Introduction
Chronic obstructive pulmonary disease (COPD) is a
prevalent, preventable and treatable chronic inflammatory airway
disease, and is the third leading cause of mortality worldwide
(1). The frequent exacerbation of
COPD (COPD-FE) phenotype is characterized by experiencing two or
more exacerbation episodes annually (2). Acute exacerbations of COPD (AECOPD)
are critical events that accelerate lung function decline, elevate
mortality (3,4) and adversely affect mental health (MH)
and quality of life in patients with COPD (5,6).
Therefore, the central focus of COPD management during stable
periods revolves around averting acute exacerbations and reducing
their frequency.
Numerous clinical predictors of acute exacerbation
risk in COPD have been assessed, including low body mass index
(BMI) (7), deteriorating lung
function (8), increased COPD
assessment test (CAT) scores (9),
modified Medical Research Council (mMRC) dyspnea scale scores
(10), and elevated
neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte
ratio (PLR) (11). However,
predicting COPD-FE risk and understanding its underlying pathogenic
mechanisms remains challenging due to the heterogeneity and
complexity of COPD. Moreover, effective preventive measures against
COPD-FE remain unsatisfactory. Therefore, a multidimensional
exploration of intrinsic COPD-FE mechanisms and the identification
of predictive biomarkers is essential for enhancing comprehensive
COPD management during stable periods.
Widely targeted metabolomics, a high-throughput
bioanalytical technique, is designed to identify and quantify
small-molecule metabolites within biological specimens (12). This method integrates the benefits
of both non-targeted and targeted metabolite detection, offering a
high-throughput, specific, sensitive and accurate approach
(13). It enables the simultaneous
analysis of hundreds to thousands of metabolites. It has broad
applications across diverse medical fields, such as drug screening,
research on metabolic diseases and exploring metabolic networks
within living organisms (14).
Although recent studies have begun to identify the metabolic
characteristics in blood samples of individuals with different COPD
phenotypes, these studies often rely on non-targeted metabolomics
or are confined to specific metabolic pathways (15–18).
Moreover, research using widely targeted metabolomics for the
COPD-FE phenotype is currently insufficient.
In the present study, a widely targeted metabolomics
approach based on ultra performance liquid chromatography tandem
mass spectrometry (UPLC-MS/MS) was used for what is considered to
be the first time, coupled with multivariate and univariate
statistical analyses, to assess the serum metabolic profile and
potential pathway changes in patients with a COPD-FE phenotype.
Furthermore, through Spearman's correlation analysis and receiver
operating characteristic (ROC) analysis, the predictive capacity of
differential metabolites for assessing the risk of COPD-FE were
evaluated. Finally, external cohort validation was performed to
further determine the reliability of the results of the present
study.
Materials and methods
Study population
This study was conducted following The Declaration
of Helsinki and was approved by the Ethics Committee of The First
Affiliated Hospital of Anhui University of Traditional Chinese
Medicine (Hefei, China; approval no. 2021AH-31). The present study
is an observational study and, during its course, no new
interventions were introduced. All participants provided written
informed consent. The present study constitutes a subset of
patients with COPD recruited from a larger unpublished cohort study
conducted by the present research group. Patients with COPD who had
previously received treatment at The First Affiliated Hospital of
Anhui University of Chinese Medicine were screened. Between April
and June 2022, 80 patients with COPD underwent preliminary
screening and exclusion, leading to the successful recruitment of
50 participants (30 for internal validation and 20 for external
validation).
The inclusion criteria were as follows: i) Fulfilled
diagnostic criteria for COPD; ii) patients were in a stable phase
of COPD, with no acute exacerbations for ≥4 weeks before enrolment
(1); iii) age range of 60–80
years; iv) had not participated in any other clinical studies
within the preceding 3 months; and v) adhered to a light diet and
regular lifestyle for ≥6 weeks before enrolment. The diagnosis of
COPD was based on the 2020 Global Initiative for Chronic
Obstructive Lung Disease (GOLD) guidelines, defining COPD as a
post-bronchodilator ratio of forced expiratory volume in 1 second
(FEV1) to forced vital capacity (FVC) FEV1/FVC ratio of <70%
(1). The exclusion criteria
included adverse habits including: Daily consumption of substantial
quantities of spicy or stimulating foods, high-sugar, high-salt
diets, selective eating (such as exclusively meat-based or
vegetarian diets), binge eating, excessive alcohol consumption and
irregular sleep patterns; coexisting diseases, including
respiratory disorders such as bronchiectasis, bronchial asthma,
active pulmonary tuberculosis and malignant tumors; metabolic
disorders including diabetes, renal disease and liver disease; and
immunodeficiency-related ailments including malignancies(such as
lung cancer, lymphoma, and leukemia), acquired immunodeficiency
syndrome and renal insufficiency.
Based on the frequency of previous acute
exacerbations, patients with stable COPD were categorized into two
groups as follows: The COPD-FE group, which included patients that
had experienced two or more exacerbations annually over the last 2
years, and the non-frequent exacerbations of COPD (COPD-NE) group,
which included patients that had experienced less than two
exacerbations per year in the same period (19). A recent acute exacerbation was
characterized as occurring ≥4 weeks after the conclusion of
treatment for the previous exacerbation or ≥6 weeks after the onset
of the exacerbation, to help differentiate between treatment
failure and new acute exacerbations (20). Trained researchers and attending
physicians strictly adhered to the inclusion and exclusion
criteria. The present study is an observational study involving
data collection from enrolled patients during a specific time
period. It incorporates retrospective elements by reviewing past
occurrences of disease exacerbations.
Clinical data collection
Physical examinations, including height, weight and
blood pressure; pulmonary function tests; chest X-rays; laboratory
tests, including complete blood counts, liver and kidney function
assessments; and electrocardiograms were conducted. Additionally, a
comprehensive questionnaire was distributed, for the collection of
demographic information, medical history, medication usage, smoking
status and results from standardized assessments, such as the CAT,
mMRC dyspnea scale and a 36-item short-form health survey (SF-36)
questionnaire. The clinical data collection included clinical
indicators, such as BMI, FEV1% predicted (FEV1% pred), CAT score,
mMRC score, NLR and PLR, which are linked to the risk of acute
exacerbations. Notably, the SF-36 questionnaire evaluated the
physical, mental and social well-being of the patients across nine
dimensions (21): Physical
function (PF), role limitations due to physical problems
(role-physical) (RP), bodily pain (BP), general health (GH),
vitality (VT), social function (SF), role limitations due to
emotional problems (role-emotional) (RE), mental health (MH) and
health transition (HT). The scoring for each dimension of the SF-36
scale is obtained through statistical analysis using an online
website (medsci.cn/).
Reagents and equipment
The following reagents and equipment were used.
QTRAP 5500 mass spectrometer (SCIEX), Nexera X2 LC-30AD UPLC system
(Shimadzu Corporation), Acquity UPLC HSS T3 column (Waters
Corporation), acetonitrile (cat. no. 1499230-935; Merck KGaA),
methanol (cat. no. 1.06007.4008; Millipore), ammonium hydroxide
solution (cat. no. 105426; Merck), acetonitrile (cat. no.
1.00030.4008; Millipore), formic acid (cat. no. 111670; Millipore),
and ammonium acetate (cat. no. 73594; Sigma).
UPLC-MS/MS analysis
Peripheral venous blood was collected from patients
in the morning, following an overnight fast lasting between 8 and
12 h, and centrifuged at 3,000 × g for 20 min at 4°C to separate
the serum. Serum samples were aliquoted into frozen tubes and
stored at −80°C. Metabolites were extracted from the serum using 1
ml pre-cooled ethanol/acetonitrile/water (v/v, 2:2:1) by sonication
at a frequency of 53 kHz for 1 h in an ice bath, followed by
incubation at −20°C for 1 h. The mixture was centrifuged at 16,000
× g for 20 min at 4°C and the supernatant was collected for
UPLC-MS/MS analysis. To ensure data quality, quality control (QC)
samples by pooling aliquots from all individual samples for
normalization. These were analyzed alongside experimental samples
in each batch. Dried extracts were dissolved in 50% acetonitrile,
filtered, and stored at −80°C until analysis.
Metabolites were analyzed using a Shimadzu Nexera X2
LC-30AD UPLC system equipped with an Acquity UPLC HSS T3 column
(1.8 µm, 2.1×50 mm) and a 5500 QTRAP triple quadruple mass
spectrometer. The UPLC HSS T3 column was maintained at 40°C with a
flow rate of 200 µl/min. The sample volume injected was 5 µl.
Mobile phase A comprises a 0.1% formic acid aqueous solution, while
mobile phase B is acetonitrile. In positive ion mode, the gradient
elution program is as follows: 0–2.5 min, 0% B; 2.5–9 min, linear
increase from 0 to 30% B; 9–10 min, linear increase from 30 to 100%
B; 10–15.4 min, holding at 100% B; 15.4–15.5 min, linear decrease
from 100 to 0% B; and 15.5–18 min, holding at 0% B. In negative ion
mode, the gradient elution program is: 0–2 min, 0% B; 2–2.5 min,
linear increase from 0 to 20% B; 2.5–3.5 min, linear increase from
20 to 40% B; 3.5–4.5 min, linear increase from 40 to 50% B; 4.5–10
min, linear increase from 50 to 100% B; 10–15.4 min, holding at
100% B; 15.4–15.5 min, linear decrease from 100 to 0% B; and
15.5–18 min, holding at 0% B. Metabolites were detected in both
electrospray negative-ionization and positive-ionization modes.
During acquisition, QC samples were intermittently injected.
Detection of transitions was performed using multiple reaction
monitoring (MRM) mode. The detailed m/z information of identified
metabolites is in Table SI.
Prior to analysis, the raw data was normalized using
cumulative sum scaling (CSS). Differential metabolites were
identified using statistically significant Variable Importance in
Projection (VIP) values derived from the Orthogonal Partial Least
Squares Discriminant Analysis (OPLS-DA) model. Subsequently,
permutation tests were performed to validate the stability and
reliability of the OPLS-DA model. A two-tailed unpaired Student's
t-test was applied to the normalized data, and in cases where the
assumptions for the t-test were not met, a non-parametric
Mann-Whitney U test was used. Metabolites with a VIP value >1
and P<0.05 were considered to have a statistically significant
difference.
Enrichment analysis
Metabolite data analysis was performed using the
Human Metabolome Database (http://www.hmdb.ca/), Kyoto Encyclopedia of Genes and
Genomes (KEGG) (http://kegg.jp), and MetaboAnalyst 5.0
(metaboanalyst.ca/). Metabolite Set Enrichment Analysis (MSEA) and
Metabolite Pathway Analysis (MetPA) were conducted with
MetaboAnalyst 5.0. Differential metabolites underwent KEGG pathway
analysis. MetPA used the relative-betweenness centrality
calculation method, while KEGG enrichment analysis used Fisher's
exact test. P<0.05 was considered to indicate a statistically
significant difference for enriched pathways.
