Introduction
Sepsis is a common and frequently fatal condition
that is one of the main causes of multiple organ failure (1). Acute kidney injury (AKI) caused by
sepsis is the most common organ failure symptom with a mortality
rate of up to 70% (2,3). It has been reported that patients
with severe AKI eventually develop renal failure, bringing serious
threaten to the life of individuals and huge economic burden
(4,5). Therefore, there is an urgent
requirement regarding effective therapeutic targets of
sepsis-induced AKI.
The pathogenesis of sepsis-induced AKI is clearly
complex and multi-factorial, including inflammation, oxidative
stress and autophagy, but definitely also involves the apoptosis of
renal tubular cells (6,7). Renal biopsy specimens in patients
with sepsis provided evidence of pronounced renal tubular
apoptosis, suggesting that sepsis-induced apoptosis is closely
associated with kidney failure (8). It was also reported that
unconscionable apoptosis of renal tubular cells can exacerbate
sepsis and increase the mortality rate of patients (9). Zhu et al (10) found that baicalin improved
sepsis-induced AKI through suppressing renal cell apoptosis in AKI
mice model. Another study demonstrated that geniposide could
ameliorate AKI through suppressing cell apoptosis in vivo
and in vitro (11).
Therefore, inhibition of renal tubular cells apoptosis may be an
effective way to improve sepsis-induced AKI.
MicroRNAs (miRNAs or miRs) are nucleotide regulatory
RNA molecules (18–24 bases in length) that regulate gene expression
post-transcriptionally via binding to the 3′-untranslated region
(UTR) of target mRNAs (12,13).
It is well-known that miRNAs are involved in almost all biological
processes via the direct post-transcriptional inhibition of target
mRNAs. They are estimated to regulate >60% of human
protein-coding genes. Importantly, miRNAs have been found to
modulate multiple target genes involved in distinct cellular
processes, including signal transduction, proliferation and
apoptosis. Thus, the dysregulation of the miRNA network contributes
to numerous pathological processes, including cancer,
cardiovascular disease and AKI (14,15).
Numerous studies have reported that miRNAs are differently
expressed in the plasma or urine of patients with AKI, such as
miR-155 and miR-21 in plasma, miR-192-5p in urine, suggesting that
they could be used as biomarkers for the diagnosis of AKI (16–18).
Furthermore, several miRNAs have been identified to have a
pathogenic or protective role in kidney injury. For example,
enhanced miR-93 led to a significant reduction in the tubular
epithelial cell apoptosis via the AKT/mTOR pathway in AKI (19). Additionally, inhibition of miR-155
could improve kidney injury by regulating apoptosis under
ischemia-reperfusion (I/R) condition (20). A previous study also reported that
upregulation of miR-21 could ameliorate I/R-induced kidney injury
by inhibiting renal tubular epithelial cell apoptosis (21). Therefore, exploring novel miRNAs as
the therapeutic targets may be the important ways to regulate
sepsis-induced AKI.
In the current study, the differentially expressed
miRNAs in microarray dataset GSE172044 were analyzed. The functions
of candidate miRNA were investigated in mouse AKI model and the
involved molecular mechanisms were further examined.
Materials and methods
Animal model
A total of 96 female C57BL6/J mice (Shanghai SLAC
Laboratory Animal Co. Ltd.), aged 10–12 weeks, weighing 18–22 g,
were housed under standard conditions (12/12-h light-dark cycle,
21±2°C, ~55% humidity) with free access to food and water. The
experimental mice were acclimatized for 7 days, anesthetized by an
intraperitoneal injection of 50 mg/kg pentobarbital sodium
(Sigma-Aldrich; Merck KGaA), and each mouse was administered
intraperitoneally with 10 mg/kg body weight lipopolysaccharide
(LPS; from Escherichia coli 0111:B4; Sigma-Aldrich; Merck
KGaA) as previously described (22,23).
The present study was approved by the Animal Experimentation Ethics
Committee of the Xinhua Hospital Affiliated to Shanghai Jiao Tong
University School of Medicine (Shanghai, China; approval no. SJTU
2022-012).
Experimental design
Animals were randomly divided into two groups: AKI
and Sham group (n=6 each group/time) were subjected to the
miR-17-5p expression using the reverse transcription-quantitative
PCR (RT-qPCR) at 6, 12, 24, 48 and 72 h after AKI.
In the following experiment, mice were randomly
divided into six groups: AKI group, Sham group, AKI +
agomir-miR-17-5p group, AKI + agomir-negative control (NC) group,
AKI + antagomir-miR-17-5p group and AKI + antagomir-NC group (n=6).
Mice in the AKI group were subjected to 200 µl LPS (10 mg/kg) in
PBS intraperitoneally (n=6/group/time), while mice that did not
receive any treatments were used as the Sham group. In AKI +
antagomir-miR-17-5p/agomir-miR-17-5p groups, each mouse was
administered antagomir or agomir-miR-17-5p (20 nM/0.1 ml) by
tail-vein injection before 24 h of LPS injection. A total of 24 h
after the last treatment, all mice were humanely killed with
intraperitoneal injection of pentobarbital sodium (50 mg/kg;
Sigma-Aldrich; Merck KGaA) to collect blood by heart puncture, as
well as urine and kidney samples.
In another animal experiment, survival outcomes of
septic mice with antagomiR-17-5p/agomir-miR-17-5p (20 nM/0.1 ml)
treatment was observed from 0 to 72 h after LPS injection using the
Kaplan Meier methods (n=10/group).
Pentobarbital sodium (50 mg/kg, intraperitoneal
injection) was used for anesthesia before each operation, and all
efforts were made to minimize animal suffering. The mice health and
behaviour were monitored twice a day. No mice succumbed during
anesthesia process. If an animal reached the defined humane
endpoints [loss of >15% of body weight in 1–2 days or an overall
reduction of >20% in body weight or displaying obvious signs of
suffering (lethargy, squinted eyes, dehydration, hunched back)],
they were humanely euthanized. Sacrifice was performed by
intraperitoneal injection of pentobarbital sodium (50 mg/kg)
followed by cervical dislocation, and animal death was confirmed by
cessation of respiration and heartbeat.
Renal function measurement
After 24 h of modeling, serum blood urea nitrogen
(BUN) and creatinine (Cre) levels were detected by using an
automated analyzer (Roche Diagnostics GmbH) and a creatinine assay
kit (cat no. E2CT-100; BioAssay Systems), respectively. The kidney
injury molecule-1 (Kim-1) and neutrophil gelatinase-associated
lipocalin (NGAL) levels of urine samples were measured by ELISA
kits (cat nos. RKM100 and DY3508; R&D Systems, Inc.) based on
the manufacturer's protocol.
Renal histopathology
The hematoxylin and eosin (H&E) staining was
used to measure the pathological changes in mouse kidney tissues.
Tissue changes was checked and scored as previously described
(24).
Detection of renal cell apoptosis
Renal tissue sections were prepared as
aforementioned; apoptosis was quantified in tissue sections by the
TUNEL assay kit (cat no. C1086; Beyotime Institute of
Biotechnology) according to the manufacturer's instructions. The
numbers of TUNEL positive cells were quantified under adjacent 10
fields using a fluorescence microscope (Olympus Corporation).
Immunohistochemistry (IHC)
Paraffin embedded sections were dewaxed with xylene
at 50°C for 3 min, hydrated by graded ethanol series, and then
incubated in 3% hydrogen peroxide at room temperature (RT) for 15
min to inactivate endogenous peroxidase. After washing with
phosphate-buffered saline (PBS), the sections were blocked with 10%
fetal bovine serum (FBS, Gibco; Thermo Fisher Scientific, Inc.) at
RT for 30 min, and subsequently incubated with primary antibodies
against cleaved caspase 3 (1:200; cat. no. ab32042), transforming
growth factor β receptor 2 (TGFβR2; 1:200; cat. no. ab186838) and
phosphorylated (p-) Smad3 (1:100; cat. no. ab52903; all from Abcam)
overnight at 4°C. After washing with PBS, the slices were incubated
with EnVision + /HRP/Rb (DAKO; Agilent Technologies, Inc.) for 30
min at 37°C. The staining was observed with 3,3′-diaminobenzidine
(DAB) matrix, and then reverse stained with hematoxylin for 30 sec.
Images of all sections were captured with Olympus BH2 microscope
(Olympus Corporation).
MicroRNA expression profile data from
Gene Expression Omnibus (GEO)
The miRNA dataset (GSE172044) was downloaded from
the GEO database in NCBI (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE172044),
and the differentially expressed miRNAs were identified using the
‘limma’ package in R (25). The
fold changes (FCs) in the expression of individual miRNAs were
calculated, and |log2FC|>1.0 and P<0.05 were regarded as the
thresholds of differentially expressed miRNAs. The heat map was
generated with the Nexus Expression (Ver.10.0. BioDiscovery
Inc.).
