Sepsis is a multiorgan dysfunction caused by the
response of the host organism to an infection due to the invasion
of pathogenic microorganisms, such as bacteria and fungi (1). It is one of the leading causes of
mortality in severely ill patients worldwide, sepsis has a
mortality rate of up to 30%, ~0.2–3 per 1,000 individuals are
affected by sepsis yearly in the developed world, resulting in
about a million cases per year in the United States (2). Over the past decade, there has been a
steady increase in the incidence and mortality of sepsis; overall,
the incidence of sepsis is increasing by 8–13% per year in the
United States (3). In addition,
sepsis treatment is costly, which places a significant financial
burden on both the health system and families of the patient
(4). The progression of sepsis is
closely associated with changes (polarization of macrophages,
infiltration of neutrophils, etc.) in immune cells such as
neutrophils, T cells and macrophages and the homeostasis is ensured
by means of macrophage polarization and neutrophil infiltration
(5–7). Previous studies on sepsis have
focused on the status of immune cells, including neutrophil
infiltration, macrophage polarization and lymphocyte deletion, but
it is currently under consideration that glycolytic enhancement is
one of the most common features of sepsis-related metabolic
disorders; therefore, the metabolic disorders of sepsis deserve
more attention (8,9).
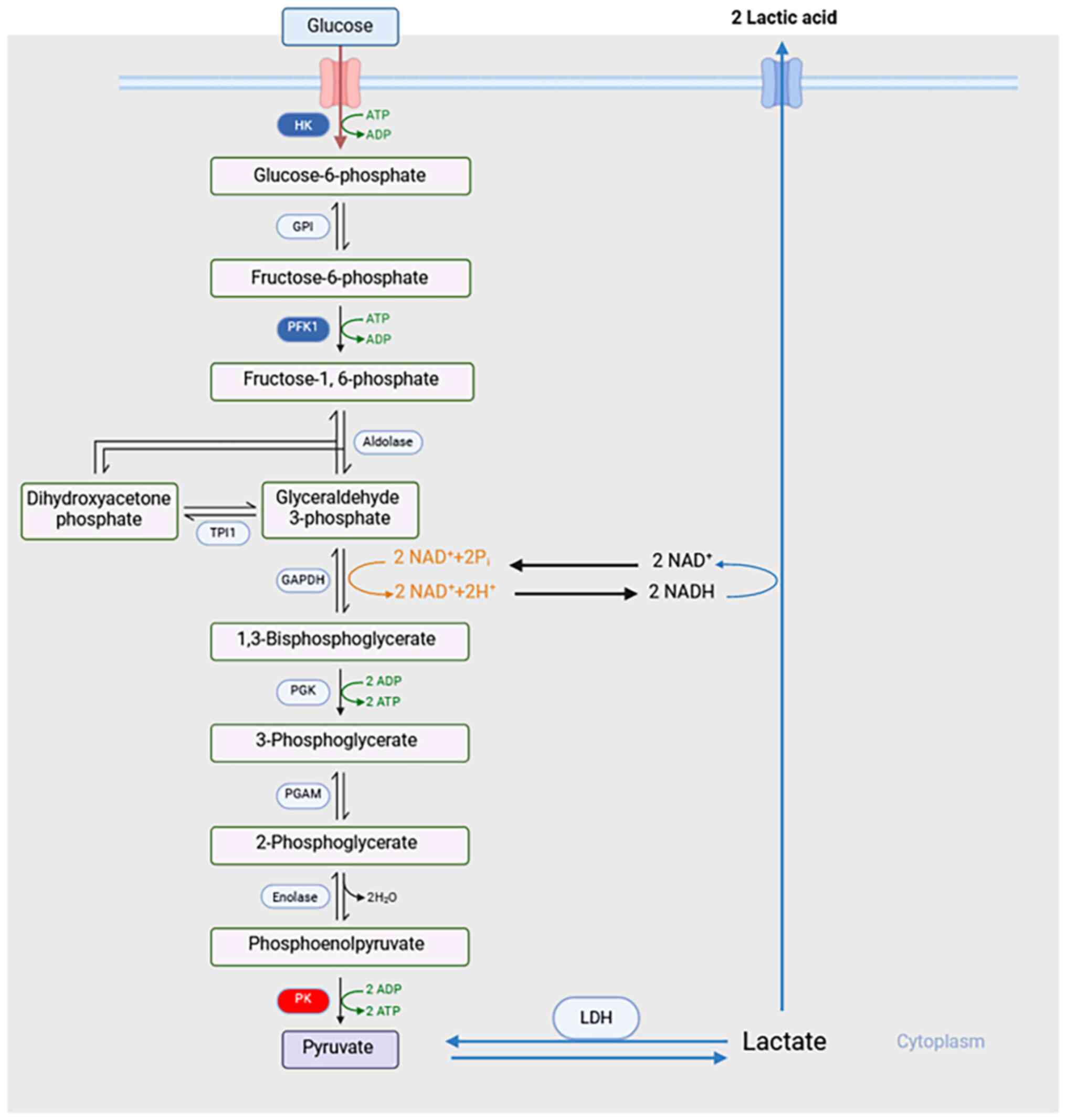
Glycolysis is a 10-step metabolic pathway that
produces pyruvate and two molecules of adenosine triphosphate (ATP)
(Fig. 1). In proliferative cells,
energy is typically only supplied through glycolysis (10). In the 1920s, Warburg (10) discovered that tumor cells exhibit
an increase in the rate of glucose uptake and lactate accumulation
even in the presence of adequate oxygen availability and fully
functioning mitochondria, a phenomenon today known as the ‘Warburg
effect’ (11,12). Glycolysis occurs in all cells of
the body. Notably, glycolysis is crucial for maintaining immune
function in macrophages (13,14).
