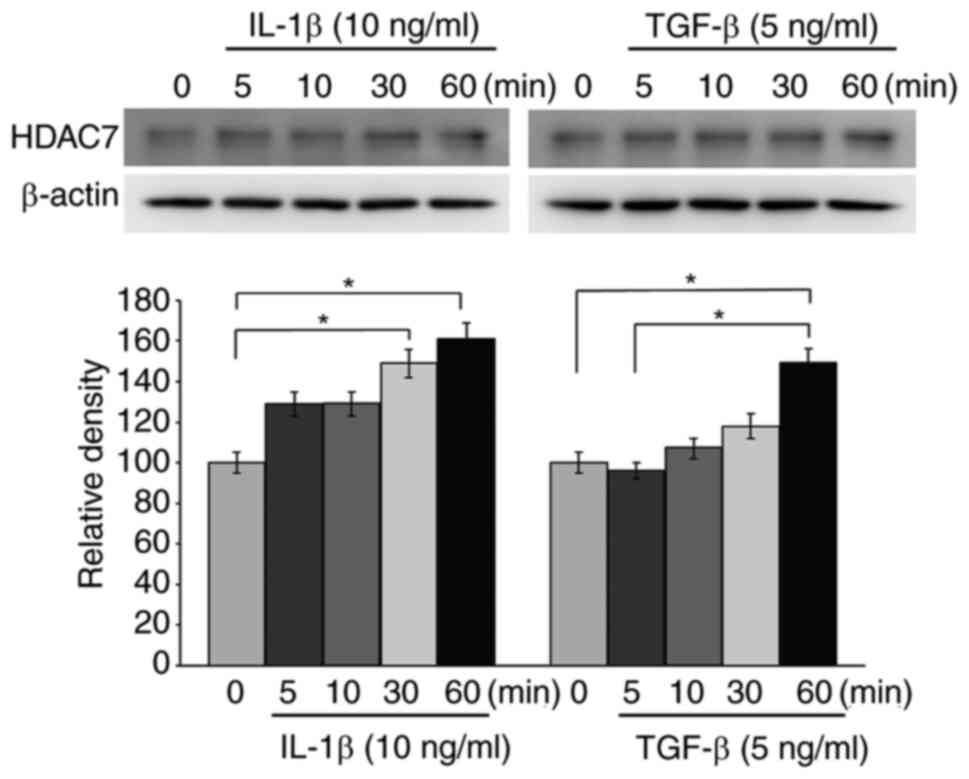
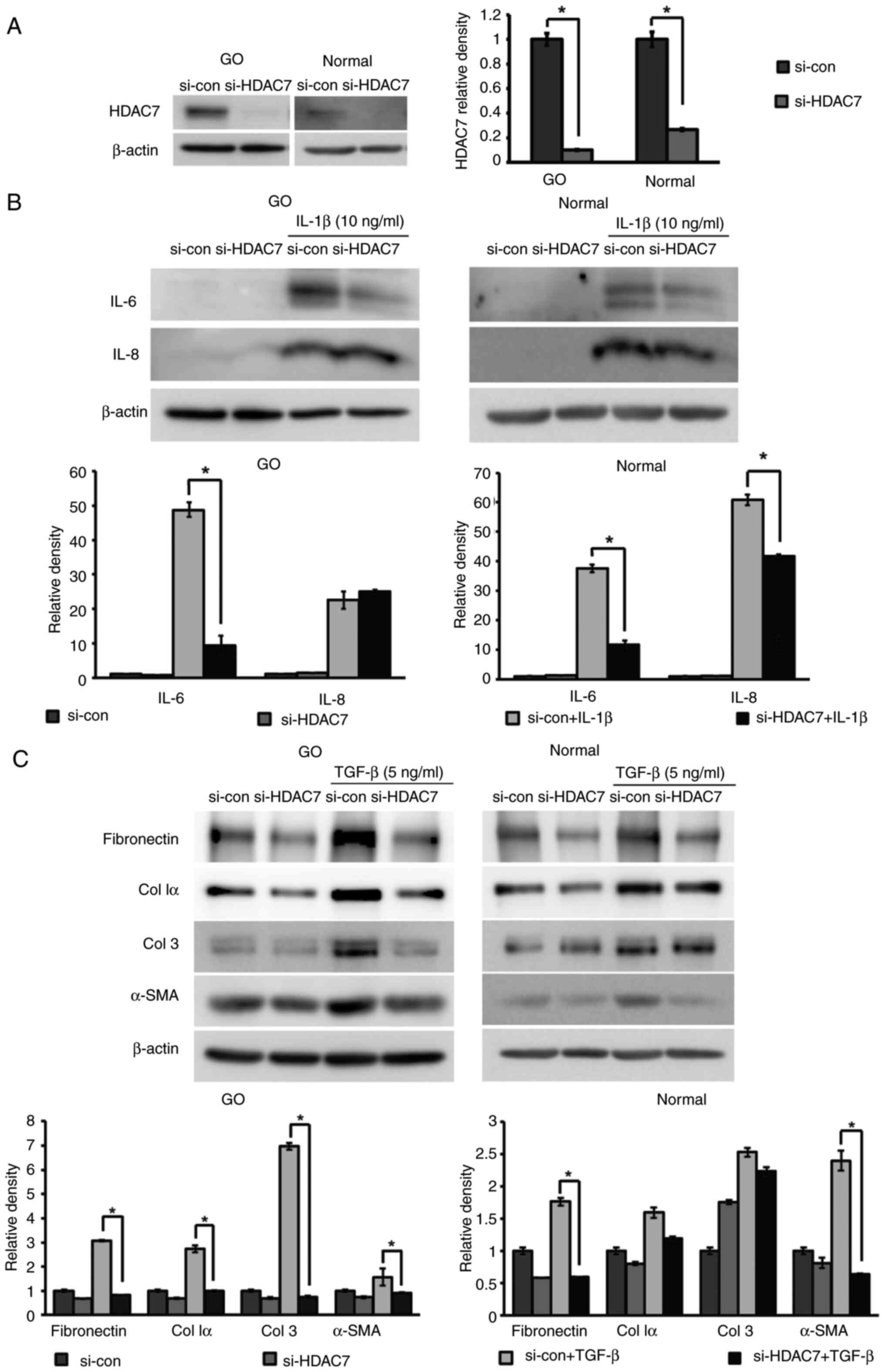
|
1
|
Lazarus JH: Epidemiology of Graves'
orbitopathy (GO) and relationship with thyroid disease. Best Pract
Res Clin Endocrinol Metab. 26:273–279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bahn RS: Graves' ophthalmopathy. N Engl J
Med. 362:726–738. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bartley GB, Fatourechi V, Kadrmas EF,
Jacobsen SJ, Ilstrup DM, Garrity JA and Gorman CA: Clinical
features of Graves' ophthalmopathy in an incidence cohort. Am J
Ophthalmol. 121:284–290. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rotondo Dottore G, Torregrossa L, Lanzolla
G, Mariotti S, Menconi F, Piaggi P, Cristofani Mencacci L,
Posarelli C, Maglionico MN, Dallan I, et al: Role of the
mononuclear cell infiltrate in Graves' orbitopathy (GO): Results of
a large cohort study. J Endocrinol Invest. 45:563–572. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang Y, Fang S, Li D, Zhou H, Li B and
Fan X: The involvement of T cell pathogenesis in thyroid-associated
ophthalmopathy. Eye (Lond). 33:176–182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yin X, Latif R, Bahn R and Davies TF:
Genetic profiling in Graves' disease: Further evidence for lack of
a distinct genetic contribution to Graves' ophthalmopathy. Thyroid.
22:730–736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hadj-Kacem H, Rebuffat S, Mnif-Féki M,
Belguith-Maalej S, Ayadi H and Péraldi-Roux S: Autoimmune thyroid
diseases: Genetic susceptibility of thyroid-specific genes and
thyroid autoantigens contributions. Int J Immunogenet. 36:85–96.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Ma XM, Wang X, Sun X, Wang LJ, Li
XQ, Liu XY and Yu HS: Emerging insights into the role of
epigenetics and gut microbiome in the pathogenesis of Graves'
