Introduction
Ovarian cancer is one of the most common malignant
tumors affecting female reproductive organs worldwide and it has
the highest mortality rate among gynecological tumors (1,2). Due
to the lack of early diagnostic methods and inconspicuous clinical
symptoms, most women are diagnosed at an advanced stage
(corresponding to stages III and IV), resulting in 5-year survival
rates ranging from 26–42% (3,4).
Despite significant advancements in medical science and technology,
the combination of surgery and chemotherapy has improved the
therapeutic outcomes for ovarian cancer in recent years, but
unfortunately, there has been no significant improvement in the
5-year survival rate (5).
Therefore, it is imperative to explore new strategies to enhance
the treatment of ovarian cancer.
The current front-line standard of care for ovarian
cancer involves surgery followed by platinum-taxane maintenance
chemotherapy (6,7). However, chemotherapy resistance
remains a significant factor contributing to the high mortality
rate in ovarian cancer (5).
Various factors, such as decreased drug accumulation, elevated
glutathione levels, increased metallothionein and enhanced DNA
repair ability, have been implicated in the development of tumor
cell resistance to paclitaxel or platinum. Among these factors, the
most crucial one is the enhanced tolerance and repair of DNA damage
through the nucleotide-excision-repair (NER) pathway (8,9). The
NER pathway is highly conserved and represents one of the major DNA
repair mechanisms in mammalian cells that counteract the formation
of genetic damage (8).
Understanding the involvement of the NER pathway in chemotherapy
resistance could provide valuable insights for developing novel
therapeutic strategies to combat ovarian cancer.
Nucleotide excision repair cross-complementary gene
1 (ERCC1) is a gene responsible for recognizing DNA damage
and cleaving the DNA chain (7).
This gene plays a crucial role in repairing the damaged platinum
and DNA compounds of tumor cells, directly affecting the ability of
tumor tissues to restore replication and proliferation (10). A number of studies have indicated
that ERCC1 serves as a marker for ovarian cancer resistance
to platinum drugs (11–13). Research findings have suggested
that individuals with low ERCC1 expression tend to have an
improved response to cisplatin chemotherapy compared with those
with high expression levels (8,14,15).
Moreover, using RNA interference (RNAi) to downregulate
ERCC1 mRNA expression in gastric cancer cells resistant to
cisplatin (DDP) has been shown to restore susceptibility to
treatment (16). Given these
findings, inhibiting ERCC1 expression emerges as a promising
therapeutic strategy for improving the treatment of ovarian cancer.
By targeting ERCC1, it may be possible to enhance the
efficacy of platinum-based chemotherapy and overcome drug
resistance in ovarian cancer patients.
Adenovirus vectors have emerged as one of the most
widely studied and applied vectors for cancer gene therapy. They
are designed to replicate preferentially in cancer cells and induce
their destruction through the natural process of lytic virus
replication (17). This approach
allows for targeted delivery of the virus to tumor cells with
specific genetic alterations or gene expression profiles, making
them applicable only to tumors with the desired characteristics
(18). However, despite the
significant increase in the number of studies on oncolytic
adenoviruses in recent years, research on ovarian cancer remains
relatively limited. For example, according to a 2023 review by
Lundstrom (19), only five out of
~150 studies involved ovarian cancer. This suggests that more
exploration and research are needed in the field of ovarian cancer
using oncolytic adenovirus therapy. In addition, the research
mechanism of using oncolytic adenovirus vectors to carry
therapeutic transgenes exhibits diversity. However, there has been
no research report on exploring the use of oncolytic adenovirus
vectors to target ERCC1 gene. Given the important role of
ERCC1 gene in the resistance of ovarian cancer, exploring
the use of oncolytic adenovirus vectors to carry interference RNA
targeting ERCC1 gene is a promising direction.
Furthermore, it is highly desirable to develop a
repertoire of vectors that can exploit microenvironmental
constraints specific to tumor growth, in addition to cell intrinsic
gene expression profiles or genetic alterations. Hypoxia,
characterized by a reduction in O2 partial pressure, is
a key element of the tumor microenvironment, commonly found in most
solid tumors irrespective of their origin, location, or genetic
makeup (20,21). Hypoxia plays a pivotal role in
conferring resistance of cancer cells to radiotherapy and
chemotherapy, promoting the selection of more aggressive tumor
clones and facilitating metastasis predisposition (22,23).
Thus, there is a critical need to devise innovative therapeutic
strategies that can specifically target hypoxic regions within
tumors. Hypoxia-inducible factor (HIF) is a heterodimeric
transcription factor that governs cellular responses to hypoxia by
binding to a hypoxia-response element (HRE) present within target
genes (20,21). The HIF/HRE regulatory system
becomes active under hypoxia or in response to genetic alterations
during cell transformation. Therefore, harnessing the HIF/HRE
system holds significant promise for selectively targeting
therapeutic gene expression to tumor tissues.
The present study genetically engineered an
oncolytic adenovirus armed with ERCC1 short interfering
(si)RNA, specifically regulated by hypoxia/HIF-dependent
mechanisms. The experiments demonstrated that the recombinant
adenovirus effectively induced sustained silencing of ERCC1
and downregulation of its oncogenic signaling in in vitro
models of ovarian cancer. Moreover, the adenovirus exhibits
targeted killing of cancer cells resistant to DDP. These findings
present a promising avenue for developing novel adjuvant
chemotherapy approaches for ovarian cancer.
Materials and methods
Cell lines and cell culture
The human ovarian cancer cell line SKOV3 was
procured from the American Type Culture Collection. The 293 cells
were acquired from Canada Microbix Biosystems Inc. All cell lines
were cultured following the instructions provided by the suppliers
in high-glucose Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% (v/v) fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). The
cultures were maintained at 37°C in a humidified atmosphere
containing 5% CO2.
Recombinant oncolytic adenoviruses
construction
The dual-regulated adenovirus
Ad-ERCC1-siRNA-hTERT-E1A-HRE-E1B (Ad-ERCC1) and its control
adenovirus Ad-negative control (NC)-siRNA-hTERT-E1A-HRE-E1B (Ad-NC)
were constructed by Shanghai GeneChem Co., Ltd. Briefly, fragments
of the specific siRNA of ERCC1 (ERCC1-siRNA) gene were obtained
which were designed based on Genbank's ERCC1 gene sequence by gene
synthesis conducted by Shanghai GeneChem Co., Ltd. The sequence of
ERCC1-siRNA was 5′-ccAAGCCCTTATTCCGATCTA-3′ and the negative
control sequence was 5′-TTCTCCGAACGTGTCACGT-3′. An overexpression
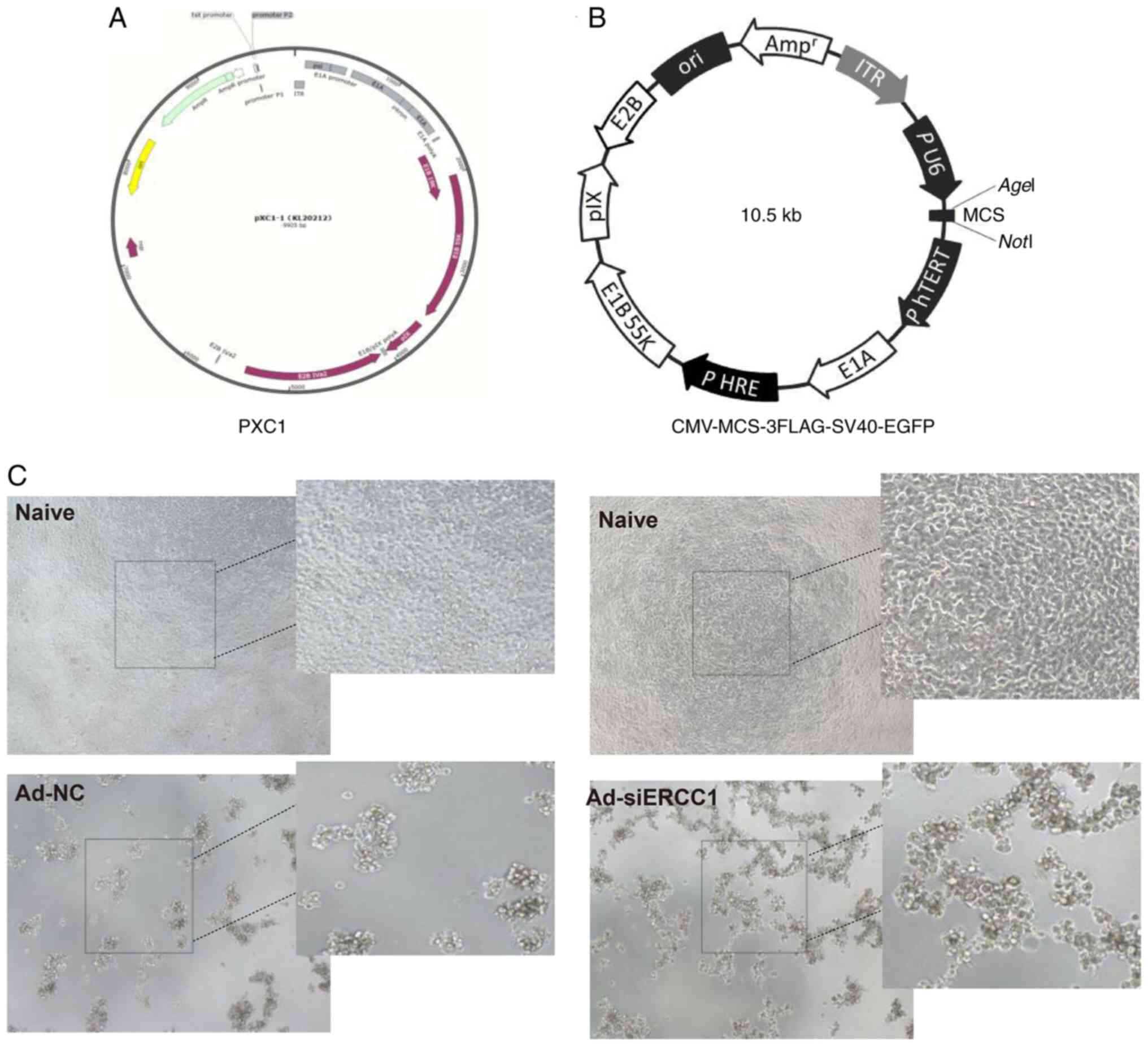
vector was first constructed using PXC1 plasmid [PXC1 (hTERTp-E1A +
HREp-E1B)], in which the human telomerase reverse transcriptase
promoter (hTERT) was used to regulate the adenovirus ELA
gene; the hypoxia regulatory element sequence (HRE) regulates the
E1B gene. (Fig. 1A and B).