Statistical analysis
Continuous variables are presented as median (IQR),
and categorical variables are presented as counts (%). The clinical
data analysis was conducted using SPSS software (version 26, IBM
Corporation). For normally distributed data with homogeneity of
variance, a two-tailed unpaired Student's t-test was applied;
otherwise, the non-parametric Mann-Whitney U test was used.
Categorical variable data were tested using Fisher's exact test.
Differential metabolites underwent cluster analysis and Spearman's
correlation analysis using R software (version 4.0.1, R Foundation
for Statistical Computing). Univariate ROC curve analysis was
conducted using the MetaboAnalyst web service (metaboanalyst.ca/).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characteristics of study
participants
The study comprised 30 patients in the internal
cohort, divided into two groups: 15 patients in the COPD-FE group
experienced a median of 3 acute exacerbations annually over the
past 2 years (IQR=1), while the COPD-NE group consisted of 15
patients with no exacerbations during the same period. No
significant differences were demonstrated between the two groups
regarding age, sex, BMI, smoking status, medication use or GOLD
spirometry grade during stable periods (Table I).
 | Table I.Characteristics and clinical
indicators of the patients. |
Table I.
Characteristics and clinical
indicators of the patients.
| Variable | COPD-NE (n=15) | COPD-FE (n=15) | P-value |
|---|
| Age, years | 71.0 (8.0) | 70.0 (5.0) | 0.812a |
| Male | 13 (86.6%) | 11 (73.3%) | 0.651 |
| BMI,
kg/m2 | 22.3 (6.8) | 23.4 (5.5) | 0.961b |
| Smoking
history |
|
|
|
| Never
smoked | 5 (33.3%) | 2 (13.3%) | 0.484 |
| Current
smoker | 3 (20.0%) | 3 (20.0%) |
|
| Former
smoker | 7 (46.7%) | 10 (66.7%) |
|
| Medication use |
|
|
|
|
LAMA | 8 (66.7%) | 6 (50.0%) | 0.794 |
|
ICS | 0 (0.0%) | 1 (8.3%) |
|
|
ICS/LABA | 2 (16.7%) | 1 (8.3%) |
|
|
ICS/LABA+LAMA | 1 (8.3%) | 2 (16.7%) |
|
|
None | 1 (8.3%) | 2 (16.7%) |
|
| Exacerbation
predictors |
|
|
|
| FEV1%
pred | 54.7 (43.1) | 48.7 (17.1) | 0.486b |
| CAT
score | 13 (8) | 26 (6) | 0.001b |
| mMRC
score | 2 (1) | 2 (1) | 0.011b |
|
NLR | 1.9 (0.9) | 2.9 (0.8) | 0.019b |
|
PLR | 108.3 (50.3) | 134.2 (67.8) | 0.325b |
| SF-36 score |
|
|
|
|
Physical functioning | 75.0 (30.0) | 40.0 (30.0) | 0.002b |
|
Role-physical | 75.0 (100.0) | 0.0 (25.0) | 0.026b |
| Bodily
pain | 84.0 (28.0) | 64.0 (32.0) | 0.116b |
| General
health | 52.0 (7.0) | 45.0 (10.0) | 0.007b |
|
Vitality | 70.0 (25.0) | 55.0 (35.0) | 0.045b |
| Social
functioning | 77.9 (22.2) | 55.6 (33.3) | 0.013b |
|
Role-emotional | 100.0 (33.3) | 100.0 (100.0) | 0.412b |
| Mental
health | 76.0 (20.0) | 68.0 (28.0) | 0.683b |
| Health
transition | 50.0 (50.0) | 25.0 (75.0) | 0.202b |
After enrolment, clinical data related to COPD
exacerbation risk were collected and analyzed. The results
demonstrated that CAT scores, mMRC scores and NLR levels were
significantly higher in the COPD-FE group compared with those in
the COPD-FE group (Table I). These
findings align with prior research in showing similar expression
trends of these indicators in COPD-FE patients (9–11).
Furthermore, the SF-36 questionnaire was used to assess disease
impact demonstrating significantly lower scores in PF, RP, GH, VT
and SF for the COPD-FE group compared with those in the COPD-NE
group; however, BP, RE, MH and HT scores did not demonstrate a
significant difference (Table I).
These findings imply a potential association between frequent
exacerbations and a decline in physical health, social function and
emotional well-being in patients with COPD.
Metabolic profiles of serum
samples
Serum samples from enrolled patients were analyzed
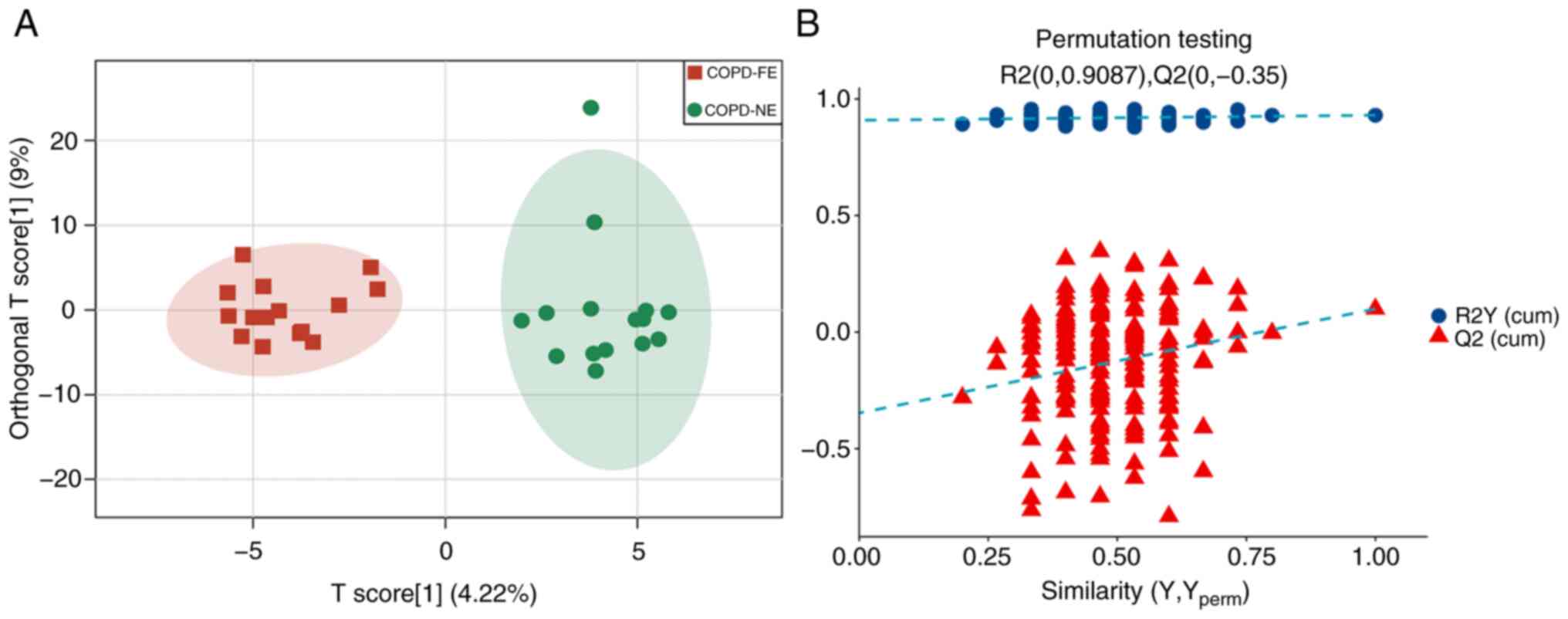
using UPLC-MS/MS with MRM scanning mode. The OPLS-DA model
demonstrated differences in serum metabolites between the COPD-FE
and COPD-NE groups, with R2Y(cum)=0.93 and Q2(cum)=0.1 (Fig. 1A). Permutation tests validated the
stability and reliability of the model. Comparison of permutation
test results with the original Q2(cum) value showed that in all 200
permutation tests, the Q2 values were lower than the original Q2
value, indicating a certain degree of stability and reliability of
the model (Fig. 1B).
