Introduction
The global prevalence of obesity and osteoporosis is
growing with the increase in the life expectancy and number of aged
individuals. Mesenchymal stem cells (MSCs) are the common
progenitor of both adipocytes and osteoblasts and delicately
balance their differentiation processes (1). An elevated shift in commitment of
MSCs toward adipogenesis increases the adipocyte population
(2,3), which may lead to osteoporosis.
Studies have shown that the number of adipocytes in the bone marrow
increases with aging, and individuals with a heightened adipocyte
count in their bone marrow typically exhibit declined bone density
(4–6).
In our previous study, next-generation RNA
sequencing (RNA-seq) of bone marrow MSCs identified differentially
expressed genes (DEGs) between patients with osteoporosis and
postmenopausal women with normal bone mineral density. Ingenuity
Pathway Analysis (IPA) of these DEGs identified NR4A1,
encoding nuclear receptor subfamily 4A member 1, as a key gene
associated with osteoporosis and adipocyte differentiation
(7).
The NR4A subfamily genes encode proteins that
regulate various cellular processes such as cell cycle, apoptosis,
steroidogenesis, adipogenesis and energy metabolism (8–10).
The expression levels of these genes have been shown to be elevated
in extreme obesity but return to normal following fat reduction,
suggesting their association with obesity (8). Additionally, the parathyroid hormone
induces the expression of NR4A family proteins in bone (11–13).
A previous study has shown an interaction between NR4A receptors
and the β-catenin signaling pathway in osteoblasts, where NR4A
receptors inhibit β-catenin-mediated transactivation, crucial for
bone tissue formation and function (14), suggesting a potential role of NR4A
family proteins in bone metabolism. However, the precise role of
the NR4A family proteins in MSCs remains to be elucidated.
The present study aimed to investigate the effects
of modulation of NR4A1 expression on differentiation of MSCs
into osteoblasts and adipocytes. Furthermore, using IPA, it sought
to clarify the common pathways and related genes involved in the
regulation of NR4A1-mediated regulation of MSC
differentiation.
Materials and methods
Cell culture
Mouse MC3T3-E1 pre-osteoblast cells (CRL-2593; ATCC)
were cultured in Minimum Essential Medium supplemented with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1%
antibiotic-antimycotic (Gibco; Thermo Fisher Scientific, Inc.).
Mouse fibroblast cell line 3T3-L1 (CL-173; ATCC) was cultured in
the high glucose Dulbecco's Modified Eagle's Medium supplemented
with 10% fetal bovine serum and 1% antibiotic-antimycotic (Gibco;
Thermo Fisher Scientific, Inc.). BMD-MSCs (passage 2; cat. no.
PCS-500-012; ATCC; expressing CD29, CD44, CD73, CD90, CD105, and
CD166 markers; not expressing CD14, CD19, CD31, CD34, and CD45
markers) were maintained in the basal medium (cat. no. PCS-500-030;
ATCC) supplemented with growth factors (cat. no. PCS-500-041;
ATCC). All cells were plated following small interfering RNA
(siRNA) or clone transfection. MC3T3-E1 cells and BMD-MSCs were
plated at a density of 1.0×104 cells/well in 48-well
plates and used for osteoblast differentiation. After 24 h of
culturing in the plates, the cells were stimulated with an
osteogenic medium containing ascorbic acid (50 µg/ml; cat. no.
50-81-7; MilliporeSigma) and β-glycerophosphate (10 mM; cat. no.
13408-09-8; MilliporeSigma) for cell adherence (day 0) and cultured
for 18 days. The cells were subjected to alkaline phosphatase (ALP)
assay on days 3, 5 and 7 and Alizarin Red S (ARS) staining on days
7, 14 and 18. 3T3-L1 cells and BMD-MSCs were seeded at a density of
5.0×104 cells/well in 30 mm plates, stimulated with the
adipogenic medium containing 1 µM dexamethasone (cat. no. 50-02-2;
MilliporeSigma), 0.5 mM isobutylmethylxanthine (cat. no.
28822-58-4; MilliporeSigma), 10 µM insulin (cat. no. 11061-68-0;
MilliporeSigma) and 200 µM indomethacin (cat. no. 53-86-1;
MilliporeSigma) for differentiation into adipocytes and cultured
for 9 and 18 days, respectively. To investigate NR4A1 expression
levels, the 3T3-L1 cells were subjected to a 9-day incubation
period, while the BMD-MSCs were incubated for 20 days. Throughout
the incubation process, the culture medium was replaced every 3
days. All cells were incubated at 37°C in a humidified environment
with 95% air and 5% CO2.
Plasmids, lentivirus packaging and
reagents
Full-length mouse Nr4a1wt (cat. no. BC004770;
pCMV-SPORT6-Nr4a1), human NR4A1wt (cat. no. NM_002135;
pCMV-SPORT6-NR4A1), and the pCMV-SPORT6 plasmid (Mock) were
provided by the Korea Human Gene Bank (Medical Genomics Research
center, KRIBB, Korea). All cells were cultured at a density of
~1×105/well in 6-well plates, and plasmids were
transfected with 100 pmol/ml siRNA using Lipofectamine®
2000 (cat. no. 11668019; Invitrogen; Thermo Fisher Scientific,
Inc.) in Opti-MEM (Gibco; Thermo Fisher Scientific, Inc.) at 37°C
for 48 h. Small interfering RNAs (siRNAs) for mouse Nr4a1
(siNr4a1, 5′-GCAAGCCUACCAUGGACCU-3′,
5′-AGGUCCAUGGUAGGCUUGC-3′), human NR4A1 (siNR4A1,
5′-GUGAAGGAAGUUGUCCGAA-3′, 5′-UUCGGACAACUUCCUUCAC-3′) and NC-siRNA
(Negative control siRNA; cat. no. SN-1002) were obtained from
Bioneer Corporation. Control and NR4A1-overexpressing
BMD-MSCs were plated at a density of 4.0×105 cells per
well in 100 mm plates and treated with 20 µM
1,1-bis(3′-indolyl)-1-(p-hydroxyphenyl) methane (DIM-C-pPhOH; cat.
no. HY-112055; MedChemExpress), an NR4A1 antagonist (15), and incubated at 37°C for 24 h
before being subjected to osteoblast or adipocyte
differentiation.
Reverse transcription-quantitative
(RT-q) PCR
RT-qPCR was performed to determine NR4A1 mRNA
expression levels, and its expression was normalized with those of
mouse or human Actb (cat. no. NM_007393)/ACTB (cat.
no. NM_001101; both Bioneer Corporation) mRNA, serving as internal
standards. Cells were cultured at a density of
~1×105/well in 6-well plates, and transfected with
plasmid or siRNA. Total RNA was isolated from cultured cells 48 h
after transfection with siRNA or plasmids using TRIzol®
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions, and its quality was assessed using a
spectrophotometer (Beckman Coulter, Inc.). Following extraction,
RNA was reverse-transcribed for 1 h at 42°C using a premix kit
containing oligo-dT as a primer (iNtRON Biotechnology). The ABI
Prism 7000 Sequence Detection System (Applied Biosystems; Thermo
Fisher Scientific, Inc.) was used for all PCR measurements. All PCR
was performed in triplicate with cycling conditions as follows:
Initial denaturation at 95°C/10 min; (ii) 40 cycles of 95°C/30 sec,
60°C/1 min; and 72°C/30 sec using the SYBR Green I qPCR kit (Takara
Bio, Inc.) in a total volume of 25 µl, containing 150 ng cDNA
according to the manufacturer's guidelines. Gene expression levels
were quantified relative to that of Actb/ACTB mRNA using the
manufacturer-recommended comparative threshold method (Applied
Biosystems). The values are expressed as fold-change from the
control levels. The relative gene expression was expressed as
2−ΔCq. The fold-change was determined to be
2−ΔΔCq (16). The
primers used for PCR are listed in Table I.
 | Table I.Primers for real-time polymerase
chain reaction. |
Table I.
