Introduction
Ischemic stroke is a devastating neurological event
that occurs when blood flow to the brain is interrupted, leading to
neuronal damage and functional impairment. Despite significant
advances in acute management and rehabilitation strategies, most
stroke survivors still experience significant impairments in motor,
sensory, and cognitive function. Effective neurorestorative
therapies are essential to promote neural plasticity and facilitate
functional recovery (1).
There are three therapeutic approaches suggested to
enhance post-stroke functional recovery. First, interventions
target common injury mechanisms for various cytoarchitectural
damages, including cortical and subcortical, gray and white matter
and the neurovascular unit, to reduce the extension of direct or
indirect damage to partially injured (penumbral) brain tissues
(2–4). The second is to use specialized
treatments that enhance neuroplasticity during the subacute phase
of ischemic stroke, including promoting neuronal sprouting, myelin
regeneration, dendritic spine density, arborization and synaptic
connections (5,6). This leads to restoring or
compensating functional deficits caused by ischemic stroke by
either enhancing the rewiring process for the damaged brain
tissues, decreasing the remote injury that may occur distal to the
ischemic territory, or even using other intact brain tissues. The
third is to introduce pluripotent stem cells into the ischemic
brain or enhance the proliferation, migration and differentiation
of endogenous progenitor cells into the damaged brain to
restoration of neuronal, axonal, and synaptic functions through
replenishing and rewiring of the damaged neural network (7,8).
Thus, treatment strategies combining neuroplasticity strategies
with neuroprotectants have been suggested (7,9).
Prothymosin α (ProT) is a small, ubiquitous protein
essential for cell proliferation and survival through its
involvement in chromatin remodeling and proapoptotic activity
(10). Furthermore, ProT
contributes to neuroprotection against cerebral and retinal
ischemia by being involved in anti-necrosis, anti-apoptosis,
immunomodulation and oxidative stress (11–15).
It has been shown that ProT and peptides derived from ProT
attenuated infarction volume and blood vessel disruption and
improved functional outcomes (16–18).
It was therefore hypothesized that ProT may have a role in
promoting neuroplasticity following ischemic injury.
The present study aimed to investigate whether ProT
improved axonal sprouting and dendrite branching in cultured
neurons exposed to oxygen-glucose deprivation and to explore its
potential of neuroplasticity effect of underlying mechanisms of
neuroplasticity following ischemia-reperfusion injury in mice.
Materials and methods
Animal preparation, anesthesia and
monitoring
All animal procedures were conducted following the
Taiwan National Institutes of Health guidelines and approved by the
Subcommittee on Research-Animal Care of National Cheng Kung
University (NCKU) Medical Center (approval no. 109184).
Sprague-Dawley rats (weight, 5–6 g; age, 1 day; n=160; obtained
from the Laboratory Animal Center of NCKU) both male and female
were used for primary cortical neuron culture, while male FVB mice
(weight, 20–25 g; age, 6–8 weeks; n=24; mice were obtained from the
Laboratory Animal Center of NCKU, and the ProT-overexpressing
transgenic mice were generated by Professor Chao-Liang Wu,
Department of Biochemistry and Molecular Biology, NCKU) were used
for middle cerebral artery (MCA) occlusion surgery. The animals
were housed in the Laboratory Animal Center of NCKU under a 12-h
light/dark cycle, with an ambient temperature of 20–26°C and
humidity maintained at 40–60%. They were provided with free access
to food and water. The animals were anesthetized with isoflurane
(induction 4–5%, maintenance 1–2%), and body temperature was
maintained at 37°C using a thermostatically controlled heating
blanket and rectal probe (Harvard Apparatus) during the surgical
procedure.
Protein preparation
ProT and ProTΔ nuclear localizing signal (NLS)
proteins preparation as previously described (18). The plasmids encoding wild-type ProT
and ProTΔNLS with glutathione-transferase (GST) tags were
transformed into BL21 E. coli for protein expression. The
transformed E. coli cultures were incubated at 37°C in
lysogeny broth medium [tryptone (Merck KGaA) 10 g, yeast extract
(Sigma-Aldrich; Merck KGaA) 5 g, sodium chloride (J.T. Baker;
Thermo Fisher Scientific, Inc.) 5 g; components dissolved in 1 l
ddH2O)]. The optical density of the medium at 600 nm
(OD600) reached 0.4–0.8. The cultured cells (1 ml) were transferred
to 250 ml of fresh culture medium for large-scale protein
expression. To induce protein expression, isopropyl
β-D-1-thiogalactopyranoside (0.1 M) was added to the culture
medium, and the cells were further incubated at 37°C for 5 h.
following induction, the cells were lysed using a sonicator and
centrifuged (10,000 × g, 4°C, 10 min). The clear supernatant was
then purified using a column designed explicitly for GST-tagged
proteins (Pierce Glutathione Superflow Agarose; Thermo Fisher
Scientific, Inc.). The column was washed with elution buffer (125
mM Tris-HCl, 150 mM sodium chloride, 10 mM reduced glutathione; pH
8.0) and regeneration buffers to ensure efficient purification.
The column was washed with elution buffer and
regeneration buffers to ensure efficient purification. The purified
protein solution was eluted by gravity flow (flow rate, ≤150 cm/h)
with 5 column volumes of elution buffer and centrifuged (700 × g,
room temperature, 2 min) using filter tubes with appropriate
molecular weight cut-offs. GST-tagged proteins were concentrated
using 10-kDa filter tubes, while ProT and ProTΔNLS were
concentrated using 30-kDa filter tubes. The protein concentration
was adjusted to 100 µg/50 µl, and the samples were stored in a
−20°C refrigerator for future use.