ERCC1-siRNA and PXC1 were digested by BamHI and KpnI
and then the products were combined by T4 DNA ligase to generate
the PXC1-ERCC1-siRNA plasmid. The connected plasmids were
transformed into competent cells, identified by PCR and sequenced
and then extracted with a TIANprep Mini Plasmid Kit (Tiangen
Biotech Co., Ltd.). The purified PXC1-ERCC1-siRNA plasmid was
transfected into 293 cells with adenovirus packing plasmid (PBHGE3)
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) as follows: 5 µg of plasmid was added gently to
the DMEM, the total volume adjusted to 50 µl and incubate at room
temperature for 5 min. Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.; 10 µl) was mixed with 50 µl of DMEM
and incubated at room temperature for 5 min. the two solutions were
gently mixed without shaking and incubated at room temperature for
20 min to form a DNA/Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) transfection complex. The
transfection complex was slowly added to the 293 cell mixing well
and incubate at 37°C and in 5% CO2. At 6 h after
incubation, the medium containing the transfection mixture was
discarded and the cells rinsed with sterile PBS and gently shaken
to wash away the residual transfection mixture. Then 5 ml of DMEM
containing 10% FBS was slowly added and incubation continued at
37°C and in 5% CO2. At 10–15 days after transfection,
single virus plaques appeared in 293 cells, which were collected
and confirmed by PCR analysis using the forward and reverse
primers. The confirmed recombinant adenovirus was designated
Ad-ERCC1. Ad-ERCC1 was amplified in 293 cells and purified by
ultracentrifugation on cesium chloride (CsCl) gradients (40,000 ×
g; 2 h; 4°C). Other viruses used were treated with the same method.
EGFP gene was cloned into the viruses to determine the infectivity.
Viral titer confirmation was performed by infecting 293 T cells
using an End-point dilution method and was calculated by
Spearman-Karber Method as follows: Viral titer=10 (x+0. 8)
plaque-forming units (PFU)/ml) (x=the sum of positive rates of
cytopathic effect in sequential dilution from 10−1 to
10−13).
Reverse transcription-quantitative
(RT-q) PCR for mRNA analysis
For quantitative RT-PCR, total RNA was extracted
from cells of different groups (6-well plate with 80% cell density)
using SuperfecTRI, Total RNA Isolation Reagent (cat. no. 3101-400;
Shanghai Pufei Biotechnology Co., Ltd.). cDNA was synthesized from
400 ng of RNA with the Promega M-MLV kit according to the
manufacturer's protocol (cat. no. M1705; Promega Corporation).
RT-qPCR) were performed in triplicate according to the kit protocol
of Bulge-Loop miRNA qRT-PCR Starter Kit (cat. no. C10211-1;
Guangzhou RiboBio Co., Ltd.) using a LightCycler 480 II (Roche
Diagnostics) with SYBR green PCR reagents. Experiments were
replicated four times and the data are shown as fold changes. The
primer sequences were: ERCC1: 5′-CTACGCCGAATATGCCATCTC-3′,
3′-GTACGGGATTGCCCCTCTG-5′; GAPDH:
5′-TGACTTCAACAGCGACACCCA-3′, 3′-CACCCTGTTGCTGTAGCCAAA-5′. The PCR
program included 95°C for 30 s, 40 cycles of 95°C for 5 sec and
60°C for 30 sec. The relative amount of mRNA for target gene was
determined by the 2−ΔΔCq method (24) and presented as changes in fold in
the target gene expression normalized to two endogenous reference
genes (GAPDH).
Detection of the inhibition rate in
ovarian cancer cells by MTT assay
The cancer cell inhibition rate was evaluated by a
standard MTT assay. Briefly, SKOV3 cells were plated in 96-well
plates at a density of 2×104 cells/well. After
incubation for 24 h, the cells were infected with of different
multiplicity of infection (MOI)s of Ad-siERCC1 (0, 0.001, 0.01,
0.1, 1, 5, 10, 50, 100 and 500 for 48 or 72 h) or different
concentrations of DDP (0, 2, 4, 6, 8, 10, 12, 14, 16 and 18 µmol
for 24 h) or both (10 MOI Ad-siERCC1 first for 24 h, 10 µmol DDP
for anther 24 h). After treatment, 20 µl (5 mg/ml) MTT solution
(cat. no. JT343; Genview Corp.) was added to each well. After 4 h
incubation at 37°C, the supernatant was discarded and 100 µl DMSO
was loaded in every well for 2–5 min. Blank control wells were also
set. The optical density (OD) of each well was measured with a
Microplate Reader (cat. no. M2009PR; Tecan infinite; Tecan Group,
Ltd.) at 490 nm. The cell growth inhibitory rate (I%) was
calculated according to the following equation: inhibitory
rate%=(ODcontrol-ODsample)/(ODcontrol-ODblank)
× 100%, where ODcontrol is the absorbance of the
untreated cells, ODsample is the absorbance of the cells
exposed to Ad-siERCC1, DDP or both and Ablank is the
absorbance of the media. A total of three reduplicate wells were
measured at each group and every experiment was performed at least
3 times.
Wound healing migration assay
Cells were counted in a Neubauer chamber slide using
the trypan blue exclusion method. Viable cells were plated at
3×105 cells/well in 6-well culture plates using growth
media containing 10% FBS for 24 h. The cells were washed with DPBS
and pretreated with either DDP or recombinant adenovirus in
serum-free media. To control wells, only serum-free media was
added. The cells were scratched perpendicular to the horizontal
line with a 200 µl sterile pipette tip to create a cell-free wound
area. After washing away suspended cells, fresh serum-free media
was added and images were captured immediately (time 0 h) using an
Olympus CX41 inverted microscope (Olympus Corporation). The cells
were cultured with 37°C and 5% CO2 and images were
captured again after 24 h at the same position. The measurement of
cell scratch was done by ImageJ software (version 1.47; National
Institutes of Health). Within each assay the experiments were
performed in triplicates. Data shown are representative of minimum
three independent experiments.
