Introduction
An odontogenic keratocyst (OKC) is a distinctive
cyst lined with a thin and flat parakeratotic squamous epithelium
composed of a palisaded basal layer and a corrugated surface.
Although the most recent World Health Organization classification
(2022) of odontogenic lesions defines OKC as a developmental cyst,
it differs from other odontogenic cysts in that it displays
potentially aggressive behavior due to the high proliferative
activity of the epithelium, and high rates of recurrence and
genetic alterations (1). In
addition, the presence of a solid variant of OKC (SOKC), which is
composed of numerous small, keratinized cysts and epithelial
islands characterized by palisaded basal cells with hyperchromatic
nuclei in the fibrous connective tissue, supports its benign
neoplastic nature (2,3). The clinical and histopathological
features of SOKC may overlap with those of keratoameloblastoma
(KA), a rare variant of ameloblastoma (AB); however, focal stellate
reticulum-like areas, subnuclear vacuolization and lamellated-type
central keratinization have been reported to be key in the
diagnosis of KA (2). Some
researchers have considered SOKC and KA as histogenetically related
entities that represent a continuous spectrum of a single tumor
type (2,3). Zhang et al (1) reported that SOKC and KA are difficult
to distinguish because both are rare lesions with similar clinical,
histopathological and biological features (1). To date, to the best of our knowledge,
two cases of SOKC and six cases of KA in the maxilla have been
reported (1). The concept of
SOKC/KA has been proposed based on the clinicopathological
similarities between these two lesions. The present study describes
a case of SOKC/KA in which primary unicystic OKC recurred as
multiple keratotic islands and microcysts; therefore, we aimed to
characterize the histogenetics of SOKC/KA by performing a genetic
analysis and a review of the literature.
Case report
Patient history
A 26-year-old man who presented with swelling and
pain in the right buccal region in February 2009 was referred to
the Department Of Oral and Maxillofacial Surgery, Hiroshima
University Hospital (Hiroshima, Japan). Radiographs showed
permeation of the root apex of a right maxillary canine. The
patient had no family history of any other diseases, and was a
nonsmoker and nondrinker. Blood tests revealed normal complete
blood count liver function, as determined by detecting alanine
transaminase and aspartate transaminase, alkaline phosphatase and
γ-glutamyl transferase, and renal function, as determined by
detecting blood urea nitrogen, creatinine and estimated glomerular
filtration rate. At the initial visit, no buccal sensory
abnormalities were observed, but bony swelling occurred from the
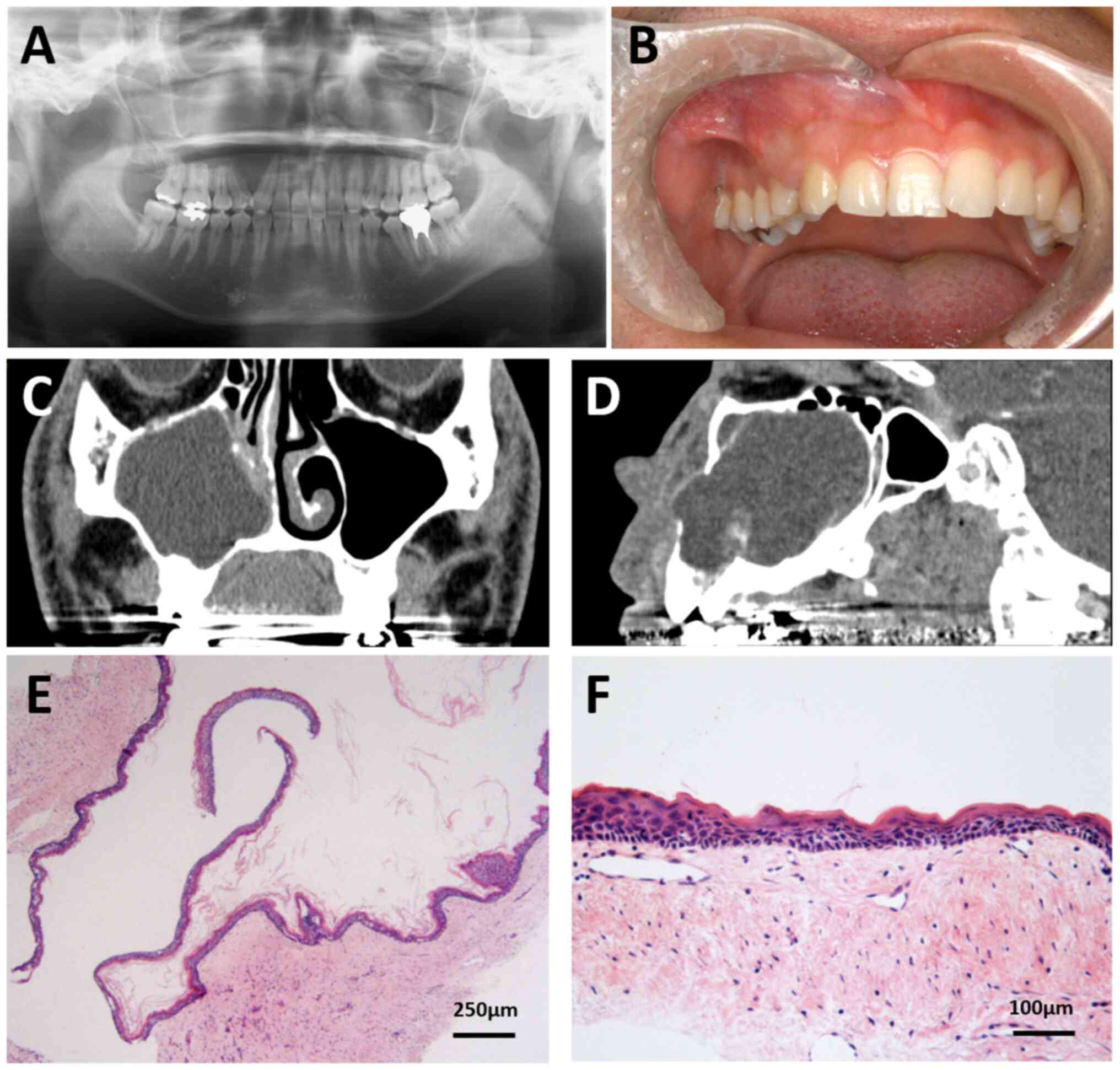
right maxillary canine to the molar region, causing root avulsion
between the canine and the first premolar (Fig. 1A and B). CT imaging before the
treatment showed that the right nasolacrimal duct was obstructed by
the lesion, and radiographs showed a lesion extending from the
right nasal cavity to the right maxillary sinus, with thinning and
bulging of the buccal cortical bone and posterior wall of the right
