Introduction
As the most common female malignant tumor in the
world, breast cancer (BC) has become a major health problem for
women due to its high mortality and morbidity rates, and 5-year
survival rate of <30% (1). The
treatment of BC includes surgery, radiotherapy, chemotherapy,
endocrine therapy and targeted therapy (1,2).
According to different conditions and needs, it is necessary to
develop personalized treatment plans to achieve the best treatment
effect (3). Surgery is the main
treatment for BC, including radical mastectomy breast-conserving
surgery and breast reconstruction (1). Surgical intervention can effectively
remove the tumor and reduce the risk of recurrence (4). After surgery, adjuvant therapy (such
as radiotherapy and chemotherapy) may be required according to
tumor stage, tumor grade, molecular typing and patient status
(3). Radiation therapy refers to
the use of high-energy rays to kill cancer cells and is often used
as an adjunct treatment after surgery to reduce the risk of
recurrence (4). For several
patients who are inoperable or at high risk of surgery, radiation
therapy can also be used as the primary treatment (5). Chemotherapy is a method of killing
cancer cells with drugs and can be used to shrink tumors before
surgery (6). Endocrine therapy is
mainly aimed at patients with hormone receptor-positive BC, which
inhibits hormones to block cancer cell growth (3,7).
Endocrine therapy drugs include anti-estrogen drugs and aromatase
inhibitors (3,7). Targeted therapies are treatments that
target specific cancer cells, such as trastuzumab for HER2-positive
BC (2), and compared with
traditional therapy, has higher pertinence and lower side effects
(2).
Endocrine therapy for BC is a long-term treatment
(7). Postoperative adjuvant
therapy for patients with early BC generally occurs over 5 years,
but in some cases may extend to 10 years (8). Risk stratification is clinically
performed for early patients with hormone receptor-positive BC.
Low-risk patients only need to be treated with a single drug for 5
years, while high-risk patients need intensive treatment (8). On the one hand, endocrine therapy for
high-risk patients is supplemented by intensive treatment with
ovarian function inhibition or CDK4/6 inhibitors (3). On the other hand, the duration of
treatment for high-risk patients was extended to 10 years (7). For medium-risk patients, it is
necessary to further determine whether endocrine therapy should
last for 5 or 10 years and whether a combination regimen or a
single drug regimen is selected according to the clinical
characteristics of patients (9).
Given that the sources of hormones in premenopausal and
postmenopausal women are different, the choice of therapeutic drugs
needs to be tailored to different populations (9). For patients with BC with a relatively
low risk of recurrence after surgery, selective estrogen receptor
modulator (SERM) therapy is often given, of which tamoxifen is a
commonly used drug (10).
Tamoxifen is a synthetic non-steroidal anti-estrogen
drug, widely used in patients with ER-positive BC, which prolongs
the survival of patients (11).
Although tamoxifen has made important clinical advances in
endocrine therapy, primary and acquired drug resistance has limited
its clinical efficacy (12).
Downregulation of ERα expression, up-regulation of ERβ expression,
the activation of signaling pathways (such as the PI3K/AKT/mTOR
signaling pathway), and the activation of certain key proteins and
RNA can all lead to tamoxifen resistance in patients with BC
(12–14).
Methylation is a common chemical modification in
organisms, which affects the expression of DNA, RNA and proteins
(15). Enzymes that catalyze DNA
methylation are termed DNA methyltransferases (DNMTs) (16). DNMTs are highly expressed in
tamoxifen-resistant patients and are important factors for
tamoxifen resistance in BC (17).
In addition, Jahangiri et al (18) found that promoters of DNMTs are
demethylated, which leads to overexpression of DNMTs, promoting
tamoxifen resistance and the recurrence of BC. Therefore, the
promoters of tamoxifen-resistant cell lines have higher methylation
levels, resulting in reduced expression of genes including nuclear
receptor-interacting protein 1, human homolog of the Drosophila
headcase and the mitochondrial fission protein 1 (19).
In the present review, methylation is introduced in
detail, including DNA methylation, RNA methylation and protein
methylation. After which, tamoxifen is described and divided into
its clinical use and tamoxifen resistance-related mechanisms. In
addition, the effect of methylation on tamoxifen resistance, and
clustering on the ER and PI3K/AKT/mTOR signaling pathways is
described. Furthermore, tamoxifen-induced methylation is also
elucidated. Finally, the clinical applications of methylation are
also described, with a focus on prognostic analysis and potential
clinical drug discovery.
Methylation
Epigenetic modifications mainly include chemical
modifications that occur on DNA, RNA and proteins (20). In general, chemical modifications
that occur on RNA are termed post-transcriptional modifications and
those that occur on proteins are termed post-translational
modifications (21). These
epigenetic modifications do not change the genetic code of genes,
but they have an important effect on gene expression (21). DNA modification is broadly divided
into DNA methylation, which silences the expression of genes, and
DNA phosphorylation, which affects the structure and function of
DNA (22). RNA modifications
include N6-methyladenosine (m6A), N6,2′-O-dimethyladenosine (m6Am)
and N1-methyladenosine (23).
Among them, m6A, the methylation of the N-6 adenosine base, is the
most widely studied, affecting splicing, output, stability,
degradation and translation, and thus affecting gene expression
(24). As for protein
modifications, phosphorylation is one of the most abundant
post-translational modifications, which is involved in the
regulation of cell signal transduction, protein-protein interaction
and gene transcription (25).
Ubiquitination, acetylation and methylation can also affect
protein-protein interactions, protein stability, subcellular
localization or enzymatic activity (26).
Methylation refers to the process of transferring
methyl groups from active methyl compounds to other compounds,
which results in the formation of various methyl compounds, or
chemical modifications in proteins or nucleic acids to form
methylated products (27,28). Based on the substrate to which the
methyl group binds, methylation is mainly divided into DNA
methylation, RNA methylation and protein methylation (Fig. 1) (28). Methyl donors are mainly derived
from one-carbon metabolism (29),
which is a one-carbon unit metabolic process, including folate
cycle, the methionine cycle and trans-vulcanization pathway,
wherein the methionine cycle is the main pathway to produce methyl
donors (29). In the methionine
cycle, one carbon unit can be used to remethylate homocysteine to
produce methionine (30).
Methionine produces s-adenosylmethionine (SAM) with the help of
methionine adenylyl transferase, and transfers methyl groups to
biomolecules, including proteins, nucleic acids and lipids
(30). The change of methylation
status is caused by the difference in the activity of
methyltransferase and demethylase (31), with SAM as the main methyl donor of
these enzymes (31). Alterations
in the methylation of proteins, nucleotides and metabolites can
lead to the occurrence of cancer (31).
DNA methylation
DNA methylation is a chemical modification process,
in which the cytosine of vertebrate CpG dinucleotides is catalyzed
by DNMTs and acquires a methyl group using SAM as the methyl donor
(27). DNA methylation occurs on
the N-6 of adenine, N-7 of guanine, and C-5 of cytosine (31). However, DNA methylation primarily
occurs on the cytosine of 5′-CpG-3′, resulting in the formation of
5-methylcytosine (27). A portion
of CpG dinucleotides is dispersed throughout the genome, while
another portion occurs in dense clusters of CpG islands (32). In normal tissues, most of the
dispersed CpG is methylated, while CpG islands tend to be
unmethylated (32). Normally, CpG
dinucleotides in relatively useless or unfavorable genomes are rare
and are always in a methylated state (33). By contrast, CpG islands rich in CpG
dinucleotides with a size of 100-1,000 bp in the genome are always
unmethylated, and CpG islands are often located near the
transcriptional regulatory region and are associated with more than
half of the genes encoded (33).
Therefore, it is necessary to study the methylation of CpG islands
in the transcription region.
The hypermethylation of CpG sites in enhancers or
promoters leads to transcriptional silencing, whereas the
hypomethylation of CpG sites usually leads to transcriptional
activation, so methylation regulates gene expression through gene
transcription (27). DNA
methylation is mainly dependent on the DNMT family, which has five
members including DNMT1, DNMT2, DNMT3A, DNMT3B and DNMT3L (27). DNMT1 is primarily involved in DNA
methylation, which is required to silence tumor suppressor genes
(16). DNMT2 is an RNA
methyltransferase that modifies cytosine residues in certain tRNA
anticodon rings (16). The key
role of DNMT3A and DNMT3B is de novo methylation (34) Although DNMT3L is a member of the
DNMT3 family, it does not have methyltransferase activity (34), however, DNMT3L can assist
DNMT3A/B-mediated de novo methylation (35). In addition, DNMT3A includes two
isoforms, while DNMT3B contains >30 isoforms, all of which have
methylation activity and conserved C-terminal domains (35). In conclusion, DNMT1 participates in
DNA methylation, whereas DNMT3A and DNMT3B, whose primary role is
de novo methylation, could also be involved in DNA
methylation (16).
DNA methylation has been detected at a very early
stage, and it is also a common epigenetic phenomenon, which plays
an important role in the maintenance of the stability of the genome
and the regulation of normal physiological functions (36). In the process of tumorigenesis,
there is a decrease in the DNA methylation level of most genes and
an increase in the methylation level of CpG islands of some genes,
including DNA repair genes, cell cycle genes and apoptosis genes
(36). Low levels of DNA
methylation not only lead to reduced genomic stability and mutation
rates, but also abnormally activate the expression of multiple
oncogenes, such as chromatin modifiers and transcription factors
(37). By contrast, high levels of
DNA methylation indirectly induce malignant tumors by decreasing
the transcriptional activity of tumor suppressor genes and then
affecting the expression (37).
RNA methylation
In addition to DNA methylation, RNA can also be
methylated to participate in the regulation of gene expression
(38). RNA methylation is a
post-transcriptional modification that transfers methyl groups from
methyl donors to RNA bases with the help of RNA methyltransferase,
thereby regulating stability, splicing, localization and
translation (38,39). RNA methylation modification is
reversible and occurs in different types of RNA, including microRNA
(miRNA), transfer RNA (tRNA), ribosomal RNA (rRNA), small nuclear
RNA (snRNA) and small nucleolar RNA (38,40).
RNA methylation occurs at the m6A methylation
modification at adenylate N6, which is the most abundant RNA
modification found in eukaryotes (41). The m6A methylation has its
methylase (writer), demethylase (eraser) and methylation
recognition protein (reader), which synergistically mediate RNA
methylation (41). The m6A
methylation in mRNA is mainly catalyzed by the METTL3/METTL14
complex (40). METTL3 primarily
acts as a catalyst, while METTL14 is an allosteric activator that
helps bind to the target RNA (40). METTL3 is mainly located in the
nucleus, but METTL3 is present in the cytoplasm in several cells
(42). The majority of m6A
methylation occurs in rRNA, mediated by the
N6-adenosine-methyltransferase zinc finger CCHC-type containing 4
and methyltransferase-like 5/tRNA methyltransferase activator
subunit 11-2 complexes (41). In
addition, m6A methylation of snRNA U6 is catalyzed by METTL16,
which also catalyzes small amounts of mRNA and other non-coding
RNA, in particular microRNAs and lncRNAs (43). The m6A demethylase is an enzyme
that converts m6A to adenylate, thereby removing m6A, and this
process is catalyzed by obesity-associated protein (FTO) and AlkB
homolog 5 (ALKBH5) (43). FTO
catalyzes the demethylation of 3-methyluridine, m6A and m6Am, but
which RNA is mainly targeted is still controversial (44). Furthermore, m6Am methylation in
snRNA may be a specific target of FTO, and its mechanism remains to
be further revealed (44).
Although ALKBH5 is a demethylase, it has no effects on m6Am
(45). As for m6A recognition
proteins, it is usually recruited by m6A methylation, which affects
mRNA functions such as localization (46). The m6A methylation recognition
proteins include the YTH domain family (YTHDF1/2/3 and YTHDC1/2),
hnRNP family (hnRNPC, hnRNPG and hnRNPA2B1) and insulin-like growth
factor 2 mRNA-binding proteins (IGF2BPs) (46). The YTH domain can directly bind to
m6A, and YTHDF2 can recruit complexes to promote the degradation of
m6A-modified mRNA (47). By
contrast, IGF2BPs can enhance the stability and translation
efficiency of m6A-modified mRNA (48). HnRNPC selectively recognizes
m6A-induced splicing in the secondary structure of mRNA (49).
Protein methylation
Protein methylation refers to the methylation of
arginine or lysine in the protein (50). Arginine can be methylated once or
twice by arginine methyltransferase, whereas lysine can be
methylated 1-3 times by lysine methyltransferase (51). Among them, arginine
methyltransferase transfers two methyl groups to the same nitrogen
atom of the arginine polypeptide to form asymmetric
dimethylarginine, and one methyl group is added to each nitrogen
terminal to form symmetric dimethylarginine (51).
Among the protein methylation, the methylation of
histones has been studied most extensively (51). Histone methylation is the
methylation of lysine or arginine residues on the N terminal of the
H3 or H4 histones (51,52). The effects of histone methylation
are mainly reflected in heterochromatin formation, gene imprinting,
X chromosome inactivation and transcriptional regulation (53). This process is catalyzed by histone
methyltransferase (HMT), which can be divided into histone lysine
methyltransferase and protein arginine methyltransferase (PRMT)
(53). H3 lysine (H3K) 4, 9, 27,
36, 79 and H4 lysine (H4K) 20 can be methylated, with histones H3K4
and H3K9 being the two most common modification sites (54). Likewise, the histone lysine
residues can undergo single, double or trimethylation, while the
arginine residues undergo only mono-methylation and di-methylation
(54). The methylation of
different sites of histone H3 and H4 and the amount of methylation
have different influences on transcriptional regulation. In most
cases, H3K9me3, H3K27me3 and H4K20me2/3 mediate transcriptional
suppression, while H3K4me1/3, H3K9me1, H3K27me1, H3K36me1/3 and
H3K79me1/3 mediate transcriptional activation (54).
In addition to HMT, there are also histone
demethylases. Histone demethylases can be divided into two
families: i) The lysine-specific demethylase (LSD) family; and ii)
the Jumonji C domain-containing (JMJD) family (55). LSD can specifically remove the
mono-methylation and di-methylation of histones H3K4 and H3K9,
while JMJD can remove the tri-methylation of lysine (55). Therefore, methylase and demethylase
together promote the stability and dynamics of histone
methylation.