Matrigel invasion assay
A total of 3×105 cells in 100 µl
serum-free medium were seeded in a Transwell chamber
(MilliporeSigma) with a coating of Matrigel (MilliporeSigma). The
Matrigel was placed on ice and thawed overnight at 4°C in the
freezer. It was applied diluted with DMEM at 1:5 to the insert cell
growth surface at 150–200 µl/cm2 at 37°C for 30 min.
Briefly, SKOV3 cells were seeded into the upper chamber in a
serum-free medium and treated with either DDP or recombinant
adenovirus. A volume of 0.5 ml of medium containing 10% FBS was
added to the lower chamber. After 24 h of incubation, the upper
surface of the membrane was wiped off with a cotton swab. Cells
that invaded the lower surface of the porous membrane were fixed
with 4% formaldehyde in PBS containing 4% sucrose for 10 min at
room temperature and stained with 0.1% crystal violet for 10 min at
room temperature. Stained cells were counted in 20 random fields
per filter, in a total of three filters (n=3). Invasion was
presented as percentage of invasion=(number treated cells/number of
control cells) ×100.
Cell cycle analysis
SKOV3 cells were transfected with Ad at an MOI of 10
for 6 h. Following DDP treatments for 24 h, cells were detached
with trypsinization, washed with Dulbecco's phosphate buffered
saline (DPBS) and the cells collected. For assessment of DNA
contents, cells were stained with propidium iodide (PI; 50 µg/ml;
cat. no. P4170; MilliporeSigma) with 0.5 mg/ml RNase (cat. no.
EN0531; Thermo Fisher Scientific, Inc.) in DPBS + 0.1% Tween (pH
7.4) in the dark for 30 min and then monitored by a
fluorescence-activated cell sorter (FACS) using a BD C6 PLUS (BD
Biosciences). The FACS data were analyzed using NovoExpress (ACEA
Biosciences, Inc.) to calculate the fraction of cells in
G1, S and G2 phases.
Cell apoptosis assay
Annexin V-APC staining and flow cytometry analysis
were used to detect cell apoptosis. Annexin V Apoptosis Detection
Kit APC (cat. no. 88-8007; eBioscience; Thermo Fisher Scientific,
Inc.) was used to measure cell apoptosis according to the
manufacturer's instructions. Briefly, after washing with PBS, cells
were resuspended in Annexin-V binding buffer, stained with the
Annexin V-APC for 15 min at room temperature in the dark and
analyzed using FACSCalibur (C6 PLUS; BD Biosciences) and the
apoptotic rate was calculated as the numbers of early + late
apoptotic cells/total numbers of cell.
Protein extraction and
immunoblotting
Cells were harvested and re-suspended in a RIPA
buffer (Beyotime Institute of Biotechnology) containing protease
inhibitor cocktail (cat. no. GK10014; GlpBio). After determining
the protein concentration using an Enhanced BCA Protein Assay kit
(Beyotime Institute of Biotechnology), the obtained lysates (20
µg/well) were subjected to 4–10% SDS-PAGE to separate the proteins,
which were subsequently transferred to PVDF membranes (cat. no.
A29562259; Cytiva). The membranes were incubated with the following
primary antibodies: anti-PI3K (1:1,000; cat. no. 4257; Cell
Signaling Technology, Inc.), phosphorylated (p)-Akt (Ser473;
1:1,000; cat. no. 4060; Cell Signaling Technology, Inc.), anti-AKT
(1:1,000; cat. no. ab179463; Abcam), anti-caspase-3 (1:1,000; cat.
no. sc-7272; Santa Cruz Biotechnology, Inc.), cleaved caspase-3
(1:1,000; cat. no. 9661S; Cell Signaling Technology, Inc.), GAPDH
(1:2,000; cat. no. sc-32233; Santa Cruz Biotechnology), β-Actin
(1:2,000; cat. no. sc-8432; Santa Cruz Biotechnology). After
blocking for 1 h at room temperature in non-fat milk in TBST
(Tris-buffered saline with 0.1% Tween-20), membranes were incubated
overnight at 4°C with primary antibodies in blocking buffer. After
rinses with TBST, membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies (goat anti-rabbit, cat.
no. 7074; goat anti-mouse, cat. no. 7076; Cell Signaling
Technology, Inc.) for 1.5 h at room temperature and detected using
20X LumiGLO reagent and 20X peroxide (cat. no. 7003; Cell Signaling
Technology, Inc.) and film exposure. Optical densities of the bands
from the original image were measured with NIH ImageJ software
(version 1.47; National Institutes of Health).
Statistical analysis
All data are expressed as mean ± SEM. The
statistical comparisons for two groups were made with unpaired
Independent-Samples t test. Multiple comparisons were made with
one-way ANOVA followed by between-group comparisons using the
Bonferroni comparisons or Dunnett's T3 comparisons post hoc tests,
according to whether the data were normally distributed or not,
using SPSS 13.0 software (SPSS, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Recombinant adenovirus can effectively
inhibit the proliferation of ovarian cancer cells
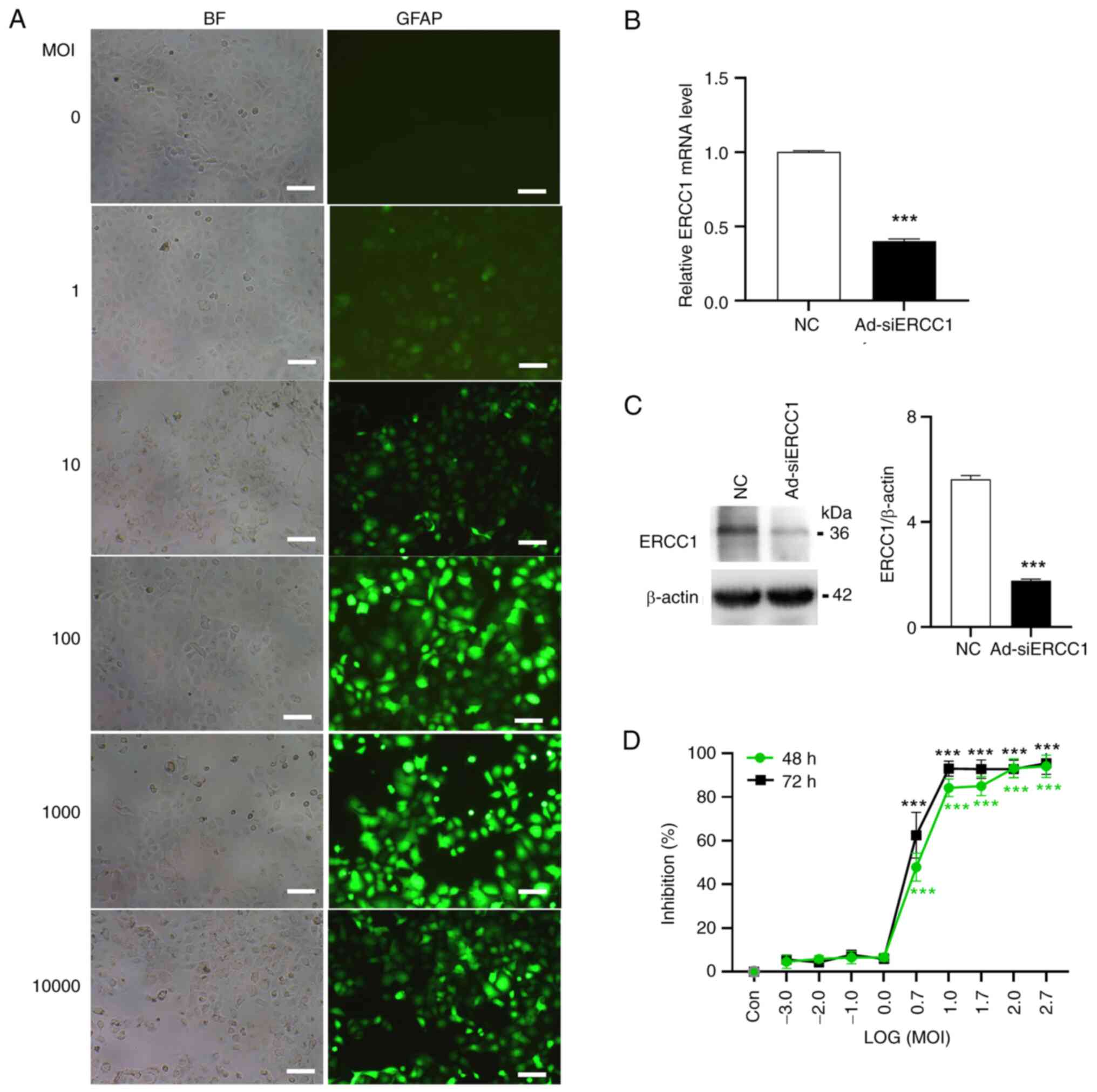
In the present study, the recombinant adenovirus
effectively infected and killed tumor cells (Fig. 1C). Subsequently, the transfection
efficiency of the recombinant adenovirus on ovarian cancer cells
was evaluated. Cultured SKOV3 cells were infected with the
recombinant adenovirus expressing green fluorescent protein (GFP)
with ERCC1-siRNA (Ad-ERCC1-siRNA, Ad-siERCC1) or the empty vector
(Ad-NC-siRNA, Ad-NC) at varying MOI levels ranging from 1–10,000.