maxillary sinus, resorption of the lateral wall of the right nasal
cavity, and thinning of the suborbital wall (Fig. 1C and D). Biopsy findings indicated
an OKC, and extraction of the right maxillary cyst and radical
maxillary sinus surgery were performed in March 2009. The tumor was
encased in a capsule and was removed in one piece, and the
superficial maxillary bone was removed with a round bur. The cyst
was macroscopically found to have a single cavity lined with a thin
wall. Microscopically, despite a careful search, the proliferation
of microcysts or solid islands with keratinization within the cyst
wall were not observed (Fig. 1E and
F). At 2 and 4 years of follow-up, the lesion recurred, and
thus, the lesion was removed twice more. A total of 7 years after
the initial visit, the recurrence was found in the right maxillary
canine, and the first and second premolar areas. Cone-beam CT
imaging showed that a lesion with well-defined but slightly uneven
margins was observed, some of which were in close proximity to the
nasal floor on the side of the maxillary sinus crest from the right
canine tooth (Fig. 2C-E). The
tumor was removed, and the first premolar was extracted. After 2
years of close follow-up (9 years after the initial visit), the
disease recurred in the same area. The recurrent tumor and
surrounding bone were then removed (Fig. 2A-E) and was histopathologically
diagnosed as SOKC/KA (Fig. 2F and
G). A total of 10 years after the initial visit, the tumor
recurred in the second premolar area; therefore, the patient
underwent tumor removal and second premolar extraction. Treatment
progress is shown in Table SI. At
present (14 years and 4 months after the initial visit), no further
recurrence has been observed, but the patient continues to undergo
strict periodic follow-ups.
Immunohistochemistry (IHC)
Immunohistochemical staining of sections from the
formalin-fixed paraffin-embedded tissue samples was performed using
Ventana BenchMark XT slide stainer (Ventana Medical Systems, Inc.).
IHC was performed to detect p53 (DO7; cat. no. 790-2912; Roche
Diagnostics K.K; prediluted), Ki-67 (30-9; cat. no. 790-4286; Roche
Diagnostics K.K; prediluted), BRAF (VE1; cat. no. 790-5095; Roche
Diagnostics K.K; prediluted), calretinin (SP65; cat. no. 790-4467;
Roche Diagnostics K.K; prediluted), β-catenin (β-catenin-1; cat.
no. GA70261-2; Agilent Technologies Japan, Ltd.; prediluted) and
CD56 (MRQ-42; cat. no. 418191; Nichirei Biosciences Inc.;
prediluted) expression in primary OKC and recurrent (10 years after
the initial diagnosis) SOKC/KA tissue specimens. In addition,
phosphorylated (p)-S6 ribosomal protein (S6) (Ser235/236; cat. no.
2211S; Cell Signaling Technology, Inc.; 1:500) and p-ERK1/2
(Thr202/Tyr204; cat. no. 20G11; 4376; Cell Signaling Technology,
Inc.; 1:500) were investigated in the same recurrent lesions. IHC
was performed on 5-µm sections of tissues fixed for 24 h at room
temperature in 10% neutral buffered formalin solution and embedded
in paraffin. After microwave-based epitope retrieval for 5 min (two
times), sections were incubated for 20 min at room temperature in
10 mM citrate buffer (pH 6.0). Next, endogenous peroxidase was
blocked with 3% hydrogen peroxide at room temperature for 15 min.
