Introduction
Myocardial infarction (MI) is one of the leading
causes of mortality worldwide (1).
Reperfusion can help minimize myocardial ischemia, prevent the
infarct size from increasing and enhance ventricular function.
However, with the restoration of blood perfusion, cardiac function
does not recover immediately, whereas reactive oxygen species
production increases, oxidative stress increases and more severe
myocardial injury ensues, known as myocardial ischemia-reperfusion
(I/R) injury (MIRI) (2,3). MIRI is characterized by myocardial
cell death, microvascular destruction and inflammation. Currently,
there is no effective therapy available for MIRI. Preventing the
death of cardiomyocytes is critical for preserving heart function
following reperfusion (4,5).
Signal transducer and activator of transcription
(STAT) is abnormally expressed in polygenic diseases and mediates
the activation of multiple signaling pathways (6). STAT4 plays a crucial role in the
initiation and progression of MI. It has been demonstrated that
IL-35 activates macrophages via the glycoprotein 130 signaling
pathway and the phosphorylation of STAT1 and STAT4, reduces cardiac
rupture following myocardial infarction, enhances wound healing and
alleviates myocardial remodeling (7). Nevertheless, the effectiveness and
mechanisms of action of STAT4 in influencing ischemia-induced
myocardial injury remain unclear. A previous study found that STAT4
promoted apoptosis via the PI3K signaling pathway in porcine
ovarian granulosa cells (8). IL-2
can activate STAT4 and PI3K/AKT signaling on melanocytes (9). Previous studies have also reported
that the activation of the PI3K/AKT signaling pathway can attenuate
the apoptosis of cardiomyocytes induced by MIRI and enhance the
proliferation of cardiomyocytes (10–12).
Thus, it was hypothesized that STAT4 may regulate apoptosis via the
PI3K/AKT signaling pathway, thereby protecting the myocardium
against I/R injury. The present study aimed to elucidate the
mechanisms of myocardial I/R injury and provide a novel strategy
for the clinical treatment of myocardial I/R.
Materials and methods
Animal models
A total of 16 male Sprague-Dawley (SD) rats (6–8
weeks old; weight, 180 g) were acquired from the Experimental
Animal Center of Zhengzhou University (Zhengzhou, China). The rats
were housed in 26°C and a humidity of 50–70%, with 12 h light/dark
cycles. Rats were adaptively fed for one week and were free to take
food and water. The rats were randomly assigned to an I/R group
(n=8) and a sham-operated group (n=8) following 1 week of routine
laboratory acclimatization. The rat model of MIRI was constructed
according to a previously described method (13). Following anesthesia with 2%
isoflurane, the chest wall of the rats was cut, and a 6–0 silk
suture was used to ligate the left anterior descending coronary
artery (LAD). Following 30 min of ischemia, the ligature was
loosened and the thoracic cavity was closed with a 6–0 silk suture
after reperfusion was resumed. The rats in the sham-operated group
underwent the same procedure as those in the I/R group, excluding
the LAD ligation. Following the surgery, the rats were sacrificed
under a 2% isoflurane gas anesthesia by cervical dislocation.
Mortality was confirmed through a physical examination for the
absence of cardiac and respiratory activity. A total of 16 rats
were used in the experiments. No rats were sacrificed due to humane
endpoints and no rats were found lifeless throughout the
experiment. All experimental procedures were approved by the Ethics
Committee of Zhengzhou Seventh People's Hospital [Zhengzhou, China;
approval no. Zheng Xin Ethics (2024) 017] and adhered to the
National Institutes of Health Guide for the Care and Use of Animal
guidelines.
Echocardiography
The left ventricular end-systolic diameter and left
ventricular end-diastolic diameter were measured using
two-dimensional M-mode echocardiography, while the rats were
sedated with 2% isoflurane. The left ventricular fractional
shortening and left ventricular ejection fraction were computed
using formulas: Left ventricular shortening fraction=(left
ventricular end-diastolic diameter-left ventricular end-systolic
diameter)/left ventricular end-diastolic diameter. Left ventricular
ejection fraction=(left ventricular end-systolic volume-left
ventricular end-diastolic volume)/left ventricular end-systolic
volume ×100%.
Immunofluorescence assays
The left ventricular tissue blocks were fixed in 4%
paraformaldehyde at 4°C for two days, rinsed with tap water for 10
min and dehydrated with alcohol. The dehydrated tissue was immersed
in xylene for 2 h. It is then soaked in paraffin for 3 h. Finally,
the tissue is placed in an embedding box, injected with paraffin,
and then moved to a cooling table to solidified the tissue blocks
with the wax solution. then sectioned at 5 µm for future use. After
the sections were deparaffinized with xylene and descending
anhydrous ethanol series, they were rinsed with PBS and placed in
sodium citrate buffer for antigen retrieval. After treating with
goat serum (Beijing Solarbio) for 30 min at 37°C, the sections were
incubated with anti-antibody (1:100; cat. no. ab284408, Abcam) at
4°C for 12 h. The sections were then incubated with Cy3-labeled
Goat Anti-Rabbit IgG (H+L; 1:100; cat. no. A0516; Beyotime
Institute of Biotechnology) were incubated at room temperature for
2 h. Subsequently, the cell nuclei were stained with DAPI
(Invitrogen; Thermo Fisher Scientific, Inc.) for 10 min at room
temperature and images were obtained using a fluorescence
microscope (Olympus Corporation; magnification, ×200).
Cell culture and treatment
H9C2 cell line (rat cardiomyocytes) was bought from
Shanghai Institute of Cell Biology (Shanghai, China). H9C2 were
cultured with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) in complete DMEM (Beyotime Institute of
Biotechnology) at 37°C and 5% CO2. To imitate the
physiological milieu of cardiac cell I/R, the cells in the hypoxia
and reoxygenation (H/R) group were grown in sugar-free and
serum-free DMEM with 95% N2 and 5% CO2 at
37°C for 2 h. The culture medium was thereafter substituted with a
full medium to simulate the reperfusion process.