Primary cortical neuronal culture
Primary cortical neurons were obtained from the
cerebral cortices of 1-day-old Sprague-Dawley rats under deep
anesthesia induced by pentobarbital [150 mg/kg; intraperitoneal
(IP)]. Following deep anesthesia, the rats were euthanized by
decapitation. The cortical tissue was minced and dissociated in a
papain solution containing DNase I [0.6 mg/ml papain and DNase I in
Hank's Balanced Salt Solution (HBSS)] at 37°C for 30 min. The
reaction was stopped with heat-inactivated horse serum (Thermo
Fisher Scientific, Inc.), and the cell suspension was centrifuged
at 800 × g, 4°C, 5 min and plated onto poly-D-lysine-coated Petri
dishes. The dissociated cells were cultured in DMEM with 10% horse
serum at 37°C in a humidified incubator with 5% CO2.
After 3 h of plating, the culture medium was replaced with a
serum-free neurobasal medium containing 25 mM glutamate, 0.5 mM
L-glutamine, and 2% B27 supplement (cat. no. 17504-044; Invitrogen;
Thermo Fisher Scientific, Inc.). The culture medium was changed
every 3 days, and the cultured cells were allowed to grow for ~6-8
days.
Oxygen and glucose deprivation
(OGD)
The OGD medium consisted of HBSS without glucose and
bubbled with N2 for 30 min. Cultured neurons were then
exposed to the OGD medium and transferred to an anaerobic chamber
with an N2-enriched atmosphere at 37°C for 2 h.
Following the deprivation period, the cultured neurons were
incubated in a neuron basal medium under normal incubator
conditions (5% CO2 at 37°C).
Transfection of primary neuron
cells
To transfect primary neuron cells, 6 cm dishes were
used for plating. A solution containing 1 µg of DNA from the ProT
plasmid, ProTΔNLS plasmid, or empty pLKO.1-GFP plasmid (sham or OGD
groups), along with Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) was prepared by diluting them in
250 µl of neurobasal medium (Invitrogen; Thermo Fisher Scientific,
Inc.) for 5 min at room temperature. The DNA and
Lipofectamine® 2000 were then mixed and incubated for 20
min at room temperature. Subsequently, the
DNA-Lipofectamine® 2000 complexes were added to each
well, and the cells were incubated at 37°C for 4 h, after which the
medium was replaced with fresh medium. After 24 h, neuron cells
were used for the OGD experiment.
Immunofluorescence staining and
quantification
Neurons cultured on coverslips were post-fixed in 4%
paraformaldehyde in PBS for 5 min at room temperature and rinsed
with PBS 3 times. Coverslips were processed with primary antibodies
at a dilution of 1:1,000 against MAP-2 (cat. no. sc-390543; Santa
Cruz Biotechnology, Inc.) or 1:100 against prothymosin α (2F11;
cat. no. ALX-804-486-C100; Enzo Life Sciences, Inc.) at 4°C
overnight. Subsequently, an appropriate secondary antibody
conjugated with biotin (1:150; cat. no. 115-065-003; Jackson
ImmunoResearch Laboratories, Inc.) was added, followed by
DTAF-conjugated streptavidin (green; 1:100; cat. no. 016-010-084;
Jackson ImmunoResearch Laboratories, Inc.) or Alexa red-conjugated
streptavidin (red; 1:100; cat, no. 016-580-084; Jackson
ImmunoResearch Laboratories, Inc.). The sections were co-incubated
with DAPI (blue; 1:1,000; cat. no. D8417; MilliporeSigma) at room
temperature for 10 min. Fluorescent photomicrographs of labeled
neurons were captured in at least three fields of view and each
result quantified at ×40 magnification using a CoolSNAP-Pro cf
digital camera (Media Cybernetics Inc.). The Digital Image Analysis
System MCID Elite (version 6.0; Imaging Research Inc.) was employed
to assess the relative intensity of ProT. ImageJ software (1.49v,
National Institutes of Health) with the Neuron J plugin was
utilized to upload MAP-2-stained images for the analysis of the
number and length of neuronal dendrite branches.
RNA isolation and PCR
Total RNA was isolated from cultured neurons using
TRIzol™ Reagent, (Invitrogen; Thermo Fisher Scientific,
Inc.), and cDNA synthesis was performed using High-Capacity cDNA
Reverse Transcription Kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
PCR amplification consisted of an initial denaturation for 5 min at
95°C, followed by 35 cycles of 30 sec denaturation at 95°C,
annealing for 45 sec at 58°C and extension for 1 min at 72°C. The
final extension step was carried out at 72°C for 10 min. RT-PCR was
carried out with specific primers for brain-derived neurotrophic
factor (BDNF), 5′-CCTCCTCTGCTCTTTCTGC-′3 (forward) and
5′-TCCCATTACACTTGGTCTCGT-3′ (reverse); GAPDH,
5′-CCAAAGTTGTCATGGATGACC-3′ (forward) and 5′-GTCTTCACCACCATGGAG-3′
(reverse), and the PCR products were separated by 2% agarose gel
electrophoresis and visualized using ethidium bromide staining. Gel
documentation was performed under UV light for analysis and
documentation of band patterns.
