Introduction
There are >1.6 million new cases of breast cancer
diagnosed each year, making it the most common malignancy among
women worldwide (1). Breast cancer
metastasis is a life-threatening occurrence that constitutes the
primary cause of breast cancer-related deaths (2). Previous studies have aimed to
identify the molecular processes that cause breast cancer
metastasis and to search for new targets that can stop its
progression (3,4). The specific underlying mechanisms
driving cell migration and invasion remain largely unknown, despite
the crucial role they serve in the metastatic development of breast
cancer.
According to previous studies, long non-coding RNAs
(lncRNAs) serve an important role in human cancer (5,6).
Numerous types of human cancer cells exhibit dysregulated
expression of these ncRNA molecules, which are ~200 nucleotides in
length and are transcribed from the corresponding gene locus
(7,8). LncRNAs exert biological functions
through the epigenetic modulation of transcriptional and
post-transcriptional regulation in physiological and pathological
activities. Notably, lncRNAs are essential regulators in human
cancer and have a substantial association with tumor prognosis
(9,10).
The lncRNA differentiation antagonizing non-protein
coding RNA (DANCR) was initially reported to be associated
with osteoclastogenesis and osteoblast differentiation in
osteoporosis (11). It has also
been reported that DANCR is overexpressed in neoplastic
tissues and functions as an oncogenic lncRNA by facilitating the
development of tumors (12).
Previous research has reported that lncRNAs are involved in the
epigenetic modification of multiple diseases, including both
transcriptional and post-transcriptional regulation (5,6). In
the present study, the association between DANCR and E2F
transcription factor 1 (E2F1) expression is assessed.
However, to the best of our knowledge, there has been no research
to date reporting the mechanism underlying the relationship between
DANCR and E2F1 in cancer; therefore, the present
study aimed to elucidate the oncogenic function of DANCR in
cancer.
Materials and methods
The Cancer Genome Atlas (TCGA) data
access and analysis
DANCR and other gene expression profiles and
clinicopathological factors were downloaded from TCGA (Data Release
v21.0; December 10, 2019) (https://tcga-data.nci.nih.gov/). To analyze the
expression levels of DANCR, the data were dichotomized using
the median expression as the cut-off point, defining ‘high’ as
expression levels at or above the median and ‘low’ as expression
levels below the median. The log-rank test was used to assess
differences in survival between different groups of patients.
Human DNA methylation profiles were determined
experimentally using the Illumina Infinium Human Methylation 450
platform (Illumina, Inc.). β-values were obtained from Johns
Hopkins University and TCGA Genome Characterization Center of the
University of Southern California (California, USA). DNA
methylation and β-values of each array probe were measured across
all samples using the BeadStudio (version 3.2; Illumina, Inc.)
software. The β-values varied from 0–1, which represented the ratio
of the methylated bead type to the combined locus intensity.
Consequently, increased β-values indicated increased DNA
methylation levels, while decreased β-values indicated decreased
DNA methylation levels. These values were treated as continuous
variables. Additionally, information about histone modifications of
the DANCR locus was retrieved from the ENCODE database
(ENCODE 3 Nov 2018) (https://www.encodeproject.org/).
Transcription factor (TF) prediction
and guilt-by-association analysis
The JASPAR database (https://Jaspar.bind.Ku.dk/) was used to predict TFs
and the chromatin immunoprecipitation (ChIP)-seq data obtained from
ENCODE served as the basis for analysis.
According to previous investigations, the
guilt-by-association methodology was adopted to discern genes that
exhibited a positive correlation with DANCR (13–16).
The pairwise Pearson correlation was applied to measure the
correlation between DANCR expression and that of other
genes. As a result, solely the genes that exhibited a favorable
association with a correlation coefficient of R≥0.3 and attained
statistical significance at a level of P<0.05 were chosen.
Subsequently, DAVID Functional Annotation Bioinformatics Microarray
Analysis (https://david.ncifcrf.gov/tools.jsp) was performed
based on Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways
and Gene Ontology (GO) terms. P<0.05 and a gene count threshold
of 4 were used to identify significant GO terms and KEGG
pathways.
Human tissue specimens and ethics
statement
The present study acquired five paired samples of
breast cancer tissues and their respective adjacent noncancerous
tissues (mean patient age, 58±13 years) as well as information on
patient sex, age, patient number and molecular subtypes from The
First Hospital of Harbin Medical University (Harbin, China).
Patients with DANCR information were eligible for the
present study, which met the ethical standards of The Declaration
of Helsinki.
For RNA isolation, tissue samples were collected
from patients with breast cancer and healthy controls (n=5/group)
and immediately cryopreserved at −80°C after resection. Before
surgery, written informed consent was obtained from all patients.
Patients with a history of adjuvant chemotherapy, immunotherapy,
radiotherapy, tumor recurrence, bilateral tumor, metastatic disease
or other previous tumors were excluded from the present study. The
Ethics Committee of the First Affiliated Hospital of Harbin Medical
University (Harbin, China) granted ethical approval after obtaining
written informed consent from patients (ethical approval no.
202438).
Cell culture
MCF7 (cat. no. HTB22), MDA-MB-231 (cat. no.
CRM-HTB-26), MCF10A (cat. no. CRL-10317) and 293T (cat. no.
ACS-4500) cell lines were obtained from the American Type Culture
Collection. MCF7, MCF10A and 293T cell lines were maintained in
DMEM (Gibco; Thermo Fisher Scientific, Inc.) and MDA-MB-231 was
maintained in L15 (Gibco; Thermo Fisher Scientific, Inc.) for
culture purposes. Before the experiments, the cells were tested to
rule out the presence of Mycoplasma. Cells were cultured at
37°C in a humidified environment with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 5% CO2.
Cell transfection
Small interfering (si)RNAs targeting DANCR,
siDANCR negative control (NC; cat. no. siN0000001- 1–5),
siRNAs targeting E2F1, siE2F1 NC, miRNA mimics, miRNA
inhibitors, mimic NC (cat. no. miR1N0000001-1-5) and inhibitor NC
(cat. no. miR2N0000001-1-5) were purchased from Guangzhou RiboBio
Co, Ltd. The DANCR plasmid (pcDNA3.1-DANCR) was
constructed by Shanghai GeneChem Co., Ltd. for DANCR
overexpression. The experimental procedure involved seeding
1×105 cells into a 6-well plate and transfecting cells
when they reached a confluence of 70–80%. Transfection was
performed using JetPRIME (Polyplus-transfection SA) and different
masses of nucleic acids as follows: DANCR overexpression
plasmid (2,000 ng); miRNA inhibitor (50 nmol); siRNA (100 nmol);
and miRNA mimic (100 nmol) for 24 h at 37°C and 5% CO2.
The transfected cells were employed for subsequent experimentations
at 24 h after transfection. The transfection efficiency was
determined using reverse transcriptase-quantitative (RT-q) PCR and
western blotting analysis at 24 and 48 h after the transfection,
respectively. The sequences used were as follows: siDANCR#1
sense, 5′-CCAACUAUCCCUUCAGUUA-3′ and antisense,
5′-UAACUGAAGGGAUAGUUGG-3′; siDANCR#2 sense,
5′-GUGCUUCAUGUUCACCUUU-3′ and antisense, 5′-AAAGGUGAACAUGAAGCAC-3′;
siE2F1 sense, 5′-GGGAGAAGUCACGCUAUGA-3′ and antisense,
5′-UCAUAGCGUGACUUCUCCC-3′; hsa-miR-34c-5p mimic sense,
5′-AGGCAGUGUAGUUAGCUGAUUGC-3′ and antisense,
5′-GCAAUCAGCUAACUACACUGCCU-3′; and hsa-miR-34c-5p inhibitor,
5′-GCAAUCAGCUAACUACACUGCCU-3′.
Lentiviral infection
DANCR-specific short-hairpin (sh)RNA-targeting
coding sequences and non-targeting negative control sequences
(Shanghai GeneChem Co., Ltd.) were cloned into GV112 vectors
(Shanghai GeneChem Co., Ltd.) to produce DANCR knockdown vectors.
The shDANCR sequence used in the experiment was as follows:
5′-GTGCTTCATGTTCACCTTT-3′. The NC of shDANCR sequence used in the
experiment was as follows: 5′-TTCTCCGAACGTGTCACGT-3'. A 3rd
generation system was used to package the lentivirus. To produce
lentiviral particles, TransIT-LT1 (Mirus Bio, LLC) was used to
co-transfect the expression vector with the packaging plasmid
pHelper 1.0 (Shanghai GeneChem Co., Ltd.) and the envelope plasmid
pHelper 2.0 (Shanghai GeneChem Co., Ltd.) into 293T cells at 37°C
and 5% CO2 for 24 h. The supernatant was collected 48 h
post-transfection and concentrated using ultracentrifugation at
75,500 × g and 4°C for 90 min and resuspended in an appropriate
volume of OptiMEM (Gibco; Thermo Fisher Scientific, Inc.). MCF7 and
MDA-MB-231 cells were seeded in 6-well plates at a density of
1×105 cells/well and cultured in DMEM with 10% FBS at 5%
CO2 at 37°C and transfecting cells when they reached a
confluence of 70–80%. The cells were transfected with 10 µg
lentiviral plasmids, 7.5 µg packaging plasmid and 5 µg envelope
plasmid at a multiplicity of infection of 10 using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) the following day when the cells were ~70%
confluent. MCF7 and MDA-MB-231 cells were cultured at 37°C for 6 h
followed by replacement of the medium. The cells were incubated at
37°C for 48 h and subsequently treated with puromycin (selection, 2
µg/ml; maintenance, 1 µg/ml; Calbiochem; Merck KGaA) at 37°C and 5%
CO2 for 72 h to select transfected clones. Stable
knockdown of DANCR was confirmed by RT-qPCR.
