Cardiovascular disease remains the predominant cause
of morbidity and mortality worldwide, accounting for almost
one-third of global mortality (1).
Despite advancements in diagnosis and treatment, effectively
managing the progression of cardiovascular disease and enhancing
patient outcomes in a timely manner continue to present significant
challenges (2). Therefore, the
early prediction and diagnosis of cardiovascular disease are
crucial for the development of effective treatment options.
Previous studies have highlighted the significant
role of chronic inflammation in the progression of cardiovascular
disease (3–5). At present, research is focused on
establishing treatment targets and regulating inflammation to
enhance cardiovascular outcomes (6–8).
Chitinase-3-like protein 1 (CHI3L1) is a pro-inflammatory protein
that plays a role in the development of chronic inflammatory
diseases in multiple systems, including the nervous, digestive and
respiratory systems. CHI3L1 exhibits potential as a biomarker for
various inflammatory diseases (9–11).
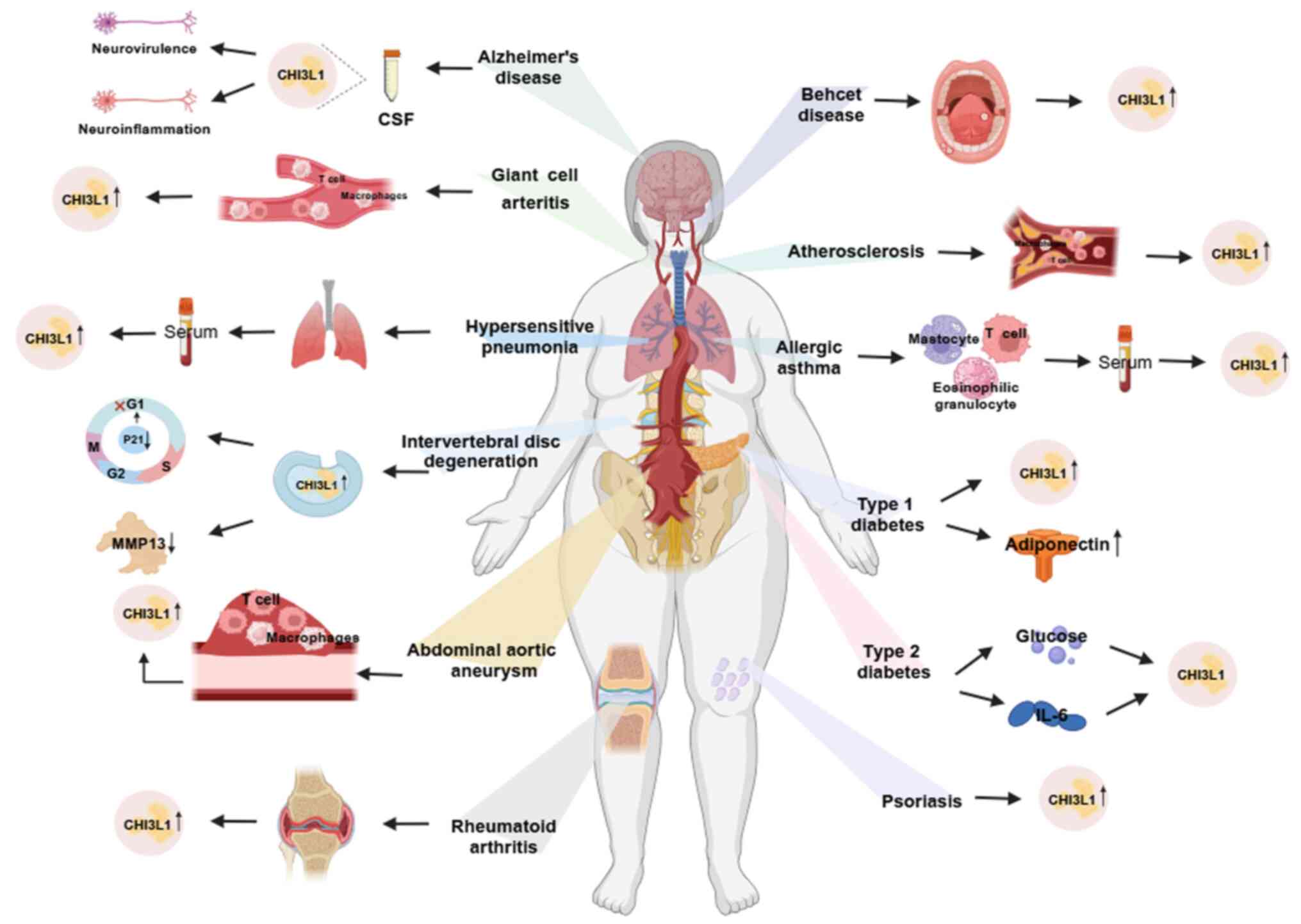
Results of previous studies revealed that CHI3L1 is closely
associated with inflammatory cardiovascular disease, such as
atherosclerosis (AS), highlighting its potential as a predictive
marker for cardiovascular disease (12,13)
(Fig. 1). The present article
systematically reviewed the role of CHI3L1 in the occurrence and
development of cardiovascular disease.
CHI3L1, also known as breast regression protein 39
in mice and YKL-40 in humans, belongs to the glycoside hydrolase 18
family and is categorized as a non-enzymatic chitinase-like
protein. In humans, CHI3L1 is encoded by the CHI3L1 gene located on
chromosomes 1q31-1q32. The gene consists of 7,498 base pairs and 10
exons, with genomic DNA that is ~8 kbp in length (14). The name ‘YKL-40’ reflects the
molecular weight of the protein, at ~40 kDa, and the presence of
the first three amino acids in the N-terminal sequence; namely,
tyrosine (Y), lysine (K) and leucine (L) (15,16).
Crystal diffraction studies revealed that CHI3L1 contains two
distinct domains; namely, a (β/α)8-barrel domain, with a
carbohydrate binding cleft of ~43 amino acids at the end of the β
chain, and a second domain composed of an α helix and six inverted
parallel β strands (17). This
structural analysis suggested that CHI3L1 interacts with heparin
and different cytokines, such as interleukin-13 receptor α2
(IL-13Rα2), CD44 (18). Despite
its ability to bind to chitin, CHI3L1 lacks chitinase activity due
to mutations in two critical catalytic residues, rendering it
incapable of breaking down chitin or any other carbohydrates
(19,20). CHI3L1 is secreted by various cell
types, including macrophages, neutrophils, chondrocytes,
synoviocytes, osteoblasts and smooth muscle cells (SMCs) (15). Although the specific function of
CHI3L1 remains to be elucidated, this protein has been implicated
in various biological processes, including cell proliferation,
tissue remodeling, extracellular matrix (ECM) turnover,
inflammation and fibrosis (21).
CHI3L1 is closely associated with inflammation and
regulates the occurrence of inflammatory responses (22). A previous study using CHI3L1-/-mice
revealed that CHI3L1 promoted the activation and enrichment of
CD4+T cells and macrophages, subsequently regulating the TH2
inflammatory response. In addition, CHI3L1 promotes the production
of the TH2 inflammatory factor, IL-13 (23). In addition, CHI3L1 induced
macrophages to secrete monocyte chemotactic protein-1 (MCP-1),
C-X-C motif chemokine ligand 2 (CXCL2), matrix metalloproteinase 9
(MMP-9) and other pro-inflammatory factors, promoting tumor growth
and metastasis in a mouse model of breast cancer (24). In addition to promoting the
production of inflammatory cytokines, CHI3L1 acts as an
inflammatory target molecule that is regulated by a variety of
other cytokines and hormones (25). For example, inflammatory factors;
namely, TNF-α and IL-1, induce the expression of CHI3L1 in
chondrocytes through the NF-κB signaling pathway (26,27).