Primers for real-time polymerase
chain reaction.
| Gene | Accession no. | Forward primer
sequence (5′→3′) | Reverse primer
sequence (5′→3′) |
|---|
| hACTB | NM_001101 |
GATGAGATTGGCATGGCTTT |
CACCTTCACCGTTCCAGTTT |
| hNR4A1 | NM_001202233 |
AGAAGATCCCTGGCTTTGCT |
CAGGGACATCGACAAGCAAG |
| mActb | NM_007393 |
GATCTGGCACCACACCTTCT |
GGGGTGTTGAAGGTCTCAAA |
| mNr4a1 | NM_001411253 |
GACTTGCTCTCTGGTTCCCT |
AGAAGGCCAGGATGTTGTCA |
| hMAML3 | NM_018717 |
CGTATATCCAGCAGCAGCAA |
TTTCTGGTCTTCGCTCAGGT |
| hJAG1 | NM_000214 |
AAGGGGTGCGGTATATTTCC |
TCCCGTGAAGCCTTTGTTAC |
| hDTX4 | NM_001300727 |
ACCCAGACTGCAAAACCATC |
CCGGAAGGTAACAGTGTCGT |
| hNOTCH3 | NM_000435 |
CTCATCCGAAACCGCTCTAC |
TCTTCCACCATGCCCTCTAC |
| hHES1 | NM_005524 |
TCAACACGACACCGGATAAA |
TCAGCTGGCTCAGACTTTCA |
| hPSEN2 | NM_000447 |
GTGCCTGTCACTCTGTGCAT |
GCGTGTAGATGAGCTGTCCA |
Western blotting
Cells, 48 h after transfection with siRNA or
plasmids, were lysed using 0.1 M NaCl, 0.01 M Tris-HCl (pH 7.6), 1
mM ethylenediaminetetraacetic acid (pH 8.0), 1 mg/ml aprotinin, and
100 mg/ml phenylmethylsulfonyl fluoride. Protein concentrations in
cell lysates were measured using the Bio-Rad protein assay.
Subsequently, 50 µg protein was denatured at 95°C for 5 min in
sodium dodecyl sulfate sample buffer, electrophoresed on 10% sodium
dodecyl sulfate-polyacrylamide gels, and transferred to
polyvinylidene difluoride membranes. The membranes were blocked in
5% skim milk for 1 h at 20–22°C, then incubated overnight at 4°C
with primary antibodies against NR4A1 (cat. no. MA5-32647, 1:500;
Thermo Fisher Scientific, Inc.) or β-Actin (cat. no. A300-491A,
1:10,000; Bethyl Laboratories, Inc.). Subsequently, the membranes
were incubated at 20–22°C for 60 min with an anti-rabbit secondary
antibody (1:5,000; Santa Cruz Biotechnology, Inc.). Protein bands
were detected using an ECF western blotting kit (Amersham
Biosciences; Cytiva) and visualized using an Automatic X-RAY Film
Processor (cat. no. JP-33; JPI Healthcare Co., Ltd.). Adobe
Photoshop 2024 (Adobe Systems, Inc.) was used for densitometry.
ALP and ARS staining
ALP staining was performed on days 3, 5, and 7, and
ARS staining was performed on days 7, 14 and 18 in control,
siNr4a1 (or siNR4A1)-treated, and Nr4a1 (or
NR4A1)-overexpressing MC3T3-E1 cells and human BMD-MSCs.
Briefly, for ALP staining, cultured cells were fixed in 10%
formalin for 10 min, permeabilized in 0.1% Triton X-100 in
phosphate-buffered saline (PBS) for 30 min, and treated for 10–30
min with nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl
phosphate at 20–22°C. Subsequently, 200 µl extraction solution was
added to the samples and incubated at 4°C overnight to determine
calcium deposition in the extracellular matrix. Total cell lysates
were homogenized in a solution containing 1 mM Tris-HCl (pH 8.8),
0.5 percent Triton X-100, 10 mM MgCl2, and 5 mM
p-nitrophenyl phosphate at 20–22°C. The absorbance at 405 nm was
then determined (BioTek Instruments, Inc.).
For ARS staining, cultured cells were fixed in 70%
ethyl alcohol for 1 h at 20–22°C. After washing with 1XPBS, the
cells were incubated for 10 min at 20–22°C with a 40 mM ARS
solution (pH 4.2; cat. no. A5533, MilliporeSigma) to stain the
calcium deposits. Prior to staining, the medium was discarded and
rinsed gently with 1XPBS. The cells were then extracted with 10%
(w/v) cetylpyridinium chloride in 10 mM sodium phosphate to
determine the degree of mineralization at pH 7.0. The concentration
was determined by measuring the absorbance at 562 nm using a
multi-plate reader (cat. no. 1681135; Bio-Rad Laboratories, Inc.)
and a standard curve obtained using ARS in the same solution. Each
value was expressed as a fold-change compared with control.
Oil Red O staining
Cells were fixed in 10% formalin and stained for 15
min at room temperature according to the manufacturer's
instructions using a Lipid (Oil Red O) Staining Kit (cat. no.
MAK194; MilliporeSigma). After staining, the cells were washed with
double distilled water and four fields were selected randomly for
observation under a fluorescence microscope at 20X or 40X
magnification using bright-field illumination (Axiovert 200FL; Carl
Zeiss AG). Oil Red O dye was extracted with 100% isopropyl alcohol,
and the absorbance was measured at 520 nm using a spectrophotometer
(SpectraMax iD3; Molecular Devices, LLC) to quantify the lipid
content.
mRNA sequencing
Total RNA was quantified using a NanoDrop8000
spectrophotometer (Thermo Fisher Scientific Inc.), and RNA quality
was determined using a 2100 Expert Bioanalyzer (Agilent
Technologies, Inc.) and an RNA 6000 Nano Kit (Agilent Technologies,
Inc.). mRNA libraries for high-throughput transcriptome sequencing
were then prepared using Illumina technology (Illumina Inc.).
Preprocessing, expression and
functional analysis
The raw sequencing data was trimmed using Cutadapt
(v.2.3; github.com/marcelm/cutadapt/releases/tag/v2.3) to eliminate
adapters and low-quality reads (Phred score 20) following a
previous study (17).
Subsequently, the high-quality reads were mapped to the reference
genome (hg38) using STAR (v.2.7.0f; Alexander Dobin, Cold Spring
Harbor Laboratory; github.com/alexdobin/STAR/tree/2.7.0f) (18), and gene counts were quantified
using quantMode option. The DEGs were determined using DESeq2
(v.1.22.1; Bioconductor;
anaconda.org/bioconda/bioconductor-deseq2/files?page=2&sort=ndownloads&sort_order=asc&type=&version=1.22.1)
software, which employs negative binomial distribution models to
analyze the raw count data. Enrichment analysis of DEGs was
performed using IPA (QIAGEN Inc.;
qiagenbioinformatics.com/products/ingenuity-pathway-analys) with
the Core Analysis feature to identify Canonical Pathways. Pathways
with a z-score absolute value of ≥2 and a P<0.05 were considered
to indicate a statistically significant difference.
Statistical analysis
The statistical analysis was conducted using one-way
ANOVA) followed by Tukey's post-hoc comparisons. P<0.05 was
considered to indicate a statistically significant difference. The
results were expressed as the mean ± standard error of the mean
(SEM; *P<0.05, **P<0.005).
