Introduction
As one of the most common complications of diabetes,
DPN refers to a condition in which patients with diabetes
experience symptoms related to peripheral nerve dysfunction after
other causes have been excluded (1). The symptoms of DPN primarily include
abnormal sensations and pain in the extremities and increase the
risk of amputation (2). Even
strict blood sugar control can only reduce the incidence of DPN in
type 1 diabetes, with little effect on type 2 diabetes (3). Currently, the main clinical approach
for managing DPN is controlling blood sugar to alleviate symptoms,
with few drugs available that specifically protect nerves (4). Therefore, finding new drugs and
treatments is crucial.
Melittin (MLT) is a basic 26-amino acid polypeptide,
making up 40–60% of dried bee venom (5). With advances in medical technology,
MLT is now purified from bee venom, removing histamine and other
harmful substances, which markedly reduces allergic reactions and
toxic side effects, thus enhancing its clinical safety. Recent
research has confirmed that MLT possesses anti-inflammatory
properties, which can alleviate symptoms of dermatitis by
inhibiting T cell-mediated inflammatory responses in mouse models
(6). Additionally, several studies
have demonstrated that MLT can reduce pain and promote nerve repair
(7,8). It was hypothesized that MLT could
also facilitate the repair of damaged nerves in DPN through these
mechanisms, potentially improving neuropathy. The present study
investigated the effects of MLT on the biological functions of SCs
and identified the potential molecular mechanisms through which MLT
enhances SC proliferation using TMT proteomic analysis, offering a
novel approach for DPN treatment.
Materials and methods
Cell culture and drug purchase
RSC96 cells were obtained from Wuhan Punosai Life
Technology Co., Ltd. These cells were cultured in DMEM (Gibco;
Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum
(Wuhan Punosai Life Technology Co., Ltd.) and maintained in a cell
incubator at 37°C with 5% CO2. MLT was sourced from
Selleck Chemicals.
Determination of glucose and MLT
concentration
RSC96 cells were seeded into a 96-well plate at a
density of 3×103 cells per well. The glucose
concentrations used were 2.8, 5.6, 7.0, 11.1, 16.9, 25, 33.3 and 50
mmol/l and the MLT concentrations were set at 0.05, 0.1, 0.2, 0.4,
0.8, 1.6 and 3.2 µg/ml. After 24, 48, and 72 h of treatment, 10 µl
of CCK-8 reagent was added to each well, and the plates were
incubated at 37°C in a 5% CO2 incubator for 1.5 h. The
OD values at 450 nm were measured using a TriStar LB 941
multifunctional plate reader and cell viability was determined
using a CCK-8 kit. Cell viability was calculated using the formula:
[(As-Ab)/(Ac-Ab)] ×100%, where As is the absorbance of the
experimental well, Ab is the absorbance of the blank well and Ac is
the absorbance of the control well.
Effect of MLT on SC cell activity in
high glucose environment as detected by CCK-8
Experimental cells were divided into six groups: i)
blank control group (NC); ii) high glucose model group (MC); iii)
MLT low dose group [0.1 µg/ml, MLT(L) group]; iv) MLT medium dose
group [0.2 µg/ml, MLT(M) group]; v) MLT high dose group [0.4 µg/ml,
MLT(H) group]; and vi) positive control group (mecobalamin, MEC
group). The experimental procedure involved adding 3×103
cells per well/100 µl into 96-well plates and incubating them at
37°C with 5% CO2. After cell adhesion, the experimental
and model groups were treated with high glucose for 24 h. The cells
were then treated according to the aforementioned groups for 48 h,
with five replicates per group. After the treatment period, 10 µl
of CCK-8 reagent was added to each well, and the plates were
incubated for 1.5 h. The OD values at 450 nm were measured using a
plate reader, and the results were graphically represented using
GraphPad Prism 8.0 software (Dotmatics).
Effects of MLT on SC cell cycle and
apoptosis in high glucose environment analyzed by flow
cytometry
The test model was established as described in
Effect of MLT on SC cell activity in high glucose environment as
detected by CCK-8. Cells were washed three times with PBS, and
the cell concentration was adjusted to 1×105/ml. A 1 ml
single-cell suspension was collected, centrifuged (112 × g; 3 min;
room temperature) and the supernatant was removed. The cells were
fixed with 500 µl of cold 70% ethanol overnight and stored at 4°C.
Prior to staining, the fixing solution was washed off with PBS, the
cell suspension was filtered through a 200-mesh screen and then the
pre-prepared PI/RNase A staining working solution was added.
Staining was conducted at room temperature, protected from light,
for 30–60 min. Finally, the red fluorescence at an excitation
wavelength of 488 nm was recorded for cell cycle analysis (ModFit
LT, v5.0.9; Verity Software House) through flow cytometry using
Invitrogen Attune NxT (Thermo Fisher Scientific, Inc.).
Cells were then treated with 5 µl of Annexin V-FITC,
followed by 5 µl of propidium iodide, mixing each time. The
reaction was allowed to proceed for 5–10 min at room temperature,
protected from light. Following this, the cells in all groups were
digested with trypsin without EDTA, washed twice with PBS, and
apoptosis was detected within 1 h. Annexin V-FITC (Ex=488 nm,
Em=530 nm) green fluorescence by FITC Channel (FL1) Detection; PI
red fluorescence (Ex=488 nm, Em ≥630 nm) was detected by PI
channels (FL2 or FL3). Then FlowJo (v10.8.1; FlowJo LLC) was used
for data analysis. After flow cytometry data is imported into
FlowJo, the software will directly calculate the proportion of
normal cells (live cells), early apoptotic cells, late apoptotic
cells and mechanically damaged cells in the total number of cells,
so as to calculate the apoptosis rate of cells in each group (early
+ late apoptotic cells).
Proteomics combined with liquid
chromatography-mass spectrometry analysis of protein expression in
Schwann cells treated with MLT in high glucose environment
After 24 h of culture in a high glucose environment
(25 mmol/l), RSC96 cells were treated with MLT (0.2 mg/ml) in the
experimental group, while the control group was treated with high
glucose medium only. After 48 h, proteins were extracted from both
groups and analyzed using 4D label-free quantitative proteomics,
with differences in protein expression identified using liquid
chromatography-mass spectrometry (LC-MS). The process is divided
into two parts: pre-experiment and formal experiment:
Pre-experiment includes protein extraction, protein quantification,
SDS-PAGE, protein enzymatic hydrolysis steps; The formal experiment
is carried out on the basis of the pre-experiment and the samples
qualified for quality control in the pre-experiment are formally
tested by using high resolution mass spectrometer to obtain the
original mass spectrum data. Samples were analyzed on a nanoElute
(Bruker, Bremen, Germany) coupled to a timsTOF Pro (Bruker, Bremen,
Germany) equipped with a CaptiveSpray source. The timsTOF Pro was
operated in PASEF mode. Mass Range 100 to 1,700 m/z, 1/K0 start
0.75 V·s/cm2 end 1.4 V·s/cm2, ramp time 100
msec, Lock Duty Cycle to 100%, Capillary Voltage 1,500V, Dry Gas 3
l/min, Dry Temp 180°C, PASEF settings: 10 MS/MS scans (total cycle
time 1.16 sec), charge range 0–5, active exclusion for 0.5 min,
Scheduling Target intensity 10,000, Intensity threshold 2,500, CID
collision energy 20–59 eV.
Bioinformatics analysis
The raw files obtained from mass spectrometry were
analyzed using database search software MaxQuant (1.6.17.0;
Max-Planck-Institute of Biochemistry). Quality control assessments
of peptide and protein levels were performed based on these search
results, focusing on the repeatability of sample quantitation
(Pearson correlation). Differential screening was then conducted
based on the quantitative data, and statistical graphs illustrating
these differences were generated. Differential proteins were
functionally classified, including Gene Ontology (GO) secondary
classification, Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway analysis and domain annotation (9). Fisher's exact test was used for
enrichment analysis, subcellular localization (WoLF PSORT,
http://wolfpsort.hgc.jp/) and transcription
factor analysis (AnimalTFDB 3.0, http://bioinfo.life.hust.edu.cn/AnimalTFDB4/) of these
proteins, as well as interaction network analysis for proteins
showing significant variations (String; http://www.string-db.org/).
mRNA expression of related proteins as
verified by reverse transcription-quantitative (RT-q) PCR
Total RNA was extracted and purified using the
Monzol Reagent Pro (cat. no. MI20201S; Mona Biotechnology Co.,
Ltd.) kit, and primers were obtained from Wuhan Jinkairui
Biotechnology Co., Ltd. Reverse transcription is then performed
using MonScript RTIII All-in-One Mix with dsDNase. (cat. no.