RT-qPCR analysis
Target miRNA was extracted from spinal tissues using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.).
miR-17-5p and mRNA were reverse transcribed using a Reverse
Transcription kit (Takara Bio, Inc.) and PrimeScript RT Reagent kit
(Takara Biotechnology Co., Ltd.) according to the manufacturer's
instructions, respectively. For detection of miR-17-5p, qPCR was
performed using TaqMan™ MicroRNA Assay kit on the ABI
PRISM 7300 system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). U6 was used as internal control. Primers used for miR-17-5p
and U6 were as follows: miR-17-5p forward,
5′-GGCAAAGTGCTTACAGTGC-3′ and reverse, 5′-GTGCAGGGTCCGAGG-3′; and
U6 forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. For mRNA detection, qPCR was
conducted using a SYBR Premix Ex Taq II kit (Takara Bio, Inc.).
Primer sequences were as follows: TGFβR2 forward,
5′-TGTGAGAAGCCGCAGGAAGTC-3′ and reverse,
5′-AGTGAAGCCGTGGTAGGTGAAC-3′; and GAPDH forward,
5′-GGCAAGTTCAACGGCACAGTCAAGG-3′ and reverse,
5′-CACGACATACTCAGCACCAGCATCAC-3′. GAPDH was used as internal
controls for detecting TGFβR2. The qPCR thermocycling conditions
were as follows: 95°C for 30 sec, followed by 40 cycles at 95°C for
5 sec and 60°C for 30 sec. The reaction volume was 25 µl. Fold
changes in expression of each gene were calculated through the
2−∆∆Cq method (26).
Measurement of cytokines
Mouse ELISA kits were used to quantify the
inflammatory cytokines, including interleukin-6 (IL-6) (cat. no.
p1326), IL-1β (cat. no. p1301), tumor necrosis factor-α (TNF-α)
(cat no. pt512) and IL-10 (cat. no. p1522) in serum according to
the manufacturer's protocols. These kits were obtained from
Beyotime Institute of Biotechnology.
Cell culture
NRK-52E cells (https://www.cellosaurus.org/CVCL_0468) were maintained
routinely in Dulbecco's modified Eagle's medium (DMEM) supplemented
with 10% bovine calf serum, 1,000 U/ml penicillin, and 1,000 µg/ml
streptomycin. For 293T cells, DMEM was supplemented with 10% FBS.
Cells were grown in a humidified atmosphere of 95% air and 5%
carbon dioxide at 37°C in a tissue culture incubator.
Bioinformatics analysis
Target genes were predicted through different
bioinformatics databases, including TargetScan 7.0 (https://www.targetscan.org/vert_80/) and miRanda
(http://www.microrna.org/microrna/home.do).
Luciferase assay
The TGFβR2 wild-type (WT) sequences or mutant (mut)
sequences in 3′-UTR containing the miR-17-5p binding site were
constructed and subcloned into the pGL3 basic plasmid (Promega
Corporation). For dual-luciferase reporter assay, when 293T cells
(1×105 per well; ATCC) reached ~60% confluence were
seeded in a 96-well plate, recombinant plasmids were co-transfected
with the miR-17-5p mimics (5′-CAAAGUGCUUACAGUGCAGGUAG-3′), mimics
NC (5′-CAGCUAGAGUAUACGCUUGAAGG-3′), miR-17-5p inhibitor
(5′-CUACCUGCACUGUAAGCACUUUG-3′) or inhibitor NC
(5′-CGUUCUAAGUCACUUCACACUGG-3′) (Shanghai GenePharma Co., Ltd.)
using Lipofectamine® 3000 (Thermo Fisher Scientific,
Inc.). After 48 h of transfection, luciferase activity was measured
using the Dual-Luciferase Reporter Assay system (Promega
Corporation) and normalized to Renilla luciferase activity.
pRL-TK plasmid was transfected as an internal control.
Western blotting
Kidney tissues were homogenized in homogenization
buffer (Thermo Fisher Scientific, Inc.) and centrifuged at 12,000 ×
g, for 10 min at 4°C, and then the protein concentrations were
measured using the BCA protein assay kit. Next, equal amounts of
proteins (50 µg/lane) were separated on 10% gels using SDS-PAGE and
electrotransferred onto polyvinylidene fluoride membranes. After
transferring on a PVDF membrane (MilliporeSigma), the membranes
were blocked with 5% skim milk for 2 h at room temperature (RT).
After washing with PBST (0.1% Tween-20) three times, membranes were
incubated with primary antibodies including cleaved caspase 3
(1:2,000; cat. no. ab32042), TGFβR2 (1:2,000; cat. no. ab186838),
p-Smad3 (1:1,000; cat. no. ab52903) and β-actin (1:1,000; cat. no.
ab6276; all from Abcam) at 4°C overnight. Subsequently, membranes
were incubated with the secondary antibody goat anti-Mouse IgG
H&L (Alexa Fluor® 488; 1:2,000; cat. no. ab150117;
Abcam) for 2 h at RT. The bands were exposed by enhanced
chemiluminescence (ECL) (Thermo Fisher Scientific, Inc.) and
analyzed by ImageJ Software (version 1.46; National Institutes of
Health).
Statistical analysis
SPSS Statistics 22.0 (IBM Corp.) was used for all
statistical analysis. All data are presented as the mean ± SD. The
comparison between multiple groups was analyzed by one-way ANOVA
followed by Tukey's post hoc test. Kaplan Meier method was used for
survival analysis, and log rank test was used to calculate the
P-value. P<0.05 was considered to indicate a statistically
significant difference.
Results
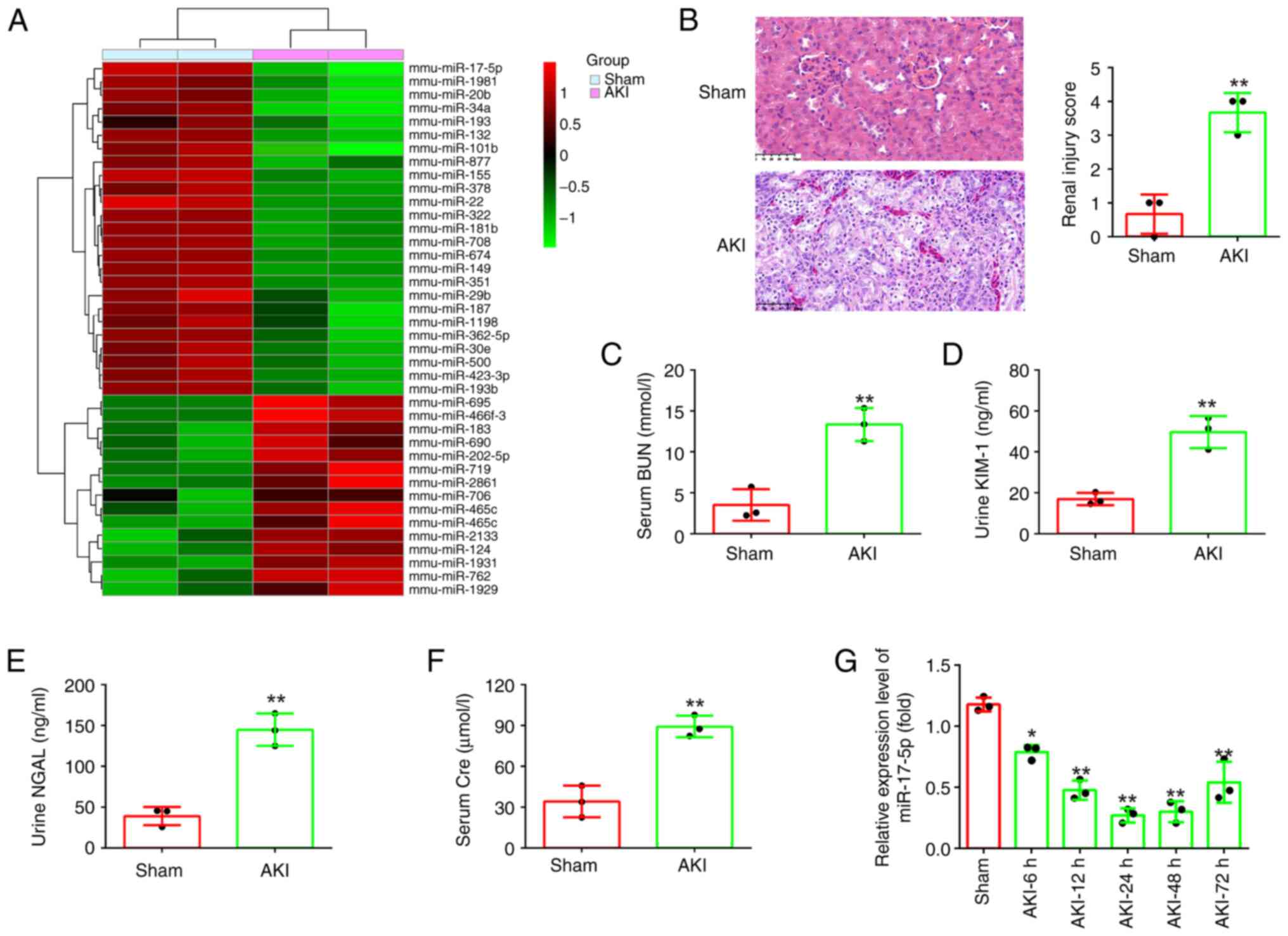
miR-17-5p is significantly
downregulated in kidney tissues of AKI mice
First, the miRNA profile (GSE172044) downloaded from
the GEO database was analyzed. Compared with the Sham group, 40
differentially expressed miRNAs were identified, among which
miR-17-5p was one of the most downregulated miRNAs (Fig. 1A). Previous studies have revealed
that miR-17-5p has a critical role in multiple organ injuries,
including cardiac I/R injury, spinal cord injury and renal I/R
injury (27–29). Of relevance, a recent study
demonstrated that miR-17-5p elevation protected renal cells from
LPS-induced injury by suppressing inflammatory response and
apoptosis in LPS-stimulated HK-2 cells (30). However, few studies have been found
regarding the function of miR-17-5p in the progression of
sepsis-induced AKI.