Experimental model and grouping
Focal cerebral ischemia was induced by
intra-arterial suture occlusion of the proximal right middle
cerebral artery (MCA) for 50 min. Briefly, a 4-0 nylon suture with
its tip rounded by heating over a flame and subsequently coated
with silicone (Merck KGaA) was inserted from the external carotid
artery into the internal carotid artery until the tip occluded the
origin of the MCA (19,20). Laser-Doppler flowmetry (LDF;
Laserflo BMP2; Vasamedics Inc.) was used for local cortical blood
perfusion (LCBF) measurement, as previously described (21). The scalp was incised along the
midline, and two areas in bilateral parietal bones were thinned 0.5
mm posterior and 7 mm lateral to the bregma to place the LDF
probes. Another area in the right parietal bone was thinned 2.0 mm
posterior and 2.5 mm lateral to the bregma for additional LCBF
measurements. LCBF was measured prior to and during MCA occlusion
and expressed as a percentage of the baseline values. Animals were
assigned to three groups: i) WT control group (n=7), ii) WT ProT
injection group (n=8) and iii) ProT transgene group (n=8). ProT
overexpression in transgenic mice has been described (22,23).
Briefly, the ProT minigene employed for transgenic mouse production
was designated pJ6Ω-ProT. This construct comprised a complete
1.2-kb murine ProT cDNA sequence, under the control of the rat
β-actin promoter and SV40 polyadenylation tail. The minigene,
isolated from pJ6Ω-ProT through PvuII and ScaI
digestions, underwent microinjection into the pronuclei of FVB
zygotes. Subsequently, the injected eggs were transferred into the
oviducts of pseudopregnant recipients. Genomic DNA from founder
mice, obtained via tail biopsies, was subjected to PCR analysis
using RAP-f and ProT-r1 primers to confirm the integration of the
transgenic ProT minigene. Normal saline or ProT protein (100 µg/kg;
IP) was injected upon reperfusion (13,18).
Neurobehavioral testing and body
weight measurements
Body weight measurements and neurobehavioral testing
were conducted both before the surgery and prior to sacrifice. A
total of two neurological grading systems, modified from previous
versions (19,24,25),
were employed to assess various aspects of neurological function:
i) Sensory Test; this examination focused on sensorimotor
integration in forelimb placing responses to visual and tactile
stimuli. The affected forelimb underwent forward and sideways
visual placing tests, with scores assigned to each test as follows:
0, complete immediate placing; 1, incomplete and/or delayed placing
(<2 sec); and 2, absence of placing. Additionally, the motor
test involved the postural reflex test to evaluate upper body
posture while the animal's tail was suspended. Scores for this test
were as follows: 0, no observable deficit; 1, forelimb flexion; 2,
forelimb flexion and decreased resistance to lateral push; 3,
forelimb flexion, decreased resistance to lateral push and
unilateral circling; and 4, forelimb flexion, making ambulation
difficult or impossible. ii) Animals were also rated using a scale
developed by Clark et al (26), with scores ranging 0–28 used for
further analysis. Higher scores indicate more severe brain defects
caused by brain injury.
Protein extraction and western blot
analysis
The brain tissue was removed after the animal was
deeply anesthetized (isoflurane 5%; inhalation), and cell lysates
were prepared with lysis buffer, containing 1% Triton X-100, 20 mM
Tris-HCl (pH 7.5), 150 mM NaCl, 0.5% sodium deoxycholate, 1 mM EDTA
and 0.1% SDS, and were centrifuged at 18,000 × g for 60 min at 4°C.
Protein concentrations were determined using a BCA protein assay
kit (Pierce; Thermo Fisher Scientific, Inc.). Subsequently, 10%
SDS-PAGE was used to separate the 25 µg protein samples, which were
then transferred onto PVDF microporous membranes. The membranes
were blocked with 5% skim milk in TBS −0.05% Tween-20 for 30 min at
room temperature and probed with primary antibodies against
growth-associated protein-43 (GAP-43; 1:1,000; cat. no. AB5220;
Chemicon; Sigma-Aldrich; Merck KGaA), postsynaptic density protein
95 (PSD-95; 1:1,000; cat. no. AB9708; Chemicon, Sigma-Aldrich;
Merck KGaA), synaptosomal-associated protein, 25 kDa (SNAP-25;
1:1,000; cat. no. AHP1124; Bio-Rad Laboratories, Inc.) and β-actin
(1:10,000; cat. no. ABT264; Chemicon; Sigma-Aldrich; Merck KGaA)
overnight at 4°C. Membranes were then incubated with horseradish
peroxidase-conjugated immunoglobulin secondary antibody (1:5,000;
cat. nos. AP106P and AP182P; Chemicon; Sigma-Aldrich; Merck KGaA)
for 30 min at room temperature. Proteins were visualized with an
Enhanced Chemiluminescence kit (GE Healthcare Bio-Sciences).
Optical densities were measured using Multi Gauge V3.0 (Fuji Photo
Film Co., Ltd.) on a Luminescent Image Analyzer (Fujifilm LAS-3000;
Fuji Photo Film Co., Ltd.).