Proliferation assay
Cell proliferation was assessed using the
5-ethynyl-2′-deoxyuridine (EdU) assay (Beyotime Institute of
Biotechnology), following the manufacturer's instructions. After a
4-h incubation with EdU, the cells were fixed in 4% formaldehyde
for a duration of 30 min at room temperature. Then, a glycine
solution (2 mg/ml) was applied for 5 min, followed by
permeabilization using 0.5% Triton X-100 for 10 min at room
temperature. A 1X reaction cocktail (from EdU kit) was then
administered and the nuclei were stained with DAPI for 10 min at
room temperature. The cells were then imaged under a fluorescence
inverted microscope.
Wound healing assay
A wound healing assay was conducted to examine MCF7
and MDA-MB-231 cell migration. Initially, 1×105 cells
were seeded in a 6-well plate and incubated until they reached
~100% confluence at 5% CO2 and 37°C. The cells were then
scratched using a pipette tip to create wounds and the
concentration of FBS in the cell culture medium was reduced from
10% to 1%. An image of the scratched area was immediately taken (0
h). The plates were then placed at 37°C and 5% CO2.
After overnight (16-h) incubation, another image was taken of the
same scratched area using light microscopy (magnification, ×10).
The width of the scratch at 24 h was calculated as a percentage of
the width at 0 h. The cell migration rate was analyzed by the
edge-finding method using Image J 1.8.0 (National Institutes of
Health).
Invasion assay
MCF7 (1×105) and MDA-MB-231
(1×105) cells were incubated in serum-free DMEM or
RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) medium using the
BRAND® Insert with Matrigel (cat. no. BR782806;
MilliporeSigma; Merck KGaA) precoated for 4 h and 37°C. Serum-free
medium was added to both upper chambers and medium containing 10%
FBS was added to the lower cell chamber. After 24 h of incubation
at 37°C, RPMI-1640 or DMEM containing 10% FBS was added to the
lower chamber and non-invading cells were removed with a cotton
swab. Cells that successfully traversed the membrane were
immobilized in 100% methanol for 30 min and subsequently dehydrated
by air drying. Furthermore, these cells were stained with a 0.5%
crystal violet solution at room temperature for 30 min and counted
manually after imaging using a light microscope.
RNA preparation and RT-qPCR
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract total RNA from cells
and tissue. Subsequently, PrimeScript RT Reagent Kit (Takara Bio,
Inc.) was used to reverse transcribe 0.5 µg total RNA. FastStart
Universal SYBR Green Master Mix (Roche Applied Science) and
gene-specific primers were used, with U6 or GAPDH
used as internal controls. The ABI 7500 Fast Real-time PCR
Detection System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) was used for RT-qPCR. To normalize the results, expression
levels relative to GAPDH and U6 were assessed using
the 2−ΔΔCq method (17). The miRNA stem-loop real-time PCR
kit (Guangzhou RiboBio Co., Ltd.) was used to quantify
miR-34c-5p and U6. The primer sequences were as
follows: DANCR forward (F), 5′-CGGAGGTGGATTCTGTTAGGGACA-3′
and reverse (R), 5′-AGAGGGCTTCGGTGTAGCAAGT-3′; E2F1 F,
5′-GGACCTGGAAACTGACCATCAG-3′ and R, 5′-CAGTGAGGTCTCATAGCGTGAC-3′;
U6 F, 5′-CTCGCTTCGGCAGCACAT-3′ and R, 5′-TTTGCGTGTCATCCTTGCG-3′;
and GAPDH F, 5′-GTCTCCTCTGACTTCAACAGCG-3′ and R,
5′-ACCACCCTGTTGCTGTAGCCAA-3′. The thermocycling conditions were as
follows: Initial denaturation at 95°C for 10 min; 40 cycles at 95°C
for 15 sec and 60°C for 30 sec.
Western blotting analysis
Protease and phosphatase inhibitors (Beyotime
Institute of Biotechnology) were added to the RIPA (Beyotime
Institute of Biotechnology) to lyse the MCF7 and MDA-MB-231 cells.
The protein concentrations were measured using a BCA Protein Assay
Kit (Beyotime Institute of Biotechnology). Subsequently, equivalent
quantities of protein (30 µg) was loaded per lane onto a 10% SDS
gel, resolved using SDS-PAGE and translocated onto nitrocellulose
membranes, followed by blocking with 5% milk in TBST (0.1% Tween)
at room temperature for 2 h. Primary antibodies targeting
E2F1 (1:1,000; cat. no. 3742; CST Biological Reagents Co.,
Ltd.) and β-actin (1:1,000; cat. no. TA09; OriGene
Technologies, Inc.) were incubated with the membranes at 4°C
overnight. Primary antibodies were then washed away with TBST (0.1%
Tween), incubated with IRDye 800CW-conjugated secondary antibodies
(1:10,000; cat. no. 92632210/92632211; LI-COR Biosciences) for 1 h
at room temperature and visualized using the Odyssey®
Imaging System and Image Studio (LI-COR Biosciences).
ChIP assay
The ChIP detection kit (cat. no. bes5001, Guangzhou
Bersinbio Co., Ltd.) was used with slight modifications to the
manufacturer's protocol (18).
Cells were crosslinked using 1% formaldehyde and the reaction was
terminated by adding glycine to a final concentration of 0.125 M.
The E2F1 antibodies (1:100) were used to immunoprecipitate
DNA from sonicated cell lysates, with IgG (BD Biosciences) as the
negative control. To detect the binding sites of E2F1, the
immunoprecipitated DNA was amplified by RT-qPCR, as aforementioned.
Subsequently, 3% agarose gel electrophoresis was used to analyze
the amplified fragments. Chromatin at a concentration of 10% was
used as a control input before immunoprecipitation. The primer
sequences were as follows: DANCR site 1 F,
5′-CGGGGATTGGTAGGTAGCC-3′ and R, 5′-CTGGAGAGGTCGGGTAGC-3′;
DANCR site 2 F, 5′-GGTGTCCCCACGAGCTTTG-3′ and R,
5′-AAATTGTTACGGTGCCCAGAC-3′; and DANCR site 3 F,
5′-CGCCCCGCTCAGGATCTTC-3′ and R, 5′-GCACTCACCGCGCAACTC-3′.
Dual-luciferase reporter assay
To produce reporter vectors with binding sites for
miRNA, the complete 3′ untranslated regions (UTRs) of human
DANCR and E2F1 were cloned. The full-length 3′UTR
fragments from DANCR and E2F1 were amplified by PCR
as aforementioned and inserted into the reporter luciferase
expression vector pmiR-RB with Not1-Xho1 sites. 293T
cells were cultured in DMEM containing 100 µg/ml
penicillin/streptomycin and 10% FBS and miR-34c-5p mimic was
used for the luciferase assay. 293T cells were transfected at
40–50% confluence using JetPRIME (Polyplus-transfection SA). For
transfection, 20 mol/l hsa-miR-34c-5p mimic or NC, alongside
0.5 mg DANCR or E2F1 plasmid (Guangzhou RiboBio Co.,
Ltd.), was utilized. The results of the luciferase activity were
determined after 48 h using a luminometer (GloMax™ 20/20; Promega
Corporation) and a dual-luciferase reporter assay kit (Promega
Corporation). The direct oligomer synthesis technique was employed
to produce a nucleotide-substitution mutation in the 3′UTRs of
DANCR and E2F1. Normalization of the firefly
luciferase results was performed by comparison with Renilla
luciferase activity.
Prediction of lncRNA localization and
fluorescence in situ hybridization (FISH)
The lncRNA subcellular localization predictor
database (lncLocator; http://www.csbio.sjtu.edu.cn/bioinf/lncLocator/)
was used to analyze the subcellular localization of DANCR.
FISH was performed using a RNA-FISH kit (Bes1002; Guangzhou
BersinBio Biotechnology Co., Ltd.) as described in the
manufacturer's instructions (19).
The lncRNA probe for DANCR was obtained from Guangzhou
Bersinbio Biotechnology Co., Ltd. MCF7 and MDA-MB-231 cells were
harvested and fixed in 4% formaldehyde. After denaturation, probes
were hybridized with cells for 20 h at 42°C. A DAPI stain was then
applied to the nuclei and cells were observed using a fluorescence
microscope (LSM800; Carl Zeiss AG).
