Introduction
Intrauterine growth restriction (IUGR) is the second
most common obstetric complication after preterm labor, affecting
5–10% of all pregnancies (1–4).
Patients with growth restricted fetuses often develop preeclampsia
(5) and fetuses with IUGR have a
5–10 times higher risk of dying in uterο. Additionally,
fetuses affected by IUGR have a higher risk for perinatal
morbidity, impaired neurodevelopment and long-term complications
such as diabetes and cardiovascular and renal disease (6–9).
IUGR occurs under the influence of fetal, maternal and placental
factors that prevent the fetus attaining the optimal growth and
placental insufficiently is the main cause for the development of
IUGR (5,10). The molecular mechanisms mediating
impaired fetal growth are unclear and therapeutic interventions in
overcoming placental insufficiency and fetal growth restriction
(FGR) remain unsatisfactory (3,11).
The human placenta is a fetal organ of embryological
origin that develops from the trophoectoderm, which is the outer
layer of the blastocyst and the extraembryonic mesoderm shortly
after blastocyst implantation. The placenta serves a key role in
ensuring optimal fetal growth and development during pregnancy
(12–14). Appropriate trophoblast
differentiation and placental structure, growth and function are
key for the maintenance of pregnancy and normal fetal growth,
development and survival (15).
Abnormal trophoblast proliferation and differentiation are
associated with severe pregnancy complications, including recurrent
miscarriage, preeclampsia and FGR (13,16,17).
In early placentation, trophoblast precursors differentiate into
highly invasive trophoblast cells, known as extravillous
trophoblasts (12,18). Extravillous trophoblast formation
occurs when placental villi attach to the uterine decidua
constituting the anchoring villi, which are also termed ‘cell
columns’. Extravillous trophoblast cells proliferate, migrate and
invade the uterine decidua, endometrial glands, spiral arteries and
inner third of the myometrium (18). One of the key roles of extravillous
trophoblasts is remodeling of the maternal spiral arteries. To
achieve this, extravillous trophoblast cells interact with maternal
immune cells, disrupt the tunica media, induce elastolysis in the
arterial wall, replace the endothelial cells and line the spiral
arteries (12). This spiral artery
remodeling provides a steady perfusion of intervillous spaces with
maternal blood, unhindered by the influence of vasoactive agents
(18). Poor spiral artery
remodeling in the uterus is associated with shallow extravillous
trophoblast cell invasion and is a key pathological feature of the
placenta in preeclampsia, IUGR, placental abruption and spontaneous
preterm premature rupture of membranes (19).
Extravillous trophoblast cell proliferation,
migration and invasion are positively or negatively regulated by
numerous molecules produced by the fetomaternal interface to
maintain a normal utero-placental homeostasis (20). These molecules include autocrine
factors produced by the trophoblast such as growth factors
[including insulin-like growth factor (IGF)-1, IGF-2, heparin
binding epidermal growth factor-like, vascular epidermal, placental
and hepatocyte growth factor], growth factor-binding proteins
[including IGF binding proteins (IGFBPs)], proteoglycans (including
decorin and biglycan), sialoproteins (including osteopontin),
cytokines (including IL-6 and IL-15), chemokines [including C-X3-C
motif chemokine ligand 1, C-C motif chemokine ligand (CCL)4 and
CCL14], lipid derivatives (including prostaglandin E2),
matrix metalloproteinases, plasminogen activator inhibitors (PAI)-1
and PAI-2 or paracrine factors produced by the decidua, such as
decidual leukocytes and immune cells (20,21).
In the third trimester of pregnancy the terminal
villous structure regulates the maternal nutrient transport and
respiratory gas exchange to fetal circulation to aid fetal growth
and development (16). Placental
chorionic tertiary villi are bathed in maternal blood, which fills
the trophoblastic lacunae. Nutrients, oxygen and waste products are
transported across the two cell layers of the tertiary villi
between the fetal and maternal blood, which are the
syncytiotrophoblast and fetal endothelial cells (16,22,23).
The syncytiotrophoblast, which is the outer layer of chorionic
villi, cannot regenerate but is formed by continuous fusion of the
underlying proliferating villous cytotrophoblasts into a continuous
multinuclear cell layer (16–25).
Following fusion of cytotrophoblasts, nuclei demonstrate signs of
degeneration (25). In the third
trimester of pregnancy, some nuclei in the syncytiotrophoblast
gather together into clusters termed syncytial trophoblastic knots
(STKs) (25–27). Notably, there is an increased
number of STKs in pregnancies complicated by IUGR and preeclampsia
(28,29). In addition, IUGR is associated with
maternal vascular malperfusion (MVM) of the placental bed and
decreased villous branching and terminal villi volume and exchange
surface area between fetal and maternal circulation (3,30–32).
Furthermore, in patients with IUGR, impaired cytotrophoblast
cell-cell fusion is associated with decreased area of the
syncytiotrophoblast within the villous and increased
syncytiotrophoblast apoptosis, interrupting placental functions
such as nutrient and oxygen transport to the fetus and the
production and release of placental hormones (33–35).
In the placenta, cellular turnover and renewal are regulated to
maintain a bilayer of chorionic villus structure (16,36,37).
The IGF system promotes trophoblast turnover by promoting both
proliferation and differentiation of the cytotrophoblast to the
syncytiotrophoblast for normal trophoblast function and fetal
growth and development (36,38,39).
Insulin, IGF-1 and IGF-2 serve a key role in fetal growth and
development (40–42). IGF-1 and IGF-2 are highly
homogenous single chain polypeptides with molecular weight of ~7
kDa that are structurally homologous to proinsulin (43,44).
Alternative splicing of exons of the IGF-1 gene produces multiple
heterogeneous IGF-1 mRNA transcripts, such as the mRNA isoforms
IGF-1Ea, IGF-1Eb and IGF-1Ec. The translation of these mRNA
isoforms produces various IGF-1 peptides (45,46).
IGF-1 isoforms are associated with various gynecological conditions
and pathologies, such as endometrial carcinoma, endometriosis and
leiomyomas (46–49). However, there is a lack of
knowledge regarding the effects of the IGF-1 isoforms, particularly
IGF-1Ea peptide, on the growth of the human placenta and the
remodeling of maternal uterine arteries by the extravillous
trophoblast. In addition, expression profile of IGF-1Ea peptide
within the endothelium of villous and endometrial blood vessels on
the maternal side of the maternal-fetal interface remains unknown,
as well as the autocrine effects of IGF-1Ea peptide in human
placenta. Therefore, the aim of the present study was to
investigate the role of IGF-1Ea expression in the placenta from
pregnancies complicated by IUGR and uneventful pregnancies.
Additionally, placental IGF-1Ea expression in IUGR pregnancies
compared with appropriate-for-gestational-age (AGA) pregnancies
were examined in connection with clinical and histopathological
parameters to improve understanding of the pathophysiology of IUGR
pregnancy.
Materials and methods
Patient population
A total of 62 placentas (15 AGA and 47 IUGR) were
obtained from patients with singleton AGA pregnancies or
pregnancies complicated by IUGR, delivered by either vaginal labor
or cesarean section at the General Maternity Hospital of Athens
‘Elena Venizelou’ (Athens, Greece). The age range of patients was
16–46 years-old and the gestational age was 26–41 weeks. Informed
written consent to participate was obtained from all patients and
the study was approved by the Scientific Committee of the General
Maternity Hospital of Athens ‘Elena Venizelou’, Athens, Greece
(approval no. 2nd Scientific Committee Meeting/8th
agenda/23-1-2018) and the Research and Bioethics Committee of the
Medical School of the National and Kapodistrian University of
Athens, Athens, Greece (approval no. 1718016683). The inclusion
criteria for the IUGR group included pregnant patients with a fetal
weight <5th percentile for gestational age and either of the
following, diagnosed via antenatal ultrasound: i) Abnormal
umbilical artery Doppler waveform with absent or reverse
end-diastolic flow velocity during pregnancy; ii) oligohydramnios,
defined as a deepest fluid pocket of ≤2 cm or an amniotic fluid
index ≤5 cm via antenatal ultrasound performed prior to delivery or
iii) asymmetric growth of the fetus with increased head to
abdominal circumference ratio >1.2. IUGR pregnancies associated
with pre-eclampsia were also included. The diagnosis of
preeclampsia was based on hypertension during pregnancy (a systolic
blood pressure ≥140 mmHg and/or a diastolic blood pressure of ≥90
mmHg) in a previously normotensive patient and proteinuria (≥300 mg
protein in a 24-h urine collection). The control patients had AGA
pregnancies without complications and delivered healthy neonates
with birth weights from the 5 to 90th percentile. All control
placentas were selected from full-term pregnancies (>37 weeks of
gestation) and were grossly normal. The exclusion criteria for both
AGA and IUGR pregnancies were abnormal oral glucose tolerance
screening test results between 24 and 28 weeks of pregnancy, use of
nutritional supplements during pregnancy, maternal hormonal
treatment, smoking or use of recreational drugs, pre-existing
maternal hypertension, liver, cardiovascular or kidney disease,
endocrinological disorders, multiple pregnancies, chorioamnionitis,
placental abruption, prolonged rupture of membranes, chromosomal
abnormality, fetal anatomical defects and intrauterine viral
infection. The appropriate gestational age was estimated using the
date of the last menstrual period and confirmed by the crown-rump
length of the fetus in the first trimester scan. The baseline
maternal and fetal demographic characteristics [including maternal
age and body mass index (BMI), gestational age, neonatal and
placental weight and fetal sex] were recorded.
Tissue sampling
The sample collection was performed from February
2018 to August 2021. All placentas were obtained <15 min after
labor and were weighed after the removal of the umbilical cord and
fetal membranes. For mRNA isolation, fresh tissue samples were
obtained from 28 placentas, excised from the medial areas, ~5 cm
apart from the insertion of the umbilical cord and excluding the
peripheral margins. These samples included villous parenchyma from
the decidua basalis to the fetal surface, avoiding areas with gross
calcification, infarcts, marked fibrin deposition and intervillous
thrombi. The samples were cut into small pieces, washed in 0.9% PBS
to remove blood contaminants and then snap-frozen and stored at
−80°C until RNA extraction. The rest of the placenta was fixed in
10% buffered formalin at room temperature for one week, for
histopathological and immunohistochemical examination.
Histopathology
Formalin-fixed paraffin-embedded sections were
obtained using an automated tissue processor (Donatello, Diapath)
and then stained with ready to use Hematoxylin H (Biognost) for 5
min and counterstained with Eosin Y 1% alcoholic (Biognost) for 10
sec, at room temperature.
The gross and histological description of the
placental lesions followed the Amsterdam criteria (50). The placentas were evaluated for the
following major histological patterns: Changes consistent with i)
maternal vascular malperfusion of the placental bed (MVM); ii)
fetal vascular malperfusion (FVM); iii) massive perivillous
fibrin/fibrinoid deposition (MPFD); iv) acute or chronic
inflammatory lesions, including chronic villitis of unknown
etiology (VUE) and v) delayed villous maturation (DVM). Other
miscellaneous lesions were also recorded.
RNA isolation and cDNA synthesis
The frozen placental samples were from 13 normal and
15 IUGR third trimester pregnancies. Total RNA was extracted from
frozen tissue samples using TRItidy (TRitidy G™ reagent; PanReac
AppliChem GmbH) according to the manufacturer's instructions. The
frozen placental tissues were cut into segments (12×8 mm) and
homogenized in 0.5 ml TRItidy G. Following addition of 500 µl
isopropanol, the resulting mixture was centrifuged (13,226 × g for
15 min at room temperature. The pellet containing the total RNA was
washed once in 75% ethanol and dissolved in 20 µl
diethylpyrocarbonate-treated water. Reverse transcription (RT) was
performed using the ProtoscriptR II First Strand cDNA synthesis kit
according to the manufacturer's instructions (New England BioLabs;
cat. no. NE, E6560L).
Quantitative PCR (qPCR)
Oligonucleotide sequences were as follows: IGF-1Ea
Forward, 5′-GTGGAGACAGGGGCTTTTATTTC-3′ and IGF-1Ea Reverse,
5′-CTTGTTTCCTGCACTCCCTCTACT-3′ generating a 251 bp product. This
set of primers was designed to lie within different exons of the
IGF-1 gene to detect and amplify only the IGF-1Ea transcript; 5′
sense primer is on exon 4, while the antisense primer is on the
exon 6. 18S ribosomal RNA was used as the housekeeping gene (sense,
5′-GGCCCTGTAATTGGAATGAGTC-3′ and antisense,
5′-CCAAGATCCAACTACGAGCTT-3′). qPCR was performed using a Thermal
Cycler (Bio-Rad iCycler Thermal Cycler IQ5 Multicolor Real-Time PCR
Detection System; Bio-Rad Laboratories, Inc.). The reaction was
conducted using 12.5 µl iQTM SYBR Green Supermix (Bio-Rad
Laboratories, Inc.), 50 ng cDNA and 0.4 µM each primer, adjusted to
a 20 µl total volume with ddH2O. A no template control
was performed in each plate to verify the absence of extraneous
nucleic acid contamination. The thermocycling conditions were as
follows: Initial denaturation at 95°C for 4 min, followed by 45
cycles of 12 sec at 95°C, 30 sec at 61°C and 30 sec at 72°C and
final extension at 72°C for 5 min. 2-ΔΔCt formula was
used to calculate the fold-differences between the IUGR and normal
pregnancy samples using 18S ribosomal RNA as the internal control
(51). All the samples were
analyzed in duplicate.
Immunohistochemistry
A total of 15 AGA and 47 IUGR
formalin-fixed-paraffin-embedded placental samples were used for
immunohistochemical analysis using EnVision FLEX+, Mouse, High pH
(Link) cat. no. K8002, Dako, Agilent Technologies. The 4 µm-thick
microtome sections were dried at 37°C overnight, de-waxed in xylene
and rehydrated in serial dilutions of ethanol. Antigen retrieval
was performed by heating at 97°C in a PT module immersed in DAKO
high ph solution (pH 9) for 20 min and cooling at room temperature.
Endogenous peroxidase activity was blocked with 3% hydrogen
peroxide (provided in the kit) for 5 min in a dark place at room
temperature, followed by two washes with distilled water and then
DAKO Wash buffer. The sections were then incubated with polyclonal
anti-Rabbit IGF-1Eα antiserum (gifted by Dr Elisabeth Barton,
University of Pennsylvania, PA, USA) at a dilution of 1:700 in Dako
Diluent and incubated for 24 h at 4°C. After rinsing in Wash
buffer, the sections were incubated with EnVision FLEX+Rabbit
(LINKER) cat. no. K8009; Dako; Agilent Technologies, Inc.) for 15
min at room temperature, followed by two rinses in Wash buffer,
followed by incubating slides in polymer Envision for 30 min and
rinsed twice in Wash buffer. Visualization of the immunocomplex was
performed by incubating the sections in DAB for 10 min (Dako;
Agilent Technologies, Inc.). The sections were stained in
hematoxylin for 5 min at room temperature, washed in distilled
water, dehydrated in serial dilutions of ethanol and xylene and
mounted in dibutyl phthalate xylate.