Tamoxifen
Tamoxifen in a clinical setting
In 1978, the FDA approved tamoxifen for the
treatment of patients with advanced BC (56). The 2010 ASCO guidelines recommend
tamoxifen as the standard for premenopausal women, and at present
has become an important drug for adjuvant endocrine therapy of BC
(57).
Currently, tamoxifen is mainly used in patients with
hormone receptor-positive BC, and it is mostly used in
premenopausal patients or postmenopausal patients who cannot
tolerate aromatase inhibitors (58). In addition, tamoxifen can also be
used in the following situations: i) To treat recurrent metastatic
BC and ovarian cancer (59); ii)
for adjuvant treatment of lymph node-negative BC after breast
surgery, and radiotherapy and chemotherapy (60); iii) it is used for the adjuvant
treatment of postmenopausal breast surgery and lymph node-positive
BC after radiotherapy and chemotherapy (60); and iv) for patients with ductal
carcinoma in situ after breast surgery and radiation as it
may reduce the risk of invasive BC (61). Moreover, clinical studies suggest
that for women with a family history of BC, the use of tamoxifen
can reduce their risk of BC by more than one-third (62–65).
Therefore, tamoxifen is also used for BC prevention. The
conventional dose of tamoxifen for BC is 20 mg, but it can also be
increased to a maximum of 40 mg/day (66), and can be taken by mouth as a
single dose or in two equal doses (66). The conventional endocrine treatment
cycle is 5 years, and the dose of the drug and the treatment drug
can also be changed after 2 years of tamoxifen treatment (67). However, long-term use of tamoxifen
leads to a series of adverse reactions, including secondary
estrogen effects, gastrointestinal reactions, neuropsychiatric
symptoms and bone marrow suppression (68). Secondary antiestrogenic effects
include facial flushing, vulvar pruritus, menstrual disorders,
amenorrhea, increased leucorrhea and vaginal bleeding (68,69).
Gastrointestinal reactions include loss of appetite, nausea,
vomiting and diarrhea (69).
Neuropsychiatric symptoms include headache, dizziness and
depression (68). Furthermore,
several patients may suffer from transient leukopenia and
thrombocytopenia (69). In
addition, large doses and long-term application can lead to visual
impairment, rash, hair loss, weight gain and liver dysfunction
(70). However, most patients
experience relatively mild symptoms, which can be overcome and will
be relieved after stopping the drug in later stages (70). However, it must be noted that the
use of tamoxifen may be associated with more serious adverse
effects including venous thrombosis and endometrial cancer (EC),
but this is less likely to occur (71). Therefore, patients should have a
gynecological examination once a year during the use of tamoxifen
(71).
Tamoxifen is an estrogen receptor modulator, and its
main target is ERα (72). ERα
promotes intracellular signaling primarily through
estrogen/ERα-mediated nucleus-initiated steroid signaling (genomic
signaling) (72). The process can
be divided into three steps. First, estrogen enters the cell
through diffusion or in situ synthesis. Second, estrogens
bind to ERα in the nucleus, which activates and forms ERα
homodimers or heterodimers (73).
Finally, the activated ERα binds to the DNA enhancer estrogen
response element (ERE), so that the ERα-ERE complex promotes the
formation of the transcription initiation complex and induces
transcription (74). In addition
to the ERE mechanism, ERα binds to other transcription factors and
then binds to the activating protein 1 at the activating region of
the target genes to regulate gene transcription (73). By binding with ERα in BC, tamoxifen
blocks the binding of estrogen to ERα, making estrogen inactive,
either blocking stimulation of transcription or weakening its
effect, thus inhibiting the occurrence and development of BC
(75). In addition, tamoxifen can
up-regulate the production of transforming growth factor β, which
makes tamoxifen suitable for patients with osteoporosis (76).
Tamoxifen resistance
After long-term use of tamoxifen, some patients will
develop tamoxifen resistance, and its specific molecular mechanism
is complex and diverse. It includes downregulation of ERα,
up-regulation of ERβ, the emergence of BC stem cells (BCSCs) and
activation of the signaling pathway (Fig. 2) (12).
 | Figure 2.Mechanism of tamoxifen resistance.
The activation of the E2/ERα signaling pathway contributes to
tamoxifen sensitivity. By contrast, the activation of the
PI3K/AKT/mTOR signaling pathway leads to tamoxifen resistance. E2,
oestrogen; ERα, estrogen receptor α; PIP2, phosphatidylinositol
4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate;
IRS, insulin receptor substrate; PTEN, phosphatase and tensin
homolog; PDK1, 3-phosphoinositide-dependent protein kinase 1; TSC,
tuberous sclerosis complex; mTORC1, target of rapamycin complex 1;
4E-BP1, eukaryotic translation initiation factor 4E binding
protein; S6K, p70/85 S6 kinase; PPARγ, peroxisome
proliferator-activated receptor gamma; Elf4e, eukaryotic initiation
factor 4E. |
A number of studies indicate that the inhibition of
ERα expression may be the main cause of resistance to endocrine
therapy (12,14). Given that the mechanism of
tamoxifen is mainly mediated by ERα and the expression of ERα is a
good predictor of response to tamoxifen, loss of expression of ERα
has been widely recognized as a major factor in tamoxifen
resistance (12). Loss of ERα
expression may be associated with methylation of CpG islands and
increased histone deacetylation, resulting in a more compact
nucleosome structure that restricts ERα transcription and thus
limits the efficacy of tamoxifen (77). Moreover, loss of ERα has been
associated with tumor invasion and suggests a poor prognosis. In
addition, a study showed that re-expression of ERα can reverse
tamoxifen resistance in MCF-7 cells, suggesting that loss of ERα
expression may be an important mechanism of tamoxifen resistance
(12). Similarly, ERα protein
point mutations (such as K303R) enhance ERα-mediated cell growth,
which is caused by increased estrogen sensitivity and alters
various cellular signaling pathways (78). Since these signaling pathways
normally downregulate ERα signaling, the ERα signaling pathway is
inhibited and patients become resistant to tamoxifen (78). There are also multiple ERα
mutations in the ligand-binding region of ERα, and in the absence
of ligands, these mutations promote ERα conformational changes that
lead to the proliferation of hormone-independent tumor cells and
resistance to tamoxifen (12). ERβ
is a product of ESR2 located at chromosome 14q.21 (79). ERβ and ERα have 96% homology in the
DNA binding region and 59% homology in the ligand binding region
(79). Interestingly, the
activation of ERβ expression enhances tamoxifen resistance, which
is mainly determined by the physiological function of ERβ (12). ERβ is highly homologous to ERα and
binds to estrogen with a similar affinity to ERα (80). ERα and ERβ respond in different
ways depending on the ligands and the responding elements (81). ERα up-regulates the expression of
genes related to cell growth (81). However, ERβ is more abundant than
ERα expression in normal breast cells (72). In ERβ knockout mice, the
proliferation of mammary cells accelerated, whereas in ERα knockout
mice, the mammary cells shrank (72). ERβ has various effects on
ERα-related regulatory genes, which include regulating the
expression of the ERα gene and enhancing or weakening the
effect of ERα (82). Overall, ERβ
inhibited ~70% of ERα-regulated genes (82). In contrast to tamoxifen-sensitive
tumors, ERβ was ~2 times higher in tamoxifen-resistant tumors than
ERα (12). Thus, ERβ expression
activated by ERβ-selective agonists, such as LY500307, may promote
tamoxifen resistance by inhibiting ERα (72). However, studies have also shown
that some ERβ splicing variants can lead to the development of
tamoxifen resistance, which is associated with a poorer prognosis
in advanced BC (79,83–85).
In addition, the expression of ERβ in tumor infiltrating leukocytes
was much higher than that of ERα in the tumor microenvironment,
suggesting that ERβ may alter the tamoxifen response by influencing
immune cells (72).
Except for ER, abnormal activation of signaling
pathways can also lead to tamoxifen resistance. The related
signaling pathways include the PI3K/AKT/mTOR signaling pathway, the
NF-κB signaling pathway and the Hedgehog signaling pathway
(75,78,86).
The most important of these is the PI3K/AKT/mTOR signaling pathway
(87). The PI3K/AKT/mTOR signaling
pathway regulates multiple biological processes, including cell
proliferation, apoptosis and metabolism (87). This signaling pathway is a complex
regulatory network involving multiple components including upstream
regulation, internal regulation and downstream regulation. Its
upstream regulation is receptor tyrosine kinase (RTK) (88). When the upstream protein is
activated by external stimuli, such as growth factors, the RTK
undergoes a conformational change that activates its tyrosine
kinase activity (88). The
activated RTK binds directly to p85, the subunit of PI3K, causing
p85 to release its inhibition on p110, thereby activating PI3K
(89). Activated PI3K converts
phosphatidylinositol diphosphate (PIP2) to phosphatidylinositol
triphosphate (PIP3) (89). After
which, PIP3 attracts PDK1 and AKT, enabling PDK1 to phosphorylate
AKT at Thr308, thereby activating AKT (90,91).
In addition to AKT, the inhibitory factor of internal regulation is
PTEN (92). PTEN can
dephosphorylate PIP3 to PIP2, which limits the activation of the
signaling pathway (92).
Therefore, the absence or abnormal function of PTEN leads to
overactivation of the PI3K/AKT/mTOR signaling pathway (93). Activated AKT phosphorylates
multiple downstream target proteins and thus participates in
cellular physiological processes. Phosphorylated proteins targeted
by AKT include mTOR, FOXO, GSK3, Bcl-2 and NF-κB (88,94–96).
Activation of the PI3K/AKT/mTOR signaling pathway was found to
cause tamoxifen-resistant cells to become resistant to DNA-damaged
chemotherapy by up-regulating BARD1 and BRCA1, suggesting that the
PI3K/AKT/mTOR signaling pathway is important in the treatment of BC
(97). In addition, high
expression of phosphorylated AKT is associated with poor prognosis,
and the inhibition of AKT expression is conducive to activation of
drug-resistant cells (87). While
multiple drugs targeting the PI3K/AKT/mTOR signaling pathway have
been used to overcome tamoxifen resistance, inhibiting this pathway
will activate the compensatory mechanism due to the complexity of
the PI3K/AKT/mTOR pathway, which limits the effects of inhibitors
(87).
The cancer stem cell model is another important
factor in BC resistance to tamoxifen (89). BCSCs refer to a small subset of BC
cytoplasmic cells that have the ability to self-renew,
differentiate and perform tumorigenesis (98). Clinically, BCSCs are considered to
be relatively resistant to radiotherapy, chemotherapy and
molecularly targeted therapy, leading to the development of drug
resistance and cancer recurrence (99). CD44+/CD24−
and ALDH+ are the most common molecular markers of
BCSCs, and other surface proteins are also considered markers of
BCSCs, such as CD133, CD61, C-X-C chemokine receptor type 4 (CXCR4)
and microsatellite instability (100,101). Ectopic expression of ERα
mutations (such as Y537S, Y537N and D538G) enriches
CD44+/CD24− cells, increases the formation of
mammospheres, and upregulates a variety of stemness genes such as
octamer-binding protein-4, SRY-box transcription factor 2, SRY-box
transcription factor 9 (SOX9) and B-cell-specific Moloney murine
leukemia virus integration site 1, thereby promoting BCSC
enrichment and leading to endocrine resistance (102). Furthermore, PI3K/AKT/mTOR, Notch,
Wnt and Hippo signaling pathways can also promote the enrichment of
BCSCs, thus leading to the generation of endocrine therapy
resistance (103). Based on the
study of these BCSCs models, a number of therapeutic options are
being gradually introduced to the clinic, including multi-drug
chemotherapy and molecular targeted therapy (100). For example, the γ-secretase
inhibitor MK-0752 in combination with tamoxifen or letrozole in
patients with early-stage BC, and the γ-secretase inhibitor
RO4929097 in combination with exemestane in patients with advanced
BC are already in clinical trials (104–107).
In addition to the aforementioned three mechanisms,
other proteins and RNAs play a crucial role in the complex network
of tamoxifen. For example, SOX9, HDAC1, SIRT1 and HIF-1αcan promote
BC resistance to tamoxifen (108–111). Furthermore, several miRNAs (such
as miR-342 and miR-375) and LncRNA can also alter the BC response
to tamoxifen through multiple mechanisms (112–114).
Effects of methylation on the tamoxifen
response
Estrogen receptor
In the majority of cases, methylation affects
tamoxifen sensitivity and resistance by altering transcription of
the ESR1 gene and ERα protein-mediated transcription
(115–118). In general, up-regulation of ERα
protein or enhanced ERα-mediated transcriptional regulation
promotes tamoxifen sensitivity (Table
I; Fig. 3) (12). By contrast, inhibition of ERα
protein expression or reduction of ERα-mediated transcriptional
regulation may promote tamoxifen resistance (Table I; Fig.
3) (12).
 | Figure 3.Effects of ERα methylation on
tamoxifen. Methylation-induced downregulation of ERα expression or
ERα signaling leads to tamoxifen resistance. MLL, mixed-lineage
leukemia protein; H3K4, histone H3 lysine 4; TET2, ten-eleven
translocation methylcytosine dioxygenase 2; ZEB1, zinc finger
E-box-binding homeobox 1; IL-1β, interleukin-1β; ESR1, estrogen
receptor 1; ID4, inhibitor of differentiation 4; miR, microRNA;
WT1, Wilms' tumor 1; EZH2, enhancer of zester homolog 2; H3K27,
histone H3 lysine 27; GREB1, gene regulated by estrogen in breast
cancer 1; UCHL1, ubiquitin C-terminal hydrolase L1; NAT1,
N-acetyltransferase type 1; ELOVL2, elongation of very long chain
fatty acids-like 2; PRA, progesterone receptor α; PTPRO, protein
tyrosine phosphatase receptor type O; PAX2, paired box 2; MMP1,
matrix metalloproteinase 1. |
 | Table I.Effect of ESR1-related
methylation on tamoxifen response. |
Table I.