It was determined that the most efficient MOI value was 10
(Fig. 2A). At MOI=10, the mRNA and
protein levels of ERCC1 were validated using RT-qPCR and
immunoblotting techniques, revealing a decrease in ERCC1 mRNA (~60%
less than control; Independent-Samples t test; t(6)=36.842; P<0.001; Fig. 2B) and protein (>3-fold less than
control) (Independent-Samples t test; t(6)=24.036; P<0.001; Fig. 2C) upon Ad-siERCC1 treatment.
Oncolytic viruses specifically target and replicate
within tumor cells, ultimately lysing them and releasing viral
progeny that can propagate among tumors, eventually leading to the
destruction of all tumors and thereby inhibiting tumor cell
proliferation and survival (16).
Considering this, it was further assessed using MTT assay whether
the recombinant adenovirus would induce inhibition of cancer cell
survival following transduction. As demonstrated in Fig. 2D, the recombinant adenovirus caused
a significant increase in cell mortality from MOI 5 (~47.87% for 48
h and 62.49% for 72 h) to MOI 500 [~94.03% for 48 h and 95.43% for
72 h; one-way ANOVA; 48 h: F (9,20)=362.834; P<0.001; vs. the
control group, LOG (5):
P<0.001; LOG (10): P<0.001;
LOG (50): P<0.001; LOG (100): P<0.001; LOG (500): P<0.001;
72 h: F (9,20)=313.047; P<0.001; vs. the
control group, LOG (5):
P<0.001; LOG (10): P<0.001;
LOG (50): P<0.001; LOG (100): P<0.001; LOG (500):
P<0.001].
ERCC1 silencing can significantly
enhance chemosensitivity to DDP of ovarian cancer cells
DDP is a widely used anticancer drug in combination
regimens and it exerts its activity by inducing the formation of
various types of DNA adducts, leading to the inhibition of DNA
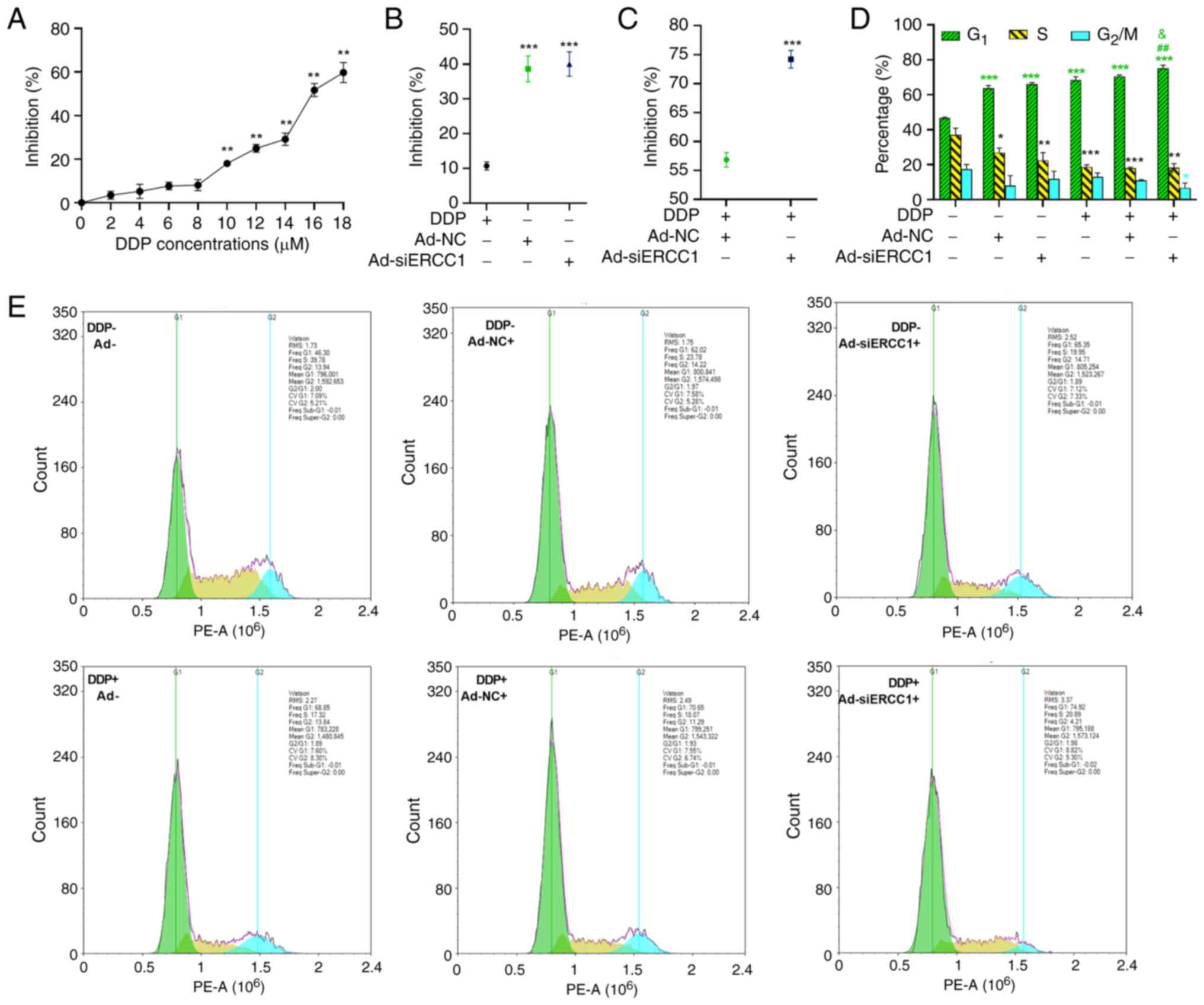
synthesis, function and transcription (25). According to our previous study
(26), a systematic inhibition of
SKOV3 cell survival was observed with increasing concentrations of
DDP from 0–18 µM after 24 h of treatment, as determined by MTT
assay (one-way ANOVA; F (9,30)=258.978;
P<0.001; Fig. 3A). Markedly,
the inhibitory effect had a significantly higher expression of
18.05, 24.98, 29.18, 51.73 and 59.75% for 10,12,14,16,18 µM DDP
(vs. the 0 µM DDP group, 10 µM DDP: P=0.001; 12 µM DDP: P=0.001; 14
µM DDP: P=0.002; 16 µM DDP: P=0.001; 18 µM DDP: P=0.001).
Therefore, this concentration was chosen for subsequent
experiments. Subsequently, the inhibitory rates were compared among
DDP, Ad-NC and Ad-siERCC1. The results demonstrated that the
recombinant adenovirus [both Ad-NC (38.63%) and Ad-siERCC1 (40%)]
exhibited greater effectiveness in inducing cell mortality vs. DDP
alone (10.7%; one-way ANOVA; F (2,9)=258.978, P<0.001; vs. DDP group,
Ad-NC: P<0.001; Ad-siERCC1: P<0.001, Fig. 3B).
