Introduction
Hepatic fibrosis (HF) is a pathological process in
which the liver performs damage repair responses to various chronic
stimuli such as chronic viral infection, alcohol consumption,
parasitic disease, genetic abnormalities and toxic damage (1,2).
Extracellular matrix (ECM) components, mainly collagen, deposited
in the liver cause contracted scarring and increased organ
stiffness (3). Hepatic stellate
cells (HSCs) represent the most important inducers of fibrosis
following liver injury. Under the action of various cytokines such
as tumor necrosis factor α, interleukin-1β, interleukin-6 and
transforming growth factor-β1 (TGF-β1), HSCs are continuously
converted into fibroblasts, producing large amounts of ECM
components that eventually cause HF (4,5). HSC
proliferation, cell phenotype transformation, and increased
synthesis of ECM and deposition, combined with neovascularization
and inflammation, represent the most prominent pathological
features in the repair and reconstruction of progressive HF
(6,7).
A significant body of evidence has shown that
signaling pathways play an important role in HF, particularly the
PI3K/Akt/mTOR pathway, which is particularly important during
inflammation and apoptosis (8,9).
Previous studies have found that in carbon tetrachloride
(CCl4)-induced animal models of HF and HSC activation,
the PI3K/Akt/mTOR pathway is activated and that inhibition of this
activation could reduce the production of ECM, thereby inhibiting
HF and HSC activation (10–13).
Wang et al (14) found that
salvianolic acid A attenuates CCl4-induced HF by
regulating the PI3K/AKT/mTOR signaling pathways. Similarly,
interleukin-22 has been found to alleviate alcohol-associated HF by
inhibiting the PI3K/AKT/mTOR pathway (12). Germacrone also improves HF by
regulating the PI3K/AKT/mTOR signaling pathway (15). Therefore, the PI3K/AKT/mTOR pathway
is particularly important for HF.
Carthamus tinctorius L. (CTL) is a cash crop with
multiple functions. The flowers and seeds of CTL are traditional
herbs that have a range of applications in China, Korea, Japan, and
other Asian countries (16,17).
CTL has been used to treat gynecological, cardiovascular, and
cerebrovascular diseases (18,19).
Numerous substances, including flavonoids and alkaloids, have been
isolated and identified from CTL (20). Water-based CTL extracts, such as
hydroxysafflor yellow A, have been developed as injections to treat
cardiovascular disease in China (21), and extracts of CTL seeds have been
used to treat osteoporosis in Korea (16). Numerous clinical and experimental
studies have focused on the therapeutic effects of CTL, with some
results indicating that CTL has potential clinical value (22–24).
However, research into the mechanisms of CTL action is scarce. By
reviewing several studies, it was found that numerous traditional
Chinese and Mongolian remedies that are used to treat liver disease
contain CTL as the main active ingredient (25–28).
However, its mechanism of action remains unclear.
In the present study, to clarify the mechanism of
action of CTL for the treatment of HF, CTL was used as an
intervention in a rat model of HF and pathological liver changes
and ECM liver tissue contents were assessed. Serum infused with CTL
was also used to treat activated HSC cells to detect the biomarkers
present in the activated cells and characterize the activity of the
PI3K/AKT/mTOR signaling pathway in both the animals and cells. The
results of the present study suggest that CTL has the potential to
inhibit PI3K and has the potential to be further developed as a
therapeutic drug for HF.
Materials and methods
Animals and treatments
A total of 40 male Sprague-Dawley rats (age,
6-weeks-old; weight, ~200 g) were purchased from Inner Mongolia
Medical University. All experiments were conducted according to The
Guidelines for the Use and Maintenance of Experimental Animals in
China and were approved by The Ethics Committee of Inner Mongolia
Medical University (Hohhot, China; approval no. YKD202201124). The
animals were acclimatized for one week, with conditions at a
controlled temperature of 22±2°C and a humidity level of 50±10% in
the designated facility, allowed unrestricted access to food and
water, and acclimated to alternating 12-h light and dark cycles.
Animals were subsequently divided into five groups (n=8/group). The
HF model rats were given intraperitoneal injections of 50%
CCl4 in olive oil twice per week for 8 consecutive
weeks, while the control rats were given the same dose of pure
olive oil over the same period. To prepare the drug, ultrafine
powder was used for the extraction of CTL. A total of three methods
for the extraction of herbal compounds were compared: i) Ultrasonic
extraction, in which CTL ultrafine powder was dissolved in 5%
sodium carboxymethylcellulose and extracted by ultrasonic treatment
at 60°C for 1 h; ii) ethanol extraction, in which CTL ultrafine
powder was dissolved in 75% ethanol and extracted for 24 h; and
iii) heat reflux extraction, in which CTL ultrafine powder was
refluxed in boiling water and extracted for 2 h (a traditional
herbal extraction method). Using mass spectrometry methods were
used for detection. Briefly, mass spectrometry analysis was
performed using a Thermo Orbitrap Exploris 120 mass spectrometer
(Thermo Fisher Scientific, Inc.) equipped with an electrospray
ionization source operating in positive and negative ionization
modes. The following parameters were utilized: Positive ion spray
voltage at 3.50 kV; negative ion spray voltage at −2.50 kV; sheath
gas flow rate at 11 l/min with 350°C; drying gas flow rate at 8
l/min with 320°C; nebulizer pressure at 40 psi; full scan mass
spectrometry analysis was conducted at a resolution of 60,000 FWHM
(full width at half maximum) over the m/z range of 100–1,000. The
three methods yielded almost the same extracts. However, in terms
of extraction efficiency, ultrasonic extraction at 60°C was similar
to ethanol extraction, but both these methods had greater
efficiency than boiling water extraction (data not shown). Finally,
the ultrasonic extraction method was selected for the subsequent
experiments.
After 4 weeks of CCl4 treatment, three
groups were selected and treated with CTL. For dosage selection,
the dosages used in another study were referred to (29), and preliminary studies with CTL
dosages of 0.1, 0.5, 1.5, 4.5, 7.5 and 15 g/kg gavage were
performed. A dose of 0.1 g/kg exhibited minimal pharmacological
activity, whereas comparison doses of 7.5–4.5 g/kg did not indicate
enhanced pharmacological efficacy (data not shown). Ultimately, to
demonstrate the dose-dependent effect of CTL, three CTL dosages,
0.5, 1.5 and 4.5 g/kg, were selected and administered for 4 weeks.
Accordingly, the five experimental groups were as follows: i)
Control group; ii) Model group; iii) CTL low-dose group (CTL-L; 0.5
g/kg); iv) CTL medium-dose group (CTL-M; 1.5 g/kg); and v) CTL
high-dose group (CTL-H; 4.5 g/kg). The rats were euthanized by
placing them in a sealed container filled with CO2
(volume displacement rate, 70% vol/min). Blood was collected from
the abdominal aorta, and centrifuged at 3,000 × g and 4°C for 15
min to collect serum using a gel/coagulant tube, and finally stored
at −80°C. The right lobe of the liver was then excised, fixed in a
4% paraformaldehyde solution at room temperature for 24 h, while
the remaining liver tissue was preserved in liquid nitrogen.
HSC culture and treatment
Primary HSCs were isolated from the livers of the
rats according to a previously described protocol (10). Rat HSC-T6 cells (Pricella; cat. no.
CL-0116) were thawed and revived according to the manufacturer's
instructions. All cells were cultured in a 5% CO2
atmosphere at 37°C in Dulbecco modified Eagle medium (DMEM) (Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(FBS) (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
and 100 U/ml streptomycin (Thermo Fisher Scientific, Inc.). Primary
HSCs were activated by culturing on plastic cell culture flasks at
37°C for 7 days. The HSC-T6 cells were then treated with 10 ng/ml
TGF-β1 (Sigma-Aldrich; Merck KGaA) at 37°C for 24 h to activate
them.
