimi M, Pourhanifeh MH, Mehdizadehkashi
A, Eftekhar T and Asemi Z: The role of inflammation, oxidative
stress, angiogenesis, and apoptosis in the pathophysiology of
endometriosis: Basic science and new insights based on gene
expression. J Cell Physiol. 234:19384–19392. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zondervan KT, Becker CM and Missmer SA:
Endometriosis. N Engl J Med. 382:1244–1256. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brasil DL, Montagna E, Trevisan CM, La
Rosa VL, Laganà AS, Barbosa CP, Bianco B and Zaia V: Psychological
stress levels in women with endometriosis: Systematic review and
meta-analysis of observational studies. Minerva Med. 111:90–102.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sullivan-Myers C, Sherman KA, Beath AP,
Duckworth TJ and Cooper MJW: Delineating sociodemographic, medical
and quality of life factors associated with psychological distress
in individuals with endometriosis. Hum Reprod. 36:2170–2180. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Nicholes K and Shih IM: The origin
and pathogenesis of endometriosis. Annu Rev Pathol. 15:71–95. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Laganà AS, Vitale SG, Salmeri FM, Triolo
O, Ban Frangež H, Vrtačnik-Bokal E, Stojanovska L, Apostolopoulos
V, Granese R and Sofo V: Unus pro omnibus, omnes pro uno: A novel,
evidence-based, unifying theory for the pathogenesis of
endometriosis. Med Hypotheses. 103:10–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dudley AC and Griffioen AW: Pathological
angiogenesis: Mechanisms and therapeutic strategies. Angiogenesis.
26:313–347. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ben Dhaou C, Mandi K, Frye M, Acheampong
A, Radi A, De Becker B, Antoine M, Baeyens N, Wittamer V and
Parmentier M: Chemerin regulates normal angiogenesis and
hypoxia-driven neovascularization. Angiogenesis. 25:159–179. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li H, Cai E, Cheng H, Ye X, Ma R, Zhu H
and Chang X: FGA Controls VEGFA secretion to promote angiogenesis
by activating the VEGFR2-FAK signalling pathway. Front Endocrinol
(Lausanne). 13:7918602022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tan Y, Flynn WF, Sivajothi S, Luo D, Bozal
SB, Davé M, Luciano AA, Robson P, Luciano DE and Courtois ET:
Single-cell analysis of endometriosis reveals a coordinated
transcriptional programme driving immunotolerance and angiogenesis
across eutopic and ectopic tissues. Nat Cell Biol. 24:1306–1318.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hon JX, Wahab NA, Karim AKA, Mokhtar NM
and Mokhtar MH: MicroRNAs in Endometriosis: Insights into
inflammation and progesterone resistance. Int J Mol Sci.
24:150012023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Potente M, Gerhardt H and Carmeliet P:
Basic and therapeutic aspects of angiogenesis. Cell. 146:873–887.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cha J, Sun X and Dey SK: Mechanisms of
implantation: Strategies for successful pregnancy. Nat Med.
18:1754–1767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tarokh M, Ghaffari Novin M, Poordast T,
Tavana Z, Nazarian H, Norouzian M and Gharesi-Fard B: Serum and
peritoneal fluid cytokine profiles in infertile women with
endometriosis. Iran J Immunol. 16:151–162. 2019.PubMed/NCBI
|
|
16
|
Laschke MW and Menger MD: Basic mechanisms
of vascularization in endometriosis and their clinical
implications. Hum Reprod Update. 24:207–224. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Symons LK, Miller JE, Kay VR, Marks RM,
Liblik K, Koti M and Tayade C: The immunopathophysiology of
endometriosis. Trends Mol Med. 24:748–762. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen S, Liu Y, Zhong Z, Wei C, Liu Y and
Zhu X: Peritoneal immune microenvironment of endometriosis: Role
and therapeutic perspectives. Front Immunol. 14:11346632023.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo F, He Y, Fan Y, Du Z, Sun H, Feng Z,
Zhang G and Xiong T: G-CSF and IL-6 may be involved in formation of
endometriosis lesions by increasing the expression of angiogenic
factors in neutrophils. Mol Hum Reprod. 27:gaab0642021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sikora J, Mielczarek-Palacz A and
Kondera-Anasz Z: Association of the precursor of interleukin-1β and
peritoneal inflammation-role in pathogenesis of endometriosis. J
Clin Lab Anal. 30:831–837. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li C, Zhao HL, Li YJ, Zhang YY, Liu HY,
Feng FZ and Yan H: The expression and significance of leukemia
inhibitory factor, interleukin-6 and vascular endothelial growth
factor in Chinese patients with endometriosis. Arch Gynecol Obstet.