The Warburg effect has previously been reported to be important for
patients with sepsis, it is affected by various metabolic disorders
such as lactic acid metabolism (15,16).
Pyruvate kinase (PK) is a kinase that catalyzes the conversion of
phosphoenolpyruvate and ADP to pyruvate and ATP during glycolysis
(Fig. 1) (17,18).
Since PK is the last rate-limiting enzyme in glycolysis, it would
be prudent to hypothesize that PK will also likely serve an
important role in metabolic disorders caused by sepsis. However,
the mechanistic role of PK in sepsis remains unclear. The present
review therefore summarizes the role of PK and discusses
potentially viable treatment strategies for patients with sepsis
treated by targeting PK through glycolytic or non-glycolytic
pathways.
The present study presents an up-to-date literature
review covering the years 2010–2024 on the role of PKM2 in sepsis,
immune cells and targeting therapy. The literature search was
performed using PubMed (https://pubmed.ncbi.nlm.nih.gov/?db=PubMed) and Google
Scholar (https://scholar.google.com.hk/?hl=zh-CN). A limited
number of studies antecedent to 2010 would also be included in the
evaluations if they contained information that could support the
up-to-date study results. The key words used for the search were
‘PKM2’, ‘sepsis’, ‘glycolysis’, ‘macrophage’, ‘T cell’, ‘NK cell’
and ‘B cell’. The studies discussing the effects of PKM2 in sepsis
and its potential as a therapeutic target were included. Of these,
studies not related to the immune and metabolic effects of sepsis
were excluded. The present review aimed to determine whether PKM2
also has a therapeutic target effect similar to that observed in
tumors, providing novel ideas for future sepsis research.
PK catalyzes the transfer of a phosphate group from
phosphoenolpyruvate (PEP) to adenosine diphosphate (ADP), yielding
one molecule of pyruvate and one molecule of ATP, rendering it the
final enzyme in the entire glycolytic process (19). This enzyme was termed PK because it
was supposed to directly catalyze pyruvate phosphorylation to
promote glycolysis. There are four different currently known
isoenzymes, namely L, R, M1 and M2, each of which has distinct
specific kinetic properties necessary to accommodate the metabolic
requirements of the cells and organs they reside in (20). These four isozymes of PK are
expressed in vertebrates as follows: L is mainly expressed in the
liver; R mainly in erythrocytes; M1 mainly in the muscle and brain
tissues; and M2 in mainly in the early fetal tissue and most adult
tissues (21). The L and R
isozymes are expressed by the gene PKLR, whereas the M1 and
M2 isozymes are expressed by the gene PKM2 (22). PKR is characterized by high
substrate affinity and promotes the glycolytic pathway by
catalyzing pyruvate phosphorylation. By contrast, PKL serves an
opposite role on PKR, causing the phosphorylation of pyruvate
kinase and inhibition of glycolysis (23). PKM serves a key role in metabolic
disorders as a result of a variety of malignant diseases like liver
cancer, glioma and lung cancer (24,25).
It has been frequently reported that tumor cells
preferentially express PKM2, leading to the metabolic reprogramming
towards the glycolysis process (31,32).
By contrast, PKM2 has been previously observed to mediate a number
of metabolic changes in sepsis, mostly in immune cells (18). The expression of PK isomers is
tissue-specific, which suggests that the expression of different
isomers meets different metabolic needs (28). Therefore, PKM2 can be regulated
through structural alterations in tumors or sepsis. Understanding
the dimer and tetramer of PKM2 facilitates the understanding of
PKM2 and its use as a therapeutic target.
