Introduction
Osteoarthritis (OA) is a type of chronic joint
disease characterized by degenerative degeneration of articular
cartilage (1,2). Chondrocytes are the only cells found
in articular cartilage and play a key role in maintaining the
stability of cartilage tissues (3,4). The
apoptosis, inflammation, extracellular matrix (ECM) degradation and
oxidative stress of chondrocytes contribute to the development of
articular cartilage disease (5,6).
Interleukin-1β (IL-1β), a major pro-inflammatory factor, is
associated with the pathogenesis of OA and is often used to
simulate the OA cell model in vitro (7,8).
Therefore, exploring the molecular mechanisms regulating
IL-1β-induced chondrocyte injury is helpful to provide potential
targets for OA treatment.
The ADAMTS5 gene encodes a protein belonging to the
ADAMTS protein family, which acts as an aggrecanase to cleave
aggrecan, thereby mediating cartilage injury in OA (9,10).
Furthermore, studies have shown that ADAMTS5 is a major aggrecanase
leading to cartilage degradation in the development of OA, which is
positively associated with the degree of articular cartilage
degradation (11,12). Notably, ADAMTS5 had been confirmed
to promote ECM degradation, inflammation and apoptosis in
IL-1β-induced chondrocytes (13–15).
Therefore, ADAMTS5 is a key regulator of the OA process, and its
molecular mechanisms are worth further investigation. The
Wnt/β-catenin pathway plays a vital function in chondrogenesis and
has been confirmed to be related to the occurrence of OA (16,17).
However, it is unclear whether ADAMTS5 mediates OA progression by
regulating the Wnt/β-catenin pathway.
The transcription factor, specific protein 1 (SP1)
belongs to the specificity protein/Kruppel-like factors family and
is a sequence-specific DNA-binding protein that regulates the
transcription process of gene promoters (18). SP1 has been found to be involved in
numerous pathological processes and plays an important role in cell
growth and tumorigenesis (19,20).
For example, it has been reported that SP1 accelerates apoptosis,
ECM degradation and inflammation in IL-1β-induced chondrocytes
(21). Furthermore, a previous
study indicated that SP1 could bind to the acyl-CoA synthetase
long-chain family member 4 (Acsl4) promoter region to increase
Acsl4 expression, thereby accelerating IL-1β-induced chondrocyte
oxidative stress and ferroptosis (22). It was hypothesized that SP1 binds
to the ADAMTS5 promoter region through bioinformatics prediction
(Jaspar software), but whether SP1 regulates the transcription of
ADAMTS5 to mediate OA process is unclear.
Therefore, the present study investigated the
hypothesis that SP1 might mediate ADAMTS5 transcription, thereby
regulating IL-1β-induced chondrocyte injury via the Wnt/β-catenin
pathway.
Materials and methods
Cell culture, treatment and
transfection
Human chondrocytes (CHON-001; American Type Culture
Collection) were cultured in DMEM containing 0.1 mg/ml geneticin
(G-418; Gibco; Thermo Fisher Scientific, Inc.) and 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C with 5% CO2.
CHON-001 cells were stimulated with different concentrations (0, 5,
10 and 15 ng/ml) of IL-1β at 37°C for 24 h to establish the OA cell
model. For transfection, cells were transfected with 50 nM ADAMTS5
siRNA [si-ADAMTS5, forward (F) 5′-AAAAUGUUUGGAUUCGUGCUC-3′; reverse
(R) 5′-GCACGAAUCCAAACAUUUUCC-3′], 50 nM SP1 siRNA (si-SP1, F
5′-ACUUGAUACUGAAUAUUAGGC-3′; R 5′-CUAAUAUUCAGUAUCAAGUAA-3′), 4.0 µg
pcDNA3.1 ADAMTS5 overexpression vector (F
5′-AAAGGGGAGAATCTGCCTGC-3′; R 5′-CCAAGATCCCCAGTTGCCAT-3′), 4.0 µg
pcDNA3.1 SP1 overexpression vector (F 5′-GTCCGCCCTCTGACCAAG-3′; R
5′-AAGGCACCACCACCATTACC-3′) or negative controls (si-NC, F
5′-GGAGUAGGGAGCAAACCUAUAGGAA-3′, R 5′-UUCCUAUAGGUUUGCUCCCUACUCC-3′;
pcDNA3.1, F 5′-CTAGAGAACCCACTGCTTAC-3′, R 5′-TAGAAGGCACAGTCGAGG-3′)
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 24 h. All siRNAs and pcDNAs were
purchased from Guangzhou RiboBio Co., Ltd. After 24 h
post-transfection, cells were treated with 10 ng/ml IL-1β for 24 h
at 37°C. To explore the role of Wnt/β-catenin pathway in the
regulation of ADAMTS5 on IL-1β-induced CHON-001 cell injury,
CHON-001 cells were transfected with 50 nM si-NC/si-ADAMTS5 for 24
h and treated with 10 ng/ml IL-1β and 40 µM Wnt/β-catenin pathway
agonist SKL2001 (MedChemExpress, Inc.) for 24 h at 37°C.
Isolation of primary chondrocytes
Fresh articular cartilages were collected from 6
patients (aged 36–49 years; male/female, 3/3) with femoral neck
fractures (underwent total hip replacement surgery) at Xiangyang
NO.1 People's Hospital, Hubei University of Medicine (Xiangyang,
China) between August 2020 and March 2021. All patients provided
written informed consent at the time of the present study and
agreed to the use of their samples in scientific research.
Cartilages were cut into 1-mm3 slices and were digested
with 4 mg/ml protease and 0.25 mg/ml collagenase P. After
centrifugation (2,000 × g for 5 min at room temperature), cell
pellets were cultured in DMEM/F12 medium (Gibco; Thermo Fisher
Scientific, Inc.) containing 5% FBS and 1% penicillin/streptomycin
at 37°C with 5% CO2. To further confirm that the
SP1/ADAMTS5 axis regulated IL-1β-induced chondrocyte injury,
primary chondrocytes were transfected with
si-NC/si-SP1/pcDNA/ADAMTS5 and then treated with 10 ng/ml IL-1β for
24 h at 37°C. The conditions for these transfections in primary
chondrocytes were the same as the transfections with CHON-001
cells. The present study was approved by The Ethics Committee of
Xiangyang NO.1 People's Hospital, Hubei University of Medicine
(Xiangyang, China; approval no. XYYYE20200082).
MTT assay
Cells were inoculated into 96-well plates (2,000
cells/well) and cultured for 24 h at 37°C. After which, cells were
treated with MTT solution (Beyotime Institute of Biotechnology) for
4 h. Following MTT incubation, the formazan was dissolved using
formazan dissolving solution (Beyotime Institute of Biotechnology).
The absorbance was measured at 570 nm to detect cell viability.
EdU assay
CHON-001 cells in 96-well plates were stained with
EdU solution and DAPI solution using the EdU Image Kit (Guangzhou
RiboBio Co., Ltd.), according to the manufacturer's instructions.
Images were captured under a fluorescent microscope (magnification,
200×) to measure the EdU-positive cell rate using ImageJ software
(version 1.8.0; National Institutes of Health).
Flow cytometry
CHON-001 cells and primary chondrocytes
(1×104 cells) were suspended with binding buffer and
then stained with Annexin V-FITC and PI using the Annexin V-FITC
Apoptosis Detection Kit (Beyotime Institute of Biotechnology). The
apoptosis rate of cells was analyzed by flow cytometry
(FACScalibur; BD Biosciences) with CellQuest Pro software (version
3.3, BD Biosciences).
