Introduction
Osteoarthritis (OA) is a common type of arthritis,
which is characterized by degenerative lesions of articular
cartilage or other joint tissues (1–3). The
incidence of OA is closely related to age (4). Prieto-Alhambra et al (4) reported that the incidence rate of
knee and hip OA continues to increase with age. Turkiewicz et
al (5) reported that the
proportion of patients with OA aged ≥45 years may increase to ~30%
by 2032. The increasing prevalence of OA will lead to a marked
social and economic burden. Clinically, drug therapy, such as
treatment with non-steroidal anti-inflammatory drugs and
glucocorticoids, surgery, such as total hip arthroplasty, and
physical therapy are commonly used methods for the treatment of OA
(6–9). However, evidence has indicated that
the long-term use of drugs used to treat OA is associated with a
number of negative side effects, including gastrointestinal
discomfort and liver function impairment (10,11).
Thus, the development of promising novel treatment methods for OA
is urgently required.
Traditional Chinese medicine (TCM) has been
practiced in China for >5,000 years (12). TCM has been shown to exert a
therapeutic effect on multiple diseases (13,14).
Quercetin (QCT) is a bioactive compound that can be isolated from
various TCM formulas, such as Panax notoginseng and
Ginkgo biloba (15,16). QCT exhibits various pharmacological
properties, including antioxidant, anti-inflammatory and
anti-bacterial activities (17,18).
Furthermore, QCT has been demonstrated to exert chondroprotective
effects in murine models of OA (19,20).
However, to the best of our knowledge, the mechanisms through which
QCT attenuates the symptoms of OA remain largely unclear.
Ferroptosis is a type of iron-dependent cell death
that is induced by iron accumulation and lipid peroxidation
(21,22). Ferroptosis serves a crucial role in
human diseases, including OA (23). Activation of ferroptosis is able to
elevate the MMP13 levels and reduce the type II collagen (collagen
II) levels in chondrocytes, suggesting that ferroptosis can
contribute to the progression of OA (23). However, whether QCT can attenuate
the development of OA by affecting ferroptosis remains largely
elusive.
In the present study, IL-1β-stimulated chondrocytes
and a mouse model of anterior cruciate ligament transection
(ACLT)-induced OA were established in order to explore the role of
QCT in the treatment of OA disease.
Materials and methods
Cell culture
The human chondrocyte cell line (CHON-001; American
Type Culture Collection) was cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) with 1% Penicillin-Streptomycin Solution
Hybri-Max™ (Sigma-Aldrich; Merck KGaA) at 37°C in an
incubator with 5% CO2. To mimic an in vitro model
of OA, CHON-001 cells were exposed to 10 ng/ml IL-1β (Novoprotein
Scientific, Inc.) for 24 h at 37°C (24). The 5′ AMP-activated protein kinase
(AMPK) inhibitor compound C, QCT and erastin were purchased from
MedChemExpress, and the cells were treated with QCT (100 µM), QCT
and erastin (5 µM) or QCT and compound C (5 µM) for 24 h at 37°C,
and then exposed to IL-1β for 24 h at 37°C. Control cells were
cultured in medium only.
Cell counting kit-8 (CCK-8) assay
CHON-001 cells were seeded (5×104
cells/well) in 96-well plates overnight. CHON-001 cells were
treated with QCT (0, 25, 50, 100 or 200 µM) for 24 h at 37°C.
Subsequently, 10 µl CCK-8 reagent (Beyotime Institute of
Biotechnology) was added to each well and the cells were incubated
for a further 2 h at 37°C. Subsequently, a microplate reader
(MULTISKAN MK3; Thermo Fisher Scientific, Inc.) was used to detect
the absorbance of each well at 450 nm. Similarly, CHON-001 cells
were treated with QCT (50 or 100 µM) for 24 h at 37°C, and then
exposed to 10 ng/ml IL-1β for 24 h at 37°C. The cell viability was
detected with the CCK-8 assay as well. In addition, CHON-001 cells
were treated with erastin (0, 1, 2, 5 or 10 µM) for 24 h at 37°C
and the cell viability was detected using a CCK-8 assay.
5-Ethynyl-2′-deoxyuridine (EdU)
staining assay
Cell proliferation was detected using an EdU
detection kit (Wuhan Servicebio Technology Co., Ltd.). The cells
were fixed in 4% paraformaldehyde (Wuhan Servicebio Technology Co.,
Ltd.) for 2 h at room temperature and then stained with EdU
Apollo567 solution for 1 h at 37°C in the dark, followed by
staining with 0.1 µg/ml DAPI (Wuhan Servicebio Technology Co.,
Ltd.) for 15 min at room temperature. Finally, the EdU-positive
cells were observed using a fluorescence microscope (Eclipse Ci-L;
Nikon Corporation). A total of three random fields were selected
and the EdU-positive cells were counted manually.
TUNEL assay
A TUNEL detection kit (G1504; Wuhan Servicebio
Technology Co., Ltd.) was applied to assess CHON-001 cell
apoptosis. The cells were fixed in 4% paraformaldehyde for 2 h at
room temperature and washed with PBS for 30 min. Subsequently, the
cells were treated with 0.2% Triton X-100 for 2 min at room
temperature. The cells were stained with the mixed solution
(recombinant TdT enzyme:CF488-dUTP labeling mix:equilibration
buffer, 1:5:50) for 1 h at 37°C. The nuclei were stained with 0.1
µg/ml DAPI for 30 min in the dark at room temperature. Polyvinyl
alcohol mounting medium with DABCO® (cat. no. 10981;
Sigma-Aldrich; Merck KGaA) was used as the mounting medium.
TUNEL-positive cells in three random fields were observed using a
fluorescence microscope (Eclipse Ci-L; Nikon Corporation).
ELISA
Human IL-6 [cat. no. ELK1156; Elk (Wuhan)
Biotechnology Co., Ltd.], TNF-α [cat. no. ELK1190; Elk (Wuhan)
Biotechnology Co., Ltd.], glutathione (GSH; cat. no. A061-1;
Nanjing Jiancheng Bioengineering Institute) and malondialdehyde
(MDA; cat. no. A003-1; Nanjing Jiancheng Bioengineering Institute)
detection kits were used to detect the IL-6, TNF-α, GSH and MDA
levels in the supernatant of CHON-001 cells according to the
manufacturers' instructions. Furthermore, the Fe2+ and
lipid reactive oxygen species (ROS) levels in CHON-001 cells were
detected using the Cell Ferrous Iron Colorimetric Assay kit (cat.
no. E-BC-K881-M; Wuhan Elabscience Biotechnology Co., Ltd.) and
BODIPY 581/591 C11 kit (cat. no. HY-D1301; MedChemExpress). All
kits were used according to the manufacturers' instructions. The
results were analyzed using a microplate reader (SMR16.1; Wuhan
USCN Business Co., Ltd.).
Western blot analysis
Total protein was extracted from CHON-001 cells
using RIPA buffer (Beyotime Institute of Biotechnology) and the
protein concentration was measured using the BCA detection assay
kit (ASPEN Biotechnology Co., Ltd.). Proteins (20 µg/lane) were
resolved using 8% SDS-PAGE and transferred to PVDF membranes. After
blocking with 5% non-fat milk for 1 h at room temperature, the
membranes were incubated with primary antibodies against
phosphorylated (p-)AMPK (cat. no. ab109402), AMPK (cat. no.
ab32047), nuclear factor erythroid 2-related factor 2 (Nrf2; cat.
no. ab31163), glutathione peroxidase 4 (Gpx4; cat. no. ab125066),
Bcl-2 (cat. no. ab182858), cleaved caspase 3 (cat. no. ab214430),
caspase 3 (cat. no. ab32351), aggrecan (cat. no. ab315486),
collagen II (cat. no. ab34712), MMP13 (cat. no. ab219620), ADAM
metallopeptidase with thrombospondin type 1 motif 5 (ADAMTS5; cat.
no. ab41037) and β-actin (cat. no. ab8227) overnight at 4°C. All
primary antibodies were purchased from Abcam and the dilution
factor was 1:1,000. The membranes were then probed with the
secondary antibody (dilution, 1:5,000; cat. no. AS1107) for 2 h at
room temperature. The secondary HRP-conjugated antibody was
purchased from ASPEN Biotechnology Co., Ltd. Finally, the bands
were visualized using ECL reagent (ASPEN Biotechnology Co., Ltd.).
Densitometry was performed using ImageJ software (version 1.8.0;
National Institutes of Health).