Effect of ESR1-related
methylation on tamoxifen response.
| Genea | Enzymeb | Typec | Numberd | ERαe |
Tamoxifenf | (Refs.) |
|---|
| ESR1 | MLL3 and SET1A | H3K4 me3 | me3 | Up | Sensitivity | (116,122) |
| ESR1 | DNMT1 | DNA promoter | NA | Down | Resistance | (191,192) |
| ESR1 | DNMT3B | DNA promoter | NA | Down | Resistance | (117) |
| ESR1 | NA | DNA promoter | NA | Down | Resistance | (118) |
| ESR1 | TET2 | demethylation | NA | Up | Sensitivity | (207) |
| ERβ | NA | DNA promoter | NA | Down | Resistance | (120) |
| ESR1 | MLL1 | H3K4me3 | me3 | Up | Sensitivity | (121) |
| ESR1 | NA | DNA enhancer | NA | Down | Resistance | (123) |
| ESR1 | EZH2 | H3K27 me3 | me3 | Down | Resistance | (124) |
| GREB1 | DNMT1, DNMT3B | DNA promoter | NA | Down | Resistance | (125) |
| UCHL1 | TET1, TET3 | Demethylation | NA | Down | Resistance | (126) |
| p21 | EZH2 | H3K27me3 | me3 | Down | Resistance | (131) |
| WT1 | NA | DNA promoter | NA | Down | Resistance | (132) |
| ID4 | NA | DNA promoter | NA | Up | Sensitivity | (133) |
| miR-27b | NA | DNA promoter | NA | Up | Resistance | (134) |
| NAT1 | NA | DNA promoter | NA | Down | Resistance | (138) |
| ELOVL2 | NA | DNA promoter | NA | Down | Resistance | (140) |
| PRA | NA | DNA promoter | NA | Down | Resistance | (141) |
| PAX2 | NA | DNA promoter | NA | Up | Resistance | (144) |
|
E-cadherin | NA | DNA promoter | NA | Up | Resistance | (145) |
| MMP1 | NA | DNA promoter | NA | Down | Sensitivity | (146) |
| PTPRO | NA | DNA promoter | NA | Up | Resistance | (149) |
Firstly, the methylation of the ESR1 promoter
affects ESR1 transcription and ERα protein expression, thus
playing an important role in BC response to tamoxifen. DNA
methyltransferase-mediated hypermethylation of the ESR1
promoter is associated with poor prognosis and indicates the
development of tamoxifen resistance (115). The methylation of H3 is also
involved in ESR1 transcription and thus affects the tamoxifen
response. MLL3 and SET1A-mediated methylation of histone H3K4
enhance ESR1 transcription, thereby promoting sensitivity to
hormone therapy (116). In
addition, ZEB1 and IL-1β also promote tamoxifen resistance by
promoting the methylation of ESR1 (117,118). ZEB1 induces hypermethylation of
the ESR1 promoter and silencing of ERα by forming the
ZEB1/DNMT3B/HDAC1 complex on the ESR1 promoter, leading to
resistance to anti-estrogenic drugs (117). IL-1β induces EMT by activating
the IL-1β/IL-1RI/β-catenin pathway, thereby enabling TWIST1 to
induce methylation of the ESR1 promoter, which leads to
reduced expression of ERα and thus increased tamoxifen resistance
(118). Similarly, loss of
demethylase also mediates tamoxifen resistance. Deletion of the DNA
demethylase TET2 promotes the methylation of ESR1, thereby
downregulating the expression of ERα and thus promoting tamoxifen
resistance (119). Interestingly,
ERβ showed the exact opposite effect to ERα in response to
tamoxifen. In tamoxifen-resistant BC, ERβ is hypomethylated,
suggesting that ERβ hypermethylation harms tamoxifen resistance
(120).
Secondly, the methylation-mediated enhanced
transcription of ERα-targeted genes can promote tamoxifen
sensitivity, and vice versa. For example, ANCCA mediates the
recruitment of MLL1 HMT at the promoters of ESR1 target genes for
H3K4 methylation associated with gene activation, which may
effectively induce tamoxifen sensitivity (121). Similarly, SETD1A is also involved
in H3K4 methylation, subsequent ERα recruitment, and transcription
of ERα target genes (122).
Conversely, inhibition of ESR1 transcription or ERα-mediated
transcription promotes BC resistance to tamoxifen. Hypermethylation
of estrogen response enhancers leads to reduced binding to ERα and
downregulation of key regulators of ERα activity, resulting in
weakened endocrine responses (123). By contrast, the hypomethylation
of enhancers plays a role in the transformation of normal breast
cells into endocrine-reactive BC (123). Overall, the methylation of the
ESR1 enhancer has potential in endocrine stratification
therapy (123). Moreover,
EZH2-mediated trimethylation of H3K27 promotes BC susceptibility
(124). The single nucleotide
polymorphism of EZH2 further promotes the methylation of H3K27,
which inhibits the transcription, thereby reducing overall survival
(OS) and progression-free survival in patients that are
ER-positive/tamoxifen-treated (124). EZH2 may also promote the
methylation of the GREB1 promoter through DNMT1 and DNMT3B,
thereby silencing GREB1 and inhibiting ERα transcription,
thus promoting tamoxifen resistance (125). Moreover, TET1 and TET3 promote
demethylation of the UCHL1 promoter, thereby promoting
UCHL1 transcription, which further downregulates ERα
expression via the UCHL1-KLF5 axis, leading to tamoxifen resistance
(126).
Finally, the methylation can also respond to
tamoxifen through promoter methylation of ERα-associated genes.
Among them, the upstream genes of ERα include p21 (127), WT1 (128) ID4 (129) and miR-27b (130). Activation of p21 activates ERα
transcription in ER-negative BC, thereby activating the ERα
signaling pathway (129). LncRNA
UCA1 interacts with the enhancer of EZH2, which inhibits p21
expression through H3K27 histone methylation on the p21
promoter, thereby promoting tamoxifen resistance (131). In the development of tamoxifen
resistance, WT1 is involved in the expression of ERα (128). Through RNA-seq and TCGA
databases, Ren et al (132) found that WT1 was
hypermethylated and upregulated in all molecular subtypes of BC,
which was closely related to the efficacy of tamoxifen in patients
with BC. By contrast, ID4 inhibits ERα expression and regulates
estrogen biosynthesis (129). In
BC resistant to tamoxifen, ID4 is hypomethylated, suggesting that
it may be the key to identifying drug resistance (133). However, there was no difference
between ID4 hypermethylation and ID4 hypomethylation on the risk of
disease progression (P=0.287) (133). As for miR-27b, it targets ERα to
exert its anti-proliferation and anti-metastasis effects (130). The methylation of the
miR-27b promoter promotes activation of HMGB3, leading to
tamoxifen resistance (134). The
downstream genes of ERα are relatively rich, including NAT1
(135), ELOVL2 (136) and PRA (137). High expression of ERα enhances
the expression of NAT1, ELOVL2 and PRA, so that the
expression level of ERα is positively associated with NAT1,
ELOVL2 and PRA (135–137). The increased methylation of these
gene promoters leads to decreased expression, which may lead to the
dysfunction of ERα and induce tamoxifen resistance (138–143). Using methylation-specific PCR and
bisulfite genomic sequencing, Kim et al (138) found that the methylation of
NAT1 was significantly enhanced in tamoxifen-resistant BC,
while the methylation of COMT, CYP1A1, CYP2D6 and
SULT1A1 was not significantly altered compared with the
control group. Therefore, hypermethylation of NAT1 may
influence the initiation of tamoxifen resistance (138). LncRNA H19 mediates methylation of
the NAT1 promoter to downregulate NAT1 expression, which
leads to tamoxifen resistance in BC (139). Similarly, the hypermethylation of
the ELOVL2 promoter and the PRA promoter downregulate
ELOVL2 and PRA to drive tamoxifen resistance
(140,141). Notably, the majority (74%) of
patients with PRA in PRA-negative BC did not exhibit
methylation status (141).
However, hypermethylation of downstream genes negatively associated
with ERα expression also promotes tamoxifen resistance, such as
PAX2 (142) and
E-cadherin (143).
Estrogen receptors promote methylation of the PAX2 promoter,
thereby downregulating its expression (142). In patients with
tamoxifen-resistant BC, abnormally elevated methylation of
PAX2 promoter leads to decreased expression of PAX2 mRNA
(144). The estrogen/ERα
signaling pathway downregulates E-cadherin to promote BC (143). The methylation of E-cadherin
leads to the downregulation of E-cadherin expression, promoting the
upregulation of Twist and the occurrence of EMT, thus resulting in
tamoxifen resistance (145). In
addition, although ERα upregulates the expression of MMP-1, the
hypomethylation of the MMP1 promoter leads to its
overexpression, thereby inducing tamoxifen resistance in BC
(146,147). As for ERβ, estrogen inhibits the
expression of PTPRO through ERβ, thus inducing the occurrence of BC
(148). The hypomethylation of
the PTPRO promoter elevates its expression, thus promoting
the sensitivity of tamoxifen (149).
PI3K/AKT/mTOR
In addition to ERα, the methylation-mediated
PI3K/AKT/mTOR signaling pathway and methylation on the
PI3K/AKT/mTOR signaling pathway also influence tamoxifen resistance
(12,14). In short, methylation-induced
activation of the PI3K/AKT/mTOR signaling pathway contributes to
tamoxifen resistance (Table II;
Fig. 4) (14). By contrast, methylation-induced
inactivation of the PI3K/AKT/mTOR signaling pathway promotes
tamoxifen sensitivity (Table II;
Fig. 4) (14).
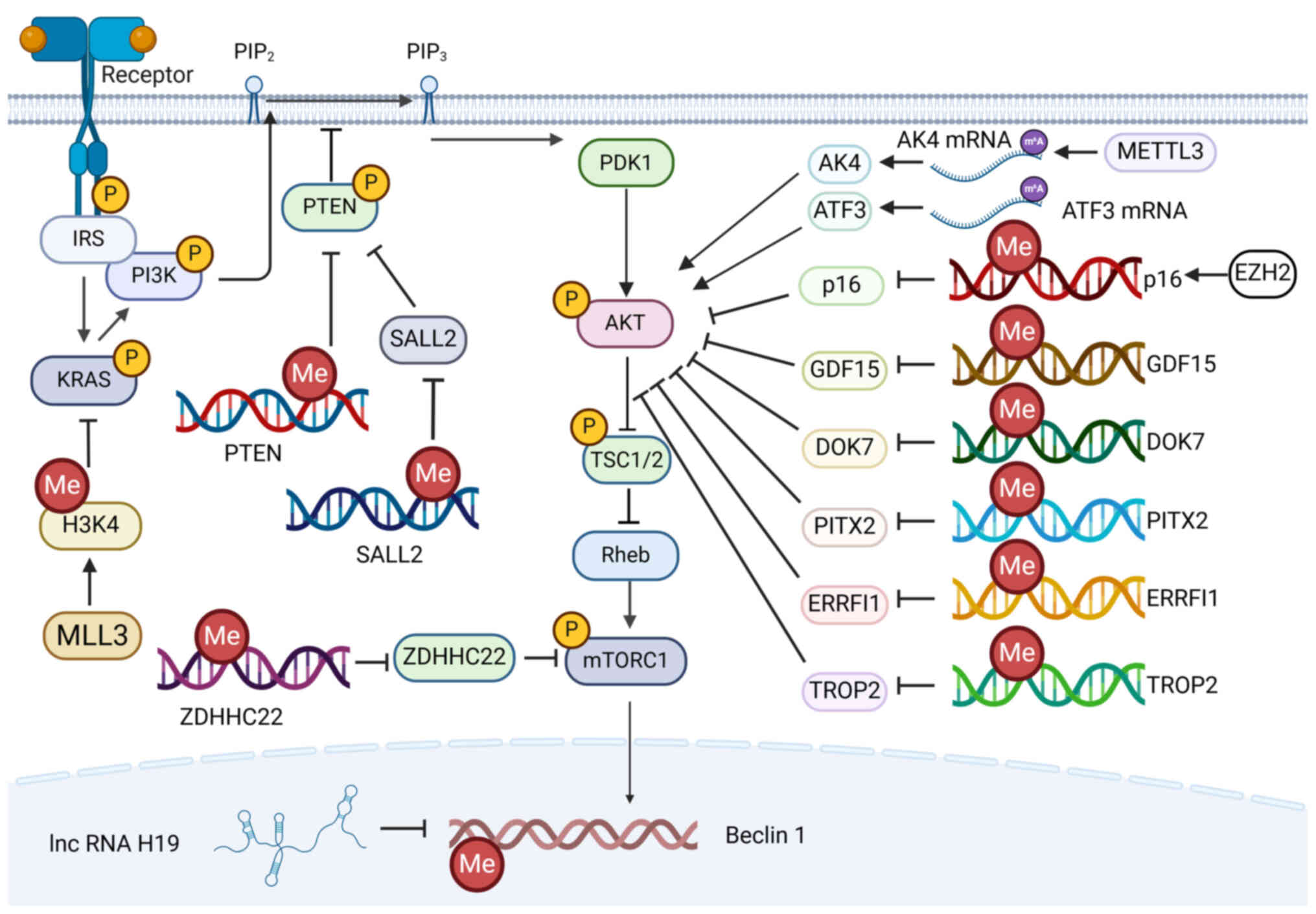 | Figure 4.Effects of methylation of the
PI3K/AKT/mTOR signaling pathway on tamoxifen. The
methylation-induced activation of the PI3K/AKT/mTOR signaling
pathway leads to tamoxifen resistance. PIP2, phosphatidylinositol
4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate;
IRS, insulin receptor substrate; PTEN, phosphatase and tensin
homolog; PDK1, 3-phosphoinositide-dependent protein kinase 1; TSC,
tuberous sclerosis complex; mTORC1, target of rapamycin complex 1;
H3K4, histone H3 lysine 4; MLL, mixed-lineage leukemia protein;
SALL2, Sal-like protein 2; ZDHHC22, Zinc finger DHHC-type
containing 22; METTL3, methyltransferase-like 3; AK4, adenylate
kinase 4; ATF3, activating transcription factor-3; EZH2, enhancer
of zester homolog 2; GDF15, Growth differentiation factor-1; DOK7,
downstream of kinase 7; PITX2, paired-like homeodomain
transcription factor 2; ERRFI1, ERBB receptor feedback inhibitor 1;
TROP2, tumor-associated calcium signal transducer 2. |
 | Table II.Effect of PI3K/AKT/mTOR-related
methylation on the tamoxifen response. |
Table II.
