Globally, stroke ranks as the second leading cause
of death, and the primary cause of disability (1). It imposes a significant burden on
patients, their families and society as a whole, with ~3 million
new cases occurring each year (2,3).
Stroke is usually referred to as an acute cerebrovascular disease,
the main mechanism of which is the sudden rupture or blockage of
blood vessels in the brain, resulting in an inability of blood to
enter the brain, leading to a lack of blood sugar (4) and oxygen (5), resulting in metabolic changes
(6), cell death (7) and brain damage. Stroke is usually
divided into two major categories; namely, ischemic stroke
(including cerebral infarction) and hemorrhagic stroke (including
cerebral hemorrhage and subarachnoid hemorrhage) (8). Concurrently, ischemia-reperfusion
injury often occurs during the treatment of stroke, especially when
reperfusion therapies, such as vascular recanalization procedures,
are employed (9). A close
association exists among stroke, ischemia-reperfusion injury and
hemorrhagic stroke, as abnormalities in cerebral blood supply are a
common feature for all of these conditions, which may lead to
either damage or death of neural cells (9). When treating stroke, it is essential
to consider these distinct types of injury mechanisms and their
corresponding therapeutic approaches. The incidence of stroke is
increasing, due to the increasing population and aging (8). Long-term disability and cognitive
impairment are considered to be major causes of stroke, which is
characterized by high morbidity and disability rates; thereby,
stroke generally requires the support of the healthcare system
(10). Therefore, exploring
different means of intervention therapy for the purposes of the
treatment of stroke remains a current international concern.
Ferroptosis is rapidly becoming understood to be one
of the key cell death mechanisms associated with stroke (11). As is well known, ferroptosis is a
type of programmed cell death that is distinct from other forms or
types of cell death; it is characterized by an increase in the
level of lipid peroxides, which are lethal substances that
ultimately lead to oxidative stress and cell death (12). Ferroptosis differs from other forms
of cell death in that morphologically, it typically manifests as
increased cell volume, mitochondrial swelling and endoplasmic
reticulum expansion (13);
physiologically, ferroptosis involves processes such as excessive
accumulation of iron ions and oxidative stress (14); and genetically, mutations or
genetic variations in genes related to iron metabolism (15) or oxidative stress (16) may affect the occurrence and
progression of ferroptosis (17).
In addition, another characteristic of ferroptosis is that it is
induced by abnormal oxidation in the intracellular
microenvironment, primarily under the influence of glutathione
peroxidase 4 (GPX4) activity (18). Decreased activity of GPX4 prevents
the metabolism of lipid peroxides via GPX4-catalyzed glutathione
(GSH) reduction reactions, resulting in the oxidation of divalent
iron ions and the generation of reactive oxygen species (ROS) in
lipids (19). As the cellular
antioxidant capacity weakens and lipid ROS accumulate, the redox
balance within cells is thereby disrupted, which induces cell death
(20). Moreover, this process has
also been shown to affect both upstream- and downstream-related
proteins (or genes), thereby exerting different effects on the
intracellular microenvironment, which ultimately influences the
outcome of ferroptosis (21). The
accumulation of iron ions is also one of the hallmark features of
ferroptosis, accompanied by an accumulation of lethal levels of
lipid peroxidation, which occurs in response to the Fenton reaction
(22). The untimely increase or
decrease in the intraorganismal level of iron, as the expression of
iron in ferroptosis is a crucial process, will have a marked impact
on the organism in question, eventually leading to the development
of various diseases such as hemochromatosis or Parkinson's disease
(15). In other words, the
majority of the changes that occur with respect to the level of
iron within organisms are primarily associated with iron metabolism
(23). The interconversion between
Fe3+ and Fe2+ generates toxic ROS that are
often detrimental to cells, and hence iron metabolism is strictly
regulated within the body (24).
When the expression of proteins associated with iron metabolism is
affected, this can influence either the intake or loss of iron,
thereby impacting ferroptosis (25). Although the presence of ferroptosis
was first demonstrated in cancer, given the increasing number of
associated studies, ferroptosis has been shown to fulfill an
important role within the nervous system (11,26–30),
and common neurological disorders, including ischemic stroke
(31), Alzheimer's disease
(32) and Parkinson's disease
(33), have been found to be
closely associated with ferroptosis, the latter two being common
neurodegenerative disorders wherein the molecular mechanism is
mainly concerned with an aggregation of iron in the hippocampal
region of the brain or in dense areas of the substantia nigra, and
these responses have been shown to be inhibited by the ferroptosis
inhibitor, ferrostatin-1 (Fer-1) (34–36).
In conclusion, a growing number of studies confirm
the link between ferroptosis and stroke. The inherent pathological
changes of stroke have been shown to be closely related to the
characteristics of ferroptosis, including iron metabolism
disorders, lipid peroxidation, and elevated ROS levels. Ferroptosis
may provide a promising therapeutic approach for treating stroke
patients. Therefore, the present review provides a comprehensive
overview of potential therapeutic targets provided by
ferroptosis-related pathways in stroke, providing new insights into
how ferroptosis can be exploited to treat stroke.
Over previous years, ferroptosis has become an area
of interest for researchers (12).
Ferroptosis is a relatively recently discovered form of regulated
cell death that is characterized by the accumulation of
iron-dependent lipid peroxides (37). It has been implicated in various
pathological conditions, including stroke (30). When the concept of ferroptosis was
initially proposed, researchers identified key features of
ferroptosis in HT-1080 cells using H2DCFDA and
C11-BODIPY fluorescent probes (38). These features included abnormal
accumulation of lipid peroxides and ROS (38). Subsequently, molecular experimental
techniques such as western blotting and polymerase chain reaction
were used to investigate the changes in ferroptosis-related
proteins and genes in stroke. The results revealed the involvement
of various pathways, including iron metabolism, lipid peroxidation
and oxidative stress in ferroptosis (39). Understanding the
ferroptosis-associated pathways in stroke can guide the development
of novel therapeutic strategies for stroke treatment (40). Targeting these pathways may help to
mitigate oxidative stress (41),
lipid peroxidation (30) and
subsequent neuronal damage (42),
ultimately improving stroke outcomes. However, further research is
needed to fully elucidate the intricate molecular mechanisms
involved in ferroptosis and their potential as therapeutic targets
in stroke.
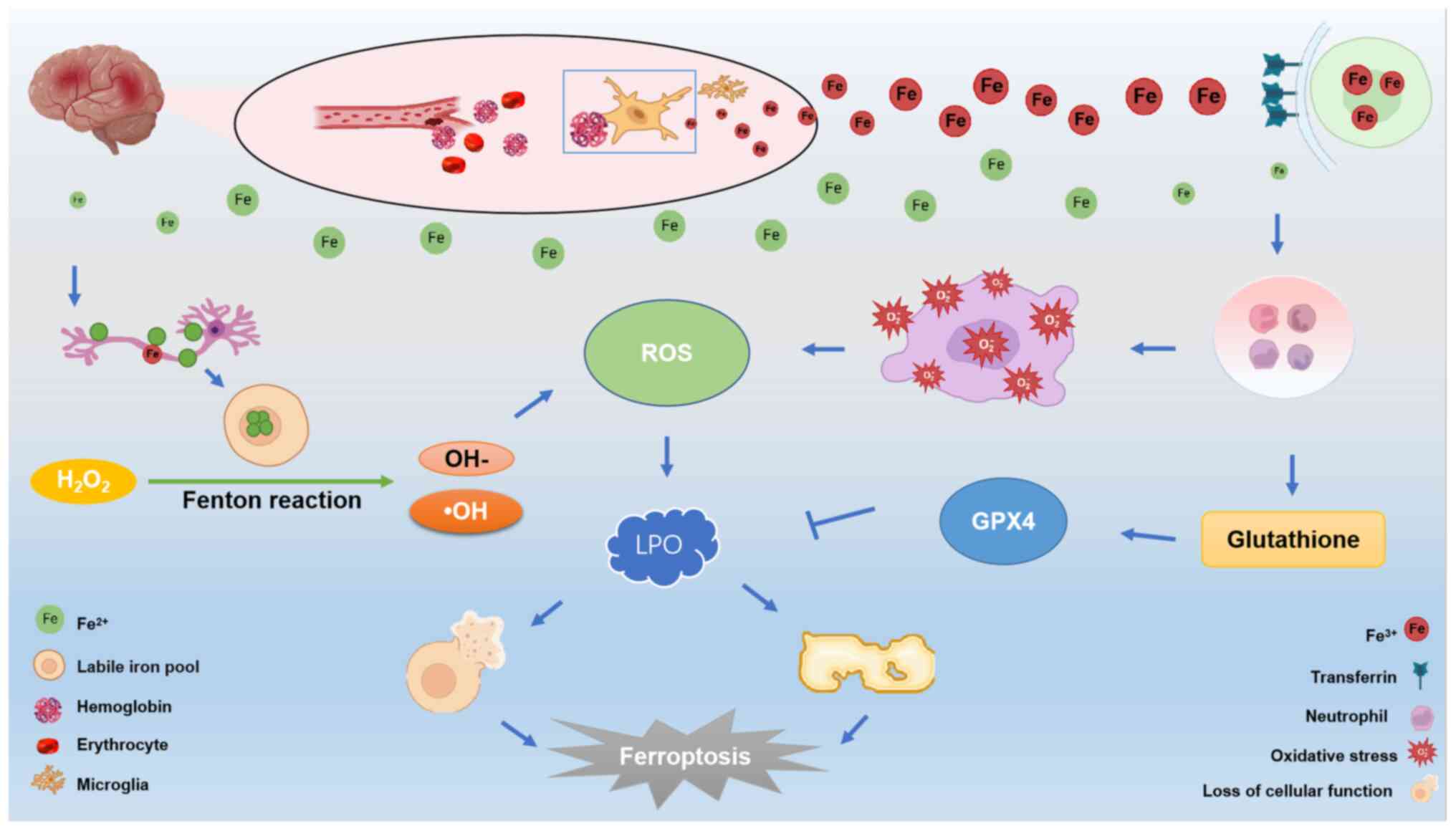
Iron metabolism is a crucial system in organisms,
and it has been shown to be closely associated with ferroptosis
(15). Ferroptosis not only
influences the organism, but also has an impact on various other
physiological processes. The overload of iron ions exacerbates the
occurrence of cerebral hemorrhage, inducing the onset of
ferroptosis (11). In the event of
cerebral hemorrhage, blood vessels rupture, leading to the release
of a substantial amount of blood and hemoglobin (43). Subsequently, microglia rapidly
engulf the released hemoglobin and metabolize
Fe2+/Fe3+ (30). The accumulation of Fe2+
and Fe3+ signifies an iron overload due to excessive
iron build-up, and this is a key factor in ferroptosis (15). Released Fe3+ ions enter
cells by binding with transferrin receptors on the cell membrane
(44). Once inside the cell,
Fe3+ can be reduced to Fe2+ by ferric
reductase, a process that is facilitated by hydroxyl radicals.
Accumulated ROS resulting from this process lead to the
peroxidization of membrane lipids, subsequently leading to a loss
of cellular function and cell death. This phenomenon represents one
of the characteristic features of ferroptosis (44). Alternatively, excess
Fe3+ can enter the unstable iron pool through solute
carrier family 39 member 14 (45),
further promoting ferroptosis. Excess Fe2+ can be
re-oxidized to Fe3+, which is subsequently moved to the
extracellular compartment, contributing to a series of iron
metabolism processes associated with ferroptosis. Moreover, the
presence of this free iron stimulates the production of lipid ROS
via participating in the inflammatory response (46), inducing oxidative stress (47), and the Fenton reaction (22). Consequently, there is an
accumulation of lipid ROS in vivo, which leads to DNA
(48), protein (49) and lipid damage (50), ultimately resulting in cell death.