STAT4 overexpression
STAT4 overexpressed plasmid, LV5-STAT4, which
contains the GFP gene was packaged into lentivirus by Gema Shanghai
Co., Ltd. The generation system used was 3rd. The 293T cell line
was used as the interim cell. Quantity of lentiviral plasmid used
20 µg for transfection, the ratio used for the lentivirus,
packaging and envelope plasmids was 1:2:1. Temperature of
transfection was 37°C. For the experiments, the cells were grouped
as follows: STAT4-NC group (transfected with control plasmid under
normal oxygen culture), STAT4-OE group (transfected with
STAT4-overexpression plasmid under normal oxygen culture), STAT4-NC
+ H/R group (transfected with control plasmid and subjected to H/R)
and the STAT4-OE + H/R group (transfected with STAT4-overexpression
plasmid and subjected to H/R).
The cells were plated in six-well plates for 12 h
and infected with 200 µl lentivirus for 48 h (MOI=90). The medium
was then replaced with fresh media and the cells were screened with
puromycin (1 µg/ml; Beyotime Institute of Biotechnology) for 1
week. Finally, the transfection efficiency of the virus was
examined using a fluorescence microscope. Time interval between
transduction and subsequent experimentation was 1 week.
Lactate dehydrogenase (LDH) and
superoxide dismutase (SOD) assays
The cells were plated in 96-well plates. LDH
(Dojindo Laboratories, Inc.) was then added, and the cells were
incubated at 37°C for 2 h, and the absorbance was determined using
a spectrophotometer (Thermo Fisher Scientific, Inc.).
For SOD assay, the cells were plated in six-well
plates. The cells were collected, PBS buffer (Beyotime Institute of
Biotechnology) was added for cell precipitation, an ultrasound (20
kHz; 10 cycles of 2 sec) was performed in an ice-water bath, and
the supernatant was then collected by centrifugation at 12,000 × g,
4°C for 10 min and appropriate reagents were added according to the
instructions provided with the SOD kit (Dojindo Laboratories,
Inc.). The SOD content was calculated by measuring the absorbance
using a spectrophotometer (Thermo Fisher Scientific, Inc.).
Flow cytometry
The apoptosis of the H9C2 cells was examined by flow
cytometry (FACSMelody, BD Biosciences) using the PE Annexin V cell
apoptosis detection kit (BD Biosciences). The H9C2 cells were
harvested and washed twice with PBS. The concentration of the cells
was then adjusted to 1×106 cells/ml and 100 µl of this
cell suspension was incubated with 5 µl PE-Annexin V in the dark at
room temperature for 15 min. The cells were washed again and
resuspended with 200 µl PBS, followed by the addition of 5 µl 7-AAD
and incubation for 5 min at room temperature. Apoptosis (early +
late apoptosis) was measured routinely by calculating the number of
cells stained with PE-Annexin V. The data were analyzed using the
Cell Quest software (version 5.1; BD Biosciences).
Western blotting
The H9C2 cells were collected and lysed in RIPA
buffer (Beyotime Institute of Biotechnology) supplemented with
protease and phosphatase inhibitor cocktails (Beyotime Institute of
Biotechnology). The supernatant was collected following
centrifugation at 4°C and 12,000 × g for 30 min. The concentration
of super albumin was determined using a bicinchoninic acid kit. The
proteins (20 µg/lane) mixed with loading buffer with 1%
β-mercaptoethanol (BME) were loaded on 10% SDS-PAGE gels and
transferred to Immune-Blot PVDF membranes at 100V for 1 h. The
membranes were blocked with 5% bovine serum albumin (Beyotime
Institute of Biotechnology) at room temperature for 1 h and
incubated with the primary antibodies [STAT4; 1:1,000; cat. no.
ab284408; phosphorylated (p-)STAT4 (phospho Y693); 1:1,000; cat.
no. ab28815; PI3K; 1:1,000; cat. no. ab302958; AKT; 1:1,000; cat.
no. ab179463; p-AKT1 (phospho S473); 1:1,000; cat. no. ab81283;
Bax; 1:1,000; cat. no. ab32503; Bcl-2; 1:1,000; cat. no. ab182858;
and β-actin; 1:1,000; cat. no. ab8227; all from Abcam] at 4°C
overnight. The membranes were washed and incubated with
HRP-conjugated goat anti-rabbit antibodies (1:1,000; cat. no.
A0208; Beyotime Biotechnology) at room temperature for 2 h. The
immunoreactive bands were detected by chemiluminescence (Bio-Rad
Laboratories, Inc.). The expression level of proteins was analyzed
semi-quantitatively using ImageJ software W10 (National Institutes
of Health) with β-actin as a control.
Statistical analysis
Data are expressed as the mean ± standard deviation.
All the experimental data were analyzed using one-way analysis of
variance followed by Tukey's multiple comparison test by SPSS 24
software (IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
I/R injury suppresses expression of
STAT4
Following the establishment of the myocardial I/R
model, cardiac function was measured using echocardiography. The
findings revealed that the ejection fraction and fractional
shortening were significantly reduced in the I/R group when
compared with the sham-operated group (P<0.01 and P<0.001,
respectively; Fig. 1A). The
results of immunofluorescence and western blotting revealed that
STAT4 expression was suppressed in the myocardium of rats with I/R
injury. Moreover, the phosphorylation activity of STAT4 was
inhibited (P<0.05 and P<0.01, respectively; Fig. 1B and C). Following H/R, the H9C2
cells exhibited an increased release of LDH, a decreased SOD
activity and increased apoptosis (P<0.001 and P<0.0001,
respectively; Fig. 1D-F).
Following H/R, the expression of STAT4 in H9C2 cells was also
decreased, and the activity of phosphorylated STAT4 was inhibited
(P<0.05 and P<0.01, respectively; Fig. 1G).