PKM2 has received increased attention in tumor
research due to its special structural regulation (the conversion
of tetramers and dimers), although its mechanism in sepsis remains
unclear. Based on its crucial role in the regulatory process in
glycolysis, it could therefore be hypothesized that targeting this
process would be viable for treating sepsis. A previous study has
reported that PKM2 oligomers can enter the nucleus, bind to the
hypoxia-inducible factor (HIF)-1α and signal transducer and
activator of transcription (STAT3), bind to the IL-1β promoter,
downregulate IL-1β, upregulate IL-10 and regulate hypoxic injury
and inflammation (37). In
addition, epidermal growth factor receptor activation has been
documented to promote the ERK1/2-dependent phosphorylation of PKM2
S37 and peptidyl-prolyl cis-trans isomerase-catalyzed PKM2
cis-trans isomerization, which binds to the input protein α5,
leading to nuclear PKM2 translocation and promoting the Warburg
effect in glioblastoma cells (38). PKM2 dimers can also mediate
non-glycolytic functions affecting inflammation. It has been
reported that PKM2 can exist in an oligomeric form in monocytes and
macrophages, where it promotes IL-6 and IL-1β production, resulting
in a proinflammatory effect (39).
Even in the absence of disease, the low catalytic activity of the
PKM2 dimer leads to the accumulation of intermediate products in
the cell. As a result, a large number of acidic intermediates such
as phosphoenolpyruvate accumulate in the cell, resulting in an
acid-base imbalance, eventually leading to metabolic disorders
(40). These aforementioned
previous studies suggest that PKM2 can serve an important role in
sepsis by converting into the dimer form and promoting inflammation
through multiple pathways.
The regulatory properties of structural changes in
PKM2 have been previously studied in the context of cancer therapy,
providing a novel avenue for the treatment of sepsis. At present,
the following two approaches have been adapted by cancer cells to
control PKM2 function: i) Impeding PKM2 nuclear translocation
through inhibition of the PKM2 dimer form; and ii) activation of
PKM2 tetramer form, thereby maintaining its normal function of
converting PEP into pyruvate (41). In conclusion, regulating the PKM2
structure may also be a potential target for the treatment of
sepsis.
During the occurrence and development of sepsis,
monocytes differentiate into macrophages and migrate to the site of
infection under the stimulation of various inflammatory substances
like pathogens, damaged cells or irritants (46). Fibrinogen-like protein 2 has been
previously shown to target PKM2 and directly exacerbate alcoholic
liver injury by downregulating macrophage glycolytic reprogramming
(47) (Fig. 2). In addition, hypoxic exosomal
PKM2 has been observed to induce M2 polarization in macrophages by
activating the 5′AMP-activated protein kinase pathway and
aggravating lung cancer (48),
although not in sepsis, regulation of macrophages by PKM2 also
provides insights into sepsis. In macrophages, the recombinant
Treponema pallidum protein Tp47 can activate the nucleotide-binding
oligomerization domain-like receptor family protein 3 inflammasome
through PKM2-dependent glycolysis and induce phagocytosis (49,50).
Sepsis is very closely related to immune cells, the effect of PKM2
on macrophages suggests its role. Digoxin can also activate the
PKM2/HIF-1α axis, reduce HIF-1α axis-sustained inflammasome
activity in macrophages and ameliorate mouse hepatitis (Fig. 2) (51). The long non-coding RNA HIF-1α
inhibitor at the transcriptional level has been previously found to
inhibit lactate production as a result of miR-106 induction and
facilitate PKM2 oligomerization, which polarizes macrophages
towards an M2-like anti-inflammatory phenotype and contributes to
immune escape (mostly in macrophages) in vivo (52). The metabolic regulation of
macrophages by PKM2 serves an important role in the regulation of
macrophage polarization and other functions like inhibiting
glycolysis in macrophages and regulating PD-L1, which may offer
potential treatment ideas for sepsis.
The role of PKM2 in other immune cells may also be
noteworthy. In natural killer (NK) cells, PKM2 mainly exists as a
monomer and tetramer, which functions through metabolic regulation,
not transcriptional regulation (61). Silencing PKM2 was found to disable
NK cell activation (61). PKM2 can
also regulate the activation by enhancing IL-12p35 expression and
metabolic function of dendritic cells through HIF-1α-dependent
pathways or by reprogramming the expression of metabolic genes such
as PKM2 (62,63). PKM2 is required to support
metabolic reprogramming (an increase in both oxidative
phosphorylation and glycolysis) for homocysteine-induced B-cell
activation and function both in vivo and in vitro,
where the shikonin compound can reverse this process and inhibit
the proliferation of B-cells (64). PKM2-dependent glycolysis is crucial
for the activation of various immune cells. The occurrence of
sepsis is closely associated with the hyperactivation of immune
cells and cytokine storms (33).
The role of PKM2 in immune cells therefore provides novel ideas for
the treatment of sepsis.