MR05101; Mona Biotechnology Co., Ltd.). The reverse transcription
reaction conditions were set as follows: Incubation at 37°C for 2
min to remove DNA contamination, followed by incubation at 55°C for
15 min; the reaction was then terminated by heating at 85°C for 5
min. MonAmp SYBR Green qPCR Mix (cat. no. MQ10101; Mona
Biotechnology Co., Ltd.) was used for qPCR; the PCR reaction
conditions included a pre-denaturation step at 95°C for 30 sec,
denaturation at 95°C for 10 sec, annealing and extension at 60°C
for 30 sec, for 40 cycles. β-actin was used as the internal
parameter, and the relative mRNA expression was calculated by
2−ΔΔCq method (10).
The primer gene sequences are shown in Table I.
 | Table I.Primers used for reverse
transcription-quantitative PCR. |
Table I.
Primers used for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5′-3′) | Annealing
temperature, °C |
|---|
| β-actin | Forward:
TGTCACCAACTGGGACGATA | 51.7 |
|
| Reverse:
GGGGTGTTGAAGGTCTCAAA | 51.7 |
| Crabp2 | Forward:
GCCTAACTTTTCTGGCAACT | 49.6 |
|
| Reverse:
CCACAGCAATCTTCCTCAT | 48.8 |
|
β-catenin | Forward:
GGTGAAAATGCTTGGGTCG | 51 |
|
| Reverse:
CTGAAGGCAGTCTGTCGTA | 51 |
| PCNA | Forward:
CTTGGAATCCCAGAACAGG | 51 |
|
| Reverse:
CTCGCAGAAAACTTCACCC | 51 |
| c-Jun | Forward:
TGGGCACATCACCACTACA | 51 |
|
| Reverse:
TGACACTGGGCAGCGTATT | 51 |
| CDK4 | Forward:
ATTGGTGTCGGTGCCTATG | 51 |
|
| Reverse:
TCACGAACTGTGCTGACGG | 49.1 |
|
Cyclind1 | Forward:
GGAGCAGAAGTGCGAAGA | 50.2 |
|
| Reverse:
GGGGCGGATAGAGTTGTC | 52.5 |
Western blotting verification of the
expression of related proteins
Cells from the specified groups were lysed using
ultrasound (20 kHz; 4°C; 1 min) and incubated on ice for 30 min.
Following centrifugation at 12,000 × g at 4°C for 10 min, the
supernatant was collected. Protein concentration was determined
using the BCA method, followed by 10% SDS-PAGE (10 µg protein
loaded per lane) electrophoresis. Proteins were then transferred to
membranes (0.45 µm PVDF; MilliporeSigma) by electrophoresis for 1.5
h and blocked with 5% skimmed milk powder at room temperature for 1
h. The primary antibodies used were cellular retinoic acid binding
protein 2 (Crabp2; cat. no. ab211927; 1:1,000; Abcam), Wnt3a (cat.
no. ab219412; 1:1,000; Abcam), β-catenin (cat. no. ab32572;
1:5,000; Abcam), c-Jun (cat. no. ab40766; 1:5,000; Abcam), CDK4
(cat. no. P24385; 1:1,000; Zen-Bio Inc.), CyclinD1 (cat. no.
P11802; 1:1,000 Zen-Bio Inc.), proliferating cell nuclear antigen
(PCNA; cat. no. R25294; 1:500; Zen-Bio Inc.) and β-Actin (cat. no.
AF7018; 1:5,000; Affinity Biosciences, Ltd.). The membranes were
incubated with the primary antibodies overnight on a shaker at 4°C.
The secondary antibody (cat. no. S0001; 1:1,000; Affinity
Biosciences, Ltd.) was incubated at room temperature for 1 h,
followed by three washes with TBST (0.1% Tween). Protein bands were
visualized using the Yase Omni-ECL (Epizyme Biotech)
ultra-sensitive chemiluminescence kit, and the grey values of each
protein band were analyzed with ImageJ (v1.8.0; National Institutes
of Health).
Statistical analysis
SPSS software (v26.0; IBM Corp.) was used to analyze
the data, with each experiment conducted independently three times.
Measurement data were expressed as mean ± standard deviation. An
unpaired Student's t-test was used for comparisons between two
groups, while one-way analysis of variance was used for comparisons
among multiple groups, with pairwise tests performed for each group
using Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Determination of glucose and MLT
concentration
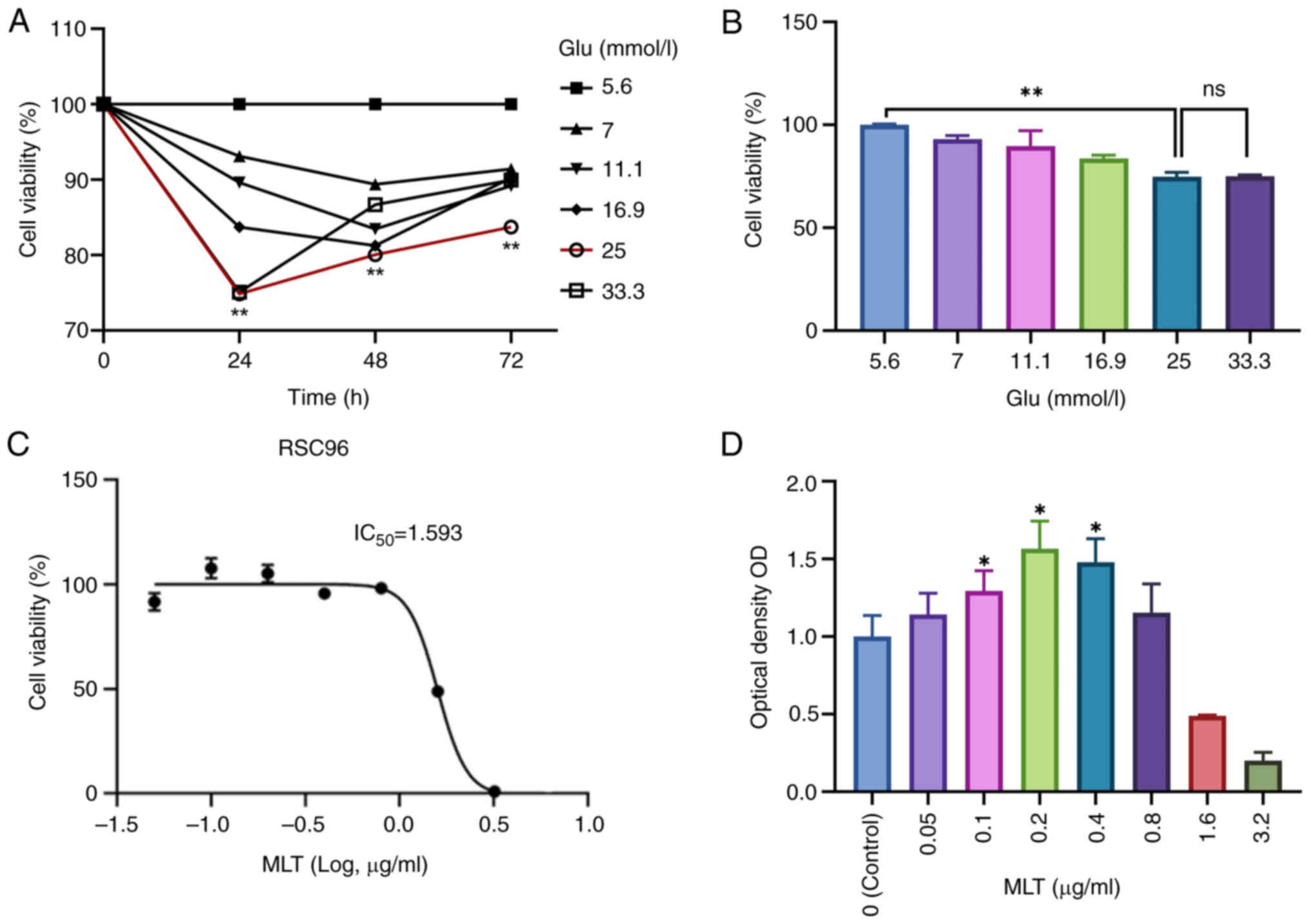
Preliminary results indicated that after 24 h, cell
activity peaked at a glucose concentration of 5.6 mmol/l. At a
glucose concentration of 25 mmol/l, the inhibition rate of cell
activity was highest (Fig. 1A).