Studies have also demonstrated that neurons require both the
ferroptosis inhibitory factor, GPX4, and genes involved in the
GPX4-synthesis pathway to survive under conditions of oxidative
stress (21,51,52).
GPX4 utilizes GSH to reduce peroxidized lipids, thereby preventing
lipoatrophy (53). This suggests
that neurons are prone to undergoing ferroptosis when exposed to
oxidative stress (54).
Additionally, the excess iron ions metabolized by microglia are
expelled through the transferrin receptor system, leading to a
significant accumulation of iron in neurons (55). Subsequently, the neurons undergo
the classical reaction of ferroptosis (the Fenton reaction)
(56). This reaction catalyzes the
generation of ROS, further promoting lipid peroxidation and leading
to lipid peroxide accumulation, ultimately inducing ferroptosis
(57) (Fig. 1).
Lipid peroxidation is a process in which lipids lose
hydrogen atoms due to the activity of free radicals or lipid
peroxidases (58). This leads to
oxidation, fragmentation and the shortening of lipid carbon chains,
resulting in the generation of lipid free radicals, lipid
hydroperoxides and reactive aldehydes (such as malondialdehyde and
4-hydroxynonenal) (59).
Ultimately, this process causes the oxidative degradation of
lipids, thereby damaging the cells. The end product of the Fenton
reaction, -OH, fulfills a crucial role in ferroptosis (60). The increase in -OH radicals induces
oxidative damage, leading to ferroptosis, and an exacerbation of
the edema response at the site of cerebral hemorrhage (61,62).
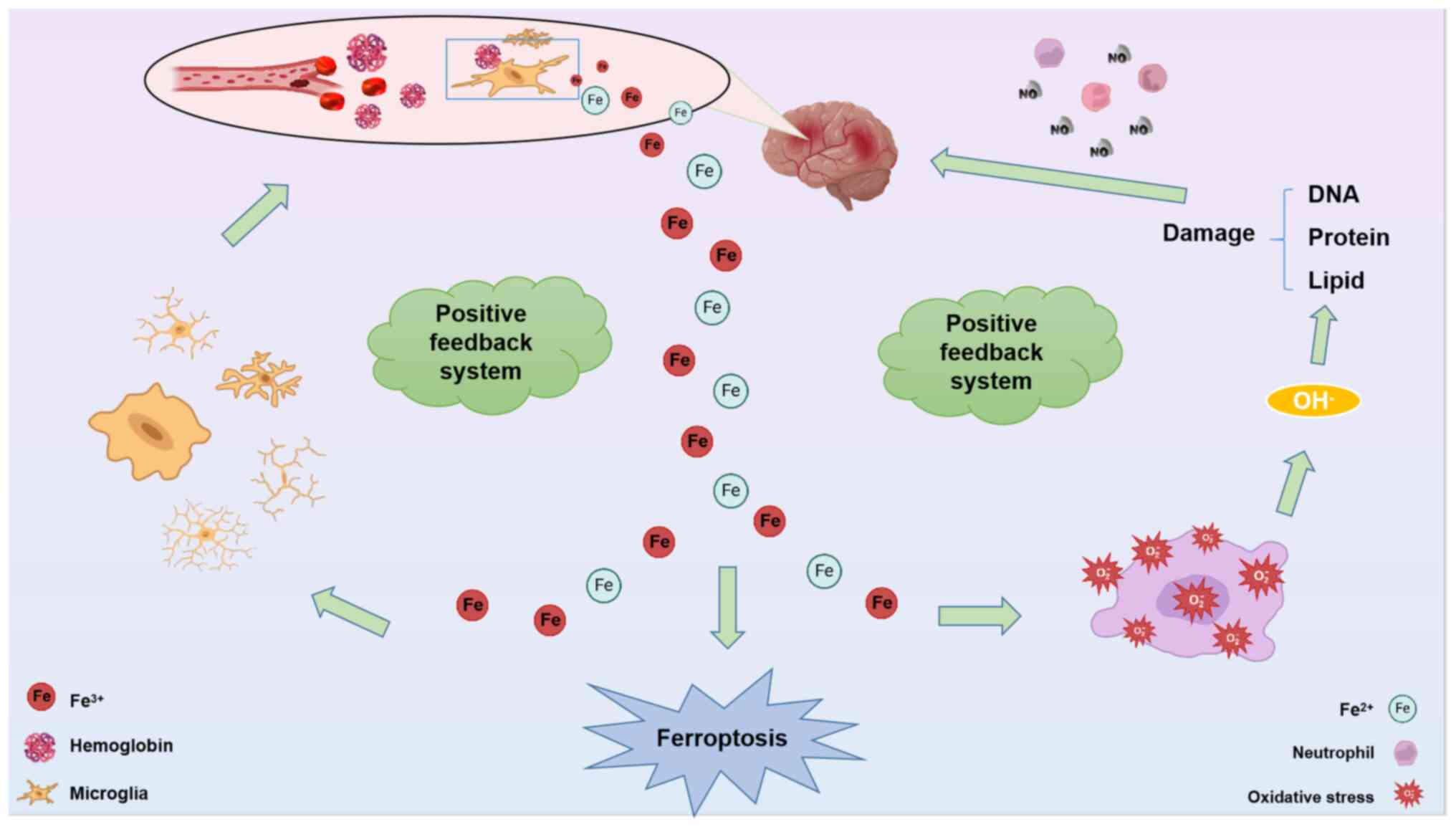
Building upon this, in the event of a cerebral hemorrhage,
ferroptosis occurring in the affected region triggers the release
of iron ions from blood cells (63). These iron ions instigate oxidative
stress reactions, leading to the production of -OH radicals that
subsequently target DNA, proteins, and lipid membranes. The
resulting damage to these components often aligns with the
manifestation of ferroptosis in the brain. Furthermore, the regions
affected by ferroptosis are subjected to significant iron
accumulation. This accumulation of iron has two subsequent effects.
First, it stimulates microglia to continue engulfing the hemoglobin
that is released from blood cells, leading to the secretion of yet
more iron ions, creating a positive feedback loop (64). Secondly, the accumulating iron
participates in various redox reactions in the brain, resulting in
an increase in the production of -OH radicals (65) (Fig.
2). In other words, when cerebral hemorrhage occurs, there can
be a positive feedback system loop promoting ferroptosis through
Fe/-OH/DNA damage/Fe and Fe/microglia/Fe interactions.
Consequently, the brain is subjected to further oxidative damage.
This oxidative damage, in the presence of interleukin and nitric
oxide, further compromises the integrity of the blood-brain
barrier, ultimately leading to the development of cerebral edema at
the site of hemorrhage (66).
Lipoxygenases (LOXs), a key component in the process
of ferroptosis, have gained significant attention in recent years
(67). One study demonstrated that
conducting diphenyl-1-pyrenylphosphine experiments using HEK-293
cells revealed the formation of lipid hydroperoxides catalyzed by
LOX (68). Based on the findings
of this study, it is hypothesized that LOX activity may enhance the
accumulation of lipid hydroperoxides within cells, thereby
promoting ferroptosis. This may be associated with a specific
ferroptotic pathway, such as the hypoxia-inducible factor-prolyl
hydroxylase domain pathway (69).
Therefore, measuring LOX activity and the levels of lipid
hydroperoxides may serve as valuable assays for assessing the
occurrence of ferroptosis (70),
given that LOX activation may drive this process (71).
It is widely recognized that elevated levels of
glutamate can exert neurotoxic effects on the brain during disease
conditions (72). In response to
this neurotoxic effect, system Xc− fulfills a crucial
function. System Xc− is a heterodimeric
cystine/glutamate antiporter protein that consists of two core
components: Solute carrier family 7 member 11 (SLC7A11), serving as
the catalytic subunit, and solute carrier family 3 member 2, which
acts as an anchoring protein (73). In the context of stroke and its
relevance to ferroptosis, particular attention has been focused on
the SLC7A11 gene, which encodes a sodium-independent member of the
anionic amino acid transport system (51). Its highly specific role in
transporting cysteine (74) and
glutamate (75) has been shown to
be of great importance. In general, system Xc−
facilitates the extracellular transport of high intracellular
concentrations of glutamate, whereas the anionic form of cysteine
is transported in exchange for glutamate (76). The intracellularly transported
cysteine is subsequently utilized for the synthesis of cysteine and
GSH (77). GSH, a tripeptide
composed of glutamate, cysteine and glycine, fulfills critical
roles in various physiological functions, including the scavenging
of free radicals, acting as an antioxidant (78) and maintaining cellular redox
balance (79). GSH also activates
various enzymes that influence cellular metabolic processes, and
serves as an essential intracellular antioxidant in brain diseases
(such as Parkinson's disease), contributing to the scavenging of
free radicals and preserving redox balance both inside and outside
of cells (80).
GPX4, a downstream target of GSH action, functions
as a unique intracellular antioxidant enzyme acting on membrane
lipid repair, which is able to directly reduce peroxidized
phospholipids produced in cell membranes (88,89),
and it converts toxic lipid ROS into non-toxic lipid alcohols with
the aid of GSH, which thereby reduces the level of oxidative damage
to cells (90). However, the
generation of large amounts of lipid ROS during stroke disrupts
this oxidative balance, resulting in both a large accumulation of
lipid ROS and the development of peroxidation, which is the main
feature of ferroptosis (42). In
addition, lipid ROS have also been shown to directly react with
polyunsaturated fatty acids (PUFAs) on lipid membranes via
oxidation, which directly leads to the occurrence of ferroptosis in
cells (91).
AMPK has a critical role in ferroptosis following
cerebral hemorrhage. AMPK is an energy sensor widely present in
various tissues and cells. For instance, when it is present in
brain tissue and microglial cells, it can alleviate secondary
damage from cerebral haemorrhage (92). It regulates cellular energy
metabolism balance (93), and
participates in the regulation of multiple biological processes,
including glycogen synthesis (94), fatty acid synthesis (95) and mitochondrial biogenesis
(96). Studies have found that
Spatholobi Caulis (SC) has the ability to activate AMPK
(97). Subsequent
2,7-dichlorodihydrofluorescein diacetate experiments using HepG2
cells revealed that the combined effect of arachidonic acid (AA)
and iron led to increased intracellular ROS production (97). However, pretreatment with SC
suppressed the production of ROS when combined with AA and iron.
This suggests that AMPK may possess antioxidant capacity (97). In in vivo experiments, using
a mouse liver injury model, it was shown that oral administration
of SC (which activates AMPK) could alleviate liver damage mediated
by acute acetaminophen (by enhancing oxidative stress and
increasing cell injury), exerting antioxidant effects (97). Therefore, both in vivo and
in vitro experiments indicate that AMPK can play a role in
protecting against oxidative damage, possibly by inhibiting
ferroptosis to provide neuroprotection (97). Furthermore, in vitro and
in vivo models of intracerebral hemorrhage were created by
treating BV2 cells with hemoglobin and intraventricular injection
of type IV collagenase into Sprague-Dawley rats, respectively.
