Introduction
Cancer is a disease characterized by the accumulation of DNA damage due to genomic instability and mutant phenotypes resulting from dysfunctional DNA repair pathways (1). It arises from a multistep, multifactorial process characterized by persistent proliferative signaling, evasion of growth suppression, immune clearance and limitless replication. Cancer screening techniques have significantly contributed to reducing cancer morbidity and mortality through enabling early diagnosis and expanding treatment options; however, traditional cancer screening methods have been shown to be associated with drawbacks, including false positives, high costs and potential complications such as tears in the colonic lining during colonoscopy (2,3). By contrast, the identification of highly specific and sensitive biomarkers as a non-invasive cancer screening approach offers significant advantages, and holds substantial potential for both improving the cancer diagnosis of patients and monitoring tumor progression (4).
In the double-helix configuration of DNA, two complementary strands are held together through hydrogen-bonding between their respective bases. These pairs twist around their longitudinal axis in a clockwise direction, thereby forming a right-handed helix. This structural design prevents the strands from disjoining, thereby safeguarding the genetic information at the DNA fiber's core, which is essential for the retention and transference of genetic data. However, accessing the base sequence in the DNA requires the helix to uncoil, necessitating the untangling of both strands (5). Additionally, the mechanisms that are involved in the unwinding and rewinding of the strands, the movement of proteins along the DNA, and the assembly of higher-order structures may lead to the emergence of topological knots and genomic instability if the relevant processes are not properly managed (6,7). Topoisomerases have been shown to fulfill an essential role in addressing these challenges through modifying the topology of DNA via the transient cleavage of one or both strands, as facilitated by an ester-exchange reaction (8,9).
Topoisomerases are primarily categorized into type I (TOP1) and type II (TOP2) enzymes, according to their structural characteristics and operational mechanisms, with an additional subclassification into subfamilies A and B. Type I enzymes are responsible for cleaving a single strand, whereas type II enzymes target both strands, resulting in interleaved breaks (6). TOP1 and TOP2 are pivotal in segregating strands to generate a DNA superhelix, and in maintaining chromosomal structural integrity (10,11). TOP1, which binds with a single-strand break (SSB), the active tyrosine site on TOP1 connects with both ends of the break, establishing a covalent bond with the 5′-phosphate at one end. This interaction allows the gap from the SSB to pass through the intact strand and reseal, releasing the enzyme and completing the cycle, thereby alleviating torsional stress and reducing super-helical twisting in the DNA (12,13).
On the other hand, TOP2 facilitates strand movement through double-stranded DNA gates (10,14,15). In eukaryotic cells, DNA TOP2 functions as a homodimer that alleviates topological stress by temporarily severing strands through adenosine 5′-triphosphate (ATP) hydrolysis-catalyzed double-stranded breaks (DSBs). Each unit slices a single strand, forming a ‘G segment’ or ‘gate segment’, enabling the transit of an intact segment termed the ‘T segment’ (12,13,15). TOP2 transiently connects to the 5′-end of the cut strand via phosphotyrosine bonds, forming complexes that shield the cleaved ends from the cellular DNA damage response, thereby allowing the smooth transfer of intact helices and subsequent DNA breaks (12,13,15) (Fig. 1).
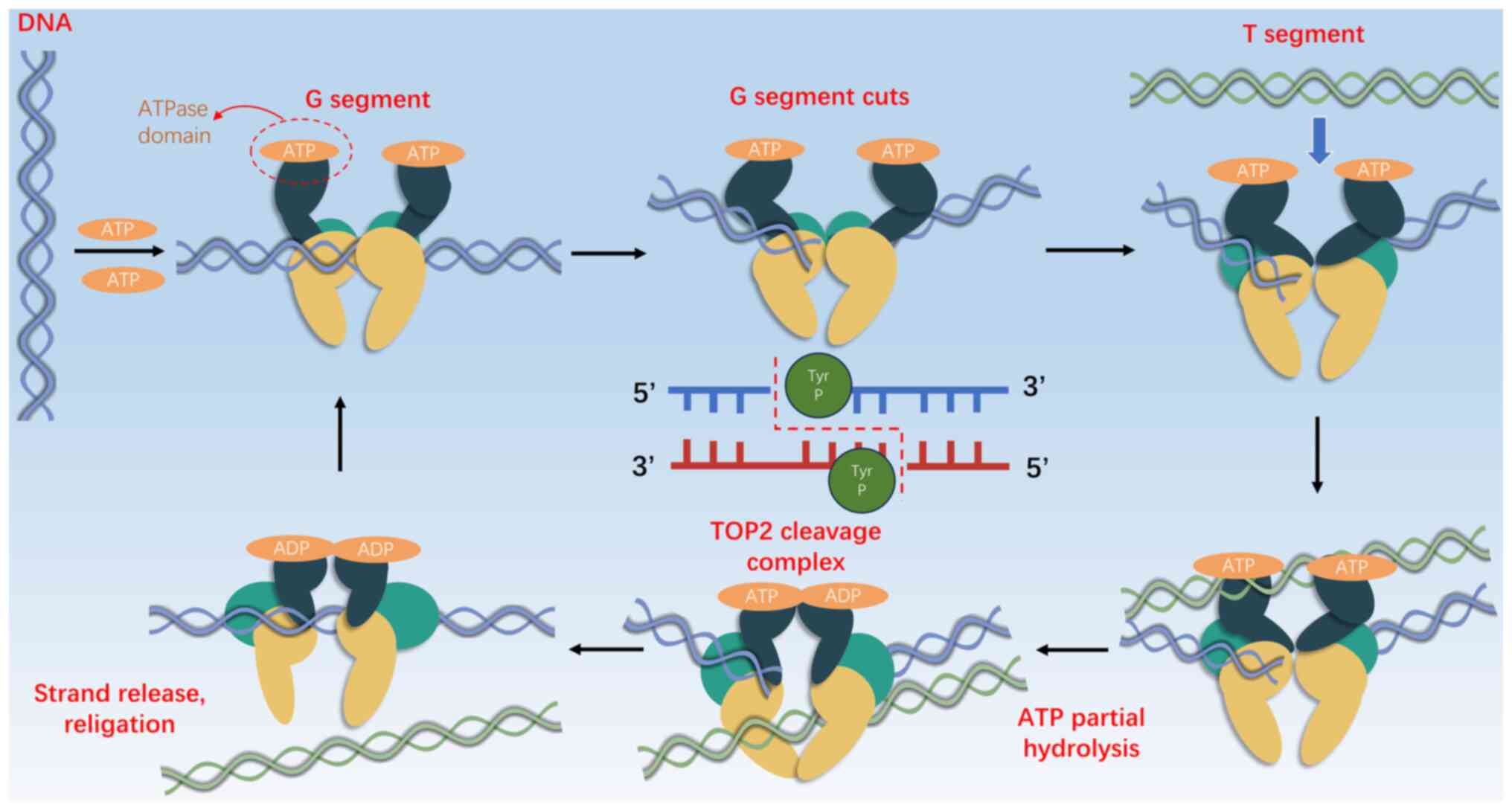 |
Figure 1.
TOP2A Illustration of basic action on DNA double strands. Each monomer cuts a single DNA strand to create a ‘G segment’ gate, allowing an uncut ‘T segment’ to pass through, with TOP2 attaching to the 5′ end via covalent phosphotyrosine bonds to form TOP2 DNA cleavage complexes. The specific steps include: i) ATP binding and capture of G segment DNA; ii) cleavage of the G segment DNA double strand; iii) capture of the T segment; iv) preparation of the T segment to pass through the G segment gate; v) formation of TOP2 cleavage complexes; and vi) re-sealing of the G segment in preparation for the next cycle. TOP2A, topoisomerase IIα.
|
TOP2 enzymes consist of three critical structural domains: The N-terminal ATPase domain, the central catalytic core responsible for binding and cleaving DNA, and the C-terminal domain (CTD) (13,14,16) (Fig. 2). The N-terminal domain performs a vital role both in terms of binding ATP and enabling the structural changes in TOP2 induced by hydrolysis (12,17,18). The central domain contains an active tyrosine site that is essential for forming a covalent bond between TOP2 and the 5′-end of the DNA DSB terminus. Finally, the CTD, characterized by nuclear localization signals, undergoes diverse post-translational modifications, which have the effect of influencing the catalytic functions of TOP2, including its interactions with other proteins and its DNA-binding capabilities (11,17,18).
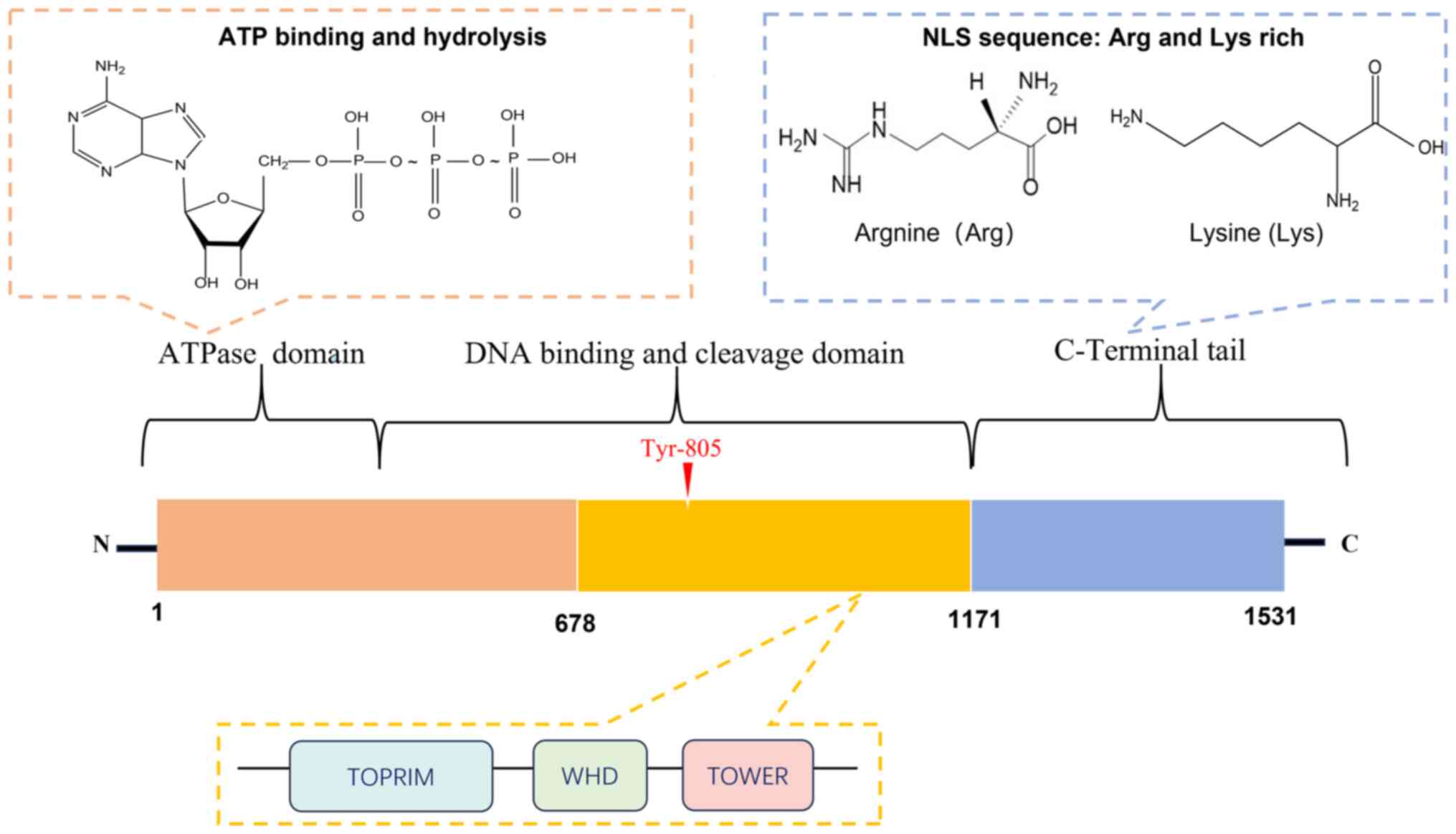 |
Figure 2.
Protein domains of TOP2A. Type II topoisomerases have three main structural domains: i) The N-terminal ATPase domain for ATP-binding and hydrolysis-mediated conformational changes; ii) the central catalytic core DNA-binding/cleavage domain with an active tyrosine site for forming covalent complexes with DNA, the central structural domain of TOP2A also includes: TOPRIM domain (Topoisomerase-primase domain), TOWER domain, and WHD domain (Winged-Helix Domain); and iii) the C-terminal domain (CTD) that includes and undergoes post-translational modifications to regulate TOP2′s catalytic activity, protein interactions and DNA binding properties. Nuclear localization sequence is rich in lysine and arginine. TOP2A, topoisomerase IIα; NLS, nuclear localization sequence; TOPRIM, topoisomerase-primase domain; TOWER, TOP2 observed with electron microscopy domain; WHD, winged-helix domain; CTD, C-terminal domain.
|
In vertebrates, the two principal type II topoisomerases, namely TOP2A and TOP2B (19), exhibit similar structural and functional attributes, and they share considerable sequence similarity with each other (19,20). However, a key difference between them exists in their CTDs, which confer distinct cellular functions, such as chromosomal binding during mitosis and the support of cell proliferation (14,15,19). Specifically, the C-terminus of TOP2A includes a specialized chromatin tethering domain that is crucial for chromosomal contact and localization within mitotic spiking granules, which serve a critical role both in terms of segregating chromosomes during mitosis and in ensuring genomic stability through the timely TOP2A-mediated separation of sister chromatids (21).
Located on chromosome 17q12-21 near to the human epidermal growth factor receptor 2 (HER2) gene, the TOP2A gene encodes the cell cycle regulator TOP2α, which has a molecular mass of 170 kDa (19). The expression of TOP2A, which varies according to the stage of the cell cycle, reaches its maximum level of expression during the G2/M phase, especially in proliferating cells. Moreover, its involvement in oncogenesis is starting to gain attention in current research efforts (22,23).
Changes in the copy number and expression levels of TOP2A have been revealed to be associated with reduced survival rates and unfavorable outcomes in patients with cancer (24,25). In a cohort of ~24,000 patients with solid tumors, ~4% of those patients exhibited TOP2A amplification, and this was found to be notably high in gall-bladder and gastroesophageal tumors, where the percentages of occurrences exceeded 10%. In a total of 4,903 analyzed samples, 129 (2.6%) displayed co-amplification of TOP2A and HER2, and the percentages of occurrences were found to be >40% in breast, ovarian, gastroesophageal and pancreatic cancer (PC). The association between TOP2A and HER2 amplifications is complex: The physical deletion of TOP2A revealed that up to 10% of breast tumors exhibited TOP2A amplification in the absence of HER2 amplification (24,25). By contrast, certain types of cancer, such as acute lymphoblastic leukemia, gastric cancer (GC) and bladder cancer (BLCA), predominantly exhibited amplification of TOP2A alone (24,25).