In order to study the role of mir-17-5p in AKI, a
mouse AKI model was established. Initially, the pathological change
in the renal tissue of mice was assessed using H&E staining
assay. The results indicated that the renal tissue of the AKI group
exhibited tubular epithelial cell edema, tubular necrosis,
telangiectasia and severe congestion/hemorrhage compared with the
sham operation group (Fig. 1B).
Meanwhile, the levels of BUN, KIM-1, NGAL and Cre that were
specific biomarkers of kidney injury (31,32),
were found to be significantly increased in the AKI group compared
with that in the sham group (Fig.
1C-F). Correspondingly, the renal injury scores were
significantly higher in the AKI group than that in the sham group
(Fig. 1B). These results indicated
that the model of mice with AKI was successful established.
Next, miR-17-5p expression was detected in AKI mice
by RT-qPCR. It was identified that miR-17-5p was continuously
decreased, and was minimal at 24 h after AKI, then its expression
was gradually increased until 72 h after injury (Fig. 1G). Collectively, these results
suggested that miR-17-5p may be involved in the pathogenesis of
AKI.
AgomiR-17-5p improves LPS-induced
kidney injury in mice
To further examine the impact of miR-17-5p in AKI,
miR-17-5p upregulation was performed in an in vivo
experiment by injecting agomiR-17-5p into mice via the caudal vein
followed by LPS stimulation for 24 h. Using RT-qPCR, it was found
that the expression levels of miR-17-5p were significantly
increased after agomiR-17-5p treatment (Fig. 2A). In addition, the mice in the
agomiR-17-5p + AKI group had a higher survival rate than that in
AKI group (Fig. 2B). Subsequently,
the altered kidney injury was evaluated. The results revealed that
miR-17-5p overexpression significantly decreased the levels of BUN,
KIM-1, NGAL and Cre expression in the septic mouse model (Fig. 2C-F). In the results from H&E
staining, injection of agomiR-17-5p significantly reduced the edema
of the glomerular tissue cells, tubular necrosis, telangiectasia,
as well as the severe congestion/hemorrhage. The renal injury
scores were significantly lower in the AKI + agomiR-17-5p group
than that in the AKI group (Fig. 2G
and H). Collectively, all data indicated that enhancing
miR-17-5p could alleviate LPS-induced kidney injury in
vivo.
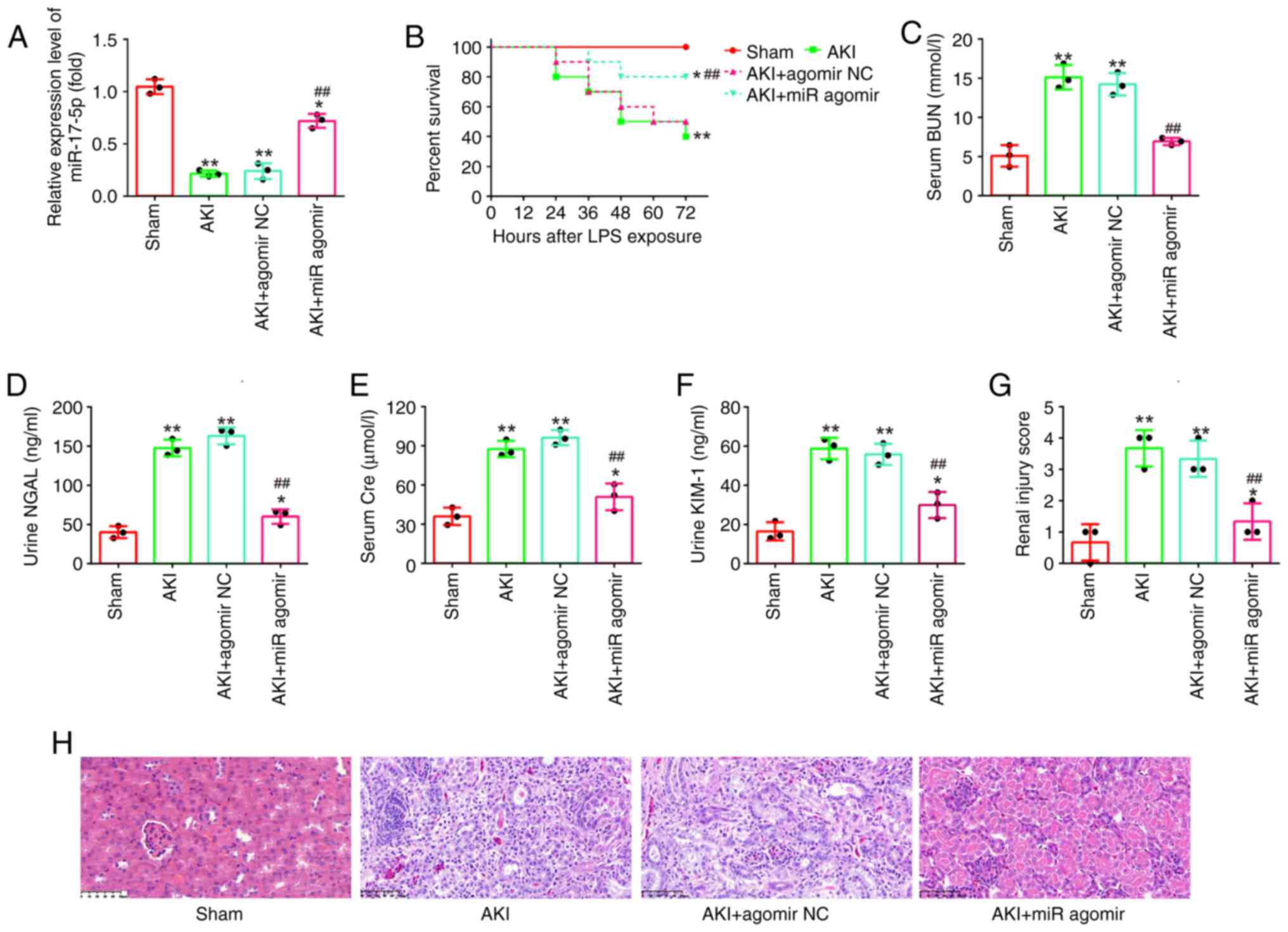 | Figure 2.AgomiR-17-5p improves LPS-induced
kidney injury in mice. AgomiR-17-5p was injected into mice via tail
vein 24 h before LPS stimulation. After 24 h post-injury, the
kidney tissues, serum and urine were collected for subsequent
experiments. (A) The expression levels of miR-17-5p were detected
using reverse transcription-quantitative PCR in the kidneys of AKI
mice. (B) Effect of agomiR-17-5p on the survival of mice. (C) Serum
BUN levels were detected by using an automated analyzer. (D and E)
The Kim-1 and NGAL levels of urine samples were measured by ELISA
kits. (F) Serum Cre levels were detected by a creatinine assay kit.