Thus, CHI3L1 demonstrates potential as a biomarker and therapeutic
target. In Alzheimer's disease, the level of CHI3L1 in
cerebrospinal fluid (CSF) is considered a biomarker of early
neuroinflammation, which may be indicative of stress-induced
neurotoxicity (28,29). CHI3L1 is also associated with the
degree of liver inflammation and fibrosis; thus, exhibiting
potential as a therapeutic target (10).
Type 2 diabetes mellitus (T2D), caused by obesity
and insulin resistance, is characterized by abnormal lipid
metabolism, which effects the occurrence of cardiovascular disease
(30,31). Clinical data suggests that obese
patients with T2D exhibit elevated CHI3L1 serum levels (Fig. 1) (32). Notably, elevated CHI3L1 levels are
associated with insulin resistance in T2D (33,34).
In addition, plasma CHI3L1 is associated with fasting plasma
glucose and plasma IL-6 levels (35) and the development of coronary
artery disease in patients with asymptomatic T2D (36).
Adiponectin is a colloidal protein secreted by
adipose tissue, with a molecular weight of 29 kDa. Plasma
adiponectin not only plays a role in obesity-related insulin
resistance, but also stimulates the phosphorylation and activation
of AMP kinase. Thus, adiponectin produces anti-inflammatory effects
and protects endothelial cells (37). Results of a previous study revealed
that CHI3L1 and adiponectin expression levels were elevated in
patients with asymptomatic T1D in a European Mediterranean
population, thus highlighting the potential of these proteins as
markers of early inflammation in diabetic patients (38).
Collectively, these results reveal that CHI3L1 may
be involved in insulin resistance, metabolic syndrome characterized
by obesity and cardiovascular and metabolic disorders (39,40).
Further research is required to fully elucidate the mechanisms
underlying these associations and to explore the potential of
CHI3L1 as a therapeutic target or biomarker for T1D/T2D and the
associated complications.
Vascular inflammation is also a common cause of
numerous cardiovascular diseases (41). Giant cell arteritis (GCA) is the
most common systemic vasculitis in adults (42), and macrophages mediate the
destruction and formation of blood vessels (43,44).
Abdominal aortic aneurysm is a vascular inflammatory disease
characterized by inflammatory cell infiltration,
neovascularization, and the production of various proteases and
cytokines. The formation of abdominal aortic aneurysm is associated
with the degeneration of aortic elastic mediators, and vascular
rupture is considered the most serious complication (45). Serum levels of CHI3L1 are elevated
in patients with GCA and abdominal aortic aneurysm (43,44,46).
AS is also a vascular inflammatory disease. The
lesion site is infiltrated by inflammatory cells, such as
macrophages and T lymphocytes, and pro-inflammatory cytokines
produced by these immune cells are a key cause of plaque rupture.
In addition, results of previous studies reveal that regulating the
gene expression of inflammatory factors affects the occurrence and
development of AS (47,48). Results of previous studies also
emphasize that AS progression is closely associated with CHI3L1
expression levels. Thus, CHI3L1 exhibits potential as a marker of
coronary AS severity and plaque instability (49,50).
Results of previous studies demonstrate that serum CHI3L1
expression levels are associated with arterial wall fibrosis and
arterial stiffness (51–53). These findings support the notion
that CHI3L1 upregulates abnormal lipid metabolism and vascular
inflammation, which are risk factors for cardiovascular disease.
Collectively, these results suggest that CHI3L1 may play a role in
accelerating the development of cardiovascular disease through
promoting the progression of these risk factors.
Previous research indicates that CHI3L1 serum levels
may affect the risk of adverse cardiovascular outcomes and
mortality (54). Results of a
previous study using clinical data reveal that CHI3L1 levels are
elevated in patients with cardiovascular disease and these elevated
levels are often associated with disease progression (55). In addition, CHI3L1 is associated
with mortality in individuals with cardiovascular disease (56). Serum CHI3L1 levels are increased in
patients with essential hypertension, which is positively
correlated with the incidence of hypertension in pre-hypertensive
subjects (57). Monitoring CHI3L1
serum levels may aid in predicting the occurrence of cardiovascular
events in patients with hypertension during long-term follow-up for
7.89±0.12 years (58). Results of
previous studies also reveal that increased CHI3L1 serum levels in
patients with aortic stenosis and peripheral artery disease are
associated with a poor prognosis (59,60).
Notably, CHI3L1 levels are elevated during the acute phase of
ischemic stroke and are independently associated with recurrent
stroke, complex vascular events and adverse functional outcomes
(61). In patients with atrial
fibrillation, CHI3L1 is highly expressed in epicardial tissue.
Thus, serum CHI3L1 levels may be used to predict the recurrence of
atrial fibrillation and may be associated with atrial fibrosis
(62,63). Assessment of serum CHI3L1 may
exhibit potential in identifying the risk of future cardiovascular
events in additional diseases, such as essential thrombocythemia
and polycythemia vera (64). In
addition, CHI3L1 may affect the progression of coronary artery
disease (CAD), affecting the stability of the fibrous cap of
atherosclerotic plaques and the occurrence of complications. Thus,
CHI3L1 may exhibit potential as significant indicator for the early
diagnosis of CAD (65,66). Results of a previous study
demonstrated a strong correlation between CHI3L1 levels and the
progression of cardiovascular disease (67). These levels not only allow for the
monitoring of disease progression, but also offer effective
prediction of mortality caused by cardiovascular events, showcasing
the potential of CHI3L1 as a valuable predictor of cardiovascular
disease. Results of previous studies also highlight the effect of
CHI3L1 on cardiovascular disease through the regulation of
cardiovascular-related cells. In disease models of AS and pulmonary
hypertension, CHI3L1 is closely associated with functions in
specific cells, including macrophages and SMCs (25,55,68,69).
During the maturation of macrophages, the expression
of CHI3L1 is upregulated due to the binding of nuclear
transcription factor sp1 to the promoter of the CHI3L1 gene. Thus,
CHI3L1 is considered a marker of macrophage maturation (70).