Adrenal carcinoma was used as a positive control for
IGF-1Ea immunostaining (obtained from archival surgical pathology
material at Aretaieion University Hospital; Medical School of the
National and Kapodistrian University of Athens). Negative controls
were incubated without the primary antibody.
Immunohistochemical evaluation was performed by
examining the tissue sections under a light microscope at 100×
magnification for the initial screening. For each specimen, 5
optical fields at 100× magnification were randomly selected and
semi-quantitative measurements were performed using a slide grid
under ×200 magnification. Measurements were simultaneously assessed
by two qualified pathologists and consensus results were recorded
for each case.
Immunostaining in the perivillous
trophoblast
Semi-quantitative scoring of IGF-1Ea immunolabeling
in the perivillous syncytiotrophoblast was used; cases were
classified into three groups according to the percentage (scores)
of positively stained perivillous syncytial areas as follows: 0,
0–10% immunopositive syncytial areas (negative); 1, 11–50%
immunopositive syncytial areas (moderate); 2, >51%
immunopositive syncytial areas (high). The staining intensity in
the perivillous syncytiotrophoblast was defined using a
semiquantitative scale: 0, negative (no staining); 1, weak; 2,
moderate and 3, strong.
Iimmunostaining in the extravillous
trophoblast
Immunostaining in the extravillous trophoblastic
cells was defined as negative (no expression) or positive (any
positive cells identified). The staining intensity in the
extravillous trophoblastic cells was graded as follows: 0, negative
(no staining); 1 weak; 2, moderate and 3, strong.
Immunostaining in the vascular
endothelium
Immunostaining in the endothelium of the maternal
decidual vessels was classified as negative (no staining) or
positive (staining observed in one maternal vessel included in the
basal plate). Similarly, immunostaining in the endothelium of the
villous fetal blood vessels was classified as negative (no
staining) or positive (staining observed in one fetal blood
vessel).
Statistical analysis
Data are presented as the mean of two independent
experimental repeats ± SD. The associations between categorical
variables were assessed using exact Pearson's χ2 or
Fisher's exact test. For continuous variables, the differences were
assessed using the Mann-Whitney U test. A two-tailed P<0.05 was
considered to indicate a statistically significant difference. Data
were analyzed using SPSS version 28.0 (IBM Corp).
Results
Clinical and pathological
findings
A total of 62 pregnant patients were recruited.
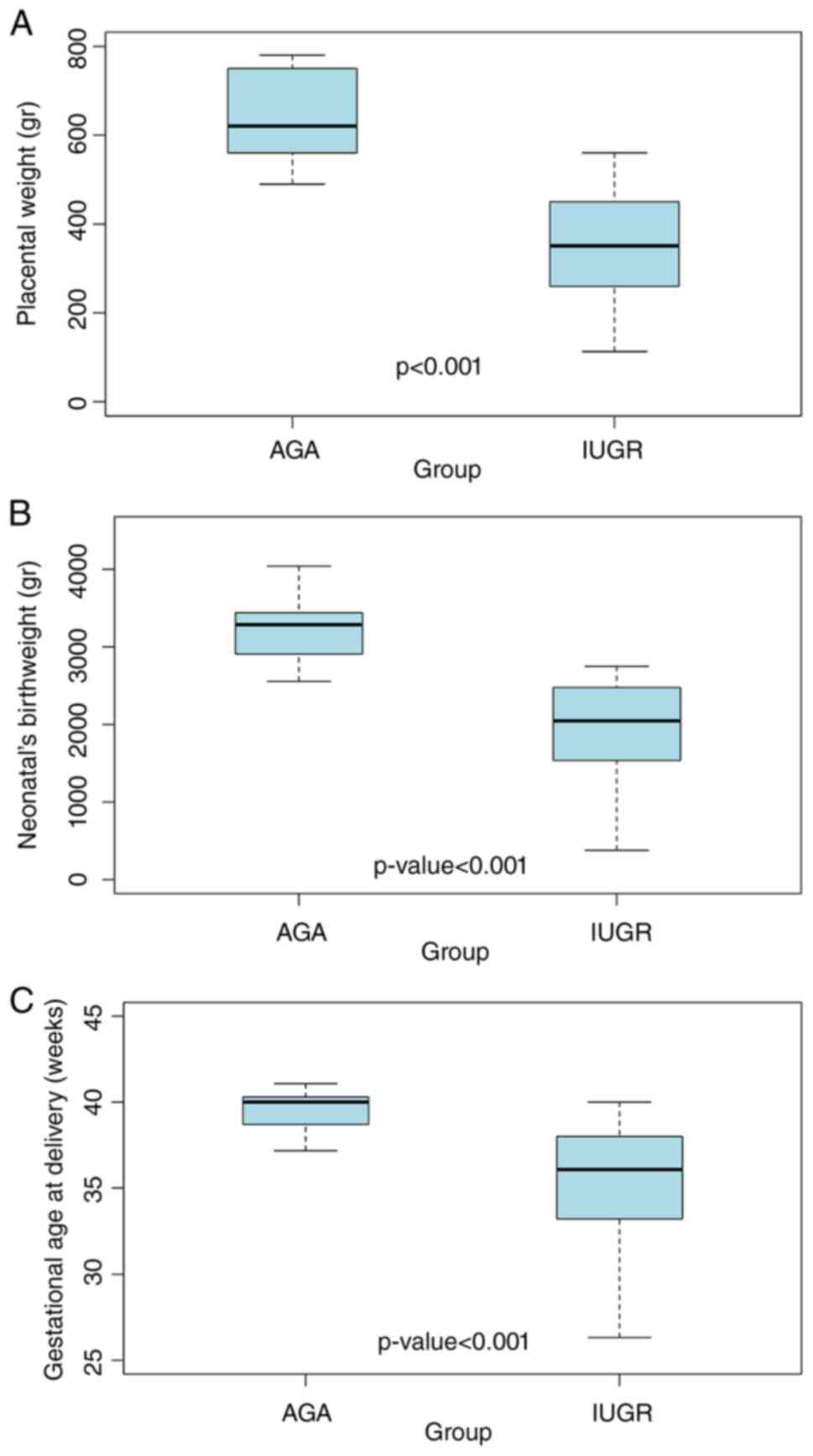
There were significant differences in the mean placental and
neonatal birth weight and the gestational age at delivery (all
P<0.001) with the IUGR group exhibiting lower values compared
with AGA pregnancies (Table I;
Fig. 1). Notably, preterm birth,
defined as gestational age at delivery of <37 weeks, was
observed only in the IUGR group (100%; Table II). All cases in the AGA group and
21 cases in the IUGR group gave birth at a gestational age of ≥37
weeks of pregnancy (Table II).
The fetal sex distribution was 15 male (34.9%) and 28 female
(65.1%; n=43; unreported in 19 IUGR cases). In the IUGR group,
fetal sex distribution was 53.3% male and 71.4% females, with a
male to female ratio of 0.746, while in the AGA group there were
46.7% male and 28.60% female fetuses with a male to female ratio of
1.633, (Table II).
 | Table I.Clinical characteristics of the study
participants. |
Table I.
Clinical characteristics of the study
participants.
| Characteristic | Group | n | Mean | Median | SD | Minimum | Maximum | P-value |
|---|
| Gestational age,
weeks | AGA | 15 | 39.5 | 40.0 | 1.40 | 37.2 | 41.1 | <0.001 |
|
| IUGR | 47 | 35.2 | 36.1 | 3.69 | 26.3 | 40.0 |
|
|
| Total | 62 | 36.2 | 37.3 | 3.76 | 26.3 | 41.1 |
|
| Maternal age,
years | AGA | 15 | 29.1 | 31.0 | 6.16 | 16.0 | 42.0 | 0.223 |
|
| IUGR | 43 | 32.0 | 31.0 | 5.92 | 22.0 | 46.0 |
|
|
| Total | 58 | 31.3 | 31.0 | 6.06 | 16.0 | 46.0 |
|
| Placental weight,
g | AGA | 15 | 644.0 | 620.0 | 103.01 | 490.0 | 780.0 | <0.001 |
|
| IUGR | 47 | 350.4 | 351.0 | 116.41 | 113.0 | 560.0 |
|
|
| Total | 62 | 421.4 | 423.5 | 169.48 | 113.0 | 780.0 |
|
| Neonate weight,
g | AGA | 15 | 3,219.7 | 3,290.0 | 447.12 | 2,555.0 | 4,040.0 | <0.001 |
|
| IUGR | 39 | 1,908.5 | 2,050.0 | 686.03 | 380.0 | 2,750.0 |
|
|
| Total | 54 | 2,272.7 | 2,380.0 | 861.19 | 380.0 | 4,040.0 |
|
| BMI,
kg/m2 | AGA | 15 | 28.0 | 27.3 | 3.06 | 22.7 | 34.7 | 0.678 |
|
| IUGR | 17 | 28.6 | 29.5 | 5.14 | 19.8 | 38.1 |
|
|
| Total | 32 | 28.3 | 29.1 | 4.24 | 19.8 | 38.1 |
|
 | Table II.Qualitative clinical characteristics
of study participants. |
Table II.
Qualitative clinical characteristics
of study participants.
|
| Group |
|
|---|
|
|
|
|
|---|
| Characteristic | Control (%) | IUGR (%) | P-value |
|---|
| Age, years |
|
|
|
|
<40 | 14 (27.5) | 37 (72.5) | 0.664 |
|
≥40 | 1 (14.3) | 6 (85.7) |
|
| Gestational age,
weeks |
|
|
|
|
<37 | 0 (0.0) | 26 (100.0) | 0.001 |
|
>37 | 15 (41.7) | 21 (58.3) |
|
| Neonate weight,
g |
|
|
|
|
<2,500 | 0 (0.0) | 29 (100.0) | <0.001 |
|
≥2,500 | 15 (60.0) | 10 (40.0) |
|
| Placenta weight,
g |
|
|
|
|
<400 | 0 (0.0) | 31 (100.0) | <0.001 |
|
≥400 | 15 (48.4) | 16 (51.6) |
|
| BMI,
kg/m2 |
|
|
|
|
<30 | 12 (52.2) | 11 (47.8) | 0.337 |
|
>30 | 3 (33.3) | 6 (66.7) |
|
| Fetal sex |
|
|
|
|
Male | 7 (46.7) | 8 (53.3) | 0.235 |
|
Female | 8 (28.6) | 20 (71.4) |
|
IUGR pregnancies were highly represented in the
group of placentas with changes of MVM (83.3%), VUE (83.3%) and DVM
(71.4%), though without any statistically significant differences
when compared with the AGA group (Table III). Notably placentas from IUGR
pregnancies showed histological changes consistent with MVM in
74.5% of cases, VUE in 10.6% and DVM in 10.6%. No differences could
be statistically confirmed between the IUGR and AGA groups for any
of the histopathological characteristics examined in our
sample.
 | Table III.Histopathological
characteristics. |
Table III.
Histopathological
characteristics.
|
| Group (%) |
|
|---|
|
|
|
|
|---|
| Characteristic | AGA | IUGR | P-value |
|---|
| MVM | 12 (25.5) | 35 (74.5) | >0.999 |
| MPFD | 0 (0.0) | 4 (100.0) | 0.564 |
| FVM | 0 (0.0) | 1 (100.0) | >0.999 |
| VUE | 1 (16.7) | 5 (83.3) | >0.999 |
| DVM | 2 (28.6) | 5 (71.4) | 0.774 |
| Othera | 0 (0.0) | 4 (100.0) | 0.564 |
Placental IGF-1Ea mRNA expression
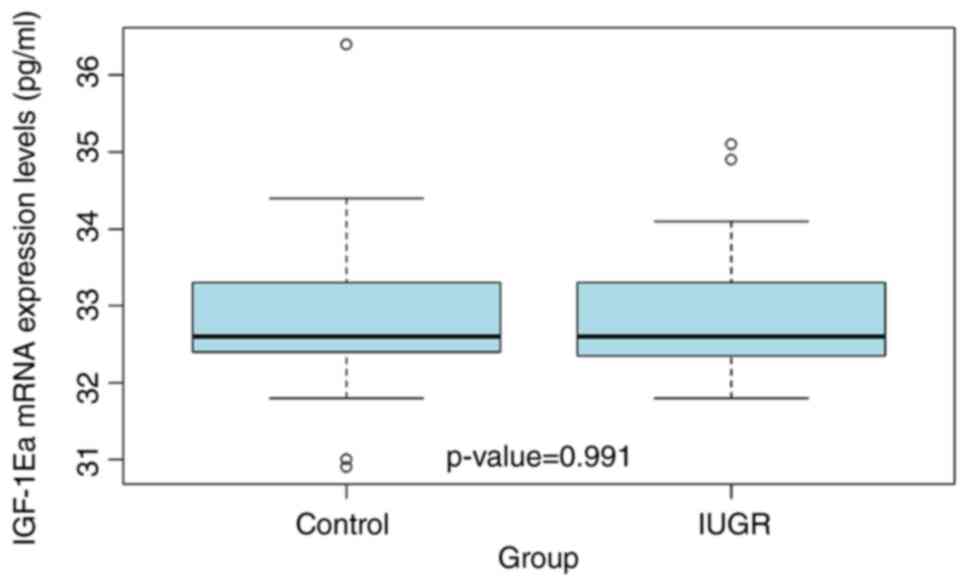
The placental IGF-1Ea mRNA expression levels were
determined by qPCR. The mean IGF-1Ea mRNA expression levels were
similar between the IUGR and AGA groups, with no significant
difference (P=0.991; Fig. 2).
Additionally, no significant associations were observed in the
placental IGF-1Ea mRNA expression between the IUGR and AGA
pregnancies in relation to the clinical parameters such as maternal
age and BMI, gestational age at delivery, fetal sex, neonatal and
placental weight at delivery or the histopathological parameters
such as MVM, MPFD, FVM, VUE, DVM and other lesions (Table IV). Furthermore, there was no
significant difference in placental IGF-1Ea mRNA expression in IUGR
vs. AGA pregnancies in the full-term cases (>37 weeks of
gestation; Table IV). Moreover,
placental IGF-1Ea mRNA expression in IUGR vs. the AGA pregnancies
was not significant in cases with fetal birth weight >2,500 g
(Table IV). Finally, placental
IGF-1Ea mRNA expression levels were similar between the IUGR and
AGA groups in cases with placental weight >400 g, with no
significant difference (Table
IV).
 | Table IV.Expression levels of IGF-1Ea mRNA in
placentas according to clinicopathological parameters. |
Table IV.