Moreover, somatic mutations in TOP2A have a critical role in the initiation and progression of cancer (26,27). A study by Boot et al (26) uncovered a novel mutation, p.K743N, in human TOP2a (hTOP2α), which generated a distinctive pattern of insertion and deletion mutations that was termed ‘ID_TOP2α’. This mutation, which is associated with a repetition of base pairs, is prevalent in tumors. These mutations diminish the efficiency of TOP2A in terms of mediating DNA cleavage and repair, resulting in the accumulation of DNA damage.
An associated mutation in yeast (yTop2-K720N) has corroborated these findings, as it produces comparable enzyme-mediated DNA damage. Analysis from the COSMIC database revealed that tumors with the ID_TOP2α mutation include insertional deletions in critical oncogenes such as PTEN and TP53, and an activating insertion in BRAF, thereby substantiating the mutation's role in cancer etiology. Given that these mutations are observed in diagnosed tumors, it may be hypothesized that they are associated with cancer progression. Therefore, mutations in hTOP2α are more likely to arise during the advanced stages of cancer than in the early stages. Specifically, these mutations may exert a pro-cancer role during tumor progression (26). In high-grade glioblastoma (GBM), the recurrent somatic mutation E948Q in TOP2A has been identified, which features the replacement of glutamate (E) at position 948 with glutamine (Q). This mutation is closely associated with accelerated tumor progression and adverse prognoses. Patients with isocitrate dehydrogenase-wild type GBM, who exhibit overexpression of TOP2A in addition to having this mutation, experience reduced lifespans. A previous study demonstrated that the E948Q mutation intensifies the superhelix relaxation function of the TOP2A protein and its capability to bind DNA, leading to heightened genomic instability and transcriptional irregularities (27). The emergence of unprocessed transcripts and the initiation of irregular transcriptional processes further substantiate the connection between overexpression of TOP2A and the accumulation of mutations in somatic cells. The E948Q mutation, which has been significantly associated with aggressive tumor behavior and diminished survival, potentially serves as a catalyst in the development of GBM, emphasizing its critical role in cancer progression (27).
Extensive research has demonstrated that aberrant expression patterns of TOP2A are closely associated with cancer prognosis (28–30). The latest biological insights into TOP2A across different types of cancer, and the elucidation of its expression patterns and mechanisms, are summarized in Fig. 3. Previous studies have expanded the understanding of its roles in cellular proliferation, invasion, migration, the immune response and resistance to treatment, all pointing towards the adverse prognosis associated with TOP2A activity in several types of cancer (28–30). Although data have been published, which indicate that gene amplification or overexpression of TOP2A is common in aggressive cancers, such as those of the breast and lung, and that this is associated with tumor aggressiveness (28,31), these findings have not definitively established that TOP2A mutations directly drive cancer progression. The amplification of TOP2A may indicate a broader functional demand for topoisomerases in cancer cells, rather than solely acting as a driver gene. Therefore, further studies are essential to determine the specific involvement of TOP2A in tumor progression. As a possible therapeutic target, medications targeting TOP2A have been extensively employed in various types of cancer treatment (32,33). Nevertheless, at present, the emergence of side effects and resistance has prompted a re-evaluation of this target's therapeutic efficacy, and the challenges facing therapies aimed at TOP2A remain under discussion. Overall, ongoing studies on the role of TOP2A in cancer and its associated therapeutic applications are crucial for advancing any comprehension of the underlying cancer mechanisms, for developing new treatment modalities, and for improving patient outcomes.
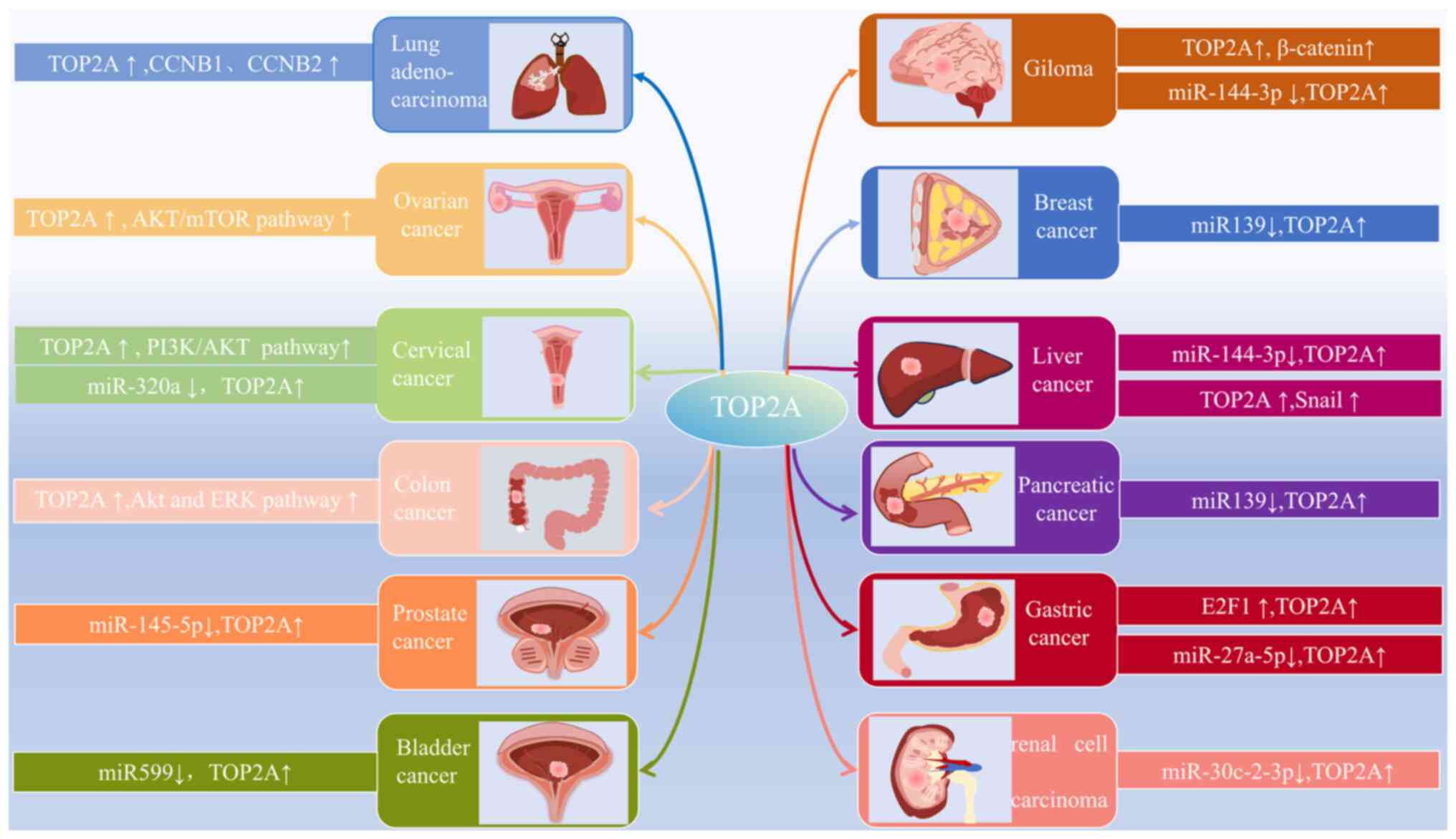 |
Figure 3.
Underlying mechanisms of TOP2A action in different cancers. The mechanism of action of TOP2A in various cancers involves the regulation of multiple transcription factors and signaling pathways. TOP2A, topoisomerase IIα.
|
Role of TOP2A in various types of cancer
Glioma
Gliomas constitute ~80% of malignant brain tumors in adults, with GBM as the most prevalent type (34). A previously published study suggested that a link exists between human cytomegalovirus (HCMV) infection and GBM, which contributes to tumor growth and metastasis (35). The conventional treatment strategy for GBM involves total tumor resection followed by chemotherapy and radiation therapy. Despite these interventions, GBM remains largely untreatable, with a median survival time for patients of 15 months (36), underscoring the importance of early biomarker detection for managing glioma.
A previous study by Yang et al (37) demonstrated that TOP2A is upregulated in GBM, and that this upregulation is associated with a negative prognosis. Go-ichi-san complex subunit 1 (GINS1), which is prominently expressed in GBM, facilitates tumor cell proliferation and migration; on the other hand, silencing GINS1 impedes these processes. Ubiquitin-specific protease 15 (USP15), an enzyme that deubiquitinates and interacts with TOP2A, enhances the malignant characteristics of GBM through decreasing the ubiquitination of TOP2A. This interaction was shown to circumvent the inhibitory effects of silencing GINS1 on tumor growth and dissemination. It has been postulated that GINS1 may drive GBM advancement via the USP15-mediated deubiquitination of TOP2A, although the detailed mechanism requires further investigation (37). In addition, the presence of β-catenin, a key element of the Wnt signaling pathway, in GBM cells is positively associated with TOP2A expression. Through the dysregulation of Wnt/β-catenin signaling, known to foster the invasion and metastasis of various types of cancer, it has been inferred that TOP2A may be dependent on the β-catenin signaling pathway to promote glioma progression (38). A previous study has highlighted the significant roles of microRNAs (miRNAs or miRs), typically considered to be tumor suppressors, in controlling tumor development, growth, migration, invasion and apoptosis (39). Interestingly, a marked difference in miR-144-3p levels was observed between HCMV-positive and HCMV-negative GBM samples. A negative association was identified between the expression levels of miR-144-3p and TOP2A. Laboratory experiments have also demonstrated that miR-144-3p promotes apoptosis and diminishes cell migration through the targeting of TOP2A, thereby inhibiting glioma cell proliferation (35). Taken together, the aforementioned studies have revealed that unraveling the complex regulatory network involving TOP2A in GBM may offer new perspectives, and provide ideas for therapeutic strategies to enhance the prognosis for patients with GBM.
Breast cancer (BC)
BC remains the most common cancer among women globally, and is the primary cause of cancer-associated mortality in women under the age of 40 (40–43). Predictions for 2024 estimate that there will be over 310,000 new diagnoses of, and more than 40,000 deaths resulting from, this disease. Since the early 2000s, there has been a notable increase in BC cases, especially in the cases of localized, early-stage and hormone receptor-positive cancer (44). The immunohistochemical (IHC) analysis of Ki67 is widely recognized as a proliferation marker in BC, offering clinical validity for prognostic evaluations made in stages I and II of the disease (45). Classification of the BC subtype relies heavily on the expression levels of HER2, progesterone receptor (PR) and estrogen receptor (ER) (46). Depending on the subtype, treatment strategies may include chemotherapy, targeted anti-HER2 therapies or endocrine treatments (42). The identification of biomarkers, whether genes, proteins or other types of molecule, is essential: i) For the early detection of the disease; ii) for making an assessment of its severity; and iii) for evaluating the potential treatment responses (47). Consequently, there is an urgent need to discover novel biomarkers that can both improve prognostic accuracy and guide therapeutic decisions.
The amplification, or heightened expression, of the TOP2A gene has been demonstrated to be closely associated with increased tumor aggressiveness, prognostic outcomes and responses to chemotherapy in BC (23,25,28,48). A previous study demonstrated that the amplification of TOP2A is associated with increased tumor size, more advanced stages of cancer, and the presence of erb-b2 receptor tyrosine kinase 2 (ERBB2) positivity, although these traits did not consistently associate with the levels of TOP2A expression (48). Additionally, the expression of the TOP2A gene was found to be increased in cancer subtypes characterized by high proliferation rates, including basal-like, luminal B and HER2-enriched tumors (48). As such, TOP2A expression acts as a proliferative marker, signaling the rapid expansion of these tumor subtypes (48). A subsequent study revealed that elevated TOP2A levels associate with poorer prognoses in patients with luminal BC (28), and the suppression of TOP2A in luminal BC cells leads to reduced cell proliferation. Furthermore, a fluorokinase reporter gene assay has demonstrated that miR-139 targets the 3′-untranslated region (3′-UTR) of TOP2A mRNA, and its overexpression led to a significant reduction in cell proliferation. On the other hand, increasing TOP2A expression was also shown to counteract the effects of miR-139 (28). Triple-negative BC (TNBC) represents the most aggressive subtype of BC, characterized by poor prognosis and a lack of ER, PR and HER2 receptors (49). A previous study demonstrated that a peptide vaccine targeting TOP2A in TNBC is highly immunogenic, inducing a strong immune response, as evidenced by significant reductions in both tumor incidence and the mean tumor volume in mouse models. This vaccine was shown to stimulate tumor-infiltrating lymphocytes through a specific T-cell receptor sequence, offering a novel and effective approach to TNBC prevention and treatment (50).
Differential sensitivity to TOP2 inhibitors, such as anthracyclines, is evident in BC, depending on whether there is amplification or deletion of TOP2A. Amplification of TOP2A results in overexpression of the TOP2A protein, enhancing tumor sensitivity to anthracycline-based treatments, whereas its deletion diminishes TOP2A levels, fostering resistance to these drugs (25,51,52) (Fig. 4).
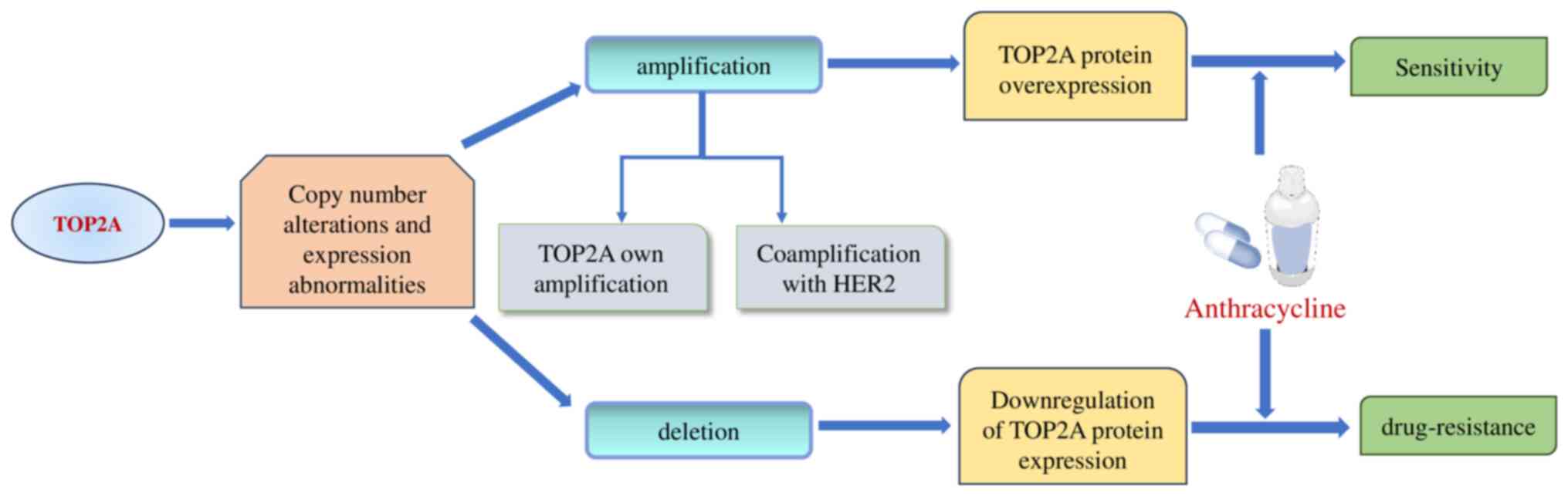 |
Figure 4.
