Immune checkpoint inhibitors (ICIs) targeting
programmed cell death protein 1 (PD-1) or programmed death ligand 1
(PD-L1) are used in the treatment of a wide range of tumors, such
as lung cancer, cervical cancer and renal cell carcinomas (1–4).
These agents are associated with a prolonged survival time
(1–4). Studies such as KEYNOTE-024 and
KEYNOTE-042 have been pivotal in establishing the role of
pembrolizumab, a PD-1 inhibitor, in improving overall survival in
patients with advanced non-small cell lung cancer (1,2). The
results of the ENGOT-cx11/GOG-3047/KEYNOTE-A18 study demonstrated
that the combination of pembrolizumab with chemoradiotherapy
improved overall survival in patients with locally advanced
cervical cancer compared with a placebo (3). Additionally, adjuvant pembrolizumab
was observed to enhance overall survival in patients with renal
cell carcinoma compared with a placebo (4). However, primary or acquired
resistance to ICIs is commonly observed (5). Therefore, research investigating the
mechanisms of PD-1/PD-L1 ICI resistance is vital to improve
clinical outcomes. There have been a number of studies examining
the mechanisms of resistance to PD-1/PD-L1 ICIs, which include loss
of tumor antigens and antigen presentation (6), T-cell exhaustion (7), lack of interferon signaling (8), and lack of PD-L1 expression (9). Furthermore, additional pathways
involved in the inhibition of immune cells within the tumor
microenvironment (TME) can lead to anti-PD-1/PD-L1 resistance
(10,11). For example, TYRO3 can increase the
responsiveness to anti-PD-1 therapy by altering the macrophage
profile towards a more M2-like state, which is facilitated by an
increase in VEGF expression (10).
Phospholipase C γ2 (PLCG2) serves a role in modulating the TME by
reducing the infiltration of CD8+ T cells and increasing
the infiltration of regulatory T cells (Treg cells), which can
suppress the immune response (11). Additionally, PLCG2 contributes to
the upregulation of PD-1 and PD-L1 expression. This dual action of
PLCG2 facilitates immune escape and is associated with resistance
to anti-PD-1 therapy (11). The
immunosuppressive TME resulting from the metabolic reprogramming of
tumor cells represents a barrier to the effectiveness of
immunotherapy (12).
Tumor cells maintain their proliferation and
cellular function through specific metabolic patterns, a process
known as metabolic reprogramming (13). Under aerobic conditions,
respiration in eukaryotic cells is mainly aerobic, providing energy
through oxidative phosphorylation. By contrast, cancer cells prefer
to generate energy through aerobic glycolysis, known as the
‘Warburg effect’, consuming large amounts of glucose and increasing
the production of lactate (14).
Lactate is subsequently released extracellularly, which results in
an acidic TME, which can facilitate tumor growth, angiogenesis and
immune evasion (14,15). Since lactate acts as a bridge
linking metabolic reprogramming to immunosuppression (16), a growing number of studies have
noted the impact of lactate on the response to anti-PD-1/PD-L1
therapy (17–19). The current review presents the
mechanisms of resistance to PD-1/PD-L1 ICIs and takes a detailed
look at the potential role of lactate in these mechanisms.
The presence of PD-L1 on cancer cells facilitates
immune evasion through its interaction with PD-1 on activated T
cells (20). This interaction
results in the phosphorylation of the immune receptor
tyrosine-based switch motif and subsequent binding to the Src
homology 2 (SH2) domains of SH2-containing phosphatase 2 (SHP2)
(21). Once activated, SHP2
dephosphorylates proximal T-cell receptor (TCR) signaling
molecules, such as ζ-associated protein of 70 kD, which is a key
component of the TCR signaling cascade. This dephosphorylation
event dampens the TCR signaling, leading to the suppression of T
cell activation (22). In
addition, PD-1 is expressed on the surface of tumor-associated
macrophages, and a study has indicated that blocking the PD-1/PD-L1
axis can increase the activity of tumor-associated macrophages
(20). PD-1/PD-L1 ICIs work by
targeting the PD-1/PD-L1 axis, which has been shown to be a
successful treatment strategy in multiple cancer types such as
melanoma, renal cell carcinoma and cervical cancer (3,4,6).
There has been much discussion about the mechanisms
of resistance to anti-PD-1/PD-L1 therapy. Firstly, as
aforementioned, anti-PD-1/PD-L1 therapy works by targeting the
PD-1/PD-L1 axis; therefore, PD-L1 expression is critical for a
response to immunotherapy (6).
Secondly, effective immune responses cannot be achieved without
antigen expression and antigen presentation (23,24).
Accordingly, a study has demonstrated that tumors with sparse
immune infiltration exhibit diminished neoantigen editing function
(25). Furthermore, it has been
demonstrated that activation of β-catenin can suppress antitumor
immune responses by impeding the recruitment of dendritic cells
(DCs) (23). Antigen presentation
leads to T-cell activation, which is a crucial method for the
immune system to attack and eliminate cancer cells (17). Furthermore, evidence shows that an
abundance of CD8+ T cells is essential for antitumor
immunity (7,26). A clinical trial of pembrolizumab in
microsatellite instability-high gastric cancer has demonstrated
that abundant tumor-infiltrating lymphocytes were associated with a
clinical benefit (27).
Furthermore, preventing the activation of T cells or other
mechanisms that affect the functioning of T cells can lead to low
response rates to anti-PD-1/PD-L1 therapy (10). It has been shown that TYRO3 protein
tyrosine kinase inhibited tumor cell ferroptosis, suppressing
T-cell attack and reducing responsiveness to anti-PD-1/PD-L1
therapy (10). In addition to the
aforementioned mechanisms, there are numerous studies on other
aspects of resistance such as genetic mutations (28), gut microbiota (29) and metabolism (17). Although progress has been achieved
in elucidating the mechanism of resistance to anti-PD-1/PD-L1
therapy, the intricate nature of the TME remains a research
limitation. This complexity arises from the interactions among
various cell types and molecular pathways, which collectively
impact the progression of resistance to anti-PD-1/PD-L1 therapy
(30). Consequently, further
research is essential to address these challenges such as tumor
heterogeneity and the interactions among various cell types, as
well as to enhance the understanding of resistance to
anti-PD-1/PD-L1 therapy.
Resistance to cancer immunotherapy is often linked
to an immunosuppressive TME in which key nutrients serve a crucial
role (17–19). Tumor cells and immune cells engage
in competition for essential nutrients, leading to reprogramming of
metabolic pathways in immune cells, which in turn suppresses
antitumor immune responses (31,32).
In recent years, a study has investigated the relationship between
tumor metabolism, immune evasion mechanisms and resistance to
immunotherapy (32). Notably,
metabolic byproducts produced by tumor cells, particularly lactic
acid, are known to contribute to the immunosuppressive nature of
the TME (33–35). A growing body of research suggests
that lactic acid in the TME is associated with resistance to
immunotherapy (33–35).
Otto Warburg noticed that cancer cells
preferentially generate energy through aerobic glycolysis, which is
a hallmark of tumor cell metabolism (36). Under aerobic conditions, normal
cells transform glucose into pyruvate via glycolysis. This pyruvate
is then transferred to the mitochondria and oxidized through the
tricarboxylic acid (TCA) cycle to generate carbon dioxide, oxygen
and adenosine triphosphate (37).