304:163–170. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barcz E, Rózewska ES, Kaminski P, Demkow
U, Bobrowska K and Marianowski L: Angiogenic activity and IL-8
concentrations in peritoneal fluid and sera in endometriosis. Int J
Gynaecol Obstet. 79:229–235. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sikora J, Smycz-Kubańska M,
Mielczarek-Palacz A, Bednarek I and Kondera-Anasz Z: The
involvement of multifunctional TGF-β and related cytokines in
pathogenesis of endometriosis. Immunol Lett. 201:31–37. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang F, Liu XL, Wang W, Dong HL, Xia YF,
Ruan LP and Liu LP: Expression of MMIF, HIF-1α and VEGF in serum
and endometrial tissues of patients with endometriosis. Curr Med
Sci. 38:499–504. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khodarahmian M, Amidi F, Moini A, Kashani
L, Salahi E, Danaii-Mehrabad S, Nashtaei MS, Mojtahedi MF,
Esfandyari S and Sobhani A: A randomized exploratory trial to
assess the effects of resveratrol on VEGF and TNF-α 2 expression in
endometriosis women. J Reprod Immunol. 143:1032482021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nanda A K T, Banerjee P, Dutta M, Wangdi
T, Sharma P, Chaudhury K and Jana SK: Cytokines, angiogenesis, and
extracellular matrix degradation are augmented by oxidative stress
in endometriosis. Ann Lab Med. 40:390–397. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singh AK, Dutta M, Chattopadhyay R,
Chakravarty B and Chaudhury K: Intrafollicular interleukin-8,
interleukin-12, and adrenomedullin are the promising prognostic
markers of oocyte and embryo quality in women with endometriosis. J
Assist Reprod Genet. 33:1363–1372. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Monsanto SP, Edwards AK, Zhou J,
Nagarkatti P, Nagarkatti M, Young SL, Lessey BA and Tayade C:
Surgical removal of endometriotic lesions alters local and systemic
proinflammatory cytokines in endometriosis patients. Fertil Steril.
105:968–977. e52016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vazgiourakis VM, Zervou MI, Papageorgiou
L, Chaniotis D, Spandidos DA, Vlachakis D, Eliopoulos E and
Goulielmos GN: Association of endometriosis with cardiovascular
disease: Genetic aspects (Review). Int J Mol Med. 51:292023.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ahn SH, Edwards AK, Singh SS, Young SL,
Lessey BA and Tayade C: IL-17A contributes to the pathogenesis of
endometriosis by triggering proinflammatory cytokines and
angiogenic growth factors. J Immunol. 195:2591–2600. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin YJ, Lai MD, Lei HY and Wing LY:
Neutrophils and macrophages promote angiogenesis in the early stage
of endometriosis in a mouse model. Endocrinology. 147:1278–1286.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan WK, Liu YN, Song SS, Kang JW, Zhang Y,
Lu L, Wei SW, Xu QX, Zhang WQ, Liu XZ, et al: Zearalenone affects
the growth of endometriosis via estrogen signaling and inflammatory
pathways. Ecotoxicol Environ Saf. 241:1138262022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gou Y, Li X, Li P, Zhang H, Xu T, Wang H,
Wang B, Ma X, Jiang X and Zhang Z: Estrogen receptor β upregulates
CCL2 via NF-κB signaling in endometriotic stromal cells and
recruits macrophages to promote the pathogenesis of endometriosis.
Hum Reprod. 34:646–658. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Laganà AS, Salmeri FM, Ban Frangež H,
Ghezzi F, Vrtačnik-Bokal E and Granese R: Evaluation of M1 and M2
macrophages in ovarian endometriomas from women affected by
endometriosis at different stages of the disease. Gynecol
Endocrinol. 36:441–444. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Q, Yuan M, Jiao X, Huang Y, Li J, Li D,
Ji M and Wang G: M1 macrophage-derived nanovesicles repolarize M2
macrophages for inhibiting the development of endometriosis. Front
Immunol. 12:7077842021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vallvé-Juanico J, Houshdaran S and Giudice
LC: The endometrial immune environment of women with endometriosis.