Amplification and deletion of TOP2A in breast cancer. TOP2A amplification results in the overexpression of TOP2A protein, making cancer more sensitive to anthracycline-based chemotherapy. Conversely, TOP2A deletion leads to downregulated expression of TOP2A protein, resulting in primary chemoresistance to TOP2 inhibitors. TOP2A, topoisomerase IIα.
|
Additionally, in terms of BC care, co-amplification of the HER2 gene with TOP2A has been shown to have prognostic significance. Both the overall survival (OS) and the progression-free survival (PFS) rates were found to improve in a group of ~5,000 patients with co-amplification of HER2 and TOP2A when administered anthracyclines, either alone or in conjunction with trastuzumab (23). A larger validation study confirmed this association, further supporting the potential of TOP2A amplification as a predictive biomarker for anthracycline-based chemotherapy (23).
Lung cancer (LC)
LC, known to have the highest mortality rate globally, is typically identified in its advanced stages (53,54). Data from the American Cancer Society have estimated that, in 2024, there will probably be 234,580 new LC cases and 125,070 fatalities resulting from LC, accounting for ~20% of all cancer-associated deaths (44). Lung adenocarcinoma (LUAD), the most common histological subtype, is frequently associated with metastasis and recurrence, thereby contributing to high mortality rates. The failure of therapy in regard to LC is often due to factors such as immune system evasion, resistance to chemotherapy and radiation, and the complexities of tumor heterogeneity, recurrence and metastasis. These factors highlight the urgent need to delineate the pathogenesis of, and the molecular mechanisms underpinning, these tumors to develop innovate targeted therapeutic approaches (55,56).
The p53 protein is instrumental in reducing the rates of tumor cell proliferation and metastasis, and its malfunction is typically associated with LUAD development and progression (57). A previous study demonstrated that silencing TOP2A led to a decrease in the proliferative, migration and invasive capabilities of LUAD cells. On the other hand, TOP2A appears to promote LUAD cell proliferation and metastasis through affecting genes within the p53 pathway, notably cyclin B1 (CCNB1) and CCNB2 (31). The ERK/JNK/p-P38/CHOP signaling pathway, known to be activated under hypoxic conditions, facilitates both LUAD cell proliferation and migration, and the resistance of LUAD cells to apoptosis, as determined by measuring the phosphorylation levels of the various components of the pathway. Du et al (29) reported that reduced TOP2A expression decreases LUAD cell proliferation and increases the rate of apoptosis, with significant upregulation of the levels of phosphorylated (p)-ERK, p-JNK, p-P38 and CHOP proteins in siTOP2A cells. These findings suggested that low levels of TOP2A expression can drive LUAD progression through the ERK/JNK/p-P38/CHOP pathway. Moreover, tumor angiogenesis is critical for tumor growth and proliferation. In non-small cell lung cancer (NSCLC), TOP2A has been shown to encourage vascular mimicry through increasing the levels of Wnt3a, thereby improving tumor cell plasticity and motility. Moreover, TOP2A was shown to increase the expression of programmed death-ligand 1 (PD-L1), which facilitated tumor immune evasion (58). Taken together, these findings have underscored the potential of TOP2A as both a biomarker and a therapeutic target in NSCLC.
Liver cancer
Primary liver cancer (PLC) is categorized into three main histological subtypes: Hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma and combined hepatocellular-intrahepatic cholangiocarcinoma (59). HCC represents the most prevalent type, constituting 70–85% of all cases of PLC (60,61). The principal risk factor for developing HCC is hepatitis B virus infection (62). The current treatment modalities for HCC include hepatectomy, liver transplantation, ablation, transarterial embolization, radiotherapy and systemic pharmacotherapy (63). Despite improvements that have been made in screening and diagnostic techniques, both the global incidence and cancer-specific mortality of HCC continue to escalate, with patients being predominantly diagnosed at advanced disease stages (64).
TOP2A fulfills a vital role in the pathology of HCC, where its overexpression is associated with enhanced tumor proliferation, metastasis and resistance to chemotherapy (30,65–67). An IHC study of 40 clinically resected HCC samples revealed a marked increase in TOP2A expression in tumor tissues (66). Moreover, array-based transcriptional profiling pinpointed a significant upregulation of TOP2A on chromosome 17q21.2, which mirrored findings identified for other types of cancer, including GC, prostate cancer (PCa) and neuroblastoma, thereby connecting this chromosomal region to tumor progression. A further study linked an increase in TOP2A expression with an earlier onset of HCC, shorter patient survival rates and increased resistance to chemotherapy (68).
The epithelial-mesenchymal transition (EMT) is a crucial process where cells assume multiple somatic cell states, facilitating tumor spread and metastasis. TOP2A is known to increase the expression level of the transcription factor Snail through the phosphorylation of ERK1/2 and SMAD2 (S425/250/255), which subsequently suppresses the expression of E-cadherin and potentially fosters HCC metastasis via the p-ERK1/2/p-SMAD2/Snail pathways (65). Additionally, miR-144-3p, typically under-expressed in HCC, has been shown to inhibit tumor cell proliferation and invasion via interacting with various signaling pathways, including the p53 pathway. Elevated miR-144-3p levels are able to mitigate the adverse effects of anomalous TOP2A expression on HCC cell proliferation, migration and invasion, and also its effects on the EMT process, thereby potentially slowing HCC progression (30). Feng et al (69) demonstrated that TOP2A, through the Hippo-Yes-associated protein (YAP) signaling pathway, is capable of advancing the growth, metastasis and osteoclastogenesis in HCC cells, marking it as a critical gene associated with HCC bone metastasis.
Regorafenib, a multikinase inhibitor that acts on the RAS/RAF/MEK/ERK signaling pathway, is utilized as a second-line treatment for advanced or metastatic HCC. Nevertheless, the emergence of primary or acquired resistance significantly hampers its effectiveness (67,70). The study by Wang et al (67) demonstrated that the suppression of TOP2A is able to alleviate resistance to regorafenib in HCC models, thereby improving therapeutic outcomes, especially when combined with doxorubicin, a well-known TOP2A inhibitor. Considering the crucial function of TOP2A in HCC, focusing attention on this gene may both lead to the identification of novel therapeutic strategies and improve the efficacy of current treatments.
PC
Among the types of cancer-associated death, PC ranks 3rd, with a 5-year OS rate of 9%, declining to a critically low 3% for metastatic cases (71,72). Pancreatic ductal adenocarcinoma (PDAC) is the most common subtype of PC, and >90% of all cases are attributed to this subtype (71). Typically, patients exhibit few clinical signs until the cancer has advanced significantly, resulting in most patients being diagnosed at advanced or metastatic stages due to the inadequacy of early detection methods (73,74). At present, surgical resection is the sole curative therapy available for PC, although enhancements in adjuvant chemotherapy have improved long-term survival rates (75). Therefore, the discovery of novel biomarkers and therapeutic targets remains a critical priority.
The regulation of TOP2A expression in PC involves complex molecular mechanisms that profoundly influence the behavior of the cancer cells (76–78). As a co-activator of β-catenin, TOP2A activates the EMT, thereby facilitating the proliferation and migration of PC cells. miR-139 has been shown to serve as a tumor suppressor, directly targeting TOP2A, thereby decreasing its expression and slowing down the malignant progression of the tumor (76). This underscores the crucial role of the miR-139/TOP2A/β-catenin axis in the aggressive development of PC (76). Additionally, DiGeorge syndrome critical region gene 5 (DGCR5), a long non-coding RNA (lncRNA), has been strongly associated with oncogenesis (79). Liu et al (77) reported that DGCR5 functions as a competitive endogenous RNA, which is acted upon by the transcription factor paired box 5 (PAX5) to modulate TOP2A, thereby activating the Wnt/β-catenin pathway, which promotes the advancement of PC via capturing miR-3163. In addition, TOP2A and its transcriptional activators, specificity protein 1 (SP1) and high-mobility group protein B2 (HMGB2), have been found to be overexpressed in clinical samples of PDAC. In PDAC cells, a number of transcriptional regulators work together to promote TOP2A expression. Combination treatment studies have shown that TOP2A knockdown increases the susceptibility of PDAC cells to the chemotherapeutic medication cisplatin (78), and TOP2A knockdown in PDAC cells reduces cell proliferation, migration and invasion.
GC
As the 3rd leading cause of cancer-associated deaths worldwide, and the 5th most common malignant tumor overall, GC poses an enormous public health problem globally (80). Helicobacter pylori infection greatly increases the likelihood of developing GC (81). Although surgical resection typically provides a relatively favorable prognosis for those diagnosed at the early stages, the often-vague initial symptoms of the disease lead to numerous patients being diagnosed at later stages, thereby reducing the effectiveness of surgical interventions and yielding a median OS rate of only 12 months (82). Therefore, the discovery of early biomarkers is critical to enable the prompt diagnosis of GC, to customize treatment strategies, and to enhance outcomes for patients with GC.
Significant overexpression of TOP2A and E2F transcription factor 1 (E2F1) has been observed in GC tissues. Studies have revealed that E2F1 is able to bind to the promoter region of TOP2A in different types of GC. In a study by Chen et al (83), in vitro analyses confirmed that the activation of TOP2A by E2F1 led to increases in GC cell viability, migration and invasion, whereas apoptosis was inhibited. lncRNAs, defined as non-coding RNAs exceeding 200 nucleotides in length, are instrumental in cancer dynamics, as they operate through modifying gene expression profiles within the transcriptome (84). Cui et al (85) demonstrated that overexpression of the lncRNA FAM230B reduces the level of miR-27a-5p in GC cells, which consequently leads to a reduction in the rates of cell proliferation, migration and invasion, and an increase in the extent of apoptosis due to a lowering of TOP2A expression. These findings suggested that lncRNA FAM230B may promote the growth and spread of GC through capturing miR-27a-5p, thereby increasing TOP2A expression. Targeting FAM230B or modulating miR-27a-5p expression could therefore provide a strategy to inhibit the upregulation of TOP2A, thereby potentially impeding tumor growth and metastasis.
Colorectal cancer (CRC)
CRC is a leading gastrointestinal malignancy worldwide, currently ranked 3rd among all cancers, and 2nd in terms of cancer-associated mortality (61). Projections for 2024 estimate that 152,810 individuals will be diagnosed with CRC, resulting in 53,010 deaths. Notably, CRC is the principal cause of cancer-associated mortality in men under the age of 50 (44). Both colon cancer and rectal cancer (RC) are categorized as subtypes of CRC, and most of the research efforts up to this point have been concerned with treating these entities collectively (86). Detection of DNA methylation levels using methylation-specific PCR is an essential early screening method for CRC, as this targets highly specific genes, including Septin9, ALX homeobox 4 (AXL4) and syndecan-2 (SDC2). This approach allows for the enhanced sensitivity of the assay through integrating the methylation status of these genes, which reflect multiple molecular pathways of tumorigenesis (87). Surgical resection offers a potentially curative option, although immunotherapy, targeted therapy and radiotherapy are also beneficial in metastatic cases. High rates of incidence and chemoresistance are major factors contributing to recurrence and poor prognosis in CRC, especially in China (88). Targeted pharmacological interventions aimed at specific signaling pathways are pivotal for enhancing treatment efficacy and reducing resistance.
In colon cancer cells, the knockdown of TOP2A has been shown to decrease cell proliferation and invasion, whereas apoptosis was promoted. Western blot assay experiments have revealed that knocking down TOP2A affects the levels of apoptosis-associated proteins (Bcl-2 and Bax) and invasion-associated proteins [matrix metalloproteinase (MMP)-2 and MMP-9], as well as diminishing the phosphorylation levels of ERK and AKT. Therefore, overexpressing TOP2A serves as a key upstream regulator that anomalously activates proliferative signaling in colon cancer cells (89).
A study by Carvalho et al (90) demonstrated that inhibitors of TOP2A, such as doxorubicin and mitoxantrone (MTX), effectively alter gene expression profiles in RC, thereby inhibiting cell proliferation. Furthermore, their therapeutic efficacy was shown to be associated with the integration of gene expression signatures from patients with RC with those induced by these drugs. The study also noted a significant association between the gene dosage or levels of TOP2A and the sensitivity towards these inhibitors. In addition, CRISPR-Cas9 and shRNA loss-of-function analyses were performed, which confirmed that a reduction in TOP2A expression leads to a significant reduction in cell proliferation, with increased TOP2A expression commonly observed in RC samples, underscoring its therapeutic potential and supporting personalized treatment strategies based on TOP2 inhibitors.
Significant overexpression of TOP2A in parental CRC cell lines has shown that TOP2A overexpression confers greater resistance to chemotherapy agents such as irinotecan (targeting TOP1) and etoposide (targeting TOP2), probably as a result of the inhibition of apoptosis (91). Additionally, combination chemotherapy involving oxaliplatin, which is commonly to treat both advanced and metastatic CRC, has encountered resistance issues. Up-frameshift protein 1 (UPF1), an mRNA surveillance factor, has been identified as a promoter of oxaliplatin resistance (88). Intriguingly, in a study by Zhu et al (88), silencing TOP2A in these experiments negated UPF1-mediated oxaliplatin resistance, suggesting that TOP2A may contribute to UPF1-induced resistance mechanisms. Furthermore, resistance to treatment in CRC is shaped by cancer stem cell (CSC)-like stemness characteristics, which are enhanced following the upregulation of UPF1. The increase in UPF1-induced stemness characteristics could be partially reduced through silencing the expression of TOP2A, suggesting that UPF1 may sustain CSC-like stemness through a TOP2A-dependent pathway. Consequently, both current research and clinical trials that are in progress have underscored the significant potential and clinical importance of TOP2A in the diagnosis and treatment of CRC (88). Therefore, targeting TOP2A expression may offer a novel therapeutic strategy for patients with oxaliplatin-resistant CRC.
Cervical cancer (CC)
Among all cancers, CC ranks 4th in prevalence worldwide. With a death rate of 3.42 per 100,000 women, it affects 11.35 per 100,000 women in China (92). A major factor in the progression of this disease is the ongoing production of the viral oncogenes Early Protein 6 (E6) and Early Protein 7 (E7), which originate from high-risk strains of human papillomavirus (HPV) (93). DNA methylation testing is an important aspect of CC screening, especially in the case of women who have tested positive for HPV. For example, the WID-qCIN test is able to detect cervical precancerous lesions by assessing the DNA methylation status of the DPP6, RALYL and GSX1 genes. This technique, combined with HPV16/18 genotyping, significantly improves the ability to predict precancerous conditions (94). However, in spite of effective screening and vaccination programs, the mortality rate for advanced CC remains high, underscoring the limitations of current treatment strategies for the cancer in its advanced stages (95).