In cancer cells, a marked proportion of the pyruvate generated
through glycolysis does not enter the mitochondria but is converted
into lactate (37). Cancer cells
regulate lactate production and secretion in the TME through
several key enzymes such as glucose transporter 1 (GLUT1),
hexokinase 2 (HK2) and pyruvate kinase 2 (PKM2) (16,38)
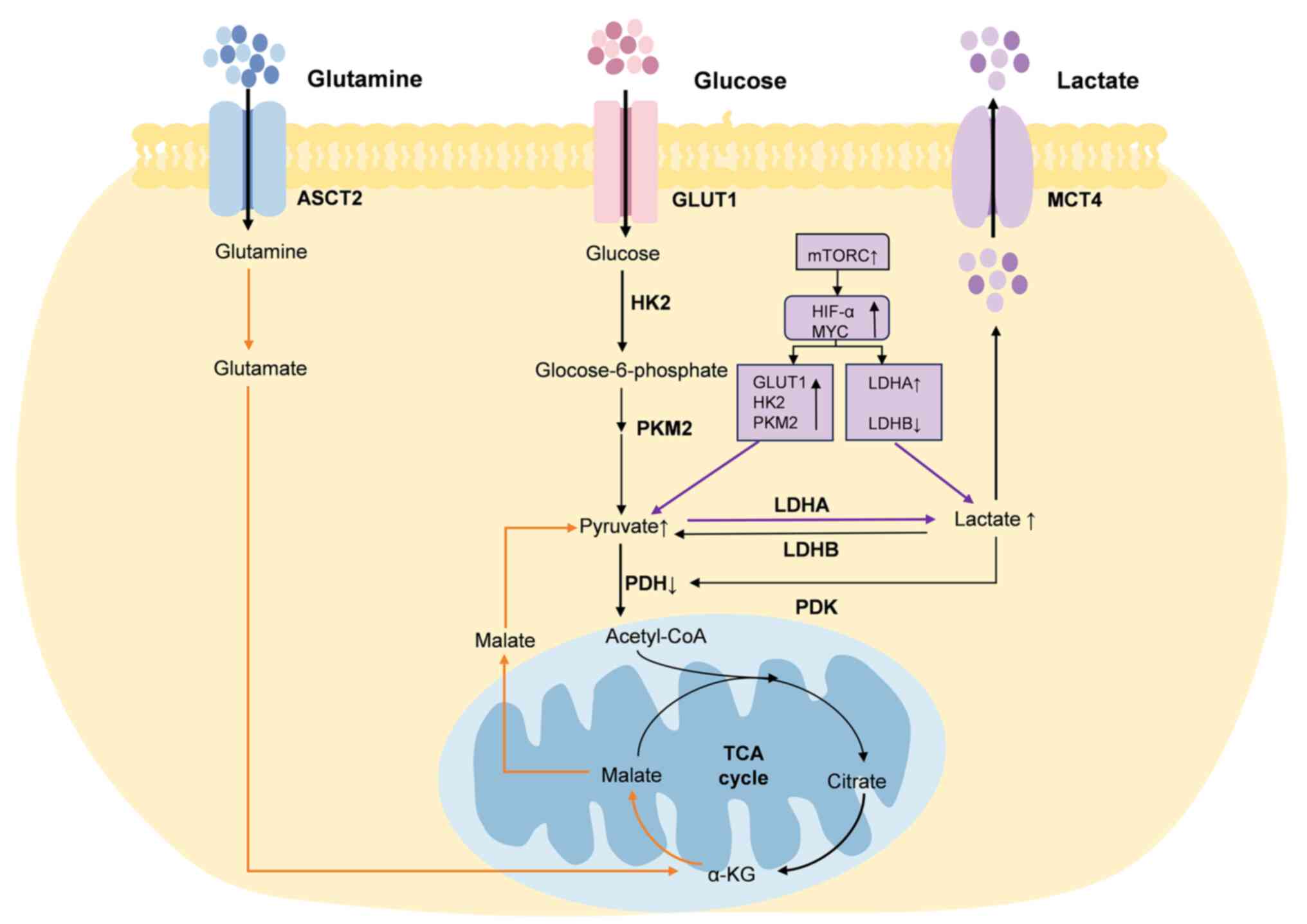
(Fig. 1).
Hypoxia-inducible factor-1α (HIF-1α) and c-Myc serve
crucial roles in regulating lactate metabolism in cancer cells, and
can be regulated by mTOR (39).
HIF-1α and c-Myc can increase pyruvate production by promoting the
activity of GLUT1, HK2 and PKM2 (16,38).
GLUT1 is responsible for transporting glucose from the
extracellular space to the intracellular space, and HK2 converts
glucose to glucose-6-phosphate (16). PKM2 is the key enzyme in the final
step of glucose conversion to pyruvate, whereas HIF-1α and c-Myc
affect lactate dehydrogenase (LDH) expression (16). LDH is divided into LDHA and LDHB,
which serve opposite roles; LDHA is responsible for catalyzing the
transformation of pyruvate into lactate (40). HIF-1α and c-Myc can increase
lactate production by upregulating LDHA expression and inhibiting
LDHB expression (16). The
secreted lactate can subsequently promote the phosphorylation of
pyruvate dehydrogenase (PDH) by PDH kinase (PDK), thereby resulting
in a greater conversion of pyruvate to lactate (41). PDK can phosphorylate PDH and
inhibit its activity (42).
In addition to glycolysis, cancer cells produce
lactate through glutaminolysis. Cancer cells uptake glutamine and
convert it to glutamate via glutaminase, which is regulated by
c-Myc. Glutamate is then converted to α-ketoglutarate, which in
turn is transformed into malate via the TCA cycle. Malate is then
translocated to the cytoplasm, where it is converted into pyruvate
by the action of malic enzyme, ultimately facilitating lactate
production (43).
Some lactate can enter the TME through the
monocarboxylate transporter (MCT) at concentrations up to 40 mM
(44). Lactate is a critical
metabolite of glycolysis and serves a crucial role in tumorigenesis
and progression of tumors (16).
In particular, lactate is not only a TCA cycle carbon source for
tumor cells (45–47), but also increases the uptake and
metabolism of glutamine by promoting the expression of glutamine
transporter and glutaminase 1 (48). In addition, lactate is an important
signaling molecule, which can influence the functions of immune
cells, and impact tumorigenesis (49) and/or tumor metastasis (50). For example, Xie et al
(51) found that lactate could
inhibit the mTOR signaling pathway and the nuclear translocation of
promyelocytic leukemia zinc-finger by decreasing the extracellular
pH, ultimately resulting in dysfunction of natural killer (NK) T
cells, particularly characterized by a reduction in the production
of IFN-γ and IL-4.