ophthalmopathy. Front Endocrinol (Lausanne). 12:7885352022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rotondo Dottore G, Bucci I, Lanzolla G,
Lanzolla G, Dallan I, Sframeli A, Torregrossa L, Casini G, Basolo
F, Figus M, et al: Genetic profiling of orbital fibroblasts from
patients with Graves' orbitopathy. J Clin Endocrinol Metab.
106:e2176–e2190. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kouzarides T: Chromatin modifications and
their function. Cell. 128:693–705. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shakespear MR, Halili MA, Irvine KM,
Fairlie DP and Sweet MJ: Histone deacetylases as regulators of
inflammation and immunity. Trends Immunol. 32:335–343. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Narita T, Weinert BT and Choudhary C:
Functions and mechanisms of non-histone protein acetylation. Nat
Rev Mol Cell Biol. 20:156–174. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gatla HR, Muniraj N, Thevkar P, Yavvari S,
Sukhavasi S and Makena MR: Regulation of chemokines and cytokines
by histone deacetylases and an update on histone decetylase
inhibitors in human diseases. Int J Mol Sci. 20:11102019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen H, Pan J, Wang JD, Liao QM and Xia
XR: Suberoylanilide hydroxamic acid, an inhibitor of histone
deacetylase, induces apoptosis in rheumatoid arthritis
fibroblast-like synoviocytes. Inflammation. 39:39–46. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bahn RS: Thyrotropin receptor expression
in orbital adipose/connective tissues from patients with
thyroid-associated ophthalmopathy. Thyroid. 12:193–195. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y and Zhang B: Trichostatin A, an
inhibitor of histone deacetylase, inhibits the viability and
invasiveness of hypoxic rheumatoid arthritis fibroblast-like
synoviocytes via PI3K/Akt signaling. J Biochem Mol Toxicol.