Hum Reprod Update. 25:564–591. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gao X, Gao H, Shao W, Wang J, Li M and Liu
S: The extracellular vesicle-macrophage regulatory axis: A novel
pathogenesis for endometriosis. Biomolecules. 13:13762023.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Suen JL, Chang Y, Shiu YS, Hsu CY, Sharma
P, Chiu CC, Chen YJ, Hour TC and Tsai EM: IL-10 from plasmacytoid
dendritic cells promotes angiogenesis in the early stage of
endometriosis. J Pathol. 249:485–497. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fainaru O, Adini A, Benny O, Adini I,
Short S, Bazinet L, Nakai K, Pravda E, Hornstein MD, D'Amato RJ and
Folkman J: Dendritic cells support angiogenesis and promote lesion
growth in a murine model of endometriosis. FASEB J. 22:522–529.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Talaat RM, Mohamed SF, Bassyouni IH and
Raouf AA: Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus
erythematosus (SLE) patients: Correlation with disease activity.
Cytokine. 72:146–153. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kang YJ, Cho HJ, Lee Y, Park A, Kim MJ,
Jeung IC, Jung YW, Jung H, Choi I, Lee HG and Yoon SR: IL-17A and
Th17 cells contribute to endometrial cell survival by inhibiting
apoptosis and NK cell mediated cytotoxicity of endometrial cells
via ERK1/2 pathway. Immune Netw. 23:e142023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Miller JE, Ahn SH, Marks RM, Monsanto SP,
Fazleabas AT, Koti M and Tayade C: IL-17A modulates peritoneal
macrophage recruitment and M2 polarization in endometriosis. Front
Immunol. 11:1082020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Apte RS, Chen DS and Ferrara N: VEGF in
signaling and disease: Beyond discovery and development. Cell.
176:1248–1264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu MH, Hsiao KY and Tsai SJ: Hypoxia: The
force of endometriosis. J Obstet Gynaecol Res. 45:532–541. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu H, Zhang Z, Xiong W, Zhang L, Xiong Y,
Li N, He H, Du Y and Liu Y: Hypoxia-inducible factor-1α promotes
endometrial stromal cells migration and invasion by upregulating
autophagy in endometriosis. Reproduction. 153:809–820. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fu JL, Hsiao KY, Lee HC, Li WN, Chang N,
Wu MH and Tsai SJ: Suppression of COUP-TFII upregulates angiogenin
and promotes angiogenesis in endometriosis. Hum Reprod.
33:1517–1527. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhou Y, Jin Y, Wang Y and Wu R: Hypoxia
activates the unfolded protein response signaling network: An
adaptive mechanism for endometriosis. Front Endocrinol (Lausanne).
13:9455782022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cheng J, Yang HL, Gu CJ, Liu YK, Shao J,
Zhu R, He YY, Zhu XY and Li MQ: Melatonin restricts the viability
and angiogenesis of vascular endothelial cells by suppressing
HIF-1α/ROS/VEGF. Int J Mol Med. 43:945–955. 2019.PubMed/NCBI
|
|
49
|
Ferrara N and Adamis AP: Ten years of
anti-vascular endothelial growth factor therapy. Nat Rev Drug
Discov. 15:385–403. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li WN, Hsiao KY, Wang CA, Chang N, Hsu PL,
Sun CH, Wu SR, Wu MH and Tsai SJ: Extracellular vesicle-associated
VEGF-C promotes lymphangiogenesis and immune cells infiltration in
endometriosis. Proc Natl Acad Sci USA. 117:25859–25868. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ureyen Ozdemir E, Adali E, Islimye Taskin
M, Yavasoglu A, Aktug H, Oltulu F and Inceboz U: Effects of
ranibizumab and zoledronic acid on endometriosis in a rat model.
Arch Gynecol Obstet. 305:267–274. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zani ACT, Valerio FP, Meola J, da Silva
AR, Nogueira AA, Candido-Dos-Reis FJ, Poli-Neto OB and Rosa-E-Silva
JC: Impact of bevacizumab on experimentally induced endometriotic
lesions: Angiogenesis, invasion, apoptosis, and cell proliferation.