Western blotting
Protein samples were collected from CHON-001 cells
and primary chondrocytes using RIPA lysis buffer (Beyotime
Institute of Biotechnology) and quantified by BCA Kit (Beyotime
Institute of Biotechnology). The protein samples (30 µg) were
separated by 10% SDS-PAGE gel and then imprinted on a PVDF
membrane. The membrane was blocked with 5% skimmed milk at 4°C for
2 h and incubated with the following primary antibodies (all
Abcam): Anti-Aggrecan (1:1,000; cat. no. ab36861), anti-Collagen II
(1:1,000; cat. no. ab34712), anti-ADAMTS5 (1:250; cat. no.
ab41037), anti-SP1 (1:10,000; cat. no. ab227383), anti-Wnt3a
(1:1,000; cat. no. ab28472), anti-β-catenin (1:4,000; cat. no.
ab16051) and anti-GAPDH (1:2,500; cat. no. ab9485) at 4°C
overnight. After which, the membranes was incubated with the
secondary antibody at room temperature for 1 h, goat anti-rabbit
IgG (1:50,000; cat. no. ab205718; Abcam). Protein signals were
detected by ECL reagent and analyzed by ImageJ software (version
1.8.0, National Institutes of Health).
ELISA
The supernatant of CHON-001 cells and primary
chondrocytes was collected to assess the concentrations of IL-6 and
TNF-α using the Human IL-6 ELISA Kit (cat. no PI325; Beyotime
Institute of Biotechnology) and Human TNF-α ELISA Kit (cat. no.
PT518; Beyotime Institute of Biotechnology), respectively,
according to the manufacturer's instructions.
Detection of oxidative stress
Superoxide dismutase (SOD) activity and
malondialdehyde (MDA) levels in cells were determined using the SOD
Assay Kit (Beyotime Institute of Biotechnology) and MDA Assay Kit
(Beyotime Institute of Biotechnology), according to the
manufacturer's instructions.
Reverse transcription-quantitative PCR
(RT-qPCR)
A TRIzol® kit (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA from CHON-001
cells, and after which, cDNA was generated using a reverse
transcription kit (Takara Bio, Inc.) following the conditions: 6
cycles of 37°C for 15 min, 85°C for 5 sec and 7 cycles of 4°C. qPCR
was performed using the SYBR Premix Ex Taq Kit (Takara Bio, Inc.)
following thermocycling conditions: 95°C for 1 min, 40 cycles of
95°C for 15 sec, 55°C for 30 sec and 72°C for 30 sec. The
2−∆∆Cq method (23) was
used for calculating the relative expression of ADAMTS5 with GAPDH
as the internal control. The primer sequences are listed in
Table I.
 | Table I.Primer sequences used for
RT-qPCR. |
Table I.
Primer sequences used for
RT-qPCR.
| Gene | Direction | Sequence
(5′-3′) |
|---|
| SP1 | Forward |
GTCCGCCCTCTGACCAAG |
|
| Reverse |
AAGGCACCACCACCATTACC |
| ADAMTS5 | Forward |
AAAGGGGAGAATCTGCCTGC |
|
| Reverse |
CCAAGATCCCCAGTTGCCAT |
| GAPDH | Forward |
AAGGCTGTGGGCAAGGTCATC |
|
| Reverse |
GCGTCAAAGGTGGAGGAGTGG |
Chromatin immunocoprecipitate (ChIP)
assay
The binding sites of SP1 in the ADAMTS5 promoter
region were predicted using the Jaspar tools (version 2024;
http://jaspar.elixir.no/). CHON-001 cells were
crosslinked with formaldehyde, and then the cell lysates were
ultrasound treated (10 cycles of 25 sec on and 30 sec off;
centrifuged 10,000 × g for 10 min at 4°C) to obtain DNA fragments.
Next, the cell lysate was incubated with anti-SP1 (1:200; cat. no.
ab227383; Abcam) or anti-IgG (1:1,000; cat. no. ab171870; Abcam) at
4°C overnight. The precipitated DNA was collected by protein
A/G-agarose beads and then used for RT-qPCR. The conditions were
the same as the aforementioned RT-qPCR.