In vitro experiments showed a phosphorylation reaction of
AMPK after initial intervention with AMPK inhibitors in BV2 cells.
Subsequently, pharmacological intervention led to upregulation of
ROS and lipid peroxidation levels (positive regulators of
ferroptosis) in BV2 cells, and silenced GPX4 (a key negative
regulator of ferroptosis) levels through the AMPK signaling pathway
(92). In in vivo
experiments, iron deposition at the site of brain injury was
observed through Perl's staining (92). So, both in vitro and in
vivo experiments have demonstrated the occurrence of
ferroptosis during cerebral hemorrhage. Additionally, the AMPK
signaling pathway, targeting GPX4 (a negative regulator of
ferroptosis), was found to be involved in neuroprotection (92). Moreover, in the field of tumor
research, it has been shown that AMPK can reduce the occurrence of
ferroptosis by regulating intracellular lipid synthesis metabolism
(98). When ferroptosis occurs,
the AMPK-mediated phosphorylation of acetyl-coenzyme A carboxylase
is considered to inhibit ferroptosis by limiting the production of
PUFAs (99). However, the specific
role and mechanism of AMPK in the field of cerebral hemorrhage and
ferroptosis have yet to be fully elucidated. When AMPK activation
protects neurons from damage caused by cerebral hemorrhage through
a series of antioxidant, anti-inflammatory and anti-apoptotic
pathways, this may result in a reduction in the occurrence of
ferroptosis (98). However,
following cerebral hemorrhage, AMPK activation may increase the
levels of intracellular free iron ions, which, in turn, may
exacerbate oxidative stress and cell damage, potentially
contributing to ferroptosis (92).
Therefore, the mechanism of action of AMPK in cerebral hemorrhage
may potentially exert an impact on ferroptosis. However, the
current research findings are both inconsistent and limited.
Further research is needed to address this issue.
The SIRT2-p53 pathway exerts neuroprotective effects
by modulating GPX4 and SCL7A11, inhibiting the occurrence of
ferroptosis (100). SIRT2 is a
NAD+-dependent deacetylase predominantly localized in the cytoplasm
and has been demonstrated to be involved in the mechanisms of
neuroinflammation- and neuroimmunology-related diseases (101). Additionally, P53, a tumor
suppressor protein, has also been implicated in ferroptosis. In the
context of ferroptosis, P53 has been shown to regulate the
expression of key genes involved in iron metabolism, lipid
peroxidation and antioxidant defense. P53 inhibits cystine uptake
and sensitizes cells to ferroptosis by inhibiting the expression of
SLC7A11, a key component of cystine/glutamate retrotransporters.
Previously, the traumatic brain injury model using the controlled
cortical impact (CCI) injury method found that knockdown of P53
could significantly block ferroptosis after CCI. In addition,
inhibition of SIRT2 led to upregulation of acetylation and
expression of P53, exacerbating ferroptosis after CCI. In other
words, P53-mediated ferroptosis is involved in the pathogenesis of
TBI, and SIRT2 exerts a neuroprotective effect on TBI by inhibiting
P53-mediated ferroptosis (100).
The overall role of NCOA4 in cerebral stroke has
been controversial, and its specific effects on ferroptosis,
contributing to this process, have yet to be fully elucidated.
Currently, studies on NCOA4 have revealed its potential dual role
in either promoting (102) or
inhibiting ferroptosis (103).
Moreover, investigations on ovarian cancer cell models with
manipulated NCOA4 expression have implicated NCOA4 in the processes
of both formation and degradation of intracellular iron storage
autophagosomes (104).
Furthermore, in ovarian cancer cells, it has been observed that
up-regulation of C-MYC (a gene regulating tumor proliferation)
leads to a significant reduction in ROS content (104). On the other hand, overexpression
of NCOA4 reverses these changes. These findings suggest that C-MYC
may exert an inhibitory effect on ferroptosis in ovarian cancer
cells through NCOA4-mediated ferritin autophagy (104). The process of NCOA4-mediated
ferritin autophagy appears to play a role in suppressing
ferroptosis in ovarian cancer cells (104). Autophagy-associated pathways
induce an excessive degradation of ferritin, leading to an increase
in the amount of free iron in neurons. Therefore, when cerebral
ischemia occurs, NCOA4 may promote the release of iron ions and
trigger ferroptosis in cells (105). Due to the large impact of NCOA4
in cerebral ischemia-reperfusion injury, it has been shown that the
autophagy-related 5 (ATG5)-ATG7-NCOA4 pathway also has an important
role in ferroptosis (106).
Notably, NCOA4 is a selective autophagy receptor that is essential
for mediating ferritin phagocytosis in certain tissues (for
example, brain tissue) and cells (for example, red blood cells)
(107,108). However, other studies have found
that, under conditions such as hypoxic-ischemic brain injury, NCOA4
may protect cells from excessive damage caused by free iron ions
via regulating intracellular iron metabolism. In this case, NCOA4
is considered as a factor that inhibits ferroptosis (109,110).
In animal models of ischemic stroke and associated
clinical specimens, it was found that, when cerebral
ischemia-reperfusion occurs, an increase in the level of iron ions
exacerbates neuronal damage (111). However, subsequent research has
indicated that there may be a close link between the increase in
the level of iron ions and ferroptosis. The increase in iron
undoubtedly exacerbates the occurrence of ferroptosis, and so, iron
may act as a catalyst for the occurrence of ferroptosis, or serve a
role as an inducer (112).
Therefore, when a stroke occurs, is it possible that some of the
inducers associated with ferroptosis may accelerate progression of
the stroke, thereby leading to more serious consequences (Table I) (113–120).
Inducers of ferroptosis may generally be grouped
into categories, based on the effects of targeted interventions at
different sites. In general, class I ferroptosis inducers that are
typically used are erastin (113)
(which inhibits Xc− cystine uptake), glutamate (121) (which inhibits glutamate transfer
to reduce Xc− activity) and sulfasalazine (122) (which inhibits Xc− in
the cell membrane). However, the most common class I ferroptosis
inducer is erastin and was first discovered before the concept of
ferroptosis came into existence (38). It was then shown that the newly
discovered compound erastin had no effect on either the apoptosis
or necrosis of cells, but it was found that lipophilic ROS were
involved in the process of ferroptosis of cells (113). After the concept of ferroptosis
had been confirmed, further studies identified that erastin
inhibited Xc− cystine uptake, and therefore it was
categorized as a class I inducer of ferroptosis for medical
research (123,124). Previous studies have revealed
that erastin-induced ferroptosis is a significant feature of
intracerebral hemorrhage. In a mouse model of intracerebral
hemorrhage (collagenase model), it was observed that the mRNA
expression of GPX4, a crucial regulator of ferroptosis, was
increased. This finding suggests that ferroptosis is regulated in
the context of intracerebral hemorrhage (125). Moreover, cell experiments were
conducted using rat PC12 cells, and the results indicated a
distinct decrease in the survival rate of these cells when treated
with erastin. This decrease in cell survival strongly suggests the
occurrence of ferroptosis in response to erastin treatment
(125). In various investigations
examining the link between stroke and ferroptosis, erastin has been
utilized to trigger ferroptosis, revealing its potential
neuroprotective effect in alleviating stroke symptoms (126). However, further experimental
investigations are needed to fully understand the precise mechanism
by which erastin induces ferroptosis during stroke. It is
hypothesized that erastin may play a valuable role in providing
neuroprotection during strokes in the future.
The commonly used class II ferroptosis inducers are
RAS-selective lethal 3 (RSL3) (127),
2-chloro-N-(3-chloro-4-methoxyphenyl)-N-(2-oxo-2-(phenethylamino)-1-(thiophen-2-yl)ethyl)acetamide
(128) and cisplatin (which binds
to GSH and inactivates GPX4) (129). However, the most commonly used
class II ferroptosis inducer is RSL3, predominantly since RSL3 is
an inducer that can either indirectly or directly induce the
occurrence of ferroptosis (130).
The main effect of RSL3 is that it can directly bind to GPX4
protein, thereby inactivating GPX4, at which point the production
of lipid ROS is increased, leading to the occurrence of ferroptosis
(131). Furthermore, it has been
shown in other studies that the protective effects against cerebral
ischemia-reperfusion injury, achieved by inhibiting ferroptosis,
are partially diminished upon induction by RSL3 (125,132). In the context of cerebral
hemorrhage ischemia-reperfusion injury, the action of RSL3 has been
found to worsen the incidence of ferroptosis (133). However, the precise underlying
mechanisms of this action have not yet been fully elucidated,
necessitating further experimental and theoretical
investigations.
There are also class III and class IV ferroptosis
inducers, which similarly intervene in the process of ferroptosis
through a number of different pathways. The more commonly used
class III ferroptosis inducers are FSP1 inhibitor (which inhibits
FSP1 activity and reduces coenzyme Q10 production) (134), statins (which inhibit the
mevalonate pathway) (135,136),
and class IV ferroptosis inducers, such as heme (which increases
the amount of intracellular iron in the unstable state) (137) and artemisinin (which induces
ferritin autophagy, causing the release of iron in the unstable
state) (138). These ferroptosis
inducers are representative in research related to cerebral stroke
and can mitigate the occurrence of cerebral stroke by inducing
ferroptosis (125,126).
Following a stroke, various forms of cell death
occur in the body, the main ones of which are apoptosis (139), necrosis (140) and autophagy (141). It has been shown that the use of
different inhibitors to inhibit apoptosis, necrosis and autophagy
is more effective than the use of any of these inhibitors in
isolation, as in the case of cerebral hemorrhage, where caspase
inhibitors have been shown to be unsuccessful in terms of
inhibiting hemoglobin-induced neuronal death (142). Therefore, when ferroptosis was
initially discovered, its inhibitors were also investigated within
the field of stroke research (143). Currently in the medical field, a
number of ferroptosis inhibitors have been identified, for which
specific information for commonly used inhibitors such as Fer-1 is
shown in Table II (144–152). In addition, with respect to the
ferroptosis inhibitors summarized in the present review and those
that are similar to them in terms of their function, their specific
target sites have been identified, as shown in Table III (146,147,153–155). Both in vitro and in
vivo experiments have shown that the levels of molecular
markers of ferroptosis are increased when cerebral hemorrhage
occurs. Experiments performed in vivo have shown that the
mortality rate may be reduced by ~80% with the use of ferroptosis
inhibitors (156,157). In vitro experiments
performed in a previously published study (140) have confirmed the occurrence of
ferroptosis without autophagy or apoptosis. Therefore, as a novel
form of cell death that is distinct from apoptosis, necrosis,
autophagy and other types of cell death, the use of certain
inhibitors targeting ferroptosis may have more pronounced
therapeutic effects with regard to the treatment of stroke
(40). The present review provided
detailed information on several different inhibitors of
ferroptosis. Deferoxamine (DFO) is an iron chelator that can act on
iron ions to inhibit the occurrence of ferroptosis (158). GPX4, as an important negative
regulator of ferroptosis (159),
may also effectively suppress the occurrence of ferroptosis
(150). Fer-1, a commonly used
inhibitor of ferroptosis, has been shown to have a significant role
in terms of inhibiting ferroptosis (146). Additionally, when combined with
inhibitors of other types of programmed cell death, Fer-1 has also
been shown to exert inhibitory effects on ferroptosis (160). These effects can alleviate
adverse reactions caused by stroke, thereby demonstrating that is
has some therapeutic potential in terms of the treatment of stroke
(161).
