Introduction
Cardiovascular diseases (CVDs) encompass a broad
spectrum of disorders affecting the heart and blood vessels,
representing a significant global health challenge (1). Among these, myocardial infarction
(MI) stands out as a critical and potentially life-threatening
event (2). MI happens when
disrupted blood flow in the ischemic heart causes a great deal of
cardiomyocyte death, eventually leading to pathological left
ventricular (LV) remodeling and heart failure (3). The inherent limited regenerative
capacity of the heart impedes self-restoration post-MI,
accentuating the lasting effect. Despite medical progress in
promptly addressing obstructed blood flow, there are no
FDA-approved drugs to regenerate the lost cardiomyocyte during
ischemia (4).
Cell transplantation using human induced pluripotent
stem cells (hiPSCs) has been proved as a promising therapeutic
approach to improve cardiac function following an ischemic event
(5). hiPSCs can be induced to
fully differentiate into cardiomyocytes (hiPSC-CM) with spontaneous
beating, expression of cardiac markers like cardiac troponin T
(cTnT) and myocardial heavy chain (MHC) and sarcomeric α actin
(SAA) (6). When transplanted into
the infarcted area, hiPSC-CMs integrate with existing cardiac
cells, contributing to the regeneration of functional myocardium
(7). This therapeutic approach
aims to enhance heart function, alleviate symptoms and reduce the
risk of heart failure post-MI. Although this method offers a novel
therapeutic perspective of restoring cardiac function, more work
needs to be conducted to optimize engraftment and improve the
long-term efficacy of hiPSC-CM transplantation.
Hypoxia-inducible factor 1-alpha (HIF-1α) is a
ubiquitously expressed, master regulator of genes that allow
adaptation to hypoxic conditions (8). Target genes of HIF-1α include
vascular endothelial growth factor (VEGF), erythropoietin,
glycolytic enzymes, glucose transporters and other factors critical
to vascularization, metabolic regulation, cell proliferation and
survival (9). Controlling
vascularization by modulating the HIF pathway may be a valuable
strategy in patients with ischemic diseases. Previous studies
report that overexpressed HIF-1α in mesenchymal stem cells and
exosomes derived from these cells mediates cardioprotection in MI
by enhanced angiogenesis, which paved the road for the use of
HIF-1a in stem cell transplantation (10,11).
In addition, HIF-1α is unstable and usually degraded by a prolyl
hydroxylase (PHD; prolyl hydroxylase domain) at two specific prolyl
residues, Pro402 and Pro564 (12).
Thus, a mutated HIF-1α (P402A, P564A) was used in the present study
to enhance its stability (13).
The present study sought to investigate the effects
of the MHC promoter driven HIF-1α overexpression in hiPSC-CM on
hypoxia-injured human umbilical vein endothelial cells (HUVECs) and
ischemic heart. It showed that the proangiogenic paracrine effects
of the iPSC-CMs were enhanced by HIF-1α overexpression, which then
resulted in the rescue of the migratory ability and angiogenic
function of hypoxia-injured HUVECs. Moreover, HIF-1α overexpressed
hiPSC-derived cardiomyocytes exhibited a strong cardioprotective
effect on MI heart by promoting neovessel formation in the ischemic
border zone. This therapeutic effect was also blocked by an
angiogenesis inhibitor, rapamycin.
Materials and methods
Culture and cardiomyocyte
differentiation of hiPSCs
Geltrex (cat. no. A1413302; Thermo Fisher
Scientific, Inc.) was diluted 1:100 in ice-cold DMEM/F-12 (Thermo
Fisher Scientific, Inc.) to coat a 6-well plate (1 ml/well). The
coated plates were incubated at 37°C for ≥1 h before use. Prior to
hiPSC cell plating, colonies were disaggregated into single cells
using Accutase (cat. no. A1110501; Thermo Fisher Scientific, Inc.)
to achieve a uniform cell suspension. Human iPSCs (passage 10) from
the American Type Culture Collection were then seeded onto
Geltrex-coated wells at a density of 2×105 cells/well,
using Essential 8 Medium (E8; cat. no. A1517001; Thermo Fisher
Scientific, Inc.) supplemented with 10 µM Rock inhibitor (Y27632;
cat. no. 1254; Tocris Bioscience) for 24 h. Subsequently, medium
was changed to freshly prepared E8 without Y27632, with daily
medium exchanges. For cardiomyocyte differentiation, GiWi protocol
(14) was used as follows: upon
reaching 80–90% confluency after two or three days, cells were
exposed to 10 µM CHIR99021 (cat. no. 4423; Tocris Bioscience) in
RPMI 1640 (cat. no. 61870; Thermo Fisher Scientific, Inc.)
supplemented with B27-without insulin (cat. no. A1895601; Thermo
Fisher Scientific, Inc.) for 24 h. The medium was changed to
RPMI-B27 without insulin for 48 h. On day 3, cells received 10 µM
IWP2 (cat. no. 3533; Tocris Bioscience) in RPMI-B27 without insulin
for 48 h. Subsequent medium changes occurred every 48 h, with
hiPSC-CM purification using RPMI-B27 with insulin supplemented with
a 4 mM DL-lactate solution over four consecutive days starting on
day 7. The Embryo Research Oversight process was not necessary for
the present study.
Cloning and generation of
HIF-1α-overexpressing hiPSC-CMs
The backbone vector aMHC-mCherry-Rex-Blasticidin
(cat. no. 21228; Addgene, Inc.) was used for the reconstruction of
a lentiviral vector containing the mutated cDNA of HIF-1α (P402A,
P564A; cat. no. 52636; Addgene, Inc.) (13). Mutation of Pro402 and Pro564 in
HIF-1α would enhance its stability under normoxic conditions. The
HIF-1α cDNA was amplified using the Q5 High-Fidelity DNA Polymerase
(New England BioLabs, Inc.), then cloned into the backbone vector
aMHC-mCherry-Rex-Blasticidin using the NEBuilder HiFi DNA Assembly
Kit (New England BioLabs, Inc.). Lentivirus (2nd generation) was
packaged using 293T cells (cat. no. CRL-3216; American Type Culture
Collection) by transfecting psPAX2 (7 µg; cat. no. 12260; Addgene,
Inc.), pMD2.G (3 µg; cat. no. 12259; Addgene, Inc.), and the
backbone vectors (10 µg) or the HIF-1α expressing vector (10 µg)
with packaging plasmids by Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to
manufacturer's instruction. Culture medium was collected at 24, 48,
and 72 h after transfection. Lentivirus was concentrated from
culture medium by PEG 8000 precipitation. HIF-1α overexpression
hiPS cell line was established by lentiviral transduction (at 5
multiplicity of infection (MOI)) and blasticidin selection (5
µg/ml; Gibco; Thermo Fisher Scientific, Inc.). The transfected
hiPSCs were maintained in mTeSR Plus medium (cat. no. 100-0276;
STEMCELL Technologies) supplemented with 5 µg/ml blasticidin and
passaged for at least 2 passages (~6 days) before conducting
further experiments. Reverse transcription-quantitative (RT-q) PCR
was performed to evaluate HIF-1α expression level in hiPSC-CM.
RT-qPCR
TRIzol® (Thermo Fisher Scientific, Inc.)
was used to isolate the total RNA from cells (at 90% confluency).
RTwas executed using the PrimeScript RT reagent kit (Takara Bio,
Inc.). HIF-1α, VEGF, angiopoietin 1 (Ang-1), fibroblast growth
factor 1 (FGF-1) and platelet-derived growth factor receptor alpha
(PDGFRA) expression levels in hiPSC-CMs were assessed through the
SYBR Green (cat. no. 12369010; Invitrogen) assay following the
manufacturer's instructions. GAPDH served as the control. RNA
extraction, cDNA synthesis, and qPCR were all performed according
to the manufacturer's protocols. Primer details are provided in
Table I. The 2−ΔΔCq
method (15) determined relative
mRNA expression and each assay was conducted in triplicate.
Thermocycling conditions were as follows: 50°C for 2 min and 95°C
for 10 min 1 cycle, 95°C for 15 sec and 60°C for 1 min 40
cycles.
 | Table I.Reverse transcription-quantitative PCR
Primers. |
Table I.
