Introduction
China has a high incidence of esophageal cancer,
with >95% of cases being squamous cell carcinoma (1,2).
Esophageal squamous cell carcinoma (ESCC) develops slowly and
presents with no obvious clinical symptoms in the early stages.
Most patients present with advanced or locally advanced stages of
ESCC when they are diagnosed, eliminating the possibility of
surgery (3). The early detection
rate of esophageal cancer has improved in recent years as China's
medical level has advanced; however, the postoperative recurrence
and metastasis rates remain high and the 5-year survival rate is
only 30%, posing a serious threat to health (4,5).
Platinum complexes are a class of chemotherapeutic drugs commonly
used in clinical practice, which form DNA adducts by cross-linking
with DNA strands, thus causing DNA damage and cytotoxicity
(6). Cis-diamminedichloroplatinum
(CDDP) was the first platinum-based chemotherapeutic drug formally
used in the clinic and it is still frequently used due to its high
efficacy and inexpensive cost (7).
CDDP-based chemotherapy has evolved into a significant component of
the comprehensive treatment strategy for ESCC, serving as the
primary choice for postoperative adjuvant therapy and the
first-line treatment for unresectable or recurrent ESCC (8). However, most patients encounter the
clinical obstacle of drug resistance, which severely limits
clinical efficacy (9); therefore,
identifying effective medications that improve tumor CDDP
resistance may markedly improve patient survival.
Herbal monomers possess antitumor properties, which
can lessen the adverse effects of chemotherapy, increase treatment
efficacy and improve the quality of life of patients (10). Triptolide (TPL), which is an
extensively researched monomer of the Chinese anticancer medicinal
tretinoin extract, was initially revealed to be a valuable
medication in the treatment of rheumatoid arthritis. TPL has
recently been discovered to have a strong inhibitory effect on a
variety of tumor cells and solid tumors, including colon, liver,
gastric, pancreatic, breast and ovarian cancer, which is linked to
a number of mechanisms, including cell proliferation inhibition,
apoptosis induction, antitumor invasion and anti-metastasis
(11,12). TPL may also have a role in
CDDP-induced DNA damage and repair and may improve tumor resistance
to radiation. Fanelli et al (13) show that the combined use of CDDP
and TPL could effectively limit DNA repair, overcoming the
resistance of osteosarcoma to CDDP. Zhu et al (14) discovered that TPL combined with
CDDP inhibits the growth of gemcitabine-resistant pancreatic cancer
PANC-1 and MIA PaCa-2 cells in vivo and in vitro. Hu
et al (15) and Huang et
al (16) reveal that TPL
markedly reduces the proliferation of CDDP-resistant human
epithelial ovarian cancer cells, enhances cell sensitivity to CDDP
and accelerates apoptosis. TPL has also recently been found to act
as an effective regulator of glycolysis and mitochondrial activity,
following extensive research into its mechanism of action (17–19).
Notably, TPL has been shown to block mitochondrial hexokinase II
(HK2) expression and aerobic glycolysis, resulting in mitochondrial
translocation of the BAX/BAD complex and cleavage of the tumor
suppressor GSDME by active cysteine 3, which can trigger head and
neck cancer cellular pyroptosis (20). Furthermore, TPL can stimulate
nasopharyngeal carcinoma cell apoptosis by inducing the degradation
of the EBV-associated tumor antigen EBNA1 through increased
susceptibility to caspase-9-dependent mitochondrial apoptosis
(21). TPL has also been shown to
markedly reduce ESCC progression (22,23).
However, the effects of TPL on CDDP resistance, glycolysis and
mitochondrial function in ESCC cells remain unknown.
The two primary routes for energy synthesis in cells
are glycolysis and oxidative phosphorylation (24); most cells can switch between these
two pathways and adapt to changes in their environment. When oxygen
supply is insufficient, glucose is converted to pyruvate, which is
converted to lactate in the cytoplasm, with net production of
protons and excretion into the extracellular medium resulting in
acidification of the medium around the cell into CO2 and
water in the mitochondria via the tricarboxylic acid cycle and the
respiratory chain when oxygen is adequately supplied (25). The energy metabolism of cancer
cells differs from that of normal cells, resulting in the Warburg
effect, in which tumor cells receive energy in a primarily
glycolytic manner in the presence of sufficient oxygen (26–28).
Although aerobic glycolysis and its offshoots are less effective
modes of energy acquisition than mitochondrial oxidative
phosphorylation, they can boost biosynthesis, offer biological raw
materials for rapid tumor cell proliferation and aid in tumor
growth (26,29,30).
Several studies have revealed that high aerobic glycolysis rates
and mitochondrial metabolism are linked to tumor development,
progression and treatment resistance (31–34).
Aurora-A can enhance CDDP resistance by decreasing cellular
senescence and inducing glycolytic metabolism in ovarian
cancer-like organs and cells (35), whereas promoting mitochondrial
reactive oxygen species (ROS) generation and triggering cell death
in rectal cancer cells can improve chemotherapeutic effectiveness
(36). Elucidating the condition
of glycolysis and mitochondrial energy metabolism is critical for
understanding how CDDP resistance can be overcome in CDDP-resistant
ESCC cells.
Based on the previously reported studies, the
present study used CDDP-resistant TE1/CDDP and KYSE30/CDDP ESCC
cells as research subjects to determine whether TPL can inhibit the
growth of drug-resistant ESCC by inhibiting cellular glycolysis and
inducing cellular mitochondrial damage. To assess this, the Cell
Counting Kit (CCK)-8 assay, flow cytometry, wound healing assay,
Transwell assay and nude mouse subcutaneous graft tumor experiments
were performed. The present study may contribute to a better
understanding of the anti-drug-resistant action of TPL in ESCC and
could provide new therapeutic options for CDDP-resistant ESCC.
Materials and methods
Construction of CDDP-resistant ESCC
cells
CDDP-resistant cells were generated using the
incremental drug concentration approach (37) with ESCC cell lines (KYSE30 and TE1)
obtained from the Cell Center of Shanghai Academy of Biological
Sciences, Chinese Academy of Sciences. CDDP (cat. no. HY-17394;
MedChem Express) was diluted to 0.2 µM in RPMI-1640 medium (cat.
no. R0883; MilliporeSigma) containing 10% fetal bovine serum (cat.
no. F0193; MilliporeSigma) and ESCC cells were grown for 48 h at
37°C in a 5% CO2 incubator. Subsequently, the cells were
rinsed three times with PBS and the culture medium (without CDDP)
was changed to continue the culture. The number and condition of
the cells were examined using a light microscope and the cells were
passaged when the density of adherent cell growth exceeded 80%.
After passaging, the cells were cultured in the same medium
containing 1.2 times the previous CDDP concentration. This
procedure was repeated until ESCC cells could be maintained in the
medium with a CDDP concentration of 10 µM, resulting in
CDDP-resistant TE1/CDDP and KYSE30/CDDP cells.
Cell viability assay
KYSE30 and TE1 cells were digested with trypsin.
After centrifugation at 300 × g, 4°C for 5 min, centrifuged and
counted and inoculated into 96-well plates at 1×104
cells/well; 200 µl culture solution (containing 0, 1, 2, 4, 8, 16
and 32 µM CDDP) was added to each well and the plates were cultured
at 37°C in a 5% CO2 incubator for 24 h. Subsequently, 20
µl CCK-8 solution (cat. no. HY-K0301; MedChem Express) was added to
each well, mixed and incubated for 2 h. The OD value of the cells
in each well at 450 nm wavelength was measured using an microplate
reader. The IC50 values were estimated, as well as the
resistance index of TE1/CDDP and KYSE30/CDDP cells to CDDP
(resistance index=IC50 of resistant
cells/IC50 of parental cells).
Similarly, KYSE30 and TE1 cells were treated with 0,
1, 2, 4, 8, 16 and 32 nM TPL and the IC50 values in
TE1/CDDP and KYSE30/CDDP cells were computed to determine the best
TPL treatment concentration (2 nM). TE1/CDDP and KYSE30/CDDP cell
treatment was continued with varying CDDP concentrations in
combination with 2 nM TPL and the IC50 values were
calculated as aforementioned.
5-ethynyl-2′-deoxyuridine (EdU) assay
for cell proliferation
The proliferation of TE-1/CDDP and KYSE30/CDDP cells
was determined using the EdU kit (cat. no. C10310-1; Guangzhou
RiboBio Co., Ltd.). Cells were inoculated in 24-well plates at
2×104 cells/well and treated with 2 nM TPL, 4 µM CDDP or
2 nM TPL + 4 µM CDDP for 24 h. The culture medium was then
discarded and the cells were rinsed in PBS and stained with 10 µM
EdU solution for 1 h at room temperature and in the dark. The cells
were rinsed again in PBS and were then fixed and permeabilized
using 4% paraformaldehyde and 0.3% Triton X-100 for 15 min at room
temperature, respectively. Subsequently, the cells were incubated
with Click reaction solution for 30 min at room temperature in the
dark, before the nuclei were stained with DAPI for 10 min at room
temperature, the slides were sealed and images were observed and
captured under a fluorescence microscope (magnification, ×200; five
fields of with uniform fluorescence distribution randomly
observed). The EdU-positive cells were analyzed using ImageJ v1.8.0
software (National Institutes of Health), and the level of cell
proliferation was determined.
Clone formation assay
The cells were randomly divided into four groups and
were inoculated in Petri dishes containing 10 ml culture medium at
a density of 100 cells/plate. The cell groups were treated with 2
nM TPL, 4 µM CDDP or 2 nM TPL + 4 µM CDDP, before being placed in
an incubator at 37°C with 5% CO2 to continue incubation
for 48 h. The colonies were fixed with 4% paraformaldehyde for 20
min at room temperature, then rinsed twice with PBS before being
stained 30 min at room temperature with 0.1% crystal violet
staining solution (cat. no. C0121; Beyotime Institute of
Biotechnology), washed with PBS and air-dried. Images of the cells
were captured under a light microscope (magnification, ×200) and
the number of clones containing >50 cells was counted.
Flow cytometry
Following various treatments, TE1/CDDP and
KYSE30/CDDP cells were collected and inoculated in cell culture
dishes at 1×105 cells/ml. PI and Annexin V-FITC staining
was performed at room temperature using the PI/Annexin V-FITC kit
(cat. no. 40302ES20; Shanghai Yeasen Biotechnology Co., Ltd.)
according to the manufacturer's instructions (incubated in the dark
for 15 min). After adding 400 µl binding buffer, the staining was
detected using a 2010284AA Flow Cytometer (Agilent Technologies,
Inc.) and the apoptosis rate (the percentage of early + late
apoptotic cells) was estimated using FlowJo v10 software (FlowJo
LLC).
Cell cycle progression (cat. no. C1052, Beyotime
Institute of Biotechnology) and ROS levels (cat. no. 50101ES01;
Shanghai Yeasen Biotechnology Co., Ltd.) were measured
respectively. The PI staining solution and ROS staining solution
(10 µM DCFH-DA) were prepared as per the kit instructions. The two
staining solutions were mixed with each group of cells
(1×106), incubated in a 6-well plate at 37°C for 30 min
in the dark (mixing every 5 min by inverting), centrifuged at 300 ×
g for 5 min at 4°C to remove the supernatant and washed and
resuspended in PBS. The cells were filtered to produce a
single-cell solution for flow cytometric analysis. Fluorescence
intensity was recorded at an excitation wavelength of 488 nm and
FlowJo v10 software (FlowJo LLC) was used to examine cell changes
in cell cycle progression and ROS content.
Hoechst 33258 staining
TE1/CDDP and KYSE30/CDDP cells were treated with 2
nM TPL, 4 µM CDDP and 2 nM TPL + 4 µM CDDP. Fluorescence was
detected using the Hoechst 33258 kit (Shanghai Yeasen Biotechnology
Co., Ltd.). According to the manufacturer's instructions, 5 µM
Hoechst 33258 staining solution was prepared in PBS and 100 µl
staining solution was added dropwise to 96-well plates containing
TE1/CDDP and KYSE30/CDDP cells. The cells were then incubated at
37°C for 20 min, after which the plates were washed twice with PBS.