By analyzing large-scale pan-cancer data, a study
has revealed that lactate metabolism-related features were
negatively associated with antitumor immunity and positively
associated with immunotherapy resistance (52). The positive response to ICIs in
patients is linked to the presence of pre-existing intratumoral
T-cell infiltration and an immunologically favorable TME
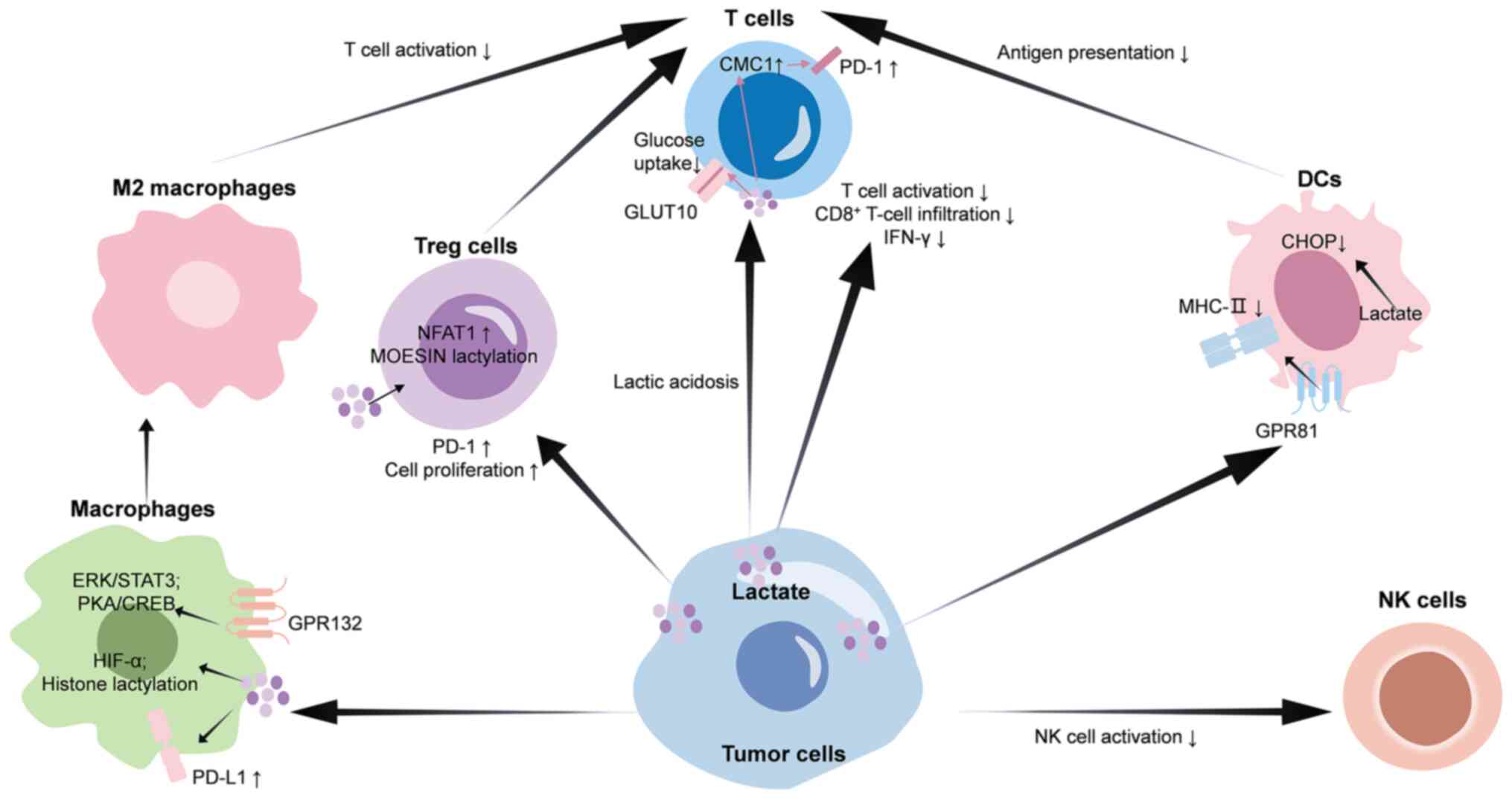
characterized as ‘hot’ or T-cell-inflamed (53). Lactate can promote an
immunosuppressive TME via its effects on immune cells (Fig. 2), which is associated with immune
cell infiltration and response to ICIs (19).
Macrophages are professional phagocytic cells that
are capable of activating T helper cells by presenting peptide
antigens through major histocompatibility complex class II (MHC-II)
(54). Macrophages can be
categorized into two distinct phenotypes, namely the classically
activated (M1) or the alternatively activated (M2) macrophages.
M1-like macrophages secrete pro-inflammatory cytokines and have the
capacity to induce tumor cell death, whereas M2-like macrophages
are known for their secretion of anti-inflammatory cytokines and
their role in facilitating tumor progression (55). M2-like macrophages can promote
malignant tumor initiation and progression (56). G protein-coupled receptor 132
(GPR132), expressed by macrophages, can sense the lactate signal in
the TME (57). Upon sensing
lactate, GPR132 activates G proteins coupled to it. This
subsequently activates protein kinase A (PKA). The activated PKA
phosphorylates cAMP response element binding protein (CREB), which
then enters the nucleus (58).
CREB binds to the promoter regions of M2 macrophage biomarkers,
including CD206, arginase 1 (ARG1) and IL10, and promotes their
expression (58). In addition,
lactate can stabilize HIF-1α protein, which induces the expression
of VEGF and ARG1, thereby leading to M2 macrophage polarization
(59). Data have also shown that
M2 macrophage polarization can be induced by lactate through the
activation of the ERK/STAT3 signaling pathway, which promotes the
expression of CD206 and ARG1 (55). Recent research has revealed that
lactate generated by tumor cells accumulated in macrophages and
induced histone H3 lysine 18 lactylation, which enhanced the
expression of M2 macrophage biomarkers such as CD206, ARG1, IL10
and adrenomedullin (60). M2
macrophages can inhibit the activity of CD8+ T cells by
secreting immunosuppressive factors such as IL10 and transforming
growth factor β1, thereby reducing the killing effect of
CD8+ T cells on tumor cells (60). The addition of lactate can increase
VEGF production by macrophages, further stimulating angiogenesis
(61). Furthermore, a recent study
found that exogenous lactate could increase PD-L1 expression in
macrophages via the activation of NF-κB (62). PD-L1 expressed on macrophages
negatively regulates T-cell function and serves a crucial role in
response to ICI therapy (63).
Thus, lactate may influence the efficacy of immunotherapy by
modulating macrophage function.
DCs are pivotal antigen-presenting cells within the
TME, and are responsible for processing and presenting antigens to
naïve T lymphocytes, thereby initiating an antigen-specific immune
response (64). DCs have been
identified as crucial players in the response to ICIs and are
promising candidates for cancer immunotherapy (64). DCs are commonly categorized into
plasmacytoid DCs (pDCs), monocyte-derived DCs and conventional DCs
(cDCs), which encompass cDC1s and cDC2s (65). pDCs are a subset of bone
marrow-derived DCs and are responsible for producing IFN-I
(53). Monocyte-derived DCs are
differentiated from monocytes in response to inflammation and are
present under steady state conditions in specific tissues such as
the gastrointestinal tract and respiratory tract (66). cDCs are derived from precursor
cells originating in the bone marrow and serve a crucial role in
inducing T-cell-dependent adaptive immunity (53).
Recent research has found that elevated
concentrations of lactic acid diminished the glucose uptake and
antitumor efficacy of CD8+ T cells by directly
interacting with the intracellular motif of GLUT10 (72). Furthermore, lactate has been shown
to enhance C-X9-C motif containing 1 (CMC1) protein expression
through the induction of ubiquitin specific peptidase 7, a
deubiquitinating enzyme that facilitates the stabilization and
deubiquitination of CMC1 protein (73). The upregulation of CMC1 expression
is associated with increased levels of T-cell surface inhibitory
receptors, including PD-1 and T-cell immunoglobulin and
mucin-domain containing-3, indicating that CMC1 may serve a role in
promoting T-cell depletion (73).
Lactate is associated with impairment of T-cell cytotoxicity and
IFN-γ secretion in liver kinase B1-deficient tumors, which affects
the anti-PD-1/PD-L1 response in vivo (19). Additionally, lactate can inhibit
CD8+ T-cell migration into tumor tissue (74). In pancreatic cancer [specifically
pancreatic ductal adenocarcinoma (PDAC)], targeting of solute
carrier family 4 member 4 can increase CD8+ T-cell
infiltration and IFN-γ production due to the reduction of lactate
production and the higher extracellular pH, which can sensitize
PDAC to anti-PD-1/PD-L1 therapy (75). In hepatocellular carcinoma,
inhibition of MCT4 reduces lactate output and alleviates TME
acidification, which suppresses tumor growth by enhancing the
infiltration and cytotoxic activity of CD8+ T cells, and
has also been found to enhance the effectiveness of anti-PD-1
therapy (76). Lactate-mediated
inactivation of NF-κB sensitizes cytotoxic T cells to
activation-induced cell death, thereby reducing cytotoxic
CD8+ T-cell infiltration and impairing the efficacy of
anti-PD-1/PD-L1 therapy (77).
