Introduction
Ischemia-reperfusion (IR) injury is a complication
that commonly occurs following medical and surgical interventions,
such as thrombolytic therapy, organ transplantation, coronary
angioplasty and cardiopulmonary bypass (1). The main issue surrounding IR injury
is microvascular dysfunction after reperfusion of ischemic tissues,
which subsequently leads to impaired endothelium-dependent
dilatation in arterioles, increased fluid filtration, leukocyte
occlusion in capillaries, leukocyte compression and plasma protein
extravasation in post-capillary venules (2). Furthermore, activated endothelial
cells in the microcirculation produce more oxygen radicals but less
nitric oxide (NO) during the first period (5–20 min) after
reperfusion and this imbalance between superoxide and NO in
endothelial cells results in the production and release of
inflammatory mediators, and increases the biosynthesis of adhesion
molecules, thus mediating leukocyte-endothelial cell adhesion
(1–3). Inflammatory mediators released as a
result of reperfusion may also activate endothelial cells in
distant organs that were not initially exposed to IR injury
(2,4). This distant response to IR can lead
to leukocyte-dependent microvascular damage, which is
characteristic of multiple organ dysfunction syndrome (1). Recently, it has been shown that
reperfusion, a term used to describe blood flow restoration after
ischemia, may place ischemic organs at a greater risk of cellular
necrosis, thus limiting the return of function (5,6).
Halogenated inhalational anesthetics are currently
the most common drugs used for the induction and maintenance of
general anesthesia. Sevoflurane is a halogenated inhalation
anesthetic widely used in general anesthesia (7), which has a positive effect on
IR-induced lung injuries through the reduction in tumor necrosis
factor-α (TNF-α) release (8).
There are numerous mechanisms that have not been
elucidated in the prevention of IR injury, especially during and
after lower extremity surgeries and the effectiveness of the
currently used methods (such as ischemic preconditioning and
antioxidant treatments) (9,10) is
limited.
It has been shown in previous studies that
sevoflurane administration is protective against IR injury;
however, to the best of our knowledge, its effect alongside
fullerenol C60, a nanoparticle, has not been determined (11,12).
The activities of nanoparticles and their uses in nanomedicine have
been subject to growing interest; thus, the present study aimed to
investigate the protective effect of fullerenol C60 in rats treated
with sevoflurane against damage to the lung and kidney tissues in
lower extremity IR. The present study investigated the effects of
fullerenol C60 and sevoflurane, alone or combined, on lung and
kidney tissue in rats with lower extremity IR injury.
Materials and methods
Animals and experimental protocol
The present study was conducted at the Gazi
University Animal Experiments Laboratory (Ankara, Turkey) in July
2021 in accordance with the ARRIVE guidelines (13). The study protocol was approved by
the Animal Research Committee of Gazi University (G.Ü.ET-21.023).
All of the animals were maintained in accordance with the
recommendations of the National Institutes of Health Guidelines for
the Care and Use of Laboratory Animals (14).
Rats were anesthetized with ketamine [50 mg/kg,
intraperitoneal (i.p.)] and Rompun® (20 mg/kg, i.p.) and
placed on a heating pad to maintain their body temperature. Rats
were kept in a temperature-controlled (21±1°C) and
humidity-controlled (45–55%) room, and were maintained under a 12-h
light/dark cycle. The animals were fed a standard pellet diet and
allowed to drink water ad libitum. The dose of ketamine and
Rompun administered to the rats was in accordance with the study by
Yesil et al (15).
A total of 30 Wistar albino male rats (Gazi
University Animal Experiments Laboratory, Ankara, Turkey) (age, 8
months; weight, 225–275 g) were used in the present study. The
animals were equally divided into the following five groups (n=6):
i) Sham; ii) IR; iii) IR-fullerenol C60 (IR-FUL); iv)
IR-sevoflurane (IR-SEVO); and v) IR-fullerenol C60-sevoflurane
(IR-FUL-SEVO). The effects of sevoflurane (16) and fullerenol C60 (17) on normal rats have been investigated
in previous studies and our ethics committee limited the number of
rats to be used in the experiment due to the 4R rule (18); therefore, the groups of the present
study were treated as follows: i) The sham group only underwent
midline laparotomy without any additional surgical intervention;
ii) the IR group was subjected to midline laparotomy and a
traumatic microvascular clamp was placed in the infrarenal
abdominal aorta for 120 min, after which it was removed and
reperfused for 120 min. Sodium heparin (500 IU/kg) was administered
through the peripheral tail vein to maintain reperfusion after
occlusion; iii) the IR-FUL group underwent the same surgical
procedures as the IR group with fullerenol C60 (Fullerene-C60; 98%;
1 g, CAS no. 99685-96-8; MilliporeSigma) administered (100 mg/kg,
i.p) (19) 30 min before the
ischemic period; iv) the IR-SEVO group underwent the same surgical
procedures as the IR group. Anesthetic gas vaporizers were
calibrated and set at a minimum alveolar concentration of
sevoflurane (2.3%). Rats were anesthetized in a transparent plastic
box (40×40×70 cm) and sevoflurane was administered at 2.3%
inspiratory concentration at a rate of 4 l/min in 100%
O2 for 4 h; and v) the IR-FUL-SEVO group underwent the
same surgical procedures as the IR group with fullerenol C60
administered 30 min before ischemia and sevoflurane administered
throughout the IR period.
Following the end of the reperfusion period, all
rats were anesthetized using ketamine (100 mg/kg) and xylazine (10
mg/kg) i.p. injection, and were sacrificed by exsanguination during
blood sample (5–10 ml) collection from the heart. After heart rate
and respiration ceased, monitoring was continued for a further 2
min to confirm death. Then, lung and kidney tissues were removed
for biochemical and histopathological analyses.
Biochemical analysis
Biochemical analyses were performed according to
protocols used in our previous publications (20,21).
Right lung and right kidney tissues were washed with cold NaCl
solution (0.154 M) to remove blood contamination and then
homogenized (Heidolph homogenizer DIAX 900; Heidolph Instruments
GMBH & CO. KG) at 1,000 U for ~3 min. After centrifugation at
10,000 × g for ~10 min at 4°C, the upper clear layer was collected
for analysis.
The thiobarbituric acid reactive substances (TBARS)
assay was performed according to the protocol described by Van Ye
et al (22). Catalase (CAT)
activity was measured using methods described by Aebi (23) and glutathione S-transferase (GST)
enzyme activity was measured according to methods described by
Habig et al (24). The
amount of sample protein was determined using the Lowry method with
BSA (MilliporeSigma) used as the standard protein (25). The results were expressed as IU/mg
protein for enzymes and nmol/mg protein for TBARS.
Histopathological assessment of kidney
and lung tissue specimens
Tissue samples taken from the periphery of the left
lung and left kidney were fixed in 10% neutral buffered formalin
for 72 h at room temperature. Following fixation, tissue samples
were processed using an increasing grade alcohol series, cleared in
xylene and embedded in paraffin blocks. Kidney and lung sections (4
µm) were obtained using a Leica RM2245 rotary microtome (Leica
Microsystems GmbH). Thereafter, sections were deparaffinized in
xylene and rehydrated through decreasing grade alcohol series, and
placed in distilled water to prepare them for histochemical and
immunohistochemical staining, and TUNEL assay.
Lung and kidney sections were stained with
hematoxylin for 12 min at room temperature and eosin for 12 min at
room temperature. Kidney sections were also stained with Periodic
acid-Schiff (PAS). To that end, sections were incubated in Periodic
acid solution and Schiff reagent at room temperature in dark for 35
and 40 min, respectively; and immersed in hematoxylin for nuclear
staining for 1 min at room temperature. The stained sections were
observed under a Leica DM4000 B light microscope (Leica
Microsystems GmbH) equipped with a computer and images were
captured using Leica LAS v4.9 (Leica Microsystems GmbH).