Differential metabolites between the
COPD-FE and COPD-NE groups
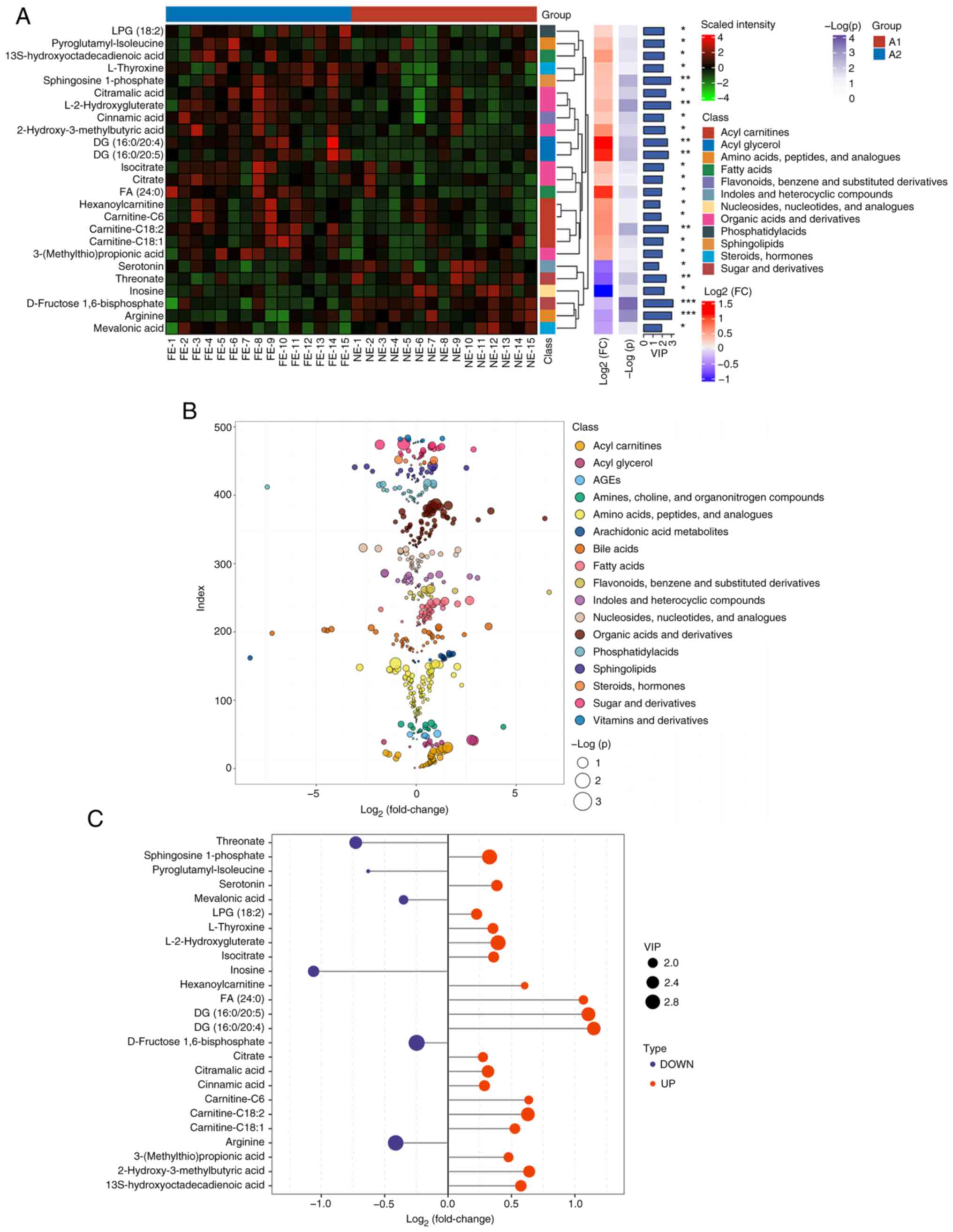
In the present study, 484 metabolites were detected
using a widely targeted metabolomics approach. The significant
differences in the levels of 25 metabolites between the COPD-FE and
COPD-NE groups were determined using multivariate analysis
(OPLS-DA) and univariate analysis methods (two-tailed t-test or
Mann-Whitney U test). In the COPD-FE group, the levels of 19
metabolites, including diacylglycerol (DG; 16:0/20:4), DG
(16:0/20:5), fatty acid (FA; 24:0) and carnitine-C6, were
significantly increased, while six metabolites, including inosine,
threonate, serotonin and arginine, were significantly reduced
compared with in the COPD-NE group. The heatmap depicts the
distribution of these differential metabolites in the samples,
demonstrating the patterns between the two groups (Fig. 2A). Additionally, scatter plots
illustrate the classification of metabolites and their differences
in expression (Fig. 2B). The
significance of the differential metabolites is visually
represented through bar graphs (Fig.
2C).
Comprehensive metabolic pathway
analysis
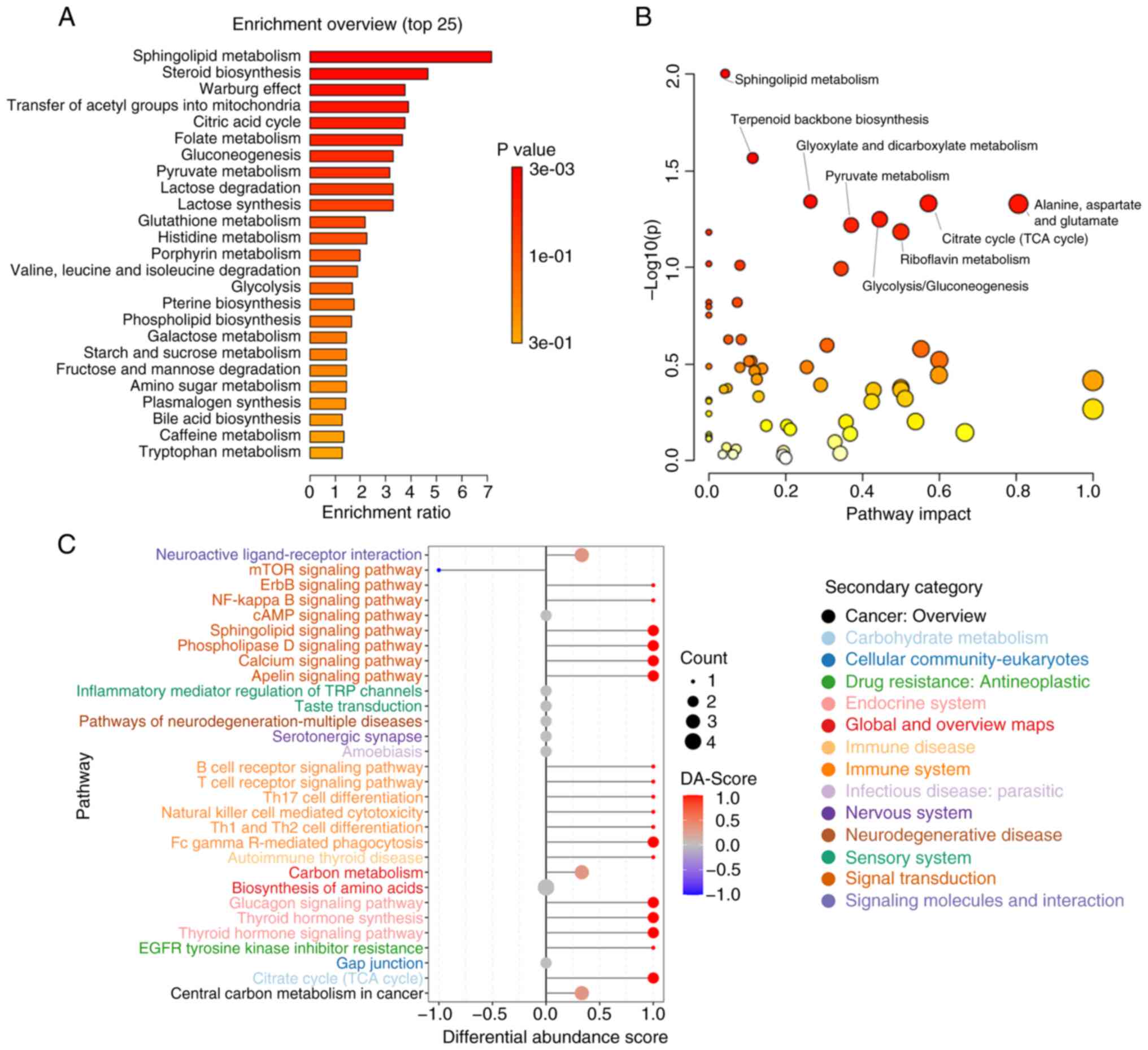
The detected metabolites were analyzed using MSEA,
MetPA and KEGG methods. The MSEA revealed significant enrichment of
multiple metabolic pathways in the COPD-FE group compared to the
COPD-NE group, including ‘Sphingolipid Metabolism’, ‘Steroid
Biosynthesis’, ‘Warburg Effect’, ‘Transfer of Acetyl Groups into
Mitochondria’, and ‘Citric Acid Cycle (TCA cycle)’ (Fig. 3A). MetPA analysis further validated
the enrichment of ‘Sphingolipid Metabolism’ and ‘Citrate cycle (TCA
cycle)’ as identified in the MSEA analysis and demonstrated
additional significant enrichment in pathways such as ‘Glyoxylate
and dicarboxylate metabolism’, as well as ‘Alanine, aspartate and
glutamate metabolism’ (Fig. 3B).
KEGG analysis corroborated the importance of ‘Sphingolipid
Metabolism’ and ‘TCA cycle’, while also revealing new significantly
enriched pathways including ‘Biosynthesis of amino acids’ and
‘Central carbon metabolism in cancer’. Moreover, pathways related
to immune inflammation, such as ‘Fc gamma R-mediated phagocytosis’
and ‘Inflammatory mediator regulation of TRP channels’ were also
significantly enriched in the COPD-FE group (Fig. 3C). This suggested the crucial role
of immune inflammation in COPD development. The detailed analysis
is listed in Table SII, Table SIII, Table SIV.
Correlation analysis between
differential metabolites and clinical indicators
Spearman's correlation analysis demonstrated
associations between metabolite levels and clinical indicators. As
shown in Fig. 4, out of the 25
differential metabolites, 12 demonstrated significant correlations
with clinical indicators of COPD exacerbation risks. Additionally,
the associations between differential metabolites and numerous
dimensions of SF-36 scores were assessed, providing insights into
the potential impact on overall health.
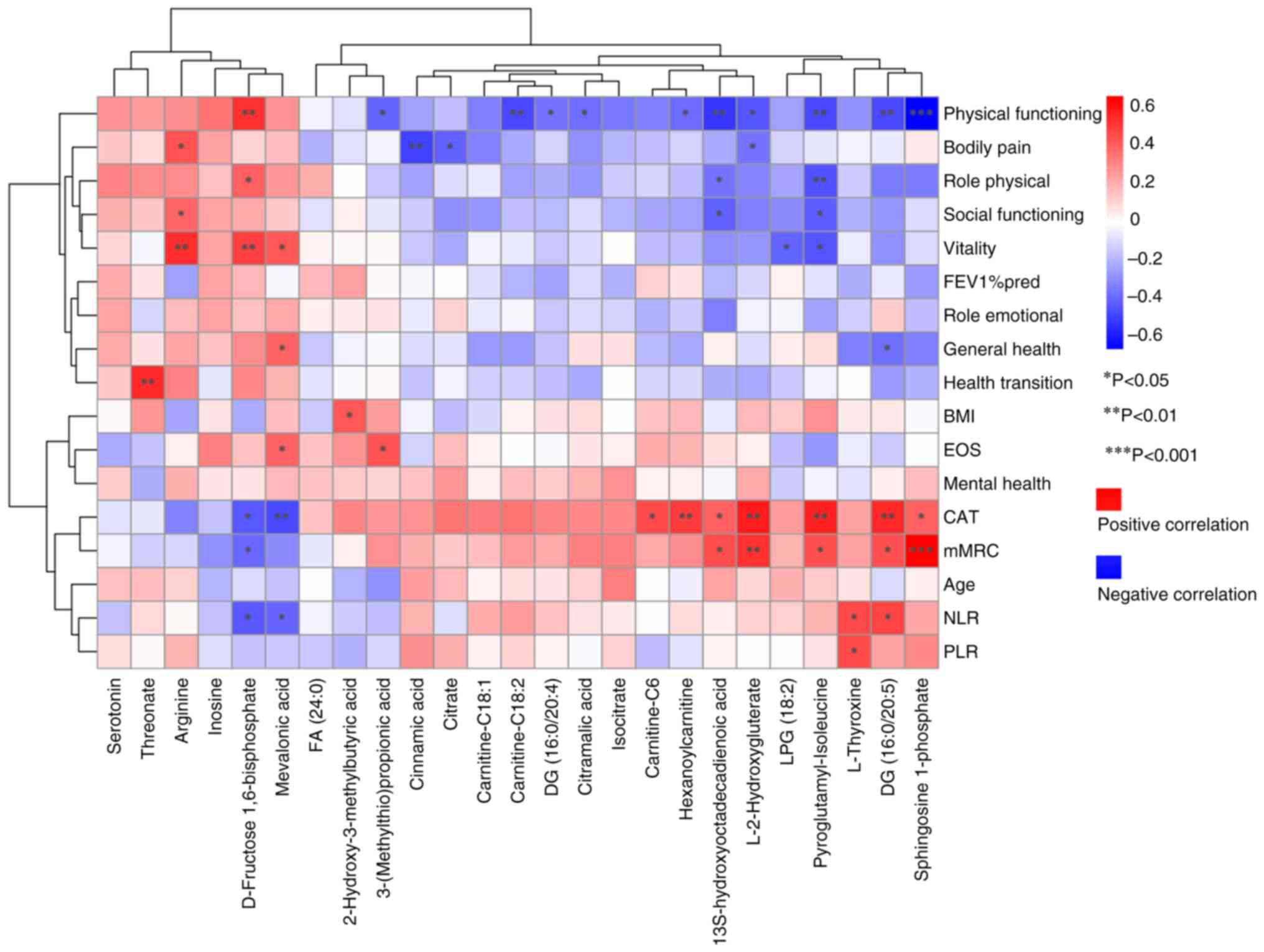 | Figure 4.Spearman's correlation analysis
between differential metabolites and clinical indicators. Red
indicates a positive correlation and blue indicates a negative
correlation; the darker the color, the greater the correlation.
