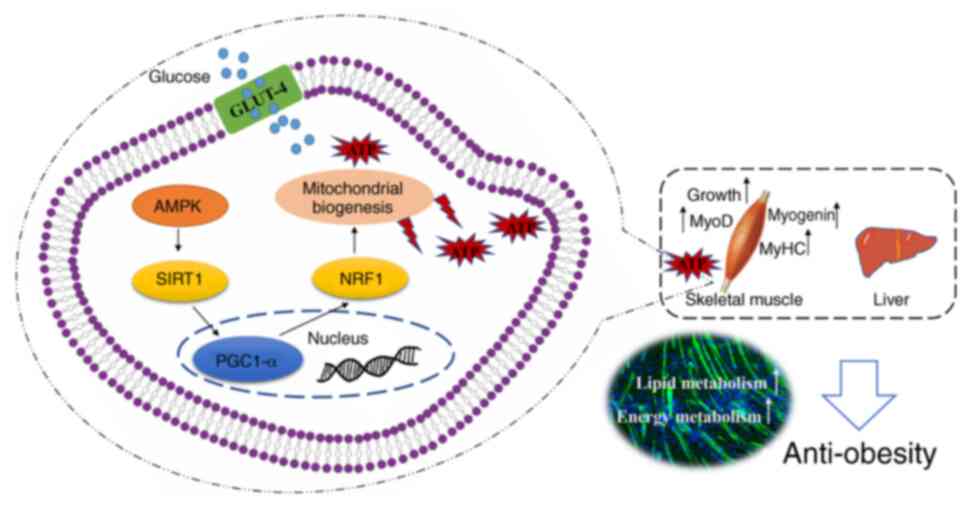
Introduction
Obesity is a metabolic disease that causes the body
to accumulate excess fat or adipose tissue. Since it is related to
cardiovascular diseases, diabetes, hypertension, atherosclerosis
and other chronic diseases, it has become an important health
problem worldwide (1). Factors
such as unhealthy dietary habits, a prevalence of high-energy-dense
foods, a lack of physical activity, and an increase in
high-pressure work and stress promote the occurrence of obesity
(2). However, classical treatment
methods for obesity often require long-term persistence and
lifestyle changes, which are difficult to practice consistently and
are often unsatisfactory (3).
Therefore, new treatment strategies for obesity are required, such
as the use of natural traditional medicine products that have
notable therapeutic effects, favorable safety profiles and clinical
evidence.
Asari Radix et Rhizoma (Asarum; xì xīn) is
the root and rhizome of Aristolochiaceae plants, such as Asarum
heterotropoides Fr. Schmidt var. mandshuricum (Maxim.),
Asarum sieboldii Miq. var. seoulense Nakai and Asarum
sieboldii Miq (4). In
traditional Chinese and Korean medicine, Asarum is a herb
with pungent and warm properties, which is believed to specifically
target the heart, lungs and kidneys by exerting its effects through
their corresponding body meridians or channels, which are believed
to have connections to internal organs. Asarum has the
herbal effects of dispelling wind, dispersing cold, relieving pain,
warming the lungs and reducing phlegm (5). Its synergistic blend with ephedra and
aconite can yield a potent effect on inducing sweating and
alleviating surface discomfort. Asarum also serves a pivotal
role in safeguarding the cardiovascular system enhancing the
circulation of blood and supporting the maintenance of normal
metabolic activity within the body. Additionally, it exhibits a
variety of pharmacological attributes, encompassing
anti-inflammatory, antioxidant and cognitive-enhancing properties
(6). The effect of Asarum
on increasing body heat production and metabolic levels may have
certain benefits for obese patients.
Molecular docking and network pharmacology analysis
is a useful technology for exploring the use of herbal medicines in
disease treatment (7), and it is
widely used to study the mechanism by which the active ingredients
of herbal medicines work in the treatment of various diseases. The
network pharmacological analysis can successfully anticipate the
active constituents, action targets and mechanisms inherent in
herbal medicines, thus reducing the cost of drug development, and
providing new ideas for analyzing the material basis and
application of herbal medicines in treating diseases (8).
The present study explored the effects of
Asarum extract on long-term high-fat diet (HFD)-induced
obese mice by predicting and analyzing its molecular targets using
network pharmacological and molecular docking analysis. In
addition, the present study investigated the mechanism of action of
Asarum extract in C2C12 myotubes.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM) and
penicillin/streptomycin (P/S) were supplied by Corning, Inc. Fetal
bovine serum (FBS; cat. no. 16000044), horse serum (HS; cat. no.
16050122), tissue protein lysis buffer (cat. no. 78510) and
radioimmunoprecipitation assay (RIPA) lysis buffer (cat. no. 89901)
were supplied by Thermo Fisher Scientific, Inc. Protein assay
buffer (cat. no. 5000006) was obtained from Bio-Rad Laboratories,
Inc. Antibodies against nuclear respiratory factor 1 (NRF1;
1:1,000; cat. no. 69432), phosphorylated (p)-AMP-activated protein
kinase (AMPK; 1:1,000; cat. no. 2537) and AMPK (1:1,000; cat. no.
2532) were purchased from Cell Signaling Technology, Inc.
Antibodies against glucose transporter type 4 (GLUT4; 1:500; cat.
no. sc-7938), MyoD (1:1,000; cat. no. sc-377460), myogenin
(1:1,000; cat. no. sc-52903) and myosin heavy chain (MyHC; 1:500;
cat. no. sc-376157) were obtained from Santa Cruz Biotechnology,
Inc. Antibodies against sirtuin 1 (SIRT1; 1:1,000; cat. no.
bs-0921R) were purchased from BIOSS. Antibodies against peroxisome
proliferator-activated receptor γ coactivator 1-α (PGC1α; 1:1,000;
cat no. NBP1-04676) were purchased from Novus Biologicals, LLC
(Bio-Techne). Horseradish peroxidase-conjugated goat anti-rabbit
immunoglobulin G (IgG) antibody (1:2,000; cat. no. BR1706515),
anti-mouse IgG antibody (1:2,000; cat. no. BR1706516) and
anti-β-actin (1:2,000; cat. no. A1978) were supplied by
MilliporeSigma. Anti-GAPDH (1:2,000; cat. no. LF-PA0018) was
supplied by Abfrontier. EveryBlot Blocking Buffer (cat. no.
12010020) was purchased from Bio-Rad Laboratories, Inc. Metformin
was obtained from MilliporeSigma (cat. no. 317240). Asarinin [cat.
no. MUST-22050912; purity assessed by high performance liquid
chromatography (HPLC), ≥98%] and sesamin (cat. no. MUST-21110513;
purity assessed by HPLC, ≥98%) were acquired from Chengdu Must
Bio-Technology Co., Ltd. Aspartate aminotransferase (AST; cat. no.
C010-2-1), alanine aminotransferase (ALT; cat. no. C009-2-1), blood
urea nitrogen (BUN; cat. no. C013-2-1) and creatinine (CRE; cat.
no. C011-2-1) were purchased from Nanjing Jiancheng Bioengineering
Institute.
Data preparation
The Traditional Chinese Medicine Systems
Pharmacology Database and Analysis Platform (TCMSP) was used to
analyze the data (http://tcmspw.com/index.php) (9); all chemical components of
Asarum were obtained by a preliminary search using ‘Asari
Radix Et Rhizoma’ as the key word. The search results were screened
for oral bioavailability (OB) ≥30%, and for drug-like properties
(DL) ≥0.18 in the chemical composition of Asarum. Based on
the aforementioned results, the protein targets of Asarum
active ingredients were systematically searched and matched using
the TCMSP database. Disease targets were searched in the Gene Cards
database (https://www.genecards.org) (10) and Online Mendelian Inheritance in
Man database (https://www.omim.org/) (11) using ‘obesity’ as the key word.
Protein-protein interaction (PPI)
network model construction and enrichment analysis
To identify the intersection points between
Asarum and obesity, the active ingredients and
obesity-related protein targets were imported to the Venn online
database (https://bioinfogp.cnb.csic.es/) (12). For PPI network diagram
construction, the original data were exported to Cytoscape (version
3.9.0; http://cytoscape.org/) from the STRING
database (https://string–db.org/) (13). Targets for treating obesity were
imported into the DAVID database (https://david.ncifcrf.gov/) (14). The biological process (BP), cell
component (CC) and molecular function (MF) terms of the targets
were then analyzed using Gene Ontology (GO) and Kyoto Encyclopedia
of Genes and Genomes (KEGG) metabolic pathway enrichment analyses.
The selection criteria were P<0.05 and gene count ≥5, ensuring
robust and meaningful results. The online drawing tool Omic Share
Tool (https://www.omicshare.com/tools/index.php/) was used
to depict the enrichment results and explain the mechanism of
action of Asarum in obesity treatment.
Molecular docking analysis
A molecular docking analysis was conducted based on
the network pharmacological prediction results. The selected core
targets and main components were employed for molecular docking
analysis. Initially, the protein structures of the selected targets
were retrieved from the Protein Data Bank (PDB; http://www.rcsb.org/). Subsequently, the necessary
ligands and proteins for molecular docking were generated. The PDB
file was preprocessed using the PyMOL software (version 2.5.5;
http://www.pymol.org/) to remove irrelevant water
molecules and unnecessary ion structures. The structures of the
main components were retrieved from PubChem (https://pubchem.ncbi.nlm.nih.gov/). Standard
processing of protein receptors and small molecule ligands was
performed using the AutoDock (version, 4.2.6) software (https://autodock.scripps.edu/). Subsequently, the
AutoDock Vina (version, 1.1.2; http://vina.scripps.edu/) software was employed for
molecular docking and the computation of binding energy (15). The obtained results were visualized
using the PyMOL software.
Asarum extract powder preparation
The dried roots of Asarum heterotropoides Fr.
Schmidt var. mandshuricum (Maxim.) Kitag. (Aristolochiaceae) were
obtained from a herbal company (Nemomedat Co. Ltd.). This herb was
recognized by Professor Y. K. Park, a herbology expert at Dongguk
University (Gyeongju, Korea) and the voucher specimen (no.
K1042200217) has been deposited in the Herbarium of College of
Korean Medicine, Dongguk University. Asarum (200 g) was
weighed and extracted with boiling water (2 l) for 3 h, the
filtrate was collected after filtration and concentrated under a
vacuum, before freeze-drying. The resulting Asarum extract
(yield, 27.82%) was kept at 4°C.
HPLC analysis
The content of the two active components in
Asarum extract was determined using an Agilent Technologies
1260 Infinity II HPLC system using asarinin and sesamin as standard
compounds. In addition, the levels of aristolochic acid A in both
Asarum and its extract were analyzed. Further details are
provided in Appendix I.
Cell culture and treatment
C2C12 myoblasts, a mouse skeletal muscle cell line,
were obtained from American Type Culture Collection (cat. no.
CRL-1772). Myoblasts were cultured in DMEM supplemented with 10%
FBS and 1% P/S. After reaching 70–80% confluence, the cells were
incubated in a 5% CO2 incubator at 37°C; the medium was
then switched to differentiation medium supplemented with 2% HS
once daily for another 4 days. Differentiated C2C12 myotubes were
classified into five groups as follows: Normal non-treated,
Asarum extract-treated (0.25, 0.5 or 1 mg/ml) and
metformin-treated (2.5 mmol/ml) groups. The appropriate dosage of
Asarum extract for treatment was determined using a cell
viability assay (Fig. S1;
Appendix I). In all groups, with
the exception of the normal group, the drugs were administered to
C2C12 myotubes for 24 h. Additionally, to assess the in
vitro nephrotoxicity of Asarum extract, 293 cells were
utilized. The 293 cells (cat. no. 84129; Korean Cell Line Bank)
were cultured in DMEM medium supplemented with 10% FBS and 1% P/S.
The cells were incubated at 37°C with 5% CO2 and 70–80%
relative humidity in a cell culture incubator. Logarithmically
proliferating 293 cells were seeded into 6-well plates
(1×105/ml) and cultured for 24 h. The concentration
range of Asarum extract was determined using a cell
viability assay (Fig. S2;
Appendix I). Different
concentrations of Asarum extract (0.25, 0.5 and 1 mg/ml)
were added to the experimental groups, while the blank group
received an equal volume of untreated medium. After 48 h of
incubation at 37°C, cell morphology was observed and images were
captured under a fluorescence microscope (Leica Microsystems GmbH)
(Fig. S3; Appendix I).