Incubation with 2.5% BSA (MilliporeSigma) in PBS for 10 min at room
temperature was used to block non-specific reactions. The sections
were exposed for 1 h at room temperature with each primary
antibody. Incubation with secondary antibodies (horseradish
peroxidase-conjugated goat anti-rabbit antibody; cat. no. ab6721;
1:1,000; or horseradish peroxidase-conjugated goat anti-mouse
antibody; cat. no. ab6789; 1:500; both Abcam]) was conducted for 1
h at room temperature and detection was performed using the Ventana
UltraView Universal DAB Detection kit (Ventana Medical Systems,
Inc.) according to the manufacturer's instructions. The sections
were observed under a light microscope (Nikon Eclipse E800
microscope; Nikon Corporation).
DNA isolation and gene panel
sequencing analysis
Extraction and purification of genomic DNA from 5-µm
tissues of the recurrent lesions diagnosed as SOKC/KA (13 years
after the initial diagnosis) was performed by an outside contractor
(Macrogen Inc.). Initially, the tissues were fixed in 10% formalin
at room temperature for 24 h and embedded in paraffin. Analysis of
the genes listed in Table SII
[single nucleotide variants (SNVs)/insertion-deletions (InDels)
(170 genes) and fusions (25 genes)] was performed using
next-generation sequencing with Axen™ Cancer Panel 2 (Macrogen
Inc.). Written informed consent was obtained from the patient,
including consent for participation and publication of the
findings. This work was approved by the Ethics Committee of
Hiroshima University (approval number: hi-72; Hiroshima,
Japan).
Primary and recurrent lesions exhibit
different histopathological features
The primary lesion was a single cyst lined with a
thin, stratified squamous epithelium of uniform thickness with no
rete ridges. It was diagnosed as OKC based on the characteristic
parakeratinized luminal surface with a focal corrugated appearance
and the palisaded basal cell layer, although orthokeratinized areas
were present in H&E (Fig. 1E and
F). Sections were prepared to a thickness of 5-µm and stained
with H&E using the Tissue-Tek® Prisma™ Plus
automated slide stainer (Sakura Finete Japan Co., Ltd.) and
observed under the light microscope. No daughter cysts or
epithelial islands were observed within the cyst walls. The six
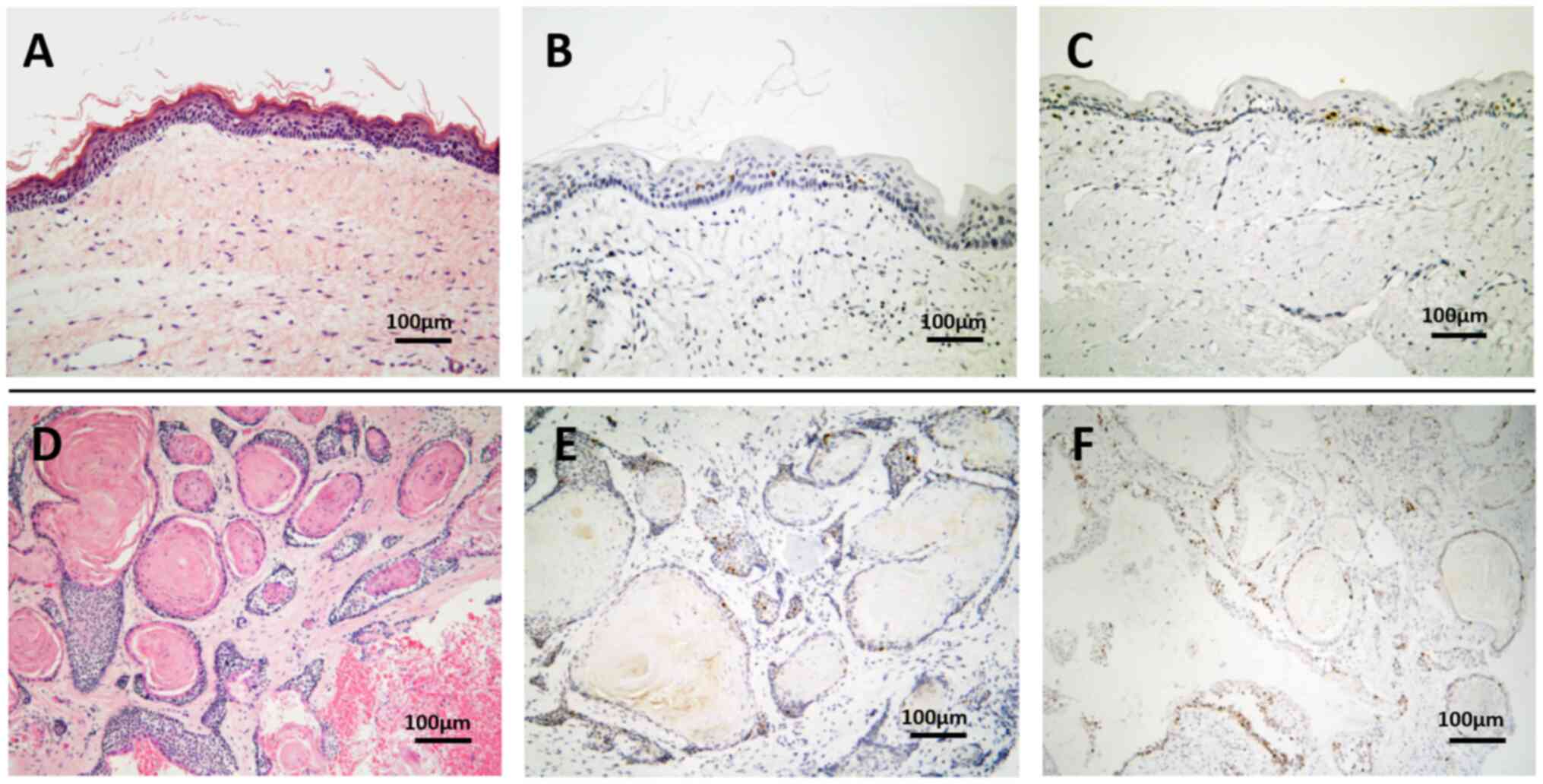
lesions that recurred over 13 years exhibited histopathological
features different from those of the primary lesion. They consisted
of a number of small cysts and solid epithelial nests with
parakeratinization and central lamellated keratin accumulation.