For preparing CTL-infused serum, the rats were
administered CTL at doses of 0.5 g/kg (CTL-L), 1.5 g/kg (CTL-M) and
4.5 g/kg (CTL-H) for 5 days. The rats were fasted on the final day,
and blood samples were collected using the abdominal aorta method
in a separation gel/coagulant tube 3 h after the last gavage
(30). The sample was centrifuged
at 3,000 × g at 4°C for 15 min to separate the serum, followed by
sterilization with a microporous filtration membrane. To
investigate the effects of CTL on the activation of primary HSCs,
CTL-infused serum samples from the three groups (CTL-L, CTL-M and
CTL-H) at a concentration of 10% of the culture medium were
collected on the second day of culturing. Activated HSCs were used
as the experimental model, and serum without CTL was used as the
blank control to compare the effects of serum components on HSCs.
The fluid was changed every other day, cells were harvested on day
7 and total proteins or RNA was extracted for subsequent analysis
of Collagen I (Colla1) and α-smooth muscle actin (SMA)
expression. To investigate the effects of CTL on the PI3K/Akt/mTOR
pathway, activated HSC-T6 cells were treated at 4°C for 2 h with 50
µg/ml 740Y-P, a PI3K agonist (Sigma-Aldrich; Merck KGaA), before
CTL-infused serum treatment. Inactivated cells served as the
controls; the cells were kept in a 5% CO2 atmosphere at
37°C in DMEM supplemented with 10% FBS. The fluid was changed every
other day, cells were harvested on day 7, and total proteins or RNA
was extracted for subsequent analysis of p-PI3K, PI3K, p-Akt, Akt,
p-mTOR, mTOR, Collagen I and α-SMA expression, with 8 replicates
per group (n=8).
Histopathological assay
Rat liver tissue specimens (1 cm3) were
fixed with 4% formaldehyde at room temperature for 24 h, embedded
in paraffin, sliced into sections of 4 µm thickness, stained with
hematoxylin (HE; 10 min) and eosin (1 min) (HE) and Masson stain
(10 min) at room temperature, and observed under a light
microscope.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from rat liver tissues or
HSCs with a mount of 1×105 colony forming unit (cfu)
using TRIzol® reagent (Thermo Fisher Scientific, Inc.).
Following this, cDNA was synthesized from total RNA samples using
the PrimeScript RT reagent Kit (Takara Bio, Inc.) for 15 min at
37°C followed by 5 sec at 85°C. qPCR was performed using SYBR
Premix Ex Taq II (Takara Bio, Inc.) with an initial denaturation
for 30 sec at 95°C, followed by 40 cycles of amplification (95°C
for 5 sec and 60°C for 34 sec), according to the manufacturer's
protocol. The expression level of the target gene was normalized to
that of β-actin and all RT-qPCR data were quantified using the
2−∆∆Cq method (31)
with the SLAN automated PCR Analysis System (8.2.2; Shanghai
Hongshi Medical Technology Co., Ltd.), with the primer sequences
listed in Table I.
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Forward primer
sequence (5′→3′) | Reverse primer
sequence (5′→3′) |
|---|
| Colla1 |
CTGCCGATGTCGCTATCC |
CCACAAGCGTGCTGTAGGT |
| α-SMA |
TCCTGACCCTGAAGTATCCG |
TCTCCAGAGTCCAGCACAAT |
| β-actin |
CGTTGACATCCGTAAAGACC |
GCTAGGAGCCAGGGCAGTA |
Western blotting
A T-PER (for tissue) or M-PER (for cell) lysis
buffer (Thermo Fisher Scientific, Inc.) containing a protease
inhibitor mixture (Thermo Fisher Scientific, Inc.) was used to
extract total proteins from liver tissues and cultured HSCs
(1×106 cfu). The protein concentration was determined
using a bicinchoninic acid-based protein assay kit (Thermo Fisher
Scientific, Inc.). Each sample, containing 100 µg protein, was
separated using 10% SDS-PAGE and transferred to a polyvinylidene
fluoride membrane. The membrane was blocked with 5% skim milk for 1
h at room temperature under 50 × g centrifugation and incubated at
4°C with primary antibodies overnight. Following incubation with
secondary antibodies for 1 h at room temperature under 50 × g
centrifugation, the membrane was observed with SuperSignal West
Pico PLUS (Pierce; Thermo Fisher Scientific, Inc.) using enhanced
chemiluminescence (TANON automated Chemiluminescence Imaging System
5200; Shanghai Tianneng Life Sciences Co., Ltd.; http://www.biotanon.com/). The detected protein levels
were normalized to β-actin expression levels. The primary
antibodies used were as follows: Anti-E-cadherin antibody (1:1,000;
cat. no. ab231303; Abcam), anti- α-SMA antibody (1:1,000; cat. no.
ab5694; Abcam), anti-SOX9 antibody (1:1,000; cat. no. ab185966;
Abcam), anti-Collagen I antibody (1:1,000; cat. no. ab34710;
Abcam), anti-phosphorylated (p-) Y607-PI3K antibody (1:1,000; cat.
no. ab182651; Abcam), anti-p-T308 AKT (1:1,000; cat. no. ab38449;
Abcam) and anti-p-S2491 mTOR (1:1,000; cat. no. ab137133; Abcam),
anti-PI3K antibody (1:1,000; cat. no. ab191606; Abcam), anti-AKT
antibody (1:2,000; cat. no. ab185633; Abcam), anti-mTOR antibody
(1:10,000; cat. no. ab134903; Abcam) and anti-β-actin antibody
(1:1,000; cat. no. ab8227; Abcam). The secondary antibodies used
were as follows: Goat anti-mouse IgG HRP (1:5,000; cat. no. ab6789;
Abcam) and goat anti-rabbit IgG HRP (1:5,000; cat. no. ab205718;
Abcam).
ELISA
For aspartate transaminase (AST) and alanine
transaminase (ALT) assays, using the Rat AST ELISA Kit (cat. no.
ab263883; Abcam) and Rat ALT ELISA Kit (cat. no. ab234579; Abcam),
were used according to the manufacturers' instructions. For the
hydroxyproline assay, 100 mg liver tissue was homogenized at 4°C
for 1 min and treated with 1 ml 10N NaOH, and heated at 120°C
hermetically for 1 h. After which, 1 ml 10N HCl was added to
neutralize NaOH, and the solution was centrifuged at 10,000 × g at
4°C for 5 min. The supernatant was collected for measurement of
hydroxyproline with a Hydroxyproline Assay kit (cat. no. ab222941;
Abcam), according to the manufacturer's instructions. Total protein
was used as an internal reference.
Statistical analysis
Data are presented as the mean ± SD and all
experiments were repeated at least three times. The data were
evaluated using one-way ANOVA, and statistical significance was
determined using the Tukey's test. P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed with GraphPad Prism (9.5; Dotmatics).
Results
CTL inhibits HF in vivo
To assess the effect of CTL in terms of inhibiting
HF in rats, HE staining, Masson staining, biochemical tests and the
detection of HF biomarkers were performed to determine the degree
of HF. According to the HE staining results, hepatocytes in the
control group were arranged neatly and exhibited normal morphology,
and appeared to be radiating from the central vein. No necrosis or
inflammatory cell infiltration was observed. By contrast, the model
group exhibited disordered hepatocyte alignment, extensive
inflammatory cell infiltration (red arrows), and substantial
fibrous tissue proliferation and bridging fibrosis around the
veins. Compared with the model group, the CTL-treated groups showed
varying degrees of improvement in the liver tissue structure and
inflammatory response, with the CTL-H group demonstrating
significant improvement (Fig. 1A).