Immunocytochemistry
To assess MyHC expression in cellular structures,
C2C12 myotubes were treated with various amounts of Asarum
extract for 24 h following myoblast differentiation into myotubes.
The experimental procedures were conducted based on the methods
outlined in our previous publication (16). MyHC-positive cells were observed
under a fluorescence microscope (Leica Microsystems GmbH) and their
lengths were quantified using the Leica Application Suite (version,
X1.1.0.12420; Leica Microsystems GmbH) software.
Preparation of an animal model of
obesity
C57BL/6 male mice (n=32; age, 6 weeks) were obtained
from a licensed supplier (Hana Experimental Animals Co.). The
temperature (24–26°C) and relative humidity (60–70%) for feeding
animals required was controlled, and the mice were maintained in
polypropylene cages under a 12/12-h light/dark cycle. All mice had
1 week for adaptation and 12 weeks of experiment duration after
adaptation. During the experiment, animal health and behavior were
observed daily; no deaths occurred with abnormal symptoms. During
the adaptation period, the mice were given free access to food and
water. After 1 week, the mice were subjected to a random allocation
process, resulting in the formation of two groups: The normal (n=8)
and HFD-induced model (n=24) groups. The normal group was fed a
basic diet, whereas the HFD-induced model group was fed a HFD
(17). Following a total of 8
weeks of continuous feeding, the HFD-induced model mice were
randomly divided into the following groups: Control (n=8),
Asarum (500 mg/kg Asarum extract, n=8) and metformin
(250 mg/kg metformin, n=8) groups. During the experiment, each
group was fed their original diet and drank freely. Asarum
and metformin were orally administered at the indicated doses once
daily for 4 weeks. The normal and control groups received the same
volume of physiological saline solution. All of the groups received
an equal volume of the drug via oral gavage. All animal experiments
including procedure and euthanasia were approved by the
Institutional Animal Care and Use Committee (IACUC) at Dongguk
University (approval. no. IACUC-2022-05). During the experiment,
mice were observed once daily for general health indicators and
twice daily for mortality and morbidity. No mice succumbed prior to
the end of the present study.
Animal status observation, fasting
blood glucose (FBG) levels and weight monitoring
Weekly measurements of FBG, body weight and water
intake were taken. FBG levels were measured using a hemoglobin
measurement system (CERA-CHEK™ Hb Plus).
Oral glucose tolerance test
(OGTT)
After a 12-h period of fasting, the mice were
subjected to an OGTT, during which a dosage of 2 g/kg body weight
of glucose was administered. Blood glucose levels were subsequently
measured at intervals of 15, 30, 60 and 120 min following the
administration of glucose. The assessment of glucose tolerance was
conducted by calculating the area under the curve (AUC) (18).
Serological analysis
At the end of the experiment, mice were euthanized
by inhalation with O2 (75%), N2O (25%) using
5% isoflurane (18) >3 min and
deaths were verified by monitoring the symptoms such as no rising
and falling of chest, no palpable heartbeat, poor mucous membrane
color, no response to toe pinch and color change in eyes. Blood
samples (0.7–0.8 ml) were immediately obtained via cardiac puncture
after sacrifice and were subsequently stored at room temperature
(RT) for 30 min and then subjected to centrifugation at 300 × g for
10 min at 4°C, resulting in the separation of serum. Serum levels
of triglycerides (TG), total cholesterol (TC), low-density
lipoprotein cholesterol (LDL-C) and high-density lipoprotein
cholesterol (HDL-C) were measured using an automated clinical
chemistry analyzer (FDC7000i; Wako Pure Chemical Corporation)
(19). To assess the potential
toxicological impact of Asarum extract, serum levels of AST,
ALT, BUN and CRE were measured by assay kits. Additionally, insulin
enzyme-linked immunosorbent assay kits (cat. no. 90080; Crystal
Chem, Inc.) were used to evaluate serum insulin levels.
Measurement of organ weight and muscle
tissue collection
After collecting blood from the mice, the inguinal
white adipose tissue (iWAT) and the back-shoulder blade brown
adipose tissue (BAT) were harvested. To clean the liver and
pancreas, they were isolated and immersed in saline. After weighing
the liver tissue, it was stored at −80°C for subsequent western
blot analysis. The gastrocnemius tissues from both hind legs were
also collected, weighed and subsequently stored at −80°C until
western blot analysis was conducted. Based on the weight of each
tissue, organ index was calculated: Organ index=organ weight/body
weight.
Histological observation
To observe the histopathological changes in the
tissues, the liver, pancreas, iWAT and BAT tissues in each group
were isolated, and three mice in each group were randomly selected
for hematoxylin and eosin (H&E) staining. The tissues were
fixed in 4% paraformaldehyde at 4°C for 24 h, and underwent ethanol
gradient dehydration, paraffin embedding and slicing into 5-µm
sections. The sections were then stained using hematoxylin and
eosin, with hematoxylin staining performed at RT for 5 min and
eosin staining for 2 min, followed by clearing using xylene at RT
for 5 min. The pathological changes in hepatocytes in the liver
tissue, degeneration and necrosis of pancreatic islet cells and
adipocytes in fat tissues were observed under an optical
microscope. A microscope was used to capture images, which were
then analyzed using ImageJ Software (version 2; National Institutes
of Health).
Western blot analysis
Cells were harvested from each group. Following the
addition of 100 µl RIPA lysis buffer, the pellets were mixed
thoroughly, vortexed for 1 min, placed on ice for 10 min and
centrifuged at 1,200 × g for 20 min at 4°C to harvest the
supernatant. In addition, gastrocnemius and liver tissues were
homogenized in tissue lysis buffer that contained protease
inhibitors (T10 basic; IKA). After being placed on ice for 20 min,
the tissues were centrifuged at 1,400 × g for 20 min at 4°C, and
the supernatant was then collected. A protein assay buffer was used
to measure the total protein concentration. The protein samples (30
µg protein/lane) were then separated by SDS-PAGE on 10% gels,
transferred onto nitrocellulose membranes, treated with EveryBlot
Blocking Buffer for 10 min at RT and incubated with the primary
antibodies (SIRT1, PGC1α, NRF1, AMPK, p-AMPK, GLUT4, MyHC, MyoD,
Myogenin, GAPDH and β-actin) at 4°C for 12 h. After 15 min of
washing with Tris-buffered saline-0.1% Tween-20 at RT, the
membranes were incubated with horseradish peroxidase-conjugated
goat anti-rabbit IgG or anti-mouse IgG secondary antibodies for 90
min with agitation at RT. After washing three times, the membranes
were visualized using a ChemiDoc MP Imaging System (Bio-Rad
Laboratories, Inc.). ImageJ software (version 1.54d; National
Institutes of Health) was used to semi-quantify the band
intensities of each target protein, and proteins were normalized to
the internal controls β-actin or GAPDH (gastrocnemius tissue), or
to AMPK (for p-AMPK).
Statistical analysis
The data are presented as the mean ± standard
deviation (n=8 for in vivo studies; n=3 for in vitro
studies). In the present study, three independent experiments were
conducted. The statistical analyses were conducted using Prism 6
software (GraphPad Software, Inc; Dotmatics). Data were analyzed
using a one-way analysis of variance and Tukey's multiple
comparisons test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Network pharmacology analysis to
explore potential targets of Asarum
To explore the potential targets of Asarum
with chemical composition, a network pharmacology-based analysis
was performed using the TCMSP, with OB and DL set at ≥30% and
≥0.18, respectively. A total of eight potential bioactive
components were identified in the analysis (Table SI; Appendix I); the structural formulas of
selected compounds are shown in Fig.
S4 and Appendix I. In
addition, 9,912 obesity-related molecular targets were obtained
from the GeneCards database and 83 active ingredient-related
targets from the TCMSP. After taking the intersection from the
Wayne diagram, 75 intersection targets of the active ingredients of
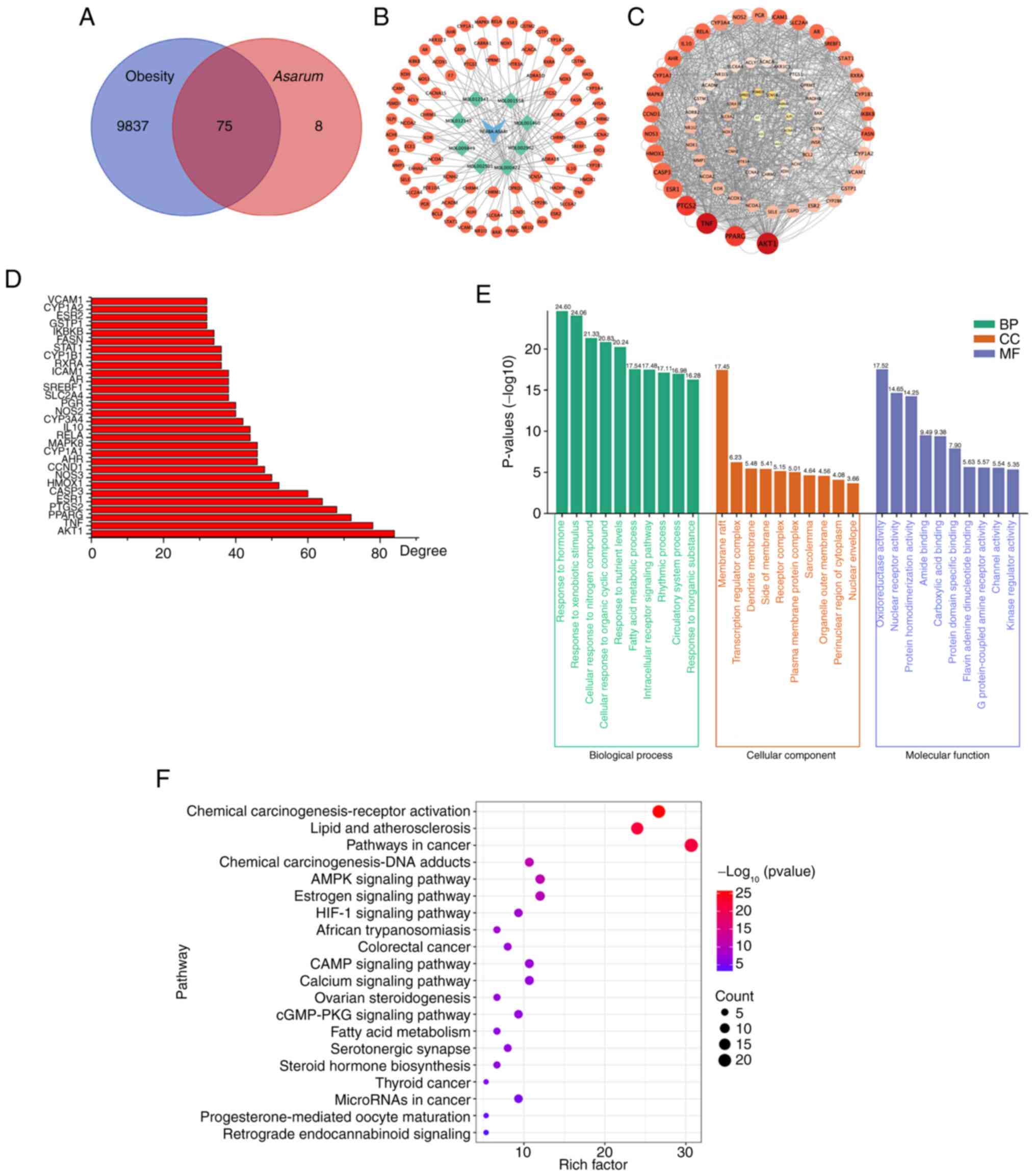
Asarum in obesity were identified (Fig. 1A). Moreover, the network diagram of
the active components associated with targets and the
component-target interaction network were visualized (Fig. 1B).
PPI network construction and gene
functional enrichment analysis
The molecular targets of Asarum and its
active ingredients in obesity were inputted into the STRING
database to construct a PPI network, which was then visualized
using the Cytoscape program (Fig.