*P<0.05, **P<0.01, ***P<0.001. FA, fatty acid; DG,
diacylglycerol; NLR, neutrophil-to-lymphocyte ratio; PLR,
platelet-to-lymphocyte ratio; CAT, COPD assessment test; COPD,
chronic obstructive pulmonary disease; BMI, body mass index; FEV1%
pred, predicted value of forced expiratory volume in 1 sec. |
Notably, L-2-hydroxyglutarate (L-2HG), sphingosine
1-phosphate (S1P), pyroglutamyl-isoleucine and
13S-hydroxyoctadecadienoic acid were significantly positively
correlated with CAT and mMRC scores, and significantly negatively
correlated with PF score. Carnitine-related differential
metabolites exhibited similar trends; DG (16:0/20:5) was
significantly positively correlated with CAT, mMRC and NLR, but
negatively correlated with PF and GH scores. Similarly, L-thyroxine
was significantly positively correlated with NLR and PLR, whereas
mevalonic acid and D-fructose 1,6-bisphosphate were significantly
negatively correlated with CAT and NLR. Threonate, arginine,
inosine and serotonin were associated with CAT and mMRC scores,
although these associations did not reach statistically significant
correlation levels (|R|<0.3). Similarly, threonate, arginine,
inosine and serotonin were associated with PF, GH, RP and SF
scores. The full results are included in Fig. 4 and Table SV.
ROC analysis of differential
metabolites
ROC analysis was used to validate the ability of
metabolites to differentiate between the COPD-NE and COPD-FE
groups. Out of the 25 differential metabolites, 18 exhibited an
area under the ROC curve (AUC) value of >0.7, demonstrating
substantial predictive capacity. Specifically, six differential
metabolites had an AUC value >0.8: D-fructose 1,6-bisphosphate
(AUC=0.871), L-2HG (AUC=0.849), arginine (AUC=0.836), DG
(16:0/20:5) (AUC=0.827), DG (16:0/20:4) (AUC=0.818) and
carnitine-C18:2 (AUC=0.804), demonstrating their predictive
capacity between the two groups. The full results are included in
Fig. 5 and Table SVI.
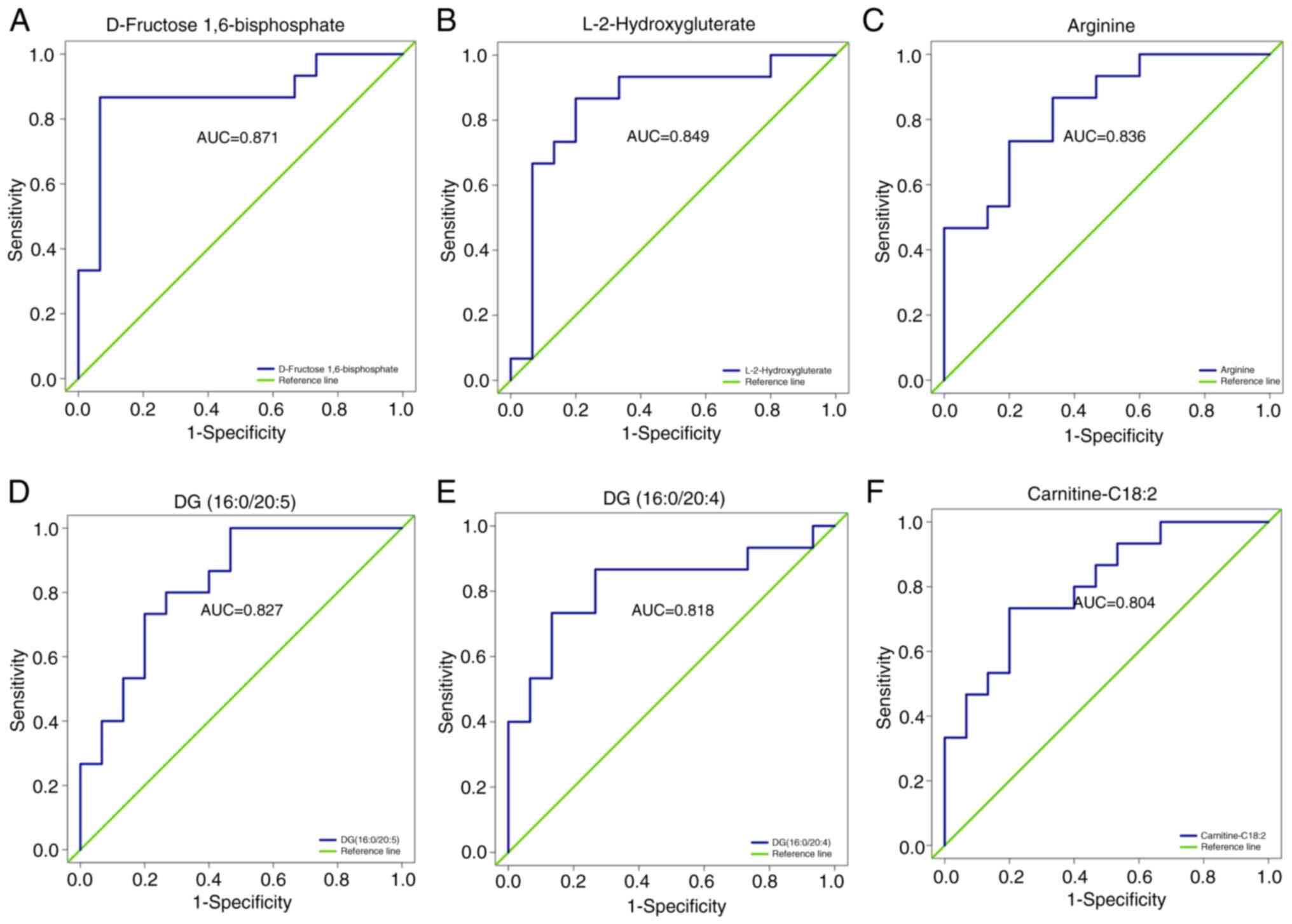 | Figure 5.ROC analysis of differential
metabolites. ROC curves for (A) D-Fructose 1,6-Bisphosphate, (B)
L-2-Hydroxyglutarate, (C) Arginine, (D) DG(16:0/20:5), (E)
DG(16:0/20:4), and (F) Carnitine C18:2 are presented to distinguish
between frequent and non-frequent exacerbation phenotypes of COPD.
COPD, chronic obstructive pulmonary disease; DG, diacylglycerol;
ROC, receiver operating characteristic; AUC, area under the
curve. |
External cohort validation of the
metabolomics results
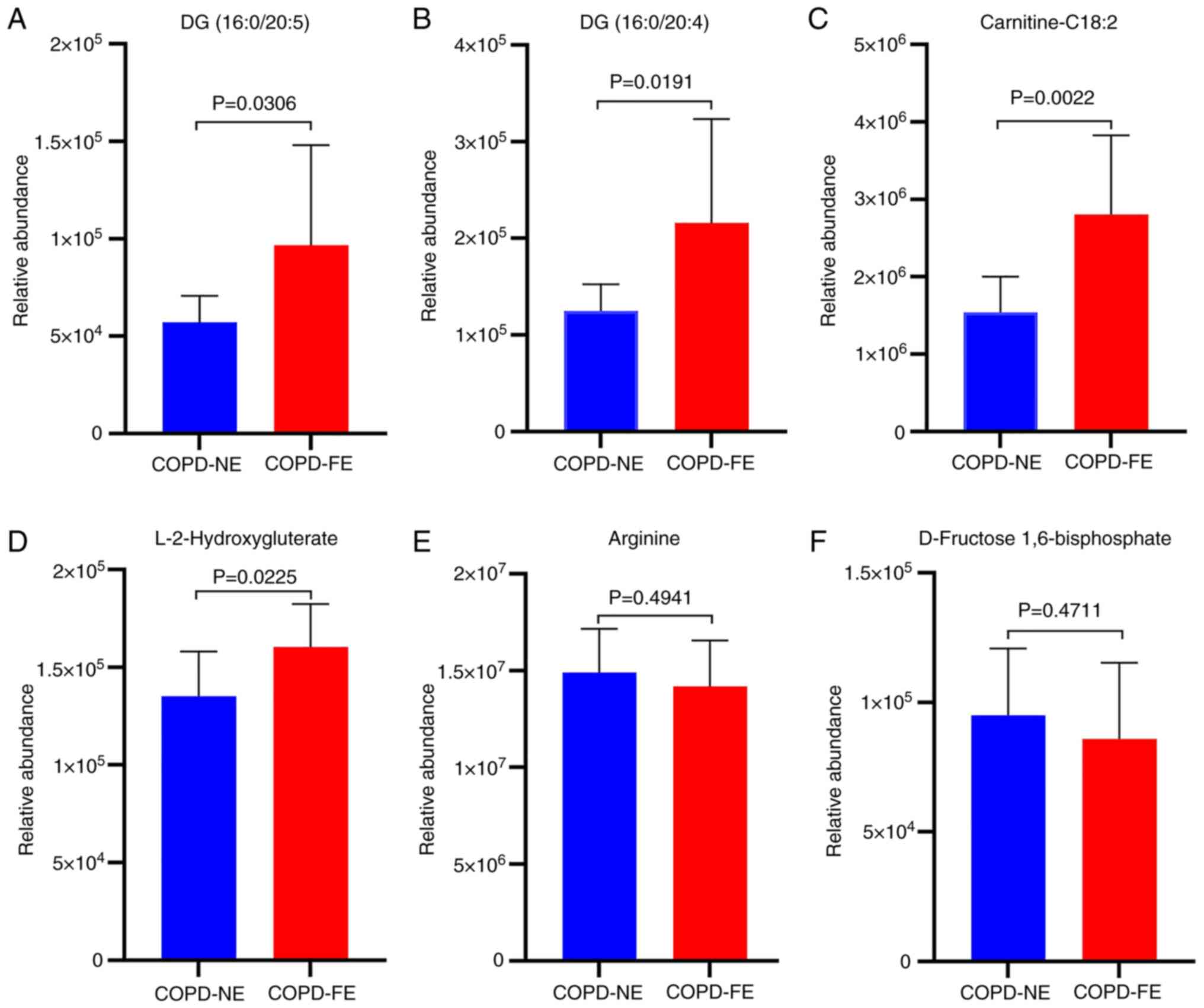
Based on the ROC analysis results, external cohort
validation was conducted for six differentially expressed
metabolites with AUC values >0.8 using UPLC-MS/MS and MRM
scanning modes. The external validation cohort consisted of 10
patients with COPD-NE and 10 patients with COPD-FE. Baseline
comparisons were performed, revealing no statistically significant
differences in age, sex, BMI, smoking history, medication usage, as
well as FEV1% pred and FEV1/FVC ratio between the two groups
(Table SVII). External validation
results demonstrated significant upregulation of DG (16:0/20:5), DG
(16:0/20:4), carnitine-C18:2 and L-2HG in the COPD-FE group
compared with in the COPD-NE group (Fig. 6). Conversely, arginine and
D-fructose 1,6-bisphosphate demonstrated a decreased expression in
the COPD-FE group; however, this difference was not statistically
significant (Fig. 6). The
extracted ion chromatograms for the six differentially expressed
metabolites are shown in Fig.