Masson staining revealed that the hepatic lobules in the control
group were intact, with only a small amount of collagen fiber
staining observed around the hepatic vein walls, indicative of the
absence of collagen fiber proliferation. In the model group, the
hepatic lobule structure was disrupted, and extensive and dense
collagen fiber deposition (black arrows) that extended to form
fibrous septa (yellow arrows) was observed around the hepatic veins
and portal tracts. In comparison, the CTL-L and CTL-M groups showed
thinning of the fibrous septa and reduced collagen fiber
expression, while the CTL-H group exhibited complete regression of
the fibrous septa and a reduction in collagen fibers (Fig. 1B). The results of both HE and
Masson staining suggest that CTL can ameliorate
CCl4-induced liver injury and collagen deposition. In
addition, AST and ALT levels were increased in the model group
compared with the control group, whereas this increase was
partially reduced on CTL administration (Fig. 1C and D). Moreover, the western
blotting results showed that compared with the control group, the
levels of the epithelial marker E-cadherin was significantly
decreased in the model group, and the levels of the HF markers
α-SMA and SOX9 were significantly increased. Following the
administration of different concentrations of CTL, E-cadherin
expression was found to be significantly increased, and α-SMA and
SOX9 expression levels were significantly decreased, all of which
occurred in a dose-dependent manner (Fig. 1E). The RT-qPCR and ELISA results
showed that CTL could attenuate the increased expression levels of
Colla1, α-SMA or hydroxyproline content induced by
CCl4 (Fig. 1F-H). The
results indicated that CTL could inhibit HF in vivo.
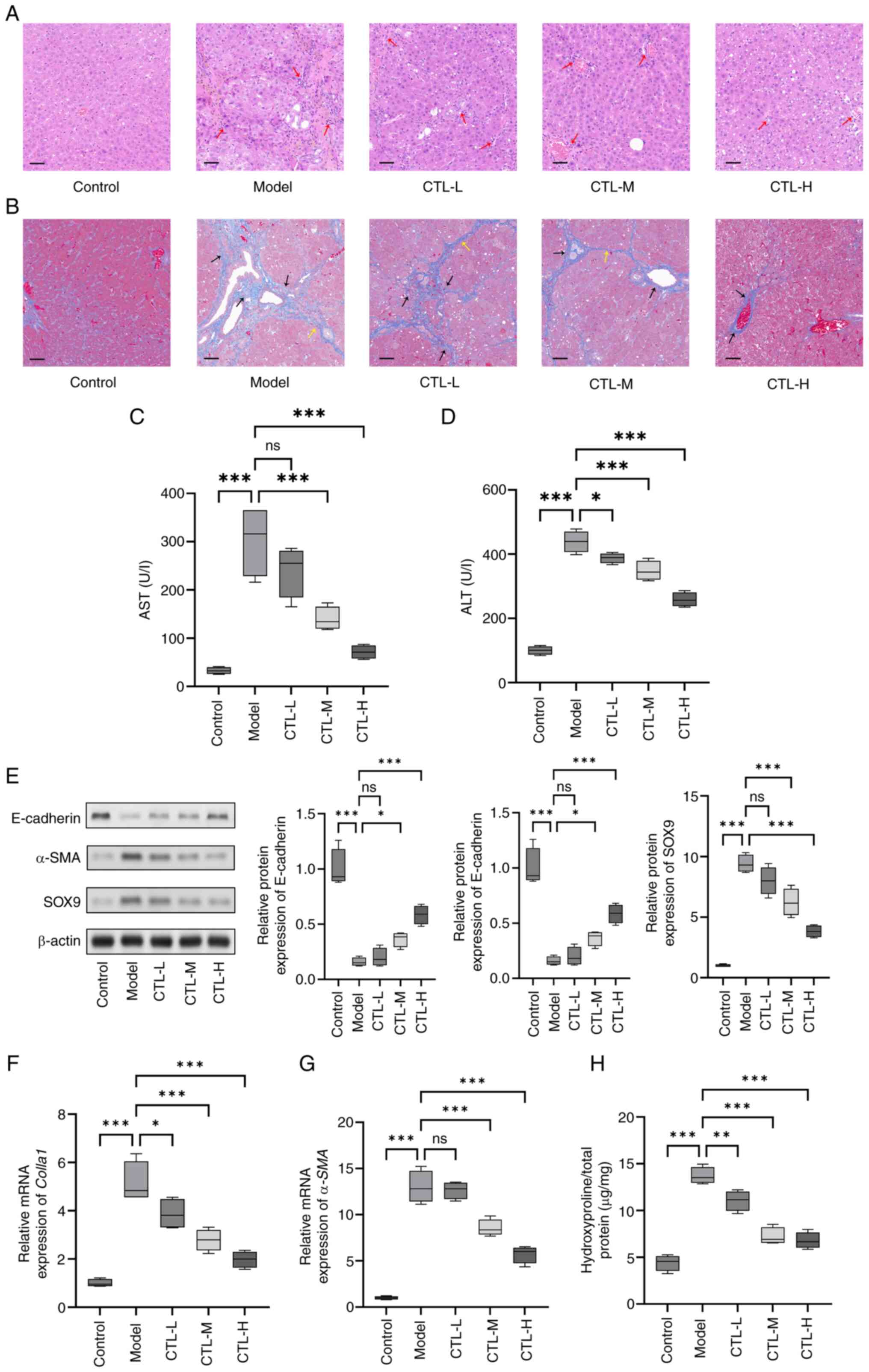 | Figure 1.Inhibition of hepatic fibrosis by CTL
under in vivo conditions. CCl4-induced rat
hepatic fibrosis models were treated with different concentrations
of CTL (0.5, 1.0 and 1.5 g/kg/day). (A) Hematoxylin and eosin
staining and (B) Masson staining showing that CTL can ameliorate
CCl4-induced liver histopathological injury. Red arrows
represent extensive inflammatory cell infiltration, black arrows
represent dense collagen fiber deposition and yellow arrows
represent fibrous septa (magnification, ×200; scale bar, 50 µm).
(C) AST and (D) ALT levels measured by ELISA. (E) Protein
expression levels of E-cadherin, α-SMA, and SOX-9, as measured
using western blot analysis. (F) Colla1 and (G) α-SMA
mRNA levels as measured using reverse transcription-quantitative
PCR. (H) Hydroxyproline level measured using ELISA. Results are
presented as the mean ± SD (n=8), using one-way ANOVA. *P<0.05,
**P<0.01 and ***P<0.001. ns, not significant; CTL,
Carthamus tinctorius L.; HE, hematoxylin and eosin; AST,
aspartate transaminase; ALT, alanine transaminase; α-SMA, α-smooth
muscle actin; Colla1, collagen I, CTL-L, CTL low-dose group;
CTL-M, CTL medium-dose group; CTL-H, CTL high-dose group. |
CTL-infused serum inhibits activation
of primary HSCs
To determine whether CTL-infused serum could inhibit
HSC activation, primary HSCs were used. Observed under a light
microscope, freshly isolated primary HSCs adhered to plastic cell
culture flasks completely within 24 h, and after seven days of
culture, they underwent a morphological change from a quiescent
phenotype to an activated phenotype. The cells in model and blank
serum group extended pseudopodia that fused and interconnected with
each other and adopted a star-shaped morphology, with some cells
already exhibiting fibroblast-like growth. By contrast, the
CTL-infused serum was found to reduce the degree of fibrosis and
alleviate the morphological changes in HSCs (Fig. 2A). The western blotting results
showed that CTL significantly inhibited the expression of collagen
I and α-SMA in activated HSCs in a dose-dependent manner (Fig. 2B). The RT-qPCR results concerning
Colla1 and α-SMA levels were consistent with the
western blotting results (Fig. 2C and
D). The results indicated that CTL could attenuate HSC
activation.