Results
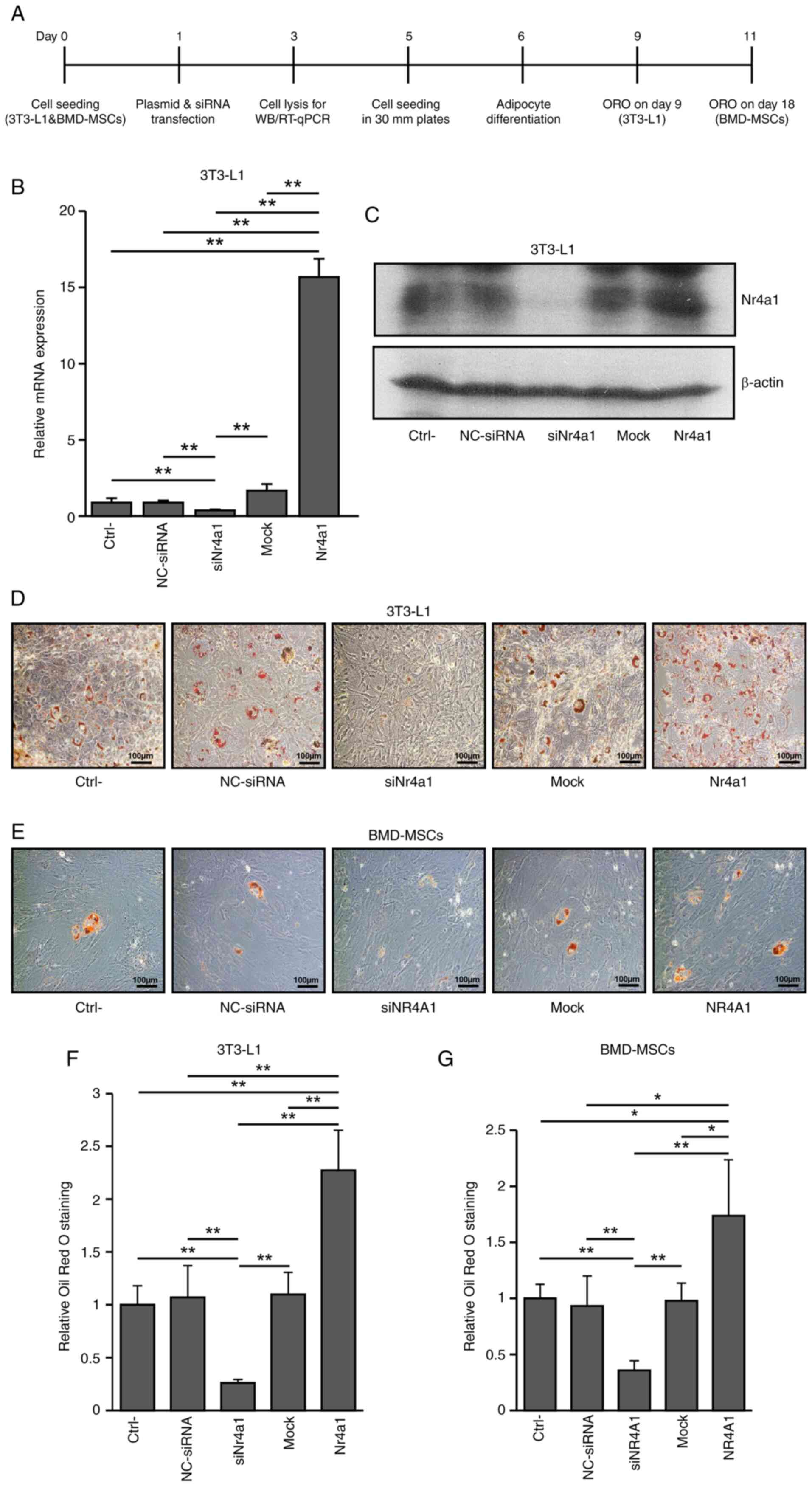
NR4A1 mediates the calcification of
pre-osteoblast cells and BMD-MSCs
In MC3T3-E1 cells and human BMD-MSCs, Nr4a1
and NR4A1, respectively, were successfully knocked down and
overexpressed (Fig. 1A, B and C, G and
H, and Fig. S1). Nr4a1
or NR4A1 knockdown tended to enhance ALP activity while it
significantly increased mineralization in both MC3T3-E1 cells and
BMD-MSCs (P<0.005), respectively compared with those in control
cells. By contrast, both ALP activity and mineralization were
significantly reduced in the respective Nr4a1 (NR4A1)
overexpressing groups [MC3T3-E1 cells (P<0.05, P<0.005) and
BMD-MSCs (P<0.005; Fig. 1D-F,
I-K; showing results of ALP and ARS staining on days 7 and 18
of culture, Fig. S2A and B;
showing results of ALP on days 3, 5, and 7, and ARS staining on
days 7, 14, and 18, respectively]. These findings suggested that
NR4A1 plays a negative role in osteoblast differentiation.
 | Figure 1.Effects of Nr4a1 or
NR4A1 knockdown or overexpression in MC3T3-E1 cells and
human BMD-MSCs. (A) Experimental design diagram. (B) Reverse
transcription-quantitative PCR of Nr4a1 (NR4A1) and
(G) mRNA expression in MC3T3-E1 cells and BMD-MSCs. (C and H)
Western blot analysis of NR4A1 expression in MC3T3-E1 cells and
BMD-MSCs. (D) ALP staining performed on day 5 and (I) Alizarin red
S staining performed on day 18 of culture. (E and J) ALP activity
measured at 405 nm using alkaline phosphatase yellow liquid
substrate system. (F and K) Alizarin red S-stained cells were
extracted using cetylpyridinium chloride, and the mineralization
level was quantified by measuring absorbance at 562 nm in control,
siNr4a1 (siNR4A1)-treated, and Nr4a1
(NR4A1)-overexpressing cells. Data are presented as the mean ±
standard errors of the mean of three biological replicates.
*P<0.05, **P<0.005 vs. control. NR4A1, nuclear receptor
subfamily 4 group A member 1; BMD-MSCs, bone marrow-derived
mesenchymal stem cells; ALP, alkaline phosphatase; Ctrl-, Control;
NC, negative control; si, small interfering; siNr4a1,
siNr4a1-treated MC3T3-E1 cells; siNR4A1, siNR4A1-treated
BMD-MSCs; Nr4a1, Nr4a1-overexpressing MC3T3-E1 cells;
NR4A1 NR4A1-overexpressing BMD-MSCs. |
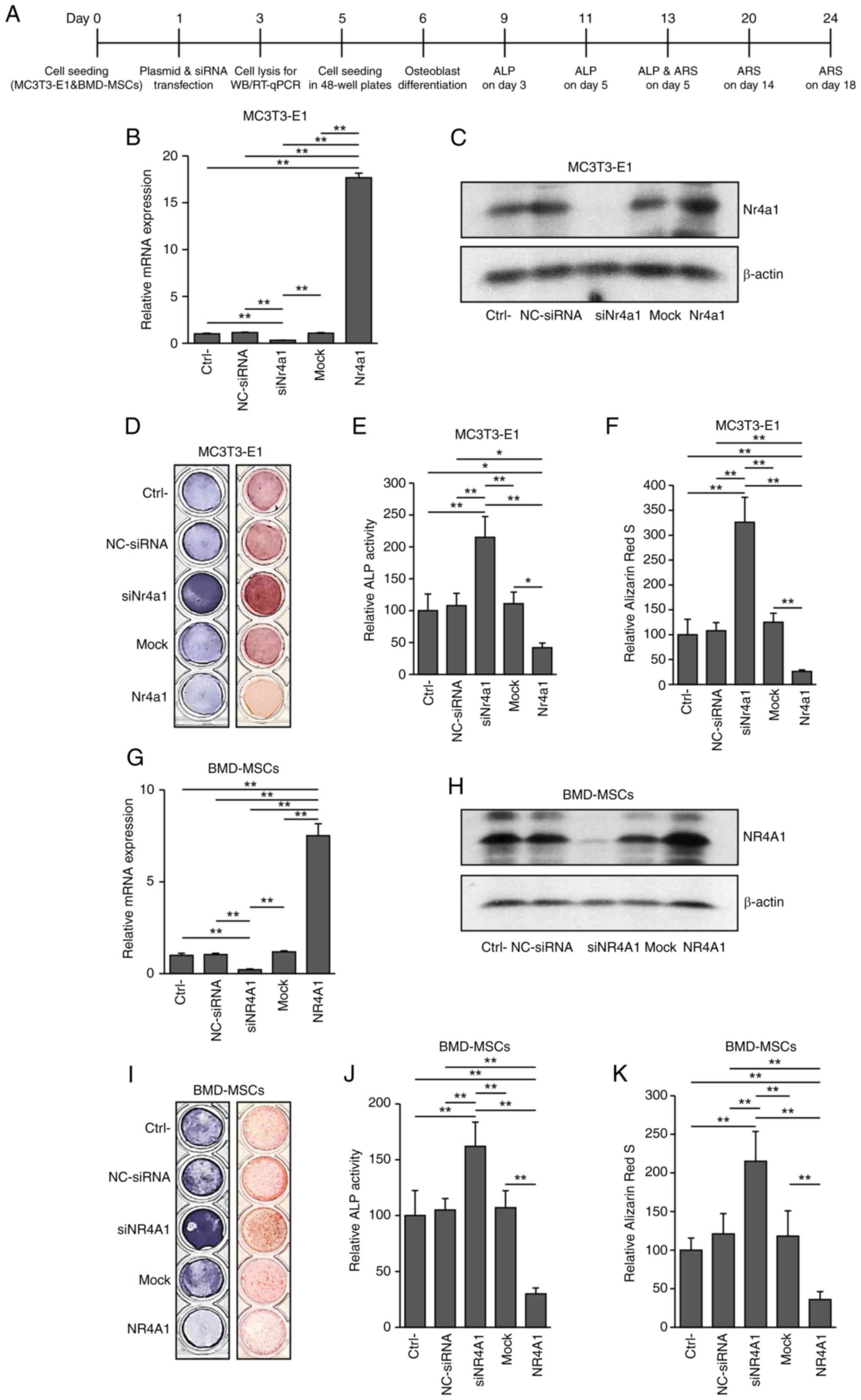
NR4A1 is associated with adipocyte
differentiation
To determine whether NR4A1 plays a role in
adipogenesis, Nr4a1 or NR4A1 overexpression and
knockdown were performed in 3T3-L1 cells and BMD-MSCs, respectively
(Fig. 2A, B and C and Fig. 1G and H and Fig. S1). Adipogenesis was significantly
increased following Nr4a1 and NR4A1 overexpression in
3T3-L1 cells (P<0.005) and BMD-MSCs, respectively (P<0.05)
compared with those in control cells. By contrast, knockdown of
Nr4a1 and NR4A1 led to significantly decreased
adipogenesis in 3T3-L1 cells (P<0.005 vs. control; Fig. 2D and F) and BMD-MSCs (P<0.005
vs. control; Fig. 2E and G),
respectively. These findings indicated that NR4A1 served a
beneficial role in adipogenesis.