Resistance, however, has limited the efficacy of
these drugs in most ovarian cancer patients. Among the various
mechanisms contributing to cisplatin (DDP) resistance, enhanced
tolerance and repair of DNA damage through the NER pathway have
been recognized as the most crucial resistance mechanism to
platinum drugs (7,8). To further investigate whether
ERCC1 silencing could enhance the cancer cell-killing effect
following chemotherapy, MTT assays were performed on SKOV3 cells
treated with recombinant adenovirus and DDP. As depicted in
Fig. 3C, in the presence of DDP,
cancer cells transduced with Ad-siERCC1 (74.19%) exhibited
significantly lower cell viability compared with those transduced
with Ad-NC (56.85%) six days post-transduction (Independent-Samples
t test; t(6)=−7.420; P<0.001).
Additionally, cell cycle analysis revealed that SKOV3 cells in all
groups were arrested in the G1 phase when compared with
the control group (control group: 46.60%; Ad-NC: 63.79%;
Ad-siERCC1: 66.05%; DDP: 68.42%; DDP + Ad-NC: 70.56%; DDP +
Ad-siERCC1: 75.12%; one-way ANOVA, G1: F (5,12)=148.758, P<0.001; vs. the
control group, Ad-NC: P<0.001; Ad-siERCC1: P<0.001; DDP:
P<0.001; DDP + Ad-NC: P<0.001; DDP + Ad-siERCC1: P<0.001).
However, the G1 phase cell block was more pronounced in
the Ad-siERCC1 combined with cisplatin group (vs. DDP group, DDP +
Ad-siERCC1: P=0.001; vs. DDP + Ad-NC group, DDP + Ad-siERCC1:
P=0.027) and the proportion of cells in the G2/M phase
was the lowest (Fig. 3D and E;
one-way ANOVA, S: F (5,12)=16.442, P<0.001; vs. the
control group, Ad-NC: P=0.036; Ad-siERCC1: P=0.001; DDP:
P<0.001; DDP + Ad-NC: P<0.001; DDP + Ad-siERCC1: P<0.001;
G2/M: F (5,12)=3.697, P=0.029; vs. the control
group, DDP + Ad-siERCC1: P<0.036).
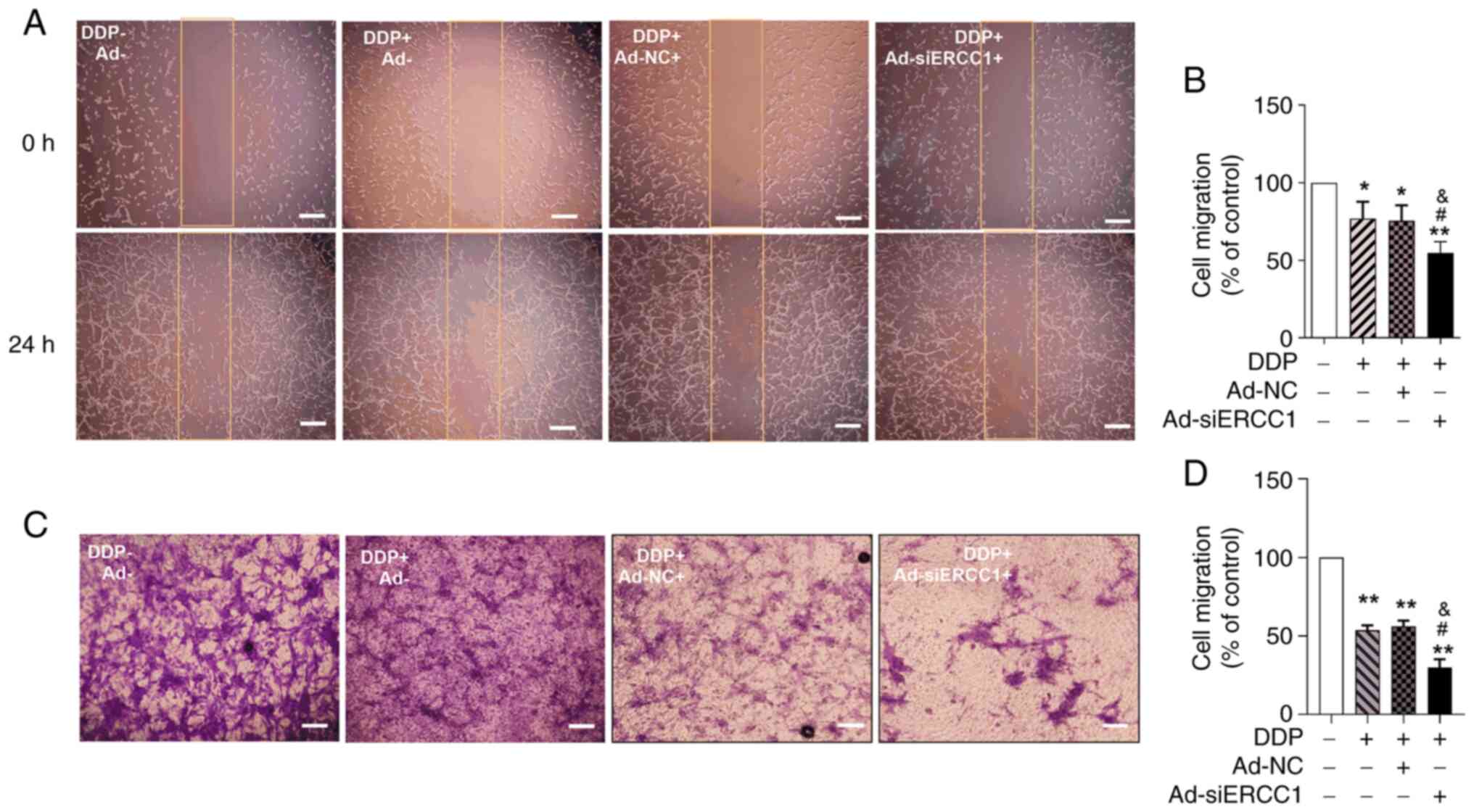
To assess the effect of the recombinant adenoviruses
on SKOV3 cell migration, a scratch wound-healing migration assay
was performed. The change in scratch width reflected cell mobility.
Ad-siERCC1 combined with DDP resulted in the most potent inhibition
of cell migration to 54.69%. (one-way ANOVA, F (3,16)=24.970, P<0.001; vs. the
control group, DDP: P=0.038; DDP + Ad-NC: P=0.024; DDP +
Ad-siERCC1: P=0.001; vs. DDP group, DDP + Ad-siERCC1: P=0.034; Vs.
DDP + Ad-NC group, DDP + Ad-siERCC1: P=0.035; Fig. 4A and B). In the Transwell invasion
assay, it was observed that the recombinant adenovirus and DDP
inhibited the migration ability of SKOV3 cells (one-way ANOVA, F
(3,16)=69.035, P<0.001; vs. the
control group, DDP: P=0.001; DDP + Ad-NC: P=0.001; DDP +
Ad-siERCC1: P=0.001; Fig. 4C and
D). Moreover, in the presence of DDP, the effect of Ad-siERCC1
was significantly superior to the DDP group (control group:
100.00%; DDP: 53.62%; DDP + Ad-NC: 56.18%; DDP + Ad-siERCC1:
29.93%; vs. DDP group, DDP + Ad-siERCC1: P=0.034; vs. DDP + Ad-NC
group, DDP + Ad-siERCC1: P=0.021). These findings indicated that
the recombinant adenovirus significantly enhanced the inhibitory
effect on migration and invasion of ovarian cancer cells and
Ad-siERCC1 can improve the chemotherapy sensitivity of DDP.
Recombinant adenovirus improves drug resistance to
DDP by enhancing apoptosis through the PI3K/AKT-caspase-3 signaling
pathways in ovarian cancer cells. Flow cytometry with Annexin V-APC
single-dye method was used to detect cells arrested in early
apoptosis. As shown in Fig. 5A and
B (one-way ANOVA, F (5,18)=892.196,
P<0.001), recombinant adenovirus and/or DDP induced apoptosis in
SKOV3 cells. The mean fluorescence intensity was 0.61± 0.07 on
control cells, 0.65±0.03 on Ad-NC cells, 1.11±0.11 on Ad-siERCC1
cells, 2.05±0.11 on DDP cells, 1.96±0.14 on DDP + Ad-NC cells and
5.68±0.21 on DDP + Ad-siERCC1 cells. (Vs. the control group, Ad-NC:
P<0.001; Ad-siERCC1: P<0.001; DDP: P<0.001; DDP + Ad-NC:
P<0.001; DDP + Ad-siERCC1: P<0.001) and Ad-siERCC1
significantly enhanced the apoptotic effect of DDP (>2.7-fold
more than control; vs. DDP group, DDP + Ad-siERCC1: P<0.001; vs.