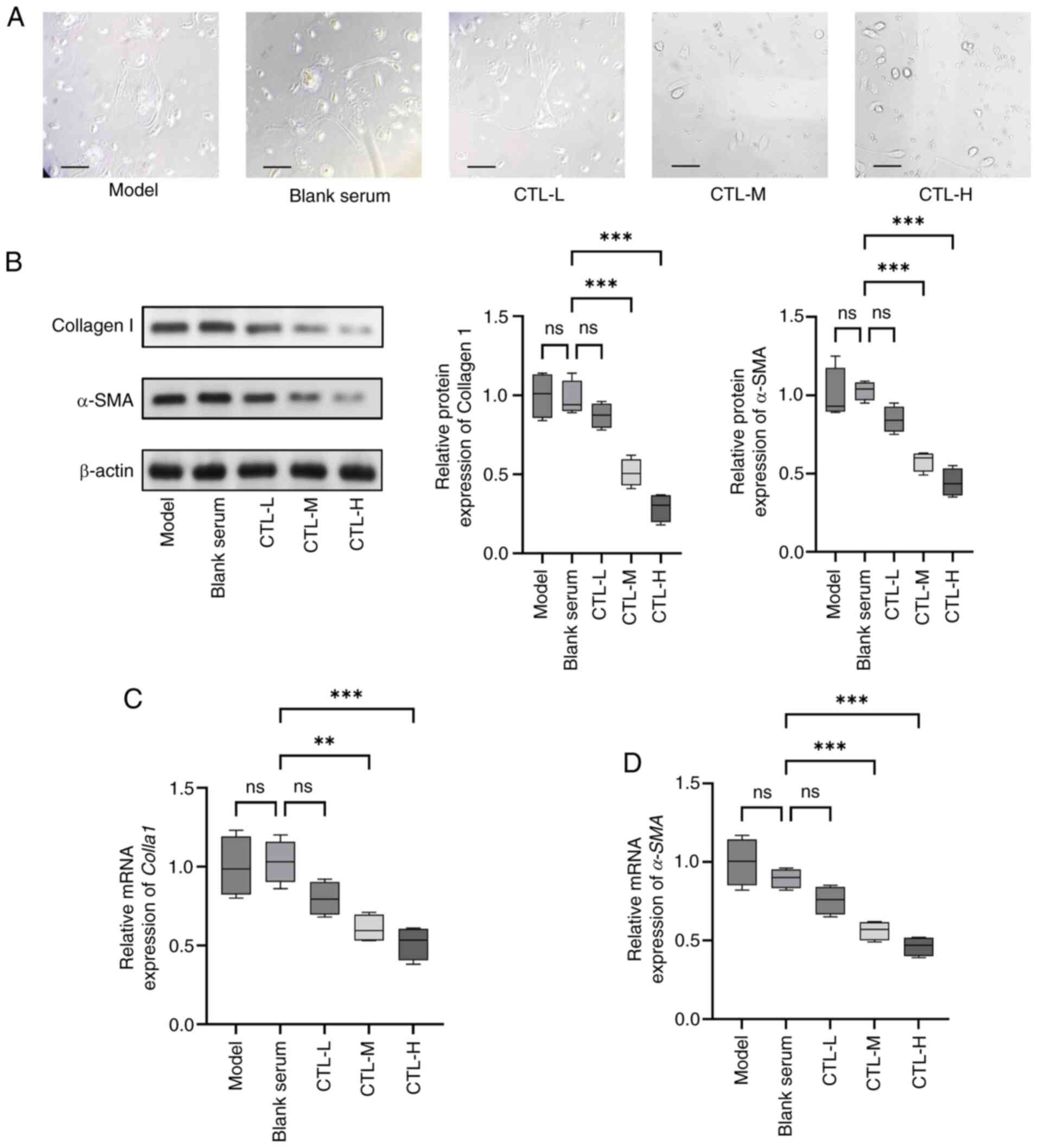 | Figure 2.Inhibition of activation of primary
HSCs by CTL-infused serum. Primary HSCs were treated with different
concentrations of CTL-infused serum (0.5, 1.0 and 1.5 g/kg/day).
(A) Morphological changes in primary HSCs (magnification, ×400;
scale bar, 50 µm). (B) Protein expression levels of collagen I and
α-SMA, as measured using western blot analysis. (C) Colla1
and (D) α-SMA mRNA levels as measured using reverse
transcription-quantitative PCR. Results are presented as the mean ±
SD (n=8), using one-way ANOVA. **P<0.01 and ***P<0.001. ns,
not significant; CTL, Carthamus tinctorius L.; α-SMA,
α-smooth muscle actin; HSCs, hepatic stellate cells; Colla1,
collagen I, CTL-L, CTL low-dose group; CTL-M, CTL medium-dose
group; CTL-H, CTL high-dose group. |
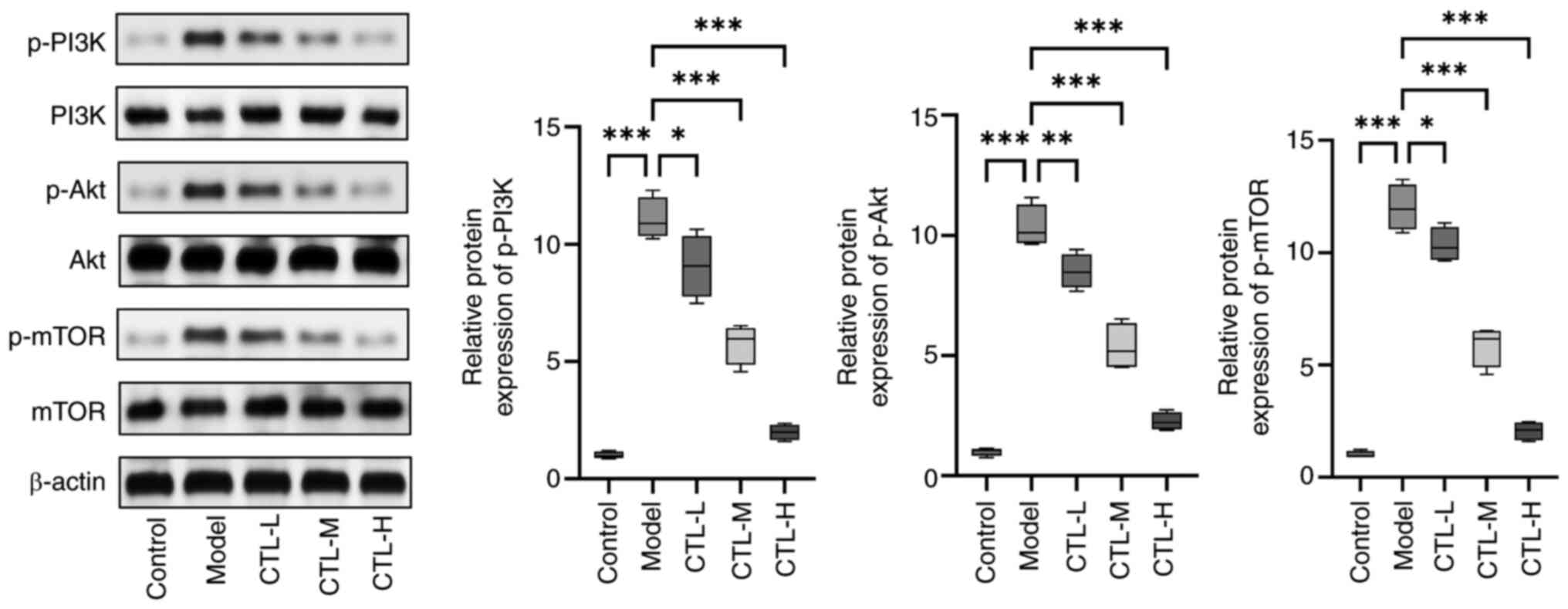
Effect of CTL on the PI3K/Akt/mTOR
pathways in rats with HF
The protein expression levels of p-PI3K, p-Akt and
p-mTOR in the liver tissues of rats with HF were measured using
western blot analysis. Compared with the control group, the
expression levels of p-PI3K, p-Akt and p-mTOR were found to be
significantly increased in the model group, which corroborated
previous findings (32,33). CTL downregulated the expression
levels of p-PI3K, p-Akt and p-mTOR in the control group in a
dose-dependent manner (Fig. 3).
These results suggest that CTL can inhibit the PI3K/Akt/mTOR
pathway.