Reverse transcription-quantitative PCR
Primers.
| Gene name | Forward primer
(5′-3′) | Reverse Primer
(5′-3′) |
|---|
| GAPDH |
GCCTCAAGATCATCAGCAATGC |
CCACGATACCAAAGTTGTCATGG |
| HIF1A |
GAACGTCGAAAAGAAAAGTCTCG |
CCTTATCAAGATGCGAACTCACA |
| PDGFRA |
TGGCAGTACCCCATGTCTGAA |
CCAAGACCGTCACAAAAAGGC |
| VEGF-A |
AGGGCAGAATCATCACGAAGT |
AGGGTCTCGATTGGATGGCA |
| Ang-1 |
AGCGCCGAAGTCCAGAAAAC |
TACTCTCACGACAGTTGCCAT |
| FGF1 |
CTCCCGAAGGATTAAACGACG |
GTCAGTGCTGCCTGAATGCT |
HUVECs culture
HUVECs were purchased from American Type Culture
Collection (cat. no. PCS-100-010; passage 3) and maintained
according to the manufacturer's protocol. In brief, cells were
cultured on a 10 cm dish and maintained in EGM-2 Endothelial Cell
Growth Medium-2 BulletKit (cat. no. CC-3162; Lonza Group Ltd.)For
hypoxic culture, HUVECs were cultured in a standard incubator
composed of 94% N2, 5% CO2 and 1%
O2 for 48 h. Hypoxia-induced HUVECs were used in the
following Matrigel and migration assays.
Microelectrode array (MEA)-based
analysis
The MEA, also referred to as multielectrode arrays,
consists of multiple small electrodes embedded in the culture
surface of the well. Electrically active cardiomyocytes were
cultured on top of these electrodes. CytoView MEA 24-well plates
(Axion BioSystems, Inc.) were pre-coated with Geltrex and incubated
at 37°C for 1 h. After dissociating cardiomyocytes from six cell
plates, 100,000 wild type hiPSCs cardiomyocytes (WT-CM) or
HIF-1α-overexpressing hiPSC-CM (HIF-CM) per well were seeded onto
the electrodes of the CytoView MEA 24-well plate and the assay
performed at 48 h later. Data were calculated using Axion
BioSystems Integrated Studio software (version 2.4; Axion
BioSystems, Inc.).
ELISA assay
The concentration of three proangiogenic factors,
VEGF, PDGF and Ang-1, were evaluated in the conditioned medium of
WT-CM and HIF-CM and blood. Cardiomyocytes were cultured in fresh
medium for 48 h and then the conditioned medium was collected for
the subsequent ELISA assay. Protein concentrations were determined
by VEGF, Ang-1 and PP775 ELISA kits purchased from Beyotime
Institute of Biotechnology (cat. nos. PV963, PA033 and PP775,
respectively) and the experiment was performed according to the
manufacturer's instructions.
Matrigel assay
HUVECs were used for in vitro tube formation
assay as described previously (16). A total of 40,000 HUVECs suspended
in HIF-1α-hiPSC-CMs conditioned medium or hiPSC-CMs conditioned
medium were applied to 100 µl of Matrigel (BD Biosciences) coated
wells (at 37°C for 1 h) in an 8-well glass chamber slide (BD
Falcon; BD Biosciences) and then incubated at 37°C for 24 h. The
number of formed tubes were counted and averaged with the help of a
computer assisted fluorescent microscope (Olympus Corporation) at
20× magnification. Five random fields were examined for each
biology replication.
Migration assay
HUVECs (5×104 cells/well) were seeded in
top chamber of the Transwell plates in FBS-free media with membrane
inserts without Matrigel coating. Then 0.6 ml DMEM supplemented
with 10% FBS as attractant was added to the well of the plate
(lower compartment). The plate was incubated at 37°C for 16 h. Then
cells that migrated to the lower surface of the membrane were fixed
with 4% paraformaldehyde at room temperature for 10 min, stained
with DAPI (room temperature for 5 min) and observed under a
fluorescence microscope (Olympus Corporation) at 20× magnification.
Five random fields were examined for each biology replication. The
migrated cells were quantified and normalized per
mm2.
Animals
A total of 10 Immunodeficient NOD/SCID mice, female,
aged 8~12-weeks-old, weighed 18~25 g were purchased from Liaoning
Changsheng Biotechnology co., Ltd., housed at Animal Facility of
the Fourth People's Hospital of Shenyang (Liaoning, China) at 18~23
°C with 40–60% humidity on a 12-h light/dark cycle with free access
to water and standard rodent food. All animal procedures were
approved by the Ethics Review Committee of the Fourth People's
Hospital of Shenyang (Liaoning, China) and were performed in
accordance with the Guidelines for the Care and Use of Research
Animals (version 2017.8) established by the Fourth People's
Hospital of Shenyang (Liaoning, China) (17).
Mouse MI model
Mouse MI model was performed according to a previous
study (18). Briefly, mice were
anesthetized with inhaled isoflurane (5% for induction, 1.5–2.0%
for maintenance), intubated and ventilated with a small animal
respirator before left thoracotomy was performed and the fourth
intercostal space was entered using scissors and blunt dissection.
An 8-0 silk suture was placed through the myocardium into the
anterolateral LV wall around the left anterior descending (LAD)
coronary artery. The suture was tied resulting in permanent
ligation of the LAD and subsequent MI was confirmed via the use of
the electrocardiogram (ECG) monitor. After LAD ligation, animals
were assigned to four treatment groups: Vehicle group, WT-CM group,
HIF-CM group and HIF-CM + Rapamycin group. Specifically, Vehicle
group received 30 µl DMEM medium; WT-CM group received
1×106 wildtype hiPSC-derived cardiomyocyte in 30 µl DMEM
medium; HIF-CM group received 1×106 HIF-1α-overexpressed
hiPSC-derived cardiomyocyte in 30 µl DMEM medium; HIF-CM +
Rapamycin group received 1×106 HIF-1α-overexpressed
hiPSC-derived cardiomyocyte together with rapamycin (10 µg/kg)
intraperitoneally administered in the mice. The injected cells were
delivered into the infarct border zone by three intramyocardial
injections (equal volume; 10 µl/site). After chest closure,
buprenorphine (0.1 mg/kg; Buprenex; Reckitt Benckiser
Pharmaceuticals Inc.) was administered subcutaneously immediately
following surgery and every 8–12 h for 72 h. Animal health was
monitored every 8–12 h for the first 72 h post-surgery and then at
least every 3 days afterwards. All study animals recovered well
from the surgery. The study was continued for 28 days before
termination. Animals were humanely sacrificed by cervical
dislocation under anesthesia with 5% isoflurane. Failure to detect
respiration and absence of a heartbeat for >5 min was used to
confirm death.
Echocardiography
The mice underwent anesthesia with 1.5% isoflurane
and was positioned supine on a heated platform equipped with
embedded ECG leads (FUJIFILM Visual Sonics) to maintain body
temperature. Ultrasound coupling gel, heated to 37°C, was applied
to the chest area. Using a linear array transducer (18–23 MHz) on
the mid-ventricular level, two-dimensional B-mode parasternal long
and short axis views were acquired using Vevo 2100 (FUJIFILM
VisualSonics). One-dimensional M-mode images were then captured in
the short-axis view to measure cardiac wall and chamber dimensions.
Offline analysis involved measuring LV chamber size and wall
thickness from at least three consecutive beats, averaging results.
Parameters included LV wall thickness at the intraventricular
septum and posterior wall during systole and diastole, as well as
LV internal dimensions (LVID) during systole and diastole. LV
percent fractional shortening (FS) and ejection fraction (EF) were
subsequently calculated based on the M-mode measurements.
Measurement of infarct size
The hearts were excised at 28 days after MI and the
infarct size was evaluated as previously described (19). In summary, the hearts were fixed
overnight in a 4% paraformaldehyde solution at 4°C, followed by
dehydration in 30% sucrose for 12 h at 4°C. Subsequently, the
hearts were embedded in optical cutting temperature compound. Then
10-µm-thick short-axis sections spanning from the base to the apex
were cut from the entire ventricles and affixed to glass slides.