Results of a previous study indicated that
individuals with Prader-Willi syndrome (PWS), a neurodevelopmental
disorder, exhibit an increased risk of obesity and cardiovascular
disease (71). The occurrence of
PWS is associated with compromised macrophage suppression and
increased ECM remodeling. Notably, patients with PWS exhibit
elevated levels of MMP-9 and myeloperoxidase, along with reduced
levels of macrophage inhibitory factor. In addition, patients with
PWS exhibit elevated CHI3L1 expression levels, highlighting the
potential association between CHI3L1 and macrophages (72). CHI3L1 expression has been detected
in CD68+ macrophages and circulating monocytes in GCA, mediated by
B cells (25). Cytokines produced
by B cells promote the transformation of macrophages into
pro-inflammatory phenotypes, and results of this study also
demonstrated that CHI3L1, IL-6, IL-1β, TNF-α and MMP-9 expression
levels were significantly increased (43). In GCA, CHI3L1 is mainly derived
from CD206+MMP9+ macrophage subsets. As an upstream regulator of
MMP-9+ macrophages, CHI3L1 binds to the IL-13Rα2, which is highly
expressed in the vascular wall of GCA layers. Notably, IL-13Rα2
mediates tissue destruction and angiogenesis. In macrophages,
CHI3L1 knockdown rescues the aforementioned effects (44). In M1 macrophages, IL-6 decreases
the expression of microRNA (miR)-24-1, and upregulates the
expression of CHI3L1 and inflammatory mediators, TNF-α and C-C
motif chemokine ligand 2 (CCL2)MCP-1 during the progression of
vascular inflammation. IL-6 mediates these effects through RelA
(p65)/Nfkb1 (p50). In addition, upregulated CHI3L1 and its
downstream inflammatory factor, CCL2, promote SMC migration through
JNK and ERK phosphorylation pathways, stimulates the expression of
vascular endothelial cell adhesion molecules, such as vascular cell
adhesion molecule-1, intercellular cell adhesion molecule-1 and
P-selectin and enhances the adhesion function of monocytes
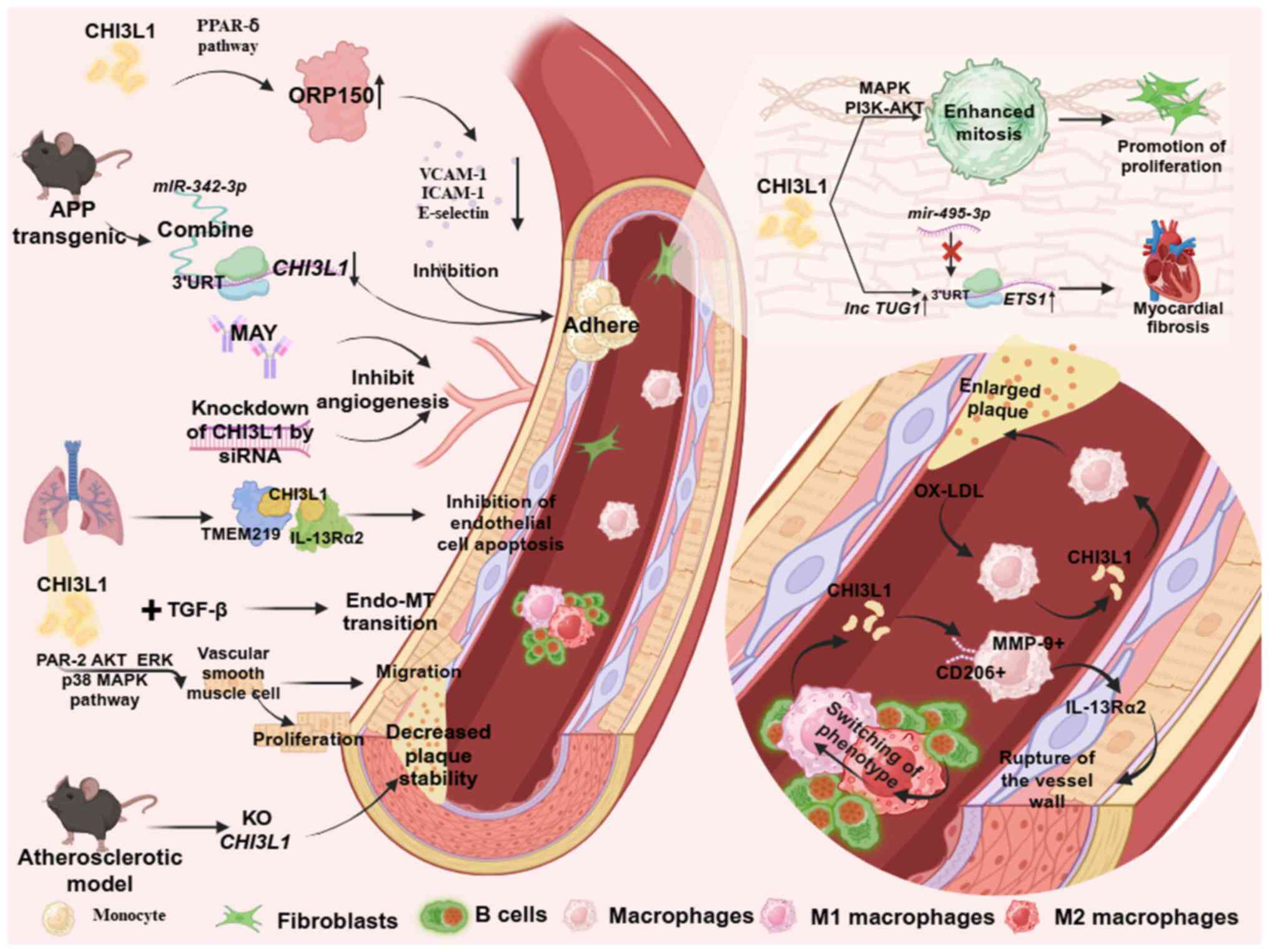
(Fig. 2) (46).
The formation of plaque following accumulation of
fat and/or fibrous material in the lining of the arteries is a
major feature of AS, which involves the phagocytosis of plasma
lipoproteins deposited in the lining of the arteries, with
macrophages transforming them into foam cells (73). Serum CHI3L1 is significantly
elevated in patients with symptomatic carotid AS (74). The initiation factor of AS,
oxidized low-density lipoprotein (OX-LDL), also stimulates
macrophages to secrete CHI3L1. These results suggest that CHI3L1
may play a role in the development of vascular diseases
characterized by macrophage/monocyte accumulation and activation
(Fig. 2) (25). Results of a previous study reveal
that CHI3L1 gene knockout suppresses the expression of
pro-inflammatory mediators, decreases plaque lipid and macrophage
levels, and increases collagen and SMC content in ApoE (−/-) mice
(75). In addition, CHI3L1
inhibits the activation of Caspase-9 and decreases the apoptosis of
macrophages, resulting in plaque fiber cap damage (76).
MCP-1 is a chemokine secreted by adipose tissue that
induces monocyte migration and macrophage infiltration and
participates in the formation of atheromatous lipostreaks and the
development of unstable plaques (77). Results of previous studies
demonstrate that patients with obesity may exhibit increased CHI3L1
expression levels (78). However,
CHI3L1 expression levels are reduced following weight loss in these
patients. These results indicate that increased CHI3L1 expression
levels induced the excessive accumulation of macrophages in obese
patients, leading to a sub-inflammatory state and the occurrence of
AS and other diseases (39,79,80).
Collectively, these results demonstrate that CHI3L1
is not only secreted by macrophages, but also acts on macrophages,
facilitating macrophage activation and inflammation. This, in turn,
leads to damage in cardiac vascular tissue. Thus, CHI3L1 may play a
key role in the advancement of AS. Targeted elimination of CHI3L1
may delay the pathological progression of AS, highlighting its
potential as a specific target in the treatment of AS, through the
inhibition of inflammation.
Results of a previous study reveal that CHI3L1
stimulated the chemotaxis and migration of human umbilical cord
vascular endothelial cells (81).
Sun et al (68) demonstrate
that CHI3L1 inhibits endothelial cell apoptosis during vascular
remodeling in pulmonary hypertension, by co-binding to the
transmembrane protein 219 (TMEM219) receptor and the corresponding
IL-13Rα2 receptor. In addition, CHI3L1 upregulates oxygen
regulatory protein through the peroxisome proliferator-activated
receptor (PPAR)-δ-dependent pathway, reducing lipopolysaccharide
(LPS)-induced phosphorylation of NFκB and inhibiting the expression
of endothelial cell adhesion molecules, such as ICAM-1, VCAM-1 and
E-selectin (Fig. 2) (82). Results of a previous study revealed
that CHI3L1 and Lp-PLA2 RNAi in combination are superior to Lp-PLA1
or CHI3L1 RNAi alone in the treatment of AS (83). In a transgenic mouse model of
amyloid precursor protein, miR-342-3p targeted the
CHI3L13′-untranslated region (UTR) to inhibit CHI3L1 expression in
endothelial cells, thereby inhibiting IL-6-induced
monocyte-endothelial cell adhesion and platelet-derived growth
factor (PDGF-BB)-induced cell migration and proliferation (Fig. 2) (69). Notably, CHI3L1 regulates
endothelial cells to promote tumor angiogenesis. Small interfering
RNA-mediated CHI3L1 knockdown inhibits tumor growth rate and blood
vessel density in the glioblastoma U87 cell line. Anti-VEGF
antibody exerts no effect on CHI3L1-mediated endothelial
angiogenesis; thus confirming that CHI3L1 promotes tumor blood
vessel formation as an angiogenic factor, independent of VEGF
(84,85). In xenograft experiments, CHI3L1
expressed by tumor-derived mural cells (GSDCs) activates neural
cadherin/β-catenin/smooth muscle α actin (SMA) and VE-cadherin/β
between GSDC and endothelial cells. The catenin/actin pathway plays
a role in mediating intercellular adhesion and permeability,
enhancing the interaction between GSDCs and endothelial cells and
stabilizing the vascular network. Results of a previous study
reveal that CHI3L1 silencing in GSDCs leads to a significant
reduction in tumor blood vessel density and stability, ultimately
inhibiting tumor growth (86). In
osteoblastoma cell lines; namely, MG-63 and U87, mouse monoclonal
anti-CHI3L1 antibodies effectively inhibit the CHI3L1-induced
activation of MAPK and ERK (1/2), thereby inhibiting the tube
formation of microvascular endothelial cells (87). CHI3L1 also interacts with TGF-β to
increase endothelial cell permeability and promote
endothelial-to-mesenchymal transition (EMT). The treatment of
bovine pulmonary artery endothelial cells with CHI3L1 in
combination with TGF-β downregulates VE-cadherin in vascular
endothelial cells and reduces the expression of α-SMA, a
mesenchymal cell marker (Fig. 2)
(68). Thus, CHI3L1 may play a
role in promoting tumor angiogenesis and in mediating endothelial
cell apoptosis and EMT. Targeting CHI3L1 may inhibit tumor growth,
thus highlighting the potential of this protein in the development
of novel treatment strategies.