DDP + Ad-NC group, DDP + Ad-siERCC1: P<0.001). To gain further
insights into this phenomenon, immunoblotting was performed to
examine the proteins involved in apoptosis. As depicted in Fig. 5C (one-way ANOVA, F (5,18)=14.040, P<0.001; vs. the control
group, Ad-NC: P=0.019; Ad-siERCC1: P=0.006; DDP: P=0.002; DDP +
Ad-NC: P=0.002; DDP + Ad-siERCC1: P<0.001; vs. DDP group,
P=0.034; vs. DDP + Ad-NC group, P=0.037). PI3K were significantly
suppressed by recombinant adenovirus and/or DDP and Ad-siERCC1
greatly enhanced the effect of DDP (DDP + Ad-siERCC1: 0.55±0.10 vs.
DDP: 0.76±0.05). Recombinant adenovirus and/or DDP reduced the
phosphorylation level of p-AKT. Ad-siERCC1 enhanced the effect of
DDP on p-AKT [DDP + Ad-siERCC1: 0.56±0.11 vs. DDP: 0.77±0.05;
one-way ANOVA, p-AKT: F (5,18)=16.062,
P<0.001; vs. the control group, Ad-siERCC1: P=0.003; DDP:
P=0.003; DDP + Ad-NC: P=0.001; DDP + Ad-siERCC1: P<0.001; vs.
DDP group, P=0.012; vs. DDP + Ad-NC group, DDP + Ad-siERCC1:
P=0.035. AKT: F (5,18)=0.107, DDP + Ad-siERCC1: P=0.989].
Concurrently, procaspase-3 levels were notably reduced after
incubation with recombinant adenovirus and/or DDP (control:
1.03±0.11 vs. DDP: 0.77±0.05 vs. DDP + Ad-NC: 0.71±0.04 vs. DDP +
Ad-siERCC1: 0.56±0.08), whereas the cleaved caspase-3, the
activated form of procaspase-3, exhibited a substantial increase
(control: 0.21±0.04 VS DDP: 0.44±0.08 vs. DDP + Ad-NC: 0.49±0.04
vs. DDP + Ad-siERCC1: 0.52±0.08; one-way ANOVA, procaspase-3: F
(5,18)=36.689, P<0.001; vs. the control
group, DDP: P<0.001; DDP + Ad-NC: P<0.001; DDP + Ad-siERCC1:
P<0.001; vs. DDP group, P=0.003. cleaved caspase-3: F (5,18)=22.502, P<0.001; vs. the control
group, DDP: P=0.001; DDP + Ad-NC: P<0.001; DDP + Ad-siERCC1:
P<0.001, Fig. 5E). These
results indicated that recombinant adenovirus ameliorates drug
resistance to DDP by promoting apoptosis through the
PI3K/AKT-caspase-3 signaling pathways in ovarian cancer cells.
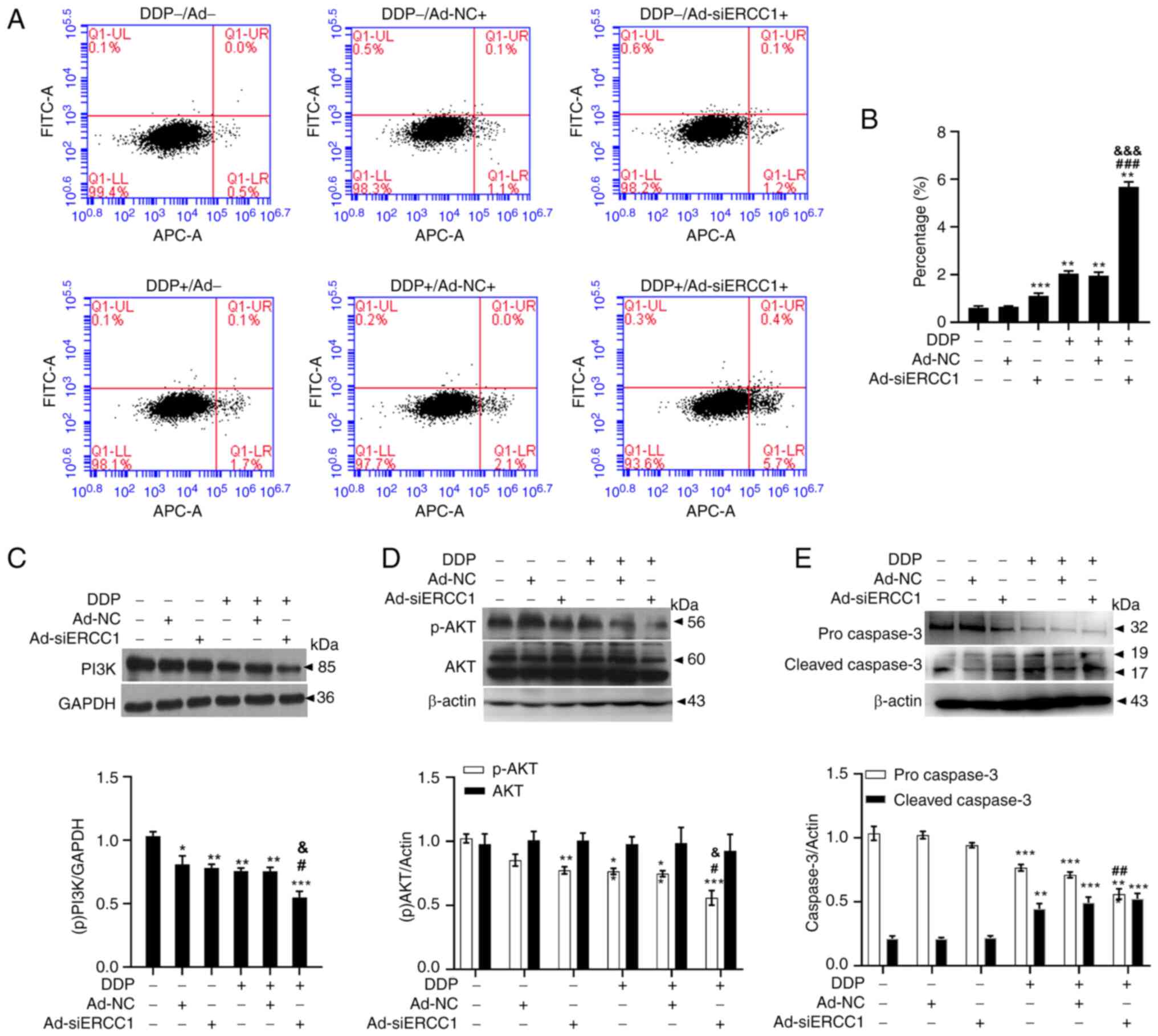 | Figure 5.Recombinant adenovirus enhancing
apoptosis through PI3K/AKT-caspase-3 ameliorates resistance to DDP
on SKOV3 cells. (A) The representative images of the Annexin V-APC
staining assay. (B) Recombinant adenovirus and/or DDP promoted the
apoptosis of ovarian cancer cells and the apoptosis rate in
combination group was significantly higher than that in DDP group
(n=4 in each group). (C) PI3K was significantly suppressed by
recombinant adenovirus and/or DDP and Ad-siERCC1 could greatly
enhanced the effect of DDP (n=4 in each group). (D) AKT was
significantly suppressed by recombinant adenovirus and (or) DDP and
Ad-siERCC1 could greatly enhanced the effect of DDP (n=4 in each
group). (E) Procaspase-3 was significantly reduced after
recombinant adenovirus and/or DDP incubation, whereas cleaved
caspase-3 was considerably increased (n=4 in each group). Data are
shown as the mean ± SEM. *P<0.05, **P<0.01, ***P<0.001 vs.
corresponding controls; #P<0.05,
##P<0.01, ###P<0.001 vs. DDP groups,
&P<0.05, &&&P<0.001 vs.
DDP combined with Ad-NC groups. DDP, cisplatin; si, short
interfering; NC, negative control; p-. phosphorylated. |
Discussion
The present study developed a recombinant oncolytic
adenovirus regulated by the hTERT/HIF dual system, which
effectively inhibited the proliferation of ovarian cancer cells.
Furthermore, using siRNA technology to downregulate ERCC1
gene expression improved drug resistance to DDP by enhancing
apoptosis through the PI3K/AKT-caspase-3 signaling pathways in
ovarian cancer cells.