1C). In the analysis, several key proteins, including
serine/threonine-protein kinase 1, tumor necrosis factor,
peroxisome proliferator activated receptor γ (PPAR-γ),
prostaglandin-endoperoxide synthase 2, estrogen receptor α,
caspase-3, heme oxygenase, nitric oxide synthase 3 and cyclin D1,
were identified as having significant roles in the mechanism of
action of Asarum on obesity (Fig. 1D). Next, from the GO enrichment
analysis, the top 10 significant CC, BP and MF terms were selected
(Fig. 1E). The most enriched BP
terms were response to hormones, xenobiotic stimuli and nitrogen
compounds, and those for CC and MF were member raft and
oxidoreductase activity. This analysis suggested that Asarum
may be involved in various biological regulatory processes in the
treatment of obesity. In particular, the KEGG metabolic pathways
were mainly involved in the AMPK signaling pathway in energy
metabolism (Fig. 1F). From the
network pharmacology analysis, the present study investigated the
effects of Asarum extract on obese mice and skeletal muscle
cells, which are important for regulating energy metabolism through
the AMPK signaling pathway.
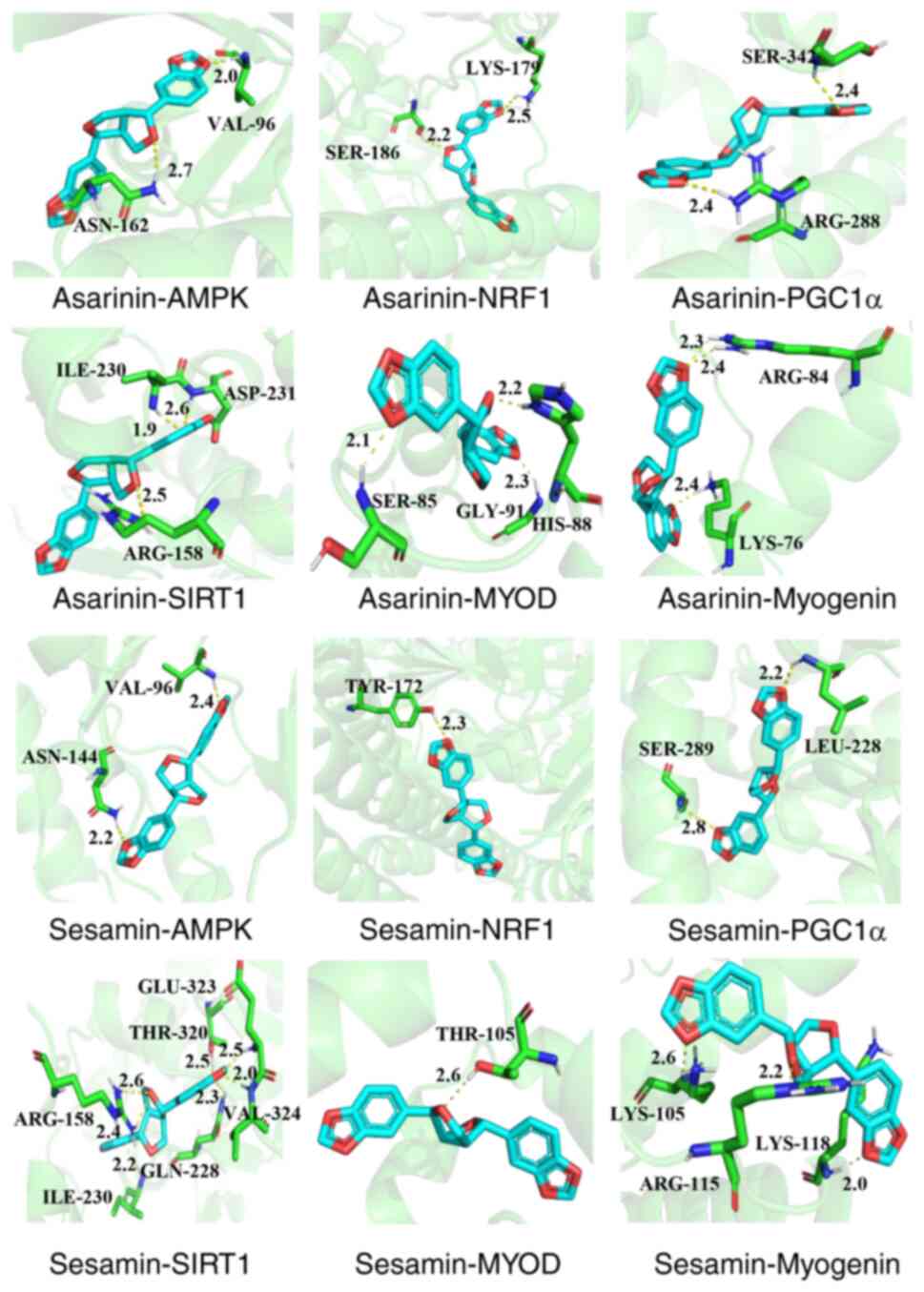
Molecular docking analysis
To gain further insights into the molecular
mechanism of Asarum in the treatment of obesity, molecular
docking experiments with key targets and main components were
conducted. According to the network pharmacology predictions,
asarinin and sesamin were identified as active ingredients
exhibiting a high correlation with the treatment of obesity.
Consequently, these compounds were chosen as core components. The
outcomes of GO and KEGG analyses demonstrated the potential
mechanism of action of Asarum in treating obesity,
highlighting the regulation of the AMPK signaling pathway.
Therefore, key targets from the AMPK signaling pathway, including
AMPK, PGC1α, NRF1 and SIRT1, were selected for molecular docking of
asarinin and sesamin. In addition, the molecular docking of the
myogenic proteins MyoD and myogenin with the two compounds was
analyzed. Generally, higher binding energy values between small
molecules and protein molecules indicate a more robust stability in
their binding (20). The results
demonstrated that asarinin-AMPK (−7.7 kcal/mol), asarinin-PGC1α
(−9.9 kcal/mol), asarinin-NRF1 (−7.8 kcal/mol), asarinin-SIRT1
(−9.0 kcal/mol), asarinin-MyoD (−7.5 kcal/mol), asarinin-myogenin
(−6.7 kcal/mol), sesamin-AMPK (−7.9 kcal/mol), sesamin-PGC1α (−9.7
kcal/mol), sesamin-NRF1 (−9.8 kcal/mol), sesamin-SIRT1 (−8.8
kcal/mol), sesamin-MyoD (−7.8 kcal/mol) and sesamin-myogenin (−6.4
kcal/mol) exhibited robust binding capabilities. The binding mode
between the core targets and the main components is depicted in
Fig. 2.
Effects of Asarum extract on the
regulation of differentiation and energy metabolism in C2C12
myotubes
From the network pharmacology analysis, the present
study further explored the molecular mechanism of Asarum
extract in the regulation of myoblast differentiation into myotubes
and energy metabolism in C2C12 cells. The results showed that
Asarum extract increased the expression of biogenic factors,
SIRT1, PGC1α, NRF1, AMPK and GLUT4, and myogenic factors, MyoD and
myogenin, in C2C12 myotubes (Fig.
3A). Treatment of C2C12 myotubes with 1 mg/ml Asarum
extract significantly increased the expression levels of SIRT1
(P<0.05; Fig. 3B), PGC1α
(P<0.01; Fig. 3C), NRF1
(P<0.05; Fig. 3D), p-AMPK
(P<0.05; Fig. 3E), GLUT4
(P<0.05; Fig. 3F), MyoD
(P<0.05; Fig. 3G) and myogenin
(P<0.05; Fig. 3H) compared with
those in the non-treated cells. In addition, treatment with 0.5
mg/ml Asarum extract significantly increased the expression
levels of SIRT1 (P<0.05), PGC1α (P<0.01) and p-AMPK
(P<0.05) in the myotubes. Asarum extract (0.25 mg/ml)
also significantly increased PGC1α expression levels (P<0.01).
These results indicated that Asarum extract may promote
muscle functions, such as differentiation and regulation of energy
metabolism, by activating the SIRT1/PGC1α/AMPK signaling
pathway.
 | Figure 3.Effects of Asarum extract on
the regulation of differentiation and energy metabolism in C2C12
myotubes. C2C12 myoblasts were differentiated into myotubes for 4
days, and were then treated with Asarum extract or MET for
24 h. (A) Protein expression was analyzed by western blotting.
(B-H) Expression levels of SIRT1 (81 kDa), PGC-1α (91 kDa), NRF1
(82 kDa), p-AMPK (60 kDa), AMPK (60 kDa), GLUT4 (55 kDa), MyoD (50
kDa) and myogenin (39 kDa) were determined by western blotting. The
histograms show the relative expression of each target compared
with β-actin (42 kDa) or AMPK (for p-AMPK). Data are presented as
the mean ± standard deviation. *P<0.05 and **P<0.01 vs. Nor
cells. SIRT1, sirtuin 1; PGC1α, peroxisome proliferator-activated
receptor γ coactivator 1-α; NRF1, nuclear respiratory factor 1; p-,
phosphorylated; AMPK, AMP-activated protein kinase; GLUT4, glucose
transporter type 4; MET, metformin; Nor, normal. |
Effects of Asarum extract on C2C12
myotube formation
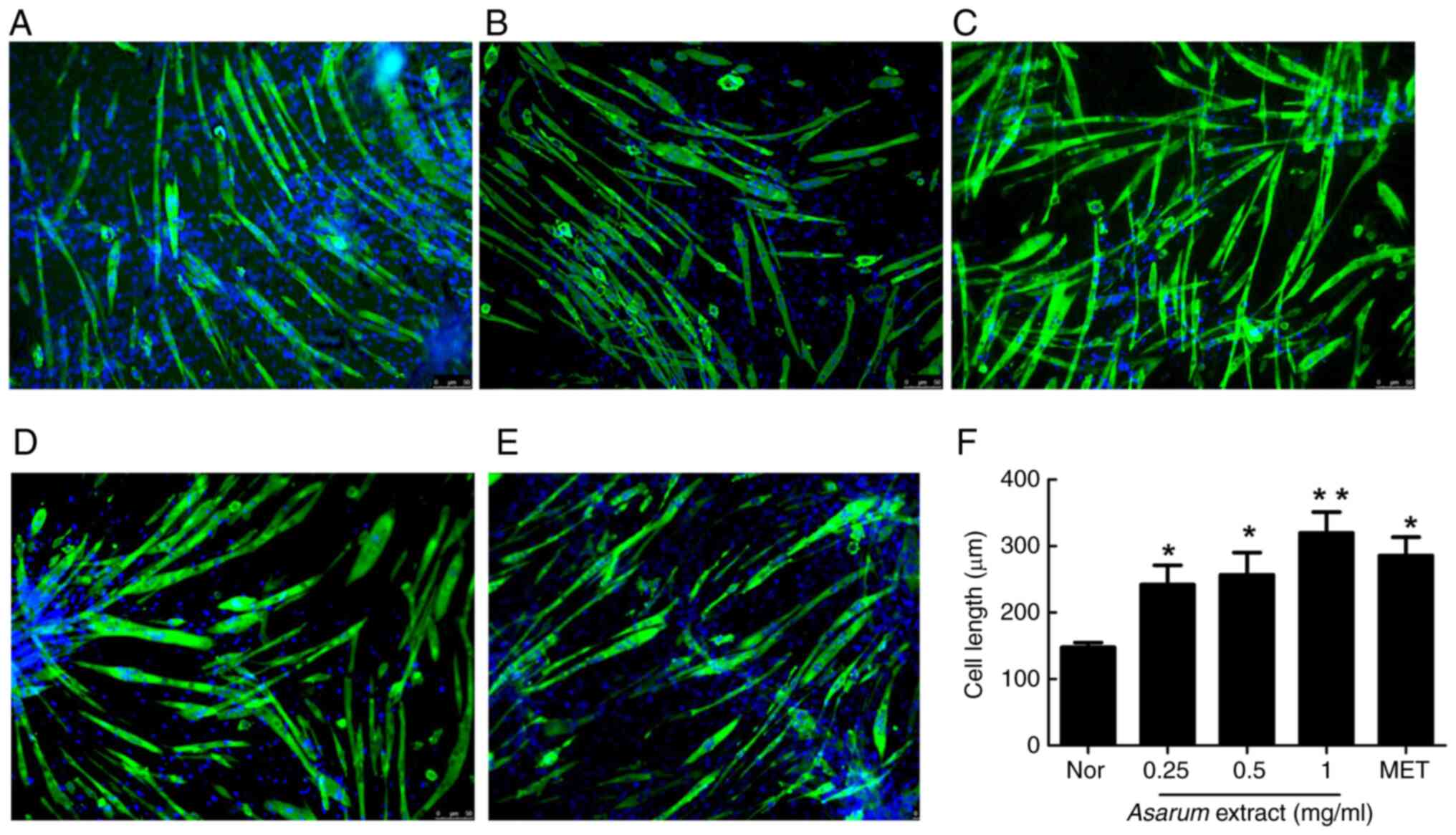
To confirm the effects of Asarum extract on
muscle differentiation, myotube formation was observed using
MyHC-immunofluorescence staining in C2C12 myotubes. Treatment with
Asarum extract stimulated the formation of MyHC-positive
myotubes with more pronounced ripples and slender cell
characteristics compared with those of non-treated cells (Fig. 4A). Moreover, treatment with
Asarum extract at 0.25 mg/ml (P<0.05; Fig. 4B), 0.5 mg/ml (P<0.05; Fig. 4C) and 1 mg/ml (P<0.01; Fig. 4D), or with metformin (P<0.05;
Fig. 4E), significantly increased
the lengths of myotubes (Fig. 4F).