The staining was visualized with a fluorescence microscope (Leica
Microsystems GmbH).
Wound healing assay
TE1/CDDP and KYSE30/CDDP cells were digested with
trypsin, resuspended in PBS and inoculated in 6-well plates with
labeled lines at 5×105 cells/well. When the cell growth
density reached 80%, a 200-µl sterile pipette tip was used to draw
a straight line perpendicular to the bottom of the well. The
detached cells were washed away with pre-cooled PBS solution and 2
ml FBS-free RPMI-1640 medium was added to the culture. The cells
were examined and images were captured under a microscope at 0 and
24 h. The width of the scratches was measured using ImageJ v1.8.0
software and relative migration rate was calculated using the
following formula: (0 h migration spacing −24 h migration
spacing)/0 h migration spacing ×100%.
Transwell detection
An 8-µm Transwell system (Corning, Inc.) was placed
in a 24-well plate and 50 µl Matrigel (cat. no. 354234; Corning,
Inc.) was added and air-dried for 4 h at room temperature.
Subsequently, 100 µl TE1/CDDP and KYSE30/CDDP cell suspensions at a
density of 1×105/ml were added to the chambers and 500
µl RPMI-1640 medium containing 10% FBS was added to each well
underneath the chambers. The plates were then incubated at 37°C in
a 5% CO2 incubator. After removing the plate, the media
from the upper and lower chambers were discarded and the cells in
the upper chamber were gently removed with a cotton swab. The cells
were then fixed with 4% paraformaldehyde for 30 min and stained
with 0.1% crystal violet (cat. no. C0121; Beyotime Institute of
Biotechnology) for 30 min at room temperature, washed twice with
PBS and air-dried and then observed and photographed under a
high-magnification microscope magnification, ×200; five fields of
with uniform fluorescence distribution randomly observed). The
number of invasive cells detected under the microscope was counted
using ImageJ v1.8.0 software.
Detection with kits
The reagents and samples (TE1/CDDP and KYSE30/CDDP
cell supernatants) were prepared according to the instructions of
the kits (all from Nanjing Jiancheng Bioengineering Institute) on
indicators including the glucose uptake level (cat. no. A154-1-1),
lactic acid production (cat. no. A019-2-1), glutathione (GSH) level
(cat. no. A006-2-1) and superoxide dismutase (SOD) level (cat. no.
A001-1-2). Indicators were all detected using the colorimetric
method. The absorbance of the cells was then measured using an
enzyme labeling instrument (cat. no. DR-200Bc; Diatek) and the
concentration of each index was estimated using the standard
curve.
Extracellular acidification rate
(ECAR) detection
ECAR is a useful indicator for assessing the acid
produced by anaerobic fermentation during cell metabolism and its
change can represent cell metabolism and growth status, whereas a
fall in pH will reduce the fluorescence intensity of the probe
(38). In the present study, ECAR
was detected using the Seahorse XF Glycolytic Stress Test Kit (cat.
no. 103020-100; Agilent Technologies, Inc. Santa Clara, CA, USA)
and Seahorse XFe24 Analyzer. Cells were treated with 2 nM TPL, 4 µM
CDDP and 2 nM TPL + 4 µM CDDP for 24 h at 37°C, according to the
experimental design. Subsequently, trypsin was used to digest
TE1/CDDP and KYSE30/CDDP cells, which were washed and collected in
PBS before being centrifuged (300 × g, 4°C for 5 min) and
resuspended. The experiment involved inoculating 1×104
cells in 96-well culture plates, as per the protocol. Cells were
grown in XF basic media without glucose and pyruvate at 37°C.
Glucose (10 mM), oligomycin (1 µM) and 2-DG (50 mM) were introduced
consecutively at particular time points according to the directions
for use. ECAR data were obtained and displayed using the Seahorse
XF24 software (Agilent Technologies, Inc.).
ATP content detection
The ATP Assay Kit (cat. no. S0026; Beyotime
Institute of Biotechnology) was used to detect ATP content.
According to the manufacturer's instructions, the standard curve
and ATP working solution were prepared. After various treatments,
TE1/CDDP and KYSE30/CDDP cells were lysed using lysis solution. The
supernatant was collected by centrifugation at 300 × g for 5 min
(4°C) and resuspended and 100 µl ATP working solution was applied
to a 6-well plate. Subsequently, 20 µl cell supernatant was added
to each well and a chemiluminescence analyzer (cat. no. 2805880;
Thermo Fisher Scientific, Inc.) was used to quickly detect the
chemiluminescence intensity of each solution. The mitochondrial ATP
content of cells in each group was determined using a standard
curve and the relative expression of the control group was
analyzed.
Mitochondrial membrane potential
detection
Following various treatments, TE1/CDDP and
KYSE30/CDDP cells were washed with PBS. According to the directions
of the Mitochondrial membrane potential assay kit (cat. no. C2006,
Beyotime Institute of Biotechnology), culture medium and JC-1
staining working solution were added to 6-well plates in a 1:1
volume ratio, carefully mixed and incubated at 37°C for 20 min in
the dark. The supernatant was then removed and the cells were
washed twice with the prepared staining buffer to eliminate any
JC-1 probe that had not attached to the cells. Subsequently, 2 ml
cell culture medium was added, the results were observed under a
fluorescence microscope and images were captured. Elevated membrane
potential indicated polarization, with JC-1 aggregates exhibiting
red fluorescence and reduced membrane potential indicates
depolarization, with JC-1 monomers exhibiting green
fluorescence.
Animal experiments
Shanghai Model Organisms Center, Inc. provided 20
5-week-old male BALB/c nude mice, which were randomly divided into
the following four groups each containing five mice: Control, TPL,
CDDP and TPL + CDDP. Mice were housed under a 12-h light/dark cycle
at a temperature of 22–26°C and relative humidity of 50–60%. To
create a tumor xenograft model, 3×106 KYSE30/CDDP cells
(100 µl) were implanted into the right axilla of nude mice. After 7
days of feeding, mice in the TPL group were administered TPL
intraperitoneally at 0.45 mg/kg/time. Mice in the CDDP group were
administered 1.5 mg/kg/time CDDP intraperitoneally. In the TPL +
CDDP group, mice were administered 0.45 mg/kg/time TPL + 1.5
mg/kg/time CDDP by gavage. All treatments were administered every 3
days for five consecutive times, whereas the control group received
saline by gavage. Tumor volume was determined every 7 days in mice
using the following formula: Volume (mm3)=1/2 × L ×
W2 (where L represents tumor length and W represents
tumor width). The mice were maintained for 28 days before being
cervically dislocated and sacrificed, with the tumors carefully
isolated, weighed and photographed. The experimental nude mice in
the present study all weighed ~25 g. According to the guidelines
for the review of humane endpoints in animal experiments (RB/T
173–2018) (39), the mouse tumors
should not be larger than 17 mm and their mass should not exceed
1.25 g (5% of body weight). The present study was approved by the
Ethics Committee of The First Affiliated Hospital of Zhengzhou
University (Henan, China; approval no. 20220096).
TUNEL apoptosis assay
Apoptosis in tumor tissues was assessed using a
TUNEL kit (cat. no. C1091; Beyotime Institute of Biotechnology)
according to the manufacturer's instructions. Mouse tumors were
fixed in 4% paraformaldehyde (4°C for 24 h), dried, embedded in
paraffin and cut into 4-µm sections. Tumor tissue sections were
deparaffinized with xylene, hydrated with a gradient series of
ethanol and then treated with 20 µg/ml DNase-free proteinase K
solution at room temperature for 30 min to promote reaction
reagents to enter the nucleus. After washing with PBS, tissue
sections were submerged in 3% H2O2 for 15 min
to deactivate the endogenous peroxidases. The sections were then
rinsed with PBS and stained with 50 µl TUNEL staining solution
(cat. no. C1005; Beyotime Institute of Biotechnology) at 37°C for
60 min, before being washed with PBS and incubated with DAPI
staining solution (cat. no. C1005; Beyotime Institute of
Biotechnology) at room temperature in the dark for 5 min. The
sections were then blocked with anti-fluorescence burst sealing
solution and the percentage of TUNEL-positive cells was calculated
under a microscope.
Immunohistochemistry
Tissue sections were dehydrated with ethanol,
underwent antigen retrieval with citrate (cat. no. C1032; Beijing
Solarbio Science & Technology Co., Ltd.) and were blocked with
avidin/biotin blocking buffer (cat. no. C-0005; Shanghai Haoran
Biotechnology Co., Ltd.) at room temperature before being incubated
with Ki67 (1:200; cat. no. ab232784; Abcam), cleaved caspase-3
(1:100; cat. no. PA5-114687; Thermo Fisher Scientific, Inc.) and
cleaved caspase-9 (1:100; cat. no. PA5-105271; Thermo Fisher
Scientific, Inc.) primary antibodies at 4°C overnight. After
washing, the appropriate secondary antibody (1:500; cat. no.
ab150077; Abcam) was applied to the tissue sections and incubated
for 1 h at room temperature. The sections were then stained for
five min at room temperature with streptavidin-horseradish
peroxidase and hematoxylin (cat. no. C0107, Beyotime Institute of
Biotechnology) and underwent dehydration in an ethanol
concentration gradient, permeabilization with xylene and section
sealing with neutral gum. The sections were observed under a light
microscope (magnification, ×200) and images were captured.
Western blot analysis
TE1/CDDP and KYSE30/CDDP cells, as well as tumor
tissues, were collected following various treatments and RIPA lysis
buffer was added to extract total proteins. The protein content in
the supernatant was measured using the BCA Protein Kit (cat. no.
P0012; Beyotime Institute of Biotechnology). A 10% SDS-PAGE gel was
then prepared and protein samples were loaded at 20 µg/well for
electrophoresis. The proteins were subsequently transferred to a
PVDF membrane and blocked with 5% bovine serum albumin (cat. no.
ST025; Beyotime Institute of Biotechnology) for 1 h at room
temperature. Afterwards, rabbit anti-cyclin-dependent kinase 4
(CDK4; 1:2,000; cat. no. ab199728), cyclin D1 (1:100; cat. no.
ab16663), Bcl-2 (1:2,000; cat. no. ab182858), cleaved caspase-3
(1:500; cat. no. ab32042), cleaved caspase-9 (1:1,000; cat. no.
9505; CST, MA, USA), E-cadherin (1:1,000; cat. no. ab212059),
Vimentin (1:1,000; cat. no. ab137321), Snail (1:1,000; cat. no.
ab216347), glucose transporter protein 1 (GLUT1; 1:100,000; cat.
no. ab115730), HK2 (1:1,000; cat. no. ab209847), lactate
dehydrogenase A (LDHA; 1:1,000; cat. no. 2012S; CST), cytochrome
c (Cytc; 1:5,000; cat. no. ab133504), COX IV (1:2,000; cat.
no. ab202554) and β-actin (1:1,000; cat. no. ab8227) primary
antibodies were added according to the experimental design and
incubated at 4°C overnight. After washing with TBST (0.05% Tween),
sheep anti-rabbit IgG (1:2,000; cat. no. ab6721) was added and
incubated at room temperature for 30 min. With the exception of the
cleaved caspase-9 and LDHA antibodies, all antibodies were
purchased from Abcam. Cytc was measured using the Cellular
Mitochondrial Isolation Kit (cat. no. C3601; Beyotime Institute of
Biotechnology), which extracted mitochondrial proteins while
eliminating cytoplasmic proteins. After washing with TBST, ECL
chemiluminescent solution (cat. no. P0018S; Beyotime Institute of
Biotechnology) was added dropwise to develop the color and
exposure. Images of the protein bands were captured with a
chemiluminescent image analysis system (5200; Tanon, Shanghai,
China) and the grayscale values of the protein bands of each group
were semi-quantitatively analyzed using ImageJ v1.8.0 software
(National Institutes of Health).
Statistical analysis
The experimental data were analyzed using SPSS 26.0
software (IBM Corp.) and are presented as mean ± standard
deviation. To analyze differences between the groups, a Student's
t-test (unpaired) or one-way ANOVA with Tukey's post hoc test was
performed. P<0.05 was considered to indicate a statistically
significant difference.