Animal experiments
C57BL/6 mice (8 weeks old; 18–22 g; female; n=24; 6
mice/group) were purchased from Beijing Vital River Laboratory
Animal Technology Co., Ltd. All mice were housed with a 12-h
light/dark cycle at 24°C with 60% humidity, with ad libitum
access to food and water. The experimental protocols were approved
by the Ethics Committee of Beijing University of Chinese Medicine
(approval no. BUCM-2023032701-2103; Beijing, China) and the animal
experiments were performed according to the institutional
guidelines. The animals were randomly divided into the following
four groups: Sham, OA, OA + QCT 20 mg/kg and OA + QCT 40 mg/kg. The
OA models were generated with ACLT surgery on the knee joints of
the mice as previously described (25). The animals were treated with QCT
(20 or 40 mg/kg) via gavage once per day for 4 weeks in the QCT
treatment group. Mouse body weight loss >20% was regarded as a
humane endpoint in the present study. The Osteoarthritis Research
Society International (OARSI) score was determined based on the
data of Safranin O/fast green staining to evaluate cartilage
degradation (26). Briefly, a 0–6
subjective scoring system (0, 0.5, 1, 2, 3, 4, 5 and 6) was applied
to all four quadrants of the joint: Medial femoral condyle, medial
tibial plateau, lateral femoral condyle and lateral tibial plateau
(Table I).
 | Table I.Semi-quantitative scoring system. |
Table I.
Semi-quantitative scoring system.
| Grade | Osteoarthritic
damage |
|---|
| 0 | Normal |
| 0.5 | Loss of Safranin-O
without structural changes |
| 1 | Small fibrillations
without loss of cartilage |
| 2 | Vertical clefts
down to the layer immediately below the superficial layer and some
loss of surface lamina |
| 3 | Vertical
clefts/erosion to the calcified cartilage extending to <25% of
the articular surface |
| 4 | Vertical
clefts/erosion to the calcified cartilage extending to 25–50% of
the articular surface |
| 5 | Vertical
clefts/erosion to the calcified cartilage extending to 51–75% of
the articular surface |
| 6 | Vertical
clefts/erosion to the calcified cartilage extending >75% of the
articular surface |
All animals were sacrificed using CO2 at
a displacement rate of 40% volume/min at 4 weeks following surgery,
and the knee joints of each mouse were collected. The toe reaction
and heartbeat of the mouse were checked to confirm animal death.
The pathological changes of articular tissues were observed using
H&E staining. Briefly, the samples were fixed in 4%
paraformaldehyde for 24 h at room temperature and then embedded in
paraffin. Subsequently, the specimens (4 µm thick) were placed in
distilled water and then stained with hematoxylin for 15 min at
room temperature. The sections were then rehydrated in alcohol at
concentrations of 90 and 70% for 15 min each. Next, the sections
were incubated with eosin staining solution for 10 min at room
temperature. Afterwards, the samples were dehydrated with 100%
alcohol and placed in in an incubator for drying. Images were
captured using a light microscope (CS31; Olympus Corporation).
Safranin O/fast green staining
assay
A modified Saffron-O and Fast Green Stain kit (cat.
no. G1371; Beijing Solarbio Science & Technology Co., Ltd.) was
used to evaluate the proteoglycan contents in the articular
tissues. The samples were fixed in 4% paraformaldehyde for 24 h at
room temperature and then embedded in paraffin. Paraffin sections
of tissue were obtained from the embedded samples. The 4-µm
paraffin sections were heated at 60°C for 1 h, dewaxed twice in
xylene solutions for 15 min each and rehydrated in a descending
alcohol series. Subsequently, the articular tissue sections were
stained with Weigert dye (Wuhan Servicebio Technology Co., Ltd.)
for 5 min at room temperature. The sections were first stained with
fast green solution for 5 min at room temperature and then stained
with safranin O solution for 5 min at room temperature. Images were
captured using a light microscope (CS31; Olympus Corporation).
Immunohistochemistry (IHC)
The samples were fixed in 4% paraformaldehyde for 24
h at room temperature and then embedded in paraffin. Paraffin
sections of tissue (4-µm) were obtained from the embedded samples
and heated in a 60°C oven. Subsequently, samples were
deparaffinized, rehydrated in a descending alcohol series and
boiled in 0.01 mol/l sodium citrate buffer (pH 6.0) in a microwave
oven for 10 min for antigen retrieval. Next, samples were blocked
with 0.3% hydrogen peroxide for 15 min at room temperature, and
washed with distilled water. The sections were blocked for 30 min
using 10% normal goat serum (Thermo Fisher Scientific, Inc.) at
room temperature, and probed with primary antibodies specific for
ADAMTS5 (1:100; cat. no. DF13268; Affinity Biosciences), MMP13
(1:300; cat. no. ab219620; Abcam), collagen II (1:200; cat. no.
ab34712; Abcam) and aggrecan (1:150; cat. no. DF7561; Affinity
Biosciences) overnight at 4°C, and then incubated with a
HRP-conjugated secondary antibody (1:200; cat. no. AS1107; ASPEN
Biotechnology Co., Ltd.) for 30 min at 37°C. The sections were
stained with 3,3′-diaminodbenzidine solution (Wuhan Servicebio
Technology Co., Ltd.). Subsequently, images were captured using a
light microscope (CS31; Olympus Corporation). ImageJ software
(version 1.8.0; National Institutes of Health) was used for
analysis.
Statistical analysis
Data are presented as the mean ± SD. Multiple
comparisons were conducted using one-way ANOVA with Tukey's post
hoc test using GraphPad Prism 7 (Dotmatics). All experiments were
repeated at least three times. P<0.05 was considered to indicate
a statistically significant difference.
Results
QCT attenuates the IL-1β-induced
apoptosis of chondrocytes
In order to determine the cytotoxic effects of QCT
on chondrocytes, a CCK-8 assay was conducted. As shown in Fig. 1A, 200 µM QCT significantly
suppressed the viability of CHON-001 cells, while 50 or 100 µM QCT
had a limited effect on CHON-001 cell viability. In addition, IL-1β
markedly reduced the viability and proliferation, and triggered the
apoptosis of CHON-001 cells; however, these changes were reversed
by 100 µM QCT (Fig. 1B-D).
Overall, QCT attenuated the IL-1β-induced injury of
chondrocytes.