Fibrosis is a tissue repair response that relies on
fibroblast activation and is characterized by the excessive
accumulation of ECM components, such as collagen and fibronectin
(88). CHI3L1 stimulates
fibroblast growth in a dose-dependent manner through MAPK and
PI3K-AKT signaling pathways. Results of a previous study reveal
that CHI3L1 mediates mitotic reactions, stimulates the
proliferation of connective tissue cells and participates in
fibrosis (89). During the wound
healing process in diabetic foot ulcer, fibroblasts overexpressing
CHI3L1 are enriched and M1-type macrophages are polarized (90). Notably, CHI3L1 is associated with
atrial fibrosis in patients with atrial fibrillation (62). Results of a previous study reveal
that CHI3L1 affects the degree of fibrosis in mouse cardiomyocytes
by modulating the long non-coding (lnc)RNA TUG1/miR-1-495-3p/ETS
proto-oncogene 1 (ETS1) axis. CHI3L1 increases the expression of
lncRNA TUG1 and reduces the expression of miR-495-3p, thereby
weakening the targeted binding of miR-495-3p to the 3′UTR sequence
of the ETS1 gene. Thus, ETS1 gene expression levels are increased
in mice, ultimately leading to increased levels of myocardial
fibrosis (Fig. 2) (91). Collectively, these studies revealed
that CHI3L1 may play a crucial role in the advancement of fibrosis
in cardiovascular patients; thus highlighting its potential in the
development of novel treatment options for fibrosis.
Collectively, these results suggest that CHI3L1 may
play a role as a crucial mediator in the development and
progression of cardiovascular disease. Prolonged nicotine
consumption exacerbates inflammatory responses through upregulation
of CHI3L1, thereby heightening the risk and advancement of
abdominal aortic aneurysm. Notably, this may be associated with
reduced microRNA-24 expression (97). In male patients with end-stage
renal disease, CHI3L1 expression is associated with vascular
calcification, indicating the sex-specific role of CHI3L1 as a
novel marker for cardiovascular disease that may affect the
development of cardiovascular comorbidities (22). Through proteomics and Mendelian
randomization, results of a previous study reveal that CHI3L1 acts
as a circulating protein that is causally associated with the
treatment of heart failure. Thus, CHI3L1 may exhibit potential in
the treatment of heart failure (98).
CHI3L1 is not only associated with the development
of cardiovascular disease, but also serves as a valuable indicator
for monitoring the prognosis of patients. Notably, CHI3L1 may
affect disease progression by modulating the functional status of
cells associated with the cardiovascular system. As a novel
predictor of cardiovascular disease, CHI3L1 exhibits potential as a
target for disease management.
As signaling proteins, chemokines bind to
corresponding receptors on the cell surface, to play key roles in
angiogenesis and in the regulation of leukocyte adhesion and
migration (99). Notably, CHI3L1
gene expression is negatively correlated with the expression of
CCL2/MCP-1. Interference with the CHI3L1 gene inhibits the
occurrence of inflammation in AS (83). In addition, CHI3L1 induces the
secretion of IL-8 and CCL2 in macrophages, promoting the migration
of macrophages and endothelial cells (100). In lung macrophages, CHI3L1
promotes CXCL2 production. Results of a previous study also
demonstrate that CHI3L1 promotes the expression of LPS-treated
macrophage angiogenesis factors, leading to further increases in
angiogenesis (101).
As an inflammatory molecule, CHI3L1 is used in
combination with VCAM-1 and ICAM-1 to evaluate the occurrence of
vascular inflammation (102).
Serum CHI3L1, VCAM-1 and ICAM-1 are significantly increased in
vascular endothelial injury and vascular inflammation induced by
high cholesterol (103). Results
of a previous study reveal that CHI3L1 promotes a decline in
endothelial barrier function by reducing the expression of
VE-cadherin (68). During the
formation of tumor blood vessels, CHI3L1 stimulates endothelial
cells to upregulate the membrane receptor sydecan-1 protein to
coordinate integrin αvβ3, triggering a signaling cascade of focal
adhesion kinase and ERK-1/2; thus promoting angiogenesis (104). Proteoglycan also plays a key role
in regulating cell adhesion and migration. Notably, CHI3L1 binds to
proteoglycans, such as chitosaccharides and hyaluronic acid; thus
playing a regulatory role in a variety of diseases (105).
ILs play a key role in inflammatory response and
regulate the progression of AS (106). In high-cholesterol rats with
vitamin D deficiency, IL-6 and CHI3L1 levels are simultaneously
increased, promoting vascular inflammation (103). Results of a previous study reveal
that CHI3L1 specifically binds to IL-13Rα2, increases the
phosphorylation of ERK1/2 and JNK, promotes the recruitment of
members of the activator protein-1 family in the nucleus, targets
the MMP family and degrades the ECM (107). In lung tissue and airway
remodeling, IL-13 upregulates the expression of CHI3L1 and plays a
key role in the inflammatory response (108).
MMPs are a class of zinc-dependent endoproteases
secreted by endothelial cells, vascular SMCs, fibroblasts,
macrophages and neutrophils. MMP expression levels are associated
with vascular remodeling and stiffening and plaque stability
(109). In AS, MMP-7 regulates
the function of macrophages, leading to the generation of
atherosclerotic unstable plaques. MMP-2, MMP-9, MMP-13, MMP-35 and
MMP-42 increase the risk of plaque rupture through degradation of
arterial elastin and increasing vascular calcification, leading to
further AS development (109,110). Results of a previous study
demonstrated that both MMP-9 and CHI3L1 were independent risk
factors for unstable plaque formation (111). Mechanical stress, including shear
force, is involved in vascular remodeling through the regulation of
MMPs. Results of a previous study reveal that vascular inflammatory
factor MMP-8 expression levels are decreased in a model of AS,
following CHI3L1 gene knockout (83).
Results of previous studies reveal that CHI3L1 is
closely associated with the occurrence and development of
cardiovascular disease; thus stressing the potential of CHI3L1 in
predicting the prognosis of patients and the management of disease.