Statistical analysis
All statistical tests were conducted utilizing R
(version 3.5.3; RStudio, Inc.). The data were presented as the mean
± standard deviation from three independent replicates. The
expression of DANCR in cancer tissues compared with normal tissues
were analyzed using a paired t-test. The unpaired Student's t-test
was used to compare variances between two groups. For evaluating
statistical differences among multiple groups, a one-way ANOVA was
performed, followed by a Tukey's Honestly Significant Difference
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Upregulation of DANCR correlates with
poor prognosis in breast cancer
TCGA data were downloaded and analyzed to evaluate
the expression levels of DANCR in human cancer. These
results demonstrated that the expression levels of DANCR
were upregulated in pan-cancer samples when compared with normal
samples (Fig. 1A; Table SI). Additionally, it was
demonstrated that the expression levels of DANCR were
notably elevated in various types of malignancy, including bladder
urothelial carcinoma (BLCA), cholangiocarcinoma, colon
adenocarcinoma, liver hepatocellular carcinoma (LIHC), lung
adenocarcinoma, lung squamous cell carcinoma, prostate
adenocarcinoma (PRAD), rectum adenocarcinoma, uterine corpus
endometrial carcinoma (UCEC) and breast invasive carcinoma (BRCA),
when compared with their noncancerous tissue counterparts (Fig. 1B; Table SI). To verify this observation,
RT-qPCR analysis of breast cancer tissues was conducted and yielded
results consistent with the aforementioned findings (Fig. 1C; Table SII).
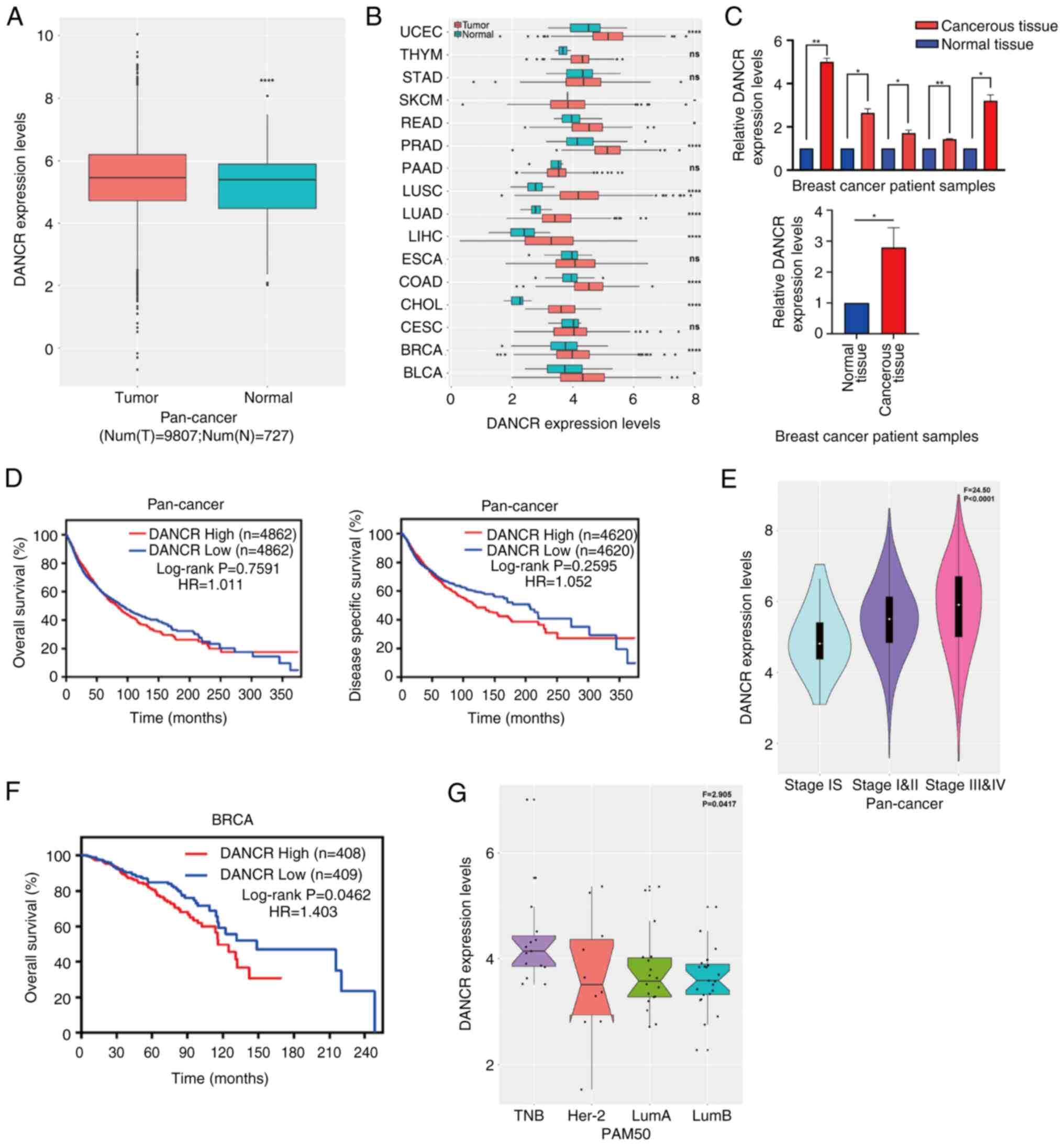 | Figure 1.Expression of DANCR is
upregulated and correlated with a poor prognosis. (A) Expression
level of DANCR was significantly higher in pan-cancer
tissues when compared with normal tissues. (B) The Cancer Genome
Atlas results demonstrated that the expression level of
DANCR was significantly higher in UCEC, SKCM, READ, PRAD,
LUSC, LUAD, LIHC, ESCA, COAD, CHOL, BRCA and BLCA and markedly
higher in THYM, STAD, PAAD and CESC compared with normal tissues.
(C) Expression level of DANCR was higher in breast cancer
tissues compared with normal tissue (n=5/group). Higher
DANCR expression level had a significant positive
association with (D) poor overall survival, a marked positive
association with poor disease-specific survival and a significant
positive association with (E) advanced stage in pan-cancer. (F)
Higher expression level of DANCR was significantly related
to poorer overall survival in BRCA. (G) DANCR expression was
upregulated in triple-negative and HER-2 enriched subtypes.
*P<0.05; **P<0.01; *****P<0.0001. DANCR, differentiation
antagonizing non-protein coding RNA; UCEC, uterine corpus
endometrial carcinoma; THYM, thymoma; STAD, stomach adenocarcinoma;
SKCM, skin cutaneous melanoma; READ, rectum adenocarcinoma; PRAD,
prostate adenocarcinoma; PAAD, pancreatic adenocarcinoma; LUSC,
lung squamous cell carcinoma; LUAD, lung adenocarcinoma; LIHC,
liver hepatocellular carcinoma; ESCA, esophageal carcinoma; COAD,
colon adenocarcinoma; CHOL, cholangiocarcinoma; CESC, cervical
squamous cell carcinoma and endocervical adenocarcinoma; BRCA,
breast invasive carcinoma; BLCA, bladder urothelial carcinoma; TNB,
triple-negative breast cancer; Her-2, human epidermal growth factor
receptor 2; LumA, luminal A; LumB, luminal B; HR, hazard ratio; PAM
50, PAM 50 molecular subtype; num(T), number of tumor tissues;
num(N), number of normal tissues. |
The relationship between DANCR expression
levels and patient survival status was further investigated using a
log-rank test and Kaplan-Meier analysis based on pan-cancer
samples. The results demonstrated that, compared with patients with
low DANCR expression, patients with high expression had
markedly lower disease-specific survival and overall survival (OS)
(Fig. 1D). Furthermore,
DANCR expression was significantly higher in advanced TNM
stages than in stage I (Fig. 1E).
Patients with high DANCR expression had significantly lower
OS compared with patients with low DANCR expression, especially in
patients with breast cancer (Fig.
1F). Furthermore, compared with other subtypes of breast
cancer, the expression of DANCR was significantly
upregulated in the triple negative subtype (Fig. 1G). In addition, various cancer
types present in the TCGA data cohort were examined. The analysis
demonstrated a notable correlation between elevated levels of
DANCR in BRCA, kidney renal clear cell carcinoma, LIHC,
sarcoma and skin cutaneous melanoma and a decline in OS rates
(Figs. 1F and S1). However, the expression of
DANCR in Head and Neck squamous cell carcinoma (HNSC),
Kidney Chromophobe, Pheochromocytoma and Paraganglioma, Stomach
adenocarcinoma, Thyroid carcinoma (THCA) and UCEC was not linked to
OS (Fig. S1).
DANCR promoted tumor growth
To elucidate the role of DANCR in the
phenotype of breast cancer cells, loss-of-function experiments were
conducted on MCF7 and MDA-MB-231 breast cancer cells. After
transfection with siRNA, the expression of DANCR in these
cells was significantly decreased compared with control cells
(Fig. 2A). In addition, after
stable silencing of DANCR, the EdU assay showed a decrease
in proliferation compared with control cells, whereas the opposite
results were determined after overexpression of DANCR
(Fig. 2B). These findings
suggested that DANCR knockdown can inhibit breast cancer
cell proliferation, implying a potential oncogenic role in
tumorigenesis.