Dual-luciferase reporter assay
The wild-type (WT) and mutant-type (MUT1, MUT3 and
MUT1+3) promoter fragments of ADAMTS5 were cloned into the
pGL3-basic vector. The 293T cells (American Type Culture
Collection) were co-transfected with the pcDNA3.1 SP1
overexpression vector and the aforementioned vectors (WT, MUT1,
MUT3 and MUT1+3) using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.), and the relative luciferase
activity (Firefly luciferase/Renilla luciferase) was detected by
Dual-Lucy Detection Assay Kit (Beyotime Institute of Biotechnology)
after 48 h.
Statistical analysis
Results are presented as the mean ± SEM. All
experiments were performed in triplicate (n=3), with each
independent experiment set 3 times to generate an average value.
Unpaired student's t-test (for 2 groups) or one-way ANOVA (for
multiple groups) followed by Tukey's post-hoc test was used to
perform statistical comparisons using GraphPad Prism 7.0 software
(Dotmatics). P<0.05 was considered to indicate a statistically
significant difference.
Results
IL-1β induces CHON-001 chondrocyte
injury
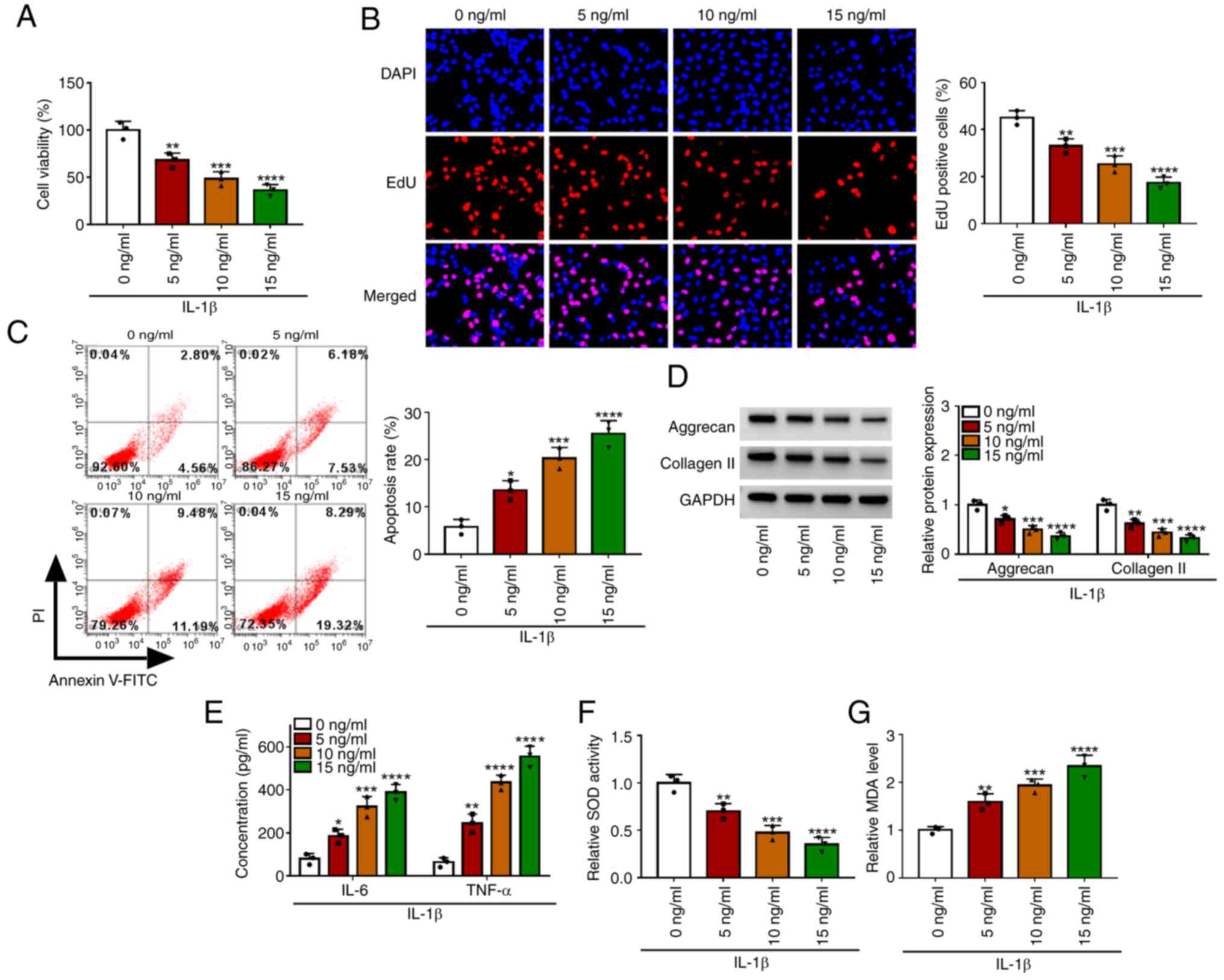
To determine the effect of IL-1β on chondrocyte
injury, CHON-001 cells were treated with different concentrations
of IL-1β. The results showed that with the increase of IL-1β
concentration, cell viability and EdU-positive cell rate were
significantly decreased, while cell apoptosis rate was markedly
increased (Fig. 1A-C). In
addition, with increasing concentrations of IL-1β, the expression
of matrix proteins (aggrecan and collagen II) and SOD activity were
reduced, while the concentrations of inflammatory factors (IL-6 and
TNF-α) and MDA levels were enhanced in a concentration-dependent
manner (Fig. 1D-G). The
aforementioned data showed that IL-1β could promote apoptosis, ECM
degradation, inflammation and oxidative stress to induce
chondrocyte injury. In the follow-up function tests, 10 ng/ml IL-1β
was used to induce the OA in vitro model.
Knockdown of ADAMTS5 alleviates
IL-1β-induced chondrocyte injury
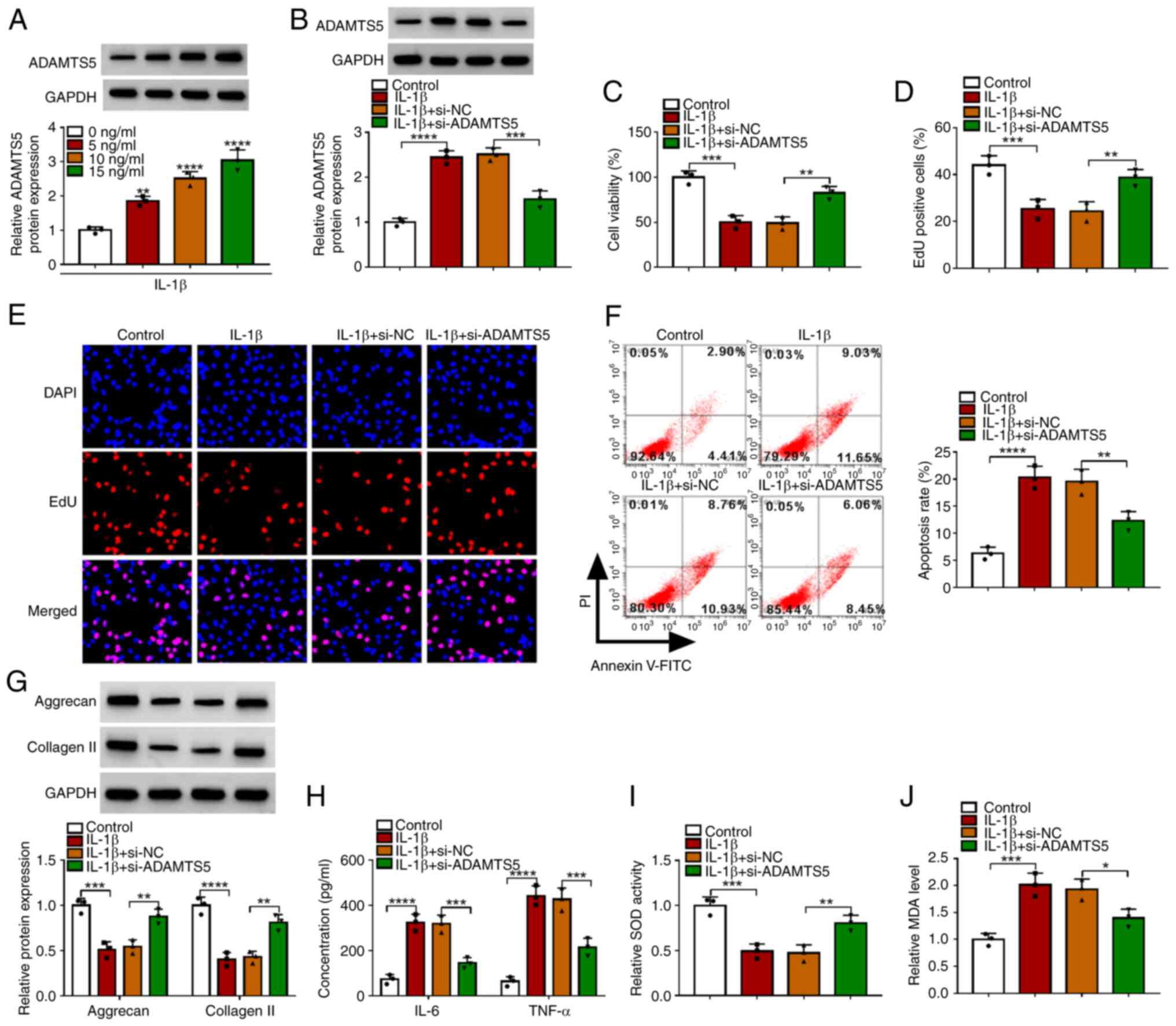
ADAMTS5 expression was detected through western
blotting and it was confirmed that ADAMTS5 was gradually enhanced
with increasing IL-1β concentration in CHON-001 cells at the
protein level (Fig. 2A). To
confirm the role of ADAMTS5 in OA progression, the effect of
ADAMTS5 knockdown on IL-1β-induced chondrocyte injury was detected.
Firstly, the transfection of si-ADAMTS5 was used to reduce ADAMTS5
protein expression in CHON-001 cells (Fig. S1A). The detection of ADAMTS5
expression showed that the promoting effect of IL-1β on ADAMTS5
protein expression was significantly reduced by si-ADAMTS5
(Fig. 2B). ADAMTS5 knockdown
enhanced the viability and EdU-positive cell rate of IL-1β-induced
CHON-001 cells (Fig. 2C-E).
Furthermore, the promoting effect of IL-1β on the apoptosis rate of
CHON-001 cells also could be suppressed by ADAMTS5 silencing
(Fig. 2F). In addition,
downregulation of ADAMTS5 increased the levels of matrix protein
(aggrecan and collagen II) and SOD activity, while decreased the
concentrations of inflammatory factors (IL-6 and TNF-α) and MDA
levels in IL-1β-induced CHON-001 cells (Fig. 2G-J). These data suggested that
ADAMTS5 knockdown relieved IL-1β-induced chondrocyte apoptosis, ECM
degradation, inflammation and oxidative stress, confirming that
ADAMTS5 might promote IL-1β-induced chondrocyte injury to
accelerate OA progression.
