Introduction
Acute myocardial infarction is a form of myocardial
necrosis resulting from acute and persistent ischemia and hypoxia
in the coronary artery and is a significant contributor to global
mortality and disability rates (1–3).
Effective treatment protocols involve restoring the blood supply to
the ischemic myocardium as soon as possible through thrombolysis
and percutaneous coronary intervention (4). However, reperfusion can further
damage the ischemic myocardium, referred to as myocardial
ischemia/reperfusion injury (MIRI) (5), which can affect the prognosis of
patients. Currently, the main clinical treatment for myocardial
ischemia is percutaneous artery intervention (PCI), however, this
treatment only provides short-term relief and also leads to
myocardial re-damage (6). Other
current treatments, such as calcium channel blockers or hypoxia
preconditioning, are used for MIRI but are not very effective, as
multiple factors are involved in the pathophysiological process of
MIRI (7,8), there is an urgent requirement to find
more therapeutic solutions for MIRI. MIRI encompasses a range of
pathophysiological mechanisms, including calcium and proton
overload, endoplasmic reticulum stress, excessive oxidative stress,
mitochondrial morphology and dysfunction, and activation of
apoptotic pathways, ultimately leading to an inflammatory cascade
within cardiac tissue (9). This
inflammatory response is intricately linked to myocardial injury
and the formation of scar tissue. Consequently, mitigating the
inflammatory response is considered important in prevention and
treatment strategies for MIRI (10).
Macrophages are a heterogeneous group of immune
cells important for regulating inflammation and immune balance
(11). Additionally, macrophages
play an important role in regulating cardiac inflammation (12). The wide range of macrophage
functions arises from their diversity and adaptability; macrophages
detect inflammation in the heart and adjust their characteristics
accordingly (13). Dependent on
the microenvironment, macrophages can polarize toward the M1 or M2
phenotype. An imbalance of the M1/M2 macrophage phenotype is a
known pathological marker of various inflammatory diseases
(14,15), including diabetic nephropathy and
atherosclerosis. Upregulated proinflammatory factor and
inflammatory mediator expression is observed with the propensity
macrophage M1 polarization, and preventing the polarization of
M1-type macrophages within a specific immune environment is vital
for regulating infections and maintaining homeostasis (16). The balance between M1- and M2-like
macrophages is a potential target for treating MIRI (17).
Increasing evidence suggests that the Janus tyrosine
kinase 2 (JAK2)/signal transducer and activator of the
transcription 3 (STAT3) pathway regulates inflammation-related
disorders, such as allergies and cardiovascular disease (18,19),
and this pathway may participate in MIRI (20). Phosphorylation of JAK2 activates
STAT3, the principal effector in the initiation and progression of
cardiac injury (21). Following
translocation from the cytoplasm to the nucleus, phosphorylated
STAT3 enhances the transcription of pro-inflammatory factors
(22). Activated JAK2/STAT3
signaling can promote the proliferation of RAW264.7 cells
stimulated by LPS and increase the secretion of
inflammation-related factors (23). This evidence suggests that
inhibiting the JAK2/STAT3 signaling pathway may promote the
transformation of macrophages from the M1 to the M2 phenotype;
thus, this pathway may play a key role in the treatment of
MIRI.
Mesenchymal stem cells (MSCs) are a unique type of
stromal cell with the potential to differentiate into multiple cell
types and the ability to regulate the phenotype and function of
immune cells (24). The use of
MSCs are considered a potential new strategy for treating
autoimmune and inflammatory diseases and have functional and
structural benefits in treating ischemic heart disease (25). The transplantation of bone marrow
MSCs (BMSCs) has been explored as a potential treatment for
repairing ischemic heart injuries, whereby BMSCs transplanted into
the ischemic heart can differentiate into endothelial, vascular
smooth muscle and myocardial-like cells (26). Nevertheless, the low cell survival
rate and potential biosafety issue reduce the effectiveness of cell
therapy (27,28).
Exosomes, the smallest extracellular vesicles with a
typical diameter of 50-150 nm, are released by various cell types
and have multiple beneficial effects such as exerted obvious
cardioprotection by increasing cardiac function and limiting
pathological remodeling, including cardiac hypertrophy and cardiac
fibrosis (29). Using exosomes
secreted by BMSCs (BMSC-Exo) instead of BMSCs to treat MIRI is a
potentially beneficial cell-free treatment strategy (30). BMSC-Exo accounts for a large
portion of circulating microvesicles (31). Although numerous studies have shown
that BMSC-Exo can alleviate MIRI (32,33),
at present, the understanding of BMSCs and BMSC-Exo is still in the
infancy stage. For example, the outcome in vivo is unknown,
the long-term implantation consequences are uncertain and the
underlying mechanisms require further exploration. Notably,
exosome-mediated cell-to-cell interactions involve various miRNAs
that play significant roles in inflammation, tissue repair and
fibrogenesis (34). For example,
miR-494 is cardioprotective in MIRI by targeting apoptosis-related
proteins (35), miR-148a
alleviates hepatic ischemia/reperfusion (I/R) injury by improving
liver function and suppressing hepatocellular apoptosis (36), and overexpression of BMSC-Exo-125b
can enhance the viability of myocardial cells after MIRI and
inhibit cell apoptosis (37).
Moreover, extracellular vesicles derived from MSCs can exert
important protective effects on the heart by overexpressing
miR-25-3p, promoting myocardial cell survival and inhibiting
inflammation in vivo and in vitro (38). However, few studies have
investigated the exact mechanism through which BMSC-Exo-25-3p
protects against inflammation during MIRI.
In the present study the therapeutic potential of
BMSC-Exo-25-3p against MIRI was explored and the underlying
mechanism was investigated by establishing an in vivo MIRI
model and an in vitro hypoxia-reoxygenation (H/R) cell
model. The present study provides a potential therapeutic approach
for MIRI, with the aim to provide new therapeutic drugs and
approaches to improve the prognosis of patients with coronary heart
disease after thrombolysis or percutaneous coronary
intervention.
Materials and methods
Experimental animals
A total of 40 Sprague-Dawley (SD) male rats (age,
8-week-old; weight, 200±20 g) were obtained from The Experimental
Animal Center of Tongji Medical College, Huazhong University of
Science and Technology (Wuhan, China; license no. SCXK 2019-0002).
The animals were housed in a controlled environment with a 12-h
light/dark cycle, at 22±2°C and 50±5% humidity, with free access to
food and water. All the experimental procedures were approved by
The Animal Care and Ethics Committee of Henan University of Science
and Technology (Luoyang, China; approval no. 2021-0392).
Extraction of primary BMSCs
In the present study, 20 male rats (age, 3-week-old;
weight, 50±5 g) were obtained from The Experimental Animal Center
of Tongji Medical College, Huazhong University of Science and
Technology (Wuhan, China; license no. SCXK 2018-0005). The animals
were housed in a controlled environment with a 12-h light/dark
cycle, at 22±2°C and 50±5% humidity, with free access to food and
water. The rats were euthanized by cervical dislocation and
immersed in 75% alcohol for 10 min. The front and back legs were
stripped, and the bone marrow cavity was cleaned by adding
serum-free culture solution via a syringe. The cell suspension was
passed through a 75-µm cell strainer to eliminate any tissue, fat
or debris. Subsequently, the mixture was centrifuged at 300 × g for
5 min at room temperature, and the resulting pellet was resuspended
in ACK lysis buffer (Beijing Solarbio Science & Technology Co.,
Ltd.) at room temperature for 2 min. The cells were washed with
minimum essential medium α (MEM; Wuhan Pricella Biotechnology Co.,
Ltd.) supplemented with 10% fetal bovine serum (FBS; Beijing
Solarbio Science & Technology Co., Ltd.) and centrifuged at 300
× g for 5 min at room temperature. After which, the cells were
collected and cultured in MEM supplemented with 10% FBS at 37°C
with 5% CO2. After 48 h of culture, the half-volume
exchange method was used whereby half of the original medium was
replaced with an equal amount of fresh medium. Subsequently, the
medium was changed every 3 days until the cells reached 90%
confluence at 37°C, at which point primary BMSCs could be obtained
(39).
Extraction and identification of
BMSC-Exo
The culture supernatant of the BMSCs was collected
when the cell density reached 80–90%. The cell supernatant was
centrifuged at 2,000 × g at 4°C for 10 min to remove the
nonadherent cells. After which, the supernatant was collected and
centrifuged at 10,000 × g at 4°C for 30 min to remove cell
fragments. The supernatant was subsequently centrifuged at 100,000
× g for 90 min at 4°C in an ultracentrifuge (Sorvall™
WX+; Thermo Fisher Scientific, Inc.). The supernatant was carefully
removed, and an appropriate volume of PBS was added to resuspend
the pellet containing the BMSC-Exo. The BMSC-Exo was filtered
(0.22-µm) and stored at −80°C for subsequent detection and use. A
total of 20 ul of the resuspended samples were added dropwise to
200-mesh copper grids. Tissue sections (0.1-µm thick) were
incubated at room temperature for 10 min, fixed in 3%
glutaraldehyde at 4°C for 2 h. After which, the grids were
negatively stained with 2% phosphotungstic acid (Shanghai Macklin
Biochemical Co., Ltd.) at room temperature for 3 min, and the
remaining liquid was removed by filter paper. BMSC-Exo morphology
was assessed via transmission electron microscopy (TEM; JEM1400;
JEOL, Ltd.; Digital Micrograph 3.5, Gatan, Inc.) at an accelerating
voltage of 80 kV. BMSC-Exo nanoparticle tracking analysis (NTA) was
performed with a Zetasizer Nano (Malvern Instruments, Ltd.), and
BMSC-Exo biomarkers were detected by western blotting.
Loading of BMSC-Exo with miR-25-3p by
electroporation
A total of 500 µl BMSC-Exo (250 µM) and 100 µg of
miR-25-3p (Shanghai GenePharma Co., Ltd.) were gently mixed in 400
µl cold electroporation buffer (MEM-α reduced serum medium; 1.15 mM
K3PO4 pH 7.2 and 25 mM KCl, conductivity and osmolarity were 300
mOsm/kg, 10 ms/cm) and incubated for 5 min at room temperature, as
previously described (40).