Consequently, 5.6 mmol/l glucose was selected for the blank control
group, and 25 mmol/l for the model group (Fig. 1B). The survival rate of SCs cells
under different concentration gradients of MLT was measured by
CCK-8 method, thereby indirectly reflecting the proliferation of
SCs. When the concentration of MLT was 0.2 µg/ml, the cell survival
rate was the highest. The survival rate was only 50% at 1.6 µg/ml,
and even lower at 3.2 µg/ml (Fig.
1D). Therefore, three concentrations of MLT within the
IC50 range were selected for the experimental group, and
cell proliferation factor was detected by western blotting and
immunofluorescence. Compared with the model group, the expression
of cell proliferation factor was increased in the MLT group, so
that MLT within a certain concentration range could promote the
proliferation of SCs in a high-sugar environment. Under high
glucose conditions, when the concentration of MLT was 0.2 µg/l and
the incubation time was 48 h, cell activity reached its maximum,
and the IC50 was calculated (Fig. 1C). Concentrations of 0.1, 0.2, and
0.4 µg/ml were ultimately chosen as the low, medium, and high
concentrations of MLT, respectively (Fig. 1D).
MLT promotes the proliferation of
Schwann cells and inhibits the apoptosis of Schwann cells in high
glucose environment
After 24 h of culturing RSC96 cells in high glucose
conditions, different concentrations of MLT were administered to
each group, and cell activity was measured using the CCK-8 assay
(Fig. 2A). The results showed that
the medium concentration of MLT had the highest cell survival rate.
PCR (Fig. 2B) and western blotting
analysis (Fig. 2C) were then used
to assess the expression of PCNA. Compared with the model group,
PCNA expression in Schwann cells treated with MLT increased,
supporting the CCK-8 findings. In western blotting analysis, the
difference in PCNA expression between the low, medium, and high
concentrations of MLT was not significant, with the MLT(M) showing
slightly higher expression than the other two groups. Flow
cytometry showed that the combined rates of early and late
apoptosis in the MLT group were lower than those in the model group
(Fig. 2D). In summary, MLT
promoted SC proliferation and reduced SC apoptosis in a high
glucose environment. Additionally, the fluorescence intensity of
Ki-67 was measured using immunofluorescence. The fluorescence
intensity of Ki-67 was significantly higher in the control group
than in the model group and it increased further in the MLT(M)
group (Fig. 2E).
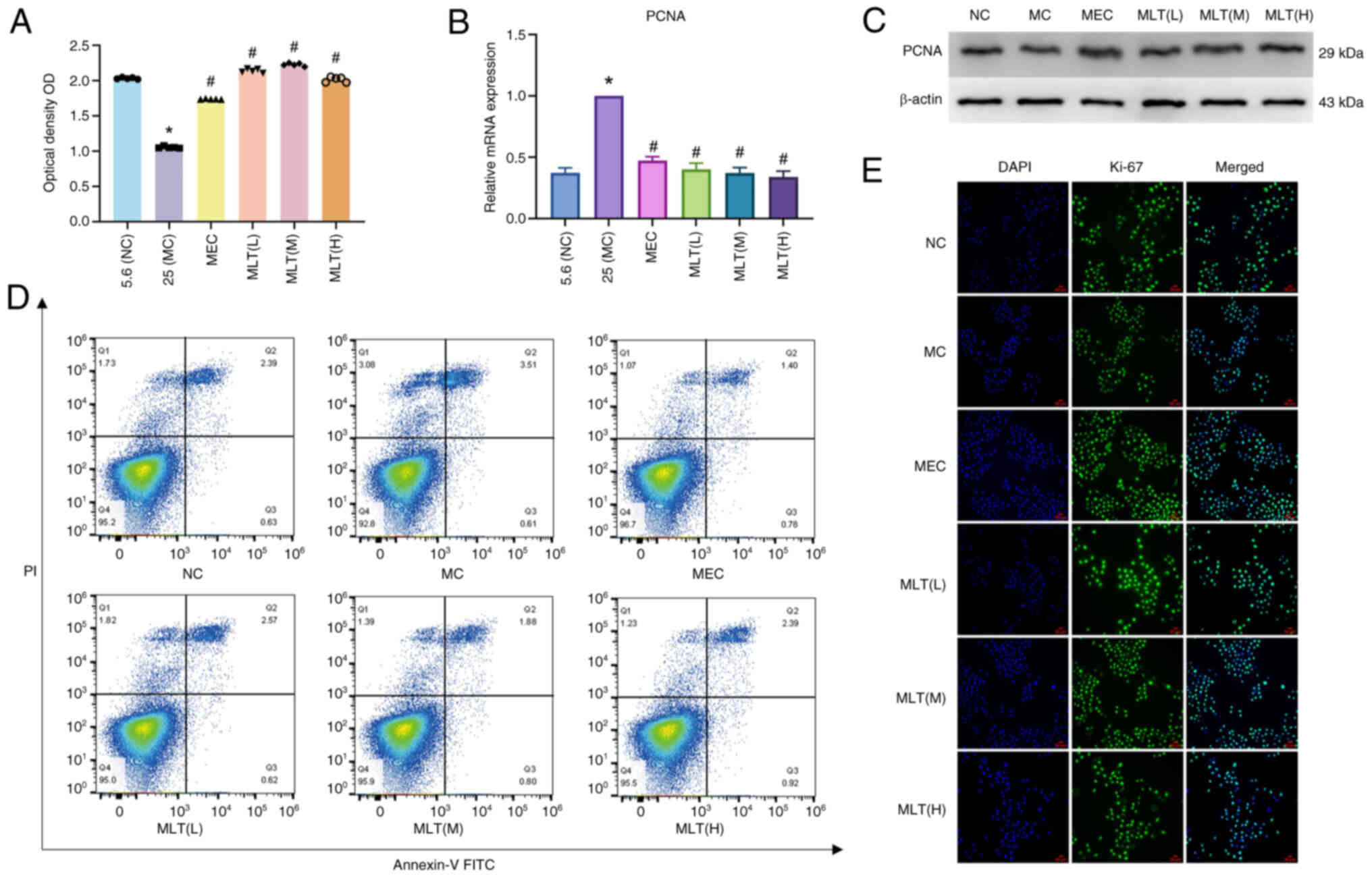 | Figure 2.Effects of MLT on Schwann cell
proliferation and apoptosis. (A) Following MLT treatment, the cell
viability of control group (Glu 5.6 mmol/l), model group (Glu 25
mmol/l), positive control group (mecobalamine) and the low, medium
and high concentrations of MLT for 48 h were determined. (B) The
mRNA expression of PCNA of RSC96 cells in each group). (C) The
protein expression level of PCNA in RSC96 cells in each group; (D
and E) Ki-67 was stained, and the subcellular localization and
expression of Ki-67 in each group were observed by confocal
microscopy (scale bar, 50 µm). *P<0.05 vs. control group;
#P<0.05 vs. model group. MLT, melittin; Glu, glucose;
MEC, mecobalamine; NC, negative control; PCNA, proliferating cell
nuclear antigen; MC, high glucose model group; NC, negative
control. |
Proteomic analysis
Consistency test of mouse Schwann cell protein
samples
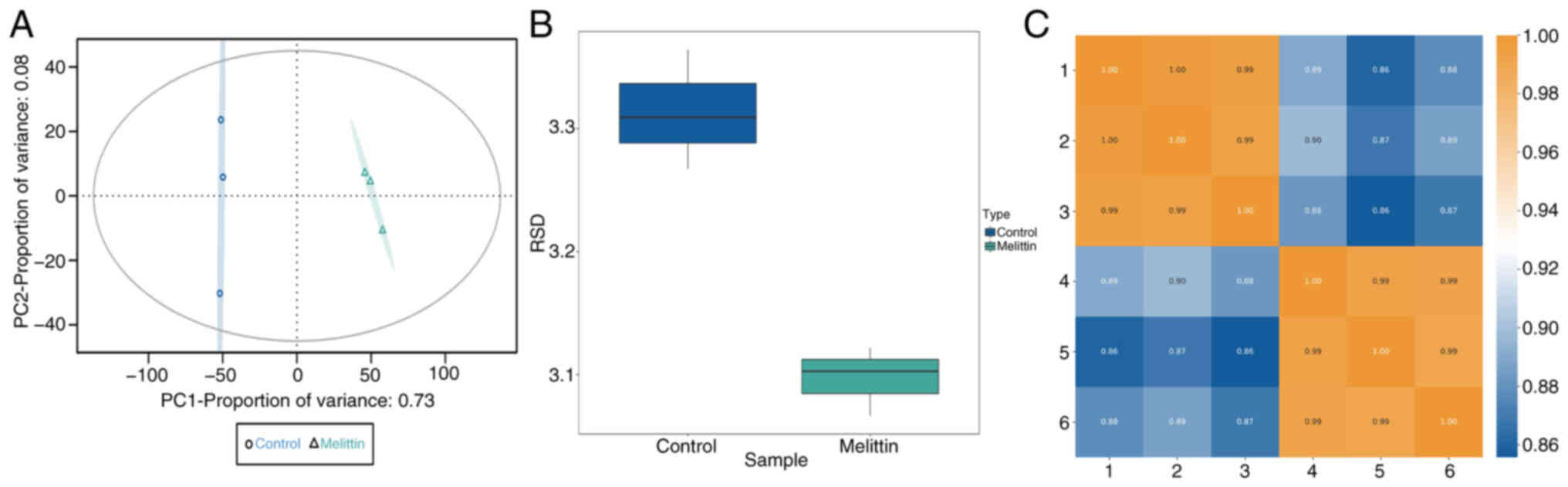
Based on the aforementioned findings, 4D label-free
quantitative proteomic analysis was conducted on MLT-treated and
untreated SCs to generate proteomic data for bioinformatics
analysis. The results from the protein quantitative principal
component analysis indicated high quantitative repeatability among
replicates, with significant differences between the two groups
(Fig. 3A). The Pearson correlation
coefficient between samples was close to 1, indicating excellent
sample repeatability (Fig. 3B).