Results
TPL increases the inhibitory effect of
CDDP on the proliferation of CDDP-resistant ESCC cells
CDDP was used to treat drug-resistant cells and
their parental cells at various concentrations (0, 1, 2, 4, 8, 16
and 32 µM). Cell viability decreased with increasing CDDP treatment
time, as measured by CCK-8 assay. The IC50 values of
CDDP in TE-1 and KYSE30 cells were 3.94 and 3.07 µM, respectively
and those in TE1/CDDP and KYSE30/CDDP cells were 19.71 and 14.57
µM, respectively; the resistance index was 5.00 and 4.75 (Fig. 1A and B), indicating successful
construction of CDDP-resistant cell lines. Based on the literature,
a CDDP action concentration of 4 µM was established. As shown in
Fig. 1C, TE1/CDDP and KYSE30/CDDP
cells were further treated with 0, 1, 2, 4, 8, 16 and 32 nM TPL and
it was discovered that cell viability gradually decreased with
increasing TPL treatment concentration, with no significant
difference in the effect of TPL on the two drug-resistant cell
lines. The experimental dose of TPL (cell viability ≥70%) was
determined to be 2 nM, which was the highest inhibitory
concentration without cytotoxicity. After treating resistant cells
with different concentrations of CDDP in combination with 2 nM TPL,
the IC50 values in TE1/CDDP and KYSE30/CDDP cells were
significantly reduced compared with CDDP alone (Fig. 1D), demonstrating that the addition
of TPL increased the inhibitory effects of CDDP on the viability of
CDDP-resistant ESCC cells. The subsequent tests established CDDP
and TPL treatment doses at 4 µM and 2 nM, respectively. EdU and
clone formation experiments both revealed that the combination of
the two inhibited the proliferation of TE1/CDDP and KYSE30/CDDP
cells more effectively than CDDP and TPL alone and the proportion
of EdU-positive cells and the number of cloned cells were found to
be even lower (Fig. 2E-I). Taken
together, the experimental results revealed that TPL considerably
increased the inhibitory effects of CDDP on the proliferation of
CDDP-resistant ESCC cells.
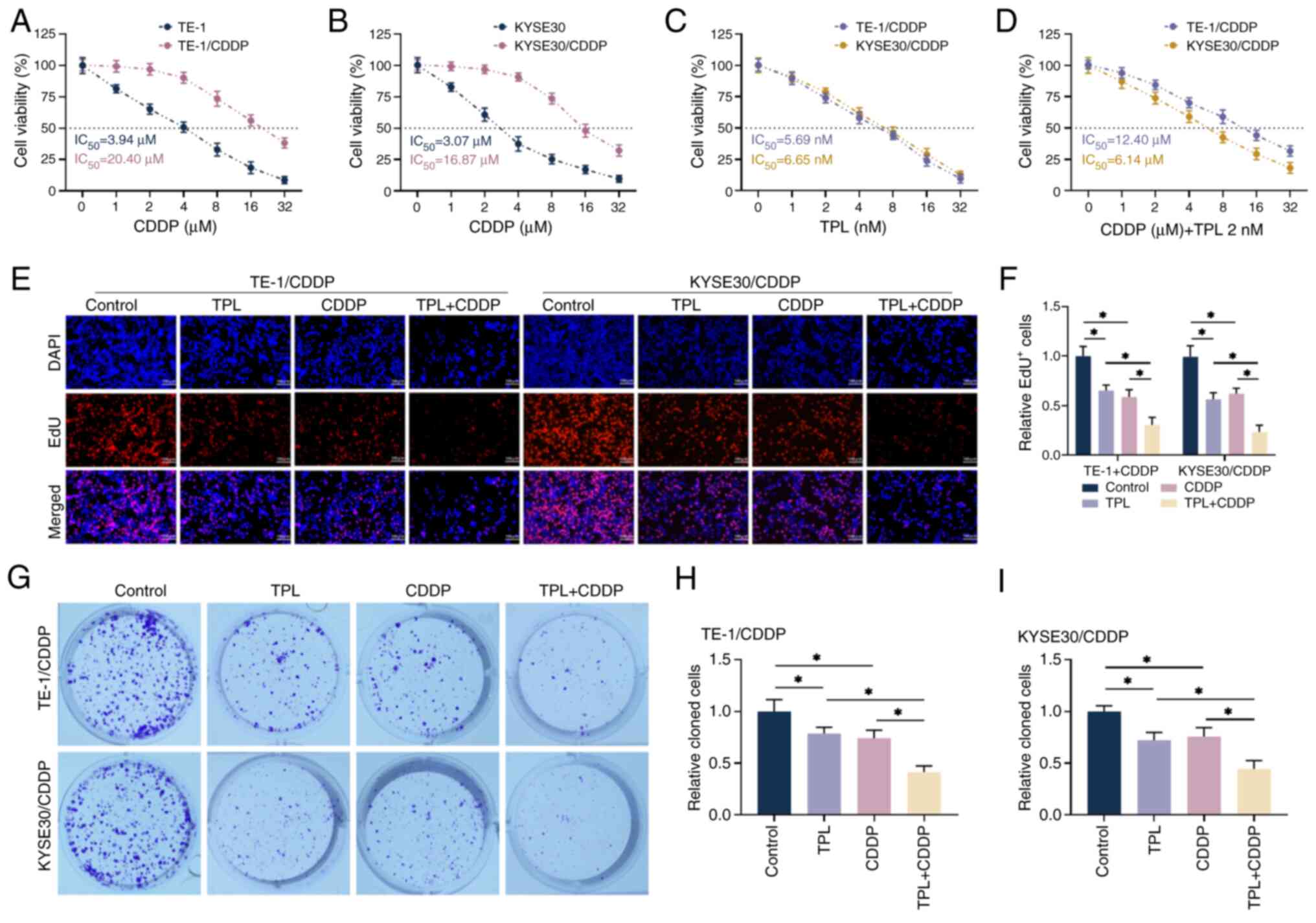 | Figure 1.TPL increases the proliferative
effect of CDDP on anti-CDDP-resistant ESCC cells. (A and B) The
cells viability was measured with CCK-8. (C) The effects of various
TPL concentrations on the proliferative viability of TE-1/CDDP and
KYSE30/CDDP cells were detected using CCK-8. (D) 2 nM TPL was
administered concurrently with CDDP treatment of TE-1/CDDP,
KYSE30/CDDP cells and viability was determined by CCK-8. (E and F)
EdU detection of proliferation in TE-1/CDDP and KYSE30/CDDP cells
(magnification, ×20; scale bar 100 µm). (G-I) Clone formation assay
to determine the formation levels of TE-1/CDDP and KYSE30/CDDP
cells. *P<0.05. TPL, triptolide; CDDP,
cis-diamminedichloro-platinum; ESCC, esophageal squamous cell
carcinoma; CCK, cell counting kit; TE-1/CDDP, cisplatin-resistant
TE-1 cells; KYSE30/CDDP, cisplatin-resistant KYSE30 cells;
IC50, median inhibition concentration; DAPI,
diamidino-phenyl-indole; EdU, 5-ethynyl-2′-deoxyuridine. |
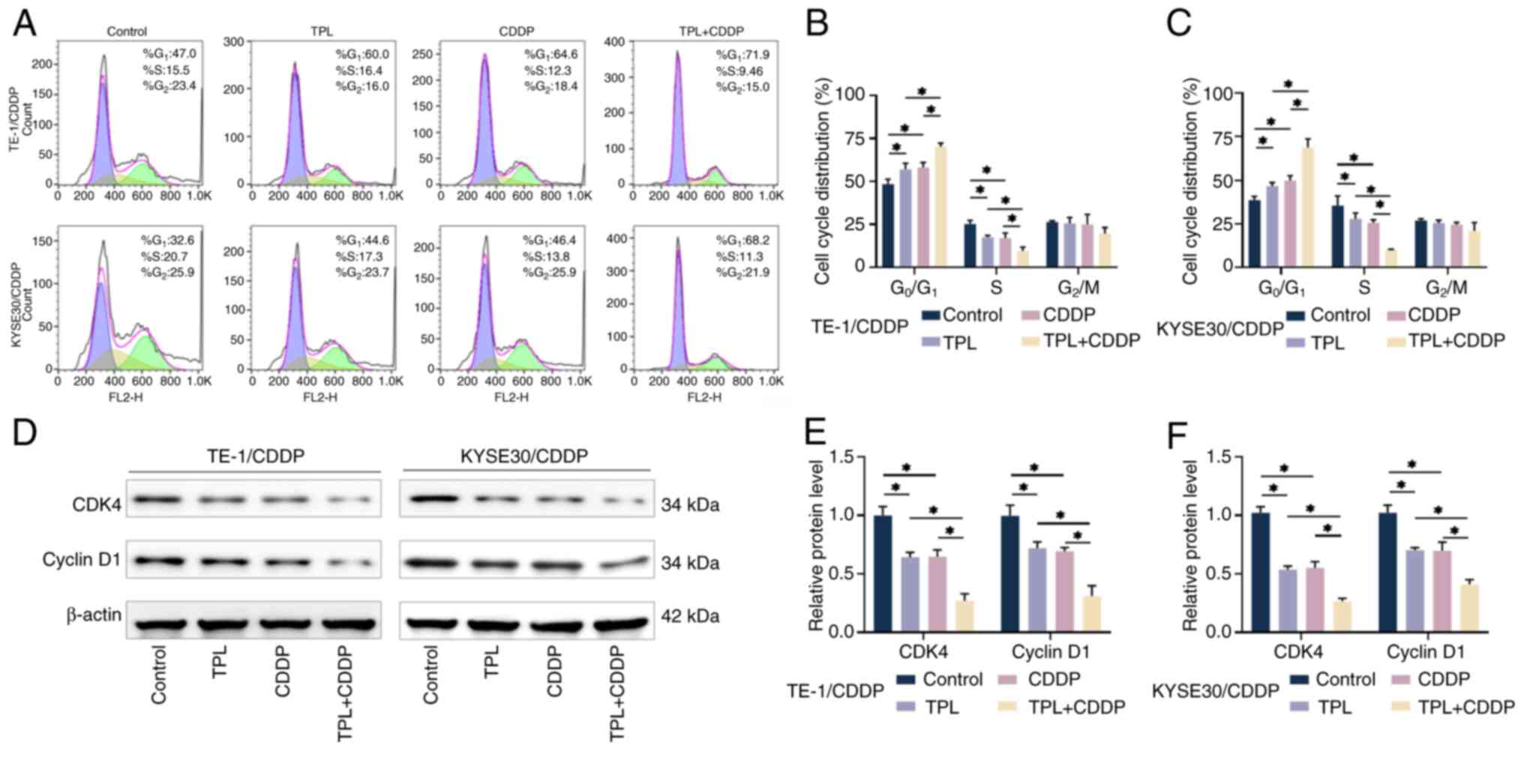
TPL promotes the effects of CDDP on
CDDP-resistant ESCC cell cycle arrest
The present study investigated the effect of TPL on
CDDP-mediated cell cycle arrest in CDDP-resistant ESCC cells using
flow cytometry. The results showed that when CDDP and TPL were used
to treat cells alone, the number of TE1/CDDP and KYSE30/CDDP cells
in the G0/G1 phase was significantly
elevated, whereas the number of cells in the S phase was
significantly decreased compared with the control group. The
combination of CDDP and TPL further accelerated the accumulation of
cells in the G0/G1 phase (Fig. 2A-C), implying that TPL may enhance
the effects of CDDP on the cell cycle arrest of CDDP-resistant ESCC
cells. Cyclin D1 and CDK4 play critical roles in cell cycle control
and proliferation (40) and the
expression of cyclin D1 and CDK4 can be evaluated to determine cell
cycle progression. The results of western blot analysis revealed
that CDDP and TPL significantly inhibited the expression of cyclin
D1 and CDK4 in TE1/CDDP and KYSE30/CDDP cells and CDDP and TPL had
a synergistic effect, preventing CDDP-resistant ESCC cells from
entering the S phase for cell proliferation (Fig. 2D-F). TPL was observed to enhance
CDDP-mediated G0/G1 phase arrest.