30:163–169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oh BR, Suh DH, Bae D, Ha N, Choi YI, Yoo
HJ, Park JK, Lee EY, Lee EB and Song YW: Therapeutic effect of a
novel histone deacetylase 6 inhibitor, CKD-L, on collagen-induced
arthritis in vivo and regulatory T cells in rheumatoid arthritis in
vitro. Arthritis Res Ther. 19:1542017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Zoeten EF, Wang L, Butler K, Beier UH,
Akimova T, Sai H, Bradner JE, Mazitschek R, Kozikowski AP, Matthias
P and Hancock WW: Histone deacetylase 6 and heat shock protein 90
control the functions of Foxp3(+) T-regulatory cells. Mol Cell
Biol. 31:2066–2078. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jones DL, Haak AJ, Caporarello N, Choi KM,
Ye Z, Yan H, Varelas X, Ordog T, Ligresti G and Tschumperlin DJ:
TGFβ-induced fibroblast activation requires persistent and targeted
HDAC-mediated gene repression. J Cell Sci. 132:jcs2334862019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Korfei M, Stelmaszek D, MacKenzie B,
Skwarna S, Chillappagari S, Bach AC, Ruppert C, Saito S, Mahavadi
P, Klepetko W, et al: Comparison of the antifibrotic effects of the
pan-histone deacetylase-inhibitor panobinostat versus the IPF-drug
pirfenidone in fibroblasts from patients with idiopathic pulmonary
fibrosis. PLoS One. 13:e02079152018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sanders YY, Hagood JS, Liu H, Zhang W,
Ambalavanan N and Thannickal VJ: Histone deacetylase inhibition
promotes fibroblast apoptosis and ameliorates pulmonary fibrosis in
mice. Eur Respir J. 43:1448–1458. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ekronarongchai S, Palaga T, Saonanon P,
Pruksakorn V, Hirankarn N, van Hagen PM, Dik WA and Virakul S:
Histone deacetylase 4 controls extracellular matrix production in
orbital fibroblasts from graves' ophthalmopathy patients. Thyroid.
31:1566–1576. 2021.PubMed/NCBI
|
|
23
|
Atadja P: Development of the pan-DAC
inhibitor panobinostat (LBH589): Successes and challenges. Cancer
Lett. 280:233–241. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shao W, Growney J, Feng Y, Wang P,
Yan-Neale Y, O'Connor G, Kwon P, Yao YM, Fawell S and Atadja P:
Potent anticancer activity of a pan-deacetylase inhibitor
panobinostat (LBH589) as a single agent in in vitro and in vivo
tumor models. Cancer Res. 68 (9 Suppl):S7352008.
|
|
25
|
Mourits MP, Koornneef L, Wiersinga WM,
Prummel MF, Berghout A and van der Gaag R: Clinical criteria for
the assessment of disease activity in Graves' ophthalmopathy: A
novel approach. Br J Ophthalmol. 73:639–644. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Byeon HJ, Chae MK, Ko J, Lee EJ, Kikkawa
DO, Jang SY and Yoon JS: The role of adipsin, complement factor D,
in the pathogenesis of Graves' orbitopathy. Invest Ophthalmol Vis
Sci. 64:132023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dokmanovic M, Perez G, Xu W, Ngo L, Clarke
C, Parmigiani RB and Marks PA: Histone deacetylase inhibitors
selectively suppress expression of HDAC7. Mol Cancer Ther.
6:2525–2534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fischle W, Dequiedt F, Fillion M, Hendzel
MJ, Voelter W and Verdin E: Human HDAC7 histone deacetylase
activity is associated with HDAC3 in vivo. J Biol Chem.
276:35826–35835. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yan N, Zhou JZ, Zhang JA, Cai T, Zhang W,
Wang Y, Muhali FS, Guan L and Song RH: Histone hypoacetylation and
increased histone deacetylases in peripheral blood mononuclear
cells from patients with Graves' disease. Mol Cell Endocrinol.