Hematoxylin and eosin (H&E)- and PAS-stained
kidney samples were examined under ×200 and ×400 magnifications and
renal injury was assessed semi-quantitatively. Swelling,
vacuolization and loss of brush borders in tubular epithelial
cells, as well as epithelial cell sloughing and hyaline cast
formation were considered indicators of kidney injury and were
evaluated in 10 randomly chosen fields from the cortex of each
kidney section. A scoring system for the ratio of injured tubules
displaying the aforementioned findings was applied as follows: 0,
no tubular injury; 1, ≤10% of tubules; 2, 10–25% of tubules; 3,
25–45% of tubules; 4, 45–75% of tubules; and 5, >75% of tubules
were involved in injury. The average score for kidney injury was
calculated for each kidney sample (26,27).
Lung injury was evaluated using the lung injury
scoring system developed by The American Thoracic Society (28). For this purpose, 20 non-overlapping
fields of H&E-stained lung sections were examined under ×200
and ×400 magnifications and the following parameters were scored
for each field: (A) Neutrophils in the alveolar space (0, none; 1,
1–5; 2, >5); (B) neutrophils in the interstitial space (0, none;
1, 1–5; 2, >5); (C) hyaline membranes (0, none; 1, 1; 2, >1);
(D) proteinaceous debris filling the airspaces (0, none; 1, 1; 2,
>1); and (E) alveolar septal thickening (0, <2×; 1, 2×-4×; 2,
>4×). The sum of the injury scores for each animal was
determined using the following formula: Score=[(20 × A) + (14 × B)
+ (7 × C) + (7 × D) + (2 × E)]/(no. of fields ×100) (28,29).
Tubular injury scores and lung injury scores were compared between
the groups.
Immunohistochemical assessment of
kidney and lung tissue specimens
For immunostaining of tissue sections,
deparaffinization and rehydration were followed by heat-induced
antigen retrieval in citrate buffer (pH 6.0) for 2 h in a water
bath adjusted to 85°C. Endogenous peroxidase activity was blocked
by incubation with 3% H2O2 for 30 min in dark
at room temperature. To perform the protein blocking to prevent
nonspecific binding of antibodies sections were incubated with
Ultra V Block solution (cat. no. TA-125-UB; Thermo Fisher
Scientific, Inc.) for 30 min at room temperature, kidney and lung
tissue sections were incubated with anti-tumor necrosis factor-α
(TNF-α; 1:100; Elabscience Biotechnology, Inc.; cat. no.
E-AB-33121), anti-interleukin 1β (IL-1β; 1:100; Elabscience
Biotechnology, Inc.; cat. no. E-AB-66749) and anti-intercellular
adhesion molecule 1 (ICAM-1; 1:100; BIOSS; cat. no. bs-0608R)
primary antibodies to investigate the inflammatory processes.
Additionally, kidney sections were incubated with anti-B-cell
lymphoma 2 (BCL-2)-associated X protein (BAX; 1:100; BIOSS; cat.
no. bs-0127R), anti-BCL-2 (1:200; BIOSS; cat. no. bs-4563R) and
anti-caspase-3 (CASP-3; 1:100; Elabscience Biotechnology, Inc.;
cat. no. E-AB-66940) primary antibodies to examine the apoptotic
processes. Incubation with primary antibodies overnight at 4°C was
followed by incubation with biotinylated secondary antibody (cat.
no. TP-125-BN; Thermo Fisher Scientific, Inc.) for 2 h at room
temperature. Afterward, HRP-labeled streptavidin (cat. no.
TS-125-HR; Thermo Fisher Scientific, Inc.) was applied to sections
for 30 min in dark at room temperature. The staining procedure was
completed using the 3,3′-diaminobenzidine (DAB) chromogen. For the
assessment of stained sections, 10 randomly chosen, non-overlapping
fields were captured under ×400 magnification using a Leica DM4000
B light microscope (Leica Microsystems GmbH) and immunopositive
staining intensity was semi-quantified using ImageJ software
(1.48v; National Institutes of Health) and expressed as % area
(30).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay for kidney samples
The processes before the TUNEL assay were the same
as those performed before histological analysis; the kidney samples
were fixed in 10% neutral buffered formalin for 72 h at room
temperature, and then processed for paraffin embedding. Tissue
sections (4 µm) were deparaffinized and rehydrated prior to the
TUNEL assay. Apoptotic epithelial cells of the kidney tubules were
determined using a TUNEL assay kit (Elabscience Biotechnology,
Inc.; cat. no. E-CK-A331-50T); the assay was performed in
accordance with the manufacturer's protocol and sections were
mounted with anhydrous mounting medium Entellan™ new (cat. no.
107961; MilliporeSigma). Tubular epithelial cells with brown nuclei
stained with DAB (0.5 mg/ml) for 5 min at room temperature were
considered TUNEL+ cells. Apoptotic cells were counted in
10 randomly chosen fields under ×400 magnification using the Leica
DM4000 B light microscope (Leica Microsystems GmbH) and the results
were expressed as number of TUNEL+ cells per field
(31).
Statistical analysis
All data are expressed as the mean ± standard
deviation. All statistical analyses were performed using SPSS
(version 20.0; IBM Corp.). The distribution of data was analyzed
using the Shapiro-Wilk test. Comparisons among >2 groups were
carried out using the Kruskal-Wallis test followed by Dunn's test
or one-way ANOVA followed by Tukey's test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Histopathological findings
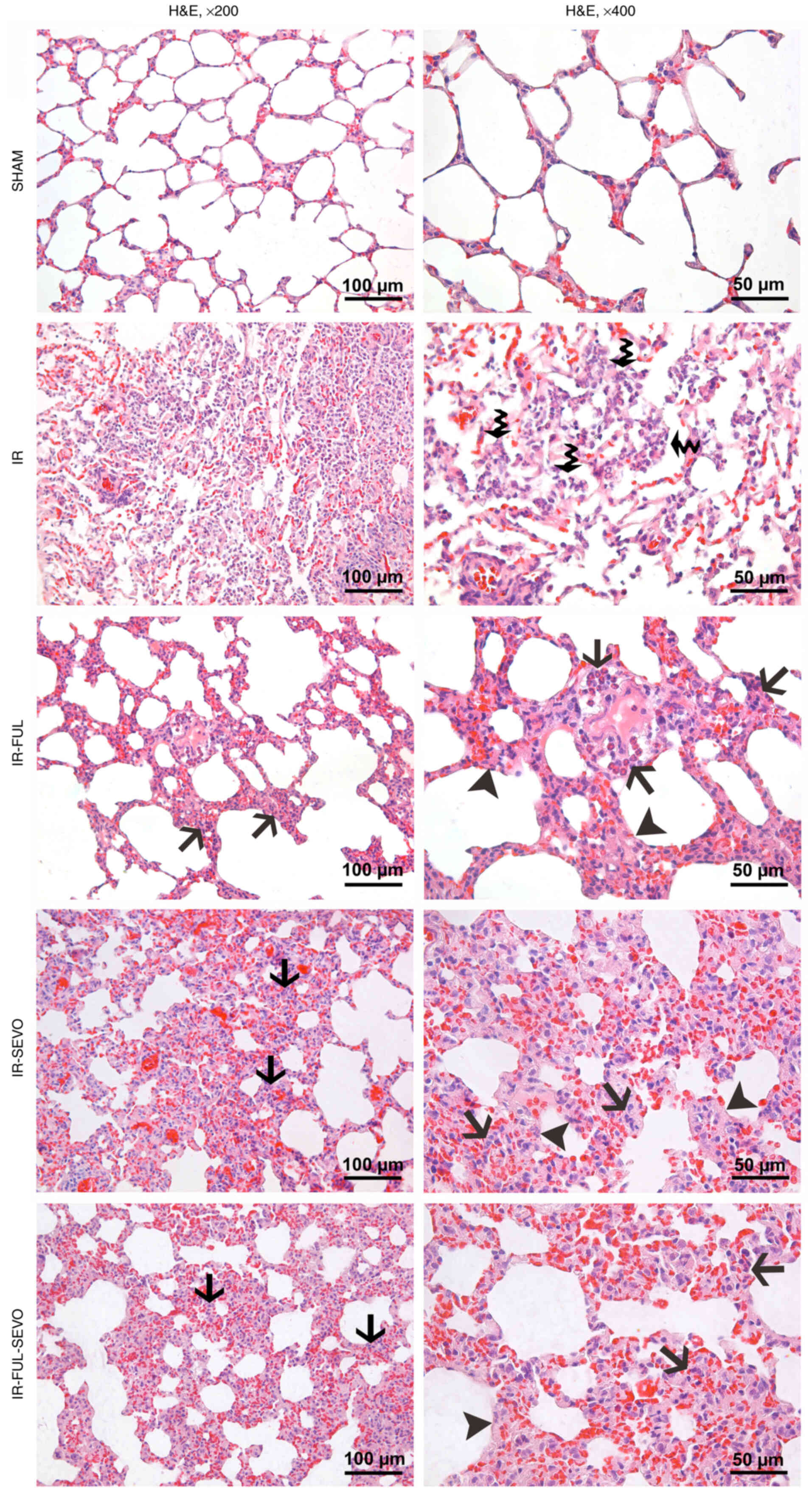
In contrast to the almost normal appearance, with
slight congestion, of the lung tissue sections from the sham group,
marked congestion and neutrophil infiltration were observed in the
lung samples of animals from the IR group. While neutrophil
infiltration in the alveolar spaces of the lung specimens from
animals in the IR group were more prominent, there was infiltration
in the interstitial space in the treatment groups, which was
accompanied by alveolar septum thickening observed in the IR-FUL,
IR-SEVO and IR-FUL-SEVO groups (Fig.