Reprod Sci. 27:1943–1950. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu Y, Wang J and Zhang X: An update on
the multifaceted role of NF-kappaB in endometriosis. Int J Biol
Sci. 18:4400–4413. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shamloo N, Taghavi N, Yazdani F, Azimian P
and Ahmadi S: Evaluation of VEGF expression correlates with COX-2
expression in pleomorphic adenoma, mucoepidermoid carcinoma and
adenoid cystic carcinoma. Dent Res J (Isfahan). 17:100–106. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Siracusa R, D'Amico R, Cordaro M, Peritore
AF, Genovese T, Gugliandolo E, Crupi R, Impellizzeri D, Cuzzocrea
S, Fusco R and Di Paola R: The Methyl Ester of
2-Cyano-3,12-Dioxooleana-1,9-Dien-28-Oic acid reduces endometrial
lesions development by modulating the NFkB and Nrf2 pathways. Int J
Mol Sci. 22:39912021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Santulli P, Marcellin L, Tosti C,
Chouzenoux S, Cerles O, Borghese B, Batteux F and Chapron C: MAP
kinases and the inflammatory signaling cascade as targets for the
treatment of endometriosis? Expert Opin Ther Targets. 19:1465–1483.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lai ZZ, Yang HL, Ha SY, Chang KK, Mei J,
Zhou WJ, Qiu XM, Wang XQ, Zhu R, Li DJ and Li MQ: Cyclooxygenase-2
in Endometriosis. Int J Biol Sci. 15:2783–2797. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hashemi Goradel N, Najafi M, Salehi E,
Farhood B and Mortezaee K: Cyclooxygenase-2 in cancer: A review. J
Cell Physiol. 234:5683–5699. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ke J, Ye J, Li M and Zhu Z: The role of
matrix metalloproteinases in endometriosis: A potential target.
Biomolecules. 11:17392021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Jana S, Chatterjee K, Ray AK, DasMahapatra
P and Swarnakar S: Regulation of matrix metalloproteinase-2
activity by COX-2-PGE2-pAKT axis promotes angiogenesis in
endometriosis. PLoS One. 11:e01635402016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Horne AW, Daniels J, Hummelshoj L, Cox E
and Cooper KG: Surgical removal of superficial peritoneal
endometriosis for managing women with chronic pelvic pain: time for
a rethink? BJOG. 126:1414–1416. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kiesel L and Sourouni M: Diagnosis of
endometriosis in the 21st century. Climacteric. 22:296–302. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kotowska M, Urbaniak J, Falęcki WJ,
Łazarewicz P, Masiak M and Szymusik I: Awareness of endometriosis
symptoms-A cross sectional survey among polish women. Int J Environ
Res Public Health. 18:99192021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ghai V, Jan H, Shakir F, Haines P and Kent
A: Diagnostic delay for superficial and deep endometriosis in the
United Kingdom. J Obstet Gynaecol. 40:83–89. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rokhgireh S, Mehdizadeh Kashi A, Chaichian
S, Delbandi AA, Allahqoli L, Ahmadi-Pishkuhi M, Khodaverdi S and
Alkatout I: The diagnostic accuracy of combined Enolase/Cr, CA125,
and CA19-9 in the detection of endometriosis. Biomed Res Int.
2020:52082792020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Harada T, Kubota T and Aso T: Usefulness
of CA19-9 versus CA125 for the diagnosis of endometriosis. Fertil
Steril. 78:733–739. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang X, Nie D, Zhang L and Liu X: Study
on diagnostic values and pathological conditions of serum HGF and
CA199 in endometriosis. Am J Transl Res. 13:2849–2857.
2021.PubMed/NCBI
|
|
68
|
Anastasiu CV, Moga MA, Elena Neculau A,
Bălan A, Scârneciu I, Dragomir RM, Dull AM and Chicea LM:
Biomarkers for the noninvasive diagnosis of endometriosis: State of
the art and future perspectives. Int J Mol Sci. 21:17502020.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Moein Mahini S, Younesi M, Mortazavi G,
Samare-Najaf M, Karim Azadbakht M and Jamali N: Non-invasive
diagnosis of endometriosis: Immunologic and genetic markers. Clin
Chim Acta. 538:70–86. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kim KH, Park JK, Choi YW, Kim YH, Lee EN,
Lee JR, Kim HS, Baek SY, Kim BS, Lee KS and Yoon S: Hexane extract
of aged black garlic reduces cell proliferation and attenuates the
expression of ICAM-1 and VCAM-1 in TNF-α-activated human
endometrial stromal cells. Int J Mol Med. 32:67–78. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Fukaya T, Sugawara J, Yoshida H, Murakami
T and Yajima A: Intercellular adhesion molecule-1 and hepatocyte
growth factor in human endometriosis: Original investigation and a
review of literature. Gynecol Obstet Invest. 47 (Suppl 1):S11–S16;
discussion 16–17. 1999. View Article : Google Scholar
|
|
72
|
Kuessel L, Wenzl R, Proestling K,
Balendran S, Pateisky P, Yotova I, Yerlikaya G, Streubel B and
Husslein H: Soluble VCAM-1/soluble ICAM-1 ratio is a promising
biomarker for diagnosing endometriosis. Hum Reprod. 32:770–779.