Additionally, the relative standard deviation of protein
quantitative values between samples was low, confirming the robust
quantitative repeatability of the proteomics data (Fig. 3C).
4D labeling free quantitative
proteomic analysis performed on the groups using proteomic
data
Samples of rat cells were analyzed using
high-throughput, label-free LC-MS/MS. Proteins with a FC greater
than 1.5 were selected, indicating statistically significant
differences in differentially expressed proteins (DEPs) between the
two groups with P<0.05.
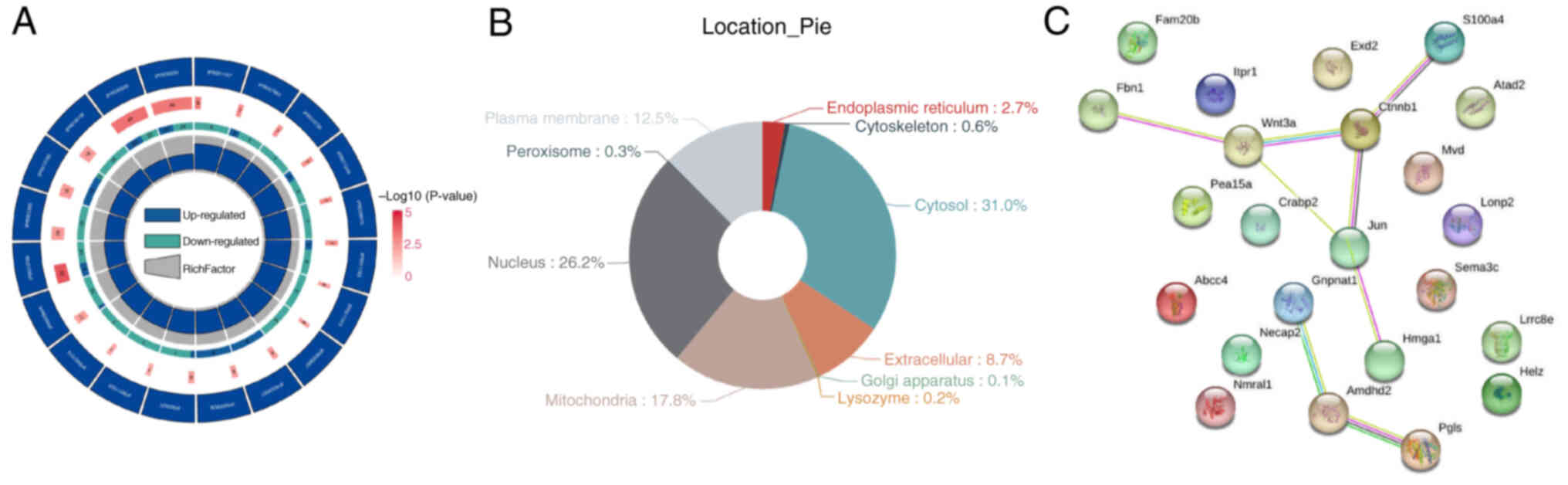
Compared with the control group, the experimental
group showed 1,784 DEPs, with 725 upregulated proteins. These
differences were statistically significant (Fig. 4A). The ten most upregulated
proteins included Crabp2, S100a4, Necap2, Pea15, Nmral1, Pgls,
Gnpnat1, Amdhd2, Helz, MvdCrabp2, S100a4, adaptin ear-binding
coat-associated protein 2, Astrocytic phosphoprotein PEA-15,
NmrA-like family domain-containing protein
1,6-phosphogluconolactonase, glucosamine-phosphate
N-acetyltransferase 1, N-acetylglucosamine-6-phosphate deacetylase,
probable helicase with zinc finger domain and diphosphomevalonate
decarboxylase, while the 10 most downregulated proteins included
high mobility group protein HMG-I/HMG-Y, fibrillin-1, Lon protease
homolog 2 peroxisomal, semaphorin 3C, glycosaminoglycan
xylosylkinase, ATPase family AAA domain containing protein 2,
ATP-binding cassette sub-family C member 4, inositol
1,4,5-trisphosphate receptor type 1, exonuclease 3′-5′
domain-containing protein 2 and volume-regulated anion channel
subunit LRRC8E. GO secondary annotation revealed that most DEPs had
binding and catalytic activities at the molecular functional level.
KEGG pathway analysis indicated that these proteins were involved
in metabolic pathways, pathways related to neurodegeneration in
multiple diseases, Parkinson's disease and Huntington's disease. GO
functional enrichment analysis showed significant trends in enzyme
regulation functions, such as GTP binding and GTPase activity
(Fig. 4B). The top 10 KEGG
pathways were illustrated (Fig.
4C). Protein domains, which are regions with specific
structures and independent functions within proteins, were also
analyzed and depicted in a circular graph (Fig. 5A). Subcellular localization
analysis using WoLF PSORT indicated that most DEPs were primarily
located in the cytoplasm (30%) and nucleus (27.9%) (Fig. 5B). Additionally, direct and
indirect interaction network analyses were conducted on 23
significantly different proteins, with the direct interaction
network plotted (Fig. 5C).