These results suggested that Asarum extract can promote
myotube elongation during differentiation.
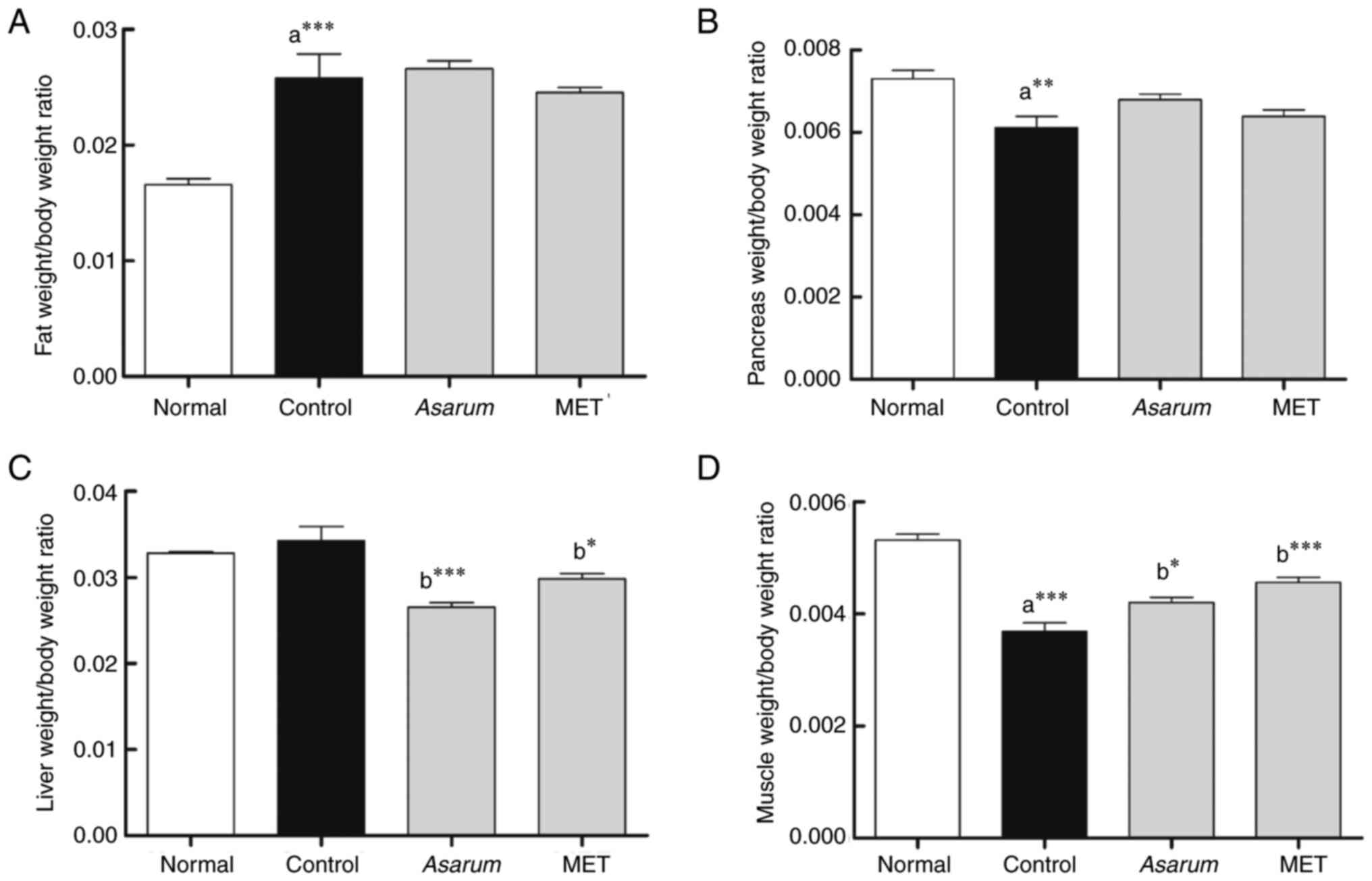
Effects of Asarum extract on
physiological changes in mice with HFD-induced obesity
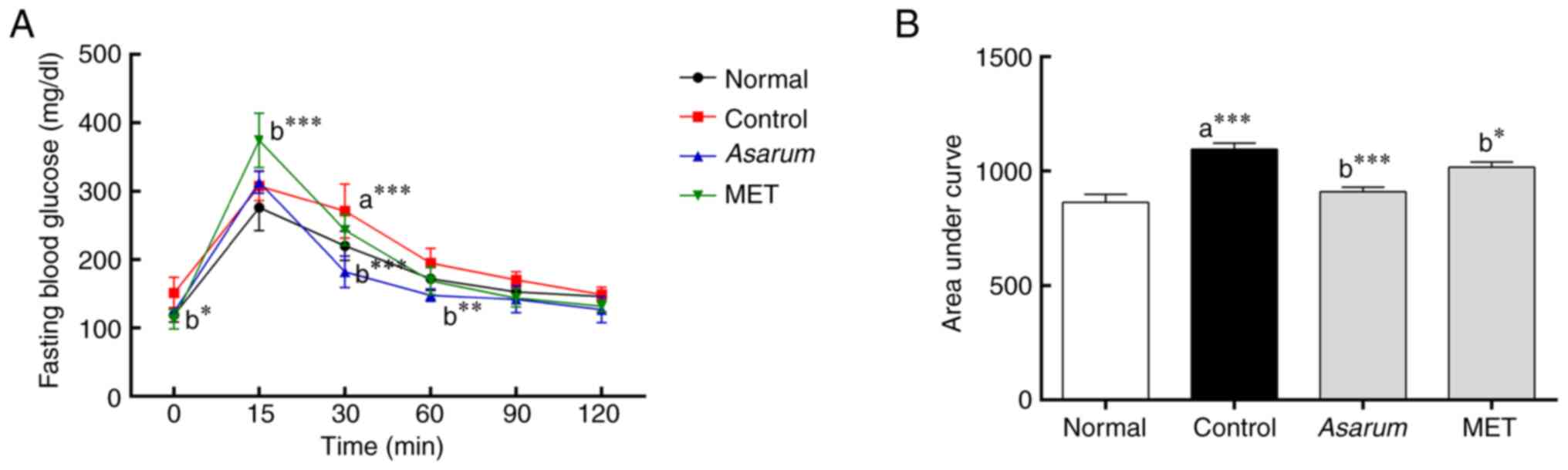
To investigate the effects of Asarum extract
on obesity-induced changes in physiological features, the body
weight and weights of organs, such as the liver, pancreas and
muscles, were measured, and an OGTT was performed in obese mice.
Firstly, the body weights of the obese mice, which were fed a HFD
for 8 weeks and included the control, Asarum and the
metformin groups, were significantly increased (P<0.001)
compared with those in the normal group (Table I). However, compared with the
control group, body weight was significantly decreased from 10
weeks (P<0.05 for metformin) to 11 weeks (P<0.05 for
metformin) after the administration of 500 mg/kg Asarum
extract or metformin for 4 weeks in obese mice. Although the
Asarum extract-treated group showed a pattern of weight
loss, it was not significant. Compared with in the control group,
the Asarum extract group exhibited a reduction in FBG levels
in obese mice (Table I). In the
OGTT, the glucose levels were significantly decreased at 30 min
(P<0.001) and 60 min (P<0.01) in the Asarum
extract-administered group compared with those in the control group
(Fig. 5A). In addition, in the AUC
analysis of impaired glucose tolerance, Asarum extract
significantly reduced (P<0.001) impaired glucose tolerance in
obese mice (Fig. 5B). Regarding
food intake, the administration of Asarum extract in obese
mice significantly decreased (P<0.001) food intake at 9 weeks
compared with that in the control group (Table I). Regarding the organ index, the
control group showed a significant increase in iWAT
(P<0.001; Fig. 6A), and
a significant decrease in pancreas (P<0.01; Fig. 6B) and gastrocnemius (P<0.001;
Fig. 6D) organ indexes compared
with those in the normal group. The administration of Asarum
extract (P<0.001) and metformin (P<0.05) significantly
decreased liver weights (Fig. 6C),
and significantly increased (P<0.001) gastrocnemius weights in
obese mice (Fig. 6D). These
findings suggested that Asarum extract may help to improve
the symptoms of obesity, such as weight gain and increased food
intake, and reduce the development of impaired glucose tolerance
and fatty liver formation.
 | Table I.Effects of Asarum extract on
physiological changes in obese mice. |
Table I.
Effects of Asarum extract on
physiological changes in obese mice.