However, it has also been proposed that lactate is an important
physiological carbon source for promoting T-cell activity and that
the intact function of LDH is critical for its cytotoxic function
(78,79). Whether lactate itself or the
resulting acidic environment mediates these different outcomes
remains to be further explored.
NK cells mediate immunity to pathogens independently
of antigen-presenting cells (80).
Lactate accumulation within the TME results in TME acidification,
leading to intracellular acidification and impaired energy
metabolism in NK cells upon uptake of lactate (81). Data show that the SIX homeobox
1/LDHA axis can promote the accumulation of tumor lactate in
pancreatic cancer, thus inhibiting the function of NK cells
(82). In breast cancer,
LDHB-associated lactic acid clearance has been found to enhance NK
cell activity (83). In addition,
lactate and low pH reduce the cytotoxic activity of NK cells.
Mechanistically, lactic acid and its dissociated hydrogen ions
(H+) can lead to intracellular acidification. The
activity of calcium-modulated phosphatase, which is sensitive to pH
variations, may be inhibited in an acidic environment, consequently
affecting the dephosphorylation and nuclear translocation of NFAT
(84). By impeding NFAT activity,
lactic acid diminishes the transcription and production of IFN-γ,
thereby impairing the effector functions of NK cells (84). Furthermore, lactic acid indirectly
hinders NK cell function by promoting the expansion of
myeloid-derived suppressor cells (81). Combination strategies encompassing
anti-NK cell and anti-PD-1/PD-L1 therapies show greater efficacy
than anti-NK cell therapies in gastric cancer (85).
Within the TME, TCRs identify tumor antigens
presented by MHC molecules, facilitating the activation of T cells
to execute cytotoxic functions and eliminate cancer cells (6). Nevertheless, tumors can progressively
evolve immune evasion strategies, including the upregulation of
PD-L1, which impairs T-cell activity through its interaction with
PD-1 receptors on T-cell surfaces (6). Anti-PD therapies employ monoclonal
antibodies to inhibit the PD-1/PD-L1 signaling pathway (6). Lactate metabolism in tumor cells has
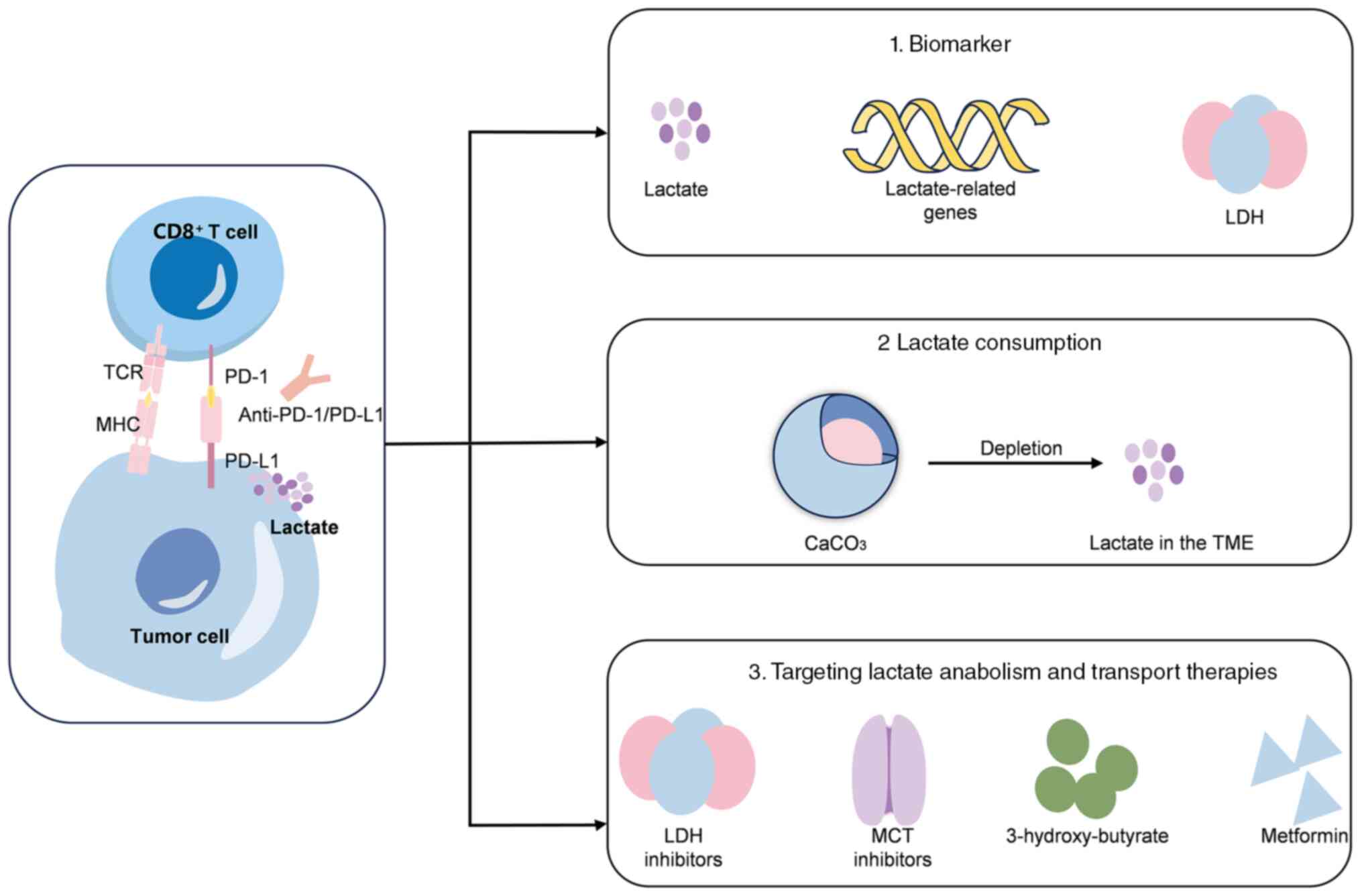
been shown to be associated with immunotherapy resistance (18). Thus, further investigation is
warranted to explore the potential of utilizing lactate as a
predictive marker for immunotherapy efficacy, as well as the
potential of targeting lactate metabolism to enhance the
effectiveness of immunotherapy (Fig.
3).
A lactate metabolism-related signature associated
with the prediction of responses to immunotherapy and related
prognosis has been identified and validated using information from
public databases; however, validation in a larger number of
patients is required (52). For
example, a prognostic signature was constructed for patients with
renal clear cell carcinoma using three lactate-associated genes,
and this could serve as a reliable predictor of prognosis and
response to immunotherapy (86).
Furthermore, a study has demonstrated that patients treated with
pembrolizumab who exhibited elevated baseline LDH levels had a
reduced overall survival compared with those with normal LDH
levels, suggesting that LDH could function as a biomarker for
predicting the efficacy of immunotherapy (87).