SP1 activates the transcription of
ADAMTS5
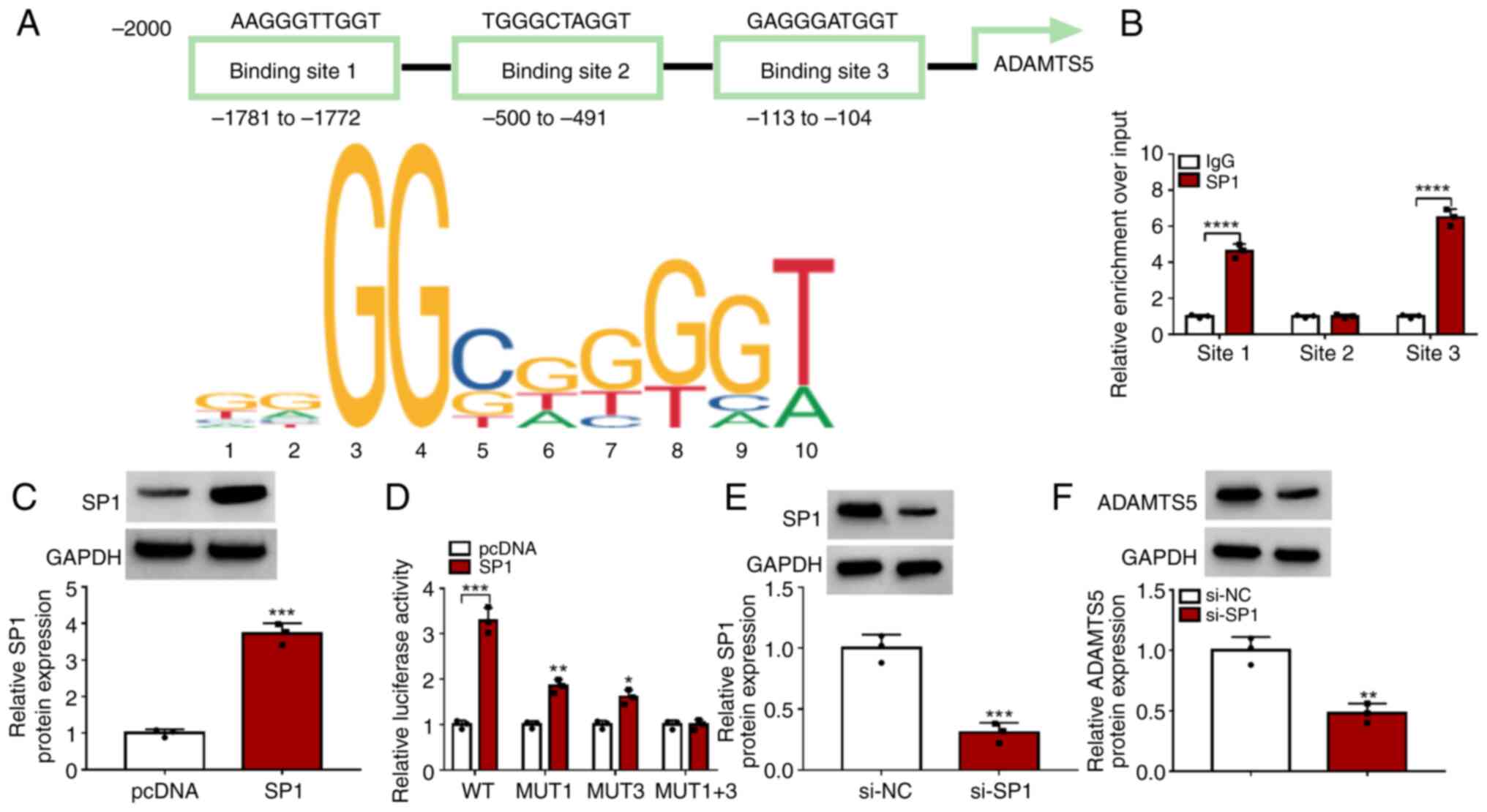
The Jaspar software predicted that there were three
binding sites between the transcription factor SP1 and ADAMTS5
promoter region (Fig. 3A). ChIP
assay results showed that SP1 could bind with the promoter sites 1
and 3 of ADAMTS5 (Fig. 3B). After
which, the WT, MUT1, MUT3 and MUT1+3 sequences for promoter site 1
and 3 of ADAMTS5 were constructed, and the SP1 overexpression
vector was used to enhance SP1 protein expression in 293T cells
(Fig. 3C). Dual-luciferase
reporter assay results revealed that SP1 overexpression enhanced
the luciferase activity of MUT1 and MUT3 and especially WT vectors,
but did not affect the MUT1+3 vector (Fig. 3D). These data confirmed that
transcription factor SP1 binds to the promoter region of ADAMTS5.
Additionally, si-SP1 was used to reduce SP1 expression in
chondrocytes (Fig. 3E). By
detecting ADAMTS5 protein levels, it was discovered that SP1
knockdown markedly reduced ADAMTS5 expression (Fig. 3F). These data indicated that the
transcription factor SP1 increased ADAMTS5 expression by binding to
its promoter region to activate its transcription.