| Group | Index | Week 8 | Week 9 | Week 10 | Week 11 | Week 12 |
|---|
| Normal | BW, g | 27.44±1.93 | 28.78±1.78 | 29.27±1.83 | 28.99±1.70 | 28.73±2.23 |
|
| FBG, mg/dl | 92.00±7.07 | 111.43±15.47 | 90.29±9.20 | 106.71±14.47 | 115.71±22.48 |
|
| FI, g |
| 28.64±1.80 | 28.15±2.16 | 283.32±5.25 | 27.31±2.10 |
| Control | BW, g |
39.16±4.10c |
40.44±3.83c |
41.32±4.05c |
41.70±3.92c |
41.47±4.04c |
|
| FBG, mg/dl |
114.29±14.07a |
135.57±15.94a |
118.00±16.86b | 121.67±23.38 |
146.00±26.88b |
|
| FI, g |
|
17.07±0.99c |
16.72±1.25c |
16.84±1.13c |
16.97±1.83c |
| Asarum (500
mg/kg) | BW, g | 39.71±3.39 | 38.41±3.21 | 38.43±3.40 | 37.78±3.61 | 37.83±3.65 |
|
| FBG, mg/dl | 111.71±13.66 | 141.43±16.49 | 113.71±9.46 | 127.57±12.22 | 124.43±10.95 |
|
| FI, g |
|
13.30±1.41e | 15.66±1.34 | 14.64±1.40 | 15.44±1.07 |
| MET (250
mg/kg) | BW, g | 39.57±3.49 | 36.59±3.26 |
35.81±3.51d |
34.36±3.19f |
34.12±3.13f |
|
| FBG, mg/dl | 112.00±15.52 | 125.43±8.46 | 115.14±5.27 | 103.71±9.12 | 123.86±23.50 |
|
| FI, g |
|
9.47±1.87f | 14.39±2.46 |
13.38±1.21d | 14.58±1.09 |
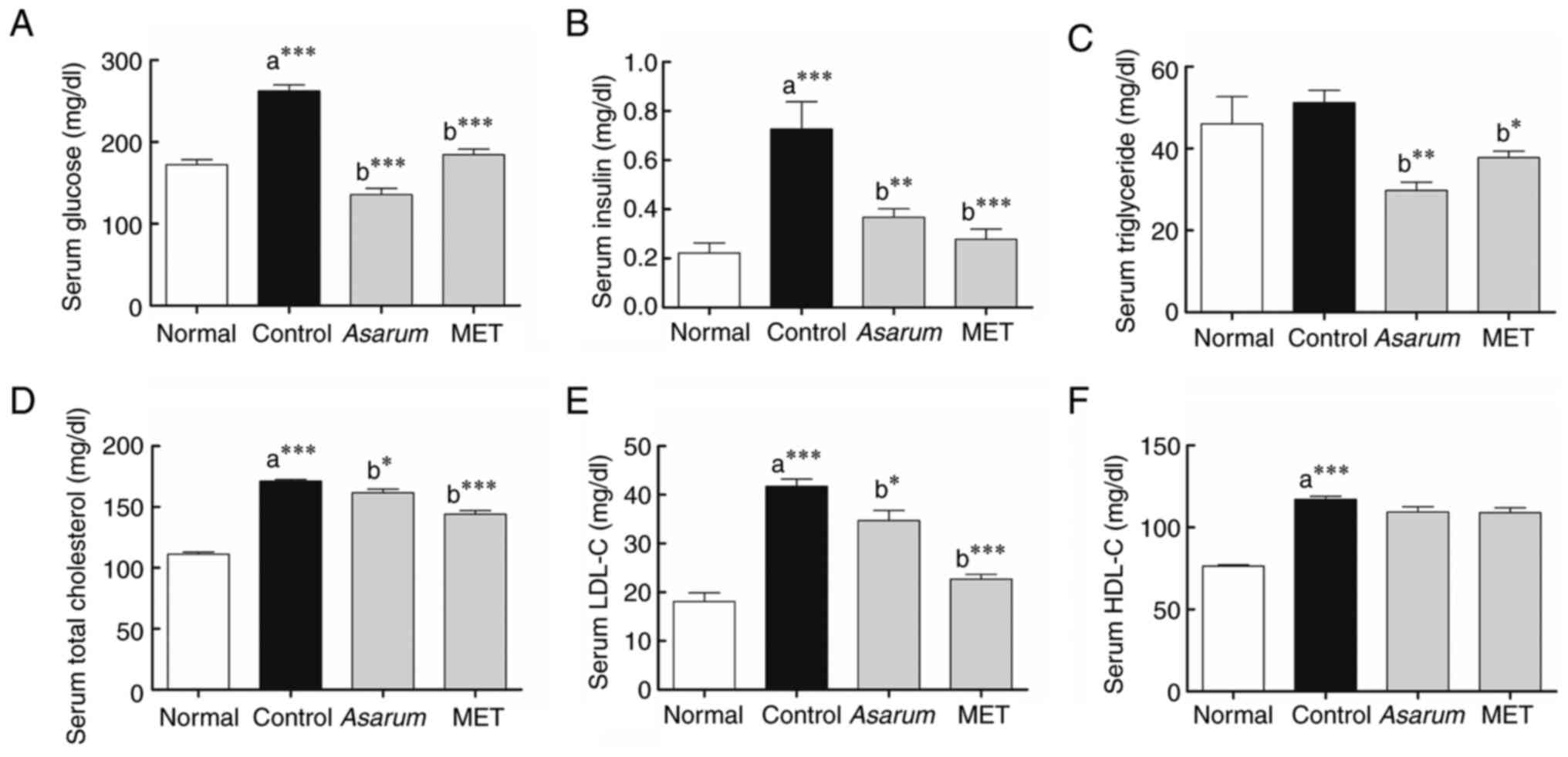
Effects of Asarum extract on
serological changes in obese mice
To examine the impact of Asarum extract on
alterations in blood composition induced by obesity, the
concentrations of serological markers in the mouse sera were
assessed. Notably, the levels of glucose (P<0.001; Fig. 7A), insulin (P<0.001; Fig. 7B), TC (P<0.001; Fig. 7D), LDL-C (P<0.001; Fig. 7E) and HDL-C (P<0.001; Fig. 7F) exhibited a significant increase
in the control group compared with those of the normal group.
Compared with those in the control group, the Asarum
extract-administered group exhibited significant decreases in the
levels of glucose (P<0.001), insulin (P<0.01), TG (P<0.01;
Fig. 7C), TC (P<0.05) and LDL-C
(P<0.05). Similarly, the metformin-administered group showed
significant decreases in glucose (P<0.001), insulin
(P<0.001), TG (P<0.05), TC (P<0.001) and LDL-C
(P<0.001) levels compared with those in the control group. These
results indicated that Asarum extract may improve the
abnormal accumulation of glucose and lipids in the blood in
obesity. Based on the results of AST, ALT, BUN and CRE analyses,
the levels of these biochemical markers in the treated mice were
lower than those observed in the control group following continuous
administration of Asarum extract at a dose of 500 mg/kg/day
for 4 weeks (Table SII and
Appendix I). These findings
suggested that no significant adverse toxicological effects were
observed in the treated mice.
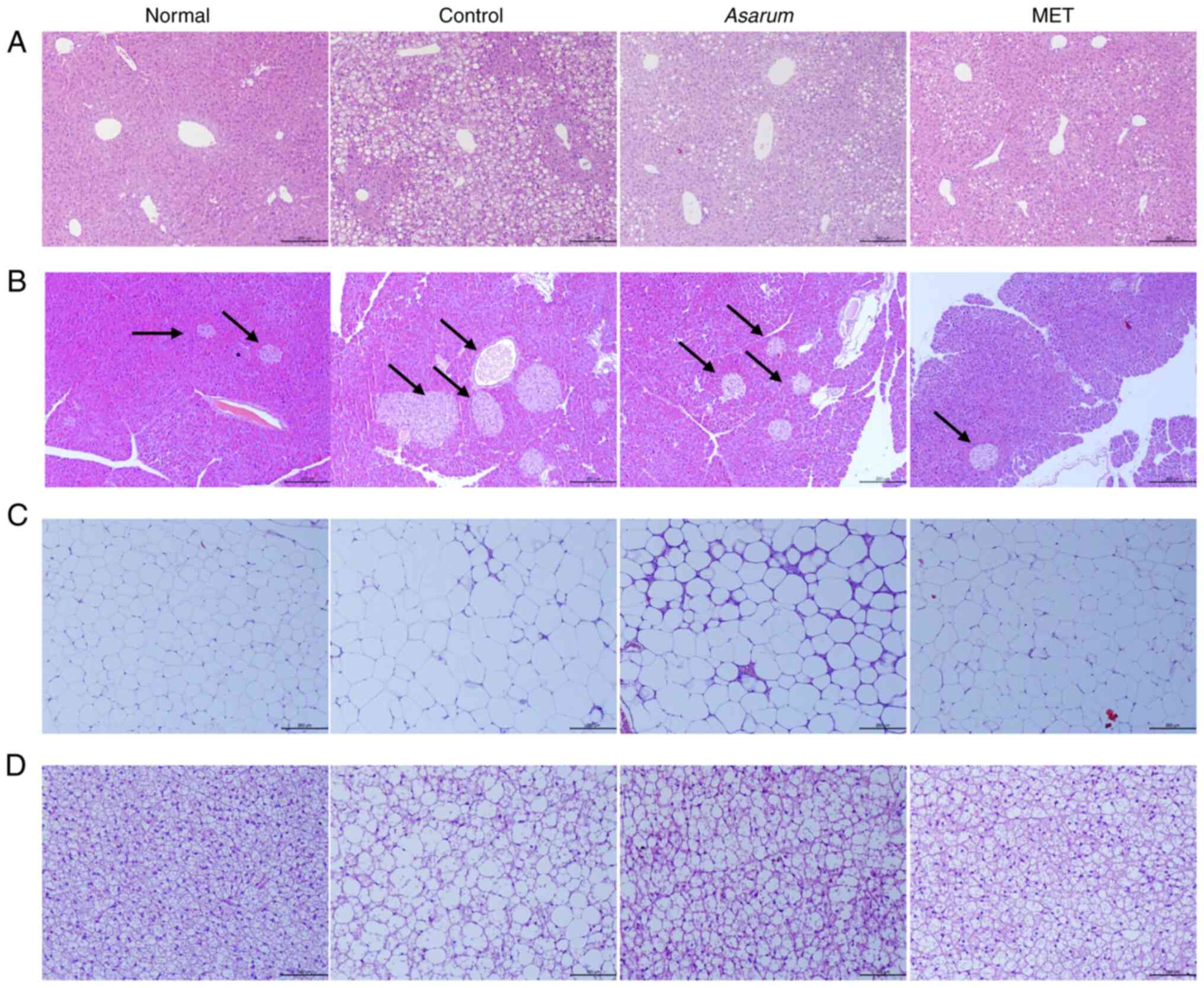
Effects of Asarum extract on
histological changes in the liver, pancreas, fat and muscle tissues
of obese mice
To investigate the effects of Asarum extract
on obesity-induced histological changes in the main metabolic
organs, the liver, pancreas, fat and gastrocnemius tissues of obese
mice were observed by H&E staining. In liver tissues, the
control group had a number of lipid droplets and exhibited
expansion of hepatic sinusoids; however, this abnormal structural
change was improved by the administration of Asarum extract
(Fig. 8A). In pancreatic tissues,
an intact structure of pancreatic islets with an elliptical shape
was observed, with neatly arranged cells, small gaps and no
significant difference in nuclear size in the normal group;
however, this was altered in the control group with a number of
vacuoles (Fig. 8B). By contrast,
Asarum extract administration in obese mice improved the
structural changes in the pancreas. In iWAT, the control group
showed a notably decreased size of adipocytes, whereas this change
was improved by Asarum extract administration to similar to
that in the normal group (Fig.
8C). In BAT, a tight arrangement of small adipocytes was shown
in the normal group, and large adipocytes with a loose arrangement
and irregular shape were observed in the control group (Fig. 8D); however, this structural change
in BAT was also improved by the administration of Asarum
extract in obese mice. These results indicated that Asarum
extract may induce energy consumption in the body by stimulating
the consumption of energy in adipose tissues in obesity.
Effects of Asarum extract on the
SIRT1/PGC1α/AMPK signaling pathway in the gastrocnemius tissues of
obese mice
The present study further investigated the effects
of Asarum extract on the gastrocnemius tissues of obese mice
(Fig. 9A). Compared with the
normal group, the control group exhibited a significant decrease in
the expression levels of SIRT1 (P<0.05; Fig. 9B), PGC1α (P<0.05; Fig. 9C), NRF1 (P<0.01; Fig. 9D), p-AMPK (P<0.01; Fig. 9E), GLUT4 (P<0.05; Fig. 9F), MyHC (P<0.05; Fig. 9G), MyoD (P<0.01; Fig. 9H) and myogenin (P<0.01; Fig. 9I) in the gastrocnemius tissues of
obese mice. The administration of Asarum extract increased
the expression of all targets, particularly the expression of
p-AMPK (P<0.05; Fig. 9E), MyHC
(P<0.05; Fig. 9G) and MyoD
(P<0.01; Fig. 9H). This result
suggested that Asarum extract may help to improve obese
conditions by promoting muscle differentiation and regulating the
expression of energy metabolism regulatory factors.