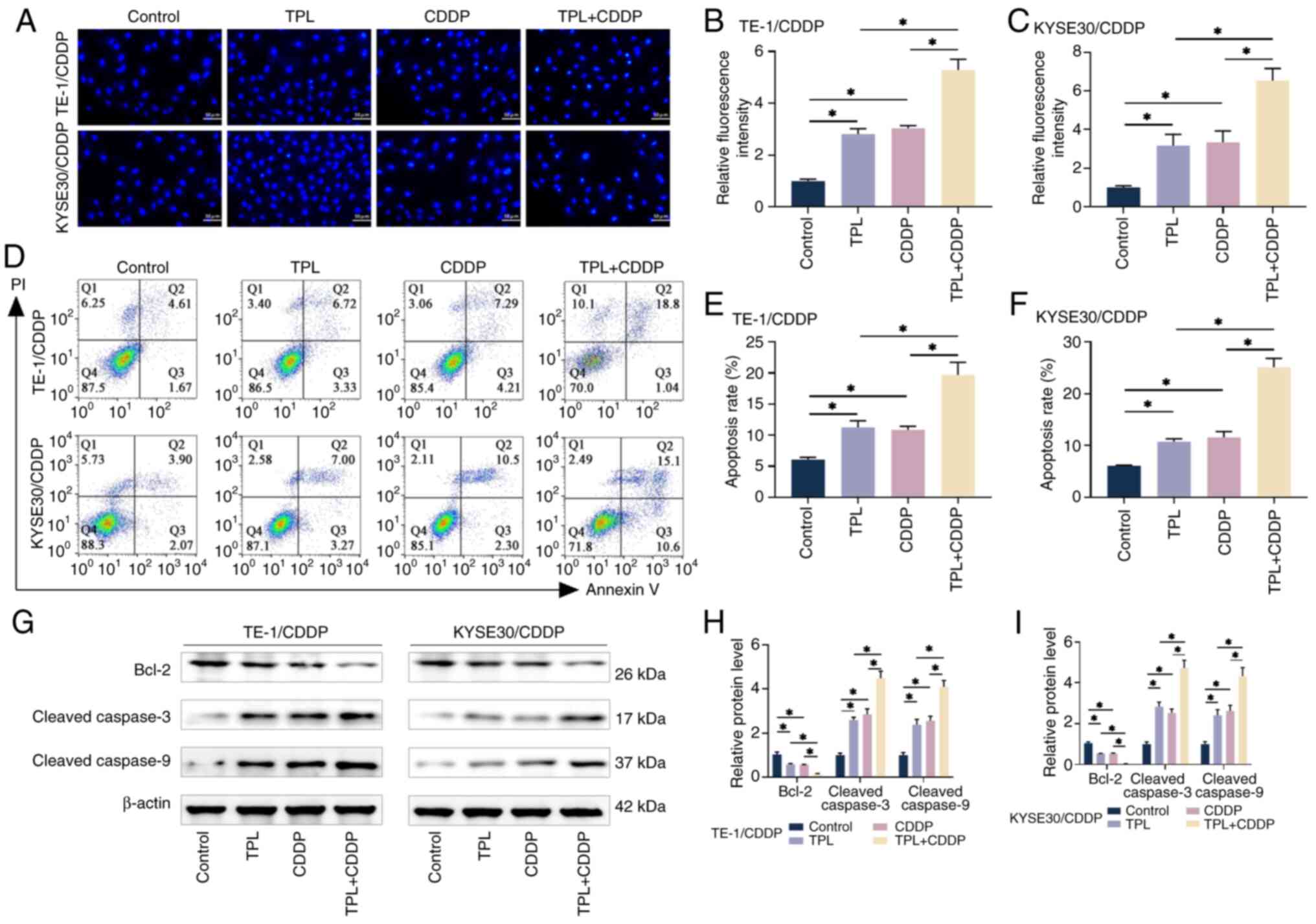
TPL promotes the CDDP-induced
apoptosis of CDDP-resistant ESCC cells
The effects of TPL on CDDP-mediated cytotoxicity in
TE1/CDDP and KYSE30/CDDP cells were further investigated. As
demonstrated by inverted fluorescence microscopy, Hoechst 33258
staining revealed that the morphology of CDDP-resistant ESCC cells
was markedly changed after CDDP and TPL treatment. As shown in
Fig. 3A-C, the nuclei of the two
drug-resistant cells in the control group exhibited light blue
fluorescence and the blue fluorescence of the nuclei was enhanced
after treatment with CDDP and TPL alone; however, the nuclei of the
cells in the group co-treated with CDDP and TPL showed a bright
blue color and the morphology of the cells showed chromatin margin
clustering, karyopyknosis, nuclear fragmentation and apoptotic body
formation. These findings suggested that TPL may enhance the
effects of CDDP and induce the apoptosis of CDDP-resistant ESCC
cells. The rates of apoptosis measured by flow cytometry showed a
consistent trend (Fig. 3D-F).
The apoptosis markers Bcl-2, cleaved caspase-3 and
cleaved caspase-9 were detected using western blotting. The results
showed that the combination of CDDP and TPL further inhibited Bcl-2
and promoted the levels of cleaved caspase-3 and cleaved caspase-9
proteins compared with the treatments alone (Fig. 3G-I). These findings indicated that
TPL could enhance the pro-apoptotic effects of CDDP on TE1/CDDP and
KYSE30/CDDP cells.
TPL increases the inhibitory effect of
CDDP on CDDP-resistant ESCC cell migration, invasion and
epithelial-mesenchymal transition (EMT)
The present study investigated CDDP-resistant ESCC
cells using cell scratch and Transwell assays. The outcomes
revealed that the CDDP combined with TPL-treated group had a
significantly lower relative migration rate and quantity of cells
entering the lower compartment than the treatment groups alone
(Fig. 4A-F). The protein levels of
EMT-related markers, E-cadherin, Vimentin and Snail, were measured
utilizing western blotting (Fig.
4G-I). The levels of the epithelial marker E-cadherin were
increased in CDDP-resistant ESCC cells in the TPL + CDDP group
compared with in the treatment groups alone, whereas the expression
levels of the mesenchymal marker Vimentin and EMT-inducing
transcription factor Snail were decreased. These findings
demonstrated that TPL could improve the sensitivity of
CDDP-resistant ESCC cells to CDDP and inhibit the occurrence of
EMT, thus suppressing the invasive and metastatic ability of the
cells.
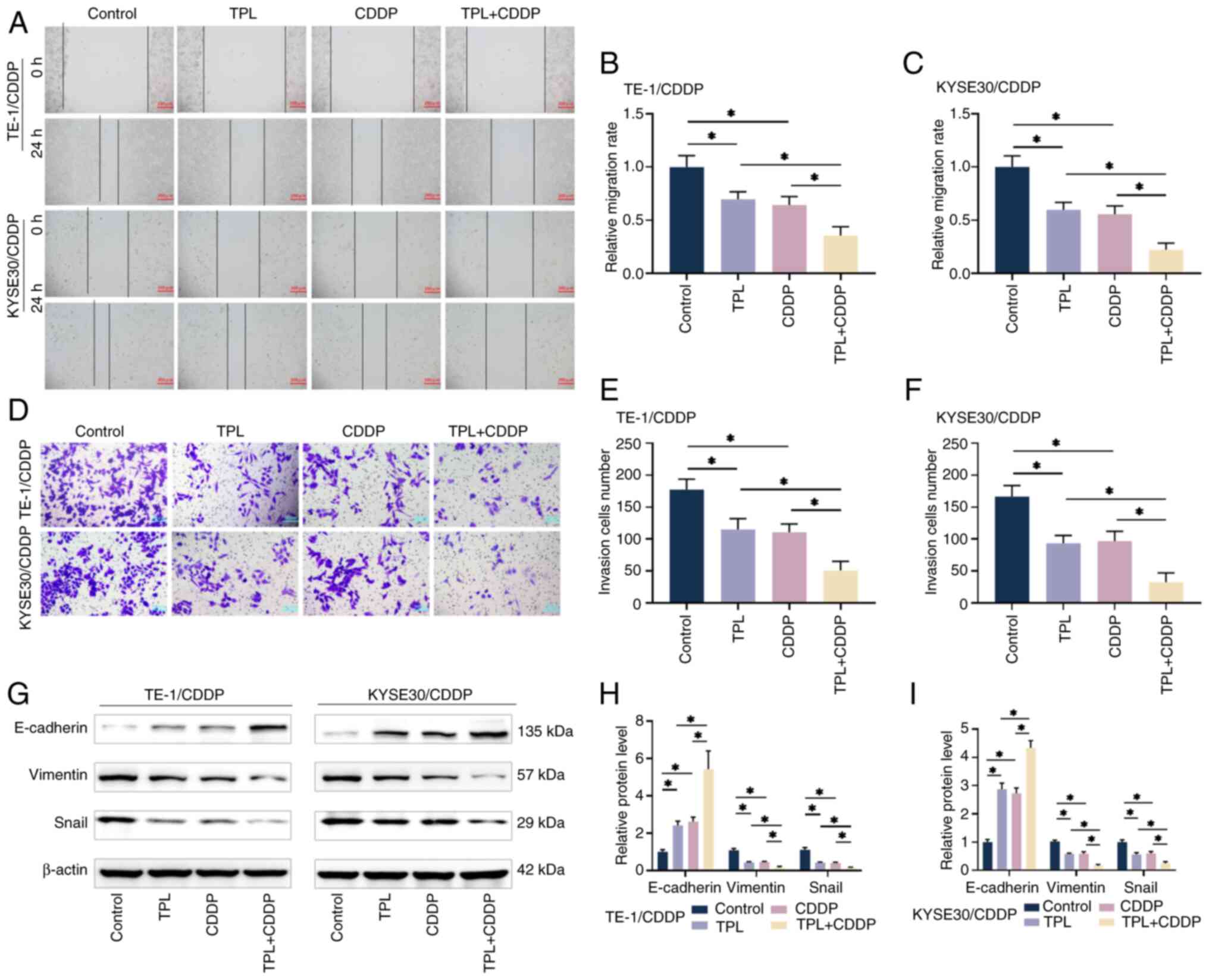 | Figure 4.TPL enhances CDDP inhibition of
CDDP-resistant ESCC cell migration, invasion and EMT effects. (A-C)
The migration levels of TE-1/CDDP and KYSE30/CDDP cells after
treatment with TPL and CDDP were determined using a scratch test
(magnification, ×10; scale bar 200 µm). (D-F) The Transwell assay
was used to determine the quantity of TE-1/CDDP and KYSE30/CDDP
cells that invaded the lower chamber following TPL and CDDP
treatment (magnification, ×40; scale bar, 50 µm). (G-I) Western
blot analysis was used to determine the expression levels of
EMT-related proteins in TE-1/CDDP and KYSE30/CDDP cells following
TPL and CDDP therapy. *P<0.05. TPL, triptolide; CDDP,
cis-diamminedichloro-platinum; ESCC, esophageal squamous cell
carcinoma; EMT, epithelial-mesenchymal transition; TE-1/CDDP,
cisplatin-resistant TE-1 cells; KYSE30/CDDP, cisplatin-resistant
KYSE30 cells. |
TPL increases the sensitivity of
CDDP-resistant ESCC cells to CDDP by suppressing glycolysis
One of the characteristics of tumor cells is the
reprogramming of energy metabolism through glycolysis and
mitochondrial oxidative phosphorylation. The present study
initially assessed the effect of each treatment condition on
glycolysis in CDDP-resistant ESCC cells. As shown in Fig. 5A-D, TPL combined with CDDP
treatment inhibited cellular glucose uptake and lactate production
more than CDDP alone, implying that TPL may further decrease
glycolysis in CDDP-resistant ESCC cells. When cellular glycolysis
creates lactate and excretes it into the environment, protons are
also ejected, resulting in extracellular acidification, making ECAR
a useful diagnostic marker for detecting glycolysis. To investigate
cellular glycolysis, ECAR was evaluated. The results of
normalization calculations are displayed in Fig. 5E and F and all four cell groups
showed the same trend of change. The ECAR values of TE1/CDDP and
KYSE30/CDDP cells rose after the injection of a saturating
concentration of glucose, reflecting the cellular glycolytic
capability at basal conditions. Further injection of oligomycin
inhibited oxidative phosphorylation, allowing cellular glycolysis
to achieve its full capacity and ECAR to rise to its peak. The
subsequent injection of 2-DG hindered glycolysis and ECAR readings
markedly dropped. The CDDP-resistant ESCC cells in the TPL and
CDDP-treated groups had considerably lower ECAR values than the
control group. The combination therapy reduced proton efflux and
ECAR values, suggesting that TPL hindered cellular glycolysis. The
expression levels of key glycolytic genes GLUT1, HK2 and LDHA were
detected by western blotting under different treatment conditions,
as shown in Fig. 5G-I. TPL
combined with CDDP suppressed the levels of these factors more
efficiently, indicating the inhibitory impact of TPL on cellular
glycolysis, which may be an important cause of enhanced CDDP
sensitivity in CDDP-resistant ESCC cells.