2017.PubMed/NCBI
|
|
73
|
O'Brien J, Hayder H, Zayed Y and Peng C:
Overview of MicroRNA biogenesis, mechanisms of actions, and
circulation. Front Endocrinol (Lausanne). 9:4022018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cosar E, Mamillapalli R, Ersoy GS, Cho S,
Seifer B and Taylor HS: Serum microRNAs as diagnostic markers of
endometriosis: A comprehensive array-based analysis. Fertil Steril.
106:402–409. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yang RQ, Teng H, Xu XH, Liu SY, Wang YH,
Guo FJ and Liu XJ: Microarray analysis of microRNA deregulation and
angiogenesis-related proteins in endometriosis. Genet Mol Res.
15:2016.
|
|
76
|
Zubrzycka A, Migdalska-Sęk M, Jędrzejczyk
S and Brzeziańska-Lasota E: Circulating miRNAs related to
epithelial-mesenchymal transitions (EMT) as the new molecular
markers in endometriosis. Curr Issues Mol Biol. 43:900–916. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Quintero-Fabián S, Arreola R,
Becerril-Villanueva E, Torres-Romero JC, Arana-Argáez V,
Lara-Riegos J, Ramírez-Camacho MA and Alvarez-Sánchez ME: Role of
matrix metalloproteinases in angiogenesis and cancer. Front Oncol.
9:13702019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hossein Razi M, Eftekhar M, Ghasemi N,
Hasan Sheikhha M and Dehghani Firoozabadi A: Expression levels of
circulatory mir-185-5p, vascular endothelial growth factor, and
platelet-derived growth factor target genes in endometriosis. Int J
Reprod Biomed. 18:347–358. 2020.PubMed/NCBI
|
|
79
|
Moustafa S, Burn M, Mamillapalli R,
Nematian S, Flores V and Taylor HS: Accurate diagnosis of
endometriosis using serum microRNAs. Am J Obstet Gynecol.
223:557.e1–557.e11. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Cho S, Mutlu L, Grechukhina O and Taylor
HS: Circulating microRNAs as potential biomarkers for
endometriosis. Fertil Steril. 103:1252–1260.e1. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Sheikhvatan M, Chaichian S and Moazzami B:
A systematic review and bioinformatics study on genes and
micro-RNAs involving the transformation of endometriosis into
ovarian cancer. Microrna. 9:101–111. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Nematian SE, Mamillapalli R, Kadakia TS,
Majidi Zolbin M, Moustafa S and Taylor HS: Systemic inflammation
induced by microRNAs: Endometriosis-Derived alterations in
circulating microRNA 125b-5p and Let-7b-5p regulate macrophage
cytokine production. J Clin Endocrinol Metab. 103:64–74. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang L, Zhang J, Sun H, Ji X and Zhang S:
Effect of miR-451 on IVF/ICSI-ET outcome in patient with
endometriosis and infertility. Am J Transl Res. 13:13051–13058.
2021.PubMed/NCBI
|
|
84
|
Nothnick WB, Falcone T, Joshi N, Fazleabas
AT and Graham A: Serum miR-451a levels are significantly elevated
in women with endometriosis and recapitulated in baboons (Papio
anubis) with experimentally-induced disease. Reprod Sci.
24:1195–1202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Dai L, Lou W, Zhu J, Zhou X and Di W:
MiR-199a inhibits the angiogenic potential of endometrial stromal
cells under hypoxia by targeting HIF-1α/VEGF pathway. Int J Clin
Exp Pathol. 8:4735–4744. 2015.PubMed/NCBI
|
|
86
|
Maged AM, Deeb WS, El Amir A, Zaki SS, El
Sawah H, Al Mohamady M, Metwally AA and Katta MA: Diagnostic
accuracy of serum miR-122 and miR-199a in women with endometriosis.
Int J Gynaecol Obstet. 141:14–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hsu CY, Hsieh TH, Tsai CF, Tsai HP, Chen
HS, Chang Y, Chuang HY, Lee JN, Hsu YL and Tsai EM: miRNA-199a-5p
regulates VEGFA in endometrial mesenchymal stem cells and
contributes to the pathogenesis of endometriosis. J Pathol.