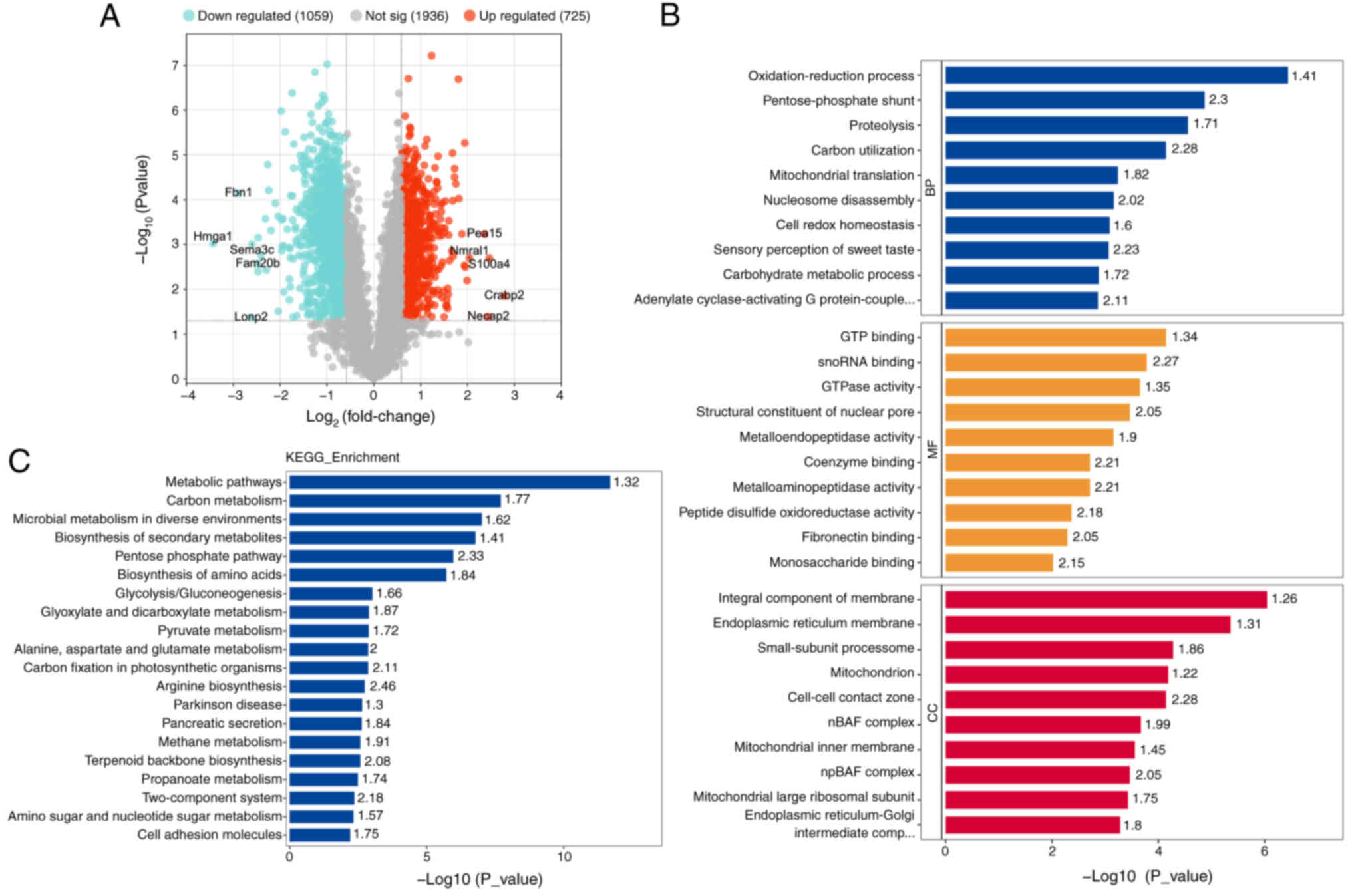 | Figure 4.Differential protein screening and
KEGG, GO enrichment analysis. (A) The results of differential
protein screening were presented in the form of volcano plot. (B)
KEGG Pathway Annotation. (C) GO Annotation. GO, Gene Ontology;
KEGG, Kyoto Encyclopedia of Genes and Genomes; Hmga1, high mobility
group protein HMG-I/HMG-Y; Fbn1, fibrillin-1; Lonp2, Lon protease
homolog 2 peroxisomal; Sema3c, semaphorin 3C; Fam20b,
glycosaminoglycan xylosylkinase; Atad2, ATPase family AAA domain
containing protein 2; Abcc4, ATP-binding cassette sub-family C
member 4; Itpr1, inositol 1,4,5-trisphosphate receptor type 1;
Exd2, exonuclease 3′-5′ domain-containing protein 2; Lrrc8e,
volume-regulated anion channel subunit LRRC8E; Crabp2, cellular
retinoic acid binding protein 2; Necap2, adaptin ear-binding
coat-associated protein 2; Pea15, astrocytic phosphoprotein PEA-15;
Nmral1, NmrA-like family domain-containing protein 1; Pgls,
6-phosphogluconolactonase; Gnpnat1, glucosamine-phosphate
N-acetyltransferase 1; Amdhd2, N-acetylglucosamine-6-phosphate
deacetylase, Helz, probable helicase with zinc finger domain; Mvd,
diphosphomevalonate decarboxylase. |
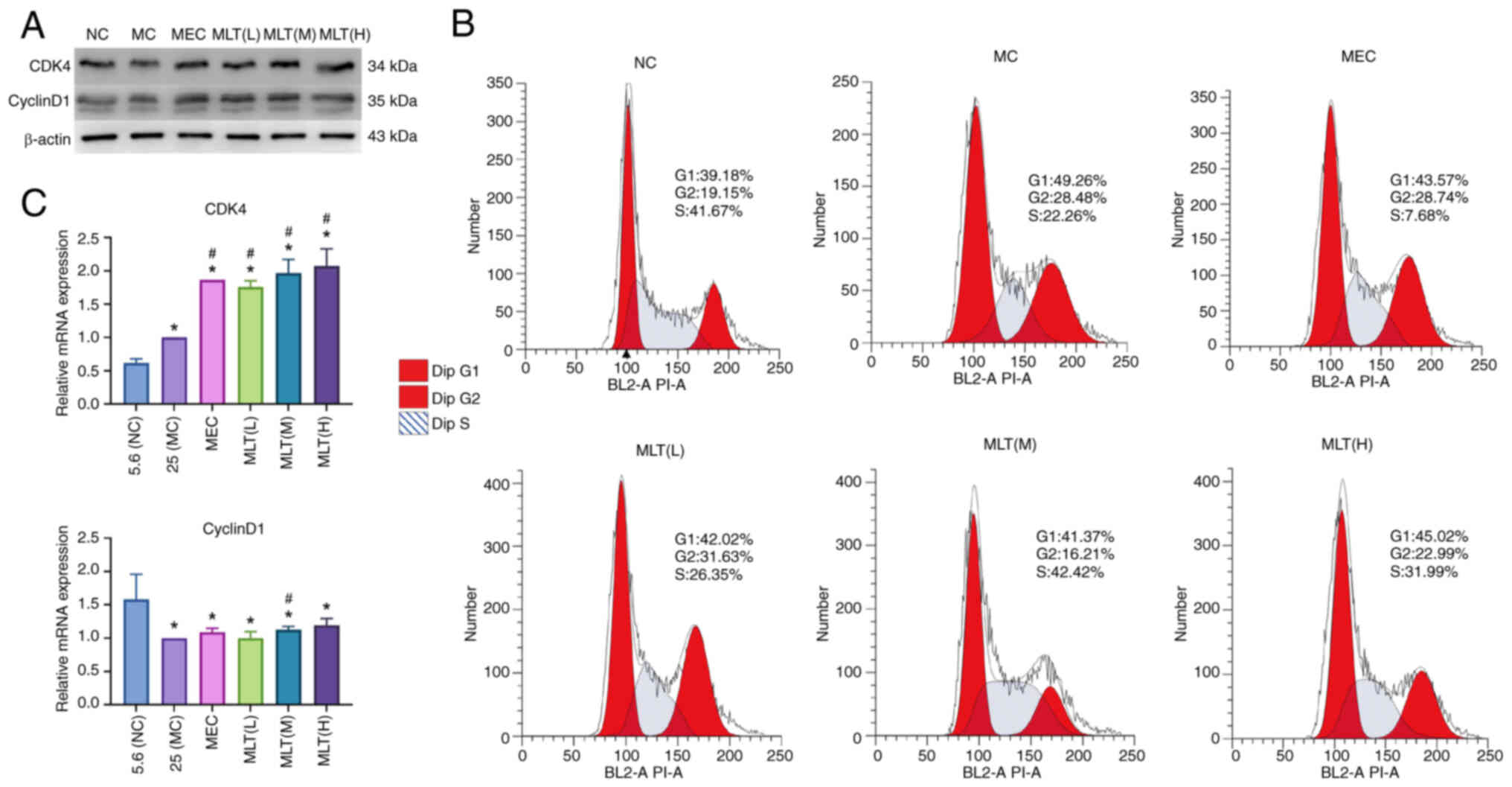
MLT prevents cell stasis by altering
the cell cycle
Western blotting (Fig.
6A) and PCR (Fig. 6C) were
used to detect cell cycle-related factors. Compared with the model
group, the expression of CDK4 and CyclinD1 was increased in the
experimental group. Flow cytometry showed that the
G2/M+S phase was prolonged in the MLT(L), MLT(M) and
MLT(H) groups vs. the model group, with the most pronounced effect
in the MLT(M) group (Fig. 6B).