1). It was found that IR significantly increased the lung
injury score compared with the sham group. Furthermore, lung injury
scores were significantly lower in the IR-FUL and IR-FUL-SEVO
groups compared with the IR group (P=0.004 and P=0.017,
respectively; Table I). In the
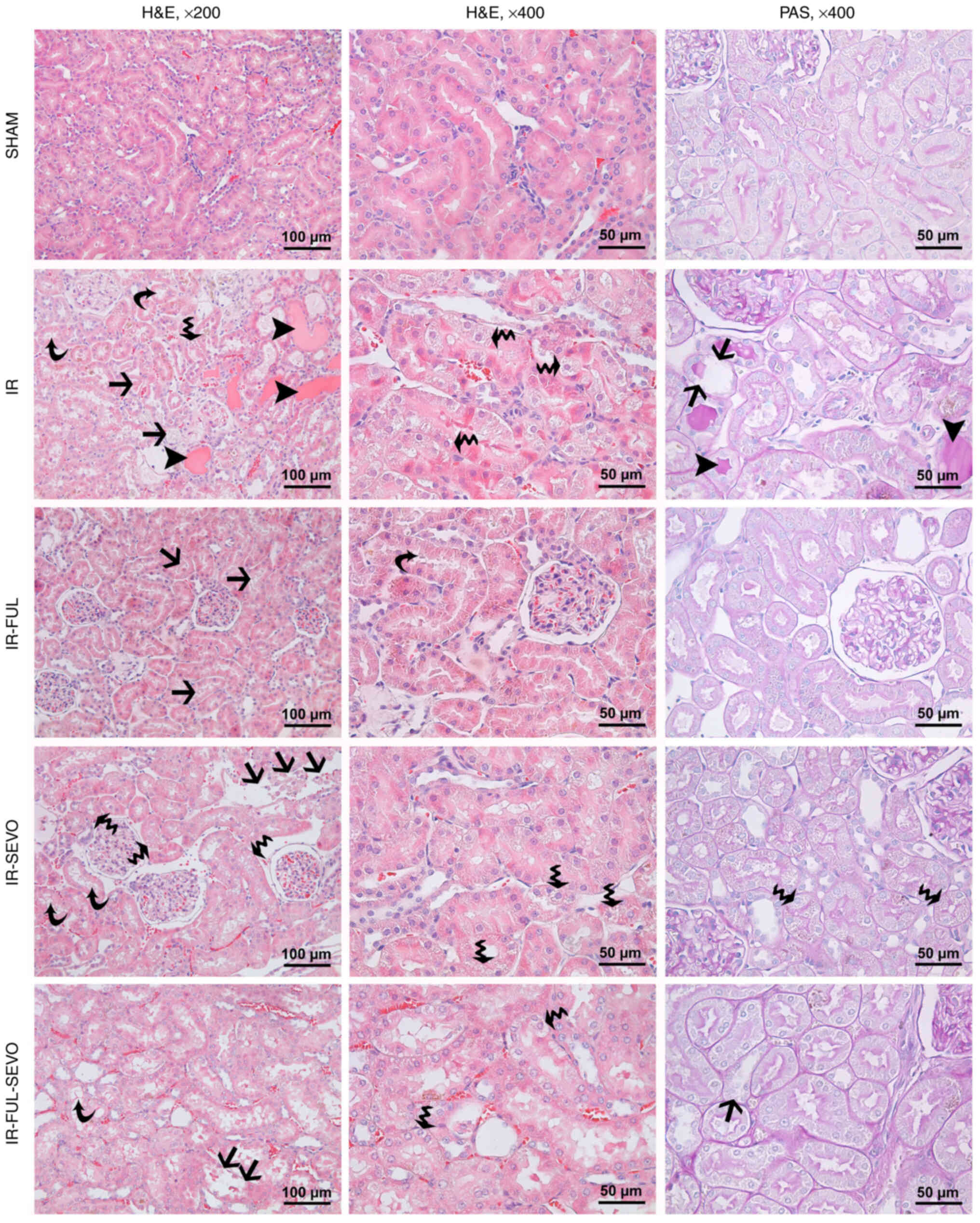
histopathological evaluation of kidney samples, varying degrees of
tubular injury, ranging from vacuolization, loss of brush border in
tubular epithelial cells to loss of tubular epithelium and hyaline
cast formation, were observed in all IR groups (Fig. 2). There was an evident increase in
the mean kidney tubule injury score in all IR groups compared with
the sham group (P<0.0001). Additionally, the mean kidney injury
score was significantly lower in the IR-FUL and IR-FUL-SEVO groups
compared with that in the IR group (P<0.0001 and P=0.014,
respectively) (Fig. 2; Table I).
 | Table I.Lung and kidney tubule injury scores
[median (IQR)]. |
Table I.
Lung and kidney tubule injury scores
[median (IQR)].