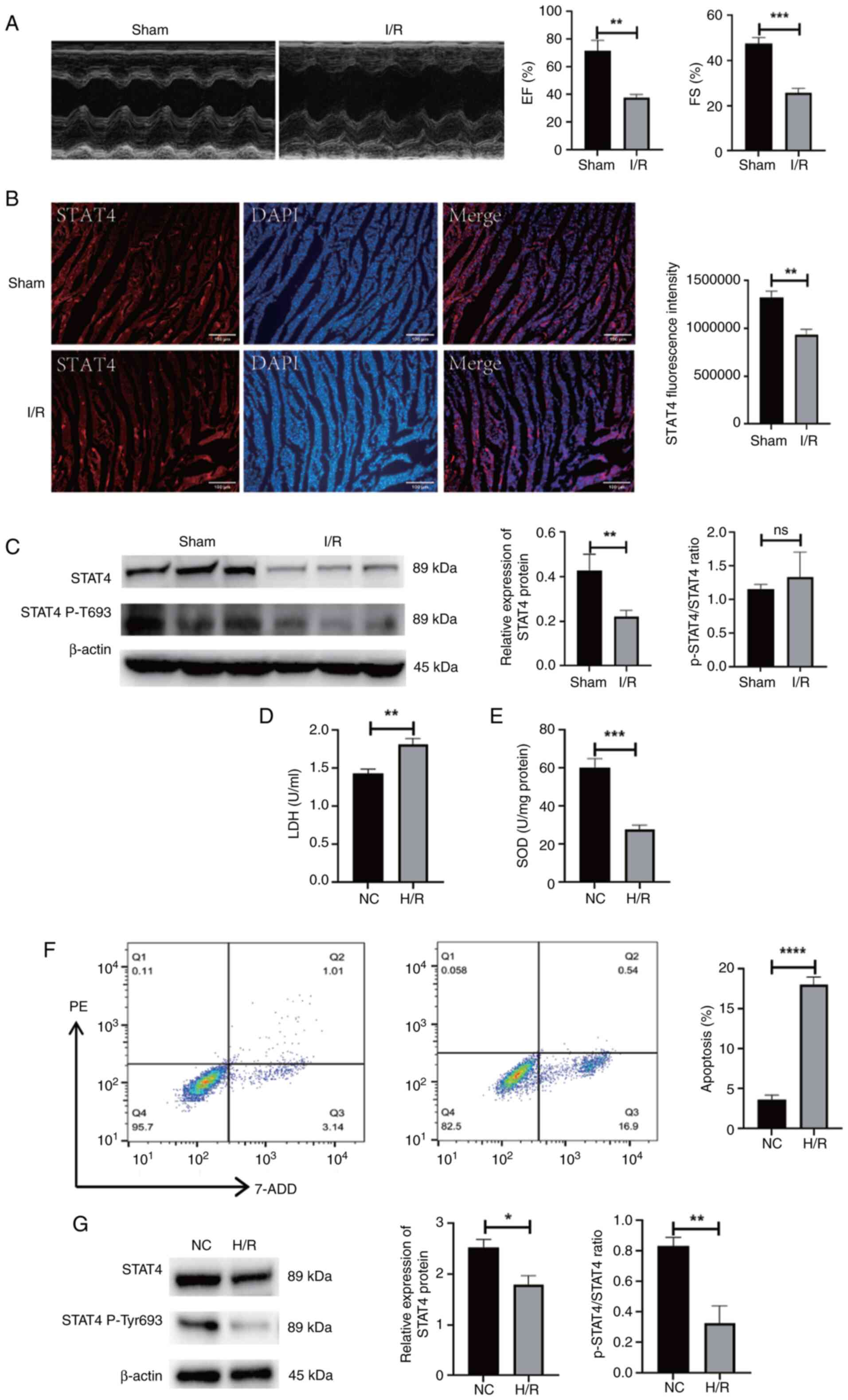 | Figure 1.The expression of STAT4 was inhibited
in the myocardium of rats suffering from I/R. (A) Cardiac function
was evaluated by echocardiography 3 h after I/R surgery.
Hemodynamic parameter alterations were noted in LVEF and LVFS. (B)
The protein expression of STAT4 in rat myocardium was detected by
immunofluorescence. (C) Protein expression of STAT4 and the
activity of phosphorylated STAT4 in rat myocardium determined using
western blotting and the quantitative values. (D) Colorimetric
detection of LDH produced by H9C2 cells. (E) SOD activity in H9C2
cells was measured using colorimetry. (F) Apoptosis of H9C2 cells
was measured by flow cytometry. (G) The protein expression of STAT4
and the activity of phosphorylated STAT4 in H9C2 cells was detected
by western blot and the quantitative values. *P<0.05,
**P<0.01, ***P<0.001, ****P<0.0001, n=3. STAT4, Signal
transducer and activator of transcription 4; I/R,
ischemia-reperfusion; LVEF, left ventricular ejection fraction;
LVFS, left ventricular fractional shortening; LDH, lactate
dehydrogenase; SOD, superoxide dismutase; EF, ejection fraction;