S1.
Discussion
The impact of frequent acute exacerbations on COPD
progression is well-established (3,4).
Assessing the mechanisms of the COPD-FE phenotype and identifying
relevant biomarkers is required for advancing COPD management
strategies. The present study used widely targeted metabolomics
techniques to analyze serum samples from patients with stable
COPD-FE and COPD-NE, identifying 484 metabolites. The subsequent
application of the OPLS-DA model demonstrated significant
disparities in serum metabolites between the two cohorts.
Permutation tests further validated these findings to ensure the
model's reliability. The present study identified 25 metabolites
with significant differences between the COPD-FE and COPD-NE
groups, demonstrating metabolic adaptations in patients with
COPD-FE and their potential role in underlying pathogenic
mechanisms.
MSEA, MetPA and KEGG analyses were conducted to
assess the enrichment of metabolic pathways in the COPD-FE
phenotype, providing insights into its underlying mechanisms. The
findings of the present study demonstrated significant enrichment
in lipid, energy and amino acid metabolism pathways. Furthermore,
KEGG analysis revealed the enrichment of immune and
inflammatory-related pathways, with all three analytical approaches
highlighting enrichment in the ‘sphingolipid metabolism’ pathway.
These findings suggested its involvement in COPD-FE pathogenesis.
COPD, characterized by persistent inflammation, immune
dysregulation and heightened oxidative stress, involves programmed
cell death, and atypical proliferation of airway and lung
parenchymal cells (22).
Sphingolipids are bioactive molecules that are crucial in numerous
biological processes (23). S1P,
ceramide-1-phosphate and ceramide form the ‘sphingolipid rheostat’,
which has garnered attention in respiratory medicine due to its
association with pulmonary inflammation and cell cycle regulation
(24–26). Sphingolipids may serve as potential
targets for predicting exacerbations of COPD (18,27),
which is consistent with our study findings. Therefore,
investigating dysregulated sphingolipid metabolism and its
potential impact on immune and inflammatory regulation in the
context of the COPD-FE phenotype may hold significant importance
for the prevention and treatment of AECOPD.
The role of energy metabolism in COPD is significant
(28). Dysregulation of
mitochondrial energy metabolism in COPD is influenced by oxidative
stress, chronic inflammation, hypoxia and heightened energy
expenditure (29,30). The present study identifies
significant enrichment of energy metabolism pathways in COPD-FE,
with the ‘TCA cycle’ pathway commonly enriched across all three
analytical methods. The TCA cycle serves a central role in cellular
metabolism, regulating bioenergetics, biosynthesis and redox
balance (31,32). A previous study detected higher
resting energy expenditure in patients with COPD compared with that
in healthy individuals (33). The
present study proposed that frequent acute exacerbations may
further increase resting energy expenditure, leading to the
accumulation of TCA cycle intermediates and the disruption of
anaerobic glycolytic pathways in patients with COPD (34,35).
Additionally, the disruption of the TCA cycle has been shown to be
associated with the severity of lung function decline and increased
mortality risk in patients with COPD (36). Consequently, a comprehensive
understanding of energy metabolism disruptions in numerous stages
and subgroups of COPD, coupled with research into the role of
metabolic reprogramming in the regulation of energy metabolism and
TCA cycle intermediates, is required for achieving personalized
management of patients with COPD.
The results from MetPA and KEGG analyses
demonstrated disruptions in amino acid metabolism within the
COPD-FE phenotype. Diminished expression of numerous amino acids,
particularly branched-chain amino acids, has been documented in
individuals with COPD, demonstrating significant differences
between acute exacerbation and stable phases (37,38).
Decreased serum concentrations of tryptophan, leucine and valine
have been independently associated with frequent acute
exacerbations in COPD (39). The
results of the present study demonstrated an enrichment of
‘Alanine, aspartate and glutamate metabolism’ and ‘Biosynthesis of
amino acids’ pathways in the COPD-FE group. These metabolic
pathways serve numerous biological functions, including amino acid
metabolism, nitrogen equilibrium, energy generation and
physiological processes related to oxidative stress (40,41),
suggesting their potential roles in the pathogenic mechanisms of
the COPD-FE phenotype. The depletion of arginine in COPD-FE is
regulated by arginase, which may be associated with factors such as
chronic hypoxia, oxidative stress and inflammation (42–44).
Previous research has demonstrated that arginine serves a role in
regulating the innate immune response in macrophages by
facilitating the activation of MAPK and the production of cytokines
(45). Furthermore, arginine
influences T-cell function by modulating the cycle of CD3ζ
internalization and subsequent re-expression (46). Administration of arginine treatment
has been reported to alleviate lung inflammation and airway
remodeling (47) and improve
cardiopulmonary health in patients with COPD (48). Therefore, increasing arginine
levels could potentially serve as a therapeutic approach for
COPD-FE.
KEGG analysis also demonstrated the enrichment of
pathways such as ‘Fc gamma R-mediated phagocytosis’ and
‘Inflammatory mediator regulation of TRP channels’. These findings
suggested that immune and inflammation-related pathways potentially
influence the pathogenesis of COPD-FE, impacting the pathological
mechanisms of the disease. The involvement of Fc gamma R (FcγR) in
facilitating antibody-antigen complexes and cellular effector
functions, as the Fc receptor for immunoglobulin G (49), is essential for mediating
phagocytosis by monocytes, macrophages and neutrophils, and is
important in immune responses and inflammation. Consequently, the
demonstrated enrichment of FcγR in patients with COPD-FE may
suggest the activation of a self-protective mechanism. TRP
channels, known for their role as regulating ion channels,
substantially influence intricate cellular signaling cascades
within the pulmonary system and serve as pathways for mediating
pulmonary toxicity. Their activation in response to stimuli such as
hypoxia and endotoxins triggers the influx of calcium ions. This
compromises the integrity of lung cell barriers, thereby
instigating immune dysregulation, inflammation, cellular demise and
edema within the lungs (50–52).
However, additional empirical evidence is necessary to substantiate
these findings and to better comprehend the specific functions of
immune and inflammation-related pathways in COPD-FE. The three
different analytical approaches used in the present study
comprehensively assessed the enrichment of metabolic pathways,
providing valuable insights for future research into the
pathological mechanisms of COPD-FE.
Through Spearman's correlation analysis,
associations between metabolites and clinical indicators of acute
exacerbations of COPD were identified. The significant positive
correlations demonstrated between S1P and L-2HG with CAT and mMRC
scores suggest their potential as biomarkers for assessing the risk
of acute exacerbations and evaluating symptoms. Furthermore, these
metabolites demonstrated negative associations with numerous
dimensions of the SF-36 questionnaire, indicating their potential
involvement in the deterioration of the overall health of patients
with COPD. S1P is a bioactive lipid mediator that is produced by
the phosphorylation of sphingosine by sphingosine kinases (SphK).
The accumulation of S1P signifies disruptions in sphingolipid
metabolism, potentially attributed to recurrent infections or acute
lung injuries (53–55). Respiratory infections are common
triggers of AECOPD. The SphK/S1P axis has been reported to regulate
the host immune system and to exert pro- or anti-viral effects in
different types of viral infections by interfering with
intracellular signaling pathways (56,57).
For example, S1P can enhance endothelial barrier function and
epithelial cell survival during respiratory syncytial virus
infection by activating the Akt/ERK signaling pathway (58,59).
Therefore, the role of S1P in respiratory infections in patients
with COPD-FE phenotype requires further investigation. CAT and mMRC
scores reflect the clinical symptoms and the severity of
breathlessness in patients. Chronic inflammation is the primary
mechanism leading to the decline in lung function and worsening of
the condition in patients with COPD. S1P modulates immune cell
recruitment, proliferation, migration and bidirectional regulation
of inflammatory processes by binding to G protein-coupled S1P
receptors (60–62). Recently, it has been reported that
in COPD, S1P inhibits histone deacetylase 1 activity, drives
alveolar macrophage polarization towards the pro-inflammatory M1
type and promotes inflammatory cytokine release (63). Elevated S1P levels have been
demonstrated to contribute to airway cholinergic
hyperresponsiveness in a mouse model of COPD, exacerbating airway
constriction and facilitating remodeling (64). These studies partially reveal the
association between S1P and worsening dyspnea and frequent acute
exacerbations in COPD. In-depth study of the expression and
function of the S1P pathway in different stages and phenotypes of
COPD, elucidation of the regulatory network between S1P and
COPD-FE, and exploration of its underlying molecular mechanisms
will be the research focus of future work.
