Introduction
The cornea, located at the front of the eye, is not
only part of the fibrous membrane of the eyeball but also a crucial
component of the refractive system and a physical barrier of the
eye. As a key refractive structure, the transparency of the cornea
is essential for maintaining normal vision. However, corneal
neovascularization (CNV) and corneal lymphangiogenesis (CL) can
lead to an increase in corneal turbidity and damage normal vision.
In addition, because these processes are integral to the immune
response in the cornea, where lymphatic vessels serve as afferent
arms for transporting antigen-presenting cells (APCs) and blood
vessels function as efferent arms, facilitating the transport of
immune effector molecules and generating an immune response, they
disrupt the inherent immune privilege of the non-vascular cornea,
ultimately reducing the survival time of transplanted corneas
(1). Nevertheless, certain studies
have indicated that CNV and lymphatic vessels also contribute to
the defense against corneal inflammation by enhancing immune
responses (2). Therefore, CNV and
CL have dual functions in the cornea.
CL shares some similarities with CNV in terms of
immune responses. For example, in animal models, CL often occurs
concurrently with CNV. The cytokines and signaling pathways that
promote or inhibit the development of CNV and/or CL also largely
act synergistically in the occurrence and progression of the
disease. However, there are differences in numerous aspects between
the two groups, such as growth characteristics and occurrence time.
The influence of cytokines on the two processes is also different,
mainly in terms of cytokine preferences and dosage sensitivity
(3).
However, due to the lack of research on CL, few
studies have systematically described the relationship between
them. Therefore, the present article reviews the research progress
on the relationships among the establishment of animal models,
growth characteristics, the influence of the cytokine pathway and
the role of disease.
The different roles that the two play in the
cornea
Under certain physical and chemical conditions, such
as alkaline burn irritation, corneal infectious diseases, immune
privileges are destroyed, neovascularization and lymphangiogenesis
appear, and the inflammatory response is intensified. CNV and CL
play different roles in this inflammatory response. As an input arm
of the immune system, corneal lymphatics produce transport APCs
that transport foreign and soluble antigen molecules to local lymph
nodes, where they activate immune effector cells such as T cells.
On the other hand, CNV acts as an output arm of the immune
response, transporting activated immune effector cells to the site
of inflammation, thereby intensifying the immune response (4) and acting as anti-inflammatory agents,
which may be related to ATP binding cassette subfamily B member 5
(5). In addition to specific
immune-related effects, CNV can also play non-specific
immune-related effects through components such as neutrophils and
macrophages (6), and can promote
corneal transparency by removing excess hyaluronic acid (HA)
(7). It has been hypothesized that
it may also help eliminate edema. In addition, it has been
suggested that moderate CNV may promote wound healing by generating
an inflammatory response to fight infection and by forming
granulation tissue to promote tissue repair (8). However, other studies have revealed
that CNV is associated with corneal edema, lipid deposition, and
further decrease in corneal clarity (9). In certain studies, CL is also
suspected to be related to the regression of corneal edema and play
a role in the fluid homeostasis of the cornea (10). The specific role of these two needs
to be further explored.
Relationship between CNV growth and
lymphangiogenesis
CNV and CL often accompany each other, but they are
regulated by different signaling systems. At the same time, their
growth patterns are different. Their processes of appearance,
growth and regression are very similar but concurrently have
numerous distinct characteristics. They play different roles in the
cornea, thus the newly formed lumen structure also has different
functions to perform the corresponding functions. It is important
to understand the relationship between the two to understand the
occurrence and development of the corresponding diseases. The
present study of CNV primarily employs CD31 staining for
identification, given that CD31 is highly expressed on vascular
endothelial cells (ECs) (11). By
contrast, CL is primarily identified through staining with
lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1), as
LYVE-1 is an analogue of CD44 and a receptor for HA, which is
abundantly expressed in the lymphatic system (12).
The difference and relationship
between the two processes
While CNV and CL often occur concurrently, they are
similar and different at the same time.
The growth patterns of CNV
The existing research on angiogenesis is relatively
thorough. Shi et al (13)
described CNV using five models (suture induction, alkali burn,
fungal infection and immunogen or tumor cell implantation) with
three key steps in its formation: Sprouting, progressive and
declination phases. Although the initial corneal edema and
vasodilatation in these five models are consistent with the
findings of Cogan (14), Shi et
al (13) provided a more)
detailed description of the pathogenesis of CNV. First, the
basement membrane (BM) of the limbal capillary network is degraded
by proteases released by ECs. Second, ECs migrate and invade the
extracellular matrix (ECM), followed by proliferation. Finally, the
lumen of new vessels forms, and the BM reshapes. An increasing
number of studies suggests that CNV and CL are initiated by the
budding of existing limbal vessels and lymphatics (3,15).
Additionally, accumulating evidence indicates that corneal edema is
not a prerequisite for the development of CNV (16,17).
The processes involved in CNV vary depending on the trigger, but
most steps are consistent. Therefore, the process of CNV can be
summarized as follows:
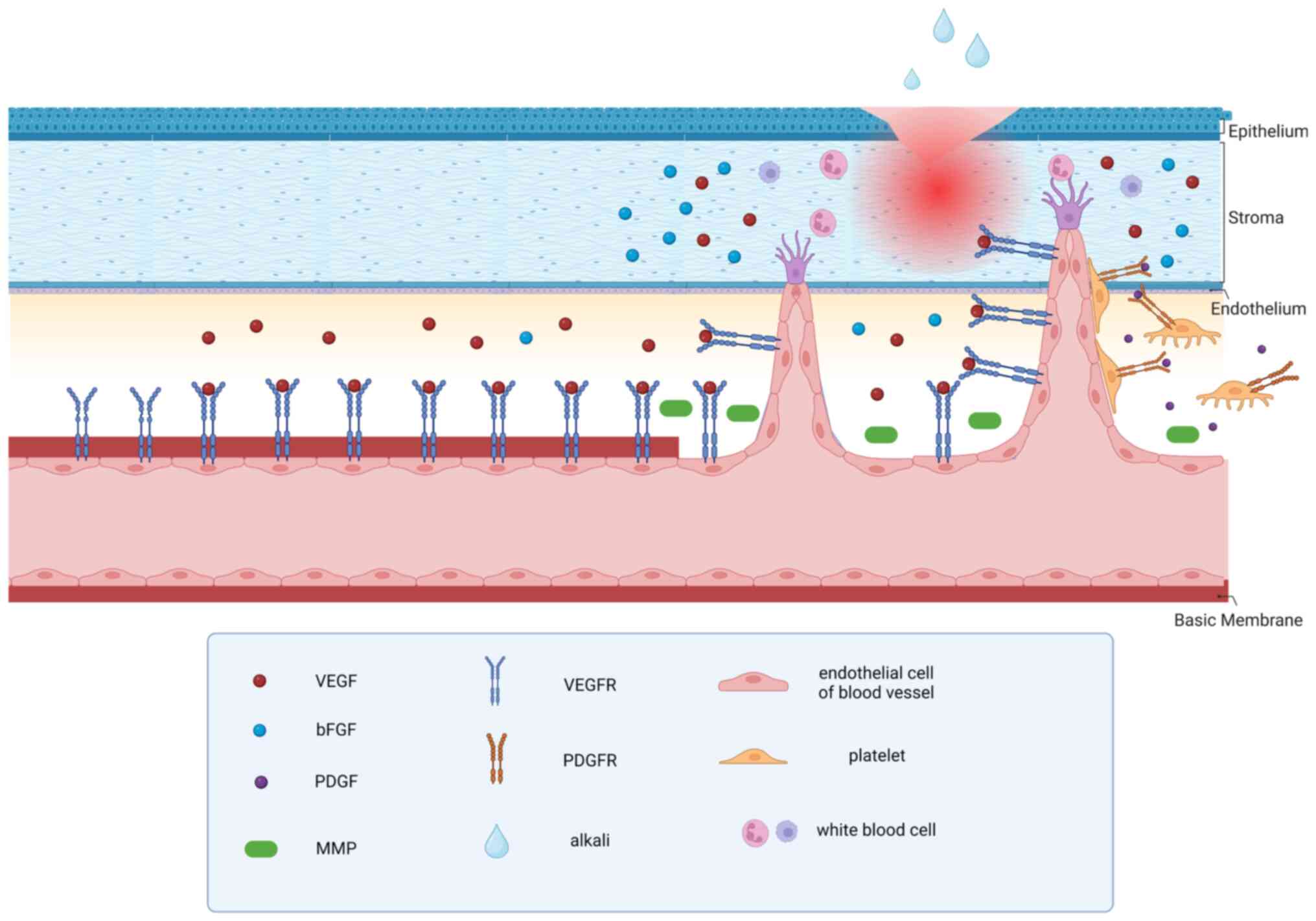
i) When corneal injury or inflammatory irritation
occurs, corneal edema, along with venules and capillary congestion,
corneal edema can also stimulate cells, such as corneal epithelial
cells, corneal ECs, vascular ECs and immune cells, to release
angiogenic cytokines, including VEGF and basic fibroblast growth
factor (bFGF). The upregulation of proangiogenic factors and the
downregulation of angiogenesis inhibitory factors, such as soluble
fms-like tyrosine kinase 1 (sFlt-1), metalloprotease 3, proteinase
inhibitors, angiostatin, endostatin, platelet response protein,
interleukin-1 receptor antagonist, pigment epithelial derived
factor, vasoactive intestinal peptide and α-melanocyte-stimulating
hormone, can occur under normal physiological conditions.
ii) Then, the combination of angiogenic factors and
receptors activates the ECs of corneal perilimbal blood vessels.