Ovarian cancer predominantly implants and
metastasizes in the pelvic cavity. However, effective drug
concentration delivery intravenously to the pelvic cavity is
challenging, resulting in poor therapeutic outcomes. Additionally,
the resistance of tumor cells to platinum drugs hinders the
anti-tumor effect, leading to a low 5-year postoperative survival
rate (3,4). Thus, the development of a new
treatment strategy that targets tumors effectively and reverses
drug resistance is a crucial research objective in gynecologic
oncology. Tumor-specific proliferative oncolytic adenovirus
carriers have garnered attention due to their high tumor targeting
and lethality. These carriers infect tumor cells, replicate,
multiply, lyse and kill tumor cells, releasing more virus that can
infect other tumor cells, resulting in a chain killing reaction.
Replication and proliferation are limited to tumor cells, with
minimal damage to normal host tissues (16). The present study constructed a
recombinant oncolytic adenovirus regulated by the hTERT/HIF dual
system, expressing GFP with ERCC1-siRNA (Ad-siERCC1). It was
demonstrated that Ad-siERCC1 effectively inhibited the
proliferation, migration, invasion and survival of ovarian cancer
cells. Furthermore, compared with DDP alone, Ad-siERCC1
significantly increased the inhibitory effect on SKOV3 cells,
possibly due to its amelioration of drug resistance to DDP.
Ovarian cancer is a heterogeneous disease
categorized into several major morphological subtypes: Epithelial
carcinoma, germ cell tumor, sex cord-stromal tumor and Krukenberg's
tumor (2,25,27).
The most common subtype is serous ovarian carcinoma (28). The present study focused on the
inhibition ratio of the recombinant oncolytic adenovirus
(Ad-siERCC1) on SKOV3 cells, derived from serous cystadenoma
carcinoma. The results revealed that Ad-siERCC1 had a marked
ability to kill tumor cells. However, the effect of Ad-siERCC1 on
other subtypes of ovarian tumors was not assessed. Considering that
the subtype of ovarian cancer varies with the age of onset and
epidemiological differences exist between races and countries due
to various factors, including genetics and economic factors
(2,25,27),
it is essential to investigate the effect of Ad-siERCC1 on
different subtypes of ovarian cancer in future research.
Viral vectors in gene therapy in the treatment of
ovarian cancer, according to a review by Lundstrom in 2023
(19); the five projects listed
involve four vector systems, including p-dpp-VSVMP [a nanoparticle
that carries the gene for the matrix protein of the vesicular
stomatitis virus (VSVMP) and encapsulates paclitaxel] (29), VSVMP (the gene for VSVMP enclosed
in a lipid complex) (30), SIN
AR339 (an RNA virus belonging to the Togavirus genus) (31) and MV-CEA (a measles virus Edmonston
vaccine strain expressing the CEA peptide) (32). The p-dpp-VSVMP was found to cause
tumor regression in both subcutaneous and orthotopic ovarian cancer
mouse models. When combined with the JAK1/2 inhibitor ruxolitinib,
the treatment outcome was further improved. The VSVMP system
demonstrated a high tumor suppression rate of 87–98% in mouse tumor
xenografts models, leading to an increase in survival. These
non-replicating vector particles induce apoptosis in cancer cells
by disrupting cell cytoskeletal elements and suppressing host cell
gene expression. Another study (31) found that intraperitoneal injection
of the oncolytic adenovirus SIN AR339 led to tumor cell killing and
regression in ovarian cancer. In clinical applications, the MV-CEA
particle has been evaluated in a phase I clinical trial for
recurrent ovarian cancer patients (32). This treatment showed no
dose-limiting toxicities and all nine patients who received the
treatment achieved disease stabilization. The median overall
survival rate for these patients was 12.15 months, twice the
expected survival time. However, this treatment is only suitable
for patients with normal CEA levels. Compared to these studies, our
research still has a long way to go, is focusing on exploring the
application of ERCC1 gene in oncolytic adenovirus therapy. It is
well known that ERCC1 is the most critical factor contributing to
platinum resistance in ovarian cancer and is one of the most
studied DNA repair genes (33).
ERCC1, located on human chromosome 19, is involved in the
cleavage and recognition of DNA chains damaged by platinum-based
drugs. The level of ERCC1 mRNA reflects the ability of tumor
tissue to repair DNA helix twist induced by platinum-based drugs
and serves as an important indicator of tumor patients prognosis
and the effectiveness of platinum-based chemotherapy (12). In the present study, interference
with ERCC1 gene expression significantly improved the
efficiency of DDP chemotherapy, consistent with numerous studies
showing that higher ERCC1 expression in patients receiving
platinum-based chemotherapy is associated with worse chemotherapy
outcomes and patient prognosis (8,14,15).
However, Bösmüller et al (34) reported that ERCC1 expression is not
related to platinum chemosensitivity in ovarian cancer patients.
The discrepancies could be attributed to limited small-scale and
small-sample research on chemotherapy and ERCC1 gene or protein
expression in ovarian cancer, lacking large-sample multicenter
clinical studies.
Although oncolytic viruses are considered a
promising new class of anticancer drugs, they present unique
challenges. Oncolytic viruses are live viruses that proliferate
upon administration, resulting in variable effective doses
(35). Currently, limited data are
available on correlating viral dose with in vivo replicative
potential and therapeutic response. Further investigations relating
viral replication to clinical response are crucial. Moreover, while
a number of clinical trials have shown that replication-defective
and replication-competent adenovirus vectors are safe and have
therapeutic activity (36–39), the replicative potential of
oncolytic viruses and immunoreaction require further study.
Additionally, the safety and immunological response to oncolytic
viruses still need to be fully evaluated. The present study
constructed a novel Dual-regulated oncolytic adenovirus carrying
ERCC1-siRNA gene that can infect and replicate in ovarian cancer
cells. This recombinant oncolytic adenoviruses effectively
suppressed cancer cell proliferation, especially when combined with
DDP. The findings of the present study suggested that the
recombinant oncolytic adenovirus exerts its anti-cancer effect by
manipulating the PI3K/Akt signaling pathway, a well-known survival
and growth pathway in cancer cells (40,41).
However, further investigation is required to confirm these results
and to address any potential safety concerns or off-target effects.
Overall, oncolytic viruses show great potential in the treatment of
ovarian cancer, but more research is needed to optimize their use
in clinical settings.
Acknowledgements
Not applicable.
Funding
The present study was supported by Jiading District Health
Commission of Shanghai Youth fund (grant no. 2018-QN-01).
Availability of data and materials
The data generated in the present study are included
in the figures of this article.
Authors' contributions
WY, YM, LX, TZ initiated and directed the study; TZ,
RZ, XZ, QS performed the research and wrote the first draft of the
manuscript; TZ, XX, WC analyzed the data. WY and YM confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang L, Xie HJ, Li YY, Wang X, Liu XX and
Mai J: Molecular mechanisms of platinum-based chemotherapy
resistance in ovarian cancer (Review). Oncol Rep. 47:822022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Penny SM: Ovarian cancer: An overview.
Radiol Technol. 91:561–575. 2020.PubMed/NCBI
|
|
3
|
Wallis B, Bowman KR, Lu P and Lim CS: The
challenges and prospects of p53-Based therapies in ovarian cancer.
Biomolecules. 13:1592023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Terp SK, Stoico MP, Dybkær K and Pedersen
IS: Early diagnosis of ovarian cancer based on methylation profiles
in peripheral blood cell-free DNA: A systematic review. Clin
Epigenetics. 15:242023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chandrasekaran A and Elias KM: Synthetic
lethality in ovarian cancer. Mol Cancer Ther. 20:2117–2128. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang XW, Wu YS, Xu TM and Cui MH: CAR-T
cells in the treatment of ovarian cancer: A promising cell therapy.
Biomolecules. 13:4652023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moufarrij S, Dandapani M, Arthofer E,
Gomez S, Srivastava A, Lopez-Acevedo M, Villagra A and Chiappinelli
KB: Epigenetic therapy for ovarian cancer: Promise and progress.