DFO induces ferroptosis during a stroke; DFO is an
iron chelator that is able to reduce the accumulation and
precipitation of iron in cells or tissues by binding to ferric
(Fe3+) ions to form a stable complex, thereby allowing
the removal of excess iron from cells (158). In the general field of cancer
research, DFO has been shown to have good antioxidant activity
(162); it acts as an
anti-proliferative agent, and can induce apoptosis in cancer cells
(163). At present, extensive
research is being performed on the use of DFO in various iron
overload-associated brain diseases (representing a class of
neurological disorders caused by excessive accumulation of iron
ions in the body, such as thalassemia). DFO has been shown to help
regulate iron balance in the body, and to reduce neurological
damage and functional impairments associated with iron overload
(164). Similarly, significant
research has also been performed on the role of DFO in
neurodegenerative diseases (such as diseases that are characterized
by neuronal cell death and functional impairments, including
Alzheimer's disease, Parkinson's disease and Huntington's disease,
which are associated with oxidative stress, abnormal
neurodevelopment and protein aggregation) (153). Upon reviewing the literature, DFO
has been shown to reduce the level of cell death through reducing
free radicals (165), and to
promote wound healing and healing in diabetic patients (166), although due to the potential
toxicity of DFO itself (167),
the research remains only at an early stage (160). In addition, animal experiments
were used to demonstrate that the treatment of mice with DFO,
wherein the aging process was simulated, led to a marked
alleviation of the occurrence of ferroptosis, with the consequent
inhibition of the increase of age spots in mice due to iron
overload, thereby achieving the desired protective effect of
delaying aging (168). In
investigating the neurological and cognitive functions of aged mice
in a study focused on the auditory cortex, the effect of
alleviating ferroptosis in the brain was achieved by treatment with
DFO, suggesting that DFO may be used to treat auditory and
cognitive impairment resulting from age-associated problems
(169). It is noteworthy that,
although DFO primarily functions as an iron chelator in ferroptosis
(158), investigations utilizing
rodent models propose that DFO could impact stroke through
gene-mediated mechanisms (69,170). Therefore, the role of DFO in
ferroptosis warrants further investigation.
Through clinical trial studies, TCM combined with
the influencing factors associated with ferroptosis has been shown
to be more effective than single-drug treatment for stroke
(177,178). It is well established that TCM
herbs have antioxidant, anti-inflammatory and blood-brain
barrier-protective effects, and that they can prevent stroke in
advance by various means (179–181). For example, Danhong injection, a
standardized injection comprising danshen (Salvia
miltiorrhiza) and saffron, has been shown in studies to improve
ferroptosis in ischemic stroke (182,183). In addition, moxibustion (a form
of therapy that entails the burning of mugwort leaves), as one of
the more frequently used treatments, has an important role in the
treatment of cerebral infarction. It has been found that
moxibustion can reduce neurological damage and neuronal death,
reduce the accumulation of ROS and inhibit ferroptosis (184). In conclusion, the effects of
certain Chinese medicines and their active ingredients on stroke
both involve multiple pathways and are multi-targeted. In addition,
the intervention of Chinese medicines on ferroptosis has been shown
to be more stable and safer to use compared with small-molecule
inducers or inhibitors of ferroptosis (185). For example, astragaloside IV can
alleviate brain injury by inhibiting the ferroptosis-associated
sequestosome-1/kelch-like ECH associated protein 1/nuclear factor
erythroid 2-related factor 2 pathway (177,186). In addition, there are studies
reporting that TCM treatment can reduce the side effects of drug
toxicity in patients via targeting ferroptosis, leading to
significant improvements in patient safety and quality of life
(187,188). The impact that specific Chinese
medicines and their active constituents have on stroke involves
multiple pathways and targets, and these are summarized in Table IV (125,182,189–191).
The present review provides a comprehensive
overview of the potential therapeutic targets of
ferroptosis-associated pathways in stroke, providing novel insights
into the application of ferroptosis in the treatment of stroke.
Ferroptosis is a relatively recently discovered form of cell death
that was first proposed by the laboratory of Brent R. Stockwell in
2012 (38). It is characterized by
an excessive accumulation of lipid peroxides, and this accumulation
is dependent on iron ions (192).
Ferroptosis distinguishes itself from other forms of cell death,
such as apoptosis, necrosis, autophagy (193,194) and pyroptosis, in terms of its
morphological and biological features, and its underlying
mechanistic regulation (44). It
is also associated with inflammation and oxidative stress, along
with other pathological processes (195). The characteristic morphological
changes observed in ferroptosis primarily include mitochondrial
atrophy, a ruptured outer membrane, reduced cristae, a compressed
inner membrane and intact nuclei (196). By contrast, apoptosis and
necrosis typically exhibit swollen mitochondria and fragmented
nuclei (197). In recent years,
the role of ferroptosis in various pathological processes has
gained significant attention. Although ferroptosis was initially
identified in studies that were associated with cancer, it has been
demonstrated to fulfill a crucial role in the progression and
toxicity of numerous neurological diseases, including stroke
(198), Parkinson's disease
(199) and Alzheimer's disease
(32). Multiple reviews have
highlighted ferroptosis as a promising target for various
neurological disorders, and these reviews have also summarized the
major regulators and associated studies in this field (178,200,201). The present review focused on
summarizing the ferroptosis-associated pathways in stroke and
discussed the potential therapeutic interventions using inhibitors
and inducers of ferroptosis, with the ultimate goal of alleviating
the impact of stroke.
The collection of studies published on ferroptosis
in stroke to date have provided a comprehensive and informative
overview of the field. By summarizing the findings from these
studies, specific research goals have been identified that should
guide targeted and precise investigations in the area of
ferroptosis in stroke. This approach allows results to be achieved
faster and more efficiently, avoiding unnecessary detours. By
summarizing this article, important signaling pathways of iron
death in stroke, and inhibitors and inductors of iron death can be
refined. Intervening in the signaling pathways of iron death and
applying inductors can slow down the occurrence of stroke. This
improved understanding will enable more accurate interventions for
stroke management, specifically targeting ferroptosis. In
conclusion, several preclinical studies (202,203) have confirmed the protective
effect of ferroptosis inhibitors in stroke, and have highlighted
the potential of these inhibitors as novel therapeutic drugs for
stroke treatment.
Although ferroptosis-associated pathways are
potential targets for the treatment of stroke, there remain certain
limitations that need to be addressed. First, although iron
overload is a key factor in ferroptosis, it remains unclear whether
other metal ions also serve a role in this process, or whether
alternative forms of cell death involving different metal ions also
have a participatory role (204).
Further investigations are needed to explore these possibilities.
Additionally, despite the numerous studies that have been published
on stroke and ferroptosis, very few of these have translated into
clinical applications. Although it has been established that
neuronal cells in the brain are particularly susceptible to
ferroptosis, the effects of ferroptosis on other cell types in the
brain have yet to be fully elucidated (51). Further studies exploring the
effects of ferroptosis on various brain cell types are necessary to
guide future research directions. In summary, targeting
ferroptosis-associated pathways represents a promising approach for
stroke treatment. However, there is a need for further research to
improve understanding of the roles and potential targets of
ferroptosis-associated pathways in stroke, which will provide
valuable insights for the prevention and treatment of stroke.
The present review focused on the impact of
ferroptosis on stroke and studied whether intervening in the
ferroptosis pathway can prevent and treat stroke. Inhibiting
ferroptosis pathways has shown promise in reducing neuronal cell
death, protecting brain tissue, and improving functional outcomes
in stroke models. This therapeutic approach not only provides new
therapeutic avenues beyond traditional approaches, but also
highlights the importance of understanding the mechanisms of
ferroptosis in developing more effective stroke therapies.
Therefore, the exploration of ferroptosis inhibitors or inducers
represents a major advance in stroke treatment and provides new
strategies for the prevention and treatment of cerebral stroke.
Not applicable.
This study was supported by The National Natural Science
Foundation of China (grant no. 82101410), The Medicine and Health
Science and Technology Development Plan Project of Shandong (grant
no. 202101040805) and Research and Innovation Plan Project of
Weifang Medical University (grant no. 2021BKQ).
Not applicable.
BGZ and MTH conceptualized the study; HD was
responsible for methodology and visualization; HD, YPM, MMC and ZHQ
were responsible for the writing, reviewing and editing of the
manuscript; and MTH and BGZ provided supervision and corrections.
All authors read and approved the final version of the manuscript.
Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare they have no competing
interests.
|
1
|
Shehjar F, Maktabi B, Rahman ZA, Bahader
GA, James AW, Naqvi A, Mahajan R and Shah ZA: Stroke: Molecular
mechanisms and therapies: Update on recent developments. Neurochem
Int. 162:1054582023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu S, Wu B, Liu M, Chen Z, Wang W,
Anderson CS, Sandercock P, Wang Y, Huang Y, Cui L, et al: Stroke in
China: Advances and challenges in epidemiology, prevention, and
management. Lancet Neurol. 18:394–405. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barthels D and Das H: Current advances in
ischemic stroke research and therapies. Biochim Biophys Acta Mol
Basis Dis. 1866:1652602020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martin S: Stroke: Does intensive blood
sugar control improve prognosis? Dtsch med Wochenschr.
137:26282012.(In German). PubMed/NCBI
|
|
5
|
Wu X, You J, Chen X, Zhou M, Ma H, Zhang T
and Huang C: An overview of hyperbaric oxygen preconditioning
against ischemic stroke. Metab Brain Dis. 38:855–872. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shin TH, Lee DY, Basith S, Manavalan B,
Paik MJ, Rybinnik I, Mouradian MM, Ahn JH and Lee G: Metabolome
changes in cerebral ischemia. Cells. 9:16302020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tuo QZ, Zhang ST and Lei P: Mechanisms of
neuronal cell death in ischemic stroke and their therapeutic
implications. Med Res Rev. 42:259–305. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Virani SS, Alonso A, Benjamin EJ,
Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR,
Cheng S, Delling FN, et al: Heart disease and stroke
statistics-2020 update: A report from the american heart
association. Circulation. 141:e139–e596. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Przykaza Ł: Understanding the connection
between common stroke comorbidities, their associated inflammation,
and the course of the cerebral ischemia/reperfusion cascade. Front
Immunol. 12:7825692021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rothwell PM, Algra A and Amarenco P:
Medical treatment in acute and long-term secondary prevention after
transient ischaemic attack and ischaemic stroke. Lancet.
377:1681–1692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo J, Tuo Q and Lei P: Iron, ferroptosis,
and ischemic stroke. J Neurochem. 165:487–520. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Cao F, Yin H, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11:882020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun
X, Kang R and Tang D: Ferroptosis: Process and function. Cell Death
Differ. 23:369–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu L, Liu Y, Chen X, Zhong H and Wang Y:
Ferroptosis in life: To be or not to be. Biomed Pharmacother.
159:1142412023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun Y, Li Q, Guo H and He Q: Ferroptosis
and iron metabolism after intracerebral hemorrhage. Cells.