Overexpression of ADAMTS5 reverses the
inhibitory effect of SP1 knockdown on IL-1β-induced chondrocyte
injury
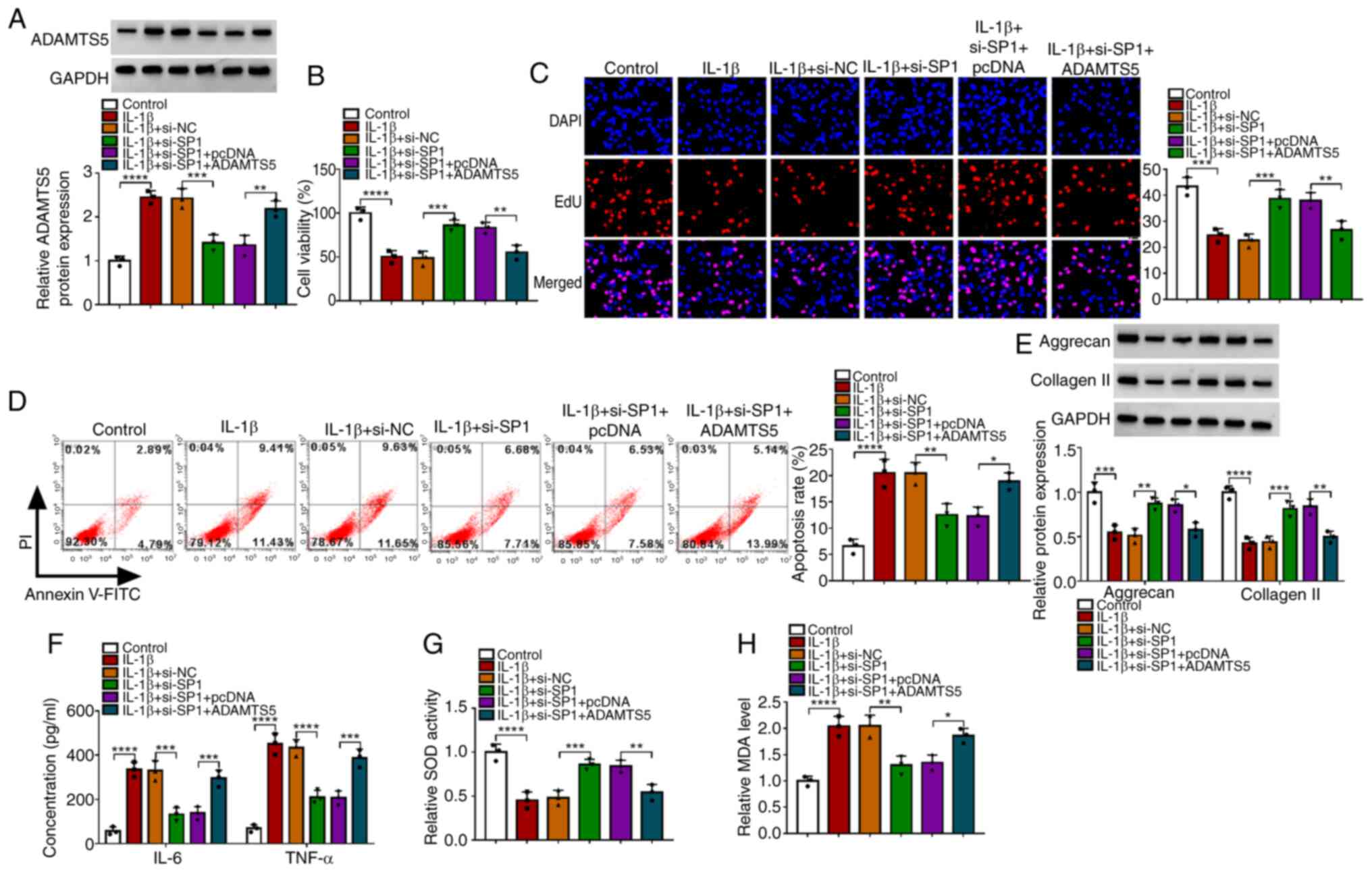
The ADAMTS5 overexpression vector was used to
enhance ADAMTS5 expression in CHON-001 cells (Fig. S1B). To explore whether SP1
regulated ADAMTS5 to mediate OA progression, IL-1β-induced CHON-001
cells were co-transfected with si-SP1 and the ADAMTS5
overexpression vector. The detection of ADAMTS5 protein expression
suggested that si-SP1 reduced ADAMTS5 expression in IL-1β-induced
CHON-001 cells, and the transfection of ADAMTS5 overexpression
vector enhanced ADAMTS5 expression (Fig. 4A). Functional experiments revealed
that SP1 knockdown promoted viability, enhanced EdU-positive cell
rate and repressed the apoptosis rate in IL-1β-induced CHON-001
cells, while these effects could be reversed by ADAMTS5
overexpression (Fig. 4B-D).
Furthermore, SP1 silencing also elevated the levels of matrix
proteins (aggrecan and collagen II), reduced the concentrations of
inflammatory factors (IL-6 and TNF-α), increased SOD activity and
decreased MDA levels in IL-1β-induced CHON-001 cells, while ADAMTS5
overexpression also abolished these effects (Fig. 4E-H). To further confirm this, the
related study in IL-1β-induced primary chondrocytes was performed.
The transfections of si-SP1 and the ADAMTS5 overexpression vector
were used to decrease SP1 protein expression and increase ADAMTS5
protein expression in primary chondrocytes, respectively (Fig. S1C and D). si-SP1 reduced ADAMTS5
protein level in IL-1β-induced primary chondrocytes, while ADAMTS5
overexpression eliminated this effect (Fig. S2A). Furthermore, the knockdown of
SP1 increased cell viability and SOD activity, while it reduced the
apoptosis rate, and IL-6, TNF-α and MDA levels in IL-1β-induced
primary chondrocytes. However, these results also were reversed by
ADAMTS5 overexpression (Fig.
S2B-G). Notably, these results confirmed that SP1 knockdown
alleviated IL-1β-induced chondrocyte injury by decreasing ADAMTS5
expression.
SP1/ADAMTS5 axis promotes the activity
of the Wnt/β-catenin pathway
The effect of SP1 and ADAMTS5 on the activity of
Wnt/β-catenin pathway was explored by measuring the protein
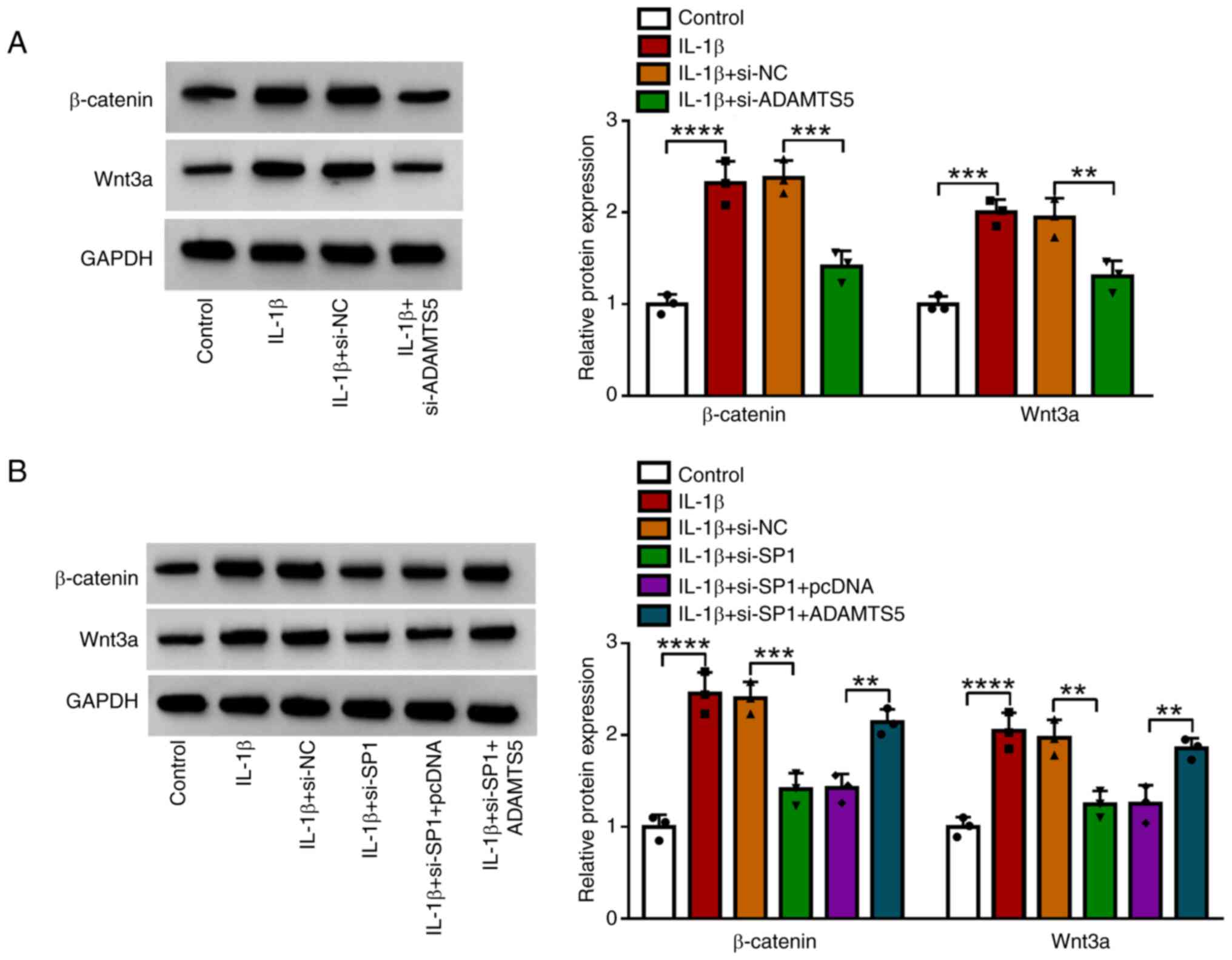
expression of β-catenin and Wnt3a. The data showed that IL-1β
treatment increased the protein expression of β-catenin and Wnt3a,
and ADAMTS5 knockdown suppressed these levels in IL-1β-induced
CHON-001 cells (Fig. 5A).