Expression levels of IGF-1Ea mRNA in
placentas according to clinicopathological parameters.
|
| Median IGF-1Ea mRNA
expression (range) |
|
|---|
|
|
|
|
|---|
| Group | AGA | IUGR | P-value |
|---|
| Total | 32.6 (30.9,
36.4) | 32.6 (31.8,
35.1) | 0.991 |
| Age, years |
|
|
|
|
<40 | 32.6 (30.9,
36.4) | 32.6 (31.8,
35.1) | 0.862 |
|
≥40 | 34.4 (34.4,
34.4) | 33.0 (32.3,
33.6) | 0.221 |
| Gestational age,
weeks |
|
|
|
|
<37 | - | 32.5 (32.3,
34.9) | - |
|
≥37 | 32.6 (30.9,
36.4) | 32.7 (31.8,
35.1) | 0.961 |
| Neonate weight,
g |
|
|
|
|
<2,500 | - | 32.5 (32.0,
34.9) | - |
|
≥2,500 | 32.6 (30.9,
36.4) | 32.7 (31.8,
35.1) | 0.759 |
| Placenta weight,
g |
|
|
|
|
<400 | - | 33.1 (32.6,
33.6) | - |
|
≥400 | 32.6 (30.9,
36.4) | 32.5 (31.8,
35.1) | 0.870 |
| BMI,
kg/m2 |
|
|
|
|
<30 | 32.6 (30.9,
36.4) | 32.7 (31.8,
35.1) | 0.570 |
|
≥30 | 33.3 (32.5,
34.4) | 32.5 (32.3,
34.1) | 0.230 |
| Fetal sex, % |
|
|
|
|
Male | 32.4 (31.1,
34.4) | 33.3 (32.3,
35.1) | 0.249 |
|
Female | 32.8 (30.9,
36.4) | 32.5 (31.8,
34.1) | 0.284 |
| Histopathology,
% |
|
|
|
|
MVM | 32.7 (30.9,
36.4) | 32.6 (31.8,
35.1) | 0.939 |
|
VUE | 33.3 (33.3,
33.3) | - | - |
|
DVM | 32.6 (32.6,
32.6) | 32.5 (32.5,
32.5) | 0.333 |
|
Other | - | 33.0 (33.0,
33.0) | - |
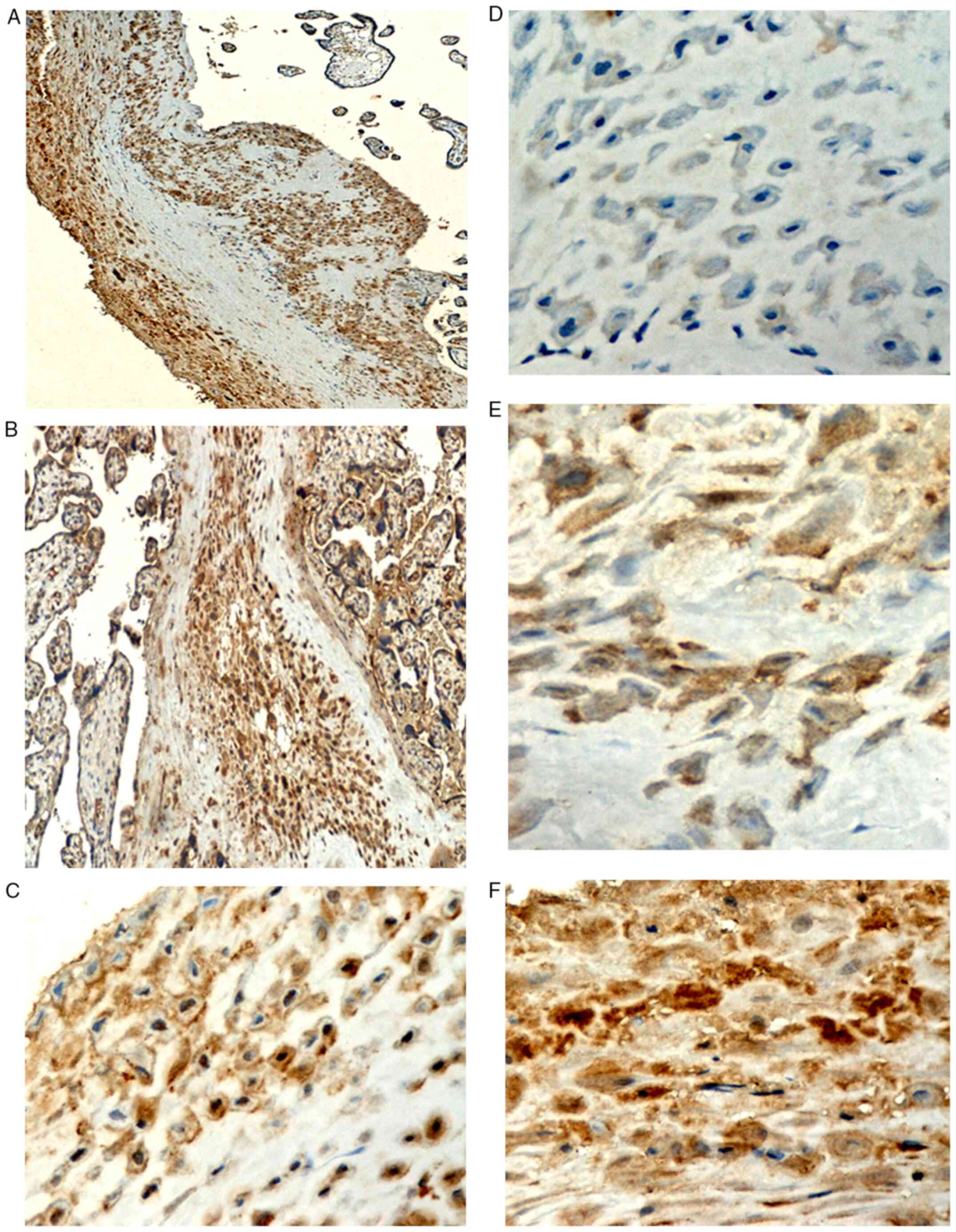
Immunostaining localization
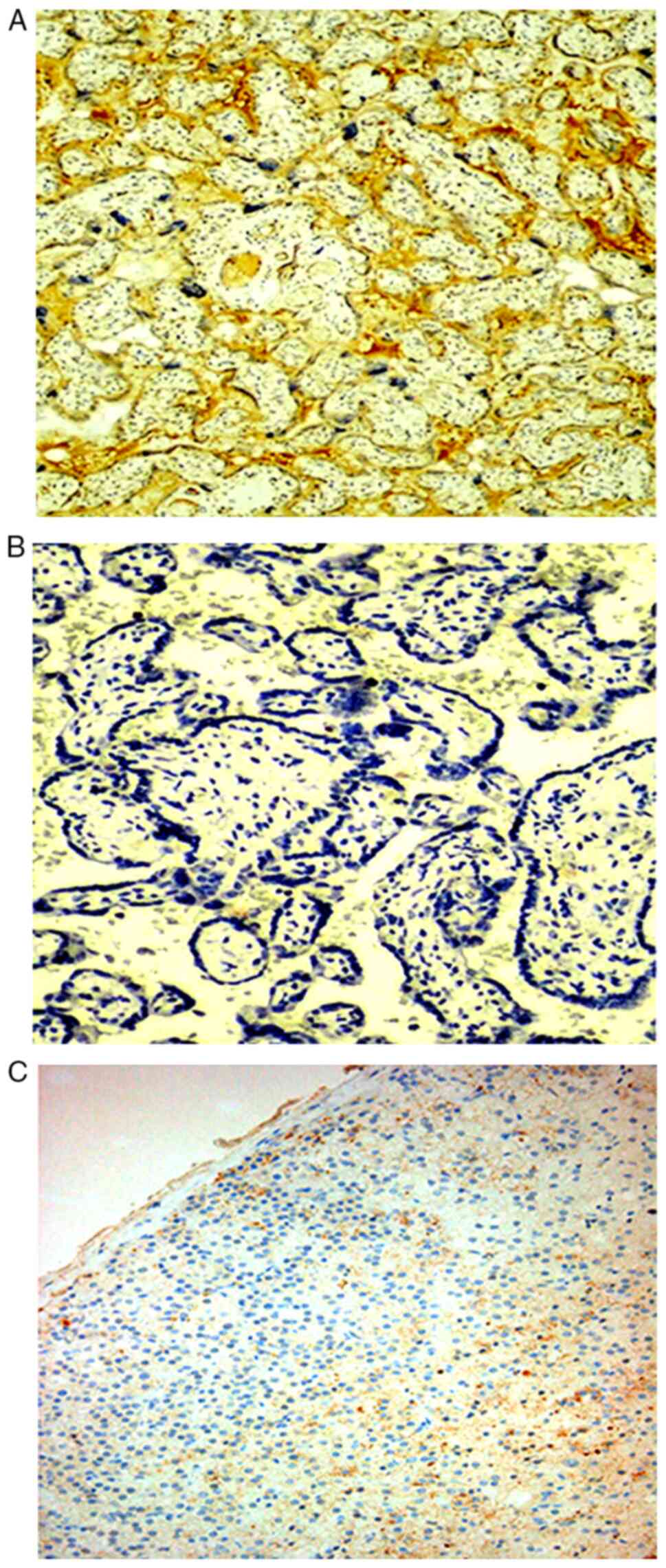
Positive expression of IGF-1Ea protein was observed
as granular or homogeneous brown staining localized to the
cytoplasm and the cytoplasmic membrane of the perivillous
syncytiotrophoblast (Figs. 3 and
4) and the extravillous
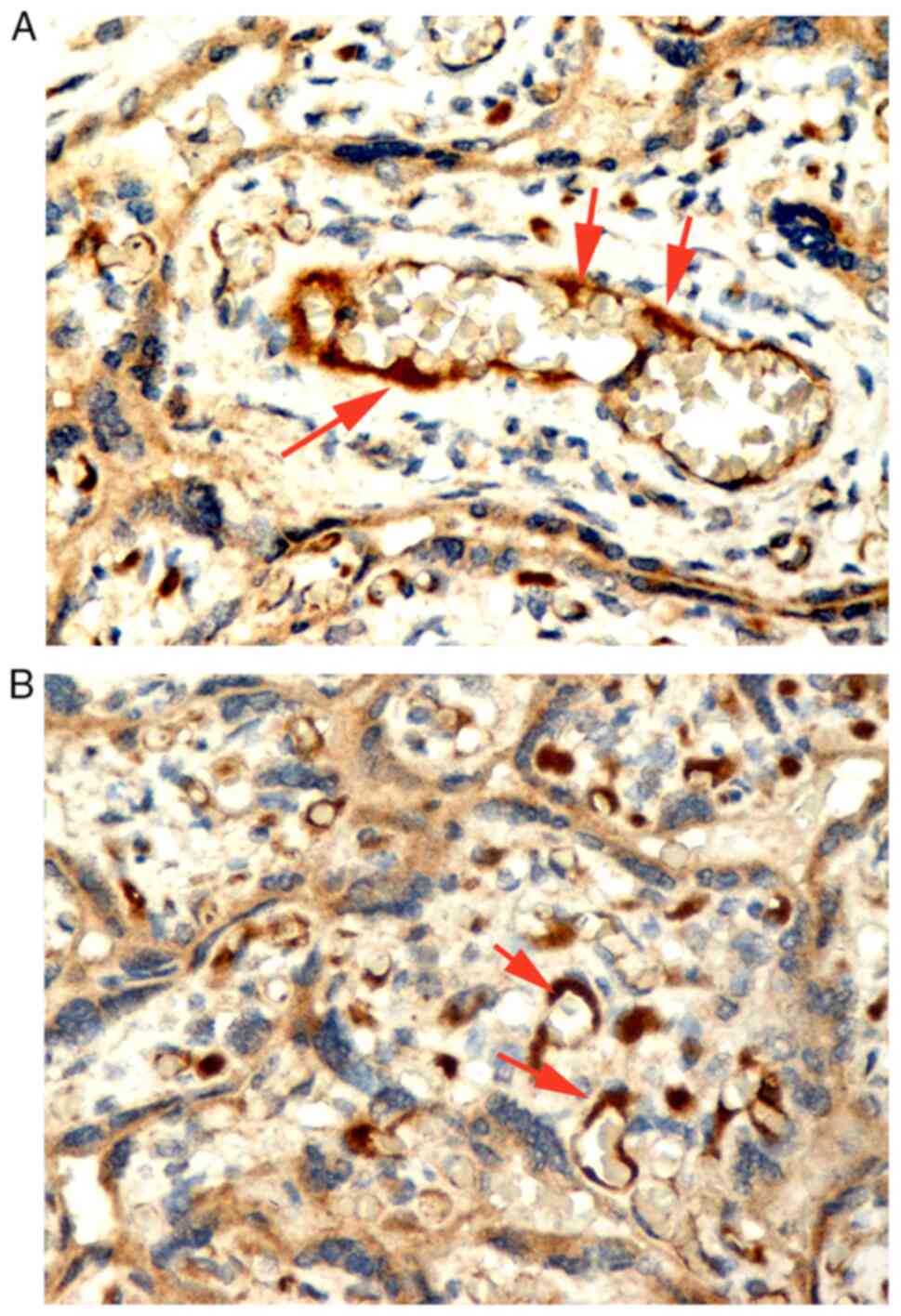
trophoblast of the basal plate and diaphragmatic columns (Fig. 5). Positive IGF-1Ea expression was
also detected in the endothelium of the fetal vessels within the
stem, intermediate and distal chorionic villi, as well as in the
endothelium of the maternal decidual vessels (Fig. 6).
IGF-1Ea isoform expression in
perivillous syncytiotrophoblast of human placentas
Of the 47 IUGR pregnancies, six (12.8%) exhibited
high expression, 15 cases (31.9%) exhibited moderate expression and
26 (55.3%) had negative IGF-1Ea expression in the perivillous
syncytiotrophoblast. Of 15 AGA pregnancies, one case (6.75%)
exhibited high expression, two (13.3%) exhibited moderate
expression and 12 cases (80%) had negative expression in the
perivillous syncytiotrophoblast. These immunohistochemical IGF-1Ea
placental expression scores were not significantly different
between the IUGR and AGA pregnancies (Fig. 7A; Table V). Additionally,
immunohistochemical IGF-1Ea expression scores of the IUGR and AGA
pregnancies were not significantly associated with the clinical
parameters such as maternal age, neonatal (>2,500 g) and
placental weight (>400 g), maternal BMI, fetal sex and the
histopathological parameters including MVM, VUE and DVM (Table V).
 | Table V.Scores of immunohistochemical IGF-1Ea
placental expression in perivillous syncytiotrophoblasts. |
Table V.