However, the present study possesses limitations. The regulatory
function of CHI3L1 in SMCs in atherosclerotic diseases is
associated with cell-cell interactions and the atherosclerotic
microenvironment. Additional negative feedback pathways may play a
role in CHI3L1 synthesis and secretion and these were not
investigated in the present study. In addition, results of previous
studies were inconsistent in demonstrating the role of CHI3L1 in
cells, which may be due to differing disease processes and
experimental environments. However, CHI3L1 may promote plaque
formation in the early stage of AS, inhibit plaque progression in
the late stage and improve plaque stability. Through the analysis
of clinical samples, results of a previous study revealed that
CHI3L1 serum levels are elevated in patients with cardiovascular
disease, suggesting that CHI3L1 may promote the development of
cardiovascular disease. Thus, further experiments are required to
determine the mechanisms underlying CHI3L1 in the prevention and
treatment of cardiovascular disease.
Not applicable.
The present study was supported by Weifang Science and
Technology Development projects (grant no. 2023YX092).
Not applicable.
ZQ, YL, YR and DX wrote and revised the manuscript,
and constructed the figures. ZG and MC conceived the study and
revised the manuscript. Data authentication is not applicable. All
authors have read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Al-Mallah MH, Sakr S and Al-Qunaibet A:
Cardiorespiratory fitness and cardiovascular disease prevention: An
update. Curr Atheroscler Rep. 20:12018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mensah GA, Fuster V and Roth GA: A
Heart-Healthy and Stroke-Free world: Using data to inform global
action. J Am Coll Cardiol. 82:2343–2349. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dhande IS and Doris PA: Genomics and
inflammation in cardiovascular disease. Compr Physiol.
11:2433–2454. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weber BN, Giles JT and Liao KP: Shared
inflammatory pathways of rheumatoid arthritis and atherosclerotic
cardiovascular disease. Nat Rev Rheumatol. 19:417–428. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Forteza MJ, Berg M, Edsfeldt A, Sun J,
Baumgartner R, Kareinen I, Casagrande FB, Hedin U, Zhang S,
Vuckovic I, et al: Pyruvate dehydrogenase kinase regulates vascular
inflammation in atherosclerosis and increases cardiovascular risk.
Cardiovasc Res. 119:1524–1536. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen R, Zhang H, Tang B, Luo Y, Yang Y,
Zhong X, Chen S, Xu X, Huang S and Liu C: Macrophages in
cardiovascular diseases: Molecular mechanisms and therapeutic
targets. Signal Transduct Target Ther. 9:1302024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wagenhauser MU, Mulorz J, Krott KJ,
Bosbach A, Feige T, Rhee YH, Chatterjee M, Petzold N, Böddeker C,
Ibing W, et al: Crosstalk of platelets with macrophages and
fibroblasts aggravates inflammation, aortic wall stiffening, and
osteopontin release in abdominal aortic aneurysm. Cardiovasc Res.
120:417–432. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kinoshita D, Suzuki K, Yuki H, Niida T,
Fujimoto D, Minami Y, Dey D, Lee H, McNulty I, Ako J, et al:
Sex-Specific association between perivascular inflammation and
plaque vulnerability. Circ Cardiovasc Imaging. 17:e0161782024.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ham HJ, Lee YS, Koo JK, Yun J, Son DJ, Han
SB and Hong JT: Inhibition of Amyloid-β (Aβ)-Induced cognitive
impairment and neuroinflammation in CHI3L1 knockout mice through
downregulation of ERK-PTX3 pathway. Int J Mol Sci. 25:55502024.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kui L, Kim AD, Onyuru J, Hoffman HM and
Feldstein AE: BRP39 regulates neutrophil recruitment in NLRP3
Inflammasome-Induced liver inflammation. Cell Mol Gastroenterol
Hepatol. 17:481–497. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferrigno I, Verzellesi L, Ottone M,
Bonacini M, Rossi A, Besutti G, Bonelli E, Colla R, Facciolongo N,
Teopompi E, et al: CCL18, CHI3L1, ANG2, IL-6 systemic levels are
associated with the extent of lung damage and radiomic features in
SARS-CoV-2 infection. Inflamm Res. 73:515–530. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song M, Zhang G, Shi H, Zhu E, Deng L and
Shen H: Serum YKL-40 in coronary heart disease: Linkage with
inflammatory cytokines, artery stenosis, and optimal cut-off value
for estimating major adverse cardiovascular events. Front
Cardiovasc Med. 10:12423392023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reilly CS, Borges AH, Baker JV, Safo SE,
Sharma S, Polizzotto MN, Pankow JS, Hu X, Sherman BT, Babiker AG,
et al: Investigation of causal effects of protein biomarkers on
cardiovascular disease in persons with HIV. J Infect Dis.
227:951–960. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Czestkowski W, Krzeminski L, Piotrowicz
MC, Mazur M, Pluta E, Andryianau G, Koralewski R, Matyszewski K,
Olejniczak S, Kowalski M, et al: Structure-Based discovery of
High-Affinity small molecule ligands and development of tool probes
to study the role of Chitinase-3-Like protein 1. J Med Chem.
67:3959–3985. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Junker N, Johansen JS, Hansen LT, Lund EL
and Kristjansen PE: Regulation of YKL-40 expression during
genotoxic or microenvironmental stress in human glioblastoma cells.
Cancer Sci. 96:183–190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao T, Su Z, Li Y, Zhang X and You Q:
Chitinase-3 like-protein-1 function and its role in diseases.
Signal Transduct Target Ther. 5:2012020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fusetti F, Pijning T, Kalk KH, Bos E and
Dijkstra BW: Crystal structure and carbohydrate-binding properties
of the human cartilage glycoprotein-39. J Biol Chem.
278:37753–37760. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao H, Huang M and Jiang L: Potential
roles and future perspectives of Chitinase 3-like 1 in macrophage
polarization and the development of diseases. Int J Mol Sci.
24:161492023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Coffman FD: Chitinase 3-Like-1 (CHI3L1): A
putative disease marker at the interface of proteomics and
glycomics. Crit Rev Clin Lab Sci. 45:531–562. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suzuki K, Okawa K, Ohkura M, Kanaizumi T,
Kobayashi T, Takahashi K, Takei H, Otsuka M, Tabata E, Bauer PO and
Oyama F: Evolutionary insights into sequence modifications
governing chitin recognition and chitinase inactivity in YKL-40
(HC-gp39, CHI3L1). J Biol Chem. 300:1073652024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu JE, Yeo IJ, Han SB, Yun J, Kim B, Yong
YJ, Lim YS, Kim TH, Son DJ and Hong JT: Significance of
chitinase-3-like protein 1 in the pathogenesis of inflammatory
diseases and cancer. Exp Mol Med. 56:1–18. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Laucyte-Cibulskiene A, Ward LJ, Ebert T,
Tosti G, Tucci C, Hernandez L, Kautzky-Willer A, Herrero MT, Norris
CM, Pilote L, et al: Role of GDF-15, YKL-40 and MMP 9 in patients
with end-stage kidney disease: Focus on sex-specific associations
with vascular outcomes and all-cause mortality. Biol Sex Differ.