PKM2 can also regulate sepsis development in several
other manners. Total PKM2 is considered to be an indicator of
sepsis diagnosis and prognosis (65). PKM2 can interact with HIF-1α and
activate the HIF-1α-dependent transcription of enzymes necessary
for aerobic glycolysis in macrophages, promoting the Warburg effect
to exacerbate sepsis (66). In a
mouse model of sepsis, sphingosine kinase 1 was found to directly
bind to PKM2, resulting in nuclear heterotopic and PKM2
phosphorylation, aggravating sepsis (Fig. 2) (67). Research on the mechanism of PKM2
has expanded the understanding of the metabolic regulation of
sepsis.
There have also been attempts to target sepsis with
PKM2 in recent years. The chemical compound Celastrol can bind to
Cys424 of PKM2, inhibiting the enzyme and suppressing aerobic
glycolysis, improving survival in an animal model of sepsis
(Fig. 2) (68). Capsaicin has also been documented
to directly bind to and inhibit PKM2 and lactate dehydrogenase A to
suppress the Warburg effect in inflammatory macrophages (69). Similarly, Lycium barbarum
polysaccharide has been observed to inhibit
lipopolysaccharide-induced inflammation by altering the glycolysis
and the differentiation of macrophages by triggering PKM2
degradation (Fig. 2) (70). Shikonin can also regulate PKM2 by
inhibiting the expression of programmed death-ligand 1 in
macrophages to control the development of sepsis (Fig. 2) (71). In addition, PKM2 was previously
found to regulate the function of platelets by PI3K/glycogen
synthase kinase 3 signaling in humans and mice (72–74),
which serve a role in sepsis and arterial thrombosis (75).
PK is the last rate-limiting enzyme in the
glycolytic pathway. Metabolic disorders are found in malignant
proliferating cells and depend on glycolysis as a means of
obtaining energy (76). In
malignant diseases, energy consumption is high, but energy
utilization is low, which harms the patient. The metabolic
characteristics of tumors can be detected at an early stage of
tumorigenesis and tumors are also considered to be a class of
metabolic diseases (76).
Therefore, several kinases involved in glycolytic metabolism like
hexokinase (HK)2, phosphofructokinase (PFKM) and lactate
dehydrogenase were also considered to be oncogenes and were used as
targets for the treatment of tumors (77,78).
HK2 has been the most studied and plays an important role in
promoting glycolysis (79). PFKM
is also one of the rate-limiting enzymes in the glycolytic pathway
and is considered as a therapeutic target (80). The role of the glycolytic pathway
in disease is promising.
Sepsis is characterized by the hyperactivation of
immune cells and proliferating immune cells are similar to tumor
cells, in that both depend on the Warburg effect for energy
(42). The presence of various
factors suggests that the Warburg effect is also important in
sepsis (15). Current research
suggests that sepsis occurs when immune cells undergo metabolic
reprogramming, leading to excessive inflammation and
immunosuppression. At the same time, the interaction of the
metabolic and immune systems further limits treatment (15). The present review summarized the
latest research progress on the role of PK in sepsis and the
regulatory effect of the conversion of tetrameric and dimeric PK
structures on glycolysis. Consistent with its role in tumors, the
PKM2 subtype serves an important role in sepsis and has the
greatest potential as a therapeutic target (Fig. 2). By targeting the Warburg effect
in immune cells, several studies have reported that PKM2 is
important for the hyperactivation of macrophages, T cells and NK
cells. In particular, macrophages serve an important role in sepsis
and warrant attention (Fig. 2).
Several studies have used drugs to promote structural changes in
PKM2 or directly regulate sepsis through the downstream HIF-1α
pathway (35,80). However, the majority of these
studies involved in vitro and in vivo experiments.
Further investigation in this area is warranted.
Metabolic disorders and even the Warburg effect have
been involved in an increasing number of diseases in recent years
(81). The present review
summarized the mechanism of PKM2 in sepsis and discussed its
potential as a therapeutic target, which may promote the
understanding of the metabolic aspects of sepsis. The present
review provides a basis for studying the mechanism of PKM2 and
developing therapeutic strategies for metabolic disorders,
including sepsis.
Not applicable.
The present study was supported by Zhejiang Provincial Natural
Science Foundation of China (grant no. LQ24H160040), the Zhejiang
Medical and Health Science and Technology Project (grant no.
2024KY501), the Jinhua Science and Technology Research Program
(grant no. 2022-3-074) and the Jinhua Municipal Central Hospital
Young and Middle-aged Science and Technology Project (grant no.
JY2022-5-03).
Not applicable.
XC designed and conceived the present review. YH,
JT, QX, ZF, RL, MY, JZ and XC wrote the draft. All authors
contributed to editorial changes in the manuscript. All authors
read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Stanski NL and Wong HR: Prognostic and
predictive enrichment in sepsis. Nat Rev Nephrol. 16:20–31. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rudd KE, Johnson SC, Agesa KM, Shackelford
KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer
S, et al: Global, regional, and national sepsis incidence and
mortality, 1990–2017: Analysis for the global burden of disease
study. Lancet. 395:200–211. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang M, Cai S and Su J: The pathogenesis
of sepsis and potential therapeutic targets. Int J Mol Sci.