| Variable | Sham (n=6) | IR (n=6) | IR-FUL (n=6) | IR-SEVO (n=6) | IR-FUL-SEVO
(n=6) | Kruskal-Wallis
P-value |
|---|
| Lung injury
score | 0.020
(0.016–0.024) | 0.25
(0.22–0.28)a | 0.19
(0.11–0.22)a,b | 0.20
(0.16–0.23)a | 0.17
(0.15–0.22)a,b | <0.0001 |
| Kidney tubules
injury score | 0.35
(0.17–0.60) | 4.40
(4.15–4.62)a | 3.05
(2.75–3.50)a | 4.40
(3.87–4.50)a | 3.85
(3.40–3.85)a | <0.0001 |
Immunohistochemical findings
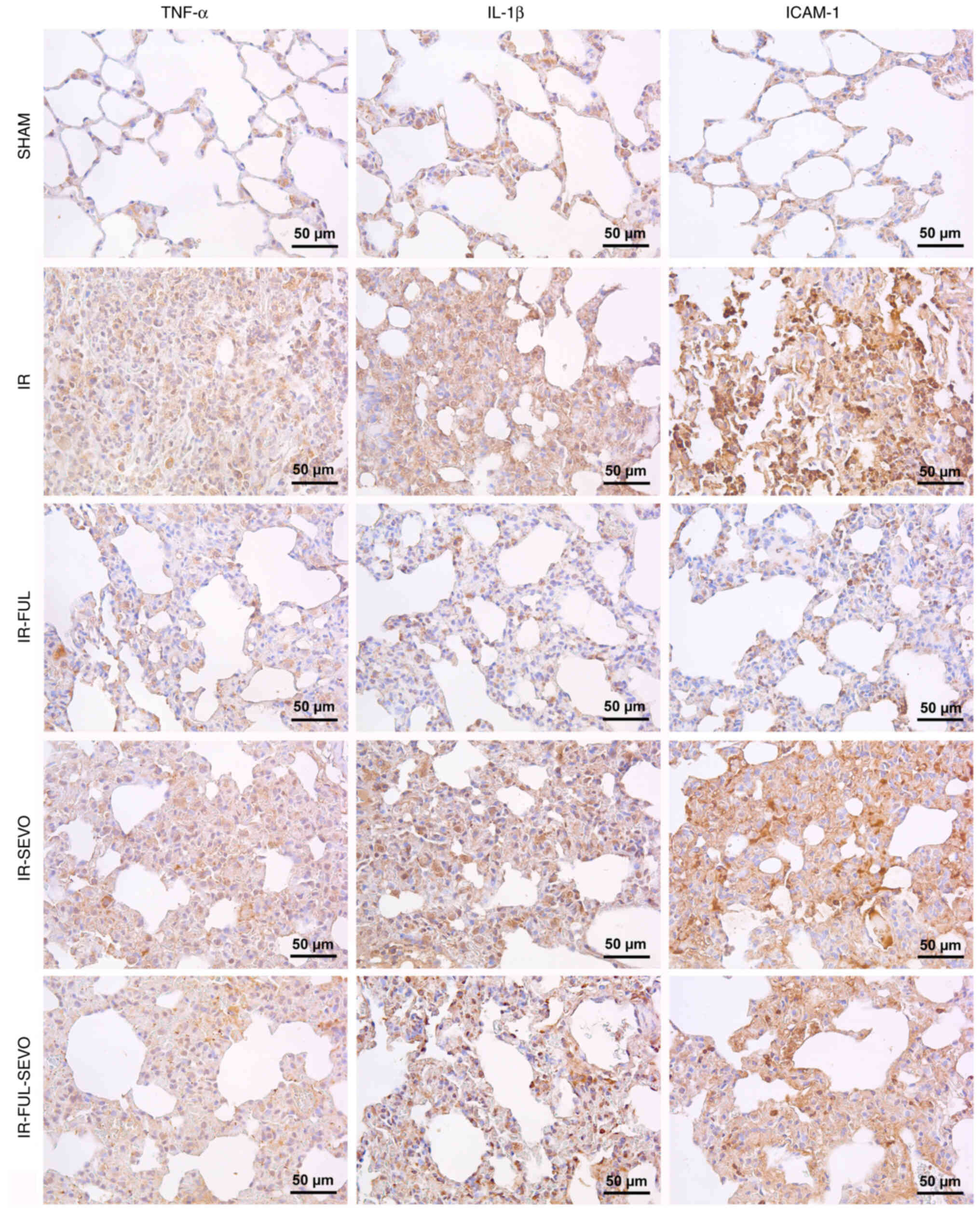
The lung tissue expression of TNF-α, IL-1β and
ICAM-1 increased significantly following hindlimb IR, whereas the
expression of these markers decreased significantly with the use of
fullerenol C60. When the improvement was compared between the
treatment groups, the effect of fullerenol C60 alone was greater
than when compared with sevoflurane alone (Table II; Fig. 3).
 | Table II.Comparison for the immunostaining
intensity of lung samples labelled with TNF-α, IL-1β and ICAM-1
antibodies (mean ± SD). |
Table II.
Comparison for the immunostaining
intensity of lung samples labelled with TNF-α, IL-1β and ICAM-1
antibodies (mean ± SD).
| Protein | Sham (n=6) | IR (n=6) | IR-FUL (n=6) | IR-SEVO (n=6) | IR-FUL-SEVO
(n=6) | ANOVA P-value |
|---|
| TNF-α | 1.91±0.48 |
9.43±2.03a |
2.84±0.59b |
4.89±1.74a–c |
3.86±1.05a,b | <0.0001 |
| IL-1β | 2.23±0.56 |
9.63±1.96a |
3.69±0.61b |
7.45±1.45a,c |
4.67±0.72a,b | <0.0001 |
| ICAM-1 | 1.66±0.54 |
21.96±8.50a |
4.87±0.86b |
14.34±3.06a–c |
9.10±3.42a,b | <0.0001 |
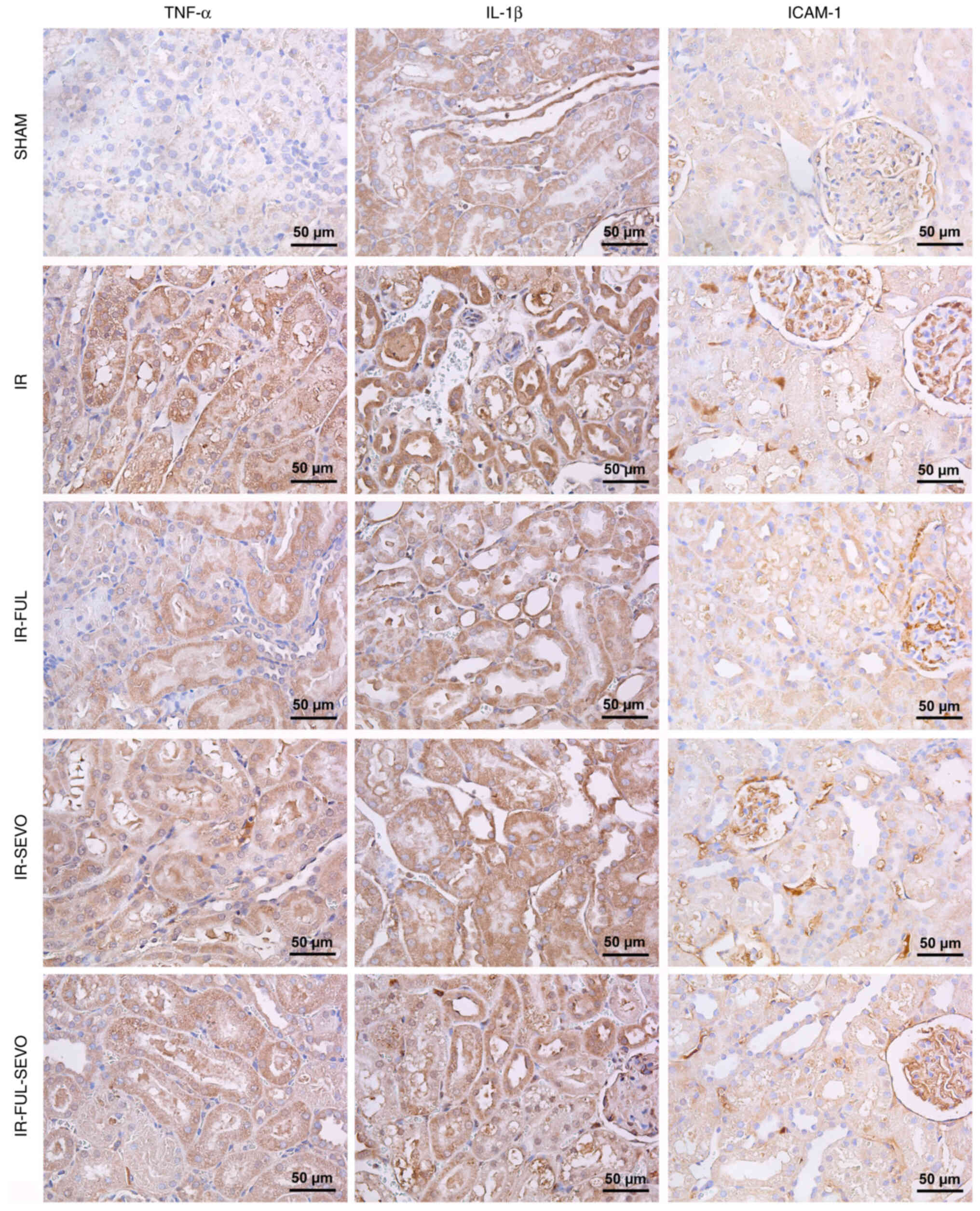
When the kidney tissue was examined for TNF-α, IL-1β
and ICAM-1 expression following hindlimb IR injury, it was observed
that elevated expression levels of these markers were significantly
improved by the administration of fullerenol 60 alone and this
effect was more prominent than that of sevoflurane alone (Table III; Fig. 4).
 | Table III.Comparison for immunostaining
intensity of kidney samples labelled with TNF-α, IL-1β and ICAM-1
antibodies (mean ± SD). |
Table III.