For each mouse, five sections were treated with Bouin's solution
and stained using 0.04% Sirius Red/0.1% Fast Green collagen
staining (MilliporeSigma). The sections of the left ventricle were
imaged by an Olympus SZ61 Stereo light microscope at 4.5×
magnification. The microscope was able to capture the whole
cross-section of the left ventricle, thus, one image was taken for
each slide. The percentage of fibrotic length was calculated using
the arc length of the fibrotic area divided by the circumference of
the left ventricle. The percentage fibrotic area was calculated
using the area of the fibrotic tissue (red) divided by the area of
the left ventricle. All these parameters were quantified by ImageJ
(National Institutes of Health, version 1.52d).
Immunostaining
Heart sections (8 µm) were fixed with 4%
paraformaldehyde at room temperature for 10 min, permeabilized with
0.1% Triton X-100 at room temperature for 3 min, blocked with 5%
donkey serum (Sigma-Aldrich, cat. no. D9663) at room temperature
for 20 min and then incubated with anti-CD31 antibody (cat. no.
ab28364, Abcam; 1:200), anti-alpha smooth muscle actin (α-SMA; cat.
no ab5694; Abcam; 1:200) and anti-cTnT (cat. no. MAB1874;
Bio-Techne; 1:50) at 4°C overnight. After washing with PBS for 3
times, sections were incubated with Alexa Fluor 488 Anti-Rabbit
secondary antibody (Thermo Fisher Scientific, Inc.) and DyLight 549
Anti-Rabbit secondary antibody (Thermo Fisher Scientific, Inc.) in
dark at room temperature for 1 h. The sections were then washed
with PBS and mounted with Vectashield (cat. no. H-1800; Vector
Laboratories, Inc.). Images were captured under a fluorescent
microscope (Olympus Corporation) at 20X magnification. Five random
fields were examined for each biology replication.
Statistical analysis
The data were expressed as mean ± SEM. Significance
was assessed using the unpaired Student's t-test for two-group
comparisons or one-way ANOVA with Tukey's multiple comparison test
for comparisons among three groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
HIF-1α is overexpressed in
hiPSC-derived cardiomyocytes
hiPSC cell line was purchased from American Type
Culture Collection and maintained in Essential 8 medium for 30
passages. To specifically overexpress HIF-1α in cardiomyocytes, but
not hiPSC, an MHC promoter was applied to drive the HIF-1α
expression and the overexpression plasmid was transduced into hiPSC
by lentiviral transduction (Fig.
1A). Cardiomyocytes differentiation was induced in both
wild-type hiPSC and HIF-1α-transduced hiPSC using the GiWi protocol
(20). Further characterization by
immunostaining with cTnT (Fig. 1B)
and RT-qPCR (Fig. 1C) showed that
cTnT and MYH6 expression levels were significantly increased in
both WT-CM and HIF-CM, indicating the successful CM
differentiation. Immunostaining also did not indicate any
morphologic changes between WT-CM and HIF-CM. HIF-1α expression was
increased by more than 4-fold in HIF-1α overexpressed hiPSC-derived
cardiomyocytes (HIF-CM) compared with WT-CM (Fig. 1D; P<0.01). Moreover, the
expression levels of pro-angiogenic factors, Vascular endothelial
growth factor A, ang-1, FGF-1 and PDGFRA, were all elevated in the
HIF-CM group compared with WT-CM (Fig.
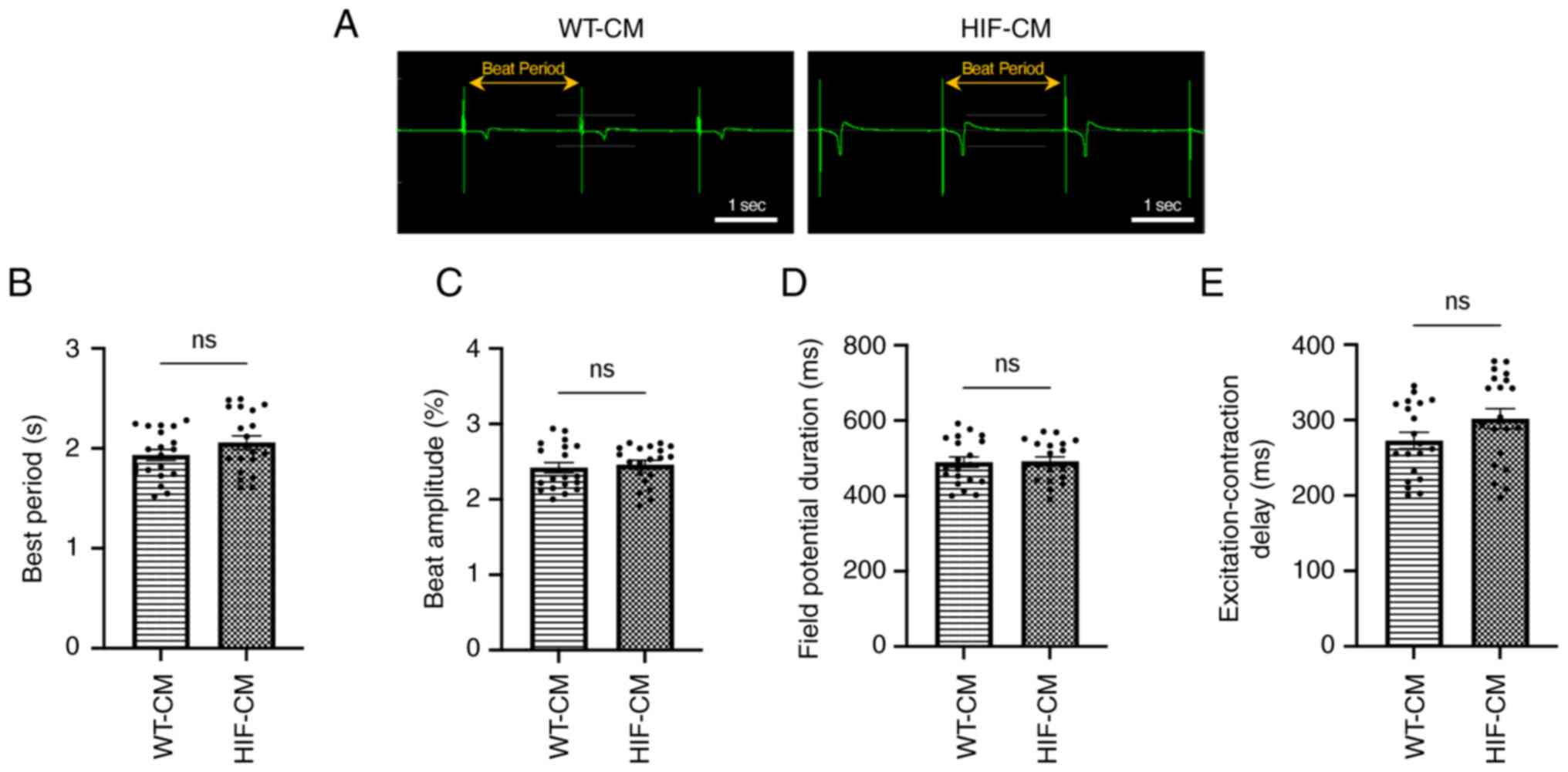
1E). In addition, MEA assay was performed to investigate the
electrophysiological properties of the hiPSC-CMs. As shown in
Fig. 2, the beat period, beat
amplitude, field potential duration and the excitation-contraction
delay were not statistically different between WT-CM and HIF-CM,
indicating the transgene did not alter the electrophysiology of the
CMs.