Targeting metabolism combined with immunotherapy can
help to increase the effectiveness of immunotherapy (88). Lactate abundance in the TME can be
reduced by affecting key enzymes in lactate metabolism such as LDH
(88) or by directly depleting
lactate (89). Accordingly,
nanovaccines are already available that deliver CaCO3 to
tumor tissue to deplete lactate (89). However, inhibition of lactic acid
production in tumor cells is also required. Evidence has shown that
lactate/GPR81 blockade (3-hydroxy-butyrate) combined with metformin
synergistically inhibited cancer cell proliferation in
vitro. Additionally, this combination has been shown to
suppress glycolysis and oxidative phosphorylation metabolism, as
well as impede tumor growth and reduce serum lactate levels in
tumor-bearing mice. Furthermore, this treatment regimen enhances
the infiltration of CD8+ T cells in tumors and augments
IFN-γ secretion in lymph nodes (90). Taken together, these findings
suggest a promising strategy to enhance patient responsiveness to
PD-1/PD-L1 inhibition (90).
A multifunctional nanoplatform has shown effective
consumption of glucose and lactate within the TME. The nanoplatform
combined three components: Glucose oxidase, laccase and CpG. These
were integrated into a zeolitic imidazolate framework-8 structure
and then coated with a red blood cell membrane. Additionally, in
conjunction with anti-PD-1/PD-L1 therapy, the nanoplatform elicited
robust systemic immunity, resulting in successful eradication of
tumors (91).
Targeting lactate metabolism and its associated
pathways offers novel strategies for cancer treatment, potentially
providing innovative therapeutic approaches to address
immunotherapy resistance. However, metabolic therapies targeting
tumors may also impact cells within the TME and compromise immune
cell function (93). Therefore,
the synergistic interaction between metabolic therapies and
antitumor immunity requires careful consideration. Given the
metabolic heterogeneity of tumors, precision and personalization
may represent the future direction for metabolic therapies in
oncology.
Briefly, the mechanisms through which lactate
influences immune cell function within the TME are as follows
(33,93,98):
i) The induction of an acidic environment that impairs the activity
of immune cells; ii) the modulation of immune cell signaling
pathways, such as NF-κB and HIF-1α; iii) the utilization of lactate
as a substrate for lactylation, which modifies proteins, including
histones, thereby impacting immune cell gene expression and
function; iv) the promotion of recruitment and stimulation of
immunosuppressive cells such as Treg cells; and v) the regulation
of the metabolic state of immune cells by either providing energy
as a metabolic substrate or affecting metabolic pathways such as
glycolysis and oxidative phosphorylation. Overall, the role of
lactate within the TME is multifaceted and diverse. Current
research mainly emphasizes the contributions of lactate to tumor
progression and immune evasion (16,50).
However, under certain conditions, lactate can also serve as an
energy source and provide survival support to immune cells. It is
expected that future studies will reveal more specific mechanisms
of the role of lactate in tumor progression and metastasis,
providing a theoretical basis for the development of novel
therapeutic strategies.
In summary, most current research indicates that
lactate within the TME may impact the efficacy of anti-PD-1/PD-L1
therapies through its role in mediating immunosuppression (71–75).
This finding implies that biomarkers associated with lactate
metabolism could serve as predictive indicators of the response to
anti-PD-1/PD-L1 treatment (99). A
study has demonstrated that immunotherapy efficacy can be altered
by modulating lactate metabolism (19). Modifying lactate metabolism might
enhance the responsiveness of patients to anti-PD-1/PD-L1
therapies. Lactate, a byproduct of tumor cell metabolism, is also
involved in the metabolic processes of immune cells. Consequently,
elucidating the metabolic crosstalk between tumor cells and immune
cells is crucial for generating novel insights and therapeutic
targets to enhance the efficacy of anti-PD-1/PD-L1 therapies.
However, research on the impact of targeting metabolic pathways in
tumor cells on immune cell metabolism within the TME remains
limited. Further studies are needed to identify novel targeted
agents capable of more effectively and selectively modulating
immune responses within the TME.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant nos. 82103004 and 82273323).
Not applicable.
YZ and YH contributed to conceptualization and
writing of the manuscript. QT, LP, JW and FT contributed to the
design of figures and revising the manuscript. XD conceptualized
the article and reviewed the manuscript. Data authentication is not
applicable. All authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho
BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK,
Bondarenko I, et al: Pembrolizumab versus chemotherapy for
previously untreated, PD-L1-expressing, locally advanced or
metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised,
open-label, controlled, phase 3 trial. Lancet. 393:1819–1830. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lorusso D, Xiang Y, Hasegawa K, Scambia G,
Leiva M, Ramos-Elias P, Acevedo A, Sukhin V, Cloven N, Pereira de
Santana Gomes AJ, et al: Pembrolizumab or placebo with
chemoradiotherapy followed by pembrolizumab or placebo for newly
diagnosed, high-risk, locally advanced cervical cancer
(ENGOT-cx11/GOG-3047/KEYNOTE-A18): Overall survival results from a
randomised, double-blind, placebo-controlled, phase 3 trial.
Lancet. 404:1321–1332. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choueiri TK, Tomczak P, Park SH, Venugopal
B, Ferguson T, Symeonides SN, Hajek J, Chang YH, Lee JL, Sarwar N,
et al: Overall survival with adjuvant pembrolizumab in renal-cell
carcinoma. N Engl J Med. 390:1359–1371. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yi M, Zheng X, Niu M, Zhu S, Ge H and Wu
K: Combination strategies with PD-1/PD-L1 blockade: Current
advances and future directions. Mol Cancer. 21:282022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vesely MD, Zhang T and Chen L: Resistance
mechanisms to anti-PD cancer immunotherapy. Annu Rev Immunol.
40:45–74. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peng DH, Rodriguez BL, Diao L, Chen L,
Wang J, Byers LA, Wei Y, Chapman HA, Yamauchi M, Behrens C, et al:
Collagen promotes anti-PD-1/PD-L1 resistance in cancer through
LAIR1-dependent CD8+ T cell exhaustion. Nature Commun. 11:45202020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu M, Peng Z, Qin M, Liu Y, Wang J, Zhang
C, Lin J, Dong T, Wang L, Li S, et al: Interferon-γ induces tumor
resistance to anti-PD-1 immunotherapy by promoting YAP phase
separation. Mol Cell. 81:1216–1230.e9. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou X, Zou L, Liao H, Luo J, Yang T, Wu
J, Chen W, Wu K, Cen S, Lv D, et al: Abrogation of HnRNP L enhances
anti-PD-1 therapy efficacy via diminishing PD-L1 and promoting CD8+
T cell-mediated ferroptosis in castration-resistant prostate
cancer. Acta Pharm Sin B. 12:692–707. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang Z, Lim SO, Yan M, Hsu JL, Yao J, Wei
Y, Chang SS, Yamaguchi H, Lee HH, Ke B, et al: TYRO3 induces
anti-PD-1/PD-L1 therapy resistance by limiting innate immunity and
tumoral ferroptosis. J Clin Invest. 131:e1394342021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou X, Lin J, Shao Y, Zheng H, Yang Y, Li
S, Fan X, Hong H, Mao Z, Xue P, et al: Targeting PLCG2 suppresses
tumor progression, orchestrates the tumor immune microenvironment
and potentiates immune checkpoint blockade therapy for colorectal
cancer. Int J Biol Sci. 20:5548–5575. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dai Y, Guo Z, Leng D, Jiao G, Chen K, Fu
M, Liu Y, Shen Q, Wang Q, Zhu L and Zhao Q: Metal-coordinated
NIR-II nanoadjuvants with nanobody conjugation for potentiating
immunotherapy by tumor metabolism reprogramming. Adv Sci (Weinh).