Effect of PI3K/AKT/mTOR-related
methylation on the tamoxifen response.
| Genea | Enzymeb | Typec | Numberd |
PI3K/AKT/mTORe |
Tamoxifenf | (Refs.) |
|---|
| PTEN | DNMT1 | DNA promoter | NA | Up | Resistance | (151) |
| AKT | SETDB1 | NA | NA | Up | Resistance | (153) |
| ERRFI1 | NA | DNA promoter | NA | Up | Resistance | (157) |
| PITX2 | NA | DNA promoter | NA | Up | Resistance | (158) |
| DOK7 | NA | DNA promoter | NA | Up | Resistance | (159) |
| AK4 | METTL3 | RNA | m6A | Up | Resistance | (161) |
| SALL2 | NA | DNA promoter | NA | Up | Resistance | (162) |
| ZDHHC22 | NA | DNA promoter | NA | Up | Resistance | (163) |
| p16 | EZH2 | DNA promoter | NA | Up | Resistance | (166) |
| GDF15 | NA | DNA promoter | NA | Up | Resistance | (167) |
| ATF3 | YTHDF2 | RNA | m6A | Up | Resistance | (169) |
| Beclin1 | DNMT3B | DNA promoter | NA | Up | Resistance | (171) |
| TROP2 | DNMT1 | DNA promoter | NA | Down | Resistance | (173) |
| Ras | NA | H3K4me1 | me1 | Down | Sensitivity | (194) |
Above all, the key proteins in the PI3K/AKT/mTOR
signaling pathway (mainly including PTEN and AKT) can be methylated
to reduce their expression, resulting in an altered response to
tamoxifen. Low expression of PTEN due to hypermethylation of
the PTEN promoter (−819 to −787 bp) predicts poor
disease-free survival (DFS) and OS in patients with
hormone-receptor-positive BC treated with tamoxifen (150). More precisely, in the −819 to
−787 bp region of the promoter, only the hypermethylation of −796
CpG islands, but not the hypermethylation of the remaining four CpG
islands, predicted shorter DFS and OS (150). A total of two sites in the
PTEN promoter can be methylated by DNMT1, thereby
downregulating the expression of PTEN and increasing
phosphorylation of AKT, leading to tamoxifen resistance (151). By contrast, miR-146b reduced
NF-κB-mediated MAT2A expression, which inhibited SAM-mediated
methylation of the PTEN promoter to restore PTEN
expression, thereby reversing tamoxifen resistance (152). Furthermore, SETDB1 regulates the
expression of ER and AKT target genes by mediating the methylation
and phosphorylation of AKT through interaction with PELP1, thus
promoting tamoxifen resistance (153).
Next, the methylation of genes involved in
inhibiting the PI3K/AKT/mTOR signaling pathway activates the
PI3K/AKT/mTOR signaling pathway, thereby inducing tamoxifen
resistance. Firstly, as for the AKT protein in PI3K/AKT/mTOR
signaling pathway, ERRFI1 (154),
PITX2 (155) and DOK7 (156) all can downregulate the expression
of phosphorylated AKT, thereby inhibiting the PI3K/AKT/mTOR
signaling pathway. The downregulated expression of ERRFI1 (157), PITX2 (158) and DOK7 (159) due to the hypermethylation of
their promoters leads to impaired inhibition of the PI3K/AKT/mTOR
signaling pathways, thereby inducing tamoxifen resistance in BC.
Moreover, the silence of AK4 can downregulate the expression of
p-AKT (160). METTL3-mediated
increased methylation at multiple m6A sites of the 5′-UTR of AK4
mRNA stabilizes AK4 mRNA and thus increases ROS and p38 levels,
leading to tamoxifen resistance (161). Secondly, inhibition of PTEN can
also activate the PI3K/AKT/mTOR signaling pathway. In
tamoxifen-resistant BCs, the SALL2 promoter is
hypermethylated, which inhibits SALL2 expression and leads
to SALL2-mediated transcription suppression of ESR1
and PTEN, thus promoting the AKT/mTOR signaling pathway and
leading to tamoxifen resistance (162). Thirdly, the activation of mTOR
also ensures the function of PI3K/AKT/mTOR. The methylation of the
ZDHHC22 promoter leads to the low expression of ZDHHC22 in
ER-negative BC, which leads to the activation of the mTOR,
resulting in tamoxifen resistance (163). In addition, the reduction of p16
(164) and GDF15 (165) inhibits the occurrence and
development of cancer through the inhibition of PI3K/AKT/mTOR
signaling pathway. EZH2 downregulates p16 by promoting methylation
of p16, which regulates the cell cycle and leads to
tamoxifen resistance (166). In
tamoxifen-resistant BC, the promoter of GDF15 is
hypermethylated, which leads to low expression and tamoxifen
resistance (167).
After which, the hypomethylation of upstream
promoter genes of the PI3K/AKT/mTOR signaling pathway promotes
tamoxifen methylation via the PI3K/AKT/mTOR pathway. For example,
ATF3 mediates the PI3K/AKT/mTOR signaling pathway by activating AKT
phosphorylation, thereby increasing radiation resistance in BC
(168). In BC, low expression of
YTHDF2 leads to reduced hypomethylation of the 5′-UTR of ATF3 mRNA,
resulting in the stabilization of ATF3 mRNA, which stimulates the
expression of ABCB1 and leads to tamoxifen resistance (169). Similarly, the hypermethylation of
downstream genes of the PI3K/AKT/mTOR signaling pathway also
promotes tamoxifen resistance. The PI3K/AKT/mTOR signaling pathway
regulates phagocytosis in macrophages through Beclin1 (170). LncRNA H19 binds and inhibits
S-adenosylhomocysteine hydrolase, which inhibits DNMT3B binding to
the Beclin1 promoter and reduces Beclin1 methylation,
thus leading to autophagy dysregulation and tamoxifen resistance in
BC (171).
Ultimately, the hypermethylation of upstream
promoter genes of the PI3K/AKT/mTOR signaling pathway leads to
tamoxifen resistance, which may be mediated by pathways other than
the PI3K/AKT/mTOR signaling pathway. For instance, TROP2 promotes
cell proliferation and migration through the PI3K/AKT/mTOR
signaling pathway (172).
However, in tamoxifen-resistant BC cells, the promoter of
TROP2 is methylated and silenced, which is meditated by
DNMT1 (173).
Others
Except for ERα protein and PI3K/AKT/mTOR signaling
pathways, the methylation of several other genes can influence BC
sensitivity and resistance to tamoxifen. This can be divided into
two components: i) Hypermethylation-mediated tamoxifen resistance
(or hypomethylation-mediated tamoxifen sensitivity); and ii)
hypomethylation-mediated tamoxifen resistance (or
hypermethylation-mediated tamoxifen sensitivity).
For the first part, hypermethylation-mediated
tamoxifen resistance mainly includes VGLL4 (174), DPYD (175), ZNF350 (176) and MAGED1 (177). HAGLR inhibits VGLL4
expression by promoting DNMT1-mediated DNA hypermethylation,
thereby promoting tamoxifen resistance (174). In tamoxifen-resistant BC cells,
methylation of the DPYD promoter region leads to a decrease
in DPYD mRNA (175). In addition,
when the methylation inhibitor was applied in tamoxifen-resistant
BC, the methylation of ZNF350 and MAGED1 promoters
was significantly reduced, suggesting that ZNF350 and
MAGED1 may play a role in tamoxifen resistance (176).
For the second part, hypomethylation-mediated
tamoxifen resistance mainly includes TSTD1 (177), LDH (178), PAST1 (179) and GNB4 (180). The hypomethylation of the
TSTD1 promoter leads to upregulation of TSTD1, which
is associated with adverse reactions to tamoxifen therapy in
patients with BC (177). In
tamoxifen-treated cells, less methylation of the LDH
promoter led to increased LDH expression, suggesting that
LDH expression could promote tamoxifen resistance (178). Similarly, PSAT1 mRNA expression
inhibited by hypermethylation of the PSAT1 promoter predicts
a good prognosis after tamoxifen treatment (179). Moreover, DNMT3B-mediated
methylation of GNB4 leads to silencing of GNB4, which
promotes tamoxifen sensitivity (180).
Effects of tamoxifen on methylation
The degree of methylation is also altered after
treatment with tamoxifen. However, there are relatively few studies
on this area and they are relatively less comprehensive. Overall,
tamoxifen reduces the level of methylation (109,181–183). Treatment of male rat embryos with
tamoxifen resulted in increased methylation of multiple imprinted
genes (such as Grb10, Igf2r, and Kcnq1), leading to downregulation
of the expression of these imprinted genes (181). In BC, tamoxifen increases
CXCL12 expression through reducing the methylation of
CXCL12 promoters, thus making cells less susceptible to
exogenous CXCL12 attraction to metastatic sites (178). In addition, tamoxifen altered the
methylation of the ESR1 promoter in patients with BC
(182). Tamoxifen induces PRMT5
to translocate to nucleus, where it methylates the ERα protein,
which causes corepressor proteins such as SMRT and HDAC1 to bind to
the target gene promoter of ERα, thereby inhibiting transcription
and cell proliferation (109). By
contrast, in tamoxifen-resistant cell lines, PRMT5 is predominantly
localized in the cytoplasm, suggesting that PRMT5 in the nucleus is
a biomarker of tamoxifen sensitivity (109). For the embryonic development of
sperm, the methylation of IGF2-H19 ICR in sperm is reduced
after tamoxifen treatment, triggering the sperm to acquire paternal
imprints and ensuring embryonic development (183). However, there was no significant
increase or decrease in the overall methylation level of the rats
(183).
It has been reported that after long-term tamoxifen
treatment, the body may induce the formation of second tumors,
especially EC, which may be related to tamoxifen-induced
methylation impairment (Table
III) (184–186). For example, the application of
tamoxifen leads to the hypomethylation of the promoters of
CXCR4 and CXCL12 by promoting the formation of DNA
methyltransferase 3B4 splice variant, thereby up-regulating the
expression of CXCR4 and CXCL12 in EC, thus promoting
cell proliferation and metastasis (185). Moreover, tamoxifen induces
hypomethylation of the PAX2 promoter, thereby activating
PAX2 expression, which induces EC (186). In addition to this, the use of
tamoxifen can also contribute to the development of liver cancer
through methylation (187).
Tryndyak et al (187)
reported that the application of tamoxifen reduces the expression
of DNMT1, DNMT3a and DNMT3b, which leads to the decrease of liver
DNA methylation, thus inducing liver cancer.
 | Table III.Potential drugs for methylation on
tamoxifen response. |
Table III.
Potential drugs for methylation on
tamoxifen response.
| Potential drug |
Methylationa | Targetb | Effectc |
Tamoxifend | Stagee | (Refs.) |
|---|
| Resveratrol,
astragalus | Inhibit | ESR1 | Promote ESR1
expression | Sensitivity | Preclinical | (190) |
| Arsenic
trioxide | Inhibit | ESR1 | Promote ESR1
expression | Sensitivity | Preclinical | (191) |
| 5-aza-cdr | Inhibit | ESR1 | Promote ESR1
expression | Sensitivity | Preclinical | (192) |
| Sodium
arsenate | Promote | ESR1, | Inhibit ESR1
and | Resistance | Preclinical | (193) |
|
|
| BRCA1 | BRCA1
expression |
|
|
|
| Procainamide | Inhibit | ERβ | Promote ERβ
expression | Resistance | Preclinical | (83) |
| Lycorine | Inhibit | VGLL4 | Promote
VGLL4 expression | Sensitivity | Preclinical | (174) |
| Luteolin | Promote | H3K4 | Inhibit Ras
expression | Sensitivity | Preclinical | (194) |
Clinical application
Given the complex crosstalk between methylation and
tamoxifen, it has multiple guidelines for clinical applications.
Specifically, this can be divided into two parts, namely, the
prediction of prognosis and recurrence of patients with BC treated
with tamoxifen, and the exploration of potential clinical
drugs.
Simply put, the higher degree of methylation, the
worse prognosis of patients and the greater risk of relapse. Using
the Illumina HumanMethylation450 BeadChip, Williams et al
(176) found that
tamoxifen-resistant cell lines share 3,000 hypermethylated and 200
hypomethylated CpG islands. For example, the proportion of patients
with PITX2 hypomethylation who did not have metastases after 10
years of tamoxifen treatment was higher than that of patients with
PITX2 hypermethylation (158).
Similarly, DOK7 CpG is hypermethylated in leukocytes of
patients which are tamoxifen-resistant, therefore the degree of
DOK7 methylation is important for early diagnosis of tamoxifen
resistance and prevention of cancer recurrence (159). Compared with the inactivation of
genes due to promoter hypermethylation, the activation of
growth-promoting genes due to promoter hypomethylation was also
observed in tamoxifen-resistant cells (188). PSAT1 mRNA expression inhibited by
hypermethylation of the PSAT1 promoter predicts a good
prognosis after tamoxifen treatment (179).
In addition, methylation of different promoters of
some genes can result in different prognoses. The methylation of
promoters in the U region of GR is associated with poorer
OS, while methylation of promoters in the C region of GR is
associated with improved OS (189). Thus, methylation of promoters in
specific regions of GR can suggest a poor prognosis in
patients who do not receive tamoxifen (189).
As for potential clinical drugs, the majority
promote BC sensitivity to tamoxifen by affecting DNMT. The
combination of resveratrol and astragalus leads to the
downregulation of DNMT activity and decreases the methylation of
the ESR1 promoter, thus promoting the expression of ERα,
which promotes tamoxifen sensitivity (190). Likewise, arsenic trioxide
(191) and 5-aza-CdR (192) also inhibit DNMT1-mediated
methylation of ESR1, thereby promoting ERα expression and
thus tamoxifen sensitivity. However, sodium arsenate induces the
recruitment of DNMT1, which increases CpG hypermethylation of
ESR1 and BRCA1, leading to tamoxifen resistance
(193). In addition, procainamide
promotes the expression of ERβ by inhibiting the methylation of the
ERβ promoter, thereby inhibiting the signaling of ERα, thus
inhibiting tamoxifen sensitivity (83). However, lycorine inhibits
DNMT1-mediated methylation of VGLL4, thereby promoting VGLL4
expression and reversing tamoxifen resistance (174). In terms of the PI3K/AKT/mTOR
signaling pathway, luteolin-induced MLL3 increases the
mono-methylation of H3K4 on the Ras enhancer and promoter,
thereby inhibiting Ras expression, which inhibits the
activation of the PI3K/AKT/mTOR signaling pathway, thereby
promoting tamoxifen sensitivity (194).