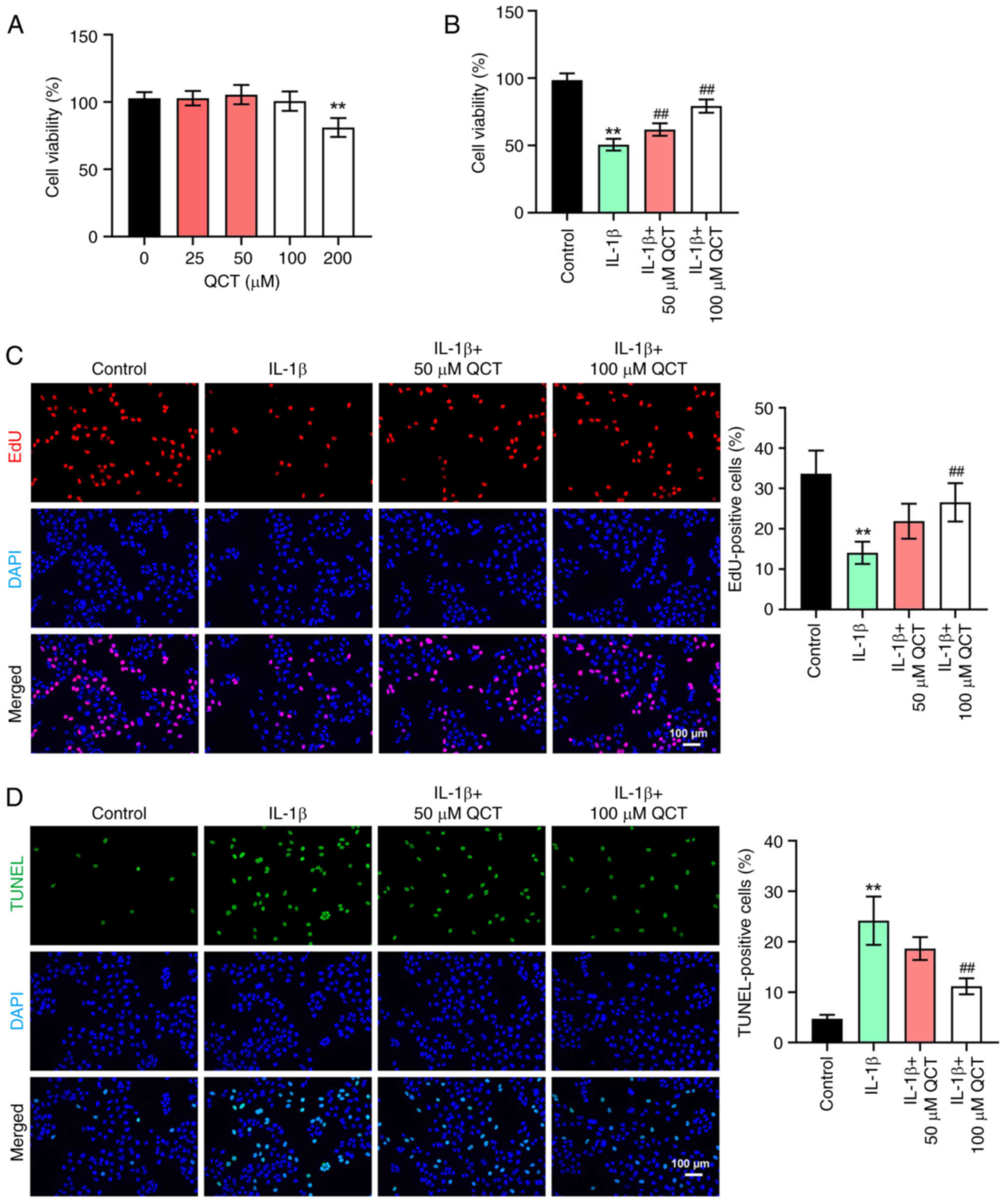 | Figure 1.QCT attenuates IL-1β-induced
apoptosis in chondrocytes. (A) CHON-001 cells were treated with QCT
(0, 25, 50, 100 or 200 µM) for 24 h at 37°C. Cell viability was
detected using a CCK-8 assay. (B) CHON-001 cells were treated with
QCT (50 or 100 µM) for 24 h at 37°C, and then exposed to IL-1β for
24 h at 37°C. Cell viability was detected using a CCK-8 assay. (C)
Cell proliferation was evaluated using an EdU staining assay.
Magnification, ×200. Scale bar, 100 µm. (D) Cell apoptosis was
assessed using a TUNEL assay. Magnification, ×200. Scale bar, 100
µm. **P<0.01 vs. no QCT treatment or control group;
##P<0.01 vs. IL-1β group. CCK-8, Cell Counting Kit-8;
EdU, 5-ethynyl-2′-deoxyuridine; QCT, quercetin. |
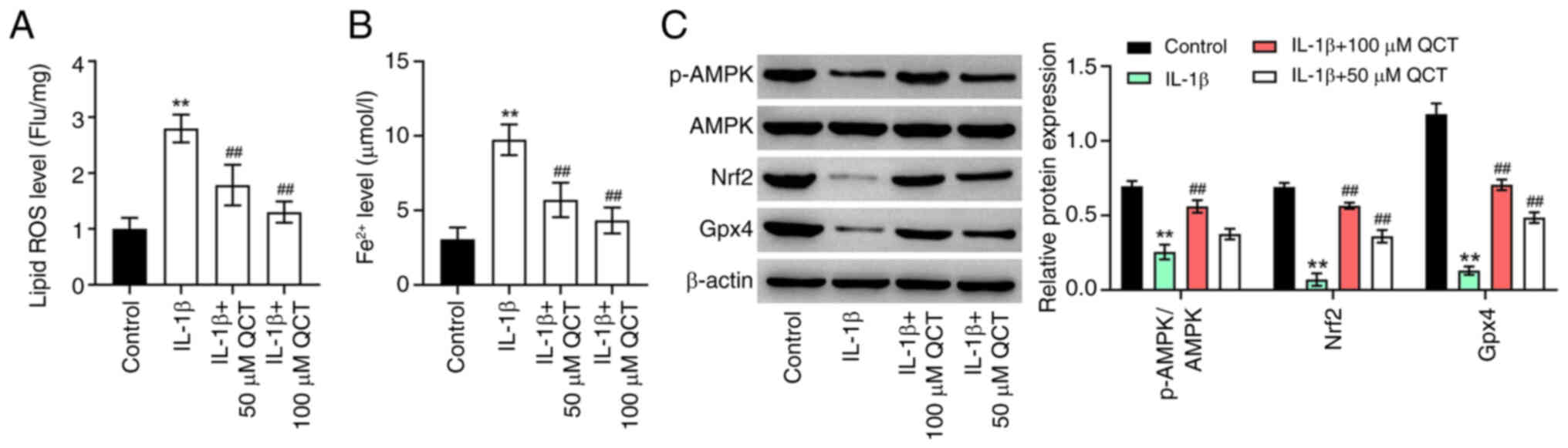
QCT inhibits the IL-1β-induced
ferroptosis of chondrocytes by activating the AMPK/Nrf2/Gpx4
signaling pathway
To explore the effects of QCT on the ferroptosis of
chondrocytes, lipid ROS and Fe2+ levels in CHON-001
cells were detected. IL-1β significantly enhanced lipid ROS and
Fe2+ levels in CHON-001 cells; however, these effects
were reversed by treatment with QCT (Fig. 2A and B). Additionally, treatment
with 100 µM QCT significantly elevated the p-AMPK, Nrf2 and Gpx4
levels in CHON-001 cells exposed to IL-1β (Fig. 2C). In summary, QCT inhibited the
IL-1β-induced ferroptosis of chondrocytes by activating the
AMPK/Nrf2/Gpx4 signaling pathway.
QCT promotes the proliferation of
IL-1β-stimulated chondrocytes by inhibiting ferroptosis
There is evidence to indicate that ferroptosis
participates in the regulation of cell proliferation (27,28).
Thus, in the present study, in order to explore whether QCT
attenuated IL-1β-induced chondrocyte injury by modulating
ferroptosis, erastin (a ferroptosis activator) was used. As shown
in Fig. 3A, 5 µM erastin reduced
CHON-001 cell viability to ~50%, with a significant difference
compared with the 0 µM treatment group. Thus, 5 µM erastin was
utilized in the subsequent experiments.
