Numerous studies have demonstrated that epigenetic
modifications regulate gene expression, thereby affecting cell
growth and differentiation, and contributing to tumor growth
(1). Epigenetic modifications,
which mainly include histone modification, RNA modification, DNA
methylation, chromatin remodeling and non-coding RNA (ncRNA)
regulation, are heritable and reversible mechanisms that regulate
gene expression and contribute to cancer progression without
inducing DNA sequence changes (2).
RNA modification involves changing the chemical properties and
structure of RNA by adding chemical groups to RNA molecules, which
regulates RNA stability, translation efficiency and recognition by
other molecules (3). The
post-transcriptional modification of RNA, including capping,
splicing and polyadenylation, is regarded as a key factor in the
regulation of protein production in mammals (4). There are >100 distinct known
chemical modifications that can be applied to RNA molecules
post-transcriptionally, and among these, messenger RNA (mRNA)
methylation has been recognized as an important mechanism of
post-transcriptional gene regulation in eukaryotes; this includes
the formation of N6-methyladenosine (m6A),
N1-methyladenosine and 5-methylcytosine (5–7).
Among these modifications, m6A RNA methylation refers to
the methylation of adenosine bases at the nitrogen-6 position,
particularly in the 3′ untranslated region (UTR) near the stop
codon and within long internal exons. This modification is
dynamically reversible and important in the regulation of precursor
mRNA stability, splicing, transport, localization, maturation,
translation and degradation (8–10).
As one of the most abundant internal modifications in eukaryotic
mRNA, m6A modification has been identified to play a
critical role in various diseases, including cancer (11,12).
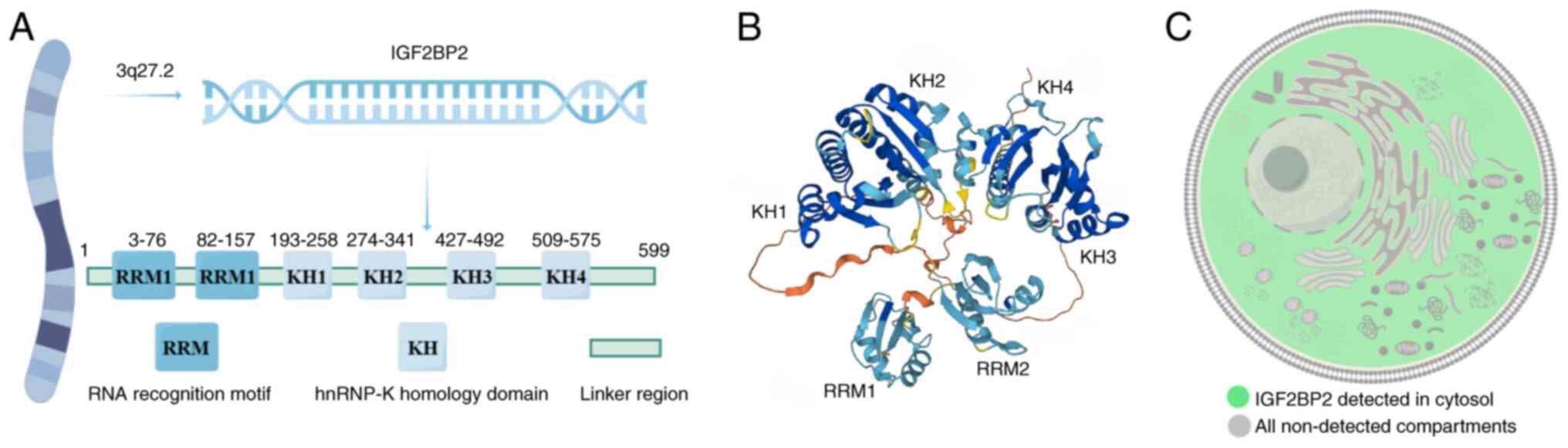
The insulin-like growth factor 2 mRNA binding
protein (IGF2BP) family, also known as the IGF-mRNA binding protein
(IMP) family was identified in 1999. These proteins attach to the
5′-UTR of mRNA and are involved in the localization, stability and
translation of target RNAs. The IGF2BP family is a unique group of
m6A readers responsible for recognizing and binding to
RNAs with m6A modification, including IGF2BP1, IGF2BP2
and IGF2BP3, which are also known as IMP1, IMP2 and IMP3,
respectively (13–16). As an RNA-binding protein, human
IGF2BP2 interacts with various types of RNA, including mRNAs,
microRNAs (miRNAs/miRs), and long ncRNAs (lncRNAs) (17). IGF2BP2 contains two N-terminal RNA
recognition motifs (RRMs) and four C-terminal K homology (KH)
domains, which bind to RNAs with very high affinity. It can bind to
the 5′-UTR, 3′-UTR or coding region of target genes, and then
assemble into granular ribonucleosomes to assist in mRNA
localization, stabilization and translation (16,18,19).
IGF2BP2 has been reported to participate in normal physiological
functions, as well as in the development of diseases such as
cancer. IGF2BP2 influences tumorigenesis and cancer progression by
affecting various biological processes in cancer cells, such as
proliferation, metastasis, angiogenesis, metabolism, cell death,
stemness and tumor immunity.
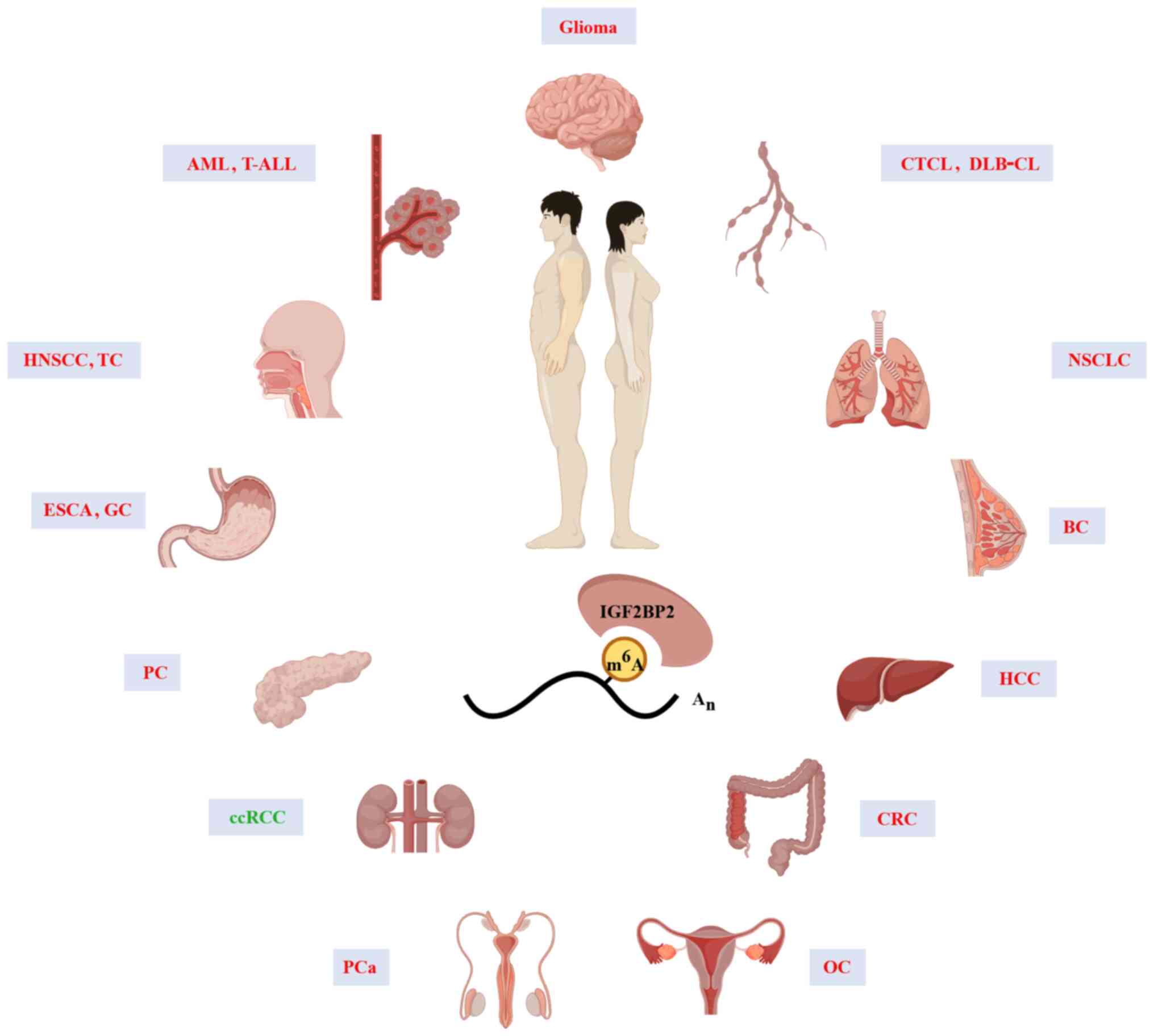
High IGF2BP2 expression has been reported to promote
the progression of various solid and hematological tumors. As shown
in Fig. 2, IGF2BP2 expression is
widely upregulated in numerous types of human tumors and only
downregulated in clear cell renal cell carcinoma (ccRCC). Thus,
IGF2BP2 predominantly acts as an oncogene, and is associated with
the tumorigenesis and development of various human cancers.
Therefore, the clinical significance of IGF2BP2 and its association
with patient survival and prognosis in different cancers are
discussed in the present review.
An association between IGF2BP2 expression and poor
prognosis has been detected in most solid tumors that have been
evaluated. For instance, IGF2BP2 expression is elevated in glioma
and contributes to the poor survival of patients with low-grade
glioma (21,22). In addition, IGF2BP2 was identified
as the core regulator among all m6A regulators in head
and neck squamous cell carcinoma (HNSCC), in which it is highly
expressed and independently predicts a poor prognosis in patients
with HNSCC (23). Deng et
al (24) were the first to
evaluate the prognostic role of IGF2BP2 in HNSCC, via Kaplan-Meier
and Cox regression analyses using public data from The Cancer
Genome Atlas (TCGA), and immunohistochemistry results from HNSCC
samples. The authors demonstrated that high expression of IGF2BP2
is associated with a poor prognosis and that IGF2BP2 has potential
as a prognostic factor. IGF2BP2 has also been indicated to be an
oncogenic factor in laryngeal squamous cell carcinoma (LSCC), and
to promote the proliferation and invasion of LSCC cells in
vitro (25). Similarly,
IGF2BP2 expression in oral squamous cell carcinoma (OSCC) exhibits
a significant association with lymph node metastasis, tumor stage
and patient survival, and IGF2BP2 protein is indicated to be a
potent predictive marker in OSCC (26). Moreover, high IGF2BP2 expression
was found to be associated with a shorter survival in patients with
esophageal adenocarcinoma or esophageal squamous cell carcinoma
(ESCC) (27). In addition,
Barghash et al (27)
suggested that IGF2BP2 might be a useful prognostic marker in
Barrett's esophageal cancer and ESCC, while Lu et al
(28) found that IGF2BP2
expression was significantly upregulated in ESCC tissues and that
its expression was closely associated with the
tumor-node-metastasis stage of ESCC.
Pancreatic ductal adenocarcinoma (PDAC) is one of
the most malignant gastrointestinal tumors, with a poor prognosis
and high relapse rate (15,29,30).
IGF2BP2 is highly expressed in pancreatic intraepithelial neoplasia
lesions, suggesting that it may serve as a marker for the early
stages of PDAC (30). Moreover, an
analysis of TCGA and Gene Expression Profiling Interaction Analysis
databases revealed that IGF2BP2 is upregulated in pancreatic cancer
(PC), and the expression level of IGF2BP2 correlates with the
overall survival of patients with PC (30). Additionally, IGF2BP2 was identified
as the IGF2BP family member with the greatest clinical relevance in
PC, with high IGF2BP2 expression associated with a poor prognosis
and an immunosuppressive microenvironment in PDAC (29).
IGF2BP2 has also been found to be associated with
the development of hepatocellular carcinoma (HCC) and colorectal
cancer (CRC). Zhang et al (31) originally isolated the autoantigen
IGF2BP2 from a patient with HCC, and Lu et al (32) further demonstrated that IGF2BP2
expression is elevated in HCC and premalignant cirrhotic nodules.
In addition, Shen et al (33) demonstrated that IGF2BP2 regulates
glycolysis in CRC cells by controlling the expression of hexokinase
and glucose transporters. Furthermore, IGF2BP2 expression was found
to be upregulated in lung adenocarcinoma (LUAD) samples, as well as
in patients with lung squamous cell carcinoma (34), while the downregulation of IGF2BP2
expression was indicated to be associated with improved survival in
patients with LUAD (35). Almawi
et al (36) investigated
the association of rs4402960 and rs1470579 variants of
IGF2BP2 with breast cancer (BC) and triple-negative BC
(TNBC). Although a positive association of rs440960 with BC was
identified, both rs4402960 and rs1470579 showed negative
associations with TNBC, suggesting that these genetic variants may
have potential diagnostic and prognostic value in BC and its
subtypes. In addition, IGF2BP2 expression was found to be
upregulated in ovarian cancer (OC) and associated with a poor
survival outcome in patients, and the knockdown of IGF2BP2
inhibited OC cell proliferation in vitro (37,38).