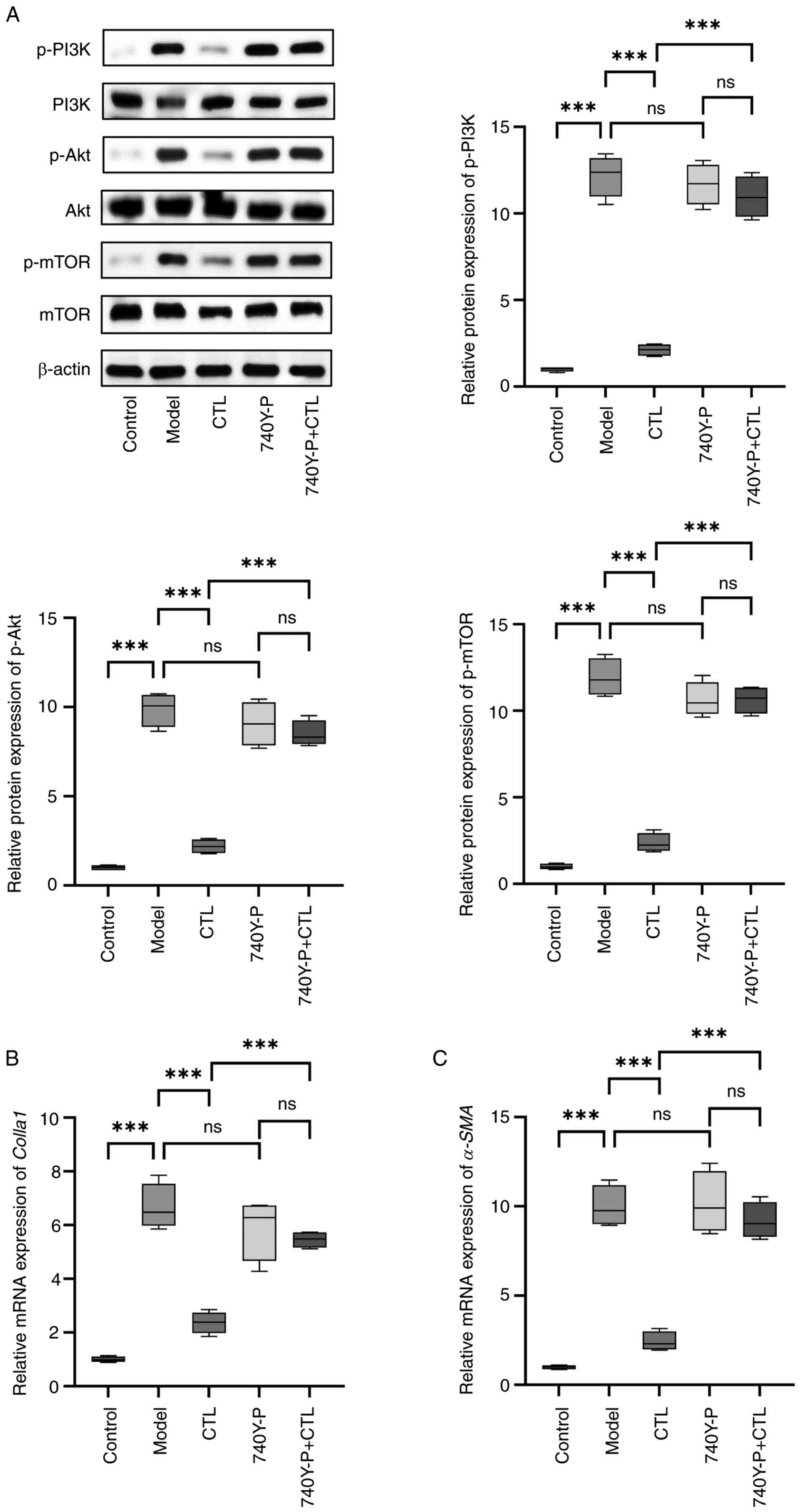
Effect of CTL-infused serum on the
PI3K/Akt/mTOR pathways in HSC-T6 cells
To determine whether CTL-infused serum could inhibit
PI3K/Akt/mTOR pathways in HSCs, the HSC-T6 cell line was used. In
preliminary experiments, primary HSCs were found to be insensitive
to the PI3K agonist 740Y-P. HSC-T6 cells were cultured in
vitro, activated using TGF-β1 and CTL-infused serum was added
to detect its inhibitory effect on the PI3K/Akt/mTOR pathway, using
blank serum as a control. The results showed that TGF-β1
significantly increased the expression levels of p-PI3K, p-Akt and
p-mTOR compared with the control. It was also observed that
CTL-infused serum significantly decreased the expression levels of
p-PI3K, p-Akt and p-mTOR. When the PI3K agonist 740Y-P was used to
counteract the effect of CTL, the results showed that 740Y-P alone
did not affect the PI3K/Akt/mTOR pathway but was able to block the
effect of CTL in reducing p-PI3K, p-Akt and p-mTOR expression
(Fig. 4A). It also blocked the
expression-lowering effect of CTL on Colla1 and α-SMA
(Fig. 4B and C). These results
indicate that CTL may directly act on PI3K and inhibit its
phosphorylation, thus inhibiting the activation of HSCs.
Discussion
Stimulated by continuous pathogenic factors, HF can
progress into hepatic cirrhosis or even hepatic carcinoma, both of
which currently lack effective therapies (34). Numerous studies have explored
various natural compounds such as Acanthus ilicifolius
alkaloid A, berberine, caffeine, capsaicin, conophylline,
evodiamine and ligustrazine in an attempt to find novel drugs to
treat HF (35,36). Traditional Chinese or Mongolian
medicine is a discipline based on practice and exploration. In
general, the therapies used in these disciplines consist of
combinations of various herbs (37). CTL is a common herb used in these
medicinal paradigms to treat liver diseases, with a curative effect
that has been well documented (38). In the present study, an animal
model of HF was constructed through intraperitoneal injection of
CCl4 in rats, after which CTL was administered as an
intervention. Through histopathological staining, Masson staining
and the detection of HF markers, it was found that CTL could
significantly relieve HF in a dose-dependent manner. In addition,
CTL-infused serum was able to inhibit the activation of primary
HSCs, and it was also demonstrated that CTL could inhibit the
activation of the PI3K/Akt/mTOR pathway in both rats and HSC-T6,
most likely by inhibiting the phosphorylation of PI3K.
The activation of HSCs is the initial and key step
that induces HF (39). Upon liver
injury or inflammation, quiescent HSCs undergo a phenotypic
transformation termed activation (39). This process involves a complex
interplay of various signaling pathways and molecular mechanisms
(40). Initially, HSCs lose their
characteristic lipid droplets and transition into an activated
state characterized by increased proliferation, contractility and
synthesis of ECM components (41).
Activation of HSCs is orchestrated by a multitude of factors
including cytokines, growth factors and oxidative stress,
ultimately leading to the deposition of excessive ECM proteins
(42). As a major component of the
ECM, collagen I is significantly upregulated during HF and serves
as a key marker of fibrogenesis (43). Accumulation of collagen I disrupts
the normal liver architecture and contributes to the formation of
fibrotic scar tissue (44).
Moreover, collagen I promotes HSC activation by inducing
profibrogenic signaling pathways such as TGF-β/Smad and PI3K/Akt
(45). Expression of α-SMA is a
hallmark of activated HSCs and is closely associated with their
contractile phenotype (46).
α-SMA-positive myofibroblasts are responsible for the increased
contractility observed in fibrotic liver tissue (47). Activation of HSCs leads to the
upregulation of α-SMA expression, facilitating their contractile
function and promoting ECM remodeling (48). Hydroxyproline is a
non-proteinogenic amino acid predominantly found in collagen
proteins Elevated levels of hydroxyproline serve as a reliable
biomarker for increased collagen turnover and fibrogenesis in the
liver. During HSC activation, there is a notable increase in
hydroxyproline content, reflecting enhanced collagen synthesis and
deposition. Measurement of hydroxyproline levels provides valuable
insights into the extent of liver fibrosis and the efficacy of
antifibrotic therapies (49).