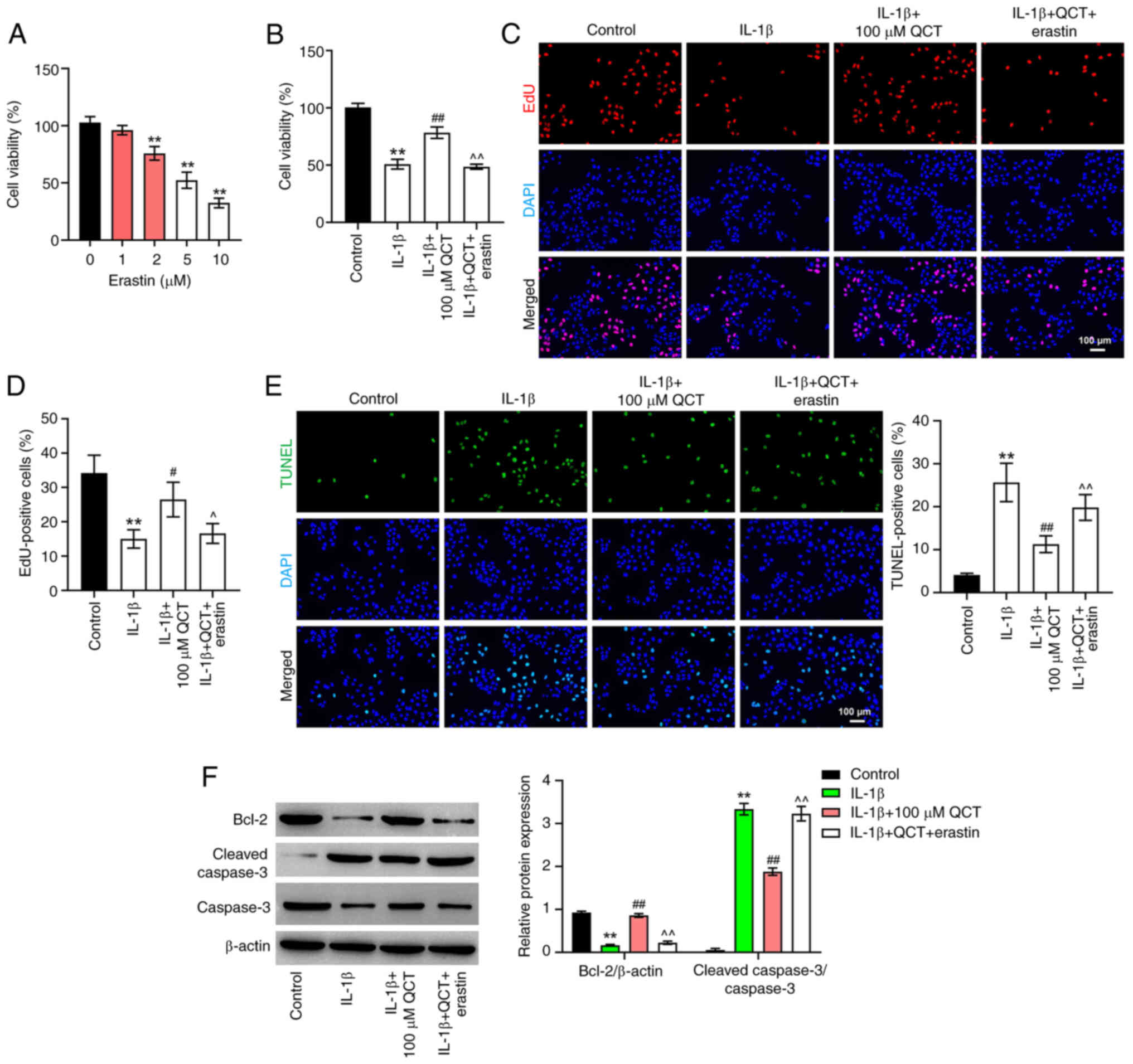 | Figure 3.QCT promotes proliferation and
inhibits apoptosis in IL-1β-treated chondrocytes by inhibiting
ferroptosis. (A) CHON-001 cells were treated with erastin (0, 1, 2,
5 or 10 μM) for 24 h at 37°C. Cell viability was detected using a
CCK-8 assay. **P<0.01 vs. erastin (0 µM) group. (B) CHON-001
cells were treated with QCT (100 µM) or QCT and erastin (5 µM) for
24 h at 37°C, and then exposed to IL-1β for 24 h at 37°C. Cell
viability was detected using a CCK-8 assay. (C) Cell proliferation
was evaluated using an EdU staining assay. (D) EdU-positive cells
were counted and quantified. Magnification, ×200. Scale bar, 100
µm. (E) Cell apoptosis was assessed using a TUNEL assay.
Magnification, ×200. Scale bar, 100 µm. (F) Western blotting was
used to detect Bcl-2, cleaved caspase 3 and caspase 3 levels in
CHON-001 cells. **P<0.01 vs. control group;
#P<0.05, ##P<0.01 vs. IL-1β group;
^P<0.05, ^^P<0.01 vs. IL-1β + QCT
group. CCK-8, Cell Counting Kit-8; EdU, 5-ethynyl-2′-deoxyuridine;
QCT, quercetin. |
As shown in Fig.
3B-E, QCT markedly elevated the proliferation and prevented the
apoptosis of IL-1β-stimulated CHON-001 cells; however, treatment
with erastin reversed these effects. Additionally, QCT
significantly elevated the Bcl-2 level and decreased the level of
cleaved caspase 3 in the IL-1β-stimulated CHON-001 cells; however,
the effects on these protein levels were reversed by erastin
(Fig. 3F). Collectively, these
results indicated that QCT promoted the proliferation of
IL-1β-stimulated chondrocytes by inhibiting ferroptosis.
QCT attenuates extracellular matrix
(ECM) degradation and inflammatory responses in IL-1β-stimulated
chondrocytes by inhibiting ferroptosis
The present study then examined the effects of QCT
on ECM degradation in IL-1β-stimulated chondrocytes. IL-1β
significantly decreased the levels of ECM proteins (collagen II and
aggrecan) and elevated the levels of ECM-degrading enzymes (MMP13
and ADAMTS5) in CHON-001 cells; however, the effects on these
protein levels (except for ADAMTS5) were reversed by treatment with
QCT (Fig. 4A and B). Conversely,
treatment with erastin reversed the QCT-induced upregulation of
collagen II and aggrecan and the downregulation of ADAMTS5 in
IL-1β-stimulated CHON-001 cells (Fig.
4A and B). Additionally, QCT significantly reduced the IL-6,
TNF-α, lipid ROS, Fe2+ and MDA levels, and increased the
GSH level in CHON-001 cells exposed to IL-1β; however, erastin
reversed these effects (Fig.
4C-H). Overall, QCT attenuated ECM degradation and inflammatory
responses in IL-1β-stimulated chondrocytes by inhibiting
ferroptosis.
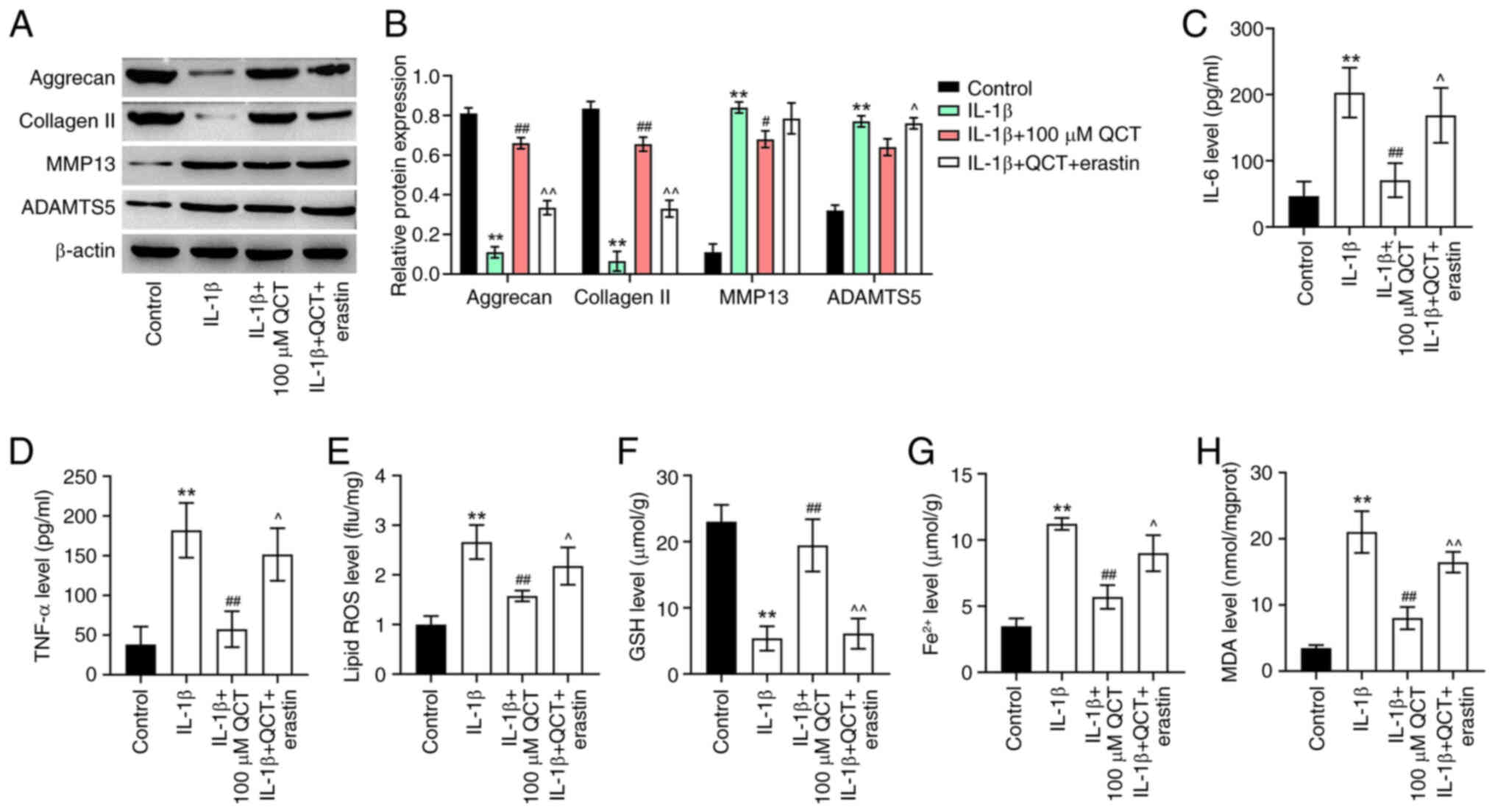 | Figure 4.QCT attenuates extracellular matrix
degradation and inflammatory responses in IL-1β-treated
chondrocytes by inhibiting ferroptosis. (A) CHON-001 cells were
treated with QCT (100 µM) or QCT and erastin (5 µM) for 24 h at
37°C, and then exposed to IL-1β for 24 h. Western blotting was used
to detect aggrecan, collagen II, MMP13 and ADAMTS5 levels in
CHON-001 cells. (B) Relative expression levels of aggrecan,
collagen II, MMP13 and ADAMTS5 were semi-quantified. CHON-001 cells
were treated with QCT (100 µM) or QCT and erastin (5 µM) for 24 h
at 37°C, and then exposed to IL-1β for 24 h at 37°C. (C) IL-6, (D)
TNF-α, (E) lipid ROS, (F) GSH, (G) Fe2+ and (H) MDA
levels in CHON-001 cells were evaluated using ELISAs. **P<0.01
vs. control group; #P<0.05, ##P<0.01
vs. IL-1β group; ^P<0.05, ^^P<0.01 vs.