These findings indicate that IGF2BP2 plays a key role and may serve
as a potential biomarker in most solid tumors.
IGF2BP2 has the potential to serve as a prognostic
biomarker and therapeutic target in acute myelocytic leukemia
(AML). He et al (39) found
that the expression of IGF2BP2 was upregulated in patients with
AML, negatively correlated with CCAAT/enhancer-binding protein a
mutation status, and positively associated with poor prognostic
factors. After analyzing gene expression datasets and performing
genome enrichment analyses, the study found that genes regulated by
IGF2BP2 were mainly enriched in pathways associated with cell
proliferation. In addition, the study revealed that the knockdown
of IGF2BP2 by a short hairpin RNA vector significantly
inhibited the growth of the KG-1a and Kasumi AML cell lines.
Similarly, Feng et al (40)
demonstrated that IGF2BP2 is highly expressed in T-cell acute
lymphoblastic leukemia (T-ALL) and directly binds to the T-ALL
oncogene NOTCH1 in an m6A-dependent manner.
Another study, performed by Zhou et al (41), revealed that the rs4402960
polymorphism in the IGF2BP2 gene was associated with an
increased risk of developing diffuse large B-cell lymphoma
(DLB-CL). These data demonstrate that IGF2BP2 plays a clinically
important role in certain hematological tumors.
Sustained proliferation, along with invasion and
metastasis, are hallmarks of human cancers that contribute to the
poor prognosis of malignant tumors (42). As an m6A modification
reader, IGF2BP2 has been reported to promote tumor cell
proliferation, metastasis and progression in a variety of cancers.
Therefore, the latest evidence regarding the effects of IGF2BP2 on
tumor proliferation, metastasis and progression is summarized in
the present review (Table I).
In AML, IGF2BP2 has been indicated to promote cancer
progression by stabilizing DEAD box helicase 21 and triggering the
expression of Unc-51-like kinase 1 (43). In glioma, Song et al
(44) revealed that IGF2BP2
mediates the upregulation of flotillin-1 via m6A
modification, thereby promoting tumor growth and metastasis. Yu
et al (45) reported that
IGF2BP2 is associated with lymphatic metastasis in patients with
HNSCC promotes and promotes the epithelial-mesenchymal transition
(EMT) of HNSCC cells by stabilizing Slug mRNA. In OSCC,
IGF2BP2 has been reported to promote the expression of MYC and the
autophagy-related gene RB1-inducible coiled-coil 1, thereby
influencing the malignant progression of OSCC (46,47).
IGF2BP2 also promotes OSCC progression by enhancing the stability
of solute carrier family 7 member 11 (SLC7A11) mRNA
(48). In addition, a recent study
showed that epiregulin functions as a downstream modulator of
IGF2BP2 and triggers EMT in OSCC, via a mechanism dependent on
activation of the FAK/Src signaling pathway (49). IGF2BP2 has been indicated to
influence the progression of thyroid cancer (TC) by regulating the
expression of lncRNA HAGLR in an m6A-dependent
manner (50), and to promote
lymphatic metastasis by the stabilization of dipeptidyl peptidase 4
mRNA in papillary thyroid carcinoma (PTC) (51). In LUAD, IGF2BP2 interacts with
circular eukaroytic elongation factor 2 and calcium-activated
nucleotidase 1 (CANT1), which stabilizes CANT1 mRNA
and promotes tumorigenesis and growth (52), while in NSCLC, IGF2BP2 promotes
proliferation by regulating the expression of lncRNA MALAT1
(53). In ESCC, IGF2BP2 promotes
the stability of 5-hydroxytryptamine receptor 3A and circular
runt-related transcription factor 1 RNA, which facilitates cell
proliferation and metastasis (54,55).
Moreover, IGF2BP2 also promotes the progression of ESCC by
increasing the mRNA stability of octamer-binding transcription
factor 4 and increasing eukaryotic initiation factor 4A1 (EIF4A1)
translation in an m6A-dependent manner (56,57).
IGF2BP2 regulates the proliferation and metastasis
of gastric cancer (GC) via the recognition of m6A
modification sites in the mRNAs of sirtuin 1, zinc finger E-box
binding homeobox 1 and high mobility group AT-hook 1 (HMGA1)
(58–60). IGF2BP2 may also promote the
progression of GC by regulating the insulin-like growth factor 1
receptor (IGF1R)-Ras homolog family member A-Rho-associated protein
kinase signaling pathway (61). In
CRC, IGF2BP2 promotes the mRNA stability and expression of SRY-box
transcription factor 2, stromal antigen 3, yes-associated protein,
cyclin D1 (CCND1) and HMGA1 via an
m6A-dependent mechanism and promotes cancer progression
(62–66). IGF2BP2 also contributes to CRC
progression by regulating the methylation of transferrin receptor 1
mRNA via methyltransferase like 4, thereby influencing iron
metabolism (67). Furthermore,
IGF2BP2 regulates RAF1 expression by blocking its
degradation by miR-195 in CRC, which promotes CRC cell
proliferation (68). In PC,
IGF2BP2 promotes cancer progression by stabilizing numerous mRNA
transcripts, including those of amyloid β precursor protein-like 2,
β-1,3-N-acetylglucosaminyltransferase 6, Toll-like receptor (TLR)4
and MYC (69–72). Furthermore, IGF2BP2 upregulates the
mRNA stability of LINC00941 and erythroblast variant
transcription factor 1 in PC, which promotes cell metastasis
(73,74). In HCC, IGF2BP2 maintains cell
division cycle 27 mRNA stability, thereby promoting both
proliferation and metastasis (75). Zhang et al (76) demonstrated that the IGF2BP2/lncRNA
TRPC7-AS1 axis promotes cell proliferation and invasion in
HCC. Additionally, IGF2BP2 activates the small Rho GTPase RAC1 in a
δ-like 1 homolog-dependent manner, which promotes
hepatocarcinogenesis by amplifying inflammation (77). Furthermore, IGF2BP2 has been shown
to facilitate the growth of liver cancer by increasing the mRNA
stability of flap endonuclease 1 (78).
During the progression of BC, IGF2BP2 increases the
stability of ubiquitin conjugating enzyme E2 D1 mRNA, which
regulates the activation of TGF-β signaling by modulating the
expression and phosphorylation levels of Smad2/3 (79). Xia et al (80) reported that IGF2BP2 drives tumor
proliferation in TNBC by recruiting EIF4A1, which promotes the
translation of m6A-modified CDK6. Additionally,
IGF2BP2 facilitates the metastasis of TNBC by promoting cell
migration and reducing cell adhesion (81). Moreover, IGF2BP2 contributes to OC
growth and metastasis by upregulating the expression of
cytoskeleton-associated protein 2-like protein in an
m6A-dependent manner (82). In endometrial cancer, IGF2BP2
stabilizes platelet-derived growth factor b and activates JAK/STAT
signaling, thereby inducing cancer progression (83). IGF2BP2 has also been shown to
promote the mRNA stability of forkhead box M1 (FOXM1) and
contribute to cervical cancer (CC) progression (84). In prostate cancer (PCa), IGF2BP2
promotes the mRNA stability of lncRNA PCAT6 and IGF1R
and facilitates the bone metastasis of cancer cells (85). In addition, IGF2BP2 stabilizes the
methylated mRNA of homeobox C6 and enhances PCa progression
(86). Furthermore, IGF2BP2
regulates the progression of cutaneous T-cell lymphoma by
recognizing and stabilizing CDK inhibitor 2A mRNA (87). In particular, IGF2BP2 promotes
tumor proliferation and metastasis by activating the PI3K/AKT
signaling pathway in numerous human cancers, such as LSCC (88,89),
HNSCC (90), PC (91) and glioblastoma (GBM) (92).
IGF2BP2 also promotes cancer progression via the
degradation of RNA transcripts. For example, IGF2BP2 has been
reported to promote the degradation of ATPase H+
transporting V1 subunit A RNA, resulting in the production of a
cellular secretome that increases tumor cell survival and
invasiveness in BC (93). In TNBC,
IGF2BP2 destabilizes the mRNA of the progesterone receptor and
promotes cancer metastasis (94).
However, several studies have suggested that IGF2BP2 inhibits
cancer progression. One study indicated that IGF2BP2 interacts with
lncRNA HCG11 and large tumor suppressor kinase 1 mRNA,
thereby mediating the methyltransferase like (METTL) 14-induced
inhibition of LUAD (95). In
addition, IGF2BP2 binds to the 3′-UTR region of snail family
transcriptional repressor 1 (SNAI1) mRNA and mediates the
fat mass and obesity-associated (FTO)-induced destabilization of
SNAI1, thereby inhibiting OC progression (96). In BC, IGF2BP2 upregulates quaking
protein expression, leading to the suppression of tumor growth
(97). IGF2BP2 suppresses ccRCC
metastasis by stabilizing the mRNA of creatine kinase B-type and
serpin family H member 1, neuropilin and tolloid-like 1 (98,99).
IGF2BP2 also binds to the m6A site of the netrin-4
transcript and promotes its expression, which suppresses the
invasion and migration of ccRCC (100). These findings suggest that
IGF2BP2 plays multiple and conflicting roles in the promotion or
inhibition of cancer cell proliferation, metastasis and
progression.
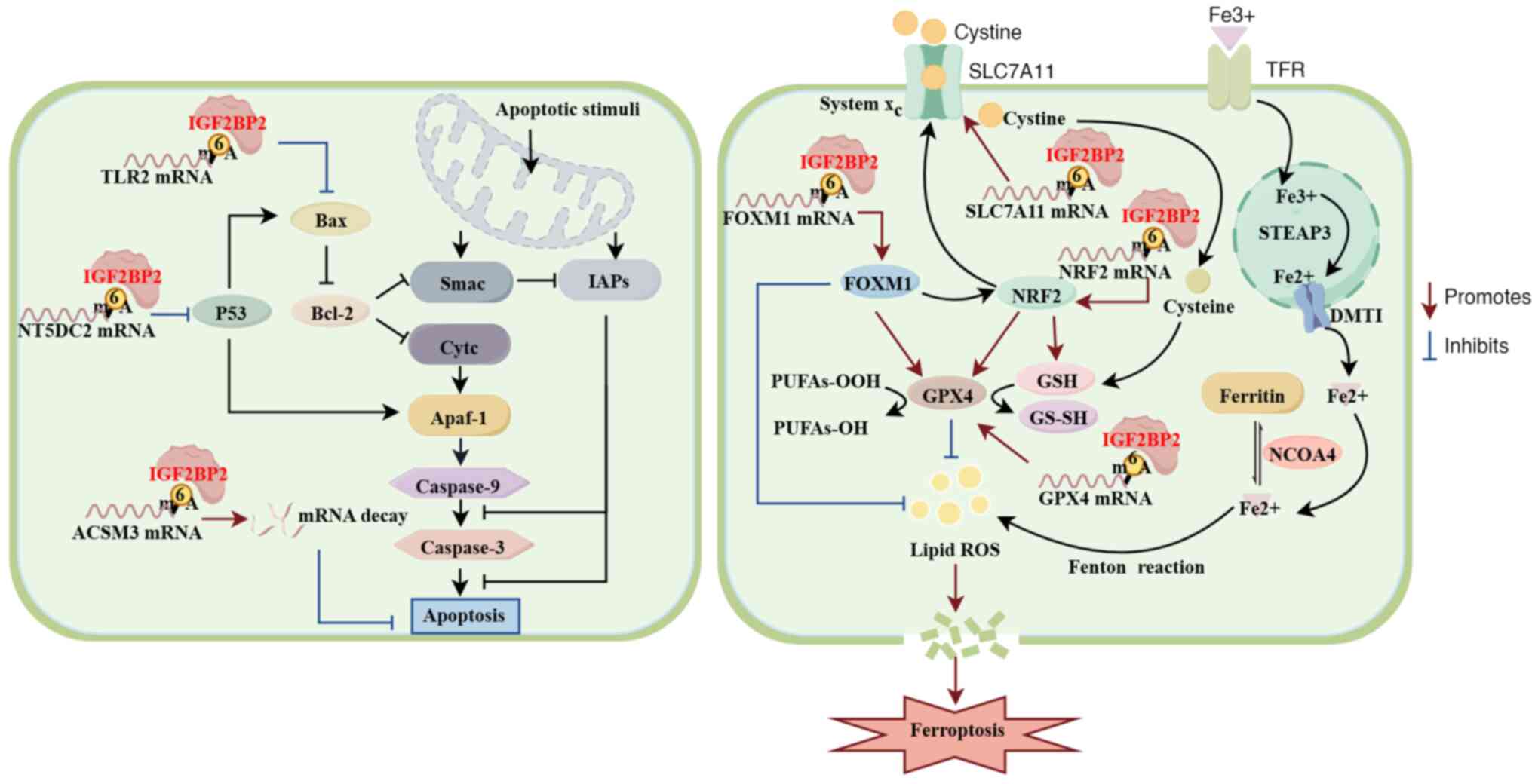
The development of chemoresistance and
radioresistance markedly hinders the efficacy of radiochemotherapy
in various cancers. However, the underlying mechanisms remain
unclear. IGF2BP2 has been shown to regulate chemotherapy
resistance, and also to lead to radiotherapy resistance. The
existing literature on the role of IGF2BP2 in tumor resistance to
chemotherapy and radiotherapy is summarized in the present review
(Table II).