Corneal limbal ECs secrete matrix metalloproteinases to breakdown
the ECM and vascular BM. Simultaneously, the combination of VEGF-A
and VEGFR2 triggers vascular migration of ECs through the
RAS-RAF-MAPK-ERK signaling cascade, the PLCγ-ERK 1/2 pathway,
Ca2+ signaling, the PI3K-AKT pathway, small G protein,
the SRC pathway, stress kinases and STATs. This enables ECs to
migrate towards the cornea through chemotaxis (12,18).
iii) Some of these vascular ECs germinate and form
vascular buds on the original blood vessels. VEGF-induced ECs
exhibit a promigratory phenotype and undergo chemotaxis toward
inflammatory sites. At those sites they form a structure that looks
like a dendritic cell with a number of pseudopod-like structures to
invade the corneal stroma. These cells are called tip cells and
stalk cells. Tip cells are motile and invasive and can extend
numerous filamentous pseudopodia that can respond to growth
factors, the ECM, and other attractive or repulsive signals.
iv) A portion of ECs will undergo a transformation
into a tip cell phenotype and initiate sprouting. Subsequently, the
ECs (stalk cells) proliferate and contribute to the maintenance of
the structural and functional integrity of neovascularization. The
tips of the aforementioned pseudopod-like structures formed by ECs
continue to secrete MMP and promote further growth of the blood
vessels. However, new blood vessels are vulnerable due to the
absence of pericyte support (19).
v) As neovascularization progresses, platelets
release platelet-derived growth factor, which binds to receptors on
pericytes, ultimately leading to their proliferation and migration.
The chemotactic movement of pericytes to new blood vessels combines
with them to stabilize new blood vessels (20).
vi) Other stimuli that can induce CNV include
inflammation, hypoxia, limbal stem cell deficiency and denervation.
Different stimuli stimulate different cells and cytokines to
generate CNV. Inflammation triggers the release of cytokines such
as VEGF, bFGF and BMP from corneal epithelial cells, corneal ECs,
vascular ECs and immune cells, which promote CNV. Hypoxia leads to
the expression of VEGF in the corneal epithelium, ECs and
endothelium of the limbal blood vessels. Limbal stem cell
deficiency recruits macrophages, a significant source of VEGF, and
causes secondary increases in TGF-β, both of which promote CNV.
Finally, the deprivation of nerves results in a reduction in
corneal angiogenesis inhibitors (21). Although the initial processes of
these conditions vary, most of their downstream processes overlap
with those caused by inflammation-induced vasculo-genesis. The
common process is demonstrated in Fig.
1.
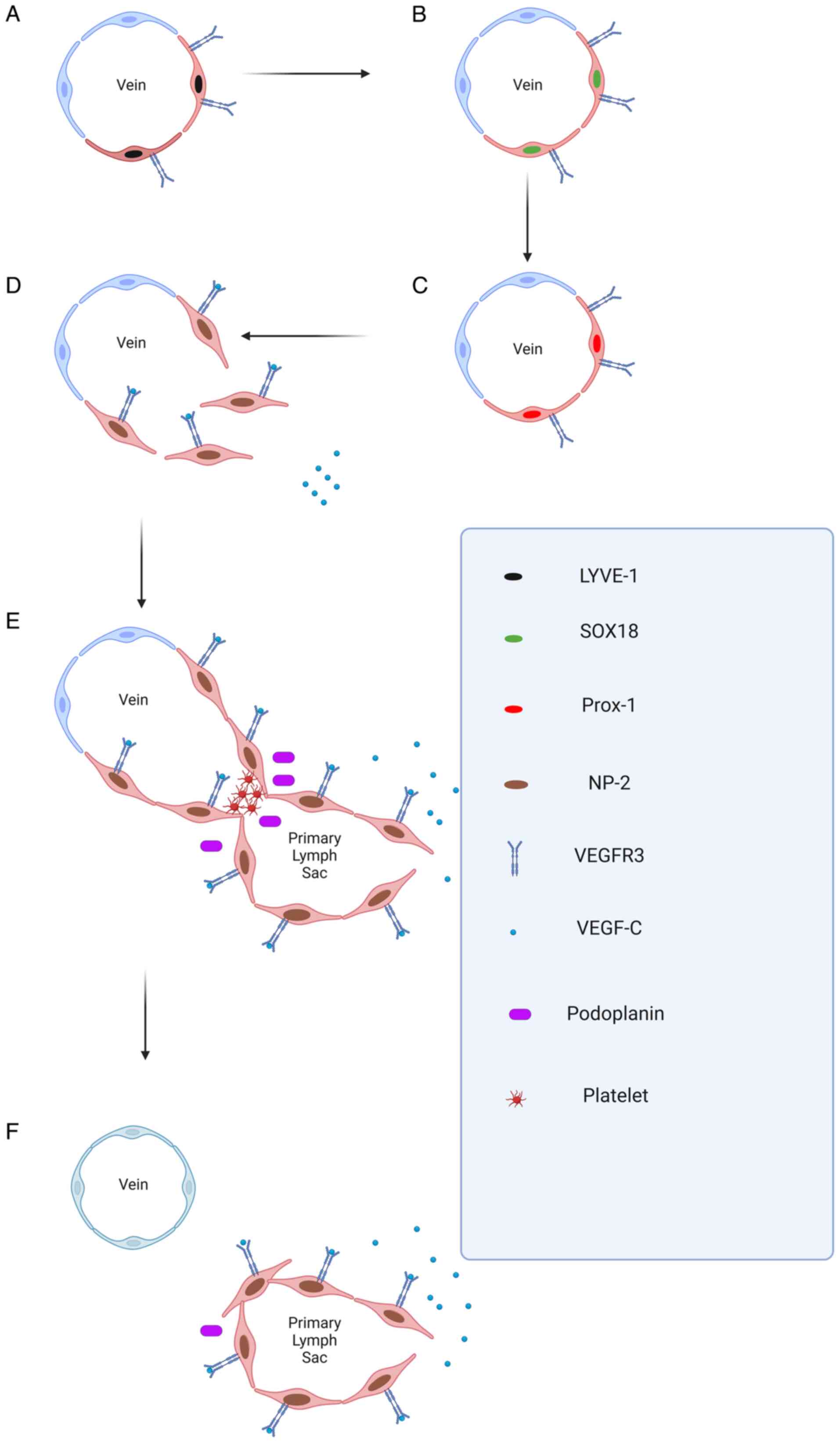
The growth patterns of CL
The formation process of new corneal lymphatic
vessels differs from that of CNV. The currently recognized
mechanism is that they sprout from the original blood vessels and
veins. The process is as follows:
i) The original venous vascular ECs express high
levels of VEGF-3 and LYVE-1, and a subset of bone marrow-derived
macrophages undergo trans-differentiation. Subsequently, both cell
types undergo the process of converting into lymphatic ECs.
ii) The original venous vascular ECs and a subset of
bone marrow-derived macrophages start to express the transcription
factor SOX18, leading to the upregulation of prospero-related
homeobox 1 (Prox-1). This represents a pivotal step in the
conversion of these cells into lymphatic ECs (22). Vascular ECs and bone marrow-derived
macrophages initially differentiate and sprout towards lymphatic
ECs (22).
iii) These two proteins express neuropilin-2 (NP-2),
and the synergistic effect of NP-2 and VEGFR-3 renders cells
sensitive to VEGF-3, which promotes the extension of lymphatic
endothelial tip cells and the continuation of sprouting (23).
iv) Afterwards, the lymphatic vessels bifurcate from
the vein. Lymphatic ECs express podophyllotoxin, which promotes
platelet aggregation, leading to the separation of
lymphangiogenesis vessels from veins.
v) VEGFR-3 activates downstream pathways such as the
Akt and p42/p44MAPK pathways, protecting lymphatic ECs from
apoptosis and further promoting EC migration (24,25).
These are the classic steps of CL. These steps are
also demonstrated in Fig. 2.
However, it is important to note that not all lymphangiogenesis
originates from the CNV. In the case of dry eye disease (DED),
there is a unique condition in which corneal neo-lymphatic vessels
are generated, but CNV is not. Multiple studies have revealed that
this may be related to the specific cytokine mechanism of dry
stress in DED (26), particularly
the secretion of IL-17 by Th-17 cells, which induces an increase in
VEGF-D (27), and the downstream
pathways that lead to an increase in VEGF-3 induced by SP (28). In addition to budding from veins,
some studies have suggested that lymphatic vessels may originate
from mesenchymal lymphatic stem cells (29), but this theory has not been
described for CL and has not been confirmed in mammals. Therefore,
it will not be discussed in the present review.
The process of generating CNV and CL are described
in detail. There are several similarities between CNV and CL in
terms of stimulating factors and activating cells, but they also
have their own unique features. The details are presented in
Table I.
 | Table I.Characteristics of CNV and CL. |
Table I.
Characteristics of CNV and CL.