IL-1β + QCT group. ADAMTS5, ADAM metallopeptidase with
thrombospondin type 1 motif 5; collagen II, type II collagen; GSH,
glutathione; MDA, malondialdehyde; QCT, quercetin; ROS, reactive
oxygen species. |
QCT protects against IL-1β-induced ECM
degradation in vitro by suppressing ferroptosis via the activation
of the AMPK/Nrf2/Gpx4 signaling pathway
AMPK/Nrf2 signaling serves a crucial role in
regulating ferroptosis (29).
Therefore, the present study explored whether QCT could attenuate
ferroptosis in chondrocytes by modulating AMPK/Nrf2 signaling.
Treatment with QCT markedly elevated the p-AMPK, Nrf2 and Gpx4
levels in IL-1β-stimulated CHON-001 cells; however, these changes
were reversed by treatment with compound C, an AMPK inhibitor
(Fig. 5A). Furthermore, treatment
with QCT markedly increased the aggrecan and collagen II levels,
and reduced the ADAMTS5 level in IL-1β-stimulated CHON-001 cells,
while compound C markedly reversed these effects (Fig. 5B). However, QCT had no effect on
the MMP13 levels in IL-1β-stimulated CHON-001 cells (Fig. 5B). Collectively, QCT protected
against IL-1β-induced ECM degradation in vitro by
suppressing ferroptosis via the activation of the AMPK/Nrf2/Gpx4
signaling pathway.
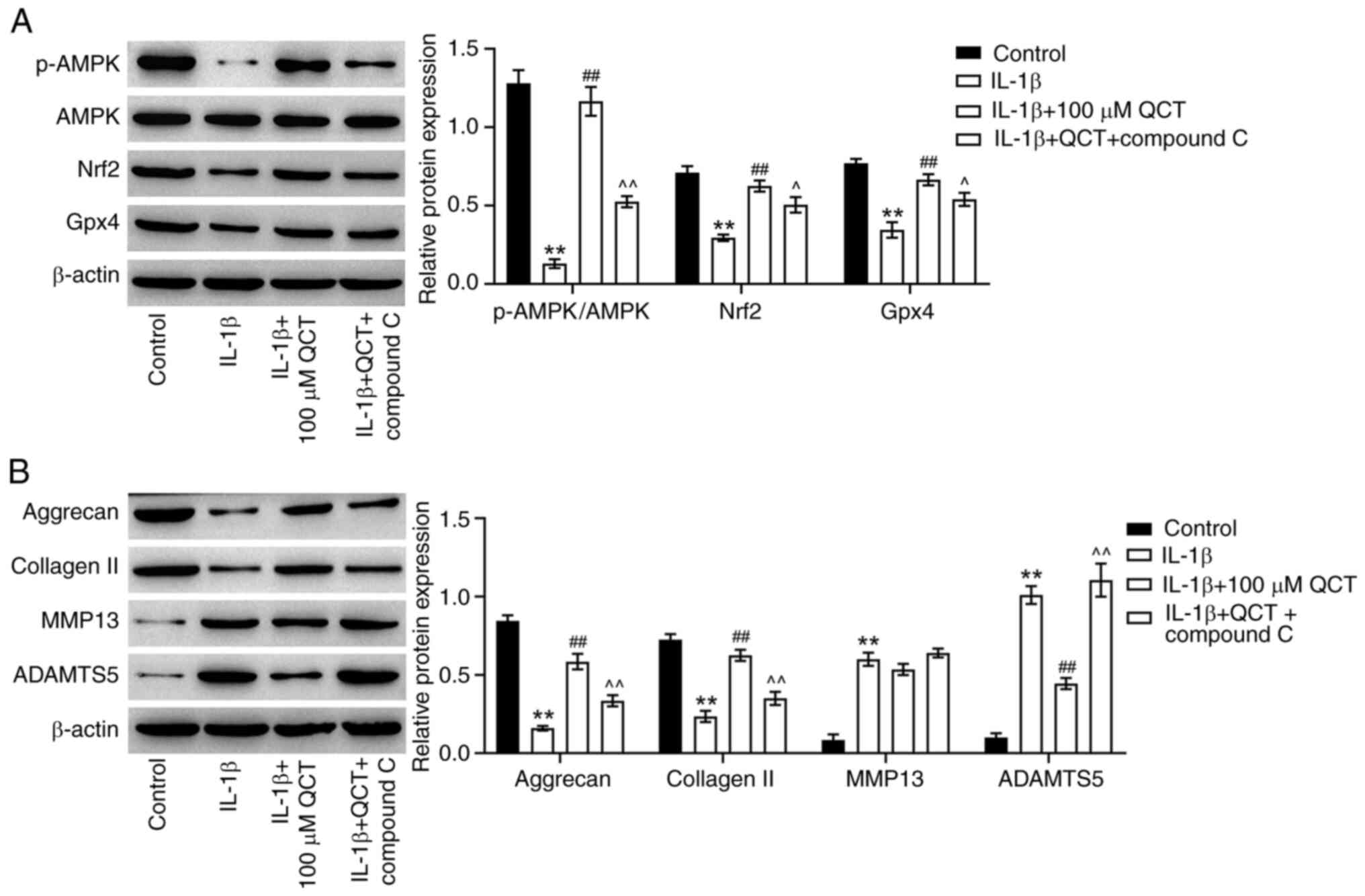 | Figure 5.QCT protects against IL-1β-induced
chondrocyte injury in vitro by suppressing ferroptosis via
the activation of AMPK/Nrf2/Gpx4 signaling. CHON-001 cells were
treated with QCT (100 µM) or QCT and compound C (5 µM) for 24 h at
37°C, and then exposed to IL-1β for 24 h at 37°C. (A) Western
blotting was used to detect p-AMPK, AMPK, Nrf2 and Gpx4 levels in
CHON-001 cells. The level of p-AMPK was normalized to that of AMPK.
(B) Western blotting was used to detect aggrecan, collagen II,
MMP13 and ADAMTS5 levels in CHON-001 cells. **P<0.01 vs. control
group; ##P<0.01 vs. IL-1β group;
^P<0.05 ^^P<0.01 vs. IL-1β + QCT group.
ADAMTS5, ADAM metallopeptidase with thrombospondin type 1 motif 5;
AMPK, 5′ AMP-activated protein kinase; collagen II, type II
collagen; Gpx4, glutathione peroxidase 4; Nrf2, nuclear factor
erythroid 2-related factor 2; p-, phosphorylated; QCT,
quercetin. |
QCT ameliorates OA in mice in vivo via
activation of the AMPK/Nrf2/Gpx4 signaling pathway
Finally, to evaluate the therapeutic effects of QCT
in vivo, a mouse model of OA was established. Disordered
chondrocytes, damaged cartilage surface and proteoglycan loss in
cartilage tissues were observed in the OA group; however, these
effects were reversed by treatment with QCT, suggesting that QCT
attenuated cartilage damage in mice with ACLT-induced OA (Fig. 6A). Additionally, the results of IHC
staining indicated that the aggrecan and collagen II levels were
reduced, and the ADAMTS5 level was markedly elevated in the
cartilage tissues of mice with OA, while these changes were
reversed by treatment with 40 mg/kg QCT (Fig. 6A). However, QCT had no effect on
the MMP13 level in the cartilage tissues of mice with OA (Fig. 6A). Furthermore, treatment with QCT
significantly elevated the p-AMPK, Nrf2 and Gpx4 levels in
cartilage tissues of mice with OA (Fig. 6B and C). The OARSI scores confirmed
that QCT significantly attenuated the progression of OA (Fig. 6D). In summary, QCT ameliorated OA
in mice in vivo via the activation of the AMPK/Nrf2/Gpx4
signaling pathway.