Electroporation was performed in a 4-mm cuvette using a 0.35-sec
pulse repeated 20 times at 0.7 kV and 50 µF (the electrode material
was a conductive polymer with a diameter range of 0.3 mm, the time
interval between each pulse was 20 sec and the repetition frequency
was 300 Hz) by an electroporation instrument (SCIENTZ-2C; Ningbo
Scientz Biotechnology, Co., Ltd.) as previously described (40). After which, the mixture was
incubated with MEM-α for 30 min at 4°C to recover the membrane
structure, and washed twice with ice-cold PBS buffer by
ultracentrifugation at 105 × g for 70 min at 4°C to
remove free miRNAs. The pellet was then resuspended in PBS and
stored at −80°C for subsequent use, and it was ensured that
reagents were used within 1 week.
Evaluating BMSC-Exo internalization in
vivo and in vitro
BMSC-Exo and DiR dye (Shanghai Aladdin Biochemical
Technology Co., Ltd.) were incubated at 37°C for 30 min and then
subjected to ultracentrifugation for 1 h at 100,000 × g at room
temperature to remove the remaining dye. Subsequently, rats were
injected with DiR-BMSC-Exo (1 ml; 100 µg/kg) through the tail vein,
and the BMSC-Exo organ distribution was dynamically observed within
24 h using a small animal imaging device (FUSION FX EDGE SPECTRA;
Vilber Lourmat) (41). Similarly,
H9C2 cells (The Cell Bank of Type Culture Collection of The Chinese
Academy of Sciences) were cocultured with Dil-labeled BMSC-Exo for
6 h at 37°C and then fixed in 4% paraformaldehyde for 20 min at
25°C. BMSC-Exo internalization by H9C2 cells was evaluated via
confocal microscopy (Nikon Corporation) (42).
Establishment of in vivo MIRI models
and animal grouping
As previously described (43), 40 rats were randomly divided into 4
groups: i) Sham; ii) I/R + PBS; iii) I/R + BMSC-Exo; and iv) I/R +
BMSC-Exo-25-3p (n=10/group). BMSC-Exo or BMSC-Exo-25-3p (100 µg/kg)
were injected through the tail vein 2 h before I/R surgery. The
rats were deeply anesthetized with pentobarbital sodium (50 mg/kg,
i.p.) and fixed on the operating table. Occasionally, 1/4 of the
initial dose was added when needed during the experiment by
observing the responses of that rats, such as muscle tension and
response to skin pinch. A ventilator was attached to keep the
animal mechanically ventilated. Electrocardiograms were recorded
continuously during the experiment using the HF-12 movable
functional experimental platform (Chengdu Taimeng Software Co.,
Ltd.). The chest cavity was opened at the fourth intercostal space,
exposing the heart. An in vivo MIRI model was established in
rats by ligating the anterior descending coronary artery for 30 min
followed by reperfusion for 120 min. In the sham group, the left
anterior descending of coronary artery was threaded without
ligation.
Triphenyl tetrazolium chloride (TTC)
staining for myocardial infarction size measurement
At the end of the experiment, all rats were
immediately euthanized by rapid injection of pentobarbital sodium
(150 mg/kg, i.p.). The hearts were immediately removed and rinsed
with saline to remove residual blood, and the remaining saline was
removed with filter paper. The hearts were frozen at −80°C for 5
min, cut into small slices, immersed in 1% TTC (BIOSS) for 20 min
at 37°C and images were captured. The area of the noninfarct
(indicated in red) and infarct (shown in white) regions were
determined using Image-Pro analysis software (version 6.0; Media
Cybernetics, Inc.). The infarct area was calculated using the
following formula: Myocardial infarct area (%)=myocardial infarct
area/total myocardial area ×100%.
Immunofluorescence staining
Left ventricular samples were fixed in 4%
paraformaldehyde at room temperature for 24 h and then embedded in
paraffin for subsequent morphological analysis. Sections (4-5-µm
thick) were obtained from paraffin-embedded samples, and then
deparaffinized using dimethylbenzene for 10 min, followed by
rehydration in descending alcohol series for 15 min at room
temperature, blocked with 3% BSA for 30 min at room temperature.
Subsequently, the sections were incubated with an anti-CD163
antibody (1:200; CST Biological Reagents Co., Ltd.) and
anti-inducible nitric oxide synthase (iNOS) antibody (1:300; CST
Biological Reagents Co., Ltd.) at 4°C overnight. Following three
10-min washes with PBS, the samples were incubated with the
fluorescent-labeled secondary antibodies (1:300; cat. no. GB21303;
Wuhan Servicebio Technology Co., Ltd.) in the dark for 1 h at room
temperature. After counter-staining with 1 µg/ml DAPI for 5 min at
room temperature, the sections were viewed under a fluorescence
microscope (Nikon Corporation).
Establishment of the H/R model in H9C2
cells
The H/R model was established as previously
described (44). H9C2 cells were
subjected to H/R in vitro to establish the MIRI model.
Specifically, hypoxia was induced using a hypoxic incubator
containing 95% nitrogen (N2) and 5% carbon dioxide
(CO2). The culture medium (90% DMEM + 10% FBS) was
replaced with serum-free medium, specifically, 100% DMEM, and the
cells were incubated for 12 h in an incubator with 95%
N2 and 5% CO2 at 37°C. After which, the
serum-free medium was replaced with a normal medium (90% DMEM + 10%
FBS), and the cells were incubated for 12 h under reoxygenation
conditions in an environment containing 95% air and 5%
CO2 at 37°C (45). The
grouping of the cell experiments was similar to that of the animal
experiments: i) Control; ii) H/R + PBS; iii) H/R + BMSC-Exo; and
iv) H/R + BMSC-Exo-25-3p. The control group was kept in a normal
culture environment without H/R treatment.
Culture of macrophages and preparation
of conditioned medium (CM)
RAW 264.7 macrophages (The Cell Bank of Type Culture
Collection of The Chinese Academy of Sciences) were used to obtain
a CM for the in vitro experiments. RAW 264.7 macrophages
were cultured in Roswell Park Memorial Institute 1640 medium
(Beijing Solarbio Science & Technology Co., Ltd.) supplemented
with 10% FBS at 37°C in a humidified atmosphere containing 5%
CO2. After seeding into 25 cm2 dishes, the
RAW 264.7 macrophages were assigned to the control, LPS + PBS, LPS
+ BMSC-Exo and LPS + BMSC-Exo-25-3p groups. Macrophages in the
control group were left untreated. In the LPS + PBS treatment, 1
µg/ml LPS (Beijing Solarbio Science & Technology Co., Ltd.) was
added for 6 h at 37°C to induce an inflammatory environment, after
which PBS was added for 48 h. In the LPS + BMSC-Exo and LPS +
BMSC-Exo-25-3p treatments, 1 µg/ml LPS was added for 6 h, after
which BMSC-Exo (200 µl; 500 µg/ml) or BMSC-Exo-25-3p (200 µl; 500
µg/ml) were added for 48 h at 37°C (46). Subsequently, the culture medium was
collected and passed through a 0.22-µm filter to obtain the CM.
H9C2 cells were then cultured with the CM, washed twice with PBS
and collected for further molecular biological analyses. The CM
includes culture supernatants from the corresponding treatments of
the LPS + PBS group, the LPS + BMSC-Exo group and the LPS +
BMSC-Exo-25-3p group, which were used as the conditioned media
termed CM1, CM2 and CM3, respectively.
Treatment of H9C2 cells with CM
H9C2 cells were treated with CM obtained as
aforementioned for 24 h at 37°C, after which the H/R model was
established as aforementioned per the experimental requirements and
different processing methods. At the end of the cell treatment, the
supernatant was collected to analyze the creatine kinase (CK) and
lactate dehydrogenase (LDH) enzyme activity, which reflect
myocardial cell damage.
Measurement of CK and LDH
activity
Myocardial injury was assessed by the activity of CK
and LDH in blood samples and the H9C2 cell culture supernatant. The
blood samples and supernatant were centrifuged at 1,716 × g at 4°C
for 15 min, and the activity of CK (Creatine Kinase Assay Kit; cat.
no. A032-1-1) and LDH (Lactate Dehydrogenase Assay Kit; cat. no.
A020-2-2) were measured following the manufacturer's instructions
(Nanjing Jiancheng Bioengineering Institute).
Reverse transcription
quantitative-polymerase chain reaction
Total RNA from the tissue and cells was isolated
using TRIzol® reagent (CoWin Biosciences) and
reverse-transcribed using a high-capacity complementary DNA RT kit
(CoWin Biosciences). The reaction mixture was incubated at 25°C for
10 min, followed by 50°C for 15 min and then 85°C for 5 min. The
sequence of collar primers for miR-25-3p reverse transcription:
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCAGAC-3′. RT-qPCR
was conducted on a 7500 Sequence Detection System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using a SYBR Green PCR
Master Mix Kit (CoWin Biosciences) following the manufacturer's
instructions, and normalized to the internal reference gene U6. The
thermocycling conditions included: Pre-denaturation at 95°C for 10
min; and annealing at 60°C for 1 min. The expression levels of
inflammatory factors, including interleukin-6 (IL-6), iNOS,
interleukin-10 (IL-10) and arginase-1 (Arg-1), were determined. The
primer sequences are shown in Tables
SI and SII. Relative gene
expression levels were quantified using the 2−ΔΔCq
method and normalized to the internal reference genes β-actin.
Western blotting
Total proteins (from exosomes, hearts and H9C2
cells) were extracted using a lysis buffer (RIPA; Beijing Solarbio
Science & Technology Co., Ltd.). The protein concentration was
measured using the BCA method, and the percentage of separation gel
was 10%. The 40 µg protein samples were loaded onto SDS-PAGE gels,
electrophoresis for 1.5 h at room temperature, maintaining a
constant voltage of 80 V (Bio-Rad Laboratories, Inc.). After
electrophoresis, the proteins were transferred onto PVDF membranes
(Millipore Sigma), maintaining a constant current of 300 mA for 2 h
at 4°C. The samples were subsequently blocked for 1 h at room
temperature with 5% BSA in TBST (0.5% Tween) buffer. The membrane
was then incubated with primary antibodies overnight in 5% BSA
(Merck KGaA) at 4°C. After being washed thrice with TBS-T, the
membrane was incubated with a secondary antibody diluted at 1:5,000
for 1 h at room temperature. The signal was detected using an
enhanced chemiluminescence detection system (Amersham; Cytiva). The
primary antibodies used were against CD9 (1:500; cat. no. WL01236,
Wanleibio Co., Ltd.), CD63 (1:1,000; cat. no. D111314-0100100;
Sangon Biotech Co., Ltd.), JAK2 (1:1,000; cat. no. 3203; Cell
Signaling Technology, Inc.), phosphorylated (p-)JAK2 (1:1,000; cat.
no. 37745; Cell Signaling Technology, Inc.), STAT3 (1:2,000; cat.
no. 9139; Cell Signaling Technology, Inc.), p-STAT3 (1:2,000; cat.
no. 9145; Cell Signaling Technology, Inc.) and GAPDH (1:5,000; cat.
no. P07486; Sangon Biotech Co., Ltd.). ImageJ software (version 6;
National Institutes of Health) was used to analyze the bands
quantitatively.