Numerous studies have reported on the overexpression of TOP2A and its associated oncogenic signaling in CC (96–98). An analysis of three raw microarray datasets from the Gene Expression Omnibus highlighted that TOP2A serves as a potential oncogene and prognostic marker in CC (96). In CC tissues, a significant upregulation of TOP2A was identified compared with the surrounding non-malignant tissues. Via stimulation of the PI3K/AKT pathway, this overexpression of TOP2A may facilitate the migration and invasion of CC cells, enabling them to undergo EMT (97). In addition, a dual-luciferase reporter gene test demonstrated that miR-320a targets the 3′-UTR of TOP2A mRNA, and it has been shown that HPV16 E6 causes a downregulation of miR-320a, which, in turn, increases the migration, invasion and proliferation rates of CC cells. The study by Zhang et al (98) has highlighted the importance of the HPV16 E6/miR-320a/TOP2A axis in the development of CC, suggesting that it may act as a novel therapeutic target through reducing HPV16 E6-induced cellular activity and increasing the extent of apoptosis. In addition, the association of Centromere Protein F, which has a vital role in chromosome segregation during cell division (99), with DNA TOP2A has been found to have synergistic effects in CC. Both proteins, significantly upregulated in CC tissues, cause the activation of genes that are associated with the cell cycle and DNA repair, linking their high expression to tumor metastasis and specific somatic mutations in genes such as TP53, MSH2 and RB1, thereby affirming their significance in cancer biology research (99).
Ovarian cancer (OC)
OC ranks as the most lethal of gynecological cancers due to its insidious development and the lack of early detection techniques, leading to late-stage diagnoses in the majority of cases. While initial chemotherapy results in remission for ~80% of affected individuals, the 5-year survival rates for patients with this cancer in its advanced stages are still discouragingly low, a consequence of significant tumor diversity and prevalent resistance to chemotherapy (100,101). Consequently, it is crucial to enhance our understanding of the pathophysiology of OC and to discover novel therapeutic targets to improve the clinical outcomes for these patients.
In OC, the intricate expression patterns of TOP2A, especially between initial and subsequent occurrences, have suggested a strong link exists between the efficacy of, and resistance to, chemotherapy (102,103). A previous study showed a heightened presence of TOP2A in tumor cells compared with adjacent stromal cells at both the protein and the mRNA level. A notable variation was observed in cases of recurrent OC where the patients were subjected to platinum-based therapies, and a reduction in TOP2A expression was noted in the epithelial cells of the tumors. By contrast, in recurrent tumors treated with carboplatin, an upsurge in TOP2A expression was identified within stromal cells adjacent to the tumors compared with primary cases (102). Similarly, IHC analysis across 50 cases of OC revealed that the levels of TOP2A and HER2 were increased in the epithelial cells of primary tumors, whereas these levels were decreased in recurrent forms of the disease, which conversely showed increased levels of TOP2A expression in the stromal cells post-platinum therapy. Hence, integrating TOP2A inhibitors with a platinum-based treatment protocol could potentially increase chemotherapy sensitivity, and reduce resistance in recurrent OC. Assessing TOP2A levels in both the tumor and the surrounding stromal cells is essential for predicting chemotherapy outcomes (103).
TOP2A has been identified as a pivotal factor in the proliferation of OC cells, where reducing its expression has been shown to inhibit cell proliferation, triggering G1-phase arrest, and thereby promoting cell death. In-depth in vitro studies have revealed that TOP2A subsequently influences the activity of transcription factors, such as c-Myc and the cyclin D1/cyclin-dependent kinase 4 (CDK4) complex, through the AKT/mTOR pathway, ultimately stimulating the proliferation of OC cells (104). Therefore, TOP2A has a crucial role in regulating the AKT/mTOR pathway, and inhibiting its expression could significantly reduce the pathway's function, thereby restraining the progression of ovarian tumors (104).
Renal cell carcinoma (RCC)
RCC originates from the renal cortex, and is identified as the 9th most prevalent cancer among men, and the 14th among women (105,106). The most common subtype, clear cell RCC (ccRCC), constitutes over 75% of all RCC cases, and is distinguished by its pronounced aggressiveness and poor prognosis (107,108). In total, ~40% of individuals with ccRCC eventually develop metastases, resulting in a dismal 5-year survival rate of only 10% (109,110). Therefore, in order to enhance the prognosis and quality of life for patients with RCC, the identification of new treatment targets and diagnostic indicators is urgently required.
The expression of TOP2A is markedly greater in RCC tissues and cell lines compared with normal cell lines and the neighboring non-cancerous tissues. Furthermore, TOP2A knockdown has been shown to lead to a significant decrease in RCC cell proliferation, with a concomitant increase in apoptosis (111). Similarly to TOP2A, miR-30c-2-3p has been shown to regulate RCC cell proliferation, thereby promoting apoptosis through activating the Fas/FasL/caspase-8/caspase-3 pathway (111). Furthermore, ccRCC tissues were found to have elevated levels of short nucleolar RNA host gene 3 (SNHG3), which performs a role in the progression of the cancer through interacting with miR-139-5p to increase TOP2A expression. Taken together, these findings have highlighted the importance of the SNHG3/miR-139-5p/TOP2A axis in the development of ccRCC (112).
Parker et al (113) investigated TOP2A expression in ccRCC, and sought to determine how it correlates with the likelihood of death from cancer. In individuals with low-risk diseases, where the mortality risk is ~3-fold higher compared with those with lower TOP2A expression, the data revealed that high levels of TOP2A are closely associated with an increased risk of cancer-specific death. This correlation has been validated across two independent cohort studies, affirming the significant prognostic utility of TOP2A following multivariate adjustments, which has highlighted its importance as a biomarker for postoperative monitoring in patients with ccRCC. According to Wang et al (114), responses to immune checkpoint inhibitor therapy vary significantly among patients with kidney renal clear cell carcinoma (KIRC). Tumors categorized within the high programmed cell death (PCD) subtype exhibit an immunosuppressive phenotype with a notable influx of regulatory T cells and tumor-associated macrophages, whereas those in the low PCD subtype respond more favorably to anti-programmed death-1 (anti-PD-1) medications. A prognostic model employing 13 PCD genes pinpointed TOP2A as a pivotal gene within this framework; its inhibition was shown to significantly impede the growth and movement of KIRC cells, emphasizing its integral role in tumoral advancement (114). Elucidating the molecular mechanisms underlying TOP2A dysregulation, and the implications of its dysregulation in KIRC, are pivotal for the development of more effective therapies.
BLCA
BLCA originates from the mucosa of the bladder, and is one of the most common and most lethal malignancies within the genitourinary system. The lack of specific diagnostic and therapeutic measures often leads to diagnosis at advanced stages, which predisposes patients to metastasis and poor outcomes (115–118). Previous studies have highlighted the diverse functions of TOP2A in BLCA, pointing to its potential as a target for therapy (119–121).
Genetic modifications in TOP2A in BLCA typically manifest as gene amplification and protein overexpression, exacerbating the malignant traits of BLCA cells and increasing their responsiveness to specific chemotherapeutic drugs. Results from an examination of 2,317 bladder tumor samples using fluorescence in situ hybridization (FISH) and IHC analyses revealed that, in muscle-invasive variants of the disease, the amplification and overexpression of TOP2A was associated with reduced survival rates. Additionally, HER2 amplification, occurring alongside TOP2A genomic changes in ~50% of these cases, suggested a common association between HER2 and TOP2A genomic alterations in BLCA (119). The gene amplification status within the 17q12-q21 chromosomal region may have clinical implications for predicting the efficacy of targeted therapies against HER2 or TOP2A (119). An increased expression of TOP2A was found to significantly enhance BLCA cell viability, migration and invasiveness. miR-599, which is markedly suppressed in BLCA, acts as an oncogenic regulator by targeting TOP2A directly (120). Similarly, Zeng et al (121) observed that the downregulation of TOP2A substantially reduced the migration and invasion of BLCA cells, promoted apoptosis, and contributed to adriamycin resistance. Given the complexity of factors that influence the responsiveness of BLCA cells to adriamycin, assessing the sensitivity to this type of chemotherapy requires a multifaceted approach that includes multiple biomarkers, not merely TOP2A expression levels alone.
PCa
PCa is a neoplastic growth that arises within the epithelial tissue of the prostate gland. It stands as the 2nd most common cancer affecting males globally, and has been identified as the 6th leading cause of mortality among men (122,123). The incidence of PCa has been increasing by ~3% annually since 2014, with diagnoses of regional and distant metastases rising at a rate of ~4.5% per year (44). The Gleason score, a critical grading system derived from the histological evaluation of prostate tissue, measures the degree of aggressiveness of PCa. Higher Gleason scores are indicative of severe cellular abnormalities and dysfunctions that drive the progression of the disease (124). The levels of prostate-specific antigen (PSA) are tightly associated with the progression of PCa, establishing PSA as an essential biomarker for early detection (125). Although a range of treatments for PCa are available, traditional approaches often lead to severe side effects that may contribute to resistance to therapy as the disease progresses (126).
In their study using tissue microarray constructs, Murphy et al (127) noted minimal levels of TOP2A gene amplification in PCa. Their multivariate analysis demonstrated that an increased protein level of TOP2A is associated with unfavorable clinical outcomes, such as advanced disease stages, high Gleason scores, HER2 amplification, androgen resistance and lower survival rates. Furthermore, increased expression levels of the TOP2A protein have been associated with higher Gleason scores and elevated preoperative PSA levels. A comprehensive study utilizing biochemical and pathological data from 193 patients with PCa, examined through IHC and FISH analyses, demonstrated that an increased expression of TOP2A was correlated with reduced biochemical recurrence-free survival, highlighting the importance of TOP2A protein assessment in prognostic evaluations (128). Furthermore, a study by Huang et al (129) demonstrated that the tumor suppressor miRNA-145-5p is downregulated in PCa tissues, whereas the mRNA and protein levels of TOP2A are markedly higher in these tissues compared with non-cancerous ones. Protein-protein interaction analysis revealed TOP2A to be a potential target of miRNA-145-5p, highlighting a significant inverse association between their expression levels in both localized and metastatic PCa settings. These findings, however, still require further experimental validation.
Advances in TOP2A and targeted drug research
TOP2 serves as a crucial target for several prominent anticancer drugs, including adriamycin, etoposide and MTX (32,130). Adriamycin, a member of the anthracycline class of chemotherapeutic agents, is extensively used to treat a variety of cancer types, including those of soft tissue. It has been further applied in the treatment of acute lymphoblastic leukemia and small-cell LC (130). Etoposide has shown efficacy against diverse malignancies (131), including LC, testicular cancer, non-Hodgkin's lymphoma, OC, leukemia and sarcoma. MTX, which has been identified as an inhibitor of TOP2A, effectively suppresses breast tumors (132).
Furthermore, agents such as adriamycin and etoposide are characteristic TOP2-directed drugs, extensively used in clinical practice as TOP2 ‘poisons’, such as, agents known for stabilizing covalent DNA TOP2 complexes, also referred to as TOP2 toxins. These poisons are commonly employed in oncology, often alongside other chemotherapeutic drugs, to address a diverse range of malignancies (130,133). The main action of TOP2 poisons involves the initiation of DNA cleavage facilitated by TOP2, resulting in both DNA SSBs and DSBs (121). Specifically, etoposide obstructs the re-ligation process of DSBs through trapping TOP2 at the 5′-terminus of the cleaved DNA, leading to the accumulation of TOP2cc and DNA DSBs (14,22,134–137). A recent study saw the introduction of a 4,5′-bithiazole derivative that curbs cancer cell proliferation through selectively inhibiting the ATPase activity of TOP2A, offering a mechanism distinct from that of traditional topoisomerase poisons, as it operates not via triggering DNA DSBs but through inhibiting cell cycle progression at the G1 phase (138)(Fig. 5). However, the clinical application of TOP2A inhibitors has encountered considerable obstacles, especially given the risk of serious adverse effects, including the onset of acute myeloid leukemia and drug-induced acute promyelocytic leukemia that is associated with compounds such as etoposide (139,140). In bone marrow cells, TOP2A inhibitors induce DNA DSBs, which subsequently hinder the proliferation and differentiation of normal hematopoietic cells, resulting in myelosuppression characterized by decreased levels of white blood cells, red blood cells and platelets. As a response to DNA damage resulting from TOP2A inhibitors, cells activate the p53-dependent apoptotic pathway and cell cycle checkpoints [such as checkpoint kinases 1 and 2 (Chk1/Chk2)]. The activation of these processes causes cell cycle arrest in the G2/M phase, ultimately leading to apoptosis (141). However, since the p53 pathway is frequently mutated in cancer cells, its capacity to induce apoptosis may be impaired, which renders normal cells more susceptible to these injuries (141). Additionally, anthracyclines, such as doxorubicin, primarily induce cardiotoxicity through iron-catalyzed generation of reactive oxygen species (ROS) and hydroxyl radicals. The resulting oxidative stress damages cardiac cellular structures, including lipids, proteins and mitochondrial DNA, leading to oxidative damage and mitochondrial dysfunction. This, along with ROS-induced lipid peroxidation, triggers cardiomyocyte apoptosis and drives the progression of cardiomyopathy and heart failure in patients (142). Therefore, it is essential to tailor drug dosages on the basis of specific disease markers to minimize the risks associated with treatment. Moreover, alterations such as mutations or fusions in the TOP2A gene may promote resistance to chemotherapy (139,140). For example, in cases of uroepithelial carcinoma of the bladder, a reduction in TOP2A expression has been linked to adriamycin resistance (121).