FS, fractional shortening; NC, negative control; H/R, hypoxia and
reoxygenation. |
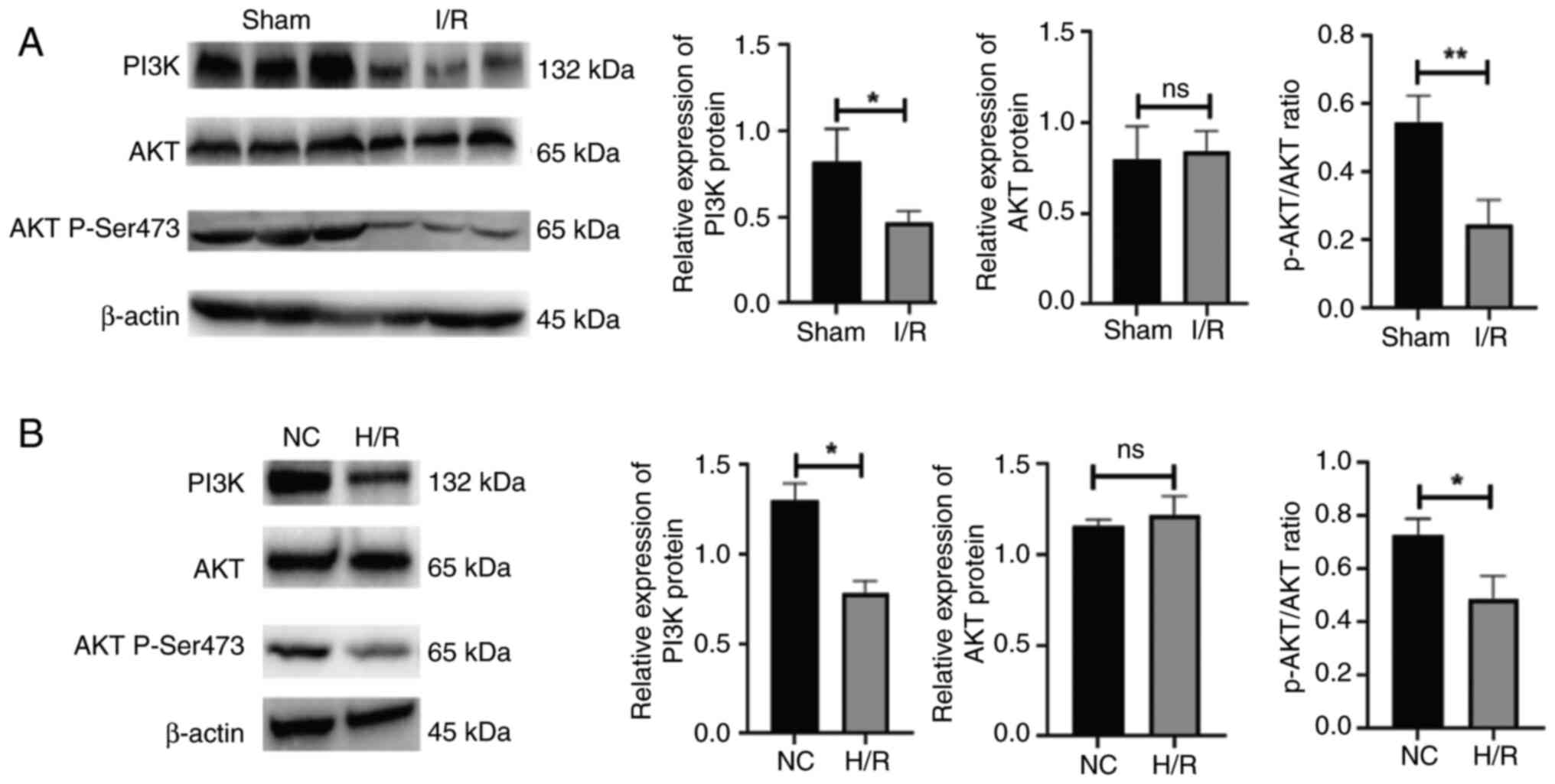
I/R injury inhibits the expression of
the PI3K/AKT signaling pathway
Following the establishment of the I/R model,
western blotting was used to examine PI3K and AKT expression in the
myocardium. The results revealed that PI3K expression was reduced
in the myocardium of rats suffering from I/R injury. Nevertheless,
AKT expression was unaffected, whereas the activity of
phosphorylated AKT was inhibited (P<0.05 and P<0.01,
respectively; Fig. 2A). In the
in vitro experiment, stimulation with H/R reduced PI3K
expression in the H9C2 cells. Likewise, AKT expression was
unaffected, while the activity of phosphorylated AKT was inhibited
(P<0.05; Fig. 2B).
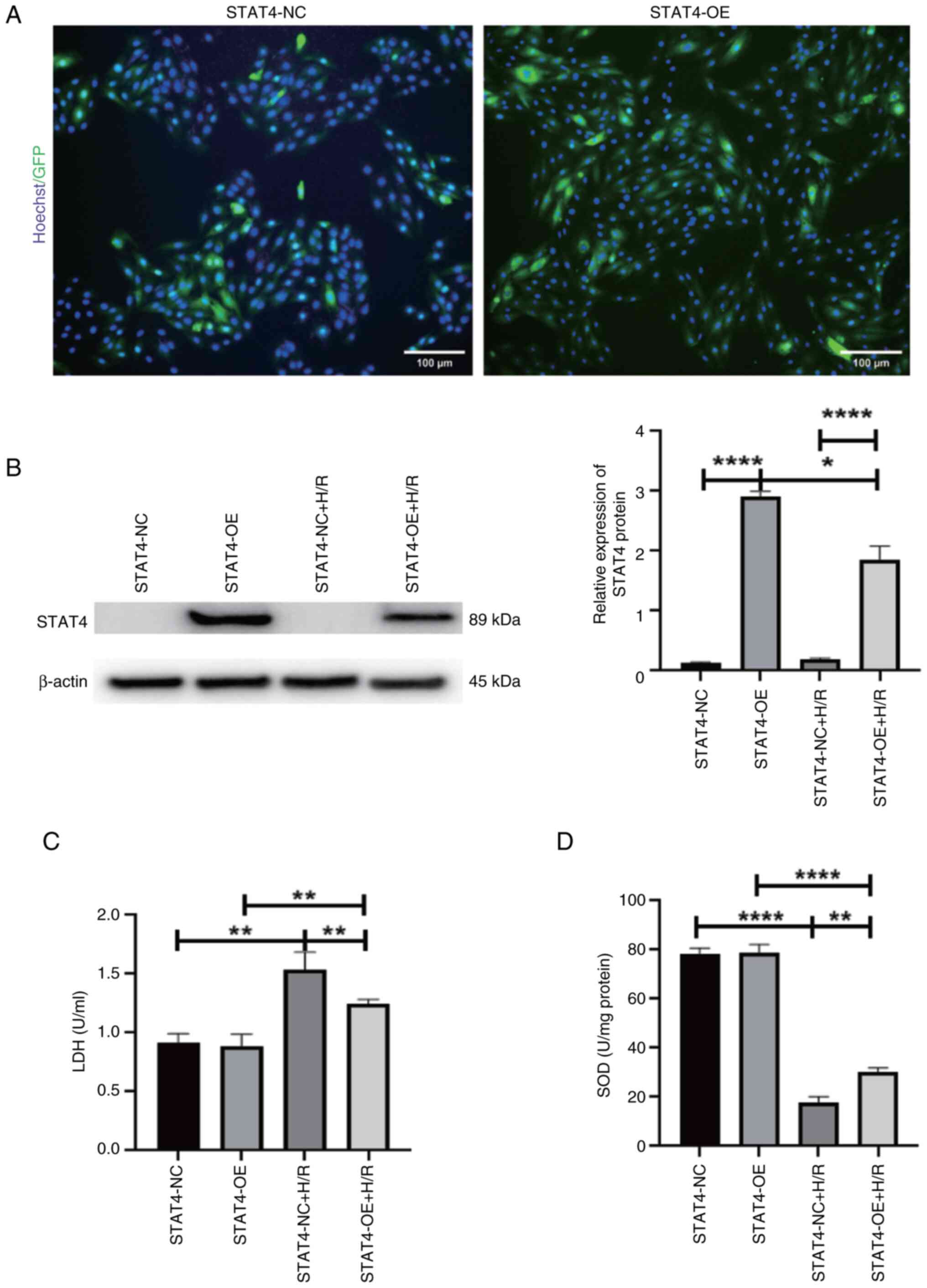
Overexpression of STAT4 mitigates H/R
injury
The fluorescence data indicated that the
transfection efficiency was ~95% (Fig.
3A). The results of western blotting demonstrated that STAT4
expression in the STAT4-OE group was considerably higher than that
in the STAT4-NC group and H/R markedly reduced STAT4 protein
expression (P<0.05 and P<0.0001, respectively; Fig. 3B). The results of colorimetric