12:902022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen GH, Song CC, Pantopoulos K, Wei XL,
Zheng H and Luo Z: Mitochondrial oxidative stress mediated
Fe-induced ferroptosis via the NRF2-ARE pathway. Free Radic Biol
Med. 180:95–107. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun Y, Chen P, Zhai B, Zhang M, Xiang Y,
Fang J, Xu S, Gao Y, Chen X, Sui X and Li G: The emerging role of
ferroptosis in inflammation. Biomed Pharmacother. 127:1101082020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, Wan Y, Jiang Y, Zhang L and Cheng
W: GPX4: The hub of lipid oxidation, ferroptosis, disease and
treatment. Biochim Biophys Acta Rev Cancer. 1878:1888902023.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X, Shen T, Lian J, Deng K, Qu C, Li
E, Li G, Ren Y, Wang Z, Jiang Z, et al: Resveratrol reduces
ROS-induced ferroptosis by activating SIRT3 and compensating the
GSH/GPX4 pathway. Mol Med. 29:1372023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Snezhkina AV, Kudryavtseva AV, Kardymon
OL, Savvateeva MV, Melnikova NV, Krasnov GS and Dmitriev AA: ROS
generation and antioxidant defense systems in normal and malignant
cells. Oxid Med Cell Longev. 2019:61758042019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fu C, Wu Y, Liu S, Luo C, Lu Y, Liu M,
Wang L, Zhang Y and Liu X: Rehmannioside A improves cognitive
impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2
and SLC7A11/GPX4 signaling pathway after ischemia. J
Ethnopharmacol. 289:1150212022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Henning Y, Blind US, Larafa S, Matschke J
and Fandrey J: Hypoxia aggravates ferroptosis in RPE cells by
promoting the Fenton reaction. Cell Death Dis. 13:6622022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kosman DJ: Redox cycling in iron uptake,
efflux, and trafficking. J Biol Chem. 285:26729–26735. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee J and Hyun DH: The interplay between
intracellular iron homeostasis and neuroinflammation in
neurodegenerative diseases. Antioxidants (Basel). 12:9182023.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Recalcati S, Gammella E and Cairo G:
Dysregulation of iron metabolism in cancer stem cells. Free Radic
Biol Med. 133:216–220. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pan F, Xu W, Ding J and Wang C:
Elucidating the progress and impact of ferroptosis in hemorrhagic
stroke. Front Cell Neurosci. 16:10675702023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weiland A, Wang Y, Wu W, Lan X, Han X, Li
Q and Wang J: Ferroptosis and its role in diverse brain diseases.
Mol Neurobiol. 56:4880–4893. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Q, Han X, Lan X, Gao Y, Wan J, Durham
F, Cheng T, Yang J, Wang Z, Jiang C, et al: Inhibition of neuronal
ferroptosis protects hemorrhagic brain. JCI Insight. 2:e907772017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Speer RE, Karuppagounder SS, Basso M,
Sleiman SF, Kumar A, Brand D, Smirnova N, Gazaryan I, Khim SJ and
Ratan RR: Hypoxia-inducible factor prolyl hydroxylases as targets
for neuroprotection by ‘antioxidant’ metal chelators: From
ferroptosis to stroke. Free Radic Biol Med. 62:26–36. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu Y, Li K, Zhao Y, Zhou L, Liu Y and Zhao
J: Role of ferroptosis in stroke. Cell Mol Neurobiol. 43:205–222.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu J, Guo ZN, Yan XL, Huang S, Ren JX,
Luo Y and Yang Y: Crosstalk between autophagy and ferroptosis and
its putative role in ischemic stroke. Front Cell Neurosci.
14:5774032020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bao WD, Pang P, Zhou XT, Hu F, Xiong W,
Chen K, Wang J, Wang F, Xie D, Hu YZ, et al: Loss of ferroportin
induces memory impairment by promoting ferroptosis in Alzheimer's
disease. Cell Death Differ. 28:1548–1562. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mahoney-Sánchez L, Bouchaoui H, Ayton S,
Devos D, Duce JA and Devedjian JC: Ferroptosis and its potential
role in the physiopathology of Parkinson's disease. Prog Neurobiol.
196:1018902021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Chen G and Shao W: Identification
of ferroptosis-related genes in Alzheimer's disease based on
bioinformatic analysis. Front Neurosci. 16:8237412022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang C, Chen S, Guo H, Jiang H, Liu H, Fu
H and Wang D: Forsythoside A mitigates Alzheimer's-like pathology
by inhibiting ferroptosis-mediated neuroinflammation via Nrf2/GPX4
axis activation. Int J Biol Sci. 18:2075–2090. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jakaria M, Belaidi AA, Bush AI and Ayton
S: Ferroptosis as a mechanism of neurodegeneration in Alzheimer's
disease. J Neurochem. 159:804–825. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee
H, Koppula P, Wu S, Zhuang L, Fang B, et al: DHODH-mediated
ferroptosis defence is a targetable vulnerability in cancer.
Nature. 593:586–590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Alim I, Caulfield JT, Chen Y, Swarup V,
Geschwind DH, Ivanova E, Seravalli J, Ai Y, Sansing LH, Ste Marie
EJ, et al: Selenium drives a transcriptional adaptive program to
block ferroptosis and treat stroke. Cell. 177:1262–1279.e25. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ren JX, Li C, Yan XL, Qu Y, Yang Y and Guo
ZN: Crosstalk between oxidative stress and ferroptosis/oxytosis in
ischemic stroke: Possible targets and molecular mechanisms. Oxid
Med Cell Longev. 2021:66433822021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Si W, Sun B, Luo J, Li Z, Dou Y and Wang
Q: Snap25 attenuates neuronal injury via reducing ferroptosis in
acute ischemic stroke. Exp Neurol. 367:1144762023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kuriakose D and Xiao Z: Pathophysiology
and treatment of stroke: Present status and future perspectives.
Int J Mol Sci. 21:76092020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu Y, Fang Y, Zhang Z, Luo Y, Zhang A,
Lenahan C and Chen S: Ferroptosis: An emerging therapeutic target
in stroke. J Neurochem. 160:64–73. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Prajapati M, Conboy HL, Hojyo S, Fukada T,
Budnik B and Bartnikas TB: Biliary excretion of excess iron in mice
requires hepatocyte iron import by Slc39a14. J Biol Chem.
297:1008352021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen Y, Fang ZM, Yi X, Wei X and Jiang DS:
The interaction between ferroptosis and inflammatory signaling
pathways. Cell Death Dis. 14:2052023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang W, Jing X, Du T, Ren J, Liu X, Chen
F, Shao Y, Sun S, Yang G and Cui X: Iron overload promotes
intervertebral disc degeneration via inducing oxidative stress and
ferroptosis in endplate chondrocytes. Free Radic Biol Med.
190:234–246. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shi F, Zhang Z, Cui H, Wang J, Wang Y,
Tang Y, Yang W, Zou P, Ling X, Han F, et al: Analysis by
transcriptomics and metabolomics for the proliferation inhibition
and dysfunction through redox imbalance-mediated DNA damage
response and ferroptosis in male reproduction of mice and TM4
Sertoli cells exposed to PM2.5. Ecotoxicol Environ Saf.
238:1135692022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lin Q, Li S, Jin H, Cai H, Zhu X, Yang Y,
Wu J, Qi C, Shao X, Li J, et al: Mitophagy alleviates
cisplatin-induced renal tubular epithelial cell ferroptosis through
ROS/HO-1/GPX4 axis. Int J Biol Sci. 19:1192–1210. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Su LJ, Zhang JH, Gomez H, Murugan R, Hong
X, Xu D, Jiang F and Peng ZY: Reactive oxygen species-induced lipid
peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med
Cell Longev. 2019:50808432019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yuan Y, Zhai Y, Chen J, Xu X and Wang H:
Kaempferol ameliorates oxygen-glucose
deprivation/reoxygenation-induced neuronal ferroptosis by
activating Nrf2/SLC7A11/GPX4 axis. Biomolecules. 11:9232021.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu H, Zhang T, Zhang WY, Huang SR, Hu Y
and Sun J: Rhein attenuates cerebral ischemia-reperfusion injury
via inhibition of ferroptosis through NRF2/SLC7A11/GPX4 pathway.
Exp Neurol. 369:1145412023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ursini F and Maiorino M: Lipid
peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic
Biol Med. 152:175–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ralhan I, Chang J, Moulton MJ, Goodman LD,
Lee NYJ, Plummer G, Pasolli HA, Matthies D, Bellen HJ and Ioannou
MS: Autolysosomal exocytosis of lipids protect neurons from
ferroptosis. J Cell Biol. 222:e2022071302023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mamais A, Kluss JH, Bonet-Ponce L, Landeck
N, Langston RG, Smith N, Beilina A, Kaganovich A, Ghosh MC,
Pellegrini L, et al: Correction: Mutations in LRRK2 linked to
Parkinson disease sequester Rab8a to damaged lysosomes and regulate
transferrin-mediated iron uptake in microglia. PLoS Biol.
20:e30016212022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Reyhani A, McKenzie TG, Fu Q and Qiao GG:
Fenton-chemistry-mediated radical polymerization. Macromol Rapid
Commun. 40:19002202019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen Y, Yang Z, Wang S, Ma Q, Li L, Wu X,
Guo Q, Tao L and Shen X: Boosting ROS-mediated lysosomal membrane
permeabilization for cancer ferroptosis therapy. Adv Healthc Mater.
12:22021502023. View Article : Google Scholar
|
|
58
|
Von Krusenstiern AN, Robson RN, Qian N,
Qiu B, Hu F, Reznik E, Smith N, Zandkarimi F, Estes VM, Dupont M,
et al: Identification of essential sites of lipid peroxidation in
ferroptosis. Nat Chem Biol. 19:719–730. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ayala A, Muñoz MF and Argüelles S: Lipid
peroxidation: Production, metabolism, and signaling mechanisms of
malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev.
2014:3604382014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhu G, Chi H, Liu M, Yin Y, Diao H, Liu Z,
Guo Z, Xu W, Xu J, Cui C, et al: Multifunctional ‘ball-rod’ Janus
nanoparticles boosting Fenton reaction for ferroptosis therapy of
non-small cell lung cancer. J Colloid Interface Sci. 621:12–23.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kajarabille N and Latunde-Dada GO:
Programmed cell-death by ferroptosis: Antioxidants as mitigators.
Int J Mol Sci. 20:49682019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li J, Jia B, Cheng Y, Song Y, Li Q and Luo
C: Targeting Molecular mediators of ferroptosis and oxidative
stress for neurological disorders. Oxid Med Cell Longev.
2022:39990832022.PubMed/NCBI
|
|
63
|
Wan J, Ren H and Wang J: Iron toxicity,
lipid peroxidation and ferroptosis after intracerebral haemorrhage.
Stroke Vasc Neurol. 4:93–95. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Garton T, Keep RF, Hua Y and Xi G: CD163,
a hemoglobin/haptoglobin scavenger receptor, after intracerebral
hemorrhage: Functions in microglia/macrophages versus neurons.
Transl Stroke Res. 8:612–616. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hare D, Ayton S, Bush A and Lei P: A
delicate balance: Iron metabolism and diseases of the brain. Front
Aging Neurosci. 5:342013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yang C, Hawkins KE, Doré S and
Candelario-Jalil E: Neuroinflammatory mechanisms of blood-brain
barrier damage in ischemic stroke. Am J Physiol Cell Physiol.
316:C135–C153. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bouchaoui H, Mahoney-Sanchez L, Garçon G,
Berdeaux O, Alleman LY, Devos D, Duce JA and Devedjian JC: ACSL4
and the lipoxygenases 15/15B are pivotal for ferroptosis induced by
iron and PUFA dyshomeostasis in dopaminergic neurons. Free Radic
Biol Med. 195:145–157. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Rock C and Moos PJ: Selenoprotein P
protects cells from lipid hydroperoxides generated by 15-LOX-1.