Scores of immunohistochemical IGF-1Ea
placental expression in perivillous syncytiotrophoblasts.
|
| AGA (%) | IUGR (%) |
|
|---|
|
|
|
|
|
|---|
| Group | Negative | Moderate | High | Negative | Moderate | High | P-value |
|---|
| Total | 12 (80.0) | 2 (13.3) | 1 (6.7) | 26 (55.3) | 15 (31.9) | 6 (12.8) | 0.231 |
| Age, years |
|
|
|
|
|
|
|
|
<40 | 11 (78.6) | 2 (14.3) | 1 (7.1) | 22 (59.5) | 10 (27.0) | 5 (13.5) | 0.444 |
|
≥40 | 1 (100.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 5 (83.3) | 0 (0.0) | >0.999 |
| Gestational age,
weeks |
|
|
|
|
|
|
|
|
<37 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 13 (50.0) | 9 (34.6) | 4 (15.4) | - |
|
≥37 | 12 (80.0) | 2 (13.3) | 1 (6.7) | 13 (61.9) | 6 (28.6) | 2 (9.5) | 0.567 |
| Neonate weight,
g |
|
|
|
|
|
|
|
|
<2,500 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 11 (37.9) | 13 (44.8) | 5 (17.2) | - |
|
≥2,500 | 12 (80.0) | 2 (13.3) | 1 (6.7) | 7 (70.0) | 2 (20.0) | 1 (10.0) | >0.999 |
| Placenta weight,
g |
|
|
|
|
|
|
|
|
<400 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 15 (48.4) | 10 (32.3) | 6 (19.4) | - |
|
≥400 | 12 (80.0) | 2 (13.3) | 1 (6.7) | 11 (68.8) | 5 (31.2) | 0 (0.0) | 0.395 |
| BMI,
kg/m2 |
|
|
|
|
|
|
|
|
<30 | 10 (83.3) | 1 (8.3) | 1 (8.3) | 7 (63.6) | 4 (36.4) | 0 (0.0) | 0.193 |
|
≥30 | 2 (66.7) | 1 (33.3) | 0 (0.0) | 3 (50.0) | 3 (50.0) | 0 (0.0) | 0.635 |
| Fetal sex, % |
|
|
|
|
|
|
|
|
Male | 6 (85.7) | 1 (14.3) | 0 (0.0) | 3 (37.5) | 3 (37.5) | 2 (25.0) | 0.139 |
|
Female | 6 (75.0) | 1 (12.5) | 1 (12.5) | 10 (50.0) | 8 (40.0) | 2 (10.0) | 0.367 |
| Histopathology,
% |
|
|
|
|
|
|
|
|
MVM | 10 (83.3) | 2 (16.7) | 0 (0.0) | 19 (54.3) | 12 (34.3) | 4 (11.4) | 0.172 |
|
MPFD | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (50.0) | 1 (25.0) | 1 (25.0) | - |
|
FVM | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | - |
|
VUE | 1 (100.0) | 0 (0.0) | 0 (0.0) | 2 (40.0) | 3 (60.0) | 0 (0.0) | 0.273 |
|
DVM | 1 (50.0) | 0 (0.0) | 1 (50.0) | 4 (80.0) | 0 (0.0) | 1 (20.0) | 0.427 |
|
Other | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 2 (50.0) | 1 (25.0) | - |
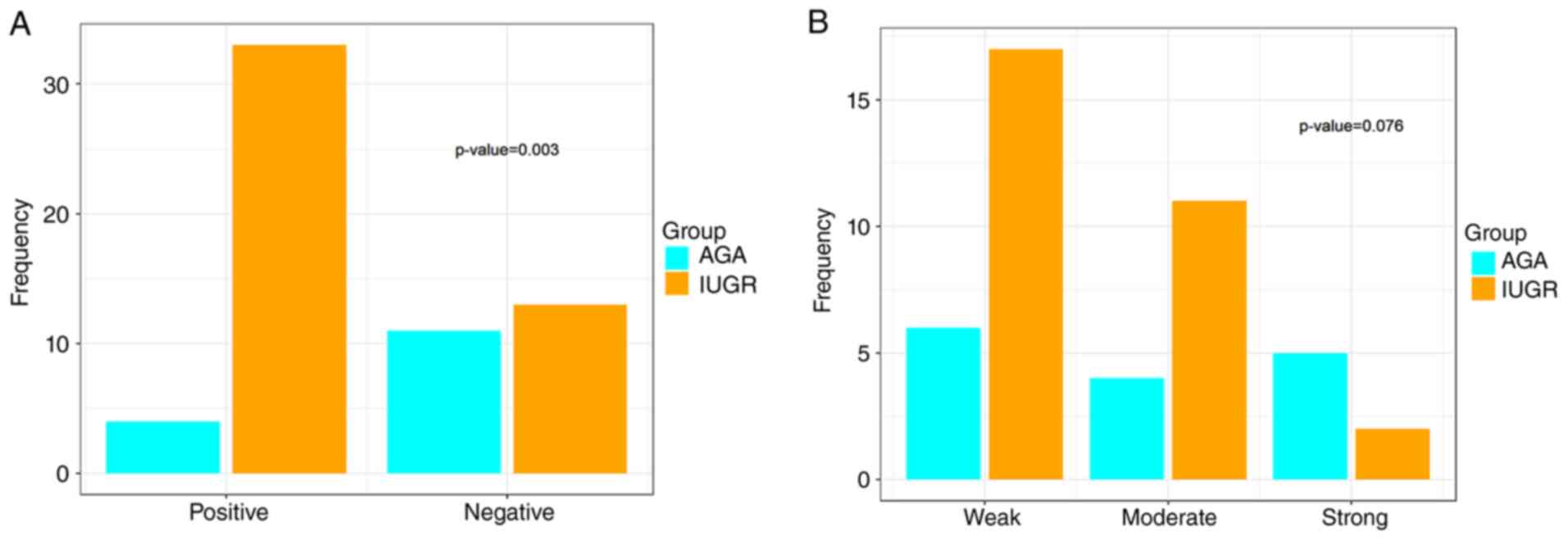
In the IUGR group, one case (2.1%) exhibited strong
IGF-1Ea intensity, nine (19.1%) exhibited moderate intensity, while
37 cases (78.7%) exhibited weak intensity. In the AGA group, no
cases exhibited strong intensity, four (26.7%) exhibited moderate
intensity, while 11 (73.3%) exhibited weak expression. The
difference in IGF-1Ea expression intensity was not significantly
different between the two groups (Fig.
7B; Table VI). Additionally,
intensity of the IGF-1Ea immunopositivity was not significantly
associated with maternal age and gestational age (>37 weeks),
neonatal birth weight (>2,500 g), placental weight (>400 g),
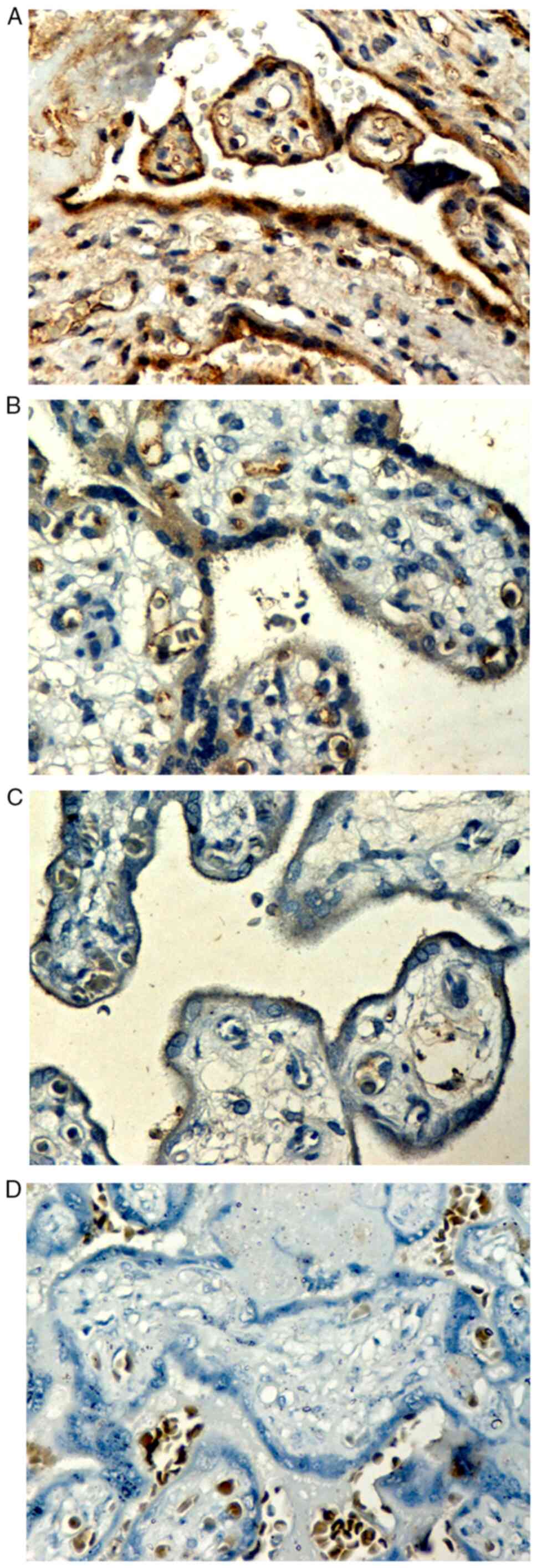
maternal BMI, fetal sex, MVM and DVM (Table VI). Figs. 3 and 4 showIGF-1Ea immunopositive expression in
the perivillous syncytiotrophoblast, respectively.
 | Table VI.Intensity of IGF-1Ea
immunohistochemical expression in perivillous syncytiotrophoblast
from AGA and IUGR human placentas from third trimester
pregnancies. |
Table VI.
Intensity of IGF-1Ea
immunohistochemical expression in perivillous syncytiotrophoblast
from AGA and IUGR human placentas from third trimester
pregnancies.
|
| AGA (%) | IUGR (%) |
|
|---|
|
|
|
|
|
|---|
| Group | Low | Moderate | Strong | Low | Moderate | Strong | P-value |
|---|
| Total | 11 (73.3) | 4 (26.7) | 0 (0.0) | 37 (78.7) | 9 (19.1) | 1 (2.1) | 0.786 |
| Age, years |
|
|
|
|
|
|
|
|
<40 | 10 (71.4) | 4 (28.6) | 0 (0.0) | 29 (78.4) | 7 (18.9) | 1 (2.4) | 0.644 |
|
≥40 | 1 (100.0) | 0 (0.0) | 0 (0.0) | 5 (83.3) | 1 (16.7) | 0 (0.0) | >0.999 |
| Gestational age,
weeks |
|
|
|
|
|
|
|
|
<37 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 19 (73.1) | 6 (23.1) | 1 (3.8) | - |
|
≥37 | 11 (73.3) | 4 (26.7) | 0 (0.0) | 18 (85.7) | 3 (14.3) | 0 (0.0) | 0.418 |
| Neonate weight,
g |
|
|
|
|
|
|
|
|
<2,500 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 21 (72.4) | 7 (24.1) | 1 (3.4) | - |
|
≥2,500 | 11 (73.3) | 4 (26.7) | 0 (0.0) | 8 (80.0) | 2 (20.0) | 0 (0.0) | >0.999 |
| Placenta weight,
g |
|
|
|
|
|
|
|
|
<400 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 24 (77.4) | 6 (19.4) | 1 (3.2) | - |
|
≥400 | 11 (73.3) | 4 (26.7) | 0 (0.0) | 13 (81.2) | 3 (18.8) | 0 (0.0) | 0.685 |
| BMI,
kg/m2 |
|
|
|
|
|
|
|
|
<30 | 9 (75.0) | 3 (25.0) | 0 (0.0) | 9 (81.8) | 2 (18.2) | 0 (0.0) | 0.692 |
|
≥30 | 2 (66.7) | 1 (33.3) | 0 (0.0) | 5 (83.3) | 1 (16.7) | 0 (0.0) | 0.571 |
| Fetal sex, % |
|
|
|
|
|
|
|
|
Male | 6 (85.7) | 1 (14.3) | 0 (0.0) | 6 (75.0) | 2 (25.0) | 0 (0.0) | 0.605 |
|
Female | 5 (62.5) | 3 (37.5) | 0 (0.0) | 15 (75.0) | 5 (25.0) | 0 (0.0) | 0.508 |
| Histopathology,
% |
|
|
|
|
|
|
|
|
MVM | 9 (75.0) | 3 (25.0) | 0 (0.0) | 27 (77.1) | 8 (22.9) | 0 (0.0) | >0.999 |
|
MPFD | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (75.0) | 1 (25.0) | 0 (0.0) | - |
|
FVM | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | - |
|
VUE | 1 (100.0) | 0 (0.0) | 0 (0.0) | 5 (100.0) | 0 (0.0) | 0 (0.0) | - |
|
DVM | 1 (50.0) | 1 (50.0) | 0 (0.0) | 4 (80.0) | 1 (20.0) | 0 (0.0) | 0.427 |
|
Other | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (75.0) | 0 (0.0) | 1 (25.0) | - |
IGF-1Ea isoform expression in
extravillous trophoblastic cells of human placenta
The positive immunohistochemical IGF-1Ea staining in
the extravillous trophoblast was significantly different between
AGA and IUGR placentas (Fig. 8A;
Table VII). In the AGA
placentas, IGF-1Ea immunopositive cells were observed in 73.3% of
cases, while in the IUGR placentas, the IGF-1Ea immunopositivity
was much lower (Fig. 8A; Table VII). Additionally, there was a
significant difference in the IGF-1Ea immunopositive cells in the
extravillous trophoblast according to maternal age <40 years.
Notably, patients <40 years in the IUGR group had negative
IGF-1Ea immunohistochemical staining in the extravillous
trophoblast in most cases; for the AGA group, positive staining was
observed in most cases. A significant difference in IGF-1Ea
immunopositive cells in the extravillous trophoblast was found
between the IUGR and AGA groups in relation to gestational age.
Notably, patients with a gestational age of 37 weeks in the IUGR
group had negative IGF-1Ea immunohistochemical staining in the
extravillous trophoblast in most cases; for AGA group,
immunopositive cells were observed in most cases. Moreover, there
was a significant difference in the IGF-1Ea immunopositive cells in
the extravillous trophoblast between the IUGR and AGA groups
according to neonatal birth weight. Newborns weighing >2,500 g
had an absence of IGF-1Ea immunopositive cells in the extravillous
trophoblast in most of IUGR placentas; in the AGA group, positive
staining was observed in 73.3% of placentas. Furthermore, there was
a significant difference in the IGF-1Ea immunopositive cells in
extravillous trophoblast between the IUGR and AGA groups in
relation to placental weight. In total, more than half of IUGR
cases with a placental weight >400 g had negative IGF-1Ea
staining in the extravillous trophoblast; for AGA, most cases had
positive staining. In addition, there was a significant difference
in IGF-1Ea immunopositive cells in the extravillous trophoblast
between IUGR and AGA groups according to maternal ΒΜI of <30
kg/m2. In total, 72.7% of IUGR cases with a maternal BMI
of <30 kg/m2 had negative IGF-1Ea staining in the
extravillous trophoblast; for AGA, most cases had positive staining
(75.0%). Furthermore, fetal female sex was associated expression of
IGF-1Ea in the extravillous trophoblastic cells. Overall, most of
IUGR cases of fetal female sex had negative IGF-1Ea staining in the
extravillous trophoblast; in AGA, in most cases had positive
staining. Additionally, there was a statistically significant
difference in IGF-1Ea immunopositive cells in the extravillous
trophoblast between the IUGR and AGA groups according to MVM of the
placental bed. IGF-1Ea expression was positive in 29.4 and negative
in 70.6% of IUGR cases with MVM, while IGF-1Ea expression was
positive in 66.7 and negative in 33.3% of AGA cases with MVM
(Table VII).
 | Table VII.Immunohistochemical IGF-1Ea
expression in extravillous trophoblast from normal and IUGR human
placentas of third trimester pregnancies. |
Table VII.
