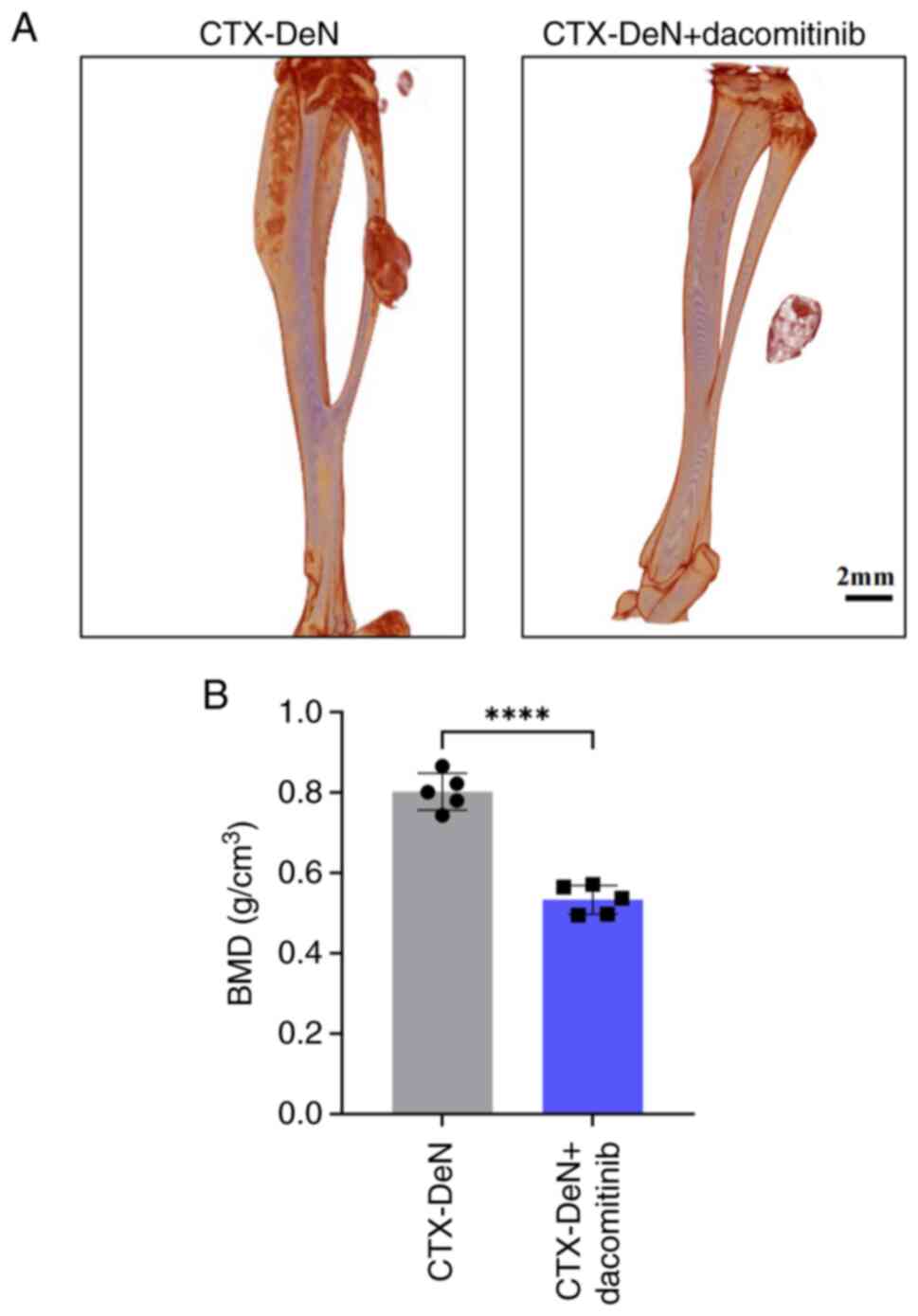
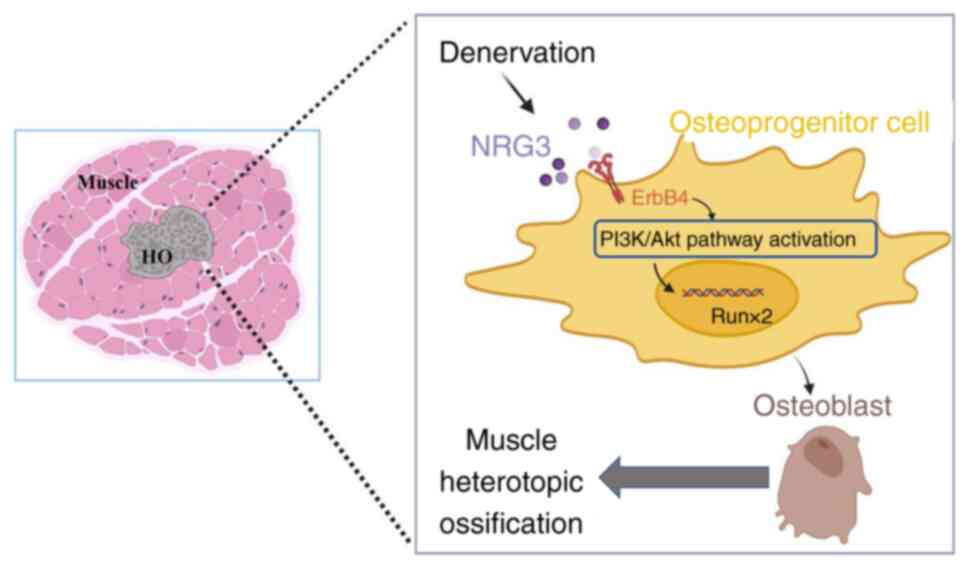
|
1
|
Edwards DS and Clasper JC: Heterotopic
ossification: A systematic review. J R Army Med Corps. 161:315–321.
2015. View Article : Google Scholar
|
|
2
|
Ranganathan K, Loder S, Agarwal S, Wong
VW, Forsberg J, Davis TA, Wang S, James AW and Levi B: Heterotopic
Ossification: Basic-Science principles and clinical correlates. J
Bone Joint Surg Am. 97:1101–1111. 2015. View Article : Google Scholar
|
|
3
|
Sturbois-Nachef N, Gatin L, Salga M,
Geffrier A, Fontaine C and Allart E: Neurogenic heterotopic
ossification in the upper limb. Hand Surg Rehabil. 41S:S167–S174.
2022. View Article : Google Scholar
|
|
4
|
Reichel LM, Salisbury E, Moustoukas MJ,
Davis AR and Olmsted-Davis E: Molecular mechanisms of heterotopic
ossification. J Hand Surg Am. 39:563–566. 2014. View Article : Google Scholar
|
|
5
|
Nauth A, Giles E, Potter BK, Nesti LJ,
O'brien FP, Bosse MJ, Anglen JO, Mehta S, Ahn J, Miclau T and
Schemitsch EH: Heterotopic ossification in orthopaedic trauma. J
Orthop Trauma. 26:684–688. 2012. View Article : Google Scholar
|
|
6
|
Moore-Lotridge SN, Li Q, Gibson BHY,
Martin JT, Hawley GD, Arnold TH, Saito M, Tannouri S, Schwartz HS,
Gumina RJ, et al: Trauma-Induced nanohydroxyapatite deposition in
skeletal muscle is sufficient to drive heterotopic ossification.
Calcif Tissue Int. 104:411–425. 2019. View Article : Google Scholar
|
|
7
|
Ji Y, Christopherson GT, Kluk MW, Amrani
O, Jackson WM and Nesti LJ: Heterotopic ossification following
musculoskeletal trauma: Modeling stem and progenitor cells in their
microenvironment. Adv Exp Med Biol. 720:39–50. 2011. View Article : Google Scholar
|
|
8
|
Sanders BS, Wilcox RB III and Higgins LD:
Heterotopic ossification of the deltoid muscle after arthroscopic
rotator cuff repair. Am J Orthop (Belle Mead NJ). 39:E67–E71.