Cisplatin (DDP) is the earliest and most effective
platinum compound used to treat various cancers. However, several
studies have shown that IGF2BP2 can contribute to DDP resistance in
tumor cells. Wu et al (101) demonstrated that IGF2BP2 plays a
role in mediating the miR-96-5p-induced resistance of CC
cells to DDP. In addition, IGF2BP2 stabilizes ATPase copper
transporting α mRNA and elevates its protein level, thereby
facilitating circular PBX homeobox 3-promoted DDP resistance in OC
cells (102). Furthermore,
IGF2BP2 stabilizes lncRNA taurine upregulated gene 1 and promotes
cell proliferation, migration, and autophagy via the
miR-195-5p/hepatoma-derived growth factor/DEAD-box helicase
5/β-catenin axis, thereby promoting the resistance of CRC to DDP
(103).
IGF2BP2 also mediates resistance to numerous other
types of chemotherapeutic agents. In gliomas, IGF2BP2 stabilizes
the lncRNA DANCR, which inhibits the FOXO1-induced
transcriptional expression of phosphotyrosine interaction domain
containing 1, thereby reducing the etoposide sensitivity of GBM
cells (104). Zhang et al
(105) elucidated the mechanism
of crizotinib resistance in NSCLC, finding that IGF2BP2 modulates
MYC expression and mediates LINC01001-induced
chemoresistance. Wang et al (106) demonstrated that IGF2BP2
upregulates p-glycoprotein in an m6A-dependent manner,
thereby contributing to Adriamycin resistance in BC. Additionally,
the IGF2BP2-dependent activation of epidermal growth factor
receptor 4 (ERBB2) signaling contributes to the acquisition of
resistance to tyrosine kinase inhibitors; the lapatinib-induced
inhibition of IGF2BP2 and ERBB2 was able to reverse the resistance
of a selumetinib-resistant PTC cell line (107). Moreover, the upregulation of
miRNA-129-5p downregulates IGF2BP2, thereby repressing
temozolomide resistance in GBM cells (108). Shi et al (109) found that IGF2BBP2 stabilizes
3-hydroxy-3-methylglutaryl-CoA synthase 1 mRNA to drive
enzalutamide resistance in PCa. In addition, IGF2BP2 mediates
sorafenib resistance in HCC by inhibiting ferroptosis via the
promotion of SLC7A11 mRNA stability (110).
Evaluation of the resistance to chemotherapeutics in
patient-derived xenografts (PDXs) and patient-derived organoids
(PDOs) revealed that IGF2BP2 expression in primary tumor tissue was
associated with resistance to selumetinib, gefitinib and
regorafenib in PDOs and to 5-fluorouracil and oxaliplatin in PDXs
in vivo (111). These
findings indicate that IGF2BP2 plays a key role in the development
of chemoresistance in various cancers.
In addition to chemoresistance, IGF2BP2 also
modulates radiosensitivity in several cancers. For example, in lung
cancer, IGF2BP2 promotes the stability and expression of
SLC7A5 mRNA, and SLC7A5 facilitates the transport of
methionine into cells, which increases H3K4me3 enrichment and
subsequently promotes IGF2BP2 expression. This IGF2BP2-SLC7A5
positive feedback loop promotes radioresistance through the
AKT/mTOR pathway, suggesting that IGF2BP2 may be a potential
therapeutic target to overcome radioresistance in lung cancer
(112). In addition, IGF2BP2 has
been shown to be involved in stabilizing deltex E3 ubiquitin ligase
3-like mRNA in HCC, thereby alleviating radiation-induced DNA
damage and increasing the radiation resistance of cancer cells
(113).
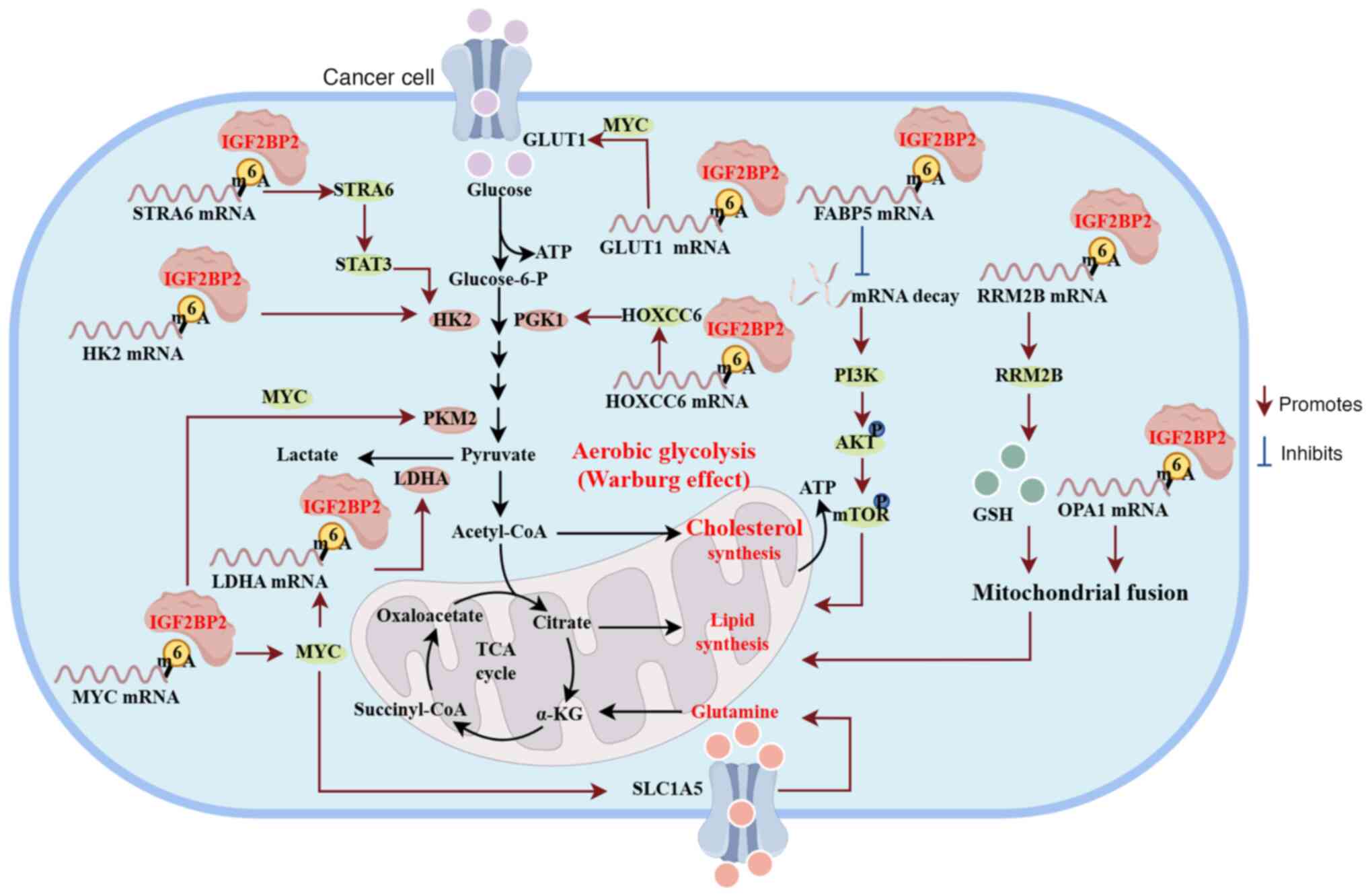
Aerobic glycolysis, also termed the Warburg effect,
has been shown to play a key role in tumor cell proliferation and
metastasis, and is an important distinguishing feature between
normal cells and malignant tumor cells (114). As shown in Fig. 3, IGF2BP2 regulates aerobic
glycolysis, glutamine metabolism and lipid synthesis by regulating
the mRNA stability of its target transcripts. For instance, IGF2BP2
promotes AML progression by regulating the expression of lncRNA
DANCR and upregulating glycolysis (115). In addition, the elevated
expression of IGF2BP2 promotes AML development and the self-renewal
of leukemia stem cells or initiation cells by regulating the
expression of key targets, such as MYC, glutamate pyruvate
transaminase 2 and solute carrier family 1 member 5, which are
involved in glutamine metabolism pathways via m6A
modification. IGF2BP2 also increases the mRNA stability of the
ribonucleotide reductase regulatory subunit M2B gene, which
promotes the generation of glutathione in CRC cells (116).
IGF2BP2 has been reported to promote aerobic
glycolysis and cell proliferation in CRC and PDAC by stabilizing
glucose transporter 1 mRNA (117). IGF2BP2 also binds to MYC
mRNA, improving its stability and expression, thereby maintaining
MYC-mediated aerobic glycolysis and proliferation of CRC cells
(118). In addition, IGF2BP2
contributes to the mRNA stability of stimulated by retinoic acid 6,
leading to activation of the STAT3/hypoxia inducible factor 1 α
axis and the subsequent upregulation of glycolysis in PDAC
(119). IGF2BP2 also promotes
MYC mRNA stability in an m6A-dependent manner and
promotes aerobic glycolysis during the progression of CC (120). Similarly, IGF2BP2 stabilizes
MYC mRNA and promotes MYC protein expression, which further
upregulates lactate dehydrogenase A expression and promotes aerobic
glycolysis in PCa (121).
Notably, the KH3-4 domains of the IGF2BP2 protein have been
identified as the key RNA-binding domains responsible for the
recognition of m6A sites (‘RGGAC/RRACH’) within the
lncRNA ZFAS1. In turn, ZFAS1 directly binds to
oxidative lethal 1 (OLA1), which promotes the ATPase activity of
OLA1, thereby mediating mitochondrial energy metabolism, including
ATP hydrolysis and glycolysis, in the progression of CRC (122).
Hexokinase 2 (HK2) is an enzyme that catalyzes the
phosphorylation of glucose to form glucose-6-phosphate, which
regulates the first committed step in glucose metabolism (123,124). The relationship between HK2
expression and tumor metabolism has been reported in numerous
studies. For example, IGF2BP2 may directly interact with HK2
mRNA by binding to the m6A site in the 3′-UTR, thereby
promoting the stability of HK2 mRNA and increasing HK2
expression (125). IGF2BP2
recognizes the m6A site of lncRNA CASC9,
enhancing its stability. The resulting IGF2BP2/CASC9 complex
increases the stability of HK2 mRNA, thereby promoting
aerobic glycolysis in GBM cells (126). The FTO-AlkB homolog
5/IGF2BP2/HK2/FOXO1 axis is involved in the mechanism underlying
the aberrant m6A modification and regulation of
glycolysis in CRC. Within this signaling pathway, IGF2BP2 regulates
HK2 mRNA expression in an m6A-dependent manner,
further promoting tumor metabolism and CRC progression (127). Additionally, IGF2BP2 increases
the expression of fatty acid binding protein 5 in an
m6A-dependent manner in pancreatic neuroendocrine
neoplasms, potentially leading to lipid metabolism disorders. This
highlights a new molecular basis for the development of therapeutic
strategies for pancreatic neuroendocrine neoplasms (128).