Immunohistochemical IGF-1Ea
expression in extravillous trophoblast from normal and IUGR human
placentas of third trimester pregnancies.
|
| AGA (%) | IUGR (%) |
|
|---|
|
|
|
|
|
|---|
| Group | Negative | Positive | Negative | Positive | P-value |
|---|
| Total | 4 (26.7) | 11 (73.3) | 33 (71.7) | 13 (28.3) | 0.003 |
| Age, years |
|
|
|
|
|
|
<40 | 4 (28.6) | 10 (71.4) | 25 (69.4) | 11 (30.6) | 0.012 |
|
≥40 | 0 (0.0) | 1 (100.0) | 4 (66.7) | 2 (33.3) | 0.429 |
| Gestational age,
weeks |
|
|
|
|
|
|
<37 | 0 (0.0) | 0 (0.0) | 15 (60.0) | 10 (40.0) | - |
|
≥37 | 4 (26.7) | 11 (73.3) | 18 (85.7) | 3 (14.3) | 0.001 |
| Neonate weight,
g |
|
|
|
|
|
|
<2,500 | 0 (0.0) | 0 (0.0) | 24 (82.8) | 5 (17.2) | - |
|
≥2,500 | 4 (26.7) | 11 (73.3) | 9 (90.0) | 1 (10.0) | 0.004 |
| Placenta weight,
g |
|
|
|
|
|
|
<400 | 0 (0.0) | 0 (0.0) | 22 (73.3) | 8 (26.7) | - |
|
≥400 | 4 (26.7) | 11 (73.3) | 11 (68.8) | 5 (31.2) | 0.032 |
| BMI,
kg/m2 |
|
|
|
|
|
|
<30 | 3 (25.0) | 9 (75.0) | 8 (72.7) | 3 (27.3) | 0.022 |
|
≥30 | 1 (33.3) | 2 (66.7) | 4 (66.7) | 2 (33.3) | 0.343 |
| Fetal sex, % |
|
|
|
|
|
|
Male | 2 (28.6) | 5 (71.4) | 5 (62.5) | 3 (37.5) | 0.189 |
|
Female | 2 (25.0) | 6 (75.0) | 16 (84.2) | 3 (15.8) | 0.003 |
| Histopathology,
% |
|
|
|
|
|
|
MVM | 4 (33.3) | 8 (66.7) | 24 (70.6) | 10 (29.4) | 0.038 |
|
MPFD | 0 (0.0) | 0 (0.0) | 2 (100.0) | 0 (0.0) | - |
|
FVM | 0 (0.0) | 0 (0.0) | 3 (100.0) | 0 (0.0) | - |
|
VUE | 0 (0.0) | 1 (100.0) | 3 (60.0) | 2 (40.0) | 0.273 |
|
DVM | 0 (0.0) | 2 (100.0) | 3 (60.0) | 2 (40.0) | 0.147 |
|
Other | 0 (0.0) | 0 (0.0) | 2 (50.0) | 2 (50.0) | - |
The intensity of IGF-1Ea expression in extravillous
trophoblastic cells was not significantly different between AGA and
IUGR placentas (Fig. 8B; Table VIII). Additionally, there was no
association with IGF-1Ea staining intensity between the two groups
according to maternal age and gestational age (>37 weeks),
neonatal birth weight (>2,500 g), placental weight (>400 g),
maternal ΒΜI, fetal male sex, MVM and DVM (Table VIII). However, fetal female sex
was associated with intensity of IGF-1Ea expression in extravillous
trophoblast of the AGA pregnancies compared with the IUGR (Table VIII). The immunohistochemical
detection of cytoplasmic IGF-1Ea in the extravillous trophoblast
from the third trimester human placentas is shown in Fig. 5.
 | Table VIII.Intensity of IGF-1Ea
immunohistochemical expression in extravillous trophoblast from
placentas at third trimester pregnancies. |
Table VIII.
Intensity of IGF-1Ea
immunohistochemical expression in extravillous trophoblast from
placentas at third trimester pregnancies.
|
| AGA | IUGR |
|
|---|
|
|
|
|
|
|---|
| Group | Weak | Moderate | Strong | Weak | Moderate | Strong | P-value |
|---|
| Total | 6 (40.0) | 4 (26.7) | 5 (33.3) | 17 (56.7) | 11 (36.7) | 2 (6.7) | 0.076 |
| Age, years |
|
|
|
|
|
|
|
|
<40 | 6 (42.9) | 3 (21.4) | 5 (35.7) | 13 (54.2) | 9 (37.5) | 2 (8.3) | 0.103 |
|
≥40 | 0 (0.0) | 1 (100.0) | 0 (0.0) | 2 (66.7) | 1 (33.3) | 0 (0.0) | >0.999 |
| Gestational age,
weeks |
|
|
|
|
|
|
|
|
<37 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (30.8) | 8 (30.8) | 2 (7.7) | - |
|
≥37 | 6 (40.0) | 4 (26.7) | 5 (33.3) | 9 (75.0) | 3 (25.0) | 0 (0.0) | 0.064 |
| Neonate weight,
g |
|
|
|
|
|
|
|
|
<2,500 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 12 (70.6) | 5 (29.4) | 0 (0.0) | - |
|
≥2,500 | 6 (40.0) | 4 (26.7) | 5 (33.3) | 5 (83.3) | 1 (16.7) | 0 (0.0) | 0.220 |
| Placenta weight,
g |
|
|
|
|
|
|
|
|
<400 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (35.7) | 8 (57.1) | 1 (7.1) | - |
|
≥400 | 6 (40.0) | 4 (26.7) | 5 (33.3) | 12 (75.0) | 3 (18.8) | 1 (6.2) | 0.093 |
| BMI,
kg/m2 |
|
|
|
|
|
|
|
|
<30 | 5 (41.7) | 3 (25.0) | 4 (33.3) | 8 (72.7) | 3 (27.3) | 0 (0.0) | 0.097 |
|
≥30 | 1 (33.3) | 1 (33.3) | 1 (33.3) | 5 (83.3) | 1 (16.7) | 0 (0.0) | 0.223 |
| Fetal sex, % |
|
|
|
|
|
|
|
|
Male | 3 (42.9) | 3 (42.9) | 1 (14.3) | 4 (50.0) | 4 (50.0) | 0 (0.0) | 0.542 |
|
Female | 3 (37.5) | 1 (12.5) | 4 (50.0) | 11 (84.6) | 2 (15.4) | 0 (0.0) | 0.017 |
| Histopathology,
% |
|
|
|
|
|
|
|
|
MVM | 6 (50.0) | 3 (25.0) | 3 (25.0) | 15 (62.5) | 7 (29.2) | 2 (8.3) | 0.530 |
|
VUE | 0 (0.0) | 0 (0.0) | 1 (100.0) | 1 (50.0) | 1 (50.0) | 0 (0.0) | - |
|
DVM | 0 (0.0) | 1 (50.0) | 1 (50.0) | 2 (40.0) | 3 (60.0) | 0 (0.0) | 0.190 |
|
Other | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (100.0) | 0 (0.0) | - |
IGF-1Ea expression in endothelium of
maternal decidual and the fetal blood vessels in the villi in human
placenta
The positive immunohistochemical IGF-1Ea staining in
endothelium of the maternal decidual vessels from human placentas
was not significantly different between the IUGR and AGA third
trimester pregnancies. However, when the placental weight was
>400 g, more IUGR placentas (81.2%) exhibited IGF-1Ea
immunopositivity in uterine vessels from the decidua basalis
compared with AGA pregnancies (33.3%; Table IX). In addition, when maternal BMI
was taken into consideration, more IUGR placentas exhibited IGF-1Ea
immunopositivity in uterine vessels compared with the AGA
pregnancies (Table IX). The
immunohistochemical positivity of IGF-1Ea in the endothelium of the
fetal vessels within the stem-intermediate and distal-peripheral
villi of the IUGR human placentas was significant compared with AGA
placentas (Table IX).
 | Table IX.Immunohistochemical immunopositivity
of insulin growth factor-1Ea in the endothelium of uterine and
fetal blood vessels in human placentas from third trimester
pregnancies. |
Table IX.
Immunohistochemical immunopositivity
of insulin growth factor-1Ea in the endothelium of uterine and
fetal blood vessels in human placentas from third trimester
pregnancies.
|
| Maternal
vessels | Fetal
stem-intermediate vessels | Fetal
distal-peripheral vessels |
|---|
|
|
|
|
|
|---|
| Group | AGA (%) | IUGR (%) | P-value | AGA (%) | IUGR (%) | P-value | AGA (%) | IUGR (%) | P-value |
|---|
| Total | 5 (33.3) | 17 (37.0) | >0.999 | 4 (26.7) | 32 (68.1) | 0.007 | 4 (26.7) | 29 (63.0) | 0.018 |
| Age, years |
|
|
|
|
|
|
|
|
|
|
<40 | 5 (35.7) | 12 (32.4) | >0.999 | 4 (28.6) | 24 (64.9) | 0.029 | 4 (28.6) | 23 (62.2) | 0.058 |
|
≥40 | 0 (0.0) | 3 (60.0) | >0.999 | 0 (0.0) | 5 (83.3) | 4 (80.0) | 0 (0.0) | 0.286 | 0.333 |
| Gestational age,
weeks |
|
|
|
|
|
|
|
|
|
|
<37 | 0 (0.0) | 8 (30.8) | - | 0 (0.0) | 18 (69.2) | - | 0 (0.0) | 15 (57.7) | - |
|
≥37 | 5 (33.3) | 9 (45.0) | 0.728 | 4 (26.7) | 14 (66.7) | 0.041 | 4 (26.7) | 14 (70.0) | 0.018 |
| Neonate weight,
g |
|
|
|
|
|
|
|
|
|
|
<2,500 | 0 (0.0) | 13 (46.4) | - | 0 (0.0) | 23 (79.3) | - | 0 (0.0) | 22 (78.6) | - |
|
≥2,500 | 5 (33.3) | 4 (40.0) | >0.999 | 4 (26.7) | 7 (70.0) | 0.048 | 4 (26.7) | 7 (70.0) | 0.048 |
| Placenta weight,
g |
|
|
|
|
|
|
|
|
|
|
<400 | 0 (0.0) | 4 (13.3) | - | 0 (0.0) | 18 (58.1) | - | 0 (0.0) | 16 (53.3) | - |
|
≥400 | 5 (33.3) | 13 (81.2) | 0.001 | 4 (26.7) | 14 (87.5) | 0.001 | 4 (26.7) | 13 (81.2) | 0.004 |
| BMI,
kg/m2 |
|
|
|
|
|
|
|
|
|
|
<30 | 5 (41.7) | 10 (90.9) | 0.013 | 4 (33.3) | 11 (100.0) | <0.001 | 4 (33.3) | 7 (63.6) | 0.146 |
|
≥30 | 0 (0.0) | 5 (83.3) | - | 0 (0.0) | 6 (100.0) | - | 0 (0.0) | 6 (100.0) | - |
| Fetal sex, % |
|
|
|
|
|
|
|
|
|
|
Male | 3 (42.9) | 7 (87.5) | 0.067 | 2 (28.6) | 8 (100.0) | 0.003 | 2 (28.6) | 6 (75.0) | 0.072 |
|
Female | 2 (25.0) | 9 (47.4) | 0.280 | 2 (25.0) | 16 (80.0) | 0.006 | 2 (25.0) | 15 (78.9) | 0.008 |
| Histopathology,
% |
|
|
|
|
|
|
|
|
|
|
MVM | 4 (33.3) | 14 (40.0) | 0.745 | 3 (25.0) | 25 (71.4) | 0.007 | 3 (25.0) | 22 (62.9) | 0.042 |
|
MPFD | 0 (0.0) | 1 (20.0) | - | 0 (0.0) | 2 (40.0) | - | 0 (0.0) | 2 (40.0) | - |
|
FVM | 0 (0.0) | 0 (0.0) | - | 0 (0.0) | 0 (0.0) | - | 0 (0.0) | 1 (100.0) | - |
|
VUE | 0 (0.0) | 2 (50.0) | - | 0 (0.0) | 2 (40.0) | - | 0 (0.0) | 2 (50.0) | - |
|
DVM | 1 (50.0) | 2 (40.0) | 0.809 | 1 (50.0) | 2 (40.0) | 0.809 | 1 (50.0) | 2 (40.0) | 0.809 |
|
Other | 0 (0.0) | 1 (25.0) | - | 0 (0.0) | 3 (75.0) | - | 0 (0.0) | 3 (75.0) | - |
As aforementioned, AGA cases were well-matched to
the IUGR cases in terms of gestational age >37 weeks and
neonatal >2,500 g and placental weight >400 g.
Immunopositivity of the IGF-1Ea peptide in the endothelium of the
fetal vessels in the stem-intermediate villi of IUGR placentas was
significantly associated with gestational age, neonatal and
placental weight, maternal ΒΜI and fetal sex compared with AGA
placentas (Table IX). In
addition, the positivity of IGF-1Ea in endothelium of the fetal
vessels within the distal villi of the IUGR human placentas was
significantly associated with gestational age, neonatal and
placental weight, maternal ΒΜI >30 kg/m2 and fetal
female sex compared with AGA placentas (Table IX). When gestational age was ≥37
weeks, more IUGR placentas showed IGF-1Ea immunopositivity in
endothelium of the fetal vessels within the stem-intermediate and
distal villi compared with the AGA placenta (Table IX). Similarly, when the neonatal
birth weight was ≥2,500 g, more frequent IGF-1Ea immunopositivity
was observed in endothelium of the fetal vessels within the
stem-intermediate and distal villi of the IUGR placentas compared
with AGA placenta. In addition, when the placental weight at birth
was ≥400 g, more frequent IGF-1Ea immunohistochemical expression
was observed in the endothelium of the fetal vessels within the
stem-intermediate and distal villi of the IUGR placentas compared
with the AGA placentas. Furthermore, there was a significant
difference in the IGF-1Ea expression in endothelium of the fetal
vessels within the stem-intermediate and distal villi of the IUGR
placentas according to MVM of the placental bed compared with AGA
placentas. There was more frequent IGF-Ea immunohistochemical
expression in endothelium of fetal vessels within the
stem-intermediate and distal villi of IUGR placentas, compared with
the AGA placentas. Endothelial immunostaining is shown in Fig. 6.