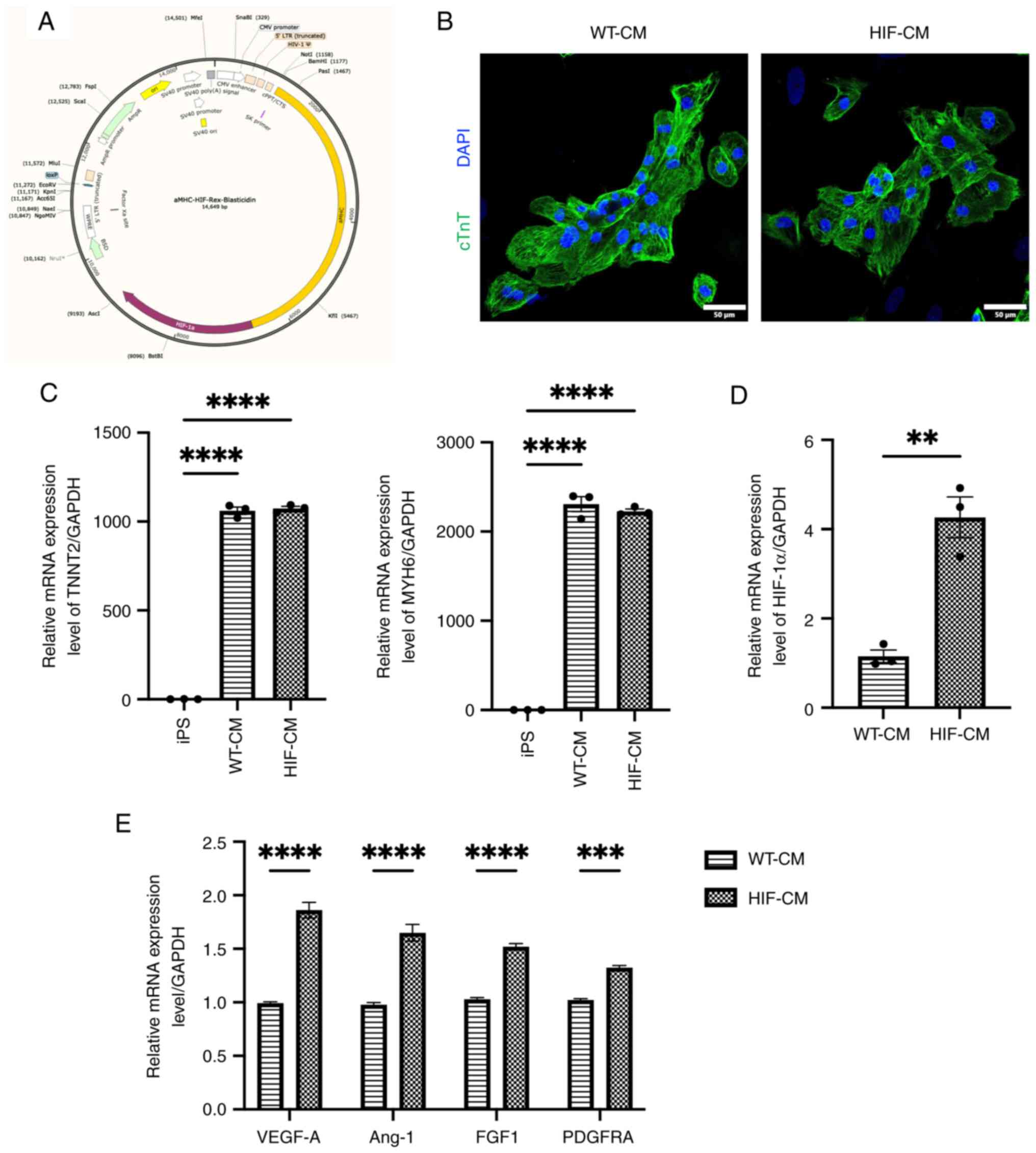 | Figure 1.HIF-1α overexpression in HIF-CM. (A)
The plasmid that express HIF-1α driven by the α-MHC promoter is
used for lentiviral transduction in hiPSC. (B) Immunofluorescent
staining against cTnT shows the successful differentiation of WT-CM
and HIF-CM. 20X magnification. (C) RT-qPCR was used to examine
cardiomyocyte specific gene TNNT2 and MYH6. (D) RT-qPCR was used to
validate the success overexpression of HIF-1α in cardiomyocytes.
(E) The expression levels of pro-angiogenic factors were evaluated
by RT-qPCR. HIF-1α, hypoxia-inducible factor 1-alpha; HIF-CM,
HIF-1α-overexpressing hiPSC-CM cardiomyocytes; α-MHC, α-myosin
heavy chain; cTnT, cardiac troponin T; WT-, wild-type-; CM,
cardiomyocytes; RT-qPCR, reverse transcription-quantitative PCR;
iPS, induced pluripotent stem cells. **P<0.01, ***P<0.001,
****P<0.0001. |
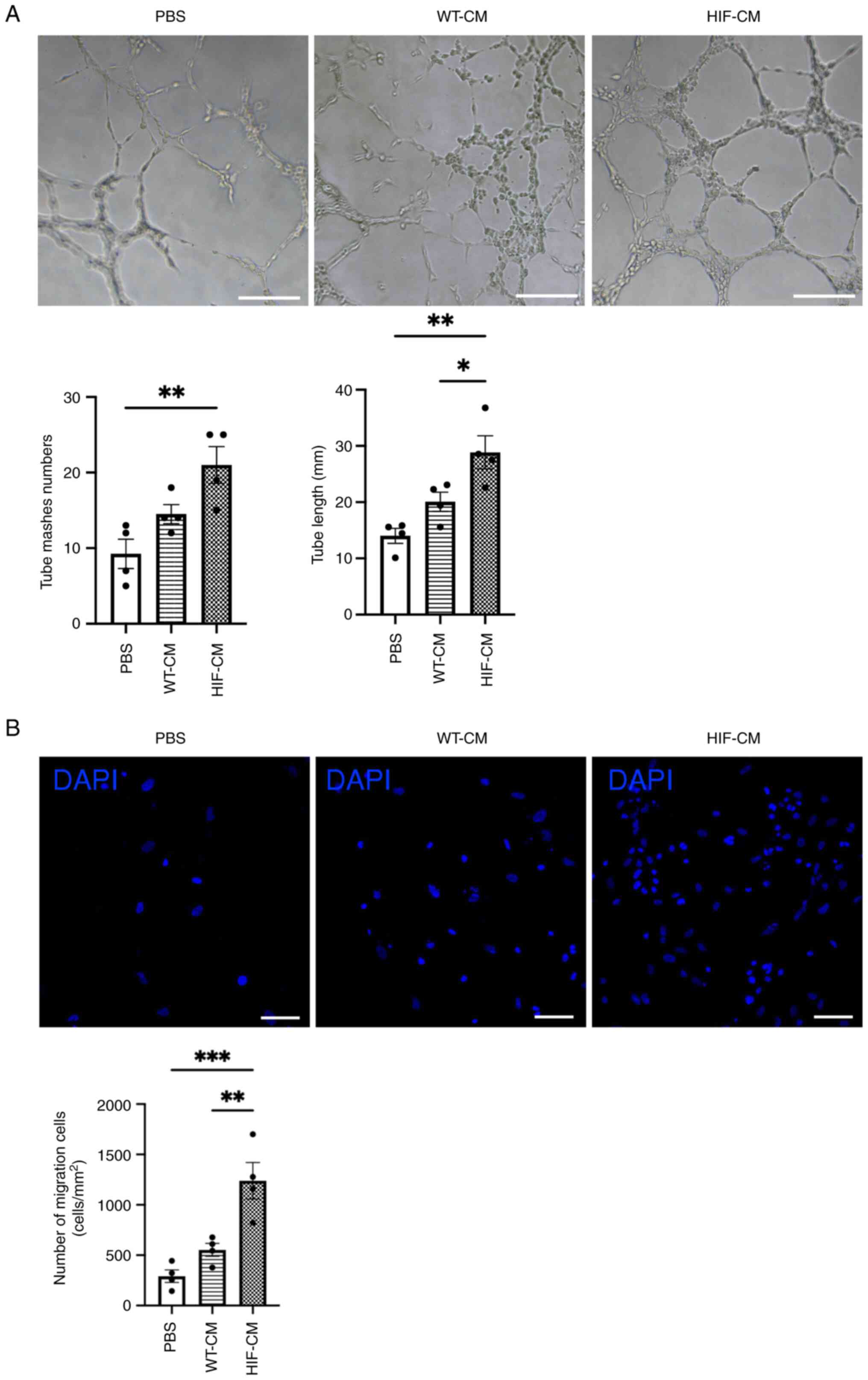
In vitro angiogenesis and migration
are rescued in hypoxia-injure HUVECs following treatment with
conditioned medium collected from HIF-1α overexpressed
cardiomyocytes
Conditioned medium were collected from HIF-CM and
WT-CM. Hypoxia-injured HUVECs were then cultured in conditioned
medium collected from HIF-CM and WT-CM for 24 h. HIF-CM derived
conditioned medium promoted tube formation of HUVECs; by contrast,
WT-CM derived conditioned medium had less effect in inducing the
tube formation (Fig. 3A). To
examine the migratory effects, HUVECs were then cultured in the
upper chamber of a Transwell plate; conditioned medium from HIF-CM
and WT-CM were added in the lower chamber of the plate. More cells
migrated towards the lower chamber with conditioned medium from
HIF-CM (Fig. 3B). In addition, to
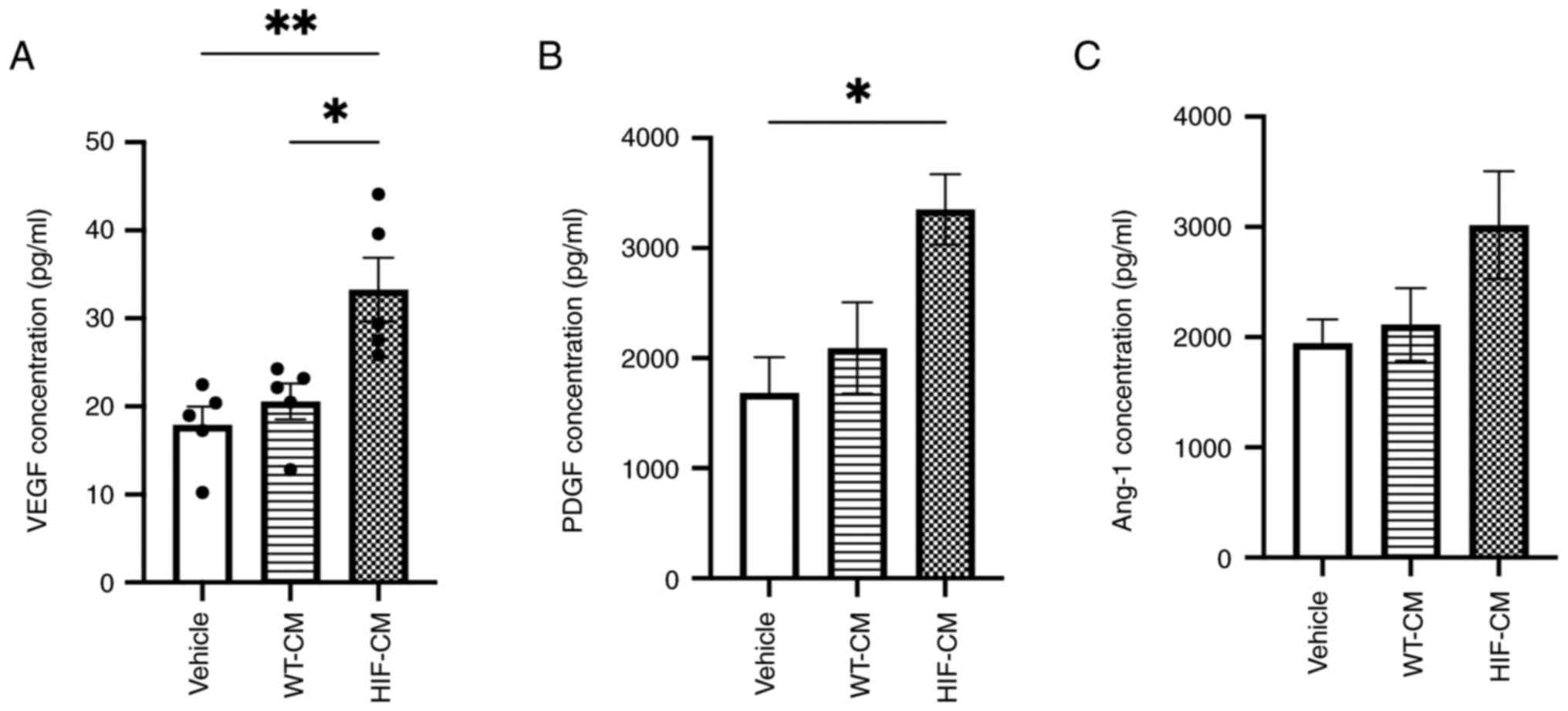
determine if these promotional effects were related to the enhanced