Clin Epigenetics. 11:72019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang C, Gao S and Hou J: ERCC1 expression
and platinum chemosensitivity in patients with ovarian cancer: A
meta-analysis. Int J Biol Markers. 35:12–19. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang M, Jia K, Wang L, Li W, Chen B, Liu
Y, Wang H, Zhao S, He Y and Zhou C: Alterations of DNA damage
response pathway: Biomarker and therapeutic strategy for cancer
immunotherapy. Acta Pharm Sin B. 11:2983–2994. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hamilton G and Rath B: Pharmacogenetics of
platinum-based chemotherapy in non-small cell lung cancer:
Predictive validity of polymorphisms of ERCC1. Expert Opin Drug
Metab Toxicol. 14:17–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zazuli Z, Vijverberg S, Slob E, Liu G,
Carleton B, Veltman J, Baas P, Masereeuw R and Maitland-van der Zee
AH: Genetic variations and cisplatin nephrotoxicity: A systematic
review. Front Pharmacol. 9:11112018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Muallem MZ, Braicu I, Nassir M, Richter R,
Sehouli J and Arsenic R: ERCC1 expression as a predictor of
resistance to platinum-based chemotherapy in primary ovarian
cancer. Anticancer Res. 34:393–399. 2014.PubMed/NCBI
|
|
13
|
Tang N, Lyu D, Zhang Y and Liu H:
Association between the ERCC1 polymorphism and platinum-based
chemotherapy effectiveness in ovarian cancer: A meta-analysis. BMC
Womens Health. 17:432017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rosell R, Taron M, Camps C and
López-Vivanco G: Influence of genetic markers on survival in
non-small cell lung cancer. Drugs Today (Barc). 39:775–786. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lenz HJ: Pharmacogenomics and colorectal
cancer. Adv Exp Med Biol. 587:211–231. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li W, Jie Z, Li Z, Liu YI, Gan Q, Mao Y
and Wang X: ERCC1 siRNA ameliorates drug resistance to cisplatin in
gastric carcinoma cell lines. Mol Med Rep. 9:2423–2428. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wilson JM and Engelhardt J: Adenovirus
vectors for gene therapy. Biotechnol Adv. 15:7691997. View Article : Google Scholar
|
|
18
|
Berkey SE, Thorne SH and Bartlett DL:
Oncolytic virotherapy and the tumor microenvironment. Adv Exp Med
Biol. 1036:157–172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lundstrom K: Viral vectors in gene
therapy: Where do we stand in 2023? Viruses. 15:6982023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stella GM, Marchiò C, Bari E, Ferrarotti
I, Bertuccio FR, Di Gennaro A, Abbott DM, Putignano P, Campo I,
Torre ML and Corsico AG: The genes-stemness-secretome interplay in
malignant pleural mesothelioma: Molecular dynamics and clinical
hints. Int J Mol Sci. 24:34962023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Strapcova S, Takacova M, Csaderova L,
Martinelli P, Lukacikova L, Gal V, Kopacek J and Svastova E:
Clinical and pre-clinical evidence of carbonic anhydrase IX in
pancreatic cancer and its high expression in pre-cancerous lesions.
Cancers (Basel). 12:20052020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bao MH and Wong CC: Hypoxia, metabolic
reprogramming and drug resistance in liver cancer. Cells.
10:17152021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kopecka J, Salaroglio IC, Perez-Ruiz E,
Sarmento-Ribeiro AB, Saponara S, De Las Rivas J and Riganti C:
Hypoxia as a driver of resistance to immunotherapy. Drug Resist
Updat. 59:1007872021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)). method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Loren P, Saavedra N, Saavedra K, De Godoy
Torso N, Visacri MB, Moriel P and Salazar LA: Contribution of
MicroRNAs in chemoresistance to cisplatin in the top five deadliest
cancer: An updated review. Front Pharmacol. 13:8310992022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao T, Bai J, Zou Q, Chen F and Xie Y:
Insulin in combination with cisplatin induces the apoptosis of
ovarian cancer cells via p53 and JNK activation. Mol Med Rep.
16:9095–9101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lheureux S, Gourley C, Vergote I and Oza
AM: Epithelial ovarian cancer. Lancet. 393:1240–1253. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lheureux S, Braunstein M and Oza AM:
Epithelial ovarian cancer: Evolution of management in the era of
precision medicine. CA Cancer J Clin. 69:280–304. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Long J, Yang Y, Kang T, Zhao W, Cheng H,
Wu Y, Du T, Liu B, Li Y, Luo F and Gou M: Ovarian cancer therapy by
VSVMP gene mediated by a paclitaxel-enhanced nanoparticle. ACS Appl
Mater Interfaces. 9:39152–39164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhong Q, Wen YJ, Yang HS, Luo H, Fu AF,
Yang F, Chen LJ, Chen X, Qi XR, Lin HG, et al: Efficient inhibition
of cisplatin-resistant human ovarian cancer growth and prolonged
survival by gene transferred vesicular stomatitis virus matrix
protein in nude mice. Ann Oncol. 19:1584–1591. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Unno Y, Shino Y, Kondo F, Igarashi N, Wang
G, Shimura R, Yamaguchi T, Asano T, Saisho H, Sekiya S and
Shirasawa H: Oncolytic viral therapy for cervical and ovarian
cancer cells by Sindbis virus AR339 strain. Clin Cancer Res.
11:4553–4560. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Galanis E, Hartmann LC, Cliby WA, Long HJ,
Peethambaram PP, Barrette BA, Kaur JS, Haluska PJ Jr, Aderca I,
Zollman PJ, et al: Phase I trial of intraperitoneal administration
of an oncolytic measles virus strain engineered to express
carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res.
70:875–882. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chebouti I, Kuhlmann JD, Buderath P, Weber
S, Wimberger P, Bokeloh Y, Hauch S, Kimmig R and Kasimir-Bauer S:
ERCC1-expressing circulating tumor cells as a potential diagnostic
tool for monitoring response to platinum-based chemotherapy and for
predicting post-therapeutic outcome of ovarian cancer. Oncotarget.
8:24303–24313. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bösmüller H, Haitchi-Petnehazy S,
Webersinke G, Marschon R, Roithmeier F, Stummvoll W, Fehm T,
Klier-Richter M, Bonzheim I, Staebler A and Fend F: Intratumoral
lymphocyte density in serous ovarian carcinoma is superior to ERCC1
expression for predicting response to platinum-based therapy.
Virchows Arch. 459:183–191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Innao V, Rizzo V, Allegra AG, Musolino C
and Allegra A: oncolytic viruses and hematological malignancies: A
new class of immunotherapy drugs. Curr Oncol. 28:159–183. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chintala NK, Choe JK, McGee E, Bellis R,
Saini JK, Banerjee S, Moreira AL, Zauderer MG, Adusumilli PS and
Rusch VW: Correlative analysis from a phase I clinical trial of
intrapleural administration of oncolytic vaccinia virus (Olvi-vec)
in patients with malignant pleural mesothelioma. Front Immunol.
14:11129602023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vijver SV, Danklmaier S, Pipperger L,
Gronauer R, Floriani G, Hackl H, Das K and Wollmann G: Prediction
and validation of murine MHC class I epitopes of the recombinant
virus VSV-GP. Front Immunol. 13:11007302023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cohn DE, Sill MW, Walker JL, O'Malley D,
Nagel CI, Rutledge TL, Bradley W, Richardson DL, Moxley KM and
Aghajanian C: Randomized phase IIB evaluation of weekly paclitaxel
versus weekly paclitaxel with oncolytic reovirus (Reolysin) in
recurrent ovarian, tubal, or peritoneal cancer: An NRG
Oncology/Gynecologic Oncology Group study. Gynecol Oncol.
146:477–483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Moreno V, Barretina-Ginesta MP,
García-Donas J, Jayson GC, Roxburgh P, Vázquez RM, Michael A,
Antón-Torres A, Brown R, Krige D, et al: Safety and efficacy of the
tumor-selective adenovirus enadenotucirev with or without
paclitaxel in platinum-resistant ovarian cancer: A phase 1 clinical
trial. J Immunother Cancer. 9:e0036452021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liao H, Zhang L, Lu S, Li W and Dong W:
KIFC3 promotes proliferation, migration, and invasion in colorectal
cancer via PI3K/AKT/mTOR signaling pathway. Front Genet.
13:8489262022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hinz N and Jücker M: Distinct functions of
AKT isoforms in breast cancer: A comprehensive review. Cell Commun
Signal. 17:1542019. View Article : Google Scholar : PubMed/NCBI
|