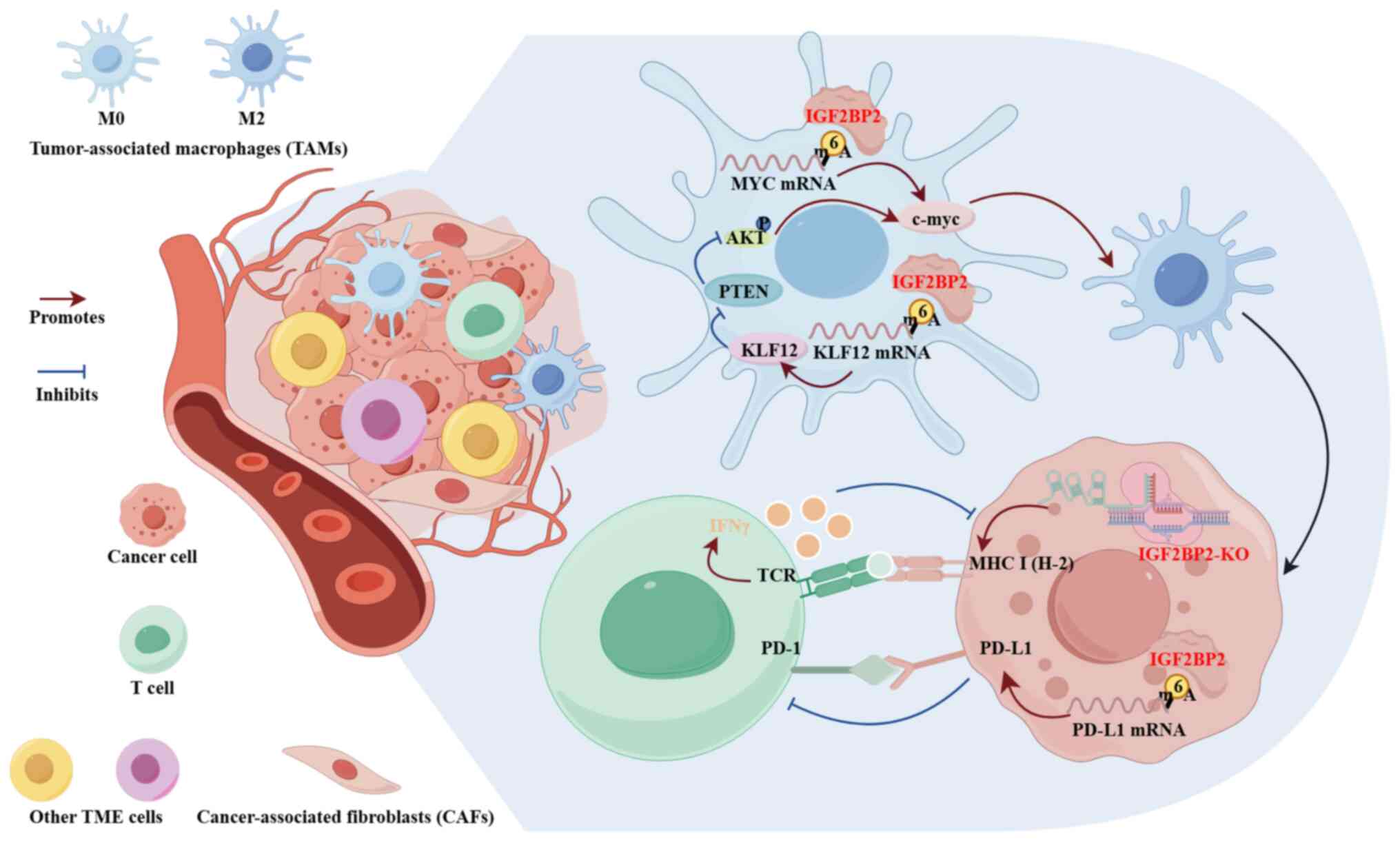
Immunotherapy acts against tumor cells that present
new antigens and exhibit pro-inflammatory activity (129). However, one feature of malignant
tumors is their ability to avoid immune destruction (42). It has been shown that the IGF2BP
family of RNA-binding proteins can regulate the innate and adaptive
immune responses to cancer cells within the tumor microenvironment
(TME). Among them, IGF2BP2 plays a crucial role in modulating the
immune microenvironment of malignant tumors. As shown in Fig. 4, IGF2BP2 knockout was found
to increase major histocompatibility complex I (H-2) expression in
mouse melanoma cells and induce intracellular IFNγ expression in
syngeneic T-lymphocytes in vitro (130). Liu et al (131) reported that the knockdown of
IGF2BP2 inhibited CRC cell proliferation and migration, and
also promoted tumor immunity by downregulation of the expression of
MYC, TNFα and IL-10. In addition, IGF2BP2 has been shown to
recognize and stabilize methylated programmed cell death ligand 1
(PD-L1) transcripts, thereby blocking T cell-mediated
antitumor activity in CRC (132).
In PDAC, IGF2BP2 increases the stability of Kruppel-like factor 12
and MYC, which induces the polarization of pro-tumor
macrophages in the TME and promotes PC progression (133).
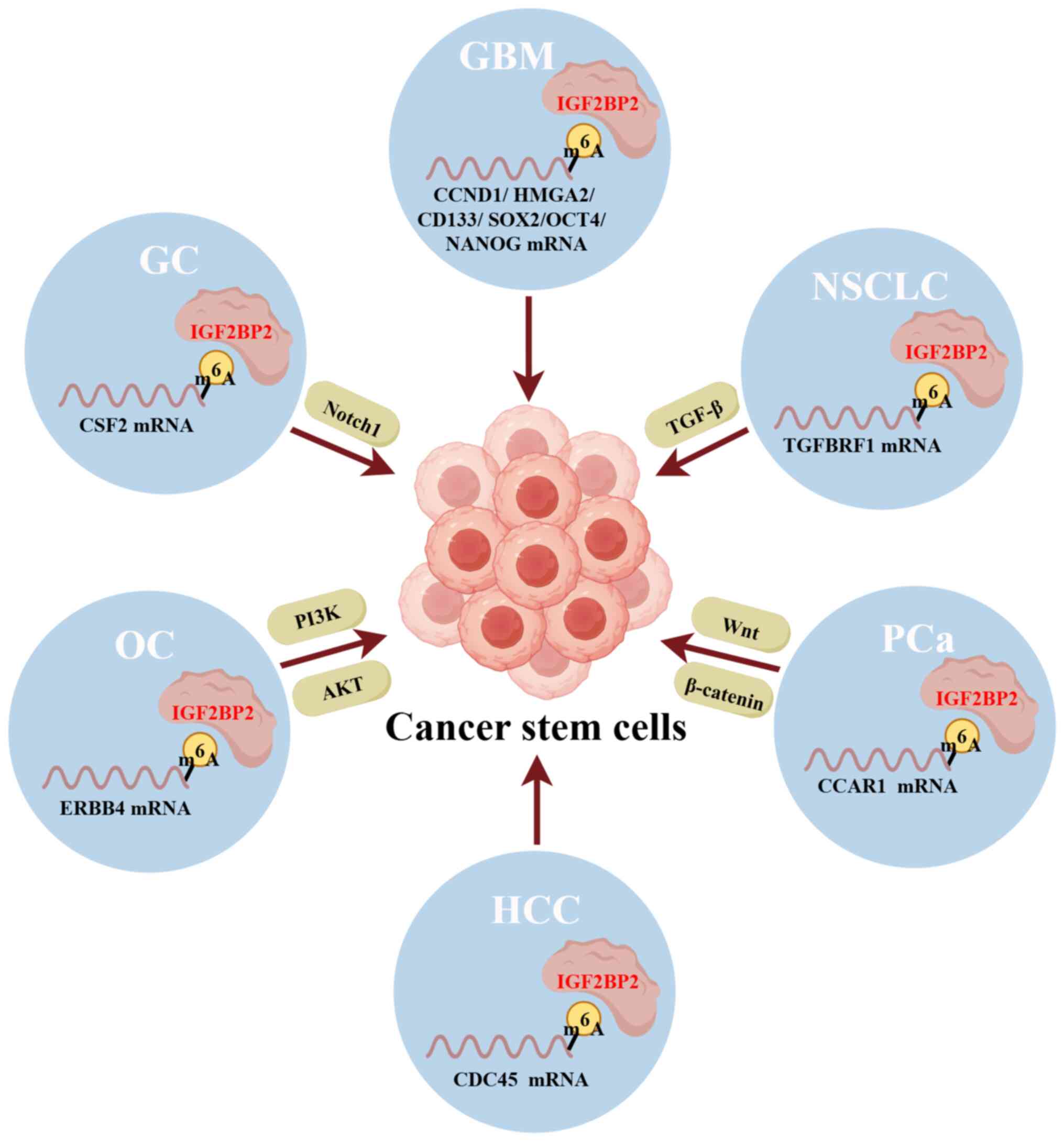
Several reports have indicated that IGF2BP2 may
affect cell stemness in various cancers. As shown in Fig. 5, IGF2BP2 stabilizes TGFb factor
receptor 1 mRNA and participates in the forkhead box P3-promoted
stemness of NSCLC cells (136).
Additionally, IGF2BP2 increases the mRNA stability and expression
of cell cycle and apoptosis regulator 1, which subsequently
upregulates the expression levels of Wnt/β-catenin target genes,
thereby promoting stemness and metastasis in PCa (137). Ji et al (138) found that the m6A
reader IGF2BP2 binds to and stabilizes colony-stimulating factor 2
(CSF2) mRNA in mesenchymal stem cells (MSCs). Consequently,
IGF2BP2 overexpression simulates the effect of CSF2 on MSCs and
promotes GC progression. In addition, the study revealed that
IGF2BP2-regulated CSF2 induces Notch1 ubiquitination to reprogram
MSCs into a tumor-promoting phenotype, including augmented tropism
towards GC cells and elevated expression of FAP, α-SMA and
inflammatory factors GM-CSF, FGF and PDGF-BB, providing a promising
therapeutic target for GC. Furthermore, an early study found that
IGF2BP2 regulates oxidative phosphorylation in primary GBM sphere
cultures and contributes to the preservation of GBM cancer stem
cells (139). Also, another study
suggested that IGF2BP2 binds to let-7 miRNA recognition
elements and inhibits the silencing of let-7 miRNA target
genes such as CCND1 and HMGA2, thereby promoting the
clonality and tumor initiation ability of glioma stem cells
(140).
The induction of angiogenesis is one of the
characteristics of malignant tumors (42), as tumor growth relies on
angiogenesis for the supply of nutrients and oxygen (152,153). IGF2BP2 increases the expression
of thymidine kinase 1, thereby promoting angiogenesis and CD31
expression (154). In addition,
IGF2BP2 activates endothelial cells and stabilizes fms-related
tyrosine kinase 4 via m6A modification, thereby
activating the PI3K/AKT signaling pathway and promoting
angiogenesis and metastasis in LUAD (155). In gallbladder cancer, IGF2BP2
recognizes the m6A modification of transient receptor
potential cation channel subfamily M member 2-antisense RNA,
stabilizes its mRNA, and promotes angiogenesis by activating Notch1
signaling (156).
In addition to traditional tumor angiogenesis,
vasculogenic mimicry (VM) is a tumor microcirculation model in
which newly formed blood vessel-like structures transport nutrients
and blood to support tumor growth (157). Both ephrin type-A receptor 2
(EphA2) and vascular endothelial growth factor (VEGFA) play crucial
roles in VM in tumors (158–161). Liu et al (162) demonstrated that IGF2BP2 mediates
the METTL3-regulated expression of EphA2 and VEGFA via an
m6A-dependent mechanism, thereby promoting VM via the
PI3K/AKT/mTOR and ERK1/2 signaling pathways in CRC. In addition,
the SUMOylation of IGF2BP2 regulates the opa-interacting
protein-antisense RNA 1/miR-495-3p axis, which promotes VM
and glioma cell growth (163).
These findings highlight the diverse mechanisms by which IGF2BP2
influences tumor angiogenesis.
Considering the multifaceted roles of IGF2BP2 in
the progression of various cancers, several IGF2BP2 inhibitors have
been explored as potential therapeutic strategies (Table III). IGF2BP2 inhibitors were
initially screened from various compound libraries using
fluorescence polarization experiments, and the screening results
were verified by thermal shift experiments and saturation transfer
difference nuclear magnetic resonance. This identified 10 compounds
in two categories, specifically 4-benzamidobenzoic acid and
ureidothiophene derivatives, of which the three compounds with the
strongest biological target-specific activities were tested and
verified. These small-molecule inhibitors targeting IGF2BP2 were
shown to have an inhibitory effect on the growth of xenograft
tumors in an in vivo experiment using zebrafish (166). Since then, CWI1-2 has been
identified as another inhibitor of IGF2BP2. CWI1-2 binds to the
K3-K4 domains of IGF2BP2 and inhibits its interaction with
m6A-modified target transcripts. Consequently, CWI1-2
induces apoptosis and differentiation and exerts an anti-leukemic
effect (167). Similarly, Feng
et al (40) identified
another IGF2BP2 inhibitor, JX5, which targets the KH3-KH4 domains
of IGF2BP2. JX5 treatment was demonstrated to inhibit T-ALL both
in vitro and in vivo. Additionally, another study
generated IGF2BP2 knockout cells using the
CRISPR/cas9-primer editing method and verified that IGF2BP2 has the
potential to serve as an anticancer target (168).
Currently, miRNAs are used in treatment regimens to
inhibit tumor progression due to their ability to directly regulate
target genes (169–171). Numerous studies have demonstrated
that ncRNAs, including miRNAs, can regulate the expression of
IGF2BP2. As shown in Table IV,
several miRNAs have been found to directly inhibit IGF2BP2
expression in different cancers. In TC, IGF2BP2 was identified as a
target of miR-204 and miR-4640-5p, which binds to the
3′-UTR of IGF2BP2 to inhibit its expression (165,172). In HCC and ESCC, miR-216b
was shown to bind to the 3′-UTR of IGF2BP2 and decrease its
expression (173,174). Similarly, miR-200b was
found to inhibit IGF2BP2 expression in ESCC (175). Additionally, miR-596 and
miR-7-5p were confirmed to downregulate IGF2BP2 expression
in HCC (176,177). In CRC, IGF2BP2 was verified as a
target of miR-133b, which directly inhibits the expression
of IGF2BP2 (178). Several
studies of gliomas demonstrated that miR-138, miR-188 and
miR-129-5p directly target and inhibit IGF2BP2 expression
(108,179,180). Furthermore, miR-98-5p,
miR-485-5p, miR141 and miR200a were shown to inhibit
IGF2BP2 expression in HNSCC (90),
NSCLC (181), PC (91) and TNBC (94), respectively. These data suggest
that the expression of IGF2BP2 can be inhibited by numerous miRNAs,
which may offer a potential therapeutic approach for the targeting
of IGF2BP2 in human cancers.