11:e24048862024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pavlova Natalya N and Thompson Craig B:
The emerging hallmarks of cancer metabolism. Cell Metab. 23:27–47.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nisar H, Sanchidrián González PM, Brauny
M, Labonté FM, Schmitz C, Roggan MD, Konda B and Hellweg CE:
Hypoxia changes energy metabolism and growth rate in non-small cell
lung cancer cells. Cancers (Basel). 15:24722023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Wenes M, Romero P, Huang SCC, Fendt
SM and Ho PC: Navigating metabolic pathways to enhance antitumour
immunity and immunotherapy. Nat Rev Clin Oncol. 16:425–441. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Zhai Z, Duan J, Wang X, Zhong J,
Wu L, Li A, Cao M, Wu Y, Shi H, et al: Lactate: The mediator of
metabolism and immunosuppression. Front Endocrinol (Lausanne).
13:9014952022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shergold AL, Millar R and Nibbs RJB:
Understanding and overcoming the resistance of cancer to PD-1/PD-L1
blockade. Pharmacol Res. 145:1042582019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao Z, Xu D, Harding J, Chen W, Liu X,
Wang Z, Wang L, Qi T, Chen S, Guo X, et al: Lactate oxidase
nanocapsules boost T cell immunity and efficacy of cancer
immunotherapy. Sci Transl Med. 15:eadd27122023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qian Y, Galan-Cobo A, Guijarro I, Dang M,
Molkentine D, Poteete A, Zhang F, Wang Q, Wang J, Parra E, et al:
MCT4-dependent lactate secretion suppresses antitumor immunity in
LKB1-deficient lung adenocarcinoma. Cancer Cell. 41:1363–1380.e7.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gordon SR, Maute RL, Dulken BW, Hutter G,
George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, et al:
PD-1 expression by tumour-associated macrophages inhibits
phagocytosis and tumour immunity. Nature. 545:495–499. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marasco M, Berteotti A, Weyershaeuser J,
Thorausch N, Sikorska J, Krausze J, Brandt HJ, Kirkpatrick J, Rios
P, Schamel WW, et al: Molecular mechanism of SHP2 activation by
PD-1 stimulation. Sci Adv. 6:eaay44582020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yokosuka T, Takamatsu M,
Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M and Saito T:
Programmed cell death 1 forms negative costimulatory microclusters
that directly inhibit T cell receptor signaling by recruiting
phosphatase SHP2. J Exp Med. 209:1201–1217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ruiz de Galarreta M, Bresnahan E,
Molina-Sánchez P, Lindblad KE, Maier B, Sia D, Puigvehi M, Miguela
V, Casanova-Acebes M, Dhainaut M, et al: β-Catenin activation
promotes immune escape and resistance to anti-PD-1 therapy in
hepatocellular carcinoma. Cancer Discov. 9:1124–1141. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou L, Mudianto T, Ma X, Riley R and
Uppaluri R: Targeting EZH2 enhances antigen presentation, antitumor
Immunity, and circumvents anti-PD-1 resistance in head and neck
cancer. Clin Cancer Res. 26:290–300. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rosenthal R, Cadieux EL, Salgado R, Bakir
MA, Moore DA, Hiley CT, Lund T, Tanić M, Reading JL, Joshi K, et
al: Neoantigen-directed immune escape in lung cancer evolution.
Nature. 567:479–485. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tumeh PC, Harview CL, Yearley JH, Shintaku
IP, Taylor EJM, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu
V, et al: PD-1 blockade induces responses by inhibiting adaptive
immune resistance. Nature. 515:568–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kwon M, An M, Klempner SJ, Lee H, Kim KM,
Sa JK, Cho HJ, Hong JY, Lee T, Min YW, et al: Determinants of
response and intrinsic resistance to PD-1 blockade in
microsatellite instability-high gastric cancer. Cancer Discov.
11:2168–2185. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Skoulidis F, Goldberg ME, Greenawalt DM,
Hellmann MD, Awad MM, Gainor JF, Schrock AB, Hartmaier RJ, Trabucco
SE, Gay L, et al: STK11/LKB1 mutations and PD-1 inhibitor
resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov.
8:822–835. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Messaoudene M, Pidgeon R, Richard C, Ponce
M, Diop K, Benlaifaoui M, Nolin-Lapalme A, Cauchois F, Malo J,
Belkaid W, et al: A natural polyphenol exerts antitumor activity
and circumvents anti-PD-1 resistance through effects on the gut
microbiota. Cancer Discov. 12:1070–1087. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lei Q, Wang D, Sun K, Wang L and Zhang Y:
Resistance mechanisms of anti-PD1/PDL1 therapy in solid tumors.
Front Cell Dev Biol. 8:6722020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Murciano-Goroff YR, Warner AB and Wolchok
JD: The future of cancer immunotherapy: Microenvironment-targeting
combinations. Cell Res. 30:507–519. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zou W and Green DR: Beggars banquet:
Metabolism in the tumor immune microenvironment and cancer therapy.
Cell Metab. 35:1101–1113. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang ZH, Peng WB, Zhang P, Yang XP and
Zhou Q: Lactate in the tumour microenvironment: From immune
modulation to therapy. EBioMedicine. 73:1036272021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jedlička M, Feglarová T, Janstová L,
Hortová-Kohoutková M and Frič J: Lactate from the tumor
microenvironment-A key obstacle in NK cell-based immunotherapies.
Front Immunol. 13:9320552022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kumagai S, Koyama S, Itahashi K,
Tanegashima T, Lin YT, Togashi Y, Kamada T, Irie T, Okumura G, Kono
H, et al: Lactic acid promotes PD-1 expression in regulatory T
cells in highly glycolytic tumor microenvironments. Cancer Cell.
40:201–218.e9. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Warburg O, Wind F and Negelein E: Über den
stoffwechsel von tumoren im körper. Klin Wochenschr. 5:829–832.
1926. View Article : Google Scholar
|
|
37
|
Paul S, Ghosh S and Kumar S: Tumor
glycolysis, an essential sweet tooth of tumor cells. Semin Cancer
Biol. 86:1216–1230. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Luo B, Song L, Chen L, Cai Y, Zhang M and
Wang S: Ganoderic acid D attenuates gemcitabine resistance of
triple-negative breast cancer cells by inhibiting glycolysis via
HIF-1alpha destabilization. Phytomedicine. 129:1556752024.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mossmann D, Park S and Hall MN: mTOR
signalling and cellular metabolism are mutual determinants in
cancer. Nat Rev Cancer. 18:744–757. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang P, Wan Y, Ma J, Gong J, Zhong Z, Cui
Y, Zhang H, Da Y, Ma J, Li C, et al: Epigenetic silencing of LDHB
promotes hepatocellular carcinoma by remodeling the tumor
microenvironment. Cancer Immunol Immunother. 73:1272024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hong SM, Lee YK, Park I, Kwon SM, Min S
and Yoon G: Lactic acidosis caused by repressed lactate
dehydrogenase subunit B expression down-regulates mitochondrial
oxidative phosphorylation via the pyruvate dehydrogenase (PDH)-PDH
kinase axis. J Biol Chem. 294:7810–7820. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yue J, Xu J, Yin Y, Shu Y, Li Y, Li T, Zou
Z, Wang Z, Li F, Zhang M, et al: Targeting the PDK/PDH axis to
reverse metabolic abnormalities by structure-based virtual
screening with in vitro and in vivo experiments. Int J Biol
Macromol. 262:1299702024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li X, Yang Y, Zhang B, Lin X, Fu X, An Y,
Zou Y, Wang JX, Wang Z and Yu T: Lactate metabolism in human health
and disease. Signal Transduct Target Ther. 7:3052022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tian LR, Lin MZ, Zhong HH, Cai YJ, Li B,
Xiao ZC and Shuai XT: Nanodrug regulates lactic acid metabolism to
reprogram the immunosuppressive tumor microenvironment for enhanced
cancer immunotherapy. Biomater Sci. 10:3892–3900. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tasdogan A, Faubert B, Ramesh V,
Ubellacker JM, Shen B, Solmonson A, Murphy MM, Gu Z, Gu W, Martin
M, et al: Metabolic heterogeneity confers differences in melanoma
metastatic potential. Nature. 577:115–120. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hui S, Ghergurovich JM, Morscher RJ, Jang
C, Teng X, Lu W, Esparza LA, Reya T, Le Zhan, Yanxiang Guo J, et
al: Glucose feeds the TCA cycle via circulating lactate. Nature.