Discussion and conclusion
In summary, methylation promotes the development of
tamoxifen resistance (115–118). The downregulation of ERα
expression and abnormal activation of the PI3K/AKT/mTOR signaling
pathway are the main causes of tamoxifen resistance by DNA
methylation (10–14). By contrast, inhibition of
methylation promotes BC sensitivity to tamoxifen (115–118). In addition, elevated methylation
levels can be used as a predictive indicator of tamoxifen
resistance (188–190). Moreover, a methylation inhibitor
combined with tamoxifen is expected to improve the efficacy of
tamoxifen (83,192–194).
Current research on methylation-induced changes in
the efficacy of tamoxifen has focused on enhanced DNA methylation,
which leads to the downregulation of the gene, resulting in
tamoxifen resistance (18,144,173,195). However, methylation is not only
limited to DNA methylation, but also m6A methylation, which is
extremely important in RNA methylation and even protein methylation
(196). In addition, methylation
promotes tamoxifen resistance mainly by downregulating ERα and
up-regulating the PI3K/AKT/mTOR signaling pathway (118,131,145,194). However, the ERα and PI3K/AKT/mTOR
pathways are not isolated. Therefore, the crosstalk between them
may have a further impact on tamoxifen resistance. ERα activates
the PI3K/AKT/mTOR signaling pathway by downregulating PTEN
expression and activating PTEN phosphorylation (197). The PI3K/AKT/mTOR signaling
pathway acts as a bridge between growth factor and ERα signal
transduction (198). However, the
specific effect of tamoxifen, whether it increases resistance or
sensitivity, needs more research.
Although drugs that target DNA methylation are
gradually being used in the clinic and may provide new therapeutic
approaches for improving tamoxifen response and cancer treatment,
they are still at an early stage and there are numerous challenges
to overcome. The precise interaction between methylation and
tamoxifen resistance needs to be further elucidated, which will
affect the accuracy of clinical applications. This includes
controlling the selective effects of methyltransferases on target
cells and the complex association between target gene methylation
and the development of tamoxifen resistance. Although new
generation sequencing can detect high-throughput methylation sites
and accurately identify various DNA/RNA methylation patterns, it
does not directly detect DNA methylation in body fluids, but relies
more on DNA extraction and PCR techniques (199,200). In addition, inhibitors or
potential drugs that inhibit the function or activity of DNMT also
face challenges. Given that DNA methylation is present not only in
tumor cells, but also in normal cells, it is a challenge to
precisely target tumor cells during treatment with inhibitors or
potential drugs targeting DNMT without affecting the epigenetic
legacy modification of normal cells (201–203). Therefore, it is urgent to develop
DNMT inhibitors that are selective for tumor cells and, more
importantly, tamoxifen-resistant BC cells. Moreover, the
combination of methylation-targeted drugs with endocrine therapy
drugs such as tamoxifen is also an aspect worth considering.
Compared with monotherapy, combination therapy can effectively
reduce drug resistance (199).
However, whether the combination therapy has a synergistic effect
and whether it can minimize adverse reactions needs to be confirmed
by further studies.
Furthermore, the present review only describes the
crosstalk between methylation and tamoxifen. However, numerous
other post-translational modifications can also affect tamoxifen
resistance (184,204,205). Ubiquitination, acetylation and
phosphorylation can also directly affect the ERα protein and the
PI3K/AKT/mTOR signaling pathway, which may mediate the response to
tamoxifen (184,204,205). Therefore, other
post-translational modifications are worth investigation. In
addition, the various post-translational modifications are not
isolated, and they also have crosstalk with each other (206,207). For example, the methylation of
histone is regulated by histone ubiquitination or by enzymes that
catalyze ubiquitination (208).
These modifications and their crosstalk play important roles in
gene expression, genome stability, heterochromatin formation and
cancer development (208).
Furthermore, HMTs and demethylases are phosphorylated, suggesting
that phosphorylation can control the initiation and extent of
histone methylation (209).
Therefore, revealing the association between various modifications
can not only improve the crosstalk between methylation and
tamoxifen resistance, but also further improve the mechanism of
tamoxifen resistance, providing a theoretical basis for improving
the efficacy of tamoxifen.
Finally, tamoxifen is only one type of SERMs, and
other endocrine drugs that are classified as SERMs have a similar
mechanism to tamoxifen. Toremifene, raloxifene, opemifene,
lasoxifene and bardoxifene are members of the SERM family (184). They are all able to interact with
ER in specific tissues, resulting in conformational changes in the
receptor, thus affecting the ERα-mediated signaling pathway
(184). In theory,
methylation-mediated downregulation of ERα could also promote
resistance to these drugs. Unfortunately, this field of research is
relatively rare and incomplete. Therefore, the research in related
fields needs to be further explored. In addition, another class of
drugs also targets the estrogen receptor, termed selective estrogen
receptor degraders, and its representative drug is fulvestrant
(210). Fulvestrant inhibits the
binding of estrogen to ERα, but it promotes the ubiquitination
degradation of ERα to inhibit ERα signaling (184). Similar to tamoxifen, loss of
ESR1 methylation leads to expression of ESR1, which
restores sensitivity to fulvestrant (211). Although AKT inhibitors or PI3K
inhibitors combined with fulvestrant are effective in ER-positive
BC with palbociclib-resistance, it is not clear whether the
PI3K/AKT/mTOR signaling pathway is one of the mechanisms of
fulvestrant resistance (212). In
addition, whether the activation of the PI3K/AKT/mTOR pathway or
other signaling pathways promoted by methylation also leads to the
development of resistance to fulvestrant remains to be further
investigated. Furthermore, the present study that aimed to discuss
the effect of methylation on tamoxifen resistance, mainly focused
on preclinical studies, with phase I, II and III clinical trials
practically absent. Therefore, future research may consider
advancing the preclinical research into clinical practical
application.
Acknowledgements
Not applicable.
Funding
This research was funded by The General Project of Hunan
Provincial Chinese Medicine Research Program (grant no.
D2022012).
Availability of data and materials
Not applicable.
Authors' contributions
HC conceived the study; JS and HC wrote the
manuscript; JS, YH and SL collated the data; and HC revised and
edited the manuscript. All authors read and approved the final
version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Katsura C, Ogunmwonyi I, Kankam HKN and
Saha S: Breast cancer: Presentation, investigation and management.
Br J Hosp Med (Lond). 83:1–7. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singh H: Role of molecular targeted
therapeutic drugs in treatment of breast cancer: A review article.
Global Med Genet. 10:79–86. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhai J, Wu Y, Ma F, Kaklamani V and Xu B:
Advances in medical treatment of breast cancer in 2022. Cancer
Innov. 2:1–17. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chatterjee A: Long term effects of modern
breast cancer surgery. Gland Surg. 7:366–370. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haque W, Butler EB and Teh BS:
Personalized radiation therapy for breast cancer. Curr Oncol.
31:1588–1599. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartsch R and Bergen E: SABCS 2017: Update
on chemotherapy, targeted therapy, and immunotherapy. Memo.
11:204–207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi L, Chen Z and Wang X: ASCO 2018:
Recent advances in endocrine therapy for advanced breast cancer.
Chin J Clin Oncol. 45:919–921. 2018.
|
|
8
|
Bhave MA and Henry NL: Extended endocrine
therapy: Is 5 years enough? Curr Oncol Rep. 19:162017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barroso-Sousa R, Silva DDAFR, Alessi JVM
and Mano MS: Neoadjuvant endocrine therapy in breast cancer:
Current role and future perspectives. Ecancermedicalscience.
10:6092016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jordan VC and O'Malley BW: Selective
estrogen-receptor modulators and antihormonal resistance in breast
cancer. J Clin Oncol. 25:5815–5824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang W, Xu J, Shi Y, Sun Q, Zhang Q and
Guan X: The novel role of miRNAs for tamoxifen resistance in human
breast cancer. Cell Mol Life Sci. 72:2575–2584. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ring A and Dowsett M: Mechanisms of
tamoxifen resistance. Endocr Relat Cancer. 11:643–658. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yao J, Deng K, Huang J, Zeng R and Zuo J:
Progress in the understanding of the mechanism of tamoxifen
resistance in breast cancer. Front Pharmacol. 11:5929122020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang M: Tamoxifen resistance in breast
cancer. Biomol Ther (Seoul). 20:256–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y: Recent advances in methylation: A
guide for selecting methylation reagents. Chemistry. 25:3405–3439.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Castillo-Aguilera O, Depreux P, Halby L,
Arimondo PB and Goossens L: DNA methylation targeting: The DNMT/HMT
crosstalk challenge. Biomolecules. 7:32017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jahangiri R, Jamialahmadi K, Gharib M,
Emami Razavi A and Mosaffa F: Expression and clinicopathological
significance of DNA methyltransferase 1, 3A and 3B in
tamoxifen-treated breast cancer patients. Gene. 685:24–31. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jahangiri R, Mosaffa F, Emami Razavi A,
Teimoori-Toolabi L and Jamialahmadi K: Altered DNA
methyltransferases promoter methylation and mRNA expression are
associated with tamoxifen response in breast tumors. J Cell
Physiol. 233:7305–7319. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin X, Li J, Yin G, Zhao Q, Elias D,
Lykkesfeldt AE, Stenvang J, Brünner N, Wang J, Yang H, et al:
Integrative analyses of gene expression and DNA methylation
profiles in breast cancer cell line models of tamoxifen-resistance
indicate a potential role of cells with stem-like properties.
Breast Cancer Res. 15:R1192013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Y, Hong T, Wang S, Mo J, Tian T and
Zhou X: Epigenetic modification of nucleic acids: From basic
studies to medical applications. Chem Soc Rev. 46:2844–2872. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu L, Davis IJ and Liu P: Regulation of
EWSR1-FLI1 function by Post-Transcriptional and Post-Translational
modifications. Cancers (Basel). 15:3822023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zaib S, Rana N and Khan I: Histone
modifications and their role in epigenetics of cancer. Curr Med
Chem. 29:2399–2411. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu WW, Zheng SQ, Li T, Fei YF, Wang C,
Zhang S, Wang F, Jiang GM and Wang H: RNA modifications in cellular
metabolism: Implications for metabolism-targeted therapy and
immunotherapy. Signal Transduct Target Ther. 9:702024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song T, Lv S, Li N, Zhao X, Ma X, Yan Y,
Wang W and Sun L: Versatile functions of RNA m6A machinery on
chromatin. J Mol Cell Biol. 14:mjac0112022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Singh V, Ram M, Kumar R, Prasad R, Roy BK
and Singh KK: Phosphorylation: Implications in Cancer. Protein J.
36:1–6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu X, Xu M, Geng M, Chen S, Little PJ, Xu
S and Weng J: Targeting protein modifications in metabolic
diseases: Molecular mechanisms and targeted therapies. Signal
Transduct Target Ther. 8:2202023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moore LD, Le T and Fan G: DNA methylation
and its basic function. Neuropsychopharmacology. 38:23–38. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Małecki JM, Davydova E and Falnes P:
Protein methylation in mitochondria. J Biol Chem. 298:1017912022.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Newman AC and Maddocks ODK: One-carbon
metabolism in cancer. Br J Cancer. 116:1499–1504. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Froese DS, Fowler B and Baumgartner MR:
Vitamin B12, folate, and the methionine remethylation
cycle-biochemistry, pathways, and regulation. J Inherit Metab Dis.
42:673–685. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Raghubeer S and Matsha TE:
Methylenetetrahydrofolate (MTHFR), the One-Carbon Cycle, and
Cardiovascular Risks. Nutrients. 13:45622021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nishiyama A and Nakanishi M: Navigating
the DNA methylation landscape of cancer. Trends Genet.
37:1012–1027. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jones PA: Functions of DNA methylation:
Islands, start sites, gene bodies and beyond. Nat Rev Genet.
13:484–492. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gros C, Fahy J, Halby L, Dufau I, Erdmann
A, Gregoire JM, Ausseil F, Vispé S and Arimondo PB: DNA methylation
inhibitors in cancer: Recent and future approaches. Biochimie.
94:2280–2296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lyko F: The DNA methyltransferase family:
A versatile toolkit for epigenetic regulation. Nat Rev Genet.
19:81–92. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kulis M and Esteller M: DNA methylation
and cancer. Adv Genet. 70:27–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Delpu Y, Cordelier P, Cho WC and Torrisani
J: DNA methylation and cancer diagnosis. Int J Mol Sci.
14:15029–15058. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang B, Wang JQ, Tan Y, Yuan R, Chen ZS
and Zou C: RNA methylation and cancer treatment. Pharmacol Res.
174:1059372021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bai Y, Zhao H, Liu H, Wang W, Dong H and
Zhao C: RNA methylation, homologous recombination repair and
therapeutic resistance. Biomed Pharmacother. 166:1154092023.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
An Y and Duan H: The role of m6A RNA
methylation in cancer metabolism. Mol Cancer. 21:142022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen XY, Zhang J and Zhu JS: The role of
m6A RNA methylation in human cancer. Mol Cancer. 18:1032019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He PC and He C: m6 A RNA
methylation: From mechanisms to therapeutic potential. EMBO J.
40:e1059772021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jiang X, Liu B, Nie Z, Duan L, Xiong Q,
Jin Z, Yang C and Chen Y: The role of m6A modification in the
biological functions and diseases. Signal Transduct Target Ther.
6:742021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Azzam SK, Alsafar H and Sajini AA: FTO m6A
demethylase in obesity and cancer: Implications and underlying
molecular mechanisms. Int J Mol Sci. 23:38002022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Qu J, Yan H, Hou Y, Cao W, Liu Y, Zhang E,
He J and Cai Z: RNA demethylase ALKBH5 in cancer: From mechanisms
to therapeutic potential. J Hematol Oncol. 15:82022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu ZX, Li LM, Sun HL and Liu SM: Link
between m6A modification and cancers. Front Bioeng Biotechnol.
6:892018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen X, Zhou X and Wang X: m6A
binding protein YTHDF2 in cancer. Exp Hematol Oncol. 11:212022.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ramesh-Kumar D and Guil S: The IGF2BP
family of RNA binding proteins links epitranscriptomics to cancer.
Semin Cancer Biol. 86:18–31. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bi Z, Liu Y, Zhao Y, Yao Y, Wu R, Liu Q,
Wang Y and Wang X: A dynamic reversible RNA N6-methyladenosine
modification: Current status and perspectives. J Cell Physiol.