The results of the present study showed that CTL can
downregulate the expression of collagen I and α-SMA and reduce the
production of hydroxyproline. Moreover, CTL also inhibited
morphological changes in primary HSCs, implying that the inhibitory
effect of CTL on HF may be related to its inhibition of HSC
activation. Activated HSCs lead to over-deposition of ECM
components, as well as the release of significant amounts of
cytokines and inflammatory factors that eventually cause HF
(50). Collagen I is the main
component of the ECM, while hydroxyproline is an important element
of collagen, and α-SMA is an important biomarker of HSC activation
(7).
The PI3K/Akt pathway is the key mechanism affecting
HF, and the inhibition of PI3K/Akt is considered to be an important
target for HF intervention (51).
Researchers have found that exosomes, miRNA, lncRNA, and natural
drugs such as isovitexin, salvianolic acid A, germacrone and
leonurine can inhibit HF by inhibiting the PI3K/Akt pathway
(14,15,52,53).
A previous study found that lncRNA GAS5 or LOC102551149 could act
as molecular sponges to absorb miR-23a and inhibit the activation
of PI3K/Akt/mTOR (32). Studies
have shown that monomeric compounds from natural plants can inhibit
the activation of the PI3K/Akt pathway in activated HSCs or animals
with HF. For example, one study showed that forsythiaside A could
downregulate the NOX4-ROS signaling pathway in HSCs, improve
oxidation imbalance, inhibit the PI3K/Akt pathway and inhibit HSC
activation (54). In addition,
Galangin has been shown to promote apoptosis of HSCs by inhibiting
the PI3K/Akt and Wnt/β-catenin pathways and increasing the
Bax/Bcl-2 ratio (55).
Furthermore, Inonotsuoxide B can inhibit HSC activation and
proliferation by inhibiting both PI3K/Akt and ERK1/2 (56). However, these studies are still in
the early stages, so few drugs have been developed based on these
findings that are currently available for clinical use.
In the present study, CTL was found to effectively
inhibit the activation of the PI3K/Akt pathway, and this effect was
blocked using the PI3K activator 740Y-P. CTL is a natural
plant-based medicine that contains a variety of biologically active
ingredients. For example, carthamin yellow can reduce ROS release
and inflammation to protect the heart against ischemia and
reperfusion (57). In addition,
Hydroxysafflor yellow A can regulate inflammation via the
inhibition of PI3K in macrophages to reduce atherosclerosis
(58). Quercetin can reduce p-PI3K
levels to alleviate chronic renal failure (59). Anhydrosafflor yellow B protects
against injuries caused by cerebral ischemia and reperfusion by
attenuating oxidative stress and apoptosis via the silent
information regulator 1 signaling pathway (60). Apigenin can inhibit the
PI3K/Akt/mTOR pathway to induce apoptosis and autophagy in
hepatocellular carcinoma cells (61). Furthermore, scutellarein can
downregulate PI3K/Akt/NF-κB signaling to inhibit HepG2 cell
proliferation and metastasis (62).
In the in vivo cellular experiments in the
present study, cells were treated with CTL-infused serum. In
general, it is hypothesized that if the components in the plants
are not absorbed into the blood, it is difficult for them to reach
the liver and exert their effects. The components of CTL extracts,
CTL-infused serum and blank serum were tested, and 207 components
in the extract of CTL and 220 components in the CTL-infuse were
identified. Subsequently, components that were also detected in the
blank serum were excluded, and 75 components remained in the
CTL-infused serum. Of the 75 components identified in the
CTL-infused serum, 20 were shared with the CTL extracts. The
remaining 55 components may be produced by the metabolism of CTL,
but they may also originate from the serum components of different
animals (data not shown). However, the active ingredients from the
75 components detected was not identified. The present study
results revealed that blank serum had no effect on the expression
of collagen I and α-SMA, whereas CTL-infused serum could
downregulate the expression of collagen I and α-SMA. These results
are largely consistent with the results of the present study.
Additionally, the present study aimed to ensure the reliability of
the results through multiple repetitions of experiments (n=8).
Therefore, the limitation of the present study is that monomeric
compounds with anti-HF activity have not yet been confirmed.
Despite this, further experiments using monomeric compounds are
required to validate their anti-HF effect.
CTL at present is regarded as a herbal medicine with
a high safety profile and is therefore, not highly regulated
(63). The present study
preliminarily demonstrated the anti-HF effect of CTL and provided a
theoretical basis for the development of CTL as a therapy for HF
treatment.
Acknowledgements
Not applicable.
Funding
The study was funded by The National Natural Science Foundation
of China (grant nos. 82160794 and 82160703); Major Project of
Natural Science Foundation of Inner Mongolia Autonomous Region
(grant nos. 2021ZD14 and 2023ZD15); Nature Science Foundation of
Inner Mongolia Autonomous Region (grant nos. 2020MS08046 and
2020MS08106); Science and Technology Planning Project of Inner
Mongolia Autonomous Region (grant nos. 2020GG0138); Program for
Young Talents of Science and Technology in Universities of Inner
Mongolia Autonomous Region (grant no. NJYT23114); Inner Mongolia
Autonomous Region ‘Prairie excellence’ Project; Western Light Young
Scholars Program of the Chinese Academy of Sciences; ‘Prairie
Talents’ Leading Talent Project in Inner Mongolia Autonomous
Region; Key Program of Inner Mongolia Medical University (grant no.
YKD2022ZD013); Health Science and Technology Program of Inner
Mongolia Health Commission (grant nos. 202201238 and 202202158);
PhD Initial Funding Project of the Affiliated Hospital of Inner
Mongolia Medical University (grant no. NYFY BS 202120); Inner
Mongolia Medical University ‘Youth Pioneering’ Team Alliance (grant
no. QNLC-2020064); Inner Mongolia Medical University Mongolian
Medicine ‘First-class Discipline’ Construction Project (grant no.
myxylxk2022019); and 2021 ‘Qihuang’ scholar supporting project.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LB and LW conceived and designed the research; ZD,
HG, LL, YZ and LW performed the experiments and analyzed the data;
and LB drafted manuscript. ZD and HG confirm the authenticity of
all the raw data. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
All experiments in the present study were approved
by The Ethics Committee of Inner Mongolia Medical University
(Hohhot, China; approval no. YKD202201124).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boursier J, Roux M, Costentin C, Chaigneau
J, Fournier-Poizat C, Trylesinski A, Canivet CM, Michalak S, Le
Bail B, Paradis V, et al: Practical diagnosis of cirrhosis in
non-alcoholic fatty liver disease using currently available
non-invasive fibrosis tests. Nat Commun. 14:52192023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roehlen N, Saviano A, El Saghire H,
Crouchet E, Nehme Z, Del Zompo F, Jühling F, Oudot MA, Durand SC,
Duong FHT, et al: A monoclonal antibody targeting nonjunctional
claudin-1 inhibits fibrosis in patient-derived models by modulating
cell plasticity. Sci Transl Med. 14:eabj42212022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ajmera V, Cepin S, Tesfai K, Hofflich H,
Cadman K, Lopez S, Madamba E, Bettencourt R, Richards L, Behling C,
et al: A prospective study on the prevalence of NAFLD, advanced
fibrosis, cirrhosis and hepatocellular carcinoma in people with
type 2 diabetes. J Hepatol. 78:471–478. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luo P, Liu D, Zhang Q, Yang F, Wong YK,
Xia F, Zhang J, Chen J, Tian Y, Yang C, et al: Celastrol induces
ferroptosis in activated HSCs to ameliorate hepatic fibrosis via
targeting peroxiredoxins and HO-1. Acta Pharm Sin B. 12:2300–2314.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Z, Wang F, Li Y, Wang X, Lu Q, Wang D,
Qi C, Li C, Li Z, Lian B, et al: Combined anti-hepatocellular
carcinoma therapy inhibit drug-resistance and metastasis via
targeting ‘substance P-hepatic stellate cells-hepatocellular
carcinoma’ axis. Biomaterials. 276:1210032021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dat NQ, Thuy LTT, Hieu VN, Hai H, Hoang
DV, Thi Thanh Hai N, Thuy TTV, Komiya T, Rombouts K, Dong MP, et
al: Hexa histidine-tagged recombinant human cytoglobin deactivates
hepatic stellate cells and inhibits liver fibrosis by scavenging
reactive oxygen species. Hepatology. 73:2527–2545. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Myojin Y, Hikita H, Sugiyama M, Sasaki Y,
Fukumoto K, Sakane S, Makino Y, Takemura N, Yamada R, Shigekawa M,
et al: Hepatic stellate cells in hepatocellular carcinoma promote
tumor growth via growth differentiation factor 15 production.