Discussion
The IGF system is composed of three ligands
(insulin, IGF-1 and IGF-2), tyrosine kinase receptors [IGF-1
receptor, (IGF-1R), insulin receptor, mannose 6-phosphate IGF-2R,
and the IR/IGF-1R hybrid], IGFBP 1–6 and downstream target proteins
including insulin receptor substrate-1, protein kinase B (Akt) and
mTOR (52–57). IGF-1 is a cell growth factor with a
molecular structure similar to insulin, which serves a key role in
normal body growth, development and maintenance, as well as cell
proliferation, differentiation, migration and survival (58,59).
IGF-1 gene contains six exons, which undergo alternative splicing
during transcription, giving rise to heterogeneous mRNAs, including
the three mRNA isoforms IGF-1Ea, IGF-1Eb and IGF-1Ec (58). The post-translational cleavage of
these pro-forms of IGF-1 results in common mature IGF-1 peptide and
extension peptides, namely Ea, Eb and Ec, which have a common 16
amino acid sequence in the N-terminal region and different amino
acid sequences in the C-terminal region (58,60).
The most predominant peptide is IGF-1Ea, which has a 35 amino acid
Ea-peptide (61). IGF-1Ea and
IGF-1Eb peptides bind strongly to the extracellular matrix,
preventing their release into the circulation and therefore exhibit
local effects (62,63). IGF-1 is secreted by the liver under
stimulation of the growth hormone (GH) (36,64,65).
IGF-1 is produced by other organs such as skeletal muscle, kidney
and brain (66–69). In addition, the three isoforms of
IGF-1 are expressed in various types of human tissue and may bind
to different receptors with different actions. IGF-1 actions are
mediated mainly via its binding to the type 1 IGF receptor
(IGF-1R), however IGF-1 signaling via insulin receptor (IR) and
hybrid IGF-1/IR is also evident. Moreover, there is evidence that
the IGF-1Ec isoform may regulate prostate cancer growth via
Ec-peptide specific and IGF-1R/IR-independent signaling (70,71).
During pregnancy, IGF-1 availability in the maternal circulation is
primarily regulated by IGF-BPs such as IGFBP-1, synthesized by the
decidua and the liver (39,72,73).
Phosphorylation of human IGFBP-1 markedly increases its binding
affinity for IGF-1 and effectively decreases IGF-1 availability and
function (39,74–76).
IGF-1 is found in human fetal serum from 15 to 23 week of gestation
(36,77). Furthermore, birth and placental
weights are positively associated with IGF-1 cord blood levels
(78,79). In addition, maternal IGF-1 levels
are positively associated with neonatal and placental weights
suggesting that higher maternal IGF-1 levels at mid- and
late-gestation indicate greater placental and fetal growth
(80). In obese patients during
pregnancy, the increased serum levels of IGF-1 and the low levels
of IGFBP-1 increase placental weight and stimulate placental mTOR
signaling, which positively regulates key placental functions,
including glucose trasporter-1 and amino acid transport as well as
mitochondrial biosynthesis, leading to fetal overgrowth (81). Conversely, IUGR pregnancies are
associated with decreased maternal serum IGF-1 and increased
IGFBP-1 levels (82,83). Additionally, higher IGFBP-1 levels
in fetal serum have been found in IUGR pregnancies (84–86).
Compared with placentas from normal pregnancies, expression of
IGF-1 in IUGR full-term placentas is either increased (87–89),
which may indicate a paracrine and/or endocrine function (87), or decreased (40,90,91),
which may contribute to inhibition of the placental insulin/IGF-1
signaling pathway (92–94). Abu-Amero et al (95) observed no significant difference in
placental IGF-1 mRNA expression in IUGR compared with AGA
pregnancies. The IGF-1Ea isoform has similar endocrine effects as
IGF-1 (96).
To the best of our knowledge, the present study is
the first to detect localization of IGF-1Ea peptide in placentas
from IUGR and AGA pregnancies, including in syncytiotrophoblast,
the extravillous cytotrophoblast and the endothelium of villous
fetal vessels, as well as the maternal spiral arteries from the
decidua basalis. The prevalence of VUE in IUGR placentas was 10.6%.
There are highly variable reported rates of incidence of VUE in
IUGR/FGR placentas, ranging from 1.6 to 86.0%, reflecting notable
heterogeneity in sample size and study design (97). The prevalence observed in the
present study (10.6%) falls within the lower limits of the reported
rates and is in accordance with Nordenvall and Sandstedt (98), which reported VUE in 7.5% of 161
FGR cases. The present study indicated a negative IGF-1Ea
expression in extravillous trophoblast in most IUGR placentas. This
suggested selective presence of IGF-1Ea peptide in extravillous
trophoblast may reflect the involvement of this isoform in normal
placentation as extravillous trophoblast serves a critical role in
spiral artery remodeling for normal maternal blood supply (19). This is further supported by the
negative IGF-1Ea expression in extravillous trophoblast in IUGR
placentas with MVM, which also included changes in decidual
vasculopathy. In patients with IUGR, negative expression of IGF-1Ea
in the extravillous trophoblast was also associated with a younger
maternal age (<40 years), gestational age, neonatal birth weight
and maternal BMI (<30 kg/m2). These findings have an
unclear mechanism and may reflect multiple and complex molecular
pathways involved in the regulation of IGF1 biological activity
(99). However, the association
between reduced IGF-1Ea expression in extravillous trophoblast and
the aforementioned clinical and pathological parameters of patients
with IUGR may implicate IGF-1Ea isoform in key pathophysiological
mechanisms underlying the development of IUGR.
In the present study, negative expression of IGF-1Ea
was associated with fetal female sex; however, there is no clear
explanation for this. Decreased or negative IGF-1Ea expression in
extravillous trophoblast in IUGR female fetuses may reflect an
inhibitory role of fetal estrogen on IGF-1Ea synthesis, either
directly via the IGF-1/GH axis or indirectly via regulation of
IGFBP-1 (100,101). Moreover, fibroblast cultures show
an interaction between IGF-1 and testosterone in controlling IGFBP
production (102). Thus, exposure
of IGF-1Ea protein to androgens or estrogens circulating in the
fetal blood may lead to decreased synthesis (selective regulation
of the synthesis) of certain IGF-1Ea binding proteins, resulting in
upregulation of IGF-1Ea expression in endothelium of fetal vessels
(102). Alternatively, other
mechanisms and complex pathways may account for sex differences in
the IGF-1/IUGR association. A potential role of IGF-1 DNA
methylation rate in the development of IUGR was investigated in a
previous study, and a fetal sex difference in IGF-1 DNA methylation
rate was noted, which is of unclear significance (103). The present hypotheses concerning
the role and interactions of the various IGF-1 binding proteins
with sex hormones in regulating IGF-1Ea synthesis, as well as the
implication of other mechanisms such as sex differences in DNA
methylation and IGF-1 gene regulation, require further elucidation.
Extravillous trophoblast serves a critical role in maternal spiral
artery remodeling (104).
Immunopositivity for IGF-1Ea peptide within the endothelium of the
villous blood vessels in IUGR indicated a potential autocrine
function of the IGF-1Ea peptide in the endothelial fetal vessels
and suggested that the presence of the IGF-1Ea peptide may induce
villous angiogenesis and hypercapillarization to compensate for
hypoxic/ischemic and malperfused villi in IUGR placentas. Moreover,
IGF-1Ea secretion in the endothelium of the villous blood vessels
from IUGR placentas may contribute to enhanced vasodilation of
fetal vasculature to enhance the compromised fetal-maternal
exchange, demonstrating an autocrine function of IGF-1Ea in
chorionic villi. In the present study, the immunopositivity of
IGF-1Ea peptide in the endothelium of the villous fetal blood
vessels was associated with MVM of the placental bed in IUGR. This
further supports the hypothesis of a reactive autocrine IGF-1Ea
secretion in malperfused villi. Cho et al (105) suggested that IGF-1 peptide
promotes angiogenesis. In addition, IGF-1 stimulates the expression
of angiogenesis-associated growth factors and promotes angiogenesis
in endothelial cells via activation of the PI3K/Akt signaling
pathway (106) or through VEGF
induction (107–109). Through autocrine, paracrine and
endocrine mechanisms, IGF-1 induces cellular activity such as DNA
synthesis, differentiation, migration and glucose uptake (110–112). In addition, IGF-1 stimulation of
adipose tissue-derived microvascular fragments enhances capacity
for vascularization (113). IGF-1
promotes vasodilation by upregulating nitric oxide synthase
activity in the endothelium and increases production of nitric
oxide (105,114,115). In humans, low serum IGF-1 levels
are associated with decreased endothelium-dependent vasodilation
(116). By contrast, upregulation
of IGF-1R decreases nitric oxide bioavailability and insulin
sensitivity in the endothelium (117). The aforementioned mechanisms may
be involved in regulating biological activity of the IGF-1Ea
isoform. The association between IGF-1Ea expression in the
endothelium of villous fetal vessels with maternal BMI, gestational
age and neonatal and placental weight in IUGR pregnancies may
suggest, as in the case of the extravillous trophoblast, that
multiple and complex molecular pathways are involved in the
regulation of IGF-1 biological activity. In addition, a significant
difference in immunohistochemically detected IGF-1Ea expression was
found in the endothelium of uterine spiral arteries of the decidua
basalis from IUGR pregnancies compared with AGA pregnancies, which
was associated with placental weight and maternal BMI. These
findings suggested that IGF-1Ea protein may promote angiogenesis
and vasodilation of maternal uterine vasculature to compensate for
maternal malperfusion in IUGR placentas. This effect appears to be
influenced by placental weight and maternal BMI.
In the present study, differences in mRNA expression
levels detected by RT-qPCR, were not consistent with the
differences at the protein levels detected immunohistochemically.
The enhanced endothelial IGF-1Ea expression in hypoxic villi from
IUGR pregnancies shown by immunohistochemistry was not detected by
RT-qPCR and immunohistochemically detected IGF-1Ea expression in
the extravillous trophoblast was observed in the placentas from AGA
pregnancies. This is likely due to the placenta being a
histologically heterogeneous organ (118), showing notable variability in
IGF-1Ea protein expression between its structures; this was
depicted in this study by the variable immunolocalization of the
protein within the optical fields where it was assessed, e.g. areas
with variable protein expression in the villous vessels or in the
perivillous syncytiotrophoblast, which could only be reflected in a
semi-quantitative way of measurement within several optical fields;
By contrast, total mRNA levels were obtained from focally resected
frozen placental samples and were overall measured (including all
histological structures, positive or negative) from only one
full-thickness site of the placenta. This procedure and the
variability of IGF-1Ea immune-detected expression in different
histological placental structures may account for the discrepancy
between mRNA and protein expression assessments in the IUGR and AGA
groups. Due to the nature of the present study (IUGR vs. AGA),
groups could not be matched for gestational age. In IUGR
pregnancies birth often takes place before the 37th week of
pregnancy, while normal pregnancies can be completed at later weeks
(up to 41 weeks). Hence, IUGR births typically have lower
gestational age compared with AGA, which is a limitation of the
present study. Additionally, the number of IUGR cases completed
after the 37th week of pregnancy was almost the same as that of AGA
pregnancies (18 and 15, respectively). Moreover, significant
associations were revealed, despite the small number of AGA cases.
In addition, demographic variables, such as maternal age, BMI,
fetal sex and numerous pathological characteristics were not
significantly different, ensuring that the differences were mainly
due to IUGR rather than other factors. Overall, the present study
demonstrated that expression of the IGF-1Ea isoform in extravillous
trophoblast was negatively associated with development of IUGR
pregnancies and that this IGF-1 isoform may be involved in
pathogenesis of placental malperfusion as well as regulation of
villous angiogenesis, in the IUGR cases. To the best of our
knowledge, the present study is the first to implicate IGF-1Ea
expression in IUGR pregnancy development and progression. However,
the potential regulatory role of the IGF-1Ea isoform in
pathophysiology of IUGR requires further investigation to confirm
whether this IGF-1 isoform could be used as a prognostic biomarker
and/or a novel therapeutic target in IUGR pregnancy. However use of
immunohistochemistry and RT-qPCR without complementary techniques
weakens the robustness of the conclusions. Mechanistic studies that
include the in vitro treatment of placental cells with
synthetic IGF-1Ea peptide may determine IGF-1Ea induced cellular
and molecular responses and the potential regulatory role of this
IGF-1 isoform in pathophysiology of IUGR.
In conclusion, the IGF-1Ea peptide is expressed in
full- and preterm placentas and may serve a role in IUGR. Negative
expression of the IGF-1Ea peptide in the extravillous trophoblast
of IUGR placentas and its association with MVM suggest involvement
in defective placentation and development of placental
insufficiency. The expression of IGF-1Ea peptide in the endothelium
of villous blood vessels may also suggest an autocrine role in
regulating villous angiogenesis and vasodilation in malperfused
villi of IUGR pregnancies. Similarly, secretion of the IGF-1Ea
peptide in endothelium of the maternal decidual vessels may also
reflect a reactive response to induce vasodilation in uterine
spiral vessels at the maternal side of IUGR placentas, influenced
by placental weight and the maternal BMI. Thus, IGF-1Ea peptide in
human placentas may mediate trophoblastic invasion and normal
placentation, contributing to development of normal pregnancies.
The negative placental expression of IGF-1Ea peptide in the
extravillous trophoblast and its positive expression within fetal
and maternal vessels of IUGR pregnancies also appears to be
influenced by clinical parameters such as maternal age (<40
years), gestational age, maternal BMI (<30 kg/m2) and
neonatal weight, likely reflecting the multiple and complex
molecular pathways that have been implicated in the various
biological functions of IGF-1.
Acknowledgements
The authors would like to thank Dr Elisabeth Barton
(University of Florida, Gainesville, FL, USA), for providing
anti-IGF-1Ea antiserum.
Funding
The present study was supported by REA Maternity Clinic (grant
no. NKUA SARG 11191).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AG performed data analysis. AF, MV, FNV, APh, DM,
VKV, EK and AEK interpreted data. AF, MV, FNV, APa, DM, VKV, EK,
AG, APh, IV, KP, MK and AEK conceived and designed the study and
wrote and revised the manuscript. All authors have read and
approved the final manuscript. AF and MV confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Scientific
Committee of the General Maternity Hospital of Athens ‘Elena
Venizelou’ (approval no. 2nd Scientific Committee Meeting/8th
agenda/23-1-2018) and the Research and Ethics Committee of the
Medical School of National and Kapodistrian University of Athens
(Athens, Greece (approval no. 1718016683). All patients provided
written informed consent before the study for participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hutter S, Hepp P, Hofmann S, Kuhn C,
Messner J, Andergassen U, Mayr D, Solano ME, Obermeier V, Mahner S,
et al: Glucocorticoid receptors α and β are modulated sex
specifically in human placentas of intrauterine growth restriction
(IUGR). Arch Gynecol Obstet. 300:323–335. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morris RK, Johnston E, Lees C, Morton V
and Smith G; Royal College of Obstetricians and Gynaecologists, :
Investigation and care of a small-for-gestational-age fetus and a
growth restricted fetus (Green-top Guideline No. 31). BJOG.