12:502021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kwak EJ, Hong JY, Kim MN, Kim SY, Kim SH,
Park CO, Kim KW, Lee CG, Elias JA, Jee HM and Sohn MH: Chitinase
3-like 1 drives allergic skin inflammation via Th2 immunity and M2
macrophage activation. Clin Exp Allergy. 49:1464–1474. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Libreros S, Garcia-Areas R, Shibata Y,
Carrio R, Torroella-Kouri M and Iragavarapu-Charyulu V: Induction
of proinflammatory mediators by CHI3L1 is reduced by chitin
treatment: Decreased tumor metastasis in a breast cancer model. Int
J Cancer. 131:377–386. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee CG, Da Silva CA, Dela Cruz CS,
Ahangari F, Ma B, Kang MJ, He CH, Takyar S and Elias JA: Role of
chitin and chitinase/chitinase-like proteins in inflammation,
tissue remodeling, and injury. Annu Rev Physiol. 73:479–501. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ling H and Recklies AD: The chitinase
3-like protein human cartilage glycoprotein 39 inhibits cellular
responses to the inflammatory cytokines interleukin-1 and tumour
necrosis factor-alpha. Biochem J. 380:651–659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Recklies AD, Ling H, White C and Bernier
SM: Inflammatory cytokines induce production of CHI3L1 by articular
chondrocytes. J Biol Chem. 280:41213–41221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Connolly K, Lehoux M, O'Rourke R, Assetta
B, Erdemir GA, Elias JA, Lee CG and Huang YA: Potential role of
chitinase-3-like protein 1 (CHI3L1/YKL-40) in neurodegeneration and
Alzheimer's disease. Alzheimers Dement. 19:9–24. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cicognola C, Mattsson-Carlgren N, van
Westen D, Zetterberg H, Blennow K, Palmqvist S, Ahmadi K,
Strandberg O, Stomrud E, Janelidze S and Hansson O: Associations of
CSF PDGFRβ with aging, Blood-Brain barrier damage,
neuroinflammation, and Alzheimer disease pathologic changes.
Neurology. 101:e30–e39. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yusuf S, Hawken S, Ounpuu S, Bautista L,
Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L,
et al: Obesity and the risk of myocardial infarction in 27,000
participants from 52 countries: A case-control study. Lancet.
366:1640–1649. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Laing SP, Swerdlow AJ, Slater SD, Burden
AC, Morris A, Waugh NR, Gatling W, Bingley PJ and Patterson CC:
Mortality from heart disease in a cohort of 23,000 patients with
insulin-treated diabetes. Diabetologia. 46:760–765. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kwon Y, Kim JH, Ha EK, Jee HM, Baek HS,
Han MY and Jeong SJ: Serum YKL-40 levels are associated with the
atherogenic index of plasma in children. Mediators Inflamm.
2020:87139082020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kyrgios I, Galli-Tsinopoulou A, Stylianou
C, Papakonstantinou E, Arvanitidou M and Haidich AB: Elevated
circulating levels of the serum acute-phase protein YKL-40
(chitinase 3-like protein 1) are a marker of obesity and insulin
resistance in prepubertal children. Metabolism. 61:562–568. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Catalan V, Gomez-Ambrosi J, Rodriguez A,
Ramírez B, Rotellar F, Valentí V, Silva C, Gil MJ, Salvador J and
Frühbeck G: Increased circulating and visceral adipose tissue
expression levels of YKL-40 in obesity-associated type 2 diabetes
are related to inflammation: Impact of conventional weight loss and
gastric bypass. J Clin Endocrinol Metab. 96:200–209. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nielsen AR, Erikstrup C, Johansen JS,
Fischer CP, Plomgaard P, Krogh-Madsen R, Taudorf S, Lindegaard B
and Pedersen BK: Plasma YKL-40: A BMI-independent marker of type 2
diabetes. Diabetes. 57:3078–3082. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim HM, Lee BW, Song YM, Kim WJ, Chang HJ,
Choi DH, Yu HT, Kang E, Cha BS and Lee HC: Potential association
between coronary artery disease and the inflammatory biomarker
YKL-40 in asymptomatic patients with type 2 diabetes mellitus.
Cardiovasc Diabetol. 11:842012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fisman EZ and Tenenbaum A: Adiponectin: A
manifold therapeutic target for metabolic syndrome, diabetes, and
coronary disease? Cardiovasc Diabetol. 13:1032014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Aguilera E, Serra-Planas E, Granada ML,
Pellitero S, Reverter JL, Alonso N, Soldevila B, Mauricio D and
Puig-Domingo M: Relationship of YKL-40 and adiponectin and
subclinical atherosclerosis in asymptomatic patients with type 1
diabetes mellitus from a European Mediterranean population.
Cardiovasc Diabetol. 14:1212015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Deng Y, Li G, Chang D and Su X: YKL-40 as
a novel biomarker in cardio-metabolic disorders and inflammatory
diseases. Clin Chim Acta. 511:40–46. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Perumalsamy S, Huri HZ, Abdullah BM,
Mazlan O, Wan Ahmad WA and Vethakkan S: Genetic markers of insulin
resistance and atherosclerosis in type 2 diabetes mellitus patients
with coronary artery disease. Metabolites. 13:4272023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sanchez-Madrid F and Sessa WC: Spotlight
on mechanisms of vascular inflammation. Cardiovasc Res. 86:171–173.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Haaversen AB, Brekke LK, Bakland G,
Rodevand E, Myklebust G and Diamantopoulos AP: Norwegian society of
rheumatology recommendations on diagnosis and treatment of patients
with giant cell arteritis. Front Med (Lausanne). 9:10826042022.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Graver JC, Jiemy WF, Altulea DHA, van
Sleen Y, Xu S, van der Geest KSM, Verstappen GMPJ, Heeringa P,
Abdulahad WH, Brouwer E, et al: Cytokine producing B-cells and
their capability to polarize macrophages in giant cell arteritis. J
Autoimmun. 140:1031112023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
van Sleen Y, Jiemy WF, Pringle S, van der
Geest KSM, Abdulahad WH, Sandovici M, Brouwer E, Heeringa P and
Boots AMH: A distinct macrophage subset mediating tissue
destruction and neovascularization in giant cell arteritis:
Implication of the YKL-40/Interleukin-13 receptor α 2 axis.
Arthritis Rheumatol. 73:2327–2337. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Haque K and Bhargava P: Abdominal aortic
aneurysm. Am Fam Physician. 106:165–172. 2022.PubMed/NCBI
|
|
46
|
Maegdefessel L, Spin JM, Raaz U, Eken SM,
Toh R, Azuma J, Adam M, Nakagami F, Heymann HM, Chernogubova E, et
al: miR-24 limits aortic vascular inflammation and murine abdominal
aneurysm development. Nat Commun. 5:52142014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kong P, Cui ZY, Huang XF, Zhang DD, Guo RJ
and Han M: Inflammation and atherosclerosis: Signaling pathways and
therapeutic intervention. Signal Transduct Target Ther. 7:1312022.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liang G, Wang S, Shao J, Jin YJ, Xu L, Yan
Y, Günther S, Wang L and Offermanns S: Tenascin-X Mediates
Flow-Induced suppression of EndMT and atherosclerosis. Circ Res.