analysis revealed that the STAT4-OE group had a reduced release of
LDH compared with the STAT4-NC group under H/R conditions. The
overall release of LDH under H/R conditions was greater than that
under normal settings (P<0.01; Fig.
3C). The results of colorimetric analysis revealed that the
STAT4-OE group had a higher SOD activity than the STAT4-NC group
under H/R conditions. H/R resulted in a decreased total SOD
activity compared with the controls (P<0.01 and P<0.0001,
respectively; Fig. 3D).
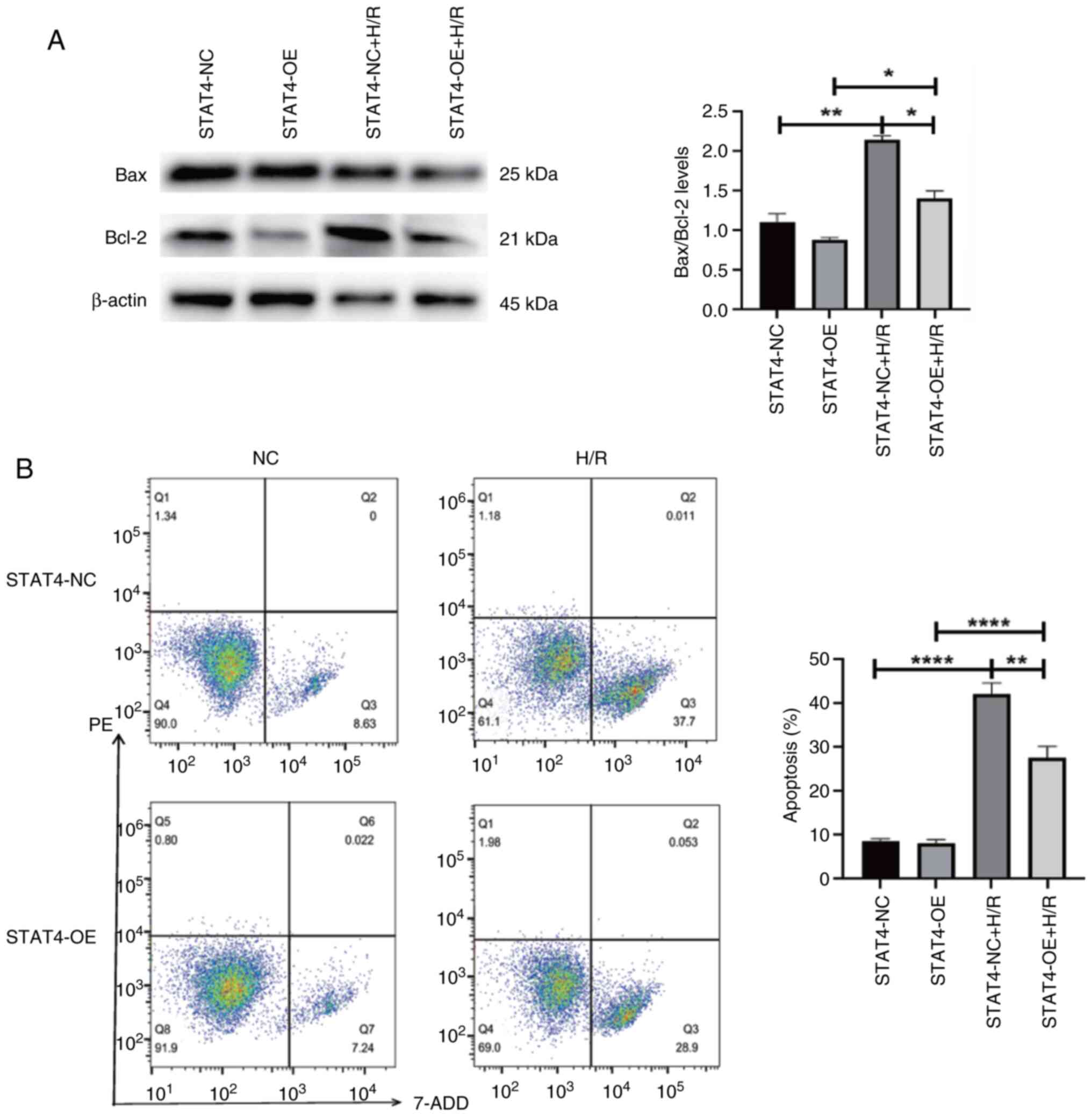
Overexpression of STAT4 mitigates the
apoptosis of H9C2 cells induced by H/R
Western blotting indicated that the STAT4-OE+H/R
group had a lower Bax/Bcl-2 ratio than the STAT4-NC + H/RSTAT4-NC
group. The total Bax/Bcl-2 ratio was higher under H/R conditions
compared with normal conditions (P<0.05 and P<0.01,
respectively; Fig. 4A). The
results of flow cytometry revealed that the STAT4-OE + H/R group
had a lower apoptotic rate than the STAT4-NC + H/R group. The
overall apoptotic rate was higher under H/R conditions compared
with normal conditions (P<0.01 and P<0.0001, respectively;
Fig. 4B).