Although IGF2BP2 expression has been found to be
upregulated in various tumors, the exact effects of IGF2BP2 on
tumor development and progression, as well as the underlying
molecular mechanisms, remain unclear in numerous cancers. Some
studies have described the association of IGF2BP2 with tumor
characteristics based solely on bioinformatic analyses using public
databases, without experimental validation in vitro or in
vivo. Therefore, further studies are necessary to understand
the roles of IGF2BP2 in different tumors and uncover the underlying
molecular mechanisms. Some studies have suggested that IGF2BP2 can
influence tumor immunity, such as tumor-associated macrophage
polarization and PD-L1 expression, by regulating the mRNA stability
and expression of target genes. However, tumor immunity is
extremely complex due to the involvement of multiple types of
immune cells and other components of the TME. Accordingly, current
knowledge of the effect of IGF2BP2 on tumor immunity is limited,
and more research is needed to fully elucidate the participation of
IGF2BP2 in TME regulation during tumorigenesis and tumor
progression. In the future, novel immunotherapy regimens targeting
IGF2BP2 may be developed to treat different tumors, particularly in
patients who are insensitive or resistant to chemotherapy and
radiotherapy.
Given the notable involvement of IGF2BP2 in various
human tumors, it is urgently necessary to develop specific
inhibitors targeting this key molecule. However, studies on IGF2BP2
inhibitors remain limited. Currently, only CWI1-2 and JX5 are being
evaluated for the treatment of AML in laboratory experiments.
Another study focused on screening IGF2BP2 inhibitors and
identified several candidate compounds. However, all existing
compounds were investigated in preclinical experiments, but lack
sufficient in vivo validation of their pharmacodynamics and
drug toxicity. It is not known whether and when these compounds
will be tested in clinical trials. All existing compounds target
the KH3-4 domains of IGF2BP2; however, other members of the IGF2BP
family, namely IGF2BP1 and IGF2BP3, also contain these domains,
which may lead to off-target effects and resistance. By contrast,
miRNAs can inhibit the expression of target genes with high
specificity, and IGF2BP2 expression can also be inhibited by
different miRNAs in cancer. Therefore, miRNAs are considered a
promising approach for the specific inhibition of IGF2BP2
expression, which could help to overcome resistance and reduce
off-target effects in human cancers. Overall, the study of IGF2BP2
inhibitors is in its early stages, but has great development
potential for clinical application.
In summary, IGF2BP2 plays a key role in the
development and progression of human cancers by regulating numerous
oncogenic processes. Therefore, it has great potential as a target
for cancer therapy. However, the effects of IGF2BP2 on certain
physiological processes and the underlying molecular mechanisms
remain poorly understood, particularly with regard to tumor
immunity. Hence, these warrant further investigations in the
future. In addition, the upstream molecular mechanisms, including
post-translational modifications, specific transcription factors
and epigenetic modifications, that regulate IGF2BP2 expression in
cancers warrant further investigation, even though certain miRNAs
have been found to regulate IGF2BP2 expression. Also, no specific
inhibitor of IGF2BP2 has been clinically used to treat tumors,
although several compounds have been identified and evaluated in
laboratory research. Accordingly, another important topic for
future research on IGF2BP2 is the development of small molecule
inhibitors, which may be valuable for treating malignant tumors in
which IGF2BP2 expression is abnormally upregulated.
Not applicable.
This study was supported by the National Natural Science
Foundation of China (grant no. 82203364).
Not applicable.
JS wrote the manuscript and drafted the figures. YD
collected literature, provided guidance and revised the manuscript.
Data authentication is not applicable. Both authors read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Zhao LY, Song J, Liu Y, Song CX and Yi C:
Mapping the epigenetic modifications of DNA and RNA. Protein Cell.
11:792–808. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Toh TB, Lim JJ and Chow EK: Epigenetics in
cancer stem cells. Mol Cancer. 16:292017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Orsolic I, Carrier A and Esteller M:
Genetic and epigenetic defects of the RNA modification machinery in
cancer. Trends Genet. 39:74–88. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen Z, Zhong X, Xia M and Zhong J: The
roles and mechanisms of the m6A reader protein YTHDF1 in tumor
biology and human diseases. Mol Ther Nucleic Acids. 26:1270–1279.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao BS, Roundtree IA and He C:
Post-transcriptional gene regulation by mRNA modifications. Nat Rev
Mol Cell Biol. 18:31–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Machnicka MA, Milanowska K, Osman Oglou O,
Purta E, Kurkowska M, Olchowik A, Januszewski W, Kalinowski S,
Dunin-Horkawicz S, Rother KM, et al: MODOMICS: A database of RNA
modification pathways-2013 update. Nucleic Acids Res. 41((Database
Issue)): D262–D267. 2013.PubMed/NCBI
|
|
7
|
Desrosiers R, Friderici K and Rottman F:
Identification of methylated nucleosides in messenger RNA from
Novikoff hepatoma cells. Proc Natl Acad Sci USA. 71:3971–3975.
1974. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ke S, Alemu EA, Mertens C, Gantman EC, Fak
JJ, Mele A, Haripal B, Zucker-Scharff I, Moore MJ, Park CY, et al:
A majority of m6A residues are in the last exons, allowing the
potential for 3′ UTR regulation. Genes Dev. 29:2037–2053. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dominissini D, Moshitch-Moshkovitz S,
Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K,
Jacob-Hirsch J, Amariglio N, Kupiec M, et al: Topology of the human
and mouse m6A RNA methylomes revealed by m6A-seq. Nature.
485:201–206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu S, Li X, Liu S, Yang R, Liu X and Wu S:
N6-methyladenosine: A novel RNA imprint in human cancer.
Front Oncol. 9:14072019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen C, Yuan W, Zhou Q, Shao B, Guo Y,
Wang W, Yang S, Guo Y, Zhao L, Dang Q, et al:
N6-methyladenosine-induced circ1662 promotes metastasis of
colorectal cancer by accelerating YAP1 nuclear localization.
Theranostics. 11:4298–4315. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roignant JY and Soller M: m6A
in mRNA: An ancient mechanism for fine-tuning gene expression.
Trends Genet. 33:380–390. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nielsen J, Christiansen J, Lykke-Andersen
J, Johnsen AH, Wewer UM and Nielsen FC: A family of insulin-like
growth factor II mRNA-binding proteins represses translation in
late development. Mol Cell Biol. 19:1262–1270. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao J, Mu Q and Huang H: The roles of
insulin-like growth factor 2 mRNA-binding protein 2 in cancer and
cancer stem cells. Stem Cells Int. 2018:42172592018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cui XH, Hu SY, Zhu CF and Qin XH:
Expression and prognostic analyses of the insulin-like growth
factor 2 mRNA binding protein family in human pancreatic cancer.
BMC Cancer. 20:11602020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dai N, Rapley J, Angel M, Yanik MF, Blower
MD and Avruch J: mTOR phosphorylates IMP2 to promote IGF2 mRNA
translation by internal ribosomal entry. Genes Dev. 25:1159–1172.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Chen L and Qiang P: The role of
IGF2BP2, an m6A reader gene, in human metabolic diseases and
cancers. Cancer Cell Int. 21:992021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Li Y and Lu H: [ARTICLE WITHDRAWN]
miR-1193 suppresses proliferation and invasion of human breast
cancer cells through directly targeting IGF2BP2. Oncol Res.
25:579–585. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu XL, Lu RY, Wang LK, Wang YY, Dai YJ,
Wang CY, Yang YJ, Guo F, Xue J and Yang DD: Long noncoding RNA
HOTAIR silencing inhibits invasion and proliferation of human colon
cancer LoVo cells via regulating IGF2BP2. J Cell Biochem.
120:1221–1231. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nielsen J, Kristensen MA, Willemoës M,
Nielsen FC and Christiansen J: Sequential dimerization of human
zipcode-binding protein IMP1 on RNA: A cooperative mechanism
providing RNP stability. Nucleic Acids Res. 32:4368–4376. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li N, Deng L, Zhang Y, Tang X, Lei B and
Zhang Q: IGF2BP2 modulates autophagy and serves as a prognostic
marker in glioma. Ibrain. 10:19–33. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lv L, Zhang X, Liu Y, Zhu X, Pan R and
Huang L: Three liquid-liquid phase separation-related genes
associated with prognosis in glioma. Pharmgenomics Pers Med.
17:171–181. 2024.PubMed/NCBI
|
|
23
|
Hu Y, Chen J, Liu M, Feng Q and Peng H:
IGF2BP2 serves as a core m6A regulator in head and neck squamous
cell carcinoma. Biosci Rep. 42:BSR202213112022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deng X, Jiang Q, Liu Z and Chen W:
Clinical significance of an m6A reader gene, IGF2BP2, in head and
neck squamous cell carcinoma. Front Mol Biosci. 7:682020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang X, Tang Q, Li S, Li M and Yang T:
IGF2BP2 acts as a m6A modification regulator in
laryngeal squamous cell carcinoma through facilitating CDK6 mRNA
stabilization. Cell Death Discov. 9:3712023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin SH, Lin CW, Lu JW, Yang WE, Lin YM, Lu
HJ and Yang SF: Cytoplasmic IGF2BP2 protein expression in human
patients with oral squamous cell carcinoma: Prognostic and clinical
implications. Int J Med Sci. 19:1198–1204. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barghash A, Golob-Schwarzl N, Helms V,
Haybaeck J and Kessler SM: Elevated expression of the IGF2 mRNA
binding protein 2 (IGF2BP2/IMP2) is linked to short survival and
metastasis in esophageal adenocarcinoma. Oncotarget. 7:49743–49750.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu F, Chen W, Jiang T, Cheng C, Wang B, Lu
Z, Huang G, Qiu J, Wei W, Yang M and Huang X: Expression profile,
clinical significance and biological functions of IGF2BP2 in
esophageal squamous cell carcinoma. Exp Ther Med. 23:2522022.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deng H, Yao H, Zhou S, He C, Huang Y, Li
Y, Chen H and Shu J: Pancancer analysis uncovers an immunological
role and prognostic value of the m6A reader IGF2BP2 in pancreatic
cancer. Mol Cell Probes. 73:1019482024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dahlem C, Barghash A, Puchas P, Haybaeck J
and Kessler SM: The insulin-like growth factor 2 mRNA binding
protein IMP2/IGF2BP2 is overexpressed and correlates with poor
survival in pancreatic cancer. Int J Mol Sci. 20:32042019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang JY, Chan EK, Peng XX and Tan EM: A
novel cytoplasmic protein with RNA-binding motifs is an autoantigen
in human hepatocellular carcinoma. J Exp Med. 189:1101–1110. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu M, Nakamura RM, Dent ED, Zhang JY,
Nielsen FC, Christiansen J, Chan EK and Tan EM: Aberrant expression
of fetal RNA-binding protein p62 in liver cancer and liver
cirrhosis. Am J Pathol. 159:945–953. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen C, Xuan B, Yan T, Ma Y, Xu P, Tian X,
Zhang X, Cao Y, Ma D, Zhu X, et al: m6A-dependent
glycolysis enhances colorectal cancer progression. Mol Cancer.
19:722020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gong L, Liu Q, Jia M and Sun X: Systematic
analysis of IGF2BP family members in non-small-cell lung cancer.
Hum Genomics. 18:632024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jia M, Shi Y, Xie Y, Li W, Deng J, Fu D,
Bai J, Ma Y, Zuberi Z, Li J and Li Z: WT1-AS/IGF2BP2 axis is a
potential diagnostic and prognostic biomarker for lung
adenocarcinoma according to ceRNA network comprehensive analysis
combined with experiments. Cells. 11:252021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Almawi WY, Zidi S, Sghaier I, El-Ghali RM,
Daldoul A and Midlenko A: Novel association of IGF2BP2 gene
variants with altered risk of breast cancer and as potential
molecular biomarker of triple negative breast cancer. Clin Breast
Cancer. 23:272–280. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yuan J, Li X, Wang F, Liu H, Guan W and Xu
G: Insulin-like growth factor 2 mRNA-binding protein 2 is a
therapeutic target in ovarian cancer. Exp Biol Med (Maywood).
248:2198–2209. 2023.PubMed/NCBI
|
|
38
|
Yang L, Liu J, Jin Y, Xing J, Zhang J,
Chen X and Yu A: Synchronous profiling of mRNA N6-methyladenosine
modifications and mRNA expression in high-grade serous ovarian
cancer: A pilot study. Sci Rep. 14:104272024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
He X, Li W, Liang X, Zhu X, Zhang L, Huang
Y, Yu T, Li S and Chen Z: IGF2BP2 overexpression indicates poor
survival in patients with acute myelocytic leukemia. Cell Physiol
Biochem. 51:1945–1956. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Feng P, Chen D, Wang X, Li Y, Li Z, Li B,
Zhang Y, Li W, Zhang J, Ye J, et al: Inhibition of the
m6A reader IGF2BP2 as a strategy against T-cell acute
lymphoblastic leukemia. Leukemia. 36:2180–2188. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou W, Gao Q, He C, Wang L, Wang Y, Feng
L, Li W, Liu W, Ma R and Liu L: Association between polymorphism in
diabetes susceptibility gene insulin-like growth factor
2mRNA-binding protein 2 and risk of diffuse large B-cell lymphoma.
Clin Med Insights Oncol. 17:117955492312011282023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhao Y, Zhou Y, Qian Y, Wei W, Lin X, Mao
S, Sun J and Jin J: m6A-dependent upregulation of DDX21
by super-enhancer-driven IGF2BP2 and IGF2BP3 facilitates
progression of acute myeloid leukaemia. Clin Transl Med.