2010.
|
|
9
|
Xu Y, Huang M, He W, He C, Chen K, Hou J,
Huang M, Jiao Y, Liu R, Zou N, et al: Heterotopic Ossification:
Clinical features, basic researches, and mechanical stimulations.
Front Cell Dev Biol. 10:7709312022. View Article : Google Scholar
|
|
10
|
Edwards DS, Kuhn KM, Potter BK and
Forsberg JA: Heterotopic Ossification: A review of current
understanding, treatment, and future. J Orthop Trauma. 30 (Suppl
3):S27–S30. 2016. View Article : Google Scholar
|
|
11
|
Peake JM, Della Gatta P, Suzuki K and
Nieman DC: Cytokine expression and secretion by skeletal muscle
cells: Regulatory mechanisms and exercise effects. Exerc Immunol
Rev. 21:8–25. 2015.
|
|
12
|
Zhang S, Sun S, He J and Shen L: NT-3
promotes osteogenic differentiation of mouse bone marrow
mesenchymal stem cells by regulating the Akt pathway. J
Musculoskelet Neuronal Interact. 20:591–599. 2020.
|
|
13
|
Feng H, Xing W, Han Y, Sun J, Kong M, Gao
B, Yang Y, Yin Z, Chen X, Zhao Y, et al: Tendon-derived cathepsin
K-expressing progenitor cells activate Hedgehog signaling to drive
heterotopic ossification. J Clin Invest. 130:6354–6365. 2020.
View Article : Google Scholar
|
|
14
|
Xu R, Hu J, Zhou X and Yang Y: Heterotopic
ossification: Mechanistic insights and clinical challenges. Bone.
109:134–142. 2018. View Article : Google Scholar
|
|
15
|
Doherty C, Lodyga M, Correa J, Di
Ciano-Oliveira C, Plant PJ, Bain JR and Batt J: Utilization of the
rat tibial nerve transection model to evaluate cellular and
molecular mechanisms underpinning denervation-mediated muscle
injury. Int J Mol Sci. 25:18472024. View Article : Google Scholar
|
|
16
|
Bertin JSF, Marques MJ, Macedo AB, de
Carvalho SC and Neto HS: Effect of photobiomodulation on
denervation-induced skeletal muscle atrophy and autophagy: A study
in mice. J Manipulative Physiol Ther. 45:97–103. 2022. View Article : Google Scholar
|
|
17
|
Rodríguez MP and Cabello-Verrugio C:
Soluble factors associated with denervation-induced skeletal muscle
atrophy. Curr Protein Pept Sci. 25:189–199. 2024. View Article : Google Scholar
|
|
18
|
Komatsu M, Nakada T, Kawagishi H, Kato H
and Yamada M: Increase in phospholamban content in mouse skeletal
muscle after denervation. J Muscle Res Cell Motil. 39:163–173.
2018. View Article : Google Scholar
|
|
19
|
Lee J, Jang SH, Lee SJ and Lee O:
Synchrotron radiation imaging analysis of neural damage in mouse
soleus muscle. Sci Rep. 10:45552020. View Article : Google Scholar
|
|
20
|
Yoshimura A, Ito M, Chikuma S, Akanuma T
and Nakatsukasa H: Negative regulation of cytokine signaling in
immunity. Cold Spring Harb Perspect Biol. 10:a0285712018.
View Article : Google Scholar
|
|
21
|
Zhou P, Zheng T and Zhao B:
Cytokine-mediated immunomodulation of osteoclastogenesis. Bone.
164:1165402022. View Article : Google Scholar
|
|
22
|
Mansurov A, Lauterbach A, Budina E, Alpar
AT, Hubbell JA and Ishihara J: Immunoengineering approaches for
cytokine therapy. Am J Physiol Cell Physiol. 321:C369–C383. 2021.