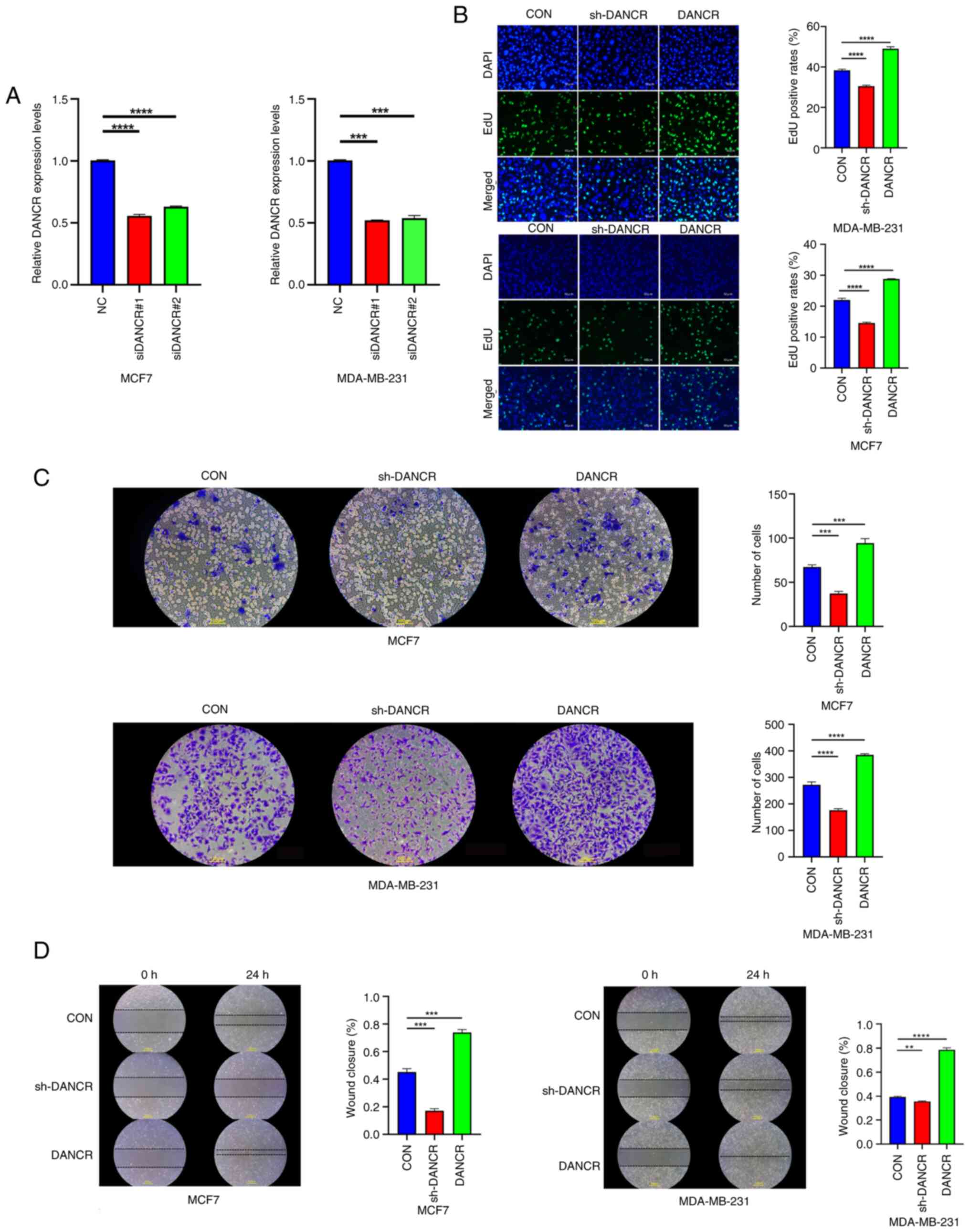 | Figure 2.DANCR promoted tumor growth.
(A) Transfection efficacy of siDANCR in MCF7 and MDA-MB-231
cells. (B) EdU assay results demonstrated that silencing
DANCR inhibited tumor cell proliferation, while
overexpression of DANCR promoted tumor cell proliferation.
Magnification, ×100. (C) Transwell invasion assay results showed
that knockdown of DANCR inhibited the invasion ability of
tumor cells, while overexpression of DANCR promoted tumor
cell invasion ability. Magnification, ×200. (D) Wound healing assay
results demonstrated that silencing DANCR suppressed tumor
cell migration, while overexpression of DANCR promoted tumor
cell migration. Magnification, ×100. Data were presented as the
mean ± standard deviation (n=3). **P<0.01; ***P<0.001;
*****P<0.0001. DANCR, differentiation antagonizing non-protein
coding RNA; si, small interfering RNA; sh, short hairpin RNA; NC,
negative control; CON, blank control group; EdU,
5-ethynyl-2′-deoxyuridine. |
Subsequently, an evaluation was conducted to
determine the impact of DANCR on breast cancer cell
migration and invasion through the implementation of wound healing
and Transwell assays. The findings demonstrated that the
introduction of shDANCR into MCF7 and MDA-MB-231 cells via
stable transfection significantly inhibited both invasive and
migratory cells compared with control cells (Fig. 2C and D). By contrast, the
overexpression of DANCR significantly enhanced the invasion
(Fig. 2C) and migration (Fig. 2D) of breast cancer cells in
vitro compared with controls.
DANCR modulated breast cancer cell
progression by regulating miR-34c-5p
The typical regulatory role of lncRNAs is to host
miRNAs and serve as a miRNA ‘sponge’. In the present study,
LncLocator and FISH were used to predict and verify the subcellular
localization of DANCR. The findings demonstrated that the
cytoplasm of both the MCF7 and MDA-MB-231 cell lines contained
DANCR (Fig. 3A and B).
Moreover, bioinformatics analysis demonstrated that
miR-34c-5p contained a sequence matching DANCR at the
3′UTR (Fig. 3C). According to the
luciferase assay results, miR-34c-5p mimics caused a
significant decrease in luciferase activity compared with negative
controls, which indicated a strong affinity between DANCR
3′UTR and miR-34c (Fig.
3C). Compared with MCF10A cells, the expression of
miR-34c-5p was significantly diminished in breast cancer
cell lines (Fig. 3D).
Additionally, the upregulation of DANCR resulted in the
downregulation of miR-34c-5p (Fig. S3A). The present study indicated
that miR-34c-5p was negatively correlated with DANCR
and targeted the 3′UTR of DANCR.
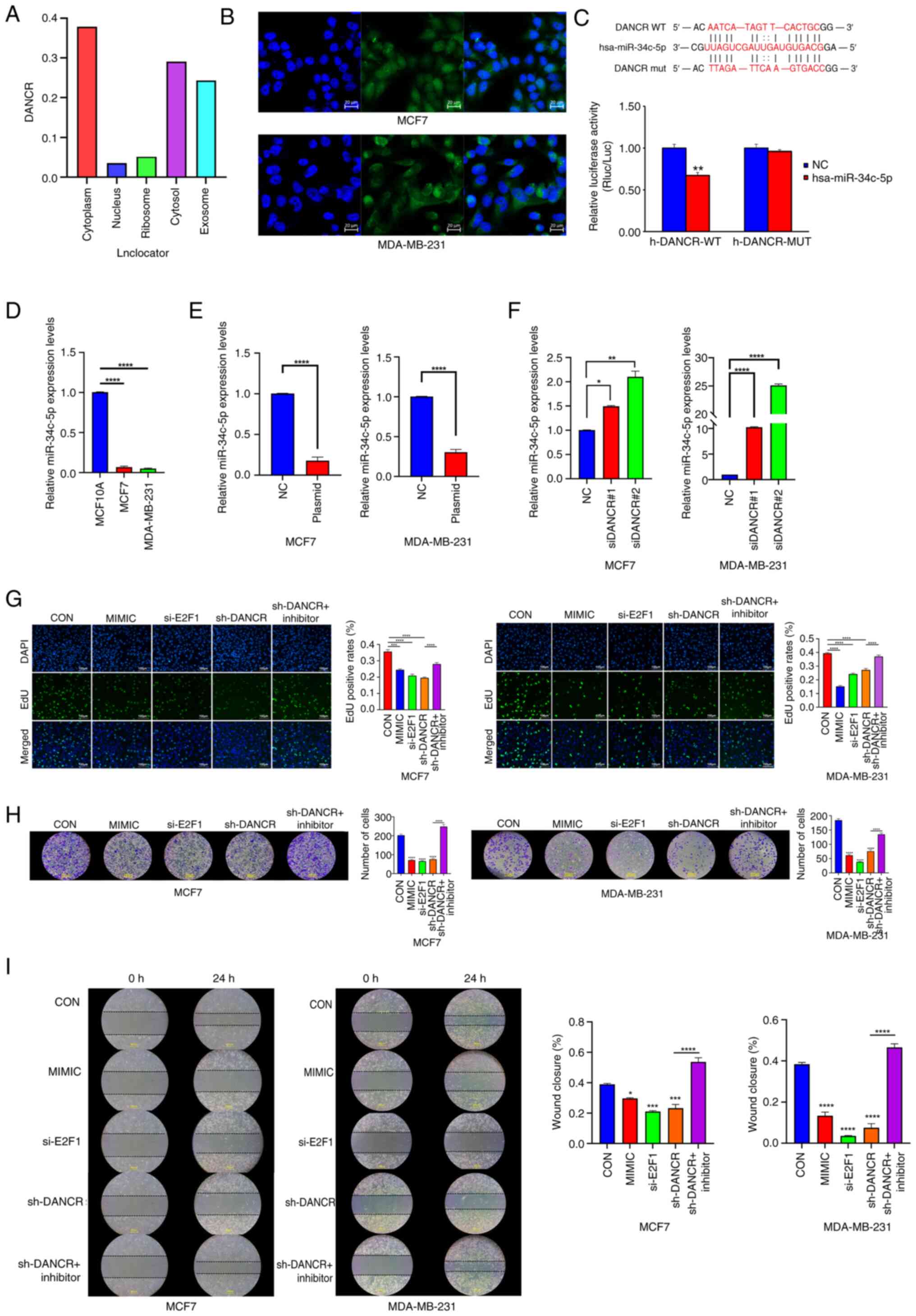 | Figure 3.DANCR modulated breast cancer
cells progression via regulating miR-34c-5p. (A) Lnclocator
predicted that DANCR was mainly located in the MCF7
cytoplasm. (B) Distribution of DANCR (green) in MCF7 and
MDA-MB-231 cells as detected by fluorescence in situ
hybridization assay (nuclei stained blue with DAPI). Magnification,
×400. (C) A luciferase reporter assay was used to assess the
interactions between miR-34c-5p and its binding sites or
mutated binding sites in the 3′ untranslated regions of
DANCR in 293T cells. (D) Expression levels of
miR-34c-5p were higher in breast cancer cell lines compared
with the MCF10A cell line. (E) Overexpression of DANCR
upregulated the expression level of miR-34c-5p. (F)
Knockdown of DANCR downregulated the expression level of
miR-34c-5p. (G) The proliferation capacity of
miR-34c-5p mimic, E2F1 siRNA, DANCR shRNA and
DANCR shRNA + miR-34c inhibitor transfected MCF7 and
MDA-MB-231 cells were assessed by EdU assay. Magnification, ×100.