proangiogenic paracrine effect of the HIF-CM, the concentration of
three proangiogenic factors, VEGF, PDGF and Ang-1, which are also
regulated by HIF-1α, were evaluated in the conditioned medium
(Fig. 4A-C). Upregulated secretion
of these three proangiogenic factors in the conditioned medium of
HIF-CM was observed, which probably contributed to the rescue of
the angiogenesis and migration in hypoxia-injured HUVECs.
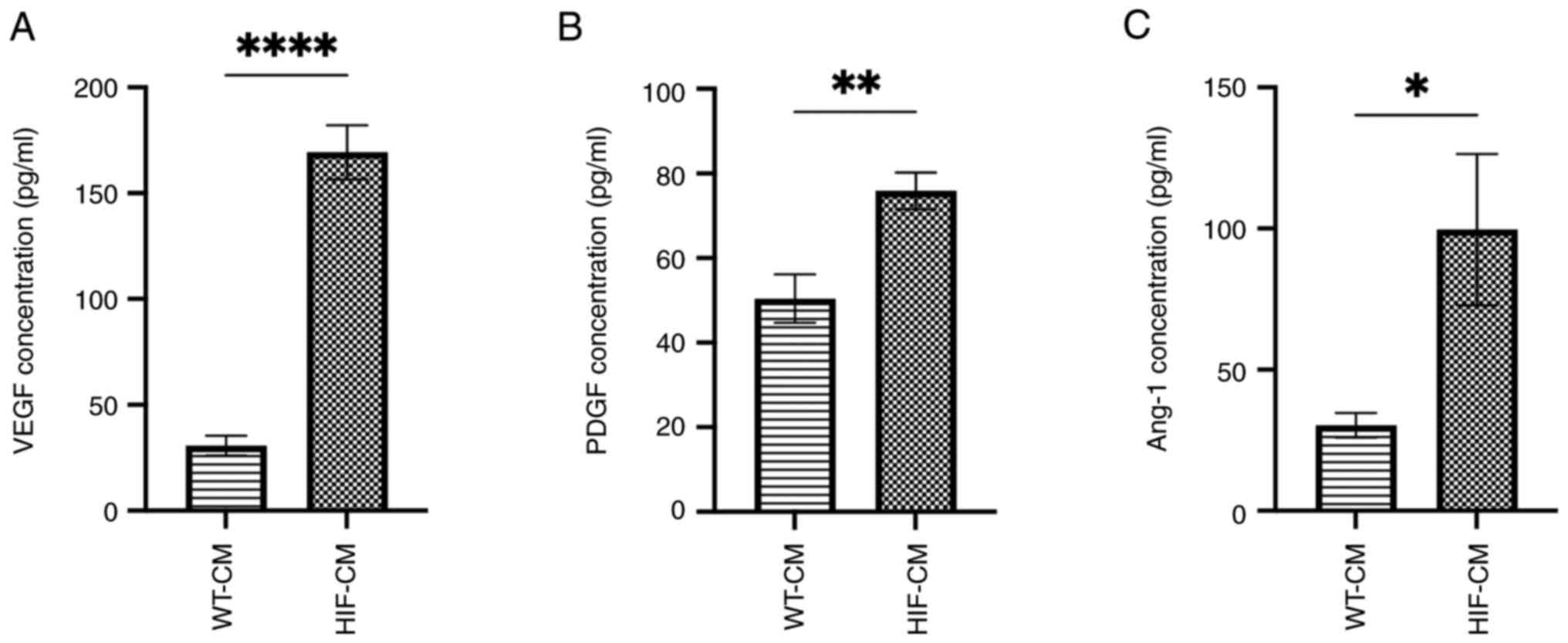 | Figure 4.Upregulated downstream proangiogenic
factors in the conditioned medium of HIF-CM. Protein expression
level of (A) VEGF, (B) PDGF and (C) Ang-1 detected by ELISA in the
conditioned medium of hiPSC-CM, respectively (n=5). *P<0.05,
**P<0.01, ****P<0.0001. HIF-CM, HIF-1α-overexpressing
hiPSC-CM cardiomyocytes; VEGF, vascular endothelial growth factor;
PDGF, platelet-derived growth factor receptor; Ang-1, angiopoietin
1; WT-, wild-type-; CM, cardiomyocytes. |
HIF-1α overexpresses cardiomyocytes
enhanced cardiac function recovery and decreased scar size
following acute MI
Transplantation of HIF-1α overexpressed
cardiomyocytes significantly improved echocardiographic parameters,
EF and FS, in mice with MI. Echocardiography performed at 1, 14 and
28 days (Fig. 5A) following cell
transplantation demonstrated that LV contractility was marked
improved in the HIF-CM-treated group compared with that in the
Vehicle and WT-CM groups (P<0.01 vs. Vehicle, WT-CM; Fig. 5B and C). Notably, a fourth group,
in which the mice received HIF-CM intramyocardially and rapamycin
(an angiogenesis inhibitor) intraperitoneally, was included to
serve as an intervention group. The therapeutic effect mediated by
HIF-CM was blocked by this angiogenesis inhibitor, indicating
HIF-CM promoted cardiac function recovery by promoting
angiogenesis.