Statistical analysis
All data were expressed as the mean ± SD. Paired
Students' t-test was used to evaluate the response to a change in
conditions, and unpaired Student's t-test/one-way ANOVA with
Fisher's protected least significant difference (LSD) and Tukey's
post hoc comparison was used to evaluate differences between
groups. Neurobehavioral scores were expressed as medians ±95%
confidence interval (CI) and analyzed using the Mann-Whitney U
test. Data were analyzed using SPSS version 17.0 software (SPSS
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
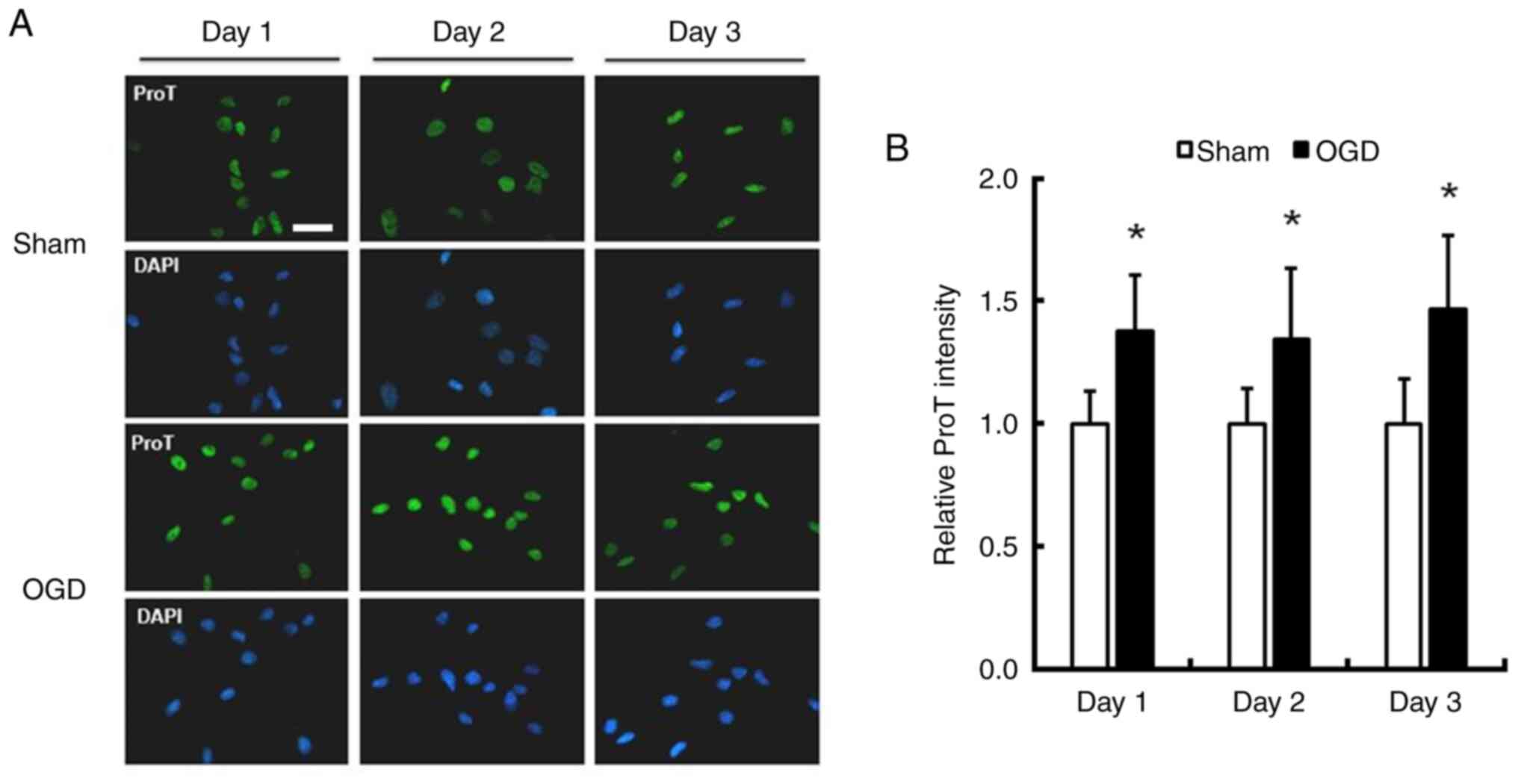
Increased ProT expression in cortical
neurons exposed to OGD
To investigate the influence of OGD on ProT protein
expression, cortical neurons obtained from 1-day-old rats were
cultured for ~1 week. Immunofluorescence staining of ProT/DAPI was
performed on cultures exposed to OGD for 1–3 days to assess changes
in ProT expression. The intensity of ProT significantly increased
by 37.9, 34.2 and 46.5% in cultured neurons 1–3 days after exposure
to OGD, respectively (Fig. 1;
P<0.05).
Transfection increases ProT gene
expression
To evaluate the effect of ProT on neuronal
plasticity in cultured cortical neurons after OGD injury,
ProT/ProTΔNLS plasmid DNA transfection was conducted prior to OGD,
while the sham and OGD group were transfected with empty plasmid.
The sham group did not exhibit an increase in ProT expression
compared with the normal group. Immunofluorescence staining of ProT
was performed on cultured neurons after OGD for 1–3 days (Fig. 2A). The relative intensity of ProT
was significantly increased compared with the sham or the OGD
control group (Fig. 2B; see
Table SI for more precise
data).
Transfection of the ProT gene
increases cultured neuron dendritic arborization following OGD
injury
To investigate the direct effect of ProT on neurite
plasticity, neurons were transfected with ProT/ProTΔNLS plasmid DNA
before being subjected to OGD injury, while the sham and OGD groups
were transfected with empty plasmid. The total length of neurites
and the number of dendritic branches were quantified by MAP-2
immunofluorescence staining (Fig.
3A). MAP2 isoforms are specialized cytoskeletal proteins found
predominantly in neurons, where they are abundant in dendrites and
perikarya. This suggests their involvement in shaping and
maintaining neuronal morphology as neurons develop. MAP2 is a
commonly employed marker for identifying neuronal cells and
visualizing dendritic processes (27,28).