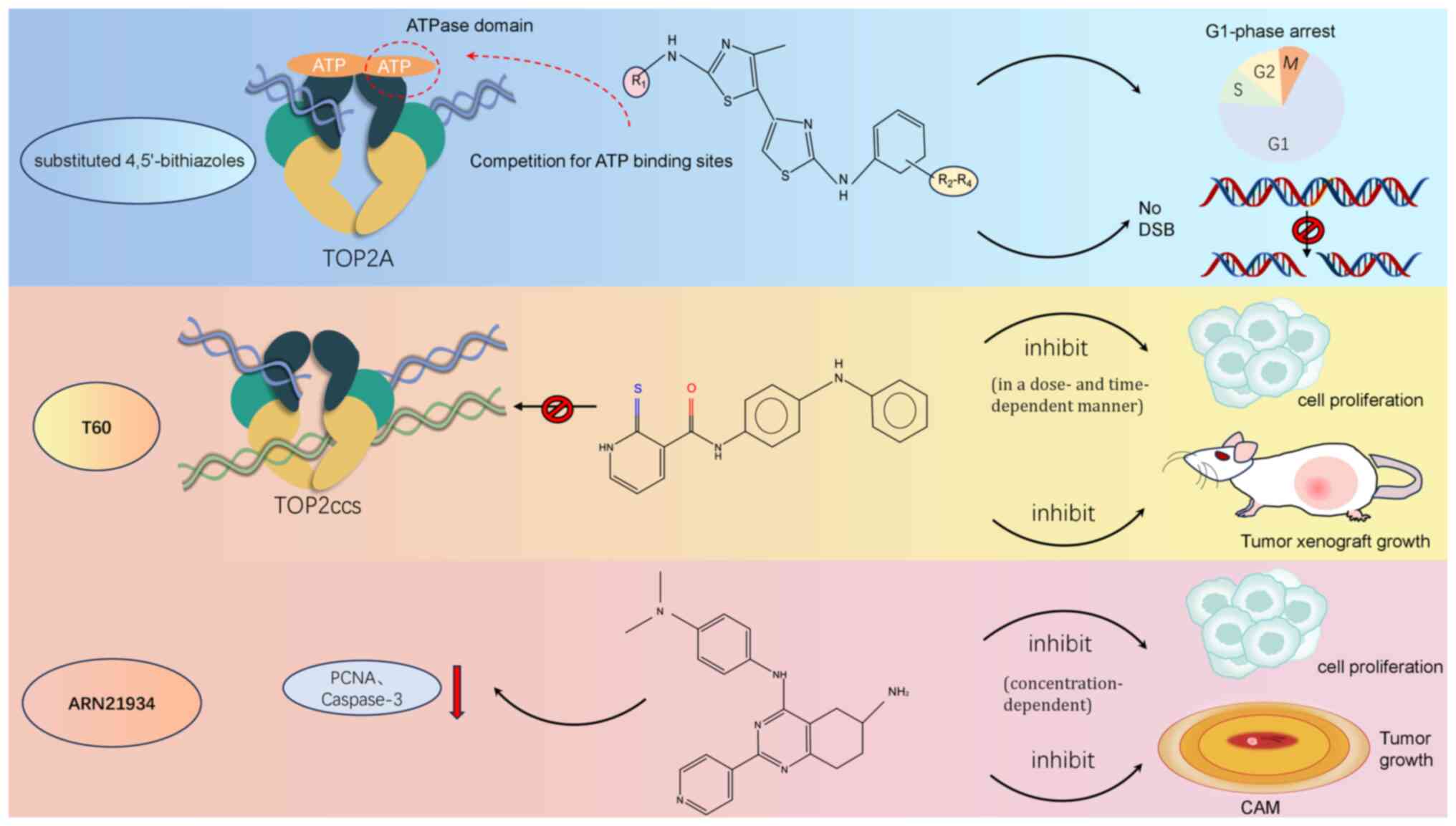 |
Figure 5.
Mechanism of action of novel TOP2A inhibitors. 4,5′-Dithiazole analogs effectively inhibit the ATP hydrolyzing activity of TOP2A by competing with ATP for binding sites, thereby blocking the proliferation process of cancer cells. In addition, the compounds do not cause DNA DSBs, but reduce cell proliferation mainly by inhibiting the G1 phase of the cell cycle. T60 acts as a catalytic inhibitor and avoids the formation of covalent TOP2Accs. T60 inhibits cell proliferation in a dose- and time-dependent manner and suppresses tumor xenograft growth; ARN21934 exerted tumor suppressive effects by decreasing the expression of PCNA mRNA and the active form of capase-3 proteins, and its antitumor activity was concentration-dependent, significantly inhibiting the proliferation of cancer cells in vitro, and a significant reduction in tumor volume was observed in the in vivo chick CAM model. TOP2A, topoisomerase IIα; DSB, double strand break; TOP2Accs; TOP2-DNA cleavage complexes; PCNA, proliferating cell nuclear antigen; CAM, chorioallantoic membrane.
|
Originally characterized as an RNA polymerase I inhibitor, CX5461 is a small molecule with a G4 ligand structure that is utilized as a chemo-genetic tool to probe the function of the RNA polymerase I complex. Subsequent studies have identified CX5461 as an effective TOP2 poison (143). Similarly, the G4 ligand pyridostatin (PDS) has been identified as a potent TOP2 poison (144). Both CX5461 and PDS are able to swiftly induce DNA DSBs and cytotoxicity via stabilizing four-stranded G-quadruplex and R-loop DNA structures, binding to TOP2, and targeting actively transcribed G-rich genomic regions. A comprehensive genetic analysis has identified TOP2A proteins as the principal effectors of cytotoxicity for these two G4 ligands, although unlike the typical TOP2 poisons that indiscriminately target both TOP2A and TOP2B, CX5461 preferentially affects TOP2B, whereas the cytotoxic and DNA-damaging actions of PDS are specifically mediated through TOP2A (143–145).
A distinct class of compounds, namely catalytic inhibitors, target various phases of the TOP2-catalyzed cycle, specifically when TOP2 binds to DNA or ATP (146). These inhibitors, especially those affecting DNA TOP2A, halt cell cycle progression at the G2/M checkpoint (147). Distinct from TOP2 poisons, inhibitors such as merbarone function by catalytically inhibiting TOP2, thereby forming stable non-covalent TOP2-DNA complexes and circumventing the generation of enzyme-mediated DSBs (12,121). TOP2 inhibitors constitute a heterogeneous group of agents that may disrupt the interaction between DNA and TOP2, stabilize non-covalent DNA-TOP2 complexes, or block ATP binding. However, their clinical application as antineoplastic agents is predominantly confined to hematological malignancies, with limited efficacy in solid tumors, as is exemplified by drugs such as azoxabicin and MST-16 (12). The tetra-hydro-quinazoline derivative ARN21934 selectively targets the α-isoform of human TOP2, and its substantial cytotoxic and growth-inhibitory effects have been identified in both in vitro and in vivo models of HPV-negative head and neck squamous cell carcinomas (148). Tumors treated with ARN21934 exhibit markedly reduced levels of proliferating cell nuclear antigen mRNA, suggesting that its mechanism of proliferation inhibition may involve the disruption of DNA synthesis (148)(Fig. 5). In contrast to typical catalytic inhibitors, the innovative TOP2 catalytic inhibitor T60 does not stabilize the TOP2A-DNA covalent complex; instead, the inhibition of TOP2A by T60 originates from its specific binding at the interface between TOP2A and DNA. T60 engages a newly discovered docking site, forming hydrogen bonds with several TOP2A amino acid residues, which results in a stable interaction that both blocks the engagement of TOP2A with DNA and suppresses its activity in a dose-dependent fashion (33)(Fig. 5).
Catalytic topoisomerase inhibitors frequently enhance the cytotoxic effects of various anticancer agents. This potentiation could occur through the inhibition of surface receptors (such as sulforaphane and neomycin), or by reducing nuclear topoisomerase activity, which, in turn, may increase the cytotoxic effects of alkylating agents such as cisplatin (133). Several clinical trials have investigated the synergistic effects of combining TOP2 inhibitors with other targeted therapies to augment their antitumor properties (Table I) (149–156). For example, in extensive-stage small-cell lung cancer, the integration of tislelizumab (an anti-PD-1 monoclonal antibody) with etoposide and platinum-based agents was found to significantly enhance both OS and PFS in a Phase III clinical trial (150). Furthermore, the strategic combination of TOP2A inhibitors with other targeted modalities has demonstrated efficacy in treating specific cancer subtypes and patient demographics (149,150,155). However, these studies also revealed variability with respect to the effectiveness of TOP2A inhibitors across different cancer types and stages, highlighting the necessity for additional clinical trials to refine and optimize therapeutic regimens. Resistance to these inhibitors may develop through changes in enzyme expression, mutations within the enzyme, or cellular adaptations, which has an impact on cytotoxic signaling and disrupts proteins that are associated with apoptosis and the cell cycle (157). A study by Liu et al (158) demonstrated that O-GlcNAc glycosylation augments the catalytic function of TOP2A, thereby enhancing its chromatin binding and catalytic capabilities, contributing to adriamycin resistance in BC cells. Specifically, glycosylation at the Ser-1469 site of TOP2A has been shown to boost its ability to unwind and cleave DNA, strengthening interactions with key cell cycle regulators such as CDK1 and UPF1, and modifying the expression of downstream cell cycle regulators, ultimately accelerating both proliferation and cell cycle progression in drug-resistant BC cells.
 |
Table I.
TOP2A inhibitors latest combination drug clinical trial information.
|
Table I.
TOP2A inhibitors latest combination drug clinical trial information.
| First author/s, year |
Identifier |
Drug name |
Indication (Phase) |
Study design |
Number of patients |
Endpoints/Main conclusions |
(Refs.) |
| Morizane et al, 2022 |
jRCTs031180005 |
Etoposide (TOP2A inhibitor) and Cisplatin (EP), Irinotecan and Cisplatin (IP) |
Advanced neuroendo-crine carcinoma of the digestive system (Phase 3) |
Multicenter, randomized, open-label, controlled trial |
170 |
Endpoints: 1. Primary endpoint: OS. 2. Secondary endpoints: Objective response rate, PFS, AEs, serious adverse events, and dose intensity of cisplatin. Main conclusions: 1. The median OS: 12.5 months (EP), 10.9 months (IP). 2. The median PFS: 5.6 months (EP), 5.1 months (IP). |
(149) |
| Cheng et al, 2024 |
NCT04005716 |
Tislelizumab Plus platinum and Etoposide (TOP2A inhibitor), |
Extensive-stage small cell lung cancer (Phase 3) |
Multicenter, double-blind, placebo-controlled, randomized. |
457 Tislelizumab (n=227) placebo n=230) |
Endpoints: 1. Primary endpoint: OS. 2. Secondary endpoints: PFS, safety, and tolerability. Main Conclusions: 1. OS benefit: The addition of tislelizumab to chemotherapy [stratified hazard ratio of 0.75 (95% CI: 0.61–0.93); one-sided P=0.0040]. 2. The median OS (tislelizumab arm): 15.5 months. The median OS (placebo arm): 13.5 months. |
(150) |
| Pollack et al, 2020 |
NCT02888665 |
Doxorubicin (TOP2A inhibitor), Pembrolizumab |
Advanced, anthracy-cline-naive sarcomas (Phase 1/2) |
Non-randomized clinical trial with a 2-stage Phase 2 design |
37 (22 men; 15 women) |
Endpoints: 1. Primary endpoint: ORR. 2. Secondary endpoints: OS, PFS and correlative studies (immunohistochemistry, gene expression and serum cytokines). Main conclusions: 1. ORR: Phase 2 patients: 13%, overall: 19%. 2. Median PFS: 8.1 months. 3. Median OS: 27.6 months. |
(151) |
| Tap et al, 2020 |
NCT02451943 |
Doxorubicin (TOP2A inhibitor), Olaratumab |
Advanced/metastatic STS (Phase 3) |
Double-blind, randomized, placebo-controlled trial |
509 (258 to doxorubicin plus olara-tumab and 251 to doxorubicin plus placebo) |
Endpoints: Dual Primary endpoint: OS (the total STS population and the leiomyosarcoma subpopulation). Main conclusions: No statistically significant difference in OS. STS (20.4 months vs. 19.7 months). LMS (21.6 months vs. 21.9 months) |
(152) |
| Abou-Alfa et al, 2019 |
NCT01015833 |
Sorafenib, Doxorubicin (TOP2A inhibitor) |
Advanced HCC (Phase 3) |
Unblinded randomized clinical trial |
356 |
Endpoints: 1. Primary endpoint: OS. 2. Secondary endpoints: PFS. Main conclusions: No statistically significant difference in median OS (9.3 months for doxorubicin plus sorafenib vs. 9.4 months for sorafenib alone). |
(153) |
| Yuan et al, 2023 |
NCT01134523 |
Epirubicin (TOP2A inhibitor), Paclitaxel Cyclophosphamide |
Operable ERBB2-Negative and Lymph Node-Positive Breast Cancer (Phase 3) |
Prospective, open-label, noninferiority randomized clinical trial |
813 |
Endpoints: 1. Primary endpoint: DFS. 2. Secondary endpoints: OS, DDFS and safety. Main Cancer (Phase 3) conclusions: The 5-year DFS for the EP group was 86.0 compared to 80.6% for the EC-P group (HR, 0.82; noninferior P=0.001). The 5-year OS for the EP and EC-P groups was 94.7% and 95.0%, respectively (HR, 0.95). |
(154) |
| Egelston et al, 2023 |
NCT02648477 |
Pembrolizumab, Doxorubicin (TOP2A inhibitor) |
mTNBC, (Phase 1) |
Open-label, single-arm, single-institution Phase I trial |
10 |
Endpoints: 1. Primary endpoint: Safety and objective response rate per RECIST 1.1. 2. Secondary endpoints: CBR, PFS, OS and safety/tolerability. Main Conclusions: Tislelizumab + chemotherapy [stratified HR of 0.75 (95% CI: 0.61–0.93); one-sided P-value of 0.0040]. Median OS: 15.5 months (tislelizumab arms). Median PFS: 0.64 (95% CI: 0.520.78); P<0.0001. (tislelizumab + chemotherapy) |
(155) |
| Livingston et al, 2021 |
NCT03056001 |
Pembrolizumab, Doxorubicin (TOP2A inhibitor) |
Metastatic and unresectable soft-tissue sarcoma (Phase 2) |
Single-center, single-arm |
30 |
Endpoints: 1. Primary endpoint: Safety. 2. Secondary endpoints: ORR, PFS, OS. Main conclusions: i) ORR: 36.7%. ii) Median PFS: 5.7 months. iii) Median OS: 17 months. |
(156) |
In cancer therapy, the levels of TOP2A expression critically influence cellular responsiveness to TOP2A-targeted interventions (22). Genetic and molecular alterations that serve to increase TOP2A expression may suggest either increased susceptibility or a more aggressive response to TOP2A-targeted inhibitors or cytotoxic agents. For example, deletions or mutations in TP53, a common genetic aberration in NSCLC, can lead to a marked increase in TOP2A levels in cancer cells by undermining its inhibitory control over TOP2A expression, thereby heightening sensitivity to TOP2A inhibitors. Additionally, shifts in the intracellular ratio of Sp1 to Sp3, as well as fluctuations in the activity of nuclear transcription factor Y (NF-Y), can also influence TOP2A expression, further modulating the sensitivity of cancer cells to TOP2A-targeted therapies (22).
To manage the toxic side effects and drug resistance associated with TOP2A toxicants/inhibitors, it is crucial to investigate their interactions with other DNA repair pathways and cell cycle regulatory networks. Additionally, a detailed analysis of the mutation and expression regulation mechanisms of TOP2A is necessary to develop more effective personalized cancer treatment strategies.
Conclusion
The progression of cancer is a multifaceted phenomenon shaped by a variety of interconnected elements, including genetic susceptibility, environmental factors and individual lifestyle decisions (159). There is considerable evidence to suggest that TOP2A is crucial for the advancement of various types of cancer. Recent studies have elucidated multiple cancer pathways that are influenced by TOP2A, primarily focusing on cell proliferation, invasion, metastasis and EMT (Table II). Despite the significant role of TOP2A in cancer, numerous aspects of the specific mechanisms associated with various cancer types and the individual variations remain largely unexplored. Developing therapeutic strategies that precisely target TOP2A is also a critical direction of ongoing research.
 |
Table II.