Comparison for immunostaining
intensity of kidney samples labelled with TNF-α, IL-1β and ICAM-1
antibodies (mean ± SD).
| Protein | Sham (n=6) | IR (n=6) | IR-FUL (n=6) | IR-SEVO (n=6) | IR-FUL-SEVO
(n=6) | ANOVA P-value |
|---|
| TNF-α | 3.75±1.54 |
23.88±3.36a |
10.36±2.60a,b |
16.49±2.92a–c |
12.84±3.27a,b | <0.0001 |
| IL-1β | 12.78±3.22 |
23.28±22.12a |
17.63±1.21a,b |
20.91±1.59a–c |
19.60±2.28a,b | <0.0001 |
| ICAM-1 | 10.79±3.41 |
25.77±3.01a |
18.66±1.03a,b |
22.59±1.15a–c |
20.69±1.12a,b | <0.0001 |
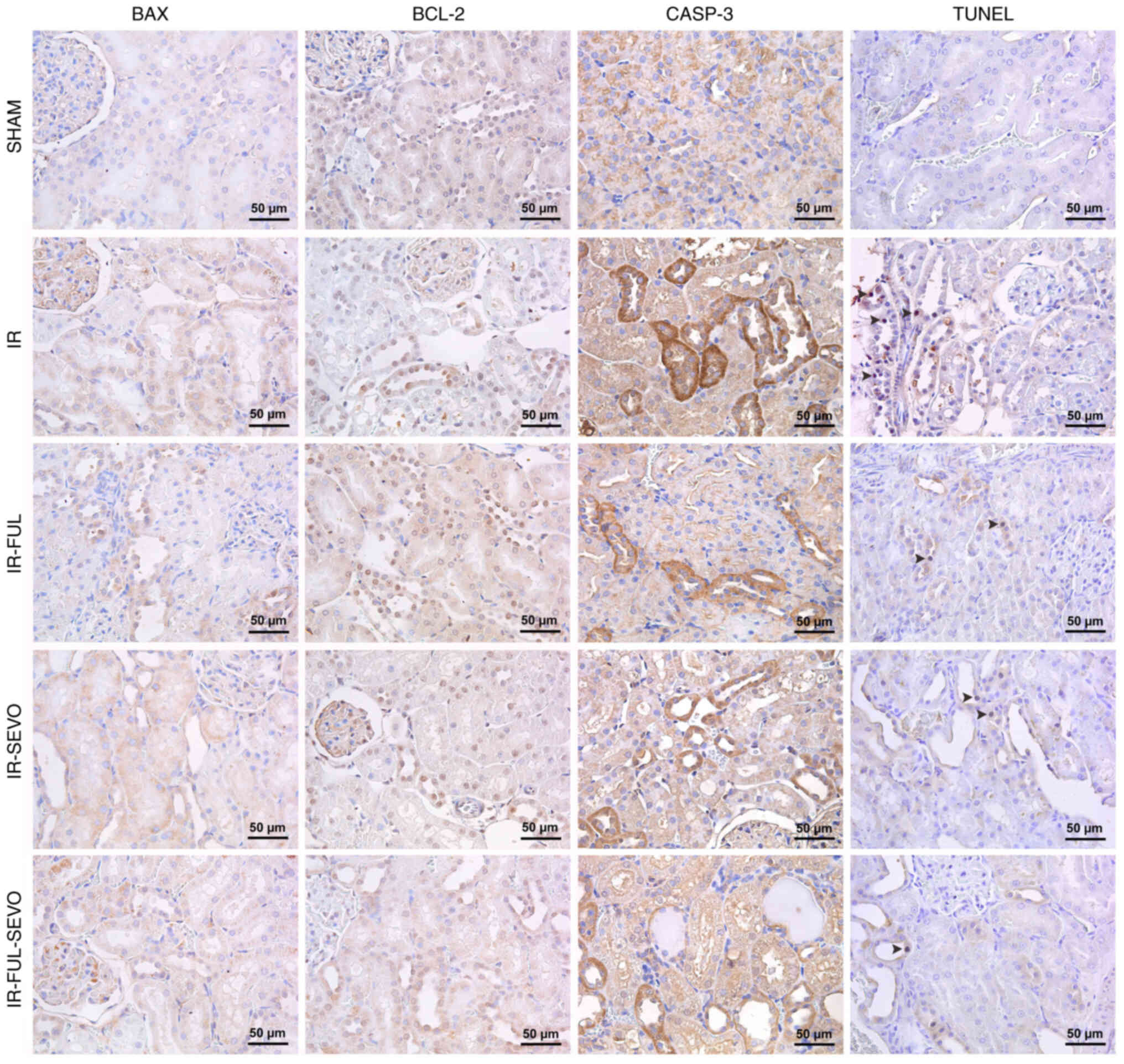
A significant elevation in BAX and CASP-3
expression, and a significant reduction in BCL-2 expression was
detected in kidney samples following hindlimb IR injury. These
alterations improved considerably in all treatment groups, although
the best outcomes were observed in the group treated with
fullerenol C60 alone (Table IV;
Fig. 5). Similarly, the TUNEL
assay revealed a considerable increase in the number of tubular
cells undergoing apoptosis following hindlimb IR injury, whereas a
significant amelioration was achieved in all treatment groups
(Table IV; Fig. 5).
 | Table IV.Comparison for immunostaining
intensity of kidney samples labelled with BAX, BCL-2 and CASP-3
antibodies, and TUNEL positivity in renal tubular cells (mean ±
SD). |
Table IV.
Comparison for immunostaining
intensity of kidney samples labelled with BAX, BCL-2 and CASP-3
antibodies, and TUNEL positivity in renal tubular cells (mean ±
SD).
| Variable | Sham (n=6) | IR (n=6) | IR-FUL (n=6) | IR-SEVO (n=6) | IR-FUL-SEVO
(n=6) | ANOVA P-value |
|---|
| BAX | 2.68±0.71 |
7.02±1.67a |
3.76±0.76b |
5.47±1.41a–c |
4.52±1.02a,b | <0.0001 |
| BCL-2 | 7.41±1.15 |
2.80±0.64a |
6.26±1.84b |
4.70±1.53a,b |
5.70±1.48b | <0.0001 |
| CASP-3 | 20.14±1.18 |
29.46±3.22a |
22.93±0.27a,b |
24.98±1.28a–c |
23.44±0.95a,b | <0.0001 |
| TUNEL | 0.05±0.02 |
3.33±0.53a |
0.11±0.04b |
0.57±0.33b |
0.32±0.26b | <0.0001 |
Biochemical findings
When the lung tissue TBARS levels were compared
between the groups, a significant difference was observed
(P<0.0001). In the IR and IR-SEVO groups, TBARS levels were
considerably higher than those in the sham group (P<0.0001 and
P=0.007, respectively). Additionally, the TBARS levels in the
IR-SEVO group were considerably higher than those in the IR-FUL
group (P=0.031). TBARS levels were significantly lower in the
IR-FUL, IR-SEVO and IR-FUL-SEVO groups compared with those in the
IR group (P<0.0001, P=0.017 and P=0.001, respectively; Table V).
 | Table V.Biochemical data of lung tissue (mean
± SD). |
Table V.