131:e31–e80. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kamphof HD, Posthuma S, Gordijn SJ and
Ganzevoort W: Fetal growth restriction: Mechanisms, epidemiology,
and management. Matern Fetal Med. 4:186–196. 2022. View Article : Google Scholar
|
|
4
|
Suhag A and Berghella V: Intrauterine
growth restriction (IUGR): Etiology and diagnosis. Curr Obstet
Gynecol Rep. 2:102–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nevo O, Many A, Xu J, Kingdom J, Piccoli
E, Zamudio S, Post M, Bocking A, Tordos T and Caniggia I: Placental
expression of soluble fms-like tyrosine kinase 1 is increased in
singletons and twin pregnancies with intrauterine growth
restriction. J Clin Endocrinol Metab. 93:285–292. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aiko Y, Askew DJ, Aramaki S, Myoga M,
Tomonaga C, Hachisuga T, Suga R, Kawamoto T, Tsuji M and Shibata E:
Differential levels of amino acid transporters system L and ASCT2,
and the mTOR protein in placenta of preeclampsia and IUGR. BMC
Pregnancy Clidbirth. 14:1812014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Menendez-Castro C, Rascher W and Hartner
A: Intrauterine growth restriction-impact on cardiovascular
diseases later in life. Mol Cell Pediatr. 5:42018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Crispi F, Crovetto F and Gratacos E:
Intrauterine growth restriction and later cardiovascular function.
Early Hum Dev. 126:23–27. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calek E, Binder J, Palmrich P,
Eibensteiner F, Thajer A, Kainz T, Harreiter K, Berger A and Binder
C: Effects of intrauterine growth restriction (IUGR) on growth and
body composition compared to constitutionally small infancts.
Nutrients. 15:41582023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Novac MV, Niculescu M, Manolea MM,
Dijmărescu AL, Iliescu DG, Novaac MB, Rotaru LT, Stonescu MF,
Tabacu MC, Tudorache Ş, et al: Placental findings in pregnancies
complicated with IUGR-histopathological and immunohistochemical
analysis. Rom J Morphol Embryol. 59:715–720. 2018.PubMed/NCBI
|
|
11
|
Bartha JL, Romero-Carmona R and
Comino-Delcado R: Inflammatory cytokines in intrauterine growth
retardation. Acta Obstet Gynecol Scand. 82:1099–1102. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McKinnon T, Chakraborty C, Gleeson LM,
Chidiac P and Lala PK: Stimulation of human extravillous
trophoblast migration by IGF-II is mediated by IGF type 2 receptor
involving inhibitory G protein(s) and phosphorylation of MAPK. J
Clin Endocrionol Metab. 86:3665–3674. 2001. View Article : Google Scholar
|
|
13
|
Horii M, Touma O, Bui T and Parast MM:
Modeling human trophoblast, the placental epithelium at the
maternal fetal interface. Reproduction. 160:R1–R11. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lawless L, Qin Y, Xie L and Zhang K:
Trophoblast differentiation: Mechanisms and implications for
pregnancy complications. Nutrients. 15:35642023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qin J, Li W, Xue Z and Xue J: Early
differentiation and gene expression characteristics of trophoblast
lineages. Biol Reprod. 108:709–719. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heazell AEP, Sharp AN, Baker PN and
Crocker IP: Intra-uterine growth restriction is associated with
increased apoptosis and altered expression of proteins in the p53
pathway in villous trophoblast. Apoptosis. 16:135–144. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dietrich B, Kunihs V, Lackner AI,
Meinhardt G, Koo BK, Pollheimer J, Haider S and Knöfler M: NOTCH3
signalling controls human trophoblast stem cell expansion and
differentiation. Development. 150:dev2021522023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Velicky P, Knöfler M and Pollheimer J:
Function and control of human invasive trophoblast subtypes:
Intrinsic vs maternal control. Cell Adh Migr. 10:154–162. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Staff AC, Fjeldstad HE, Fosheim IK, Moe K,
Turowski G, Johnsen GM, Alnaes-Katjavivi P and Sugulle M: Failure
of physiological transformation and spiral artery atherosis: Their
roles in preeclampsia. Am J Obstet Gynecol. 226:S895–S906. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lala PK and Nandi P: Mechanisms of
trophoblast migration, endometrial angiogenesis in preeclampsia:
The role of decorin. Cell Adh Mig. 10:111–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lala PK and Graham CH: Editorial: Cellular
and molecular determinants of pregnancy success at the
fetal-maternal interface in health and disease. Front Cell Dev
Biol. 11:12404812023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fuchs R and Ellinger I: Endocytic and
transcytotic processes in villous syncytiotrophoblast: Role in
nutrient transport to the human fetus. Traffic. 5:725–738. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Knöfler M, Haider S, Saleh L, Pollheimer
J, Gamage TKJB and James J: Human placenta and trophoblast
development: Key molecular mechanisms and model systems. Cell Mol
Life Sci. 76:3479–3496. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Celik O, Saglam A, Baysal B, Derwig IE,
Celik N, Ak M, Aslan SN, Ulas M, Ersahin A, Tayyar AT, et al:
Factors preventing materno-fetal trasmission of SARS-CoV-2.
Placenta. 97:1–5. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coleman SJ, Gerza L, Jones CJP, Aplin JD
and Heazell AEP: Syncytial nuclear aggregates in normal placenta
show increased nuclear condensation, but apoptosis and cytoskeletal
redistribution are uncommon. Placenta. 34:449–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huppertz B and Kingdom JC: Apoptosis in
the trophoblast-role of apoptosis in placental morphogenesis. J Soc
Gynecol Invest. 11:353–362. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Calvert SJ, Longtime MS, Cotter S, Jones
CJP, Sibley CP, Aplin JD, Nelson DM and Heazell AEP: Studies of the
dynamics of nuclear clustering in human syncytiotrophoblast.
Reproduction. 151:657–671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park SY, Kim MY, Kim YJ, Chun YK, Kim HS,
Kim HS and Hong SR: Placental pathology in intrauterine growth
retardation. Korea J Pathol. 36:30–37. 2002.
|
|
29
|
Corrêa RRM, Gilio DB, Cavellani CL,
Paschoini MC, Oliveira FA, Peres LC, Reis MA, Teixeira VPA and
Castro ECC: Placental morphometrical and histopathology changes in
the different clinical presentations of hypertensive syndromes in
pregnancy. Arch Gynecol Obstet. 277:201–206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Resnik R: Intrauterine growth restriction.
Obstet Gynecol. 99:490–496. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sankaran S and Kyle PM: Aetiology and
pathogenesis of IUGR. Best Pract Res Clin Obstet Gynaecol.
23:765–777. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Burnett B, Street L, Quinn K and Denney
JM: Early onset fetal growth restriction: Does path to diagnosis
impact outcomes and pathology? Arch Obstet Gynaecol. 1:5–12.
2020.PubMed/NCBI
|
|
33
|
Scifres CM and Nelson DM: Intrauterine
growth restriction, human placental development and trophoblast
cell death. J Physiol. 587:3453–3458. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Roland CS, Hu J, Ren CE, Chen H, Li J,
Varvoutis MS, Leaphart LW, Byck DB, Zhu X and Jiang SW:
Morphological changes of placental syncytium and their implications
for the pathogenesis of preeclampsia. Cell Mol Life Sci.
73:365–376. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou H, Zha C, Wang P, Yang W, Zhu H and
Zhang S: Regulators involved in trophoblast syncytialization in the
placenta of intrauterine growth restriction. Front Endocrinol
(Lausanne). 14:11071822023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Carter AM: Maintaining the integrity of
trophoblast during growth of the placenta. Focus on ‘Insulin-like
growth factor I and II regulate the life cycle of trophoblast in
the developing human placenta’. Am J Physiol Cell Physiol.
294:C1303–C1304. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pérez-Pérez A, Toro A, Vilariño-García T,
Maymó J, Guadix P, Dueñas JL, Fernández-Sánchez M, Varone C and
Sánchez-Margalet V: Leptin action in normal and pathological
pregnancies. J Cell Mol Med. 22:716–727. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Forbes K, Westwood M, Baker PN and Aplin
JD: Insulin-like growth factor I and II regulate the life cycle of
trophoblast in the developing human placenta. Am J Physiol Cell
Physiol. 294:C1313–C1322. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dumolt JH, Powell TL and Jansson T:
Placental function and the development of fetal overgrowth and
fetal growth restriction. Obstet Gynecol Clin North Am. 48:247–266.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee MH, Jeon YJ, Lee SM, Park MH, Jung SC
and Kim YJ: Placental gene expression is related to glucose
metabolism and fetal cord blood levels of insulin and insulin-like
growth factors in intrauterine growth restriction. Early Hum Dev.
86:45–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Giabicani E, Chantot-Bastaraud S, Bonnard
A, Rachid M, Whalen S, Netchine I and Brioude F: Roles of type 1
insulin-like growth factor (IGF) receptor and IGF-II in growth
regulation: Evidence from a patient carrying both an 11p paternal
dublication and 15q deletion. Front Endocrinol (Lausanne).
10:2632019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
LeRoith D, Werner H, Beitner-Johnson D and
Roberts C Jr: Molecular and cellular aspects of the insulin-like
growth factor I receptor. Endocr Rev. 16:143–163. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schmid C: Insulin-like growth factors.
Cell Biol Int. 19:445–457. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bowman CJ, Steck RD and Chapin RE:
Maternal-placental insulin-like growth factor (IGF) signaling and
its importance to normal embryo-fetal development. Birth Defects
Res B Dev Reprod Toxicol. 89:339–349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kasprzak A and Szaflaski W: Role of
alternatively spliced messenger RNA (mRNA) isoforms of the
insulin-like growth factor 1 (IGF1) in selected human tumors. Int J
Mol Sci. 21:69952020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Stavropoulos A, Varras M, Philippou A,
Vasilakaki T, Varra VK, Varra FN, Tsavari A, Lazaris AC and
Koutsilieris M: Immunohistochemical expression of insulin-like
growth factor-1Ec in primary endometrial carcinoma: Association
with PTEN, p53 and survivin expression. Oncol Lett. 20:3952020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Milingos DS, Philippou A, Armakolas A,
Papageorgiou E, Sourla A, Protopapas A, Liapi A, Antsaklis A,
Mastrominas M and Koutsilieris M: Insulinlike growth factor-1Ec
(MGF) expression in eutopic and ectopic endometrium:
Characterization of the MGF E-peptide actions in vitro. Mol Med.
17:21–28. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gkioka E, Msaouel P, Philippou A,
Vlachogiannis NI, Voskou CT, Margiolis A and Koutsilieris M:
Review: The role of insulin-like growth factor-1 signaling pathways
in uterine leiomyoma. In Vivo. 29:637–649. 2015.PubMed/NCBI
|
|
49
|
Blontzos N, Mavrogianni D, Ntzeros K,
Kathopoulis N, Moustogiannis A, Philippou A, Koutsilieris M and
Protopapas A: Differential expression of insulin growth factor 1
(IGF-1) isoforms in different types of endometriosis: Preliminary
results of a single-center study. Biomolecules. 14:72023.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Khong TY, Mooney EE, Ariel I, Balmus NCM,
Boyd TK, Brundler MA, Derricott H, Evans MJ, Faye-Petersen OM,
Gillan JE, et al: Sampling and definitions of placental lesions:
Amsterdam placental workshop group consensus statement. Arch Pathol
Lab Med. 140:698–713. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using realtime quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Scotlandi K and Belfiore A: Targeting the
insulin-like growth factor (IGF) system is not as simple as just
targeting the type 1 IGF receptor. Am Soc Clin Oncol Educ Book.
2012:599–604. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Griffeth RJ, Bianda V and Nef S: The
emerging role of insulin-like growth factors in testis development
and function. Basic Clin Androl. 24:122014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Nawathe AR, Christian M, Kim SH, Johnson
M, Savvidou MD and Terzidou V: Insulin-like growth factor axis in
pregnancies affected by fetal growth disorders. Clin Epigenetics.
8:112016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
LeRoith D, Holly JMP and Forbes BE:
Insulin-like growth factors: Ligands, binding proteins, and
receptors. Mol Metab. 52:1012452021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Werner H: The IGF1 signaling pathway: From
basic concepts to therapeutic opportunities. Int J Mol Sci.
24:148822023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Philippou A, Halapas A, Maridaki M and
Koutsilieris M: Type I insulin-like growth factor receptor
signaling in skeletal muscle regeneration and hypertrophy. J
Musculoskelet Neuronal Interact. 7:208–218. 2007.PubMed/NCBI
|
|
58
|
Philippou A, Maridaki M, Pneumaticos S and
Koutsilieris M: The complexity of the IGF1 gene splicing,
posttranslational modification and bioactivity. Mol Med.
20:202–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gong H, Wang H, Wang L, Liu Y, Wang J, Lv
Q, Pang H, Zhang Q and Wang Z: Inhibition of IGF-1 receptor kinase
blocks the differentiation into cardiomyocyte-like cells of BMSCs
induced by IGF-1. Mol Med Rep. 16:787–793. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Philippou A, Maridaki M, Halapas A and
Koutsilieris M: The role of the insulin-like growth factor 1
(IGF-1) in skeletal muscle physiology. In Vivo. 21:45–54.
2007.PubMed/NCBI
|
|
61
|
Annibalini G, Bielli P, De Santi M,
Agostini D, Guescini M, Sisti D, Contarelli S, Brandi G, Villarini
A, Stocchi V, et al: MIR retroposon exonization promotes
evolutionary variability and generates species-specific expression
of IGF-1 splice variants. Biochim Biophys Acta. 1859:757–768. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hede MS, Salimova E, Piszczek A, Perlas E,
Winn N, Nastasi T and Rosenthal N: E-peptides control
bioavailability of IGF-1. PLoS One. 7:e511522012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Gallego-Colon E, Sampson RD, Sattler S,
Schneider MD, Rosenthal N and Tonkin J: Cardiac-restricted IGF-1Ea
overexpression reduces the early accumulation of inflammatory
myeloid cells and mediates expression of extracellular matrix
remodeling genes after myocardial infarction. Mediators Imflamm.
2015:4843572015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Adamek A and Kasprzak A: Insulin-like
growth factor (IGF) system in liver disease. Int J Mol Sci.