234:7948–7956. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Malbeteau L, Pham HT, Eve L, Stallcup MR,
Poulard C and Le Romancer M: How protein methylation regulates
steroid receptor function. Endocr Rev. 43:160–197. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zheng K, Chen S, Ren Z and Wang Y: Protein
arginine methylation in viral infection and antiviral immunity. Int
J Biol Sci. 19:5292–5318. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Black JC, Van Rechem C and Whetstine JR:
Histone lysine methylation dynamics: Establishment, regulation, and
biological impact. Mol Cell. 48:491–507. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li Y, Chen X and Lu C: The interplay
between DNA and histone methylation: Molecular mechanisms and
disease implications. EMBO Rep. 22:e518032021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang Y, Sun Z, Jia J, Du T, Zhang N, Tang
Y, Fang Y and Fang D: Overview of histone modification. Adv Exp Med
Biol. 1283:1–16. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang H, Guo B and Guo X: Histone
demethylases in neurodevelopment and neurodegenerative diseases.
Int J Neurosci. 1–11. 2023.doi: 10.1080/00207454.2023.2276656.
View Article : Google Scholar
|
|
56
|
Harvey HA, Kimura M and Hajba A:
Toremifene: An evaluation of its safety profile. Breast.
15:142–157. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Burstein HJ, Temin S, Anderson H, Buchholz
TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Rowden D, Solky
AJ, et al: Adjuvant endocrine therapy for women with hormone
receptor-positive breast cancer: American society of clinical
oncology clinical practice guideline focused update. J Clin Oncol.
32:2255–2269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Montagna E, Cancello G and Colleoni M: The
aromatase inhibitors (plus ovarian function suppression) in
premenopausal breast cancer patients: Ready for prime time? Cancer
Treat Rev. 39:886–890. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Crump M, Sawka CA, DeBoer G, Buchanan RB,
Ingle JN, Forbes J, Meakin JW, Shelley W and Pritchard KI: An
individual patient-based meta-analysis of tamoxifen versus ovarian
ablation as first line endocrine therapy for premenopausal women
with metastatic breast cancer. Breast Cancer Res Treat. 44:201–210.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tsoutsou PG, Koukourakis MI, Azria D and
Belkacemi Y: Optimal timing for adjuvant radiation therapy in
breast cancer A comprehensive review and perspectives. Crit Rev
Oncol Hematol. 71:102–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Nakhlis F, Lazarus L, Hou NJ, Acharya S,
Khan SA, Staradub VL, Rademaker AW and Morrow M: Tamoxifen use in
patients with ductal carcinoma in situ and T1a/b N0 invasive
carcinoma. J Am Coll Surg. 201:688–694. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nichols HB, DeRoo LA, Scharf DR and
Sandler DP: Risk-Benefit profiles of women using tamoxifen for
chemoprevention. J Natl Cancer Inst. 107:3542014.PubMed/NCBI
|
|
63
|
Anderson C, Nichols HB, House M and
Sandler DP: Risk versus benefit of chemoprevention among raloxifene
and tamoxifen users with a family history of breast cancer. Cancer
Prev Res. 12:801–808. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chlebowski RT: Current concepts in breast
cancer chemoprevention. Pol Arch Med Wewn. 124:191–199. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Nichols HB, Sturmer T, Lee VS, Anderson C,
Lee JS, Roh JM, Visvanathan K, Muss H and Kushi LH: Breast cancer
chemoprevention in an integrated health care setting. JCO Clin
Cancer Inform. 1:1–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Friedl A and Jordan VC: What do we know
and what don't we know about tamoxifen in the human uterus. Breast
Cancer Res Treat. 31:27–39. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
DeCensi A, Johansson H, Helland T, Puntoni
M, Macis D, Aristarco V, Caviglia S, Webber TB, Briata IM, D'Amico
M, et al: Association of CYP2D6 genotype and tamoxifen metabolites
with breast cancer recurrence in a low-dose trial. NPJ Breast
Cancer. 7:342021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Buijs SM, Koolen SLW, Mathijssen RHJ and
Jager A: Tamoxifen dose De-escalation: An effective strategy for
reducing adverse effects? Drugs. 84:385–401. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Reinhorn D, Yerushalmi R, Moore A,
Desnoyers A, Saleh RR, Amir E and Goldvaser H: Evolution in the
risk of adverse events of adjuvant endocrine therapy in
postmenopausal women with early-stage breast cancer. Breast Cancer
Res Treat. 182:259–266. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wibowo E, Pollock PA, Hollis N and
Wassersug RJ: Tamoxifen in men: A review of adverse events.
Andrology. 4:776–788. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sestak I and Cuzick J: Endometrial cancer
risk in postmenopausal breast cancer patients treated with
tamoxifen or aromatase inhibitors. Expert Rev Endocrinol Metab.
11:425–432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Huang B, Warner M and Gustafsson JA:
Estrogen receptors in breast carcinogenesis and endocrine therapy.
Mol Cell Endocrinol. 418:240–244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ye H, Dudley SZ and Shaw IC: Intimate
estrogen receptor-α/ligand relationships signal biological
activity. Toxicology. 408:80–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ge Y, Ni X, Li J, Ye M and Jin X: Roles of
estrogen receptor α in endometrial carcinoma (Review). Oncol Lett.
26:5302023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Nass N and Kalinski T: Tamoxifen
resistance: From cell culture experiments towards novel biomarkers.
Pathol Res Pract. 211:189–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ramchand SK, Cheung YM, Yeo B and
Grossmann M: The effects of adjuvant endocrine therapy on bone
health in women with breast cancer. J Endocrinol. 241:R111–R124.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Mansouri S, Farahmand L, Hosseinzade A,
Eslami SZ and Majidzadeh AK: Estrogen can restore Tamoxifen
sensitivity in breast cancer cells amidst the complex network of
resistance. Biomed Pharmacother. 93:1320–1325. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
BachmannMoisson N, BarberiHeyob M and
Merlin JL: Molecular mechanisms of tamoxifen resistance. Bull Du
Cancer. 84:69–75. 1997.
|
|
79
|
Kulkoyluoglu E and Madak-Erdogan Z:
Nuclear and extranuclear-initiated estrogen receptor signaling
crosstalk and endocrine resistance in breast cancer. Steroids.
114:41–47. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Balfe PJ, McCann AH, Welch HM and Kerin
MJ: Estrogen receptor beta and breast cancer. Eur J Surg Oncol.
30:1043–1050. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Khalid AB and Krum SA: Estrogen receptors
alpha and beta in bone. Bone. 87:130–135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hartman J, Stroem A and Gustafsson JA:
Current concepts and significance of estrogen receptor β in
prostate cancer. Steroids. 77:1262–1266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Altundag O, Altundag K and Gunduz M: DNA
methylation inhibitor, procainamide, may decrease the tamoxifen
resistance by inducing overexpression of the estrogen receptor beta
in breast cancer patients. Med Hypotheses. 63:684–687. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Levy N, Paruthiyil S, Zhao X, Vivar OI,
Saunier EF, Griffin C, Tagliaferri M, Cohen I, Speed TP and Leitman
DC: Unliganded estrogen receptor-β regulation of genes is inhibited
by tamoxifen. Mol Cell Endocrinol. 315:201–207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Tee MK, Rogatsky I, Tzagarakis-Foster C,
Cvoro A, An J, Christy RJ, Yamamoto KR and Leitman DC: Estradiol
and selective estrogen receptor modulators differentially regulate
target genes with estrogen receptors alpha and beta. Mole Biol
Cell. 15:1262–1272. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Mansouri S, Feizi N, Mahdi A, Majidzadeh
AK and Farahmand L: A review on the role of VEGF in tamoxifen
resistance. Anticancer Agents Med Chem. 18:2006–2009. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Provenzano A, Kurian S and Abraham J:
Overcoming endocrine resistance in breast cancer: Role of the PI3K
and the mTOR pathways. Expert Rev Anticancer Ther. 13:143–147.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Mohite R and Doshi G: Elucidation of the
role of the epigenetic regulatory mechanisms of PI3K/Akt/mTOR
signaling pathway in human malignancies. Curr Cancer Drug Targets.
24:231–244. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Luo Q, Du R, Liu W, Huang G, Dong Z and Li
X: PI3K/Akt/mTOR signaling pathway: Role in esophageal squamous
cell carcinoma, regulatory mechanisms and opportunities for
targeted therapy. Front Oncol. 12:8523832022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Su H, Peng C and Liu Y: Regulation of
ferroptosis by PI3K/Akt signaling pathway: A promising therapeutic
axis in cancer. Front Cell Dev Biol. 12:13723302024. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Bertacchini J, Heidari N, Mediani L,
Capitani S, Shahjahani M, Ahmadzadeh A and Saki N: Targeting
PI3K/AKT/mTOR network for treatment of leukemia. Cell Mol Life Sci.
72:2337–2347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Hashemi M, Etemad S, Rezaei S, Ziaolhagh
S, Rajabi R, Rahmanian P, Abdi S, Koohpar ZK, Rafiei R, Raei B, et
al: Progress in targeting PTEN/PI3K/Akt axis in glioblastoma
therapy: Revisiting molecular interactions. Biomed Pharmacother.
158:1142042023. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Sadrkhanloo M, Paskeh MDA, Hashemi M,
Raesi R, Bahonar A, Nakhaee Z, Entezari M, Beig Goharrizi MAS,
Salimimoghadam S, Ren J, et al: New emerging targets in
osteosarcoma therapy: PTEN and PI3K/Akt crosstalk in
carcinogenesis. Pathol Res Pract. 251:1549022023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Xiang Y, Yang Y, Liu J and Yang X:
Functional role of MicroRNA/PI3K/AKT axis in osteosarcoma. Front
Oncol. 13:12192112023. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Huang C, Li J and Ma TT: PI3K/Akt
signaling pathway and liver fibrosis. Zhongguo Yaolixue Tongbao.
27:1037–1041. 2011.
|
|
96
|
Li L, Han C and Liu X: Regulation of
PI3K-Akt-mTORC1 signal pathway in the cell growth and
proliferation. J Biology. 31:75–77. 2014.
|
|
97
|
Minami A, Nakanishi A, Ogura Y, Kitagishi
Y and Matsuda S: Connection between tumor suppressor BRCA1 and PTEN
in damaged DNA repair. Front Oncol. 4:318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhou J, Chen Q, Zou Y, Chen H, Qi L and
Chen Y: Stem cells and cellular origins of breast cancer: Updates
in the rationale, controversies, and therapeutic implications.
Front Oncol. 9:8202019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zheng Q, Zhang M, Zhou F, Zhang L and Meng
X: The breast cancer stem cells traits and drug resistance. Front
Pharmacol. 11:5999652021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Telang N: Stem cell models for cancer
therapy. Int J Mol Sci. 23:70552022. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Haiduk TS, Sicking M, Brücksken KA,
Espinoza-Sánchez NA, Eder KM, Kemper B, Eich HT, Götte M, Greve B
and Troschel FM: Dysregulated stem cell markers Musashi-1 and
Musashi-2 are associated with therapy resistance in inflammatory
breast cancer. Arch Med Res. 54:1028552023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Gelsomino L, Panza S, Giordano C, Barone
I, Gu G, Spina E, Catalano S, Fuqua S and Andò S: Mutations in the
estrogen receptor alpha hormone binding domain promote stem cell
phenotype through notch activation in breast cancer cell lines.
Cancer Lett. 428:12–20. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Ghasemi F, Sarabi PZ, Athari SS and
Esmaeilzadeh A: Therapeutics strategies against cancer stem cell in
breast ca ncer. Int J Biochem Cell Biol. 109:76–81. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Albain KS, Czerlanis C, Zlobin A,
Covington KR, Rajan P, Godellas C, Bova D, Lo SS, Robinson P,
Sarker S, et al: S1-5: Modulation of cancer and stem cell
biomarkers by the notch inhibitor MK-0752 added to endocrine
therapy for early stage ER + breast cancer. Cancer Res. 71 (Suppl
24):S1–S5. 2011. View Article : Google Scholar
|
|
105
|
Schott AF, Landis MD, Dontu G, Griffith
KA, Layman RM, Krop I, Paskett LA, Wong H, Dobrolecki LE, Lewis MT,
et al: Preclinical and clinical studies of gamma secretase
inhibitors with docetaxel on human breast tumors. Clin Cancer Res.
19:1512–1524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wang D, Xu J, Liu B, He X, Zhou L, Hu X,
Qiao F, Zhang A, Xu X, Zhang H, et al: IL6 blockade potentiates the
anti-tumor effects of γ-secretase inhibitors in Notch3-expressing
breast cancer. Cell Death Differ. 25:330–339. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Means-Powell JA, Mayer IA, Ismail-Khan R,
Del Valle L, Tonetti D, Abramson VG, Sanders MS, Lush RM,
Sorrentino C, Majumder S and Miele L: A Phase ib dose escalation
trial of RO4929097 (a γ-secretase inhibitor) in combination with
exemestane in patients with er plus metastatic breast cancer (MBC).
Clin Breast Cancer. 22:103–114. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Jeselsohn R, Cornwell M, Pun M, Buchwalter
G, Nguyen M, Bango C, Huang Y, Kuang Y, Paweletz C, Fu X, et al:
Embryonic transcription factor SOX9 drives breast cancer endocrine
resistance. Proc Natl Acad Sci USA. 114:E4482–E4491. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Poulard C, Pham TH, Drouet Y, Jacquemetton
J, Surmielova A, Kassem L, Mery B, Lasset C, Reboulet J, Treilleux
I, et al: Nuclear PRMT5 is a biomarker of sensitivity to tamoxifen
in ERα+ breast cancer. Embo Mol Med. 15:e172482023. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhou J, Xu M, Le K, Ming J, Guo H, Ruan S
and Huang T: SRC promotes tamoxifen resistance in breast cancer via
Up-Regulating SIRT1. Onco Targets Ther. 13:4635–4647. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Jogi A, Ehinger A, Hartman L and Alkner S:
Expression of HIF-1α is related to a poor prognosis and tamoxifen
resistance in contralateral breast cancer. PLoS One.