Statistical analysis
The data are presented as the mean ± SD (whole
animal experiments, n=8; molecular biology experiment and the cell
experiment; n=4). All the experimental data were analyzed using
one-way analysis of variance followed by Tukey's multiple
comparison test by GraphPad Prism 9.0 (Dotmatics). P<0.05 was
considered to indicate a statistically significant difference.
Results
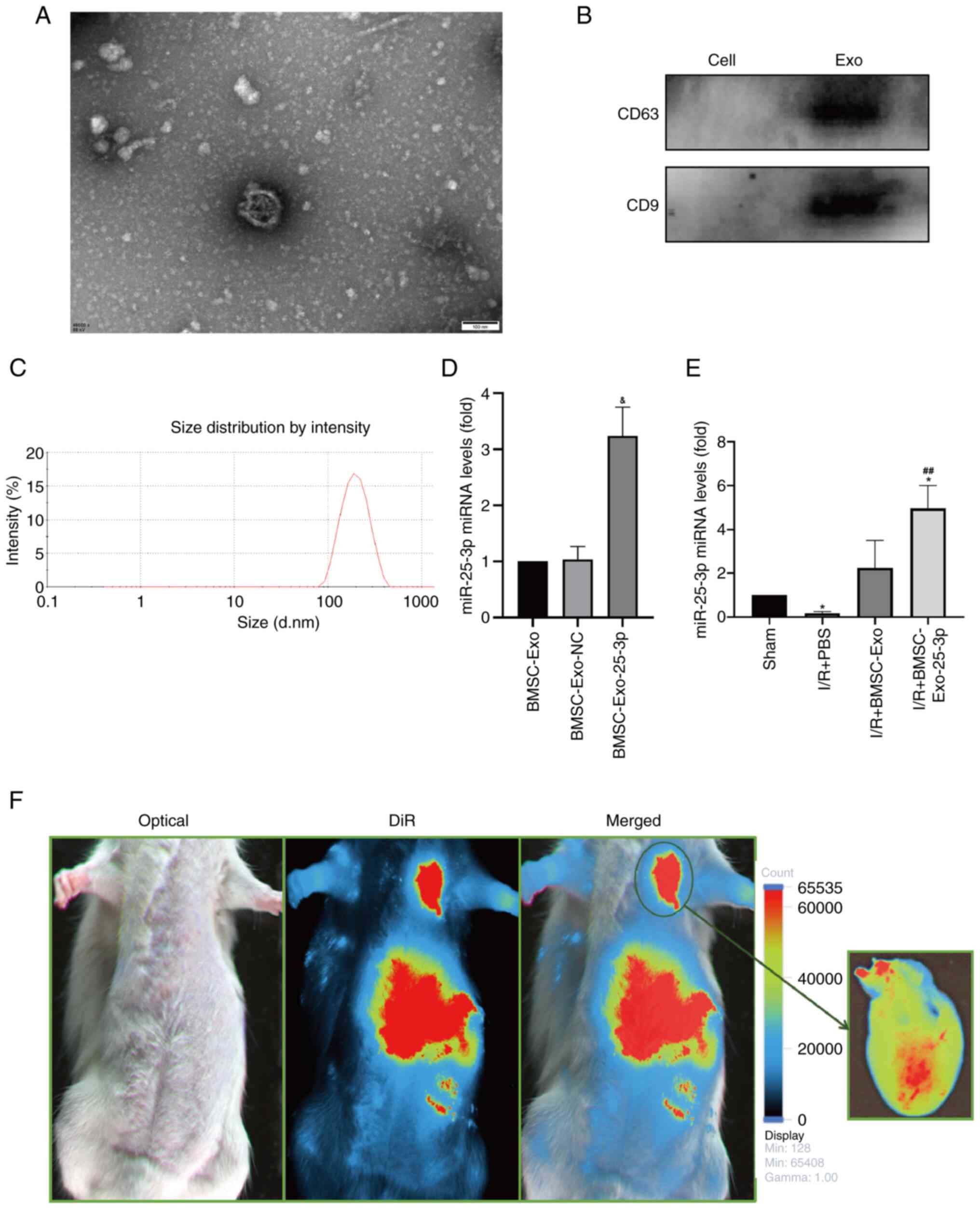
BMSC-Exo identification
TEM, NTA and surface protein marker analysis were
used as the basic criteria for BMSC-Exo identification (28). TEM revealed circular bilayer lipid
vesicles in BMSC-Exo (Fig. 1A).
Western blotting demonstrated that CD63 and CD9 were highly
expressed in the isolated particles and weakly or not expressed in
the cells (Fig. 1B). NTA also
revealed that the BMSC-Exo diameter peaked at 130-150 nm (Fig. 1C). These data revealed that the
particles extracted from the BMSCs were BMSC-Exo.
BMSC-Exo internalization into the
heart in vivo
MiR-25-3p plays an important protective role in
myocardial infarction (47,48).
To determine the biological role and mechanism of exogenous
miR-25-3p, an exosome delivery system was designed containing
miR-25-3p via BMSC-Exo, referred to as BMSC-Exo-25-3p. The loading
efficiency of miR-25-3p in exosomes was evaluated via RT-qPCR and,
as shown in Fig. 1D, miR-25-3p
expression increased significantly in BMSC-Exo-25-3p compared with
BMSC-Exo or BMSC-Exo loaded with a miR-25-3p random sequence as a
negative control. A rat model of MIRI was generated via treatment
with BMSC-Exo-25-3p, and the results showed that the expression of
the miR-25-3p gene in the heart decreased in the I/R model rats but
increased significantly after the delivery of BMSC-Exo-25-3p
(Fig. 1E). These results indicated
that the heart has a high affinity for BMSC-Exo-25-3p, and cardiac
delivery of miR-25-3p was successful.
In vivo imaging was performed to observe the
distribution of BMSC-Exo-25-3p in rats. Imaging within 12 h after
intravenous injection showed that BMSC-Exo-25-3p was ingested by
multiple organs, including the heart. Following these findings, the
heart was removed for imaging and the fluorescence intensity was
considerable (Fig. 1F). The
results showed that the heart had a high affinity for BMSC-Exo.
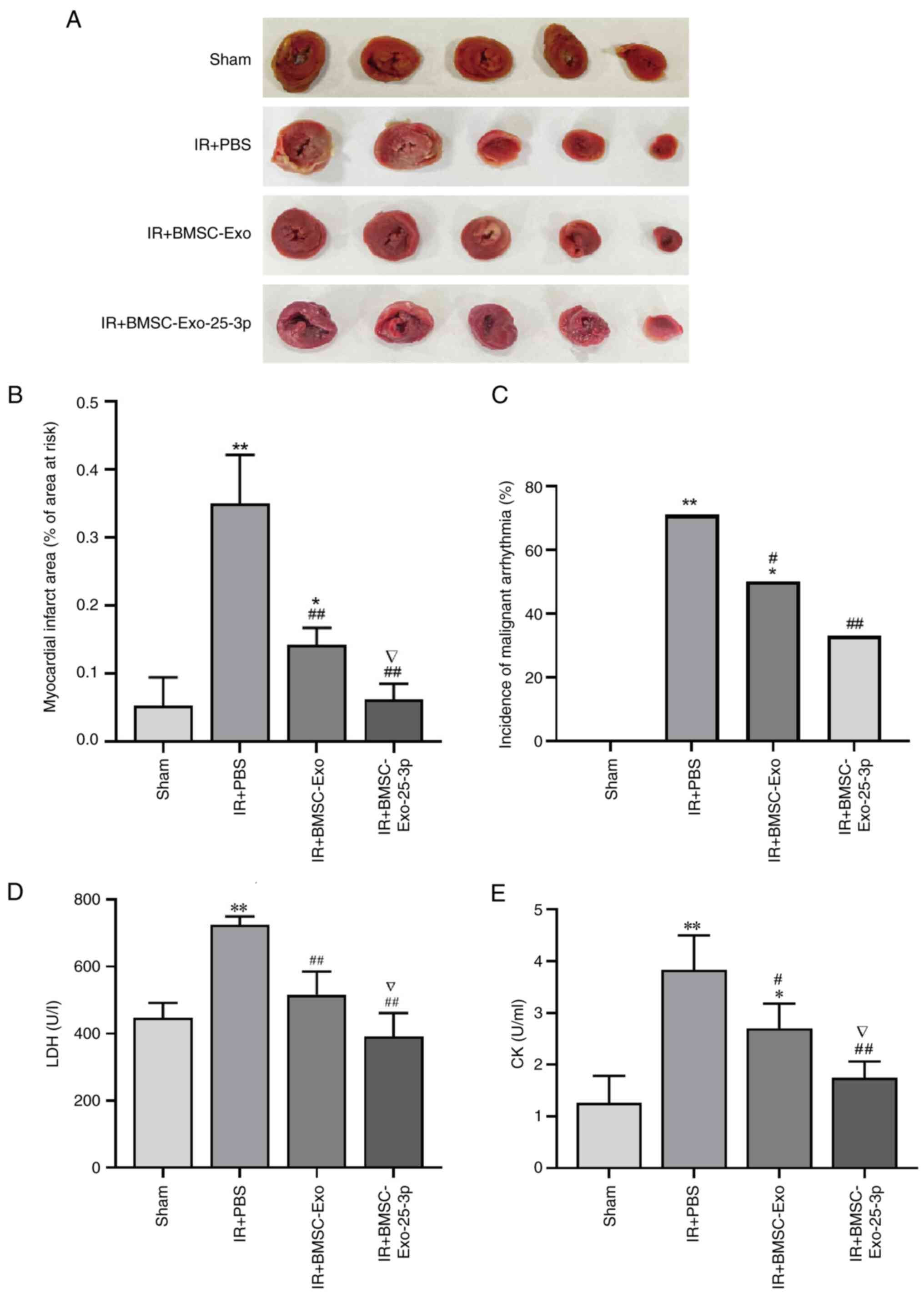
BMSC-Exo-25-3p alleviated MIRI in
vivo
To explore whether BMSC-Exo-25-3p has a protective
effect on MIRI in vivo, myocardial infarction size was
detected by TTC staining, and the incidence of malignant arrhythmia
was calculated via electrocardiogram. Moreover, myocardial enzyme
activity was measured as aforementioned. As shown in Fig. 2, BMSC-Exo-25-3p exerted
cardioprotective effects by decreasing the cardiac infarct size
(Fig. 2A and B). The incidence of
malignant arrhythmias was significantly greater in the I/R group
than in the sham group and significantly lower in the
BMSC-Exo-25-3p treatment group (Fig.