NR4A1 antagonist modulates
osteogenesis and adipogenesis in BMD-MSCs
Subsequently, control and
NR4A1-overexpressing BMD-MSCs were treated with DIM-C-pPhOH
to confirm the effect of NR4A1 on osteogenesis and adipogenesis.
The ALP assay was performed on days 3, 5 and 7, while ARS staining
was performed on days 7, 14 and 18 of culture. ALP activities
tended to increase in both normal and NR4A1-overexpressing
BMD-MSCs treated with DIM-C-pPhOH compared with those in untreated
normal control cells (Fig. 3A-D,
Fig. S2C; showing results of ALP
on days 3, 5 and 7, and ARS staining on days 7, 14 and 18,
respectively). Additionally, adipocyte differentiation was
significantly enhanced in the NR4A1-overexpressing group
compared with that in the control group (P<0.005; Fig. 3E and F). By contrast, DIM-C-pPhOH
treatment in both normal and NR4A1-overexpressing groups
significantly reduced adipocyte differentiation compared with those
in their respective untreated control groups (P<0.005). These
results indicated that the effects of the treatment with
DIM-C-pPhOH were comparable to those of NR4A1 knockdown in
normal cells (Fig. 3E and F).
Together, these findings suggested that NR4A1 downregulated
osteoblastogenesis but promoted adipogenesis.
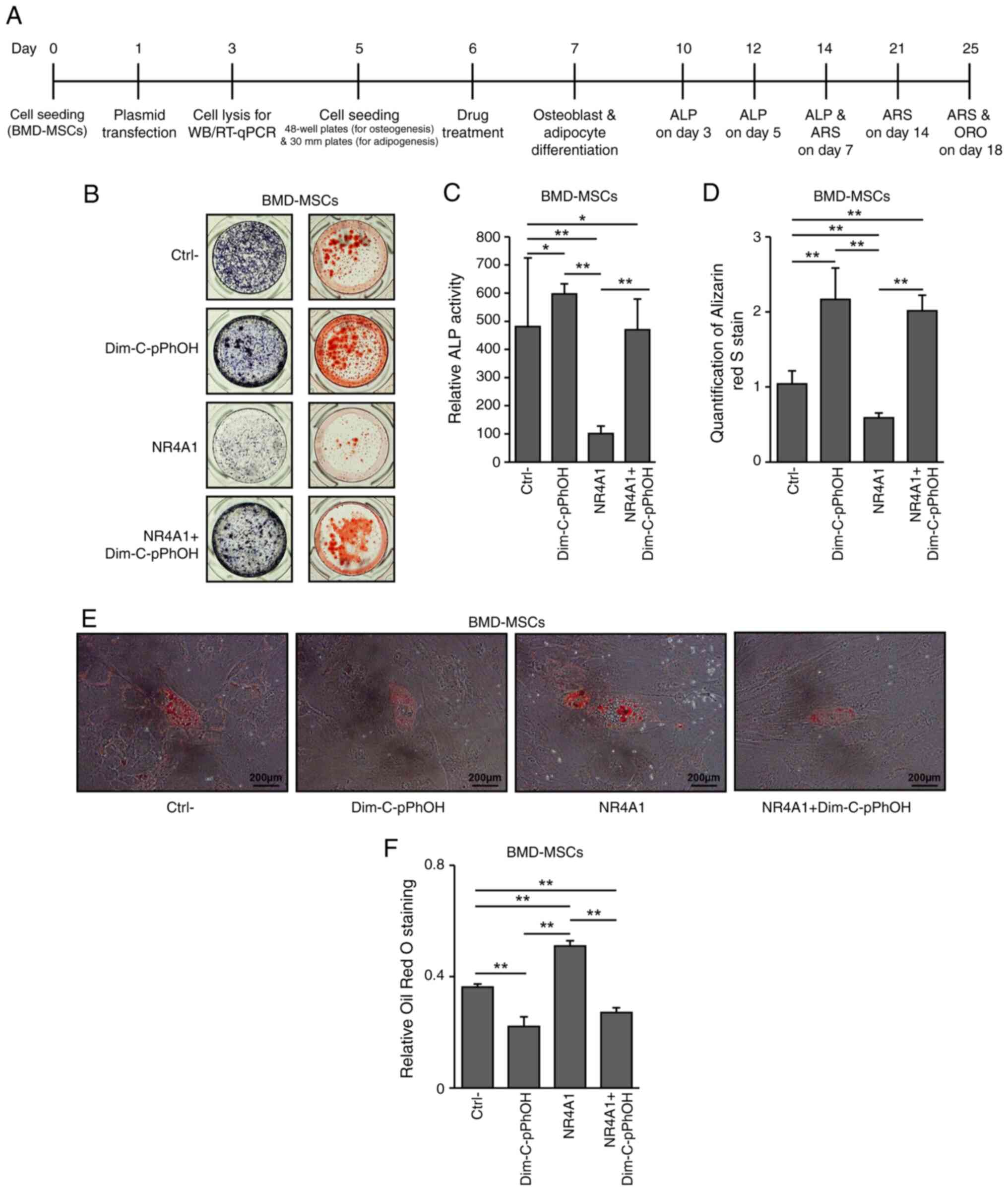 | Figure 3.DIM-C-pPhOH and NR4A1
knockdown demonstrate similar effects on adipogenesis in BMD-MSCs.