130:1647–1659. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Michelsen AE, Rathcke CN, Skjelland M,
Holm S, Ranheim T, Krohg-Sørensen K, Klingvall MF, Brosstad F, Oie
E, Vestergaard H, et al: Increased YKL-40 expression in patients
with carotid atherosclerosis. Atherosclerosis. 211:589–595. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sciborski K, Kuliczkowski W, Karolko B,
Bednarczyk D, Protasiewicz M, Mysiak A and Negrusz-Kawecka M:
Plasma YKL-40 levels correlate with the severity of coronary
atherosclerosis assessed with the SYNTAX score. Pol Arch Intern
Med. 128:644–648. 2018.PubMed/NCBI
|
|
51
|
Xu Q, Sun L, Wang Y, Wang R, Jia Y, Guo D,
Shi M, Yang P, Zhang Y and Zhu Z: Causal effects of YKL-40 on
ischemic stroke and its subtypes: A 2-Sample mendelian
randomization study. J Am Heart Assoc. 12:e0290002023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kjaergaard AD, Bojesen SE, Johansen JS and
Nordestgaard BG: Elevated plasma YKL-40 levels and ischemic stroke
in the general population. Ann Neurol. 68:672–680. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ma WH, Wang XL, Du YM, Wang YB, Zhang Y,
Wei DE, Guo LL and Bu PL: Association between human cartilage
glycoprotein 39 (YKL-40) and arterial stiffness in essential
hypertension. BMC Cardiovasc Disord. 12:352012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Schroder J, Jakobsen JC, Winkel P, Hilden
J, Jensen GB, Sajadieh A, Larsson A, Ärnlöv J, Harutyunyan M,
Johansen JS, et al: Prognosis and reclassification by YKL-40 in
stable coronary artery disease. J Am Heart Assoc. 9:e0146342020.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wu S, Hsu LA, Cheng ST, Teng MS, Yeh CH,
Sun YC, Huang HL and Ko YL: Circulating YKL-40 level, but not
CHI3L1 gene variants, is associated with atherosclerosis-related
quantitative traits and the risk of peripheral artery disease. Int
J Mol Sci. 15:22421–22437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wallentin L, Eriksson N, Olszowka M,
Grammer TB, Hagström E, Held C, Kleber ME, Koenig W, März W,
Stewart RAH, et al: Plasma proteins associated with cardiovascular
death in patients with chronic coronary heart disease: A
retrospective study. PLoS Med. 18:e10035132021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xu T, Zhong C, Wang A, Guo Z, Bu X, Zhou
Y, Tian Y, HuangFu X, Zhu Z and Zhang Y: YKL-40 is a novel
biomarker for predicting hypertension incidence among
prehypertensive subjects: A population-based nested case-control
study in China. Clin Chim Acta. 472:146–150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Çetin M, Erdoğan T, Kırış T, Özer S,
Çinier G, Emlek N, Durak H and Şatıroğlu Ö: Elevated serum YKL40
level is a predictor of MACE during the long-term follow up in
hypertensive patients. Clin Exp Hypertens. 42:271–274. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Arain F, Abraityte A, Bogdanova M, Solberg
OG, Michelsen AE, Lekva T, Aakhus S, Holm S, Halvorsen B, Finsen
AV, et al: YKL-40 (Chitinase-3-Like protein 1) serum levels in
aortic stenosis. Circ Heart Fail. 13:e0066432020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hobaus C, Tscharre M, Herz CT, Pesau G,
Wrba T, Koppensteiner R and Schernthaner GH: YKL-40 levels increase
with declining ankle-brachial index and are associated with
long-term cardiovascular mortality in peripheral arterial disease
patients. Atherosclerosis. 274:152–156. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chen XL, Li Q, Huang WS, Lin YS, Xue J,
Wang B, Jin KL and Shao B: Serum YKL-40, a prognostic marker in
patients with large-artery atherosclerotic stroke. Acta Neurol
Scand. 136:97–102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang Q, Shen H, Min J, Gao Y, Liu K, Xi W,
Yang J, Yin L, Xu J, Xiao J and Wang Z: YKL-40 is highly expressed
in the epicardial adipose tissue of patients with atrial
fibrillation and associated with atrial fibrosis. J Transl Med.
16:2292018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Michelakakis N, Neroutsos GJ, Perpinia AS,
Farmakis D, Voukouti EG, Karavidas AJ, Parissis J, Georgiakaki MT
and Pyrgakis VN: Chitinase-3-like protein-1 (YKL-40) before and
after therapy in supraventricular arrhythmias. J Cardiovasc Med
(Hagerstown). 18:650–654. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Krečak I, Gverić-Krečak V, Lapić I,
Rončević P, Gulin J, Fumić K, Krečak F, Holik H and Duraković N:
Circulating YKL-40 in Philadelphia-negative myeloproliferative
neoplasms. Acta Clin Belg. 76:32–39. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Xing Y, Guo J, Gai L, Liu B and Luo D:
Serum YKL-40 is associated with the severity of coronary artery
disease and hypertension. Asian J Surg. 43:1121–1122. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Song CL, Bin L, Diao HY, Wang JH, Shi YF,
Lu Y, Wang G, Guo ZY, Li YX, Liu JG, et al: Diagnostic value of
serum YKL-40 level for coronary artery disease: A Meta-Analysis. J
Clin Lab Anal. 30:23–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zheng JL, Lu L, Hu J, Zhang RY, Zhang Q,
Chen QJ and Shen WF: Increased serum YKL-40 and C-reactive protein
levels are associated with angiographic lesion progression in
patients with coronary artery disease. Atherosclerosis.
210:590–595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sun X, Nakajima E, Norbrun C, Sorkhdini P,
Yang AX, Yang D, Ventetuolo CE, Braza J, Vang A, Aliotta J, et al:
Chitinase 3 like 1 contributes to the development of pulmonary
vascular remodeling in pulmonary hypertension. JCI Insight.
7:e1595782022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Jung YY, Kim KC, Park MH, Seo Y, Park H,
Park MH, Chang J, Hwang DY, Han SB, Kim S, et al: Atherosclerosis
is exacerbated by chitinase-3-like-1 in amyloid precursor protein
transgenic mice. Theranostics. 8:749–766. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Rehli M, Niller HH, Ammon C, Langmann S,
Schwarzfischer L, Andreesen R and Krause SW: Transcriptional
regulation of CHI3L1, a marker gene for late stages of macrophage
differentiation. J Biol Chem. 278:44058–44067. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Thomas C, Mandilaras G, Rabenhorst D,
Oberhoffer FS, Fischer M, Haas NA and Fernandez Rodriguez S: Vagal
asystoles in a boy with Prader-Willi syndrome. Pediatrics.
152:e20220582162023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hope S, Naerland T, Olav Kolset S, Ueland
T, Andreassen OA and Nordstrom M: Systemic immune profile in
Prader-Willi syndrome: Elevated matrix metalloproteinase and
myeloperoxidase and reduced macrophage inhibitory factor. Orphanet
J Rare Dis. 18:1852023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Libby P, Buring JE, Badimon L, Hansson GK,
Deanfield J, Bittencourt MS, Tokgözoğlu L and Lewis EF:
Atherosclerosis. Nat Rev Dis Primers. 5:562019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Boot RG, van Achterberg TA, van Aken BE,
Renkema GH, Jacobs MJ, Aerts JM and de Vries CJ: Strong induction
of members of the chitinase family of proteins in atherosclerosis:
Chitotriosidase and human cartilage gp-39 expressed in lesion
macrophages. Arterioscler Thromb Vasc Biol. 19:687–694. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Gong Z, Xing S, Zheng F and Xing Q:
Increased expression of chitinase 3-like 1 in aorta of patients
with atherosclerosis and suppression of atherosclerosis in
apolipoprotein E-knockout mice by chitinase 3-like 1 gene
silencing. Mediators Inflamm. 2014:9054632014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Huan W, Yandong L, Chao W, Sili Z, Jun B,
Mingfang L, Yu C and Lefeng Q: YKL-40 aggravates early-stage
atherosclerosis by inhibiting macrophage apoptosis in an
Aven-dependent Way. Front Cell Dev Biol. 9:7527732021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
de Lemos JA, Morrow DA, Sabatine MS,
Murphy SA, Gibson CM, Antman EM, McCabe CH, Cannon CP and Braunwald
E: Association between plasma levels of monocyte chemoattractant
protein-1 and long-term clinical outcomes in patients with acute
coronary syndromes. Circulation. 107:690–695. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ahangari F, Sood A, Ma B, Takyar S,
Schuyler M, Qualls C, Dela Cruz CS, Chupp GL, Lee CG and Elias JA:
Chitinase 3-like-1 regulates both visceral fat accumulation and
asthma-like Th2 inflammation. Am J Respir Crit Care Med.
191:746–757. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hempen M, Kopp HP, Elhenicky M, Höbaus C,
Brix JM, Koppensteiner R, Schernthaner G and Schernthaner GH:
YKL-40 is elevated in morbidly obese patients and declines after
weight loss. Obes Surg. 19:1557–1563. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kopp HP, Kopp CW, Festa A, Krzyzanowska K,
Kriwanek S, Minar E, Roka R and Schernthaner G: Impact of weight
loss on inflammatory proteins and their association with the
insulin resistance syndrome in morbidly obese patients.