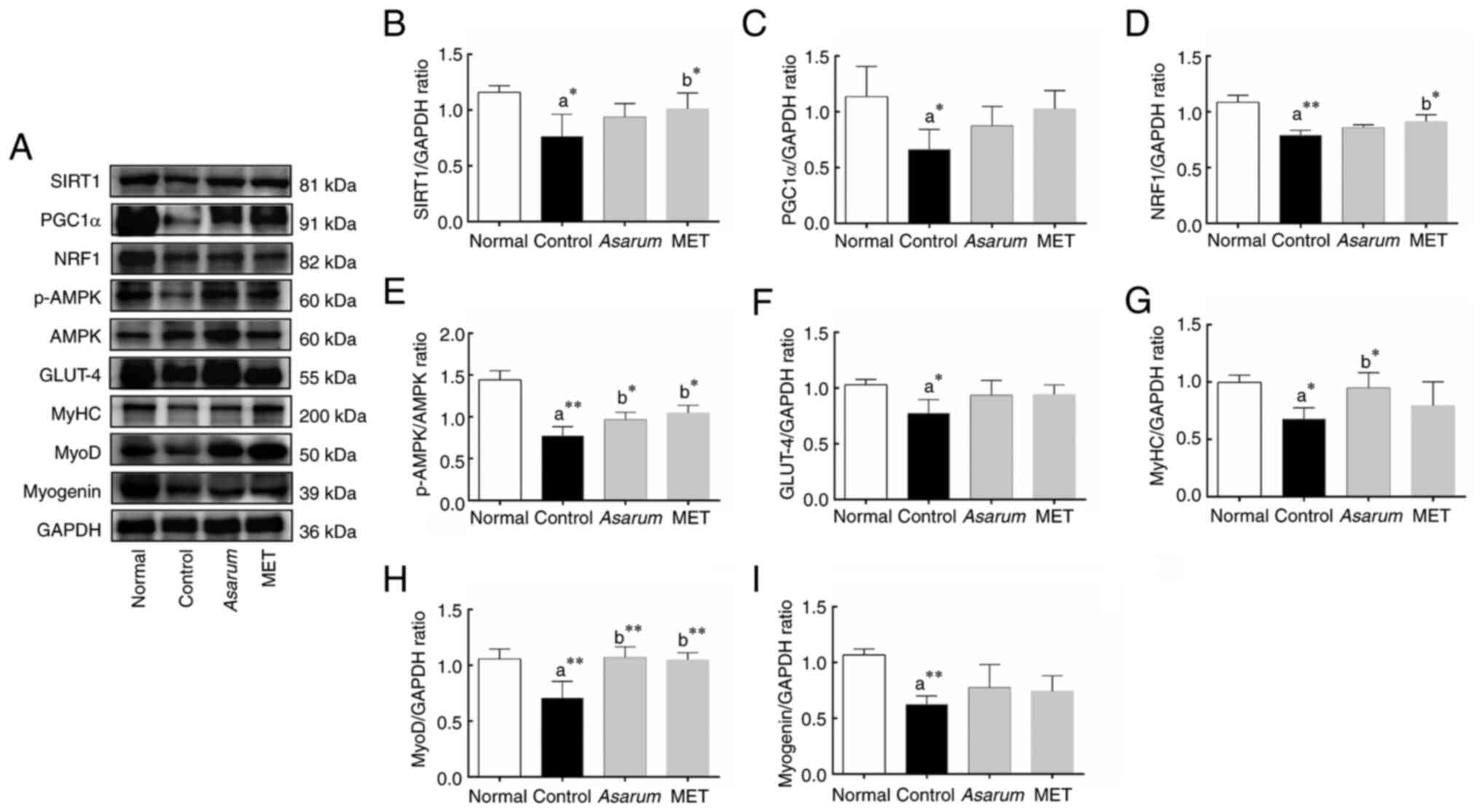 | Figure 9.Effects of Asarum extract on
the SIRT1/PGC1α/AMPK signaling pathway in the gastrocnemius tissues
of obese mice. (A) Protein expression was analyzed by western
blotting. (B-I) Protein expression levels of (B) SIRT1 (81 kDa),
(C) PGC-1α (91 kDa), (D) NRF1 (82 kDa), (E) p-AMPK (60 kDa) and
AMPK (60 kDa), (F) GLUT4 (55 kDa), (G) MyHC (200 kDa), (H) MyoD (50
kDa) and (I) myogenin (39 kDa) in gastrocnemius tissues were
determined by western blotting. The histograms show the relative
expression of each target compared with GAPDH (36 kDa) or AMPK (for
p-AMPK). Data are presented as the mean ± standard deviation.
*P<0.05 and **P<0.01 vs. (a) normal or (b) control groups.
SIRT1, sirtuin 1; PGC1α, peroxisome proliferator-activated receptor
γ coactivator 1-α; NRF1, nuclear respiratory factor 1; p-,
phosphorylated; AMPK, AMP-activated protein kinase; GLUT4, glucose
transporter type 4; MyHC, myosin heavy chain; MET, metformin. |
Effects of Asarum extract on the
SIRT1/PGC1α/AMPK signaling pathway in the liver tissues of obese
mice
To further validate the effects of Asarum
extract on obese mice, western blot analysis was conducted on the
liver tissues of obese mice (Fig.
10A). Compared with the normal group, the control group
exhibited a significant decrease in the expression levels of SIRT1
(P<0.01; Fig. 10B), PGC1α
(P<0.05; Fig. 10C), NRF1
(P<0.05; Fig. 10D), p-AMPK
(P<0.05; Fig. 10E) and GLUT4
(P<0.05; Fig. 9F), in the liver
tissues of obese mice. After the administration of Asarum
extract, the expression levels of these proteins, particularly
SIRT1 (P<0.001; Fig. 10B),
PGC1α (P<0.05; Fig. 10C) and
p-AMPK (P<0.05; Fig. 10E),
were reversed. This result indicated that Asarum extract can
also activate the SIRT1/PGC1α/AMPK signaling pathway in liver
tissue, regulating metabolic levels.
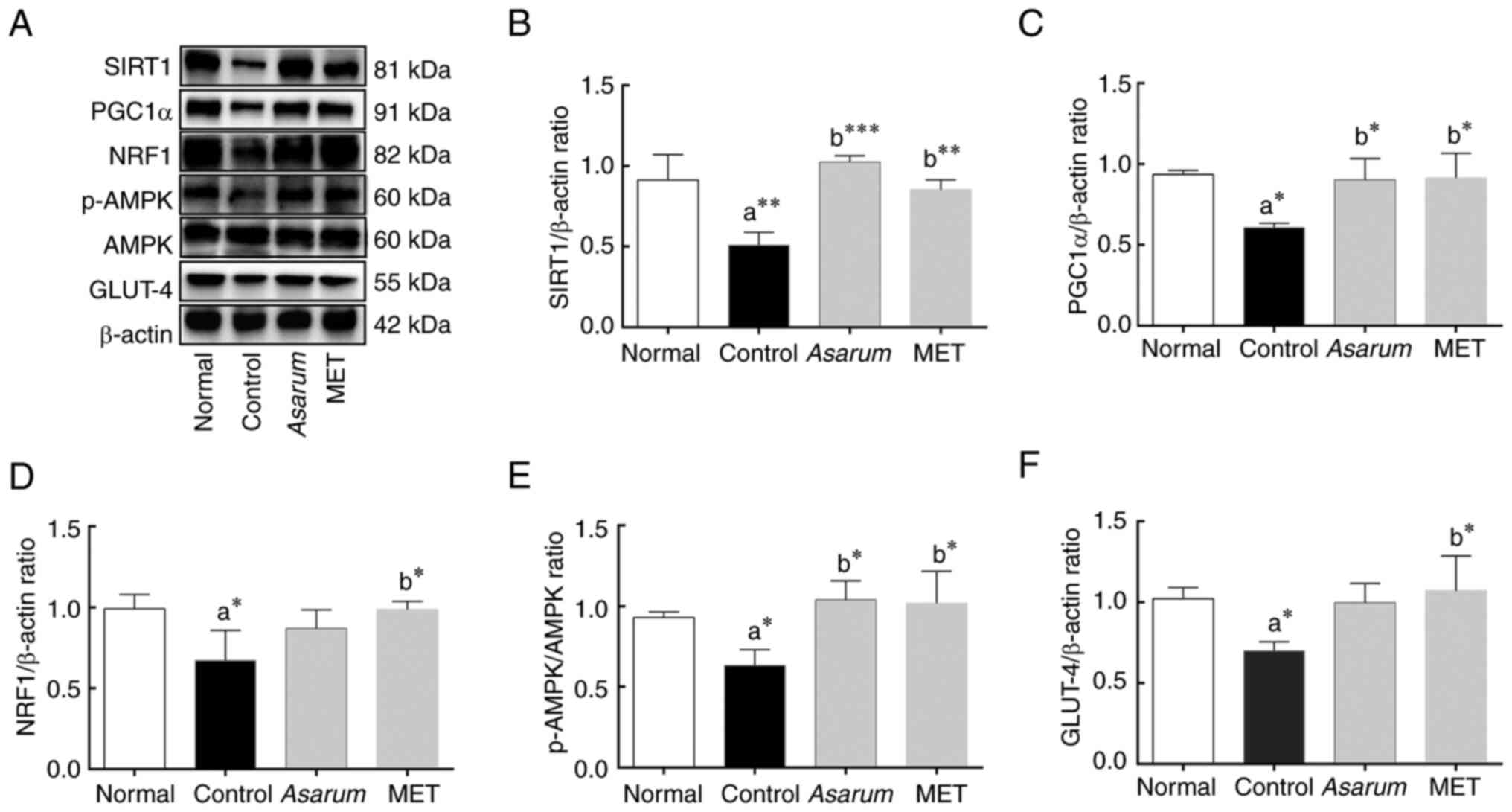 | Figure 10.Effects of Asarum extract on
the SIRT1/PGC1α/AMPK signaling pathway in the liver tissues of
obese mice. (A) Protein expression was analyzed by western
blotting. (B-F) Protein expression levels of (B) SIRT1 (81 kDa),
(C) PGC-1α (91 kDa), (D) NRF1 (82 kDa), (E) p-AMPK (60 kDa) and
AMPK (60 kDa) and (F) GLUT4 (55 kDa) in liver tissues were
determined by western blotting. The histograms show the relative
expression of each target compared with β-actin (42 kDa) or AMPK
(for p-AMPK). Data are presented as the mean ± standard deviation.
*P<0.05, **P<0.01 and ***P<0.001 vs. (a) normal or (b)
control groups. SIRT1, sirtuin 1; PGC1α, peroxisome
proliferator-activated receptor γ coactivator 1-α; NRF1, nuclear
respiratory factor 1; p-, phosphorylated; AMPK, AMP-activated
protein kinase; GLUT4, glucose transporter type 4; MET,
metformin. |
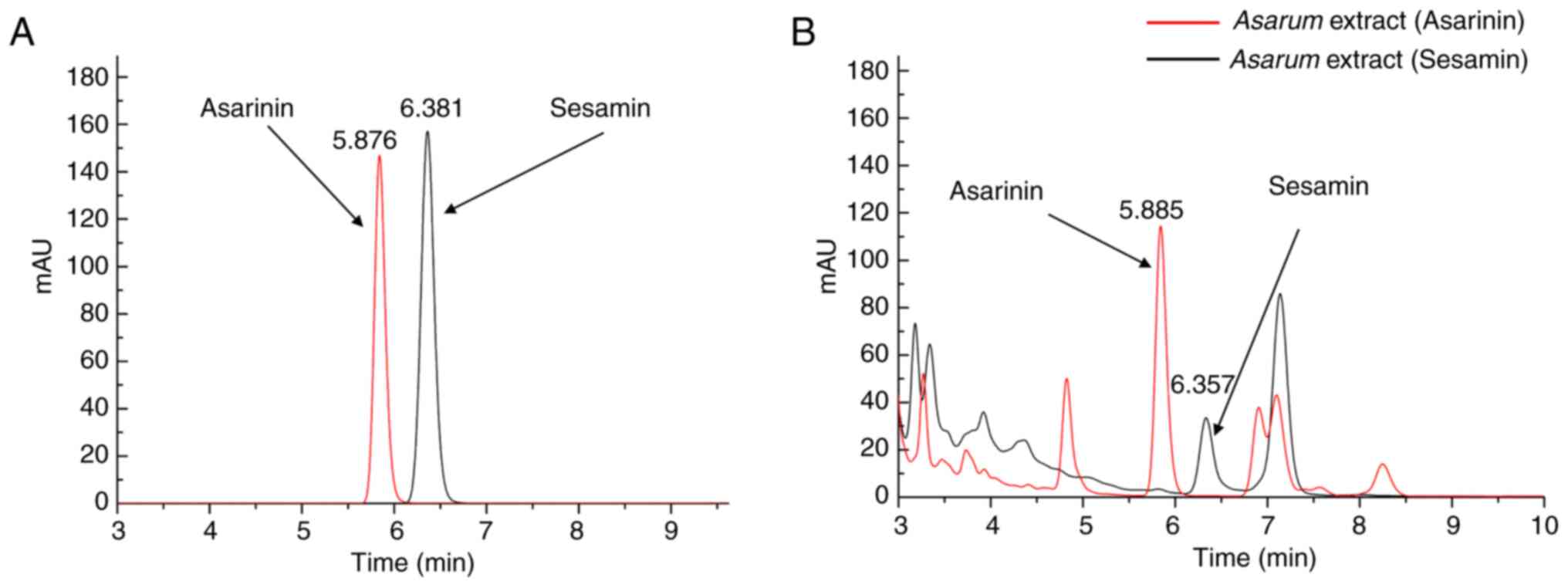
HPLC analysis
In the HPLC analysis of the main compounds in
Asarum extract, the amounts of asarinin and sesamin were
1.082±0.006 and 0.220±0.001 mg/g, respectively (Fig. 11). Standard curves (Fig. 11A) with linear ranges and
chromatographic patterns of asarinin and sesamin (Fig. 11B) are presented in Table SIII and Appendix I. In addition, the
chromatographic patterns of the Asarum herb and its extract
revealed that aristolochic acid A was not detected in either
sample. The detailed results are shown in Fig. S5 and Appendix I.