In the OGD group, neurites exhibited rupture and shortening
compared with the sham control, resulting in decreased total length
and number of branches. However, compared with the OGD group,
transfection with ProT/ProTΔNLS plasmid DNA significantly increased
the total length and number of branches, indicating enhanced
neurite plasticity in cultured primary cortical neurons following
OGD injury (Fig. 3B; Table SII).
ProT treatment increases cultured
neuron dendritic arborization after OGD injury
To ascertain the role of ProT in neuronal
plasticity, the cultured neurons were pre-treated with
ProT/ProTΔNLS protein before OGD injury. The total length of
neurites and the number of dendritic branches were calculated by
MAP-2 immunofluorescence staining (Fig. 4A). Compared with the sham control
group, in the OGD group, neurites were ruptured and shortened and
the total length and number of branches significantly decreased.
However, compared with that of the OGD group, the total length and
number of branches significantly increased in the ProT/ProTΔNLS
protein-treated group upon OGD injury. These data suggested that
ProT/ProTΔNLS protein treatment facilitated neurite plasticity in
cultured primary cortical neurons after OGD injury (Fig. 4B; Table SIII).
ProT treatment increases BDNF mRNA
levels after OGD injury
To examine whether ProT/ProTΔNLS treatment would
virtually promote BDNF levels in the neurons after exposure to OGD.
Treatment of ProT/ProTΔNLS protein significantly increased the
level of BDNF mRNA level by 40.0 and 60.7%, respectively (Fig. 5).
In vivo stroke animal model
To further investigate the underlying mechanism of
the neuroplastic effect of ProT in vivo, focal cerebral
ischemia was employed on FVB wild-type mice and FVB ProT
overexpression in transgenic mice by intra-arterial suture
occlusion of the proximal right MCA for 50 min. LCBF was measured
prior to and during the MCA occlusion and after the onset of
reperfusion (Table SIV). Body
temperature was kept constantly at 37°C.
ProT improves neurologic behavior
score
To evaluate whether ProT treatment improves
neurobehavioral outcomes, a neurologic behavior test was conducted.
The ProT-treated and ProT overexpression in transgenic mice groups
had a less severe neurologic behavior score than the control group,
especially significant in the 28-point clinical scale (Table I) (26). This result indicated that ProT
ameliorated neurobehavioral recovery after ischemic insults.
 | Table I.Weight loss and sensorimotor
behavioral scores. |
Table I.
Weight loss and sensorimotor
behavioral scores.
|
|
|
| Neurologic behavior
score |
|---|
|
|
|
|
|
|---|
| Group | n | Weight loss
(g) | Motor | Sensory | 28-point clinical
scale |
|---|
| WT | 7 | 3.5±0.5 | 1.7 (1.2–2.2) | 3.0 (2.4–3.6) | 20.6
(19.4–21.7) |
| WT ProT (IP) | 8 | 3.6±0.9 | 1.4 (0.9–1.9) | 2.6 (1.9–3.4) | 17.9
(16.4–19.3)a |
| ProT overexpression
in transgenic mice | 8 | 3.8±0.5 | 1.5 (1.0–2.0) | 2.6 (2.1–3.1) | 18.3
(16.8–19.7)a |
ProT increases
neuroplasticity-associated proteins at the penumbra
SNAP-25 is a protein related to neuronal signal
transmission. It primarily operates at presynaptic terminals,
aiding in the regulation of neurotransmitter release, especially
between neurons at synapses. The function of SNAP-25 is essential
for normal neuronal transmission and it participates in
inter-neuronal messaging (9,29).
In presynaptic area analysis, SNAP-25 significantly increased in
the ProT injection group and ProT overexpression in transgenic mice
group compared with the control group at the penumbra (Fig. 6). However, there were no
significant differences at the contralateral area and ischemic
core. Concerning postsynaptic area analysis, PSD-95 is a protein
located at the postsynaptic density of neurons and is typically
involved in inter-neuronal signal transmission. It plays a crucial
role at the postsynaptic density in regulating connections and
communication between neurons. It is a synaptic protein that helps
maintain the stability of neuronal connections (20,30).
PSD-95 significantly increased in the ProT injection group and ProT
overexpression in transgenic mice group compared with the control
group at the penumbra. There was no significant difference between
the contralateral area and the ischemic core.
 | Figure 6.Western immunoblot analysis for
neuroplasticity-associated proteins in the ischemic brain after
ischemic insults for 24 h. (A) Images show typical changes in the
protein expressions of GAP-43, SNAP-25, and PSD-95 in the
contralateral hemisphere, ischemic core and penumbral cortices 24 h
after ischemia. (B) The ProT injection group (100 µg/kg, n=8) and
ProT overexpression in transgenic mice group (n=8) showed an
increase in the level of SNAP-25 vs. the control group (n=7) in the
penumbra area (*P<0.05), but not an increase in the level of
GAP-43. GAP-43, growth-associated protein-43 SNAP-25,
synaptosomal-associated protein, 25 kDa; PSD-95, postsynaptic
density protein 95; WT, wild-type. |
Discussion
Stroke has attracted significant attention due to
the high mortality rate and the effect on the lifestyle of stroke
patients. The development of a good neuroprotectant is essential
for clinical therapies. The present study demonstrated an increase
in endogenous ProT expression following OGD injury. Previous
reports have suggested that ProT is neuroprotective by preventing
necrosis and apoptosis after stroke (13,14).
However, the role of ProT in promoting neuroplasticity remains
unclear.