20:53762019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rocheteau P, Chatre L, Briand D, Mebarki
M, Jouvion G, Bardon J, Crochemore C, Serrani P, Lecci PP, Latil M,
et al: Sepsis induces long-term metabolic and mitochondrial muscle
stem cell dysfunction amenable by mesenchymal stem cell therapy.
Nat Commun. 6:101452015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu CL, Wang Y, Liu Q, Li HR, Yu CM, Li P,
Deng XM and Wang JF: Dysregulation of neutrophil death in sepsis.
Front Immunol. 13:9639552022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zou S, Jie H, Han X and Wang J: The role
of neutrophil extracellular traps in sepsis and sepsis-related
acute lung injury. Int Immunopharmacol. 124:1104362023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chaplin DD: Overview of the immune
response. J Allergy Clin Immunol. 125 (Suppl 2):S3–S23. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wasyluk W and Zwolak A: Metabolic
alterations in sepsis. J Clin Med. 10:24122021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiao M, Liu D, Xu Y, Mao W and Li W: Role
of PFKFB3-driven glycolysis in sepsis. Ann Med. 55:1278–1289. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zlacká J and Zeman M: Glycolysis under
circadian control. Int J Mol Sci. 22:136662021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li X, Yang Y, Zhang B, Lin X, Fu X, An Y,
Zou Y, Wang JX, Wang Z and Yu T: Lactate metabolism in human health
and disease. Signal Transduct Target Ther. 7:3052022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen X, Sun N, Li R, Sang X, Li X, Zhao J,
Han J, Yang J and Ikezoe T: Targeting HLA-F suppresses the
proliferation of glioma cells via a reduction in hexokinase
2-dependent glycolysis. Int J Biol Sci. 17:1263–1276. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuan Y, Fan G, Liu Y, Liu L, Zhang T, Liu
P, Tu Q, Zhang X, Luo S, Yao L, et al: The transcription factor
KLF14 regulates macrophage glycolysis and immune function by
inhibiting HK2 in sepsis. Cell Mol Immunol. 19:504–515. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng Z, Ma H, Zhang X, Tu F, Wang X, Ha
T, Fan M, Liu L, Xu J, Yu K, et al: Enhanced glycolytic metabolism
contributes to cardiac dysfunction in polymicrobial sepsis. J
Infect Dis. 215:1396–1406. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bar-Or D, Carrick M, Tanner A II, Lieser
MJ, Rael LT and Brody E: Overcoming the Warburg effect: Is it the
key to survival in sepsis? J Crit Care. 43:197–201. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Wang Z and Tang D: Aerobic
exercise improves LPS-induced sepsis via regulating the Warburg
effect in mice. Sci Rep. 11:177722021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu S, Guo Y, Zhang X, Liu H, Yin M, Chen
X and Peng C: Pyruvate kinase M2 (PKM2) in cancer and cancer
therapeutics. Cancer Lett. 503:240–248. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alquraishi M, Puckett DL, Alani DS,
Humidat AS, Frankel VD, Donohoe DR, Whelan J and Bettaieb A:
Pyruvate kinase M2: A simple molecule with complex functions. Free
Radic Biol Med. 143:176–192. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gupta V and Bamezai RN: Human pyruvate
kinase M2: A multifunctional protein. Protein Sci. 19:2031–2044.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Swint-Kruse L, Dougherty LL, Page B, Wu T,
O'Neil PT, Prasannan CB, Timmons C, Tang Q, Parente DJ, Sreenivasan
S, et al: PYK-SubstitutionOME: An integrated database containing
allosteric coupling, ligand affinity and mutational, structural,
pathological, bioinformatic and computational information about
pyruvate kinase isozymes. Database (Oxford). 2023:baad0302023.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Buneeva O, Kopylov A, Gnedenko O,
Medvedeva M, Veselovsky A, Ivanov A, Zgoda V and Medvedev A:
Proteomic profiling of mouse brain pyruvate kinase binding
proteins: A hint for moonlighting functions of PKM1? Int J Mol Sci.
24:76342023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Du D, Liu C, Qin M, Zhang X, Xi T, Yuan S,
Hao H and Xiong J: Metabolic dysregulation and emerging
therapeutical targets for hepatocellular carcinoma. Acta Pharm Sin
B. 12:558–580. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Battisti UM, Gao C, Akladios F, Kim W,
Yang H, Bayram C, Bolat I, Kiliclioglu M, Yuksel N, Tozlu OO, et
al: Ellagic acid and its metabolites as potent and selective
allosteric inhibitors of liver pyruvate kinase. Nutrients.