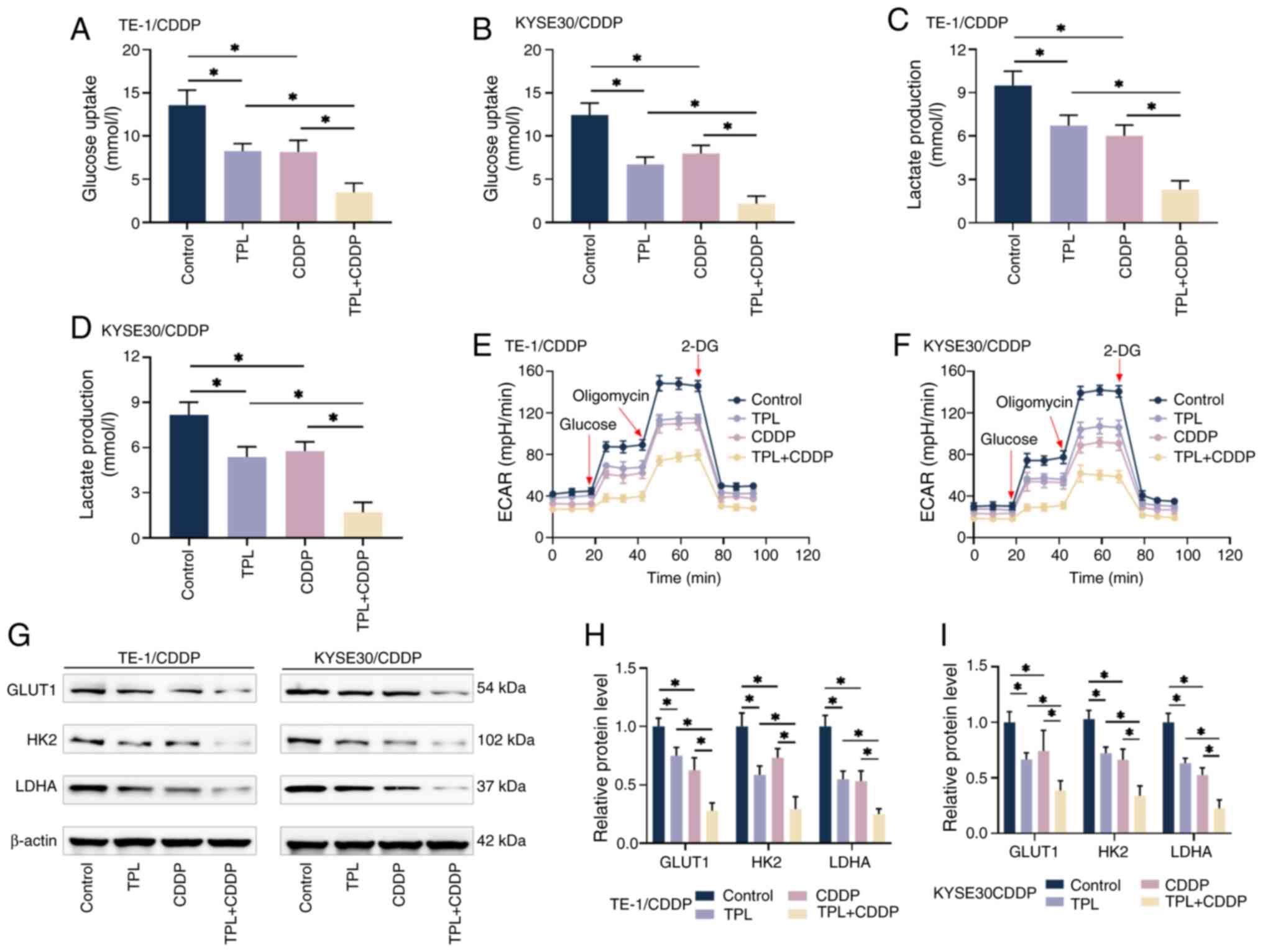 | Figure 5.TPL enhances CDDP sensitivity of
CDDP-resistant ESCC cells via inhibiting glycolysis. (A-D) Kits
were used to detect glucose absorption and lactate generation in
TE-1/CDDP and KYSE30/CDDP cells from each group. (E and F) Kits
were used to detect ECAR in TE-1/CDDP and KYSE30/CDDP cells from
each group in order to assess cellular energy metabolic activity.
(G-I) Western blot analysis revealed changes in the levels of key
glycolysis regulators GLUT1, HK2 and LDHA in TE-1/CDDP and
KYSE30/CDDP cells from each group. *P<0.05. TPL, triptolide;
CDDP, cis-diamminedichloro-platinum; ESCC, esophageal squamous cell
carcinoma; TE-1/CDDP, cisplatin-resistant TE-1 cells; KYSE30/CDDP,
cisplatin-resistant KYSE30 cells; GLUT1, glucose transporter type
1; ECAR, extracellular acidification rate; HK2, hexokinase 2; LDHA,
lactate dehydrogenase A. |
TPL increases the CDDP sensitivity of
CDDP-resistant ESCC cells by generating mitochondrial
dysfunction
The effect of TPL on mitochondrial oxidative
phosphorylation was further investigated. The combined treatment of
2 nM TPL and 4 µM CDDP significantly lowered ATP levels in TE1/CDDP
and KYSE30/CDDP cells, indicating poor mitochondrial energy
metabolism (Fig. 6A). Mitochondria
are the primary intracellular source of ATP and defective
mitochondrial energy metabolism generates a considerable amount of
ROS. The ROS fluorescent probe DCFH-DA was used to measure ROS
levels in CDDP-resistant ESCC cells. As shown in Fig. 6B and C, DCFH-DA fluorescence
signals in TE1/CDDP and KYSE30/CDDP cells were increased in
response to TPL and CDDP treatment alone. Furthermore, the DCFH-DA
fluorescence signal was significantly boosted after combining the
two cell treatments. In addition, a kit was used to investigate
changes in GSH and SOD concentration in the cells. GSH and SOD can
inactivate ROS, thereby protecting mitochondria from free radicals,
maintaining mitochondrial structure and function (41). The results showed that TPL combined
with CDDP significantly decreased the levels of GSH and SOD
compared with the two treatments alone (Fig. 6D and E), indicating that TPL
exacerbated CDDP-induced mitochondrial dysfunction. The effect of
TPL on the mitochondrial membrane potential of CDDP-resistant ESCC
cells was detected using JC-1 probe dye and flow cytometry
(Fig. 6F and G). TE1/CDDP and
KYSE30/CDDP cells treated with 2 nM TPL + 4 µM CDDP showed a
significant decrease in red fluorescence and an increase in green
fluorescence compared with the treatment groups alone. This
suggested a decrease in mitochondrial membrane potential, which may
be one of the important causes of cell death. Cytc is an electron
carrier in the mitochondrial electron transport chain that is
released into the cytosol when the cell is injured, initiating the
creation of apoptotic bodies and resulting in apoptosis (42,43).
As shown in Fig. 6H-J,
CDDP-resistant ESCC cells were treated with various conditions that
resulted in a large amount of Cytc being released into the cytosol
and the Cytc content in the cytosol and mitochondria increased and
decreased, respectively, with the TPL + CDDP treatment group having
the most significant effect. These findings indicated that TPL may
inhibit glycolysis and produce mitochondrial dysfunction, which
alters energy metabolism; this may be the primary explanation for
its ability to increase CDDP sensitivity in CDDP-resistant ESCC
cells.
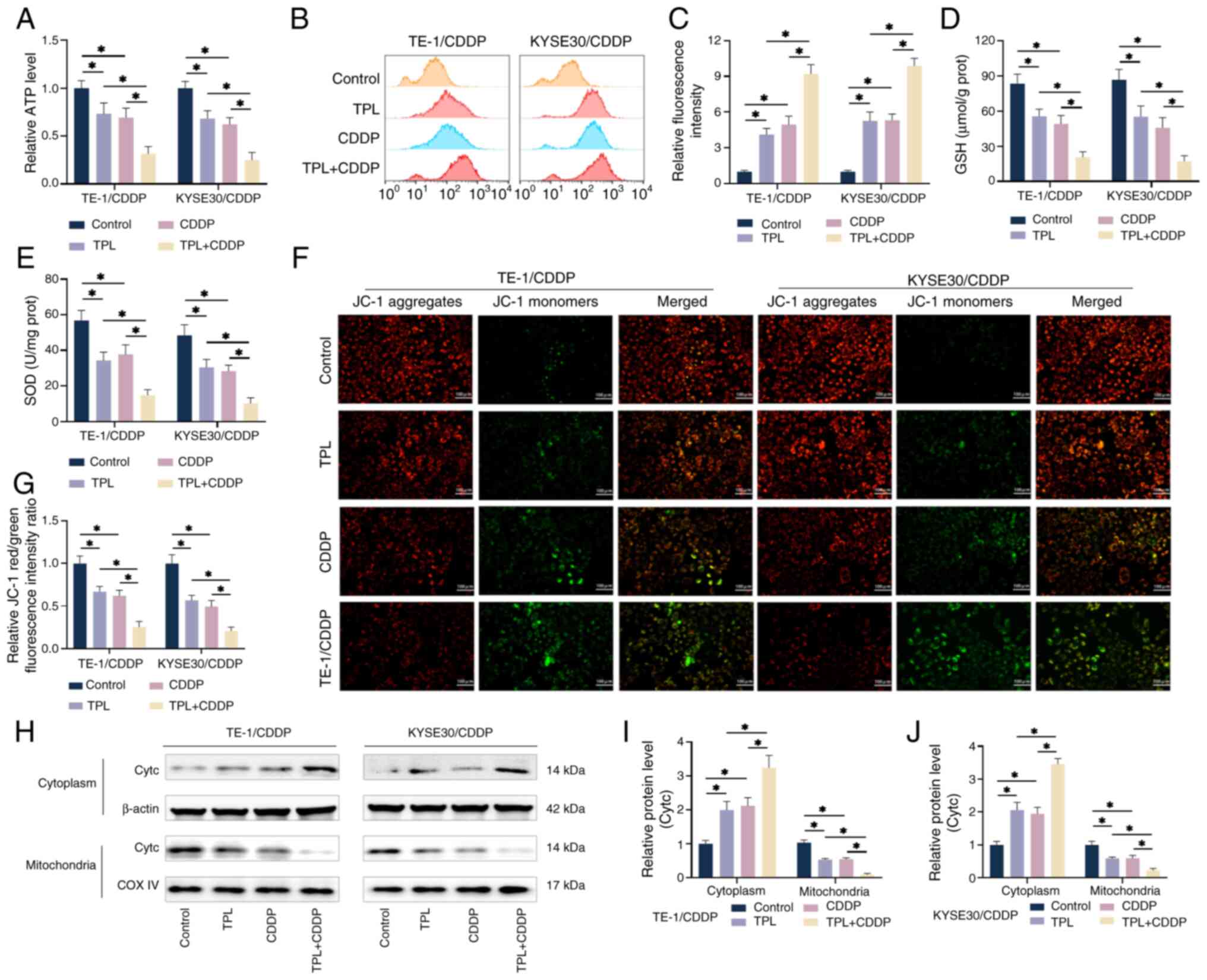 | Figure 6.TPL increases the CDDP sensitivity of
CDDP-resistant ESCC cells via generating mitochondrial dysfunction.