Biochemical data of lung tissue (mean
± SD).
| Variable | Sham (n=6) | IR (n=6) | IR-FUL (n=6) | IR-SEVO (n=6) | IR-FUL-SEVO
(n=6) | ANOVA P-value |
|---|
| TBARS
(nmol/mg.pro) | 0.43±0.07 |
1.04±0.10a |
0.50±0.09b |
0.76±0.05a–c |
0.63±0.10b | <0.0001 |
| CAT
(IU/mg.pro) | 60.25±3.48 |
25.32±1.76a |
56.58±3.49b |
43.83±5.41a–c |
47.32±4.18a,b | <0.0001 |
| GST
(IU/mg.pro) | 0.90±0.14 |
0.28±0.04a |
0.77±0.12b |
0.66±0.07a,b |
0.82±0.06b | <0.0001 |
A significant difference was found between the
groups in terms of CAT enzyme activity in lung tissue
(P<0.0001). CAT enzyme activity was significantly decreased in
the IR, IR-SEVO and IR-FUL-SEVO groups compared with that in the
sham group (P<0.0001, P=0.006 and P=0.025, respectively).
Additionally, CAT activity was significantly lower in the IR-SEVO
group than in the IR-FUL group (P=0.027). CAT enzyme activity was
significantly higher in the IR-FUL, IR-SEVO and IR-FUL-SEVO groups
than in the IR group (P<0.0001, P=0.002, P<0.0001,
respectively; Table V).
There was a significant difference between the
groups in terms of GST activity in lung tissue (P<0.0001). GST
activity was significantly lower in the IR and IR-SEVO groups than
in the sham group (P<0.0001 and P=0.048, respectively). GST
enzyme activity was significantly higher in the IR-FUL, IR-SEVO and
IR-FUL-SEVO groups than in the IR group (P=0.001, P=0.010,
P<0.0001, respectively; Table
V).
In kidney tissue, TBARS levels were considerably
higher in the IR and IR-SEVO groups, than those in the sham group
(P<0.0001 and P<0.0001, respectively). Also, it was
considerably higher in the IR-SEVO group compared with that in the
IR-FUL group (P=0.004). However, TBARS levels were significantly
lower in IR-FUL, IR-SEVO and IR-FUL-SEVO groups compared with those
in the IR group (P<0.0001, P=0.016 and P<0.0001,
respectively; Table VI).
 | Table VI.Biochemical data of kidney tissue
(mean ± SD). |
Table VI.
Biochemical data of kidney tissue
(mean ± SD).
| Variable | Sham (n=6) | IR (n=6) | IR-FUL (n=6) | IR-SEVO (n=6) | IR-FUL-SEVO
(n=6) | ANOVA P-value |
|---|
| TBARS
(nmol/mg.pro) | 0.83±0.10 |
1.83±0.14a |
1.01±0.12b |
1.46±0.09a–c |
1.11±0.10b | <0.0001 |
| CAT
(IU/mg.pro) | 70.47±3.62 |
44.28±2.10a |
63.58±4.34b |
54.65±2.30a,b |
57.98±4.18a,b | <0.0001 |
| GST
(IU/mg.pro) | 1.75±0.10 |
0.78±0.06a |
1.74±0.15b |
1.37±0.13a–c |
1.50±0.09b | <0.0001 |
CAT enzyme activity was significantly decreased in
the IR, IR-SEVO and IR-FUL-SEVO groups compared with that in the
sham group (P<0.0001, P=0.003 and P=0.016, respectively). CAT
enzyme activity was significantly higher in the IR-FUL, IR-SEVO and
IR-FUL-SEVO groups compared to the IR group (P=0.001, P=0.043,
P=0.009, respectively) (Table
VI).
GST activity was significantly lower in the IR and
IR-SEVO groups compared with the sham group (P<0.0001 and
P=0.018, respectively). Additionally, GST activity was
significantly lower in the IR-SEVO group compared with that in the
IR-FUL group (P=0.019). GST enzyme activity was significantly
higher in the IR-FUL, IR-SEVO and IR-FUL-SEVO groups compared with
that in the IR group (all P<0.0001) (Table VI).
Discussion
During IR, serious damage occurs to tissues with
inflammatory mediators released during the reperfusion period
leading to damage by activating endothelial cells in distant organs
(4). Furthermore, this response to
IR can lead to leukocyte-dependent microvascular damage (4). Restoring blood flow in the ischemic
limb may save the limb; however, multisystem organ failure may
develop, which can be fatal (10,32).
The findings of the present study support the idea that oxidative
damage due to ischemia of the lower extremity results in damage to
lung and kidney tissue with reperfusion. However, fullerenol C60
administered 30 min before ischemia and sevoflurane administered
during IR were observed to be protective against this damage.
Restoring the blood flow in the ischemic limb may
save the limb; however, multisystem organ failure may develop,
which can be fatal (33). The
severity of the inflammatory response in tissues after ischemia may
be similar in distant organs. Pulmonary vasoconstriction and
respiratory dysfunction have been shown to occur in humans after
aortic replacement, independent of capillary wedge pressure,
following aortic clamping and reperfusion of the lower extremities
(34). IR in the lower extremities
may cause pulmonary damage and dysfunction, characterized by
interstitial edema, requiring prolonged ventilatory and inotropic
support in some patients (10,32).
Surgery of the infrarenal aorta and the great arteries of the lower
extremities may cause rhabdomyolysis in the skeletal muscle, which
may result in remote kidney damage (35).
A number of studies have shown that volatile
anesthetics are protective against IR damage by reducing
inflammation (36–38). It has been proven that the
administration of sevoflurane protects distant organs, such as the
heart (39), lungs (40) and kidneys (36) against IR damage. Lee et al
(36) examined the protective
effects of volatile anesthetics against IR damage, but it was not
clear at what stage they were effective. By contrast, in a previous
study, 42 patients [classified as American Society of
Anesthesiologists physical status 1 (40 men, 2 women] who were
scheduled to undergo dental or orthopedic surgery that was expected
to last ≥4 h were studied. Patients who showed evidence of abnormal
hepatic or renal function, based on medical history, physical
examination or laboratory tests, were excluded from the study. The
results revealed that low-flow sevoflurane anesthesia may cause
proteinuria; however, the observed proteinuria was not associated
with any changes in blood urea nitrogen, creatinine or creatinine
clearance in patients without pre-existing kidney disease (41). In the present study, the protective
effect of sevoflurane against IR damage was supported by both
histopathological and biochemical data.
Fullerenol, a new nanoparticle, is used in medicine,
as well as in numerous branches of science. Owing to its spherical
molecules with 30 carbon double bonds, fullerenol C60 can easily
react with free radicals, thus acting as an effective free radical
scavenger that can be labeled a ‘radical sponge’ (42). Chen et al (43) showed that fullerenol C60 has
antioxidant activities at low concentrations and protects the lung
tissue from IR injury. Since the cytotoxic effect of high
concentration fullerenol C60 was proven in a previous study, low
concentrations of fullerenol C60 were used in the present study
(the maximum dose of fullerenol C60 was 100 µg/ml to avoid
cytotoxicity) (44,45). As aforementioned, fullerenes
exhibit strong antioxidant effects. It has been proven by studies
that they are protective against renal IR injury induced by
oxidative stress through this mechanism (46,47).
In addition, in a study using fullerenol C60 in IR injury of
skeletal muscle, it was determined that both intramuscularly and
intravenously administered fullerene treated ischemic pathologies
without showing a cytotoxic effect (48). In another study, it was revealed
that fullerenol C60 ameliorated ischemic renal failure by reducing
the formation of apoptosis; pretreatment with fullerenol C60 into
the kidneys diminished apoptosis (as determined by TUNEL staining,
the detection of apoptotic particles, and assesment of BCL-xl mRNA
and protein expression), and DNA laddering (49). In the present study, kidney damage
score was significantly lower in the group receiving fullerenol C60
compared with the IR group.