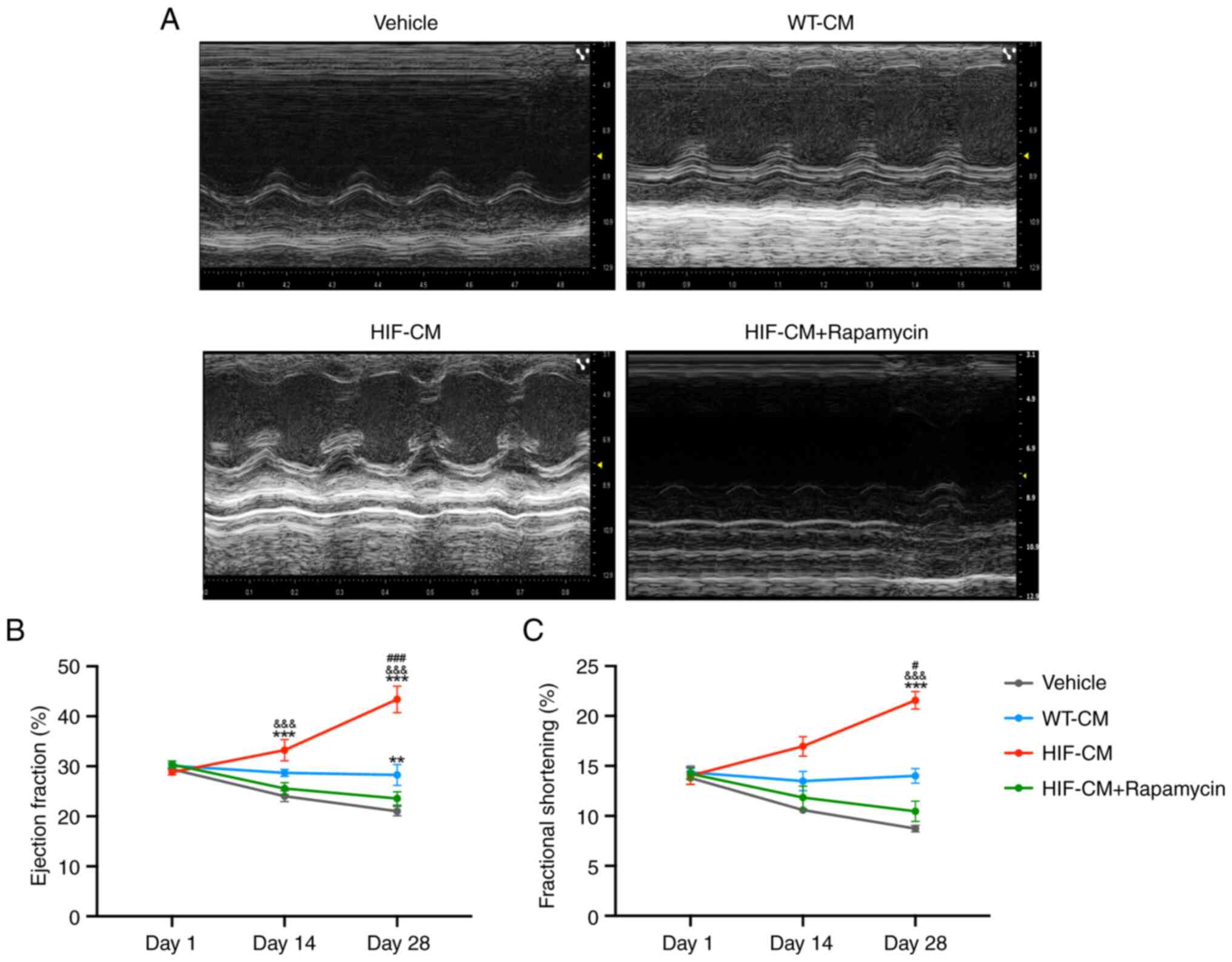 | Figure 5.HIF-CM transplantation promotes
cardiac function following MI. (A) Echocardiographic images were
acquired from mice in Vehicle group, WT-CM group, HIF-CM group and
HIF-CM + rapamycin group on days 1, 14 and 28 after MI induction
and used to calculate (B) LVEF and (C) LVFS. n=5. **P<0.01 vs.
Vehicle, ***P<0.001 vs. Vehicle;
&&&P<0.01 vs. HIF-CM + Rapamycin;
#P<0.05 vs. WT-CM, ###P<0.01 vs. WT-CM.
HIF-CM, HIF-1α-overexpressing hiPSC-CM cardiomyocytes; MI,
myocardial infarction; WT-, wild-type-; CM, cardiomyocytes; LVEF,
left ventricular ejection fraction; LVFS, left ventricular
fractional shortening. |
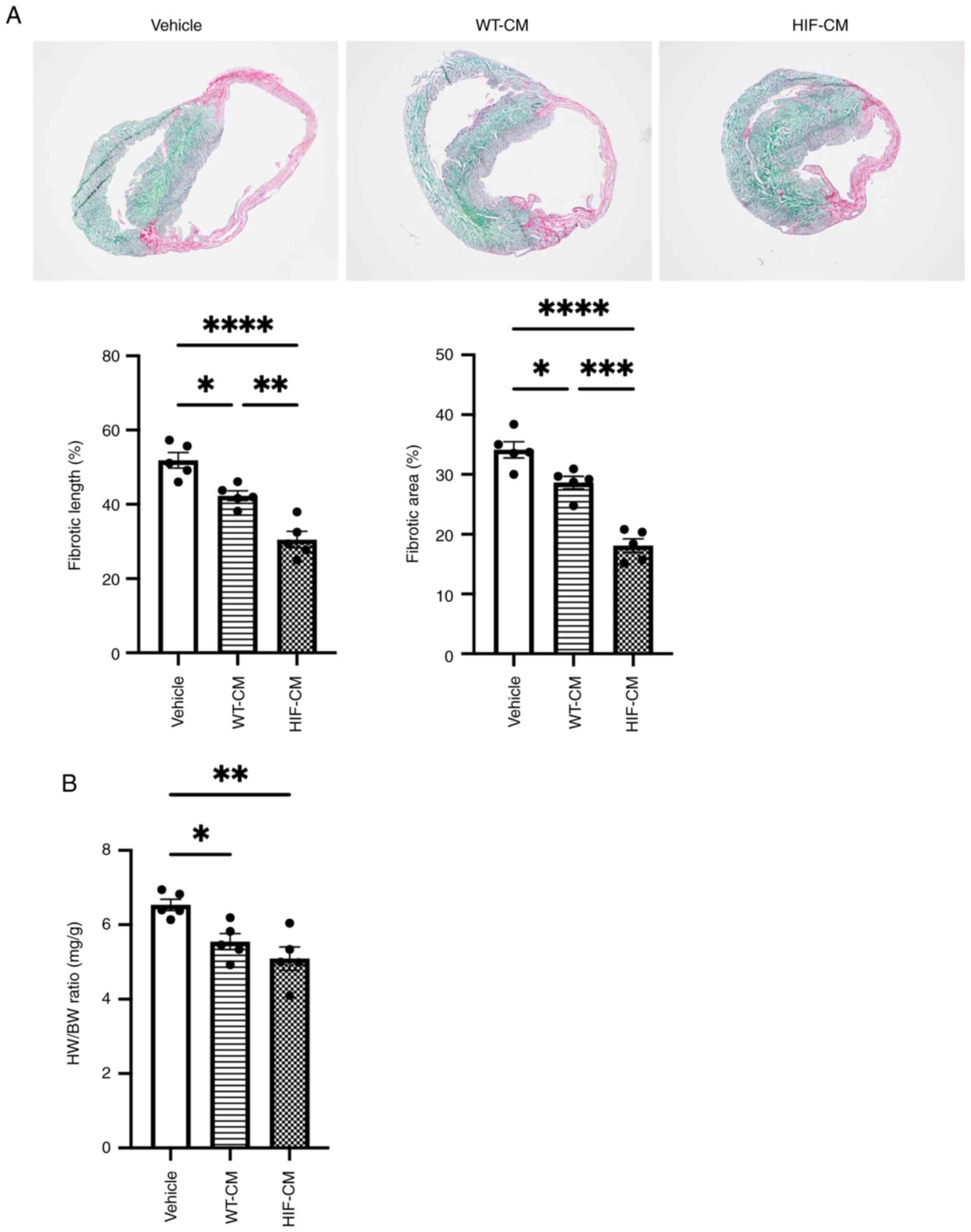
Scar size was also evaluated 4 weeks after MI
induction by Fast Green/Sirius Red staining to indicate fibrotic
tissue (Fig. 6A). Although WT-CM
transplantation could decrease scar size compared with Vehicle
treatment, HIF-CM further decreased scar size when compared with
WT-CM. Heart weight was recorded after sacrificing the mice to
calculate heart /body weight ratio (Fig. 6B). The results showed that HIF-CM
significantly reduced the Heart Weight/Body Weight ratio compared
with other two groups, suggesting that HIF-CM could prevent the
cardiac hypertrophy following MI. Altogether, transplantation of
HIF-1α overexpressed cardiomyocytes presented significant LV
functional recovery compared with WT-CM group following MI.