Expression and regulatory mechanism of TOP2A in different cancers.
|
Table II.
Expression and regulatory mechanism of TOP2A in different cancers.
| First author/s, year |
Cancer type |
Expression |
Gene overexpression/Knockdown |
Effect in vitro/ Cell behaviors |
Effect in vivo |
Regulatory Mechanisms |
Role |
(Refs.) |
| Liu et al, 2022 |
GBM |
Upregulation |
Knockdown |
Proliferation↓, migration↓, invasion↓ |
Tumor growth↓ |
TOP2A/β-catenin |
Oncogene |
(38) |
| Hua et al, 2015 |
BRCA |
Upregulation |
Knockdown |
Proliferation↓ |
- |
TOP2A/miR-139 |
Oncogene |
(28) |
| Kou et al, 2020 |
LUAD |
Upregulation |
Knockdown |
Proliferation↓, migration↓, invasion↓ |
- |
TOP2A/CCNB1, CCNB2 |
Oncogene |
(31) |
| Du et al, 2020 |
|
Upregulation |
Knockdown |
Proliferation↓, apoptosis↑ |
- |
TOP2A/ERK/JNK/p-P38/CHOP |
Oncogene |
(29) |
| Dong et al, 2021 |
HCC |
Upregulation |
Overexpression |
Proliferation↑, migration↑, invasion↑ and EMT↑ |
Tumor growth↑ lung metastasis↑ |
TOP2A/p-ERK1/2/p-SMAD2 (S425/250/255)/Snail |
Oncogene |
(65) |
| Wang et al, 2022 |
|
Upregulation |
Overexpression |
Proliferation↑, migration↑, invasion↑ and EMT ↑ |
Tumor growth↑ |
TOP2A/miR-144-3p |
Oncogene |
(30) |
| Feng et al, 2023 |
|
Upregulation |
Knockdown |
Proliferation↓, migration↓, invasion↓ |
- |
TOP2A/Hippo-YAP signaling pathway |
Oncogene |
(69) |
| Pei et al, 2018 |
PC |
Upregulation |
Overexpression |
Proliferation↑, migration↑, EMT↑ |
Tumor growth↑ |
miR-139/TOP2A/β-catenin axis |
Oncogene |
(76) |
| Chen et al, 2022 |
GC |
Upregulation |
Overexpression |
Viability↑, migration↑, invasion↑ and apoptosis↓ |
- |
E2F1/TOP2A |
Oncogene |
(83) |
| Zhang et al, 2018 |
CRC |
Upregulation |
Knockdown |
Proliferation↓, invasion↓ and apoptosis↑ |
- |
TOP2A/Akt and ERK signaling pathways |
Oncogene |
(89) |
| Wang et al, 2020 |
CC |
Upregulation |
Overexpression |
Migration↑, invasion↑ and EMT ↑ |
- |
PI3K/AKT signaling pathway |
Oncogene |
(97) |
| Zhang et al, 2024 |
OC |
Upregulation |
Knockdown |
Proliferation↓, aptosis↑ |
- |
AKT/mTOR pathway |
Oncogene |
(104) |
| Huang et al, 2023 |
RCC |
Upregulation |
Overexpression |
Proliferation↑, apoptosis↓ |
- |
TOP2A/miR-30c-2-3p |
Oncogene |
(111) |
| Zhang et al, 2021 |
BC |
Upregulation |
Overexpression |
Viability↑, migration↑, invasion↑ |
- |
TOP2A/miR-599 |
Oncogene |
(120) |
The mechanism of TOP2A in cancer potentially involves multiple signaling pathways and regulatory networks. TOP2A has the potential to enhance the swift proliferation of cancer cells through its influence on cell cycle proteins and regulatory mechanisms. Furthermore, it has the potential to promote the invasion and metastasis of cancer cells via modulating the expression of cell adhesion molecules and matrix MMPs. During EMT, TOP2A overexpression may result in a loss of polarity and intercellular junctions in epithelial cells, which thereby acquire mesenchymal properties that enhance their migratory and invasive capabilities.
Both the expression level and genetic status of TOP2A serve as biomarkers for monitoring cancer progression and predicting treatment efficacy. Single-cell sequencing technology, a valuable tool for studying cells aberrantly expressing TOP2A, has revealed further biomarkers in highly expressing cells, and the technology is able to characterize the interaction partners of TOP2A and its upstream regulators or downstream effectors (160). The use of multiple markers may improve predictive accuracy regarding tumor progression and the therapeutic response.
Inhibitors targeting TOP2A have demonstrated promising clinical applications, facilitated by ongoing efforts to overcome severe toxic side effects and resistance to conventional inhibitors. In addition, clinical trials combining TOP2A inhibitors with other therapeutic agents, including targeted therapies and immunotherapies, have shown enhanced efficacy. The development of new-generation TOP2A inhibitors that selectively target cancer cells while minimizing adverse effects has also represented a promising research avenue. Moreover, investigating TOP2A-associated gene mutations and epigenetic modifications, such as the O-GlcNAc glycosylation, may provide novel insights into overcoming drug resistance. Existing studies have shown that mutations in TOP2A significantly influence both its function and drug responsiveness. Future research should focus on a more systematic analysis of the TOP2A mutation spectrum in order to elucidate variations across different cancer types and to investigate potential links between these mutations and specific cancer subtypes. Additionally, a thorough examination of how specific mutations impact the enzymatic activity of TOP2A and its drug sensitivity is essential for predicting therapeutic responses and identifying novel therapeutic targets.
While existing studies have provided a preliminary understanding of the role of TOP2A in cancer progression, the specific underlying molecular mechanisms and regulatory networks require further investigation. Given the variability of TOP2A's role across different cancer types, multilevel and multidimensional studies are required to elucidate these differences. Additionally, individual differences act as crucial factors that affect the role of TOP2A, and future research efforts should integrate genomic, transcriptomic and proteomic data to clarify the differential roles of TOP2A in various individuals.
In summary, as a significant molecular target, TOP2A potentially holds considerable value in cancer research and therapy. Future studies on TOP2A are expected to yield further evidence of its regulatory roles in carcinogenesis and drug resistance in vivo, thereby advancing precision medicine and enhancing treatment options for patients with cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Shanxi Applied Fundamental Research Program (grant no. 201901D111408).
Availability of data and materials
Not applicable.
Authors' contributions
TZ and YN wrote and edited the article. TZ drew pictures and tables. YL reviewed and supervised the writing of the manuscript. All authors read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Samadder P, Aithal R, Belan O and Krejci L: Cancer TARGETases: DSB repair as a pharmacological target. Pharmacol Ther. 161:111–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Crosby D, Bhatia S, Brindle KM, Coussens LM, Dive C, Emberton M, Esener S, Fitzgerald RC, Gambhir SS, Kuhn P, et al: Early detection of cancer. Science. 375:eaay90402022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ and Watanabe T: Colorectal cancer. Nat Rev Dis Primers. 1:150652015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wahab MRA, Palaniyandi T, Ravi M, Viswanathan S, Baskar G, Surendran H, Gangadharan SGD and Rajendran BK: Biomarkers and biosensors for early cancer diagnosis, monitoring and prognosis. Pathol Res Pract. 250:1548122023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bizard AH and Hickson ID: The many lives of type IA topoisomerases. J Biol Chem. 295:7138–7153. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang JC: Cellular roles of DNA topoisomerases: A molecular perspective. Nat Rev Mol Cell Biol. 3:430–440. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schoeffler AJ and Berger JM: DNA topoisomerases: Harnessing and constraining energy to govern chromosome topology. Q Rev Biophys. 41:41–101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Forterre P and Gadelle D: Phylogenomics of DNA topoisomerases: Their origin and putative roles in the emergence of modern organisms. Nucleic Acids Res. 37:679–692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spakman D, Bakx JAM, Biebricher AS, Peterman EJG, Wuite GJL and King GA: Unravelling the mechanisms of Type 1A topoisomerases using single-molecule approaches. Nucleic Acids Res. 49:5470–5492. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Uusküla-Reimand L and Wilson MD: Untangling the roles of TOP2A and TOP2B in transcription and cancer. Sci Adv. 8:eadd49202022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vos SM, Tretter EM, Schmidt BH and Berger JM: All tangled up: How cells direct, manage and exploit topoisomerase function. Nat Rev Mol Cell Biol. 12:827–841. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang JC, Caron PR and Kim RA: The role of DNA topoisomerases in recombination and genome stability: A double-edged sword? Cell. 62:403–406. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Champoux JJ: DNA topoisomerases: Structure, function, and mechanism. Annu Rev Biochem. 70:369–413. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen SH, Chan NL and Hsieh TS: New mechanistic and functional insights into DNA topoisomerases. Annu Rev Biochem. 82:139–170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Laponogov I, Veselkov DA, Crevel IMT, Pan XS, Fisher LM and Sanderson MR: Structure of an ‘open’ clamp type II topoisomerase-DNA complex provides a mechanism for DNA capture and transport. Nucleic Acids Res. 41:9911–9923. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roca J and Wang JC: The capture of a DNA double helix by an ATP-dependent protein clamp: A key step in DNA transport by type II DNA topoisomerases. Cell. 71:833–840. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Massé E and Drolet M: Escherichia coli DNA topoisomerase I inhibits R-loop formation by relaxing transcription-induced negative supercoiling. J Biol Chem. 274:16659–16664. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nitiss JL: DNA topoisomerase II and its growing repertoire of biological functions. Nat Rev Cancer. 9:327–337. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Linka RM, Porter ACG, Volkov A, Mielke C, Boege F and Christensen MO: C-terminal regions of topoisomerase IIalpha and IIbeta determine isoform-specific functioning of the enzymes in vivo. Nucleic Acids Res. 35:3810–3822. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jenkins JR, Ayton P, Jones T, Davies SL, Simmons DL, Harris AL, Sheer D and Hickson ID: Isolation of cDNA clones encoding the beta isozyme of human DNA topoisomerase II and localisation of the gene to chromosome 3p24. Nucleic Acids Res. 20:5587–5592. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang M, Liang C, Chen Q, Yan H, Xu J, Zhao H, Yuan X, Liu J, Lin S, Lu W and Wang F: Histone H2A phosphorylation recruits topoisomerase IIα to centromeres to safeguard genomic stability. EMBO J. 39:e1018632020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen T, Sun Y, Ji P, Kopetz S and Zhang W: Topoisomerase IIα in chromosome instability and personalized cancer therapy. Oncogene. 34:4019–4031. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Press MF, Sauter G, Buyse M, Bernstein L, Guzman R, Santiago A, Villalobos IE, Eiermann W, Pienkowski T, Martin M, et al: Alteration of topoisomerase II-alpha gene in human breast cancer: Association with responsiveness to anthracycline-based chemotherapy. J Clin Oncol. 29:859–867. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heestand GM, Schwaederle M, Gatalica Z, Arguello D and Kurzrock R: Topoisomerase expression and amplification in solid tumours: Analysis of 24,262 patients. Eur J Cancer. 83:80–87. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ren L, Liu J, Gou K and Xing C: Copy number variation and high expression of DNA topoisomerase II alpha predict worse prognosis of cancer: A meta-analysis. J Cancer. 9:2082–2092. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boot A, Liu M, Stantial N, Shah V, Yu W, Nitiss KC, Nitiss JL, Jinks-Robertson S and Rozen SG: Recurrent mutations in topoisomerase IIα cause a previously undescribed mutator phenotype in human cancers. Proc Natl Acad Sci USA. 119:e21140241192022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gielniewski B, Poleszak K, Roura AJ, Szadkowska P, Jacek K, Krol SK, Guzik R, Wiechecka P, Maleszewska M, Kaza B, et al: Targeted sequencing of cancer-related genes reveals a recurrent TOP2A variant which affects DNA binding and coincides with global transcriptional changes in glioblastoma. Int J Cancer. 153:1003–1015. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hua W, Sa KD, Zhang X, Jia LT, Zhao J, Yang AG, Zhang R, Fan J and Bian K: MicroRNA-139 suppresses proliferation in luminal type breast cancer cells by targeting topoisomerase II alpha. Biochem Biophys Res Commun. 463:1077–1083. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Du X, Xue Z, Lv J and Wang H: Expression of the topoisomerase II alpha (TOP2A) gene in lung adenocarcinoma cells and the association with patient outcomes. Med Sci Monit. 26:e9291202020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang T, Lu J, Wang R, Cao W and Xu J: TOP2A promotes proliferation and metastasis of hepatocellular carcinoma regulated by miR-144-3p. J Cancer. 13:589–601. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kou F, Sun H, Wu L, Li B, Zhang B, Wang X and Yang L: TOP2A promotes lung adenocarcinoma cells' malignant progression and predicts poor prognosis in lung adenocarcinoma. J Cancer. 11:2496–2508. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bau JT and Kurz EU: Sodium salicylate is a novel catalytic inhibitor of human DNA topoisomerase II alpha. Biochem Pharmacol. 81:345–354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matias-Barrios VM, Radaeva M, Song Y, Alperstein Z, Lee AR, Schmitt V, Lee J, Ban F, Xie N, Qi J, et al: Discovery of new catalytic topoisomerase II inhibitors for anticancer therapeutics. Front Oncol. 10:6331422021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, et al: The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 23:1231–1251. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song J, Ma Q, Hu M, Qian D, Wang B and He N: The inhibition of miR-144-3p on cell proliferation and metastasis by targeting TOP2A in HCMV-positive glioblastoma cells. Molecules. 23:32592018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang W, Blank A, Kremenetskaia I, Nitzsche A, Acker G, Vajkoczy P and Brandenburg S: CD13 expression affects glioma patient survival and influences key functions of human glioblastoma cell lines in vitro. BMC Cancer. 24:3692024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang H, Liu X, Zhu X, Zhang M, Wang Y, Ma M and Lv K: GINS1 promotes the proliferation and migration of glioma cells through USP15-mediated deubiquitination of TOP2A. iScience. 25:1049522022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Y, Ma J, Song JS, Zhou HY, Li JH, Luo C, Geng X and Zhao HX: DNA topoisomerase II alpha promotes the metastatic characteristics of glioma cells by transcriptionally activating β-catenin. Bioengineered. 13:2207–2216. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang B, Pan X, Cobb GP and Anderson TA: microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fazeli S, Sakala M, Rakow-Penner R and Ojeda-Fournier H: Cancer in pregnancy: Breast cancer. Abdom Radiol (NY). 48:1645–1662. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A and Bray F: Cancer statistics for the year 2020: An overview. Int J Cancer. Apr 5–2021.(Epub ahead of print). View Article : Google Scholar
|
|
42
|