(G and H) Effect of agomiR-17-5p on renal morphologic changes of
mice (magnification, ×200). Data are presented as the mean ± SD of
three individual experiments. *P<0.05 and **P<0.01 vs. the
Sham group; ##P<0.01 vs. AKI group. LPS,
lipopolysaccharide; AKI, acute kidney injury; miR, microRNA; BUN,
blood urea nitrogen; Kim-1, kidney injury molecule-1; NGAL,
neutrophil gelatinase-associated lipocalin; Cre, creatinine; NC,
negative control. |
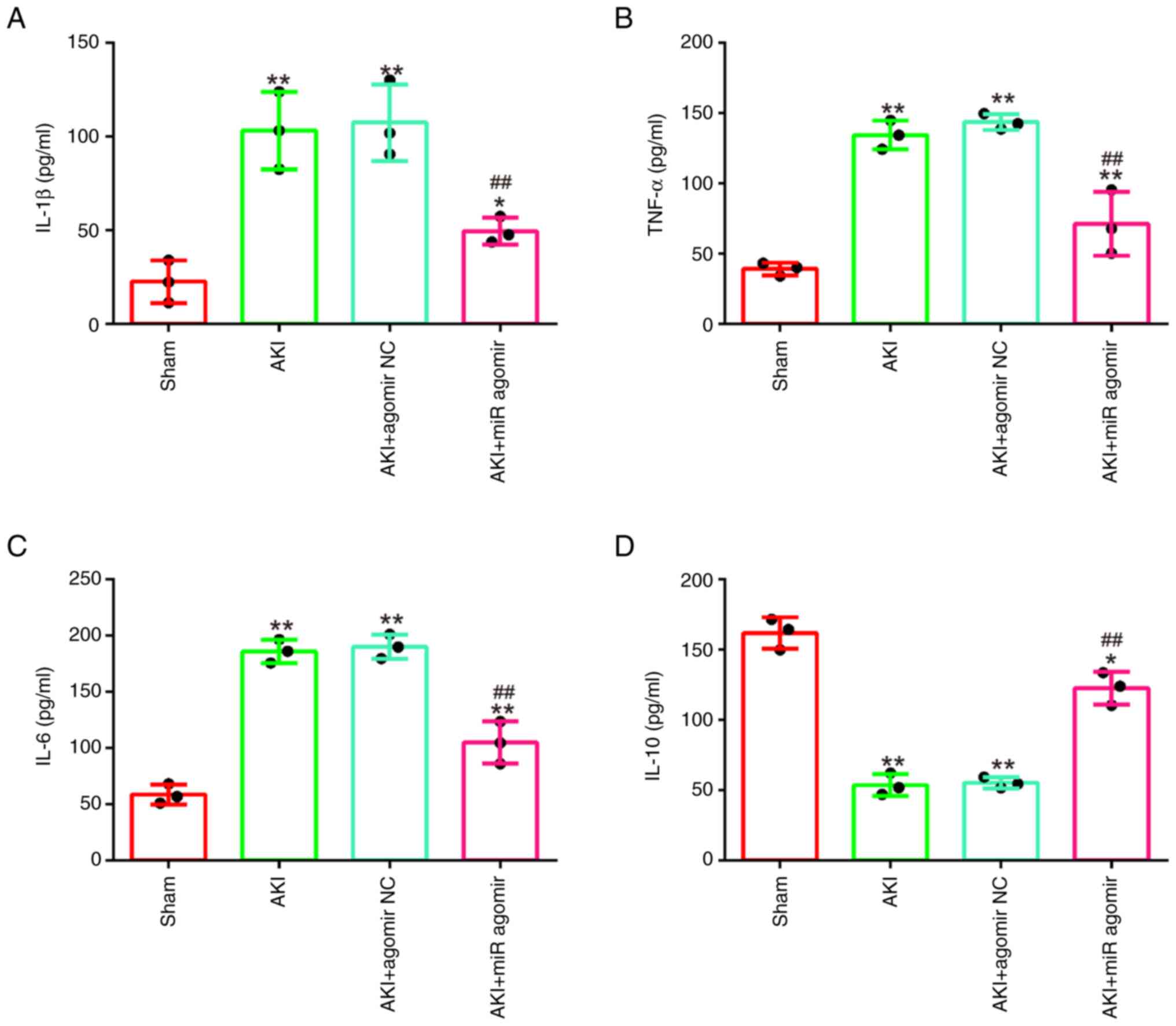
AgomiR-17-5p alleviates the
inflammatory response in LPS-induced AKI mouse model
It was further examined whether miR-17-5p affects
the inflammatory response in AKI mice model. As demonstrated in
Fig. 3A-D, LPS stimulation
significantly increased the levels of IL-1β, TNF-α and IL-6, and
decreased IL-10 levels compared with the sham group, which
suggested that excessive inflammatory response occurred in mice
during AKI. On the contrary, agomiR-17-5p significantly decreased
the production of IL-1β, TNF-α and IL-6 and increased IL-10
expression levels compared with the AKI group. All these data
indicated that enhancing miR-17-5p exerts protective role through
alleviating inflammatory response in AKI.
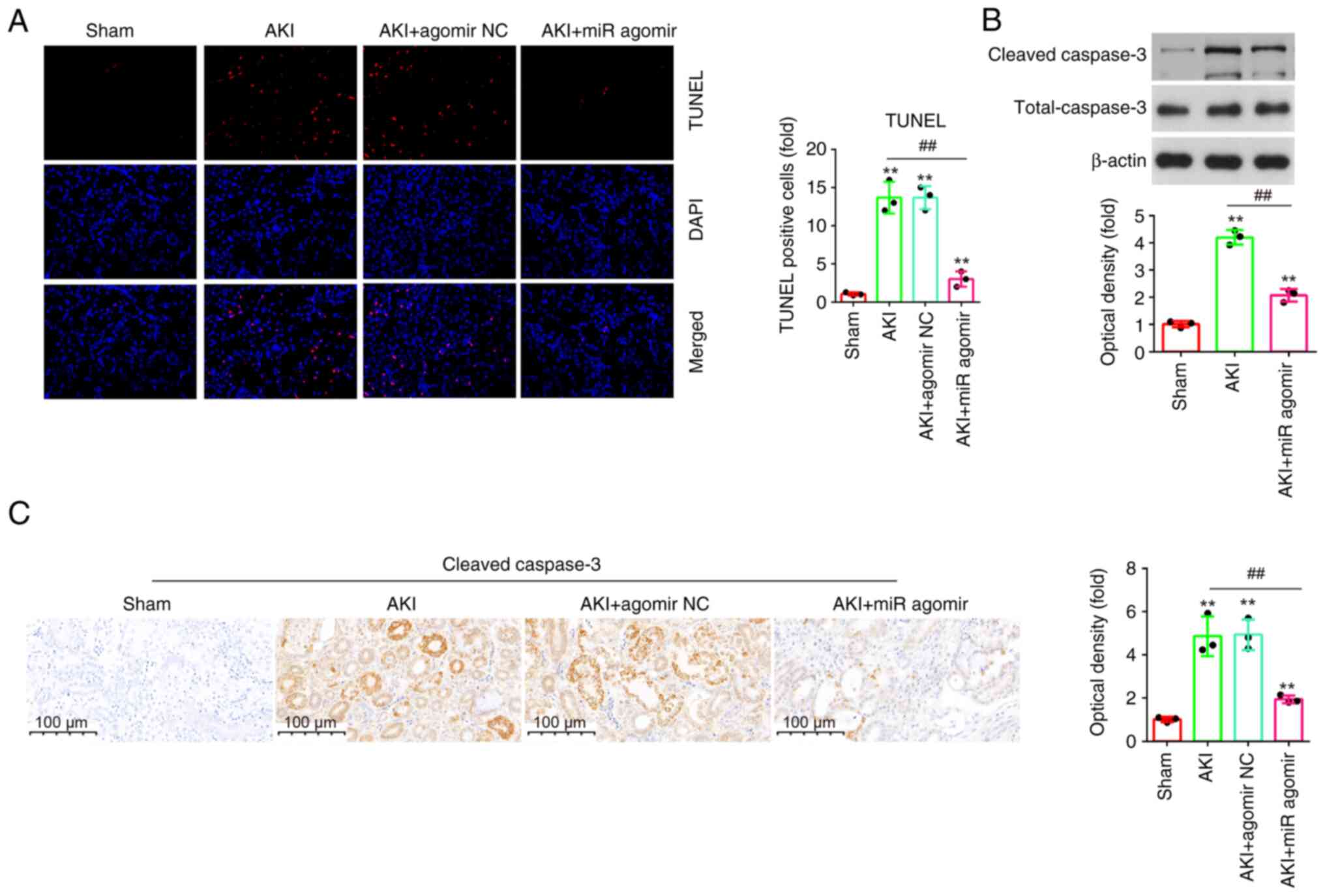
AgomiR-17-5p suppresses apoptosis in
LPS-induced AKI mouse models
Apoptosis is an important characteristic of
sepsis-induced AKI, and it was also reported that inhibition of
apoptosis improves renal injury (33). To investigate the effect of
miR-17-5p on the apoptosis in AKI, the apoptosis in kidney tissue
sections was measured using TUNEL assay. As demonstrated in
Fig. 4A, the number of TUNEL
positive cells was significantly elevated in the AKI group
(Fig. 4A). However, the number of
TUNEL positive cells was significantly reduced by agomiR-17-5p.