Angiogenesis is significantly improved
following transplantation of HIF-1α overexpressed
cardiomyocytes
Supported by the improved cardiac function and
reduced scar size, the present study sought to evaluate the
mechanism of this therapeutic effect. It first revealed that
concentration of angiogenic factors (VEGF and PDGF) in the
circulation was elevated at day 7 post MI with HIF-CM
transplantation (Fig. 7), which is
in accordance with the in vitro study. On day 28, mice were
sacrificed and the angiogenesis in the peri-infarction area of the
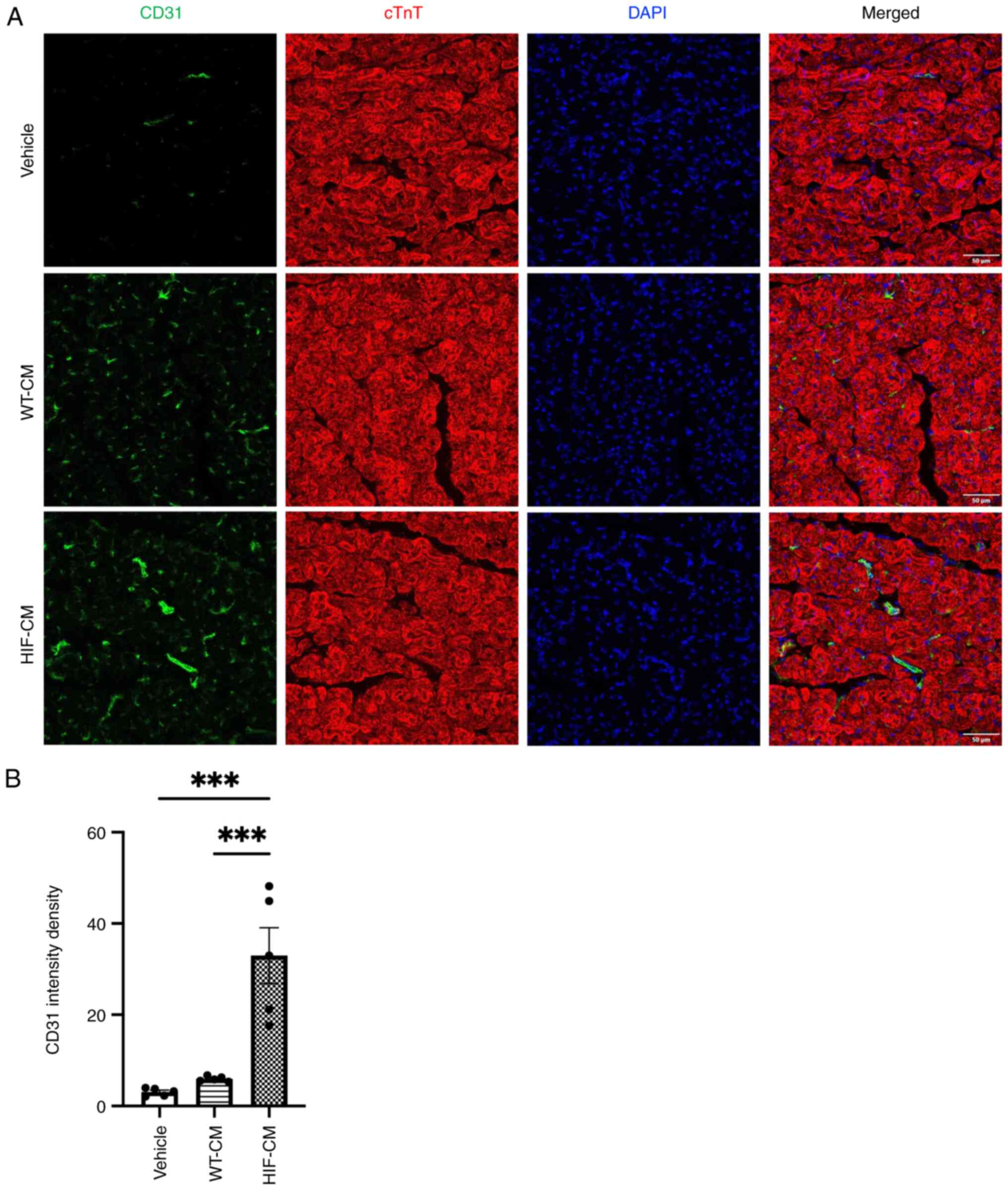
infarcted hearts evaluated. Immunostaining with CD31 reflected
neovascularization in the peri-infarct myocardium four weeks after
MI (Fig. 8A). The averaged
fluorescent intensity density in the ischemic border zones of left
ventricle was significantly greater in the HIF-CM-transplanted
group compared with the WT-CM-transplanted group and Vehicle group
(Fig. 8B).
Discussion
Previous studies have demonstrated the therapeutic
effects of hiPSC-derived cardiomyocytes in a murine infarction
model (5,21). Although these cells were able to
exert myocardial protection to a certain degree, the therapeutic
efficacy of the transplanted cells still needs to be improved
(22). Previous studies using
HIF-1α overexpressed stem cells exhibited sufficient
cardioprotection in MI (10,23),
which prompted the present study to genetically engineer
iPSC-derived cardiomyocytes as well by HIF-1α overexpression.
The present study genetically engineered an iPSC
clone overexpressing mutated HIF-1α (P402A and P564A), which could
enhance its stability under normoxia (13). The iPSC clone carries HIF-1α gene
that is expressed under control of a cardiac-specific promoter, MHC
promoter (24). After cardiac
differentiation, the present study was able to obtain contracting
CMs with a purity of 85%. CMs showed a clear mature phenotype with
strong expression of cTnT and MHC. Moreover, iPSC-derived CMs also
showed high expression of HIF-1α as determined by RT-qPCR.
Conditioned medium was collected from cardiomyocytes differentiated
from HIF-CM and WT-CM. In the tube formation assay the present
study was able to effectively enhance the tube formation of
hypoxia-injured HUVECs using conditioned medium from HIF-CM
compared with WT-CM. The data also showed that conditioned medium
from HIF-CM promoted the migration of hypoxia-injured HUVECs in
Transwell assay. These effects were probably caused by the enhanced
secretion of proangiogenic factors, VEGF, Ang-1 and PDGF. These
findings showed the paracrine effects of HIF-CM, which are also in
accordance with previous reports (10,11,25).