The results of the present study indicated that ProT
gene overexpression enhanced the length and dendritic branch
recovery of neurites damaged by OGD toxicity. Having established
the involvement of the ProT gene in neurite recovery, the present
study proceeded to investigate the direct effects of ProT protein
treatment. It revealed that ProT protein promoted neurite recovery,
consistent with the transfection results. ProT possesses
immunological functions at the nuclear localization signal (NLS),
deleted in ProTΔNLS (31). A
previous study reported that ProTΔNLS attenuates the
proinflammatory activity and enhances the neuroprotective effects
of ProT in ischemic injuries (18). Therefore, the present study also
treated cells with ProTΔNLS through transfection and protein
administration following OGD injury. The results indicated that
ProTΔNLS also exerted neuroplasticity effects, but the improvement
was not significantly better compared with ProT. Numerous studies
have shown that ischemic stress induces a complete release of ProT
in a serum- and supplement-free system (32–34).
In serum-free conditions, neurons in low-density cultures undergo
necrosis, while neurons cultured at high density exhibit an
elevated ProT release, accompanied by increased apoptotic features
and enhanced survival activity (35). When the conditioned medium from
neurons in the high-density culture, which contained ProT, was
added to the low-density culture, the survival activity markedly
increased (35). In the present
study, the neural cell culture medium initially contained serum and
supplements, which were subsequently removed during the 2 h of OGD.
The addition of ProT to the culture medium with serum and
supplements resulted in a notable upregulation of BDNF mRNA
expression and an increase in the cell survival rate.
A previous study mentioned that in vitro
experiments on neurons cultured in hypoxic and low-glucose
solutions, extracellular ProT changes the cell death mode from
necrosis to apoptosis through putative Gi-coupled ProT receptor
activation and delays neuronal cell death. During this period,
neurotrophic factors, such as BDNF, are produced and inhibit
apoptosis (36). BDNF plays an
important role in neuronal survival and growth, serves as a
neurotransmitter modulator and participates in neuronal plasticity
(37). The current study
demonstrated that the administration of ProT functions similarly to
extracellular ProT, inducing an increase in BDNF mRNA expression.
Therefore, to investigate the impact of ProT on neuronal
plasticity, exploring neuroplasticity-related proteins associated
with BDNF, such as GAP-43, PSD-95 and SNAP-25 (9,20,38),
becomes crucial.
To further investigate the neuroplastic effect of
ProT in vivo, the present study employed intra-arterial
suture occlusion of the proximal right MCA on FVB wild-type mice
and FVB ProT overexpression in transgenic mice in an animal model.
The results demonstrated that ProT treatment or ProT overexpression
in transgenic mice improved neurobehavioral outcomes compared with
the control group, suggesting that ProT improves motor-sensory
functional recovery following stroke. To investigate the molecular
mechanism of the neuroplasticity effect of ProT after ischemic
injury, the present study assessed the expression of several
plasticity-associated proteins. The results revealed that ProT
injection or the ProT overexpression in transgenic mice group
exhibited increased expression of PSD-95 and SNAP-25, leading to
improved neurite outgrowth and arborization. The expression of
neuroplasticity-associated proteins were analyzed in three regions:
Contralateral, penumbra and ischemic core. The contralateral area,
corresponding to the healthy left brain, showed similar levels of
plasticity-associated protein expression among the control group,
ProT injection group and ProT overexpression in transgenic mice
group. In the penumbra, ischemic damage is reversible through
reperfusion and the levels of plasticity-associated proteins were
significantly higher in the ProT injection and ProT overexpression
in transgenic mice groups compared with the control group. However,
no differences were observed in the ischemic core, where necrosis
occurred.
Based on the aforementioned analysis and
discussions, in conjunction with findings from previous studies and
the results of the present study, it was concluded that ProT
facilitated the enhancement of dendritic branch and length regrowth
by increasing the expression of plasticity-associated proteins
in vitro. Additionally, ProT treatment and ProT
overexpression in transgenic mice resulted in less severe
neurological behavioral outcomes following stroke. ProT treatment
and ProT overexpression in transgenic mice exhibited increased
protein levels of neuroplasticity-associated proteins compared with
the control group. However, the detailed mechanism by which ProT
promotes neuron regrowth remains unclear. It has been reported that
deletion of the NLS from ProT decreases the expression of activated
MMP-2 and MMP-9 72 h after ischemia/reperfusion injury (18). Another study has indicated that MMP
enzymes also play essential roles in neuroplasticity and brain
remodeling during the subacute stage of stroke (20,39,40).
Further research is needed to elucidate these mechanisms.
Nevertheless, there are several potential
limitations in the present study. First, although it demonstrated
the potential positive effects of ProT on neuroplasticity following
ischemia-reperfusion injury, it primarily focused on the cellular
level and used mouse models. Further investigations are warranted
in other animal models and clinical trials to determine the
feasibility of applying ProT to human brain injuries. Second, the
in vitro study used immunofluorescence staining to represent
protein expression. For subsequent studies, alternative
biotechnological assessments should be considered. Third, the
present study predominantly concentrated on specific
neuroplasticity-related proteins such as BDNF, SNAP-25 and PSD-95.
However, neuroplasticity is a complex process regulated by various
molecular mechanisms. Future investigations could expand to explore
other related proteins and pathways to gain a more comprehensive
understanding of ProT on neuroplasticity. Finally, the results of
the present study were obtained in a controlled laboratory setting
and real clinical situations may be more complex. Therefore, future
research needs to consider the actual treatment conditions of
patients to determine the clinical applicability of ProT and its
potential limitations.