15:5772023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang S, Liao Z, Li S and Luo Y:
Non-metabolic enzyme function of PKM2 in hepatocellular carcinoma:
A review. Medicine (Baltimore). 102:e355712023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang X, Li C and Chen Y: Phosphoserine
aminotransferase 1: A metabolic enzyme target of cancers. Curr
Cancer Drug Targets. 23:171–186. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Noguchi T, Inoue H and Tanaka T: The M1-
and M2-type isozymes of rat pyruvate kinase are produced from the
same gene by alternative RNA splicing. J Biol Chem.
261:13807–13812. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dombrauckas JD, Santarsiero BD and Mesecar
AD: Structural basis for tumor pyruvate kinase M2 allosteric
regulation and catalysis. Biochemistry. 44:9417–9429. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prakasam G, Iqbal MA, Bamezai RNK and
Mazurek S: Posttranslational modifications of pyruvate kinase M2:
Tweaks that benefit cancer. Front Oncol. 8:222018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang YC, Cheng TY, Huang SM, Su CY, Yang
PW, Lee JM, Chen CK, Hsiao M, Hua KT and Kuo ML: Cytosolic PKM2
stabilizes mutant EGFR protein expression through regulating
HSP90-EGFR association. Oncogene. 35:3387–3398. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu WR, Tian MX, Yang LX, Lin YL, Jin L,
Ding ZB, Shen YH, Peng YF, Gao DM, Zhou J, et al: PKM2 promotes
metastasis by recruiting myeloid-derived suppressor cells and
indicates poor prognosis for hepatocellular carcinoma. Oncotarget.
6:846–861. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chaneton B and Gottlieb E: Rocking cell
metabolism: Revised functions of the key glycolytic regulator PKM2
in cancer. Trends Biochem Sci. 37:309–316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bailleul J, Ruan Y, Abdulrahman L, Scott
AJ, Yazal T, Sung D, Park K, Hoang H, Nathaniel J, Chu FI, et al:
M2 isoform of pyruvate kinase rewires glucose metabolism during
radiation therapy to promote an antioxidant response and
glioblastoma radioresistance. Neuro Oncol. 25:1989–2000. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Z, Le Y, Chen H, Zhu J and Lu D: Role
of PKM2-mediated immunometabolic reprogramming on development of
cytokine storm. Front Immunol. 12:7485732021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luo W, Hu H, Chang R, Zhong J, Knabel M,
O'Meally R, Cole RN, Pandey A and Semenza GL: Pyruvate kinase M2 is
a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell.
145:732–744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Malla A, Gupta S and Sur R: Glycolytic
enzymes in non-glycolytic web: Functional analysis of the key
players. Cell Biochem Biophys. Jan 9–2024.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liang N, Mi L, Li J, Li T, Chen J, Dionigi
G, Guan H and Sun H: Pan-cancer analysis of the oncogenic and
prognostic role of PKM2: A potential target for survival and
immunotherapy. Biomed Res Int. 2023:33751092023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Palsson-McDermott EM, Curtis AM, Goel G,
Lauterbach MAR, Sheedy FJ, Gleeson LE, van den Bosch MWM, Quinn SR,
Domingo-Fernandez R, Johnston DGW, et al: Pyruvate kinase M2
regulates hif-1alpha activity and IL-1beta induction and is a
critical determinant of the Warburg effect in LPS-activated
macrophages. Cell Metab. 21:3472015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo
F, Lyssiotis CA, Aldape K, Cantley LC and Lu Z: ERK1/2-dependent
phosphorylation and nuclear translocation of PKM2 promotes the
Warburg effect. Nat Cell Biol. 14:1295–1304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shirai T, Nazarewicz RR, Wallis BB, Yanes
RE, Watanabe R, Hilhorst M, Tian L, Harrison DG, Giacomini JC,
Assimes TL, et al: The glycolytic enzyme PKM2 bridges metabolic and
inflammatory dysfunction in coronary artery disease. J Exp Med.
213:337–354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lv L, Li D, Zhao D, Lin R, Chu Y, Zhang H,
Zha Z, Liu Y, Li Z, Xu Y, et al: Acetylation targets the M2 isoform
of pyruvate kinase for degradation through chaperone-mediated
autophagy and promotes tumor growth. Mol Cell. 42:719–730. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chhipa AS and Patel S: Targeting pyruvate
kinase muscle isoform 2 (PKM2) in cancer: What do we know so far?
Life Sci. 280:1196942021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Palsson-McDermott EM and O'Neill LA: The
Warburg effect then and now: From cancer to inflammatory diseases.