MLT promotes SCs proliferation through
up-regulation of Crabp2/Wnt/β-catenin signaling pathway
Proteomic analysis revealed that Crabp2 was
significantly upregulated among various proteins. Western blotting
(Fig. 7A), immunofluorescence
(Fig. 7B) and RT-qPCR (Fig. 7C) confirmed the increased
expression of Crabp2 in MLT-treated SCs, particularly in the MLT(M)
group. Crabp2 has been shown to activate the Wnt/β-catenin pathway
(11). PCR and western blotting
were used to detect factors related to the Wnt/β-catenin pathway,
revealing that MLT treatment increased the expression of Wnt3a,
β-catenin, and c-Jun in SCs, thereby activating the Wnt/β-catenin
pathway. The results were consistent across the MLT groups, with no
significant differences observed. Further verification of Crabp2
expression post-MLT treatment using immunofluorescence confirmed
these findings.
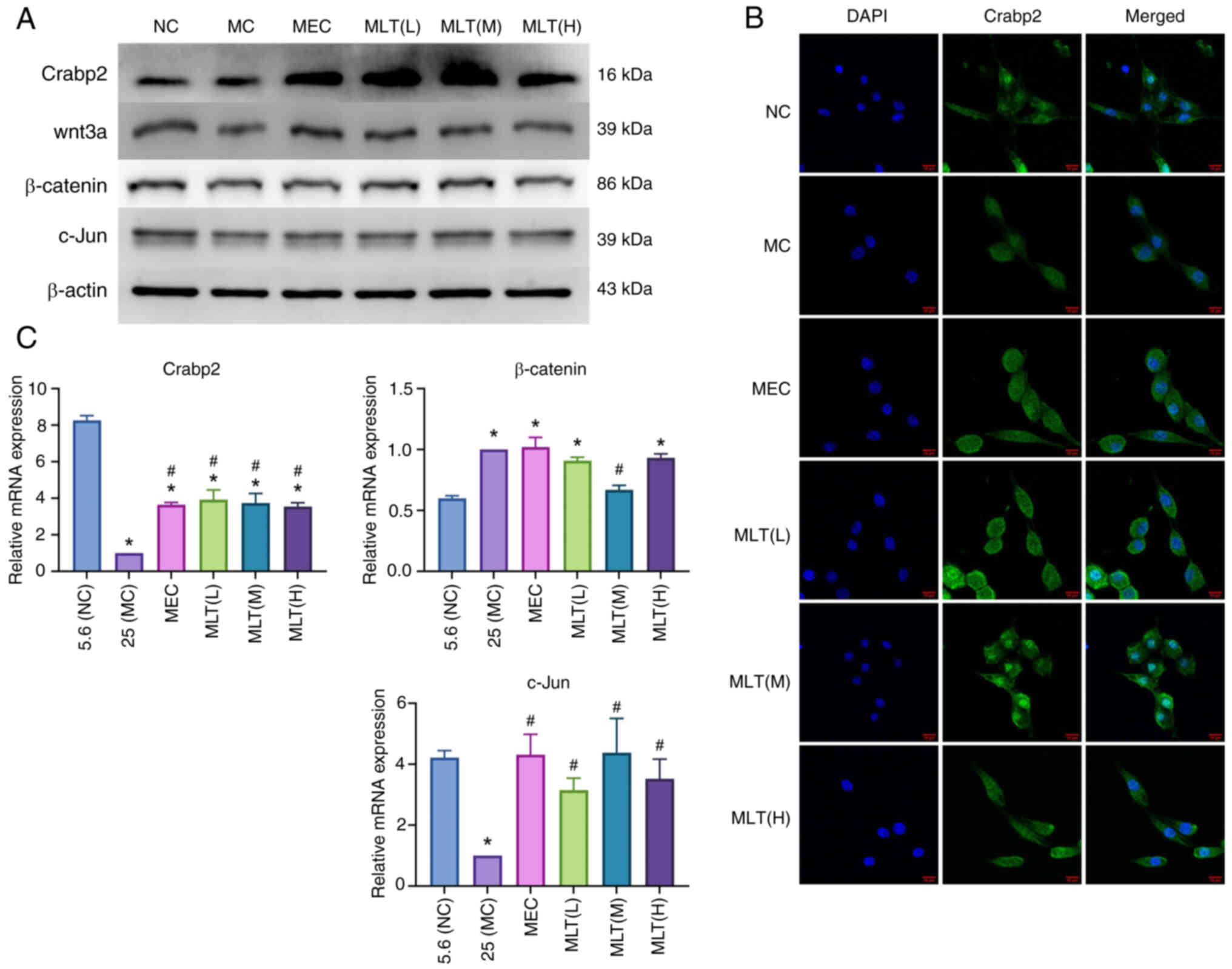 | Figure 7.The mechanism of MLT promoting cell
proliferation. (A) The protein expression level of Crabp2, Wnt3a,
β-catenin and c-Jun in RSC96 cells in each group. (B) The
subcellular localization and expression of Crabp2 in each group
were observed by confocal microscopy, scale bar, 10 µm. (C) The
mRNA expression level of Crabp2, Wnt3a, β-catenin and c-Jun in
RSC96 cells in each group (*P<0.05 vs. control group,
#P<0.05 vs. model group. Crabp2, cellular retinoic
acid; NC, negative control; MC, high glucose model group; MEC,
mecobalamine; MLT, melittin. |
Discussion
Numerous studies have explored diabetic peripheral
neuropathy (DPN), but the precise mechanisms remain unclear.
Previous research suggests links to various metabolic pathways,
including the polyol pathway, hexosamine pathway, advanced
glycosylation end products, oxidative stress and nerve growth
factor (12–14). These factors are important for
understanding the pathological processes in peripheral neurons
under diabetic conditions. Notably, most clinical and basic
research on DPN has focused on the neuronal aspects, viewing
neurons as the primary signal-transmitting elements. However,
extensive data on the development and regeneration of the
peripheral nervous system emphasize the critical role of glial
cells in supporting neuronal structure and function, nourishing
axons (15–17) and aiding in survival and growth
after injury (18).
SCs play a crucial role in nerve regeneration
following peripheral nerve injury. They enhance the repair
capabilities of various tissues, dedifferentiate after injury, and
secrete neurotrophic factors such as Glial cell line-derived
neurotrophic factor (GDNF), nerve growth factor (NGF), brain
derived neurotrophic factor (BDNF) and ciliary neurotrophic factor
(CNTF), which support nerve fiber regeneration (19). SCs also interact with other cells,
such as fibroblasts and macrophages, to clear myelin debris from
damaged nerves, create paths for regeneration and repair nerves
(20,21). Although it remains debated whether
the primary lesion in DPN is demyelination or axon loss, SCs are
now recognized as central to the pathogenesis of DPN (22).
MLT, a water-soluble cationic peptide derived from
bee venom, is widely used in the treatment of various cancers
(23–25). Its anti-inflammatory and analgesic
properties have also been applied to treat peripheral neuropathy
caused by chemotherapy (26–28).
The mechanism involves the activation of intracellular signaling
pathways, promotion of cell proliferation and inhibition of
apoptosis.
The present study first examined cell viability in
response to varying glucose concentrations over different time
periods. It was found that the effect of high glucose
concentrations on cell viability was most pronounced at 25 and 33.3
mmol/l after 24 h, but this effect diminished after 48 and 72 h.
This reduction may be due to nutrient depletion, such as glucose,
during prolonged cell growth, leading to lower glucose levels in
the medium than initially provided. Therefore, the 24-h time point
was chosen as it accurately and efficiently reflects cell vitality
under high glucose conditions. Three concentrations of MLT within
its IC50 range were then selected for the experimental
group. The results showed that MLT promotes SC proliferation and
inhibits apoptosis in vitro, suggesting a potential
therapeutic mechanism for MLT in treating DPN.
To further explore the underlying mechanisms, TMT
labeling quantitative proteomics and bioinformatics analysis were
used. The proteomic analysis identified 1,784 DEPs after MLT
treatment, with 725 upregulated and 1,059 downregulated. GO
annotation and functional enrichment analysis indicated that a
number of these proteins were involved in protein binding, RNA
binding, GTP binding, GTPase activity, and redox processes, all of
which are closely associated with the onset of DPN (29). KEGG pathway analysis suggested that
the effects of MLT might be linked to various metabolic pathways,
including amino sugar and nucleotide sugar metabolism,
neurodegenerative disease pathways, and protein processing in the
endoplasmic reticulum, all of which are important in the
development of DPN (30,31).