Existing research has suggested that hypoxia and
mitochondrial stress contribute to increased L-2HG levels (65,66).
The demonstrated positive association between L-2HG levels and the
intensity of breathlessness implies its potential role as an
adaptive reaction to hypoxia. L-2HG alleviates hypoxia-induced
mitochondrial damage by inhibiting glycolysis and regulating
oxidative phosphorylation (67).
Moreover, L-2HG levels influence immune cells, specifically
promoting Th17 cell differentiation, enhancing the stability of
HIF-1α to facilitate the activation of inflammatory macrophages,
thereby promoting the expression of inflammatory factors (68,69).
A more comprehensive investigation is necessary to elucidate the
exact involvement of L-2HG in the pathological mechanisms of
COPD-FE. The present study demonstrated an elevation in carnitine
metabolite levels among patients with COPD-FE, showing positive
associations with CAT scores and negative associations with
Physical Functioning. This abnormal metabolic profile highlights
disruptions in mitochondrial energy processes (70), potentially contributing to a
decline in lung function (71).
Research has indicated that L-carnitine can mitigate apoptosis in
alveolar type II (ATII)-like LA4 cells induced by PPE and H2O2
(72). Furthermore, carnitine
metabolism is recognized for its significant regulatory function in
processes associated with inflammation and oxidative stress
(73,74). These mechanisms could provide
valuable insights into the reported associations.
Clinical studies have demonstrated that NLR and PLR
are valuable predictive indicators for COPD prognosis and the
likelihood of acute exacerbations (11). Furthermore, evidence has suggested
an association between thyroid function and COPD severity and
prognosis (75–77). The results of the present study
demonstrated a significant positive correlation between L-thyroxine
levels with NLR and PLR, suggesting the potential of L-thyroxine as
a predictive factor for frequent exacerbations in individuals with
COPD. In contrast to individuals with COPD-NE, the increased
concentrations of L-thyroxine in patients with COPD-FE may indicate
physiological adjustments in response to recurrent acute
exacerbations. Although thyroid hormones are suggested to serve a
role in energy metabolism, inflammation and airway remodeling
(78,79), their specific influence in the
pathogenesis of COPD-FE remains uncertain, requiring additional
research to clarify underlying mechanisms. DG, produced through
phosphatidylinositol metabolism, is a lipid secondary messenger
activated by extracellular stimuli (80). The innate and adaptive immune
systems are crucial mechanisms for protecting against external
pathogens. In the intricate signaling pathways, DG, under the
regulation of DG kinase, serves a significant role, particularly in
modulating immune responses such as antimicrobial autophagy,
Th1/Th17 cell differentiation, neutrophil function and
proliferation (81–83). The outcomes of the present study
demonstrate a positive association between DG (16:0/20:5) and NLR
and PLR, highlighting the requirement for further investigation
into the role of DG (16:0/20:5) in modulating immune responses in
COPD-FE. Furthermore, the levels of D-fructose 1,6-bisphosphate
demonstrated a significant negative correlation with NLR, CAT and
mMRC scores, while displaying a significant positive correlation
with PF and VT scores in patients with COPD-FE. This correlation
could be associated with diminished D-fructose 1,6-bisphosphate
levels in the physiological system of patients with COPD-FE,
attributable to factors including hypoxia, disrupted glycolysis and
oxidative stress (84). Diminished
D-fructose 1,6-bisphosphate levels may disrupt sugar metabolism and
energy provision, potentially impacting the quality of life of
patients. Nevertheless, further investigation is essential to fully
comprehend the underlying mechanisms. In summary, the application
of Spearman's correlation analysis provided initial insights into
the intricate correlation between metabolic disturbances and the
propensity for recurrent acute exacerbations in individuals with
COPD. Subsequent investigations should prioritize elucidating the
precise molecular mechanisms underlying these connections and
exploring their potential applications in COPD treatment.
ROC analysis was performed on the 25 identified
differential metabolites. Among them, six metabolites, including
D-fructose 1,6-bisphosphate, arginine, L-2HG, DG (16:0/20:5), DG
(16-0/20:4) and carnitine-C18:2 demonstrated AUC values >0.8,
indicating their discriminatory ability between COPD-FE and COPD-NE
groups. External validation of these metabolites demonstrated a
significant upregulation of DG (16:0/20:5), DG (16:0/20:4),
carnitine-C18:2 and L-2HG in patients with COPD-FE compared with in
those with COPD-NE. While D-fructose 1,6-bisphosphate and arginine
did not exhibit a significant decrease in expression in the
external validation cohort, their trends aligned with the internal
validation cohort. These consistent trends underscore the relevance
of these metabolites in predicting the risk of exacerbations. The
concordance between internal metabolomics analysis results and
external validation supports these identified metabolites as
potential biomarkers for COPD-FE, guiding future research into
their roles and pathways, contributing to developing more refined
predictive models and increasing the understanding of COPD
pathophysiology. In the external cohort, the results were
consistent with those of the internal cohort. This served as a
partial alleviation of the limitations identified in the internal
cohort, thereby offering supplementary evidence for the
dependability and replicability of the present research
results.
The present study possesses both limitations and
strengths. Despite controlled inclusion criteria to minimize
confounding factors, the limited sample size may have reduced the
statistical power and robustness of the ROC results. However, the
consistency between external validation results and internal cohort
findings enhances the reliability of the conclusions of the present
study. Furthermore, the interpretability of the findings supports
publication, as the present study represents a primary and
exploratory effort aiming to provide foundational knowledge for
future research. A history of previous acute exacerbations is a
recognized independent risk factor for predicting future
exacerbations (2,85). However, the present study excluded
patients with COPD experiencing only one acute exacerbation
annually, potentially limiting the understanding of differences
among patients with varied exacerbation frequencies and reducing
the comprehensiveness of the study. Additionally, the study
exclusively included Chinese Han participants, limiting the
generalization of the findings. Future research will focus on
expanding the sample size, investigating pathological differences
among patients with COPD with diverse exacerbation frequencies and
severity, and include more representative populations to enhance
the applicability of these findings.
The present study used the comprehensive targeted
metabolomics technique based on UPLC-MS/MS to detect serum
differential metabolites in patients with stable-phase COPD-FE.
This differs from previous research in terms of the technology
used, the disease stage of the subjects (stable phase instead of
acute exacerbation), and the specific focus on the COPD-FE
phenotype (15,17,39,86).
In comparison to prior studies, the present study used more
comprehensive bioinformatics methods, including MSEA, MetPA and
KEGG analyses. This allowed the present analysis to cover both
differentially and non-differentially expressed metabolites,
contributing to a more comprehensive understanding of potential
changes in metabolic pathways. The present study analyzed the
correlation between differential metabolites and clinical
indicators predicting acute exacerbations. Through ROC analysis and
external cohort validation, the potential of these metabolites as
biomarkers and therapeutic targets was emphasized. Furthermore, by
examining the correlation between differential metabolites and
SF-36 scores, a comprehensive exploration of the association
between metabolites, and both physical and psychological health was
provided.
In conclusion, the present study highlights the
significant differences in metabolite expression between COPD-FE
and COPD-NE groups. Disruptions in lipid, energy and amino acid
metabolism pathways were revealed to be the primary defining
features of the COPD-FE phenotype. Importantly, metabolites such as
DG (16:0/20:5), DG (16:0/20:4), carnitine-C18:2 and L-2HG were
demonstrated as potential biomarkers for predicting the risk of
COPD-FE. The present study lays the foundation for further
pathological investigations, developing risk prediction models and
implementing personalized management approaches targeting the
COPD-FE phenotype.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Dr Qi Yaoxin from
Shanghai Bioprofile Technology Co., Ltd., Shanghai, China, for
providing valuable technical support in mass spectrometry.
Funding
This study was financially supported by the National Natural
Science Foundation of China Joint Key Project (grant no. U20A20398)
and the 2022 Anhui Provincial Natural Science Foundation, China
(grant no. 2208085QH264).
Availability of data and materials
The data generated and analyzed during the current
study are available in the MetaboLights repository under the
accession number MTBLS9119. The data can be accessed at the
following URL: www.ebi.ac.uk/metabolights/MTBLS9119.
Authors' contributions
ZGL and HZD conceived the study. HZD and HW designed
the methodology and wrote the manuscript. HZD, HW, FCZ and DW
analyzed data. HZD, HW, DW, YTG and JBT performed experiments. HZD,
DW and JZ interpreted data. HZD, HW and JZ reviewed the manuscript.
HZD, HW and FCZ visualized data. JBT and ZGL supervised the study.
DW and ZGL confirm the authenticity of all the raw data. All
authors have read approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Anhui Provincial Hospital of Traditional Chinese Medicine (Hefei,
China; approval no. 2021AH-31) and was conducted in accordance with
the guidelines outlined in The Declaration of Helsinki. All
patients gave their written informed consent to the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AECOPD
|
acute exacerbations of COPD
|
|
BMI
|
body mass index
|
|
BP
|
bodily pain
|
|
CAT
|
COPD assessment test
|
|
COPD
|
chronic obstructive pulmonary
disease
|
|
COPD-FE
|
frequent exacerbation of COPD
|
|
COPD-NE
|
non-frequent exacerbation of COPD
|
|
DG
|
diacylglycerol
|
|
FEV1
|
forced expiratory volume in 1
second
|
|
FVC
|
forced vital capacity
|
|
GH
|
general health
|
|
GOLD
|
Global Initiative for Chronic
Obstructive Lung Disease
|
|
HT
|
health transition
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
L-2HG
|
L-2-hydroxyglutarate
|
|
MetPA
|
Metabolite Pathway Analysis
|
|
MH
|
mental health
|
|
MRM
|
multiple reaction monitoring
|
|
MSEA
|
Metabolite Set Enrichment Analysis
|
|
OPLS-DA
|
Orthogonal Partial Least Squares
Discriminant Analysis
|
|
PF
|
physical functioning
|
|
PLR
|
platelet-to-lymphocyte ratio
|
|
RE
|
role emotional
|
|
ROC
|
receiver operating characteristic
|
|
RP
|
role physical
|
|
S1P
|
sphingosine-1-phosphate
|
|
SF
|
social functioning
|
|
SF-36
|
36-item short-form health survey
|
|
UPLC-MS/MS
|
ultra performance liquid
chromatography tandem mass spectrometry
|
|
VIP
|
Variable Importance in Projection
|
|
VT
|
vitality
|
References
|
1
|
Global Initiative for Chronic Obstructive
Lung Disease (GOLD), . Global strategy for the diagnosis,
management and prevention of chronic obstructive pulmonary disease:
2020 report. GOLD; Fontana, WI: 2020, https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdfFebruary
15–2020
|
|
2
|
Hurst JR, Vestbo J, Anzueto A, Locantore
N, Müllerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee
W, et al: Susceptibility to exacerbation in chronic obstructive
pulmonary disease. N Engl J Med. 363:1128–1138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dransfield MT, Kunisaki KM, Strand MJ,
Anzueto A, Bhatt SP, Bowler RP, Criner GJ, Curtis JL, Hanania NA,
Nath H, et al: Acute exacerbations and lung function loss in
smokers with and without chronic obstructive pulmonary disease. Am
J Respir Crit Care Med. 195:324–330. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wedzicha JA and Seemungal TA: COPD
exacerbations: Defining their cause and prevention. Lancet.