Bioessays. 35:965–973. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Karnovsky ML: The metabolism of
leukocytes. Semin Hematol. 5:156–165. 1968.PubMed/NCBI
|
|
44
|
Kelly B and O'Neill LA: Metabolic
reprogramming in macrophages and dendritic cells in innate
immunity. Cell Res. 25:771–784. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Palmer CS, Ostrowski M, Balderson B,
Christian N and Crowe SM: Glucose metabolism regulates T cell
activation, differentiation, and functions. Front Immunol. 6:12015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jakubzick CV, Randolph GJ and Henson PM:
Monocyte differentiation and antigen-presenting functions. Nat Rev
Immunol. 17:349–362. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hu X, Wan X, Diao Y, Shen Z, Zhang Z, Wang
P, Hu D, Wang X, Yan W, Yu C, et al: Fibrinogen-like protein 2
regulates macrophage glycolytic reprogramming by directly targeting
PKM2 and exacerbates alcoholic liver injury. Int Immunopharmacol.
124:1109572023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou S, Lan Y, Li Y, Li Z, Pu J and Wei L:
Hypoxic tumor-derived exosomes induce M2 macrophage polarization
via PKM2/AMPK to promote lung cancer progression. Cell Transplant.
31:96368972211069982022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zheng YW, Wang M, Xie JW, Chen R, Wang XT,
He Y, Yang TC, Liu LL and Lin LR: Recombinant Treponema pallidum
protein Tp47 promoted the phagocytosis of macrophages by activating
NLRP3 inflammasome induced by PKM2-dependent glycolysis. J Eur Acad
Dermatol Venereol. 37:2067–2079. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zheng XQ, Li Z, Meng QQ, Li W, Li QL, Xie
L, Xiao Y, Xu QY and Chen YY: Treponema pallidum recombinant
protein Tp47 activates NOD-like receptor family protein 3
inflammasomes in macrophages via glycolysis. Int Immunopharmacol.
126:1112042024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhao P, Han SN, Arumugam S, Yousaf MN, Qin
Y, Jiang JX, Torok NJ, Chen Y, Mankash MS, Liu J, et al: Digoxin
improves steatohepatitis with differential involvement of liver
cell subsets in mice through inhibition of PKM2 transactivation. Am
J Physiol Gastrointest Liver Physiol. 317:G387–G397. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhao K, Wang X, Zhao D, Lin Q, Zhang Y and
Hu Y: lncRNA HITT inhibits lactate production by repressing PKM2
oligomerization to reduce tumor growth and macrophage polarization.
Research (Wash D C). 2022:98549042022.PubMed/NCBI
|
|
53
|
Zhu J and Paul WE: CD4 T cells: Fates,
functions, and faults. Blood. 112:1557–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bettencourt IA and Powell JD: Targeting
metabolism as a novel therapeutic approach to autoimmunity,
inflammation, and transplantation. J Immunol. 198:999–1005. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pearce EL and Pearce EJ: Metabolic
pathways in immune cell activation and quiescence. Immunity.
38:633–643. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Angiari S, Runtsch MC, Sutton CE,
Palsson-McDermott EM, Kelly B, Rana N, Kane H, Papadopoulou G,
Pearce EL, Mills KHG and O'Neill LAJ: Pharmacological activation of
pyruvate kinase M2 inhibits CD4+ T cell pathogenicity
and suppresses autoimmunity. Cell Metab. 31:391–405.e8. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jiang S: Tetrameric PKM2 activation Curbs
CD4+ T cell overactivation. Trends Endocrinol Metab.
31:393–395. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Damasceno LEA, Prado DS, Veras FP, Fonseca
MM, Toller-Kawahisa JE, Rosa MH, Públio GA, Martins TV, Ramalho FS,
Waisman A, et al: PKM2 promotes Th17 cell differentiation and
autoimmune inflammation by fine-tuning STAT3 activation. J Exp Med.
217:e201906132020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Moreno-Fernandez ME, Giles DA, Oates JR,
Chan CC, Damen MSMA, Doll JR, Stankiewicz TE, Chen X, Chetal K,
Karns R, et al: PKM2-dependent metabolic skewing of hepatic Th17
cells regulates pathogenesis of non-alcoholic fatty liver disease.
Cell Metab. 33:1187–1204.e9. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen C, Zhang W, Zhou T, Liu Q, Han C,
Huang Z, Chen S, Mei Q, Zhang C, Zhang K, et al: Vitamin B5 rewires
Th17 cell metabolism via impeding PKM2 nuclear translocation. Cell
Rep. 41:1117412022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Walls JF, Subleski JJ, Palmieri EM,
Gonzalez-Cotto M, Gardiner CM, McVicar DW and Finlay DK: Metabolic
but not transcriptional regulation by PKM2 is important for natural
killer cell responses. Elife. 9:e591662020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jin X, Zhang W, Wang Y, Liu J, Hao F, Li
Y, Tian M, Shu H, Dong J, Feng Y and Wei M: Pyruvate kinase M2
promotes the activation of dendritic cells by enhancing IL-12p35
expression. Cell Rep. 31:1076902020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Guak H, Al Habyan S, Ma EH, Aldossary H,
Al-Masri M, Won SY, Ying T, Fixman ED, Jones RG, McCaffrey LM and
Krawczyk CM: Glycolytic metabolism is essential for CCR7
oligomerization and dendritic cell migration. Nat Commun.