Furthermore, there was a significant decrease of the
cleaved-caspase-3 expression levels in response to LPS treatment
when miR-17-5p expression was evaluated in kidney tissues compared
with the AKI group (Fig. 4B).
Additionally, similar results were observed in IHC staining
(Fig. 4C). Taken together, these
results suggested that miR-17-5p upregulation could improve
LPS-induced apoptosis in AKI mouse model.
AntagomiR-17-5p aggravates LPS-induced
kidney injury in mice
To determine the effects of inhibition of miR-17-5p
on the kidney injury, antagomiR-17-5p/antagomir-NC were injected
into AKI mice via the tail vein. As shown in Fig. 5A, miR-17-5p expression levels in
kidney tissues were significantly decreased in the AKI +
antagomiR-17-5p group compared with the AKI group. Kaplan-Meier
survival analysis revealed that the survival rate in the AKI +
antagomiR-17-5p group was significantly lower than that in the AKI
group (Fig. 5B). The pathological
change of injury in mice was evaluated by H&E staining. It was
observed that these pathological lesions were evidently aggravated
by antagomiR-17-5p injection, accompanied by significantly higher
renal injury scores (Fig. 5C and
D). Furthermore, the inflammatory cytokine production was also
determined using ELISA. As demonstrated in Fig. 5E-H, antagomiR-17-5p enhanced the
production of IL-1β, TNF-α and IL-6 and resulted in a robust
decline in IL-10 expression compared with AKI the group.
Collectively, miR-17-5p knockdown aggravated LPS-induced kidney
injury in mice, suggesting the important role of miR-17-5p in
sepsis-induced AKI.
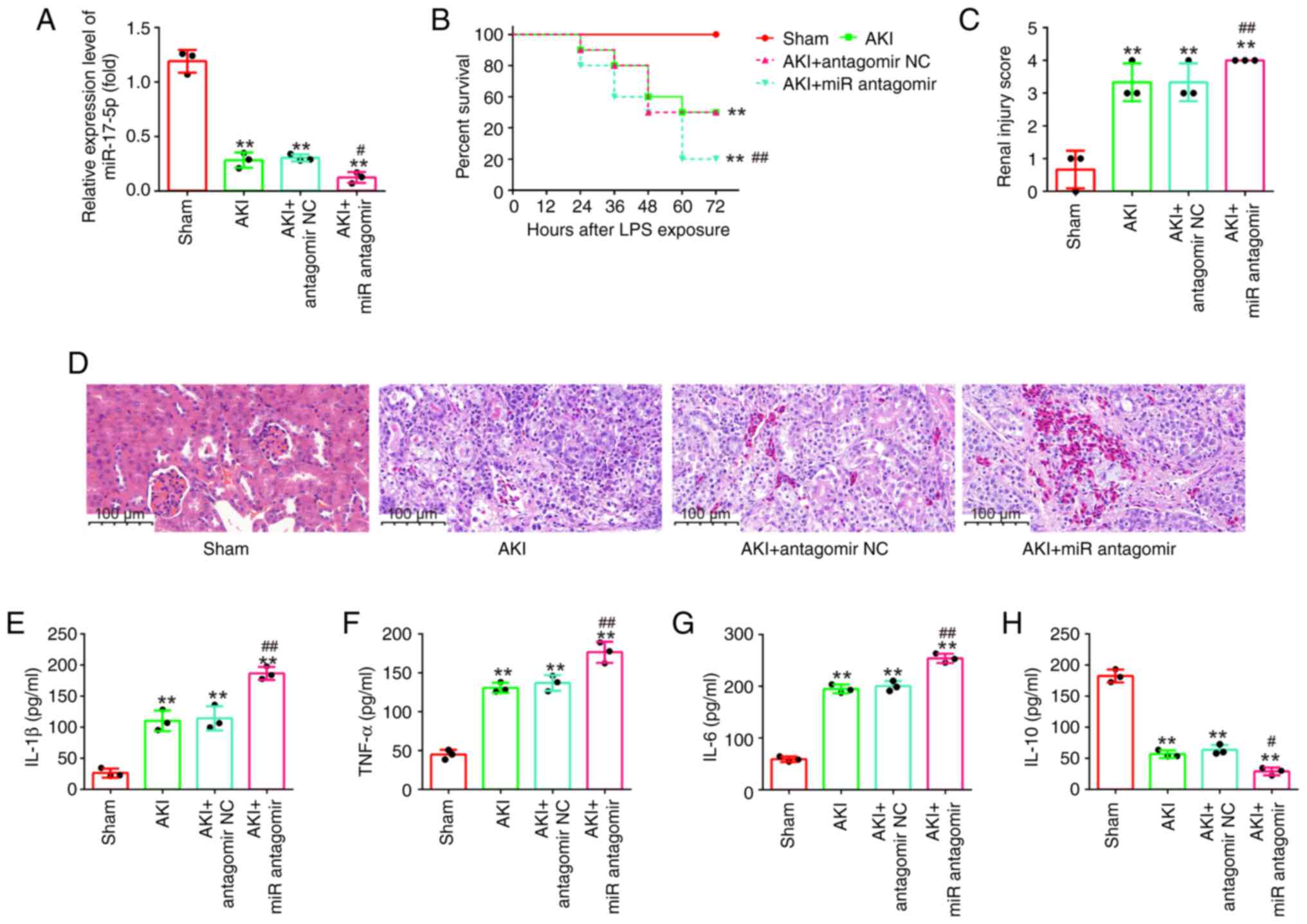 | Figure 5.AntagomiR-17-5p aggravates
LPS-induced kidney injury in mice. AntagomiR-17-5p was injected
into mice via the tail vein 24 h before LPS stimulation. After 24 h
post-injury, the kidney tissues, serum and urine were collected for
subsequent experiments. (A) The expression levels of miR-17-5p were
detected using reverse transcription-quantitative PCR. (B) Effect
of antagomiR-17-5p on the survival of mice. (C and D)
Hematoxylin-eosin staining of kidney tissues of mice from
LPS-induced or sham groups (magnification, ×200). (E-H) The
production of (E) IL-1β, (F) TNF-α, (G) IL-6 and (H) IL-10 were
determined by ELISA assays. Data are presented as the mean ± SD of
three individual experiments. **P<0.01 vs. the Sham group;
#P<0.05, ##P<0.01 vs. the AKI +
antagomir-NC group. LPS, lipopolysaccharide; miR, microRNA; IL,
interleukin; TNF-a, tumor necrosis factor-α; AKI, acute kidney
injury; NC, negative control. |
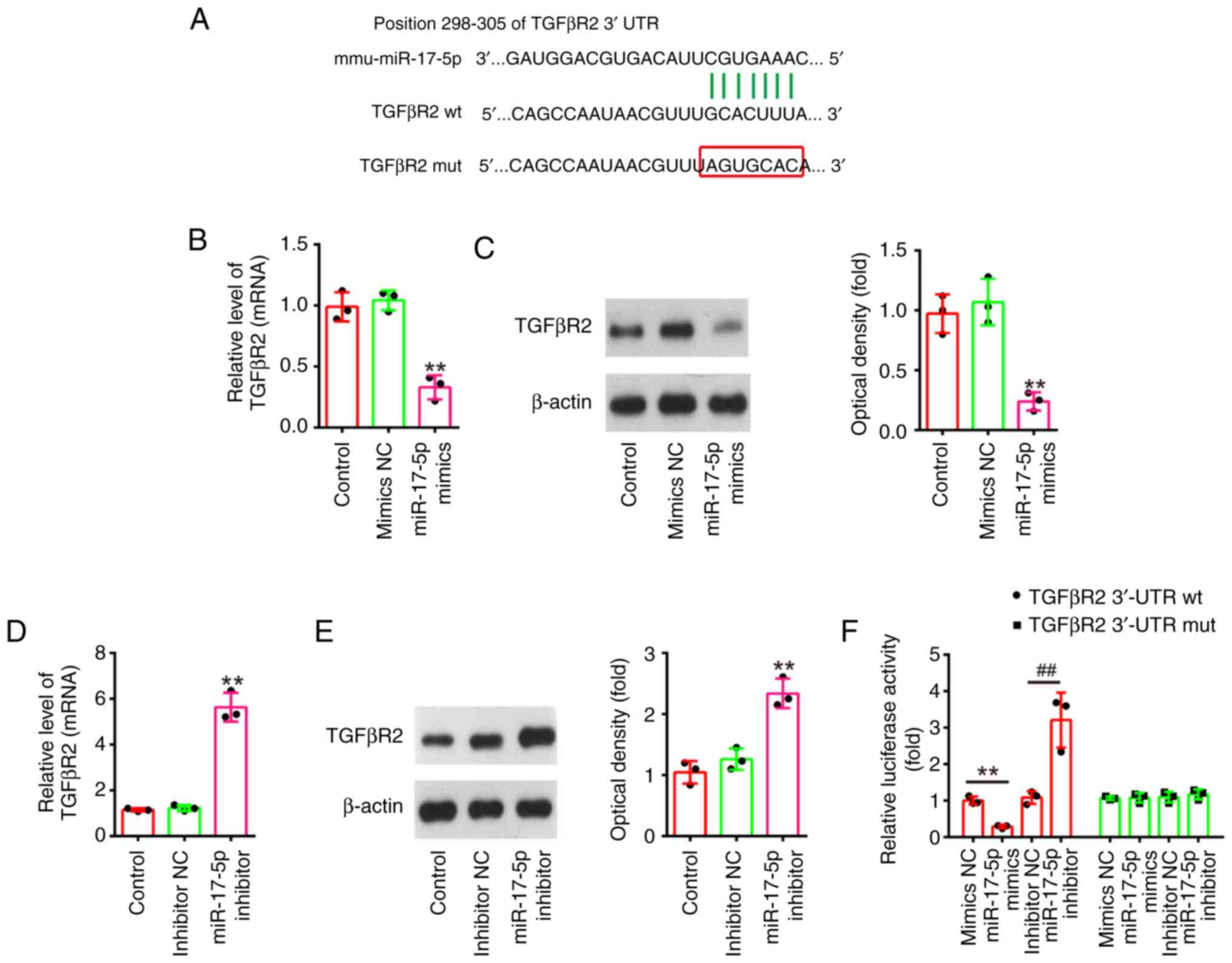
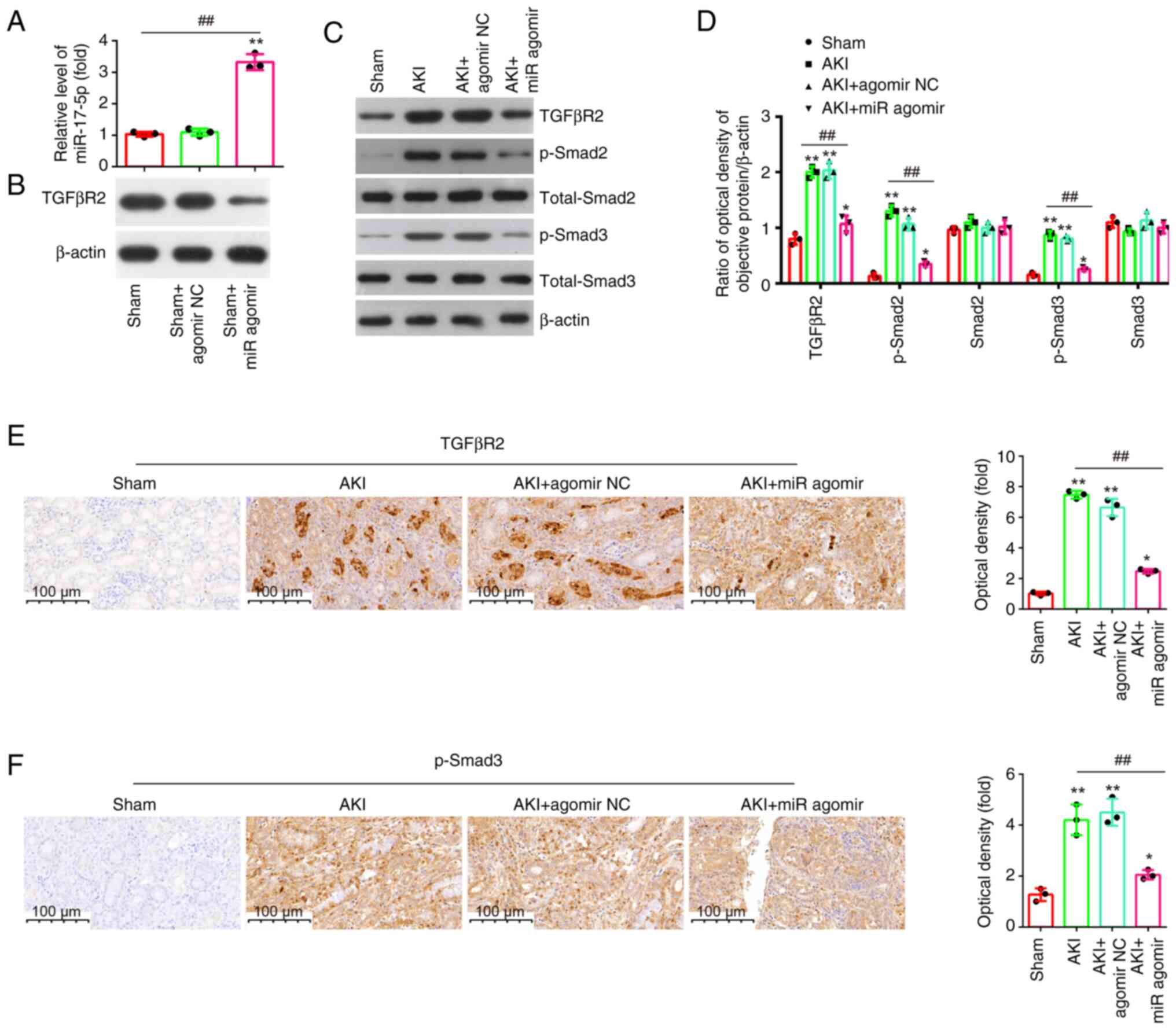
TGFβR2 is a direct target of
miR-17-5p
To explore the potential mechanisms in which
miR-17-5p improves the apoptosis and inflammatory response induced
by LPS, TargetScan 7.0 (https://www.targetscan.org/vert_80/) and miRanda
(http://www.microrna.org/microrna/home.do) were used to