14:e16282024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Song T, Hu Z, Zeng C, Luo H and Liu J:
FLOT1, stabilized by WTAP/IGF2BP2 mediated N6-methyladenosine
modification, predicts poor prognosis and promotes growth and
invasion in gliomas. Heliyon. 9:e162802023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu D, Pan M, Li Y, Lu T, Wang Z, Liu C and
Hu G: RNA N6-methyladenosine reader IGF2BP2 promotes lymphatic
metastasis and epithelial-mesenchymal transition of head and neck
squamous carcinoma cells via stabilizing slug mRNA in an
m6A-dependent manner. J Exp Clin Cancer Res. 41:62022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liang J, Cai H, Hou C, Song F, Jiang Y,
Wang Z, Qiu D, Zhu Y, Wang F, Yu D and Hou J: METTL14 inhibits
malignant progression of oral squamous cell carcinoma by targeting
the autophagy-related gene RB1CC1 in an m6A-IGF2BP2-dependent
manner. Clin Sci (Lond). 137:1373–1389. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Leng F, Miu YY, Zhang Y, Luo H, Lu XL,
Cheng H and Zheng ZG: A micro-peptide encoded by HOXB-AS3 promotes
the proliferation and viability of oral squamous cell carcinoma
cell lines by directly binding with IGF2BP2 to stabilize c-Myc.
Oncol Lett. 22:6972021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xu L, Li Q, Wang Y, Wang L, Guo Y, Yang R,
Zhao N, Ge N, Wang Y and Guo C: m6A methyltransferase
METTL3 promotes oral squamous cell carcinoma progression through
enhancement of IGF2BP2-mediated SLC7A11 mRNA stability. Am J Cancer
Res. 11:5282–5298. 2021.PubMed/NCBI
|
|
49
|
Lin CW, Yang WE, Su CW, Lu HJ, Su SC and
Yang SF: IGF2BP2 promotes cell invasion and epithelial-mesenchymal
transition through Src-mediated upregulation of EREG in oral
cancer. Int J Biol Sci. 20:818–830. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dong L, Geng Z, Liu Z, Tao M, Pan M and Lu
X: IGF2BP2 knockdown suppresses thyroid cancer progression by
reducing the expression of long non-coding RNA HAGLR. Pathol Res
Pract. 225:1535502021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang W, Ding Y, Zhao Y and Li X: m6A
reader IGF2BP2 promotes lymphatic metastasis by stabilizing DPP4 in
papillary thyroid carcinoma. Cancer Gene Ther. 31:285–299. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zheng H, Cao Z, Lv Y and Cai X:
WTAP-mediated N6-methyladenine modification of circEEF2 promotes
lung adenocarcinoma tumorigenesis by stabilizing CANT1 in an
IGF2BP2-dependent manner. Mol Biotechnol. Apr 15–2024.(Epub ahead
of print). View Article : Google Scholar
|
|
53
|
Han L, Lei G, Chen Z, Zhang Y, Huang C and
Chen W: IGF2BP2 regulates MALAT1 by serving as an
N6-methyladenosine reader to promote NSCLC proliferation. Front Mol
Biosci. 8:7800892022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Huang GW, Chen QQ, Ma CC, Xie LH and Gu J:
linc01305 promotes metastasis and proliferation of esophageal
squamous cell carcinoma through interacting with IGF2BP2 and
IGF2BP3 to stabilize HTR3A mRNA. Int J Biochem Cell Biol.
136:1060152021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang C, Zhou M, Zhu P, Ju C, Sheng J, Du
D, Wan J, Yin H, Xing Y, Li H, et al: IGF2BP2-induced circRUNX1
facilitates the growth and metastasis of esophageal squamous cell
carcinoma through miR-449b-5p/FOXP3 axis. J Exp Clin Cancer Res.
41:3472022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhao R, Li T, Zhao X, Yang Z, Ma L and
Wang X: The m6A reader IGF2BP2 promotes the progression of
esophageal squamous cell carcinoma cells by increasing the
stability of OCT4 mRNA. Biochem Cell Biol. 102:169–178. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li Y, Xiao Z, Wang Y, Zhang D and Chen Z:
The m6A reader IGF2BP2 promotes esophageal cell carcinoma
progression by enhancing EIF4A1 translation. Cancer Cell Int.
24:1622024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang Z, Xing Y, Gao W, Yang L, Shi J,
Song W and Li T: N6-methyladenosine (m6A)
reader IGF2BP2 promotes gastric cancer progression via targeting
SIRT1. Bioengineered. 13:11541–11550. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shen H, Zhu H, Chen Y, Shen Z, Qiu W, Qian
C and Zhang J: ZEB1-induced LINC01559 expedites cell proliferation,
migration and EMT process in gastric cancer through recruiting
IGF2BP2 to stabilize ZEB1 expression. Cell Death Dis. 12:3492021.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ouyang J, Li J, Li D, Jiang J, Hao T, Xia
Y, Lu X, Zhang C and He Y: IGF2BP2 promotes epithelial to
mesenchymal transition and metastasis through stabilizing HMGA1
mRNA in gastric cancer. Cancers(Basel). 14:53812022.PubMed/NCBI
|
|
61
|
Liu D, Xia AD, Wu LP, Li S, Zhang K and
Chen D: IGF2BP2 promotes gastric cancer progression by regulating
the IGF1R-RhoA-ROCK signaling pathway. Cell Signal. 94:1103132022.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li T, Hu PS, Zuo Z, Lin JF, Li X, Wu QN,
Chen ZH, Zeng ZL, Wang F, Zheng J, et al: METTL3 facilitates tumor
progression via an m6A-IGF2BP2-dependent mechanism in
colorectal carcinoma. Mol Cancer. 18:1122019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yi J, Peng F, Zhao J and Gong X:
METTL3/IGF2BP2 axis affects the progression of colorectal cancer by
regulating m6A modification of STAG3. Sci Rep. 13:172922023.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cui J, Tian J, Wang W, He T, Li X, Gu C,
Wang L, Wu J and Shang A: IGF2BP2 promotes the progression of
colorectal cancer through a YAP-dependent mechanism. Cancer Sci.
112:4087–4099. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bian Y, Wang Y, Xu S, Gao Z, Li C, Fan Z,
Ding J and Wang K: m6A Modification of Long Non-Coding
RNA HNF1A-AS1 Facilitates Cell Cycle Progression in Colorectal
Cancer via IGF2BP2-Mediated CCND1 mRNA Stabilization. Cells.
11:30082022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hou P, Meng S, Li M, Lin T, Chu S, Li Z,
Zheng J, Gu Y and Bai J: Correction to: LINC00460/DHX9/IGF2BP2
complex promotes colorectal cancer proliferation and metastasis by
mediating HMGA1 mRNA stability depending on m6A modification. J Exp
Clin Cancer Res. 40:3652021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liu TY, Hu CC, Han CY, Mao SY, Zhang WX,
Xu YM, Sun YJ, Jiang DB, Zhang XY, Zhang JX, et al: IGF2BP2
promotes colorectal cancer progression by upregulating the
expression of TFRC and enhancing iron metabolism. Biol Direct.
18:192023. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ye S, Song W, Xu X, Zhao X and Yang L:
IGF2BP2 promotes colorectal cancer cell proliferation and survival
through interfering with RAF-1 degradation by miR-195. FEBS Lett.
590:1641–1650. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liu K, Wei C, Yu H, Zhang Q and Du Z:
HMGA2 overexpression activates IGF2BP2 to stabilize APLP2 via m6A
modification and promote pancreatic cancer progression. Heliyon.
10:e272682024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Cao P, Wu Y, Sun D, Zhang W, Qiu J, Tang
Z, Xue X and Qin L: IGF2BP2 promotes pancreatic carcinoma
progression by enhancing the stability of B3GNT6 mRNA via m6A
methylation. Cancer Med. 12:4405–4420. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Cai H, Zhao J, Zhang Q, Wu H, Sun Y, Guo
F, Zhou Y, Qin G, Xia W, Zhao Y, et al: Ubiquitin ligase TRIM15
promotes the progression of pancreatic cancer via the upregulation
of the IGF2BP2-TLR4 axis. Biochim Biophys Acta Mol Basis Dis.
1870:1671832024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Peng WX, Liu F, Jiang JH, Yuan H, Zhang Z,
Yang L and Mo YY: N6-methyladenosine modified LINC00901 promotes
pancreatic cancer progression through IGF2BP2/MYC axis. Genes Dis.
10:554–567. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lu J, Yu L, Xie N, Wu Y and Li B: METTL14
facilitates the metastasis of pancreatic carcinoma by stabilizing
LINC00941 in an m6A-IGF2BP2-dependent manner. J Cancer.
14:1117–1131. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Weng H, Feng W, Li F, Huang D, Lin L and
Wang Z: Transcription factor ETV1-induced lncRNA MAFG-AS1 promotes
migration, invasion, and epithelial-mesenchymal transition of
pancreatic cancer cells by recruiting IGF2BP2 to stabilize ETV1
expression. Growth Factors. 41:152–164. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhao C, Sun J, Dang Z, Su Q and Yang J:
Circ_0000775 promotes the migration, invasion and EMT of hepatic
carcinoma cells by recruiting IGF2BP2 to stabilize CDC27. Pathol
Res Pract. 235:1539082022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang X, Li Z, Nie H, Huang Y, Du J, Xi Y,
Guo C, Mu M, Li X, Zheng X, et al: The IGF2BP2-lncRNA TRPC7-AS1
axis promotes hepatocellular carcinoma cell proliferation and
invasion. Cell Signal. 117:1110782024. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kessler SM, Laggai S, Barghash A,
Schultheiss CS, Lederer E, Artl M, Helms V, Haybaeck J and Kiemer
AK: IMP2/p62 induces genomic instability and an aggressive
hepatocellular carcinoma phenotype. Cell Death Dis. 6:e18942015.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Pu J, Wang J, Qin Z, Wang A, Zhang Y, Wu
X, Wu Y, Li W, Xu Z, Lu Y, et al: IGF2BP2 promotes liver cancer
growth through an m6A-FEN1-dependent mechanism. Front Oncol.
10:5788162020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Guan XQ, Yuan XN, Feng KX, Shao YC, Liu Q,
Yang ZL, Chen YY, Deng J, Hu MS, Li J, et al: IGF2BP2-modified
UBE2D1 interacts with Smad2/3 to promote the progression of breast
cancer. Am J Cancer Res. 13:2948–2968. 2023.PubMed/NCBI
|
|
80
|
Xia T, Dai XY, Sang MY, Zhang X, Xu F, Wu
J, Shi L, Wei JF and Ding Q: IGF2BP2 drives cell cycle progression
in triple-negative breast cancer by recruiting EIF4A1 to promote
the m6A-modified CDK6 translation initiation process. Adv Sci
(Weinh). 11:e23051422024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Li Y, Francia G and Zhang JY: p62/IMP2
stimulates cell migration and reduces cell adhesion in breast
cancer. Oncotarget. 6:32656–32668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Shi Y, Xiong X, Sun Y, Geng Z, Chen X, Cui
X, Lv J, Ge L, Jia X and Xu J: IGF2BP2 promotes ovarian cancer
growth and metastasis by upregulating CKAP2L protein expression in
an m6 A-dependent manner. FASEB J. 37:e231832023.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Shi R, Zhao R, Shen Y, Wei S, Zhang T,
Zhang J, Shu W, Cheng S, Teng H and Wang H: IGF2BP2-modified
circular RNA circCHD7 promotes endometrial cancer progression via
stabilizing PDGFRB and activating JAK/STAT signaling pathway.
Cancer Gene Ther. 31:1221–1236. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ji F, Lu Y, Chen S, Yu Y, Lin X, Zhu Y and
Luo X: IGF2BP2-modified circular RNA circARHGAP12 promotes cervical
cancer progression by interacting m6A/FOXM1 manner. Cell Death
Discov. 7:2152021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Lang C, Yin C, Lin K, Li Y, Yang Q, Wu Z,
Du H, Ren D, Dai Y and Peng X: m6 A modification of
lncRNA PCAT6 promotes bone metastasis in prostate cancer through
IGF2BP2-mediated IGF1R mRNA stabilization. Clin Transl Med.
11:e4262021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
He P, Liu X, Yu G, Wang Y, Wang S, Liu J
and An Y: METTL3 facilitates prostate cancer progression via
inducing HOXC6 m6A modification and stabilizing its expression
through IGF2BP2-dependent mechanisms. Mol Cell Biochem.
479:1707–1720. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang X, Hu M, Yu L, Wang X, Jiang X, Zhang
G and Ding K: The ‘m6A writer’ METTL3 and the ‘m6A reader’ IGF2BP2
regulate cutaneous T-cell lymphomas progression via CDKN2A. Hematol
Oncol. 40:567–576. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Li J, Cao H, Yang J and Wang B: CircCDK1
blocking IGF2BP2-mediated m6A modification of CPPED1 promotes
laryngeal squamous cell carcinoma metastasis via the PI3K/AKT
signal pathway. Gene. 884:1476862023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Li J, Cao H, Yang J and Wang B:
IGF2BP2-m6A-circMMP9 axis recruits ETS1 to promote TRIM59
transcription in laryngeal squamous cell carcinoma. Sci Rep.