9:24632018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Deng J, Lü S, Liu H, Liu B, Jiang C, Xu Q,
Feng J and Wang X: Homocysteine activates B cells via regulating
PKM2-dependent metabolic reprogramming. J Immunol. 198:170–183.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang L, Tang D and Zhang P: Changes of
serum pyruvate kinase M2 level in patients with sepsis and its
clinical value. Infect Drug Resist. 16:6437–6449. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yang L, Xie M, Yang M, Yu Y, Zhu S, Hou W,
Kang R, Lotze MT, Billiar TR, Wang H, et al: PKM2 regulates the
Warburg effect and promotes HMGB1 release in sepsis. Nat Commun.
5:44362014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li S, Xue X, Zhang H, Jiang L, Zhang Y,
Zhu X and Wang Y: Inhibition of sphingosine kinase 1 attenuates
LPS-induced acute lung injury by suppressing endothelial cell
pyroptosis. Chem Biol Interact. 390:1108682024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ni L, Lin B, Shen M, Li C, Hu L, Fu F,
Chen L, Yang J and Shi D: PKM2 deficiency exacerbates gram-negative
sepsis-induced cardiomyopathy via disrupting cardiac calcium
homeostasis. Cell Death Discov. 8:4962022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang Q, Luo P, Xia F, Tang H, Chen J,
Zhang J, Liu D, Zhu Y, Liu Y, Gu L, et al: Capsaicin ameliorates
inflammation in a TRPV1-independent mechanism by inhibiting
PKM2-LDHA-mediated Warburg effect in sepsis. Cell Chem Biol.
29:1248–1259.e6. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ding H, Wang JJ, Zhang XY, Yin L and Feng
T: Lycium barbarum polysaccharide antagonizes LPS-induced
inflammation by altering the glycolysis and differentiation of
macrophages by triggering the degradation of PKM2. Biol Pharm Bull.
44:379–388. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yuan L, Wang Y, Chen Y, Chen X, Li S and
Liu X: Shikonin inhibits immune checkpoint PD-L1 expression on
macrophage in sepsis by modulating PKM2. Int Immunopharmacol.
121:1104012023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhao X, Wu X, Si Y, Xie J, Wang L, Liu S,
Duan C, Wang Q, Wu D, Wang Y, et al: D-DI/PLT can be a prognostic
indicator for sepsis. PeerJ. 11:e159102023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Fu G, Deng M, Neal MD, Billiar TR and
Scott MJ: Platelet-monocyte aggregates: Understanding mechanisms
and functions in sepsis. Shock. 55:156–166. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Greco E, Lupia E, Bosco O, Vizio B and
Montrucchio G: Platelets and multi-organ failure in sepsis. Int J
Mol Sci. 18:22002017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Nayak MK, Ghatge M, Flora GD, Dhanesha N,
Jain M, Markan KR, Potthoff MJ, Lentz SR and Chauhan AK: The
metabolic enzyme pyruvate kinase M2 regulates platelet function and
arterial thrombosis. Blood. 137:1658–1668. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zu XL and Guppy M: Cancer metabolism:
Facts, fantasy, and fiction. Biochem Biophys Res Commun.
313:459–465. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhou Y, Guo Y and Tam KY: Targeting
glucose metabolism to develop anticancer treatments and therapeutic
patents. Expert Opin Ther Pat. 32:441–453. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhao M, Wei F, Sun G, Wen Y, Xiang J, Su
F, Zhan L, Nian Q, Chen Y and Zeng J: Natural compounds targeting
glycolysis as promising therapeutics for gastric cancer: A review.
Front Pharmacol. 13:10043832022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Shan W, Zhou Y and Tam KY: The development
of small-molecule inhibitors targeting hexokinase 2. Drug Discov
Today. 27:2574–2585. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Xu JQ, Fu YL, Zhang J, Zhang KY, Ma J,
Tang JY, Zhang ZW and Zhou ZY: Targeting glycolysis in non-small
cell lung cancer: Promises and challenges. Front Pharmacol.
13:10373412022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zuo J, Tang J, Lu M, Zhou Z, Li Y, Tian H,
Liu E, Gao B, Liu T and Shao P: Glycolysis rate-limiting enzymes:
Novel potential regulators of rheumatoid arthritis pathogenesis.
Front Immunol. 12:7797872021. View Article : Google Scholar : PubMed/NCBI
|