Focal areas of loosely arranged polygonal epithelial cells
resembling the stellate reticulum of the enamel organ and reverse
nuclear polarity of the basal cells were also observed. Moreover,
the recurrent lesion consisted of OKC, AB with prominent central
keratinization, and epithelium with features of both. Although the
staining for p53 in recurrent lesions was slightly higher than that
in primary lesion and the Ki-67 labeling index of the epithelial
cells in the recurrent lesions (~10%) was higher than that in the
primary lesion (~4%), cellular and nuclear pleomorphisms were not
prominent in any lesion and malignant transformation was ruled out
due to the absence of cellular atypia (Fig. 3). The recurrent lesions were
diagnosed as SOKC/KA. IHC results showed that calretinin, CD56 and
BRAFV600E were not expressed in either the primary or recurrent
lesions (data not shown). These findings indicated that this lesion
is different from typical AB or OKC.
Cancer panel sequencing analysis shows
mutations in adenomatous polyposis coli (APC) and Kirsten rat
sarcoma viral oncogene homolog (KRAS)
SNVs/InDels (170 genes) and fusions (25 genes) were
analyzed in the recurrent lesions diagnosed as SOKC/KA. Data from
the ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/), CancerVar
(https://cancervar.wglab.org/) and
OncoKB™ (https://www.oncokb.org/) databases
indicated that the detected mutation in APC
(NM_000038:c.2626C>T; p.Arg876*) may be clinically pathogenic,
the detected mutation in KRAS (NM_004985:c.38G>A;
p.Gly13Asp) may be oncogenic, and the detected missense variant of
TP53 (NM_000546:c.91G>A; p.Val31Ile) may be clinically
pathogenic. Other gene mutations that were detected are shown in
Table I. In addition, since MAPK
and mTOR are known to be activated downstream of KRAS, p-S6
and p-ERK1/2 expression was detected; IHC of the recurrent lesions
showed focal positivity for p-S6 and p-ERK1/2 in the tumor nests
(Fig. S1).
 | Table I.Results of the cancer panel gene
analysis. |
Table I.
Results of the cancer panel gene
analysis.