14:e02261502019. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Ward A, Balwierz A, Zhang JD, Küblbeck M,
Pawitan Y, Hielscher T, Wiemann S and Sahin Ö: Re-expression of
microRNA-375 reverses both tamoxifen resistance and accompanying
EMT-like properties in breast cancer. Oncogene. 32:1173–1182. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Cittelly DM, Das PM, Spoelstra NS,
Edgerton SM, Richer JK, Thor AD and Jones FE: Downregulation of
miR-342 is associated with tamoxifen resistant breast tumors. Mol
Cancer. 9:3172010. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Xu E, Hu M, Ge R, Tong D, Fan Y, Ren X and
Liu Y: LncRNA-42060 regulates tamoxifen sensitivity and tumor
development via regulating the miR-204-5p/SOX4 axis in canine
mammary gland tumor cells. Front Vet Sci. 8:6546942021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Sukocheva OA, Lukina E, Friedemann M,
Menschikowski M, Hagelgans A and Aliev G: The crucial role of
epigenetic regulation in breast cancer anti-estrogen resistance:
Current findings and future perspectives. Semin Cancer Biol.
82:35–59. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Kim SS, Lee MH and Lee MO: Histone
methyltransferases regulate the transcriptional expression of ERα
and the proliferation of tamoxifen-resistant breast cancer cells.
Breast Cancer Res Treat. 180:45–54. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Zhang J, Zhou C, Jiang H, Liang L, Shi W,
Zhang Q, Sun P, Xiang R, Wang Y and Yang S: ZEB1 induces ER-α
promoter hypermethylation and confers antiestrogen resistance in
breast cancer. Cell Death Dis. 8:e27322017. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Jiménez-Garduño AM, Mendoza-Rodríguez MG,
Urrutia-Cabrera D, Domínguez-Robles MC, Pérez-Yépez EA,
Ayala-Sumuano JT and Meza I: IL-1β induced methylation of the
estrogen receptor ERα gene correlates with EMT and chemoresistance
in breast cancer cells. Biochem Biophys Res Commun. 490:780–785.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Kim MR, Wu MJ, Zhang Y, Yang JY and Chang
CJ: TET2 directs mammary luminal cell differentiation and endocrine
response. Nat Commun. 11:46422020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Chang HG, Kim SJ, Chung KW, Noh DY, Kwon
Y, Lee ES and Kang HS: Tamoxifen-resistant breast cancers show less
frequent methylation of the estrogen receptor beta but not the
estrogen receptor alpha gene. J Mol Med (Berl). 83:132–139. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Zou JX, Duan Z, Wang J, Sokolov A, Xu J,
Chen CZ, Li JJ and Chen HW: Kinesin family deregulation coordinated
by bromodomain protein ANCCA and histone methyltransferase MLL for
breast cancer cell growth, survival, and tamoxifen resistance. Mol
Cancer Res. 12:539–549. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Jin ML, Kim YW, Jin HL, Kang H, Lee EK,
Stallcup MR and Jeong KW: Aberrant expression of SETD1A promotes
survival and migration of estrogen receptor α-positive breast
cancer cells. Int J Cancer. 143:2871–2883. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Stone A, Zotenko E, Locke WJ, Korbie D,
Millar EK, Pidsley R, Stirzaker C, Graham P, Trau M, Musgrove EA,
et al: DNA methylation of oestrogen-regulated enhancers defines
endocrine sensitivity in breast cancer. Nat Commun. 6:77582015.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Gautam N, Verma H, Choudhary S, Kaur S and
Silakari O: Functional relationship of SNP (Ala490Thr) of an
epigenetic gene EZH2 results in the progression and poor survival
of ER+/tamoxifen treated breast cancer patients. J Genet.
100:862021. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Wu Y, Zhang Z, Cenciarini ME, Proietti CJ,
Amasino M, Hong T, Yang M, Liao Y, Chiang HC, Kaklamani VG, et al:
Tamoxifen resistance in breast cancer is regulated by the
EZH2-ERα-GREB1 transcriptional axis. Cancer Res. 78:671–684. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Li J, Liang Y, Zhou S, Chen J and Wu C:
UCHL1 contributes to insensitivity to endocrine therapy in
triple-negative breast cancer by deubiquitinating and stabilizing
KLF5. Breast Cancer Res. 26:442024. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Redeuilh G, Attia A, Mester J and Sabbah
M: Transcriptional activation by the oestrogen receptor α is
modulated through inhibition of cyclin-dependent kinases. Oncogene.
21:5773–5782. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Wang L, Zhang X and Wang ZY: The Wilms'
tumor suppressor WT1 regulates expression of members of the
epidermal growth factor receptor (EGFR) and estrogen receptor in
acquired tamoxifen resistance. Anticancer Res. 30:3637–3642.
2010.PubMed/NCBI
|
|
129
|
Best SA, Hutt KJ, Fu NY, Vaillant F, Liew
SH, Hartley L, Scott CL, Lindeman GJ and Visvader JE: Dual roles
for Id4 in the regulation of estrogen signaling in the mammary
gland and ovary. Development. 141:3159–3164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Mobini K, Tamaddon G, Fardid R, Keshavarzi
M and Mohammadi-Bardbori A: Aryl hydrocarbon-estrogen alpha
receptor-dependent expression of miR-206, miR-27b, and miR-133a
suppress cell proliferation and migration in MCF-7 cells. J Biochem
Mol Toxicol. 33:e223042019. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Li Z, Yu D, Li H, Lv Y and Li S: Long
non-coding RNA UCA1 confers tamoxifen resistance in breast cancer
endocrinotherapy through regulation of the EZH2/p21 axis and the
PI3K/AKT signaling pathway. Int J Oncol. 54:1033–1042.
2019.PubMed/NCBI
|
|
132
|
Ren C, Tang X and Lan H: Comprehensive
analysis based on DNA methylation and RNA-seq reveals
hypermethylation of the up-regulated WT1 gene with potential
mechanisms in PAM50 subtypes of breast cancer. PeerJ. 9:e113772021.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Zhang Y, Zhang B, Fang J and Cao X:
Hypomethylation of DNA-binding inhibitor 4 serves as a potential
biomarker in distinguishing acquired tamoxifen-refractory breast
cancer. Int J Clin Exp Pathol. 8:9500–9505. 2015.PubMed/NCBI
|
|
134
|
Li X, Wu Y, Liu A and Tang X: MiR-27b is
epigenetically downregulated in tamoxifen resistant breast cancer
cells due to promoter methylation and regulates tamoxifen
sensitivity by targeting HMGB3. Biochem Biophys Res Commun.
477:768–773. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zhang X, Carlisle SM, Doll MA, Martin RCG,
States JC, Klinge CM and Hein DW: High N-Acetyltransferase 1
expression is associated with estrogen receptor expression in
breast tumors, but is not Under Direct Regulation by Estradiol,
5α-androstane-3β, 17β-Diol, or dihydrotestosterone in breast cancer
cells. J Pharmacol Exp Ther. 365:84–93. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
González-Bengtsson A, Asadi A, Gao H,
Dahlman-Wright K and Jacobsson A: Estrogen enhances the expression
of the polyunsaturated fatty acid elongase Elovl2 via ERα in breast
cancer cells. PLoS One. 11:e01642412016. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Rickard DJ, Waters KM, Ruesink TJ, Khosla
S, Katzenellenbogen JA, Katzenellenbogen BS, Riggs BL and Spelsberg
TC: Estrogen receptor isoform-specific induction of progesterone
receptors in human osteoblasts. J Bone Miner Res. 17:580–592. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Kim SJ, Kang HS, Jung SY, Min SY, Lee S,
Kim SW, Kwon Y, Lee KS, Shin KH and Ro J: Methylation patterns of
genes coding for drug-metabolizing enzymes in tamoxifen-resistant
breast cancer tissues. J Mol Med (Berl). 88:1123–1131. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Sun H, Wang G, Cai J, Wei X, Zeng Y, Peng
Y and Zhuang J: Long non-coding RNA H19 mediates
N-acetyltransferase 1 gene methylation in the development of
tamoxifen resistance in breast cancer. Exp Ther Med. 23:122022.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Kim HW, Baek M, Jung S, Jang S, Lee H,
Yang SH, Kwak BS and Kim SJ: ELOVL2-AS1 suppresses tamoxifen
resistance by sponging miR-1233-3p in breast cancer. Epigenetics.
18:22763842023. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Pathiraja TN, Shetty PB, Jelinek J, He R,
Hartmaier R, Margossian AL, Hilsenbeck SG, Issa JP and Oesterreich
S: Progesterone receptor isoform-specific promoter methylation:
Association of PRA promoter methylation with worse outcome in
breast cancer patients. Clin Cancer Res. 17:4177–4186. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Chen H, Li L, Liu H, Qin P, Chen R, Liu S,
Xiong H, Li Y, Yang Z, Xie M, et al: PAX2 is regulated by
estrogen/progesterone through promoter methylation in endometrioid
adenocarcinoma and has an important role in carcinogenesis via the
AKT/mTOR signaling pathway. J Pathol. 262:467–479. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Oesterreich S, Deng W, Jiang S, Cui X,
Ivanova M, Schiff R, Kang K, Hadsell DL, Behrens J and Lee AV:
Estrogen-mediated down-regulation of E-cadherin in breast cancer
cells. Cancer Res. 63:5203–5208. 2003.PubMed/NCBI
|
|
144
|
Jahangiri R, Mosaffa F, Emami Razavi A,
Teimoori-Toolabi L and Jamialahmadi K: PAX2 promoter methylation
and AIB1 overexpression promote tamoxifen resistance in breast
carcinoma patients. J Oncol Pharm Pract. 28:310–325. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Wang Q, Gun M and Hong XY: Induced
tamoxifen resistance is mediated by increased methylation of
E-Cadherin in estrogen receptor-expressing breast cancer cells. Sci
Rep. 9:141402019. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Kim HW, Park JE, Baek M, Kim H, Ji HW, Yun
SH, Jeong D, Ham J, Park S, Lu X, et al: Matrix Metalloproteinase-1
(MMP1) upregulation through promoter hypomethylation enhances
tamoxifen resistance in breast cancer. Cancers (Basel).
14:12322022. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Jung HH, Park YH, Jun HJ, Kong J, Kim JH,
Kim JA, Yun J, Sun JM, Won YW, Lee S, et al: Matrix
Metalloproteinase-1 expression can be upregulated through
mitogen-activated protein kinase pathway under the influence of
human epidermal growth factor Receptor 2 synergized with estrogen
receptor. Mol Cancer Res. 8:1037–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Ren W, Yi H, Bao Y, Liu Y and Gao X:
Oestrogen inhibits PTPRO to prevent the apoptosis of renal
podocytes. Exp Ther Med. 17:2373–2380. 2019.PubMed/NCBI
|
|
149
|
Ramaswamy B, Majumder S, Roy S, Ghoshal K,
Kutay H, Datta J, Younes M, Shapiro CL, Motiwala T and Jacob ST:
Estrogen-mediated suppression of the gene encoding protein tyrosine
phosphatase PTPRO in human breast cancer: Mechanism and role in
tamoxifen sensitivity. Mol Endocrinol. 23:176–187. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Fan Y, Xie G, Wang Z, Wang Y, Wang Y,
Zheng H and Zhong X: PTEN promoter methylation predicts 10-year
prognosis in hormone receptor-positive early breast cancer patients
who received adjuvant tamoxifen endocrine therapy. Breast Cancer
Res Treat. 192:33–42. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Phuong NT, Kim SK, Lim SC, Kim HS, Kim TH,
Lee KY, Ahn SG, Yoon JH and Kang KW: Role of PTEN promoter
methylation in tamoxifen-resistant breast cancer cells. Breast
Cancer Res Treat. 130:73–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Phuong NT, Kim SK, Im JH, Yang JW, Choi
MC, Lim SC, Lee KY, Kim YM, Yoon JH and Kang KW: Induction of
methionine adenosyltransferase 2A in tamoxifen-resistant breast
cancer cells. Oncotarget. 7:13902–13916. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Liu Z, Liu J, Ebrahimi B, Pratap UP, He Y,
Altwegg KA, Tang W, Li X, Lai Z, Chen Y, et al: SETDB1 interactions
with PELP1 contributes to breast cancer endocrine therapy
resistance. Breast Cancer Res. 24:262022. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Schomann T, Mirzakhani K, Kallenbach J, Lu
J, Rasa SMM, Neri F and Baniahmad A: Androgen-Induced MIG6
regulates phosphorylation of retinoblastoma protein and AKT to
counteract Non-Genomic AR signaling in prostate cancer cells.
Biomolecules. 12:10482022. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Wang Q, Li J, Wu W, Shen R, Jiang H, Qian
Y, Tang Y, Bai T, Wu S, Wei L, et al: Smad4-dependent suppressor
pituitary homeobox 2 promotes PPP2R2A-mediated inhibition of Akt
pathway in pancreatic cancer. Oncotarget. 7:11208–11222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Yue C, Bai Y, Piao Y and Liu H: DOK7
inhibits cell proliferation, migration, and invasion of breast
cancer via the PI3K/PTEN/AKT pathway. J Oncol. 2021:40352572021.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Barkovskaya A, Seip K, Prasmickaite L,
Mills IG, Moestue SA and Itkonen HM: Inhibition of O-GlcNAc
transferase activates tumor-suppressor gene expression in
tamoxifen-resistant breast cancer cells. Sci Rep. 10:169922020.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Maier S, Nimmrich I, Koenig T,
Eppenberger-Castori S, Bohlmann I, Paradiso A, Spyratos F, Thomssen
C, Mueller V, Nährig J, et al: DNA-methylation of the homeodomain
transcription factor PITX2 reliably predicts risk of distant
disease recurrence in tamoxifen-treated, node-negative breast
cancer patients-Technical and clinical validation in a multi-centre
setting in collaboration with the European Organisation for
Research and Treatment of Cancer (EORTC) PathoBiology group. Eur J
Cancer. 43:1679–1686. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Gowdini E, Aleyasin SA, Ramezani N, Nafisi
N and Tutuni M: DOK7 CpG hypermethylation in blood leukocytes as an
epigenetic biomarker for acquired tamoxifen resistant in breast
cancer. J Hum Genet. 68:33–38. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Wujak M, Veith C, Wu CY, Wilke T, Kanbagli
ZI, Novoyatleva T, Guenther A, Seeger W, Grimminger F, Sommer N, et
al: Adenylate Kinase 4-A key regulator of proliferation and
metabolic shift in human pulmonary arterial smooth muscle cells via
Akt and HIF-1α signaling pathways. Int J Mol Sci. 22:103712021.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Liu X, Gonzalez G, Dai X, Miao W, Yuan J,
Huang M, Bade D, Li L, Sun Y and Wang Y: Adenylate Kinase 4
modulates the resistance of breast cancer cells to tamoxifen
through an m6A-Based epitranscriptomic mechanism. Mol Ther.