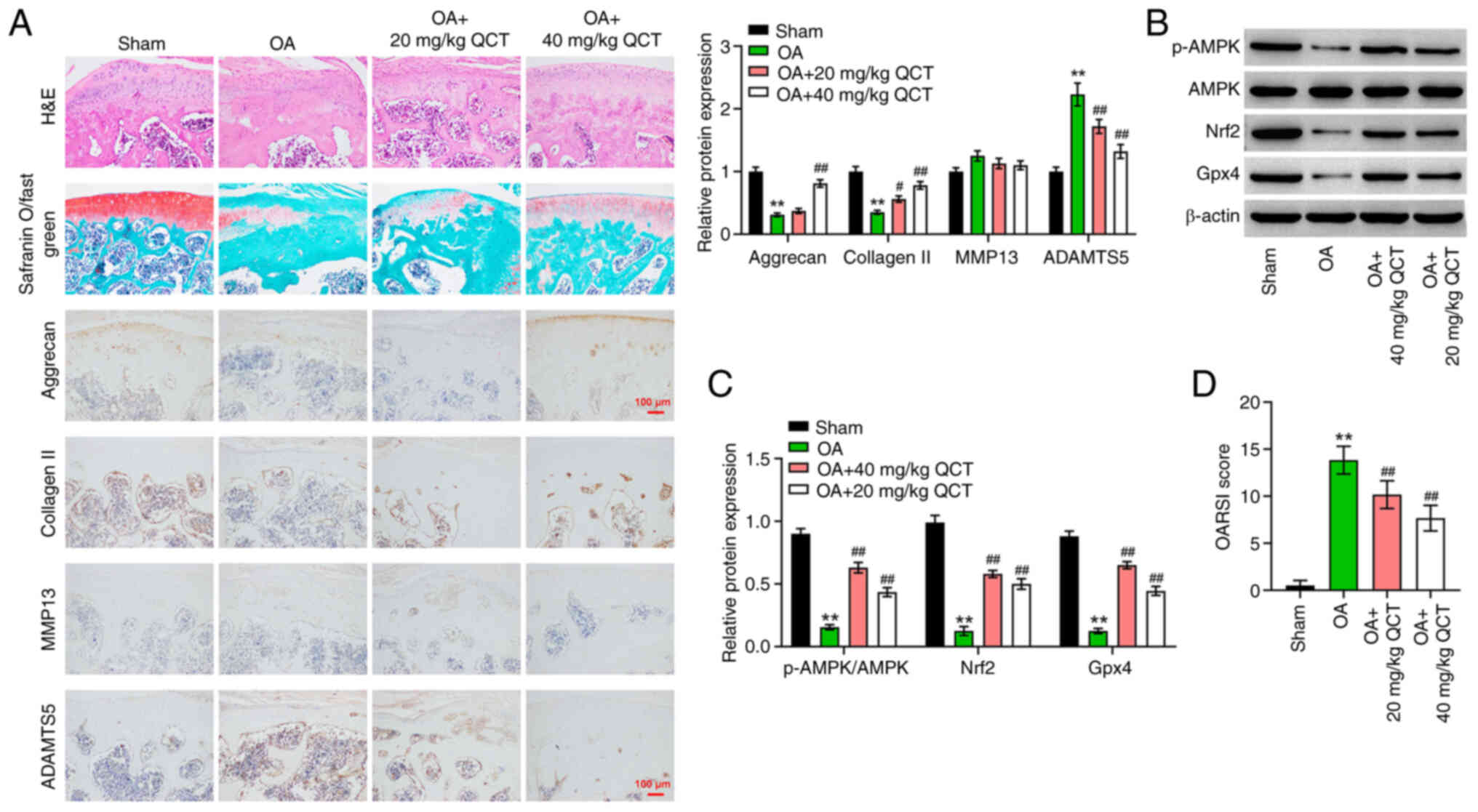 | Figure 6.QCT ameliorates OA in mice in
vivo via activation of AMPK/Nrf2/Gpx4 signaling. (A) Pathologic
changes of mouse cartilage tissue were evaluated using H&E
staining and safranin O/fast green assays. Immunohistochemistry was
used to assess aggrecan, collagen II, MMP13 and ADAMTS5 levels in
mouse cartilage tissues. Magnification, ×200. Scale bar, 100 µm.
(B) Western blotting was used to detect p-AMPK, AMPK, Nrf2 and Gpx4
levels in mouse cartilage tissues. (C) The level of p-AMPK was
normalized to that of AMPK and the other proteins were normalized
to β-actin. (D) OARSI score in each group. **P<0.01 vs. sham
group; #P<0.05, ##P<0.01 vs. OA group.
ADAMTS5, ADAM metallopeptidase with thrombospondin type 1 motif 5;
AMPK, 5′ AMP-activated protein kinase; collagen II, type II
collagen; Gpx4, glutathione peroxidase 4; Nrf2, nuclear factor
erythroid 2-related factor 2; OA, osteoarthritis; OARSI,
Osteoarthritis Research Society International; p-, phosphorylated;
QCT, quercetin. |
Discussion
It has been demonstrated that QCT exerts beneficial
effects against multiple diseases, including diabetes mellitus,
Alzheimer's and other neurodegenerative diseases and OA (30–32).
Hu et al (19) found that
QCT prevented the progression of OA in rats by attenuating
cartilage degradation and suppressing chondrocyte apoptosis. Feng
et al (33) indicated that
QCT attenuated damage to rat chondrocytes by suppressing oxidative
stress, endoplasmic reticulum stress and cell apoptosis. Qiu et
al (20) found that QCT
restored mitochondrial dysfunction and inhibited ECM degradation in
rats with OA by activating AMPK/sirtuin 1 signaling, thereby
attenuating OA progression. These findings demonstrate the critical
roles of QCT in the development of OA. The present study revealed a
novel mechanism that underlies the chondroprotective effects of QCT
in OA. In the present study, QCT markedly attenuated articular
cartilage injury in mice with OA. Additionally, 100 µM QCT
significantly enhanced the proliferation and reduced the apoptosis
of IL-1β-stimulated chondrocytes. Furthermore, to the best of our
knowledge, the present study was the first to demonstrate that QCT
notably suppressed the ferroptosis of IL-1β-stimulated
chondrocytes, as demonstrated by the reduced lipid ROS and
Fe2+ levels. These results demonstrated that QCT
attenuated the symptoms of OA by suppressing ferroptosis.
Chondrocyte ferroptosis has been found to aggravate
the progression of OA (34).
Ferroptosis is characterized by iron overload and the accumulation
of lipid ROS (35). Furthermore,
iron overload and lipid peroxidation are key pathological
characteristics of OA (36,37).
Iron overload is often observed in the tissues of elderly
individuals, including knee joint tissues (38,39).
Iron overload has been found to elevate the expression levels of
the matrix-degrading enzymes MMP13 and ADAMTS5 in cartilage tissues
(40,41). In the present study, lipid ROS
production and the Fe2+ levels were markedly elevated in
IL-1β-stimulated chondrocytes, suggesting that IL-1β induced
ferroptosis in chondrocytes. However, treatment with QCT
significantly reversed these effects. These data suggested that QCT
possesses anti-ferroptosis properties in OA. Notably, the
inhibition of chondrocyte ferroptosis has been found to attenuate
the development of OA. For example, Zhou et al (42) found that curcumin exerted
protective effects against erastin-induced ferroptosis in
chondrocytes through the upregulation of Nrf2. He et al
(43) indicated that biochanin A
markedly attenuated articular cartilage injury in a mouse model of
iron overload-associated OA by suppressing iron levels and
activating Nrf2/Gpx4 signaling. Xu et al (44) found that tanshinone IIA was able to
inhibit ECM degeneration in chondrocytes by inhibiting ferroptosis.
The results of the present study demonstrated that treatment with
QCT notably increased the aggrecan and collagen II levels in
IL-1β-stimulated chondrocytes; however, the activation of
ferroptosis evidently reversed these phenomena. Furthermore, the
inhibitory effects of QCT on the apoptosis and inflammatory
responses of IL-1β-stimulated chondrocytes were reversed by the
activation of ferroptosis. Thereby, the present study indicated
that QCT could prevent apoptosis, inflammation and ECM degradation
in OA by suppressing ferroptosis.