In conclusion, ProT is a promising neuroplasticity
agent for treating ischemic stroke models in vitro and in
vivo. The treatment of ProT in both gene transfection and
protein addition increased the expression of
neuroplasticity-associated proteins BDNF. In addition, ProT
injection stroke mice and ProT overexpression in transgenic mice
had a less severe outcome than the control group in the neurologic
test. Furthermore, the expression of neuroplasticity-associated
proteins PSD-95 and SNAP-25 increased at the penumbra area,
indicating the repair and growth of ischemic damage neurons. The
findings suggested that ProT has a potent neuroplastic effect in
ischemic stroke. However, the detailed mechanism remains unclear.
Further investigating ProT in developing novel therapy against
ischemic stroke may be worthwhile.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by a grant from the
Ministry of Science and Technology of Taiwan, R.O.C. (grant no.
MOST 109-2314-B-006-035).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
Conceptualization was performed by EL and CW. ST and
AL designed the experiments. The evaluation and confirmation of the
experimental methodologies employed in the study were validated by
ST. AL and SH analyzed and interpreted the data. EL AL and YC
performed the experiments. Resources were from CW and EL. SH was
responsible for data curation. AL and SH wrote the original draft.
EL and CW wrote, reviewed and edited the manuscript. YC created
figures, tables and visual representations of the data. CW and EL
supervised the study. Project administration was done by LC. EL and
AL confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed on experimental animals
were approved by the Subcommittee on Research Animal Care of the
National Cheng Kung University Medical Center (approval no.
109184).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Su F and Xu W: Enhancing brain plasticity
to promote stroke recovery. Front Neurol. 11:5540892020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barone FC and Feuerstein GZ: Inflammatory
mediators and stroke: New opportunities for novel therapeutics. J
Cereb Blood Flow Metab. 19:819–834. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clemens JA: Cerebral ischemia: Gene
activation, neuronal injury, and the protective role of
antioxidants. Free Radic Biol Med. 28:1526–1531. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gürsoy-Ozdemir Y, Can A and Dalkara T:
Reperfusion-induced oxidative/nitrative injury to neurovascular
unit after focal cerebral ischemia. Stroke. 35:1449–1453. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
del Zoppo GJ: Stroke and neurovascular
protection. N Engl J Med. 354:553–555. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Romero JR, Babikian VL, Katz DI and
Finklestein SP: Neuroprotection and stroke rehabilitation:
Modulation and enhancement of recovery. Behav Neurol. 17:17–24.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Legos JJ and Barone FC: Update on
pharmacological strategies for stroke: Prevention, acute
intervention and regeneration. Curr Opin Investig Drugs. 4:847–858.
2003.PubMed/NCBI
|
|
8
|
Bang OY, Kim EH, Cha JM and Moon GJ: Adult
stem cell therapy for stroke: Challenges and progress. J Stroke.
18:256–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen HY, Hung YC, Chen TY, Huang SY, Wang
YH, Lee WT, Wu TS and Lee EJ: Melatonin improves presynaptic
protein, SNAP-25, expression and dendritic spine density and
enhances functional and electrophysiological recovery following
transient focal cerebral ischemia in rats. J Pineal Res.
47:260–270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Freire M, Sarandeses CS, Covelo G and
Díaz-Jullien C: Phosphorylation of prothymosin α. An approach to
its biological significance. Vitam Horm. 102:73–99. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujita R, Ueda M, Fujiwara K and Ueda H:
Prothymosin-alpha plays a defensive role in retinal ischemia
through necrosis and apoptosis inhibition. Cell Death Differ.
16:349–358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Teixeira A, Yen B, Gusella GL, Thomas AG,
Mullen MP, Aberg J, Chen X, Hoshida Y, van Bakel H, Schadt E, et
al: Prothymosin α variants isolated from CD8+ T cells and
cervicovaginal fluid suppress HIV-1 replication through type I
interferon induction. J Infect Dis. 211:1467–1475. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fujita R and Ueda H: Prothymosin-alpha1
prevents necrosis and apoptosis following stroke. Cell Death
Differ. 14:1839–1842. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ueda H: Prothymosin alpha plays a key role
in cell death mode-switch, a new concept for neuroprotective
mechanisms in stroke. Naunyn Schmiedebergs Arch Pharmacol.
377:315–323. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karapetian RN, Evstafieva AG, Abaeva IS,
Chichkova NV, Filonov GS, Rubtsov YP, Sukhacheva EA, Melnikov SV,
Schneider U, Wanker EE and Vartapetian AB: Nuclear oncoprotein
prothymosin alpha is a partner of Keap1: Implications for
expression of oxidative stress-protecting genes. Mol Cell Biol.