(A) Experimental design diagram. (B) ALP staining and Alizarin red
S staining were performed on days 7 and 18 of culture,
respectively. (C) ALP activity measured at 405 nm using alkaline
phosphatase yellow liquid substrate system. (D) Alizarin red
S-stained cells were extracted using cetylpyridinium chloride, and
the mineralization level was quantified by measuring absorbance at
562 nm in control, DIM-C-pPhOH-treated,
NR4A1-overexpressing, and
NR4A1-overexpressing/DIM-C-pPhOH-treated BMD-MSCs. (E)
Adipocyte differentiation and (F) quantification of Oil red O
staining in human BMD-MSCs. *P<0.05, **P<0.005. DIM-C-pPhOH,
1,1-bis(3′-indolyl)-1-(p-hydroxyphenyl) methane; NR4A1, nuclear
receptor subfamily 4 group A member 1; BMD-MSCs, bone
marrow-derived mesenchymal stem cells; ALP, alkaline phosphatase;
Ctrl-, Control; NC, negative control; si, small interfering;
NR4A1 NR4A1-overexpressing BMD-MSCs. |
NR4A1 effect is related to the Notch
signaling pathway
To evaluate the mechanism by which NR4A1 modulated
osteoblastogenesis and adipogenesis, DEGs were identified in
BMD-MSCs in which NR4A1 was either knocked down or
overexpressed. IPA revealed the association between the changes in
NR4A1 expression and Notch signaling in osteoblastogenesis
and adipogenesis. In particular, IPA demonstrated that cells
treated with siNR4A1 had reduced expression of the
Mastermind-like transcriptional coactivator 3 (MAML3) and
elevated expression levels of Jagged canonical Notch ligand 1
(JAG1), Deltex E3 ubiquitin ligase 4 (DTX4), Notch
receptor 3 (NOTCH3), Hes family bHLH transcription factor 1
(HES1), and Presenilin 2 (PSEN2). RT-qPCR data
indicated similar results for MAML3 in NR4A1
overexpressing (P<0.05) and NR4A1 knocked down
(P<0.005) cells (Fig. 4A and
B). Additionally, manipulating the expression of NR4A1
did not significantly affect expression levels of NR4A2 or
NR4A3 (Fig. S3).
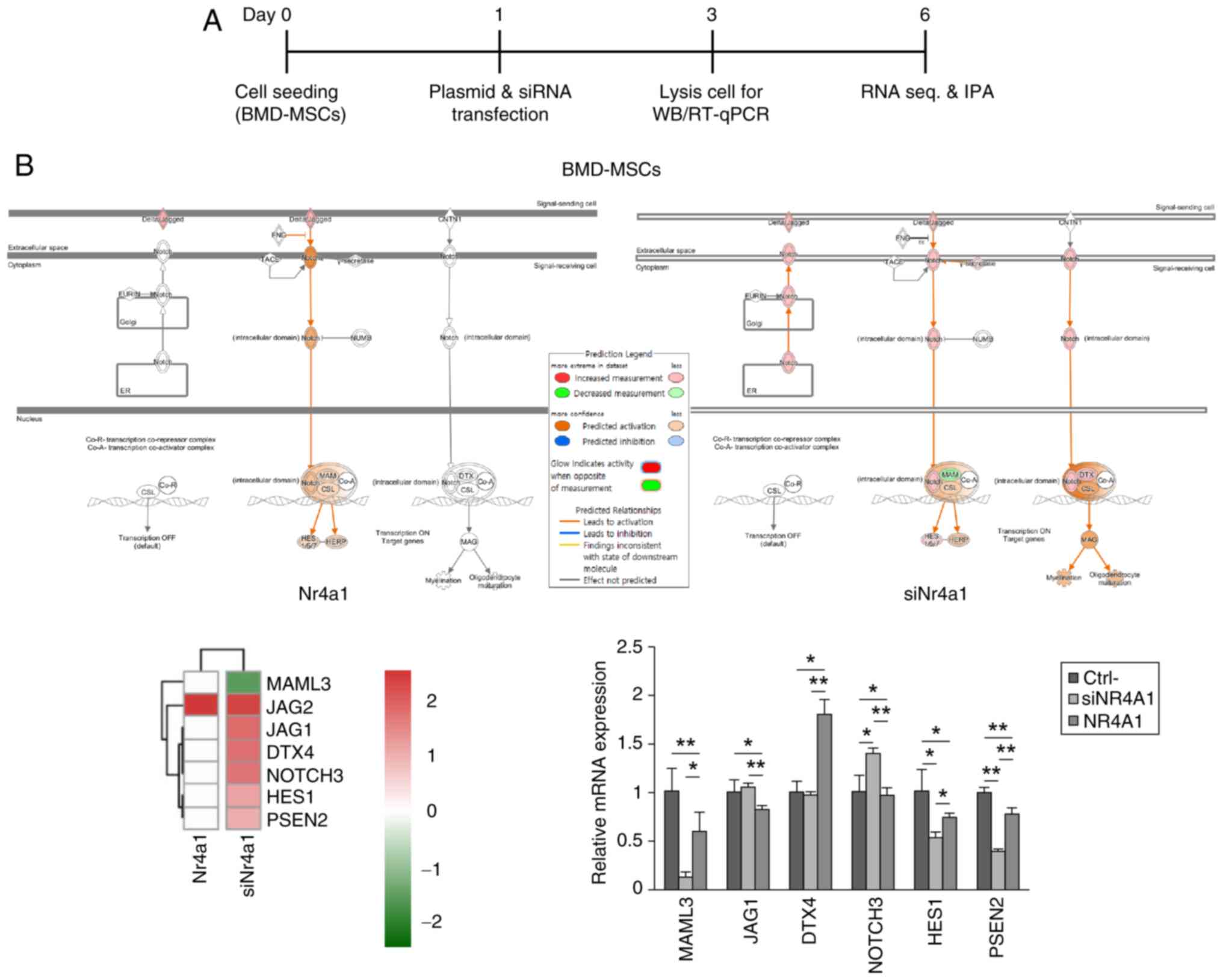 | Figure 4.Notch signaling mediates the effects
of NR4A1 on osteoblastogenesis and adipogenesis in BMD-MSCs. (A)
Experimental design diagram. (B) IPA revealed that the expression
of the gene encoding the Notch signaling pathway component MAML3
was decreased, whereas those of JAG1, DTX4, NOTCH3, HES1 and
PSEN2 were increased in siNR4A1-treated cells.
RT-qPCR data of the representative genes showing altered expression
in IPA. Similar changes were observed in MAML3 mRNA
expression. Data are presented as the mean ± standard errors of the
mean of three biological replicates. *P<0.05, **P<0.005.
NR4A1, nuclear receptor subfamily 4 group A member 1; BMD-MSCs,
bone marrow-derived mesenchymal stem cells; IPA, ingenuity pathway
analysis; MAML3, Mastermind-like transcriptional coactivator 3;
RT-qPCR, reverse transcription-quantitative PCR; WB, western blot
analysis; Ctrl-, Control; si, small interfering; siNr4a1,
siNr4a1-treated MC3T3-E1 cells; siNR4A1, siNR4A1-treated
BMD-MSCs; Nr4a1, Nr4a1-overexpressing MC3T3-E1 cells. |
Discussion
The NR4A subfamily of nuclear receptors serves an
important role in various cellular processes. Expression levels of
members of the NR4A subfamily of nuclear receptors have been shown
to be upregulated in human obesity (8). Furthermore, in 3T3-L1 adipocytes,
insulin and insulin sensitizers, such as thiazolidinediones, have
been shown to induce the expression of both Nur77 (NR4A1) and Nor-1
(NR4A3), suggesting their potential involvement in adipogenesis
(19). The present study
investigated whether and how NR4A1 regulated osteoblast and
adipocyte differentiation of BMD-MSCs.
Numerous studies have reported a relationship
between Nr4a1 expression and adipogenesis; however, their
findings are not consistent. For instance, Nagai et al
(20) demonstrate an indirect
association between Nr4a1 and adipogenesis. Based on this study,
estrogen enhances the expression of NR4A1 in muscle cells, leading
to an increase in ATP and mitochondrial DNA. Their work suggests an
indirect association between adipogenesis and overall energy
metabolism in muscle cells rather than directly proving the
significance of NR4A1 in adipocyte differentiation. The present
study specifically examined adipocyte precursor cells and presented
direct evidence of the involvement of NR4A1 in the process of
adipocyte development. Qin et al (21) report that permanently increased
NR4A1 expression reduces adipogenesis in 3T3-L1 cells and that
Nr4a1 knockdown mice are prone to obesity, suggesting the
association between NR4A1 and dysregulation of adipocyte
differentiation. Moreover, several Nr4a1-responsive genes, such as
gap-junction protein 1 and tolloid-like 1, have been shown to
generally suppress adipocyte differentiation (22,23).