search the target genes of miR-17-5p. Bioinformatics analysis
indicated that the 3′-UTR of TGFβR2 was a potential miR-17-5p
binding site (Fig. 6A). It has
been previously reported that TGFβR2 could regulate the TGF-β/Smad
signaling pathway, and promotes renal cell apoptosis (34,35).
Thus, it was selected for subsequent study. Next, it was also found
that miR-17-5p overexpression significantly decreased the mRNA and
protein levels of TGFβR2 (Fig. 6B and
C), while miR-17-5p inhibition significantly increased TGFβR2
expression levels in NRK-52E cells (Fig. 6D and E). Furthermore, the results
of luciferase reporter assay revealed that the miR-17-5p
overexpression decreased the luciferase activity, while miR-17-5p
inhibition increased the luciferase activity of the reporter
containing wild-type 3′-UTR, but not that of the mutant reporter
(Fig. 6F). Collectively, these
findings indicated that TGFβR2 may be a functional target of
miR-17-5p in renal cells.
Effect of miR-17-5p overexpression on
the TGFβR2/TGF-β/Smad3 signaling pathway in kidney tissues of AKI
mice
It is well-known that TGFβR2 is a typical reporter
of TGF-β signaling pathway (36),
and it has been proved to be a direct target of miR-17-5p.
Therefore, it was investigated whether miR-17-5p affects apoptosis
and inflammation via the TGFβR2/TGF-β/Smad3 pathway in vivo.
First, the expression of miR-17-5p was detected after agomir-17-5p
treatment in AKI mice, and the data identified an upregulation of
miR-17-5p levels in kidney tissue upon agomir-17-5p injection
(Fig. 7A). On the contrary, a
downregulation of TGFβR2 levels was observed in kidney tissue upon
agomir-17-5p injection (Fig. 7B).
Moreover, it was found that LPS stimulation led to a significant
increase in the protein expression levels of TGFβR2, p-smad2 and
p-smad3, while the overexpression of miR-17-5p weakened the
promoting effects of LPS on TGFβR2, p-Smad2 and p-Smad3 expression
levels (Fig. 7C and D). Similar
results of the expression of TGFβR2 and p-Smad3 were observed in
IHC staining (Fig. 7E and F).
These results suggested that miR-17-5p may exert anti-apoptotic and
anti-inflammatory effects through TGFβR2/Smad3 pathway in AKI
mice.
Discussion
In the present study, a number of differentially
expressed miRNAs was identified through retrieving the GSE172044
dataset, and it was found that miR-17-5p exhibited the highest
change fold. Furthermore, agomir-miR-17-5p improves renal function,
reduces inflammation and suppresses apoptosis, while
antagomir-miR-17-5p aggravated this injury, with the involvement of
TGFβR2/TGF-β/Smad3 pathway in AKI mice. The present findings
demonstrated that miR-17-5p has a key role in pathogenesis of AKI,
and enhanced miR-17-5p expression improves sepsis-induced AKI.
Emerging research has revealed that several miRNAs
possess important roles in regulating the sepsis-induced AKI in
animal or cell models (37,38).