| Points of
comparison | CNV | CL | (Refs.) |
|---|
| Stimulating
factor | Infection, chemical
burns, corneal sutures, immune response | Infection, chemical
burns, corneal sutures, immune response | (2,36,40,46) |
| Stimulating
cytokines | Ang-1,2 | VEGF-A, C, D, bFGF,
Ang-1 | (33,83,84,112) |
| Activating
cells | Vascular
endothelial cells | Primary venous
vascular endothelial cells, bone marrow-derived macrophages | (18,22) |
| Activation
pathway | VEGF-A/VEGFR-1,2
VEGF-C/VEGFR-2 | VEGF-C,
D/VEGFR-3 | (33) |
| Migratory
route | Hydrolyses the
basement membrane, invading the ECM and forming a lumen | The original venous
endothelial cells and bone marrow-derived macrophages are
transformed into lymphatic endothelial cells, and the blood vessels
emit the anterior lymphatic tip. After the lumen sprouts and
bifurcates, the new lymphatic vessels are separated from the veins
to form lymphatic valves | (13,18–25) |
| Cells produced by
the process | Vascular
endothelial cell | Lymphatic
endothelial cells | (30,31) |
| Key steps in
development | Sprouting,
progressive and declination phases | Germination,
branching, proliferation, differentiation and remodeling
processes | (13,18–25) |
| Luminal
markers | Endoglin (membrane
glycoprotein), neuropilin-1 (transmembrane receptor), collagen IV
(ECM molecule) | Prox1
(transcription factor), podoplanin (transmembrane glycoprotein),
LYVE1 (transmembrane receptor), VEGFR3 (receptor tyrosine kinase),
CCL21 (expressed in secondary lymphoid organs) | (112) |
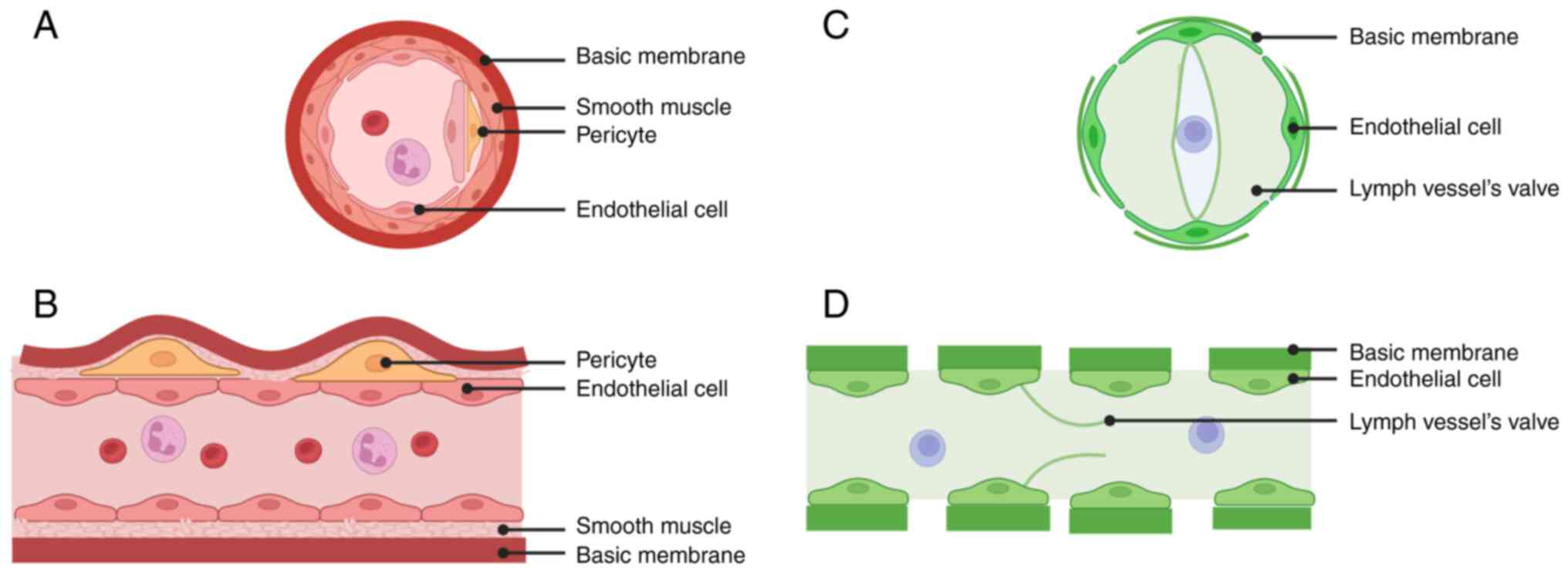
Tubular morphology
CNV is a tubular structure surrounded by ECs, with a
wall composed of a single layer of ECs surrounded by smooth muscle
cells and peripheral cells and an incomplete BM on the outermost
layer. CNV arises from the sprouting of veins behind existing
capillaries, and the process of its formation can be explained by
the tips and stalk cells. In addition, CL involves a tubular
structure surrounded by a single layer of lymphatic ECs. The ECs of
the lymphatic vessels are surrounded by an incomplete BM, while the
lumen is protected from reflux by lymphatic flaps. The formation of
CNV occurs by sprouting from existing veins (30–32)
Their morphologies are shown in Fig.
3.
Both structures share several similarities in terms
of luminal morphology. They are both surrounded by a single layer
of ECs, have discontinuous BMs, and sprout from pre-existing veins.
However, there are also numerous differences:
i) CL involves the presence of a lymphatic valve
that assists the entry and exit of APCs, but it does not involve
the presence of blood vessels;
ii) The wall involved in CNV contains pericytes and
smooth muscle cells in addition to ECs. On the other hand, the wall
of the corneal lymphatic vessel consists of a single layer of cells
with a large lumen;
iii) CNV clearly involves red blood cells, while CL
does not (30,31). However, in addition to the
difference between CNV and the formation of new lymphatic vessels,
there are also differences in the signaling pathways that induce
new blood vessels and lymphatic vessels; (iv) Studies on CNV
indicate that lymphatic vessels affected by VEGF-A/VEGFR-2 are
structurally and functionally different from those affected by
VEGFR-3 ligands. These vessels exhibit a relatively dilated, leaky,
and poorly functional phenotype (26). In terms of new corneal lymphatic
vessels, Cao et al (33)
reported that the lymphatic vessels affected by bFGF and VEGF-C
were larger than those affected by bFGF alone, which may be related
to the synergistic effects of different cytokines.
Temporal regularity of
angiogenesis
As early as 2002, Cursiefen et al (34,35)
reported that although CL occurs simultaneously with CNV, CL
degrades earlier than CNV. This result was obtained again in 2006.
Although CNV and lymphangiogenesis generally occur together, subtle
differences exist in the temporal regularity of angiogenesis in
different models.
CNV coexists with corneal lymphatic
vessels
In the case of bacterial keratitis, CNV resulted in
significant proliferation on the seventh day of modelling, but CL
increased more slowly on the fourteenth day than did CNV (2). In the case of alkaline burns,
although CL vessels grow parallel to CNV vessels, CL vessels sprout
approximately at the third day after neovascularization occurs, but
at the same time point of detection, the density and length of CL
vessels are smaller than those of neovascularization vessels, and
there is still a certain lag compared with CNV vessels (36). In the corneal suture model, the
situation of CL vessels and CNV is similar to that of alkali burns.
However, the generation of CL vessels is greater than that of
alkali burns, and even on the third day, the area of CL vessel
proliferation is greater than that of CNV (36,37).
Although CNV and CL have distinct characteristics in these three
types of patients, they are still concomitant overall.
Only corneal lymphatics are
present
In DED, only CL occurs, without CNV. This suggests
that corneal neogenesis and lymphatic vessels grow in parallel or
that the preference for blood vessels over lymphatic vessels is not
universally applicable. In DED, this phenomenon has been proven to
be related to specific cytokine pathways; for example, TH-17 cells
secrete IL-17 directly, leading to an increase in VEGF-D, which
promotes lymphatic vessel growth (27). SP promotes an increase in
downstream VEGF-3 secretion in signaling pathways (28). There is currently no consensus on
why CNV does not occur in DED. However, according to the
experiments conducted by Chang et al (3), when the dose of bFGF was reduced to
12.5 ng, lymphatic vessel proliferation was significant,
accompanied by only a small amount of neovascularization. The
process of CNV is highly sensitive to dose, therefore it can be
inferred that this phenomenon may be related to the concentration
of cytokines that stimulate corneal growth. This is despite the
fact that there are now cases where only CL are present. However,
by reviewing the relevant articles, no case was found where only
CNV occurs without CL. This idea is very novel and provides a
direction for the future research.
Methods of animal modelling
In most cases, due to the coexistence of CNV and CL
vessels, the modelling of CNV and CL vessels is universal.
Currently, the most commonly used animal models are alkali burn and
corneal suture models. In addition, there are specific models
developed for different situations, such as the infectious
keratitis model, which explores the generation of corneal blood
vessels and lymphatic vessels during corneal infection; the low-
and high-risk corneal transplantation model, which explores the
impact of neovascularization and lymphatic vessels on the success
rate of corneal transplantation; and the corneal stromal
implantation bFGF model, which explores the effect of bFGF on CNV
and lymphatic vessel generation.
Alkali burn model
The alkali burn model is a frequently employed model
for studying chemical eye trauma in experiments. As early as 2010,
Shi et al (36) confirmed
the presence of blood vessels and lymphatic vessels in a corneal
alkali burn model. The modelling method calculates the dosage
according to the different animals, and then an appropriate dose of
sodium hydroxide solution is used to directly drip into the eyes or
soak the sodium hydroxide solution of this concentration on the
upper filter paper and stick it on the animal cornea. After a
period of time, the alkaline solution is discharged, or the filter
paper is removed (35). Although
this model is primarily used to investigate the treatment of
corneal alkali burns, it is also utilized to examine the impact of
various substances on blood vessels and lymphangiogenesis following
an alkali burn. For example, Song et al (38) utilized this model to study the
impact of leucine rich α-2-glycoprotein 1 (LRG-1) on CNV and
lymphangiogenesis in the cornea (38). Compared with other methods, the
model offers several advantages. First, the modelling method is
simple and has minimal requirements for reagents and resources.
Second, it is reproducible and can significantly reduce
interference from factors other than variables. Third, it provides
high controllability. Finally, the corneal alkali burn model can
effectively simulate the condition of the cornea during alkali
burns, which is very useful for studying the regulatory mechanism
of corneal angiogenesis, the process of corneal inflammation and
fibrosis, and the effects of related drugs. This method also has
several limitations, such as its consistency being affected by
equipment and drug specifications, the absorption of indoor air
CO2 by NaOH solution impacting the results, and other
factors. Overall, this method has more advantages than
disadvantages and remains a common technique for mold preparation
(39).
Corneal suture model
Corneal suturing is another widely used method to
study the relationship between the two parameters. It was first
used to induce CNV and was later also used for studying CNV and the
formation of new lymphatic vessels in the cornea (40). Its main molding method involves
placing three stitches in the corneal stromal layer using a 10/0
nylon suture under a surgical microscope. The needle is then
inserted into the center of the pupil from a distance slightly
<2.0 mm from the corneal limbus (specific methods may vary for
different animals). It is important to note that the specific
method used may vary between animals. At present, this modelling
method is mainly applied to study the roles of different cytokines
in CNV and CL vessels. For example, Maier et al (41) used a corneal suture model to study
the effect of TNF-α receptor deficiency on mouse CNV and
lymphangiogenesis. This method is simple to use and prevents
corneal damage caused by chemical reagents. The incidence of
postoperative infection is low, but it can be influenced by the
skill level of the operator and the size of the suture line and may
induce protective eye rubbing in experimental animals, leading to
model failure. Further research is needed to determine how to avoid
these shortcomings.
Infectious keratitis model
Infection by various pathogens can lead to corneal
inflammation, which in turn triggers the formation of new lymphatic
vessels and blood vessels. At present, the most common model of
bacterial keratitis is Pseudomonas aeruginosa-induced
keratitis (42), while the most
common model of viral keratitis is caused by the herpes simplex
virus type 1 (HSV-1) (43).
Currently, these two models are primarily used to investigate the
impact of corneal blood vessels and corneal lymphatic vessels on
the prognosis of patients with infectious keratitis. For example,
Narimatsu et al (2) used
Pseudomonas aeruginosa-induced infectious keratitis to study
the impact of CL vessels on the prognosis of infectious keratitis.