370:786–796. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
France G, Orme MW, Greening NJ, Steiner
MC, Chaplin EJ, Clinch L and Singh SJ: Cognitive function following
pulmonary rehabilitation and post-discharge recovery from
exacerbation in people with COPD. Respir Med. 176:1062492021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Camac ER, Voelker H and Criner GJ; COPD
Clinical Research Network and the Canadian Institutes of Health
Research, : Impact of COPD exacerbations leading to hospitalization
on general and disease-specific quality of life. Respir Med.
186:1065262021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hallin R, Koivisto-Hursti UK, Lindberg E
and Janson C: Nutritional status, dietary energy intake and the
risk of exacerbations in patients with chronic obstructive
pulmonary disease (COPD). Respir Med. 100:561–567. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Asai N, Ohkuni Y, Ohashi W and Kaneko N:
Modified MRC assessment and FEV1.0 can predict frequent acute
exacerbation of COPD: An observational prospective cohort study at
a single-center in Japan. Respir Med. 212:1072182023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cen J and Weng L: Comparison of peak
expiratory Flow(PEF) and COPD assessment test (CAT) to assess COPD
exacerbation requiring hospitalization: A prospective observational
study. Chron Respir Dis. 19:147997312210818592022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu JJ, Xu HR, Zhang YX, Li YX, Yu HY,
Jiang LD, Wang CX and Han M: The characteristics of the frequent
exacerbator with chronic bronchitis phenotype and non-exacerbator
phenotype in patients with chronic obstructive pulmonary disease: A
meta-analysis and system review. BMC Pulm Med. 20:1032020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu X, Ge H, Feng X, Hang J, Zhang F, Jin
X, Bao H, Zhou M, Han F, Li S, et al: The combination of hemogram
indexes to predict exacerbation in stable chronic obstructive
pulmonary disease. Front Med (Lausanne). 7:5724352020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen W, Gong L, Guo Z, Wang W, Zhang H,
Liu X, Yu S, Xiong L and Luo J: A novel integrated method for
large-scale detection, identification, and quantification of widely
targeted metabolites: Application in the study of rice
metabolomics. Mol Plant. 6:1769–1780. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Q and Song J: Analysis of widely
targeted metabolites of the euhalophyte Suaeda salsa under saline
conditions provides new insights into salt tolerance and
nutritional value in halophytic species. BMC Plant Biol.
19:3882019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun T, Ding ZX, Luo X, Liu QS and Cheng Y:
Blood exosomes have neuroprotective effects in a mouse model of
Parkinson's disease. Oxid Med Cell Longev. 2020:38074762020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peng L, You H, Xu MY, Dong ZY, Liu M, Jin
WJ and Zhou C: A novel metabolic score for predicting the acute
exacerbation in patients with chronic obstructive pulmonary
disease. Int J Chron Obstruct Pulmon Dis. 18:785–795. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim J, Suresh B, Lim MN, Hong SH, Kim KS,
Song HE, Lee HY, Yoo HJ and Kim WJ: Metabolomics reveals
dysregulated sphingolipid and amino acid metabolism associated with
chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon
Dis. 17:2343–2353. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gai X, Guo C, Zhang L, Zhang L, Abulikemu
M, Wang J, Zhou Q, Chen Y, Sun Y and Chang C: Serum
glycerophospholipid profile in acute exacerbation of chronic
obstructive pulmonary disease. Front Physiol. 12:6460102021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X, Zhang H, Si Y, Du Y, Wu J and Li J:
High-coverage lipidomics analysis reveals biomarkers for diagnosis
of acute exacerbation of chronic obstructive pulmonary disease. J
Chromatogr B Analyt Technol Biomed Life Sci. 1201–1202.
1232782022.PubMed/NCBI
|
|
19
|
Singanayagam A, Loo SL, Calderazzo M,
Finney LJ, Trujillo Torralbo MB, Bakhsoliani E, Girkin J, Veerati
P, Pathinayake PS, Nichol KS, et al: Antiviral immunity is impaired
in COPD patients with frequent exacerbations. Am J Physiol Lung
Cell Mol Physiol. 317:L893–L903. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lenferink A, Brusse-Keizer M, van der Valk
PD, Frith PA, Zwerink M, Monninkhof EM, van der Palen J and Effing
TW: Self-management interventions including action plans for
exacerbations versus usual care in patients with chronic
obstructive pulmonary disease. Cochrane Database Syst Rev.
8:CD0116822017.PubMed/NCBI
|
|
21
|
Wang R, Wu C, Zhao Y, Yan X, Ma X, Wu M,
Liu W, Gu Z, Zhao J and He J: Health related quality of life
measured by SF-36: A population-based study in Shanghai, China. BMC
Public Health. 8:2922008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Christenson SA, Smith BM, Bafadhel M and
Putcha N: Chronic obstructive pulmonary disease. Lancet.
399:2227–2242. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hannun YA and Obeid LM: Sphingolipids and
their metabolism in physiology and disease. Nat Rev Mol Cell Biol.
19:175–191. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ammit AJ, Hastie AT, Edsall LC, Hoffman
RK, Amrani Y, Krymskaya VP, Kane SA, Peters SP, Penn RB, Spiegel S
and Panettieri RA Jr: Sphingosine 1-phosphate modulates human
airway smooth muscle cell functions that promote inflammation and
airway remodeling in asthma. FASEB J. 15:1212–1214. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jolly PS, Rosenfeldt HM, Milstien S and
Spiegel S: The roles of sphingosine-1-phosphate in asthma. Mol
Immunol. 38:1239–1245. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Helke K, Angel P, Lu P, Garrett-Mayer E,
Ogretmen B, Drake R and Voelkel-Johnson C: Ceramide synthase 6
deficiency enhances inflammation in the DSS model of colitis. Sci
Rep. 8:16272018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bowler RP, Jacobson S, Cruickshank C,
Hughes GJ, Siska C, Ory DS, Petrache I, Schaffer JE, Reisdorph N
and Kechris K: Plasma sphingolipids associated with chronic
obstructive pulmonary disease phenotypes. Am J Respir Crit Care
Med. 191:275–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Belchamber KBR, Singh R, Batista CM, Whyte
MK, Dockrell DH, Kilty I, Robinson MJ, Wedzicha JA, Barnes PJ and
Donnelly LE; COPD-MAP consortium, : Defective bacterial
phagocytosis is associated with dysfunctional mitochondria in COPD
macrophages. Eur Respir J. 54:18022442019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haji G, Wiegman CH, Michaeloudes C, Patel
MS, Curtis K, Bhavsar P, Polkey MI, Adcock IM and Chung KF; COPDMAP
consortium, : Mitochondrial dysfunction in airways and quadriceps
muscle of patients with chronic obstructive pulmonary disease.
Respir Res. 21:2622020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou WC, Qu J, Xie SY, Sun Y and Yao HW:
Mitochondrial dysfunction in chronic respiratory diseases:
Implications for the pathogenesis and potential therapeutics. Oxid
Med Cell Longev. 2021:51883062021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martínez-Reyes I and Chandel NS:
Mitochondrial TCA cycle metabolites control physiology and disease.
Nat Commun. 11:1022020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pålsson-McDermott EM and O'Neill LAJ:
Targeting immunometabolism as an anti-inflammatory strategy. Cell
Res. 30:300–314. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Finamore P, Lattanzi G, Pedone C, Poci S,
Alma A, Scarlata S, Fontana DO, Khazrai YM and Incalzi RA: Energy
expenditure and intake in COPD: The extent of unnoticed unbalance
by predicting REE. Respir Med. 201:1069512022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Naz S, Kolmert J, Yang M, Reinke SN,
Kamleh MA, Snowden S, Heyder T, Levänen B, Erle DJ, Sköld CM, et
al: Metabolomics analysis identifies sex-associated metabotypes of
oxidative stress and the autotaxin-lysoPA axis in COPD. Eur Respir
J. 49:16023222017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xue M, Zeng Y, Lin R, Qu HQ, Zhang T,
Zhang XD, Liang Y, Zhen Y, Chen H, Huang Z, et al: Metabolomic
profiling of anaerobic and aerobic energy metabolic pathways in
chronic obstructive pulmonary disease. Exp Biol Med (Maywood).
246:1586–1596. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pinto-Plata V, Casanova C, Divo M,
Tesfaigzi Y, Calhoun V, Sui J, Polverino F, Priolo C, Petersen H,
de Torres JP, et al: Plasma metabolomics and clinical predictors of
survival differences in COPD patients. Respir Res. 20:2192019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou J, Li Q, Liu C, Pang R and Yin Y:
Plasma metabolomics and lipidomics reveal perturbed metabolites in
different disease stages of chronic obstructive pulmonary disease.
Int J Chron Obstruct Pulmon Dis. 15:553–565. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Engelen MPKJ and Schols AMWJ: Altered
amino acid metabolism in chronic obstructive pulmonary disease: New
therapeutic perspective? Curr Opin Clin Nutr Metab Care. 6:73–78.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Labaki WW, Gu T, Murray S, Curtis JL,
Yeomans L, Bowler RP, Barr RG, Comellas AP, Hansel NN, Cooper CB,
et al: Serum amino acid concentrations and clinical outcomes in
smokers: SPIROMICS metabolomics study. Sci Rep. 9:113672019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pouw EM, Schols AM, Deutz NE and Wouters
EF: Plasma and muscle amino acid levels in relation to resting
energy expenditure and inflammation in stable chronic obstructive
pulmonary disease. Am J Respir Crit Care Med. 158:797–801. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sivakumar R, Babu PV and Shyamaladevi CS:
Aspartate and glutamate prevents isoproterenol-induced cardiac
toxicity by alleviating oxidative stress in rats. Exp Toxicol
Pathol. 63:137–142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rus A, Peinado MA, Castro L and Del Moral
ML: Lung eNOS and iNOS are reoxygenation time-dependent upregulated
after acute hypoxia. Anat Rec (Hoboken). 293:1089–1098. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Caldwell RB, Toque HA, Narayanan SP and
Caldwell RW: Arginase: An old enzyme with new tricks. Trends
Pharmacol Sci. 36:395–405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bulau P, Zakrzewicz D, Kitowska K, Leiper
J, Gunther A, Grimminger F and Eickelberg O: Analysis of
methylarginine metabolism in the cardiovascular system identifies
the lung as a major source of ADMA. Am J Physiol Lung Cell Mol
Physiol. 292:L18–L24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mieulet V, Yan L, Choisy C, Sully K,
Procter J, Kouroumalis A, Krywawych S, Pende M, Ley SC, Moinard C
and Lamb RF: TPL-2-mediated activation of MAPK downstream of TLR4
signaling is coupled to arginine availability. Sci Signal.