Prostaglandins Leukot Essent Fatty Acids. 83:203–210. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Karuppagounder SS, Alim I, Khim SJ,
Bourassa MW, Sleiman SF, John R, Thinnes CC, Yeh TL, Demetriades M,
Neitemeier S, et al: Therapeutic targeting of oxygen-sensing prolyl
hydroxylases abrogates ATF4-dependent neuronal death and improves
outcomes after brain hemorrhage in several rodent models. Sci
Transl Med. 8:328ra292016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wang H, Xu L, Tang X, Jiang Z and Feng X:
Lipid peroxidation-induced ferroptosis as a therapeutic target for
mitigating neuronal injury and inflammation in sepsis-associated
encephalopathy: Insights into the hippocampal PEBP-1/15-LOX/GPX4
pathway. Lipids Health Dis. 23:1282024. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shah R, Shchepinov MS and Pratt DA:
Resolving the role of lipoxygenases in the initiation and execution
of ferroptosis. ACS Cent Sci. 4:387–396. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Martami F and Holton KF: Targeting
glutamate neurotoxicity through dietary manipulation: Potential
treatment for migraine. Nutrients. 15:39522023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Saini KK, Chaturvedi P, Sinha A, Singh MP,
Khan MA, Verma A, Nengroo MA, Satrusal SR, Meena S, Singh A, et al:
Loss of PERK function promotes ferroptosis by downregulating
SLC7A11 (System Xc-) in colorectal cancer. Redox Biol.
65:1028332023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Koppula P, Zhuang L and Gan B: Cystine
transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient
dependency, and cancer therapy. Protein Cell. 12:599–620. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Dahlmanns M, Dahlmanns JK, Savaskan N,
Steiner HH and Yakubov E: Glial glutamate transporter-mediated
plasticity: System xc-/xCT/SLC7A11 and EAAT1/2 in brain
diseases. Front Biosci (Landmark Ed). 28:572023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Albrecht P, Lewerenz J, Dittmer S, Noack
R, Maher P and Methner A: Mechanisms of oxidative glutamate
toxicity: The glutamate/cystine antiporter system xc-as a
neuroprotective drug target. CNS Neurol Disord Drug Targets.
9:373–382. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Puka-Sundvall M, Eriksson P, Nilsson M,
Sandberg M and Lehmann A: Neurotoxicity of cysteine: interaction
with glutamate. Brain Res. 705:65–70. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yuan Y, Yucai L, Lu L, Hui L, Yong P and
Haiyang Y: Acrylamide induces ferroptosis in HSC-T6 cells by
causing antioxidant imbalance of the XCT-GSH-GPX4 signaling and
mitochondrial dysfunction. Toxicol Lett. 368:24–32. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang W, Niu C, Liu Y and Chen B:
Glutathione redox balance in hibernating Chinese soft-shelled
turtle Pelodiscus sinensis hatchlings. Comp Biochem Physiol B
Biochem Mol Biol. 207:9–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Iskusnykh IY, Zakharova AA and Pathak D:
Glutathione in brain disorders and aging. Molecules. 27:3242022.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Miladinovic T and Singh G: Spinal
microglia contribute to cancer-induced pain through system
xC−-mediated glutamate release. Pain Rep.
4:e7382019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Shen SY, Yu R, Li W, Liang LF, Han QQ,
Huang HJ, Li B, Xu SF, Wu GC, Zhang YQ and Yu J: The
neuroprotective effects of GPR55 against hippocampal
neuroinflammation and impaired adult neurogenesis in CSDS mice.
Neurobiol Dis. 169:1057432022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chen Z and Trapp BD: Microglia and
neuroprotection. J Neurochem. 136 (Suppl 1):S10–S17. 2016.
View Article : Google Scholar
|
|
84
|
Frank D, Gruenbaum BF, Grinshpun J,
Melamed I, Severynovska O, Kuts R, Semyonov M, Brotfain E, Zlotnik
A and Boyko M: Measuring post-stroke cerebral edema, infarct zone
and blood-brain barrier breakdown in a single set of rodent brain
samples. J Vis Exp. 2020:e613092020.
|
|
85
|
Raper DMS and Abla AA: Commentary:
Encephalodu-roarteriosynangiosis averts stroke in atherosclerotic
patients with border-zone infarct: Post hoc analysis from a
performance criterion phase II trial. Neurosurgery. 88:E319–E320.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Fan G, Liu M, Liu J and Huang Y: The
initiator of neuroexcitotoxicity and ferroptosis in ischemic
stroke: Glutamate accumulation. Front Mol Neurosci. 16:11130812023.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bridges R, Lutgen V, Lobner D and Baker
DA: Thinking outside the cleft to understand synaptic activity:
Contribution of the cystine-glutamate antiporter (System xc-) to
normal and pathological glutamatergic signaling. Pharmacol Rev.
64:780–802. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Seibt TM, Proneth B and Conrad M: Role of
GPX4 in ferroptosis and its pharmacological implication. Free Radic
Biol Med. 133:144–152. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Liu J, Yang G and Zhang H:
Glyphosate-triggered hepatocyte ferroptosis via suppressing
Nrf2/GSH/GPX4 axis exacerbates hepatotoxicity. Sci Total Environ.
862:1608392023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Jin M, Shi C, Li T, Wu Y, Hu C and Huang
G: Solasonine promotes ferroptosis of hepatoma carcinoma cells via
glutathione peroxidase 4-induced destruction of the glutathione
redox system. Biomed Pharmacother. 129:1102822020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Delesderrier E, Monteiro JDC, Freitas S,
Pinheiro IC, Batista MS and Citelli M: Can iron and polyunsaturated
fatty acid supplementation induce ferroptosis? Cell Physiol
Biochem. 57:24–41. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Xie G, Liang Y, Gao W, Wu L, Zhang Y, Ye Z
and Qin C: Artesunate alleviates intracerebral haemorrhage
secondary injury by inducing ferroptosis in M1-polarized microglia
and suppressing inflammation through AMPK/mTORC1/GPX4 pathway.
Basic Clin Pharmacol Toxicol. 132:369–383. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Steinberg GR and Hardie DG: New insights
into activation and function of the AMPK. Nat Rev Mol Cell Biol.
24:255–272. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Muraleedharan R and Dasgupta B: AMPK in
the brain: Its roles in glucose and neural metabolism. FEBS J.
289:2247–2262. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Taghiyar S, Pourrajab F and Aarabi MH:
Astaxanthin improves fatty acid dysregulation in diabetes by
controlling the AMPK-SIRT1 pathway. EXCLI J. 22:502–515.
2023.PubMed/NCBI
|
|
96
|
Malik N, Ferreira BI, Hollstein PE, Curtis
SD, Trefts E, Weiser Novak S, Yu J, Gilson R, Hellberg K, Fang L,
et al: Induction of lysosomal and mitochondrial biogenesis by AMPK
phosphorylation of FNIP1. Science. 380:eabj55592023. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Bae SJ, Bak SB and Kim YW: Coordination of
AMPK and YAP by Spatholobi caulis and procyanidin B2
provides antioxidant effects in vitro and in vivo. Int J Mol Sci.
23:137302022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Lee H, Zandkarimi F, Zhang Y, Meena JK,
Kim J, Zhuang L, Tyagi S, Ma L, Westbrook TF, Steinberg GR, et al:
Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat
Cell Biol. 22:225–234. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chen X, Kang R, Kroemer G and Tang D:
Broadening horizons: The role of ferroptosis in cancer. Nat Rev
Clin Oncol. 18:280–296. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Gao J, Li Y and Song R: SIRT2 inhibition
exacerbates p53-mediated ferroptosis in mice following experimental
traumatic brain injury. Neuroreport. 32:1001–1008. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lu W, Ji H and Wu D: SIRT2 plays complex
roles in neuroinflammation neuroimmunology-associated disorders.
Front Immunol. 14:11741802023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wu H, Liu Q, Shan X, Gao W and Chen Q: ATM
orchestrates ferritinophagy and ferroptosis by phosphorylating
NCOA4. Autophagy. 19:2062–2077. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Mi Y, Wei C, Sun L, Liu H, Zhang J, Luo J,
Yu X, He J, Ge H and Liu P: Melatonin inhibits ferroptosis and
delays age-related cataract by regulating SIRT6/p-Nrf2/GPX4 and
SIRT6/NCOA4/FTH1 pathways. Biomed Pharmacother. 157:1140482023.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Jin Y, Qiu J, Lu X and Li G: C-MYC
inhibited ferroptosis and promoted immune evasion in ovarian cancer
cells through NCOA4 mediated ferritin autophagy. Cells.
11:41272022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Li C, Sun G, Chen B, Xu L, Ye Y, He J, Bao
Z, Zhao P, Miao Z, Zhao L, et al: Nuclear receptor coactivator
4-mediated ferritinophagy contributes to cerebral ischemia-induced
ferroptosis in ischemic stroke. Pharmacol Res. 174:1059332021.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Santana-Codina N, Gikandi A and Mancias
JD: The role of NCOA4-mediated ferritinophagy in ferroptosis.
Ferroptosis: Mechanism and Diseases. Vol. 1301. Florez AF and
Alborzinia H: Springer International Publishing; Cham: pp. 41–57.
2021, PubMed/NCBI
|
|
107
|
Fang Y, Chen X, Tan Q, Zhou H, Xu J and Gu
Q: Inhibiting ferroptosis through disrupting the NCOA4-FTH1
interaction: A new mechanism of action. ACS Cent Sci. 7:980–989.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Santana-Codina N, Gableske S, Quiles del
Rey M, Małachowska B, Jedrychowski MP, Biancur DE, Schmidt PJ,
Fleming MD, Fendler W, Harper JW, et al: NCOA4 maintains murine
erythropoiesis via cell autonomous and non-autonomous mechanisms.
Haematologica. 104:1342–1354. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Bellelli R, Federico G, Matte' A,
Colecchia D, Iolascon A, Chiariello M, Santoro M, De Franceschi L
and Carlomagno F: NCOA4 deficiency impairs systemic iron
homeostasis. Cell Rep. 14:411–421. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Nai A, Lidonnici MR, Federico G, Pettinato
M, Olivari V, Carrillo F, Geninatti Crich S, Ferrari G, Camaschella
C, Silvestri L and Carlomagno F: NCOA4-mediated ferritinophagy in
macrophages is crucial to sustain erythropoiesis in mice.
Haematologica. 106:795–805. 2021.PubMed/NCBI
|
|
111
|
Xu W, Guo ZN and Shao A: Editorial:
Ferroptosis in stroke, neurotrauma and neurodegeneration, volume
II. Front Cell Neurosci. 17:12384252023. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Rochette L, Dogon G, Rigal E, Zeller M,
Cottin Y and Vergely C: Lipid peroxidation and iron metabolism: Two
corner stones in the homeostasis control of ferroptosis. Int J Mol
Sci. 24:4492022. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wang L, Liu Y, Du T, Yang H, Lei L, Guo M,
Ding HF, Zhang J, Wang H, Chen X and Yan C: ATF3 promotes
erastin-induced ferroptosis by suppressing system Xc. Cell Death
Differ. 27:662–675. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Verbruggen L, Sprimont L, Bentea E,
Janssen P, Gharib A, Deneyer L, De Pauw L, Lara O, Sato H, Nicaise
C and Massie A: Chronic sulfasalazine treatment in mice induces
system xc−-independent adverse effects. Front Pharmacol.