AMPK can serve as an energy sensor that participates
in a number of signal transduction pathways, including ferroptosis
(45,46). The activation of AMPK signaling can
inhibit ferroptotic cell death (46). Furthermore, the activation of AMPK
signaling has been found to inhibit the development of OA in a
mouse model of OA (47,48). There is evidence to indicate that
AMPK can act as an activator of Nrf2 and Gpx4, two negative
regulators of ferroptosis (49–51),
suggesting that the activation of AMPK/Nrf2/Gpx4 signaling can
suppress ferroptosis (52). Wan
et al (53) revealed that
baicalein was able to reduce chondrocyte ferroptosis by activating
AMPK/Nrf2 signaling. Consistent with these previous findings, the
present study demonstrated that treatment with QCT elevated the
p-AMPK, Nrf2 and Gpx4 levels in the cartilage tissues of mice with
OA. Similarly, QCT significantly elevated the p-AMPK, Nrf2 and Gpx4
levels in IL-1β-stimulated chondrocytes; however, these changes
were reversed by treatment with compound C, an AMPK inhibitor.
Collectively, QCT suppressed ferroptosis in vitro and in
vivo by activating the AMPK/Nrf2/Gpx4 signaling pathway.
However, whether QCT activation of AMPK/Nrf2/Gpx4
signaling was direct or indirect in the current study remains
unclear. In addition, only one cell line was used in the present
study, and the results should be validated using primary
chondrocytes. Furthermore, the treatment duration in the animal
experiment was relatively short (4 weeks), and it should be
explored whether long-term effects could be observed. Although
increasing evidence suggests that QCT protects against OA in
vitro and in vivo, clinical testing of QCT is rare
(54,55). Thus, it is difficult to suggest the
advantages of QCT compared with other established anti-OA drugs
such as oral non-steroidal anti-inflammatory drugs at present
(6,7).
In conclusion, the findings of the present study
demonstrated that QCT prevented the development of OA in
vitro and in vivo by suppressing ferroptosis via the
activation of AMPK/Nrf2/Gpx4 signaling. The findings illustrated
that QCT could suppress chondrocyte ferroptosis via the activation
of the AMPK/Nrf2/Gpx4 signaling pathway, providing novel insights
into the regulatory mechanisms of QCT in OA. Additionally, these
findings further support the potential use of QCT in the treatment
of OA.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SD designed the overall study with contributions
from XL. SD designed and carried out experiments, collected data
and wrote the draft. XL, GX and LC carried out experiments and
analyzed the data. SD, LC and JZ discussed and edited the paper. JZ
supervised the study, designed experiments and cowrote the paper.
SD and JZ confirm the authenticity of all the raw data. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The protocols for animal care and use of laboratory
animals were approved by the Ethics Committee of Beijing University
of Chinese Medicine (approval no. BUCM-2023032701-2103; Beijing,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Collins DP, Elsouri KN and Demory Beckler
M: Osteoarthritis: Can we do better? Cureus.
14:e315052022.PubMed/NCBI
|
|
2
|
Tavallaee G, Rockel JS, Lively S and
Kapoor M: MicroRNAs in synovial pathology associated with
osteoarthritis. Front Med (Lausanne). 7:3762020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiménez G, Cobo-Molinos J, Antich C and
López-Ruiz E: Osteoarthritis: Trauma vs disease. Adv Exp Med Biol.
1059:63–83. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prieto-Alhambra D, Judge A, Javaid MK,
Cooper C, Diez-Perez A and Arden NK: Incidence and risk factors for
clinically diagnosed knee, hip and hand osteoarthritis: Influences
of age, gender and osteoarthritis affecting other joints. Ann Rheum
Dis. 73:1659–1664. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Turkiewicz A, Petersson IF, Björk J,
Hawker G, Dahlberg LE, Lohmander LS and Englund M: Current and
future impact of osteoarthritis on health care: A population-based
study with projections to year 2032. Osteoarthritis Cartilage.
22:1826–1832. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ishijima M, Nakamura T, Shimizu K, Hayashi
K, Kikuchi H, Soen S, Omori G, Yamashita T, Uchio Y, Chiba J, et
al: Intra-articular hyaluronic acid injection versus oral
non-steroidal anti-inflammatory drug for the treatment of knee
osteoarthritis: A multi-center, randomized, open-label,
non-inferiority trial. Arthritis Res Ther. 16:R182014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Q, Mol MF, Bos PK, Dorleijn DMJ, Vis
M, Gussekloo J, Bindels PJE, Runhaar J and Bierma-Zeinstra SMA:
Effect of intramuscular vs intra-articular glucocorticoid injection
on pain among adults with knee osteoarthritis: The KIS randomized
clinical trial. JAMA Netw Open. 5:e2248522022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Umpierres CS, Ribeiro TA, Marchisio ÂE,
Galvão L, Borges ÍN, Macedo CA and Galia CR: Rehabilitation
following total hip arthroplasty evaluation over short follow-up
time: Randomized clinical trial. J Rehabil Res Dev. 51:1567–1578.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Safran-Norton CE, Sullivan JK, Irrgang JJ,
Kerman HM, Bennell KL, Calabrese G, Dechaves L, Deluca B, Gil AB,
Kale M, et al: A consensus-based process identifying physical
therapy and exercise treatments for patients with degenerative
meniscal tears and knee OA: The TeMPO physical therapy
interventions and home exercise program. BMC Musculoskelet Disord.
20:5142019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ghouri A and Conaghan PG: Prospects for
therapies in osteoarthritis. Calcif Tissue Int. 109:339–350. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie Z, Wang L, Chen J, Zheng Z, Srinual S,
Guo A, Sun R and Hu M: Reduction of systemic exposure and side
effects by intra-articular injection of anti-inflammatory agents
for osteoarthritis: What is the safer strategy? J Drug Target.
31:596–611. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Siow YL, Gong Y, Au-Yeung KKW, Woo CWH,
Choy PC and O K: Emerging issues in traditional Chinese medicine.
Can J Physiol Pharmacol. 83:321–334. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan HHL and Ng T: Traditional Chinese
medicine (TCM) and allergic diseases. Curr Allergy Asthma Rep.
20:672020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun H, Qu W, Chen G, Sun X, Zhang D and
Shao S: Efficacy and safety of traditional Chinese patent medicine
on carotid artery atherosclerosis in adults: A network
meta-analysis protocol. Medicine (Baltimore). 100:e244062021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ginkgo, . Drugs and Lactation Database
(LactMed®) [Internet]. National Institute of Child
Health and Human Development; Bethesda, MD: 2006
|
|
16
|
Li Y, Zhang N, Peng X, Ma W, Qin Y, Yao X,
Huang C and Zhang X: Network pharmacology analysis and clinical
verification of Jishe Qushi capsules in rheumatoid arthritis
treatment. Medicine (Baltimore). 102:e348832023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qi W, Qi W, Xiong D and Long M: Quercetin:
Its antioxidant mechanism, antibacterial properties and potential
application in prevention and control of toxipathy. Molecules.
27:65452022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Beken B, Serttas R, Yazicioglu M, Turkekul
K and Erdogan S: Quercetin improves inflammation, oxidative stress,
and impaired wound healing in atopic dermatitis model of human
keratinocytes. Pediatr Allergy Immunol Pulmonol. 33:69–79. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu Y, Gui Z, Zhou Y, Xia L, Lin K and Xu
Y: Quercetin alleviates rat osteoarthritis by inhibiting
inflammation and apoptosis of chondrocytes, modulating synovial
macrophages polarization to M2 macrophages. Free Radic Biol Med.
145:146–160. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qiu L, Luo Y and Chen X: Quercetin
attenuates mitochondrial dysfunction and biogenesis via upregulated
AMPK/SIRT1 signaling pathway in OA rats. Biomed Pharmacother.
103:1585–1591. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11:882020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen X, Kang R, Kroemer G and Tang D:
Ferroptosis in infection, inflammation, and immunity. J Exp Med.
218:e202105182021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miao Y, Chen Y, Xue F, Liu K, Zhu B, Gao
J, Yin J, Zhang C and Li G: Contribution of ferroptosis and GPX4′s
dual functions to osteoarthritis progression. EBioMedicine.