14:30142024. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yu D, Xiao Z, Zou Z, Lin L, Li J, Tan J
and Chen W: IGF2BP2 promotes head and neck squamous carcinoma cell
proliferation and growth via the miR-98-5p/PI3K/Akt signaling
pathway. Front Oncol. 13:12529992023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Xu X, Yu Y, Zong K, Lv P and Gu Y:
Up-regulation of IGF2BP2 by multiple mechanisms in pancreatic
cancer promotes cancer proliferation by activating the PI3K/Akt
signaling pathway. J Exp Clin Cancer Res. 38:4972019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Mu Q, Wang L, Yu F, Gao H, Lei T, Li P,
Liu P, Zheng X, Hu X, Chen Y, et al: Imp2 regulates GBM progression
by activating IGF2/PI3K/Akt pathway. Cancer Biol Ther. 16:623–633.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Latifkar A, Wang F, Mullmann JJ, Panizza
E, Fernandez IR, Ling L, Miller AD, Fischbach C, Weiss RS, Lin H,
et al: IGF2BP2 promotes cancer progression by degrading the RNA
transcript encoding a v-ATPase subunit. Proc Natl Acad Sci USA.
119:e22004771192022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kim HY, Ha Thi HT and Hong S: IMP2 and
IMP3 cooperate to promote the metastasis of triple-negative breast
cancer through destabilization of progesterone receptor. Cancer
Lett. 415:30–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Mao J, Qiu H and Guo L: LncRNA HCG11
mediated by METTL14 inhibits the growth of lung adenocarcinoma via
IGF2BP2/LATS1. Biochem Biophys Res Commun. 580:74–80. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Sun M, Zhang X, Bi F, Wang D, Zhou X, Li X
and Yang Q: FTO inhibits epithelial ovarian cancer progression by
destabilising SNAI1 mRNA through IGF2BP2. Cancers (Basel).
14:52182022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yan Y, Ma J, Chen Q, Zhang T, Fan R and Du
J: GAS5 regulated by FTO-mediated m6A modification suppresses cell
proliferation via the IGF2BP2/QKI axis in breast cancer. Discov
Oncol. 15:1822024. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ren J, Huang B, Li W, Wang Y, Pan X, Ma Q,
Liu Y, Wang X, Liang C, Zhang Y, et al: RNA-binding protein IGF2BP2
suppresses metastasis of clear cell renal cell carcinoma by
enhancing CKB mRNA stability and expression. Transl Oncol.
42:1019042024. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Pan X, Huang B, Ma Q, Ren J, Liu Y, Wang
C, Zhang D, Fu J, Ran L, Yu T, et al: Circular RNA circ-TNPO3
inhibits clear cell renal cell carcinoma metastasis by binding to
IGF2BP2 and destabilizing SERPINH1 mRNA. Clin Transl Med.
12:e9942022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wang G, Zhuang T, Zhen F, Zhang C, Wang Q,
Miao X, Qi N and Yao R: IGF2BP2 inhibits invasion and migration of
clear cell renal cell carcinoma via targeting Netrin-4 in an
m6A-dependent manner. Mol Carcinog. 63:1572–1587. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wu EY, Huang LP and Bao JH: miR-96-5p
regulates cervical cancer cell resistance to cisplatin by
inhibiting lncRNA TRIM52-AS1 and promoting IGF2BP2. Kaohsiung J Med
Sci. 38:1178–1189. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Fu L, Zhang D, Yi N, Cao Y, Wei Y, Wang W
and Li L: Circular RNA circPBX3 promotes cisplatin resistance of
ovarian cancer cells via interacting with IGF2BP2 to stabilize
ATP7A mRNA expression. Hum Cell. 35:1560–1576. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Xia C, Li Q, Cheng X, Wu T, Gao P and Gu
Y: Insulin-like growth factor 2 mRNA-binding protein 2-stabilized
long non-coding RNA Taurine up-regulated gene 1 (TUG1) promotes
cisplatin-resistance of colorectal cancer via modulating autophagy.
Bioengineered. 13:2450–2469. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Han J, Yu X, Wang S, Wang Y, Liu Q, Xu H
and Wang X: IGF2BP2 induces U251 glioblastoma cell chemoresistance
by inhibiting FOXO1-mediated PID1 expression through stabilizing
lncRNA DANCR. Front Cell Dev Biol. 9:6592282022. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhang M, Wang Q, Ke Z, Liu Y, Guo H, Fang
S and Lu K: LINC01001 promotes progression of crizotinib-resistant
NSCLC by modulating IGF2BP2/MYC axis. Front Pharmacol.
12:7592672021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wang J, Xu J and Zheng J: A1BG-AS1
promotes adriamycin resistance of breast cancer by recruiting
IGF2BP2 to upregulate ABCB1 in an m6A-dependent manner. Sci Rep.
13:207302023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Sa R, Liang R, Qiu X, He Z, Liu Z and Chen
L: IGF2BP2-dependent activation of ERBB2 signaling contributes to
acquired resistance to tyrosine kinase inhibitor in differentiation
therapy of radioiodine-refractory papillary thyroid cancer. Cancer
Lett. 527:10–23. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Wang X, Li X, Zhou Y, Huang X and Jiang X:
Long non-coding RNA OIP5-AS1 inhibition upregulates microRNA-129-5p
to repress resistance to temozolomide in glioblastoma cells via
downregulating IGF2BP2. Cell Biol Toxicol. 38:963–977. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Shi SJ, Han DH, Zhang JL, Li Y, Yang AG
and Zhang R: VIM-AS1 promotes proliferation and drives enzalutamide
resistance in prostate cancer via IGF2BP2-mediated HMGCS1 mRNA
stabilization. Int J Oncol. 62:342023. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Dong FL, Xu ZZ, Wang YQ, Li T, Wang X and
Li J: Exosome-derived circUPF2 enhances resistance to targeted
therapy by redeploying ferroptosis sensitivity in hepatocellular
carcinoma. J Nanobiotechnology. 22:2982024. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Kendzia S, Franke S, Kröhler T,
Golob-Schwarzl N, Schweiger C, Toeglhofer AM, Skofler C, Uranitsch
S, El-Heliebi A, Fuchs J, et al: A combined computational and
functional approach identifies IGF2BP2 as a driver of
chemoresistance in a wide array of pre-clinical models of
colorectal cancer. Mol Cancer. 22:892023. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Zhou Z, Zhang B, Deng Y, Deng S, Li J, Wei
W, Wang Y, Wang J, Feng Z, Che M, et al: FBW7/GSK3β mediated
degradation of IGF2BP2 inhibits IGF2BP2-SLC7A5 positive feedback
loop and radioresistance in lung cancer. J Exp Clin Cancer Res.
43:342024. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Hu P, Lin L, Huang T, Li Z, Xiao M, Guo H,
Chen G, Liu D, Ke M, Shan H, et al: Circular RNA circEYA3 promotes
the radiation resistance of hepatocellular carcinoma via the
IGF2BP2/DTX3L axis. Cancer Cell Int. 23:3082023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Kim J and DeBerardinis RJ: Mechanisms and
implications of metabolic heterogeneity in cancer. Cell Metab.
30:434–446. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Wu S, Chi C, Weng S, Zhou W and Liu Z:
IGF2BP2 promotes lncRNA DANCR stability mediated glycolysis and
affects the progression of FLT3-ITD + acute myeloid leukemia.
Apoptosis. 28:1035–1047. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhou J, Zhang H, Zhong K, Tao L, Lin Y,
Xie G, Tan Y, Wu Y, Lu Y, Chen Z, et al: N6-methyladenosine
facilitates mitochondrial fusion of colorectal cancer cells via
induction of GSH synthesis and stabilization of OPA1 mRNA. Natl Sci
Rev. 11:nwae0392024. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wang J, Zhu M, Zhu J, Li J, Zhu X, Wang K,
Shen K, Yang K, Ni X, Liu X, et al: HES1 promotes aerobic
glycolysis and cancer progression of colorectal cancer via
IGF2BP2-mediated GLUT1 m6A modification. Cell Death Discov.
9:4112023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Wang Y, Lu JH, Wu QN, Jin Y, Wang DS, Chen
YX, Liu J, Luo XJ, Meng Q, Pu HY, et al: LncRNA LINRIS stabilizes
IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer.
Mol Cancer. 18:1742019. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Yang K, Zhong Z, Zou J, Liao JY, Chen S,
Zhou S, Zhao Y, Li J, Yin D, Huang K and Li Y: Glycolysis and tumor
progression promoted by the m6A writer VIRMA via
m6A-dependent upregulation of STRA6 in pancreatic ductal
adenocarcinoma. Cancer Lett. 590:2168402024. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Hu C, Liu T, Han C, Xuan Y, Jiang D, Sun
Y, Zhang X, Zhang W, Xu Y, Liu Y, et al: HPV E6/E7 promotes aerobic
glycolysis in cervical cancer by regulating IGF2BP2 to stabilize
m6A-MYC expression. Int J Biol Sci. 18:507–521. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Jiang X, Guo S, Wang S, Zhang Y, Chen H,
Wang Y, Liu R, Niu Y and Xu Y: EIF4A3-induced circARHGAP29 promotes
aerobic glycolysis in docetaxel-resistant prostate cancer through
IGF2BP2/c-Myc/LDHA signaling. Cancer Res. 82:831–845. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Lu S, Han L, Hu X, Sun T, Xu D, Li Y, Chen
Q, Yao W, He M, Wang Z, et al: N6-methyladenosine reader IMP2
stabilizes the ZFAS1/OLA1 axis and activates the Warburg effect:
Implication in colorectal cancer. J Hematol Oncol. 14:1882021.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Qiu X, Xu Q, Liao B, Hu S, Zhou Y and
Zhang H: Circ-CCS regulates oxaliplatin resistance via targeting
miR-874-3p/HK2 axis in colorectal cancer. Histol Histopathol.
38:1145–1156. 2023.PubMed/NCBI
|
|
124
|
Wang Z, Wang MM, Geng Y, Ye CY and Zang
YS: Membrane-associated RING-CH protein (MARCH8) is a novel
glycolysis repressor targeted by miR-32 in colorectal cancer. J
Transl Med. 20:4022022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Xu K, Dai X, Wu J and Wen K:
N6-methyladenosine (m6A) reader IGF2BP2
stabilizes HK2 stability to accelerate the Warburg effect of oral
squamous cell carcinoma progression. J Cancer Res Clin Oncol.
148:3375–3384. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Liu H, Qin S, Liu C, Jiang L, Li C, Yang
J, Zhang S, Yan Z, Liu X, Yang J and Sun X: m6A reader
IGF2BP2-stabilized CASC9 accelerates glioblastoma aerobic
glycolysis by enhancing HK2 mRNA stability. Cell Death Discov.
7:2922021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ye M, Chen J, Lu F, Zhao M, Wu S, Hu C, Yu
P, Kan J, Bai J, Tian Y and Tang Q: Down-regulated FTO and ALKBH5
co-operatively activates FOXO signaling through m6A methylation
modification in HK2 mRNA mediated by IGF2BP2 to enhance glycolysis
in colorectal cancer. Cell Biosci. 13:1482023. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Chen J, Ye M, Bai J, Gong Z, Yan L, Gu D,
Hu C, Lu F, Yu P, Xu L, et al: ALKBH5 enhances lipid metabolism
reprogramming by increasing stability of FABP5 to promote
pancreatic neuroendocrine neoplasms progression in an
m6A-IGF2BP2-dependent manner. J Transl Med. 21:7412023. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Chandra J, Hansen M, Labarriere N, Marigo
I, Souza-Fonseca-Guimaraes F, Vujanovic L, Koguchi Y and Jacquelot
N: Editorial: Cancer immunotherapies: From efficacy to resistance
mechanisms. Front Immunol. 13:9397892022. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Elcheva IA, Gowda CP, Bogush D,
Gornostaeva S, Fakhardo A, Sheth N, Kokolus KM, Sharma A, Dovat S,
Uzun Y, et al: IGF2BP family of RNA-binding proteins regulate
innate and adaptive immune responses in cancer cells and tumor
microenvironment. Front Immunol. 14:12245162023. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Liu T, Han C, Hu C, Mao S, Sun Y, Yang S
and Yang K: Knockdown of IGF2BP2 inhibits colorectal cancer cell
proliferation, migration and promotes tumor immunity by
down-regulating MYC expression. Xi Bao Yu Fen Zi Mian Yi Xue Za
Zhi. 39:303–310. 2023.(In Chinese). PubMed/NCBI
|
|
132
|
Liu QZ, Zhang N, Chen JY, Zhou MJ, Zhou
DH, Chen Z, Huang ZX, Xie YX, Qiao GL and Tu XH: WTAP-induced
N6-methyladenosine of PD-L1 blocked T-cell-mediated
antitumor activity under hypoxia in colorectal cancer. Cancer Sci.
115:1749–1762. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Liu Y, Shi M, He X, Cao Y, Liu P, Li F,
Zou S, Wen C, Zhan Q, Xu Z, et al: LncRNA-PACERR induces pro-tumour
macrophages via interacting with miR-671-3p and m6A-reader IGF2BP2
in pancreatic ductal adenocarcinoma. J Hematol Oncol. 15:522022.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Li S, Wu Q, Liu J and Zhong Y:
Identification of two m6A Readers YTHDF1 and IGF2BP2 as immune
biomarkers in head and neck squamous cell carcinoma. Front Genet.