2C). Similarly, compared with those in the sham group, the LDH
and CK levels in the serum were significantly greater after I/R,
whereas BMSC-Exo-25-3p treatment reversed these trends (Fig. 2D and E).
BMSC-Exo-25-3p constrains M1-like
macrophage polarization and inhibits the JAK2/STAT3 signaling
pathway activation in vivo
Activated macrophages can be divided into two main
subtypes: M1-like (classically activated macrophages) and M2-like
(alternatively activated macrophages) (49). First, immunofluorescence staining
was used to detect macrophage subtypes using different macrophage
markers of iNOS (M1-like) and CD163 (M2-like). As shown in Fig. 3A and B, BMSC-Exo-25-3p constrained
M1-like macrophage polarization and promoted M2-like macrophage
polarization. M1-like macrophages can secrete various
proinflammatory cytokines, chemokines and inflammatory mediators,
such as interleukin-1β (IL-1β) and interleukin-6 (IL-6) (50), while M2-like macrophages can
release anti-inflammatory factors, such as IL-10 and Arg-1.
Therefore, the level of inflammatory factors can indirectly reflect
differences in macrophage polarization. RT-qPCR revealed that
BMSC-Exo-25-3p delivery notably decreased the mRNA expression of
IL-1β and IL-6 in myocardial tissue (Fig. 3C and D). Moreover, BMSC-Exo-25-3p
delivery increased the mRNA expression of IL-10 and Arg-1 in
myocardial tissue (Fig. 3E and F).
JAK2/STAT3 signaling pathway activation is closely related to
inflammation (51). It was found
that JAK2/STAT3 signaling pathway activation increased after MIRI,
and BMSC-Exo-25-3p delivery reversed this effect. The
aforementioned results indicated that overexpression of miR-25-3p
alleviates the I/R-induced inflammatory response and inhibits the
JAK2/STAT3 signaling pathway (Fig.
3G-I).
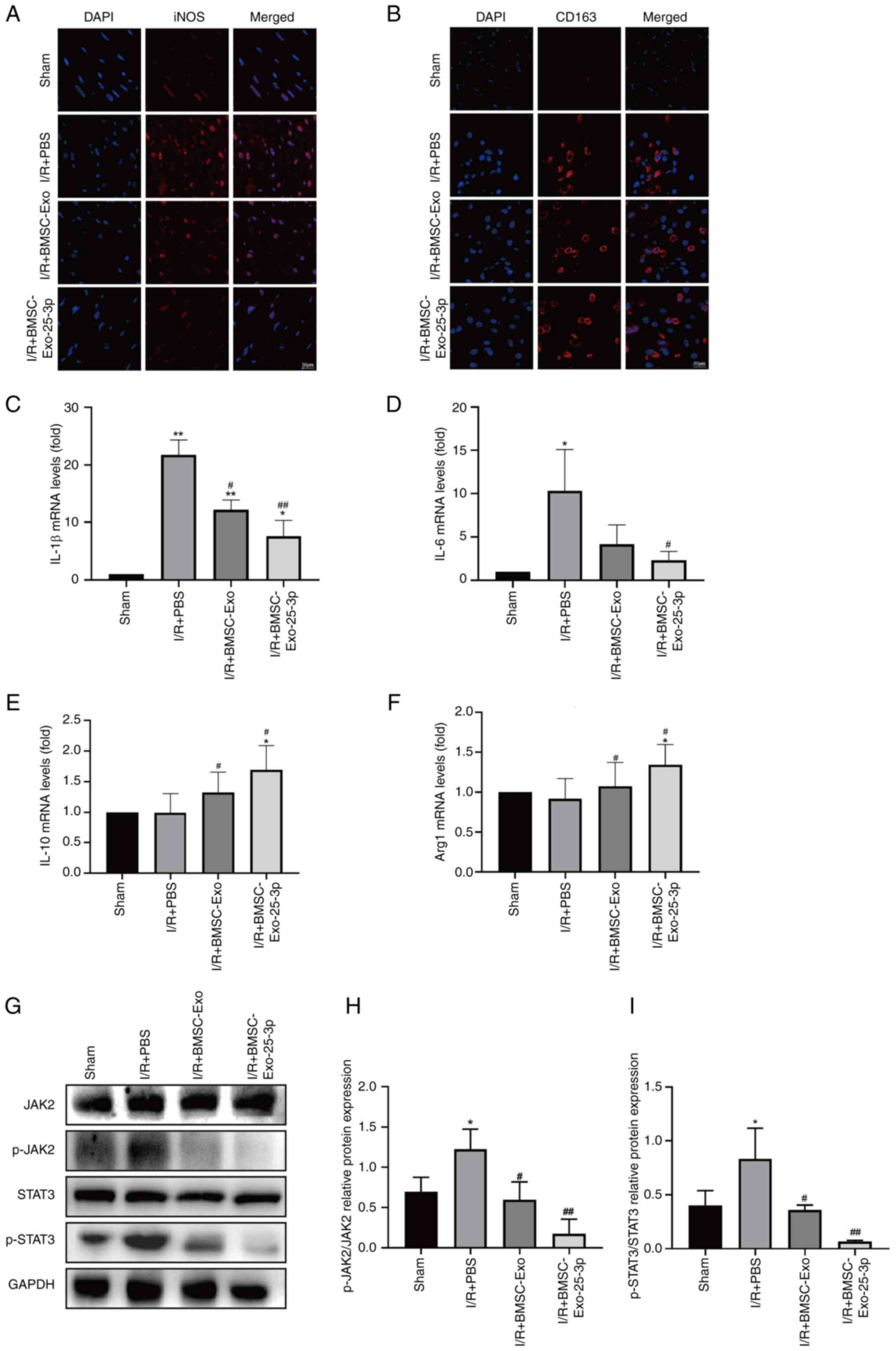 | Figure 3.BMSC-Exo-25-3p inhibited I/R-induced
inflammatory response in rats. Immunofluorescence staining of (A)
iNOS and (B) CD163 of myocardial tissue (scale bar, 50 µm). Gene
expression of proinflammatory cytokines (C) IL-1β and (D) IL-6 in
myocardial tissue. Gene expression of anti-inflammatory cytokines
(E) IL-10 and (F) Arg-1 in myocardial tissue. (G) Representative
western blotting images of the JAK2/STAT3 signaling pathway.
Quantitative analysis of (H) p-JAK2 and (I) p-STAT3. **P<0.01,
*P<0.05 vs. Sham group. ##P<0.01,
#P<0.05 vs. I/R + PBS group. All data were presented
as mean ± SD. Exo, exosome; I/R, ischemia/reperfusion; BMSC, bone
marrow mesenchymal stem cells; IL, interleukin; Arg-1, Arginase-1;
JAK2, Janus kinase 2; p-, phosphorylated; STAT3, signal transducer
and activator of transcription 3. |
Uptake of BMSC-Exo-25-3p by H9C2 cells
in vitro
H9C2 cells were cocultured with BMSC-Exo-25-3p
labeled with Dil. Endocytotic BMSC-Exo generated from H9C2 cells
were observed via confocal microscopy (Fig. 4A). RT-qPCR revealed that miR-25-3p
expression decreased after H/R. Intracellular miR-25-3p expression
increased significantly in the normal H9C2 cells and the H9C2 cells
with H/R-induced injury after BMSC-Exo delivery (Fig. 4B and C), indicating that
BMSC-Exo-25-3p was engulfed by H9C2 cells and that BMSC-Exo
successfully delivered miR-25-3p to H9C2 cells.
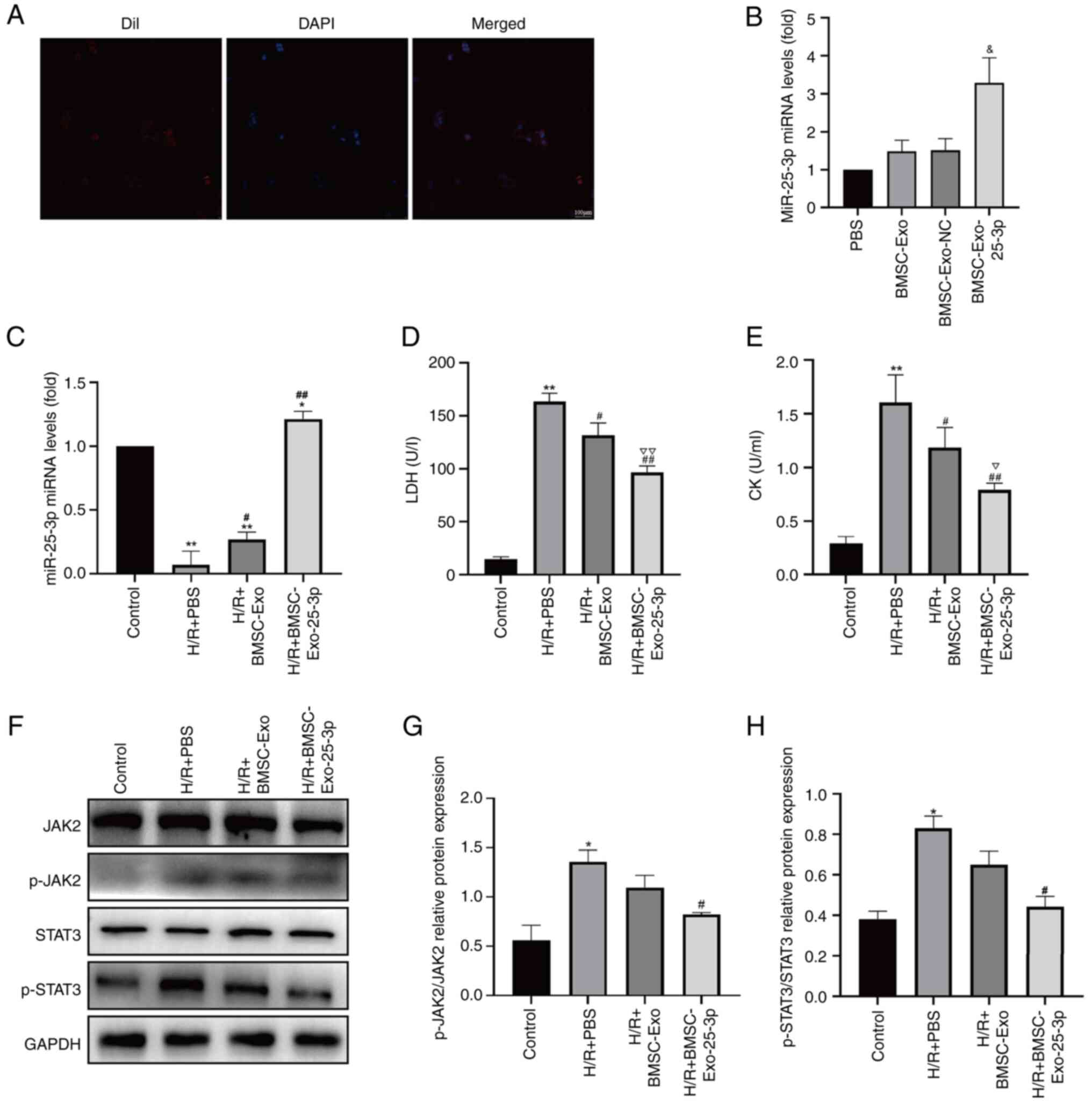 | Figure 4.BMSC-Exo-25-3p alleviated H/R-induced
injury in H9C2 cells. (A) Phagocytosis of Dil-labeled BMSC-Exo in
H9C2 cells observed by a confocal microscope (scale bar, 50 µm).