25:1089–1099. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ueda H: Prothymosin alpha and cell death
mode switch, a novel target for the prevention of cerebral
ischemia-induced damage. Pharmacol Ther. 123:323–333. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Halder SK, Sugimoto J, Matsunaga H and
Ueda H: Therapeutic benefits of 9-amino acid peptide derived from
prothymosin alpha against ischemic damages. Peptides. 43:68–75.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang LC, Wu CL, Cheng YY and Tsai KJ:
Deletion of nuclear localizing signal attenuates proinflammatory
activity of prothymosin-alpha and enhances its neuroprotective
effect on transient ischemic stroke. Mol Neurobiol. 54:582–593.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Belayev L, Alonso OF, Busto R, Zhao W and
Ginsberg MD: Middle cerebral artery occlusion in the rat by
intraluminal suture. Neurological and pathological evaluation of an
improved model. Stroke. 27:1616–1623. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Juan WS, Huang SY, Chang CC, Hung YC, Lin
YW, Chen TY, Lee AH, Lee AC, Wu TS and Lee EJ: Melatonin improves
neuroplasticity by upregulating the growth-associated protein-43
(GAP-43) and NMDAR postsynaptic density-95 (PSD-95) proteins in
cultured neurons exposed to glutamate excitotoxicity and in rats
subjected to transient focal cerebral ischemia even during a
long-term recovery period. J Pineal Res. 56:213–223. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee EJ, Wu TS, Lee MY, Chen TY, Tsai YY,
Chuang JI and Chang GL: Delayed treatment with melatonin enhances
electrophysiological recovery following transient focal cerebral
ischemia in rats. J Pineal Res. 36:33–42. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li KJ, Shiau AL, Chiou YY, Yo YT and Wu
CL: Transgenic overexpression of prothymosin alpha induces
development of polycystic kidney disease. Kidney Int. 67:1710–1722.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Su BH, Tseng YL, Shieh GS, Chen YC, Shiang
YC, Wu P, Li KJ, Yen TH, Shiau AL and Wu CL: Prothymosin α
overexpression contributes to the development of pulmonary
emphysema. Nat Commun. 4:19062013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang SY, Chang CH, Hung HY, Lin YW and
Lee EJ: Neuroanatomical and electrophysiological recovery in the
contralateral intact cortex following transient focal cerebral
ischemia in rats. Neurol Res. 40:130–138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bederson JB, Pitts LH, Tsuji M, Nishimura
MC, Davis RL and Bartkowski H: Rat middle cerebral artery
occlusion: Evaluation of the model and development of a neurologic
examination. Stroke. 17:472–476. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Clark WM, Rinker LG, Lessov NS, Hazel K,
Hill JK, Stenzel-Poore M and Eckenstein F: Lack of interleukin-6
expression is not protective against focal central nervous system
ischemia. Stroke. 31:1715–1720. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caceres A, Banker G, Steward O, Binder L
and Payne M: MAP2 is localized to the dendrites of hippocampal
neurons which develop in culture. Brain Res. 315:314–318. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
DeGiosio RA, Grubisha MJ, MacDonald ML,
McKinney BC, Camacho CJ and Sweet RA: More than a marker: Potential
pathogenic functions of MAP2. Front Mol Neurosci. 15:9748902022.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tomasoni R, Repetto D, Morini R, Elia C,
Gardoni F, Di Luca M, Turco E, Defilippi P and Matteoli M: SNAP-25
regulates spine formation through postsynaptic binding to p140Cap.
Nat Commun. 4:21362013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoo KS, Lee K, Oh JY, Lee H, Park H, Park
YS and Kim HK: Postsynaptic density protein 95 (PSD-95) is
transported by KIF5 to dendritic regions. Mol Brain. 12:972019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Skopeliti M, Iconomidou VA, Derhovanessian
E, Pawelec G, Voelter W, Kalbacher H, Hamodrakas SJ and Tsitsilonis
OE: Prothymosin alpha immunoactive carboxyl-terminal peptide
TKKQKTDEDD stimulates lymphocyte reactions, induces dendritic cell
maturation and adopts a beta-sheet conformation in a
sequence-specific manner. Mol Immunol. 46:784–792. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ueda H: Non-vesicular release of alarmin
prothymosin α complex associated with annexin-2 flop-out. Cells.
12:15692023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ueda H, Fujita R, Yoshida A, Matsunaga H
and Ueda M: Identification of prothymosin-alpha1, the
necrosis-apoptosis switch molecule in cortical neuronal cultures. J
Cell Biol. 176:853–862. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Matsunaga H and Ueda H: Stress-induced
non-vesicular release of prothymosin-α initiated by an interaction
with S100A13, and its blockade by caspase-3 cleavage. Cell Death
Differ. 17:1760–1772. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fujita R, Yoshida A, Mizuno K and Ueda H:
Cell density-dependent death mode switch of cultured cortical
neurons under serum-free starvation stress. Cell Mol Neurobiol.
21:317–324. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ueda H: Prothymosin α plays role as a
brain guardian through Ecto-F1 ATPase-P2Y12
complex and TLR4/MD2. Cells. 12:4962023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang EJ and Reichardt LF: Neurotrophins:
Roles in neuronal development and function. Annu Rev Neurosci.
24:677–736. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tai SH, Huang SY, Chao LC, Lin YW, Huang
CC, Wu TS, Shan YS, Lee AH and Lee EJ: Lithium upregulates
growth-associated protein-43 (GAP-43) and postsynaptic density-95
(PSD-95) in cultured neurons exposed to oxygen-glucose deprivation
and improves electrophysiological outcomes in rats subjected to
transient focal cerebral ischemia following a long-term recovery
period. Neurol Res. 44:870–878. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Michaluk P, Wawrzyniak M, Alot P, Szczot
M, Wyrembek P, Mercik K, Medvedev N, Wilczek E, De Roo M,
Zuschratter W, et al: Influence of matrix metalloproteinase MMP-9
on dendritic spine morphology. J Cell Sci. 124:3369–3380. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Oliveira-Silva P, Jurgilas PB, Trindade P,
Campello-Costa P, Perales J, Savino W and Serfaty CA: Matrix
metalloproteinase-9 is involved in the development and plasticity
of retinotectal projections in rats. Neuroimmunomodulation.
14:144–149. 2007. View Article : Google Scholar : PubMed/NCBI
|