28:2593–2604. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Ye L, Lin C, Wang X, Li Q, Li Y, Wang M,
Zhao Z, Wu X, Shi D, Xiao Y, et al: Epigenetic silencing of SALL2
confers tamoxifen resistance in breast cancer. EMBO Mol Med.
11:e106382019. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Huang J, Li J, Tang J, Wu Y, Dai F, Yi Z,
Wang Y, Li Y, Wu Y, Ren G and Xiang T: ZDHHC22-mediated mTOR
palmitoylation restrains breast cancer growth and endocrine therapy
resistance. Int J Biol Sci. 18:2833–2850. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Al-Ansari MM, Hendrayani SF, Tulbah A,
Al-Tweigeri T, Shehata AI and Aboussekhra A: p16INK4A represses
breast stromal fibroblasts migration/invasion and their
VEGF-A-dependent promotion of angiogenesis through Akt inhibition.
Neoplasia. 14:1269–1277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Zhou J and Chen C: Suppression of
malignant melanoma by knocking down growth differentiation
factor-15 via inhibiting PTEN/PI3K/AKT signaling pathway. J Cancer.
15:1115–1123. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Chen S, Yao F, Xiao Q, Liu Q, Yang Y, Li
X, Jiang G, Kuno T and Fang Y: EZH2 inhibition sensitizes
tamoxifen-resistant breast cancer cells through cell cycle
regulation. Mol Med Rep. 17:2642–2650. 2018.PubMed/NCBI
|
|
167
|
Stone A, Valdés-Mora F, Gee JM, Farrow L,
McClelland RA, Fiegl H, Dutkowski C, McCloy RA, Sutherland RL,
Musgrove EA and Nicholson RI: Tamoxifen-induced epigenetic
silencing of oestrogen-regulated genes in anti-hormone resistant
breast cancer. PLoS One. 7:e404662012. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Zhao W, Sun M, Li S, Chen Z and Geng D:
Transcription factor ATF3 mediates the radioresistance of breast
cancer. J Cell Mol Med. 22:4664–4675. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Liu X, Yuan J, Zhang X, Li L, Dai X, Chen
Q and Wang Y: ATF3 modulates the resistance of breast cancer cells
to tamoxifen through an N6-Methyladenosine-Based epitranscriptomic
mechanism. Chem Res Toxicol. 34:1814–1821. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Lv Y, Fang L, Ding P and Liu R:
PI3K/Akt-Beclin1 signaling pathway positively regulates
phagocytosis and negatively mediates NF-κB-dependent inflammation
in Staphylococcus aureus-infected macrophages. Biochem Biophys Res
Commun. 510:284–289. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Wang J, Xie S, Yang J, Xiong H, Jia Y,
Zhou Y, Chen Y, Ying X, Chen C, Ye C, et al: The long noncoding RNA
H19 promotes tamoxifen resistance in breast cancer via autophagy. J
Hematol Oncol. 12:812019. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Gu Q-Z, Nijiati A, Gao X, Tao KL, Li CD,
Fan XP and Tian Z: TROP2 promotes cell proliferation and migration
in osteosarcoma through PI3K/AKT signaling. Mol Med Rep.
8:1782–1788. 2018.PubMed/NCBI
|
|
173
|
Zimmers SM, Browne EP, Williams KE, Jawale
RM, Otis CN, Schneider SS and Arcaro KF: TROP2 methylation and
expression in tamoxifen-resistant breast cancer. Cancer Cell Int.
18:942018. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Zhai J, Jiang JF and Shi L: Lycorine
weakens tamoxifen resistance of breast cancer via abrogating
HAGLR-mediated epigenetic suppression on VGLL4 by DNMT1. Kaohsiung
J Med Sci. 39:278–289. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Watanabe T, Oba T, Tanimoto K, Shibata T,
Kamijo S and Ito KI: Tamoxifen resistance alters sensitivity to
5-fluorouracil in a subset of estrogen receptor-positive breast
cancer. PLoS One. 16:e02528222021. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Williams KE, Anderton DL, Lee MP,
Pentecost BT and Arcaro KF: High-density array analysis of DNA
methylation in Tamoxifen-resistant breast cancer cell lines.
Epigenetics. 9:297–307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Ansar M, Thu LTA, Hung CS, Su CM, Huang
MH, Liao LM, Chung YM and Lin RK: Promoter hypomethylation and
overexpression of TSTD1 mediate poor treatment response in breast
cancer. Front Oncol. 12:10042612022. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Pietkiewicz PP, Lutkowska A, Lianeri M and
Jagodzinski PP: Tamoxifen epigenetically modulates CXCL12
expression in MCF-7 breast cancer cells. Biomed Pharmacother.
64:54–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Martens JW, Nimmrich I, Koenig T, Look MP,
Harbeck N, Model F, Kluth A, Bolt-de Vries J, Sieuwerts AM,
Portengen H, et al: Association of DNA methylation of phosphoserine
aminotransferase with response to endocrine therapy in patients
with recurrent breast cancer. Cancer Res. 65:4101–4117. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Wang B, Li D, Rodriguez-Juarez R, Farfus
A, Storozynsky Q, Malach M, Carpenter E, Filkowski J, Lykkesfeldt
AE and Kovalchuk O: A suppressive role of guanine
nucleotide-binding protein subunit beta-4 inhibited by DNA
methylation in the growth of anti-estrogen resistant breast cancer
cells. BMC cancer. 18:8172018. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Kedia-Mokashi NA, Kadam L, Ankolkar M,
Dumasia K and Balasinor NH: Aberrant methylation of multiple
imprinted genes in embryos of tamoxifen-treated male rats.
Reproduction. 146:155–168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Liggett TE, Melnikov AA, Marks JR and
Levenson VV: Methylation patterns in cell-free plasma DNA reflect
removal of the primary tumor and drug treatment of breast cancer
patients. Int J Cancer. 128:492–499. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Pathak S, Kedia-Mokashi N, Saxena M,
D'Souza R, Maitra A, Parte P, Gill-Sharma M and Balasinor N: Effect
of tamoxifen treatment on global and insulin-like growth factor
2-H19 locus-specific DNA methylation in rat spermatozoa and its
association with embryo loss. Fertil Steril. 91:2253–2263. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Ge Y, Zhan Z, Ye M and Jin X: The
crosstalk between ubiquitination and endocrine therapy. J Mol Med
(Berl). 101:461–486. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Kubarek Ł and Jagodzinski PP: Epigenetic
up-regulation of CXCR4 and CXCL12 expression by 17 beta-estradiol
and tamoxifen is associated with formation of DNA methyltransferase
3B4 splice variant in Ishikawa endometrial adenocarcinoma cells.
FEBS Lett. 581:1441–1448. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Wu H, Chen Y, Liang J, Shi B, Wu G, Zhang
Y, Wang D, Li R, Yi X, Zhang H, et al: Hypomethylation-linked
activation of PAX2 mediates tamoxifen-stimulated endometrial
carcinogenesis. Nature. 438:981–987. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Tryndyak VP, Muskhelishvili L, Kovalchuk
O, Rodriguez-Juarez R, Montgomery B, Churchwell MI, Ross SA, Beland
FA and Pogribny IP: Effect of long-term tamoxifen exposure on
genotoxic and epigenetic changes in rat liver: Implications for
tamoxifen-induced hepatocarcinogenesis. Carcinogenesis.
27:1713–1720. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Fan M, Yan PS, Hartman-Frey C, Chen L,
Paik H, Oyer SL, Salisbury JD, Cheng AS, Li L, Abbosh PH, et al:
Diverse gene expression and DNA methylation profiles correlate with
differential adaptation of breast cancer cells to the antiestrogens
tamoxifen and fulvestrant. Cancer Res. 66:11954–11966. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Snider H, Villavarajan B, Peng Y, Shepherd
LE, Robinson AC and Mueller CR: Region-specific glucocorticoid
receptor promoter methylation has both positive and negative
prognostic value in patients with estrogen receptor-positive breast
cancer. Clin Epigenetics. 11:1552019. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Kala R and Tollefsbol TO: A novel
combinatorial epigenetic therapy using resveratrol and
pterostilbene for restoring estrogen Receptor-α (ERα) expression in
ERα-Negative breast cancer cells. PLoS One. 11:e01550572016.
View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Zhang WJ, Xu DF, Fan QX, Wu XA, Wang F,
Wang R and Wang LX: Arsenic trioxide restores ERα expression in
ERα-negative human breast cancer cells and its treatment efficacy
in combination with tamoxifen in xenografts in nude mice. Zhonghua
Zhong Liu Za Zhi. 34:645–651. 2012.(In Chinese). PubMed/NCBI
|
|
192
|
Tang B, Peng ZH and Jiang J: The
synergistic inhibitory effect of 5-aza-2-deoxycytidine and
Tamoxifen on estrogen receptor alpha negative breast cancer cell
lines in vitro. Zhonghua Wai Ke Za Zhi. 43:1545–1549. 2005.(In
Chinese). PubMed/NCBI
|
|
193
|
Selmin OI, Donovan MG, Skovan B,
Paine-Murieta GD and Romagnolo DF: Arsenic-induced BRCA1 CpG
promoter methylation is associated with the downregulation of ERα
and resistance to tamoxifen in MCF7 breast cancer cells and mouse
mammary tumor xenografts. Int J Oncol. 54:869–878. 2019.PubMed/NCBI
|
|
194
|
Wu HT, Liu YE, Hsu KW, Wang YF, Chan YC,
Chen Y and Chen DR: MLL3 induced by luteolin causes apoptosis in
Tamoxifen-Resistant breast cancer cells through H3K4
monomethylation and suppression of the PI3K/AKT/mTOR Pathway. Am J
Chin Med. 48:1221–1241. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Jin K, Lan H, Zhang J, Lv J, Chen Y, Yu K
and Wang W: UBASH3B promotes tamoxifen resistance and could be
negatively regulated by ESR1. Oncotarget. 9:8326–8333. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Jayasree PJ, Dutta S, Karemore P and
Khandelia P: Crosstalk between m6A RNA methylation and miRNA
biogenesis in cancer: An unholy nexus. Mol Biotechnol. Oct
13–2023.doi: 10.1007/s12033-023-00921-w. View Article : Google Scholar
|
|
197
|
Noh EM, Lee YR, Chay KO, Chung EY, Jung
SH, Kim JS and Youn HJ: Estrogen receptor α induces down-regulation
of PTEN through PI3-kinase activation in breast cancer cells. Mol
Med Rep. 4:215–219. 2011.PubMed/NCBI
|
|
198
|
Khatpe AS, Adebayo AK, Herodotou CA, Kumar
B and Nakshatri H: Nexus between PI3K/AKT and estrogen receptor
signaling in breast cancer. Cancers (Basel). 13:3692021. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Zhu D, Zeng S, Su C, Li J, Xuan Y, Lin Y,
Xu E and Fan Q: The interaction between DNA methylation and tumor
immune microenvironment: From the laboratory to clinical
applications. Clin Epigenetics. 16:242024. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Sina AA, Carrascosa LG and Trau M: DNA
Methylation-based point-of-care cancer detection: Challenges and
possibilities. Trends Mol Med. 25:955–966. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Wang B, Wang M, Lin Y, Zhao J, Gu H and Li
X: Circulating tumor DNA methylation: A promising clinical tool for
cancer diagnosis and management. Clin Chem Lab Med. Mar 7–2024.doi:
10.1515/cclm-2023-1327. View Article : Google Scholar
|
|
202
|
Garcia-Ortiz MV, Cano-Ramirez P,
Toledano-Fonseca M, Aranda E and Rodriguez-Ariza A: Diagnosing and
monitoring pancreatic cancer through cell-free DNA methylation:
Progress and prospects. Biomark Res. 11:882023. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Fan S and Chi W: Methods for genome-wide
DNA methylation analysis in human cancer. Brief Funct Genomics.
15:432–442. 2016.PubMed/NCBI
|
|
204
|
Liu B, Wang T, Wang H, Zhang L, Xu F, Fang
R, Li L, Cai X, Wu Y, Zhang W and Ye L: Oncoprotein HBXIP enhances
HOXB13 acetylation and co-activates HOXB13 to confer tamoxifen
resistance in breast cancer. J Hematol Oncol. 11:262018. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Geter PA, Ernlund AW, Bakogianni S, Alard
A, Arju R, Giashuddin S, Gadi A, Bromberg J and Schneider RJ:
Hyperactive mTOR and MNK1 phosphorylation of eIF4E confer tamoxifen
resistance and estrogen independence through selective mRNA
translation reprogramming. Genes Dev. 31:2235–2249. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Filipčík P, Curry JR and Mace PD: When
Worlds Collide-mechanisms at the interface between phosphorylation
and ubiquitination. J Mol Biol. 429:1097–1113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Bailey LT, Northall SJ and Schalch T:
Breakers and amplifiers in chromatin circuitry: Acetylation and
ubiquitination control the heterochromatin machinery. Curr Opin
Struct Biol. 71:156–163. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Shukla A, Chaurasia P and Bhaumik SR:
Histone methylation and ubiquitination with their cross-talk and
roles in gene expression and stability. Cell Mol Life Sci.
66:1419–1433. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Separovich RJ, Pang CNI and Wilkins MR:
Controlling the controllers: Regulation of histone methylation by
phosphosignalling. Trends Biochem Sci. 45:1035–1048. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Blackburn SA, Parks RM and Cheung KL:
Fulvestrant for the treatment of advanced breast cancer. Expert Rev
Anticancer Ther. 18:619–628. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Intabli H, Gee JM, Oesterreich S, Yeoman
MS, Allen MC, Qattan A and Flint MS: Glucocorticoid induced loss of
oestrogen receptor alpha gene methylation and restoration of
sensitivity to fulvestrant in triple negative breast cancer. Gene.
851:1470222023. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Hopcroft L, Wigmore EM, Williamson SC, Ros
S, Eberlein C, Moss JI, Urosevic J, Carnevalli LS, Talbot S,
Bradshaw L, et al: Combining the AKT inhibitor capivasertib and
SERD fulvestrant is effective in palbociclib-resistant ER+ breast
cancer preclinical models. NPJ Breast Cancer. 9:642023. View Article : Google Scholar : PubMed/NCBI
|


