|
|
|
| ToMMo |
|
| Clinical
significance |
|---|
|
|
|
|
|
|
|
|
|---|
| Gene | Transcript ID (exon
ID) | DNA change/Protein
change | 38KJPN AF | gnomAD global AF | gnomAD EAS AF | gnomAD PopMax AF | AF, %
(Alt/Total) | Exonic effect | Axen Cancer Panel 2
report | CancerVar | ClinVar | OncoKB |
|---|
| APC | NM_000038
(16/16) | c.2626C>T;
p.Arg876* | None | None | None | None | 4.8 (110/2,303) | Stop gain | Pathogenic | Pathogenic | Pathogenic | Likely oncogenic,
likelyloss-of-function |
| RUNX1 | NM_001754 (9/9) | c.1270T>G;
p.Ser424Ala | 0.004919 | 0.0002 | 0.000936 | 0.001702 | 11.7 (25/214) | Missense variant | Likely
pathogenic | Pathogenic | Likely
pathogenic | Unknown oncogenic
effect |
| NTRK3 | NM_001012338
(3/20) | c.61G>T;
p.Val21Phe | 0.014036 | 0.000291 | 0.005068 | 0.005068 | 33.2 (265/797) | Missense variant | Likely benign | Benign | Likely benign | Unknown oncogenic
effect |
| APC | NM_000038
(12/16) | c.1488A>T;
p.Thr496Thr | 0.001628 | 0.00023 | 0.00672 | 0.00672 | 34 (803/2360) | Synonymous
variant | Benign/likely
benign | Benign | Benign/likely
benign | Unknown oncogenic
effect |
| IDH2 | NM_002168 (7/11) | c.939A>G;
p.Gly313Gly | 0.06619 | 0.002263 | 0.055898 | 0.055898 | 24.5 (551/2,253) | Synonymous
variant | Benign/likely
benign | Benign | Benign/likely
benign | Unknown oncogenic
effect |
| STK11 | NM_000455 (8/10) | c.1062C>G;
p.Phe354Leu | 0.045247 | 0.00396 | 0.040749 | 0.040749 | 25.8 (628/2437) | Missense
variant | Benign/likely
benign | Benign | Benign/likely
benign | Unknown oncogenic
effect |
| RUNX1 | NM_001754
(9/9) | c.1415T>C;
p.Leu472Pro | 0.00983 | 0.000079 | 0.002138 | 0.002138 | 33.8 (71/210) | Missense
variant | Benign/likely
benign | Benign | Benign | Unknown oncogenic
effect |
| MAP3K1 | NM_005921
(1/20) |
c.233_234delTCinsCT; p.Leu78Pro | 0.007529 | 0.001662 | 0.004073 | 0.011671 | 44.6 (95/213) | Missense
variant | Benign | - | Benign | Unknown oncogenic
effect |
| NOTCH4 | NM_004557
(1/30) |
c.36_47delGCTGCTGCTGCT;
p.Leu13_Leu16del | 0.163822 | 0.108437 | 0.175256 | 0.175256 | 98.2(496/505) | Disruptive inframe
deletion | Benign | - | Benign | Unknown oncogenic
effect |
| ROS1 | NM_001378902
(9/43) | c.883+3A>G;
- | 0.012953 | 0.001604 | 0.037943 | 0.037943 | 35.7
(616/1,725) | Splice variant
intron variant | Benign | - | Benign | Unknown oncogenic
effect |
| SMO | NM_005631
(3/12) | c.582A>G;
p.Glu194Glu | 0.065299 | 0.003659 | 0.090979 | 0.090979 | 24.7
(406/1,646) | Synonymous
variant | Benign | Unknown or
conflict | Benign | Unknown oncogenic
effect |
| NTRK2 | NM_006180
(3/19) | c.249C>T;
p.Asn83Asn | 0.011311 | 0.000105 | 0.002699 | 0.002699 | 31.2
(681/2,184) | Synonymous
variant | Benign | Unknown or
conflict | Benign | Unknown oncogenic
effect |
| PTEN | NM_000314
(1/9) | c.-366delT; - | 0.999522 | 0.99926 | 0.99842 | 0.999738 | 99.1 (453/457) | 5′ UTR variant | Benign | - | - | Unknown oncogenic
effect |
| PTEN | NM_000314
(1/9) | c.-326G>C;
- | 0.999341 | 0.999921 | 1.00000 | 1.000000 | 100 (319/319) | 5′ UTR variant | Benign | - | Benign | Unknown oncogenic
effect |
| CDKN1B | NM_004064
(1/3) | c.165G>A;
p.Ala55Ala | 0.052425 | 0.001426 | 0.02812 | 0.02812 | 51.4
(636/1,237) | Synonymous
variant | Benign | Unknown or
conflict | Benign | Synonymous
mutation |
| BRCA2 | NM_000059
(11/27) | c.2350A>G;
p.Met784Val | 0.095501 | 0.000611 | 0.017514 | 0.017514 | 39.5
(736/1,865) | Missense
variant | Benign | Benign | Benign | Conflicting
(inconclusive) |
| BRCA2 | NM_000059
(18/27) | c.8187G>T;
p.Lys2729Asn | 0.01728 | 0.00046 | 0.009808 | 0.009808 | 14.8
(399/2,687) | Missense
variant | Benign | Pathogenic | Benign | Likely neutral |
| RNF43 | NM_017763
(6/10) | c.597G>A;
p.Val199Val | 0.034683 | 0.000815 | 0.010978 | 0.010978 | 46.5 (435/936) | Synonymous
variant | Benign | Unknown or
conflict | Benign | Unknown oncogenic
effect |
| ALK | NM_004304
(3/29) | c.941A>G;
p.Glu314Gly | 0.001201 | 0.000007 | 0.000193 | 0.000193 | 15.4
(186/1,206) | Missense
variant | VUS | Benign | VUS | Synonymous
mutation |
| RAD50 | NM_005732
(12/25) | c.1924T>G;
p.Leu642Val | 0.003913 | 0.000046 | 0.001346 | 0.001346 | 50.2
(710/1,415) | Missense
variant | VUS | Pathogenic | VUS | Unknown oncogenic
effect |
| ERBB2 | NM_004448
(27/27) | c.3430G>C;
p.Asp1144His | None | None | None | None | 37.6 (183/487) | Missense
variant | VUS | Benign | VUS | Unknown oncogenic
effect |
| KRAS | NM_004985
(2/5) | c.38G>A;
p.Gly13Asp | None | 0.00002 | None | 0.000044 | 10.3
(189/1,834) | Missense
variant | Conflicting
interpretations of pathogenicity | Pathogenic | Conflicting