The present study focused on the beneficial effects
of fullerenol C60 administration with sevoflurane; however, there
were a number of limitations. Firstly, theoretically, a single dose
of fullerenol C60 may not be sufficient to prevent lower extremity
IR injury (50). The effects of IR
injury persist over a long period of time; however, the animal
models in the present study were euthanized at the end of the
experiment (51). It would have
been beneficial to include rats that were euthanized at different
time points after fullerenol C60 administration and thus, this
requires further investigation. In addition, only a single
concentration of fullerenol C60 was used in the present study.
Finally, the results of the present study should have been
supported by experimental methods, such as western blotting;
however, due to funding restrictions, this was not feasible. Future
studies will include these methods to further validate the
results.
In conclusion, the present study demonstrated the
effectiveness of fullerenol C60 in the lung and kidney tissues of
rats under sevoflurane anesthesia after lower extremity IR through
reduction of oxidative and histopathological damage in the lungs
and kidneys. Reducing the oxidative damage associated with IR has
become a target for drug studies in this area and a number of
molecules (albumin nanoparticles, PLGA nanoparticles, exosomes,
chitosan nanoparticles, polymeric micelles) that inhibit oxidative
stress have been previously investigated (52–54).
The results of the present study indicated that fullerenol C60 may
be considered as a potential inhibitor of lower-extremity IR injury
and, to the best of our knowledge, the present study was the first
to investigate the effects of fullerenol C60 on distant organ
damage in a lower-extremity IR model under sevoflurane anesthesia.
Therefore, considering the limitations of the present study, future
studies with additional methods of analysis would positively
contribute to the literature and support these results.
Acknowledgements
Not applicable.
Funding
This study was supported by the Gazi University BAP coordination
unit within the scope of the project numbered TGA-2021-7231.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MA, AHA and AK designed the study, and analyzed and
interpreted data. VŞ, MA and ZK performed the experiments. MA, AHA
and ZK confirm the authenticity of all the raw data. ZK, AK, MA and
ZY provided scientific and technical assistance, and critically
revised the article for important intellectual content. VŞ and MA
collected samples. ZY, SÖAD and MK performed cellular and molecular
experiments. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Ethical approval for the study was obtained from
Animal Research Committee of Gazi University (Ankara, Turkey;
approval no. G.Ü.ET-21.023).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Banz Y and Rieben R: Role of complement
and perspectives for intervention in ischemia-reperfusion damage.
Ann Med. 44:205–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Granger DN: Ischemia-reperfusion:
Mechanisms of microvascular dysfunction and the influence of risk
factors for cardiovascular disease. Microcirculation. 6:167–178.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kalogeris T, Baines CP, Krenz M and
Korthuis RJ: Cell biology of ischemia/reperfusion injury. Int Rev
Cell Mol Biol. 298:229–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carden DL and Granger DN: Pathophysiology
of ischaemia-reperfusion injury. J Pathol. 190:255–266. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao T, Wu W, Sui L, Huang Q, Nan Y, Liu J
and Ai K: Reactive oxygen species-based nanomaterials for the
treatment of myocardial ischemia reperfusion injuries. Bioact
Mater. 7:47–72. 2021.PubMed/NCBI
|
|
6
|
Linfert D, Chowdhry T and Rabb H:
Lymphocytes and ischemia-reperfusion injury. Transplant Rev
(Orlando). 23:1–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Safari S, Motavaf M, Seyed Siamdoust SA
and Alavian SM: Hepatotoxicity of halogenated inhalational
anesthetics. Iran Red Crescent Med J. 16:e201532014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu R, Ishibe Y and Ueda M:
Isoflurane-sevoflurane administration before ischemia attenuates
ischemia-reperfusion-induced injury in isolated rat lungs.
Anesthesiology. 92:833–840. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herrero de la Parte B, Roa-Esparza J,
Cearra I, Ruiz Montesinos I, Alonso-Alconada D, Alonso-Varona A,
Mar Medina C, Iturrizaga Correcher S and García-Alonso I: The
prevention of ischemia-reperfusion injury in elderly rats after
lower limb tourniquet use. Antioxidants (Basel). 11:19362022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harkin DW, Barros D'Sa AA, McCallion K,
Hoper M and Campbell FC: Ischemic preconditioning before lower limb
ischemia-reperfusion protects against acute lung injury. J Vasc
Surg. 35:1264–1273. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lucchinetti E, Ambrosio S, Aguirre J,
Herrmann P, Härter L, Keel M, Meier T and Zaugg M: Sevoflurane
inhalation at sedative concentrations provides endothelial
protection against ischemia-reperfusion injury in humans.
Anesthesiology. 106:262–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chappell D, Heindl B, Jacob M, Annecke T,
Chen C, Rehm M, Conzen P and Becker BF: Sevoflurane reduces
leukocyte and platelet adhesion after ischemia-reperfusion by
protecting the endothelial glycocalyx. Anesthesiology. 115:483–491.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Percie du Sert N, Hurst V, Ahluwalia A,
Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl
U, et al: The ARRIVE guidelines 2.0: Updated guidelines for
reporting animal research. Exp Physiol. 105:1459–1466. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
National Research Council (US), .
Committee for the Update of the Guide for the Care and Use of
Laboratory Animals: Guide for the care and use of laboratory
animals. 8th edition. National Academies Press; Washington, DC:
2011
|
|
15
|
Yesil S, Ozdemir C, Arslan M, Gundogdu AC,
Kavutcu M and Atan A: Protective effect of cerium oxide on
testicular function and oxidative stress after torsion/detorsion in
adult male rats. Exp Ther Med. 25:12022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Conzen PF, Vollmar B, Habazettl H, Frink
EJ, Peter K and Messmer K: Systemic and regional hemodynamics of
isoflurane and sevoflurane in rats. Anesth Analg. 74:79–88. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Namdar F, Bahrami F, Bahari Z, Ghanbari B,
Elahi SA and Mohammadi MT: Evaluation of the effects of fullerene
C60 nanoparticles on oxidative stress parameters in normal rats
liver and brain. J Adv Med Biomed Res. 27:8–15. 2019. View Article : Google Scholar
|
|
18
|
Tüfek H and Özcan Ö: 4R rule in laboratory
animal science. Comm J Biol. 2:55–60. 2018. View Article : Google Scholar
|
|
19
|
Sivgin V, Yalcin G, Kucuk A, Sezen SC,
Afandiyeva N and Arslan M: Effects of fullerenol nanoparticles on
kidney tissue in sevoflurane-treated rats. Bratisl Lek Listy.
121:117–121. 2020.PubMed/NCBI
|
|
20
|
Ozdemirkan A, Kurtipek AC, Kucuk A,
Ozdemir C, Yesil S, Sezen SC, Kavutcu M and Arslan M: Effect of
cerium oxide on kidney and lung tissue in rats with testicular
torsion/detorsion. Biomed Res Int. 2022:31764552022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kartal S, Kip G, Küçük A, Atan A, Erdem Ö
and Kavutçu M: The efficacy of dexmedetomidine on lung injury
induced by renal ischemia/reperfusion in diabetic rats. Anaesth.
pain intensive care. 24:272–277. 2020.
|
|
22
|
Van Ye TM, Roza AM, Pieper GM, Henderson J
Jr, Johnson CP and Adams MB: Inhibition of intestinal lipid
peroxidation does not minimize morphological damage. J Surg Res.