Furthermore, SP1 knockdown reduced the protein expression of
β-catenin and Wnt3a in IL-1β-induced CHON-001 cells, and this
effect was be eliminated by ADAMTS5 overexpression (Fig. 5B). These data showed that SP1 might
regulate ADAMTS5 to promote the activity of the Wnt/β-catenin
pathway.
SKL2001 reverses the
si-ADAMTS5-mediated inhibitory effect on IL-1β-induced chondrocyte
injury
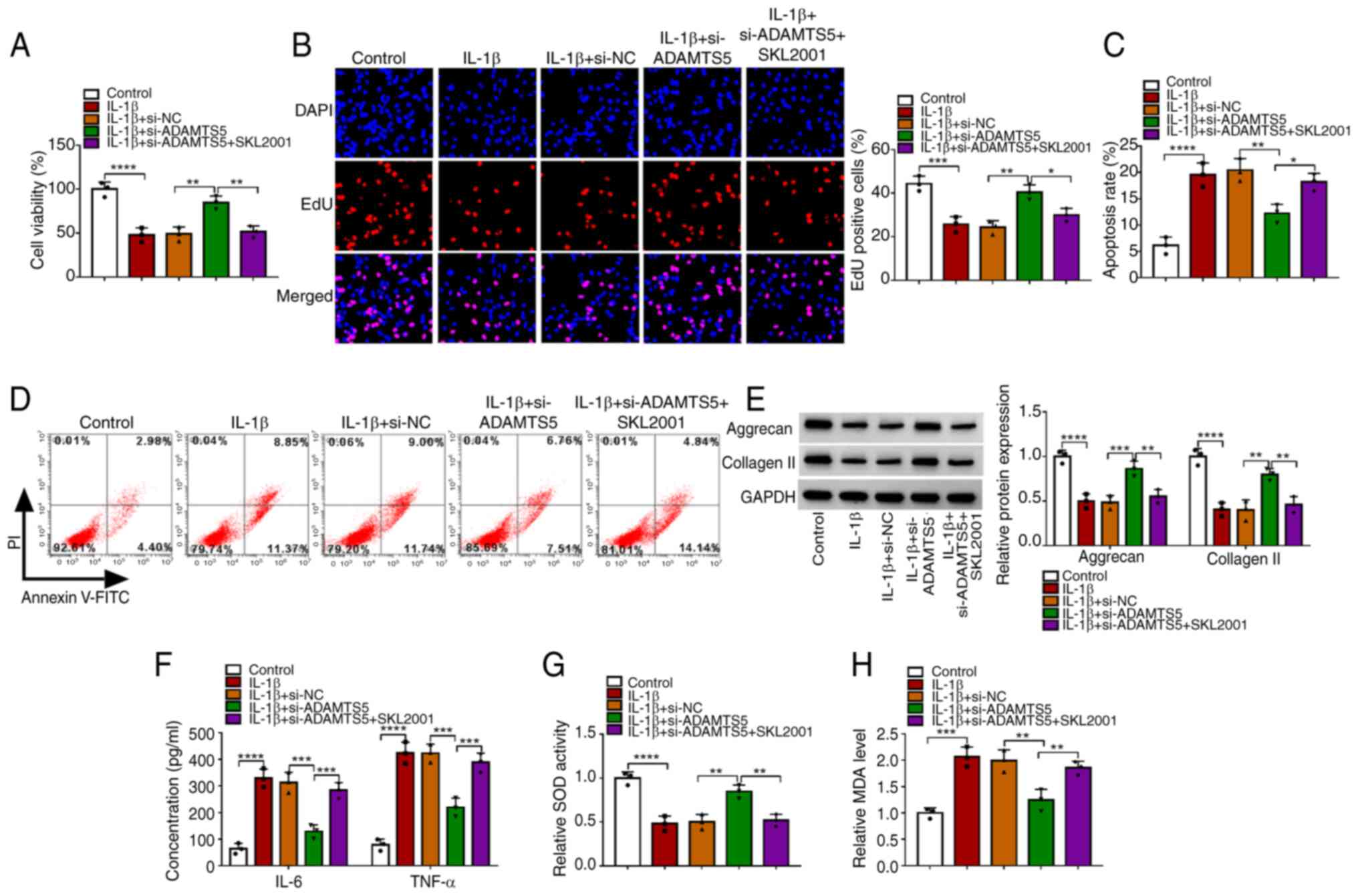
To further confirm the role of the Wnt/β-catenin
pathway in the regulation of ADAMTS5 on IL-1β-induced chondrocyte
injury, IL-1β-induced CHON-001 cells were transfected with
si-ADAMTS5 and treated with the Wnt/β-catenin pathway agonist
SKL2001. The data showed that the promoting effect of si-ADAMTS1 on
the viability and EdU-positive cell rate, as well as the inhibiting
effect on the apoptosis rate of IL-1β-induced CHON-001 cells, could
be abolished by SKL2001 (Fig.
6A-D). Furthermore, SKL2001 treatment also eliminated the
si-ADAMTS5-mediated increasing effect on the levels of matrix
proteins (aggrecan and collagen II) and SOD activity, and the
decreasing effect on the concentrations of inflammatory factors
(IL-6 and TNF-α) and MDA levels in IL-1β-induced CHON-001 cells
(Fig. 6E-H). These data suggested
that ADAMTS5 knockdown alleviated IL-1β-induced chondrocyte injury
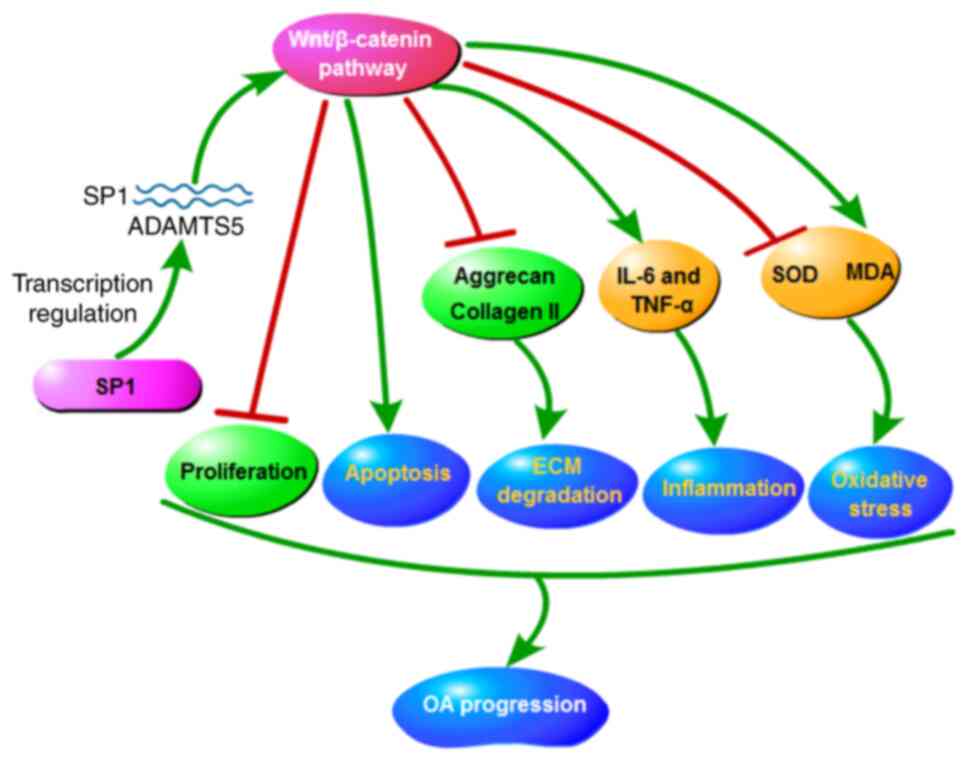
by inhibiting the activity of the Wnt/β-catenin pathway. To
conclude, the data showed that SP1/ADAMTS5 suppressed
proliferation, while accelerated apoptosis, ECM degradation,
inflammation and oxidative stress in IL-1β-induced chondrocytes by
promoting the activity of the Wnt/β-catenin pathway, thereby
facilitating OA progression (Fig.