Among the identified proteins, Crabp2 was the most
significantly upregulated. Crabp2 belongs to the intracellular
lipid-binding protein family and acts as a shuttle protein between
the cytoplasm and nucleus. It is primarily found in the skin,
uterus, ovary and nerve choroid (32). Crabp2 is closely linked to
neurological disorders (33). It
can activate the Wnt/β-catenin signaling pathway, promoting the
proliferation of Hu sheep dermal papilla cells (DPCs) (11). The Wnt/β-catenin signaling pathway
plays a vital role in the development of nervous system-related
diseases (34,35). While this pathway typically becomes
inactive after embryonic development, it can reactivate in adults
to promote nerve repair after injury (36). For example, injecting Wnt3a into
the vitreous of mice has been shown to facilitate the regeneration
of retinal ganglion cell axons, indicating that Wnt3a activates the
Wnt/β-catenin pathway to aid nerve repair when ganglion cells are
damaged (37). In diabetes,
hyperglycemia can lead to the demyelination of peripheral nerve
fibers, activating the Wnt/β-catenin pathway. However, prolonged
hyperglycemia can result in damage that outpaces repair, leading to
peripheral nerve demyelination and axonal degeneration, which
contribute to DPN (38).
In the present study, MLT treatment of SCs led to a
significant increase in Crabp2 expression and the upregulation of
the Wnt/β-catenin signaling pathway. Inhibition of GSK-3β prevents
the degradation of β-Catenin, allowing it to enter the nucleus and
initiate the transcription of downstream genes, including c-Jun.
c-Jun directly regulates the transcription of CyclinD1, promoting
cell proliferation by facilitating the G1 phase of the
cell cycle (39–41). In the present study, the expression
of the cell cycle-dependent complex CDK4/CyclinD1, essential for
the transition from G1 to S phase, was increased. Flow
cytometry results indicated that MLT treatment significantly
decreased the proportion of cells in the
G0/G1 phase, while increasing the number in
the G2/M and S phases. This shift suggested that MLT
inhibited the transition from proliferation to differentiation,
thereby promoting cell proliferation (42,43).
In summary, MLT may protect cells from hyperglycemic toxicity and
enhance SC proliferation by upregulating Crabp2 expression,
activating the Wnt/β-catenin signaling pathway and shortening the
cell cycle.
Currently, research on MLT therapy is limited both
domestically and internationally, with few studies examining its
role in DPN. The present study introduced the innovative use of
proteomics to analyze the expression of DEPs in SCs treated with
MLT under high-glucose conditions. It explored the mechanism by
which MLT may treat DPN, providing valuable experimental insights
for further research in MLT-related fields and laying a theoretical
foundation for its clinical application in DPN treatment. In the
future, it is possible that MLT could be administered through
acupuncture points or topical applications to alleviate the
symptoms of DPN. However, the present study has limitations due to
its in vitro nature; further animal experiments are
necessary to validate its findings. Additionally, due to financial
constraints, only the RSC96 cell line was used. According to
literature, the RSC96 cell line is commonly used in peripheral
neuropathy research, giving it a degree of representativeness. In
future studies, more cell lines may be included.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Guangxi Natural Science
Foundation project (grant no. 2020JJA140216), Guangxi medical and
health self-financing plan (grant no. Z20170240).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Author's contributions
QZ was responsible conceptualization, writing the
original draft, software and diagrams, reviewing and editing. YC
was responsible for software and formal analysis. WH was
responsible for software, resources, data curation and acquisition
of data. JZ was responsible for software and analysis and
interpretation of data. DY was responsible for conceptualization,
methodology, writing, review and editing, supervision, project
administration and funding acquisition. QZ and DY confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Selvarajah D, Kar D, Khunti K, Davies MJ,
Scott AR, Walker J and Tesfaye S: Diabetic peripheral neuropathy:
Advances in diagnosis and strategies for screening and early
intervention. Lancet Diabetes Endocrinol. 7:938–948. 2019.
View Article : Google Scholar
|
|
2
|
Sun H, Saeedi P, Karuranga S, Pinkepank M,
Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, et
al: IDF diabetes atlas: Global, regional and country-level diabetes
prevalence estimates for 2021 and projections for 2045. Diabetes
Res Clin Pract. 183:1091192022. View Article : Google Scholar
|
|
3
|
Calcutt NA: Diabetic neuropathy and
neuropathic pain: A (con)fusion of pathogenic mechanisms? Pain. 161
(Suppl 1):S65–S86. 2020. View Article : Google Scholar
|
|
4
|
Sloan G, Selvarajah D and Tesfaye S:
Pathogenesis, diagnosis and clinical management of diabetic
sensorimotor peripheral neuropathy. Nat Rev Endocrinol. 17:400–420.
2021. View Article : Google Scholar
|
|
5
|
Memariani H and Memariani M: Melittin as a
promising anti-protozoan peptide: Current knowledge and future
prospects. AMB Express. 11:692021. View Article : Google Scholar
|
|
6
|
Liu Z, Fan Z, Liu J, Wang J, Xu M, Li X,
Xu Y, Lu Y, Han C and Zhang Z: Melittin-carrying nanoparticle
suppress T cell-driven immunity in a murine allergic dermatitis
model. Adv Sci (Weinh). 10:e22041842023. View Article : Google Scholar
|
|
7
|
Choi S, Chae HK, Heo H, Hahm DH, Kim W and
Kim SK: Analgesic effect of melittin on oxaliplatin-induced
peripheral neuropathy in rats. Toxins (Basel). 11:3962019.
View Article : Google Scholar
|
|
8
|
Shaik RA, Alotaibi MF, Nasrullah MZ,
Alrabia MW, Asfour HZ and Abdel-Naim AB: Cordycepin-melittin
nanoconjugate intensifies wound healing efficacy in diabetic rats.
Saudi Pharm J. 31:736–745. 2023. View Article : Google Scholar
|
|
9
|
Dennis G, Sherman BT, Hosack DA, Yang J,
Gao W, Lane HC and Lempicki RA: DAVID: Database for annotation,
visualization, and integrated discovery. Genome Biol. 4:P32003.
View Article : Google Scholar
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
11
|
He M, Lv X, Cao X, Yuan Z, Quan K,
Getachew T, Mwacharo JM, Haile A, Li Y, Wang S and Sun W: CRABP2
promotes the proliferation of dermal papilla cells via the
Wnt/β-catenin pathway. Animals (Basel). 13:20332023. View Article : Google Scholar
|
|
12
|
Baum P, Toyka KV, Blüher M, Kosacka J and
Nowicki M: Inflammatory mechanisms in the pathophysiology of
diabetic peripheral neuropathy (DN)-new aspects. Int J Mol Sci.
22:108352021. View Article : Google Scholar
|
|
13
|
Eftekharpour E and Fernyhough P: Oxidative
stress and mitochondrial dysfunction associated with peripheral
neuropathy in type 1 diabetes. Antioxid Redox Signal. 37:578–596.
2022. View Article : Google Scholar
|
|
14
|
Rumora AE, Kim B and Feldman EL: A Role
for fatty acids in peripheral neuropathy associated with type 2
diabetes and prediabetes. Antioxid Redox Signal. 37:560–577. 2022.
View Article : Google Scholar
|
|
15
|
Yang C, Zhao X, An X, Zhang Y, Sun W,
Zhang Y, Duan Y, Kang X, Sun Y, Jiang L and Lian F: Axonal
transport deficits in the pathogenesis of diabetic peripheral
neuropathy. Front Endocrinol (Lausanne). 14:11367962023. View Article : Google Scholar
|
|
16
|
Majd H, Amin S, Ghazizadeh Z, Cesiulis A,
Arroyo E, Lankford K, Majd A, Farahvashi S, Chemel AK, Okoye M, et
al: Deriving Schwann cells from hPSCs enables disease modeling and
drug discovery for diabetic peripheral neuropathy. Cell Stem Cell.