3:ra612010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zea AH, Rodriguez PC, Culotta KS,
Hernandez CP, DeSalvo J, Ochoa JB, Park HJ, Zabaleta J and Ochoa
AC: L-Arginine modulates CD3zeta expression and T cell function in
activated human T lymphocytes. Cell Immunol. 232:21–31. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pera T, Zuidhof AB, Smit M, Menzen MH,
Klein T, Flik G, Zaagsma J, Meurs H and Maarsingh H: Arginase
inhibition prevents inflammation and remodeling in a guinea pig
model of chronic obstructive pulmonary disease. J Pharmacol Exp
Ther. 349:229–238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rathor VPS, Chugh P, Ali R, Bhatnagar A,
Haque SE, Bhatnagar A and Mittal G: Formulation, preclinical and
clinical evaluation of a new submicronic arginine respiratory fluid
for treatment of chronic obstructive pulmonary disorder. Saudi
Pharm J. 24:49–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Anderson CL, Shen L, Eicher DM, Wewers MD
and Gill JK: Phagocytosis mediated by three distinct Fc gamma
receptor classes on human leukocytes. J Exp Med. 171:1333–1345.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dietrich A, Steinritz D and Gudermann T:
Transient receptor potential (TRP) channels as molecular targets in
lung toxicology and associated diseases. Cell Calcium. 67:123–137.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Takahashi N, Kozai D, Kobayashi R, Ebert M
and Mori Y: Roles of TRPM2 in oxidative stress. Cell Calcium.
50:279–287. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Khalil M, Alliger K, Weidinger C, Yerinde
C, Wirtz S, Becker C and Engel MA: Functional role of transient
receptor potential channels in immune cells and epithelia. Front
Immunol. 9:1742018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ebenezer DL, Fu P, Suryadevara V, Zhao Y
and Natarajan V: Epigenetic regulation of pro-inflammatory cytokine
secretion by sphingosine 1-phosphate (S1P) in acute lung injury:
Role of S1P lyase. Adv Biol Regul. 63:156–166. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wadgaonkar R, Patel V, Grinkina N, Romano
C, Liu J, Zhao Y, Sammani S, Garcia JG and Natarajan V:
Differential regulation of sphingosine kinases 1 and 2 in lung
injury. Am J Physiol Lung Cell Mol Physiol. 296:L603–L613. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Feng A, Rice AD, Zhang Y, Kelly GT, Zhou T
and Wang T: S1PR1-associated molecular signature predicts survival
in patients with sepsis. Shock. 53:284–292. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mohammed S, Bindu A, Viswanathan A and
Harikumar KB: Sphingosine 1-phosphate signaling during infection
and immunity. Prog Lipid Res. 92:1012512023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang L, Liu J, Xiao E, Han Q and Wang L:
Sphingosine-1-phosphate related signalling pathways manipulating
virus replication. Rev Med Virol. 33:e24152023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Monick MM, Cameron K, Powers LS, Butler
NS, McCoy D, Mallampalli RK and Hunninghake GW: Sphingosine kinase
mediates activation of extracellular signal-related kinase and Akt
by respiratory syncytial virus. Am J Respir Cell Mol Biol.
30:844–852. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Thomas KW, Monick MM, Staber JM,
Yarovinsky T, Carter AB and Hunninghake GW: Respiratory syncytial
virus inhibits apoptosis and induces NF-kappa B activity through a
phosphatidylinositol 3-kinase-dependent pathway. J Biol Chem.
277:492–501. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Obinata H and Hla T: Sphingosine
1-phosphate and inflammation. Int Immunol. 31:617–625. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lee H, Deng J, Kujawski M, Yang C, Liu Y,
Herrmann A, Kortylewski M, Horne D, Somlo G, Forman S, et al:
STAT3-induced S1PR1 expression is crucial for persistent STAT3
activation in tumors. Nat Med. 16:1421–1428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hou L, Zhang Z, Yang L, Chang N, Zhao X,
Zhou X, Yang L and Li L: NLRP3 inflammasome priming and activation
in cholestatic liver injury via the sphingosine 1-phosphate/S1P
receptor 2/Gα(12/13)/MAPK signaling pathway. J Mol Med
(Berl). 99:273–288. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang M, Hei R, Zhou Z, Xiao W, Liu X and
Chen Y: Macrophage polarization involved the inflammation of
chronic obstructive pulmonary disease by S1P/HDAC1 signaling. Am J
Cancer Res. 13:4478–4489. 2023.PubMed/NCBI
|
|
64
|
De Cunto G, Brancaleone V, Riemma MA,
Cerqua I, Vellecco V, Spaziano G, Cavarra E, Bartalesi B,
D'Agostino B, Lungarella G, et al: Functional contribution of
sphingosine-1-phosphate to airway pathology in cigarette
smoke-exposed mice. Br J Pharmacol. 177:267–281. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Oldham WM, Clish CB, Yang Y and Loscalzo
J: Hypoxia-mediated increases in L-2-hydroxyglutarate coordinate
the metabolic response to reductive stress. Cell Metab. 22:291–303.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Intlekofer AM, Dematteo RG, Venneti S,
Finley LW, Lu C, Judkins AR, Rustenburg AS, Grinaway PB, Chodera
JD, Cross JR and Thompson CB: Hypoxia induces production of
L-2-hydroxyglutarate. Cell Metab. 22:304–311. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Intlekofer AM, Wang B, Liu H, Shah H,
Carmona-Fontaine C, Rustenburg AS, Salah S, Gunner MR, Chodera JD,
Cross JR and Thompson CB: L-2-Hydroxyglutarate production arises
from noncanonical enzyme function at acidic pH. Nat Chem Biol.
13:494–500. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Steinert EM, Vasan K and Chandel NS:
Mitochondrial metabolism regulation of T cell-mediated immunity.
Annu Rev Immunol. 39:395–416. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Williams NC, Ryan DG, Costa ASH, Mills EL,
Jedrychowski MP, Cloonan SM, Frezza C and O'Neill LA: Signaling
metabolite L-2-hydroxyglutarate activates the transcription factor
HIF-1α in lipopolysaccharide-activated macrophages. J Biol Chem.
298:1015012022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Almannai M, Alfadhel M and El-Hattab AW:
Carnitine inborn errors of metabolism. Molecules. 24:32512019.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang Y, Li P, Cao Y, Liu C, Wang J and Wu
W: Skeletal muscle mitochondrial dysfunction in chronic obstructive
pulmonary disease: Underlying mechanisms and physical therapy
perspectives. Aging Dis. 14:33–45. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Conlon TM, Bartel J, Ballweg K, Günter S,
Prehn C, Krumsiek J, Meiners S, Theis FJ, Adamski J, Eickelberg O
and Yildirim AÖ: Metabolomics screening identifies reduced
L-carnitine to be associated with progressive emphysema. Clin Sci
(Lond). 130:273–287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Nicholas DA, Proctor EA, Agrawal M,
Belkina AC, Van Nostrand SC, Panneerseelan-Bharath L, Jones AR IV,
Raval F, Ip BC, Zhu M, et al: Fatty Acid metabolites combine with
reduced β oxidation to activate Th17 inflammation in human type 2
diabetes. Cell Metab. 30:447–461.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Aguer C, McCoin CS, Knotts TA, Thrush AB,
Ono-Moore K, McPherson R, Dent R, Hwang DH, Adams SH and Harper ME:
Acylcarnitines: Potential implications for skeletal muscle insulin
resistance. FASEB J. 29:336–345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Okutan O, Kartaloglu Z, Onde ME, Bozkanat
E and Kunter E: Pulmonary function tests and thyroid hormone
concentrations in patients with chronic obstructive pulmonary
disease. Med Princ Pract. 13:126–128. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Alshamari AHI, Deli F, Kadhum HI and
Kadhim IJ: Assessment of thyroid function tests in patients with
chronic obstructive pulmonary disease. J Med Life. 15:1532–1535.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Dimopoulou I, Ilias I, Mastorakos G,
Mantzos E, Roussos C and Koutras DA: Effects of severity of chronic
obstructive pulmonary disease on thyroid function. Metabolism.
50:1397–1401. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Dekkers BGJ, Naeimi S, Bos IST, Menzen MH,
Halayko AJ, Hashjin GS and Meurs H: L-thyroxine promotes a
proliferative airway smooth muscle phenotype in the presence of
TGF-β1. Am J Physiol Lung Cell Mol Physiol. 308:L301–L306. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Mancini A, Di Segni C, Raimondo S,
Olivieri G, Silvestrini A, Meucci E and Currò D: Thyroid hormones,
oxidative stress, and inflammation. Mediators Inflamm.
2016:67571542016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Baldanzi G and Malerba M: DGKα in
neutrophil biology and its implications for respiratory diseases.
Int J Mol Sci. 20:56732019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Shahnazari S, Namolovan A, Klionsky DJ and
Brumell JH: A role for diacylglycerol in antibacterial autophagy.
Autophagy. 7:331–333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Yang J, Wang HX, Xie J, Li L, Wang J, Wan
ECK and Zhong XP: DGK α and ζ activities control TH1 and
TH17 cell differentiation. Front Immunol. 10:30482020.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Cooke M and Kazanietz MG: Overarching
roles of diacylglycerol signaling in cancer development and
antitumor immunity. Sci Signal. 15:eabo02642022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Fernie AR, Carrari F and Sweetlove LJ:
Respiratory metabolism: Glycolysis, the TCA cycle and mitochondrial
electron transport. Curr Opin Plant Biol. 7:254–261. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Al-ani S, Spigt M, Hofset P and Melbye H:
Predictors of exacerbations of asthma and COPD during one year in
primary care. Fam Pract. 30:621–628. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Cruickshank-Quinn CI, Jacobson S, Hughes
G, Powell RL, Petrache I, Kechris K, Bowler R and Reisdorph N:
Metabolomics and transcriptomics pathway approach reveals
outcome-specific perturbations in COPD. Sci Rep. 8:171322018.
View Article : Google Scholar : PubMed/NCBI
|