Harbeck N and Gnant M: Breast cancer. Lancet. 389:1134–1150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Winters S, Martin C, Murphy D and Shokar NK: Breast cancer epidemiology, prevention, and screening. Prog Mol Biol Transl Sci. 151:1–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Siegel RL, Giaquinto AN and Jemal A: Cancer statistics, 2024. CA Cancer J Clin. 74:12–49. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nielsen TO, Leung SCY, Rimm DL, Dodson A, Acs B, Badve S, Denkert C, Ellis MJ, Fineberg S, Flowers M, et al: Assessment of Ki67 in breast cancer: Updated recommendations from the international Ki67 in breast cancer working group. J Natl Cancer Inst. 113:808–819. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee Y, Ni J, Beretov J, Wasinger VC, Graham P and Li Y: Recent advances of small extracellular vesicle biomarkers in breast cancer diagnosis and prognosis. Mol Cancer. 22:332023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Anderson DC and Kodukula K: Biomarkers in pharmacology and drug discovery. Biochem Pharmacol. 87:172–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Romero A, Martín M, Cheang MC, López García-Asenjo JA, Oliva B, He X, de la Hoya M, García Sáenz JÁ, Arroyo Fernández M, Díaz Rubio E, et al: Assessment of topoisomerase II α status in breast cancer by quantitative PCR, gene expression microarrays, immunohistochemistry, and fluorescence in situ hybridization. Am J Pathol. 178:1453–1460. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hu H, Tong K, Tsang JY, Ko CW, Tam F, Loong TC and Tse GM: Subtyping of triple-negative breast cancers: Its prognostication and implications in diagnosis of breast origin. ESMO Open. 9:1029932024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lee SB, Pan J, Xiong D, Palen K, Johnson B, Lubet RA, Shoemaker RH, Green JE, Fernando RI, Sei S, et al: Striking efficacy of a vaccine targeting TOP2A for triple-negative breast cancer immunoprevention. NPJ Precis Oncol. 7:1082023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Järvinen TAH and Liu ET: Topoisomerase IIalpha gene (TOP2A) amplification and deletion in cancer-more common than anticipated. Cytopathology. 14:309–313. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang J, Xu B, Yuan P, Zhang P, Li Q, Ma F and Fan Y: TOP2A amplification in breast cancer is a predictive marker of anthracycline-based neoadjuvant chemotherapy efficacy. Breast Cancer Res Treat. 135:531–537. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chaudhary P, Janmeda P and Pareek A, Chuturgoon AA, Sharma R and Pareek A: Etiology of lung carcinoma and treatment through medicinal plants, marine plants and green synthesized nanoparticles: A comprehensive review. Biomed Pharmacother. 173:1162942024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Monteiro Lde S, Bastos KX, Barbosa-Filho JM, de Athayde-Filho PF, Diniz Mde F and Sobral MV: Medicinal plants and other living organisms with antitumor potential against lung cancer. Evid Based Complement Alternat Med. 2014:6041522014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Reya T, Morrison SJ, Clarke MF and Weissman IL: Stem cells, cancer, and cancer stem cells. Nature. 414:105–111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, et al: International association for the study of lung cancer/american thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kaiser AM, Gatto A, Hanson KJ, Zhao RL, Raj N, Ozawa MG, Seoane JA, Bieging-Rolett KT, Wang M, Li I, et al: p53 governs an AT1 differentiation programme in lung cancer suppression. Nature. 619:851–859. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wu J, Zhang L, Li W, Wang L, Jia Q, Shi F, Li K, Liao L, Shi Y and Wu S: The role of TOP2A in immunotherapy and vasculogenic mimicry in non-small cell lung cancer and its potential mechanism. Sci Rep. 13:109062023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xue R, Zhang Q, Cao Q, Kong R, Xiang X, Liu H, Feng M, Wang F, Cheng J, Li Z, et al: Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nature. 612:141–147. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen Y, Tang L, Huang W, Abisola FH, Zhang Y, Zhang G and Yao L: Identification of a prognostic cuproptosis-related signature in hepatocellular carcinoma. Biol Direct. 18:42023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang J, Chenivesse X, Henglein B and Bréchot C: Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature. 343:555–557. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A and Roberts LR: A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 16:589–604. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Global Burden of Disease Liver Cancer Collaboration, . Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, et al: The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: Results from the global burden of disease study 2015. JAMA Oncol. 3:1683–1691. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Dong Y, Sun X, Zhang K, He X, Zhang Q, Song H, Xu M, Lu H and Ren R: Type IIA topoisomerase (TOP2A) triggers epithelial-mesenchymal transition and facilitates HCC progression by regulating Snail expression. Bioengineered. 12:12967–12979. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Panvichian R, Tantiwetrueangdet A, Angkathunyakul N and Leelaudomlipi S: TOP2A amplification and overexpression in hepatocellular carcinoma tissues. Biomed Res Int. 2015:3816022015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang Z, Zhu Q, Li X, Ren X, Li J, Zhang Y, Zeng S, Xu L, Dong X and Zhai B: TOP2A inhibition reverses drug resistance of hepatocellular carcinoma to regorafenib. Am J Cancer Res. 12:4343–4360. 2022.PubMed/NCBI
|
|
68
|
Wong N, Yeo W, Wong WL, Wong NL, Chan KY, Mo FK, Koh J, Chan SL, Chan AT, Lai PB, et al: TOP2A overexpression in hepatocellular carcinoma correlates with early age onset, shorter patients survival and chemoresistance. Int J Cancer. 124:644–652. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Feng J, Wei X, Liu Y, Zhang Y, Li G, Xu Y, Zhou P, Zhang J, Han X, Zhang C, et al: Identification of topoisomerase 2A as a novel bone metastasis-related gene in liver hepatocellular carcinoma. Aging (Albany NY). 15:13010–13040. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shi W, Zhang S, Ma D, Yan D, Zhang G, Cao Y, Wang Z, Wu J and Jiang C: Targeting SphK2 reverses Acquired resistance of regorafenib in hepatocellular carcinoma. Front Oncol. 10:6942020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Krebs N, Klein L, Wegwitz F, Espinet E, Maurer HC, Tu M, Penz F, Küffer S, Xu X, Bohnenberger H, et al: Axon guidance receptor ROBO3 modulates subtype identity and prognosis via AXL-associated inflammatory network in pancreatic cancer. JCI Insight. 7:e1544752022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang CY, Liu S and Yang M: Clinical diagnosis and management of pancreatic cancer: Markers, molecular mechanisms, and treatment options. World J Gastroenterol. 28:6827–6845. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kawai M, Fukuda A, Otomo R, Obata S, Minaga K, Asada M, Umemura A, Uenoyama Y, Hieda N, Morita T, et al: Early detection of pancreatic cancer by comprehensive serum miRNA sequencing with automated machine learning. Br J Cancer. 131:1158–1168. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Waleleng BJ, Adiwinata R, Wenas NT, Haroen H, Rotty L, Gosal F, Rotty L, Winarta J, Waleleng A and Simadibrata M: Screening of pancreatic cancer: Target population, optimal timing and how? Ann Med Surg (Lond). 84:1048142022.PubMed/NCBI
|
|
75
|
Mizrahi JD, Surana R, Valle JW and Shroff RT: Pancreatic cancer. Lancet. 395:2008–2020. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Pei YF, Yin XM and Liu XQ: TOP2A induces malignant character of pancreatic cancer through activating β-catenin signaling pathway. Biochim Biophys Acta Mol Basis Dis. 1864:197–207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Liu SL, Cai C, Yang ZY, Wu ZY, Wu XS, Wang XF, Dong P and Gong W: DGCR5 is activated by PAX5 and promotes pancreatic cancer via targeting miR-3163/TOP2A and activating Wnt/β-catenin pathway. Int J Biol Sci. 17:498–513. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tanaka T, Okada R, Hozaka Y, Wada M, Moriya S, Satake S, Idichi T, Kurahara H, Ohtsuka T and Seki N: Molecular pathogenesis of pancreatic ductal adenocarcinoma: Impact of miR-30c-5p and miR-30c-2-3p regulation on oncogenic genes. Cancers (Basel). 12:27312020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Fang C, He W, Xu T, Dai J, Xu L and Sun F: Upregulation of lncRNA DGCR5 correlates with better prognosis and inhibits bladder cancer progression via transcriptionally facilitating P21 expression. J Cell Physiol. 234:6254–6262. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Siegel RL, Miller KD, Wagle NS and Jemal A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhou C, Bisseling TM, van der Post RS and Boleij A: The influence of Helicobacter pylori, proton pump inhibitor, and obesity on the gastric microbiome in relation to gastric cancer development. Comput Struct Biotechnol J. 23:186–198. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhang XY and Zhang PY: Gastric cancer: Somatic genetics as a guide to therapy. J Med Genet. 54:305–312. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chen YU, Yu Y, Lv M, Shi Q and Li X: E2F1-mediated up-regulation of TOP2A promotes viability, migration, and invasion, and inhibits apoptosis of gastric cancer cells. J Biosci. 47:842022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Liu SJ, Dang HX, Lim DA, Feng FY and Maher CA: Long noncoding RNAs in cancer metastasis. Nat Rev Cancer. 21:446–460. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cui Y, Pu R, Ye J, Huang H, Liao D, Yang Y, Chen W, Yao Y and He Y: LncRNA FAM230B promotes gastric cancer growth and metastasis by regulating the miR-27a-5p/TOP2A axis. Dig Dis Sci. 66:2637–2650. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kneis B, Wirtz S, Weber K, Denz A, Gittler M, Geppert C, Brunner M, Krautz C, Siebenhüner AR, Schierwagen R, et al: Colon cancer microbiome landscaping: Differences in right- and left-sided colon cancer and a tumor microbiome-Ileal microbiome association. Int J Mol Sci. 24:32652023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Li Y, Li B, Jiang R, Liao L, Zheng C, Yuan J, Zeng L, Hu K, Zhang Y, Mei W, et al: A novel screening method of DNA methylation biomarkers helps to improve the detection of colorectal cancer and precancerous lesions. Cancer Med. 12:20626–20638. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhu C, Zhang L, Zhao S, Dai W and Xu Y, Zhang Y, Zheng H, Sheng W and Xu Y: UPF1 promotes chemoresistance to oxaliplatin through regulation of TOP2A activity and maintenance of stemness in colorectal cancer. Cell Death Dis. 12:5192021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhang R, Xu J, Zhao J and Bai JH: Proliferation and invasion of colon cancer cells are suppressed by knockdown of TOP2A. J Cell Biochem. 119:7256–7263. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Carvalho RF, do Canto LM, Cury SS, Frøstrup Hansen T, Jensen LH and Rogatto SR: Drug repositioning based on the reversal of gene expression signatures identifies TOP2A as a therapeutic target for rectal cancer. Cancers (Basel). 13:54922021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Coss A, Tosetto M, Fox EJ, Sapetto-Rebow B, Gorman S, Kennedy BN, Lloyd AT, Hyland JM, O'Donoghue DP, Sheahan K, et al: Increased topoisomerase IIalpha expression in colorectal cancer is associated with advanced disease and chemotherapeutic resistance via inhibition of apoptosis. Cancer Lett. 276:228–238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zou Q, Wu Y, Zhang S, Li S, Li S, Su Y, Zhang L, Li Q, Zou H, Zhang X, et al: Escherichia coli and HPV16 coinfection may contribute to the development of cervical cancer. Virulence. 15:23199622024. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Meng Q, Zhang Y, Sun H, Yang X, Hao S, Liu B, Zhou H, Wang Y and Xu ZX: Human papillomavirus-16 E6 activates the pentose phosphate pathway to promote cervical cancer cell proliferation by inhibiting G6PD lactylation. Redox Biol. 71:1031082024. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Schreiberhuber L, Barrett JE, Wang J, Redl E, Herzog C, Vavourakis CD, Sundström K, Dillner J and Widschwendter M: Cervical cancer screening using DNA methylation triage in a real-world population. Nat Med. 30:2251–2257. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lin Z, Li X, Shi H, Cao R, Zhu L, Dang C, Sheng Y, Fan W, Yang Z and Wu S: Decoding the tumor microenvironment and molecular mechanism: Unraveling cervical cancer subpopulations and prognostic signatures through scRNA-Seq and bulk RNA-seq analyses. Front Immunol. 15:13512872024. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhao Q, Li H, Zhu L, Hu S, Xi X, Liu Y, Liu J and Zhong T: Bioinformatics analysis shows that TOP2A functions as a key candidate gene in the progression of cervical cancer. Biomed Rep. 13:212020.PubMed/NCBI
|
|
97
|