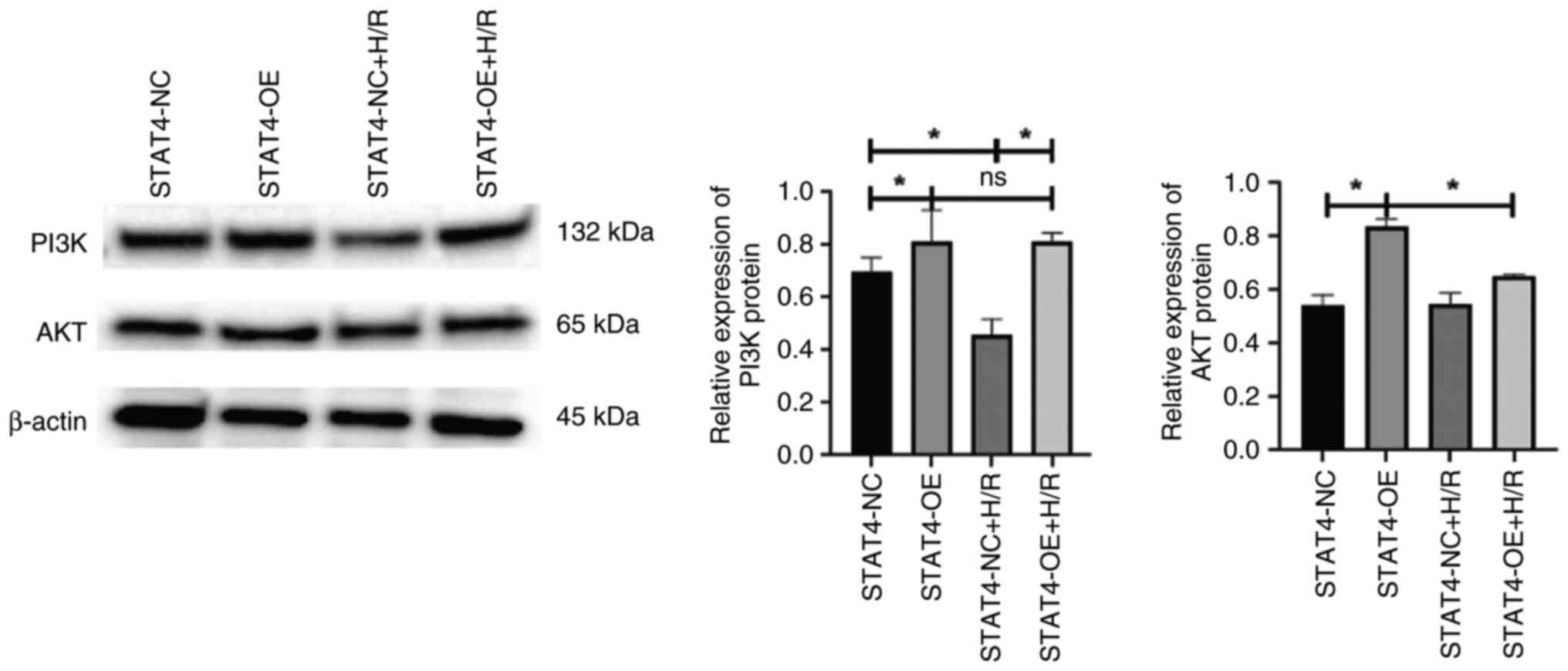
Overexpression of STAT4 activates the
PI3K/AKT signaling pathway in H9C2 cells
The results of western blotting demonstrated that
PI3K protein expression in the STAT4-OE group was considerably
higher than that in the STAT4-NC group and H/R considerably
decreased PI3K protein expression. Nevertheless, AKT protein
expression in the STAT4-OE group was higher than that in the
STAT4-NC group, and H/R did not modify the change in AKT protein
expression (P<0.05; Fig.
5).
Discussion
Ischemic injury from various causes initially
results in hypoxia and the malnutrition of tissues and cells. When
prolonged ischemia occurs, metabolite aggregation in cells
increases and causes metabolic acidosis. When the blood supply is
re-established, local inflammation and reactive oxygen species
production increase, resulting in apoptosis, autophagy and necrosis
(14). Currently, molecular
mechanisms responsible for I/R injury to the myocardium are not yet
fully understood.