19:13082018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Blum WF, Alherbish A, Alsagheir A, Awwa
AE, Kaplan W, Koledova E and Savage MO: The growth
hormone-insulin-like growth factor-I axis in the diagnosis and
treatment of growth disorders. Endocr Connect. 7:R212–R222. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Karagiannis AK, Philippou A,
Tseleni-Balafouta S, Zevolis E, Nakouti T, Tsopanomichalou-Gklotsou
M, Psarras V and Koutsilieris M: IGF-IEc expression is associated
with advanced differentiated thyroid cancer. Anticancer Res.
39:2811–2819. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Philippou A, Papageorgiou E, Bogdanis G,
Halapas A, Sourla A, Maridaki M, Pissimissis N and Koutsilieris M:
Expression of IGF-1 isoforms after exercise-induced muscle damage
in humans: Characterization of the MGF E peptide actions in vitro.
In Vivo. 23:567–575. 2009.PubMed/NCBI
|
|
68
|
Ahtiainen JP, Hulmi JJ, Lehti M, Kraemer
WJ, Nyman K, Selänne H, Alen M, Komulainen J, Kovanen V, Mero AA,
et al: Effects of resistance training on expression of IGF-I splice
variants in younger and older men. Eur J Sport Sci. 16:1055–1063.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bikle DD, Tahimic C, Chang W, Wang Y,
Philippou A and Barton ER: Role of IGF-I signaling in muscle bone
interactions. Bone. 80:79–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Christopoulos PF, Philippou A and
Koutsilieris M: Pattern of IGF-1 variants' expression in human
cancer cell lines using a novel q-RT-PCR approach. Anticancer Res.
35:107–115. 2015.PubMed/NCBI
|
|
71
|
Philippou A, Armakolas A and Koutsilieris
M: Evidence for the possible biological significance of the igf-1
gene alternative splicing in prostate cancer. Front Endocrinol
(Lausanne). 4:312013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Martina NA, Kim E, Chitkara U, Wathen NC,
Chard T and Giudice LC: Gestational age-dependent expression of
insulin-like growth factor-binding protein-1 (IGFBP-1)
phosphoisoforms in human extraembryonic cavities, maternal serum,
and decidua suggests decidua as the primary source of IGFBP-1 in
these fluids during early pregnancy. J Clin Endocrinol Metab.
82:1894–1898. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Han VK and Certer AM: Spatial and temporal
patterns of expression of messenger RNA for insulin-like growth
factors and their binding proteins in the placenta of man and
laboratory animals. Placenta. 21:289–305. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gupta MB: The role and regulation of
IGFBP-1 phosphorylation in fetal growth restriction. J Cell Commun
Signal. 9:111–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Malkani N, Biggar K, Shahab MA, Li SS,
Jansson T and Gupta MB: Increased IGFBP-1 phosphorylation in
response to leucine deprivation is mediated by CK2 and PKC. Mol
Cell Endocrinol. 425:48–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shehab MA, Biggar K, Kakadia JH, Dhruv M,
Jain B, Nandi P, Nygard K, Jansson T and Gupta MB: Inhibition of
decidual IGF-1 signaling in response to hypoxia and leucine
deprivation is mediated by mTOR and AAR pathways and increased
IGFBP-1 phosphorylation. Mol Cell Endocrinol. 512:1108652020.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ashton IK, Zapf J, Einschenk I and
MacKenzie IZ: Insulin-like growth factors (IGF) 1 and 2 in human
foetal plasma and relationship to gestational age and foetal size
during midpregnancy. Acta Endocrinol (Norway). 110:558–563.
1985.PubMed/NCBI
|
|
78
|
Giudice LC, de Zegher F, Gargosky SE,
Dsupin BA, de las Fuentes L, Crystal RA, Hintz RL and Rosenfeld RG:
Insulin-like growth factors and their binding proteins in the term
and preterm human fetus and neonate with normal and extremes of
intrauterine growth. J Clin Endocrinol Metab. 80:1548–1555. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ong K, Kratzsch J, Kiess W, Costello M,
Scott C and Dunger D: Size at birth and cord blood levels of
insulin, insulin-like growth factor I (IGF-I), IGF-II, IGF-binding
protein-1 (IGFBP-1), IGFBP-3, and the soluble
IGF-II/mannose-6-phosphate receptor in term human infants. The
ALSPAC study team. Avon longitudinal study of pregnancy and
childhood. J Clin Endocrinol Metab. 85:4266–4269. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Luo ZC, Nuyt AM, Delvin E, Audibert F,
Girard I, Shatenstein B, Cloutier A, Cousineau J, Djemli A, Deal C,
et al: Maternal and fetal IGF-I and IGF-II levels, fetal growth,
and gestational diabetes. J Clin Endocrinol Metab. 97:1720–1728.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Soliman A, Hamed N, Alyafei F, Alaaraj N,
Ahmad S, Itani M and Soliman N: Insulin like growth factor 1
(IGF-1) and IGF binding proteins in obese pregnant women and their
babies: Potential effects on placental function and fetal growth.
World J Adv Res Rev. 17:93–100. 2023. View Article : Google Scholar
|
|
82
|
Olausson H, Löf M, Brismar K, Forsum E and
Sohlström A: Maternal serum concentrations of insulin-like growth
factor (IGF)-I and IGF binding protein-1 before and during
pregnancy in relation to maternal body weight and composition and
infant birth weight. Br J Nutr. 104:842–848. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chassen S and Jansson T: Complex,
coordinated and highly regulated changes in placental signaling and
nutrient transport capacity in IUGR. Biochim Biophys Acta Mol Basis
Dis. 1866:1653732020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wang HS, Lim J, English J, Irvine L and
Chard T: The concentration of insulin-like growth factor-I and
insulin-like growth factor-binding protein-1 in human umbilical
cord serum at delivery: Relation to fetal weight. J Endocrinol.
129:459–464. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Tapanainen PJ, Bang P, Wilson K, Unterman
TG, Vreman HJ and Rosenfeld RG: Maternal hypoxia as a model for
intrauterine growth retardation: Effects on insulin-like growth
factors and their binding proteins. Pediatr Res. 36:152–158. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Verhaeghe J, Van Herck E, Billen J,
Moerman P, Van Assche FA and Giudice LC: Regulation of insulin-like
growth factor-I and insulin-like growth factor binding protein-1
concentrations in preterm fetuses. Am J Obstet Gynecol.
188:485–491. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Dalçik H, Yardimoğlu M, Vural B, Dalçik C,
Filiz S, Gonca S, Köktürk S and Ceylan S: Expression of
insulin-like growth factor in the placenta of intrauterine
growth-retarded human fetuses. Acta Histochem. 103:195–207. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Sheikh S, Satoskar P and Bhartiya D:
Expression of insulin-like growth factor-I and placental growth
hormone mRNA in placentae: A comparison between normal and
intrauterine growth retardation pregnancies. Mol Hum Reprod.
7:287–292. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ozkan S, Vural B, Dalçik C, Taş A and
Dalçik H: Placental expression of insulin-like growth factor-I,
fibroblast growth factor-basic and neural cell adhesion molecule in
pregnancies with small for gestational age fetuses. J Perinatol.
28:468–474. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Calvo MT, Romo A, Gutiérrez JJ, Relaño E,
Barrio E and Ferrández Longás A: Study of genetic expression of
intrauterine growth factors IGF-I and EGFR in placental tissue from
pregnancies with intrauterine growth retardation. J Pediatr
Endocrinol Metab. 17 (Suppl 3):S445–S450. 2004.
|
|
91
|
Koutsaki M, Sifakis S, Zaravinos A,
Koutroulakis D, Koukoura O and Spandidos DA: Decreased placental
expression of hPGH, IGF-I and IGFBP-1 in pregnancies complicated by
fetal growth restriction. Growth Horm IGF Res. 21:31–36. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Laviola L, Perrini S, Belsanti G,
Natalicchio A, Montrone C, Leonardini A, Vimercati A, Scioscia M,
Selvaggi L, Giorgino R, et al: Intrauterine growth restriction in
humans is associated with abnormalities in placental insulin-like
growth factor signaling. Endocrinology. 166:1498–1505. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Yung HW, Calabrese S, Hynx D, Hemmings BA,
Cetin I, Charnock-Jones DS and Burton GJ: Evidence of placental
translation inhibition and endoplasmic reticulum stress in the
etiology of human intrauterine growth restriction. Am J Pathol.
173:451–462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Street ME, Viani I, Ziveri MA, Volta C,
Smerieri A and Bernasconi S: Impairment of insulin receptor signal
transduction in placentas of intra-uterine growth-restricted
newborns and its relationship with fetal growth. Eur J Endocrinol.
164:45–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Abu-Amero SN, Ali Z, Bennett P, Vaughan JI
and Moore GE: Expression of the insulin-like growth factors and
their receptors in term placentas: A comparison between normal and
IUGR births. Mol Reprod Dev. 49:229–235. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Milingos D, Katopodis H, Milingos S,
Protopapas A, Creatsas G, Michalas S, Antsaklis A and Koutsilieris
M: Insulin-like growth factor-1 isoform mRNA expression in women
with endometriosis: Eutopic endometrium versus endometriotic cyst.
Ann N Y Acad Sci. 1092:434–439. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Mekinian Α, Kolanska Κ, Cheloufi Μ,
Coulomb Α, Cohen J, Abisror N, Bornes M, Kayem G, Alijotas-Reig J
and Fain O: Chronic villitis of unknown etiology (VUE): Obstetrical
features, outcome and treatment. J Reprod Immunol. 148:1034382021.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Nordenvall M and Sandstedt B: Placental
villitis and intrauterine growth retardation in a Swedish
population. APMIS. 98:19–24. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Martín-Estal I, de la Garza RG and
Castilla-Cortázar I: Intrauterine growth retardation (IUGR) as a
novel condition of insulin-like growth factor-1 (IGF-1) deficiency.
Reviews of Physiology, Biochemistry and Pharmacology. Vol. 170.
Nilius B, de Tombe P, Gudermann T, Jahn R, Lill R and Petersen O:
Reviews of Physiology, Biochemistry and Pharmacology; vol 170.
Springer, Cham: pp. 1–35. 2016, View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Leung KC, Johannsson G, Leong GM and Ho
KKY: Estrogen regulation of growth hormone action. Endocr Rev.
25:693–721. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Isotton AL, Wender MCO, Casagrande A,
Rollin G and Czepielewski MA: Effects of oral and transdermal
estrogen on IGF1, IGFBP3, IGFBP1, serum lipids, and glucose in
patients with hypopituitarism during GH treatment: A randomized
study. Eur J Endocrinol. 166:207–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Yoshizawa A and Clemmons DR: Testosterone
and insulin-like growth factor (IGF) I interact in controlling
IGF-binding protein production in androgen-responsive foreskin
fibroblasts. J Clin Endocrinol Metab. 85:1627–1633. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Li X, Yu B, Wu X, Zhang J, Jia C, Wang Z,
Zhou Q, Zhou H, Yi G, Chen X and Fu S: Associations between
placental insulin-like growth factor-1 gene expression, DNA
methylation and intrauterine growth restriction. Health.
12:270–280. 2020. View Article : Google Scholar
|
|
104
|
Wei XW, Zhang YC, Wu F, Tian FJ and Lin Y:
The role of extravillous trophoblasts and uterine NK cells in
vascular remodeling during pregnancy. Front Immunol. 13:9514822022.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Cho YL, Hur SM, Kim JY, Kim JH, Lee DK,
Choe J, Won MH, Ha KS, Jeoung D, Han S, et al: Specific activation
of insulin-like growth factor-1 receptor by ginsenoside Rg5
promotes angiogenesis and vasorelaxation. J Biol Chem. 290:467–477.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Lin S, Zhang Q, Shao X, Zhang T, Xue C,
Shi S, Zhao D and Lin Y: IGF-1 promotes angiogenesis in endothelial
cells/adipose-derived stem cells co-culture system with activation
of PI3K/Akt signal pathway. Cell Prolif. 50:e123902017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Reinmuth N, Fan F, Liu W, Parikh AA,
Stoeltzing O, Jung YD, Bucana CD, Radinsky R, Gallick GE and Ellis
LM: Impact of insulin-like growth factor receptor-I function on
angiogenesis, growth, and metastasis of colon cancer. Lab Invest.
82:1377–1389. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Slomiany MG and Rosenzweig SA:
IGF-1-induced VEGF and IGFBP-3 secretion correlates with increased
HIF-1 alpha expression and activity in retinal pigment epithelial
cell line D407. Invest Ophthalmol Vis Sci. 45:2838–2847. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ferrara N and Davis-Smyth T: The biology
of vascular endothelial growth factor. Endocr Rev. 18:4–25. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Saltiel AR and Kahn CR: Insulin signalling
and the regulation of glucose and lipid metabolism. Nature.
414:799–806. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Kondo T, Vicent D, Suzuma K, Yanagisawa M,
King GL, Holzenberger M and Kahn CR: Knockout of insulin and IGF-1
receptors on vascular endothelial cells protects against retinal
neovascularization. J Clin Invest. 111:1835–1842. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Arjunan A, Sah DK, Woo M and Song J:
Identification of the molecular mechanism of insulin-like growth
factor-1 (IGF-1): A promising therapeutic target for
neurodegenerative diseases associated with metabolic syndrome. Cell
Biosci. 13:162023. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Laschke MW, Kontaxi E, Scheuer C, Heß A,
Karscnia P and Menger MD: Insulin-like growth factor 1 stimulates
the angiogenic activity of adipose tissue-derived microvascular
fragments. J Tissue Eng. 10:20417314198798372019. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Tsukahara H, Gordienko DV, Tonshoff B,
Gelato MC and Goligorsky MS: Direct demonstration of insulin-like
growth factor-I-induced nitric oxide production by endothelial
cells. Kidney Int. 45:598–604. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Higashi Y, Gautam S, Delafontaine P and
Sukhanov S: IGF-1 and cardiovascular disease. Growth Horm IGF Res.
45:6–16. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Perticone F, Sciacqua A, Perticone M,
Laino I, Miceli S, Carè I, Galiano Leone G, Andreozzi F, Maio R and
Sesti G: Low-plasma insulin-like growth factor-I levels are
associated with impaired endothelium-dependent vasodilatation in a
cohort of untreated, hypertensive Caucasian subjects. J Clin
Endocrinol Metab. 93:2806–2810. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Imrie H, Viswambharan H, Sukumar P, Abbas
A, Cubbon RM, Yuldasheva N, Gage M, Smith J, Galloway S, Skromna A,
et al: Novel role of the IGF-1 receptor in endothelial function and
repair: Studies in endothelium-targeted IGF-1 receptor trangenic
mice. Diabetes. 61:2359–2368. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Li H, Huang Q, Liu Y and Garmire LX:
Single cell transcriptome research in human placenta. Reproduction.
160:R155–R167. 2000. View Article : Google Scholar : PubMed/NCBI
|