Gastroenterology. 160:1741–1754.e16. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li WX, Chen X, Yang Y, Huang HM, Li HD,
Huang C, Meng XM and Li J: Hesperitin derivative-11 suppress
hepatic stellate cell activation and proliferation by targeting
PTEN/AKT pathway. Toxicology. 381:75–86. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parsons CJ, Takashima M and Rippe RA:
Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol
Hepatol. 22 (Suppl 1):S79–S84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shamsan E, Almezgagi M, Gamah M, Khan N,
Qasem A, Chuanchuan L and Haining F: The role of PI3k/AKT signaling
pathway in attenuating liver fibrosis: A comprehensive review.
Front Med (Lausanne). 11:13893292024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin X, Wei Y, Li Y, Xiong Y, Fang B, Li C,
Huang Q, Huang R and Wei J: Tormentic acid ameliorates hepatic
fibrosis in vivo by inhibiting glycerophospholipids metabolism and
PI3K/Akt/mTOR and NF-κB pathways: Based on transcriptomics and
metabolomics. Front Pharmacol. 13:8019822022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meng YX, Zhao R and Huo LJ: Interleukin-22
alleviates alcohol-associated hepatic fibrosis, inhibits autophagy,
and suppresses the PI3K/AKT/mTOR pathway in mice. Alcohol Clin Exp
Res (Hoboken). 47:448–458. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang X, Liu H, Wang Y, Wang P, Yi Y, Lin Y
and Li X: Novel protein C6ORF120 promotes liver fibrosis by
activating hepatic stellate cells through the PI3K/Akt/mTOR
pathway. J Gastroenterol Hepatol. 39:1422–1430. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang R, Song F, Li S, Wu B, Gu Y and Yuan
Y: Salvianolic acid A attenuates CCl4-induced liver
fibrosis by regulating the PI3K/AKT/mTOR, Bcl-2/Bax and
caspase-3/cleaved caspase-3 signaling pathways. Drug Des Devel
Ther. 13:1889–1900. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ji D, Zhao Q, Qin Y, Tong H, Wang Q, Yu M,
Mao C, Lu T, Qiu J and Jiang C: Germacrone improves liver fibrosis
by regulating the PI3K/AKT/mTOR signalling pathway. Cell Biol Int.
45:1866–1875. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang LL, Tian K, Tang ZH, Chen XJ, Bian
ZX, Wang YT and Lu JJ: Phytochemistry and pharmacology of
Carthamus tinctorius L. Am J Chin Med. 44:197–226. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Delshad E, Yousefi M, Sasannezhad P,
Rakhshandeh H and Ayati Z: Medical uses of Carthamus tinctorius
L. (safflower): A comprehensive review from traditional
medicine to modern medicine. Electron Physician. 10:6672–6681.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okuyama H, Yamada K, Miyazawa D, Yasui Y
and Ohara N: Dietary lipids impacts on healthy ageing. Lipids.
42:821–825. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suzuki K, Tsubaki S, Fujita M, Koyama N,
Takahashi M and Takazawa K: Effects of safflower seed extract on
arterial stiffness. Vasc Health Risk Manag. 6:1007–1014. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang Y and Wang L: Carthamus
tinctorius L.: A natural neuroprotective source for
anti-Alzheimer's disease drugs. J Ethnopharmacol. 298:1156562022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ao H, Feng W and Peng C: Hydroxysafflor
yellow A: A promising therapeutic agent for a broad spectrum of
diseases. Evid Based Complement Alternat Med. 2018:82592802018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bai J, Wang X, Du S, Wang P, Wang Y, Quan
L and Xie Y: Study on the protective effects of danshen-honghua
herb pair (DHHP) on myocardial ischaemia/reperfusion injury (MIRI)
and potential mechanisms based on apoptosis and mitochondria. Pharm
Biol. 59:335–346. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Du SB, Zhou HH, Wang PF, Wang XP, Xue ZP,
Li J, Gao S, Li N, Bai JQ and Xie LH: Modulation effects of
danshen-honghua herb pair on gut microbiota of acute myocardial
ischemia model rat. FEMS Microbiol Lett. 369:fnac0362022.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wan H, Yang Y, Li Z, Cheng L, Ding Z, Wan
H, Yang J and Zhou H: Compatibility of ingredients of Danshen
(Radix Salviae Miltiorrhizae) and Honghua (Flos Carthami) and their
protective effects on cerebral ischemia-reperfusion injury in rats.
Exp Ther Med. 22:8492021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Zhou B and Ding X: Informatic study
of cada prescription for the treatment of liver disease. TCM
Pharmacol Clin. 39:104–110. 2023.
|
|
26
|
Meng X, Zhou B and Liu Y: Data mining of
the ‘Bashaga’ class (Qumai) prescription prescription and its
action mechanism analysis for the treatment of liver disease. Chin
Mod TCM. 25:1266–1279. 2023.
|
|
27
|
Urig Wang Y and Nao M: Progress in
experimental research on Mongolian drug therapy for liver injury.
World Sci Technol-Modern Tradit Chin Med. 22:416–422. 2020.
|
|
28
|
Yang T, Liang S and Zhou B: Progress in
the treatment of liver fibrosis. Chin Ethnic Folk Med. 28:68–71.
2019.
|
|
29
|
Chang LL, Li C, Li ZL, Wei ZL, Jia XB,
Pang ST, An YQ, Gu JF and Feng L: Carthamus tinctorius L:
Extract ameliorates cerebral ischemia-reperfusion injury in rats by
regulating matrix metalloproteinases and apoptosis. Indian J
Pharmacol. 52:108–116. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Greenfield EA: Sampling and preparation of
mouse and rat serum. Cold Spring Harb Protoc. 2017.pdb.prot100271,
2017. View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong Z, Li S, Si L, Ma R, Bao L and Bo A:
Identification lncRNA LOC102551149/miR-23a-5p pathway in hepatic
fibrosis. Eur J Clin Invest. 50:e132432020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dong Z, Li S, Wang X, Si L, Ma R, Bao L
and Bo A: lncRNA GAS5 restrains CCl4-induced hepatic
fibrosis by targeting miR-23a through the PTEN/PI3K/Akt signaling
pathway. Am J Physiol Gastrointest Liver Physiol. 316:G539–G550.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ali MH, Talha M and Hussain SAS: The role
of hepatic stellate cells and the Gas6/Axl axis in liver fibrosis
and hepatocellular carcinoma. J Clin Exp Hepatol. 14:1014002024.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shan L, Liu Z, Ci L, Shuai C, Lv X and Li
J: Research progress on the anti-hepatic fibrosis action and
mechanism of natural products. Int Immunopharmacol. 75:1057652019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shan L, Wang F, Zhai D, Meng X, Liu J and
Lv X: New drugs for hepatic fibrosis. Front Pharmacol.
13:8744082022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li D, Tao L, Chen Z, Cai W and Shen W:
Treatment of peripheral facial paralysis after COVID-19 infection
with traditional chinese medicine therapies: A case report. Cureus.