55:553–558. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aebi H: Catalase. Methods of Enzymatic
Analysis. Bergmeyer HU: Academic Press; London: pp. 673–677. 1974,
View Article : Google Scholar
|
|
24
|
Habig WH, Pabst MJ and Jakoby WB:
Glutathione S-transferases. The first enzymatic step in mercapturic
acid formation. J Biol Chem. 249:7130–7139. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lowry OH, Rosenbraugh NJ, Farr AL and
Randall RJ: Protein measurement with folin phenol reagent. J Biol
Chem. 193:265–275. 1951. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Garbaisz D, Turoczi Z, Aranyi P, Fulop A,
Rosero O, Hermesz E, Ferencz A, Lotz G, Harsanyi L and Szijarto A:
Attenuation of skeletal muscle and renal injury to the lower limb
following ischemia-reperfusion using mPTP inhibitor NIM-811. PLoS
One. 9:e1010672014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shih JM, Shih YM, Hou YC, Pai MH, Yeh CL
and Yeh SL: Effects of fish oil-based lipid emulsion on
inflammation and kidney injury in mice subjected to unilateral hind
limb ischemia/reperfusion. Cytokine. 111:49–57. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matute-Bello G, Downey G, Moore BB,
Groshong SD, Matthay MA, Slutsky AS and Kuebler WM; Acute Lung
Injury in Animals Study Group, : An Official American Thoracic
Society Workshop Report: Features and Measurements of Experimental
Acute Lung Injury in Animals. Am J Respir Cell Mol Biol.
44:725–738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huwae TECJ, Santoso ARB, Kesuma W, Sujuti
H, Ratnawati R, Sukmajaya WP and Hidayat M: Reperfusion interval as
a prevention of lung injury due to limb ischemia-Reperfusion after
application of tourniquet in murine experimental study. Indian J
Orthop. 54:704–710. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang C, Zhang L, Shi Y, Yi H, Zhao Y,
Chen J, Pollock CA and Chen XM: The KCa3. 1 blocker TRAM34 reverses
renal damage in a mouse model of established diabetic nephropathy.
PLoS One. 13:e01928002018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen M, Ai G, Zhou J, Mao W, Li H and Guo
J: circMTO1 promotes tumorigenesis and chemoresistance of cervical
cancer via regulating miR-6893. Biomed Pharmacother.
117:1090642019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vlastos D, Zeinah M, Ninkovic-Hall G,
Vlachos S, Salem A, Asonitis A, Chavan H, Kalampalikis L, Al
Shammari A, Alvarez Gallesio JM, et al: The effects of ischaemic
conditioning on lung ischaemia-reperfusion injury. Respir Res.
23:3512022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Woodruff TM, Arumugam TV, Shiels IA, Reid
RC, Fairlie DP and Taylor SM: Protective effects of a potent C5a
receptor antagonist on experimental acute limb ischemia-reperfusion
in rats. J Surg Res. 116:81–90. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schofield ZV, Woodruff TM, Halai R, Wu MC
and Cooper MA: Neutrophils-a key component of ischemia-reperfusion
injury. Shock. 40:463–470. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yassin MM, Harkin DW, Barros D'Sa AA,
Halliday MI and Rowlands BJ: Lower limb ischemia-reperfusion injury
triggers a systemic inflammatory response and multiple organ
dysfunction. World J Surg. 26:115–121. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee HT, Ota-Setlik A, Fu Y, Nasr SH and
Emala CW: Differential protective effects of volatile anesthetics
against renal ischemia-reperfusion injury in vivo. Anesthesiology.
101:1313–1324. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee HT, Kim M, Jan M and Emala CW:
Anti-inflammatory and antinecrotic effects of the volatile
anesthetic sevoflurane in kidney proximal tubule cells. Am J
Physiol Renal Physiol. 291:F67–F78. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
De Hert SG, Van der Linden PJ, Cromheecke
S, Meeus R, Nelis A, Van Reeth V, ten Broecke PW, De Blier IG,
Stockman BA and Rodrigus IE: Cardioprotective properties of
sevoflurane in patients undergoing coronary surgery with
cardiopulmonary bypass are related to the modalities of its
administration. Anesthesiology. 101:299–310. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu P, Zhang J, Yu S, Luo Z, Hua F, Yuan L,
Zhou Z, Liu Q, Du X, Chen S, et al: Protective effect of
sevoflurane postconditioning against cardiac ischemia/reperfusion
injury via ameliorating mitochondrial impairment, oxidative stress
and rescuing autophagic clearance. PLoS One. 10:e01346662015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu G, Wang X, Xiong Y, Ma X and Qu L:
Effect of sevoflurane pretreatment in relieving liver
ischemia/reperfusion-induced pulmonary and hepatic injury. Acta Cir
Bras. 34:e2019008052019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Higuchi H, Sumita S, Wada H, Ura T,
Ikemoto T, Nakai T, Kanno M and Satoh T: Effects of sevoflurane and
isoflurane on renal function and on possible markers of
nephrotoxicity. Anesthesiology. 89:307–322. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Davıd WIF, Ibberson RM, Dennıs TJS, Hare
JP and Prassıdes K: Structural phase transitions in the fullerene
C60. Europhy Lett. 18:735–736. 1992. View Article : Google Scholar
|
|
43
|
Chen YW, Hwang KC, Yen CC and Lai YL:
Fullerene derivatives protect against oxidative stress in RAW 264.7
cells and ischemia-reperfused lungs. Am J Physiol Regul Integr Comp
Physiol. 287:R21–R26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Djordjević A, Bogdanović G and Dobrić S:
Fullerenes in biomedicine. J BUON. 11:391–404. 2006.PubMed/NCBI
|
|
45
|
Harhaji L, Isakovic A, Raicevic N,
Markovic Z, Todorovic-Markovic B, Nikolic N, Vranjes-Djuric S,
Markovic I and Trajkovic V: Multiple mechanisms underlying the
anticancer action of nanocrystalline fullerene. Eur J Pharmacol.
568:89–98. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chien CT, Chen CF, Hsu SM, Chiang LY and
Lai MK: Forced expression of bcl-2 and bcl-xL by novel
water-soluble fullerene, C60 (glucosamine) 6, reduces renal
ischemia/reperfusion-induced oxidative stress. Fullerene Sci
Technol. 9:77–88. 2007. View Article : Google Scholar
|
|
47
|
Chien CT, Lee PH, Chen CF, Ma MC, Lai MK
and Hsu SM: De novo demonstration and co-localization of
free-radical production and apoptosis formation in rat kidney
subjected to ischemia/reperfusion. J Am Soc Nephrol. 12:973–982.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nozdrenko DN, Prylutskyy YI, Ritter U and
Scharff P: Protective effect of water-soluble pristine C 60
fullerene in ischemia-reperfusion injury of skeletal muscle. Int J
Phys Pathophysiol. 5:97–104. 2014. View Article : Google Scholar
|
|
49
|
Chien CT, Chen CF, Chiang LY and Lai MK:
Novel water-soluble hexa (sulfobutyl) fullerene attenuates
apoptosis formation after ischemic renal failure. Fullerene Sci
Technol. 7:529–540. 1999. View Article : Google Scholar
|
|
50
|
Monteiro-Riviere NA, Linder KE, Inman AO,
Saathoff JG, Xia XR and Riviere JE: Lack of hydroxylated fullerene
toxicity after intravenous administration to female Sprague-Dawley
rats. J Toxicol Environ Health A. 75:367–373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gueler F, Gwinner W, Schwarz A and Haller
H: Long-term effects of acute ischemia and reperfusion injury.
Kidney Int. 66:523–527. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zang X, Zhou J, Zhang X, Han Y and Chen X:
Ischemia reperfusion injury: Opportunities for nanoparticles. ACS
Biomater Sci Eng. 6:6528–6539. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Trujillo-Rangel WÁ, García-Valdés L,
Méndez-Del Villar M, Castañeda-Arellano R, Totsuka-Sutto SE and
García-Benavides L: Therapeutic targets for regulating oxidative
damage induced by ischemia-reperfusion injury: A study from a
pharmacological perspective. Oxid Med Cell Longev.
2022:86243182022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhou L, Tang S, Li F, Wu Y, Li S, Cui L,
Luo J, Yang L, Ren Z, Zhang J, et al: Ceria nanoparticles
prophylactic used for renal ischemia-reperfusion injury treatment
by attenuating oxidative stress and inflammatory response.
Biomaterials. 287:1216862022. View Article : Google Scholar : PubMed/NCBI
|