Arterioscler Thromb Vasc Biol. 23:1042–1047. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Malinda KM, Ponce L, Kleinman HK,
Shackelton LM and Millis AJ: Gp38k, a protein synthesized by
vascular smooth muscle cells, stimulates directional migration of
human umbilical vein endothelial cells. Exp Cell Res. 250:168–173.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jung TW, Park HS, Choi GH, Kim D, Jeong JH
and Lee T: Chitinase-3-like protein 1 ameliorates atherosclerotic
responses via PPARdelta-mediated suppression of inflammation and ER
stress. J Cell Biochem. 119:6795–6805. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhang H, Zhou W, Cao C, Zhang W, Liu G and
Zhang J: Amelioration of atherosclerosis in apolipoprotein
E-deficient mice by combined RNA interference of
lipoprotein-associated phospholipase A2 and YKL-40. PLoS One.
13:e02027972018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ngernyuang N, Yan W, Schwartz LM, Oh D,
Liu YB, Chen H and Shao R: A heparin binding motif rich in arginine
and lysine is the functional domain of YKL-40. Neoplasia.
20:182–192. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Shao R, Hamel K, Petersen L, Cao QJ,
Arenas RB, Bigelow C, Bentley B and Yan W: YKL-40, a secreted
glycoprotein, promotes tumor angiogenesis. Oncogene. 28:4456–4468.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Francescone R, Ngernyuang N, Yan W,
Bentley B and Shao R: Tumor-derived mural-like cells coordinate
with endothelial cells: Role of YKL-40 in mural cell-mediated
angiogenesis. Oncogene. 33:2110–2122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Faibish M, Francescone R, Bentley B, Yan W
and Shao R: A YKL-40-neutralizing antibody blocks tumor
angiogenesis and progression: A potential therapeutic agent in
cancers. Mol Cancer Ther. 10:742–751. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Henderson NC, Rieder F and Wynn TA:
Fibrosis: From mechanisms to medicines. Nature. 587:555–566. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Recklies AD, White C and Ling H: The
chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39)
stimulates proliferation of human connective-tissue cells and
activates both extracellular signal-regulated kinase- and protein
kinase B-mediated signalling pathways. Biochem J. 365:119–126.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Theocharidis G, Thomas BE, Sarkar D, Mumme
HL, Pilcher WJR, Dwivedi B, Sandoval-Schaefer T, Sîrbulescu RF,
Kafanas A, Mezghani I, et al: Single cell transcriptomic landscape
of diabetic foot ulcers. Nat Commun. 13:1812022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Sun Y, Shan X, Guo J, Liu X and Ma D:
CHI3L1 promotes myocardial fibrosis via regulating lncRNA
TUG1/miR-495-3p/ETS1 axis. Apoptosis. 28:1436–1451. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Shackelton LM, Mann DM and Millis AJ:
Identification of a 38-kDa heparin-binding glycoprotein (gp38k) in
differentiating vascular smooth muscle cells as a member of a group
of proteins associated with tissue remodeling. J Biol Chem.
270:13076–13083. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Bara I, Ozier A, Girodet PO, Carvalho G,
Cattiaux J, Begueret H, Thumerel M, Ousova O, Kolbeck R, Coyle AJ,
et al: Role of YKL-40 in bronchial smooth muscle remodeling in
asthma. Am J Respir Crit Care Med. 185:715–722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Tang H, Sun Y, Shi Z, Huang H, Fang Z,
Chen J, Xiu Q and Li B: YKL-40 induces IL-8 expression from
bronchial epithelium via MAPK (JNK and ERK) and NF-κB pathways,
causing bronchial smooth muscle proliferation and migration. J
Immunol. 190:438–446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lambert J and Jorgensen HF: Vascular
smooth muscle cell phenotypic switching and plaque stability: A
role for CHI3L1. Cardiovasc Res. 117:2691–2693. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Tsantilas P, Lao S, Wu Z, Eberhard A,
Winski G, Vaerst M, Nanda V, Wang Y, Kojima Y, Ye J, et al:
Chitinase 3 like 1 is a regulator of smooth muscle cell physiology
and atherosclerotic lesion stability. Cardiovasc Res.
117:2767–2780. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Mulorz J, Spin JM, Mulorz P, Wagenhäuser
MU, Deng A, Mattern K, Rhee YH, Toyama K, Adam M, Schelzig H, et
al: E-cigarette exposure augments murine abdominal aortic aneurysm
development: Role of Chil1. Cardiovasc Res. 119:867–878. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Henry A, Gordillo-Maranon M, Finan C,
Schmidt AF, Ferreira JP, Karra R, Sundström J, Lind L, Ärnlöv J,
Zannad F, et al: Therapeutic targets for heart failure identified
using proteomics and mendelian randomization. Circulation.
145:1205–1217. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Sadeghi M, Dehnavi S, Asadirad A, Xu S,
Majeed M, Jamialahmadi T, Johnston TP and Sahebkar A: Curcumin and
chemokines: Mechanism of action and therapeutic potential in
inflammatory diseases. Inflammopharmacology. 31:1069–1093. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kawada M, Seno H, Kanda K, Nakanishi Y,
Akitake R, Komekado H, Kawada K, Sakai Y, Mizoguchi E and Chiba T:
Chitinase 3-like 1 promotes macrophage recruitment and angiogenesis
in colorectal cancer. Oncogene. 31:3111–3123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Libreros S, Garcia-Areas R, Keating P,
Carrio R and Iragavarapu-Charyulu VL: Exploring the role of CHI3L1
in ‘pre-metastatic’ lungs of mammary tumor-bearing mice. Front
Physiol. 4:3922013. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Janelidze S, Mattsson N, Stomrud E,
Lindberg O, Palmqvist S, Zetterberg H, Blennow K and Hansson O: CSF
biomarkers of neuroinflammation and cerebrovascular dysfunction in
early Alzheimer disease. Neurology. 91:e867–e877. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Kocabas R: Effect of Vitamin D on YKL-40:
Rat hypercholesterolemia model. Korean Circ J. 53:92–102. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Francescone RA, Scully S, Faibish M,
Taylor SL, Oh D, Moral L, Yan W, Bentley B and Shao R: Role of
YKL-40 in the angiogenesis, radioresistance, and progression of
glioblastoma. J Biol Chem. 286:15332–15343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kognole AA and Payne CM: Inhibition of
mammalian glycoprotein YKL-40: identification of the physiological
ligand. J Biol Chem. 292:2624–2636. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Henein MY, Vancheri S, Longo G and
Vancheri F: The role of inflammation in cardiovascular disease. Int
J Mol Sci. 23:129062022. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Chen Y, Zhang S, Wang Q and Zhang X:
Tumor-recruited M2 macrophages promote gastric and breast cancer
metastasis via M2 macrophage-secreted CHI3L1 protein. J Hematol
Oncol. 10:362017. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Lee CG, Hartl D, Lee GR, Koller B,
Matsuura H, Da Silva CA, Sohn MH, Cohn L, Homer RJ, Kozhich AA, et
al: Role of breast regression protein 39 (BRP-39)/chitinase
3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J
Exp Med. 206:1149–1166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Olejarz W, Lacheta D and
Kubiak-Tomaszewska G: Matrix metalloproteinases as biomarkers of
atherosclerotic plaque instability. Int J Mol Sci. 21:39462020.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Liu SF, Nambiar Veetil N, Li Q, Kucherenko
MM, Knosalla C and Kuebler WM: Pulmonary hypertension: Linking
inflammation and pulmonary arterial stiffening. Front Immunol.
13:9592092022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Jiao Y, Qin Y, Zhang Z, Zhang H, Liu H and
Li C: Early identification of carotid vulnerable plaque in
asymptomatic patients. BMC Cardiovasc Disord. 20:4292020.
View Article : Google Scholar : PubMed/NCBI
|