(A) The kit identified ATP generation in TE-1/CDDP and KYSE30/CDDP
cells from each group. (B and C) The ROS generation levels of
TE-1/CDDP and KYSE30/CDDP cells in each group were detected via by
flow cytometry utilizing the DCFH-DA technique. (D and E) The kit
measured the GSH and SOD levels in TE-1/CDDP and KYSE30/CDDP cells
from each group. (F and G) Each group's TE-1/CDDP and KYSE30/CDDP
cells were tested for mitochondrial membrane potential using the
JC-1 assay (magnification, ×20; scale bar 100 µm). (H-J) Western
blot analysis was used to assess cytochrome c levels in the
mitochondria and cytoplasm of TE-1/CDDP and KYSE30/CDDP cells under
various treatment conditions. *P<0.05. TPL, triptolide; CDDP,
cis-diamminedichloro-platinum; ESCC, esophageal squamous cell
carcinoma; TE-1/CDDP, cisplatin-resistant TE-1 cells; KYSE30/CDDP,
cisplatin-resistant KYSE30 cells; GSH, glutathione; SOD, superoxide
dismutase; Cytc, cytochrome c; COX IV, cytochrome c
oxidase IV. |
TPL increases the susceptibility of
CDDP-resistant ESCC cells to CDDP in vivo
To determine whether the addition of TPL had similar
effects on CDDP in vivo as it did in vitro,
5-week-old nude mice were divided into four groups: Control, TPL,
CDDP and TPL + CDDP for the subcutaneous tumor transplantation
experiment (Fig. 7A). KYSE30/CDDP
cells were injected subcutaneously into the right abdomen of naked
mice for 1 week before a subcutaneous tumor developed. After 21
days of various administration regimens, it was revealed that all
administration groups effectively inhibited tumor volume and weight
compared with in the control group (Fig. 7B-D). Notably, the combined
treatment of TPL and CDDP had the highest inhibitory effect on
tumor volume and weight compared with TPL and CDDP alone (Fig. 7B-D). TUNEL staining revealed that
the proportion of positive apoptotic cells in tumor tissues was
considerably higher in the TPL + CDDP group than in the groups
administered TPL and CDDP alone, implying that TPL increased the
apoptosis induced by CDDP administration (Fig. 7E). Furthermore, immunohistochemical
staining revealed that the combination of TPL and CDDP markedly
decreased the levels of the proliferative protein Ki-67 and
promoted the levels of the apoptotic proteins cleaved caspase-3 and
cleaved caspase-9 in the tumor tissues, compared with in the group
administered TPL or CDDP alone (Fig.
7G). The levels of important glycolysis regulators GLUT1, HK2
and LDHA, as well as the ratio of mitochondrial red/green
fluorescence, followed a similar pattern; i.e., control
group>TPL group>CDDP group>TPL + CDDP group, with
statistical significance detected across all groups (Fig. 7H-K). These findings suggested that
TPL may inhibit the glycolysis of CDDP-resistant ESCC cells in
vivo and generate a drop in mitochondrial membrane potential to
promote apoptosis, which may be the primary reasons that TPL
enhances the sensitivity of resistant cells to CDDP.
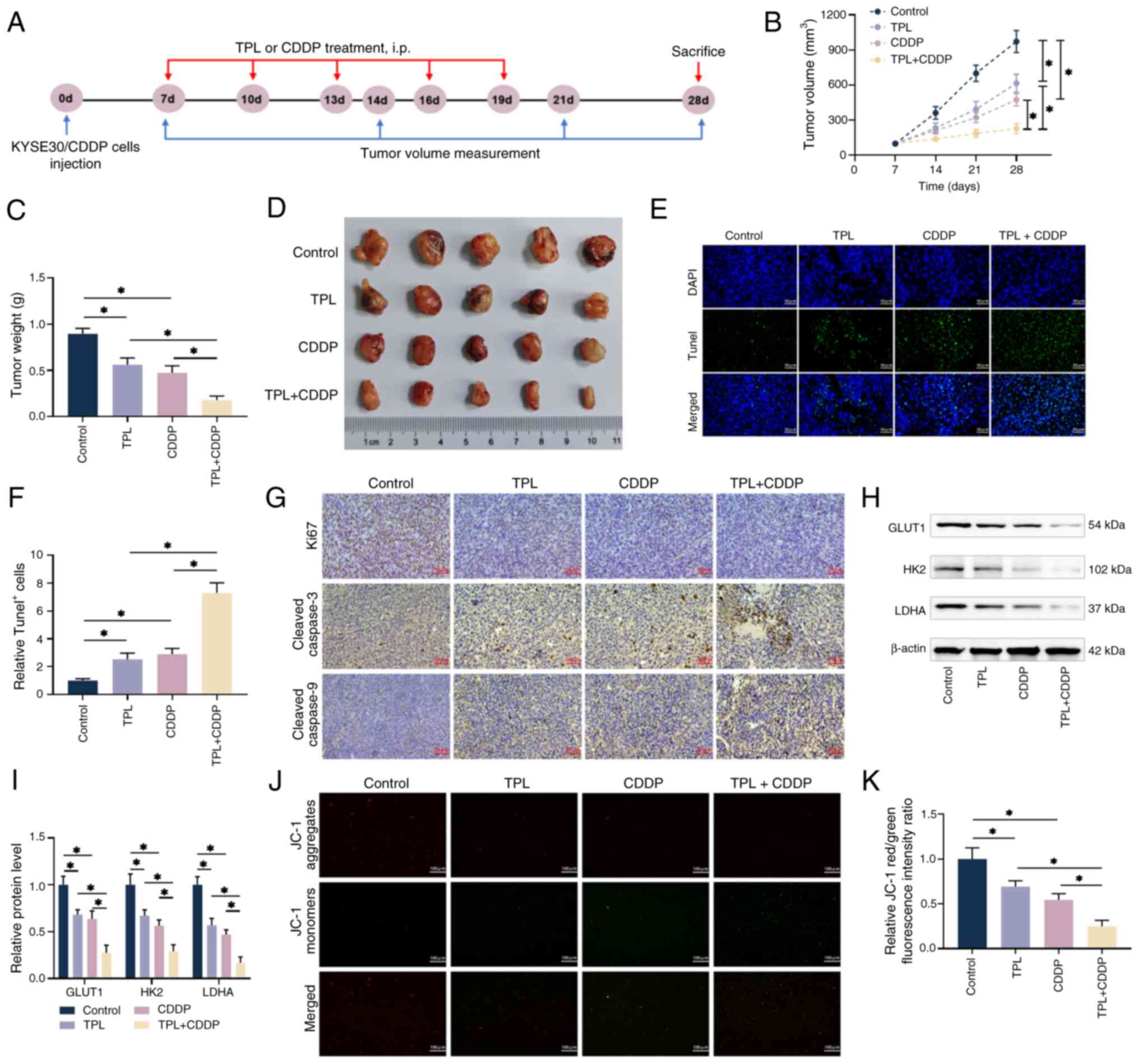 | Figure 7.TPL increases the susceptibility of
CDDP-resistant ESCC cells to CDDP in vivo. (A) Animal models
were constructed according to the timeline. (B-D) Subcutaneous
tumor volumes were measured using vernier calipers and the mice
were killed on the 28th day of feeding, with the isolated tumors
weighed and photographed. (E and F) Tissue sections from the
isolated tumors were produced and apoptosis was identified using
the Tunel method (magnification, ×40; scale bar, 50 µm). (G)
Immunohistochemistry was used to identify the expression levels of
Ki67, cleaved caspase-3 and cleaved caspase-9 in each group's tumor
sections (magnification, ×40; scale bar, 50 µm). (H and I) Western
blot analysis of tumor tissues from each group revealed the
expression levels of major glycolysis regulators GLUT1, HK2 and
LDHA. (J and K) The intensity of red/green fluorescence in tumor
tissues from each group was measured using the JC-1 method to
quantify mitochondrial membrane potential alterations
(magnification, ×20; scale bar 100 µm). *P<0.05. TPL,
triptolide; CDDP, cis-diamminedichloro-platinum; ESCC, esophageal
squamous cell carcinoma; KYSE30/CDDP, cisplatin-resistant KYSE30
cells; DAPI, diamidino-phenyl-indole; GLUT1, glucose transporter
type 1; HK2, hexokinase 2; LDHA, lactate dehydrogenase A. |
Discussion
The efficacy and low toxicity of Chinese herbs make
them highly favorable and potentially useful in clinical cancer
treatment and they have become a research hotspot for several types
of tumor immunotherapy (44). TPL,
an epoxy diterpene lactone compound with multiple biological
activities and a molecular weight of 360.4 g/mol, was first
isolated and named in 1972 from the root of Tripterygium
wilfordii and has been suggested as a promising candidate for
the clinical treatment of tumors and autoimmune diseases (45). TPL overdose affects various tissues
and organs in the body and causes substantial difficulties
associated with toxicity, severely limiting its therapeutic use
(12). As a result, the present
study used the CCK-8 assay to investigate the effect of various
concentrations of TPL on the viability of CDDP-resistant ESCC cells
and the highest active concentration of TPL that did not cause
cytotoxicity was identified (2 nM). At this concentration, the
regulatory effects of TPL on the malignant growth of CDDP-resistant
ESCC cells were evaluated, as well as its effects on glycolysis and
mitochondrial metabolism, in order to demonstrate its function in
CDDP sensitivity and to understand the molecular mechanisms.
One of the most distinguishing aspects of ESCC is
its high invasiveness and metastatic potential. Cancer cells of
epithelial origin can spread locally and regionally by EMT before
entering arteries and resulting in distant metastasis; therefore,
the presence of EMT in cancer cells is seen as a significant phase
in tumor invasion and metastatic progression (46). In the present study, treatment with
TPL and CDDP (particularly a combination of these therapies)
markedly increased the epithelial marker E-cadherin, while
decreasing the mesenchymal marker Vimentin and the EMT
transcription factor Snail, indicating that the process of EMT was
suppressed. The combined treatment of TPL and CDDP also
significantly reduced the cell scratch migration rate and the
number of cells invading the lower chamber of the Transwell system,
indicating that TPL could impede the invasion and metastasis of
CDDP-resistant ESCC cells by suppressing EMT. EMT has also been
proven to alter tumor chemosensitivity. It has been observed that
EMT causes resistance to chemotherapeutic drugs, such as tamoxifen,
CDDP and paclitaxel, in breast cancer cells and that it is
correlated with radioresistance in breast cancer tumors (47). Yu et al (48) reveal that baicalein could inhibit
EMT and promote apoptosis in CDDP-resistant lung adenocarcinoma
cells via the PI3K/Akt/NF-κB pathway; this increases the
susceptibility of drug-resistant lung adenocarcinoma cells to CDDP.
Zhao et al (49) found that
microRNA-128-3p inhibits EMT by downregulating the levels of
EMT-transforming proteins (c-Met, PDGFRα, Notch1 and Slug), making
glioblastoma more sensitive to temozolomide treatment. The
aforementioned research findings indicate that EMT plays a crucial
role in tumor chemosensitivity that cannot be overlooked. In the
present study, treatment with 2 nM TPL + 4 µM CDDP inhibited EMT in
TE1/CDDP and KYSE30/CDDP cells, resulting in increased E-cadherin
and decreased Vimentin and Snail expression. Under this condition,
cell proliferation was suppressed as measured by EdU and clone
formation assays and flow cytometry and western blot analysis
detected elevated rates of apoptosis and severe
G0/G1 cell cycle arrest, indicating that
CDDP-resistant ESCC cells are more sensitive to CDDP. EMT was shown
to be an essential component of acquired CDDP resistance in
CDDP-resistant ESCC cells and TPL could efficiently reverse CDDP
chemoresistance by suppressing the EMT process.