Discussion
In traditional Chinese and Korean medicine
Asarum is a herb that is believed to release wind and
disperse cold in the body. It is classified under the category of
releasing exterior in the Chinese Materia Medica, and is widely
used for treating the common cold, symptoms of which include
headache, molar pain, a blocked nose and increased mucus production
(6). Moreover, it has been
reported to have various pharmacological effects on diseases such
as arthritis (21), allergic
rhinitis (22), H1N1 influenza
virus infection (23), brain
inflammation (24) and cancer,
such as ovarian cancer (25),
melanoma (26) and prostate cancer
(27). The present study
investigated the anti-obesity effects of Asarum extract and
its mechanism of action in a long-term HFD-induced mouse model of
obesity and in C2C12 mouse myoblast cells. The molecular targets of
Asarum extract in obesity were first predicted using network
pharmacology and molecular docking analysis, and its effects on
in vitro and in vivo models, and on the
SIRT1/PGC1α/AMPK signaling pathway, were investigated. The present
study also identified the main compounds of Asarum extract,
asarinin and sesamin, by HPLC analysis.
Obesity is characterized by an increase in body fat
and abnormal levels of blood lipids (28). The present study prepared an
experimental mouse model of obesity using a long-term HFD for 12
weeks, which exhibited the typical symptoms of obesity, such as
increased appetite, dyslipidemia, insulin resistance, elevated
blood sugar levels, increased visceral fat and fatty liver
formation. Asarum extract reduced body weight gain, and
increases in calorie intake, blood glucose and lipid levels, as
well as the development of insulin resistance in obese mice. From
the network pharmacology analysis, it was hypothesized that the
pharmacological action of Asarum extract in obesity may be
related to the regulation of energy metabolism in the body by
promoting energy expenditure and controlling food intake (29).
The liver is an important metabolic organ that
mainly participates in endogenous fat synthesis and transportation
(30). If a dynamic imbalance in
lipid metabolism occurs, TG and TC levels increase, leading to fat
degradation in the liver and the increased risk of disease
development (31). In the liver,
LDL-C is a type of lipoprotein responsible for transporting
cholesterol (32). Most lipids in
plasma exist in the form of HDL-C, which contributes to the reverse
transport of cholesterol from peripheral tissues to the liver, and
acts as an antagonist of atherosclerosis formation (33). Therefore, obesity can lead to an
imbalance of lipid metabolism by increasing the levels of the lipid
metabolites TC, TG and LDL-C. Moreover, obese patients often suffer
from various diseases, such as diabetes with insulin resistance and
glucose tolerance, which lead to an increase in blood glucose
levels, thereby stimulating fat synthesis/storage in the liver
(34). The objective of the
present study was to assess the potential benefits of Asarum
extract in alleviating the symptoms of obesity through the
regulation of abnormal lipid metabolism in mice. The administration
of Asarum extract resulted in a significant reduction in the
serum levels of TG, TC, LDL-C, glucose and insulin, while
simultaneously increasing HDL-C levels in obese mice. In the
analysis of organ index, it was observed that the administration of
Asarum extract hindered the accumulation of fat in the
liver, while simultaneously augmenting the weights of the pancreas
and muscle tissues in mice with obesity. These findings indicated
that Asarum extract may potentially ameliorate symptoms
associated with obesity by modulating the dysregulation of glucose
and lipid metabolism.
Energy metabolism refers to the energy consumption
of the body by exercise/movement and metabolism, as well as the
generation of energy through food (35). Obesity is caused when energy intake
exceeds energy expenditure; therefore, improving body energy
metabolism and increasing energy expenditure can reduce the energy
stored in body fat, thereby improving obesity (36). Skeletal muscles consume more energy
than fat, and an increase in muscle mass in the body indicates an
improvement in the energy metabolism ability of the body (37). In the present study, Asarum
extract administration increased the muscle-to-body weight ratio
and BAT mass, and reduced white fat gain with fatty liver formation
and pancreatic structural damage in obese mice. Furthermore,
Asarum extract administration significantly increased the
expression levels of AMPK, MyHC and MyoD in the gastrocnemius
tissues of obese mice, suggesting that Asarum extract may
improve the symptoms of obesity by stimulating energy metabolism.
In C2C12 myotubes, a significant increase of myotube length was
also observed by Asarum treatment, suggesting that this herb
may help to enhance muscle mass and function in obese mice. It is
known that increases in myotube length and muscle weight improve
muscle strength, which could contribute to an enhanced overall
metabolic capacity in obesity.
During glucose metabolism, most ATP is synthesized
in the mitochondria via oxidative phosphorylation (38). AMPK is an intracellular protein
kinase that serves a key role in regulating cellular energy
metabolism (39), and its
signaling pathway promotes glucose uptake and oxidation, and
increases the expression of GLUT4, which stimulates glucose entry
into cells and improves the efficiency of glucose utilization
(40). In addition, SIRT1, an
NAD+-dependent histone deacetylase, is an important
regulator of energy metabolism. PGC1α promotes the biosynthesis and
functional enhancement of mitochondria (41). NRF1, a transcription factor, exerts
a pivotal influence on the production of mitochondrial proteins,
thereby assuming a critical function in the preservation of
cellular energy metabolism and mitochondrial functionality
(42). In the present study,
Asarum extract increased the phosphorylation of AMPK, and
the expression of SIRT1, PGC1α, NRF1 and GLUT4, in C2C12 myotubes.
These findings suggested that Asarum extract may help to
enhance energy metabolism in obesity.
Transcription factors, such as MyoD and myogenin,
belong to the MyoD family and promote the differentiation and
maturation of myoblasts with the formation of muscle fibers
(43). The MyHC gene is closely
related to muscle expression (44). More muscle cells and fibers
indicate higher energy expenditure, thereby helping to regulate
overall energy metabolism. The molecular docking results indicated
that Asarum had a strong binding affinity with the myogenic
proteins, MyoD and myogenin, suggesting that this extract may
regulate muscle differentiation by increasing the expression of
these proteins. In addition, it has been reported that AMPK is
involved in enhancing myogenesis and muscle regeneration (45). During myocyte differentiation,
energy is required to sustain the process and activation of the
AMPK/PGC1-α signaling pathway in muscle cells, to promote
mitochondrial activity and subsequently enhance muscle regeneration
under pathological conditions (46). Furthermore, AMPK, alongside PGC1-α
and SIRT1, is involved in the regulation of muscle growth. However,
the present study did not directly assess whether activation or
inhibition of the AMPK signaling pathway directly affects
biological processes, such as muscle differentiation, energy
production and mitochondrial function; this will be investigated in
our future studies.
In the present study, Asarum extract
increased not only the expression levels of MyoD, myogenin and MyHC
in C2C12 myotubes, but also stimulated differentiation into
MyHC-positive myotubes. These findings indicated that Asarum
extract may stimulate mature muscle growth and enhance energy
metabolism by activating the SIRT1/PGC1α/AMPK signaling pathway.
Additionally, western blot analysis was conducted on the liver
tissues of obese mice, and it was demonstrated that Asarum
extract significantly increased the expression of SIRT1 and PGC1α,
as well as the p-AMPK/AMPK ratio. This finding suggested that
Asarum extract may also activate the SIRT1/PGC1α/AMPK
signaling pathway in liver tissue, thereby enhancing metabolic
levels. Liver tissue serves a crucial role in maintaining energy
balance and metabolic homeostasis in the body, particularly in
regulating lipid metabolism. Thus, the mechanism predicted to be
responsible for the anti-obesity effects of Asarum extract
by the network pharmacology analysis, the AMPK signaling pathway,
was verified.
Asarum extract contains various compounds,
such as lignans, including sesamin and asarinin (6). Asarinin is a standard compound for
the quality control of Asarum in the Korean and Chinese
pharmacopeia. In the present HPLC analysis, sesamin and asarinin
were identified in Asarum extract. Asarinin has been
reported to possess various pharmacological activities, including
antidepressant, anti-inflammatory, lipid-lowering and
thrombosis-preventing effects (47). In addition, sesamin, a stereoisomer
of asarinin, has been shown to exert antioxidative and
anti-inflammatory effects via the regulation of lipid metabolism
(48). Sesamin also activates the
AMPK signaling pathway, which serves a crucial role in the
regulation of glucose uptake and utilization (49). Asarinin is also known to activate
PPAR-γ and to promote cell proliferation, and it has been indicated
that sesamin shares a similar spatial structure and biological
activity (50). Therefore,
Asarum extract may exert anti-obesity effects by modulating
energy metabolism within the body. In particular, the mechanism
underlying the effect of Asarum extract on weight loss in
obese mice could be further identified by measuring metabolic
parameters, such as oxygen consumption, heat production and
activity. The results of the present study were obtained using
skeletal muscle cells and a mouse model of obesity. Although these
models provide valuable insights into the underlying molecular
mechanisms, the applicability of the findings to other biological
systems may be somewhat limited. Therefore, to further validate the
broader applicability of these results, future studies should
involve more complex models, including primary cells derived from
human tissues, muscle cells from different species such as L6 cells
and clinical samples from humans. These studies will help further
confirm the universality of the proposed mechanisms and their
potential clinical relevance.
Asarum is well-known for its acute and
long-term toxicities in the human kidney, mainly via aristolochic
acid analogs; therefore, it is being considered in low doses for
clinical trials and experimental research (51,52).
The present study detected aristolochic acid, a toxic component in
Asarum extract, using HPLC analysis, and assessed its
toxicities in 293 cells, and the serum levels of AST, ALT, BUN and
creatinine in mice. In the present study, no significant toxicity
was demonstrated in vitro and in vivo in
Asarum extract. Moreover, aristolochic acid was not detected
in Asarum extract and Asarum herb. These findings
indicated that the doses of Asarum extract used in the
present study were within a safe range.
In conclusion, Asarum extract has a
multi-pathway/-target regulatory mechanism in HFD-induced obese
mice, where it can improve the symptoms of obesity, such as weight
gain and fatty liver formation, reduce blood glucose, insulin and
lipid levels and prevent pancreatic damage by regulating the
SIRT1/PGC1α/AMPK signaling pathway in muscle tissues and cells
(Fig. 12). Asarum extract
may be used in the development of an anti-obesity drug and could
have potential in the treatment of muscle atrophy in the
future.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Research
Foundation of Korea (grant nos. 2021R1A2C1012267 and
RS-2024-00340898).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HWJ and SYK designed the study. CZL, HFS, DGK and
SYK performed the experiments. CZL conducted the statistical
analysis and wrote the original draft of the manuscript. All
authors revised the manuscript. HWJ, SYK and CZL confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee (IACUC) of Dongguk
University (approval no. IACUC-2022-05; Gyeongju, Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NRF1
|
nuclear respiratory factor 1
|
|
AMPK
|
AMP-activated protein kinase
|
|
SIRT1
|
sirtuin 1
|
|
GLUT4
|
glucose transporter type 4
|
|
MyHC
|
myosin heavy chain
|
|
PGC1α
|
peroxisome proliferator-activated
receptor γ coactivator 1-α
|
|
HFD
|
high-fat diet
|
|
DMEM
|
Dulbecco's modified Eagle's
medium
|
|
P/S
|
penicillin/streptomycin
|
|
TCMSP
|
Traditional Chinese Medicine Systems
Pharmacology Database and Analysis Platform
|
|
OB
|
oral bioavailability
|
|
DL
|
drug-like property
|
|
PPI
|
protein-protein interaction
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
BP
|
biological processes
|
|
CC
|
cell components
|
|
MF
|
molecular functions
|
|
FBG
|
fasting blood glucose
|
|
OGTT
|
oral glucose tolerance test
|
|
AUC
|
area under the curve
|
|
RT
|
room temperature
|
|
HPLC
|
high performance liquid
chromatography
|
|
TC
|
total cholesterol
|
|
TG
|
triglycerides
|
|
LDL-C
|
low-density lipoprotein
cholesterol
|
|
HDL-C
|
high-density lipoprotein
cholesterol
|
|
AST
|
aspartate aminotransferase
|
|
ALT
|
alanine aminotransferase
|
|
BUN
|
blood urea nitrogen
|
|
CRE
|
creatinine
|
|
iWAT
|
inguinal white adipose tissue
|
|
BAT
|
brown adipose tissue
|
|
H&E
|
hematoxylin and eosin
|
References
|
1
|
Rhee EJ: The influence of obesity and
metabolic health on vascular health. Endocrinol Metab (Seoul).