Moreover, these two models also have applications in studying the
role of macrophages in neovascular and lymphatic vessels, as well
as the factors that influence the timing of their formation
(44). This method can effectively
simulate the conditions of infectious keratitis. Because keratitis
caused by different pathogens may require different treatment
methods, the current approach is not reproducible and cannot
establish a standard process. Therefore, further improvement is
still needed.
The cytokine particle implantation
model
In this novel modelling method, cytokine particles
related to CNV and the development of CL vessels are implanted at
the corneal edge to study the corresponding roles of these
cytokines. The specific methods used are as follows: The sucralfate
and cytokines are placed in a centrifuge tube and completely
dissolved. A certain amount of hydron dissolved in anhydrous
ethanol is added, and the mixture is dried on a nylon mesh to
prepare particles containing cytokines. Cytokine particles are
implanted into the corneal edge within 1 mm of the corneal stroma.
At present, bFGF particles have been successfully implanted into
the corneal margin in experimental research, and it has been
verified that they can promote the production of blood vessels and
lymphatic vessels (45).
The keratoplasty model
With the widespread use of keratoplasty, there is an
increasing interest in studying the role of neovascularization and
lymphatic vessels in this procedure. The use of animals to simulate
human corneal transplantation is also one of the currently
available methods for inducing CNV and lymphatic vessel formation.
Its main modelling method is to simulate the situation of corneal
transplantation. For example, the specific process of simulating
penetrating keratoplasty involves replacing and removing the
corneas of both the donor and recipient mice, suturing the corneas
of the donor mice to the eyes of the recipient mice, using nylon
thread sutures, injecting sterile air and performing antibacterial
treatment, and finally removing the thread. Similar to other types
of surgical methods, the process of human corneal transplantation
can be reproduced in animals. The application of this method has
focused primarily on studying the relationship between the two in
the context of corneal transplantation (46).
Other
In addition to the aforementioned model, there are
other models used to induce CNV and CL, such as the incision model
and the limbal injury model. The incision model simulates corneal
trauma by using a surgical blade after central cyclin labelling of
the mouse cornea (47). The limbal
injury model involves debridement after corneal injury to disrupt
the integrity of the limbal epithelium to study its effect on
corneal repair (48), although
this approach is less commonly used.
Effects of different cytokine signaling
pathways on CNV and lymphangiogenesis
Vascular endothelial growth factor (VEGF)
family
Direct role of VEGF in CNV and
lymphangiogenesis
VEGF and its associated receptor VEGFR are important
cytokines that maintain the growth of corneal neovascular ECs and
CL cells. The main members of the VEGF family include VEGF-A,
VEGF-B, VEGF-C and VEGF-D, all of which are associated with the
generation of CNV or CL vessels. They act by stimulating VEGFR1-3,
in which VEGF-C plays a role in promoting the growth of corneal
lymphatic vessels in newborns primarily by stimulating VEGFR-3, but
it can also occasionally stimulate VEGFR-2. Moreover, VEGF-C can
stimulate CNV by stimulating VEGFR-2. VEGF-D is generally similar
to VEGF-C. The main function of VEGF-A is to stimulate
neovascularization, and it can specifically stimulate VEGFR-1 and
VEGFR-2, promoting corneal vascular growth (1).
In addition, VEGF-A activation of VEGFR-2 also plays
a role in promoting lymphangiogenesis. VEGF-A stimulation of
VEGFR-2 leads to the formation of lymphatic vessels that differ in
morphology from those formed by VEGF-C. Compared with those induced
by the VEGFR-3 ligand, VEGF-A/VEGFR-2-induced vessels exhibit a
dilated, leaky and poorly functional phenotype (49). However, in keratitis caused by
HSV-1 infection, VEGF-A, which is driven by the immediate early
gene product of HSV-1-infected cell polypeptide 4, also activates
corneal lymphatic vessel growth promoted by VEGFR-2, which is the
primary pathway for corneal lymphatic vessel growth (50,51).
Since VEGF itself is closely related to the
formation of CNV and CL, drugs targeting VEGF and its receptors
have been widely used in clinical ophthalmic diseases to reduce the
formation of CNV and CL. Faricimab, as a novel specific antibody
against VEGF-A, has been widely used in the treatment of macular
degeneration, but there is no study on its clinical application in
CNV (52). In addition to
Faricimab, anti-VEGF-A drugs currently on the market also include
aflibercept, Ranibizumab and Brolucizumab, which are mainly applied
to retinal and choroidal diseases such as retinal vascular
obstruction and macular degeneration (53–56).
Except these drugs, there are numerous anti-VEGF drugs on the
market, such as Ivonescimab for small cell lung cancer (57). However, these anti-VEGF drugs have
not been used in the clinical trials' field of inhibiting CNV and
CL.
Cytokines mediated by VEGF in CNV and
CL
The effects of numerous inflammatory cells on CNV
and CL can be attributed to the secretion of VEGF. For example, Cho
et al (58) reported that
mast cells can secrete VEGF-A to promote angiogenesis and can
specifically secrete high levels of VEGF-D to promote
lymphangiogenesis. The effects of other cytokines on CL and
neovascularization also depend on this pathway. TNF-α can drive CL
and CNV (59), primarily by
activating the NF-κB signaling pathway, which stimulates
macrophages to produce VEGF-C (60) or corneal dendritic cells and
macrophages to express VEGF-C/VEGFR-3 (61,62).
Interleukins such as IL-1β and IL-10 promote CNV by attracting
neutrophils and macrophages and enhancing VEGF-A and VEGF-C
expression in macrophages (63–65).
IL-1β mainly attracts macrophages through IL-1β secretion and the
IL-1RI receptor (66). The
chemokine receptor CXCR-3 on macrophages recruits marrow-derived
macrophages to sites of inflammation, which is closely associated
with CNV. Its absence can increase VEGF levels and drive corneal
angiogenesis (67). In 2019,
Narimatsu et al (2)
demonstrated that VEGF also plays an important role in bacterial
keratitis caused by Pseudomonas aeruginosa. Specifically,
bacterial lipopolysaccharide (LPS) stimulates F4/80-positive
macrophages to promote VEGF-C release through the Toll-like
receptor (TLR) 4 pathway, promoting the growth of new corneal
lymphatic vessels and neovascularization (2). Song et al (38) reported that LRG-1, an emerging
factor that promotes CNV and CL, is also related to VEGF. The
authors hypothesized that LRG-1 induces VEGF secretion through
TGF-β/Smad signaling or HIF-1α activation. However, the underlying
mechanism still needs to be investigated (38,68,69).
Signaling pathways that function
through VEGF
At present, Notch pathway is the signaling pathway
that plays a role through VEGF. Current studies on Notch pathway
have found that the Notch pathway and VEGF system interact; VEGF
can stimulate the Notch pathway, and the Notch pathway stimulates
VEGF-A expression through a feedback loop. In addition, Hu et
al (70) reported that the
bone morphogenetic protein 4 (BMP4/Smad pathway can enhance the
Notch pathway through hairy and enhancer of split 1 (HES1)
expression, and that overexpression of HES1 can inhibit CNV.
Previously, BMP4 has been revealed to affect CNV by binding to
BMPR-I/II, causing c-Src phosphorylation and activation of VEGFR-2
(71).
In addition to the Notch pathway, numerous of the
signaling pathways that promote CNV and CL can ultimately be
attributed to the influence of the VEGF family, therefore numerous
therapeutic approaches also focus on blocking or inhibiting the
signaling pathways acting through VEGF to reduce the production of
CNV and CL. Shokirova et al (72) found that local proliferation of
corneal vascular ECs during CNV could lead to upregulation of KOR
expression. They also found that nalfurafine-activated KOR can
inhibit VEGF production by inhibiting the g-αi/o pathway of
adenylate cyclase. Liu et al (73) inhibited the IL-1/IL-1RI/ERK
signaling pathway through AS-1 to reduce the production of
pro-inflammatory cytokines, recruitment of neutrophils and
macrophages, and thus reduce pro-angiogenic factors. The pathway of
TLR2/NF-κB inducing macrophage production of VEGF-C and VEGF-D has
also been confirmed by Song et al (74). This pathway can be inhibited by the
secretion of TNF α-induced protein 6 (TSG-6) by mesenchymal stem
cells (MSCs). In addition, it was found that TSG6 may indirectly
reduce macrophage recruitment by regulating TLR/NF-κB signaling in
laparoscopic endoscopic cooperative surgery. In addition to these
studies, Bai et al (75)
found that Wilms' tumor 1-associated protein could regulate
N6-methyladenosine modification to influence C-C motif chemokine
ligand 2, consequently promoting recruitment of macrophages to
secrete VEGF to promote CNV and CL.
The use of inhibitory molecules acting on VEGF and
its receptors to reduce CNV and CL is also a research direction.
Zhang et al (76,77) and Le et al (78) focused on studying the VEGF receptor
TrapR1R2, which can bind to VEGF to inactivate it. They found that
the use of eye drops effectively inhibited CNV and CL and improved
survival after transplantation. Salabarria et al (79) inhibited the effect of VEGF-C/D
through VEGFC/DTrap and inhibited the growth of corneal
lymphangiogenic vessels, but the results did not show an
improvement in graft survival. In addition, synthetic L-783277
derivatives have also been reported to inhibit VEGFR-2 and VEGFR-3
(80). Cho et al (81,82)
reported that both the β-blocker timolol and the insecticide
resistant albendazole inhibited CNV and CL by blocking the
VEGF-VEGFR pathway.
bFGF
The mechanism by which bFGF affects CNV and CL is
complex. In general, the pathways that promote CL depend not only
on VEGF-C and VEGF-D acting on VEGFR-3 (83,84)
but also on dose-specific regulation of CNV and CL (85). When the dose of bFGF was reduced to
12.5 ng, lymphatic vessel hyperplasia became evident, and only a
small amount of neovascularization was observed (3). According to the findings of Xie et
al (45), the Notch/Dll4
pathway can activate the bFGF system, while the Notch pathway and
the VEGF system simultaneously interact. VEGF can stimulate the
Notch pathway, which can stimulate the expression of VEGF-A through
a feedback loop (86,87). This finding demonstrated the
intricate relationship between the VEGF family and bFGF. However,
in some cases, such as HSV-1 infection, bFGF can act as a master
regulator of other proangiogenic and lymphangiogenic factors. This
has profound effects on HSV-1-induced CNV and is associated with
improved visual outcomes (44).