For example, in septic AKI mouse model, miR-21 silencing improved
renal damage through suppressing renal cell apoptosis via targeting
cyclin-dependent kinase 6 (CDK6) (39). Qin et al (40) revealed that miR-191-5p upregulation
was able to improve renal function in septic rat models by
targeting oxidative stress responsive 1. These data indicated the
potential values of miRNAs in septic AKI. In the present study,
miR-17-5p was one of the major miRNAs that were downregulated in
AKI mice. Moreover, certain studies have demonstrated the
protective roles of miR-17-5p in different organ injuries,
including brain and heart (41,42).
In addition, miR-17-5p displayed potent anti-apoptotic properties,
which was closely associated with the cellular mechanisms of renal
damage (30). In the resent study,
an AKI mouse model was established to explore the regulatory role
of miR-17-5p in AKI pathogenesis. More importantly, miR-17-5p
downregulation was also validated in AKI mouse model. Considering
these previous studies, miR-17-5p might be involved in the
pathological process of AKI.
Some miRNAs have been demonstrated to be involved in
AKI via regulating the renal tubular cell apoptosis (43,44).
For example, Yan et al (45) found that miR-214 targeted
mitofusin-2 to promote renal tubular cell apoptosis in AKI mice.
Song et al (44) revealed
that overexpression of miR-21 protected the kidney from AKI by
suppressing epithelial cell apoptosis in mice. Zhang et al
(46) identified that weakened
miR-17-5p expression could inhibit the apoptosis of human renal
podocytes through the upregulation of ActA, Smad2 and Smad3 in
nephrotic syndrome. Hao et al (29) reported that miR-17-5p directly
targeted the expression of death receptor 6 to improve renal I/R
injury through suppressing apoptosis. In addition, a previous study
reported that renal tubular apoptosis was an important regulator in
the progression of AKI, and inhibition of apoptosis has been shown
to protect against LPS-induced acute renal failure in mice
(47). In the present study, it
was found that miR-17-5p upregulation obviously improved
LPS-induced renal dysfunction in mice. The present findings further
demonstrated that LPS-stimulated apoptosis was attenuated by
miR-17-5p upregulation, while knockdown of miR-17-5p conferred
contrasting effects. Collectively, the current results indicated
that miR-17-5p may improve LPS-induced AKI through suppressing cell
apoptosis.
In addition to renal cellular apoptosis, excessive
inflammation has been recognized as another feature of septic AKI
(48). The inflammatory factors
levels including TNF-α, IL-1β, IL-6 and IL-10, have been proved to
be rich during septic AKI (49).
Moreover, inhibiting the production of these inflammatory factors
has been found to diminish the severity of septic kidney injury. In
this context, miR-17-5p overexpression in kidney was found to
repress the inflammatory response in AKI rat model.
In the present study, TGFβR2, a transmembrane
receptor of the TGF-β/Smad signaling, was identified as a direct
target of miR17-5p. Upon stimulation, TGFβR2 first binds to TGF-β,
which promotes the formation of Smad2/3/4 complex, activates
transcription of TGFβ-downstream genes, finally affecting
biological characteristics of inflammation and apoptosis (34,50,51).
For example, targeting TGFβR2 inhibited
hypoxia-reoxygenation-induced renal cellular apoptosis via
regulating the TGF-β/Smad3 pathway activation (35). Additionally, Sun et al
(52) found that silencing THBS1
protected mice against sepsis-induced AKI through suppressing the
inflammation by inhibit the TGF-β/Smad3 pathway. Another study
reported that miR-211 alleviated I/R-induced inflammatory response
by targeting the TGFβR2/TGFβ/Smad3 pathway in kidney injury mice
(35). Considering the association
between TGFβR2 and the TGF-β/Smad3 pathway, the influence of
miR-17-5p in the TGFβR2/Smad3 pathway in AKI mouse model was
examined. The expression of p-Smad3 was significantly upregulated
in AKI mice; however, the increased p-Smad3 expression was
attenuated by miR-17-5p overexpression, suggesting that miR-17-5p
overexpression inactivated TGF-β/Smad3 signaling through the
degradation of TGFβR2 transcription in AKI mice.
However, there are certain limitations to the
present study. First, only one dataset from the GEO database was
used to detect the expression pattern of miR-17-5p, while the
samples from patients could not be used. Further research on this
point shall be conducted by the authors. In addition, the mechanism
of AKI is complex, and TGFβR2 may not be the unique element during
the protective role of miR-17-5p against AKI. In future research,
the relationship between miR-17-5p and TGFβR2 shall be verified by
the authors through overexpressing both miR-17-5p and TGFβR2 in AKI
mice model.
In conclusion, miR-17-5p was downregulated in an
experimental model of AKI in mice. Moreover, the enforced miR-17-5p
expression was found to improve renal function by regulating the
TGFβR2/TGF-β/Smad3 signaling pathway, while knockdown of miR-17-5p
conferred contrasting effects. Therefore, miR-17-5p might be a
possible therapeutic target for the treatment of septic AKI.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The datasets generated
and/or analyzed during the current study are available in the Gene
Expression Omnibus repository (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE172044).
Authors' contributions
JS, LN, YW, GZ, LT and JJ performed all the
experiments and collected the data. XG and SP conceived and
designed the study. JS and LN wrote the main manuscript and
analyzed the data. XG and SP confirm the authenticity of all the
raw data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All animal care and experimental procedures were
approved by the Animal Experimentation Ethics Committee of the
Xinhua Hospital Affiliated to Shanghai Jiao Tong University School
of Medicine (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hoste EA, Bagshaw SM, Bellomo R, Cely CM,
Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, et
al: Epidemiology of acute kidney injury in critically ill patients:
The multinational AKI-EPI study. Intensive Care Med. 41:1411–1423.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Poston JT and Koyner JL: Sepsis associated
acute kidney injury. BMJ. 364:k48912019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Uchino S, Kellum JA, Bellomo R, Doig GS,
Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, et al:
Acute renal failure in critically ill patients: A multinational,
multicenter study. JAMA. 294:813–818. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jia Y, Li Z, Feng Y, Cui R, Dong Y, Zhang
X, Xiang X, Qu K, Liu C and Zhang J: Methane-rich saline
ameliorates sepsis-induced acute kidney injury through
anti-inflammation, antioxidative, and antiapoptosis effects by
regulating endoplasmic reticulum stress. Oxid Med Cell Longev.
2018:47568462018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gameiro J, Carreiro C, Fonseca JA, Pereira
M, Jorge S, Gouveia J and Lopes JA: Acute kidney disease and
long-term outcomes in critically ill acute kidney injury patients
with sepsis: a cohort analysis. Clin Kidney J. 14:1379–1387. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalantari K and Rosner MH: Recent advances
in the pharmacological management of sepsis-associated acute kidney
injury. Expert Rev Clin Pharmacol. 14:1401–1411. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Suo L, Fu Z, Li G and Zhang J:
Pivotal role of endothelial cell autophagy in sepsis. Life Sci.
276:1194132021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lerolle N, Nochy D, Guérot E, Bruneval P,
Fagon JY, Diehl JL and Hill G: Histopathology of septic shock
induced acute kidney injury: Apoptosis and leukocytic infiltration.
Intensive Care Med. 36:471–478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ozkok A and Edelstein CL: Pathophysiology
of cisplatin-induced acute kidney injury. Biomed Res Int.
2014:9678262014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu Y, Fu Y and Lin H: Baicalin inhibits
renal cell apoptosis and protects against acute kidney injury in
pediatric sepsis. Med Sci Monit. 22:5109–5115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J, Zhao N, Shi G and Wang H:
Geniposide ameliorated sepsis-induced acute kidney injury by
activating PPARγ. Aging (Albany NY). 12:22744–22758.
2020.PubMed/NCBI
|
|
12
|
Betel D, Wilson M, Gabow A, Marks DS and
Sander C: The microRNA.org resource: Targets and expression.
Nucleic Acids Res. 36((Database Issue)): D149–D153. 2008.PubMed/NCBI
|
|
13
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ao X, Ding W, Li X, Xu Q, Chen X, Zhou X,
Wang J and Liu Y: Non-coding RNAs regulating mitochondrial function
in cardiovascular diseases. J Mol Med (Berl). 101:501–526. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Ding W, Wang J, Ao X and Xue J:
Non-coding RNA-mediated modulation of ferroptosis in cardiovascular
diseases. Biomed Pharmacother. 164:1149932023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du J, Cao X, Zou L, Chen Y, Guo J, Chen Z,
Hu S and Zheng Z: MicroRNA-21 and risk of severe acute kidney
injury and poor outcomes after adult cardiac surgery. PLoS One.
8:e633902013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zou YF, Wen D, Zhao Q, Shen PY, Shi H,
Zhao Q, Chen YX and Zhang W: Urinary MicroRNA-30c-5p and
MicroRNA-192-5p as potential biomarkers of
ischemia-reperfusion-induced kidney injury. Exp Biol Med (Maywood).