Tumor growth rate is a crucial determinant
influencing patient prognosis and improper energy metabolism has an
impact on tumor growth rate. Reversing the energy metabolism of
tumor cells can make them more chemosensitive (50). One of the most common
characteristics in tumor cells is elevated activation of aerobic
glycolysis, which is known as a cancer hallmark (51). Cancer cell metabolism is largely
dependent on glycolysis and even under normal oxygen concentrations
and with fully functional mitochondria, most cancer cells produce
energy via glycolysis, with the glycolytic pathway accounting for
~50% of ATP production; this is also known as the Warburg effect or
aerobic glycolysis (52). This
metabolic alteration causes larger amounts of lactic acid in the
tumor microenvironment, resulting in tumor acidosis, which aids
cancer cell adaption to hypoxic circumstances and promotes tumor
development and invasiveness (53). The use of aerobic glycolysis or
oxidative phosphorylation by cancer cells is determined by a number
of parameters, including cancer cell stage, tumor microenvironment
and cell growth rate. Rapidly reproducing tumor cells may use
aerobic glycolysis to create ATP for intracellular anabolism, but
slowly growing drug-resistant cancer cells are more likely to rely
on oxidative phosphorylation for survival (54). In the present study, the viability
of TE1/CDDP and KYSE30/CDDP resistant cells treated with different
concentrations of CDDP was much higher than that of parental cells,
implying that the energy metabolism of the two types of cells are
not same, TE1/CDDP and KYSE30/CDDP cells may rely primarily on
aerobic glycolysis to maintain their proliferation. After
co-treatment with TPL and CDDP, CDDP-resistant ESCC cells
proliferated at a slower rate, with considerably more cells in the
G0/G1 phase than the S phase. In addition,
the rate of extracellular acidification caused by glycolysis was
reduced, mitochondrial ATP was depleted, oxidative stress ensued
and substantial amounts of Cytc were released into the cytoplasm.
These findings suggested that TPL combined with CDDP impaired both
energy metabolism modes, which could be the primary explanation for
the effects of TPL on the increased sensitivity of CDDP-resistant
ESCC cells to CDDP. Wang et al (55) discovered that TPL blocks the
nucleotide excision repair pathway in melanoma, increases tumor
cell sensitivity to carboplatin in vitro and in vivo
and decreases tumor development. Zhu et al (56) demonstrate that TPL increases the
susceptibility of non-small cell lung cancer cells to CDDP,
etoposide and epirubicin by blocking the Nrf2-ARE pathway,
decreasing cancer cell proliferation and transplanted tumor growth
in mice in vivo.
The present study also assessed the effects of TPL
on tumor growth in CDDP-resistant mice in vivo. The results
revealed that the tumor volume and mass in the TPL + CDDP group
were significantly lower than those in the treatment only groups.
After the tumor tissues were cut into sections, TUNEL and
immunohistochemical staining were performed to assess apoptosis and
proliferation and the results showed that compared with in the
treatment groups alone, the apoptosis level (TUNEL-positive cells
and cleaved caspase-3 and cleaved caspase-9 staining) of the
sections in the TPL + CDDP combined treatment group was
significantly upregulated, while the proliferation level (Ki67) was
significantly downregulated, indicating that TPL had a sensitizing
effect on CDDP-resistant ESCC tumors, inducing apoptosis and
inhibiting cell proliferation. In addition, TPL hindered glycolysis
and caused mitochondrial damage in vivo, as evidenced by
alterations in major glycolysis regulators and mitochondrial
membrane potential measurements. The present study elucidated the
effect of TPL on CDDP-resistant cells using in vivo and
in vitro experiments. In light of the current findings, it
may be hypothesized that TPL has the potential to be a therapeutic
medication for the treatment of chemotherapy-resistant malignant
tumors.
TPL has been shown to sensitize CDDP-resistant ESCC
cells to CDDP by inhibiting glycolysis and promoting mitochondrial
dysfunction. However, the factors that influence the sensitization
of CDDP-resistant ESCC cells in vivo are complex and include
DNA repair, apoptosis, the tumor microenvironment and tumor stem
cells. In the future, it will be necessary to explore and further
identify the TPL sensitization pathway.
In conclusion, the present study revealed that TPL
may reduce the development of CDDP-resistant ESCC cells in
vitro and in vivo. The CDDP-sensitizing effect of TPL
could be due to the inhibition of the glycolytic pathway and the
induction of mitochondrial impairment in ESCC cells, which impairs
the proliferation, migration, invasion and EMT processes of
CDDP-resistant ESCC cells and promotes apoptosis to inhibit ESCC
tumor growth (Fig. 8). The
findings of the present study may serve as a theoretical foundation
for the future development of CDDP-sensitizing medicines. For
energy metabolism, it is necessary to further observe the changes
in ESCC cell proliferation and the tumor microenvironment under
different conditions of chemotherapeutic drugs and TPL, as well as
to analyze the energy metabolism of ESCC cells under different
conditions, in order to further identify the chemonsensitizing
mechanism of TPL. Furthermore, in the future, it is necessary to
optimize the concentration of TPL and apply it in clinical trials,
to conduct more and longer-term follow-up investigations, to
analyze the effect of TPL on the prognosis of patients with ESCC
treated with radiotherapy and to assess the practical value of its
supportive clinical application. Due to their multi-component
nature, herbal mixtures can act on multiple pathways and targets in
the human body, making them more conducive to improving
chemotherapeutic efficacy while reducing toxic side effects.
However, the specific clinical effects of ATL-I are still unclear
and, in the future, it will be necessary to further improve the
toxicity testing and clinical trials and to use gene editing
technology combined with transcriptomics, epigenetics and other
methods to explore the transcriptional regulation mechanism of
ATL-I metabolism, transport, immunity and other related genes.
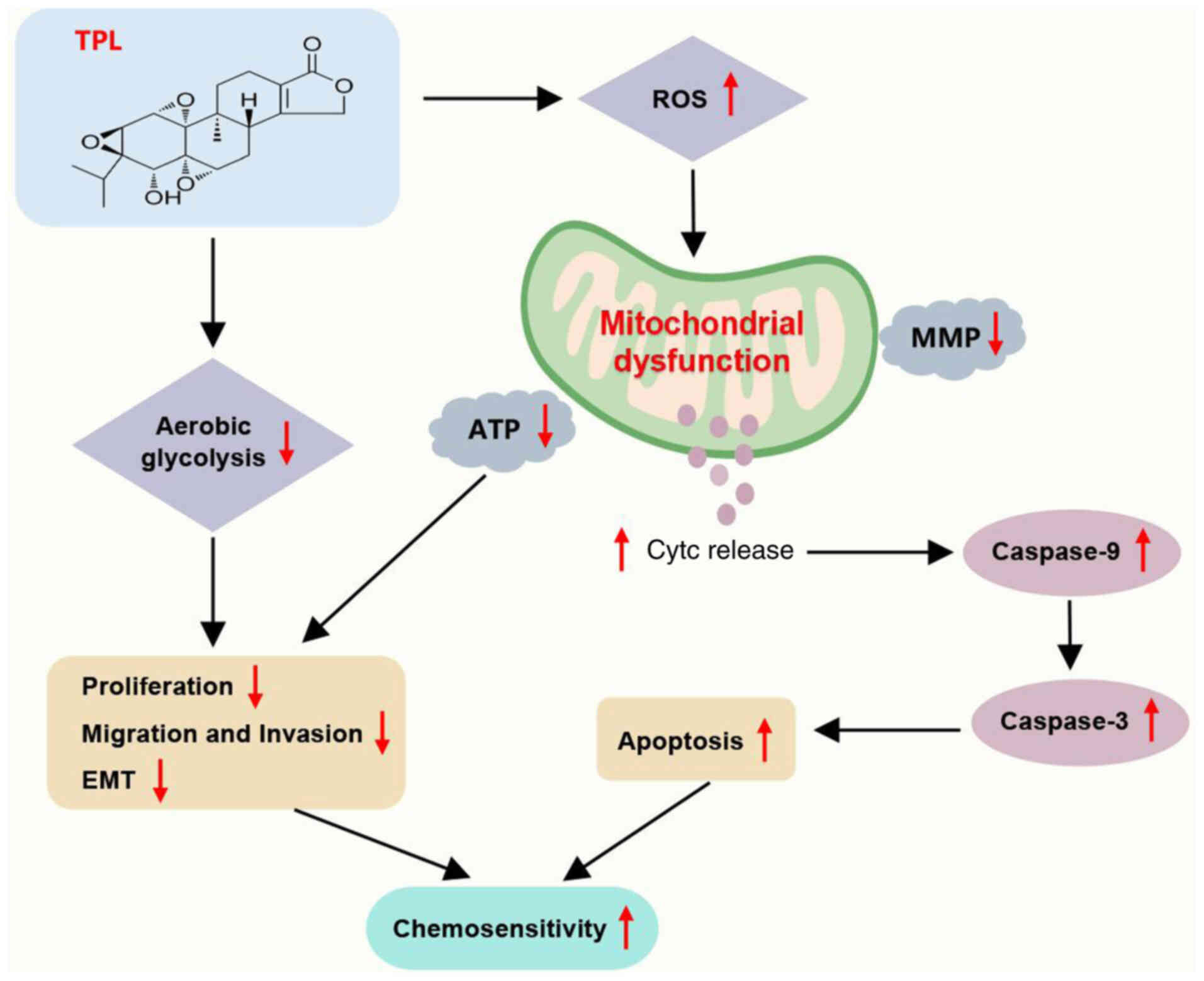 | Figure 8.Diagram of the action mechanism. TPL
inhibits glycolysis and causes mitochondrial dysfunction (elevated
ROS, decreased mitochondrial membrane potential and ATP and Cytc
release), which further inhibits ESCC cell proliferation,
migration, invasion and EMT, as well as promotes apoptosis
(elevated caspase-9 and caspase-3), thereby inhibiting ESCC cell
resistance to CDDP. TPL, triptolide; ROS, reactive oxygen species;
Cytc, cytochrome c; ESCC, esophageal squamous cell
carcinoma; EMT, epithelial-mesenchymal transition; MMP,
mitochondrial membrane potential; CDDP,
cis-diamminedichloro-platinum. |
TPL may be used in the future, in conjunction with
other herbal components, to establish efficient biological
screening and formulation research based on herbal components, as
well as clinical applications and industrial needs to fully explore
the essence of traditional Chinese medicine.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KL and JiaL conceived and designed the research,
conducted experiments and analyzed data as well as drafting and
revising the manuscript critically for important intellectual
content and confirmed the authenticity of all the raw data. TM, NW
and JuL contributed to the acquisition, analysis and interpretation
of data and provided substantial intellectual input during the
drafting and revision of the manuscript. MQ, LD and JinL
participated in the conception and design of the present study and
played a key role in data interpretation and manuscript
preparation. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by Department of
Thoracic Surgery, The First Affiliated Hospital of Zhengzhou
University (approval no. 20220096).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Conway E, Wu H and Tian L: Overview of
risk factors for esophageal squamous cell carcinoma in China.
Cancers (Basel). 15:56042023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li J, Xu J, Zheng Y, Gao Y, He S, Li H,
Zou K, Li N, Tian J, Chen W and He J: Esophageal cancer:
Epidemiology, risk factors and screening. Chin J Cancer Res.
33:535–547. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen R, Zheng R, Zhang S, Wang S, Sun K,
Zeng H, Li L, Wei W and He J: Patterns and trends in esophageal
cancer incidence and mortality in China: An analysis based on
cancer registry data. J Natl Cancer Cent. 3:21–27. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang CS and Chen XL: Research on
esophageal cancer: With personal perspectives from studies in China
and Kenya. Int J Cancer. 149:264–276. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li H, Cheng S, Zhai J, Lei K, Zhou P, Cai
K and Li J: Platinum based theranostics nanoplatforms for antitumor
applications. J Mater Chem B. 11:8387–8403. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang C, Xu C, Gao X and Yao Q:
Platinum-based drugs for cancer therapy and anti-tumor strategies.