37:1–8. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Agyemang K, Banstola A, Pokhrel S and
Anokye N: Determinants of physical activity and dietary habits
among adults in Ghana: A cross-sectional study. Int J Environ Res
Public Health. 19:46712022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hall KD and Kahan S: Maintenance of lost
weight and long-term management of obesity. Med Clin North Am.
102:183–197. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu YS, Zhang JQ, Xu M, Yang HY, Liu CX, Li
Y, Bi QR, Yang Y, Chen QH and Guo DA: Chemical profiling and
comparative analysis of different parts of Asarum heterotropoides
using SPME-GC-QTOF-MS and LC- Orbitrap -MS. J Pharm Biomed Anal.
252:1165022025. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu M, Wang L, Meng J, Zheng B, Qin S and
Liang A: Chemical Constituents, Pharmacology, and Toxicology of
Asari Radix et Rhizoma: A Review. Chin J Exp Trad Med Form.
10:224–234. 2023.(In Chinese).
|
|
6
|
Liu H and Wang C: The genus Asarum: A
review on phytochemistry, ethnopharmacology, toxicology and
pharmacokinetics. J Ethnopharmacol. 282:1146422022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mukherjee PK, Banerjee S and Kar A:
Molecular combination networks in medicinal plants: Understanding
synergy by network pharmacology in Indian traditional medicine.
Phytochemistry Reviews. 20:693–703. 2021. View Article : Google Scholar
|
|
8
|
Noor F, Qamar MT, Ashfaq UA, Albutti A,
Alwashmi ASS and Aljasir MA: Network pharmacology approach for
medicinal plants: Review and assessment. Pharmaceuticals (Basel).
15:5722022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ru JL, Li P, Wang JN, Zhou W, Li B, Huang
C, Li P, Guo Z, Tao W, Yang Y, et al: TCMSP: A database of systems
pharmacology for drug discovery from herbal medicines. J
Cheminform. 6:132014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stelzer G, Rosen N, Plaschkes I, Zimmerman
S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y, et
al: The GeneCards suite: From gene data mining to disease genome
sequence analyses. Curr Protoc Bioinformatics. 54:1.30.31–31.30.33.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamosh A, Scott AF, Amberger JS, Bocchini
CA and McKusick VA: Online Mendelian Inheritance in Man (OMIM), a
knowledgebase of human genes and genetic disorders. Nucleic Acids
Res. 33:D514–D517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bardou P, Mariette J, Escudié F, Djemiel C
and Klopp C: jvenn: An interactive Venn diagram viewer. BMC
Bioinformatics. 15:2932014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Szklarczyk D, Kirsch R, Koutrouli M,
Nastou K, Mehryary F, Hachilif R, Gable AL, Fang T, Doncheva NT,
Pyysalo S, et al: The STRING database in 2023: Protein-protein
association networks and functional enrichment analyses for any
sequenced genome of interest. Nucleic Acids Res. 51:D638–D646.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dennis G Jr, Sherman BT, Hosack DA, Yang
J, Gao W, Lane HC and Lempicki RA: DAVID: Database for annotation,
visualization, and integrated discovery. Genome Biol. 4:P32003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jaghoori MM, Bleijlevens B and Olabarriaga
SD: 1001 ways to run AutoDock vina for virtual screening. J Comput
Aided Mol Des. 30:237–249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang P, Kang SY, Kim SJ, Park YK and Jung
HW: Monotropein improves dexamethasone-induced muscle atrophy via
the AKT/mTOR/FOXO3a signaling pathways. Nutrients. 14:18592022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hariri N and Thibault L: High-fat
diet-induced obesity in animal models. Nutr Res Rev. 23:270–299.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagy C and Einwallner E: Study of in vivo
glucose metabolism in high-fat diet-fed mice using oral glucose
tolerance test (OGTT) and insulin tolerance test (ITT). J Vis Exp.
7:566722018.
|
|
19
|
Wang P, Liu Y, Zhang T, Yin C, Kang SY,
Kim SJ, Park YK and Jung HW: Effects of root extract of Morinda
officinalis in mice with high-fat-diet/streptozotocin-induced
diabetes and C2C12 myoblast differentiation. ACS Omega.
6:26959–26968. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cai X, Wang S and Wang H, Liu S, Liu G,
Chen H, Kang J and Wang H: Naringenin inhibits lipid accumulation
by activating the AMPK pathway in vivo and vitro. Food Science and
Human Wellness. 12:1174–1183. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang W, Zhang J, Zhang M and Nie L:
Protective effect of Asarum extract in rats with adjuvant
arthritis. Exp Ther Med. 8:1638–1642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choi S, Jung MA, Hwang YH, Pyun BJ, Lee
JY, Jung DH, Ji KY and Kim T: Anti-allergic effects of Asarum
heterotropoides on an ovalbumin-induced allergic rhinitis murine
model. Biomed Pharmacother. 141:1119442021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang J, Fu YP, Du BX, Yang Y and Rong R:
Protective effect of Asarum polysaccharide on H1N1 influenza virus
infection and expression of inflammatory factors. Zhongguo Zhong
Yao Za Zhi. 46:412–419. 2021.(In Chinese). PubMed/NCBI
|
|
24
|
Han AR, Kim HJ, Shin M, Hong M, Kim YS and
Bae H: Constituents of Asarum sieboldii with inhibitory activity on
lipopolysaccharide (LPS)-induced NO production in BV-2 microglial
cells. Chem Biodivers. 5:346–351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jeong M, Kim HM, Lee JS, Choi JH and Jang
DS: (−)-Asarinin from the roots of Asarum sieboldii induces
apoptotic cell death via caspase activation in human ovarian cancer
cells. Molecules. 23:18492018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park KH, Choi JH, Song YS, Kim GC and Hong
JW: Ethanol extract of asiasari radix preferentially induces
apoptosis in G361 human melanoma cells by differential regulation
of p53. BMC Complement Altern Med. 19:2312019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jeong SJ, Choi JY, Dong MS, Seo CS and
Shin HK: Trichosanthes kirilowii exerts androgenic activity via
regulation of PSA and KLK2 in 22Rv1 prostate cancer cells.
Pharmacogn Mag. 13:153–158. 2017.PubMed/NCBI
|
|
28
|
Yoshikawa T, Shimano H, Amemiya-Kudo M,
Yahagi N, Hasty AH, Matsuzaka T, Okazaki H, Tamura Y, Iizuka Y,
Ohashi K, et al: Identification of liver X receptor-retinoid X
receptor as an activator of the sterol regulatory element-binding
protein 1c gene promoter. Mol Cell Biol. 21:2991–3000. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tremblay A and Bellisle F: Nutrients,
satiety, and control of energy intake. Appl Physiol Nutr Metab.
40:971–979. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lim S: Journal of Obesity & Metabolic
Syndrome: A new international journal targeting the pathophysiology
and treatment of obesity and metabolic syndrome. J Obes Metab
Syndr. 26:81–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reccia I, Kumar J, Akladios C, Virdis F,
Pai M, Habib N and Spalding D: Non-alcoholic fatty liver disease: A
sign of systemic disease. Metabolism. 72:94–108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fabbrini E, Sullivan S and Klein S:
Obesity and nonalcoholic fatty liver disease: Biochemical,
metabolic, and clinical implications. Hepatology. 51:679–689. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Paththinige CS, Sirisena ND and
Dissanayake V: Genetic determinants of inherited susceptibility to
hypercholesterolemia-a comprehensive literature review. Lipids
Health Dis. 16:1032017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luscher TF, Landmesser U, von Eckardstein
A and Fogelman AM: High-density lipoprotein: vascular protective
effects, dysfunction, and potential as therapeutic target. Circ
Res. 114:171–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Galgani J and Ravussin E: Energy
metabolism, fuel selection and body weight regulation. Int J Obes
(Lond). 32 (Suppl 7):S109–S119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hill JO, Wyatt HR and Peters JC: Energy
balance and obesity. Circulation. 126:126–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McPherron AC, Guo T, Bond ND and Gavrilova
O: Increasing muscle mass to improve metabolism. Adipocyte.
2:92–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Misrani A, Tabassum S and Yang L:
Mitochondrial dysfunction and oxidative stress in Alzheimer's
disease. Front Aging Neurosci. 13:6175882021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Long YC and Zierath JR: AMP-activated
protein kinase signaling in metabolic regulation. J Clin Invest.
116:1776–1783. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Habegger KM, Hoffman NJ, Ridenour CM,
Brozinick JT and Elmendorf JS: AMPK enhances insulin-stimulated
GLUT4 regulation via lowering membrane cholesterol. Endocrinology.
153:2130–2141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Canto C and Auwerx J: PGC-1alpha, SIRT1
and AMPK, an energy sensing network that controls energy
expenditure. Curr Opin Lipidol. 20:98–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kiyama T, Chen CK, Wang SW, Pan P, Ju Z,
Wang J, Takada S, Klein WH and Mao CA: Essential roles of
mitochondrial biogenesis regulator Nrf1 in retinal development and
homeostasis. Mol Neurodegener. 13:562018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bi J, Jing H, Zhou C, Gao P, Han F, Li G
and Zhang S: Regulation of skeletal myogenesis in C2C12 cells
through modulation of Pax7, MyoD, and myogenin via different
low-frequency electromagnetic field energies. Technol Health Care.
30:371–382. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hoh JFY: Developmental, physiologic and
phylogenetic perspectives on the expression and regulation of
myosin heavy chains in mammalian skeletal muscles. J Comp Physiol
B. 193:355–382. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fu X, Zhao JX, Liang J, Zhu MJ, Foretz M,
Viollet B and Du M: AMP-activated protein kinase mediates myogenin
expression and myogenesis via histone deacetylase 5. Am J Physiol
Cell Physiol. 305:C887–C895. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yan Y, Li M, Lin J, Ji Y, Wang K, Yan D,
Shen Y, Wang W, Huang Z, Jiang H, et al: Adenosine monophosphate
activated protein kinase contributes to skeletal muscle health
through the control of mitochondrial function. Front Pharmacol.
13:9473872022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chellian R, Pandy V and Mohamed Z:
Pharmacology and toxicology of α- and β-Asarone: A review of
preclinical evidence. Phytomedicine. 32:41–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang Y, Liu F, Lin Y, Li L, Chen M and Ni
L: A comprehensive review on distribution, pharmacological
properties, and mechanisms of action of sesamin. J Chemistry.
2022:1–17. 2022. View Article : Google Scholar
|
|
49
|
Cai J, Tian X, Ren J, Lu S and Guo J:
Synergistic effect of sesamin and γ-tocotrienol on promoting
osteoblast differentiation via AMPK signaling. Natural Product
Communications. 17:32022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zeng Q, Zhou TT, Huang WJ, Huang XT, Huang
L, Zhang XH, Sang XX, Luo YY, Tian YM, Wu B, et al: Asarinin
attenuates bleomycin-induced pulmonary fibrosis by activating
PPARgamma. Sci Rep. 13:147062023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu M, Wang L, Qin S, Zhao Y, Liu S, Yi Y,
Li C, Tian J, Liu C, Meng J, et al: Long-term oral administration
of Asarum heterotropoides f. mandshuricum (Maxim.) Kitag. decoction
and its aristolochic acid analogs do not cause renal toxicity in
mice. J Ethnopharmacol. 307:1162022023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Min SE, Gu EY, Jung J, Back SM, Kim W, Min
BS, Kim YB and Han KH: Evaluating the toxicity of the roots of
Asarum heterotropoides var. mandshuricum extracted using the
decoction method: Genotoxicity, single-dose toxicity, and 13-week
repeated-dose toxicity studies. J Ethnopharmacol. 325:1177832024.
View Article : Google Scholar : PubMed/NCBI
|