16:e570472024.PubMed/NCBI
|
|
38
|
Xi S, Yue L, Shi M, Peng Y, Xu Y, Wang X,
Li Q, Kang Z, Li H and Wang Y: The effects of taoren-honghua herb
pair on pathological microvessel and angiogenesis-associated
signaling pathway in mice model of CCl4-induced chronic liver
disease. Evid Based Complement Alternat Med. 2016:29742562016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wan S, Liu X, Sun R, Liu H, Jiang J and Wu
B: Activated hepatic stellate cell-derived Bmp-1 induces liver
fibrosis via mediating hepatocyte epithelial-mesenchymal
transition. Cell Death Dis. 15:412024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hwang CH, Jang E and Lee JH:
Pharmacological benefits and underlying mechanisms of Salvia
miltiorrhiza against molecular pathology of various liver diseases:
A review. Am J Chin Med. 51:1675–1709. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tuohetahuntila M, Molenaar MR, Spee B,
Brouwers JF, Wubbolts R, Houweling M, Yan C, Du H, VanderVen BC,
Vaandrager AB and Helms JB: Lysosome-mediated degradation of a
distinct pool of lipid droplets during hepatic stellate cell
activation. J Biol Chem. 292:12436–12448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sharma A, Verma AK, Kofron M, Kudira R,
Miethke A, Wu T, Wang J and Gandhi CR: Lipopolysaccharide reverses
hepatic stellate cell activation through modulation of cMyb, small
mothers against decapentaplegic, and CCAAT/enhancer-binding protein
C/EBP transcription factors. Hepatology. 72:1800–1818. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li R, Zhang J, Liu Q, Tang Q, Jia Q, Xiong
Y, He J and Li Y: CREKA-modified liposomes target activated hepatic
stellate cells to alleviate liver fibrosis by inhibiting collagen
synthesis and angiogenesis. Acta Biomater. 168:484–496. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yin L, Zhang Y, Shi H, Feng Y, Zhang Z and
Zhang L: Proteomic profiling of hepatic stellate cells in alcohol
liver fibrosis reveals proteins involved in collagen production.
Alcohol. 86:81–91. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang M, Wu Z, Salas SS, Aguilar MM,
Trillos-Almanza MC, Buist-Homan M and Moshage H: Arginase 1
expression is increased during hepatic stellate cell activation and
facilitates collagen synthesis. J Cell Biochem. 124:808–817. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ezquerro S, Tuero C, Becerril S, Valentí
V, Moncada R, Landecho MF, Catalán V, Gómez-Ambrosi J, Mocha F,
Silva C, et al: Antagonic effect of ghrelin and LEAP-2 on hepatic
stellate cell activation and liver fibrosis in obesity-associated
nonalcoholic fatty liver disease. Eur J Endocrinol. 188:564–577.
2023.PubMed/NCBI
|
|
47
|
Jokl E, Llewellyn J, Simpson K, Adegboye
O, Pritchett J, Zeef L, Donaldson I, Athwal VS, Purssell H, Street
O, et al: Circadian disruption primes myofibroblasts for
accelerated activation as a mechanism underpinning fibrotic
progression in non-alcoholic fatty liver disease. Cells.
12:15822023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hussein KH, Park KM, Yu L, Kwak HH and Woo
HM: Decellularized hepatic extracellular matrix hydrogel attenuates
hepatic stellate cell activation and liver fibrosis. Mater Sci Eng
C Mater Biol Appl. 116:1111602020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bissoondial TL, Han Y, Mullan S, Pabla AK,
Spahn K, Shi S, Zheng L, Zhou P, Jiang K, Prakash N, et al: Liver
biopsy hydroxyproline content is a diagnostic for hepatocellular
carcinoma in murine models of nonalcoholic steatohepatitis.
Diagnostics (Basel). 10:7842020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhou L, Liang Q, Li Y, Cao Y, Li J, Yang
J, Liu J, Bi J and Liu Y: Collagenase-I decorated co-delivery
micelles potentiate extracellular matrix degradation and hepatic
stellate cell targeting for liver fibrosis therapy. Acta Biomater.
152:235–254. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lopez-Sanchez I, Dunkel Y, Roh YS, Mittal
Y, De Minicis S, Muranyi A, Singh S, Shanmugam K, Aroonsakool N,
Murray F, et al: GIV/Girdin is a central hub for profibrogenic
signalling networks during liver fibrosis. Nat Commun. 5:44512014.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Huang Y, Luo W, Chen S, Su H, Zhu W, Wei
Y, Qiu Y, Long Y, Shi Y and Wei J: Isovitexin alleviates hepatic
fibrosis by regulating miR-21-mediated PI3K/Akt signaling and
glutathione metabolic pathway: Based on transcriptomics and
metabolomics. Phytomedicine. 121:1551172023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yu Y, Zhou S, Wang Y, Di S, Wang Y, Huang
X and Chen Y: Leonurine alleviates acetaminophen-induced acute
liver injury by regulating the PI3K/AKT signaling pathway in mice.
Int Immunopharmacol. 120:1103752023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhou M, Zhao X, Liao L, Deng Y, Liu M,
Wang J, Xue X and Li Y: Forsythiaside A regulates activation of
hepatic stellate cells by inhibiting NOX4-dependent ROS. Oxid Med
Cell Longev. 2022:99383922022.PubMed/NCBI
|
|
55
|
Xiong Y, Lu H and Xu H: Galangin reverses
hepatic fibrosis by inducing HSCs apoptosis via the PI3K/Akt,
Bax/Bcl-2, and Wnt/β-catenin pathway in LX-2 cells. Biol Pharm
Bull. 43:1634–1642. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jin J, Yang H, Hu L, Wang Y, Wu W, Hu C,
Wu K, Wu Z, Cheng W and Huang Y: Inonotsuoxide B suppresses hepatic
stellate cell activation and proliferation via the PI3K/AKT and
ERK1/2 pathway. Exp Ther Med. 23:4172022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lu QY, Ma JQ, Duan YY, Sun Y, Yu S, Li B
and Zhang GM: Carthamin yellow protects the heart against
ischemia/reperfusion injury with reduced reactive oxygen species
release and inflammatory response. J Cardiovasc Pharmacol.
74:228–234. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Feng X, Du M, Li S, Zhang Y, Ding J, Wang
J, Wang Y and Liu P: Hydroxysafflor yellow A regulates
lymphangiogenesis and inflammation via the inhibition of PI3K on
regulating AKT/mTOR and NF-κB pathway in macrophages to reduce
atherosclerosis in ApoE-/- mice. Phytomedicine. 112:1546842023.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tu H, Ma D, Luo Y, Tang S, Li Y, Chen G,
Wang L, Hou Z, Shen C, Lu H, et al: Quercetin alleviates chronic
renal failure by targeting the PI3k/Akt pathway. Bioengineered.
12:6538–6558. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Fangma Y, Zhou H, Shao C, Yu L, Yang J,
Wan H and He Y: Hydroxysafflor yellow A and anhydrosafflor yellow B
protect against cerebral ischemia/reperfusion injury by attenuating
oxidative stress and apoptosis via the silent information regulator
1 signaling pathway. Front Pharmacol. 12:7398642021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yang J, Pi C and Wang G: Inhibition of
PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy
in hepatocellular carcinoma cells. Biomed Pharmacother.
103:699–707. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ha SE, Kim SM, Vetrivel P, Kim HH, Bhosale
PB, Heo JD, Lee HJ and Kim GS: Inhibition of cell proliferation and
metastasis by scutellarein regulating PI3K/Akt/NF-κB signaling
through PTEN activation in hepatocellular carcinoma. Int J Mol Sci.
22:88412021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Shuang R, QirigeerWurihan, Bai M,
Laxinamujila and Han X: Toxicity of Carthamus tinctorius L.
Water Extract. World Trad Chin Med. 18:979–982. 2023.(In
Chinese).
|