12:6256992021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
de Baat A, Meier DT, Fontana A,
Böni-Schnetzler M and Donath MY: Cystine/Glutamate antiporter
system xc- deficiency impairs macrophage glutathione metabolism and
cytokine production. PLoS One. 18:e02919502023. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Sui X, Zhang R, Liu S, Duan T, Zhai L,
Zhang M, Han X, Xiang Y, Huang X, Lin H and Xie T: RSL3 drives
ferroptosis through GPX4 inactivation and ROS production in
colorectal cancer. Front Pharmacol. 9:13712018. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Cui C, Yang F and Li Q: Post-translational
modification of GPX4 is a promising target for treating
ferroptosis-related diseases. Front Mol Biosci. 9:9015652022.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Zhou J, Zhang L, Yan J, Hou A, Sui W and
Sun M: Curcumin induces ferroptosis in A549 CD133+ cells
through the GSH-GPX4 and FSP1-CoQ10-NAPH pathways. Discov Med.
35:251–263. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Almahi WA, Yu KN, Mohammed F, Kong P and
Han W: Hemin enhances radiosensitivity of lung cancer cells through
ferroptosis. Exp Cell Res. 410:1129462022. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ma S, Henson ES, Chen Y and Gibson SB:
Ferroptosis is induced following siramesine and lapatinib treatment
of breast cancer cells. Cell Death Dis. 7:e23072016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Zhang X, Yu K, Ma L, Qian Z, Tian X, Miao
Y, Niu Y, Xu X, Guo S, Yang Y, et al: Endogenous glutamate
determines ferroptosis sensitivity via ADCY10-dependent YAP
suppression in lung adenocarcinoma. Theranostics. 11:5650–5674.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Sun S, Guo C, Gao T, Ma D, Su X, Pang Q
and Zhang R: Hypoxia enhances glioma resistance to
sulfasalazine-induced ferroptosis by upregulating SLC7A11 via
PI3K/AKT/HIF-1α axis. Oxid Med Cell Longev. 2022:78624302022.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zhao Y, Li Y, Zhang R, Wang F, Wang T and
Jiao Y: The role of erastin in ferroptosis and its prospects in
cancer therapy. Onco Targets Ther. 13:5429–5441. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zhang Y, Tan H, Daniels JD, Zandkarimi F,
Liu H, Brown LM, Uchida K, O'Connor OA and Stockwell BR: Imidazole
ketone erastin induces ferroptosis and slows tumor growth in a
mouse lymphoma model. Cell Chem Biol. 26:623–633.e9. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Duan L, Zhang Y, Yang Y, Su S, Zhou L, Lo
PC, Cai J, Qiao Y, Li M, Huang S, et al: Baicalin inhibits
ferroptosis in intracerebral hemorrhage. Front Pharmacol.
12:6293792021. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Li J, Wei G, Song Z, Chen Z, Gu J, Zhang L
and Wang Z: SIRT5 regulates ferroptosis through the Nrf2/HO-1
signaling axis to participate in ischemia-reperfusion injury in
ischemic stroke. Neurochem Res. 49:998–1007. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Li Y, Bao Y, Li Y, Duan X, Dong S, Lin J,
Chang X, Tan Y, Zhang H and Shan H: RSL3 inhibits porcine epidemic
diarrhea virus replication by activating ferroptosis. Viruses.
15:20802023. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Cheff DM, Huang C, Scholzen KC, Gencheva
R, Ronzetti MH, Cheng Q, Hall MD and Arnér ESJ: The ferroptosis
inducing compounds RSL3 and ML162 are not direct inhibitors of GPX4
but of TXNRD1. Redox Biol. 62:1027032023. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Hu J, Gu W, Ma N, Fan X and Ci X:
Leonurine alleviates ferroptosis in cisplatin-induced acute kidney
injury by activating the Nrf2 signalling pathway. Br J Pharmacol.
179:3991–4009. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Yang J, Mo J, Dai J, Ye C, Cen W, Zheng X,
Jiang L and Ye L: Cetuximab promotes RSL3-induced ferroptosis by
suppressing the Nrf2/HO-1 signalling pathway in KRAS mutant
colorectal cancer. Cell Death Dis. 12:10792021. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Li S, He Y, Chen K, Sun J, Zhang L, He Y,
Yu H and Li Q: RSL3 drives ferroptosis through NF-κB pathway
activation and GPX4 depletion in glioblastoma. Oxid Med Cell
Longev. 2021:29150192021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Sun X, Huang N, Li P, Dong X, Yang J,
Zhang X, Zong WX, Gao S and Xin H: TRIM21 ubiquitylates GPX4 and
promotes ferroptosis to aggravate ischemia/reperfusion-induced
acute kidney injury. Life Sci. 321:1216082023. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Li M, Meng Z, Yu S, Li J, Wang Y, Yang W
and Wu H: Baicalein ameliorates cerebral ischemia-reperfusion
injury by inhibiting ferroptosis via regulating GPX4/ACSL4/ACSL3
axis. Chem Biol Interact. 366:1101372022. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Nakamura T, Hipp C, Santos Dias Mourão A,
Borggräfe J, Aldrovandi M, Henkelmann B, Wanninger J, Mishima E,
Lytton E, Emler D, et al: Phase separation of FSP1 promotes
ferroptosis. Nature. 619:371–377. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Yao X, Xie R, Cao Y, Tang J, Men Y, Peng H
and Yang W: Simvastatin induced ferroptosis for triple-negative
breast cancer therapy. J Nanobiotechnology. 19:3112021. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Shu X and Wu J, Zhang T, Ma X, Du Z, Xu J,
You J, Wang L, Chen N, Luo M and Wu J: Statin-induced
geranylgeranyl pyrophosphate depletion promotes ferroptosis-related
senescence in adipose tissue. Nutrients. 14:43652022. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Miyamoto HD, Ikeda M, Ide T, Tadokoro T,
Furusawa S, Abe K, Ishimaru K, Enzan N, Sada M, Yamamoto T, et al:
Iron overload via heme degradation in the endoplasmic reticulum
triggers ferroptosis in myocardial ischemia-reperfusion injury.
JACC Basic Transl Sci. 7:800–819. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Chen GQ, Benthani FA, Wu J, Liang D, Bian
ZX and Jiang X: Artemisinin compounds sensitize cancer cells to
ferroptosis by regulating iron homeostasis. Cell Death Differ.
27:242–254. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Pei X, Tian M, Wang Y, Xin Y, Jiang J,
Wang Y and Gong Y: Advances in the knowledge on the role of
apoptosis repressor with caspase recruitment domain in hemorrhagic
stroke. J Intensive Med. 3:138–143. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Zille M, Karuppagounder SS, Chen Y, Gough
PJ, Bertin J, Finger J, Milner TA, Jonas EA and Ratan RR: Neuronal
death after hemorrhagic stroke in vitro and in vivo shares features
of ferroptosis and necroptosis. Stroke. 48:1033–1043. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Fu K, Xu W, Lenahan C, Mo Y, Wen J, Deng
T, Huang Q, Guo F, Mo L and Yan J: Autophagy regulates inflammation
in intracerebral hemorrhage: Enemy or friend? Front Cell Neurosci.
16:10363132023. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Wang X, Mori T, Sumii T and Lo EH:
Hemoglobin-induced cytotoxicity in rat cerebral cortical neurons:
Caspase activation and oxidative stress. Stroke. 33:1882–1888.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Xu Y, Liu Y, Li K, Yuan D, Yang S, Zhou L,
Zhao Y, Miao S, Lv C and Zhao J: COX-2/PGE2 pathway inhibits the
ferroptosis induced by cerebral ischemia reperfusion. Mol
Neurobiol. 59:1619–1631. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Dendorfer A, Heidbreder M, Hellwig-Bürgel
T, Jöhren O, Qadri F and Dominiak P: Deferoxamine induces prolonged
cardiac preconditioning via accumulation of oxygen radicals. Free
Radic Biol Med. 38:117–124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Wang C, Xie L, Xing Y, Liu M, Yang J, Gao
N and Cai Y: Iron-overload-induced ferroptosis in mouse cerebral
toxoplasmosis promotes brain injury and could be inhibited by
Deferiprone. PLoS Negl Trop Dis. 17:e00116072023. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Miotto G, Rossetto M, Di Paolo ML, Orian
L, Venerando R, Roveri A, Vučković AM, Bosello Travain V, Zaccarin
M, Zennaro L, et al: Insight into the mechanism of ferroptosis
inhibition by ferrostatin-1. Redox Biol. 28:1013282020. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Gao Z, Zhang Z, Gu D, Li Y, Zhang K, Dong
X, Liu L, Zhang J, Chen J, Wu D and Zeng M: Hemin mitigates
contrast-induced nephropathy by inhibiting ferroptosis via
HO-1/Nrf2/GPX4 pathway. Clin Exp Pharma Physio. 49:858–870. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Mishima E, Ito J, Wu Z, Nakamura T, Wahida
A, Doll S, Tonnus W, Nepachalovich P, Eggenhofer E, Aldrovandi M,
et al: A non-canonical vitamin K cycle is a potent ferroptosis
suppressor. Nature. 608:778–783. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Sun W, Lv Z, Li W, Lu J, Xie Y, Wang P,
Jiang R, Dong J, Guo H, Liu Z, et al: XJB-5-131 protects
chondrocytes from ferroptosis to alleviate osteoarthritis
progression via restoring Pebp1 expression. J Orthop Translat.
44:114–124. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al:
The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature. 575:688–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Doll S, Proneth B, Tyurina YY, Panzilius
E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A,
et al: ACSL4 dictates ferroptosis sensitivity by shaping cellular
lipid composition. Nat Chem Biol. 13:91–98. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Liu Y, Wang W, Li Y, Xiao Y, Cheng J and
Jia J: The 5-lipoxygenase inhibitor zileuton confers
neuroprotection against glutamate oxidative damage by inhibiting
ferroptosis. Biol Pharm Bull. 38:1234–1239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Kosyakovsky J, Fine JM, Frey WH II and
Hanson LR: Mechanisms of intranasal deferoxamine in
neurodegenerative and neurovascular disease. Pharmaceuticals
(Basel). 14:952021. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Huang Z and Huang S: Reposition of the
fungicide ciclopirox for cancer treatment. Recent Pat Anticancer
Drug Discov. 16:122–135. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Averill-Bates DA: The antioxidant
glutathione. Vitam Horm. 121:109–141. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Chen J, Yang L, Geng L, He J, Chen L, Sun
Q, Zhao J and Wang X: Inhibition of Acyl-CoA synthetase long-chain
family member 4 facilitates neurological recovery after stroke by
regulation ferroptosis. Front Cell Neurosci. 15:6323542021.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Shen L, Lin D, Li X, Wu H, Lenahan C, Pan
Y, Xu W, Chen Y, Shao A and Zhang J: Ferroptosis in acute central
nervous system injuries: The future direction? Front Cell Dev Biol.
8:5942020. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Guo Z, Lin J, Sun K, Guo J, Yao X, Wang G,
Hou L, Xu J, Guo J and Guo F: Deferoxamine alleviates
osteoarthritis by inhibiting chondrocyte ferroptosis and activating
the Nrf2 pathway. Front Pharmacol. 13:7913762022. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Xue Q, Yan D, Chen X, Li X, Kang R,
Klionsky DJ, Kroemer G, Chen X, Tang D and Liu J: Copper-dependent
autophagic degradation of GPX4 drives ferroptosis. Autophagy.