551:115–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Faubert B, Li KY, Cai L, Hensley CT, Kim
J, Zacharias LG, Yang C, Do QN, Doucette S, Burguete D, et al:
Lactate metabolism in human lung tumors. Cell. 171:358–371.e9.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pérez-Escuredo J, Dadhich RK, Dhup S,
Cacace A, Van Hée VF, De Saedeleer CJ, Sboarina M, Rodriguez F,
Fontenille MJ, Brisson L, et al: Lactate promotes glutamine uptake
and metabolism in oxidative cancer cells. Cell Cycle. 15:72–83.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gu J, Zhou J, Chen Q, Xu X, Gao J, Li X,
Shao Q, Zhou B, Zhou H, Wei S, et al: Tumor metabolite lactate
promotes tumorigenesis by modulating MOESIN lactylation and
enhancing TGF-β signaling in regulatory T cells. Cell Rep.
39:1109862022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ippolito L, Comito G, Parri M, Iozzo M,
Duatti A, Virgilio F, Lorito N, Bacci M, Pardella E, Sandrini G, et
al: Lactate rewires lipid metabolism and sustains a
metabolic-epigenetic axis in prostate cancer. Cancer Res.
82:1267–1282. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xie D, Zhu S and Bai L: Lactic acid in
tumor microenvironments causes dysfunction of NKT cells by
interfering with mTOR signaling. Sci China Life Sci. 59:1290–1296.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen D, Liu P, Lu X, Li J, Qi D, Zang L,
Lin J, Liu Y, Zhai S, Fu D, et al: Pan-cancer analysis implicates
novel insights of lactate metabolism into immunotherapy response
prediction and survival prognostication. J Exp Clin Cancer Res.
43:1252024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Marciscano AE and Anandasabapathy N: The
role of dendritic cells in cancer and anti-tumor immunity. Semin
Immunol. 52:1014812021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Christofides A, Strauss L, Yeo A, Cao C,
Charest A and Boussiotis VA: The complex role of tumor-infiltrating
macrophages. Nat Immunol. 23:1148–1156. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mu X, Shi W, Xu Y, Xu C, Zhao T, Geng B,
Yang J, Pan J, Hu S, Zhang C, et al: Tumor-derived lactate induces
M2 macrophage polarization via the activation of the ERK/STAT3
signaling pathway in breast cancer. Cell Cycle. 17:428–438. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang A, Xu Y, Xu H, Ren J, Meng T, Ni Y,
Zhu Q, Zhang WB, Pan YB, Jin J, et al: Lactate-induced M2
polarization of tumor-associated macrophages promotes the invasion
of pituitary adenoma by secreting CCL17. Theranostics.
11:3839–3852. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen P, Zuo H, Xiong H, Kolar MJ, Chu Q,
Saghatelian A, Siegwart DJ and Wan Y: Gpr132 sensing of lactate
mediates tumor-macrophage interplay to promote breast cancer
metastasis. Proc Natl Acad Sci USA. 114:580–585. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jiang H, Wei H, Wang H, Wang Z, Li J, Ou
Y, Xiao X, Wang W, Chang A, Sun W, et al: Zeb1-induced metabolic
reprogramming of glycolysis is essential for macrophage
polarization in breast cancer. Cell Death Dis. 13:2062022.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Colegio OR, Chu NQ, Szabo AL, Chu T,
Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC,
Phillips GM, et al: Functional polarization of tumour-associated
macrophages by tumour-derived lactic acid. Nature. 513:559–563.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cai J, Song L, Zhang F, Wu S, Zhu G, Zhang
P, Chen S, Du J, Wang B, Cai Y, et al: Targeting SRSF10 might
inhibit M2 macrophage polarization and potentiate anti-PD-1 therapy
in hepatocellular carcinoma. Cancer Commun (Lond). 44:1231–1260.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang J, Muri J, Fitzgerald G, Gorski T,
Gianni-Barrera R, Masschelein E, D'Hulst G, Gilardoni P, Turiel G,
Fan Z, et al: Endothelial lactate controls muscle regeneration from
ischemia by inducing M2-like macrophage polarization. Cell Metab.
31:1136–1153.e7. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Morrissey SM, Zhang F, Ding C,
Montoya-Durango DE, Hu X, Yang C, Wang Z, Yuan F, Fox M, Zhang HG,
et al: Tumor-derived exosomes drive immunosuppressive macrophages
in a pre-metastatic niche through glycolytic dominant metabolic
reprogramming. Cell Metab. 33:2040–2058.e10. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Tang H, Liang Y, Anders RA, Taube JM, Qiu
X, Mulgaonkar A, Liu X, Harrington SM, Guo J, Xin Y, et al: PD-L1
on host cells is essential for PD-L1 blockade-mediated tumor
regression. J Clin Invest. 128:580–588. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Del Prete A, Salvi V, Soriani A,
Laffranchi M, Sozio F, Bosisio D and Sozzani S: Dendritic cell
subsets in cancer immunity and tumor antigen sensing. Cell Mol
Immunol. 20:432–447. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
See P, Dutertre CA, Chen J, Gunther P,
McGovern N, Irac SE, Gunawan M, Beyer M, Händler K, Duan K, et al:
Mapping the human DC lineage through the integration of
high-dimensional techniques. Science. 356:eaag30092017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rigamonti A, Villar J and Segura E:
Monocyte differentiation within tissues: A renewed outlook. Trends
Immunol. 44:999–1013. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Peng X, He Y, Huang J, Tao Y and Liu S:
Metabolism of dendritic cells in tumor microenvironment: for
immunotherapy. Front Immunol. 12:6134922021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Monti M, Vescovi R, Consoli F, Farina D,
Moratto D, Berruti A, Specchia C and Vermi W: Plasmacytoid
dendritic cell impairment in metastatic melanoma by lactic
acidosis. Cancers (Basel). 12:20852020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Brown TP, Bhattacharjee P, Ramachandran S,
Sivaprakasam S, Ristic B, Sikder MOF and Ganapathy V: The lactate
receptor GPR81 promotes breast cancer growth via a paracrine
mechanism involving antigen-presenting cells in the tumor
microenvironment. Oncogene. 39:3292–3304. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Plebanek MP, Xue Y, Nguyen YV, DeVito NC,
Wang X, Holtzhausen A, Beasley GM, Theivanthiran B and Hanks BA: A
lactate-SREBP2 signaling axis drives tolerogenic dendritic cell
maturation and promotes cancer progression. Sci Immunol.
9:eadi41912024. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang Z, Xu F, Hu J, Zhang H, Cui L, Lu W,
He W, Wang X, Li M, Zhang H, et al: Modulation of lactate-lysosome
axis in dendritic cells by clotrimazole potentiates antitumor
immunity. J Immunother Cancer. 9:e0021552021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Liu Y, Wang F, Peng D, Zhang D, Liu L, Wei
J, Yuan J, Zhao L, Jiang H, Zhang T, et al: Activation and
antitumor immunity of CD8+ T cells are supported by the glucose
transporter GLUT10 and disrupted by lactic acid. Sci Transl Med.