7).
Discussion
OA is a common disease and can cause joint pain and
stiffness deformity, which heavily impacts human health (24). At present, OA pathogenesis has not
been fully elucidated, but abnormal expression of some
disease-related genes has been confirmed to be related to OA
development (25). Fisch et
al (26) found a total of
1,332 differentially expressed genes between OA and non-OA tissues
samples by RNA sequencing analysis. Furthermore, Huang et al
(27) analyzed the GEO database
(dataset no. GSE48556) and noted that there were 1,696
differentially expressed genes in OA samples compared with normal
tissues. Studies have shown that ADAMTS5 is under expressed in the
normal articular cartilage matrix, but abnormal expression of
ECM-related cytokines in OA has led to increased ADAMTS5
expression, which accelerates ECM degradation and cartilage injury
(28,29). It has been reported that ADAMTS5
overexpression contributes to the decreased viability and promoted
ECM degradation in IL-1β-induced chondrocytes (13). Yu et al (14) reported that ADAMTS5 accelerated
IL-1β-treated CHON-001 cell injury, including inflammation and
apoptosis. Li et al (15)
suggested that ADAMTS5 overexpression enhanced inflammation,
apoptosis and ECM degradation in IL-1β-treated chondrocytes.
Moreover, ADAMTS5 had elevated expression in OA tissues and its
upregulation could induce apoptosis and ECM degradation in
IL-1β-treated chondrocytes (30).
Consistent with previous studies (13–15,30),
the present study used IL-1β to induce chondrocyte apoptosis, ECM
degradation and inflammation, and revealed that ADAMTS5 knockdown
reversed the inducing effect of IL-1β on chondrocyte injury. In
addition, it was also found that ADAMTS5 knockdown inhibited
IL-1β-induced chondrocyte oxidative stress. These findings offer
new evidence for ADAMTS5 as a potential target for OA therapy.
The transcription factor SP1 has been shown to
mediate OA progression through promoting chondrocyte injury
(21,22). Notably, SP1 can regulate the
expression of genes that have GC-rich promoters, thereby mediating
numerous cellular functions, including cell proliferation,
apoptosis and differentiation (31). Kong et al (32) reported that SP1 could upregulate
the promoter activity of the cancer-associated biomarker CD147,
thus mediating lung cancer progression. Furthermore, the
transcription factor SP1 might be an effective target for
inhibiting disc-related ECM loss, and its inhibitor could reduce
ADAMTS5 promoter activity (33).
In the present study, it was found that SP1 could bind to the
promoter region of ADAMTS5 at −1,781 to −1,772 and −113 to −104 bp
to enhance ADAMTS5 expression. Moreover, downregulation of SP1
alleviated IL-1β induced-chondrocyte injury via reducing ADAMTS5
expression, confirming that SP1-mediated ADAMTS5 transcription
regulated OA progression. To the best of our knowledge, the present
study is the first to reveal the interaction between SP1 and
ADAMTS5 in OA progression and indicates that the SP1/ADAMTS5 axis
mediates OA progression by regulating the Wnt/β-catenin
pathway.
Wnt/β-catenin is a unique pathway that regulates the
development of OA and affects OA process by influencing chondrocyte
function (34). Studies have shown
that the Wnt/β-catenin pathway is overactivated in IL-1β-treated
chondrocytes, and inhibition of Wnt/β-catenin activity could
significantly relieve chondrocyte injury (35,36).
In the present study it was confirmed that IL-1β treatment
activated the Wnt/β-catenin pathway, and this effect could be
abolished by the silencing of ADAMTS5 and SP1. In the protection of
chondrocytes mediated by si-ADAMTS5, activation of the
Wnt/β-catenin signal used SKL2001 to alter the inhibitory effect of
si-ADAMTS5 on chondrocyte injury. These results further confirmed
that ADAMTS5 promoted OA progression by mediating Wnt/β-catenin
pathway activity, which provided new evidence for Wnt/β-catenin
pathway-mediated chondrocyte injury. However, the present study
also had some limitations. At present, only the expression of Wnt3a
and β-catenin has been examined, and no data are currently
available on the phosphorylation status of β-catenin. Future
research will continue to explore the specific proteins regulated
by Wnt/β-catenin and reveal its downstream pathways to further
improve the experimental results. In addition, studies were only
carried out at the cellular level, and no animal experiments have
been performed. Future endeavors will consider conducting animal
experiments to further confirm the findings of the present
study.
To conclude, the present study indicated that
ADAMTS5, mediated by the transcription factor SP1, promoted
IL-1β-induced chondrocyte injury via activating the Wnt/β-catenin
pathway. The proposed SP1/ADAMTS5/Wnt/β-catenin axis provides a new
theoretical basis for developing molecular targets of OA
therapy.
Supplementary Material
Supporting Data
Acknowledgεments
Not applicable.
Funding
This work was funded by the Xiangyang Medical and Health Field
Science and Technology Project (grant no. 2022YL31B).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XH collected the data, performed the data analysis
and drafted the manuscript; and XW conceived and designed the
study, drafted the manuscript and supervised the study. Both
authors read approved the final version of the manuscript. XH and
XW confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
This study was approved by The Ethics Committee of
Xiangyang No.1 People's Hospital, Hubei University of Medicine
(Xiangyang, China; approval no. XYYYE20200082).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pereira D, Ramos E and Branco J:
Osteoarthritis. Acta Med Port. 28:99–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abramoff B and Caldera FE: Osteoarthritis:
Pathology, diagnosis, and treatment options. Med Clin North Am.
104:293–311. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schulze-Tanzil G: Activation and
dedifferentiation of chondrocytes: Implications in cartilage injury
and repair. Ann Anat. 191:325–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shen H, He Y, Wang N, Fritch MR, Li X, Lin
H and Tuan RS: Enhancing the potential of aged human articular
chondrocytes for high-quality cartilage regeneration. FASEB J.
35:e214102021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mao H, Han B, Li H, Tao Y and Wu W: FABP4
knockdown suppresses inflammation, apoptosis and extracellular
matrix degradation in IL-1β-induced chondrocytes by activating
PPARγ to regulate the NF-κB signaling pathway. Mol Med Rep.