View Article : Google Scholar
|
|
23
|
Schaible HG, Del Rosso A and
Matucci-Cerinic M: Neurogenic aspects of inflammation. Rheum Dis
Clin North Am. 3177–101. (ix)2005. View Article : Google Scholar
|
|
24
|
Salisbury E, Rodenberg E, Sonnet C, Hipp
J, Gannon FH, Vadakkan TJ, Dickinson ME, Olmsted-Davis EA and Davis
AR: Sensory nerve induced inflammation contributes to heterotopic
ossification. J Cell Biochem. 112:2748–2758. 2011. View Article : Google Scholar
|
|
25
|
Zhang D, Sliwkowski MX, Mark M, Frantz G,
Akita R, Sun Y, Hillan K, Crowley C, Brush J and Godowski PJ:
Neuregulin-3 (NRG3): A novel neural tissue-enriched protein that
binds and activates ErbB4. Proc Natl Acad Sci USA. 94:9562–9567.
1997. View Article : Google Scholar
|
|
26
|
Jullien N, Maudinet A, Leloutre B, Ringe
J, Haupl T and Marie PJ: Downregulation of ErbB3 by Wnt3a
contributes to wnt-induced osteoblast differentiation in
mesenchymal cells. J Cell Biochem. 113:2047–2056. 2012. View Article : Google Scholar
|
|
27
|
Achilleos A and Trainor PA: Neural crest
stem cells: Discovery, properties and potential for therapy. Cell
Res. 22:288–304. 2012. View Article : Google Scholar
|
|
28
|
Lazard ZW, Olmsted-Davis EA, Salisbury EA,
Gugala Z, Sonnet C, Davis EL, Beal E II, Ubogu EE and Davis AR:
Osteoblasts Have a Neural Origin in Heterotopic Ossification. Clin
Orthop Relat Res. 473:2790–2806. 2015. View Article : Google Scholar
|
|
29
|
Joe AW, Yi L, Natarajan A, Le Grand F, So
L, Wang J, Rudnicki MA and Rossi FM: Muscle injury activates
resident fibro/adipogenic progenitors that facilitate myogenesis.
Nat Cell Biol. 12:153–163. 2010. View Article : Google Scholar
|
|
30
|
Helmbacher F and Stricker S: Tissue cross
talks governing limb muscle development and regeneration. Semin
Cell Dev Biol. 104:14–30. 2020. View Article : Google Scholar
|
|
31
|
Contreras O, Rossi FMV and Theret M:
Origins, potency, and heterogeneity of skeletal muscle
fibro-adipogenic progenitors-time for new definitions. Skelet
Muscle. 11:162021. View Article : Google Scholar
|
|
32
|
Vallecillo-Garcia P, Orgeur M, Vom
Hofe-Schneider S, Stumm J, Kappert V, Ibrahim DM, Börno ST, Hayashi
S, Relaix F, Hildebrandt K, et al: Odd skipped-related 1 identifies
a population of embryonic fibro-adipogenic progenitors regulating
myogenesis during limb development. Nat Commun. 8:12182017.
View Article : Google Scholar
|
|
33
|
Wosczyna MN, Biswas AA, Cogswell CA and
Goldhamer DJ: Multipotent progenitors resident in the skeletal
muscle interstitium exhibit robust BMP-dependent osteogenic
activity and mediate heterotopic ossification. J Bone Miner Res.
27:1004–1017. 2012. View Article : Google Scholar
|
|
34
|
Lees-Shepard JB, Yamamoto M, Biswas AA,
Stoessel SJ, Nicholas SE, Cogswell CA, Devarakonda PM, Schneider MJ
Jr, Cummins SM, Legendre NP, et al: Activin-dependent signaling in
fibro/adipogenic progenitors causes fibrodysplasia ossificans
progressiva. Nat Commun. 9:4712018. View Article : Google Scholar
|
|
35
|
Eisner C, Cummings M, Johnston G, Tung LW,
Groppa E, Chang C and Rossi FM: Murine tissue-resident PDGFRα+
fibro-adipogenic progenitors spontaneously acquire osteogenic
phenotype in an altered inflammatory environment. J Bone Miner Res.