(H) The invasion capacity of miR-34c-5p mimic, E2F1
siRNA, DANCR shRNA and DANCR shRNA plus miR-34c
inhibitor transfected MCF7 and MDA-MB-231 cells were assessed by
Transwell assay. Magnification, ×200. (I) The migratory capacity of
miR-34c-5p mimic, E2F1 siRNA, DANCR shRNA and
DANCR shRNA + miR-34c inhibitor transfected MCF7 and
MDA-MB-231 cells were assessed by wound healing assay.
Magnification, ×100. Data were presented as the mean ± standard
deviation (n=3). *P<0.05; **P<0.01; ***P<0.001;
*****P<0.0001. DANCR, differentiation antagonizing non-protein
coding RNA; E2F1, E2F transcription factor 1; WT, wild type; mut,
mutant; miR, microRNA; si, small interfering RNA; sh, short hairpin
RNA; NC, negative control; CON, blank control group. |
Given that DANCR has previously been
identified as an oncogenic lncRNA in breast cancer (20), how it interacts with
miR-34c-5p to control pathological course was studied.
Loss-of-function and rescue tests were conducted in MCF7 and
MDA-MB-231 cells. According to these experiments, siDANCR
transfection of MCF7 and MDA-MB-231 cells significantly enhanced
the expression of miR-34c-5p compared with negative controls
(Fig. 3E and F). In addition, EdU
analysis demonstrated that, compared with in the shDANCR
transfection group, shDANCR co-transfection with the
miR-34c-5p inhibitor significantly reversed the reduced
proliferation of MCF7 and MDA-MB-231 breast cancer cells (Fig. 3G). Furthermore, DANCR
knockdown significantly reduced cell migration and invasion rates
compared with controls and this effect was significantly reversed
by the miR-34c-5p inhibitor (Fig. 3H and I). Overall, these findings
suggested a potential antagonistic relationship between
DANCR and miR-34c-5p in breast cancer, whereby
miR-34c-5p inhibition may help to lessen the inhibitory
effect of DANCR knockdown on cell proliferation, migration
and invasion.
E2F1 acted as the target of
miR-34c-5p
DANCR expression was shown to be
significantly positively correlated with E2F1 gene
expression by bioinformatics analysis (Fig. 4A) and downstream gene pathways,
such as E2F1, were found to be significantly related to cell
cycle and cell division (Fig. 4B).
Additionally, it was expected that the 3′UTR of E2F1
contained binding sites complementary to miR-34c-5p
(Fig. 4C). The binding of
miR-34c-5p to the 3′UTR of E2F1 was confirmed using a
luciferase reporter assay (Fig.
4C). These results showed that transfection with the
miR-34c inhibitor significantly increased E2F1
expression in MCF7 and MDA-MB-231 cells compared with negative
controls (Fig. 4D). In addition,
when knocking down DANCR, both E2F1 mRNA and protein
expression levels were significantly decreased compared with
controls (Fig. 4E and F). However,
this effect was reversed when cells were co-transfected with
shDANCR and miR-34c-5p inhibitor (Fig. 4E and F).
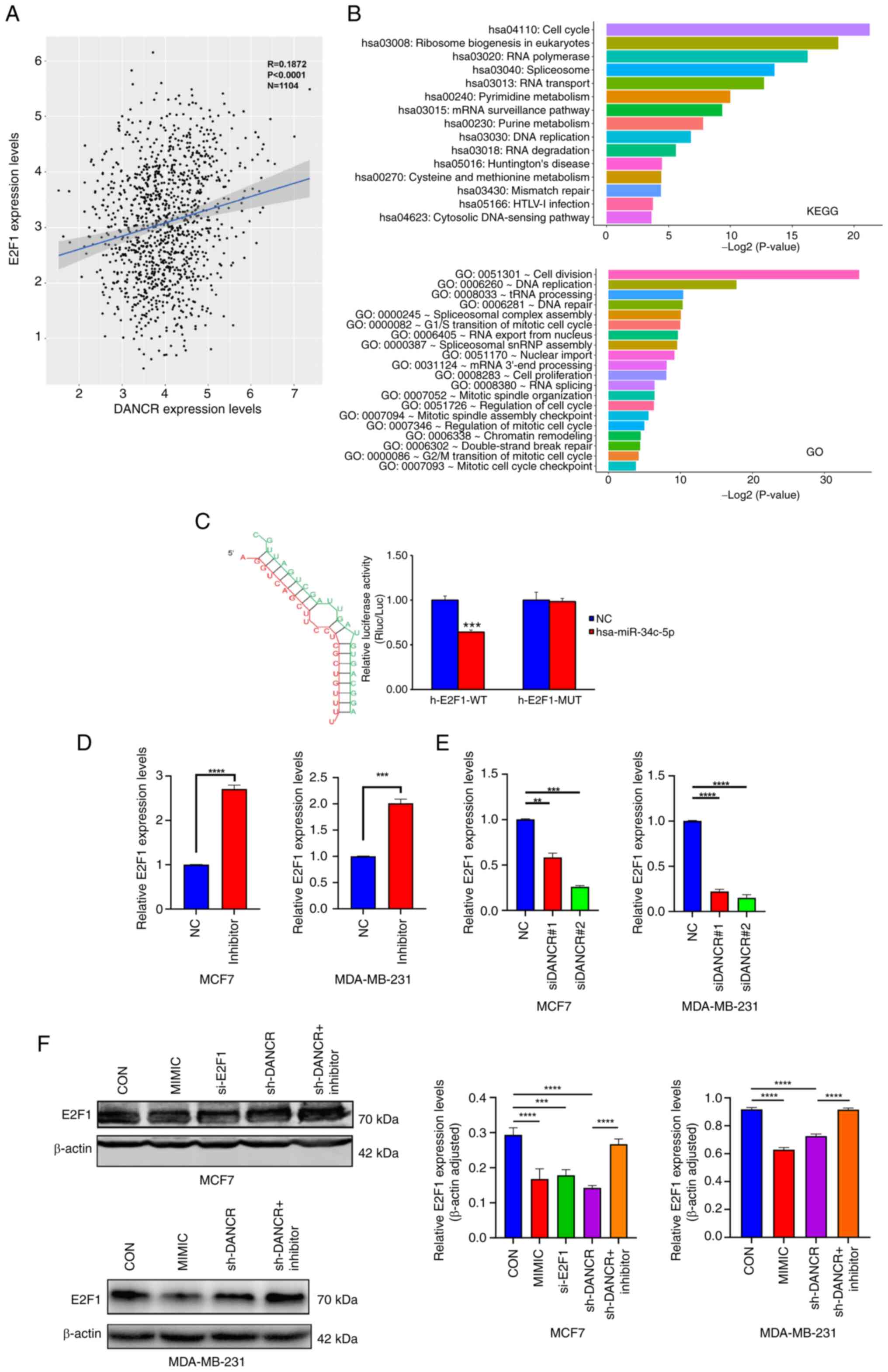 | Figure 4.E2F1 acted as the target of
miR-34c-5p. (A) The Cancer Genome Atlas data showed that
E2F1 positively correlated with DANCR in breast
cancer. (B) GO and KEGG enrichment analysis of key genes of
DANCR downstream pathway. (C) A luciferase reporter assay
was used to assess the interactions between miR-34c-5p and
its binding sites or mutated binding sites in the 3′ untranslated
regions of E2F1 in 293T cells. (D) Downregulated
miR-34c-5p promoted E2F1 expression. (E) Knockdown of
DANCR upregulated the expression levels of E2F1. (F)
E2F1 expression changes cells transfected with in miR-34c
mimic, DANCR shRNA and DANCR shRNA + miR-34c
inhibitor as detected by Western blotting. Data were presented as
the mean ± standard deviation (n=3). **P<0.01; ***P<0.001;
*****P<0.0001. DANCR, differentiation antagonizing non-protein
coding RNA; E2F1, E2F transcription factor 1; WT, wild type; MUT,
mutant; miR, microRNA; si, small interfering RNA; sh, short hairpin
RNA; KEGG, Kyoto Encyclopedia of Genes and Genomes; GO, Gene
Ontology; NC, negative control; CON, blank control group. |
These findings showed a negative association between
the expression of miR-34c-5p and the expression of
E2F1 and DANCR. There was also a strong positive
association between E2F1 expression and DANCR
expression. Consequently, this study potentially elucidated a
regulatory pathways of DANCR/miR-34c-5p/E2F1
in breast cancer.