Because bFGF is beneficial to the recovery of corneal injury, it
has the potential to be applied to promote the recovery of corneal
surface injury. However, bFGF-related drugs have not been approved
by FDA (88). In China, although
bFGF is also prohibited for injection, topical gel has been
approved for clinical trials of injury healing, especially
stomatology-related injuries (89). At present, there are no reported
drugs that inhibit CNV and CL by inhibiting bFGF. In general, the
current clinical application of bFGF is focused on promoting
corneal injury recovery.
Neuropirim
Neuropilin-1 and neuropilin-2 are the coreceptors of
VEGFR-2 and VEGFR-3, respectively. Currently, researchers are
exploring their role in inhibiting CNV and CL. The class 3
semaphorin family is a soluble ligand of neuropilin, which can
inhibit its ability to promote CNV and CL. Sema3F is a new member
of this family and has the same function (32,67).
Calcitonin gene-related peptide
(CGRP)
CGRP is a 37-amino-acid neuropeptide that is
extensively expressed in both the central and peripheral nervous
systems. The trigeminal ganglion in the cornea has also been
revealed to secrete CGRP, mainly by forming heterodimers with the
calcitonin receptor and receptor activity-modifying proteins to
send signals (90). Zhu et
al (91) reported that
although CGRP reduced the production of the inflammatory cytokines
TNF-α and IL-1β in the cornea after CD45+ leukocyte
infiltration and mechanical injury in vivo, CGRP promoted
VEC proliferation, migration and blood vessel formation in
vitro. The effect of CGRP on lymphangiogenesis is similar to
that on angiogenesis; CGRP increases the formation and branching of
lymphatic vessels in the cornea. According to Majima et al
(92) VEGF is the downstream
signaling molecule of CGRP. Zhu et al (91) demonstrated that changes in VEGF-A
expression correlate with changes in CGRP signaling in vivo.
However, further research is needed to determine the exact
relationship between these changes and their mechanism of
action.
Chemokines
Chemokines play a crucial role in CL and CNV, and
there is currently considerable research on them. For example, Li
et al (93) recently found
that the cell surface chemokine receptor CXCR-3 can attract
marrow-derived macrophages to sites of inflammation, and its
reduction leads to increased VEGF, promoting corneal angiogenesis.
There is also a class of atypical chemokine receptors known as
decoy receptors for chemokines. These receptors have the ability to
neutralize chemokines and inhibit their effects (94). Chevigné et al (95)reported that ACKR2 can bind CC
chemokines and CXCL10 to inhibit CNV and CL in an HSK mouse model.
The mechanism may be related to its ability to indirectly limit
corneal angiogenesis by clearing macrophages. Muramatsu et
al (96) reported that
SDF-1/CXCL12 and its receptor system are associated with corneal
opacity and promote CNV and the formation of new lymphatic vessels,
while DSCR-1 can inhibit this process.
Other cytokines
At present, studies on the cytokine pathway involved
in CNV and CL have focused mainly on VEGF and bFGF. Most studies on
other factors, such as BMP and TGF, primarily attribute their
effects to VEGF. The remaining factors, such as integrins, HA and
IL-17, are not currently available, therefore they will not be
discussed again.
Diseases related to both
Corneal transplantation
There are typically no blood vessels present in the
normal cornea. Due to its avascular nature, the cornea has immune
privilege and a lower rejection rate. However, when the cornea is
damaged, CNV and CL vessels are produced, forming the immune arms
of the cornea. The CL vessels act as the afferent arms,
transporting antigen substances and sensitizing lymph nodes, while
CNV acts as the efferent arms, promoting activated immune cells to
infiltrate the inflammatory site, which undermines the immune
privilege of the cornea and reduces the success rate of corneal
transplantation (1). According to
the study by Zhang et al (77), CNV and CL are more prevalent in
cases of corneal transplantation than in other models. This finding
also highlights the significance of CNV and CL in the rejection of
corneal transplantation.
At present, numerous researchers are also focusing
on various methods to inhibit the generation and development of CNV
and lymphangiogenesis vessels to improve the success rate of
corneal transplantation. Reuer et al (67) utilized the combination of Sema3F
and neuropirin to inhibit the activation of VEGFR and the
production of CNV and lymphatic vessels, and especially after a
period of treatment cessation, the graft survival rate was also
very high, indicating that this treatment method has certain
clinical application value. Ren et al (97) reported that myeloid-derived
suppressor cells increase the expression of the antiangiogenic
factor Tsp-1 and decrease the expression of the proangiogenic
factors VEGF-A and VEGF-C through the iNOS pathway, inhibiting the
generation of CNV and CL vessels. Ren et al (98) found that promoting the effect of
myeloid suppressor cells through rapamycin can improve the success
rate of corneal transplantation, while Wei et al (99) proposed that Cannabidiol can also
enhance the effect of myeloid suppressor cells. In addition to
myeloid suppressant cells, Kang et al (100) found that human umbilical cord
MCSs and human adipose MSCs could also inhibit the formation of
neovascular lymphatic vessels and corneal neovascularity to improve
the survival rate of corneal transplantation, which was stronger
than human adipose MSCs. Concurrently, Yu et al (101) found that insulin-like growth
factor-1 modified mRNA can enhance the role of human adipose MSCs
and promote corneal injury repair. Intravenous injection of
cytokine-treated bone marrow cells can improve the survival rate of
corneal transplant grafts (97).
Zhu et al (102) also
reported that isolated induced myeloid-derived suppressor cells
could inhibit CNV through the iNOS pathway, improving the success
rate of corneal transplantation. Shokirova et al (72) found that inhibiting adenylyl
cyclase through Gαi/o using the κ opioid receptor agonist
nanofuranin could suppress the production of VEGF and produce
analgesic effects similar to those of opioid receptor agonists, but
it does not have side effects caused by MOR activation, making it
an ideal treatment for inflammation after corneal transplantation.
Zhu et al (103) proposed
that membrane collagen could be crosslinked with riboflavin and UVA
to combat corneal inflammation. Subsequently, Hou et al
(104) further confirmed that
this therapy can promote the regression of mature CNV and CL
vessels after transplantation and can be used to improve the
survival rate of corneal grafts. Zhang et al (76) proposed that local application of
VEGFTrapR1R2 can further inhibit CNV and CL vessels by specifically
inhibiting VEGF, thereby improving the survival rate of corneal
transplantation (98). Unlike
Zhang et al (76), the
soluble form of VEGF-C/D used by Salabarria et al (79) is VEGFR-3. It can inhibit the
formation of new lymphatic vessels and angiogenesis in the cornea
after transplantation, but it cannot promote corneal graft
survival. In addition to acting on VEGF itself and its upstream
pathway, blocking its downstream pathway is also feasible at
present. Dietrich-Ntoukas et al (105) found that CNV can be inhibited by
blocking Integrin-α5β1 downstream of VEGF.
Infectious keratitis
Infectious keratitis is caused mainly by infections
caused by bacteria, viruses and fungi. Pseudomonas
aeruginosa is a common pathogen of bacterial keratitis and can
cause keratitis in individuals who wear contact lenses for a long
time. LPS from Pseudomonas aeruginosa can activate VEGF-C
through the TLR4 pathway and subsequently induce lymphangiogenesis.
The main pathway by which Pseudomonas aeruginosa promotes CL
is also VEGF-C/VEGFR-3. However, research has shown that in
bacterial keratitis, lymphatic vessels not only play a
proinflammatory role but also, to some extent, reduce macrophage
infiltration and alleviate corneal edema (2,47,63).
Herpetic keratitis is a type of infectious keratitis
caused by HSV-1 (106). It is the
primary cause of infectious corneal blindness in developed
countries. Unlike bacterial keratitis caused by Pseudomonas
aeruginosa, the lymphangiogenesis effect of HSV-1 does not rely
on VEGF-3, VEGFR-3, or macrophage infiltration but mainly relies on
VEGF-A and VEGFR-2. VEGF-A can also promote CNV. Studies have
revealed that lymphangiogenesis promotes the progression of
herpetic keratitis, resulting in visual damage by directing
destructive inflammatory mediators to infected areas. Compared with
the visual impairment caused by HSV-1-induced CNV, CL is more
harmful (44,107). In addition to affecting VEGF,
HSV-1-induced keratitis is also closely related to chemokines,
especially CXCL-10, which is also related to CC chemokines but not
as closely related to CXCL-10. Both factors affect the infiltration
of leukocytes, which in turn affects the generation of CNV and CL
vessels (108).
At present, in the treatment of infectious
keratitis, researchers are exploring new treatments in addition to
the traditional approach of using antibiotics for anti-inflammatory
purposes and using glucocorticoids to inhibit CNV and CL. Zhu et
al (103) proposed that
corneal collagen can be cross-linked with riboflavin and UVA to
treat infectious keratitis, which has the advantages of direct
sterilization, reducing inflammatory cell infiltration, and
inhibiting CNV and CL vessel formation. Shokirova et al
(72) reported that κ opioid
receptor agonists can also be used to combat infectious keratitis.
This is due to their ability to inhibit transplanted CNV, promote
lymphangiogenesis, and provide analgesic effects. For herpetic
keratitis, the most commonly used method is to inhibit the
generation of VEGF-A. Gurung et al (44) reported that regulating FGF-2 can
indirectly inhibit the generation of VEGF-A, and this method can
partially preserve the vision of HSV-1-infected mice, which has
certain clinical value. However, inhibiting FGF-2 can also lead to
nerve degeneration and has potential side effects. However, further
research is needed to determine its effectiveness for clinical
application. The CC chemokine ligand ACKR-2 is considered to be an
important treatment for HSV-1 keratitis. It specifically binds to
CC chemokines, reducing the number of free CC chemokines and
promoting the resolution of inflammation. Recent findings have
shown that it can also bind to CXCL-10, a factor involved in the
pathogenesis of HSV-1 keratitis, further demonstrating that ACKR-2
can effectively inhibit HSV-1-induced keratitis (95,108).