Following intramyocardial injection into ischemic
myocardium, the HIF-CM group exhibited enhanced neovascularization
compared with the WT-CM group. It was hypothesized that enhanced
neovascularization was induced by the overexpression of HIF-1α.
HIF-1α has been described as a master regulator of genes including
VEGF, erythropoietin and other factors critical to vascularization
(26). In the MI model of the
present study, HIF-CMs significantly improved cardiac functions
following injection into the infarct area. The echocardiographic
data suggested that following transplantation of HIF-CM enhanced
angiogenesis could be a critical reason for the survival of the
surrounding cardiomyocytes in the ischemic myocardium. Future
experiments will have to establish whether HIF-1α overexpression
may improve the myocardial connection in our model.
In summary, the present study demonstrated that the
direct intramyocardial transplantation of HIF-1α-engineered
iPSC-derived cardiomyocytes led to substantial functional
improvement and mitigated adverse remodeling 28 days post-acute MI,
as evidenced by both echocardiography and histological morphology
assessment. The preservation of LV thickness in the infarct zone
prevented progressive LV dilatation. Future investigations delving
into the mechanical mechanisms by which transplanted cells confer
therapeutic effects would enhance our understanding and potentially
advance stem cell therapy for ischemic heart diseases.
Acknowledgements
Not applicable.
Funding
The present study was supported by Natural Science Foundation of
Liaoning Province (grant no. 20170540527).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JD and LX conceived and designed the project. JD and
TW acquired and analyzed data. JD and LX wrote and revised the
manuscript. All authors read and approved the final manuscript. JD
and LX confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by The Fourth
People's Hospital of Shenyang Ethics Review Committee (Liaoning,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maiorino E and Loscalzo J: Phenomics and
robust multiomics data for cardiovascular disease subtyping.
Arterioscler Thromb Vasc Biol. 43:1111–1123. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Toscano O, Cosentino N, Campodonico J,
Bartorelli AL and Marenzi G: Acute myocardial infarction during the
covid-19 pandemic: An update on clinical characteristics and
outcomes. Front Cardiovasc Med. 8:6482902021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Severino P, D'Amato A, Pucci M, Infusino
F, Adamo F, Birtolo LI, Netti L, Montefusco G, Chimenti C, Lavalle
C, et al: Ischemic heart disease pathophysiology paradigms
overview: From plaque activation to microvascular dysfunction. Int
J Mol Sci. 21:81182020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Uygur A and Lee RT: Mechanisms of cardiac
regeneration. Dev Cell. 36:362–374. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nelson TJ, Martinez-Fernandez A, Yamada S,
Perez-Terzic C, Ikeda Y and Terzic A: Repair of acute myocardial
infarction by human stemness factors induced pluripotent stem
cells. Circulation. 120:408–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao M, Tang Y, Zhou Y and Zhang J:
Deciphering role of wnt signalling in cardiac mesoderm and
cardiomyocyte differentiation from human iPSCs: Four-dimensional
control of Wnt pathway for hiPSC-CMs differentiation. Sci Rep.
9:193892019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lalit PA, Hei DJ, Raval AN and Kamp TJ:
Induced pluripotent stem cells for post-myocardial infarction
repair: Remarkable opportunities and challenges. Circ Res.
114:1328–1345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Malkov MI, Lee CT and Taylor CT:
Regulation of the hypoxia-inducible factor (HIF) by
Pro-inflammatory cytokines. Cells. 10:23402021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng L, Yu H, Yan N, Lai K and Xiang M:
Hypoxia-inducible factor-1α target genes contribute to retinal
neuroprotection. Front Cell Neurosci. 11:202017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun J, Shen H, Shao L, Teng X, Chen Y, Liu
X, Yang Z and Shen Z: HIF-1α overexpression in mesenchymal stem
cell-derived exosomes mediates cardioprotection in myocardial
infarction by enhanced angiogenesis. Stem Cell Res Ther.
11:3732020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palomaki S, Pietila M, Laitinen S, Pesala
J, Sormunen R, Lehenkari P and Koivunen P: HIF-1α is upregulated in
human mesenchymal stem cells. Stem Cells. 31:1902–1909. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Masson N, Willam C, Maxwell PH, Pugh CW
and Ratcliffe PJ: Independent function of two destruction domains
in hypoxia-inducible factor-alpha chains activated by prolyl
hydroxylation. EMBO J. 20:5197–5206. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kageyama Y, Koshiji M, To KK, Tian YM,
Ratcliffe PJ and Huang LE: Leu-574 of human HIF-1alpha is a
molecular determinant of prolyl hydroxylation. FASEB J.
18:1028–1030. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lian X, Zhang J, Azarin SM, Zhu K,
Hazeltine LB, Bao X, Hsiao C, Kamp TJ and Palecek SP: Directed
cardiomyocyte differentiation from human pluripotent stem cells by
modulating Wnt/beta-catenin signaling under fully defined
conditions. Nat Protoc. 8:162–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
DeCicco-Skinner KL, Henry GH, Cataisson C,
Tabib T, Gwilliam JC, Watson NJ, Bullwinkle EM, Falkenburg L,
O'Neill RC, Morin A and Wiest JS: Endothelial cell tube formation
assay for the in vitro study of angiogenesis. J Vis Exp.
1:e513122014.PubMed/NCBI
|
|
17
|
Huang L, Chen S, Fan H, Ai F and Sheng W:
BZW2 promotes the malignant progression of colorectal cancer via
activating the ERK/MAPK pathway. J Cell Physiol. 235:4834–4842.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lugrin J, Parapanov R, Krueger T and
Liaudet L: Murine myocardial infarction model using permanent
ligation of left anterior descending coronary artery. J Vis Exp.
16:2019.PubMed/NCBI
|
|
19
|
Chen W, Pretorius D, Zhou Y, Nakada Y,
Yang J and Zhang J: TT-10-loaded nanoparticles promote
cardiomyocyte proliferation and cardiac repair in a mouse model of
myocardial infarction. JCI Insight. 6:e1519872021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lian X, Hsiao C, Wilson G, Zhu K,
Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ and Palecek SP:
Robust cardiomyocyte differentiation from human pluripotent stem
cells via temporal modulation of canonical Wnt signaling. Proc Natl
Acad Sci USA. 109:E1848–E1857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu W, Zhao M, Mattapally S, Chen S and
Zhang J: CCND2 overexpression enhances the regenerative potency of
human induced pluripotent stem cell-derived cardiomyocytes:
Remuscularization of injured ventricle. Circ Res. 122:88–96. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang J, Bolli R, Garry DJ, Marban E,
Menasche P, Zimmermann WH, Kamp TJ, Wu JC and Dzau VJ: Basic and
translational research in cardiac repair and regeneration: JACC
state-of-the-art review. J Am Coll Cardiol. 78:2092–2105. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Datta Chaudhuri R, Banik A, Mandal B and
Sarkar S: Cardiac-specific overexpression of HIF-1alpha during
acute myocardial infarction ameliorates cardiomyocyte apoptosis via
differential regulation of hypoxia-inducible pro-apoptotic and
anti-oxidative genes. Biochem Biophys Res Commun. 537:100–108.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Molkentin JD, Jobe SM and Markham BE:
Alpha-myosin heavy chain gene regulation: Delineation and
characterization of the cardiac muscle-specific enhancer and
muscle-specific promoter. J Mol Cell Cardiol. 28:1211–1225. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ai X, Yan B, Witman N, Gong Y, Yang L, Tan
Y, Chen Y, Liu M, Lu T, Luo R, et al: Transient secretion of VEGF
protein from transplanted hiPSC-CMs enhances engraftment and
improves rat heart function post MI. Mol Ther. 31:211–229. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rodriguez D, Watts D, Gaete D, Sormendi S
and Wielockx B: Hypoxia pathway proteins and their impact on the
blood vasculature. Int J Mol Sci. 22:91912021. View Article : Google Scholar : PubMed/NCBI
|