E2F1 regulated the DANCR promoter
region and activated its expression
The present study proposed that TF binding to the
promoter region of the lncRNA DANCR enhanced its expression.
To validate this, the online database JASPAR was used to predict TF
binding to the DANCR promoter region (Fig. 5A). In addition, GO and KEGG
analyses were performed to identify the basic functions and
pathways of DANCR promoter region-binding TFs via DAVID
Functional Annotation Bioinformatics Microarray Analysis. These
results suggested that the transcription factor E2F1, which
may serve an important role in breast cancer, could bind to the
functional region of the DANCR promoter (Fig. 5B). E2F1 may bind to the
promoter region of DANCR in MCF7 and MDA-MB-231 breast
cancer cell lines. This theory was supported by ChIP-seq data for
E2F1 from ENCODE (Fig. 5C).
Additionally, the ChIP data demonstrated that the DANCR
promoter had a far higher affinity for E2F1 than for IgG
(Fig. 5D).
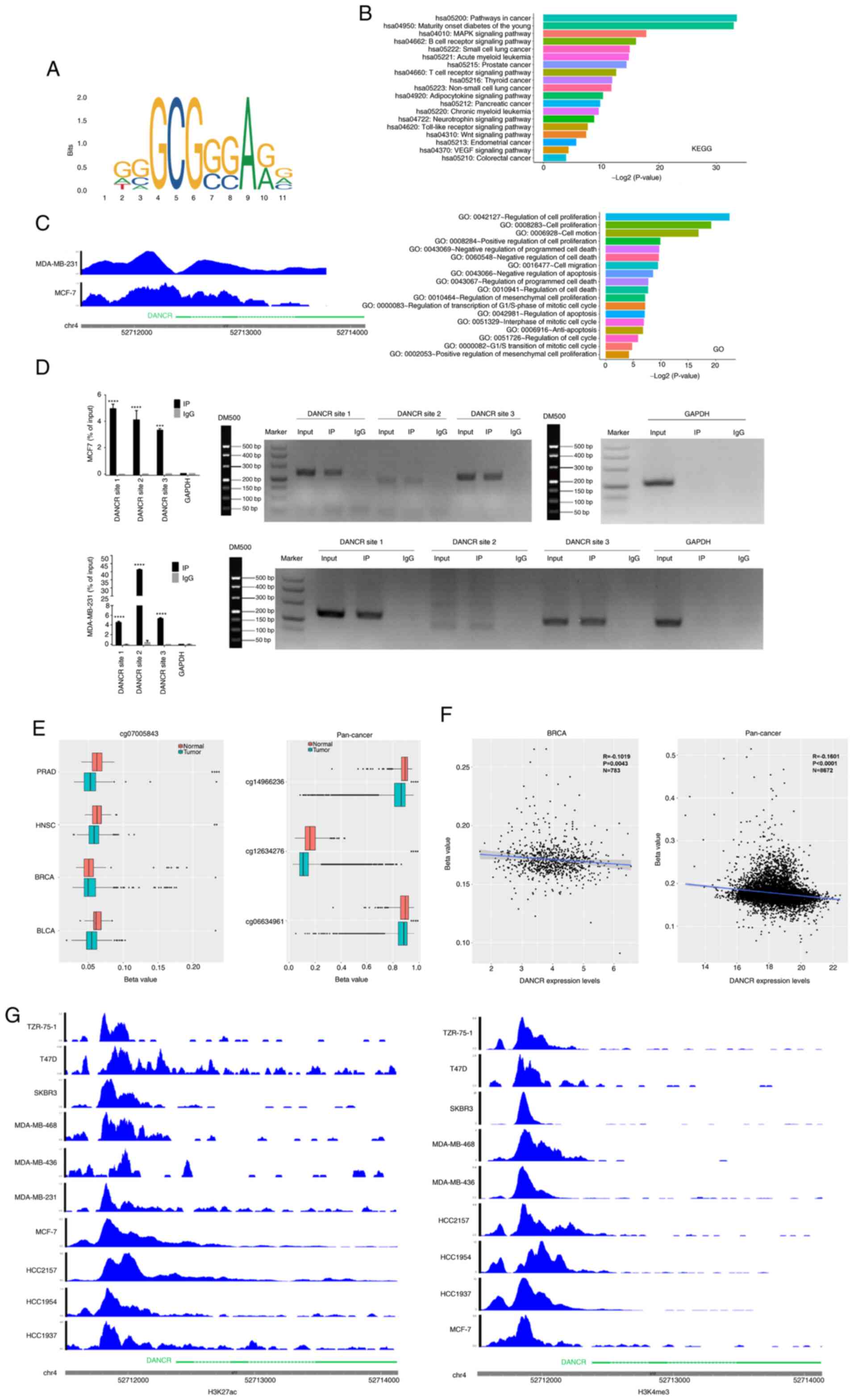 | Figure 5.E2F1 regulated the
DANCR promoter region and activates its expression. (A)
JASPAR predicted that E2F1 could bind with the promoter of
DANCR. (B) GO/KEGG enrichment analysis of transcription
factors predicted to bind to DANCR. (C) ENCODE validated the
binding region of E2F1 to the DANCR promoter. (D)
Chromatin immunoprecipitation assay showed the binding of
E2F1 and DANCR promoter region in MCF7 and MDA-MB-231
cells. Data were presented as the mean ± standard deviation (n=3).
(E) Hypomethylation occurred at the DANCR promoter locus in
breast and pan-cancer tissues. (F) The methylation level at the
DANCR promoter site was inversely proportional to
DANCR expression in breast and pan-cancer samples. (G)
H3K27ac and H3K4me3 were significantly enriched at the DANCR
locus in breast cancer cell lines. *P<0.05; **P<0.01;
***P<0.001; ****P<0.0001. DANCR, differentiation antagonizing
non-protein coding RNA; E2F1, E2F transcription factor 1; KEGG,
Kyoto Encyclopedia of Genes and Genomes; GO, Gene Ontology; PRAD,
prostate adenocarcinoma; HNSC, head and neck squamous cell
carcinoma; BRCA, breast invasive carcinoma; BLCA, bladder
urothelial carcinoma; bp, base pairs; IP, immunoprecipitation
group; DM500, DNA marker. |
The pathway by which DANCR is upregulated in
human cancer was also investigated and it was predicted that cancer
samples would have less DNA methylation enrichment at the
DANCR promoter locus compared with normal samples. In
contrast to non-cancer tissues, these findings demonstrated that
the DANCR promoter region was significantly hypomethylated
in pan-cancer, BRCA, BLCA, PRAD and HNSC samples (Figs. 5E and S2; Table
SIII). Moreover, in pan-cancer samples and most types of
malignancies tested, the degree of DANCR promoter region
methylation was significantly inversely correlated with
DANCR expression (Figs. 5F
and S2; Tables SIII and SIV).
Additionally, the role of H3K27ac and H3K4me3
modifications in upregulating DANCR expression in human
cancer was investigated. These results demonstrated that both
modifications were significantly enriched at the DANCR locus
in breast cancer cell lines (Fig.
5G), which indicated their potential contribution to the
upregulation of DANCR in breast cancer. Taken together,
these findings suggested that TF binding, hypomethylation and
H3K27ac and H3K4me3 modifications may collectively contribute to
the upregulation of DANCR in breast cancer.
Discussion
In human esophageal squamous cell carcinoma and
osteosarcoma, the lncRNA DANCR serves a crucial role,
according to previously published studies (21,22).
DANCR upregulation has been identified as a prognostic
biomarker in both pancreatic and colorectal cancer (23,24).
In our previous research, we reported that DANCR serves a
tumor-promoting role both in vivo and in vitro in
breast cancer (20).
Mechanistically, DANCR targets miR-216a-5p, thereby
regulating the expression of proteins such as Nanog, SOX2 and OCT4
to promote breast cancer progression. In the present study, the
results demonstrated that partial inhibition of DANCR
significantly reduced the proliferation, migration and invasion of
MDA-MB-231 and MCF7 cells, which may have an impact on the tumor
cell cycle.
Previous research has indicated that lncRNAs are
involved in the epigenetic modification of multiple diseases,
including via both transcriptional and post-transcriptional
regulation (25,26). Notably, it has been reported that
lncRNAs serve a crucial role in the etiology, proliferation,
metastasis and recurrence of tumors (27–30).