76:1038472022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiao P, Zhu X, Sun J, Zhang Y, Qiu W, Li J
and Wu X: MicroRNA-613 alleviates IL-1β-induced injury in
chondrogenic CHON-001 cells by targeting fibronectin 1. Am J Transl
Res. 12:5308–5319. 2020.PubMed/NCBI
|
|
25
|
Kamekura S, Hoshi K, Shimoaka T, Chung U,
Chikuda H, Yamada T, Uchida M, Ogata N, Seichi A, Nakamura K and
Kawaguchi H: Osteoarthritis development in novel experimental mouse
models induced by knee joint instability. Osteoarthritis Cartilage.
13:632–641. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Glasson SS, Chambers MG, Van Den Berg WB
and Little CB: The OARSI histopathology initiative-recommendations
for histological assessments of osteoarthritis in the mouse.
Osteoarthritis Cartilage. 18 (Suppl 3):S17–S23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin Z, Liu J, Kang R, Yang M and Tang D:
Lipid metabolism in ferroptosis. Adv Biol (Weinh). 5:e21003962021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng Q, Li P, Zhou X, Qiang Y, Fan J, Lin
Y, Chen Y, Guo J, Wang F, Xue H, et al: Deficiency of the
X-inactivation escaping gene KDM5C in clear cell renal cell
carcinoma promotes tumorigenicity by reprogramming glycogen
metabolism and inhibiting ferroptosis. Theranostics. 11:8674–8691.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu Q, Yang L, Xiao JJ, Liu Q, Ni L, Hu JW,
Yu H, Wu X and Zhang BF: Empagliflozin attenuates the renal tubular
ferroptosis in diabetic kidney disease through AMPK/NRF2 pathway.
Free Radic Biol Med. 195:89–102. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zu G, Sun K, Li L, Zu X, Han T and Huang
H: Mechanism of quercetin therapeutic targets for Alzheimer disease
and type 2 diabetes mellitus. Sci Rep. 11:229592021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bayazid AB and Lim BO: Quercetin is an
active agent in berries against neurodegenerative diseases
progression through modulation of Nrf2/HO1. Nutrients. 14:51322022.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Aleebrahim-Dehkordi E, Soveyzi F, Arian
AS, Hamedanchi NF, Hasanpour-Dehkordi A and Rafieian-Kopaei M:
Quercetin and its role in reducing the expression of
pro-inflammatory cytokines in osteoarthritis. Antiinflamm
Antiallergy Agents Med Chem. 21:153–165. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feng K, Chen Z, Pengcheng L, Zhang S and
Wang X: Quercetin attenuates oxidative stress-induced apoptosis via
SIRT1/AMPK-mediated inhibition of ER stress in rat chondrocytes and
prevents the progression of osteoarthritis in a rat model. J Cell
Physiol. 234:18192–18205. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou X, Zheng Y, Sun W, Zhang Z and Liu J,
Yang W, Yuan W, Yi Y, Wang J and Liu J: D-mannose alleviates
osteoarthritis progression by inhibiting chondrocyte ferroptosis in
a HIF-2α-dependent manner. Cell Prolif. 54:e131342021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu X, Li Y, Zhang S and Zhou X:
Ferroptosis as a novel therapeutic target for cardiovascular
disease. Theranostics. 11:3052–3059. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang S, Xu J, Si H, Wu Y, Zhou S and Shen
B: The role played by ferroptosis in osteoarthritis: Evidence based
on iron dyshomeostasis and lipid peroxidation. Antioxidants
(Basel). 11:16682022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dai T, Xue X, Huang J, Yang Z, Xu P, Wang
M, Xu W, Feng Z, Zhu W, Xu Y, et al: SCP2 mediates the transport of
lipid hydroperoxides to mitochondria in chondrocyte ferroptosis.
Cell Death Discov. 9:2342023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Burton LH, Radakovich LB, Marolf AJ and
Santangelo KS: Systemic iron overload exacerbates osteoarthritis in
the strain 13 guinea pig. Osteoarthritis Cartilage. 28:1265–1275.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gozzelino R and Arosio P: Iron homeostasis
in health and disease. Int J Mol Sci. 17:1302016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jing X, Du T, Li T, Yang X, Wang G, Liu X,
Jiang Z and Cui X: The detrimental effect of iron on OA
chondrocytes: Importance of pro-inflammatory cytokines induced iron
influx and oxidative stress. J Cell Mol Med. 25:5671–5680. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jing X, Lin J, Du T, Jiang Z, Li T, Wang
G, Liu X, Cui X and Sun K: Iron overload is associated with
accelerated progression of osteoarthritis: The role of DMT1
mediated iron homeostasis. Front Cell Dev Biol. 8:5945092021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou Y, Jia Z, Wang J, Huang S, Yang S,
Xiao S, Xia D and Zhou Y: Curcumin reverses erastin-induced
chondrocyte ferroptosis by upregulating Nrf2. Heliyon.
9:e201632023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
He Q, Yang J, Pan Z, Zhang G, Chen B, Li
S, Xiao J, Tan F, Wang Z, Chen P and Wang H: Biochanin A protects
against iron overload associated knee osteoarthritis via regulating
iron levels and NRF2/System xc-/GPX4 axis. Biomed Pharmacother.
157:1139152023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu J, Zhi X, Zhang Y and Ding R:
Tanshinone IIA alleviates chondrocyte apoptosis and extracellular
matrix degeneration by inhibiting ferroptosis. Open Life Sci.
18:202206662023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ge Y, Zhou M, Chen C, Wu X and Wang X:
Role of AMPK mediated pathways in autophagy and aging. Biochimie.
195:100–113. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee H, Zandkarimi F, Zhang Y, Meena JK,
Kim J, Zhuang L, Tyagi S, Ma L, Westbrook TF, Steinberg GR, et al:
Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat
Cell Biol. 22:225–234. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li J, Zhang B, Liu WX, Lu K, Pan H, Wang
T, Oh CD, Yi D, Huang J, Zhao L, et al: Metformin limits
osteoarthritis development and progression through activation of
AMPK signalling. Ann Rheum Dis. 79:635–645. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jin Z, Chang B, Wei Y, Yang Y, Zhang H,
Liu J, Piao L and Bai L: Curcumin exerts chondroprotective effects
against osteoarthritis by promoting AMPK/PINK1/Parkin-mediated
mitophagy. Biomed Pharmacother. 151:1130922022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ding X, Jian T, Li J, Lv H, Tong B, Li J,
Meng X, Ren B and Chen J: Chicoric acid ameliorates nonalcoholic
fatty liver disease via the AMPK/Nrf2/NFκB signaling pathway and
restores gut microbiota in high-fat-diet-fed mice. Oxid Med Cell
Longev. 2020:97345602020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ge MH, Tian H, Mao L, Li DY, Lin JQ, Hu
HS, Huang SC, Zhang CJ and Mei XF: Zinc attenuates ferroptosis and
promotes functional recovery in contusion spinal cord injury by
activating Nrf2/GPX4 defense pathway. CNS Neurosci Ther.
27:1023–1040. 2021.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang Y, Wu Q, Liu J, Zhang Z, Ma X, Zhang
Y, Zhu J, Thring RW, Wu M, Gao Y and Tong H: Sulforaphane
alleviates high fat diet-induced insulin resistance via
AMPK/Nrf2/GPx4 axis. Biomed Pharmacother. 152:1132732022.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lu H, Xiao H, Dai M, Xue Y and Zhao R:
Britanin relieves ferroptosis-mediated myocardial
ischaemia/reperfusion damage by upregulating GPX4 through
activation of AMPK/GSK3β/Nrf2 signalling. Pharm Biol. 60:38–45.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wan Y, Shen K, Yu H and Fan W: Baicalein
limits osteoarthritis development by inhibiting chondrocyte
ferroptosis. Free Radic Biol Med. 196:108–120. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Samadi F, Kahrizi MS, Heydari F,
Arefnezhad R, Roghani-Shahraki H, Mokhtari Ardekani A and
Rezaei-Tazangi F: Quercetin and osteoarthritis: A mechanistic
review on the present documents. Pharmacology. 107:464–471. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yamaura K, Nelson AL, Nishimura H,
Rutledge JC, Ravuri SK, Bahney C, Philippon MJ and Huard J:
Therapeutic potential of senolytic agent quercetin in
osteoarthritis: A systematic review and meta-analysis of
preclinical studies. Ageing Res Rev. 90:1019892023. View Article : Google Scholar : PubMed/NCBI
|