DED
The order of formation of CNV and CL is different,
but they often occur together in corneal diseases. However, in DED,
only lymphangiogenesis occurs. Currently, the mechanism underlying
the selective generation of corneal lymphatic vessels in DED is not
fully understood. The results of the experiments conducted by Chang
et al (3) may be related to
differences in the sensitivity of corneal lymphatic vessels and CNV
to cytokine dosage. Current treatment methods also mostly exert
their effects through the side effects of anti-CNV and anti-CL
(28).
Corneal alkali burn
Corneal alkali burn injury is a commonly used
technique for establishing mouse CNV and CL models. It is detected
in some laboratories and chemical plants and can cause accidental
damage. Due to the lipophilicity of alkali, it often leads to more
severe keratitis than acid burns (109). Li et al (93) reported that knockout of the CXCR-3
gene in an alkali burn model exacerbates CNV and CL in mice. This
may be related to the gene's regulation of VEGF function. Song
et al (38) reported that
LRG1 also regulates the expression of VEGF and VEGFR, as well as
CNV and CL, in alkaline burn models. Additionally, Oh et al
(110) targeted DDR1 through
microRNA-199a/b-5p and inhibited DDR1 expression, ultimately
reducing lymphangiogenesis and neovascularization in alkaline burn
models. However, the mechanism by which DDR1 promotes CNV and the
formation of new corneal lymphatic vessels remains unclear. At
present, for the treatment of CNV and CL after alkali burn, Li
et al (111) proposed that
shark cartilage-derived anti-angiogenic peptide could be used,
because it could inhibit VEGF, MMP1 and other factors.
Others
In addition to the aforementioned diseases, CNV and
CL also play significant roles in the development of other
conditions, such as allergic keratitis and corneal edema, which are
associated with angiogenesis and lymphangiogenesis. However, recent
advancements in research on this topic have been limited, and the
present topic will not be further discussed.
Summary
In recent years, with the progress of research on
the mechanisms of corneal diseases, our understanding of the
mechanisms of CNV and CL, regulatory factors, and their
relationship with diseases has become increasingly comprehensive.
Relevant animal models can be created and treatment plans can be
proposed based on these mechanisms. Currently, VEGF factor blockers
are widely used in clinical practice to improve the success rate of
corneal transplantation, and other therapies are in different
stages of development.
However, due to the complex mechanisms related to
CNV and CL, which involve numerous cells and cytokines, further
research is required. Further understanding of the mechanisms by
which various cytokines influence each other and the relationship
between CNV and CL are needed to improve the treatment of related
diseases.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of Jilin (grant no. YDZJ202301zyTS067).
Availability of data and materials
Not applicable.
Authors' contributions
ZZ conceived and wrote the main manuscript. RZ
prepared figures and helped write the manuscript. YH helped write
the manuscript and provided valuable advice. XW and MY helped to
write the manuscript. Data authentication is not applicable. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhong W, Montana M, Santosa SM, Isjwara
ID, Huang YH, Han KY, O'Neil C, Wang A, Cortina MS, de la Cruz J,
et al: Angiogenesis and lymphangiogenesis in corneal
transplantation-A review. Surv Ophthalmol. 63:453–479. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Narimatsu A, Hattori T, Koike N, Tajima K,
Nakagawa H, Yamakawa N, Usui Y, Kumakura S, Matsumoto T and Goto H:
Corneal lymphangiogenesis ameliorates corneal inflammation and
edema in late stage of bacterial keratitis. Sci Rep. 9:29842019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang LK, Garcia-Cardeña G, Farnebo F,
Fannon M, Chen EJ, Butterfield C, Moses MA, Mulligan RC, Folkman J
and Kaipainen A: Dose-dependent response of FGF-2 for
lymphangiogenesis. Proc Natl Acad Sci USA. 101:11658–11663. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dietrich T, Bock F, Yuen D, Hos D,
Bachmann BO, Zahn G, Wiegand S, Chen L and Cursiefen C: Cutting
edge: Lymphatic vessels, not blood vessels, primarily mediate
immune rejections after transplantation. J Immunol. 184:535–539.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meshko B, Volatier TLA, Hadrian K, Deng S,
Hou Y, Kluth MA, Ganss C, Frank MH, Frank NY, Ksander B, et al:
ABCB5+ limbal epithelial stem cells inhibit developmental but
promote inflammatory (Lymph) angiogenesis while preventing corneal
inflammation. Cells. 12:17312023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dana MR: Angiogenesis and
lymphangiogenesis-implications for corneal immunity. Semin
Ophthalmol. 21:19–22. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cursiefen C, Chen L, Dana MR and Streilein
JW: Corneal lymphangiogenesis: Evidence, mechanisms, and
implications for corneal transplant immunology. Cornea. 22:273–281.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Di Zazzo A, Gaudenzi D, Yin J, Coassin M,
Fernandes M, Dana R and Bonini S: Corneal angiogenic privilege and
its failure. Exp Eye Res. 204:1084572021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Benayoun Y, Casse G, Forte R, Dallaudière
B, Adenis JP and Robert PY: Corneal neovascularization:
epidemiological, physiopathological, and clinical features. J Fr
Ophtalmol. 36:627–639. 2013.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hadrian K and Cursiefen C: The role of
lymphatic vessels in corneal fluid homeostasis and wound healing. J
Ophthalmic Inflamm Infect. 14:42024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kimyon Comert G, Basaran D, Ergin Akkoz H,
Celik B, Sinaci S, Turkmen O, Karalok A, Kandemir O and Turan T:
Blood Vessel Invasion in Endometrial Cancer Is One of the
Mechanisms of Spread to the Cervix. Pathol Oncol Res. 25:1431–1436.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jackson DG, Prevo R, Clasper S and Banerji
S: LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends
Immunol. 22:317–321. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi W, Liu J, Li M, Gao H and Wang T:
Expression of MMP, HPSE, and FAP in stroma promoted corneal
neovascularization induced by different etiological factors. Curr
Eye Res. 35:967–977. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cogan DG: Vascularization of the cornea;
ats experimental induction by small lesions and a new theory of its
pathogenesis. Arch Ophthal. 41:406–416. 1949. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cursiefen C, Chen L, Borges LP, Jackson D,
Cao J, Radziejewski C, D'Amore PA, Dana MR, Wiegand SJ and
Streilein JW: VEGF-A stimulates lymphangiogenesis and
hemangiogenesis in inflammatory neovascularization via macrophage
recruitment. J Clin Invest. 113:1040–1050. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gothard TW, Hardten DR, Lane SS, Doughman
DJ, Krachmer JH and Holland EJ: Clinical findings in Brown-McLean
syndrome. Am J Ophthalmol. 115:729–737. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kenyon BM, Voest EE, Chen CC, Flynn E,
Folkman J and D'Amato RJ: A model of angiogenesis in the mouse
cornea. Invest Ophthalmol Vis Sci. 37:1625–1632. 1996.PubMed/NCBI
|
|
18
|
Simons M, Gordon E and Claesson-Welsh L:
Mechanisms and regulation of endothelial VEGF receptor signalling.
Nat Rev Mol Cell Biol. 17:611–625. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Viallard C and Larrivée B: Tumor
angiogenesis and vascular normalization: Alternative therapeutic
targets. Angiogenesis. 20:409–426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nicholas MP and Mysore N: Corneal
neovascularization. Exp Eye Res. 202:1083632021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roshandel D, Eslani M, Baradaran-Rafii A,
Cheung AY, Kurji K, Jabbehdari S, Maiz A, Jalali S, Djalilian AR
and Holland EJ: Current and emerging therapies for corneal
neovascularization. Ocul Surf. 16:398–414. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wigle JT, Harvey N, Detmar M, Lagutina I,
Grosveld G, Gunn MD, Jackson DG and Oliver G: An essential role for
Prox1 in the induction of the lymphatic endothelial cell phenotype.
EMBO J. 21:1505–1513. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu Y, Yuan L, Mak J, Pardanaud L, Caunt M,
Kasman I, Larrivée B, Del Toro R, Suchting S, Medvinsky A, et al:
Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together
with VEGFR3. J Cell Biol. 188:115–130. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abdelfattah NS, Amgad M, Zayed AA, Hussein
H and Abd El-Baky N: Molecular underpinnings of corneal
angiogenesis: Advances over the past decade. Int J Ophthalmol.
9:768–779. 2016.PubMed/NCBI
|
|
25
|
Mäkinen T, Veikkola T, Mustjoki S,
Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H,
Kerjaschki D, et al: Isolated lymphatic endothelial cells transduce
growth, survival and migratory signals via the VEGF-C/D receptor
VEGFR-3. EMBO J. 20:4762–4773. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goyal S, Chauhan SK, El Annan J, Nallasamy
N, Zhang Q and Dana R: Evidence of corneal lymphangiogenesis in dry
eye disease: A potential link to adaptive immunity? Arch
Ophthalmol. 128:819–824. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chauhan SK, Jin Y, Goyal S, Lee HS,
Fuchsluger TA, Lee HK and Dana R: A novel pro-lymphangiogenic
function for Th17/IL-17. Blood. 118:4630–4634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee SJ, Im ST, Wu J, Cho CS, Jo DH, Chen
Y, Dana R, Kim JH and Lee SM: Corneal lymphangiogenesis in dry eye
disease is regulated by substance P/neurokinin-1 receptor system
through controlling expression of vascular endothelial growth
factor receptor 3. Ocul Surf. 22:72–79. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wilting J, Neeff H and Christ B: Embryonic
lymphangiogenesis. Cell Tissue Res. 297:1–11. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee HK, Lee SM and Lee DI: Corneal
Lymphangiogenesis: Current pathophysiological understandings and
its functional role in ocular surface disease. Int J Mol Sci.
22:116282021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sáinz-Jaspeado M and Claesson-Welsh L:
Cytokines regulating lymphangiogenesis. Curr Opin Immunol.
53:58–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Patnam M, Dommaraju SR, Masood F, Herbst
P, Chang JH, Hu WY, Rosenblatt MI and Azar DT: Lymphangiogenesis
guidance mechanisms and therapeutic implications in pathological
states of the cornea. Cells. 12:3192023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cao R, Ji H, Feng N, Zhang Y, Yang X,
Andersson P, Sun Y, Tritsaris K, Hansen AJ, Dissing S and Cao Y:
Collaborative interplay between FGF-2 and VEGF-C promotes
lymphangiogenesis and metastasis. Proc Natl Acad Sci USA.