Currently, the most well-known mechanism by which lncRNAs
participate in disease etiology is by serving as ceRNAs for miRNAs
(31,32). In the present study, through
computational approaches, it was determined that miR-34c-5p
bound to the 3′UTR of DANCR. Furthermore, functional
experiments showed the ability of miR-34c-5p to inhibit the
oncogenic effects of DANCR on breast cancer cells, which
suggests that the regulatory effect of DANCR on these cells
may be mediated by its sequestration of miR-34c-5p. The
regulation of gene expression by miRNAs occurs through binding to
particular locations in the 3′UTR of target mRNAs and lncRNA-miRNA
crosstalk is essential for the indirect control of gene expression
(33–36). When lncRNAs are in the cytoplasm,
they participate in modulating mRNA stability, regulating mRNA
translation, serving as ceRNAs and functioning as precursors of
miRNAs (31). In the present
study, the subcellular grading confirmed that DANCR is mainly
located in the cytoplasm of breast cancer cell lines. Furthermore,
the inhibitory effect of shDANCR on E2F1 could be reversed
by miR-34c-5p inhibitor, which suggests that DANCR could
potentially enhance the expression of E2F1 by absorbing
miR-34c-5p as ceRNA in cancer. Additionally, the present study
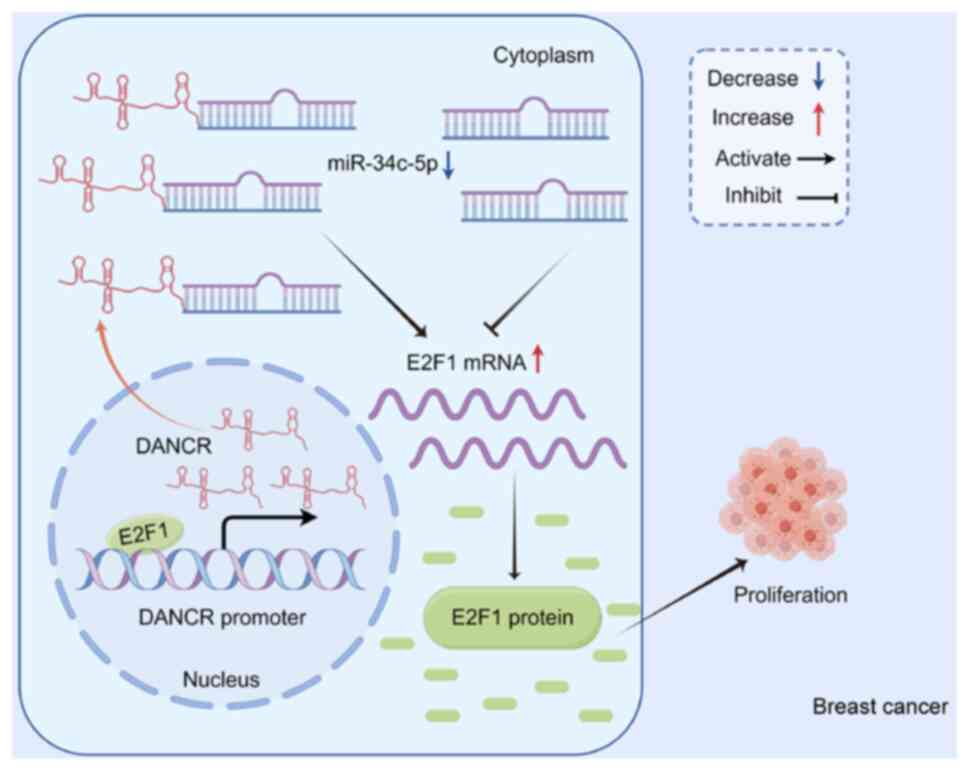
demonstrated the involvement of the
DANCR/miR-34c-5p/E2F1 feedback loop in the
occurrence and development of breast cancer (Fig. 6). By modulating the expression of
the oncogene E2F1, DANCR may serve as a potential
therapeutic target for breast cancer. The results of the present
study suggested that manipulating the expression of DANCR,
as a ceRNA, may competitively regulate the expression of
E2F1, thereby regulating the biological function of breast
cancer cells. However, in the future, additional in vivo
experiments and clinical trials are necessary to clarify the
potential of DANCR as a therapeutic target for breast
cancer.
The results of the present study showed that
knocking down E2F1 reduced cell migration and invasion.
Therefore, E2F1, as a protein regulated by DANCR, may
be involved in the G1/S transition of the mitochondrial
cell cycle, regulation of the cell cycle, regulation of the
mitochondrial cell cycle and G2/M transition of the
mitochondrial cell cycle. E2F1, as a TF that binds to the
DANCR promoter region, may be involved in the regulation of
transcription of G1/S phase of the mitotic cell cycle,
interphase of the mitotic cell cycle, cell cycle regulation,
G1/S transition of the mitotic cell cycle and the
G1 phase of the mitotic cell cycle.
Based on the analysis of the binding region between
the TF E2F1 and DANCR, further analysis was performed
on biological behaviors that E2F1 may be involved in by
activating DANCR transcription. These results demonstrated
that the regulation of DANCR by the TF E2F1 may be
involved in various types of cancer, such as small cell lung
cancer, prostate cancer and thyroid cancer, as well as signaling
pathways, such as the MAPK signaling pathway, the Toll-like
receptor pathway and the Wnt signaling pathway. Furthermore,
E2F1 may be involved in regulating cell proliferation, cell
death and the cell cycle. Therefore, it could potentially be
hypothesized that the TF E2F1 binds to the DANCR
promoter functional region and serves an important role in
cancer.
Our previous study reported that miR-34c-5p
can mediate liver and lung metastasis of breast cancer by
regulating G protein-coupled receptor kinase interacting protein 1
(GIT1) (37). In addition, GIT1
can mediate the development of estrogen receptor-negative breast
cancer by regulating the Notch pathway (38). Another study reported that UBTF
promotes melanoma cell proliferation and cell cycle progression by
promoting GIT1 transcription, thereby activating MEK1/2-ERK1/2
signaling pathways (39).
Therefore, E2F1 and miR-34c-5p may regulate the
progression of breast cancer by affecting the cell cycle.
Further validation of the present findings are
required to address a number of limitations of the present study.
Firstly, it is imperative to note that the assessment of
DANCR expression levels in a limited sample size of breast
cancer specimens requires further investigation with a more
extensive sample size to establish a definitive correlation between
the expression of DANCR/miR-34c/E2F1 and
clinical parameters. Furthermore, it is necessary to confirm the
protein concentration of E2F1 and the expression level of
DANCR across a wider range of cell models and in vivo
studies. Finally, the genes of interest identified in the present
study via bioinformatics analysis, which may have the potential to
become significant contributors to the development of breast
cancer, warrant further investigation to validate the results.
Nevertheless, the present study demonstrated the
potential role of DANCR in breast cancer progression. The
formation of the DANCR/miR-34c/E2F1 feedback
loop, facilitated by the binding of E2F1 to the DANCR
promoter region, may provide a promising avenue for the precise
treatment of breast cancer in the future.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the First Affiliated Hospital of
Harbin Medical University Fund for Distinguished Young Medical
Scholars (grant no. 2021J17), the Beijing Medical Award Foundation
(grant no. YXJL-2021-0302-0287), and the Postgraduate Research and
Practice Innovation Program of Harbin Medical University (grant no.
YJSCX2023-63HYD).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
SY and WT confirm the authenticity of all the raw
data. SY and WT designed and directed experimental studies. SY, LT,
JD, LJ, PX, WZ and WT performed sequencing data analysis. SY, LT,
JD, LJ, PX and WZ performed experimental studies. SY, LJ and WT
acquired patient samples. WT provided financial support. SY and WT
provided project guidance. SY, LT, JD, LJ, PX, WZ and WT wrote the
manuscript, which all authors reviewed. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The First Hospital of Harbin Medical University
granted ethical approval for the present study and all patients
provided their written informed consent (approval no.: 202438;
Harbin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ramamoorthi G, Kodumudi K, Gallen C,
Zachariah NN, Basu A, Albert G, Beyer A, Snyder C, Wiener D, Costa
RLB and Czerniecki BJ: Disseminated cancer cells in breast cancer:
Mechanism of dissemination and dormancy and emerging insights on
therapeutic opportunities. Semin Cancer Biol. 78:78–89. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu L, Jiang S, Yu S, Liu X, Pu S, Xie P,
Chen H, Liao X, Wang K and Wang B: Increased SIX-1 expression
promotes breast cancer metastasis by regulating
lncATB-miR-200s-ZEB1 axis. J Cell Mol Med. 24:5290–5303. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu L, Tian Q, Gao H, Wu K, Wang B, Ge G,
Jiang S, Wang K, Zhou C, He J, et al: PROX1 promotes breast cancer
invasion and metastasis through WNT/β-catenin pathway via
interacting with hnRNPK. Int J Biol Sci. 18:2032–2046. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang W, Xia J, Xie S, Zou R, Pan S, Wang
ZW, Assaraf YG and Zhu X: Long non-coding RNAs as a determinant of
cancer drug resistance: Towards the overcoming of chemoresistance
via modulation of lncRNAs. Drug Resist Updat. 50:1006832020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chu Z, Huo N, Zhu X, Liu H, Cong R, Ma L,
Kang X, Xue C, Li J, Li Q, et al: FOXO3A-induced LINC00926
suppresses breast tumor growth and metastasis through inhibition of
PGK1-mediated Warburg effect. Mol Ther. 29:2737–2753. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang Y, Mo W, Ding X and Ding Y: Long
non-coding RNAs in breast cancer stem cells. Med Oncol. 40:1772023.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karthikeyan SK, Xu N, Ferguson Rd JE,
Rais-Bahrami S, Qin ZS, Manne U, Netto GJ, S Chandrashekar D and
Varambally S: Identification of androgen response-related lncRNAs
in prostate cancer. Prostate. 83:590–601. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen X, Luo R, Zhang Y, Ye S, Zeng X, Liu
J, Huang D, Liu Y, Liu Q, Luo ML, et al: Long noncoding RNA DIO3OS
induces glycolytic-dominant metabolic reprogramming to promote
aromatase inhibitor resistance in breast cancer. Nat Commun.