35:1525–1534. 2020. View Article : Google Scholar
|
|
36
|
Akhter ET, Rotterman TM, English AW and
Alvarez FJ: Sciatic nerve cut and repair using fibrin glue in adult
mice. Bio Protoc. 9:e33632019. View Article : Google Scholar
|
|
37
|
Engelman JA, Zejnullahu K, Gale CM,
Lifshits E, Gonzales AJ, Shimamura T, Zhao F, Vincent PW, Naumov
GN, Bradner JE, et al: PF00299804, an irreversible pan-ERBB
inhibitor, is effective in lung cancer models with EGFR and ERBB2
mutations that are resistant to gefitinib. Cancer Res.
67:11924–11932. 2007. View Article : Google Scholar
|
|
38
|
Kang X, Yang MY, Shi YX, Xie MM, Zhu M,
Zheng XL, Zhang CK, Ge ZL, Bian XT, Lv JT, et al: Interleukin-15
facilitates muscle regeneration through modulation of
fibro/adipogenic progenitors. Cell Commun Signal. 16:422018.
View Article : Google Scholar
|
|
39
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
40
|
Zhao SJ, Kong FQ, Jie J, Li Q, Liu H, Xu
AD, Yang YQ, Jiang B, Wang DD, Zhou ZQ, et al: Macrophage MSR1
promotes BMSC osteogenic differentiation and M2-like polarization
by activating PI3K/AKT/GSK3β/β-catenin pathway. Theranostics.
10:17–35. 2020. View Article : Google Scholar
|
|
41
|
Pan JM, Wu LG, Cai JW, Wu LT and Liang M:
Dexamethasone suppresses osteogenesis of osteoblast via the
PI3K/Akt signaling pathway in vitro and in vivo. J Recept Signal
Transduct Res. 39:80–86. 2019. View Article : Google Scholar
|
|
42
|
Davis EL, Davis AR, Gugala Z and
Olmsted-Davis EA: Is heterotopic ossification getting nervous?: The
role of the peripheral nervous system in heterotopic ossification.
Bone. 109:22–27. 2018. View Article : Google Scholar
|
|
43
|
Hwang CD, Pagani CA, Nunez JH, Cherief M,
Qin Q, Gomez-Salazar M, Kadaikal B, Kang H, Chowdary AR, Patel N,
et al: Contemporary perspectives on heterotopic ossification. JCI
insight. 7:e1589962022. View Article : Google Scholar
|
|
44
|
Yuasa M, Mignemi NA, Nyman JS, Duvall CL,
Schwartz HS, Okawa A, Yoshii T, Bhattacharjee G, Zhao C, Bible JE,
et al: Fibrinolysis is essential for fracture repair and prevention
of heterotopic ossification. J Clin Invest. 125:3117–3131. 2015.
View Article : Google Scholar
|
|
45
|
Wan QQ, Qin WP, Ma YX, Shen MJ, Li J,
Zhang ZB, Chen JH, Tay FR, Niu LN and Jiao K: Crosstalk between
bone and nerves within bone. Adv Sci (Weinh). 8:20033902021.
View Article : Google Scholar
|
|
46
|
Qureshi AT, Crump EK, Pavey GJ, Hope DN,
Forsberg JA and Davis TA: Early characterization of blast-related
heterotopic ossification in a rat model. Clin Orthop Relat Res.
473:2831–2839. 2015. View Article : Google Scholar
|
|
47
|
Olmsted-Davis EA, Salisbury EA, Hoang D,
Davis EL, Lazard Z, Sonnet C, Davis TA, Forsberg JA and Davis AR:
Progenitors in peripheral nerves launch heterotopic ossification.