109:15894–15899. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cursiefen C, Schlötzer-Schrehardt U,
Küchle M, Sorokin L, Breiteneder-Geleff S, Alitalo K and Jackson D:
Lymphatic vessels in vascularized human corneas:
Immunohistochemical investigation using LYVE-1 and podoplanin.
Invest Ophthalmol Vis Sci. 43:2127–2135. 2002.PubMed/NCBI
|
|
35
|
Cursiefen C, Maruyama K, Jackson DG,
Streilein JW and Kruse FE: Time course of angiogenesis and
lymphangiogenesis after brief corneal inflammation. Cornea.
25:443–447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shi W, Ming C, Liu J, Wang T and Gao H:
Features of corneal neovascularization and lymphangiogenesis
induced by different etiological factors in mice. Graefes Arch Clin
Exp Ophthalmol. 249:55–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Giacomini C, Ferrari G, Bignami F and Rama
P: Alkali burn versus suture-induced corneal neovascularization in
C57BL/6 mice: An overview of two common animal models of corneal
neovascularization. Exp Eye Res. 121:1–4. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Song S, Cheng J, Yu BJ, Zhou L, Xu HF and
Yang LL: LRG1 promotes corneal angiogenesis and lymphangiogenesis
in a corneal alkali burn mouse model. Int J Ophthalmol. 13:365–373.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ammassam Veettil R, Li W, Pflugfelder SC
and Koch DD: A Mouse Model for Corneal Neovascularization by Alkali
Burn. J Vis Exp. 2023. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Park JH, Joo CK and Chung SK: Comparative
study of tacrolimus and bevacizumab on corneal neovascularization
in rabbits. Cornea. 34:449–455. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Maier AK, Reichhart N, Gonnermann J,
Kociok N, Riechardt AI, Gundlach E, Strauß O and Joussen AM:
Effects of TNFα receptor TNF-Rp55- or TNF-Rp75- deficiency on
corneal neovascularization and lymphangiogenesis in the mouse. PLoS
One. 16:e02451432021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ung L and Chodosh J: Foundational concepts
in the biology of bacterial keratitis. Exp Eye Res. 209:1086472021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Koganti R, Yadavalli T, Naqvi RA, Shukla D
and Naqvi AR: Pathobiology and treatment of viral keratitis. Exp
Eye Res. 205:1084832021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gurung HR, Carr MM, Bryant K,
Chucair-Elliott AJ and Carr DJ: Fibroblast growth factor-2 drives
and maintains progressive corneal neovascularization following
HSV-1 infection. Mucosal Immunol. 11:172–185. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xie F, Zhang X, Luo W, Ge H, Sun D and Liu
P: Notch signaling pathway is involved in bFGF-induced corneal
lymphangiogenesis and hemangiogenesis. J Ophthalmol.
2019:96139232019.PubMed/NCBI
|
|
46
|
Li S, Li L, Zhou Q, Gao H, Liu M and Shi
W: Blood vessels and lymphatic vessels in the cornea and iris after
penetrating keratoplasty. Cornea. 38:742–747. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hos D, Bukowiecki A, Horstmann J, Bock F,
Bucher F, Heindl LM, Siebelmann S, Steven P, Dana R, Eming SA and
Cursiefen C: Transient ingrowth of lymphatic vessels into the
physiologically avascular cornea regulates corneal edema and
transparency. Sci Rep. 7:72272017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gao X, Guo K, Santosa SM, Montana M,
Yamakawa M, Hallak JA, Han KY, Doh SJ, Rosenblatt MI, Chang JH and
Azar DT: Application of corneal injury models in dual fluorescent
reporter transgenic mice to understand the roles of the cornea and
limbus in angiogenic and lymphangiogenic privilege. Sci Rep.
9:123312019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Goyal S, Chauhan SK and Dana R: Blockade
of prolymphangiogenic vascular endothelial growth factor C in dry
eye disease. Arch Ophthalmol. 130:84–89. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wuest TR and Carr DJ: VEGF-A expression by
HSV-1-infected cells drives corneal lymphangiogenesis. J Exp Med.
207:101–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wuest T, Zheng M, Efstathiou S, Halford WP
and Carr DJ: The herpes simplex virus-1 transactivator infected
cell protein-4 drives VEGF-A dependent neovascularization. PLoS
Pathog. 7:e10022782011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Thangamathesvaran L, Kong J, Bressler SB,
Singh M, Wenick AS, Scott AW, Arévalo JF and Bressler NM: Severe
intraocular inflammation following intravitreal faricimab. JAMA
Ophthalmol. 142:365–370. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jaggi D, Nagamany T, Wolf S, Zinkernagel
MS and Heussen FM: Aflibercept for central retinal vein occlusions:
Long-term outcomes of a ‘Treat-and-Extend’ regimen. BMJ Open
Ophthalmol. 9:e0016592024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Limon DU, Kaplan FB, Saygın I, Önder Tokuç
E, Kutlutürk Karagöz I, Kanar HS, Sevik MO, Yayla U, Çelik E,
Sönmez A, et al: One-Year functional and morphological prognosis
after intravitreal injection treatments according to different
morphological patterns of diabetic macular edema in real-life:
MARMASIA Study Group Report No.13. Semin Ophthalmol. 39:460–467.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang XM, Li QP, Wang ZH and Zhang MN:
Comparison of ranibizumab and conbercept treatment in type 1
prethreshold retinopathy of prematurity in zone II. BMC Pediatr.
24:5562024. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hong SH and Kim HD: Central retinal artery
occlusion after intravitreal brolucizumab injection for
treatment-naïve neovascular age-related macular degeneration; a
case report. BMC Ophthalmol. 24:2002024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
HARMONi-A Study Investigators, . Fang W,
Zhao Y, Luo Y, Yang R, Huang Y, He Z, Zhao H, Li M, Li K, et al:
Ivonescimab Plus Chemotherapy in Non-Small Cell Lung Cancer With
EGFR Variant: A Randomized Clinical Trial. JAMA. 332:561–570. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cho W, Mittal SK, Elbasiony E and Chauhan
SK: Ocular surface mast cells promote inflammatory
lymphangiogenesis. Microvasc Res. 141:1043202022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ferrari G, Bignami F, Giacomini C,
Franchini S and Rama P: Safety and efficacy of topical infliximab
in a mouse model of ocular surface scarring. Invest Ophthalmol Vis
Sci. 54:1680–1688. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang Q, Lu Y, Proulx ST, Guo R, Yao Z,
Schwarz EM, Boyce BF and Xing L: Increased lymphangiogenesis in
joints of mice with inflammatory arthritis. Arthritis Res Ther.
9:R1182007. View
Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ji RC: Macrophages are important mediators
of either tumor- or inflammation-induced lymphangiogenesis. Cell
Mol Life Sci. 69:897–914. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ji RC: Lymphatic endothelial cells,
inflammatory lymphangiogenesis, and prospective players. Curr Med
Chem. 14:2359–2368. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hos D, Bucher F, Regenfuss B, Dreisow ML,
Bock F, Heindl LM, Eming SA and Cursiefen C: IL-10 indirectly
regulates corneal lymphangiogenesis and resolution of inflammation
via macrophages. Am J Pathol. 186:159–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Nakao S, Kuwano T, Tsutsumi-Miyahara C,
Ueda S, Kimura YN, Hamano S, Sonoda KH, Saijo Y, Nukiwa T, Strieter
RM, et al: Infiltration of COX-2-expressing macrophages is a
prerequisite for IL-1 beta-induced neovascularization and tumor
growth. J Clin Invest. 115:2979–2991. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lu P, Li L, Liu G, Zhang X and Mukaida N:
Enhanced experimental corneal neovascularization along with
aberrant angiogenic factor expression in the absence of IL-1
receptor antagonist. Invest Ophthalmol Vis Sci. 50:4761–4768. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Doz E, Noulin N, Boichot E, Guénon I, Fick
L, Le Bert M, Lagente V, Ryffel B, Schnyder B, Quesniaux VF and
Couillin I: Cigarette smoke-induced pulmonary inflammation is
TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol.
180:1169–1178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Reuer T, Schneider AC, Cakir B, Bühler AD,
Walz JM, Lapp T, Lange C, Agostini H, Schlunck G, Cursiefen C, et
al: Semaphorin 3F Modulates Corneal Lymphangiogenesis and Promotes
Corneal Graft Survival. Invest Ophthalmol Vis Sci. 59:5277–5284.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang X, Abraham S, McKenzie JAG, Jeffs N,
Swire M, Tripathi VB, Luhmann UFO, Lange CAK, Zhai Z, Arthur HM, et
al: LRG1 promotes angiogenesis by modulating endothelial TGF-β
signalling. Nature. 499:306–311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang J, Zhu L, Fang J, Ge Z and Li X:
LRG1 modulates epithelial-mesenchymal transition and angiogenesis
in colorectal cancer via HIF-1α activation. J Exp Clin Cancer Res.
35:292016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hu H, Wang S, He Y, Shen S, Yao B, Xu D,
Liu X and Zhang Y: The role of bone morphogenetic protein 4 in
corneal injury repair. Exp Eye Res. 212:1087692021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Rezzola S, Di Somma M, Corsini M, Leali D,
Ravelli C, Polli VAB, Grillo E, Presta M and Mitola S: VEGFR2
activation mediates the pro-angiogenic activity of BMP4.
Angiogenesis. 22:521–533. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Shokirova H, Inomata T, Saitoh T, Zhu J,
Fujio K, Okumura Y, Yanagawa A, Fujimoto K, Sung J, Eguchi A, et
al: Topical administration of the kappa opioid receptor agonist
nalfurafine suppresses corneal neovascularization and inflammation.
Sci Rep. 11:86472021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Liu Y, Shu Y, Yin L, Xie T, Zou J, Zhan P,
Wang Y, Wei T, Zhu L, Yang X, et al: Protective roles of the
TIR/BB-loop mimetic AS-1 in alkali-induced corneal
neovascularization by inhibiting ERK phosphorylation. Exp Eye Res.
207:1085682021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Song HB, Park SY, Ko JH, Park JW, Yoon CH,
Kim DH, Kim JH, Kim MK, Lee RH, Prockop DJ and Oh JY: Mesenchymal
Stromal Cells Inhibit Inflammatory Lymphangiogenesis in the Cornea
by Suppressing Macrophage in a TSG-6-Dependent Manner. Mol Ther.