(B) Expression of miR-25-3p in H9C2 cells after co-incubation
assessed by RT-qPCR. (C) Expression of miR-25-3p in H/R-induced
injury in H9C2 cells. (D) LDH and (E) CK activity in cell culture
supernatant. (F) Representative western blotting images of the
JAK2/STAT3 signaling pathway. Quantity analysis of (G) p-JAK2 and
(H) p-STAT3 in H9C2 cells. **P<0.01, *P<0.05 vs. control
group. ##P<0.01, #P<0.05 vs. H/R + PBS
group. ▽P<0.05 vs. H/R + BMSC-Exo group.
&P<0.01 vs. PBS group, BMSC-Exo group or
BMSC-Exo-NC group. All data were presented as mean ± SD. Exo,
exosome; BMSC, bone marrow mesenchymal stem cells; NC, negative
control; H/R, hypoxia/reoxygenation; LDH, lactate dehydrogenase;
CK, creatine kinase; JAK2, Janus kinase 2; p-, phosphorylated;
STAT3, signal transducer and activator of transcription 3. |
BMSC-Exo-25-3p alleviated H/R-induced
H9C2 cell injury
Given the in vivo results, enzymatic activity
testing was conducted on the culture medium of H9C2 cells. It was
found that, after H/R treatment, the enzymatic activity of CK and
LDH increased significantly compared with that in the control
group, whereas BMSC-Exo-25-3p pretreatment reversed these trends
and reduced the levels of CK and LDH after H/R (Fig. 4D and E). These results indicated
that miR-25-3p delivered by BMSC-Exo has a direct protective effect
on H9C2 cell injury caused by H/R.
Consistent with the in vivo experiments, a
trend toward JAK2/STAT3 signaling pathway activation after H/R
treatment was observed in H9C2 cells, while this trend was weakened
after overexpression of miR-25-3p, suggesting that BMSC-Exo-25-3p
directly inhibited the JAK2/STAT3 signaling pathway in H9C2 cells
(Fig. 4F-H).
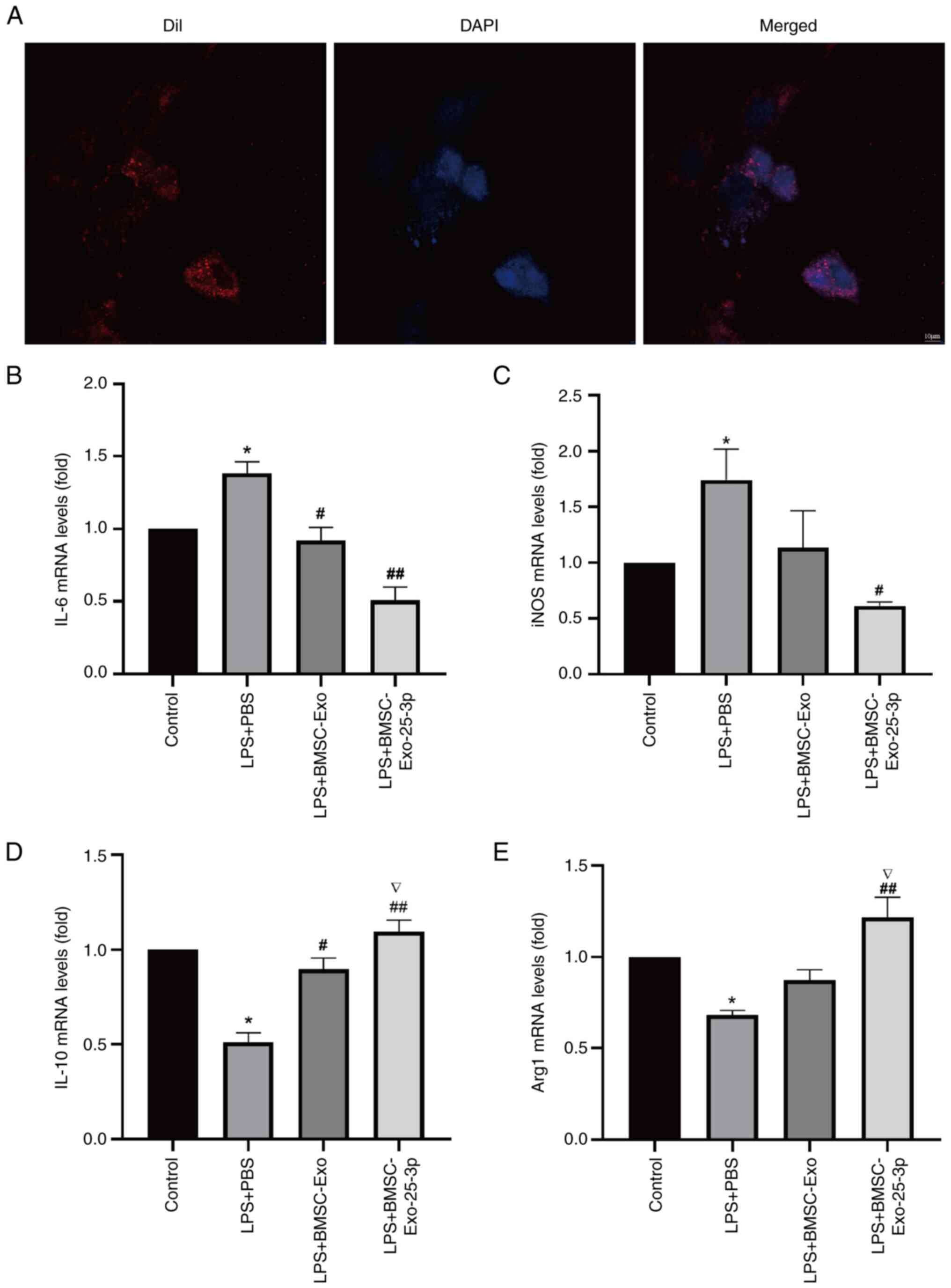
Uptake of BMSC-Exo-25-3p by RAW 264.7
macrophages in vitro
To confirm that BMSC-Exo-25-3p has an indirect
protective effect on heart injury, whether the protective effect of
BMSC-Exo-25-3p on the heart was related to the regulation of
macrophage polarization was examined. BMSC-Exo-25-3p labeled with
Dil was cocultured with RAW 264.7 macrophages, and endocytosis by
RAW 264.7 macrophages was observed via confocal microscopy
(Fig. 5A).
BMSC-Exo-25-3p constrains M1-like
macrophage polarization
After RAW 264.7 macrophages were stimulated with
LPS, RT-qPCR was used to measure the gene expression of
M1-characterizing cytokines, including IL-6 and iNOS, and
M2-characterizing cytokines, including IL-10 and Arg-1. The results
showed that the levels of IL-6 and iNOS in the LPS treatment group
were significantly increased. However, pretreatment with
BMSC-Exo-25-3p reversed these effects, reducing the expression of
IL-6 and iNOS (Fig. 5B and C) and
increasing the expression of IL-10 and Arg-1 significantly in RAW
264.7 macrophages after LPS stimulation (Fig. 5D and E). These results suggested
that BMSC-Exo-25-3p inhibits M1-like macrophage polarization and
accelerates M2-like polarization.
Mechanistically, western blotting was performed to
measure the expression levels of proteins related to the JAK2/STAT3
signaling pathway, given its involvement in regulating macrophage
polarization in several pathological processes including regulation
of the immune response and involvement in disease recovery in the
late inflammatory phase (52). The
results showed that the JAK2/STAT3 signaling pathway was activated
in LPS-stimulated RAW 264.7 macrophages, as shown by the
significant increase in the phosphorylation of JAK2 and STAT3.
However, BMSC-Exo-25-3p inhibited the activation of this signaling
pathway and significantly reduced the phosphorylation of JAK2 and
STAT3 (Fig. 6A-C).