242:657–667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saikumar J, Hoffmann D, Kim TM, Gonzalez
VR, Zhang Q, Goering PL, Brown RP, Bijol V, Park PJ, Waikar SS and
Vaidya VS: Expression, circulation, and excretion profile of
microRNA-21, −155, and −18a following acute kidney injury. Toxicol
Sci. 129:256–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhan Y, Zhu M, Liu S, Lu J, Ni Z, Cai H
and Zhang W: MicroRNA-93 inhibits the apoptosis and inflammatory
response of tubular epithelial cells via the PTEN/AKT/mTOR pathway
in acute kidney injury. Mol Med Rep. 24:6662021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang XB, Chen X, Li DJ, Qi GN, Dai YQ, Gu
J, Chen MQ, Hu S, Liu ZY and Yang ZM: Inhibition of miR-155
ameliorates acute kidney injury by apoptosis involving the
regulation on TCF4/Wnt/β-catenin pathway. Nephron. 143:135–147.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang W and Shu L: Upregulation of miR-21
by ghrelin ameliorates ischemia/reperfusion-induced acute kidney
injury by inhibiting inflammation and cell apoptosis. DNA Cell
Biol. 35:417–425. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miao S, Lv C, Liu Y, Zhao J, Li T, Wang C,
Xu Y, Wang X, Xiao X and Zhang H: Pharmacologic blockade of 15-PGDH
protects against acute renal injury induced by LPS in mice. Front
Physiol. 11:1382020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu Y, Wei SW, Ding A, Zhu WP, Mai MF, Cui
TX, Yang H and Zhang H: The long noncoding RNA ANRIL promotes cell
apoptosis in lipopolysaccharide-induced acute kidney injury
mediated by the TLR4/nuclear factor-kappa B pathway. Kidney Blood
Press Res. 45:209–221. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang C, Han H, Yan M, Zhu S, Liu J, Liu Z,
He L, Tan J, Liu Y, Liu H, et al: PINK1-PRKN/PARK2 pathway of
mitophagy is activated to protect against renal
ischemia-reperfusion injury. Autophagy. 14:880–897. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi J, Bei Y, Kong X, Liu X, Lei Z, Xu T,
Wang H, Xuan Q, Chen P, Xu J, et al: miR-17-3p contributes to
exercise-induced cardiac growth and protects against myocardial
ischemia-reperfusion injury. Theranostics. 7:664–676. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yue XH, Guo L, Wang ZY and Jia TH:
Inhibition of miR-17-5p promotes mesenchymal stem cells to repair
spinal cord injury. Eur Rev Med Pharmacol Sci. 23:3899–3907.
2019.PubMed/NCBI
|
|
29
|
Hao J, Wei Q, Mei S, Li L, Su Y, Mei C and
Dong Z: Induction of microRNA-17-5p by p53 protects against renal
ischemia-reperfusion injury by targeting death receptor 6. Kidney
Int. 91:106–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuan W, Xiong X, Du J, Fan Q, Wang R and
Zhang X: LncRNA PVT1 accelerates LPS-induced septic acute kidney
injury through targeting miR-17-5p and regulating NF-κB pathway.
Int Urol Nephrol. 53:2409–2419. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schrezenmeier EV, Barasch J, Budde K,
Westhoff T and Schmidt-Ott KM: Biomarkers in acute kidney
injury-pathophysiological basis and clinical performance. Acta
Physiol (Oxf). 219:554–572. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han WK, Bailly V, Abichandani R, Thadhani
R and Bonventre JV: Kidney injury molecule-1 (KIM-1): A novel
biomarker for human renal proximal tubule injury. Kidney Int.
62:237–244. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kang K, Gao Y, Wang SC, Liu HT, Kong WL,
Zhang X, Huang R, Qi ZD, Zheng JB, Qu JD, et al: Dexmedetomidine
protects against lipopolysaccharide-induced sepsis-associated acute
kidney injury via an α7 nAChR-dependent pathway. Biomed
Pharmacother. 106:210–216. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goumans MJ, Valdimarsdottir G, Itoh S,
Rosendahl A, Sideras P and ten Dijke P: Balancing the activation
state of the endothelium via two distinct TGF-beta type I
receptors. EMBO J. 21:1743–1753. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shang J, Sun S, Zhang L, Hao F and Zhang
D: miR-211 alleviates ischaemia/reperfusion-induced kidney injury
by targeting TGFβR2/TGF-β/SMAD3 pathway. Bioengineered. 11:547–557.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Erbüyün K, Tok D, Vatansever S, Ok G,
Türköz E, Aydede H, Erhan Y and Tekin I: Levosimendan up-regulates
transforming growth factor-beta and smad signaling in the aorta in
the early stage of sepsis. Ulus Travma Acil Cerrahi Derg.
16:293–299. 2010.PubMed/NCBI
|
|
37
|
Cheng Q and Wang L: LncRNA XIST serves as
a ceRNA to regulate the expression of ASF1A, BRWD1M, and PFKFB2 in
kidney transplant acute kidney injury via sponging hsa-miR-212-3p
and hsa-miR-122-5p. Cell Cycle. 19:290–299. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang J, Song J, Li Y, Shao J, Xie Z and
Sun K: Down-regulation of LncRNA CRNDE aggravates kidney injury via
increasing MiR-181a-5p in sepsis. Int Immunopharmacol.
79:1059332020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wei W, Yao YY, Bi HY, Zhai Z and Gao Y:
miR-21 protects against lipopolysaccharide-stimulated acute kidney
injury and apoptosis by targeting CDK6. Ann Transl Med. 8:3032020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qin Y, Wang G and Peng Z: MicroRNA-191-5p
diminished sepsis-induced acute kidney injury through targeting
oxidative stress responsive 1 in rat models. Biosci Rep.
39:BSR201905482019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gamdzyk M, Doycheva DM, Kang R, Tang H,
Travis ZD, Tang J and Zhang JH: GW0742 activates miR-17-5p and
inhibits TXNIP/NLRP3-mediated inflammation after hypoxic-ischaemic
injury in rats and in PC12 cells. J Cell Mol Med. 24:12318–12330.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao L, Jiang S, Wu N, Shi E, Yang L and
Li Q: MiR-17-5p-mediated endoplasmic reticulum stress promotes
acute myocardial ischemia injury through targeting Tsg101. Cell
Stress Chaperones. 26:77–90. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wei Q, Sun H, Song S, Liu Y, Liu P,
Livingston MJ, Wang J, Liang M, Mi QS, Huo Y, et al: MicroRNA-668
represses MTP18 to preserve mitochondrial dynamics in ischemic
acute kidney injury. J Clin Invest. 128:5448–5464. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Song N, Zhang T, Xu X, Lu Z, Yu X, Fang Y,
Hu J, Jia P, Teng J and Ding X: miR-21 protects against
ischemia/reperfusion-induced acute kidney injury by preventing
epithelial cell apoptosis and inhibiting dendritic cell maturation.
Front Physiol. 9:7902018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yan Y, Ma Z, Zhu J, Zeng M, Liu H and Dong
Z: miR-214 represses mitofusin-2 to promote renal tubular apoptosis
in ischemic acute kidney injury. Am J Physiol Renal Physiol.
318:F878–F887. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang YR, Wu YF, Wang H, Lin XM and Zhang
XM: Role of microRNA-17-5p in the pathogenesis of pediatric
nephrotic syndrome and related mechanisms. Zhongguo Dang Dai Er Ke
Za Zhi. 22:958–963. 2020.(In Chinese). PubMed/NCBI
|
|
47
|
Guo R, Wang Y, Minto AW, Quigg RJ and
Cunningham PN: Acute renal failure in endotoxemia is dependent on
caspase activation. J Am Soc Nephrol. 15:3093–3102. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang S, Ma J, Sheng L, Zhang D, Chen X,
Yang J and Wang D: Total coumarins from hydrangea paniculata show
renal protective effects in lipopolysaccharide-induced acute kidney
injury via anti-inflammatory and antioxidant activities. Front
Pharmacol. 8:8722017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huang Y, Zhou LS, Yan L, Ren J, Zhou DX
and Li SS: Alpinetin inhibits lipopolysaccharide-induced acute
kidney injury in mice. Int Immunopharmacol. 28:1003–1008. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Weinstein M, Yang X and Deng C: Functions
of mammalian Smad genes as revealed by targeted gene disruption in
mice. Cytokine Growth Factor Rev. 11:49–58. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen G, Deng C and Li YP: TGF-β and BMP
signaling in osteoblast differentiation and bone formation. Int J
Biol Sci. 8:272–288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sun J, Ge X, Wang Y, Niu L, Tang L and Pan
S: USF2 knockdown downregulates THBS1 to inhibit the TGF-β
signaling pathway and reduce pyroptosis in sepsis-induced acute
kidney injury. Pharmacol Res. 176:1059622022. View Article : Google Scholar : PubMed/NCBI
|