19:1982–1996. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Wang L, Zhang X, Xu M, Zheng G, Chen J, Li
S, Cui J and Zhang S: Implication of ferroptosis in hepatic
toxicity upon single or combined exposure to polystyrene
microplastics and cadmium. Environ Pollut. 334:1222502023.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Chen Y, He W, Wei H, Chang C, Yang L, Meng
J, Long T, Xu Q and Zhang C: Srs11-92, a ferrostatin-1 analog,
improves oxidative stress and neuroinflammation via Nrf2 signal
following cerebral ischemia/reperfusion injury. CNS Neurosci Ther.
29:1667–1677. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Holden P and Nair LS: Deferoxamine: An
angiogenic and antioxidant molecule for tissue regeneration. Tissue
Eng Part B Rev. 25:461–470. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Kim JL, Lee DH, Na YJ, Kim BR, Jeong YA,
Lee SI, Kang S, Joung SY, Lee SY, Oh SC and Min BW: Iron
chelator-induced apoptosis via the ER stress pathway in gastric
cancer cells. Tumor Biol. 37:9709–9719. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Lin S, Gao W, Zhu C, Lou Q, Ye C, Ren Y,
Mehmood R, Huang B and Nan K: Efficiently suppress of ferroptosis
using deferoxamine nanoparticles as a new method for retinal
ganglion cell protection after traumatic optic neuropathy. Biomater
Adv. 138:2129362022. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
You H, Wang D, Wei L, Chen J, Li H and Liu
Y: Deferoxamine inhibits acute lymphoblastic leukemia progression
through repression of ROS/HIF-1α, Wnt/β-catenin, and p38MAPK/ERK
pathways. J Oncol. 2022:82812672022. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Abdul Y, Li W, Ward R, Abdelsaid M, Hafez
S, Dong G, Jamil S, Wolf V, Johnson MH, Fagan SC and Ergul A:
Deferoxamine treatment prevents post-stroke vasoregression and
neurovascular unit remodeling leading to improved functional
outcomes in type 2 male diabetic rats: Role of endothelial
ferroptosis. Transl Stroke Res. 12:615–630. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Jones G, Zeng L and Kim J: Mechanism-based
pharmacokinetic modeling of absorption and disposition of a
deferoxamine-based nanochelator in rats. Mol Pharm. 20:481–490.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Chen X, Li D, Sun H, Wang W, Wu H and Kong
W and Kong W: Relieving ferroptosis may partially reverse
neurodegeneration of the auditory cortex. FEBS J. 287:4747–4766.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Tuo Q, Lei P, Jackman KA, Li XL, Xiong H,
Li XL, Liuyang ZY, Roisman L, Zhang ST, Ayton S, et al:
Tau-mediated iron export prevents ferroptotic damage after ischemic
stroke. Mol Psychiatry. 22:1520–1530. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Kannan M, Sil S, Oladapo A, Thangaraj A,
Periyasamy P and Buch S: HIV-1 Tat-mediated microglial ferroptosis
involves the miR-204-ACSL4 signaling axis. Redox Biol.
62:1026892023. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Li Y, Zeng X, Lu D, Yin M, Shan M and Gao
Y: Erastin induces ferroptosis via ferroportin-mediated iron
accumulation in endometriosis. Hum Reprod. 36:951–964. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Xie B, Wang Y, Lin Y, Mao Q, Feng J, Gao G
and Jiang J: Inhibition of ferroptosis attenuates tissue damage and
improves long-term outcomes after traumatic brain injury in mice.
CNS Neurosci Ther. 25:465–475. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Carbonell T and Rama R: Iron, oxidative
stress and early neurological deterioration in ischemic stroke.
Curr Med Chem. 14:857–874. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Silver MK, Lozoff B and Meeker JD: Blood
cadmium is elevated in iron deficient U.S. children: A
cross-sectional study. Environ Health. 12:1172013. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Zhang Q, Luo C, Li Z, Huang W, Zheng S,
Liu C, Shi X, Ma Y, Ni Q, Tan W, et al: Astaxanthin activates the
Nrf2/Keap1/HO-1 pathway to inhibit oxidative stress and
ferroptosis, reducing triphenyl phosphate (TPhP)-induced
neurodevelopmental toxicity. Ecotoxicol Environ Saf.
271:1159602024. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Liu X, Du Y, Liu J, Cheng L, He W and
Zhang W: Ferrostatin-1 alleviates cerebral ischemia/reperfusion
injury through activation of the AKT/GSK3β signaling pathway. Brain
Res Bull. 193:146–157. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Chen B and Jin W: A comprehensive review
of stroke-related signaling pathways and treatment in western
medicine and traditional Chinese medicine. Front Neurosci.
17:12000612023. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Lou Y, Ma M, Jiang Y, Xu H, Gao Z, Gao L
and Wang Y: Ferroptosis: A new strategy for traditional Chinese
medicine treatment of stroke. Biomed Pharmacother. 156:1138062022.
View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Li J, Zhao X, Zhang Y, Wan H, He Y, Li X,
Yu L and Jin W: Comparison of traditional Chinese medicine in the
long-term secondary prevention for patients with ischemic stroke: A
systematical analysis. Front Pharmacol. 12:7229752021. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Zhu T, Wang L, Wang L and Wan Q:
Therapeutic targets of neuroprotection and neurorestoration in
ischemic stroke: Applications for natural compounds from medicinal
herbs. Biomed Pharmacother. 148:1127192022. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Wang J, Hu J, Chen X, Lei X, Feng H, Wan F
and Tan L: Traditional Chinese medicine monomers: Novel strategy
for endogenous neural stem cells activation after stroke. Front
Cell Neurosci. 15:6281152021. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Zhan S, Liang J, Lin H, Cai J, Yang X, Wu
H, Wei J, Wang S and Xian M: SATB1/SLC7A11/HO-1 axis ameliorates
ferroptosis in neuron cells after ischemic stroke by danhong
injection. Mol Neurobiol. 60:413–427. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Ko G, Kim J, Jeon YJ, Lee D, Baek HM and
Chang KA: Salvia miltiorrhiza alleviates memory deficit
induced by ischemic brain injury in a transient MCAO mouse model by
inhibiting ferroptosis. Antioxidants (Basel). 12:7852023.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Zhang J, Cai W, Wei X, Shi Y, Zhang K, Hu
C, Wan J, Luo K and Shen W: Moxibustion ameliorates cerebral
ischemia-reperfusion injury by regulating ferroptosis in rats. Clin
Exp Pharmacol Physiol. 50:779–788. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Liu J, Jiang G, He P, Du X, Hu Z and Li F:
Mechanism of ferroptosis in traditional chinese medicine for
clinical treatment: A review. Front Pharmacol. 13:11088362023.
View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Wang L, Liu C, Wang L and Tang B:
Astragaloside IV mitigates cerebral ischaemia-reperfusion injury
via inhibition of P62/Keap1/Nrf2 pathway-mediated ferroptosis. Eur
J Pharmacol. 944:1755162023. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Jiang Y, Zhao S, Zhou Y and Wei Z:
Research progress of traditional Chinese medicine in
ferroptosis-related diseases. Med Nov Technol Devices.
16:1001932022. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Yang K, Zeng L, Zeng J, Deng Y, Wang S, Xu
H, He Q, Yuan M, Luo Y, Ge A and Ge J: Research progress in the
molecular mechanism of ferroptosis in Parkinson's disease and
regulation by natural plant products. Ageing Res Rev.
91:1020632023. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Wu C, Duan F, Yang R, Dai Y, Chen X and Li
S: 15, 16-Dihydrotanshinone I protects against ischemic stroke by
inhibiting ferroptosis via the activation of nuclear factor
erythroid 2-related factor 2. Phytomedicine. 114:1547902023.
View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Bai X, Zheng E, Tong L, Liu Y, Li X and
Yang H, Jiang J, Chang Z and Yang H: Angong Niuhuang Wan inhibit
ferroptosis on ischemic and hemorrhagic stroke by activating
PPARγ/AKT/GPX4 pathway. J Ethnopharmacol. 321:1174382024.
View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Jin Z, Gao W, Guo F, Liao S, Hu M, Yu T,
Yu S and Shi Q: Astragaloside IV alleviates neuronal ferroptosis in
ischemic stroke by regulating fat mass and
obesity-associated-N6-methyladenosine-acyl-CoA synthetase
long-chain family member 4 axis. J Neurochem. 166:328–345. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Hirata Y, Cai R, Volchuk A, Steinberg BE,
Saito Y, Matsuzawa A, Grinstein S and Freeman SA: Lipid
peroxidation increases membrane tension, Piezo1 gating, and cation
permeability to execute ferroptosis. Curr Biol. 33:1282–1294.e5.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Gao W, Wang X, Zhou Y, Wang X and Yu Y:
Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor
immunotherapy. Sig Transduct Target Ther. 7:1962022. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Zhou Y, Liao J, Mei Z, Liu X and Ge J:
Insight into crosstalk between ferroptosis and necroptosis: Novel
therapeutics in ischemic stroke. Oxid Med Cell Longev.
2021:99910012021. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Yu Y, Yan Y, Niu F, Wang Y, Chen X, Su G,
Liu Y, Zhao X, Qian L, Liu P and Xiong Y: Ferroptosis: A cell death
connecting oxidative stress, inflammation and cardiovascular
diseases. Cell Death Discov. 7:1932021. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Battaglia AM, Chirillo R, Aversa I, Sacco
A, Costanzo F and Biamonte F: Ferroptosis and cancer: Mitochondria
meet the ‘iron maiden’ cell death. Cells. 9:15052020. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Jeong SY and Seol DW: The role of
mitochondria in apoptosis. BMB Rep. 41:11–22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Cui Y, Zhang Y, Zhao X, Shao L, Liu G, Sun
C, Xu R and Zhang Z: ACSL4 exacerbates ischemic stroke by promoting
ferroptosis-induced brain injury and neuroinflammation. Brain Behav
Immun. 93:312–321. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Wang ZL, Yuan L, Li W and Li JY:
Ferroptosis in Parkinson's disease: Glia-neuron crosstalk. Trends
Mol Med. 28:258–269. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Ren JX, Sun X, Yan XL, Guo ZN and Yang Y:
Ferroptosis in neurological diseases. Front Cell Neurosci.
14:2182020. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Chen Y, Long T, Xu Q and Zhang C:
Bibliometric analysis of ferroptosis in stroke from 2013 to 2021.
Front Pharmacol. 12:8173642022. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Jin Y, Zhuang Y, Liu M, Che J and Dong X:
Inhibiting ferroptosis: A novel approach for stroke therapeutics.
Drug Discov Today. 26:916–930. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Yang K, Zeng L, Yuan X, Wang S, Ge A, Xu
H, Zeng J and Ge J: The mechanism of ferroptosis regulating
oxidative stress in ischemic stroke and the regulation mechanism of
natural pharmacological active components. Biomed Pharmacother.
154:1136112022. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Xing G, Meng L, Cao S, Liu S, Wu J, Li Q,
Huang W and Zhang L: PPARα alleviates iron overload-induced
ferroptosis in mouse liver. EMBO Rep. 23:e522802022. View Article : Google Scholar : PubMed/NCBI
|