26:162–172. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Bai Y, Jiao X, Hu J, Xue W, Zhou Z and
Wang W: WTAP promotes macrophage recruitment and increases VEGF
secretion via N6-methyladenosine modification in corneal
neovascularization. Biochim Biophys Acta Mol Basis Dis.
1869:1667082023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang W, Schönberg A, Bock F and Cursiefen
C: Posttransplant VEGFR1R2 Trap Eye Drops Inhibit Corneal
(Lymph)angiogenesis and Improve Corneal Allograft Survival in Eyes
at High Risk of Rejection. Transl Vis Sci Technol. 11:62022.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang W, Schönberg A, Hamdorf M, Georgiev
T, Cursiefen C and Bock F: Preincubation of donor tissue with a
VEGF cytokine trap promotes subsequent high-risk corneal transplant
survival. Br J Ophthalmol. 106:1617–1626. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Le VNH, Hos D, Hou Y, Witt M, Barkovskiy
M, Bock F and Cursiefen C: VEGF TrapR1R2 Suspended in the
Semifluorinated Alkane F6H8 Inhibits Inflammatory Corneal Hem- and
Lymphangiogenesis. Transl Vis Sci Technol. 9:152020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Salabarria AC, Koch M, Schönberg A, Zinser
E, Hos D, Hamdorf M, Imhof T, Braun G, Cursiefen C and Bock F:
Topical VEGF-C/D Inhibition Prevents Lymphatic Vessel Ingrowth into
Cornea but Does Not Improve Corneal Graft Survival. J Clin Med.
9:12702020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Han Y, Sengupta S, Lee BJ, Cho H, Kim J,
Choi HG, Dash U, Kim JH, Kim ND, Kim JH and Sim T: Identification
of a Unique Resorcylic Acid Lactone Derivative That Targets Both
Lymphangiogenesis and Angiogenesis. J Med Chem. 62:9141–9160. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Cho YK, Shin EY, Uehara H and Ambati B:
The Effect of 0.5% Timolol Maleate on Corneal(Lymph)Angiogenesis in
a Murine Suture Model. J Ocul Pharmacol Ther. 34:403–409. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Cho YK, Shin EY, Uehara H and Ambati B:
Antiangiogenesis Effect of Albendazole on the Cornea. J Ocul
Pharmacol Ther. 35:254–261. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ellenberg D, Azar DT, Hallak JA, Tobaigy
F, Han KY, Jain S, Zhou Z and Chang JH: Novel aspects of corneal
angiogenic and lymphangiogenic privilege. Prog Retin Eye Res.
29:208–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Tzeng HE, Chang AC, Tsai CH, Wang SW and
Tang CH: Basic fibroblast growth factor promotes VEGF-C-dependent
lymphangiogenesis via inhibition of miR-381 in human chondrosarcoma
cells. Oncotarget. 7:38566–38578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Shin JW, Min M, Larrieu-Lahargue F, Canron
X, Kunstfeld R, Nguyen L, Henderson JE, Bikfalvi A, Detmar M and
Hong YK: Prox1 promotes lineage-specific expression of fibroblast
growth factor (FGF) receptor-3 in lymphatic endothelium: A role for
FGF signaling in lymphangiogenesis. Mol Biol Cell. 17:576–584.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hellström M, Phng LK and Gerhardt H: VEGF
and Notch signaling: the yin and yang of angiogenic sprouting. Cell
Adh Migr. 1:133–136. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kofler NM, Shawber CJ, Kangsamaksin T,
Reed HO, Galatioto J and Kitajewski J: Notch signaling in
developmental and tumor angiogenesis. Genes Cancer. 2:1106–1116.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Food and Drug Administration, . Drug
Approvals and Databases. https://www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databasesOctober
20–2024
|
|
89
|
Imamura K, Yoshida W, Seshima F, Aoki H,
Yamashita K, Kitamura Y, Murakami T, Ambiru M, Bizenjima T,
Katayama A, et al: Periodontal regenerative therapy using
recombinant human fibroblast growth factor (rhFGF)-2 in combination
with carbonate apatite granules or rhFGF-2 alone: 12-month
randomized controlled trial. Clin Oral Investig. 28:5742024.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Clark AJ, Mullooly N, Safitri D, Harris M,
de Vries T, MaassenVanDenBrink A, Poyner DR, Gianni D, Wigglesworth
M and Ladds G: CGRP, adrenomedullin and adrenomedullin 2 display
endogenous GPCR agonist bias in primary human cardiovascular cells.
Commun Biol. 4:7762021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhu S, Zidan A, Pang K, Musayeva A, Kang Q
and Yin J: Promotion of corneal angiogenesis by sensory
neuron-derived calcitonin gene-related peptide. Exp Eye Res.
220:1091252022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Majima M, Ito Y, Hosono K and Amano H:
CGRP/CGRP Receptor Antibodies: Potential adverse effects due to
blockade of neovascularization? Trends Pharmacol Sci. 40:11–21.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Li S, Shi S, Xia F, Ha Y, Luisi J, Gupta
PK, Merkley KH, Motamedi M, Liu H and Zhang W: CXCR3 deletion
aggravates corneal neovascularization in a corneal alkali-burn
model. Exp Eye Res. 225:1092652022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Nibbs RJ and Graham GJ: Immune regulation
by atypical chemokine receptors. Nat Rev Immunol. 13:815–829. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chevigné A, Janji B, Meyrath M, Reynders
N, D'Uonnolo G, Uchański T, Xiao M, Berchem G, Ollert M, Kwon YJ,
et al: CXCL10 Is an Agonist of the CC Family Chemokine Scavenger
Receptor ACKR2/D6. Cancers (Basel). 13:10542021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Muramatsu M, Osawa T, Miyamura Y, Nakagawa
S, Tanaka T, Kodama T, Aburatani H, Sakai J, Ryeom S and Minami T:
Loss of Down syndrome critical region-1 leads to cholesterol
metabolic dysfunction that exaggerates hypercholesterolemia in
ApoE-null background. J Biol Chem. 296:1006972021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ren Y, Dong X, Zhao H, Feng J, Chen B,
Zhou Y, Peng Y, Zhang L, Zhou Q, Li Y, et al: Myeloid-derived
suppressor cells improve corneal graft survival through suppressing
angiogenesis and lymphangiogenesis. Am J Transplant. 21:552–566.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ren Y, Dong X, Liu Y, Kang H, Guan L,
Huang Y, Zhu X, Tian J, Chen B, Jiang B and He Y: Rapamycin
antagonizes angiogenesis and lymphangiogenesis through
myeloid-derived suppressor cells in corneal transplantation. Am J
Transplant. 23:1359–1374. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wei C, Mi Y, Sun L, Luo J, Zhang J, Gao Y,
Yu X, Ge H and Liu P: Cannabidiol alleviates suture-induced corneal
pathological angiogenesis and inflammation by inducing
myeloid-derived suppressor cells. Int Immunopharmacol.
137:1124292024. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kang H, Feng J, Peng Y, Liu Y, Yang Y, Wu
Y, Huang J, Jie Y, Chen B and He Y: Human mesenchymal stem cells
derived from adipose tissue showed a more robust effect than those
from the umbilical cord in promoting corneal graft survival by
suppressing lymphangiogenesis. Stem Cell Res Ther. 14:3282023.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Yu F, Gong D, Yan D, Wang H, Witman N, Lu
Y, Fu W and Fu Y: Enhanced adipose-derived stem cells with
IGF-1-modified mRNA promote wound healing following corneal injury.
Mol Ther. 31:2454–2471. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhu J, Inomata T, Fujimoto K, Uchida K,
Fujio K, Nagino K, Miura M, Negishi N, Okumura Y, Akasaki Y, et al:
Ex Vivo-Induced bone marrow-derived myeloid suppressor cells
prevent corneal allograft rejection in mice. Invest Ophthalmol Vis
Sci. 62:32021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhu Y, Reinach PS, Ge C, Li Y, Wu B, Xie
Q, Tong L and Chen W: Corneal collagen cross-linking pretreatment
mitigates injury-induced inflammation, hemangiogenesis and
lymphangiogenesis in vivo. Transl Vis Sci Technol. 10:112021.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hou Y, Le VNH, Tóth G, Siebelmann S,
Horstmann J, Gabriel T, Bock F and Cursiefen C: UV light
crosslinking regresses mature corneal blood and lymphatic vessels
and promotes subsequent high-risk corneal transplant survival. Am J
Transplant. 18:2873–2884. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Dietrich-Ntoukas T, Bock F, Onderka J, Hos
D, Bachmann BO, Zahn G and Cursiefen C: Selective, Temporary
Postoperative Inhibition of Lymphangiogenesis by Integrin α5β1
blockade improves allograft survival in a murine model of high-risk
corneal transplantation. J Clin Med. 13:44182024. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Farooq AV and Shukla D: Herpes simplex
epithelial and stromal keratitis: An epidemiologic update. Surv
Ophthalmol. 57:448–462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Park PJ, Chang M, Garg N, Zhu J, Chang JH
and Shukla D: Corneal lymphangiogenesis in herpetic stromal
keratitis. Surv Ophthalmol. 60:60–71. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Yu T, Schuette F, Christofi M, Forrester
JV, Graham GJ and Kuffova L: The atypical chemokine receptor-2
fine-tunes the immune response in herpes stromal keratitis. Front
Immunol. 13:10542602022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Anderson C, Zhou Q and Wang S: An
alkali-burn injury model of corneal neovascularization in the
mouse. J Vis Exp. 86:511592014.
|
|
110
|
Oh S, Seo M, Choi JS, Joo CK and Lee SK:
MiR-199a/b-5p Inhibits Lymphangiogenesis by Targeting Discoidin
Domain Receptor 1 in Corneal Injury. Mol Cells. 41:93–102.
2018.PubMed/NCBI
|
|
111
|
Li Y, Chen A, Hong A, Xiong S, Chen X and
Xie Q: Shark Cartilage-Derived Anti-Angiogenic Peptide Inhibits
Corneal Neovascularization. Bioengineering (Basel). 11:6932024.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Adams RH and Alitalo K: Molecular
regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell
Biol. 8:464–478. 2007. View Article : Google Scholar : PubMed/NCBI
|