232:330–343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lin SC, Wang CC, Wu MH, Yang SH, Li YH and
Tsai SJ: Hypoxia-induced microRNA-20a expression increases ERK
phosphorylation and angiogenic gene expression in endometriotic
stromal cells. J Clin Endocrinol Metab. 97:E1515–1523. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lei Z, Li B, Yang Z, Fang H, Zhang GM,
Feng ZH and Huang B: Regulation of HIF-1alpha and VEGF by miR-20b
tunes tumor cells to adapt to the alteration of oxygen
concentration. PLoS One. 4:e76292009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ramón LA, Braza-Boïls A, Gilabert-Estellés
J, Gilabert J, España F, Chirivella M and Estellés A: microRNAs
expression in endometriosis and their relation to angiogenic
factors. Hum Reprod. 26:1082–1090. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Marí-Alexandre J, García-Oms J,
Barceló-Molina M, Gilabert-Aguilar J, Estellés A, Braza-Boíls A and
Gilabert-Estellés J: MicroRNAs and angiogenesis in endometriosis.
Thromb Res. 135 (Suppl 1):S38–S40. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
van Solingen C, Seghers L, Bijkerk R,
Duijs JM, Roeten MK, van Oeveren-Rietdijk AM, Baelde HJ, Monge M,
Vos JB, de Boer HC, et al: Antagomir-mediated silencing of
endothelial cell specific microRNA-126 impairs ischemia-induced
angiogenesis. J Cell Mol Med. 13((8A)): 1577–1585. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Panda H, Pelakh L, Chuang TD, Luo X,
Bukulmez O and Chegini N: Endometrial miR-200c is altered during
transformation into cancerous states and targets the expression of
ZEBs, VEGFA, FLT1, IKKβ, KLF9, and FBLN5. Reprod Sci. 19:786–796.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Rekker K, Saare M, Roost AM, Kaart T,
Sõritsa D, Karro H, Sõritsa A, Simón C, Salumets A and Peters M:
Circulating miR-200-family micro-RNAs have altered plasma levels in
patients with endometriosis and vary with blood collection time.
Fertil Steril. 104:938–946.e2. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Misir S, Hepokur C, Oksasoglu B, Yildiz C,
Yanik A and Aliyazicioglu Y: Circulating serum miR-200c and
miR-34a-5p as diagnostic biomarkers for endometriosis. J Gynecol
Obstet Hum Reprod. 50:1020922021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Papari E, Noruzinia M, Kashani L and
Foster WG: Identification of candidate microRNA markers of
endometriosis with the use of next-generation sequencing and
quantitative real-time polymerase chain reaction. Fertil Steril.
113:1232–1241. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Nisenblat V, Sharkey DJ, Wang Z, Evans SF,
Healey M, Ohlsson Teague EMC, Print CG, Robertson SA and Hull ML:
Plasma miRNAs display limited potential as diagnostic tools for
endometriosis. J Clin Endocrinol Metab. 104:1999–2022. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wang WT, Zhao YN, Han BW, Hong SJ and Chen
YQ: Circulating microRNAs identified in a genome-wide serum
microRNA expression analysis as noninvasive biomarkers for
endometriosis. J Clin Endocrinol Metab. 98:281–289. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zafari N, Tarafdari AM, Izadi P, Noruzinia
M, Yekaninejad MS, Bahramy A and Mohebalian A: A Panel of Plasma
miRNAs 199b-3p, 224-5p and Let-7d-3p as non-invasive diagnostic
biomarkers for endometriosis. Reprod Sci. 28:991–999. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Marí-Alexandre J, Carcelén AP, Agababyan
C, Moreno-Manuel A, García-Oms J, Calabuig-Fariñas S and
Gilabert-Estellés J: Interplay Between MicroRNAs and oxidative
stress in ovarian conditions with a focus on ovarian cancer and
endometriosis. Int J Mol Sci. 20:53222019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhou S, Huang C, Wang W and Liu J:
MiR-370-3p inhibits the development of human endometriosis by
downregulating EDN1 expression in endometrial stromal cells. Cell
Biol Int. 45:1183–1190. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Khan M, Shah PM, Khan IA, Islam SU, Ahmad
Z, Khan F and Lee Y: IoMT-Enabled Computer-Aided Diagnosis of
pulmonary embolism from computed tomography scans using deep
learning. Sensors (Basel). 23:14712023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Mohd Aman AH, Hassan WH, Sameen S,
Attarbashi ZS, Alizadeh M and Latiff LA: IoMT amid COVID-19
pandemic: Application, architecture, technology, and security. J
Netw Comput Appl. 174:1028862021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Wang F, Wang H, Jin D and Zhang Y: Serum
miR-17, IL-4, and IL-6 levels for diagnosis of endometriosis.