24:8552021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zahan OM, Serban O, Gherman C and Fodor D:
The evaluation of oxidative stress in osteoarthritis. Med Pharm
Rep. 93:12–22. 2020.PubMed/NCBI
|
|
7
|
Jin J, Lv X, Wang B, Ren C, Jiang J, Chen
H, Chen X, Gu M, Pan Z, Tian N, et al: Limonin Inhibits
IL-1β-induced inflammation and catabolism in chondrocytes and
ameliorates osteoarthritis by activating Nrf2. Oxid Med Cell
Longev. 2021:72925122021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei J, Gao C, Hu K, Li M, Li J, Shen M and
Zhang S: Knockdown of DAPK1 attenuates IL-1β-induced extracellular
matrix degradation and inflammatory response in osteoarthritis
chondrocytes via regulating the p38 MAPK-signaling pathway.
Allergol Immunopathol (Madr). 50:169–175. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mead TJ and Apte SS: ADAMTS proteins in
human disorders. Matrix Biol. 71–72. 225–239. 2018.
|
|
10
|
Li T, Peng J, Li Q, Shu Y, Zhu P and Hao
L: The mechanism and role of ADAMTS protein family in
osteoarthritis. Biomolecules. 12:9592022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chu X, You H, Yuan X, Zhao W, Li W and Guo
X: Protective effect of lentivirus-mediated siRNA targeting
ADAMTS-5 on cartilage degradation in a rat model of osteoarthritis.
Int J Mol Med. 31:1222–1228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Glasson SS, Askew R, Sheppard B, Carito B,
Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, et al:
Deletion of active ADAMTS5 prevents cartilage degradation in a
murine model of osteoarthritis. Nature. 434:644–648. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu C, Ren S, Zhao S and Wang Y: LncRNA
MALAT1/MiR-145 Adjusts IL-1β-induced chondrocytes viability and
cartilage matrix degradation by regulating ADAMTS5 in human
osteoarthritis. Yonsei Med J. 60:1081–1092. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu Z, Cong F, Zhang W, Song T, Zhang S and
Jiang R: Circular RNA circ_0020014 contributes to osteoarthritis
progression via miR-613/ADAMTS5 axis. Bosn J Basic Med Sci.
22:716–727. 2022.PubMed/NCBI
|
|
15
|
Li N, Wang Y and Wu X: Knockdown of
Circ_0037658 alleviates IL-1β-induced osteoarthritis progression by
serving as a sponge of miR-665 to regulate ADAMTS5. Front Genet.
13:8868982022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Y, Wang Y, Jia H, Li B, Xing D and Li
JJ: MicroRNA-1 modulates chondrocyte phenotype by regulating FZD7
of Wnt/β-catenin signaling pathway. Cartilage. 13
(2_Suppl):1019S–1029S. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu L, Luo D, Zhang H and He L: Polydatin
inhibits IL-1β-mediated chondrocyte inflammation and ameliorates
cartilage degradation: Involvement of the NF-κB and Wnt/β-catenin
pathways. Tissue Cell. 78:1018652022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
O'Connor L, Gilmour J and Bonifer C: The
role of the ubiquitously expressed transcription factor Sp1 in
tissue-specific transcriptional regulation and in disease. Yale J
Biol Med. 89:513–525. 2016.PubMed/NCBI
|
|
19
|
Shelake S, Sankpal UT, Paul Bowman W, Wise
M, Ray A and Basha R: Targeting specificity protein 1 transcription
factor and survivin using tolfenamic acid for inhibiting Ewing
sarcoma cell growth. Invest New Drugs. 35:158–165. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wong CF, Barnes LM, Dahler AL, Smith L,
Popa C, Serewko-Auret MM and Saunders NA: E2F suppression and Sp1
overexpression are sufficient to induce the
differentiation-specific marker, transglutaminase type 1, in a
squamous cell carcinoma cell line. Oncogene. 24:3525–3534. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Xie W, Zheng Y, Li H, Wen Z, Wang C,
Chen S and Deng Z: The miR-548d-5p/SP1 signaling axis regulates
chondrocyte proliferation and inflammatory responses in
osteoarthritis. Int Immunopharmacol. 110:1090292022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He W, Lin X and Chen K: Specificity
protein 1-mediated ACSL4 transcription promoted the osteoarthritis
progression through suppressing the ferroptosis of chondrocytes. J
Orthop Surg Res. 18:1882023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barra GB, Santa Rita TH, Almeida ALSC,
Jácomo RH and Nery LFA: Serum Has higher proportion of Janus kinase
2 V617F Mutation compared to paired EDTA-whole blood sample: A
model for somatic mutation quantification using qPCR and the
2−∆∆Cq method. Diagnostics (Basel). 10:1532020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ebell MH: Osteoarthritis: Rapid evidence
review. Am Fam Physician. 97:523–526. 2018.PubMed/NCBI
|
|
25
|
Zhu N, Zhang P, Du L, Hou J and Xu B:
Identification of key genes and expression profiles in
osteoarthritis by co-expressed network analysis. Comput Biol Chem.
85:1072252020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fisch KM, Gamini R, Alvarez-Garcia O,
Akagi R, Saito M, Muramatsu Y, Sasho T, Koziol JA, Su AI and Lotz
MK: Identification of transcription factors responsible for
dysregulated networks in human osteoarthritis cartilage by global
gene expression analysis. Osteoarthritis Cartilage. 26:1531–1538.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang PY, Wu JG, Gu J, Zhang TQ, Li LF,
Wang SQ and Wang M: Bioinformatics analysis of miRNA and mRNA
expression profiles to reveal the key miRNAs and genes in
osteoarthritis. J Orthop Surg Res. 16:632021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang CY, Chanalaris A and Troeberg L:
ADAMTS and ADAM metalloproteinases in osteoarthritis-looking beyond
the ‘usual suspects’. Osteoarthritis Cartilage. 25:1000–1009. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun K, Luo J, Jing X, Xiang W, Guo J, Yao
X, Liang S, Guo F and Xu T: Hyperoside ameliorates the progression
of osteoarthritis: An in vitro and in vivo study. Phytomedicine.
80:1533872021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li G, Luo H, Ding Z, Liang H, Lai Z, Chen
S and Huang Y: Silencing of circ_0000205 mitigates
interleukin-1β-induced apoptosis and extracellular matrix
degradation in chondrocytes via targeting miR-766-3p/ADAMTS5 axis.
Innate Immun. 28:79–90. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaczynski J, Cook T and Urrutia R: Sp1-
and Kruppel-like transcription factors. Genome Biol. 4:2062003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kong LM, Liao CG, Fei F, Guo X, Xing JL
and Chen ZN: Transcription factor Sp1 regulates expression of
cancer- associated molecule CD147 in human lung cancer. Cancer Sci.
101:1463–1470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu K, Wang X, Zhang Q, Liang A, Zhu H,
Huang D, Li C and Ye W: Sp1 downregulates proinflammatory
cytokine-induced catabolic gene expression in nucleus pulposus
cells. Mol Med Rep. 14:3961–3968. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou Y, Wang T, Hamilton JL and Chen D:
Wnt/β-catenin signaling in osteoarthritis and in other forms of
arthritis. Curr Rheumatol Rep. 19:532017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu X, Wang L, Ma C, Wang G, Zhang Y and
Sun S: Exosomes derived from platelet-rich plasma present a novel
potential in alleviating knee osteoarthritis by promoting
proliferation and inhibiting apoptosis of chondrocyte via
Wnt/β-catenin signaling pathway. J Orthop Surg Res. 14:4702019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Z, Yang P, Wang C and Tian R: LncRNA
CRNDE hinders the progression of osteoarthritis by epigenetic
regulation of DACT1. Cell Mol Life Sci. 79:4052022. View Article : Google Scholar : PubMed/NCBI
|