13:9036342022. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zhou L, Li H, Cai H, Liu W, Pan E, Yu D
and He S: Upregulation of IGF2BP2 promotes oral squamous cell
carcinoma progression that is related to cell proliferation,
metastasis and tumor-infiltrating immune cells. Front Oncol.
12:8095892022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Zhu L, Liu Y, Tang H and Wang P: FOXP3
activated-LINC01232 accelerates the stemness of non-small cell lung
carcinoma by activating TGF-β signaling pathway and recruiting
IGF2BP2 to stabilize TGFBR1. Exp Cell Res. 413:1130242022.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Huang C, Xu R, Zhu X and Jiang H:
m6A-modified circABCC4 promotes stemness and metastasis of prostate
cancer by recruiting IGF2BP2 to increase stability of CCAR1. Cancer
Gene Ther. 30:1426–1440. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Ji R, Wu C, Yao J, Xu J, Lin J, Gu H, Fu M
and Zhang X, Li Y and Zhang X: IGF2BP2-meidated m6A
modification of CSF2 reprograms MSC to promote gastric cancer
progression. Cell Death Dis. 14:6932023. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Janiszewska M, Suvà ML, Riggi N,
Houtkooper RH, Auwerx J, Clément-Schatlo V, Radovanovic I, Rheinbay
E, Provero P and Stamenkovic I: Imp2 controls oxidative
phosphorylation and is crucial for preserving glioblastoma cancer
stem cells. Genes Dev. 26:1926–1944. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Degrauwe N, Schlumpf TB, Janiszewska M,
Martin P, Cauderay A, Provero P, Riggi N, Suvà ML, Paro R and
Stamenkovic I: The RNA binding protein IMP2 preserves glioblastoma
stem cells by preventing let-7 target gene silencing. Cell Rep.
15:1634–1647. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Cui Y, Wen Y, Lv C, Zhao D, Yang Y, Qiu H
and Wang C: Decreased RNA-binding protein IGF2BP2 downregulates
NT5DC2, which suppresses cell proliferation, and induces cell cycle
arrest and apoptosis in diffuse large B-cell lymphoma cells by
regulating the p53 signaling pathway. Mol Med Rep. 26:2862022.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Ye J, Wu Y, Chen Y, Ren Y, Jiang X, Dong
Z, Zhang J, Jin M, Chen X, Wang Z and Xiao M: ALKBH5 promotes
hypopharyngeal squamous cell carcinoma apoptosis by targeting TLR2
in a YTHDF1/IGF2BP2-mediated manner. Cell Death Discov. 9:3082023.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Yang X and Liu J: Targeting PD-L1
(Programmed death-ligand 1) and inhibiting the expression of
IGF2BP2 (Insulin-like growth factor 2 mRNA-binding protein 2)
affect the proliferation and apoptosis of hypopharyngeal carcinoma
cells. Bioengineered. 12:7755–7764. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Kessler SM, Pokorny J, Zimmer V, Laggai S,
Lammert F, Bohle RM and Kiemer AK: IGF2 mRNA binding protein
p62/IMP2-2 in hepatocellular carcinoma: Antiapoptotic action is
independent of IGF2/PI3K signaling. Am J Physiol Gastrointest Liver
Physiol. 304:G328–G336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Zheng X, Wu J, Song L and Huang B: ACSM3
suppresses proliferation and induces apoptosis and cell cycle
arrest in acute myeloid leukemia cells via the regulation of
IGF2BP2. Exp Ther Med. 25:1772023. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Dixon SJ: Ferroptosis: Bug or feature?
Immunol Rev. 277:150–157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11:882020. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Yang R, Wan J, Ma L, Zhou F, Yang Z, Li Z,
Zhang M and Ming L: TMEM44-AS1 promotes esophageal squamous cell
carcinoma progression by regulating the IGF2BP2-GPX4 axis in
modulating ferroptosis. Cell Death Discov. 9:4312023. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Ye J, Chen X, Jiang X, Dong Z, Hu S and
Xiao M: RNA demethylase ALKBH5 regulates hypopharyngeal squamous
cell carcinoma ferroptosis by posttranscriptionally activating
NFE2L2/NRF2 in an m6 A-IGF2BP2-dependent manner. J Clin
Lab Anal. 36:e245142022. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Bian Y, Xu S, Gao Z, Ding J, Li C, Cui Z,
Sun H, Li J, Pu J and Wang K: m6A modification of lncRNA
ABHD11-AS1 promotes colorectal cancer progression and inhibits
ferroptosis through TRIM21/IGF2BP2/FOXM1 positive feedback loop.
Cancer Lett. 596:2170042024. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Jung YD, Ahmad SA, Liu W, Reinmuth N,
Parikh A, Stoeltzing O, Fan F and Ellis LM: The role of the
microenvironment and intercellular cross-talk in tumor
angiogenesis. Semin Cancer Biol. 12:105–112. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Wang Y, Sun H, Zhang D, Fan D, Zhang Y,
Dong X, Liu S, Yang Z, Ni C, Li Y, et al: TP53INP1 inhibits
hypoxia-induced vasculogenic mimicry formation via the ROS/snail
signalling axis in breast cancer. J Cell Mol Med. 22:3475–3488.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Ma YS, Shi BW, Guo JH, Liu JB, Yang XL,
Xin R, Shi Y, Zhang DD, Lu GX, Jia CY, et al: microRNA-320b
suppresses HNF4G and IGF2BP2 expression to inhibit angiogenesis and
tumor growth of lung cancer. Carcinogenesis. 42:762–771. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Fang H, Sun Q, Zhou J, Zhang H, Song Q,
Zhang H, Yu G, Guo Y, Huang C, Mou Y, et al: m6A
methylation reader IGF2BP2 activates endothelial cells to promote
angiogenesis and metastasis of lung adenocarcinoma. Mol Cancer.
22:992023. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
He Z, Zhong Y, Regmi P, Lv T, Ma W, Wang
J, Liu F, Yang S, Zhong Y, Zhou R, et al: Exosomal long non-coding
RNA TRPM2-AS promotes angiogenesis in gallbladder cancer through
interacting with PABPC1 to activate NOTCH1 signaling pathway. Mol
Cancer. 23:652024. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Xu S, Bai J, Zhuan Z, Li B, Zhang Z, Wu X,
Luo X and Yang L: EBV-LMP1 is involved in vasculogenic mimicry
formation via VEGFA/VEGFR1 signaling in nasopharyngeal carcinoma.
Oncol Rep. 40:377–384. 2018.PubMed/NCBI
|
|
159
|
Yang Z, Sun B, Li Y, Zhao X, Zhao X, Gu Q,
An J, Dong X, Liu F and Wang Y: ZEB2 promotes vasculogenic mimicry
by TGF-β1 induced epithelial-to-mesenchymal transition in
hepatocellular carcinoma. Exp Mol Pathol. 98:352–359. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Taddei ML, Parri M, Angelucci A, Bianchini
F, Marconi C, Giannoni E, Raugei G, Bologna M, Calorini L and
Chiarugi P: EphA2 induces metastatic growth regulating amoeboid
motility and clonogenic potential in prostate carcinoma cells. Mol
Cancer Res. 9:149–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Yao X, Ping Y, Liu Y, Chen K, Yoshimura T,
Liu M, Gong W, Chen C, Niu Q, Guo D, et al: Vascular endothelial
growth factor receptor 2 (VEGFR-2) plays a key role in vasculogenic
mimicry formation, neovascularization and tumor initiation by
glioma stem-like cells. PLoS One. 8:e571882013. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Liu X, He H, Zhang F, Hu X, Bi F, Li K, Yu
H, Zhao Y, Teng X, Li J, et al: m6A methylated EphA2 and VEGFA
through IGF2BP2/3 regulation promotes vasculogenic mimicry in
colorectal cancer via PI3K/AKT and ERK1/2 signaling. Cell Death
Dis. 13:4832022. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Li H, Wang D, Yi B, Cai H, Wang Y, Lou X,
Xi Z and Li Z: SUMOylation of IGF2BP2 promotes vasculogenic mimicry
of glioma via regulating OIP5-AS1/miR-495-3p axis. Int J Biol Sci.
17:2912–2930. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Sa R, Liang R, Qiu X, He Z, Liu Z and Chen
L: Targeting IGF2BP2 promotes differentiation of radioiodine
refractory papillary thyroid cancer via destabilizing RUNX2 mRNA.
Cancers (Basel). 14:12682022. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Sa R, Guo M, Liu D and Guan F: AhR
antagonist promotes differentiation of papillary thyroid cancer via
regulating circSH2B3/miR-4640-5P/IGF2BP2 axis. Front Pharmacol.
12:7953862021. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Dahlem C, Abuhaliema A, Kessler SM,
Kröhler T, Zoller BGE, Chanda S, Wu Y, Both S, Müller F, Lepikhov
K, et al: First small-molecule inhibitors targeting the RNA-binding
protein IGF2BP2/IMP2 for cancer therapy. ACS Chem Biol. 17:361–375.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Weng H, Huang F, Yu Z, Chen Z, Prince E,
Kang Y, Zhou K, Li W, Hu J, Fu C, et al: The m6A reader
IGF2BP2 regulates glutamine metabolism and represents a therapeutic
target in acute myeloid leukemia. Cancer Cell. 40:1566–1582.e10.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Chanda S, Lepikhov K, Dahlem C, Schymik
HS, Hoppstädter J, Geber AK, Wagner K, Kessler SM, Empting M and
Kiemer AK: Gene editing and small molecule inhibitors of the RNA
binding protein IGF2BP2/IMP2 show its potential as an anti-cancer
drug target. Front Biosci (Landmark Ed). 29:412024. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Winkle M, El-Daly SM, Fabbri M and Calin
GA: Noncoding RNA therapeutics-challenges and potential solutions.
Nat Rev Drug Discov. 20:629–651. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Toden S, Zumwalt TJ and Goel A: Non-coding
RNAs and potential therapeutic targeting in cancer. Biochim Biophys
Acta Rev Cancer. 1875:1884912021. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Ye M, Dong S, Hou H, Zhang T and Shen M:
Oncogenic role of long noncoding RNAMALAT1 in thyroid cancer
progression through regulation of the miR-204/IGF2BP2/m6A-MYC
signaling. Mol Ther Nucleic Acids. 23:1–12. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Liu FY, Zhou SJ, Deng YL, Zhang ZY, Zhang
EL, Wu ZB, Huang ZY and Chen XP: MiR-216b is involved in
pathogenesis and progression of hepatocellular carcinoma through
HBx-miR-216b-IGF2BP2 signaling pathway. Cell Death Dis.
6:e16702015. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Xiao Y, Tang J, Yang D, Zhang B, Wu J, Wu
Z, Liao Q, Wang H, Wang W and Su M: Long noncoding RNA LIPH-4
promotes esophageal squamous cell carcinoma progression by
regulating the miR-216b/IGF2BP2 axis. Biomark Res. 10:602022.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Wu X, Fan Y, Liu Y, Shen B, Lu H and Ma H:
Long non-coding RNA CCAT2 promotes the development of esophageal
squamous cell carcinoma by inhibiting miR-200b to upregulate the
IGF2BP2/TK1 axis. Front Oncol. 11:6806422021. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Fen H, Hongmin Z, Wei W, Chao Y, Yang Y,
Bei L and Zhihua S: RHPN1-AS1 drives the progression of
hepatocellular carcinoma via regulating miR-596/IGF2BP2 axis. Curr
Pharm Des. 25:4630–4640. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Zhu X, Yu H, Li H, Zhang W, Sun L, Dou T,
Wang Z, Zhao H and Yang H: lncRNA SNHG1 promotes the progression of
hepatocellular carcinoma by regulating the miR-7-5p/IGF2BP2 axis.
Heliyon. 10:e276312024. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Yao B, Zhang Q, Yang Z, An F, Nie H, Wang
H, Yang C, Sun J, Chen K, Zhou J, et al: CircEZH2/miR-133b/IGF2BP2
aggravates colorectal cancer progression via enhancing the
stability of m6A-modified CREB1 mRNA. Mol Cancer.
21:1402022. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Yang Y, Liu X, Cheng L, Li L, Wei Z, Wang
Z, Han G, Wan X, Wang Z, Zhang J and Chen C: Tumor suppressor
microRNA-138 suppresses low-grade glioma development and metastasis
via regulating IGF2BP2. Onco Targets Ther. 13:2247–2260. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Ding L, Wang L and Guo F: microRNA-188
acts as a tumour suppressor in glioma by directly targeting the
IGF2BP2 gene. Mol Med Rep. 16:7124–7130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Huang RS, Zheng YL, Li C, Ding C, Xu C and
Zhao J: MicroRNA-485-5p suppresses growth and metastasis in
non-small cell lung cancer cells by targeting IGF2BP2. Life Sci.
199:104–111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Liao S, Sun H and Xu C: YTH domain: A
family of N6-methyladenosine (m6A) readers.
Genomics Proteomics Bioinformatics. 16:99–107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Wang Q, Geng W, Guo H, Wang Z, Xu K, Chen
C and Wang S: Emerging role of RNA methyltransferase METTL3 in
gastrointestinal cancer. J Hematol Oncol. 13:572020. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Jumper J, Evans R, Pritzel A, Green T,
Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A,
Potapenko A, et al: Highly accurate protein structure prediction
with AlphaFold. Nature. 596:583–589. 2021. View Article : Google Scholar : PubMed/NCBI
|