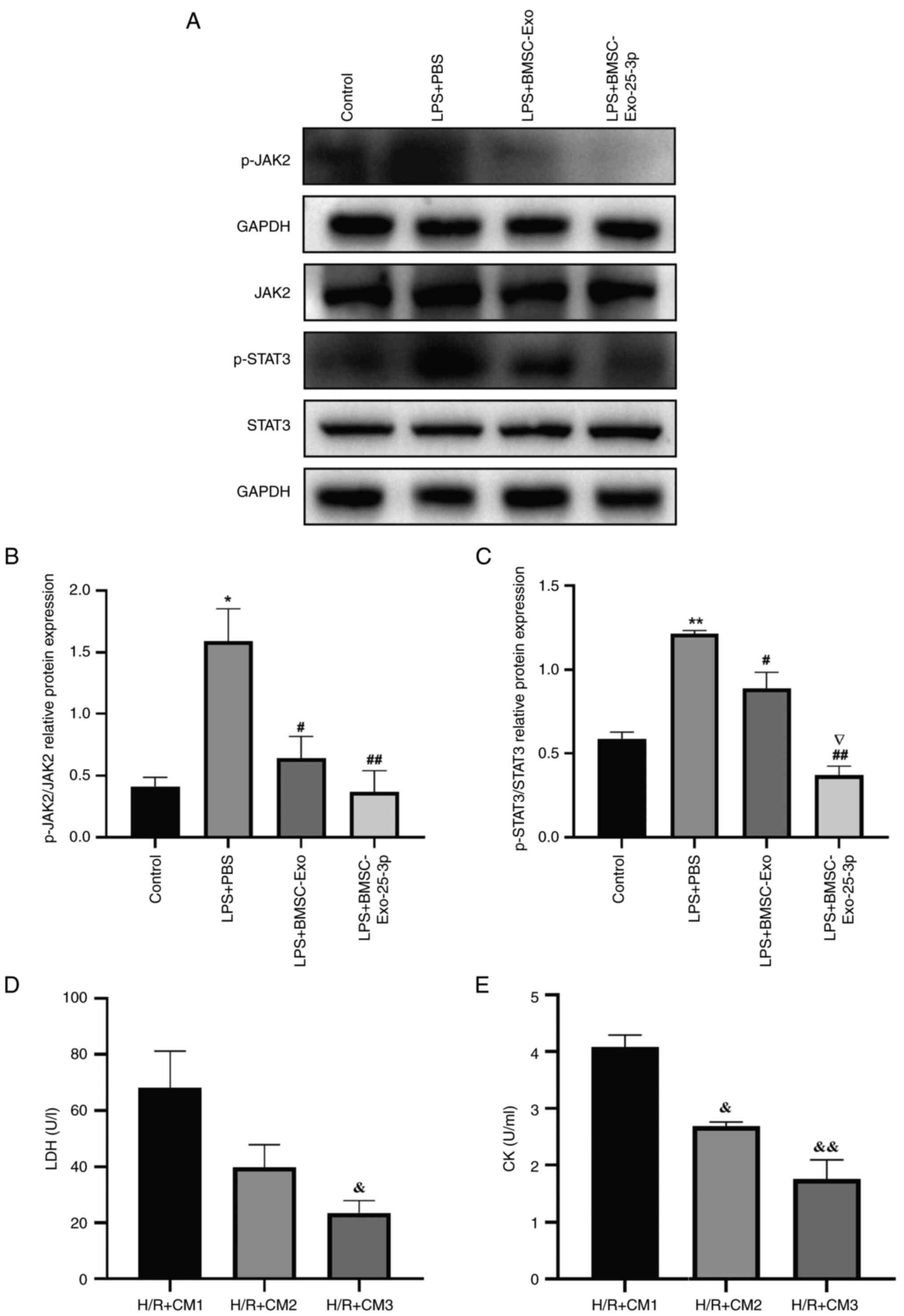 | Figure 6.LPS-stimulated RAW 264.7 macrophage
cocultured with H/R-induced H9C2 cells. (A) Representative western
blotting images of the JAK2/STAT3 signaling pathway in
LPS-stimulated RAW 264.7 cells. Quantitative analysis of (B) p-JAK2
and (C) p-STAT3. (D) LDH and (E) CK activity in cell culture
supernatant of H9C2 cells pretreated with CM obtained from
LPS-stimulated RAW 264.7 macrophages. **P<0.01, *P<0.05 vs.
Control group. ##P<0.01, #P<0.05 vs.
LPS + PBS group. ▽P<0.05 vs LPS + BMSC-Exo group.
&&P<0.01, &P<0.05 vs. H/R +
CM1 group. All data were presented as mean ± SD. Exo, exosome;
BMSC, bone marrow mesenchymal stem cells; LPS, lipopolysaccharide;
JAK2, Janus kinase 2; p-, phosphorylated; STAT3, signal transducer
and activator of transcription 3; LDH, lactate dehydrogenase; CK,
creatine kinase; CM, conditioned medium. |
LPS-stimulated RAW 264.7 macrophage
coculture with H/R-induced H9C2 cells
H9C2 cells were treated with CM obtained from
LPS-stimulated RAW 264.7 macrophages as aforementioned to explore
the role of the macrophage phenotype in H/R-induced H9C2 cell
injury, and H9C2 cell culture supernatant was collected to analyze
the enzymatic activity of LDH and CK. Compared with the PBS group,
CM derived from BMSC-Exo-25-3p pre-intervention of RAW 264.7
macrophages significantly decreased the activities of LDH and CK in
H/R-induced H9C2 cells (Fig. 6D and
E).
Discussion
MIRI occurs after the ischemic myocardium restoring
blood supply, and morbidity rates associated with MIRI have
steadily increased since 2004 (53). Previous studies have shown that
BMSCs promote wound healing and tissue repair by regulating the
immune response and inhibiting inflammation and apoptosis (54,55).
Several recent studies have indicated that BMSC-Exo could alleviate
MIRI by inhibiting the expression of inflammatory factors or
inflammatory mediators in the earlier inflammatory phase or the
healing phase (56,57). miRNAs are considered important
components of exosomes and largely determine their effects on
receptor cells (58). Extensive
research has confirmed that exosomes play a crucial role in
intercellular communication by transporting miRNAs and proteins and
have been found to reduce I/R-induced damage (53,59,60).
Studies have reported that miR-25-3p in BMSC-Exo ameliorates liver
I/R injury through PTEN (61,62).
Exosomal miR-25-3p derived from MSCs has been shown to alleviate
myocardial infarction by targeting proapoptotic proteins and
enhancer of zeste homolog 2 (48).
Although previous studies have shown that delivering miR-25-3p
through stem cell extracellular vesicles can improve cardiac
function and inhibit MIRI, the specific mechanism remains to be
determined.
The present study explored the mechanism of
miR-25-3p overexpression in BMSC-Exo in MIRI. An in vivo
MIRI model in rats was estabilshed by ligating the anterior
descending left coronary artery and an in vitro H/R model in
H9C2 cells. BMSC-Exo carrying miR-25-3p protected against MIRI, as
demonstrated by the finding that BMSC-Exo-25-3p reduced the
incidence of arrhythmia and the myocardial infarction area in rats.
Cardiac enzymes such as LDH and CK are released after myocardial
injury (63) and are often used as
markers of myocardial damage. Furthermore, the present study
revealed a significant decrease in cardiac enzymatic activity
following the overexpression of miR-25-3p.
After the onset of I/R, various injury-related
factors are released to induce an inflammatory cascade in the
heart. The inflammatory response involves the infiltration of
inflammatory cells, including lymphocytes, neutrophils and
macrophages, leading to the accumulation of proinflammatory
cytokines and, ultimately, severe cardiac dysfunction (64). Inflammatory cell infiltration and
the accumulation of proinflammatory cytokines can not only clear
cell debris but also cause further damage and stress in surviving
myocardial cells (65,66). Inflammatory monocytes enter the
damaged surrounding area and transform into macrophages, which are
the core mediators of cardiac inflammation and regulate tissue
damage and repair (67). M1-like
macrophages infiltrate damaged heart tissue, triggering the release
of proinflammatory cytokines and local inflammatory responses. In
contrast, M2-like macrophages have anti-inflammatory properties and
are involved in tissue regeneration and remodeling (68,69),
promoting wound healing and scar formation (49). In addition, promoting M2-type
macrophage polarization has been shown to prevent I/R damage
(49,70). BMSC-Exo plays an immunoprotective
role in inflammatory reactions (71). The present study showed that the
mRNA expression levels of IL-1 β and IL-6 increased after MIRI
in vivo, and BMSC-Exo-25-3p reversed this effect. This
finding suggested that BMSC-Exo, especially BMSC-Exo-25-3p,
restricts M1-like macrophage polarization and promotes the
polarization of M2-like macrophages after MIRI in vivo. The
results were consistent with those reported in the literature
(65).
Since the JAK/STAT signaling pathway is widely
involved in the regulation of inflammation and immune responses,
which play an important role in the progression of I/R (72), inhibiting JAK/STAT signaling
pathway activation might alleviate inflammatory response and tissue
damage in I/R (73,74). The results of the present study
showed that BMSC-Exo-25-3p could decrease phosphorylated JAK2 and
STAT3 in myocardial tissue, suggesting that BMSC-Exo-25-3p
inhibited JAK2/STAT3 signaling pathway activation.
To verify that BMSC-Exo-25-3p regulates the
polarization of macrophages concerning the JAK2/STAT3 signaling
pathway, RAW264.7 macrophages were cultured in vitro and
cocultured with H9C2 cells. After LPS stimulation, the mRNA
expression levels of iNOS and IL-6 increased, suggesting that LPS
induced M1-like macrophage polarization. BMSC-Exo, particularly
BMSC-Exo-25-3p, reversed this effect, restricting M1-like
macrophage polarization and promoting the polarization of M2-like
macrophages after LPS stimulation. Similarly, the phosphorylation
levels of the JAK2 and STAT3 proteins increased after LPS
stimulation, while BMSC-Exo, particularly BMSC-Exo-25-3p,
effectively reduced the phosphorylation of JAK2 and STAT3.
Moreover, H9C2 cells were treated with CM obtained from
LPS-stimulated RAW 264.7 macrophages to explore the role of the
macrophage phenotype in H/R-induced H9C2 cell injury. The results
showed that CM derived from BMSC-Exo-25-3p preintervention in RAW
264.7 macrophages significantly decreased the activities of LDH and
CK in H/R-induced H9C2 cells. The present study demonstrated that
BMSC-Exo, especially BMSC-Exo-25-3p, inhibited JAK2/STAT3 signaling
pathway activation and induced the transformation of macrophages
from the M1 to the M2 phenotype, reducing the inflammatory response
and protecting against MIRI.
In addition to the indirect protective effects of
BMSC-25-3p, whether BMSC-Exo-25-3p has a direct effect on
H/R-induced H9C2 cells was investigated. Consistent with the
literature, BMSC-Exo-25-3p uptake resulted in a significant
increase in miR-25-3p in H9C2 cells and protected H9C2 cells from
H/R-induced injury. Notably, it was also observed that
BMSC-Exo-25-3p had a certain regulatory effect on the JAK2/STAT3
signaling pathway in H9C2 cells, which requires further
investigation.
The present study has some limitations. First, the
current understanding of macrophage polarization behavior is
limited, and extensive research is required to fully comprehend the
complexity of this process. Second, although BMSC-Exo-25-3p can
regulate the polarization of macrophages through the JAK2/STAT3
signaling pathway, the direct mechanism of action of miRNA-25-3p
remains to be further studied. Third, since promoting the
polarization of M2-type macrophages is crucial for the
cardioprotective effect of BMSC-Exo-25-3p, it is necessary to
investigate how polarized macrophages protect the heart. Finally,
although TTC-staining of myocardial tissue was performed to reflect
myocardial injury, the present study was unable to assess the
effects of miR-25-3p on heart injury in situ.