30:632–647.e10. 2023. View Article : Google Scholar
|
|
17
|
Wang X, Xu G, Liu H, Chen Z, Huang S, Yuan
J, Xie C and Du L: Inhibiting apoptosis of Schwann cell under the
high-glucose condition: A promising approach to treat diabetic
peripheral neuropathy using Chinese herbal medicine. Biomed
Pharmacother. 157:1140592023. View Article : Google Scholar
|
|
18
|
Cheng Y, Liu J, Luan Y, Liu Z, Lai H,
Zhong W, Yang Y, Yu H, Feng N, Wang H, et al: Sarm1 gene deficiency
attenuates diabetic peripheral neuropathy in mice. Diabetes.
68:2120–2130. 2019. View Article : Google Scholar
|
|
19
|
Wang Q, Chen FY, Ling ZM, Su WF, Zhao YY,
Chen G and Wei ZY: The effect of Schwann cells/schwann cell-like
cells on cell therapy for peripheral neuropathy. Front Cell
Neurosci. 16:8369312022. View Article : Google Scholar
|
|
20
|
Kalinski AL, Yoon C, Huffman LD, Duncker
PC, Kohen R, Passino R, Hafner H, Johnson C, Kawaguchi R, Carbajal
KS, et al: Analysis of the immune response to sciatic nerve injury
identifies efferocytosis as a key mechanism of nerve debridement.
Elife. 9:e602232020. View Article : Google Scholar
|
|
21
|
Qu WR, Zhu Z, Liu J, Song DB, Tian H, Chen
BP, Li R and Deng LX: Interaction between Schwann cells and other
cells during repair of peripheral nerve injury. Neural Regen Res.
16:93–98. 2021. View Article : Google Scholar
|
|
22
|
Chen CZ, Neumann B, Förster S and Franklin
RJM: Schwann cell remyelination of the central nervous system: Why
does it happen and what are the benefits? Open Biol. 11:2003522021.
View Article : Google Scholar
|
|
23
|
Han IH, Jeong C, Yang J, Park SH, Hwang DS
and Bae H: Therapeutic effect of melittin-dKLA targeting
tumor-associated macrophages in melanoma. Int J Mol Sci.
23:30942022. View Article : Google Scholar
|
|
24
|
Yu X, Dai Y, Zhao Y, Qi S, Liu L, Lu L,
Luo Q and Zhang Z: Melittin-lipid nanoparticles target to lymph
nodes and elicit a systemic anti-tumor immune response. Nat Commun.
11:11102020. View Article : Google Scholar
|
|
25
|
Ombredane AS, de Andrade LR, Bonadio RS,
Pinheiro WO, de Azevedo RB and Joanitti GA: Melittin sensitizes
skin squamous carcinoma cells to 5-fluorouracil by affecting cell
proliferation and survival. Exp Dermatol. 30:710–716. 2021.
View Article : Google Scholar
|
|
26
|
Fan XG, Pei SY, Zhou D, Zhou PC, Huang Y,
Hu XW, Li T, Wang Y, Huang ZB and Li N: Melittin ameliorates
inflammation in mouse acute liver failure via inhibition of
PKM2-mediated Warburg effect. Acta Pharmacol Sin. 42:1256–1266.
2021. View Article : Google Scholar
|
|
27
|
Tender T, Rahangdale RR, Balireddy S,
Nampoothiri M, Sharma KK and Raghu Chandrashekar H: Melittin, a
honeybee venom derived peptide for the treatment of
chemotherapy-induced peripheral neuropathy. Med Oncol. 38:522021.
View Article : Google Scholar
|
|
28
|
Er-Rouassi H, Bakour M, Touzani S,
Vilas-Boas M, Falcão S, Vidal C and Lyoussi B: Beneficial effect of
bee venom and its major components on facial nerve injury induced
in mice. Biomolecules. 13:6802023. View Article : Google Scholar
|
|
29
|
Mandel N, Büttner M, Poschet G, Kuner R
and Agarwal N: SUMOylation modulates reactive oxygen species (ROS)
levels and acts as a protective mechanism in the type 2 model of
diabetic peripheral neuropathy. Cells. 12:25112023. View Article : Google Scholar
|
|
30
|
Pang B, Zhang LL, Li B, Sun FX and Wang
ZD: BMP5 ameliorates diabetic peripheral neuropathy by augmenting
mitochondrial function and inhibiting apoptosis in Schwann cells.
Biochem Biophys Res Commun. 643:69–76. 2023. View Article : Google Scholar
|
|
31
|
Hu Y, Chen C, Liang Z, Liu T, Hu X, Wang
G, Hu J, Xie X and Liu Z: Compound Qiying Granules alleviates
diabetic peripheral neuropathy by inhibiting endoplasmic reticulum
stress and apoptosis. Mol Med. 29:982023. View Article : Google Scholar
|
|
32
|
Larange A, Takazawa I, Kakugawa K, Thiault
N, Ngoi S, Olive ME, Iwaya H, Seguin L, Vicente-Suarez I, Becart S,
et al: A regulatory circuit controlled by extranuclear and nuclear
retinoic acid receptor α determines T cell activation and function.
Immunity. 56:2054–2069.e10. 2023. View Article : Google Scholar
|
|
33
|
Khazeem MM, Casement JW, Schlossmacher G,
Kenneth NS, Sumbung NK, Chan JYT, McGow JF, Cowell IG and Austin
CA: TOP2B is required to maintain the adrenergic neural phenotype
and for ATRA-induced differentiation of SH-SY5Y neuroblastoma
cells. Mol Neurobiol. 59:5987–6008. 2022. View Article : Google Scholar
|
|
34
|
Kim TW, Piao J, Koo SY, Kriks S, Chung SY,
Betel D, Socci ND, Choi SJ, Zabierowski S, Dubose BN, et al:
Biphasic activation of WNT signaling facilitates the derivation of
midbrain dopamine neurons from hESCs for translational use. Cell
Stem Cell. 28:343–355.e5. 2021. View Article : Google Scholar
|
|
35
|
Sun X, Peng X, Cao Y, Zhou Y and Sun Y:
ADNP promotes neural differentiation by modulating Wnt/β-catenin
signaling. Nat Commun. 11:29842020. View Article : Google Scholar
|
|
36
|
Gao J, Liao Y, Qiu M and Shen W:
Wnt/β-catenin signaling in neural stem cell homeostasis and
neurological diseases. Neuroscientist. 27:58–72. 2021. View Article : Google Scholar
|
|
37
|
Jang E, Jin S, Cho KJ, Kim D, Rho CR and
Lyu J: Wnt/β-catenin signaling stimulates the self-renewal of
conjunctival stem cells and promotes corneal conjunctivalization.
Exp Mol Med. 54:1156–1164. 2022. View Article : Google Scholar
|
|
38
|
El-Sawaf ES, Saleh S, Abdallah DM, Ahmed
KA and El-Abhar HS: Vitamin D and rosuvastatin obliterate
peripheral neuropathy in a type-2 diabetes model through modulating
Notch1, Wnt-10α, TGF-β and NRF-1 crosstalk. Life Sci.
279:1196972021. View Article : Google Scholar
|
|
39
|
Kullmann MK, Pegka F, Ploner C and Hengst
L: Stimulation of c-Jun/AP-1-activity by the cell cycle inhibitor
p57Kip2. Front Cell Dev Biol. 9:6646092021. View Article : Google Scholar
|
|
40
|
Requejo-Aguilar R: Cdk5 and aberrant cell
cycle activation at the core of neurodegeneration. Neural Regen
Res. 18:1186–1190. 2023. View Article : Google Scholar
|
|
41
|
Chen Z, Xie Y, Luo H, Song Y, Que T, Hu R,
Huang H, Luo K, Li C, Qin C, et al: NAP1L1 promotes proliferation
and chemoresistance in glioma by inducing CCND1/CDK4/CDK6
expression through its interaction with HDGF and activation of
c-Jun. Aging (Albany NY). 13:26180–26200. 2021. View Article : Google Scholar
|
|
42
|
Lange C, Huttner WB and Calegari F:
Cdk4/cyclinD1 overexpression in neural stem cells shortens G1,
delays neurogenesis, and promotes the generation and expansion of
basal progenitors. Cell Stem Cell. 5:320–331. 2009. View Article : Google Scholar
|
|
43
|
Gao S, Tan H and Gang J: Inhibition of
hepatocellular carcinoma cell proliferation through regulation of
the cell cycle, AGE-RAGE, and Leptin signaling pathways by a
compound formulation comprised of andrographolide, wogonin, and
oroxylin A derived from andrographis paniculata(Burm.f.) nees. J
Ethnopharmacol. 329:1180012024. View Article : Google Scholar
|