STAT4 is a member of the STAT family and localizes
to the cytoplasm. STAT4 is phosphorylated after a variety of
cytokines bind to the membrane, and then dimerized STAT4
translocates to the nucleus to regulate gene expression (15). A key function of STAT4 is its
pro-inflammatory activity. In chronic inflammatory diseases, the
presence of STAT4 is not considered to be beneficial. It has been
reported that atherosclerosis is a chronic inflammatory process;
thus, STAT4 deficiency can reduce inflammation around blood vessels
and visceral adipose tissue, which can reduce the formation of
atherosclerosis (16,17). Autoimmune myocarditis is also an
inflammatory disease of the heart muscle and is one of the leading
causes of heart failure. STAT4 expression is upregulated in
autoimmune myocarditis, and the silencing of STAT4 can reduce the
degree of inflammatory cell infiltration in myocardial tissue and
improve cardiac function (18).
However, studies have found that STAT4 plays a crucial role in
cardiovascular disease caused by I/R injury (7). In the present study, it was
discovered that STAT4 expression was inhibited in the rat
myocardium following I/R injury. Moreover, H/R reduced the
expression of STAT4 in H9C2 cells. However, the overexpression of
STAT4 effectively alleviated H/R-induced myocardial cell injury and
attenuated apoptosis. STAT4 plays differential roles in various
heart diseases; thus, its role in diseases warrants further
exploration.
Previous research has demonstrated that STAT4
expression is downregulated in liver cancer, cutaneous T-cell
lymphoma, breast cancer, gastric cancer and ovarian cancer, and the
high expression of STAT4 is beneficial for prognosis and
rehabilitation (19–22). A previous study by the authors also
found that STAT4 expression was reduced in diabetic cardiomyopathy,
and the overexpression of STAT4 attenuated the high sugar-induced
apoptosis of cardiomyocytes (23).
Genetic variations of STAT4 have also been identified in studies to
enhance the risk of myocardial infarction in patients with systemic
lupus erythematosus (24). These
findings suggest that STAT4 is critical for healing cell damage and
increasing cell function. Combined with the experimental results of
the present study, STAT4 has a substantial protective influence on
I/R-induced myocardial cell injury, which represents a novel
experimental addition to the protective effects of STAT4 on the
myocardium.
The PI3K/AKT signaling pathway is a fundamental
signaling pathway, which plays a vital role in cell viability,
apoptosis, oxidative stress and the regulation of downstream
molecules (25). The indirect
upregulation of the PI3K/AKT signaling pathway through the
activation of JAK2/STAT3 has been found to protect against cerebral
I/R injury (26). STAT3 and STAT4
can form heterodimers to transduce signals in response to IL-23
(27). A previous study found that
STAT4 promotes apoptosis via the PI3K signaling pathway in porcine
ovarian granulosa cells (8). As a
result, it was hypothesized that STAT4 plays a role in regulating
the PI3K/AKT signaling pathway. In the present study, it was
discovered that PI3K expression was suppressed in the rat
myocardium following I/R injury. Furthermore, H/R suppressed the
expression of PI3K in H9C2 cells. Additionally, STAT4
overexpression increased PI3K expression under both normal and H/R
conditions. However, the expression of AKT was not affected in the
myocardium of rats with I/R injury and in H9C2 cells subjected to
H/R; however, the phosphorylation level of AKT was significantly
decreased. Perhaps STAT4 cannot bind to the promoter region of AKT,
which affects the expression of AKT. However, I/R and H/R can
affect the phosphorylation level of AKT and thus affect cell
apoptosis. These findings indicate that STAT4 protects against
myocardial I/R injury in rats via the PI3K/AKT signaling
pathway.
In conclusion, the present study demonstrated that
in rats with myocardial I/R injury, the expression level of STAT4
was reduced and the expression level of PI3K was downregulated. The
overexpression of STAT4 increased the expression level of PI3K,
decreased the ratio of Bax/Bcl-2, reduced the release of LDH,
increased the activity of SOD, and suppressed the H/R-induced
apoptosis of H9C2 cells. However, whether there is an interaction
between the PI3K/AKT signaling pathway and STAT4 remains unclear.
Thus, further investigations are required to fully elucidate this
matter.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Joint Construction
Project of Medical Science and Technology Research Plan of Henan
Province (grant no. LHGJ20230735).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MH was responsible for conceptualization, data
curation, formal analysis, methodology, project administration,
supervision, validation, visualization, writing the original draft.
reviewing and editing. YY was responsible for data curation, formal
analysis, methodology and validation. SN and JH were responsible
for data curation and formal analysis. ZG designed the study. YY
and ZG confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Patient consent for publication
Not applicable.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Ethics Committee of Zhengzhou Seventh People's Hospital [Henan,
China; approval no. Zheng Xin Ethics (2024) 017].
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
I/R
|
ischemia-reperfusion
|
|
H/R
|
hypoxia/reoxygenation
|
|
PI3K
|
phosphatidylinositol-3-hydroxykinase
|
|
AKT
|
serine/threonine kinase
|
|
MI
|
myocardial infarction
|
|
SD
|
Sprague-Dawley
|
|
LAD
|
left anterior descending coronary
artery
|
|
LDH
|
lactate dehydrogenase
|
|
SOD
|
superoxide dismutase
|
|
JAK2
|
Janus Kinase 2
|
References
|
1
|
Kuppe C, Ramirez Flores RO, Li Z, Hayat S,
Levinson RT, Liao X, Hannani MT, Tanevski J, Wunnemann F, Nagai JS,
et al: Spatial multi-omic map of human myocardial infarction.