interpretations of pathogenicity: Pathogenic (6); uncertain significance (1) | Oncogenic/gain of
function |
| BRCA2 | NM_000059
(10/27) | c.964A>C;
p.Lys322Gln | 0.011909 | 0.000079 | 0.002307 | 0.002307 | 48.5
(544/1,122) | Missense
variant | Conflicting
interpretations of pathogenicity | Pathogenic | Conflicting
interpretations of pathogenicity : Likely pathogenic (1); uncertain significance (2); benign (4); likely benign (9) | Unknown oncogenic
effect |
| TP53 | NM_000546
(3/11) | c.91G>A;
p.Val31Ile | 0.00643 | 0.000099 | 0.002901 | 0.002901 | 8.6 (31/361) | Missense
variant | Conflicting
interpretations of pathogenicity | Pathogenic | Conflicting
interpretations of pathogenicity : Likely pathogenic (1); uncertain significance (2); benign (1); likely benign (8) | Unknown oncogenic
effect |
| STK11 | NM_000455
(6/10) | c.842C>T;
p.Pro281Leu | 0.012138 | 0.000125 | 0.002499 | 0.002499 | 21.6
(269/1,248) | Missense
variant | Conflicting
interpretations of pathogenicity | Benign | Conflicting
interpretations of pathogenicity : Uncertain significance (3); benign (3); likely benign (6) | Unknown oncogenic
effect |
Discussion
In the present case, a primary unicystic lesion was
readily diagnosed as OKC based on the characteristic lining of a
thin and flat parakeratotic squamous epithelium composed of a
palisaded basal layer and a corrugated surface. The complex
histopathology of the recurrent lesions, which consisted of a solid
proliferation of numerous small cysts and epithelial islands with
prominent keratinization, stellate reticulum-like configuration and
subnuclear vacuolization of basal cells, complicated the diagnosis.
Although the proliferative activity in the recurrent lesions was
higher than that in the primary lesion, malignant transformation
was ruled out due to the absence of cellular atypia.
In the 2000s, SOKC, an extremely rare form of OKC,
was identified (4,5). SOKC is macroscopically solid and
microscopically composed of multiple keratinizing microcysts and
epithelial islands with central keratinization, each resembling
OKC, in collagenous stroma. SOKC reportedly has aggressive clinical
and radiographic features, including multilocular appearance,
cortical expansion, infiltration into the bone marrow and soft
tissues, and a tendency to recur (4–9).
Based on these characteristics, OKC may consist of a spectrum of
clinicopathological features from simple individual cysts, to cysts
with multiple daughter cysts/epithelial islands, and to solid
lesions recognized as true benign neoplasms. However, SOKC remains
poorly defined because of unclear histopathological criteria due to
the small number of reported cases (2,3,5,7,10).
Given their overlapping pathological features, SOKC is difficult to
distinguish from KA, a rare variant of AB with extensive
keratinization in epithelial islands. Various histopathological
features have been reported in cases of KA: i) Simple histology
(follicular AB with extensive keratinization); ii) simple histology
with OKC-like features; and iii) complex histology (simple
histology with OKC-like features, epithelial follicles packed with
parakeratin, and epithelial ribbons forming lamellar stacks of
parakeratin extruded into the stroma) (10). The diagnostic differences between
KA and SOKC are the stellate reticulum-like appearance of focal
areas, subnuclear vacuolization of basal cells and lamellated-type
central keratinization, which are characteristic of KA (11). However, a lesion with histological
features resembling those of both SOKC and KA has been reported
under the name of SOKC with ameloblastomatous transformation
(7), and SOKC and KA may fall into
a similar histological spectrum of odontogenic tumors. Ide et
al (10) also suggested that
SOKC and KA share a histogenetic relationship and form a
clinicopathological spectrum, indicating that they should not
necessarily be separated into different entities. Therefore, the
term ‘SOKC/KA’ seemed appropriate for the diagnosis of recurrent
lesions reported on in the present case report. The present case
supports the idea that conventional OKC may be a histogenetic
source of SOKC/KA, thus suggesting that a close histogenetic
relationship exists among them.
Given the high recurrence rate in the present case,
a genetic mutation analysis was performed. SNVs/InDels (170 genes)
and fusions (25 genes) were analyzed in the recurrent lesions
diagnosed as SOKC/KA. Data from the ClinVar, Cancer Var and OncoKB
databases indicated that the mutation in APC
(NM_000038:c.2626C>T; p.Arg876*) may be clinically pathogenic.