Medicine (Baltimore). 97:e108532018. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Menzhinskaya IV, Pavlovich SV, Melkumyan
AG, Chuprynin VD, Yarotskaya EL and Sukhikh GT: Potential
significance of serum autoantibodies to endometrial antigens,
α-Enolase and hormones in non-invasive diagnosis and pathogenesis
of endometriosis. Int J Mol Sci. 24:155782023. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Mathiasen M, Egekvist AG, Kesmodel US,
Knudsen UB and Seyer-Hansen M: Similar evolution of pain symptoms
and quality of life in women with and without endometriosis
undergoing assisted reproductive technology (ART). Acta Obstet
Gynecol Scand. 98:77–85. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Gao Y, Liu P and Shi R: Anlotinib as a
molecular targeted therapy for tumors. Oncol Lett. 20:1001–1014.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
He Y, Hung SW, Liang B, Zhang R, Gao Y,
Chu CY, Zhang T, Xu H, Chung JPW and Wang CC: Receptor tyrosine
kinase inhibitor sunitinib as novel immunotherapy to inhibit
myeloid-derived suppressor cells for treatment of endometriosis.
Front Immunol. 12:6412062021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Yildiz C, Kacan T, Akkar OB, Karakus S,
Kacan SB, Ozer H and Cetin A: Effects of pazopanib, sunitinib, and
sorafenib, Anti-VEGF agents, on the growth of experimental
endometriosis in rats. Reprod Sci. 22:1445–1451. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Iampietro C, Brossa A, Canosa S, Tritta S,
Croston GE, Reinheimer TM, Bonelli F, Carosso AR, Gennarelli G,
Cosma S, et al: Quinagolide treatment reduces invasive and
angiogenic properties of endometrial mesenchymal stromal cells. Int
J Mol Sci. 23:17752022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Pellicer N, Galliano D, Herraiz S, Bagger
YZ, Arce JC and Pellicer A: Use of dopamine agonists to target
angiogenesis in women with endometriosis. Hum Reprod. 36:850–858.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Hamid AM, Madkour WA, Moawad A, Elzaher MA
and Roberts MP: Does cabergoline help in decreasing endometrioma
size compared to LHRH agonist? A prospective randomized study. Arch
Gynecol Obstet. 290:677–682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Mendoza-Torreblanca JG, Cárdenas-Rodríguez
N, Carro-Rodríguez J, Contreras-García IJ, Garciadiego-Cázares D,
Ortega-Cuellar D, Martínez-López V, Alfaro-Rodríguez A,
Evia-Ramírez AN, Ignacio-Mejía I, et al: Antiangiogenic effect of
dopamine and dopaminergic agonists as an adjuvant therapeutic
option in the treatment of cancer, endometriosis, and
osteoarthritis. Int J Mol Sci. 24:101992023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Olivares C, Ricci A, Bilotas M, Barañao RI
and Meresman G: The inhibitory effect of celecoxib and
rosiglitazone on experimental endometriosis. Fertil Steril.
96:428–433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zhang S, Zhuang L, Liu Q, Yu X, Min Q,
Chen M and Chen Q: Rosiglitazone affects the progression of
surgically-induced endometriosis in a rat model. Mol Med Rep.
23:352021.PubMed/NCBI
|
|
116
|
Nenicu A, Körbel C, Gu Y, Menger MD and
Laschke MW: Combined blockade of angiotensin II type 1 receptor and
activation of peroxisome proliferator-activated receptor-γ by
telmisartan effectively inhibits vascularization and growth of
murine endometriosis-like lesions. Hum Reprod. 29:1011–1024. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Nenicu A, Gu Y, Körbel C, Menger MD and
Laschke MW: Combination therapy with telmisartan and parecoxib
induces regression of endometriotic lesions. Br J Pharmacol.
174:2623–2635. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Nasu K, Nishida M, Ueda T, Yuge A, Takai N
and Narahara H: Application of the nuclear factor-kappaB inhibitor
BAY 11-7085 for the treatment of endometriosis: An in vitro study.