Stem Cells Transl Med. 6:1109–1119. 2017. View Article : Google Scholar
|
|
48
|
Alfieri KA, Forsberg JA and Potter BK:
Blast injuries and heterotopic ossification. Bone Joint Res.
1:192–197. 2012. View Article : Google Scholar
|
|
49
|
Smith JK, Miller ME, Carroll CG, Faillace
WJ, Nesti LJ, Cawley CM and Landau ME: High-resolution ultrasound
in combat-related peripheral nerve injuries. Muscle Nerve.
54:1139–1144. 2016. View Article : Google Scholar
|
|
50
|
Edwards DS, Clasper JC and Patel HD:
Heterotopic ossification in victims of the London 7/7 bombings. J R
Army Med Corps. 161:345–347. 2015. View Article : Google Scholar
|
|
51
|
Wang X, Li F, Xie L, Crane J, Zhen G,
Mishina Y, Deng R, Gao B, Chen H, Liu S, et al: Inhibition of
overactive TGF-β attenuates progression of heterotopic ossification
in mice. Nat Commun. 9:5512018. View Article : Google Scholar
|
|
52
|
Li L, Jiang Y, Lin H, Shen H, Sohn J,
Alexander PG and Tuan RS: Muscle injury promotes heterotopic
ossification by stimulating local bone morphogenetic protein-7
production. J Orthop Translat. 18:142–153. 2019. View Article : Google Scholar
|
|
53
|
O'Brien EJ, Frank CB, Shrive NG,
Hallgrímsson B and Hart DA: Heterotopic mineralization
(ossification or calcification) in tendinopathy or following
surgical tendon trauma. Int J Exp Pathol. 93:319–331. 2012.
View Article : Google Scholar
|
|
54
|
Wei X, Nicoletti C and Puri PL:
Fibro-Adipogenic Progenitors: Versatile keepers of skeletal muscle
homeostasis, beyond the response to myotrauma. Semin Cell Dev Biol.
119:23–31. 2021. View Article : Google Scholar
|
|
55
|
Gallardo FS, Cordova-Casanova A,
Bock-Pereda A, Rebolledo DL, Ravasio A, Casar JC and Brandan E:
Denervation Drives YAP/TAZ activation in muscular fibro/adipogenic
progenitors. Int J Mol Sci. 24:55852023. View Article : Google Scholar
|
|
56
|
Mejias Rivera L, Shore EM and Mourkioti F:
Cellular and molecular mechanisms of heterotopic ossification in
fibrodysplasia ossificans progressiva. Biomedicines. 12:7792024.
View Article : Google Scholar
|
|
57
|
Tumolo MR, Panico A, De Donno A, Mincarone
P, Leo CG, Guarino R, Bagordo F, Serio F, Idolo A, Grassi T and
Sabina S: The expression of microRNAs and exposure to environmental
contaminants related to human health: A review. Int J Environ
Health Res. 32:332–354. 2022. View Article : Google Scholar
|
|
58
|
Fisher MC, Clinton GM, Maihle NJ and Dealy
CN: Requirement for ErbB2/ErbB signaling in developing cartilage
and bone. Dev Growth Differ. 49:503–513. 2007. View Article : Google Scholar
|
|
59
|
Linder M, Hecking M, Glitzner E, Zwerina
K, Holcmann M, Bakiri L, Ruocco MG, Tuckermann J, Schett G, Wagner
EF and Sibilia M: EGFR controls bone development by negatively
regulating mTOR-signaling during osteoblast differentiation. Cell
Death Differ. 25:1094–1106. 2018. View Article : Google Scholar
|
|
60
|
Shirley M: Dacomitinib: First global
approval. Drugs. 78:1947–1953. 2018. View Article : Google Scholar
|
|
61
|
Zheng HC: The molecular mechanisms of
chemoresistance in cancers. Oncotarget. 8:59950–59964. 2017.
View Article : Google Scholar
|