As a key medium of intercellular communication, and
as a potential drug delivery system, exosomes will play an
important therapeutic role in the future, especially in the area of
heart repair, however at present, the clinical transformation of
exosomes faces numerous problems and challenges. Currently, no
exosome therapy has been approved for marketing, and methods of
isolating exosomes with high purity, large quantities and a stable
quality remain to be solved in the future.
In conclusion, in the present study, the protective
mechanism of BMSC-Exo in MIRI was investigated. The results
revealed that BMSC-Exo delivery of miR-25-3p caused a shift in the
macrophage phenotype from the proinflammatory/M1 to the
anti-inflammatory/M2 phenotype, reducing the inflammatory response
and protecting against MIRI. The polarization of macrophages may be
related to the JAK2/STAT3 signaling pathway. These findings
provided a theoretical foundation for the use of BMSC-Exo as
carriers of therapeutic drugs or genes in MIRI medical
therapeutics, which may affect the prognosis of patients with
coronary heart disease after thrombolysis or percutaneous coronary
intervention.
Supplementary Material
Supporting Data
Acknowledgments
Not applicable.
Funding
The present study was supported by The Luoyang City Social
Development Public Welfare Project (grant no. 2302001A) and The
Henan Province Scientific and Technology Research Project (grant
no. 222103810051).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YD and JS developed the animal pathological models
and cell models, and completed the molecular biological
experiments. HS and CX assisted with the biochemical tests and
in vivo imaging. YH was responsible for analyzing the data.
SZ and SW assisted in the establishment of animal pathological
models. JD designed the study and revised the manuscript. YD and JS
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
All the experimental procedures were approved by The
Animal Care and Ethics Committee of Henan University of Science and
Technology (Luoyang, China; approval no. 2021-0392). and adhered to
the guidelines for the care and use of laboratory animals outlined
by the US National Institutes of Health.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gibbons RJ: Myocardial ischemia in the
management of chronic coronary artery disease: Past and present.
Circ Cardiovasc Imaging. 14:e0116152021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Safiri S, Karamzad N, Singh K,
Carson-Chahhoud K, Adams C, Nejadghaderi SA, Almasi-Hashiani A,
Sullman MJM, Mansournia MA, Bragazzi NL, et al: Burden of ischemic
heart disease and its attributable risk factors in 204 countries
and territories, 1990-2019. Eur J Prev Cardiol. 29:420–431. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asaria P, Elliott P, Douglass M, Obermeyer
Z, Soljak M, Majeed A and Ezzati M: Acute myocardial infarction
hospital admissions and deaths in England: A national follow-back
and follow-forward record-linkage study. Lancet Public Health.
2:e191–e201. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fan Q, Tao R, Zhang H, Xie H, Lu L, Wang
T, Su M, Hu J, Zhang Q, Chen Q, et al: Dectin-1 contributes to
myocardial ischemia/reperfusion injury by regulating macrophage
polarization and neutrophil infiltration. Circulation. 139:663–678.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu R, Liu H, Yuan D, Chen Y, Tang X,
Zhang C, Zhu P, Yang T, Zhang Y, Li H, et al: For patients with
prior coronary artery bypass grafting and recurrent myocardial
ischemia, percutaneous coronary intervention on bypass graft or
native coronary artery?-A 5-year follow-up cohort study. Clin
Cardiol. 46:680–688. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Y, Du J, Zheng L, Wang Z, Zhang Z, Wu
Z, Zhu X and Xiong JW: Chemical screening links disulfiram with
cardiac protection after ischemic injury. Cell Regen. 12:252023.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miri R, Howlett SE and Knaus EE: Synthesis
and calcium channel modulating effects of isopropyl
1,4-dihydro-2,6-dimethyl-3-nitro-4-(thienyl)-5-pyridinecarboxylates.
Arch Pharm (Weinheim). 330:290–294. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Z, Gao J, Sun D, Jiao Q, Ma J, Cui W,
Lou Y, Xu F, Li S and Li H: LncRNA MEG3: Potential stock for
precision treatment of cardiovascular diseases. Front Pharmacol.
13:10455012022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Algoet M, Janssens S, Himmelreich U, Gsell
W, Pusovnik M, Van den Eynde J and Oosterlinck W: Myocardial
ischemia-reperfusion injury and the influence of inflammation.
Trends Cardiovasc Med. 33:357–366. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hassanshahi A, Moradzad M, Ghalamkari S,
Fadaei M, Cowin AJ and Hassanshahi M: Macrophage-mediated
inflammation in skin wound healing. Cells. 11:29532022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jia D, Chen S, Bai P, Luo C, Liu J, Sun A
and Ge J: Cardiac resident macrophage-derived legumain improves
cardiac repair by promoting clearance and degradation of apoptotic
cardiomyocytes after myocardial infarction. Circulation.
145:1542–1556. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu M, Li X and Song L: Baicalin regulates
macrophages polarization and alleviates myocardial
ischaemia/reperfusion injury via inhibiting JAK/STAT pathway. Pharm
Biol. 58:655–663. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Louiselle AE, Niemiec SM, Zgheib C and
Liechty KW: Macrophage polarization and diabetic wound healing.
Transl Res. 236:109–116. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Calle P and Hotter G: Macrophage phenotype
and fibrosis in diabetic nephropathy. Int J Mol Sci. 21:28062020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu B, Xiong Y, Sha Z, Xue W, Xu B, Tan S,
Guo D, Lin F, Wang L, Ji J, et al: SEPTIN2 suppresses an
IFN-γ-independent, proinflammatory macrophage activation pathway.
Nat Commun. 14:74412023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wan E, Yeap XY, Dehn S, Terry R, Novak M,
Zhang S, Iwata S, Han X, Homma S, Drosatos K, et al: Enhanced
efferocytosis of apoptotic cardiomyocytes through
myeloid-epithelial-reproductive tyrosine kinase links acute
inflammation resolution to cardiac repair after infarction. Circ
Res. 113:1004–1012. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mahdiani S, Omidkhoda N, Rezaee R, Heidari
S and Karimi G: Induction of JAK2/STAT3 pathway contributes to
protective effects of different therapeutics against myocardial
ischemia/reperfusion. Biomed Pharmacother. 155:1137512022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu L, Tan JL, Wang ZH, Chen YX, Gao L, Liu
JL, Shi YH, Endoh M and Yang HT: ROS generated during early
reperfusion contribute to intermittent hypobaric hypoxia-afforded
cardioprotection against postischemia-induced Ca(2+) overload and
contractile dysfunction via the JAK2/STAT3 pathway. J Mol Cell
Cardiol. 81:150–161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liao Y, Hu X, Guo X, Zhang B, Xu W and
Jiang H: Promoting effects of IL-23 on myocardial ischemia and
reperfusion are associated with increased expression of IL-17A and
upregulation of the JAK2-STAT3 signaling pathway. Mol Med Rep.
16:9309–9316. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yin Q, Zhao B, Zhu J, Fei Y, Shen W, Liang
B, Zhu X and Li Y: JLX001 improves myocardial ischemia-reperfusion
injury by activating Jak2-Stat3 pathway. Life Sci. 257:1180832020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sopjani M, Morina R, Uka V, Xuan NT and
Dërmaku-Sopjani M: JAK2-mediated intracellular signaling. Curr Mol
Med. 21:417–425. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu J and Jiang B: Sphk1 promotes
ulcerative colitis via activating JAK2/STAT3 signaling pathway. Hum
Cell. 33:57–66. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen Z, Huang W, Liu J, Tian J, Wang S and
Rui K: Effects of mesenchymal stem cell-derived exosomes on
autoimmune diseases. Front Immunol. 12:7491922021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuan Y, Fan X, Guo Z, Zhou Z and Gao W:
Metformin protects against spinal cord injury and cell pyroptosis
via AMPK/NLRP3 inflammasome pathway. Anal Cell Pathol (Amst).
2022:36349082022.PubMed/NCBI
|
|
26
|
Harrell CR, Jovicic N, Djonov V,
Arsenijevic N and Volarevic V: Mesenchymal stem cell-derived
exosomes and other extracellular vesicles as new remedies in the
therapy of inflammatory diseases. Cells. 8:16052019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang SJ, Song XY, He M and Yu SB: Effect
of TGF-β1/SDF-1/CXCR4 signal on BM-MSCs homing in rat heart of
ischemia/perfusion injury. Eur Rev Med Pharmacol Sci. 20:899–905.
2016.PubMed/NCBI
|
|
28
|
Hatzistergos KE, Quevedo H, Oskouei BN, Hu
Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP,
Rodriguez JE, et al: Bone marrow mesenchymal stem cells stimulate
cardiac stem cell proliferation and differentiation. Circ Res.
107:913–922. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Davidson SM and Yellon DM: Exosomes and
cardioprotection-A critical analysis. Mol Aspects Med. 60:104–114.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lai RC, Arslan F, Lee MM, Sze NS, Choo A,
Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, et al:
Exosome secreted by MSC reduces myocardial ischemia/reperfusion
injury. Stem Cell Res. 4:214–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McDonald MK, Tian Y, Qureshi RA, Gormley
M, Ertel A, Gao R, Aradillas Lopez E, Alexander GM, Sacan A,
Fortina P and Ajit SK: Functional significance of
macrophage-derived exosomes in inflammation and pain. Pain.
155:1527–1539. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun XH, Wang X, Zhang Y and Hui J:
Exosomes of bone-marrow stromal cells inhibit cardiomyocyte
apoptosis under ischemic and hypoxic conditions via miR-486-5p
targeting the PTEN/PI3K/AKT signaling pathway. Thromb Res.