Am J Physiol Endocrinol Metab. 293:E16–E23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zhang JJ, Xu ZM, Chang H, Zhang CM, Dai
HY, Ji XQ, Li C and Wang XF: Pyrrolidine dithiocarbamate attenuates
nuclear factor-ĸB activation, cyclooxygenase-2 expression and
prostaglandin E2 production in human endometriotic epithelial
cells. Gynecol Obstet Invest. 72:163–168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Huang R, Chen S, Zhao M, Li Z and Zhu L:
Ginsenoside Rg3 attenuates endometriosis by inhibiting the
viability of human ectopic endometrial stromal cells through the
nuclear factor-kappaB signaling pathway. J Gynecol Obstet Hum
Reprod. 49:1016422020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Vallée A and Lecarpentier Y: Curcumin and
Endometriosis. Int J Mol Sci. 21:E24402020. View Article : Google Scholar
|
|
122
|
Chowdhury I, Banerjee S, Driss A, Xu W,
Mehrabi S, Nezhat C, Sidell N, Taylor RN and Thompson WE: Curcumin
attenuates proangiogenic and proinflammatory factors in human
eutopic endometrial stromal cells through the NF-κB signaling
pathway. J Cell Physiol. 234:6298–6312. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Wei X and Shao X: Nobiletin alleviates
endometriosis via down-regulating NF-κB activity in endometriosis
mouse model. Biosci Rep. 38:BSR201804702018. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Uludag SZ, Demirtas E, Sahin Y and Aygen
EM: Dienogest reduces endometrioma volume and endometriosis-related
pain symptoms. J Obstet Gynaecol. 41:1246–1251. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Keleş CD, Vural B, Filiz S, Vural F, Gacar
G, Eraldemir FC and Kurnaz S: The effects of etanercept and
cabergoline on endometriotic implants, uterus and ovaries in rat
endometriosis model. J Reprod Immunol. 146:1033402021. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Ren XU, Wang Y, Xu G and Dai L: Effect of
rapamycin on endometriosis in mice. Exp Ther Med. 12:101–106. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Grammatis AL, Georgiou EX and Becker CM:
Pentoxifylline for the treatment of endometriosis-associated pain
and infertility. Cochrane Database Syst Rev.
8:CD0076772021.PubMed/NCBI
|
|
128
|
Dicitore A, Castiglioni S, Saronni D,
Gentilini D, Borghi MO, Stabile S, Vignali M, Di Blasio AM, Persani
L and Vitale G: Effects of human recombinant type I IFNs (IFN-α2b
and IFN-β1a) on growth and migration of primary endometrial stromal
cells from women with deeply infiltrating endometriosis: A
preliminary study. Eur J Obstet Gynecol Reprod Biol. 230:192–198.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Söderman L, Böttiger Y, Edlund M,
Järnbert-Pettersson H and Marions L: Adjuvant use of melatonin for
pain management in endometriosis-associated pelvic pain-A
randomized double-blinded, placebo-controlled trial. PLoS One.
18:e02861822023. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Vašková J, Klepcová Z, Špaková I, Urdzík
P, Štofilová J, Bertková I, Kľoc M and Rabajdová M: The importance
of natural antioxidants in female reproduction. Antioxidants
(Basel). 12:9072023. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Schwertner A, Conceição Dos Santos CC,
Costa GD, Deitos A, de Souza A, de Souza IC, Torres IL, da Cunha
Filho JS and Caumo W: Efficacy of melatonin in the treatment of
endometriosis: A phase II, randomized, double-blind,
placebo-controlled trial. Pain. 154:874–881. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Santanam N, Kavtaradze N, Murphy A,
Dominguez C and Parthasarathy S: Antioxidant supplementation
reduces endometriosis-related pelvic pain in humans. Transl Res.
161:189–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Li Y, Zeng X, Lu D, Yin M, Shan M and Gao
Y: Erastin induces ferroptosis via ferroportin-mediated iron
accumulation in endometriosis. Hum Reprod. 36:951–964. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Bouquet de Joliniere J, Fruscalzo A,
Khomsi F, Stochino Loi E, Cherbanyk F, Ayoubi JM and Feki A:
Antiangiogenic therapy as a new strategy in the treatment of
endometriosis? The first case report. Front Surg. 8:7916862021.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Bodnar RJ: Anti-Angiogenic Drugs:
Involvement in cutaneous side effects and wound-healing
complication. Adv Wound Care (New Rochelle). 3:635–646. 2014.
View Article : Google Scholar : PubMed/NCBI
|

