Moreover, c.1488A>T; p.Thr496Thr may be a synonymous variant
detected in exon 12 of APC in this case. APC is a
multifunctional tumor suppressor gene that not only regulates
β-catenin degradation in the Wnt signaling pathway but also
controls cytoskeletal movement, regulates the cell cycle, and
influences cell proliferation and division (12). Activation of the Wnt pathway is
also important for tumor initiation and development, and mutations
in APC cause regulatory dysfunction, which is closely linked
to tumor initiation and development (12). Defects in APC induce β-catenin
accumulation in the nucleus, leading to activation of the
transcription factors TCF and LEF, which consequently activate the
classical Wnt/β-catenin/TCF signaling pathway (12).
The present case report identified mutations in
KRAS G13D, a mutation frequently detected in colorectal
cancer (13). This mutation is
considered the reason why the EGFR inhibitor cetuximab is highly
effective for colorectal cancer, and the KRAS G13D mutation
results in constant activation of the RAS/MAPK and MEKK/SEK/JNK
pathways, allowing cancer cells to continue to invade and
proliferate regardless of cell surface EGF stimulation (14). To the best of our knowledge, no
KRAS G13D mutations in benign tumors arising from the oral
cavity have been reported. However, adjacent KRAS G12V/R
mutations have been reported in adenomatoid odontogenic tumor and
AB in the oral cavity (15,16).
In the present case, a KRAS mutation was detected, and the
results of IHC showed focal positivity for p-S6 and p-ERK1/2 in the
tumor nests. These results suggested that KRAS aberrations
may activate downstream signaling and result in positive staining
of p-S6 (16), which may reflect
abnormal cell proliferation due to genetic mutations detected in
the gene panel. In a previous case, the time to relapse of the
SOKC/KA lesion was short, and an increased Ki-67 nuclear reaction
in areas of cytologic atypia was observed, which may suggest
possible malignant transformation, or proliferation of cells to
form new neoplastic follicles and nests (17).
Treatment for AB includes jaw osteotomy with an
emphasis on cure, and conservative surgical treatment aimed at
preserving oral function, such as enucleation and fenestration. In
the latter treatment, bone surface removal or cryotherapy may also
be performed to increase the curative effect. SOKC/KA has been
suggested to be more aggressive than purely cystic cases due to its
infiltrative growth pattern and strong tendency to recur after
resection (6). In a previous
report, the mean recurrence rates for SOKC and KA cases were 12.5
and 41.7%, respectively, depending on the treatment method
(3). In this previous study,
SOKC/KA was reported to be treated similarly to AB, with
conservative surgical therapy as the initial treatment in numerous
cases. In the present case, conservative surgical therapy and bone
surface removal were performed to preserve function in all
procedures due to the patient age and extent of the lesion. Among
the six cases of SOKC/KA (3) in
which enucleation was performed, as in the present case, recurrence
was observed in one of the five cases in the mandible and in the
one case in the maxilla. Since SOKC/KA has been suggested to be
more invasive than pure cystic cases, maxillary resection should
also be considered. However, in the present case, the patient was
young, and preferred enucleation and fenestration as a conservative
surgical treatment, due to concerns about aesthetics and reduced
quality of life. If clinical and imaging findings suggest a shorter
recurrence period or malignant transformation, we plan to perform
immediate radical treatment, such as jaw osteotomy.
The present study describes the case of a unicystic
OKC that transformed into a hyperkeratotic SOKC/KA during long-term
follow-up. The recurrent lesion was solid and consisted of OKC, AB
with prominent central keratinization, and epithelium with features
of both. Recently, the concept of SOKC/KA has been proposed based
on the clinicopathological similarity between the two lesions
(10,11). In accordance with this concept, the
lesion in the present case was diagnosed as originating from OKC
and becoming SOKC/KA upon recurrence. Notably, the
immunohistochemical staining of calretinin, CD56, β-catenin and
BRAFV600E was negative in the recurrent lesions, unlike in AB, and
mutations in APC and KRAS were observed. Moreover, no
mutations were observed in BRAF and SMO, which are
generally common in OKC and AB. Thus, the clinical course and
histological transformation suggested the possibility of a novel
subtype. In the present study, genomic DNA could not be extracted
from the primary lesion and compared with recurrent lesions because
of the long clinical course of the disease. In the future, it may
be necessary to perform sequencing analysis at an earlier stage in
recurrent cases to investigate APC and KRAS
mutations.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Dr Ikuko Ogawa
(Hiroshima University) for pathological analysis and advice, and Dr
Yasutaka Hayashido and Dr Kensaku Matsui (Hiroshima University) for
clinical support.
Funding
This research was partially supported by a Grant-in-Aid for
Scientific Research from the Ministry of Education, Science,
Sports, and Culture of Japan (grant no. 18K09723).
Availability of data and materials
The sequencing data generated in the present study
may be found in the DDBJ BioProject database under accession number
(DRR519302) or at the following URL: https://ddbj.nig.ac.jp/resource/biosample/SAMD00664639.
The other data generated in the present study may be requested from
the corresponding author.
Authors' contributions
SaY, TS and TA conceived the case presentation and
drafted the manuscript. SaY, TS and SoY participated in the
treatment of the patient. TA and MM performed pathological
analysis. SaY and TS confirm the authenticity of all the raw data.