177:23–32. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Q, Liu Y, Ding X, Li Q, Qiu F, Wang
M, Shen Z, Zheng H and Fu G: Bone marrow mesenchymal stem
cell-secreted exosomes carrying microRNA-125b protect against
myocardial ischemia reperfusion injury via targeting SIRT7. Mol
Cell Biochem. 465:103–114. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cui H, He Y, Chen S, Zhang D, Yu Y and Fan
C: Macrophage-derived miRNA-containing exosomes induce
peritendinous fibrosis after tendon injury through the
miR-21-5p/Smad7 pathway. Mol Ther Nucleic Acids. 14:114–130. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiong W, Qu Y, Chen H and Qian J: Insight
into long noncoding RNA-miRNA-mRNA axes in myocardial
ischemia-reperfusion injury: The implications for mechanism and
therapy. Epigenomics. 11:1733–1748. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng D, Li Z, Wei X, Liu R, Shen A, He D,
Tang C and Wu Z: Role of miR-148a in mitigating hepatic
ischemia-reperfusion injury by repressing the TLR4 signaling
pathway via targeting CaMKIIα in vivo and in vitro. Cell Physiol
Biochem. 49:2060–2072. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang X, Zhang X, Ren XP, Chen J, Liu H,
Yang J, Medvedovic M, Hu Z and Fan GC: MicroRNA-494 targeting both
proapoptotic and antiapoptotic proteins protects against
ischemia/reperfusion-induced cardiac injury. Circulation.
122:1308–1318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu JB, Ye XH, Xian SX and Dong MG:
Expressions of SERCA2a and miR-25-3p/5p in myocardium of rats with
heart failure and therapeutic effects of Xiefei Lishui recipe.
Zhongguo Ying Yong Sheng Li Xue Za Zhi. 33:146–150. 2017.(In
Chinese). PubMed/NCBI
|
|
39
|
Wu D, Kang L, Tian J, Wu Y, Liu J, Li Z,
Wu X, Huang Y, Gao B, Wang H, et al: Exosomes derived from bone
mesenchymal stem cells with the stimulation of
Fe3O4 nanoparticles and static magnetic field
enhance wound healing through upregulated miR-21-5p. Int J
Nanomedicine. 15:7979–7993. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang Z, Shi J, Xie J, Wang Y, Sun J, Liu
T, Zhao Y, Zhao X, Wang X, Ma Y, et al: Large-scale generation of
functional mRNA-encapsulating exosomes via cellular nanoporation.
Nat Biomed Eng. 4:69–83. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Khan AA, Man F, Faruqu FN, Kim J,
Al-Salemee F, Carrascal-Miniño A, Volpe A, Liam-Or R, Simpson P,
Fruhwirth GO, et al: PET imaging of small extracellular vesicles
via [89Zr]Zr(oxinate)4 direct radiolabeling.
Bioconjug Chem. 33:473–485. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim CJ, Kuczler MD, Dong L, Kim J, Amend
SR, Cho YK and Pienta KJ: Extracellular vesicle uptake assay via
confocal microscope imaging analysis. J Vis Exp. 2022.
|
|
43
|
Chen G, Wang M, Ruan Z, Zhu L and Tang C:
Mesenchymal stem cell-derived exosomal miR-143-3p suppresses
myocardial ischemia-reperfusion injury by regulating autophagy.
Life Sci. 280:1197422021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen X, Li X, Zhang W, He J, Xu B, Lei B,
Wang Z, Cates C, Rousselle T and Li J: Activation of AMPK inhibits
inflammatory response during hypoxia and reoxygenation through
modulating JNK-mediated NF-κB pathway. Metabolism. 83:256–270.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Du J, Li H, Song J, Wang T, Dong Y, Zhan
A, Li Y and Liang G: AMPK activation alleviates myocardial
ischemia-reperfusion injury by regulating Drp1-mediated
mitochondrial dynamics. Front Pharmacol. 13:8622042022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee SM, Son KN, Shah D, Ali M,
Balasubramaniam A, Shukla D and Aakalu VK: Histatin-1 attenuates
LPS-induced inflammatory signaling in RAW264.7 macrophages. Int J
Mol Sci. 22:78562021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bush EW and van Rooij E: miR-25 in heart
failure. Circ Res. 115:610–612. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Peng Y, Zhao JL, Peng ZY, Xu WF and Yu GL:
Exosomal miR-25-3p from mesenchymal stem cells alleviates
myocardial infarction by targeting pro-apoptotic proteins and EZH2.
Cell Death Dis. 11:3172020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yunna C, Mengru H, Lei W and Weidong C:
Macrophage M1/M2 polarization. Eur J Pharmacol. 877:1730902020.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong
MH, Yoon SH, Kim YS and Ahn Y: Mesenchymal stem cells reciprocally
regulate the M1/M2 balance in mouse bone marrow-derived
macrophages. Exp Mol Med. 46:e702014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yu L, Zhang Y, Chen Q, He Y, Zhou H, Wan H
and Yang J: Formononetin protects against inflammation associated
with cerebral ischemia-reperfusion injury in rats by targeting the
JAK2/STAT3 signaling pathway. Biomed Pharmacother. 149:1128362022.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Song M, Cui X, Zhang J, Li Y, Li J, Zang
Y, Li Q, Yang Q, Chen Y, Cai W, et al: Shenlian extract attenuates
myocardial ischaemia-reperfusion injury via inhibiting M1
macrophage polarization by silencing miR-155. Pharm Biol.
60:2011–2024. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Vicencio JM, Yellon DM, Sivaraman V, Das
D, Boi-Doku C, Arjun S, Zheng Y, Riquelme JA, Kearney J, Sharma V,
et al: Plasma exosomes protect the myocardium from
ischemia-reperfusion injury. J Am Coll Cardiol. 65:1525–1536. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fu X, Liu G, Halim A, Ju Y, Luo Q and Song
AG: Mesenchymal stem cell migration and tissue repair. Cells.
8:7842019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang Z, He C, He J, Chu J, Liu H and Deng
X: Curcumin-mediated bone marrow mesenchymal stem cell sheets
create a favorable immune microenvironment for adult full-thickness
cutaneous wound healing. Stem Cell Res Ther. 9:212018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhao D, Bu Y, Shao H, Wang J, Li W and Li
Q: Protective effect of exosomes derived from bone marrow
mesenchymal stem cells on hypoxia reperfusion injury of
cardiomyocytes. Cell Mol Biol (Noisy-le-grand). 70:73–80. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Feng Y, Bao X, Zhao J, Kang L, Sun X and
Xu B: MSC-derived exosomes mitigate myocardial ischemia/reperfusion
injury by reducing neutrophil infiltration and the formation of
neutrophil extracellular traps. Int J Nanomedicine. 19:2071–2090.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wei Z, Qiao S, Zhao J, Liu Y, Li Q, Wei Z,
Dai Q, Kang L and Xu B: miRNA-181a over-expression in mesenchymal
stem cell-derived exosomes influenced inflammatory response after
myocardial ischemia-reperfusion injury. Life Sci. 232:1166322019.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Davidson SM, Riquelme JA, Takov K,
Vicencio JM, Boi-Doku C, Khoo V, Doreth C, Radenkovic D, Lavandero
S and Yellon DM: Cardioprotection mediated by exosomes is impaired
in the setting of type II diabetes but can be rescued by the use of
non-diabetic exosomes in vitro. J Cell Mol Med. 22:141–151. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chang D, Fan T, Gao S, Jin Y, Zhang M and
Ono M: Application of mesenchymal stem cell sheet to treatment of
ischemic heart disease. Stem Cell Res Ther. 12:3842021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li H, Lin W, Zhang G, Liu R, Qu M, Zhang J
and Xing X: BMSC-exosomes miR-25-3p regulates the p53 signaling
pathway through PTEN to inhibit cell apoptosis and ameliorate liver
ischemia-reperfusion injury. Stem Cell Rev Rep. 19:2820–2836. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kim MJ, Lim SG, Cho DH, Lee JY, Suk K and
Lee WH: Regulation of inflammatory response by LINC00346 via
miR-25-3p-mediated modulation of the PTEN/PI3K/AKT/NF-κB pathway.
Biochem Biophys Res Commun. 709:1498282024. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Gao S, Li L, Li L, Ni J, Guo R, Mao J and
Fan G: Effects of the combination of tanshinone IIA and puerarin on
cardiac function and inflammatory response in myocardial ischemia
mice. J Mol Cell Cardiol. 137:59–70. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liu S, Chen J, Shi J, Zhou W, Wang L, Fang
W, Zhong Y, Chen X, Chen Y, Sabri A and Liu S: M1-like
macrophage-derived exosomes suppress angiogenesis and exacerbate
cardiac dysfunction in a myocardial infarction microenvironment.
Basic Res Cardiol. 115:222020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lafuse WP, Wozniak DJ and Rajaram MVS:
Role of cardiac macrophages on cardiac inflammation, fibrosis and
tissue repair. Cells. 10:512020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mack M: Inflammation and fibrosis. Matrix
Biol. 68–69. 106–121. 2018.PubMed/NCBI
|
|
67
|
Wynn TA and Vannella KM: Macrophages in
tissue repair, regeneration, and fibrosis. Immunity. 44:450–462.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Alvarez-Argote S and O'Meara CC: The
evolving roles of cardiac macrophages in homeostasis, regeneration,
and repair. Int J Mol Sci. 22:79232021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Choi JW, Kwon MJ, Kim IH, Kim YM, Lee MK
and Nam TJ: Pyropia yezoensis glycoprotein promotes the M1 to M2
macrophage phenotypic switch via the STAT3 and STAT6 transcription
factors. Int J Mol Med. 38:666–674. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Alam S, Liu Q, Liu S, Liu Y, Zhang Y, Yang
X, Liu G, Fan K and Ma J: Up-regulated cathepsin C induces
macrophage M1 polarization through FAK-triggered p38 MAPK/NF-κB
pathway. Exp Cell Res. 382:1114722019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhai Q, Chen X, Fei D, Guo X, He X, Zhao
W, Shi S, Gooding JJ, Jin F, Jin Y and Li B: Nanorepairers rescue
inflammation-induced mitochondrial dysfunction in mesenchymal stem
cells. Adv Sci (Weinh). 9:e21038392022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Mascareno E, El-Shafei M, Maulik N, Sato
M, Guo Y, Das DK and Siddiqui MA: JAK/STAT signaling is associated
with cardiac dysfunction during ischemia and reperfusion.
Circulation. 104:325–329. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang WY, Zhang QL and Xu MJ: Effects of
propofol on myocardial ischemia reperfusion injury through
inhibiting the JAK/STAT pathway. Eur Rev Med Pharmacol Sci.
23:6339–6345. 2019.PubMed/NCBI
|
|
74
|
Chen Y, Wu J, Zhu J, Yang G, Tian J, Zhao
Y and Wang Y: Artesunate provides neuroprotection against cerebral
ischemia-reperfusion injury via the TLR-4/NF-κB pathway in rats.
Biol Pharm Bull. 44:350–356. 2021. View Article : Google Scholar : PubMed/NCBI
|