The present study, for the first time to the best of the authors'
knowledge, examined the effects of NR4A1 on cell fate in
stem cells and demonstrated the crucial role of NR4A1 in
enhancing adipocyte differentiation in MSCs. However, the
adipogenesis-promoting effect of Nr4a1 in BMD-MSCs was found to be
less pronounced than in 3T3-L1, which may be due to different
regulatory networks or additional factors specific to BMD-MSCs.
Furthermore, the observations of the present study indicated a
marginal increase in Nr4a1 (NR4A1) expression in siNr4a1
(siNR4A1)-treated cells and a decrease in Nr4a1 (NR4A1)
overexpressing cells over time (Fig.
S4). These fluctuations in expression levels are commonly seen
in transient transfection and would not have been detectable with
permanent transfection. The relatively reduced effect in BMD-MSCs
cells can be ascribed, to some extent, to the transient nature of
our transfection method. This transient transfection probably had
its main effect during the initial stages of adipogenesis, which
may have limited its long-term influence on the overall
differentiation process. Methodological variations and gene
expression mechanisms may account for the differences observed in
the present study compared with those of Qin et al (21) and Chao et al (22). While the aforementioned studies
used stable cell lines infected with lentivirus for permanent
transfection, the present study employed transient transfection,
which can cause fluctuations in NR4A1 expression. This transient
expression may lead to varying effects on adipogenesis,
particularly in the early stages, but may not fully reflect
long-term effects. Additionally, NR4A1 interactions with other
factors could influence adipogenesis differently. Qin et a
(21) noted that increased GATA2
and p53 expression might inhibit adipogenesis, while Chao et
al (22) found that Nur77,
Nurr1 and Nor1, including NR4A1, generally suppress adipocyte
differentiation. By contrast, the present study showed that NR4A1
promoted adipocyte differentiation, possibly due to different
regulatory networks or other factors specific to MSCs. The marginal
fluctuations in NR4A1 expression in the present study, owing to the
transient transfection method, could further explain the divergence
in findings compared with studies using permanent transfection. The
experiments of the present study employed transient transfection to
manipulate NR4A1 expression, a methodological choice for capturing
the dynamic nature of adipogenesis and calcification processes.
Permanent transfection was not considered because it has the
potential to obscure dynamic changes, compromise cell viability,
and introduce artifacts by continuously driving gene expression,
which may not accurately reflect natural cellular regulation
(24). Instead, transient
transfection was chosen to capture the temporal dynamics crucial
for the analysis and more effectively ensure the integrity of our
research process.
It is worth noting that a previous study
demonstrated that siRNA constitutive expression of Nur77 (Nr4a1)
prevents adipogenesis, whereas its transient overexpression
increases adipogenesis in NIH-3T3 cells (25). That study also shows that Nur77
siRNA and constitutive expression delay adipogenesis in 3T3-L1
cells, accompanied by prolonged mitotic clonal expansion (25). It also suggests that Nur77 promotes
adipocyte differentiation by clonal expansion during the initial
phases of adipocyte development and the regulation of the
progression of the cell cycle (25). Based on the previous research and
the present study, it can be inferred that NR4A1 plays a role in
directing MSCs toward adipocytes in the stem cell phase and
promoting transient clonal expansion in the early preadipocyte
stage. Many complex regulatory mechanisms are involved in the
adipogenesis process. For instance, the RNA-seq analysis of the
present study revealed that fatty acid binding protein 4
(FABP4) expression increased in NR4A1 overexpressing
cells, while it was decreased in siNR4A1-treated cells. By
contrast, the expression of other key adipogenesis-related genes,
including CCAAT/enhancer binding proteins (CEBPs) and
peroxisome proliferator-activated receptor gamma (PPARγ),
did not alter (Fig. S5). However,
changes in the expression of these genes did not qualify for
differential expression analysis, suggesting NR4A1
influences adipogenesis through some distinct mechanisms.
Therefore, further studies are required to elucidate the precise
role and function of NR4A1 in this intricate process.
Fewer studies have explored the potential role of
NR4A1 in osteoblast differentiation compared with the number of
studies on its role in adipocyte differentiation. NR4A1 has been
suggested to be a critical regulator of osteoclast biology and bone
remodeling, making this nuclear receptor an attractive target for
osteoporosis therapy (26).
Parathyroid hormone injection has been shown to rapidly and
transiently enhance the expression of all NR4A family members in
target tissues in vivo and NUR77 activated by PTH influences
osteoblast development by increasing cAMP-PKA signaling (12,27).
Although the role of NR4A1 in bone formation is not fully
understood, these studies indicate that NR4A1 may affect
osteoblastogenesis. In the present study, calcification was
increased in human BMD-MSCs with NR4A1 knockdown, whereas it
was decreased in NR4A1-overexpressing BMD-MSCs. These
findings suggested that NR4A1 negatively regulates
osteoblastogenesis in MSCs.
DIM-C-pPhOH binds to NR4A1 and acts as an
antagonist, inhibiting NR4A1-dependent transactivation and showing
antineoplastic activity. DIM-C-pPhOH causes changes in gene
expression comparable to those of NR4A1 knockdown by RNAi
(15,28–30).
For instance, treatment with DIM-C-pPhOH suppresses NR4A1
overexpression and cancer cell proliferation and promotes apoptosis
in breast, pancreatic and lung cancers (15,28,31).
Similarly, when BMD-MSCs were treated with DIM-C-pPhOH,
anticipating an antagonistic effect on NR4A1, identical outcomes
were observed in the cells treated with DIM-C-pPhOH and
siNR4A1. These findings confirmed that NR4A1 had a negative
effect on osteoblastogenesis, whereas it apparently stimulated
adipogenesis in BMD-MSCs. Together, these data suggested that
DIM-C-pPhOH could be a new therapeutic agent targeting both
osteoporosis and obesity. Nevertheless, further research is needed
to confirm this hypothesis.
The present study examined the effects of
NR4A1 up- and downregulation of genes in BMD-MSCs. The IPA
analysis indicated the presence of multiple interconnected pathways
such as Notch signaling, Sonic Hedgehog Signaling, Gαs Signaling
and Gαq Signaling (Data not shown). Of all the pathways, the Notch
pathway exhibited contrasting effects on adipogenesis and
osteoblastogenesis, which aligned with the present study. Notch
signaling has been speculated to interact with Wingless-type MMTV
integration site family or bone morphogenetic protein pathways
directly or indirectly in osteoblasts, osteocytes, and osteoclasts,
regulating skeletal tissue development (32). The commitment of MSCs to the
osteoblastic lineage is inhibited by Notch1, which suppresses the
transactivation activity of Runt-related transcription factor 2
(Runx2) (33) and Notch2
inactivation, specifically in osteoblasts (Notch2fl/fl/Runx2-Cre),
leads to increased trabecular bone formation and enhances
osteogenic capacity, underscoring Notch2 as a key inhibitor of
osteoblast differentiation (34).
Mesenchymal progenitor cell proliferation and differentiation are
controlled by Notch signaling, which is dependent on the
recombination signal binding protein-J (35). When expressed in immature
osteoblasts, Notch inhibits their development, resulting in
osteopenia. By contrast, Notch expression in osteocytes initially
inhibits bone resorption and increases bone volume in mice
(36). Notch also regulates the
expression of transcription factors triggered by fatty acids and is
essential for adipogenesis (37).
Notch decreases the levels of Hes-1, which is necessary for the
initial phase of adipogenesis (38). Notch signaling increases osteogenic
differentiation but inhibits adipogenesis in primary human MSCs
(39). Taken together, Notch
signaling inhibits adipogenesis by decreasing the expression of
adipogenic transcription factors such as PPARγ and C/EBPα,
particularly through the activation of Notch1 and Jagged1 (38,39).
In the present study, IPA showed that MAML3 expression was
significantly reduced in NR4A1-knocked down BMD-MSCs.
Furthermore, RT-qPCR findings showed most similar patterns of
alterations in the expression of MAML3 and other genes
related to Notch signaling. MAML3 is a member of the Notch
signaling pathway, which is conserved throughout metazoans and
essential for cell proliferation, differentiation and death
(40). Human MAML3 stabilizes the
DNA-binding complex of RBP-J/CBF-1 protein and the Notch
intracellular domains, which act as signaling intermediates
(41). RBP represses coactivation
by NF-kB and another cellular transcription factor, C/EBP-b
(42). These observations are
consistent with the literature and the present study, where Notch
signaling promotes bone-forming cells (osteoblasts) but inhibits
fat cell formation (adipocytes). This dual functionality
underscores the significant role of Notch in determining cell fate
within the MSC population.