Theranostics. 12:2115–2132. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang J, Liu X, Cao S, Dong X, Rao S and
Cai K: Understanding Esophageal Cancer: The challenges and
opportunities for the next decade. Front Oncol. 10:17272020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ashrafizadeh M: Cell death mechanisms in
human cancers: Molecular pathways, therapy resistance and
therapeutic perspective. J Cancer Biol Ther. 1:17–40. 2024.
|
|
10
|
Lv H, Jiang L, Zhu M, Li Y, Luo M, Jiang
P, Tong S, Zhang H and Yan J: The genus Tripterygium: A
phytochemistry and pharmacological review. Fitoterapia.
137:1041902019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng K, Li X, Bai Y, Zhang D and Tian L:
Mechanisms of cancer cell death induction by triptolide: A
comprehensive overview. Heliyon. 10:e243352024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song J, He GN and Dai L: A comprehensive
review on celastrol, triptolide and triptonide: Insights on their
pharmacological activity, toxicity, combination therapy, new dosage
form and novel drug delivery routes. Biomed Pharmacother.
162:1147052023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fanelli M, Tavanti E, Patrizio MP, Vella
S, Fernandez-Ramos A, Magagnoli F, Luppi S, Hattinger CM and Serra
M: Cisplatin resistance in osteosarcoma: In vitro validation of
candidate DNA repair-related therapeutic targets and drugs for
tailored treatments. Front Oncol. 10:3312020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu W, Li J, Wu S, Li S, Le L, Su X, Qiu
P, Hu H and Yan G: Triptolide cooperates with Cisplatin to induce
apoptosis in gemcitabine-resistant pancreatic cancer. Pancreas.
41:1029–1038. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu H, Zhu S, Tong Y, Huang G, Tan B and
Yang L: Antitumor activity of triptolide in SKOV3 cells and
SKOV3/DDP in vivo and in vitro. Anticancer Drugs. 31:483–491. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang G, Hu H, Zhang Y, Zhu Y, Liu J, Tan
B and Chen T: Triptolide sensitizes cisplatin-resistant human
epithelial ovarian cancer by inhibiting the phosphorylation of AKT.
J Cancer. 10:3012–3020. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hamdi AM, Jiang ZZ, Guerram M, Yousef BA,
Hassan HM, Ling JW and Zhang LY: Biochemical and computational
evaluation of Triptolide-induced cytotoxicity against NSCLC. Biomed
Pharmacother. 103:1557–1566. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li L, Wang C, Qiu Z, Deng D, Chen X, Wang
Q, Meng Y, Zhang B, Zheng G and Hu J: Triptolide inhibits
intrahepatic cholangiocarcinoma growth by suppressing glycolysis
via the AKT/mTOR pathway. Phytomedicine. 109:1545752023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Su Y, Yang S, Xiao Z, Wang W, Okunieff P
and Zhang L: Triptolide alters mitochondrial functions. Adv Exp Med
Biol. 599:139–146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cai J, Yi M, Tan Y, Li X, Li G, Zeng Z,
Xiong W and Xiang B: Natural product triptolide induces
GSDME-mediated pyroptosis in head and neck cancer through
suppressing mitochondrial hexokinase-IotaIota. J Exp Clin Cancer
Res. 40:1902021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou H, Liu Y, Wang C, Liu L, Wang H,
Zhang Y, Long C and Sun X: Triptolide inhibits Epstein-Barr nuclear
antigen 1 expression by increasing sensitivity of mitochondria
apoptosis of nasopharyngeal carcinoma cells. J Exp Clin Cancer Res.
37:1922018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang H, Che W, Peng F, Chen H, Xie X and
Wu B: Triptolide inhibits esophageal squamous cell carcinoma
progression by regulating the circNOX4/miR-153-3p/SATB1 signaling
pathway. Thorac Cancer. 15:538–549. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yanchun M, Yi W, Lu W, Yu Q, Jian Y,
Pengzhou K, Ting Y, Hongyi L, Fang W, Xiaolong C and Yongping C:
Triptolide prevents proliferation and migration of esophageal
squamous cell cancer via MAPK/ERK signaling pathway. Eur J
Pharmacol. 851:43–51. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu L, Zhu X and Wu Y: Effects of glucose
metabolism, lipid metabolism, and glutamine metabolism on tumor
microenvironment and clinical implications. Biomolecules.
12:5802022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rigoulet M, Bouchez CL, Paumard P, Ransac
S, Cuvellier S, Duvezin-Caubet S, Mazat JP and Devin A: Cell energy
metabolism: An update. Biochim Biophys Acta Bioenerg.
1861:1482762020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liberti MV and Locasale JW: The warburg
effect: How does it benefit cancer cells? Trends Biochem Sci.
41:211–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reinfeld BI, Rathmell WK, Kim TK and
Rathmell JC: The therapeutic implications of immunosuppressive
tumor aerobic glycolysis. Cell Mol Immunol. 19:46–58. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y and Patti GJ: The Warburg effect: A
signature of mitochondrial overload. Trends Cell Biol.
33:1014–1020. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang CH, Qiu J, O'Sullivan D, Buck MD,
Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ,
et al: Metabolic competition in the tumor microenvironment is a
driver of cancer progression. Cell. 162:1229–1241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu F, Ma F, Wang Y, Hao L, Zeng H, Jia C,
Wang Y, Liu P, Ong IM, Li B, et al: PKM2 methylation by CARM1
activates aerobic glycolysis to promote tumorigenesis. Nat Cell
Biol. 19:1358–1370. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bai R and Cui J: Mitochondrial immune
regulation and anti-tumor immunotherapy strategies targeting
mitochondria. Cancer Lett. 564:2162232023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Paul S, Ghosh S and Kumar S: Tumor
glycolysis, an essential sweet tooth of tumor cells. Semin Cancer
Biol. 86:1216–1230. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie Y, Wang M, Xia M, Guo Y, Zu X and
Zhong J: Ubiquitination regulation of aerobic glycolysis in cancer.
Life Sci. 292:1203222022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zong WX, Rabinowitz JD and White E:
Mitochondria and cancer. Mol Cell. 61:667–676. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun H, Wang H and Wang X, Aoki Y and Wang
X, Yang Y, Cheng X, Wang Z and Wang X: Aurora-A/SOX8/FOXK1
signaling axis promotes chemoresistance via suppression of cell
senescence and induction of glucose metabolism in ovarian cancer
organoids and cells. Theranostics. 10:6928–6945. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yun J, Mullarky E, Lu C, Bosch KN,
Kavalier A, Rivera K, Roper J, Chio II, Giannopoulou EG, Rago C, et
al: Vitamin C selectively kills KRAS and BRAF mutant colorectal
cancer cells by targeting GAPDH. Science. 350:1391–1396. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yan F, Zhao W, Xu X, Li C, Li X, Liu S,
Shi L and Wu Y: LncRNA DHRS4-AS1 inhibits the stemness of NSCLC
cells by sponging miR-224-3p and upregulating TP53 and TET1. Front
Cell Dev Biol. 8:5852512020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lane AN, Higashi RM and Fan TW: Metabolic
reprogramming in tumors: Contributions of the tumor
microenvironment. Genes Dis. 7:185–198. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
China National Certificatino and
Accreditation Administration: The People's Republic of China
Certification and Accreditation Industry Standards (RB/T 173-2018):
Guideline of Assessment for Humane Endpoints in Animal Experiments.
2018. http://rbtest.cnca.cn/cnca_kfs/file/read/c61b11ea7094db88fc82793142dbb977April
21–2024
|
|
40
|
Simoneschi D, Rona G, Zhou N, Jeong YT,
Jiang S, Milletti G, Arbini AA, O'Sullivan A, Wang AA, Nithikasem
S, et al: CRL4(AMBRA1) is a master regulator of D-type cyclins.
Nature. 592:789–793. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jomova K, Alomar SY, Alwasel SH,
Nepovimova E, Kuca K and Valko M: Several lines of antioxidant
defense against oxidative stress: Antioxidant enzymes,
nanomaterials with multiple enzyme-mimicking activities, and
low-molecular-weight antioxidants. Arch Toxicol. 98:1323–1367.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kalpage HA, Wan J, Morse PT, Zurek MP,
Turner AA, Khobeir A, Yazdi N, Hakim L, Liu J, Vaishnav A, et al:
Cytochrome c phosphorylation: Control of mitochondrial electron
transport chain flux and apoptosis. Int J Biochem Cell Biol.
121:1057042020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Morse PT, Arroum T, Wan J, Pham L,
Vaishnav A, Bell J, Pavelich L, Malek MH, Sanderson TH, Edwards BFP
and Hüttemann M: Phosphorylations and acetylations of cytochrome c
control mitochondrial respiration, mitochondrial membrane
potential, energy, ROS, and Apoptosis. Cells. 13:4932024.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nie J, Zhao C, Deng LI, Chen J, Yu B, Wu
X, Pang P and Chen X: Efficacy of traditional Chinese medicine in
treating cancer. Biomed Rep. 4:3–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Noel P, Von Hoff DD, Saluja AK, Velagapudi
M, Borazanci E and Han H: Triptolide and its derivatives as cancer
therapies. Trends Pharmacol Sci. 40:327–341. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xian D and Zhao Y: LncRNA KCNQ1OT1
enhanced the methotrexate resistance of colorectal cancer cells by
regulating miR-760/PPP1R1B via the cAMP signalling pathway. J Cell
Mol Med. 23:3808–3823. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hashemi M, Arani HZ, Orouei S, Fallah S,
Ghorbani A, Khaledabadi M, Kakavand A, Tavakolpournegari A, Saebfar
H, Heidari H, et al: EMT mechanism in breast cancer metastasis and
drug resistance: Revisiting molecular interactions and biological
functions. Biomed Pharmacother. 155:1137742022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yu M, Qi B, Xiaoxiang W, Xu J and Liu X:
Baicalein increases cisplatin sensitivity of A549 lung
adenocarcinoma cells via PI3K/Akt/NF-ĸB pathway. Biomed
Pharmacother. 90:677–685. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhao C, Guo R, Guan F, Ma S, Li M, Wu J,
Liu X, Li H and Yang B: MicroRNA-128-3p enhances the
chemosensitivity of temozolomide in glioblastoma by targeting c-Met
and EMT. Sci Rep. 10:94712020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu G and Song G: Regulation of tumor cell
glycometabolism and tumor therapy. Sheng Wu Yi Xue Gong Cheng Xue
Za Zhi. 36:691–695. 2019.(In Chinese). PubMed/NCBI
|
|
51
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bhattacharya B, Mohd Omar MF and Soong R:
The Warburg effect and drug resistance. Br J Pharmacol.
173:970–979. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Andreucci E, Peppicelli S, Ruzzolini J,
Bianchini F, Biagioni A, Papucci L, Magnelli L, Mazzanti B, Stecca
B and Calorini L: The acidic tumor microenvironment drives a
stem-like phenotype in melanoma cells. J Mol Med (Berl).
98:1431–1446. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ippolito L, Marini A, Cavallini L, Morandi
A, Pietrovito L, Pintus G, Giannoni E, Schrader T, Puhr M, Chiarugi
P and Taddei ML: Metabolic shift toward oxidative phosphorylation
in docetaxel resistant prostate cancer cells. Oncotarget.
7:61890–61904. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang G, Guo H, Ren Y, Chen W, Wang Y, Li
J, Liu H, Xing J, Zhang Y and Li N: Triptolide enhances
carboplatin-induced apoptosis by inhibiting nucleotide excision
repair (NER) activity in melanoma. Front Pharmacol. 14:11574332023.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhu J, Wang H, Chen F, Lv H, Xu Z, Fu J,
Hou Y, Xu Y and Pi J: Triptolide enhances chemotherapeutic efficacy
of antitumor drugs in non-small-cell lung cancer cells by
inhibiting Nrf2-ARE activity. Toxicol Appl Pharmacol. 358:1–9.
2018. View Article : Google Scholar : PubMed/NCBI
|