13:71602022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou L, Jiang J, Huang Z, Jin P, Peng L,
Luo M, Zhang Z, Chen Y, Xie N, Gao W, et al: Hypoxia-induced lncRNA
STEAP3-AS1 activates Wnt/β-catenin signaling to promote colorectal
cancer progression by preventing m6A-mediated
degradation of STEAP3 mRNA. Mol Cancer. 21:1682022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tong X, Gu PC, Xu SZ and Lin XJ: Long
non-coding RNA-DANCR in human circulating monocytes: A potential
biomarker associated with postmenopausal osteoporosis. Biosci
Biotechnol Biochem. 79:732–737. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gan X, Ding D, Wang M, Yang Y, Sun D, Li
W, Ding W, Yang F, Zhou W and Yuan S: DANCR deletion retards the
initiation and progression of hepatocellular carcinoma based on
gene knockout and patient-derived xenograft in situ hepatoma mice
model. Cancer Lett. 550:2159302022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lamere AT and Li J: Inference of gene
co-expression networks from single-cell RNA-sequencing data.
Methods Mol Biol. 1935:141–153. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo ZH, Walid AA, Xie Y, Long H, Xiao W,
Xu L, Fu Y, Feng L and Xiao B: Construction and analysis of a
dysregulated lncRNA-associated ceRNA network in a rat model of
temporal lobe epilepsy. Seizure. 69:105–114. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruan Y, Li Y, Liu Y, Zhou J, Wang X and
Zhang W: Investigation of optimal pathways for preeclampsia using
network-based guilt by association algorithm. Exp Ther Med.
17:4139–4143. 2019.PubMed/NCBI
|
|
16
|
Thiel D, Conrad ND, Ntini E, Peschutter
RX, Siebert H and Marsico A: Identifying lncRNA-mediated regulatory
modules via ChIA-PET network analysis. BMC Bioinformatics.
20:2922019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhong G, Su S, Li J, Zhao H, Hu D, Chen J,
Li S, Lin Y, Wen L, Lin X, et al: Activation of Piezo1 promotes
osteogenic differentiation of aortic valve interstitial cell
through YAP-dependent glutaminolysis. Sci Adv. 9:eadg04782023.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiao YF, Li BS, Liu JJ, Wang SM, Liu J,
Yang H, Hu YY, Gong CL, Li JL and Yang SM: Role of lncSLCO1C1 in
gastric cancer progression and resistance to oxaliplatin therapy.
Clin Transl Med. 12:e6912022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tao W, Wang C, Zhu B, Zhang G and Pang D:
LncRNA DANCR contributes to tumor progression via targetting
miR-216a-5p in breast cancer: lncRNA DANCR contributes to tumor
progression. Biosci Rep. 39:BSR201816182019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bi Y, Guo S, Xu X, Kong P, Cui H, Yan T,
Ma Y, Cheng Y, Chen Y, Liu X, et al: Decreased ZNF750 promotes
angiogenesis in a paracrine manner via activating
DANCR/miR-4707-3p/FOXC2 axis in esophageal squamous cell carcinoma.
Cell Death Dis. 11:2962020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan Z, Wu C, Li Y, Li H, An Y, Wang G, Dai
J and Wang Q: LncRNA DANCR silence inhibits SOX5-medicated
progression and autophagy in osteosarcoma via regulating
miR-216a-5p. Biomed Pharmacother. 122:1097072020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu X, Peng WX, Zhou H, Jiang J, Zhou X,
Huang D, Mo YY and Yang L: IGF2BP2 regulates DANCR by serving as an
N6-methyladenosine reader. Cell Death Differ. 27:1782–1794. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiong M, Wu M, Peng D, Huang W, Chen Z, Ke
H, Chen Z, Song W, Zhao Y, Xiang AP, et al: LncRNA DANCR represses
Doxorubicin-induced apoptosis through stabilizing MALAT1 expression
in colorectal cancer cells. Cell Death Dis. 12:242021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang X, Xie K, Zhou H, Wu Y, Li C, Liu Y,
Liu Z, Xu Q, Liu S, Xiao D and Tao Y: Role of non-coding RNAs and
RNA modifiers in cancer therapy resistance. Mol Cancer. 19:472020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang ZJ, Liu R, Han XJ, Qiu CL, Dong GL,
Liu ZQ, Liu LH, Luo Y and Jiang LP: Knockdown of the long
non-coding RNA MALAT1 ameliorates TNF-α-mediated endothelial cell
pyroptosis via the miR-30c-5p/Cx43 axis. Mol Med Rep. 27:902023.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi SJ, Wang LJ, Yu B, Li YH, Jin Y and
Bai XZ: LncRNA-ATB promotes trastuzumab resistance and
invasion-metastasis cascade in breast cancer. Oncotarget.
6:11652–11663. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xue X, Yang YA, Zhang A, Fong KW, Kim J,
Song B, Li S, Zhao JC and Yu J: LncRNA HOTAIR enhances ER signaling
and confers tamoxifen resistance in breast cancer. Oncogene.
35:2746–2755. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ghafouri-Fard S, Khoshbakht T, Hussen BM,
Baniahmad A, Taheri M and Samadian M: A review on the role of DANCR
in the carcinogenesis. Cancer Cell Int. 22:1942022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu Y, Bao Y, Qiu G, Ye H, He M and Wei X:
METTL3 promotes proliferation and migration of colorectal cancer
cells by increasing SNHG1 stability. Mol Med Rep. 28:2172023.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xue ST, Zheng B, Cao SQ, Ding JC, Hu GS,
Liu W and Chen C: Long non-coding RNA LINC00680 functions as a
ceRNA to promote esophageal squamous cell carcinoma progression
through the miR-423-5p/PAK6 axis. Mol Cancer. 21:692022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ghaemi Z, Mowla SJ and Soltani BM: Novel
splice variants of LINC00963 suppress colorectal cancer cell
proliferation via miR-10a/miR-143/miR-217/miR-512-mediated
regulation of PI3K/AKT and Wnt/β-catenin signaling pathways.
Biochim Biophys Acta Gene Regul Mech. 1866:1949212023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang S, Wang X, Zhou X, Hou L, Wu J, Zhang
W, Li H, Gao C and Sun C: ncRNA-mediated ceRNA regulatory network:
Transcriptomic insights into breast cancer progression and
treatment strategies. Biomed Pharmacother. 162:1146982023.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou Y, Meng X, Chen S, Li W, Li D, Singer
R and Gu W: IMP1 regulates UCA1-mediated cell invasion through
facilitating UCA1 decay and decreasing the sponge effect of UCA1
for miR-122-5p. Breast Cancer Res. 20:322018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang N, Wang X, Xie X, Liao Y, Liu N, Liu
J, Miao N, Shen J and Peng T: lncRNA DANCR promotes tumor
progression and cancer stemness features in osteosarcoma by
upregulating AXL via miR-33a-5p inhibition. Cancer Lett. 405:46–55.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu G, Li Y, Ma Y, Lu J, Chen Y, Jiang Q,
Qin Q, Zhao L, Huang Q, Luo Z, et al: Long noncoding RNA LINC00511
contributes to breast cancer tumourigenesis and stemness by
inducing the miR-185-3p/E2F1/Nanog axis. J Exp Clin Cancer Res.
37:2892018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tao WY, Wang CY, Sun YH, Su YH, Pang D and
Zhang GQ: MicroRNA-34c suppresses breast cancer migration and
invasion by targeting GIT1. J Cancer. 7:1653–1662. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang S, Miyakawa A, Wickström M, Dyberg
C, Louhivuori L, Varas-Godoy M, Kemppainen K, Kanatani S, Kaczynska
D, Ellström ID, et al: GIT1 protects against breast cancer growth
through negative regulation of Notch. Nat Commun. 13:15372022.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang J, Zhang J, Liu W, Ge R, Gao T, Tian
Q, Mu X, Zhao L and Li X: UBTF facilitates melanoma progression via
modulating MEK1/2-ERK1/2 signalling pathways by promoting GIT1
transcription. Cancer Cell Int. 21:5432021. View Article : Google Scholar : PubMed/NCBI
|