In a previous study, NR4A1 was suggested to
be important for osteoporosis and adipogenesis (7). The present study found that
osteoblastogenesis was increased in the NR4A1 knockdown
group and decreased in the NR4A1 overexpression group.
Significantly increased adipogenesis was observed in the
NR4A1 overexpression group, whereas decreased adipogenesis
was observed in the NR4A1 knockdown group. Thus, the
anticipated functions of NR4A1 in human BMD-MSCs were confirmed in
our experiments. According to the RNA-seq and IPA that compared
gene expression in control, siNR4A1-treated, and NR4A1
overexpressing cells, Notch signaling was expected to be the common
pathway of NR4A1, related to both osteoblastogenesis and
adipogenesis. Numerous studies have linked Notch signaling to
osteogenesis or adipogenesis in stem cells (33,35).
In this study, analysis of the expression of associated Notch
signaling genes using real-time PCR revealed similar alterations in
the expression of several genes, including MAML3, as shown
by the IPA data. Various scientific investigations have established
that the NR4A family exhibits interactive regulation of comparable
target genes. For instance, Philips et al (43) and Carpentier et al (44) demonstrate that NR4A1 and
NR4A2 (Nurr1) could potentially interact with adipogenic
signaling pathways, such as Wnt pathways and glucocorticoid
receptors. The expression of NR4A1 and NR4A3 is
concomitantly reduced in cases of myelodysplastic syndromes
(45). Hence, there is a plausible
hypothesis that NR4A1 may indirectly associate with the
NOTCH pathway via NR4A2 and NR4A3. Nevertheless, the
present study suggested that altering expression of NR4A1 did not
result in notable changes in the expression levels of NR4A2
or NR4A3. Therefore, in human BMD-MSCs, NR4A1 may regulates
osteoblastogenesis and adipogenesis via MAML3, a component of Notch
signaling. The modulation of Notch signaling in mice, specifically
targeting adipose tissue, leads to the induction of browning in
white adipose tissue. This process promotes increased energy
expenditure, enhances metabolic parameters and confers resistance
to obesity (46). Brown adipose
tissue (BAT) serves as an energy reservoir and plays a role in
thermogenesis, leading to increased caloric expenditure (47). A notable upregulation of
NR4A1 in BAT has been reported (48). Hence, an association between NR4A1
and beige adipocytes is possible. However, the precise mechanisms
underpinning the relationship between Notch and NR4A1 should be
investigated in subsequent studies.
In the present study, the Nr4a1 (NR4A1)
overexpressing group showed more noticeable changes, especially in
BMD-MSCs. However, an analysis of IPA showed that only a few genes
had changes in expression linked to the Notch signaling pathway in
the group that had increased NR4A1 levels, which could be
attributed to several factors. Typically, a P-value ≤0.05 is used
for DEG analysis, whereas in the present study, DEGs were evaluated
using a stricter P-value threshold (P<0.01), identifying a
limited number of genes associated with the differences in gene
expression patterns in cells undergoing genetic manipulation. This
is evident from the identification of additional genes using a
lenient P-value ≤0.05 (Fig. S6).
The most represented processes, including canonical pathways,
networks, upstream regulators, illnesses and biological functions,
were listed by IPA following enrichment analysis. These findings
suggested that it is essential to make subjective decisions about
which data to use and how to integrate them for the required output
(49). Even though the present
study chose to focus on the Notch pathway, which encourages the
formation of fat cells and hinders the formation of bone cells, the
chance of another pathway, which was not thoroughly investigated,
cannot be entirely dismissed. Therefore, further investigation is
required.
In conclusion, NR4A1 has a negative role in
osteoblastogenesis and a positive role in adipogenesis in MSCs. In
addition, Nr4a1 may affect the progression of osteoporosis and
adipogenesis via the Notch signaling pathway (Fig. 5). Additional in vivo studies
are needed to elucidate the role of NR4A1 in osteoblastogenesis and
adipogenesis in MSCs.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the National
Research Foundation, Korea (grant nos. NRF-2019R1F1A1063188 and
NRF-2022R1C1C1006818), and Ajou University Medical Center, Korea
(grant no. 2023-C0460-00098).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article. The datasets
generated or analyzed during the current study are available in the
NCBI SRA database repository [BioProject: PRJNA941109; http://www.ncbi.nlm.nih.gov/bioproject/?term=(PRJNA941109)%20AND%20bioproject_sra[filter]].
Authors' contributions
Conceptualization was by YJ, YS, IS, YSC and YJC.
Methodology was by YJ, YS, and YJC. Validation was by YJ and YJC.
Formal analysis was by YJ, YS and YJC. Investigation was by YJ.
Data curation, original draft preparation and review and editing
was by YJ. Reviewing and editing was by YJC. Visualization was by
YJ. Supervision and project administration were by YJC. YJC and YJ
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rosen CJ and Klibanski A: Bone, fat, and
body composition: Evolving concepts in the pathogenesis of
osteoporosis. Am J Med. 122:409–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kelly OJ, Gilman JC, Kim Y and Ilich JZ:
Long-chain polyunsaturated fatty acids may mutually benefit both
obesity and osteoporosis. Nutr Res. 33:521–533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maurin AC, Chavassieux PM, Vericel E and
Meunier PJ: Role of polyunsaturated fatty acids in the inhibitory
effect of human adipocytes on osteoblastic proliferation. Bone.
31:260–266. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sheu Y, Amati F, Schwartz AV, Danielson
ME, Li X, Boudreau R and Cauley JA; Osteoporotic Fractures in Men
(MrOS) Research Group, : Vertebral bone marrow fat, bone mineral
density and diabetes: The Osteoporotic Fractures in Men (MrOS)
study. Bone. 97:299–305. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scheller EL, Doucette CR, Learman BS,
Cawthorn WP, Khandaker S, Schell B, Wu B, Ding SY, Bredella MA,
Fazeli PK, et al: Region-specific variation in the properties of
skeletal adipocytes reveals regulated and constitutive marrow
adipose tissues. Nat Commun. 6:78082015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bredella MA, Fazeli PK, Miller KK, Misra
M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ and Klibanski A:
Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol
Metab. 94:2129–2136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi YJ, Song I, Jin Y, Jin HS, Ji HM,
Jeong SY, Won YY and Chung YS: Transcriptional profiling of human
femoral mesenchymal stem cells in osteoporosis and its association
with adipogenesis. Gene. 632:7–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Veum VL, Dankel SN, Gjerde J, Nielsen HJ,
Solsvik MH, Haugen C, Christensen BJ, Hoang T, Fadnes DJ, Busch C,
et al: The nuclear receptors NUR77, NURR1 and NOR1 in obesity and
during fat loss. Int J Obes (Lond). 36:1195–1202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pearen MA and Muscat GE: Minireview:
Nuclear hormone receptor 4A signaling: Implications for metabolic
disease. Mol Endocrinol. 24:1891–1903. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Y and Bruemmer D: NR4A orphan nuclear
receptors: Transcriptional regulators of gene expression in
metabolism and vascular biology. Arterioscler Thromb Vasc Biol.
30:1535–1541. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tetradis S, Bezouglaia O and Tsingotjidou
A: Parathyroid hormone induces expression of the nuclear orphan
receptor Nurr1 in bone cells. Endocrinology. 142:663–670. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tetradis S, Bezouglaia O, Tsingotjidou A
and Vila A: Regulation of the nuclear orphan receptor Nur77 in bone
by parathyroid hormone. Biochem Biophys Res Commun. 281:913–916.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pirih FQ, Nervina JM, Pham L, Aghaloo T
and Tetradis S: Parathyroid hormone induces the nuclear orphan
receptor NOR-1 in osteoblasts. Biochem Biophys Res Commun.