Nature. 608:766–777. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen M, Li X, Yang H, Tang J and Zhou S:
Hype or hope: Vagus nerve stimulation against acute myocardial
ischemia-reperfusion injury. Trends Cardiovasc Med. 30:481–488.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li T, Tan Y, Ouyang S, He J and Liu L:
Resveratrol protects against myocardial ischemia-reperfusion injury
via attenuating ferroptosis. Gene. 808:1459682022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xing X, Guo S, Zhang G, Liu Y, Bi S, Wang
X and Lu Q: miR-26a-5p protects against myocardial
ischemia/reperfusion injury by regulating the PTEN/PI3K/AKT
signaling pathway. Braz J Med Biol Res. 53:e91062020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cai W, Liu L, Shi X, Liu Y, Wang J, Fang
X, Chen Z, Ai D, Zhu Y and Zhang X: Alox15/15-HpETE aggravates
myocardial ischemia-reperfusion injury by promoting cardiomyocyte
ferroptosis. Circulation. 147:1444–1460. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Philips RL, Wang Y, Cheon H, Kanno Y,
Gadina M, Sartorelli V, Horvath CM, Darnell JE Jr, Stark GR and
O'Shea JJ: The JAK-STAT pathway at 30: Much learned, much more to
do. Cell. 185:3857–3876. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jia D, Jiang H, Weng X, Wu J, Bai P, Yang
W, Wang Z, Hu K, Sun A and Ge J: Interleukin-35 promotes macrophage
survival and improves wound healing after myocardial infarction in
mice. Circ Res. 124:1323–1336. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang Y, Xin X, Pan X, Zhang A, Zhang Z,
Li J and Yuan X: STAT4 targets KISS1 to promote the apoptosis of
ovarian granulosa cells. J Ovarian Res. 13:1352020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Byrne-Hoffman CN, Deng W, McGrath O, Wang
P, Rojanasakul Y and Klinke DJ II: Interleukin-12 elicits a
non-canonical response in B16 melanoma cells to enhance survival.
Cell Commun Signal. 18:782020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ajzashokouhi AH, Rezaee R, Omidkhoda N and
Karimi G: Natural compounds regulate the PI3K/Akt/GSK3β pathway in
myocardial ischemia-reperfusion injury. Cell Cycle. 22:741–757.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Syed Abd Halim SA, Abd Rashid N, Woon CK
and Abdul Jalil NA: Natural Products Targeting PI3K/AKT in
myocardial ischemic reperfusion injury: A scoping review.
Pharmaceuticals (Basel). 16:7392023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cui ZH, Zhang XJ, Shang HQ, Wang X and
Rong D: Glutamine protects myocardial ischemia-reperfusion injury
in rats through the PI3K/Akt signaling pathway. Eur Rev Med
Pharmacol Sci. 24:444–451. 2020.PubMed/NCBI
|
|
13
|
Mao S, Tian S, Luo X, Zhou M, Cao Z and Li
J: Overexpression of PLK1 relieved the myocardial
ischemia-reperfusion injury of rats through inducing the mitophagy
and regulating the p-AMPK/FUNDC1 axis. Bioengineered. 12:2676–2687.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng
YL, Cheng PW, Li CY and Li CJ: Current mechanistic concepts in
ischemia and reperfusion injury. Cell Physiol Biochem.
46:1650–1667. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang C, Mai H, Peng J, Zhou B, Hou J and
Jiang D: STAT4: An immunoregulator contributing to diverse human
diseases. Int J Biol Sci. 16:1575–1585. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dobrian AD, Hatcher MA, Brotman JJ,
Galkina EV, Taghavie-Moghadam P, Pei H, Haynes BA and Nadler JL:
STAT4 contributes to adipose tissue inflammation and
atherosclerosis. J Endocrinol. 227:13–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taghavie-Moghadam PL, Gjurich BN, Jabeen
R, Krishnamurthy P, Kaplan MH, Dobrian AD, Nadler JL and Galkina
EV: STAT4 deficiency reduces the development of atherosclerosis in
mice. Atherosclerosis. 243:169–178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pan A, Tan Y, Wang Z and Xu G: STAT4
silencing underlies a novel inhibitory role of microRNA-141-3p in
inflammation response of mice with experimental autoimmune
myocarditis. Am J Physiol Heart Circ Physiol. 317:H531–H540. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang G, Chen JH, Qiang Y, Wang DZ and Chen
Z: Decreased STAT4 indicates poor prognosis and enhanced cell
proliferation in hepatocellular carcinoma. World J Gastroenterol.
21:3983–3993. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Litvinov IV, Cordeiro B, Fredholm S, Odum
N, Zargham H, Huang Y, Zhou Y, Pehr K, Kupper TS, Woetmann A and
Sasseville D: Analysis of STAT4 expression in cutaneous T-cell
lymphoma (CTCL) patients and patient-derived cell lines. Cell
Cycle. 13:2975–2982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishi M, Batsaikhan BE, Yoshikawa K,
Higashijima J, Tokunaga T, Takasu C, Kashihara H, Ishikawa D and
Shimada M: High STAT4 expression indicates better disease-free
survival in patients with gastric cancer. Anticancer Res.
37:6723–6729. 2017.PubMed/NCBI
|
|
22
|
Gong X and Liu X: In-depth analysis of the
expression and functions of signal transducers and activators of
transcription in human ovarian cancer. Front Oncol. 12:10546472022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He M, Li M and Guo Z: STAT4 regulates
cardiomyocyte apoptosis in rat models of diabetic cardiomyopathy.
Acta Histochem. 124:1518722022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reid S, Hagberg N, Sandling JK, Alexsson
A, Pucholt P, Sjowall C, Lerang K, Jonsen A, Gunnarsson I, Syvanen
AC, et al: Interaction between the STAT4 rs11889341(T) risk allele
and smoking confers increased risk of myocardial infarction and
nephritis in patients with systemic lupus erythematosus. Ann Rheum
Dis. 80:1183–1189. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu F, Na L, Li Y and Chen L: Roles of the
PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and
tumours. Cell Biosci. 10:542020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hou Y, Wang K, Wan W, Cheng Y, Pu X and Ye
X: Resveratrol provides neuroprotection by regulating the
JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats. Genes Dis.
5:245–255. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee PW, Smith AJ, Yang Y, Selhorst AJ, Liu
Y, Racke MK and Lovett-Racke AE: IL-23R-activated STAT3/STAT4 is
essential for Th1/Th17-mediated CNS autoimmunity. JCI Insight.
2:e916632017. View Article : Google Scholar : PubMed/NCBI
|