All authors contributed to the discussion and critical comments.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from this
patient, including consent to participate. Gene analysis was
approved by the Ethics Committee of Hiroshima University (approval
number: hi-72).
Patient consent for publication
Written informed consent was obtained from this
patient, including consent for publication of the findings.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
KA
|
keratoameloblastoma
|
|
OKC
|
odontogenic keratocyst
|
|
SOKC
|
solid variant of OKC
|
|
AB
|
ameloblastoma
|
|
APC
|
adenomatous polyposis coli
|
|
KRAS
|
Kirsten rat sarcoma viral oncogene
homolog
|
References
|
1
|
Zhang R, Yang J, Zhang J, Hong Y, Xie X
and Li T: Should the solid variant of odontogenic keratocyst and
keratoameloblastoma be classified as the same entity? A
clinicopathological analysis of nine cases and a review of the
literature. Pathology. 53:478–486. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ide F, Mishima K and Saito I: Solid-cystic
tumor variant of odontogenic keratocyst: an aggressive but benign
lesion simulating keratoameloblastoma. Virchows Arch. 442:501–503.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vered M, Buchner A, Dayan D, Shteif M and
Laurian A: Solid variant of odontogenic keratocyst. J Oral Pathol
Med. 33:125–128. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Daley TD, Multari J and Darling MR: A case
report of a solid keratocystic odontogenic tumor: Is it the missing
link? Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
103:512–515. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Geng N, Lv D, Chen QM, Zhu ZY, Wu RQ, He
ZX and Chen Y: Solid variant of keratocystic odontogenic tumor with
ameloblastomatous transformation: A case report and review of the
literature. Oral Surg Oral Med Oral Pathol Oral Radiol.
114:223–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kahraman D, Gunhan O and Celasun B: A
series of 240 odontogenic keratocysts: Should we continue to use
the terminology of ‘keratocystic odontogenic tumor’ for the solid
variant of odontogenic keratocyst? J Craniomaxillofac Surg.
46:942–946. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Santana DCP, Xavier FCDA, Santos JND and
Henriques ÁCG: Is the solid variant of odontogenic keratocyst the
neoplastic counterpart of the lesion? Oral Dis. 28:2215–2218. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Whitt JC, Dunlap CL, Sheets JL and
Thompson ML: Keratoameloblastoma: A tumor sui generis or a chimera?
Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 104:368–376.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Robinson L, Smit C, Fonseca FP, Abrahão
AC, Romañach MJ, Khurram SA, Hunter KD, Speight PM and van Heerden
WFP: Keratoameloblastoma: A report of seven new cases and review of
literature. Head Neck Pathol. 16:1103–1113. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ide F, Ito Y, Muramatsu T, Saito I and
Abiko Y: Histogenic relations between keratoameloblastoma and solid
variant of odontogenic keratocyst. Oral Surg Oral Med Oral Pathol
Oral Radiol. 114:812–814. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ide F, Ito Y, Nishimura M, Ogawa I and
Kikuchi K: Keratoameloblastomatous transformation of a recurrent
unicystic ameloblastoma: A novel case raising diagnostic and
classification difficulties. Pathology. 54:386–388. 2012.
View Article : Google Scholar
|
|
12
|
Hankey W, Frankel WL and Groden J:
Functions of the APC tumor suppressor protein dependent and
independent of canonical WNT signaling: Implications for
therapeutic targeting. Cancer Metastasis Rev. 37:159–172. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kennedy SA, Jarboui MA, Srihari S, Raso C,
Bryan K, Dernayka L, Charitou T, Bernal-Llinares M,
Herrera-Montavez C, Krstic A, et al: Extensive rewiring of the EGFR
network in colorectal cancer cells expressing transforming levels
of KRASG13D. Nat Commun. 11:4992020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Coura BP, Bernardes VF, de Sousa SF,
França JA, Pereira NB, Pontes HAR, Gomes CC, da Cruz Perez DE,
Albuquerque Junior RLC, de Souza LB, et al: KRAS mutations drive
adenomatoid odontogenic tumor and are independent of
clinicopathological features. Modern Pathol. 32:799–806. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guimarães LM, Coura BP, Gomez RS and Gomes
CC: The molecular pathology of odontogenic tumors: Expanding the
spectrum of MAPK pathway driven tumors. Front Oral Health.
2:7407882022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chaisuparat R, Yodsanga S, Montaner S and
Jham BC: Activation of the Akt/mTOR pathway in dentigerous cysts,
odontogenic keratocysts, and ameloblastomas. Oral Surg Oral Med
Oral Pathol Oral Radiol. 116:336–342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stojanov IJ, Ho D, Huss J, Gopalakrishnan
R, Yoest JM and Koutlas IG: An unusual gingival (Peripheral) tumor
with features of keratoameloblastoma with cytologic atypia or
possible malignant transformation exhibiting ARID1A mutation. Head
Neck Pathol. 17:808–814. 2023. View Article : Google Scholar : PubMed/NCBI
|