306:144–150. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rajalin AM and Aarnisalo P: Cross-talk
between NR4A orphan nuclear receptors and β-catenin signaling
pathway in osteoblasts. Arch Biochem Biophys. 509:44–51. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee SO, Abdelrahim M, Yoon K,
Chintharlapalli S, Papineni S, Kim K, Wang H and Safe S:
Inactivation of the orphan nuclear receptor TR3/Nur77 inhibits
pancreatic cancer cell and tumor growth. Cancer Res. 70:6824–6836.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martin M: Cutadapt removes adapter
sequences from high-throughput sequencing reads. EMBnet J.
17:10–12. 2011. View Article : Google Scholar
|
|
18
|
Dobin A, Davis CA, Schlesinger F, Drenkow
J, Zaleski C, Jha S, Batut P, Chaisson M and Gingeras TR: STAR:
Ultrafast universal RNA-seq aligner. Bioinformatics. 29:15–21.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu Y, Luo L, Luo N, Zhu X and Garvey WT:
NR4A orphan nuclear receptors modulate insulin action and the
glucose transport system: Potential role in insulin resistance. J
Biol Chem. 282:31525–31533. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagai S, Ikeda K, Horie-Inoue K, Takeda S
and Inoue S: Estrogen signaling increases nuclear receptor
subfamily 4 group A member 1 expression and energy production in
skeletal muscle cells. Endocr J. 65:1209–1218. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qin DD, Yang YF, Pu ZQ, Liu D, Yu C, Gao
P, Chen JC, Zong C, Zhang YC, Li X, et al: NR4A1 retards adipocyte
differentiation or maturation via enhancing GATA2 and p53
expression. J Cell Mol Med. 22:4709–4720. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chao LC, Bensinger SJ, Villanueva CJ,
Wroblewski K and Tontonoz P: Inhibition of adipocyte
differentiation by Nur77, Nurr1, and Nor1. Mol Endocrinol.
22:2596–2608. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martínez-González J, Rius J, Castelló A,
Cases-Langhoff C and Badimon L: Neuron-derived orphan receptor-1
(NOR-1) modulates vascular smooth muscle cell proliferation. Circ
Res. 92:96–103. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chong ZX, Yeap SK and Ho WY: Transfection
types, methods and strategies: A technical review. PeerJ.
9:e111652021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fumoto T, Yamaguchi T, Hirose F and Osumi
T: Orphan nuclear receptor Nur77 accelerates the initial phase of
adipocyte differentiation in 3T3-L1 cells by promoting mitotic
clonal expansion. J Biochem. 141:181–192. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Scholtysek C, Ipseiz N, Böhm C,
Krishnacoumar B, Stenzel M, Czerwinski T, Palumbo-Zerr K, Rothe T,
Weidner D, Klej A, et al: NR4A1 regulates motility of osteoclast
precursors and serves as target for the modulation of systemic bone
turnover. J Bone Miner Res. 33:2035–2047. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pirih FQ, Aghaloo TL, Bezouglaia O,
Nervina JM and Tetradis S: Parathyroid hormone induces the NR4A
family of nuclear orphan receptors in vivo. Biochem Biophys
Res Commun. 332:494–503. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee SO, Andey T, Jin UH, Kim K, Singh M
and Safe S: The nuclear receptor TR3 regulates mTORC1 signaling in
lung cancer cells expressing wild-type p53. Oncogene. 31:3265–3276.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee SO, Li X, Hedrick E, Jin UH, Tjalkens
RB, Backos DS, Li L, Zhang Y, Wu Q and Safe S: Diindolylmethane
analogs bind NR4A1 and are NR4A1 antagonists in colon cancer cells.
Mol Endocrinol. 28:1729–1739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hedrick E, Lee SO, Kim G, Abdelrahim M,
Jin UH, Safe S and Abudayyeh A: Nuclear receptor 4A1 (NR4A1) as a
drug target for renal cell adenocarcinoma. PLoS One.
10:e01283082015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hedrick E, Lee SO, Doddapaneni R, Singh M
and Safe S: NR4A1 antagonists inhibit β1-integrin-dependent breast
cancer cell migration. Mol Cell Biol. 36:1383–1394. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Regan J and Long F: Notch signaling and
bone remodeling. Curr Osteoporos Rep. 11:126–129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Engin F, Yao Z, Yang T, Zhou G, Bertin T,
Jiang MM, Chen Y, Wang L, Zheng H, Sutton RE, et al: Dimorphic
effects of Notch signaling in bone homeostasis. Nat Med.
14:299–305. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yorgan T, Vollersen N, Riedel C, Jeschke
A, Peters S, Busse B, Amling M and Schinke T: Notch2 inactivation
specifically in osteoblasts (Notch2fl/fl/Runx2-Cre) leads to
increased trabecular bone formation and enhanced osteogenic
capacity, underscoring Notch2 as a key inhibitor of osteoblast
differentiation. Bone. 87:136–146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dong Y, Jesse AM, Kohn A, Gunnell LM,
Honjo T, Zuscik MJ, O'Keefe RJ and Hilton MJ: RBPjkappa-dependent
Notch signaling regulates mesenchymal progenitor cell proliferation
and differentiation during skeletal development. Development.
137:1461–1471. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Canalis E, Parker K, Feng JQ and Zanotti
S: Osteoblast lineage-specific effects of notch activation in the
skeleton. Endocrinology. 154:623–634. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Garcés C, Ruiz-Hidalgo MJ, Font de Mora
JF, Park C, Miele L, Goldstein J, Bonvini E, Porrás A and Laborda
J: Notch-1 controls the expression of fatty acid-activated
transcription factors and is required for adipogenesis. J Biol
Chem. 272:29729–29734. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ross DA, Rao PK and Kadesch T: Dual roles
for the Notch target gene Hes-1 in the differentiation of 3T3-L1
preadipocytes. Mol Cell Biol. 24:3505–3513. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ugarte F, Ryser M, Thieme S, Fierro FA,
Navratiel K, Bornhäuser M and Brenner S: Notch signaling enhances
osteogenic differentiation while inhibiting adipogenesis in primary
human bone marrow stromal cells. Exp Hematol. 37:867–875.e1. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang X, Bledsoe KL, Graham RP, Asmann YW,
Viswanatha DS, Lewis JE, Lewis JT, Chou MM, Yaszemski MJ, Jen J, et
al: Recurrent PAX3-MAML3 fusion in biphenotypic sinonasal sarcoma.
Nat Genet. 46:666–668. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lin SE, Oyama T, Nagase T, Harigaya K and
Kitagawa M: Identification of new human mastermind proteins defines
a family that consists of positive regulators for notch signaling.
J Biol Chem. 277:50612–50620. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kannabiran C, Zeng X and Vales LD: The
mammalian transcriptional repressor RBP (CBF1) regulates
interleukin-6 gene expression. Mol Cell Biol. 17:1–9. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Philips A, Maira M, Mullick A, Chamberland
M, Lesage S, Hugo P and Drouin J: Antagonism between Nur77 and
glucocorticoid receptor for control of transcription. Mol Cell
Biol. 17:5952–5959. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Carpentier R, Sacchetti P, Ségard P,
Staels B and Lefebvre P: The glucocorticoid receptor is a
co-regulator of the orphan nuclear receptor Nurr1. J Neurochem.
104:777–789. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mullican SE, Zhang S, Konopleva M, Ruvolo
V, Andreeff M, Milbrandt J and Conneely OM: Abrogation of nuclear
receptors Nr4a3 and Nr4a1 leads to development of acute myeloid
leukemia. Nat Med. 13:730–735. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bi P, Shan T, Liu W, Yue F, Yang X, Liang
XR, Wang J, Li J, Carlesso N, Liu X, et al: Inhibition of Notch
signaling promotes browning of white adipose tissue and ameliorates
obesity. Nat Med. 20:911–918. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gaspar RC, Pauli JR, Shulman GI and Muñoz
VR: An update on brown adipose tissue biology: A discussion of
recent findings. Am J Physiol Endocrinol Metab. 320:E488–E495.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hampton M, Melvin RG and Andrews MT:
Transcriptomic analysis of brown adipose tissue across the
physiological extremes of natural hibernation. PLoS One.
8:e851572013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cirillo E, Parnell LD and Evelo CT: A
review of pathway-based analysis tools that visualize genetic
variants. Front Genet. 8:1742017. View Article : Google Scholar : PubMed/NCBI
|