414:143–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tan C, Xuan L, Cao S, Yu G, Hou Q and Wang
H: Decreased histone deacetylase 2 (HDAC2) in peripheral blood
monocytes (PBMCs) of COPD patients. PLoS One. 11:e01473802016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Y, Zhou M, Lv X, Song L, Zhang D, He Y,
Wang M, Zhao X, Yuan X, Shi G and Wang D: Reduced activity of HDAC3
and increased acetylation of histones H3 in peripheral blood
mononuclear cells of patients with rheumatoid arthritis. J Immunol
Res. 2018:73135152018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie K: Interleukin-8 and human cancer
biology. Cytokine Growth Factor Rev. 12:375–391. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sampson AP: The role of eosinophils and
neutrophils in inflammation. Clin Exp Allergy. 30 (Suppl
1):S22–S27. 2000. View Article : Google Scholar
|
|
35
|
Gu LQ, Jia HY, Zhao YJ, Liu N, Wang S, Cui
B and Ning G: Association studies of interleukin-8 gene in Graves'
disease and Graves' ophthalmopathy. Endocrine. 36:452–456. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Antonelli A, Fallahi P, Elia G, Ragusa F,
Paparo SR, Ruffilli I, Patrizio A, Gonnella D, Giusti C, Virili C,
et al: Graves' disease: Clinical manifestations, immune
pathogenesis (cytokines and chemokines) and therapy. Best Pract Res
Clin Endocrinol Metab. 34:1013882020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Virakul S, van Steensel L, Dalm VASH,
Paridaens D, van Hagen PM and Dik WA: Platelet-derived growth
factor: A key factor in the pathogenesis of graves' ophthalmopathy
and potential target for treatment. Eur Thyroid J. 3:217–226. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheng T, Kiser K, Grasse L, Iles L,
Bartholomeusz G, Samaniego F, Orlowski RZ and Chandra J: Expression
of histone deacetylase (HDAC) family members in
bortezomib-refractory multiple myeloma and modulation by
panobinostat. Cancer Drug Resist. 4:888–902. 2021.PubMed/NCBI
|
|
39
|
Hemmatazad H, Rodrigues HM, Maurer B,
Brentano F, Pileckyte M, Distler JH, Gay RE, Michel BA, Gay S,
Huber LC, et al: Histone deacetylase 7, a potential target for the
antifibrotic treatment of systemic sclerosis. Arthritis Rheum.
60:1519–1529. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huber LC, Distler JHW, Moritz F,
Hemmatazad H, Hauser T, Michel BA, Gay RE, Matucci-Cerinic M, Gay
S, Distler O and Jüngel A: Trichostatin A prevents the accumulation
of extracellular matrix in a mouse model of bleomycin-induced skin
fibrosis. Arthritis Rheum. 56:2755–2764. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kang DH, Yin GN, Choi MJ, Song KM, Ghatak
K, Minh NN, Kwon MH, Seong DH, Ryu JK and Suh JK: Silencing histone
deacetylase 7 alleviates transforming growth factor-β1-induced
profibrotic responses in fibroblasts derived from peyronie's
plaque. World J Mens Health. 36:139–146. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hua HS, Wen HC, Weng CM, Lee HS, Chen BC
and Lin CH: Histone deacetylase 7 mediates endothelin-1-induced
connective tissue growth factor expression in human lung
fibroblasts through p300 and activator protein-1 activation. J
Biomed Sci. 28:382021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Saito S, Zhuang Y, Shan B, Danchuk S, Luo
F, Korfei M, Guenther A and Lasky JA: Tubastatin ameliorates
pulmonary fibrosis by targeting the TGFβ-PI3K-Akt pathway. PLoS
One. 12:e01866152017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu Y, Wang R, Han H and Li L: Tubastatin
A suppresses the proliferation of fibroblasts in epidural fibrosis
through phosphatidylinositol-3-kinase/protein kinase B/mammalian
target of rapamycin (PI3K/AKT/mTOR) signalling pathway. J Pharm
Pharmacol. 74:426–434. 2022. View Article : Google Scholar
|
|
45
|
Guo W, Shan B, Klingsberg RC, Qin X and
Lasky JA: Abrogation of TGF-beta1-induced fibroblast-myofibroblast
differentiation by histone deacetylase inhibition. Am J Physiol
Lung Cell Mol Physiol. 297:L864–L870. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bao Y, Kim D, Cho YH, Ku CR, Yoon JS and
Lee EJ: Cre-loxP system-based mouse model for investigating Graves'
disease and associated orbitopathy. Thyroid. 33:1358–1367. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang Y, Abrol R, Mak JYW, Das Gupta K,
Ramnath D, Karunakaran D, Fairlie DP and Sweet MJ: Histone
deacetylase 7: A signalling hub controlling development,
inflammation, metabolism and disease. FEBS J. 290:2805–2832. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu L, Dong L, Bourguet E and Fairlie DP:
Targeting class IIa HDACs: Insights from phenotypes and inhibitors.
Curr Med Chem. 28:8628–8672. 2021. View Article : Google Scholar : PubMed/NCBI
|