16:eadk73992024. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen Y, Gao J, Ma M, Wang K, Liu F, Yang
F, Yang F, Zou X, Cheng Z and Wu D: The potential role of CMC1 as
an immunometabolic checkpoint in T cell immunity. Oncoimmunology.
13:23449052024. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sasaki K, Nishina S, Yamauchi A, Fukuda K,
Hara Y, Yamamura M, Egashira K and Hino K: Nanoparticle-mediated
delivery of 2-deoxy-D-glucose induces antitumor immunity and
cytotoxicity in liver tumors in mice. Cell Mol Gastroenterol
Hepatol. 11:739–762. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Cappellesso F, Orban MP, Shirgaonkar N,
Berardi E, Serneels J, Neveu MA, Di Molfetta D, Piccapane F,
Caroppo R, Debellis L, et al: Targeting the bicarbonate transporter
SLC4A4 overcomes immunosuppression and immunotherapy resistance in
pancreatic cancer. Nat Cancer. 3:1464–1483. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Fang Y, Liu W, Tang Z, Ji X, Zhou Y, Song
S, Tian M, Tao C, Huang R, Zhu G, et al: Monocarboxylate
transporter 4 inhibition potentiates hepatocellular carcinoma
immunotherapy through enhancing T cell infiltration and immune
attack. Hepatology. 77:109–123. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Liu H, Liang Z, Cheng S, Huang L, Li W,
Zhou C, Zheng X, Li S, Zeng Z and Kang L: Mutant KRAS drives immune
evasion by sensitizing cytotoxic T-cells to activation-induced cell
death in colorectal cancer. Adv Sci (Weinh). 10:e22037572023.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kaymak I, Luda KM, Duimstra LR, Ma EH,
Longo J, Dahabieh MS, Faubert B, Oswald BM, Watson MJ,
Kitchen-Goosen SM, et al: Carbon source availability drives
nutrient utilization in CD8(+) T cells. Cell Metab.
34:1298–1311.e6. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Notarangelo G, Spinelli JB, Perez EM,
Baker GJ, Kurmi K, Elia I, Stopka SA, Baquer G, Lin JR, Golby AJ,
et al: Oncometabolite d-2HG alters T cell metabolism to impair
CD8(+) T cell function. Science. 377:1519–1529. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang H, Grzywacz B, Sukovich D, McCullar
V, Cao Q, Lee AB, Blazar BR, Cornfield DN, Miller JS and Verneris
MR: The unexpected effect of cyclosporin A on CD56+CD16- and
CD56+CD16+ natural killer cell subpopulations. Blood.
110:1530–1539. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Miao L, Lu C, Zhang B, Li H, Zhao X, Chen
H, Liu Y and Cui X: Advances in metabolic reprogramming of NK cells
in the tumor microenvironment on the impact of NK therapy. J Transl
Med. 22:2292024. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ge W, Meng L, Cao S, Hou C, Zhu X, Huang
D, Li Q, Peng Y and Jiang K: The SIX1/LDHA axis promotes lactate
accumulation and leads to NK cell dysfunction in pancreatic cancer.
J Immunol Res. 2023:68916362023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Luo Z, Huang X, Xu X, Wei K, Zheng Y, Gong
K and Li W: Decreased LDHB expression in breast tumor cells causes
NK cell activation and promotes tumor progression. Cancer Biol Med.
21:513–540. 2024.PubMed/NCBI
|
|
84
|
Brand A, Singer K, Koehl GE, Kolitzus M,
Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, et
al: LDHA-associated lactic acid production blunts tumor
immunosurveillance by T and NK cells. Cell Metab. 24:657–671. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Abdolahi S, Ghazvinian Z, Muhammadnejad S,
Ahmadvand M, Aghdaei HA, Ebrahimi-Barough S, Ai J, Zali MR, Verdi J
and Baghaei K: Adaptive NK cell therapy modulated by anti-PD-1
antibody in gastric cancer model. Front Pharmacol. 12:7330752021.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Sun Z, Tao W, Guo X, Jing C, Zhang M, Wang
Z, Kong F, Suo N, Jiang S and Wang H: Construction of a
lactate-related prognostic signature for predicting prognosis,
tumor microenvironment, and immune response in kidney renal clear
cell carcinoma. Front Immunol. 13:8189842022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wagner NB, Forschner A, Leiter U, Garbe C
and Eigentler TK: S100B and LDH as early prognostic markers for
response and overall survival in melanoma patients treated with
anti-PD-1 or combined anti-PD-1 plus anti-CTLA-4 antibodies. Br J
Cancer. 119:339–346. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Heuser C, Renner K, Kreutz M and Gattinoni
L: Targeting lactate metabolism for cancer immunotherapy-a matter
of precision. Semin Cancer Biol. 88:32–45. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ding Y, Yang J, Wei H, Wang J, Huang S,
Yang S, Guo Y, Li B and Shuai X: Construction of pH-sensitive
nanovaccines encapsulating tumor cell lysates and immune adjuvants
for breast cancer therapy. Small. 19:e23014202023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Chen S, Zhou X, Yang X, Li W, Li S, Hu Z,
Ling C, Shi R, Liu J, Chen G, et al: Dual blockade of lactate/GPR81
and PD-1/PD-L1 pathways enhances the anti-tumor effects of
metformin. Biomolecules. 11:13732021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ji P, Jin XK, Deng XC, Zhang SM, Liang JL,
Li QR, Chen WH and Zhang XZ: Metabolic regulation-mediated
reversion of the tumor immunosuppressive microenvironment for
potentiating cooperative metabolic therapy and immunotherapy. Nano
Lett. 24:4691–701. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Renner K, Bruss C, Schnell A, Koehl G,
Becker HM, Fante M, Menevse AN, Kauer N, Blazquez R, Hacker L, et
al: Restricting glycolysis preserves T Cell effector functions and
augments checkpoint therapy. Cell Rep. 29:135–150.e9. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zheng Y, Xu R, Chen X, Lu Y, Zheng J, Lin
Y, Zheng J, Lin Y, Lin P, Zhao X and Cui L: Metabolic gatekeepers:
Harnessing tumor-derived metabolites to optimize T cell-based
immunotherapy efficacy in the tumor microenvironment. Cell Death
Dis. 15:7752024. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Liu Y, Zhao Y, Song H, Li Y, Liu Z, Ye Z,
Zhao J, Wu Y, Tang J and Yao M: Metabolic reprogramming in tumor
immune microenvironment: Impact on immune cell function and
therapeutic implications. Cancer Lett. 597:2170762024. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Li J, Zhao J, Tian C, Dong L, Kang Z, Wang
J, Zhao S, Li M and Tong X: Mechanisms of regulation of glycolipid
metabolism by natural compounds in plants: Effects on short-chain
fatty acids. Nutr Metab (Lond). 21:492024. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wang J, Yang Y, Shao F, Meng Y, Guo D, He
J and Lu Z: Acetate reprogrammes tumour metabolism and promotes
PD-L1 expression and immune evasion by upregulating c-Myc. Nat
Metab. 6:914–932. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Bose S, Ramesh V and Locasale JW: Acetate
metabolism in physiology, cancer, and beyond. Trends Cell Biol.
29:695–703. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Burgdorf S, Porubsky S, Marx A and Popovic
ZV: Cancer acidity and hypertonicity contribute to dysfunction of
tumor-associated dendritic cells: Potential impact on antigen
cross-presentation machinery. Cancers (Basel). 12:24032020.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Shang S, Wang MZ, Xing Z, He N and Li S:
Lactate regulators contribute to tumor microenvironment and predict
prognosis in lung adenocarcinoma. Front Immunol. 13:10249252022.
View Article : Google Scholar : PubMed/NCBI
|