Wang B, Shen Y, Zou Y, Qi Z, Huang G, Xia S, Gao R, Li F and Huang Z: TOP2A promotes cell migration, invasion and epithelial-mesenchymal transition in cervical cancer via activating the PI3K/AKT signaling. Cancer Manag Res. 12:3807–3814. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhang J, Yu X, Guo Y and Wang D: HPV16 E6 promoting cervical cancer progression through down-regulation of miR-320a to increase TOP2A expression. Cancer Med. 13:e68752024. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Yu B, Chen L, Zhang W, Li Y, Zhang Y, Gao Y, Teng X, Zou L, Wang Q, Jia H, et al: TOP2A and CENPF are synergistic master regulators activated in cervical cancer. BMC Med Genomics. 13:1452020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Katsumata N, Yasuda M, Isonishi S, Takahashi F, Michimae H, Kimura E, Aoki D, Jobo T, Kodama S, Terauchi F, et al: Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): A randomised, controlled, open-label trial. Lancet Oncol. 14:1020–1026. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Geng D, Zhou Y and Wang M: Advances in the role of GPX3 in ovarian cancer (Review). Int J Oncol. 64:312024. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Chekerov R, Klaman I, Zafrakas M, Könsgen D, Mustea A, Petschke B, Lichtenegger W, Sehouli J and Dahl E: Altered expression pattern of topoisomerase IIalpha in ovarian tumor epithelial and stromal cells after platinum-based chemotherapy. Neoplasia. 8:38–45. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Chekerov R, Koensgen D, Klaman I, Rosenthal A, Oskay-Oezcelik G, Mustea A, Lightenegger W, Dahl E and Sehouli J: Tumor- and stromal cell-specific expression of topoisomerase IIα and HER-2/neu in primary and recurrent ovarian cancer: Results of a prospective study. Mol Med Rep. 2:1011–1016. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhang K, Zheng X, Sun Y, Feng X, Wu X, Liu W, Gao C, Yan Y, Tian W and Wang Y: TOP2A modulates signaling via the AKT/mTOR pathway to promote ovarian cancer cell proliferation. Cancer Biol Ther. 25:23251262024. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Gudbrandsdottir G, Aarstad HH, Bostad L, Hjelle KM, Aarstad HJ, Bruserud Ø, Tvedt THA and Beisland C: Serum levels of the IL-6 family of cytokines predict prognosis in renal cell carcinoma (RCC). Cancer Immunol Immunother. 70:19–30. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Piao XM, Byun YJ, Zheng CM, Song SJ, Kang HW, Kim WT and Yun SJ: A new treatment landscape for RCC: Association of the human microbiome with improved outcomes in RCC. Cancers (Basel). 15:9352023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Huang B, Ren J, Ma Q, Yang F, Pan X, Zhang Y, Liu Y, Wang C, Zhang D, Wei L, et al: A novel peptide PDHK1-241aa encoded by circPDHK1 promotes ccRCC progression via interacting with PPP1CA to inhibit AKT dephosphorylation and activate the AKT-mTOR signaling pathway. Mol Cancer. 23:342024. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Ren J, Huang B, Li W, Wang Y, Pan X, Ma Q, Liu Y, Wang X, Liang C, Zhang Y, et al: RNA-binding protein IGF2BP2 suppresses metastasis of clear cell renal cell carcinoma by enhancing CKB mRNA stability and expression. Transl Oncol. 42:1019042024. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhang L, Jin GZ and Li D: Tat-hspb1 suppresses clear cell renal cell carcinoma (ccRCC) growth via lysosomal membrane permeabilization. Cancers (Basel). 14:57102022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhang X, Zhang G, Xu L, Bai X, Zhang J, Chen L, Lu X, Yu S, Jin Z and Sun H: Prediction of World Health Organization/international society of urological pathology (WHO/ISUP) pathological grading of clear cell renal cell carcinoma by dual-layer spectral CT. Acad Radiol. 30:2321–2328. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Huang X, Jia Y, Shi H, Fan H, Sun L, Zhang H, Wang Y, Chen J, Han J, Wang M, et al: miR-30c-2-3p suppresses the proliferation of human renal cell carcinoma cells by targeting TOP2A. Asian Biomed (Res Rev News). 17:124–135. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Zhang C, Qu Y, Xiao H, Xiao W and Liu J, Gao Y, Li M and Liu J: LncRNA SNHG3 promotes clear cell renal cell carcinoma proliferation and migration by upregulating TOP2A. Exp Cell Res. 384:1115952019. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Parker AS, Eckel-Passow JE, Serie D, Hilton T, Parasramka M, Joseph RW, Wu KJ, Cheville JC and Leibovich BC: Higher expression of topoisomerase II alpha is an independent marker of increased risk of cancer-specific death in patients with clear cell renal cell carcinoma. Eur Urol. 66:929–935. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Wang Q, Liu J, Li R, Wang S, Xu Y, Wang Y, Zhang H, Zhou Y, Zhang X, Chen X, et al: Assessing the role of programmed cell death signatures and related gene TOP2A in progression and prognostic prediction of clear cell renal cell carcinoma. Cancer Cell Int. 24:1642024. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Ashrafizadeh M, Hushmandi K, Hashemi M, Akbari ME, Kubatka P, Raei M, Koklesova L, Shahinozzaman M, Mohammadinejad R, Najafi M, et al: Role of microRNA/epithelial-to-mesenchymal transition axis in the metastasis of bladder cancer. Biomolecules. 10:11592020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A and Bray F: Bladder cancer incidence and mortality: A global overview and recent trends. Eur Urol. 71:96–108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wang X, Luo L, Xu J, Lu Q, Xia H, Huang Y, Zhang L, Xie L, Jiwa H, Liang S, et al: Echinatin inhibits tumor growth and synergizes with chemotherapeutic agents against human bladder cancer cells by activating p38 and suppressing Wnt/β-catenin pathways. Genes Dis. 11:1050–1065. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Hou J, Huang H, Xie J, Yu W, Hao H and Li H: KLHDC7B as a novel diagnostic biomarker in urine exosomal mRNA promotes bladder urothelial carcinoma cell proliferation and migration, inhibits apoptosis. Mol Carcinog. 63:286–300. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Simon R, Atefy R, Wagner U, Forster T, Fijan A, Bruderer J, Wilber K, Mihatsch MJ, Gasser T and Sauter G: HER-2 and TOP2A coamplification in urinary bladder cancer. Int J Cancer. 107:764–772. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zhang F and Wu H: MiR-599 targeting TOP2A inhibits the malignancy of bladder cancer cells. Biochem Biophys Res Commun. 570:154–161. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Zeng S, Liu A, Dai L, Yu X, Zhang Z, Xiong Q, Yang J, Liu F, Xu J, Xue Y, et al: Prognostic value of TOP2A in bladder urothelial carcinoma and potential molecular mechanisms. BMC Cancer. 19:6042019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Schatten H: Brief overview of prostate cancer statistics, grading, diagnosis and treatment strategies. Adv Exp Med Biol. 1095:1–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ilic D, Neuberger MM, Djulbegovic M and Dahm P: Screening for prostate cancer. Cochrane Database Syst Rev. 2013:Cd0047202013.PubMed/NCBI
|
|
124
|
Temilola DO, Wium M, Paccez J, Salukazana AS, Out HH, Carbone GM, Kaestner L, Cacciatore S and Zerbini LF: Potential of miRNAs in plasma extracellular vesicle for the stratification of prostate cancer in a South African population. Cancers (Basel). 15:39682023. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Moradi A, Srinivasan S, Clements J and Batra J: Beyond the biomarker role: Prostate-specific antigen (PSA) in the prostate cancer microenvironment. Cancer Metastasis Rev. 38:333–346. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Sekhoacha M, Riet K, Motloung P, Gumenku L, Adegoke A and Mashele S: Prostate cancer review: Genetics, diagnosis, treatment options, and alternative approaches. Molecules. 27:57302022. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Murphy AJ, Hughes CA, Barrett C, Magee H, Loftus B, O'Leary JJ and Sheils O: Low-level TOP2A amplification in prostate cancer is associated with HER2 duplication, androgen resistance, and decreased survival. Cancer Res. 67:2893–2898. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
de Resende MF, Vieira S, Chinen LTD, Chiappelli F, da Fonseca FP, Guimarães GC, Soares FA, Neves I, Pagotty S, Pellionisz PA, et al: Prognostication of prostate cancer based on TOP2A protein and gene assessment: TOP2A in prostate cancer. J Transl Med. 11:362013. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Huang ZG, Sun Y, Chen G, Dang YW, Lu HP, He J, Cheng JW, He ML and Li SH: MiRNA-145-5p expression and prospective molecular mechanisms in the metastasis of prostate cancer. IET Syst Biol. 15:1–13. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Sritharan S and Sivalingam N: A comprehensive review on time-tested anticancer drug doxorubicin. Life Sci. 278:1195272021. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Fathi-Karkan S, Arshad R, Rahdar A, Ramezani A, Behzadmehr R, Ghotekar S and Pandey S: Recent advancements in the targeted delivery of etoposide nanomedicine for cancer therapy: A comprehensive review. Eur J Med Chem. 259:1156762023. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Guan J, Tan X, Jiao J, Lai S, Zhang H, Kan Q, He Z, Sun M and Sun J: Iron ion-coordinated carrier-free supramolecular co-nanoassemblies of dual DNA topoisomerase-targeting inhibitors for tumor suppression. Acta Biomater. 144:121–131. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Larsen AK, Escargueil AE and Skladanowski A: Catalytic topoisomerase II inhibitors in cancer therapy. Pharmacol Ther. 99:167–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Ratain MJ, Kaminer LS, Bitran JD, Larson RA, Le Beau MM, Skosey C, Purl S, Hoffman PC, Wade J, Vardiman JW, et al: Acute nonlymphocytic leukemia following etoposide and cisplatin combination chemotherapy for advanced non-small-cell carcinoma of the lung. Blood. 70:1412–1417. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Montecucco A, Zanetta F and Biamonti G: Molecular mechanisms of etoposide. EXCLI J. 14:95–108. 2015.PubMed/NCBI
|
|
136
|
Choudhari AS, Mandave PC, Deshpande M, Ranjekar P and Prakash O: Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front Pharmacol. 10:16142019. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Pedersen-Bjergaard J, Philip P, Larsen SO, Andersson M, Daugaard G, Ersbøll J, Hansen SW, Hou-Jensen K, Nielsen D, Sigsgaard TC, et al: Therapy-related myelodysplasia and acute myeloid leukemia. Cytogenetic characteristics of 115 consecutive cases and risk in seven cohorts of patients treated intensively for malignant diseases in the Copenhagen series. Leukemia. 7:1975–1986. 1993.PubMed/NCBI
|
|
138
|
Bergant Loboda K, Janežič M, Štampar M, Žegura B, Filipič M and Perdih A: Substituted 4,5′-bithiazoles as catalytic inhibitors of human DNA topoisomerase IIα. J Chem Inf Model. 60:3662–3678. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Murphy MB, Kumar P, Bradley AM, Barton CE, Deweese JE and Mercer SL: Synthesis and evaluation of etoposide and podophyllotoxin analogs against topoisomerase IIα and HCT-116 cells. Bioorg Med Chem. 28:1157732020. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Li J, Sun P, Huang T, He S, Li L and Xue G: Individualized chemotherapy guided by the expression of ERCC1, RRM1, TUBB3, TYMS and TOP2A genes versus classic chemotherapy in the treatment of breast cancer: A comparative effectiveness study. Oncol Lett. 21:212021. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Hartmann JT and Lipp HP: Camptothecin and podophyllotoxin derivatives: Inhibitors of topoisomerase I and II-mechanisms of action, pharmacokinetics and toxicity profile. Drug Saf. 29:209–230. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Xu X, Persson HL and Richardson DR: Molecular pharmacology of the interaction of anthracyclines with iron. Mol Pharmacol. 68:261–271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Bruno PM, Lu M, Dennis KA, Inam H, Moore CJ, Sheehe J, Elledge SJ, Hemann MT and Pritchard JR: The primary mechanism of cytotoxicity of the chemotherapeutic agent CX-5461 is topoisomerase II poisoning. Proc Natl Acad Sci USA. 117:4053–4060. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Bossaert M, Pipier A, Riou JF, Noirot C, Nguyên LT, Serre RF, Bouchez O, Defrancq E, Calsou P, Britton S and Gomez D: Transcription-associated topoisomerase 2α (TOP2A) activity is a major effector of cytotoxicity induced by G-quadruplex ligands. eLife. 10:e651842021. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Olivieri M, Cho T, Álvarez-Quilón A, Li K, Schellenberg MJ, Zimmermann M, Hustedt N, Rossi SE, Adam S, Melo H, et al: A genetic map of the response to DNA damage in human cells. Cell. 182:481–496.e21. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Li TK and Liu LF: Tumor cell death induced by topoisomerase-targeting drugs. Annu Rev Pharmacol Toxicol. 41:53–77. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Luo K, Yuan J, Chen J and Lou Z: Topoisomerase IIalpha controls the decatenation checkpoint. Nat Cell Biol. 11:204–210. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Sarogni P, Brindani N, Zamborlin A, Gonnelli A, Menicagli M, Mapanao AK, Munafò F, De Vivo M and Voliani V: Tumor growth-arrest effect of tetrahydroquinazoline-derivative human topoisomerase II-alpha inhibitor in HPV-negative head and neck squamous cell carcinoma. Sci Rep. 14:91502024. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Morizane C, Machida N, Honma Y, Okusaka T, Boku N, Kato K, Nomura S, Hiraoka N, Sekine S, Taniguchi H, et al: Effectiveness of etoposide and cisplatin vs irinotecan and cisplatin therapy for patients with advanced neuroendocrine carcinoma of the digestive system: The TOPIC-NEC phase 3 randomized clinical trial. JAMA Oncol. 8:1447–1455. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Cheng Y, Fan Y, Zhao Y, Huang D, Li X, Zhang P, Kang M, Yang N, Zhong D, Wang Z, et al: Tislelizumab plus platinum and etoposide versus placebo plus platinum and etoposide as first-line treatment for extensive-stage SCLC (RATIONALE-312): A multicenter, double-blind, placebo-controlled, randomized, phase 3 clinical trial. J Thorac Oncol. 19:1073–1085. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Pollack SM, Redman MW, Baker KK, Wagner MJ, Schroeder BA, Loggers ET, Trieselmann K, Copeland VC, Zhang S, Black G, et al: Assessment of doxorubicin and pembrolizumab in patients with advanced anthracycline-naive sarcoma: A phase 1/2 nonrandomized clinical trial. JAMA Oncol. 6:1778–1782. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Tap WD, Wagner AJ, Schöffski P, Martin-Broto J, Krarup-Hansen A, Ganjoo KN, Yen CC, Abdul Razak AR, Spira A, Kawai A, et al: Effect of doxorubicin plus olaratumab vs doxorubicin plus placebo on survival in patients with advanced soft tissue sarcomas: The ANNOUNCE randomized clinical trial. JAMA. 323:1266–1276. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Abou-Alfa GK, Shi Q, Knox JJ, Kaubisch A, Niedzwiecki D, Posey J, Tan BR Jr, Kavan P, Goel R, Lammers PE, et al: Assessment of treatment with sorafenib plus doxorubicin vs sorafenib alone in patients with advanced hepatocellular carcinoma: Phase 3 CALGB 80802 randomized clinical trial. JAMA Oncol. 5:1582–1588. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Yuan P, Kang Y, Ma F, Fan Y, Wang J, Wang X, Yue J, Luo Y, Zhang P, Li Q and Xu B: Effect of epirubicin plus paclitaxel vs epirubicin and cyclophosphamide followed by paclitaxel on disease-free survival among patients with operable ERBB2-negative and lymph node-positive breast cancer: A randomized clinical trial. JAMA Netw Open. 6:e2301222023. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Egelston CA, Guo W, Yost SE, Ge X, Lee JS, Frankel PH, Cui Y, Ruel C, Schmolze D, Murga M, et al: Immunogenicity and efficacy of pembrolizumab and doxorubicin in a phase I trial for patients with metastatic triple-negative breast cancer. Cancer Immunol Immunother. 72:3013–3027. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Livingston MB, Jagosky MH, Robinson MM, Ahrens WA, Benbow JH, Farhangfar CJ, Foureau DM, Maxwell DM, Baldrige EA, Begic X, et al: Phase II study of pembrolizumab in combination with doxorubicin in metastatic and unresectable soft-tissue sarcoma. Clin Cancer Res. 27:6424–6431. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Singh V, Afshan T, Tyagi P, Varadwaj PK and Sahoo AK: Recent development of multi-targeted inhibitors of human topoisomerase II enzyme as potent cancer therapeutics. Int J Biol Macromol. 226:473–484. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Liu Y, Yu K, Zhang K, Niu M, Chen Q, Liu Y, Wang L, Zhang N, Li W, Zhong X, et al: O-GlcNAcylation promotes topoisomerase IIα catalytic activity in breast cancer chemoresistance. EMBO Rep. 24:e564582023. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Amicuzi U, Grillo M, Stizzo M, Olivetta M, Tammaro S, Napolitano L, Reccia P, De Luca L, Rubinacci A, Della Rosa G, et al: Exploring the multifactorial landscape of penile cancer: A comprehensive analysis of risk factors. Diagnostics (Basel). 14:17902024. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Heumos L, Schaar AC, Lance C, Litinetskaya A, Drost F, Zappia L, Lücken MD, Strobl DC, Henao J, Curion F, et al: Best practices for single-cell analysis across modalities. Nat Rev Genet. 24:550–572. 2023. View Article : Google Scholar : PubMed/NCBI
|



















